Abstract
Cubital tunnel syndrome is the second most common peripheral mononeuropathy in the upper extremity. However, the diagnosis and treatment of cubital tunnel syndrome remains controversial without a standard algorithm. Although diagnosis can often be made from the patient’s history and physical examination alone, electrodiagnostic studies, ultrasound, computed tomography (CT), and magnetic resonance image (MRI) can also be useful in diagnosing the disease and selecting the most appropriate treatment option. Treatment options include conservative nonoperative techniques as well as various surgical options, including in situ decompression with or without transposition, medial epicondylectomy, and nerve transfer in advanced disease. The purpose of this review is to summarize the most up-to-date literature regarding cubital tunnel syndrome and propose a treatment algorithm to provide clarity about the challenges of treating this complex patient population.
Key words: Electrodiagnostic testing, Cubital tunnel syndrome, Nerve compression syndrome, Nerve transfer, Ulnar nerve
Cubital tunnel syndrome (CuTS) is the second most common peripheral mononeuropathy in the upper extremity,1 with an estimated prevalence of 1.8% in the US population.2 Despite this relatively high occurrence, modern diagnosis and treatment of CuTS remains controversial. This is in part because of the heterogeneity of patient clinical presentation and disease severity across different age groups,3 as well as a lack of standardized diagnostic tools and treatment algorithm. This has led to significant variation in practice patterns both in the United States4,5 and abroad.6
Controversy regarding the diagnosis and treatment of CuTS is abundant: more than 500 articles on CuTS have been published in the past decade. This research has led to an improvement in our understanding of the disease and has spurred the development of several diagnostic and treatment options. However, the impact of these developments on the modern treatment of CuTS remains relatively unknown. Therefore, the purpose of this review is to discuss the diagnosis and treatment of CuTS, incorporate emerging and recent evidence, and highlight controversial topics that confound current clinical practice.
Anatomy
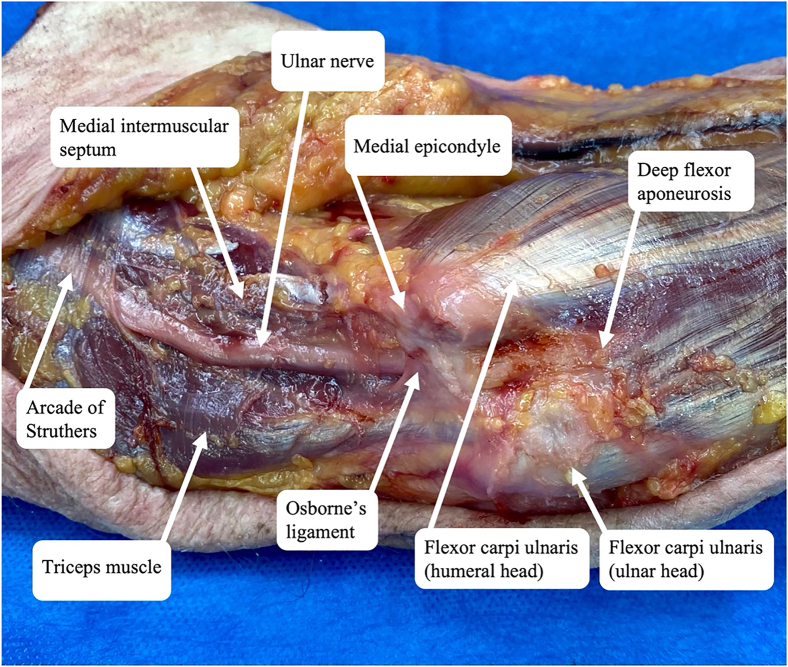
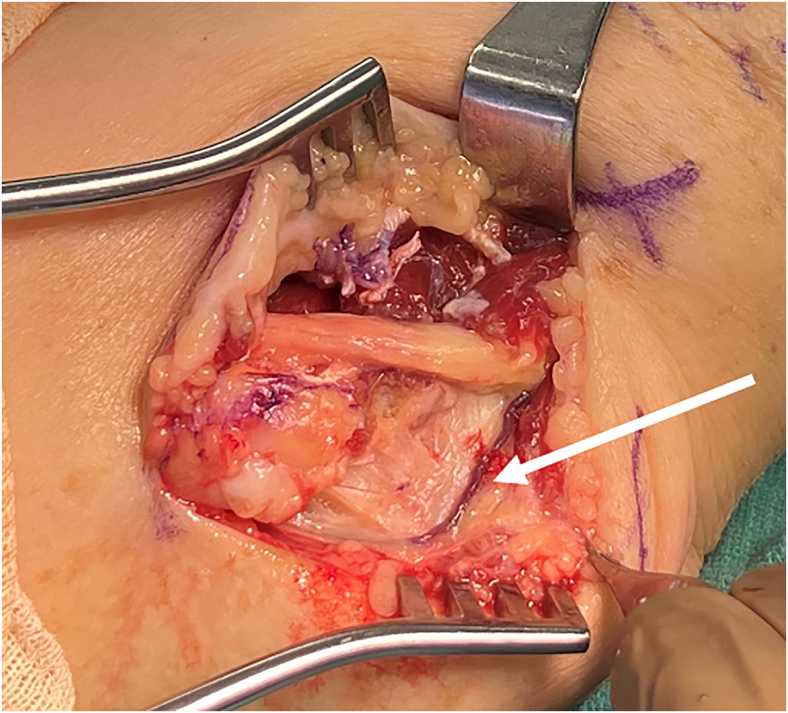
Ulnar nerve compression about the elbow, commonly referred to as CuTS, is caused by compression, traction, or friction of the ulnar nerve as it courses behind the elbow. The nerve provides motor innervation to the forearm and hand as well as sensory innervation to the hand. Previously described points of compression (from proximal to distal to the elbow) include the medial intermuscular septum, arcade of Struthers, medial epicondyle, Osborne’s ligament, flexor carpi ulnaris (FCU) fascia, and flexor-pronator aponeurosis (Fig. 1).1 An anomalous anconeus epitrochlearis muscle is present in as much as 20% of the population and has also been implicated in some studies as a point of compression associated with CuTS (Fig. 2).7 Although most patients with anconeus epitrochlearis muscle do not have CuTS,8 faster and more reliable symptom improvement has been reported after surgical release than in those without the anomalous muscle.9
Figure 1.
Points of ulnar nerve compression in CuTS.
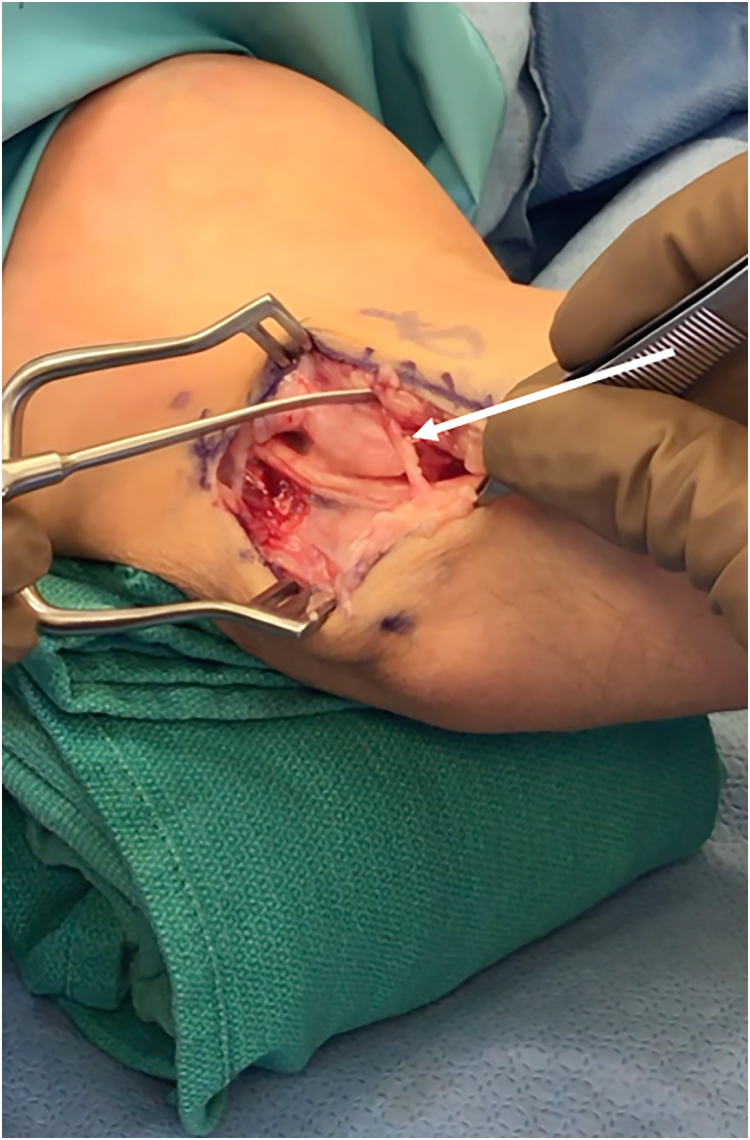
Figure 2.
Intraoperative photograph of right elbow depicting an anomalous anconeus epitrochlearis (arrow) present in a patient with CuTS.
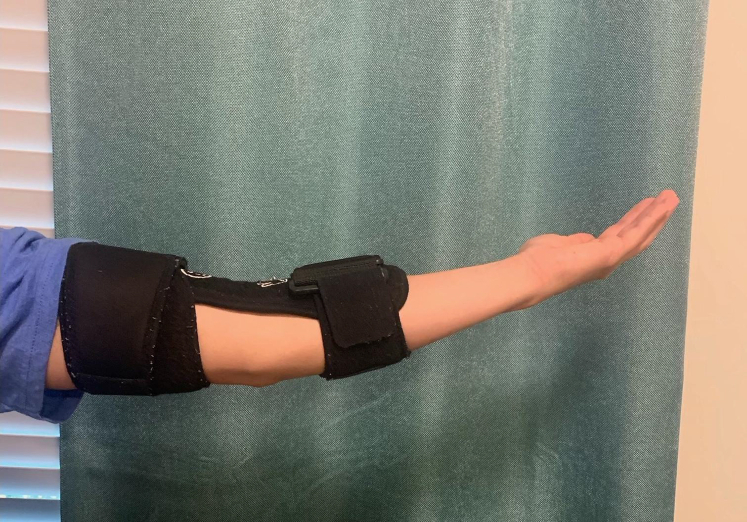
In addition to static sites of compression, previous studies have also shown dynamic compression of the ulnar nerve with elbow motion.10,11 In 1998, Gelberman et al10 found a 30% to 41% reduction in cubital tunnel volume as the elbow was moved from full extension to 135° of flexion with a corresponding 7-fold increase in intraneural pressure. Furthermore, their study showed minimum intraneural pressures within the cubital tunnel at 30°–60° of flexion. This report established the basis of extension splinting for conservative treatment for CuTS (Fig. 3). Subsequent studies also demonstrated that an ulnar nerve excursion of 21.9 and 23.2 mm was required at the elbow and wrist for normal upper extremity motion, respectively. In addition, an ulnar nerve strain of 15% or greater can occur during elbow flexion and wrist extension and radial deviation.12
Figure 3.
Elbow extension splinting.
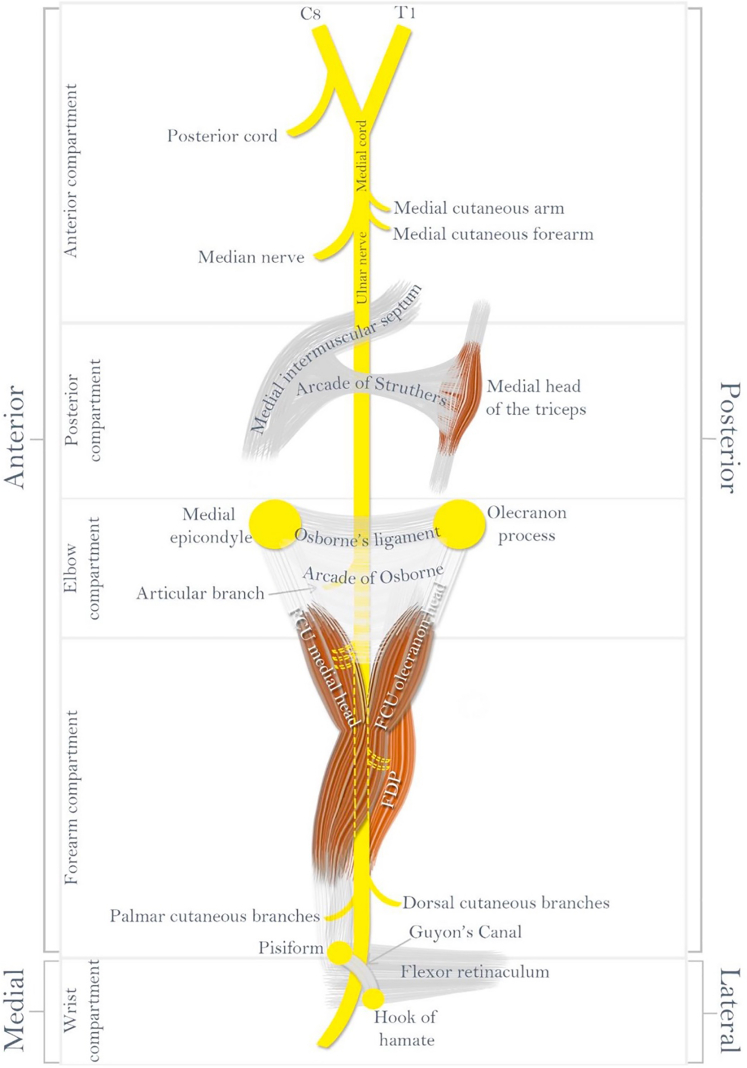
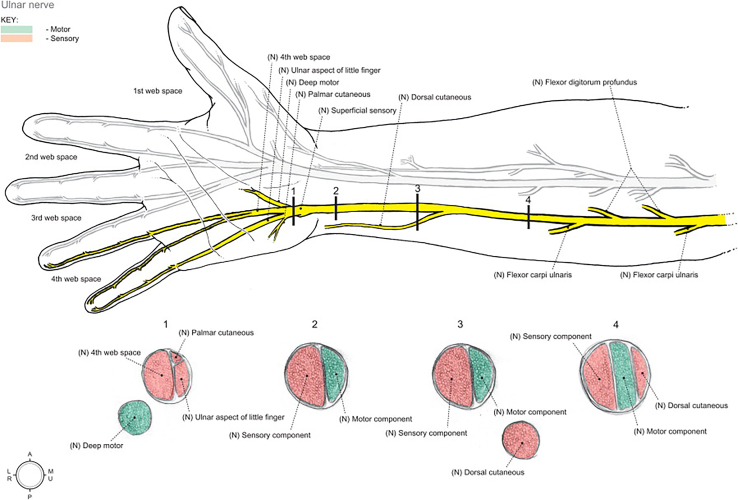
Ulnar nerve branching is highly variable in number and level.13 At the level of the medial epicondyle, 1–2 posterior articular branches are typically present, followed by 2–3 distal motor branches (Fig. 4).14 The most proximal motor branch typically arises 1.6 cm distal to the epicondyle and innervates the FCU alone, whereas a second motor branch typically arises 3.6 cm on average to the medial epicondyle and innervates the FCU, flexor digitorum profundus, or both.13 Intrafascicular dissection of these nerve branches has been shown to facilitate increased nerve excursion anteriorly during transposition.13 At the cubital tunnel, 3 main intrafascicular groups are present, which are divided into distinct patterns as they travel down the forearm to innervate the hand. The location of these groups within the nerve is often referred to as the “intraneural topography,” which may suggest as to why certain signs/symptoms prelude others. In the proximal forearm, the following intrafascicular groups can be identified: the dorsal cutaneous (ulnar), motor (central), and sensory (radial) nerves (Fig. 5).15 This topography is crucial when performing a supercharged nerve transfer during advanced surgery for CuTS.15
Figure 4.
Ulnar nerve branching patterns at the elbow and wrist. Used with permission from Andrews et al.22
Figure 5.
Topography of the ulnar nerve in the forearm and hand from Moore et al.126 Used with permission from Elsevier.
As the ulnar nerve enters the hand, it passes through another possible point of compression: Guyon’s canal. The boundaries of Guyon’s canal include the volar carpal ligament volar and the transverse carpal ligament dorsal. The pisiform, pisohamate ligament, and abductor digiti minimi form the border medially and the hook of the hamate laterally.16 The dorsal branch of the ulnar cutaneous nerve arises about 5 cm proximally to Guyon’s canal to provide sensation to the medial dorsal hand. This anatomy can be important in distinguishing between distal compression at Guyon’s canal and proximal compression in the case of CuTS.
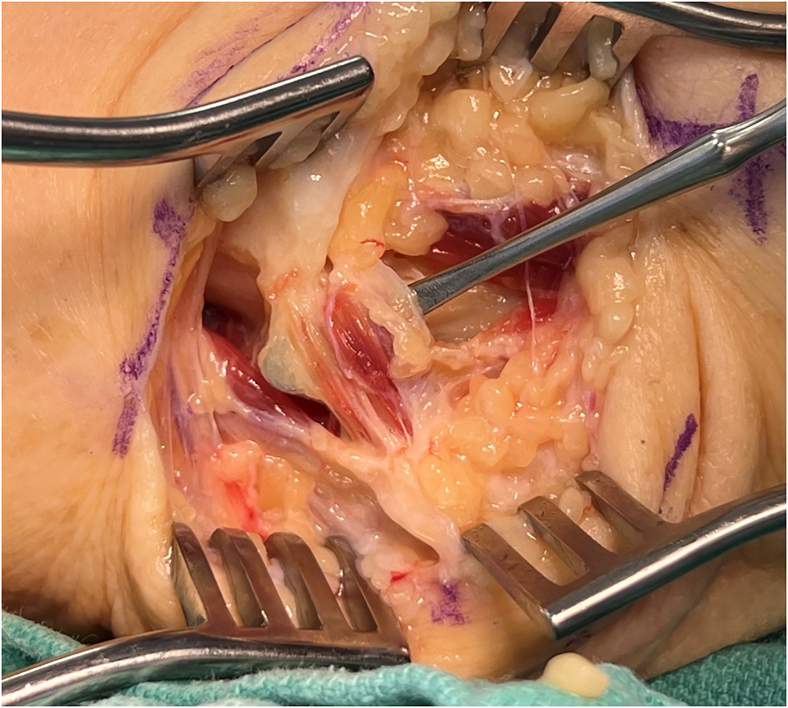
Three main arteries contribute to the blood supply of the ulnar nerve at the elbow: the superior ulnar collateral artery, the inferior ulnar collateral artery, and the posterior ulnar recurrent artery.17 When possible, these branches should be preserved during decompression and/or transposition (Fig. 6). Previous studies have reported that the ulnar nerve can be transposed up to 2.5 cm anteriorly without significantly disrupting its vascular supply.17 Disruption of vascular supply has been shown to cause segmental nerve ischemia,18 which in animal models have led to decreased nerve conduction.19
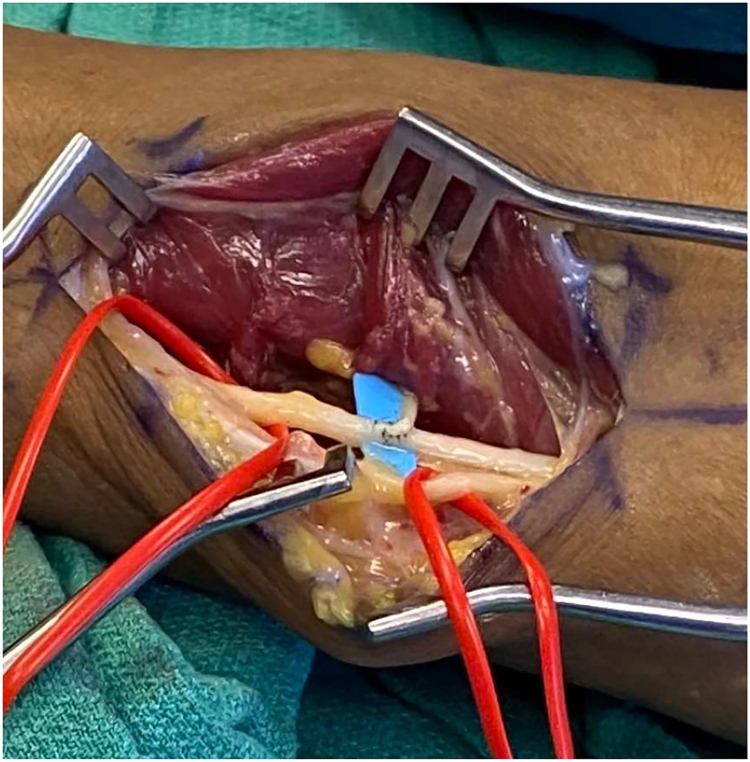
Figure 6.
Intraoperative photograph of the right elbow depicting preservation of blood supply during anterior transposition of the nerve for treatment of CuTS. The arrow indicates the intact posterior recurrent ulnar artery.
Diagnosis
Patients with CuTS often delay seeking treatment and are more likely to present with advanced disease.20 Specific risk factors associated with advanced disease at presentation include older patients, patients with higher body mass index, diabetes mellitus, and economic distress.21 White females in their fourth and fifth decade of life comprise the most common patient demographic associated with carpal tunnel syndrome.2 The most common signs and symptoms of CuTS are intermittent numbness and tingling in the ulnar ring and small fingers. Symptoms can be exacerbated by activities requiring elbow flexion, such as talking on the phone.22 Additional signs/symptoms may include hand weakness, atrophy, diminished dexterity, and pain of the medial elbow, forearm, or wrist.1,23 Consideration of differential diagnoses such as cervical radiculopathy, Pancoast tumor, thoracic outlet syndrome, medial epicondylitis, elbow arthritis, FCU tendinitis, ulnar tunnel syndrome, and hypothenar hammer syndrome must be assessed during the history and physical examination (Table 1).
Table 1.
Differential Diagnosis of CuTS
| Differential Diagnosis for CuTS | Special Considerations |
|---|---|
| Cervical radiculopathy |
|
| Pancoast tumor |
|
| Thoracic outlet syndrome |
|
| Medial epicondylitis |
|
| Elbow arthritis |
|
| FCU tendinitis |
|
| Ulnar tunnel syndrome |
|
| Hypothenar hammer syndrome |
|
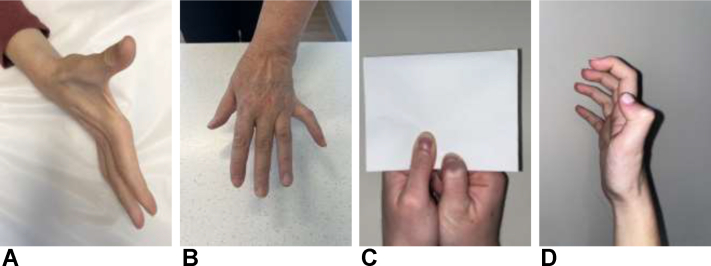
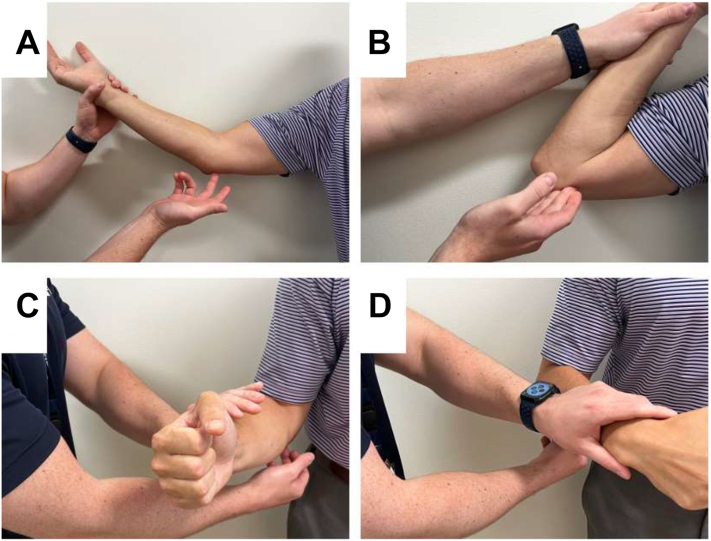
Common physical examination findings associated with CuTS include impaired sensation in ulnar nerve distribution, muscular atrophy of first dorsal interosseus muscle, as well as Wartenberg, Froment, and Jeanne signs (Fig. 7).1 Clawing of the fourth and fifth fingers is a result of weakened intrinsics (third and fourth lumbricals) being overpowered by the flexor digitorum profundus tendons. This is considered a late finding owing to the axonal loss of the intrinsics of the hand.24 Provocative maneuvers that may be positive in patients with CuTS include Tinel sign test with percussion at the retrocondylar groove, as well as elbow flexion and flexion compression tests (Fig. 8).1 Novak et al25 previously reported higher sensitivity of elbow flexion compression test (91%) compared with Tinel sign test (70%) and elbow flexion test (32%) at 30 seconds. More recently, the glenohumeral internal rotation test has been shown to demonstrate positive findings in less than 5 seconds and is 87% sensitive and 98% specific.26,27 The scratch collapse test can also be performed and has been shown to have a sensitivity of 69% (Fig. 8).28 However, recently, the sensitivity and interrater reliability of the scratch collapse test have been questioned.29
Figure 7.
Physical examination findings and signs in patients with CuTS. A Ulnar claw hand with atrophy of the FDI. B Wartenberg sign. C Froment sign. D Jeanne sign.
Figure 8.
Provocative physical examination maneuvers that are useful in the diagnosis of CuTS. A Tinel sign test. B Flexion compression test. C Scratch collapse test before collapse. D Scratch collapse test after collapse.
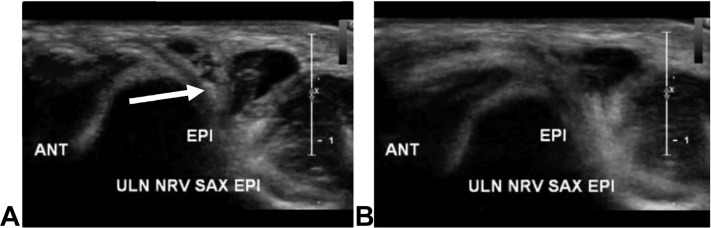
Nerve instability should be assessed on clinical examination with palpation of the ulnar nerve in the retrocondylar groove as the elbow is moved from extension to full flexion.30 “Subluxation” is thought to occur when the nerve moves anteriorly out of the groove and perches on the medial epicondyle and “dislocate” when it fully translocates anterior to the medial epicondyle. However, definitions of degrees of ulnar nerve instability are not uniformly agreed upon.30 In addition, hypermobility of the ulnar nerve has found to be present and asymptomatic in one-third of the general population (Fig. 9).31 More recently, preoperative dynamic ultrasound (US) has been shown to more accurately predict the degree of ulnar nerve instability following in situ decompression compared with physical examination (88% vs 12%).32
Figure 9.
High-resolution ultrasound depicting dynamic instability of a hypermobile ulnar nerve (arrow). A The elbow in extension with the ulnar nerve reduced. B The elbow in hyperflexion with the ulnar nerve subluxated and no longer visible.
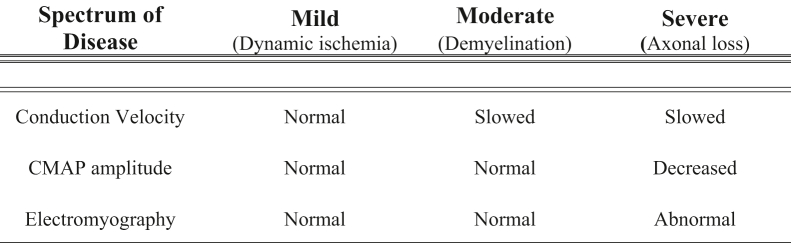
Electrodiagnostic studies (EDX) can aid in the diagnosis of CuTS as the disease progresses from dynamic ischemia to demyelination and finally axonal loss.26 In the early stages of the disease, EDX can present as normal as the dynamic ischemia from intermittent nerve compression has not yet reduced perfusion enough to decrease nerve conduction velocity in the fastest-conducting nerve fibers.33 Once demyelination has occurred, nerve conduction will slow to the point of ulnar nerve compression. According to the American Association of Neuromuscular and Electrodiagnostic Medicine, the greatest strength of evidence for the diagnosis of CuTS is a conduction velocity of <50 m/s across the elbow or when conduction velocity from the above-elbow to below-elbow segment is slowed to >10 m/s compared with the below-elbow to wrist segment.34 However, the diagnostic accuracy of EDX in CuTS is highly variable and secondary to biological (ie, low elbow temperature) and technical factors.35 Shubert et al36 previously reported that the application of American Association of Neuromuscular and Electrodiagnostic Medicine criteria increased the sensitivity of EDX in CuTS from 33.3% to 87.5%. However, this variable sensitivity is the reason why EDX is not considered the gold standard for diagnosing CuTS, especially in early disease. In their study consisting of 118 patients with clinically diagnosed CuTS, Shubert et al36 demonstrated that cubital tunnel release provided considerable relief in 94% of patients, even though EDX reports showed that only 11% of patients had clear CuTS, 23% had ulnar neuropathy, and 66% had negative findings.
Axonal loss occurs as a result of severe and prolonged nerve ischemia and manifests itself as EDX changes with a decrease in amplitude, which can reflect the overall decrease in the number of functioning nerve fibers.33 Electromyography shows abnormal activity during the insertional phase (indicating muscle denervation), fibrillations during the resting phase, and the presence of motor unit action potentials during the recruitment phase (indicating attempted reinnervation by either collateral sprouting or axonal reinnervation; Fig. 10).33 Friedrich et al37 previously showed that stringent EDX findings have prognostic value following surgical treatment. They found that recovery (86%) was strongly associated with a combination of conduction block across the elbow to the first dorsal interosseous (FDI) and normal distal compound muscle action potential (CMAP) amplitude from the abductor digiti minimi.37 Conversely, reduced FDI amplitude has been associated with preoperative weakness in grip and key pinch strength, indicating more severe disease with less predictable recovery potential.38
Figure 10.
EDX findings in CuTS.
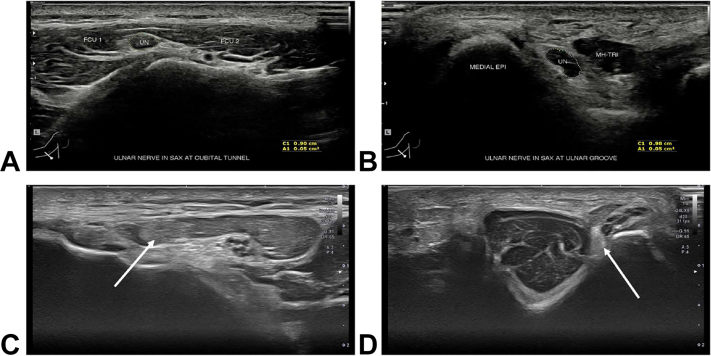
Table 2 provides a summary of the presentation, physical examination, ultrasound, and EDX parameters used in the diagnosis of CuTS. Additional imaging modalities, such as US, have been incorporated recently for diagnosing CuTS. High-resolution US enables a relatively fast, convenient, well-tolerated, and accurate evaluation of the ulnar nerve (Fig. 11).26,39,40 Volpe et al41 have previously shown that the maximum cross-sectional area (CSA), defined as the largest CSA of the ulnar nerve and recorded 4 cm proximal and distal to the medial epicondyle, was 14.6 mm2 in patients with EDX-confirmed ulnar neuropathy at the elbow versus 7.1 mm2 in control patients. In addition, a positive relationship was observed between the disease severity and nerve size of CuTS. In their study, patients with axonal loss demonstrated a mean CSA of 18.3 mm2, whereas patients with less severe symptoms, such as demyelination, demonstrated a CSA of 11.1 mm.2,41 The distance between the medial epicondyle and ulnar nerve at full elbow extension has also been found to be diagnostic for CuTS, with a distance of 0.53 cm having good sensitivity (71%) and specificity (90.7%).42 US has also been used to diagnose patients with CuTS with negative EDX43 as well as in patients with recurrent disease.44,45 However, compared with EDX, US has been shown to have lower sensitivity for detecting CuTS in the primary setting (71% vs 89%).46
Table 2.
Presentation, Physical Examination, Ultrasound,41 and Electrodiagnostic Parameters36 for the Diagnosis of CuTS
| Spectrum of Disease | Mild (Dynamic ischemia) | Moderate (Demyelination) | Severe (Axonal loss) |
|---|---|---|---|
| Presentation | Intermittent paresthesias in ulnar nerve distribution with elbow flexion | Sensory symptoms more constant, some motor weakness | Hand weakness, clawing |
| Physical examination | Positive findings on provocative testing (elbow flexion test, Tinel sign, scratch collapse test) | Decreased 2-point discrimination, weakness on strength testing (grip and apposition pinch) | Atrophy of intrinsic hand muscles with profound sensory disturbances |
| US CSA of ulnar nerve | 11.1 ± 3.4 mm2 | 15.8 ± 3.8 mm2 | 18.3 ± 5.1 mm2 |
| Electromyography | Normal | Normal | Abnormal |
| Conduction velocity | Normal | Slowed | Slowed |
Figure 11.
High-resolution ultrasound for diagnosis in CuTS. A The ulnar nerve (UN) as it courses between the 2 bellies of the FCU. B The UN as it travels around the elbow in the ulnar groove between the medial epicondyle (MEDIAL EPI) of the humerus and the medial head of the triceps (MH-TRI). C The UN (arrows) in a patient which CuTS at the FCU. D The UN (arrows) in a patient with CuTS at the FCU and perched on the medial epicondyle. The nerve’s morphological appearance is abnormally hypoechoic and has lost its normal fascicular echo pattern.
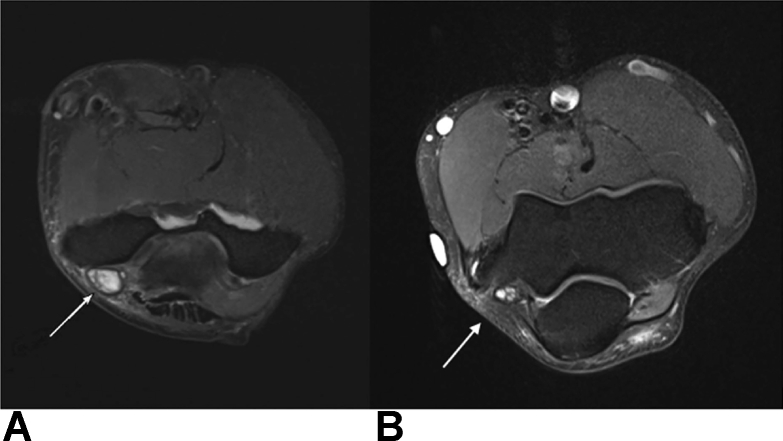
In addition to US, CT and MRI have also been used in the diagnosis of CuTS. Computed tomography scans of the cubital tunnel have shown decreased CSA compared with normal controls as well as differences in bony morphology that may increase the odds of developing CuTS.14 Magnetic resonance image studies have been shown to have higher diagnostic sensitivities than EDX in some studies,47 especially in patients with nonlocalizing neurophysiological response. The most frequent MRI findings include a combination of high signal intensity and nerve enlargement (63%), followed by nerve compression (27%) and isolated high signal intensity (23%) or nerve enlargement (2%; Fig. 12).47 In addition, an increase in T2 signal intensity and nerve caliber enlargement have been shown to positively correlate with disease severity and progression over time.48
Figure 12.
Axial MRI using T2-weighted imaging of the right elbow. A An enlarged and high signal intensity ulnar nerve in a patient with CuTS. B Comparison MRI in a patient without CuTS depicting the internal topography of the ulnar nerve fascicles.
Treatment
Nonoperative treatment
Patients with intermittent symptoms and clinical examination consistent with CuTS without evidence of axon loss, weakness, or atrophy are candidates for nonoperative treatment. Previous studies have reported 50% to 88% success using nonoperative approaches2,49,50 and found a positive correlation between symptomatic relief and improved EDX findings.49 Successful nonoperative treatment is lower in pediatric and adolescent patients but should be attempted prior to surgical intervention.51 Nonoperative approaches include orthoses to prevent elbow flexion, elbow pads, avoidance of triceps exercises, postural/behavior modification, nonsteroidal anti-inflammatory drugs, night splints, physical therapy, US, pulsed signal therapy, ergonomics education, and corticosteroid injections.22,52 In a randomized trial, Svernlöv et al53 found 90% clinical improvement in patients with early disease regardless of whether they received education separately, wore nighttime orthosis, or performed nerve glides.
Surgical treatment
Although the surgical treatment of CuTS is increasing,1 there remains a lack of consensus or standardized algorithm for treatment. Modern treatment strategies fall into one of 2 categories: in situ decompression or transposition, with multiple technical options and nuances inclusive to both. Proponents of simple decompression cite equivalent clinical54 and electrodiagnostic outcomes55 with lower morbidity,56 whereas proponents of transposition cite improved clinical outcomes in some studies57 with less revision for postoperative nerve instability58 and decreased reoperation rate in the long term (12% vs 25%).59 Furthermore, biomechanical evidence of decreased ulnar nerve pressure60 and strain,61 especially in elbow flexion, has historically favored transposition relative to in situ release. This dilemma is currently being researched as part of a clinical trial funded by the National Institutes of Health (clinicaltrials.gov identifier NCT04254185).
In a recent large systematic review, outcomes from 30 studies were evaluated, in which 8 different surgical techniques were utilized on more than 2800 elbows. The study found an improvement rate of 87% with surgery, 3% risk of complication, and 2% recurrence rate.62 Comparative analysis of surgical treatment techniques revealed in situ decompression to be the most effective procedure with the least amount complications and lowest risk of reoperation and recurrence.62 It should be noted that transpositions were not performed randomly in these studies, creating a important selection bias.
In situ decompression
In situ decompression (Fig. 13) was first described by Buzzard63 in 1922 and later by Osborne64 in 1957. This technique is relatively faster than transposition,5 more cost-effective,65 and can be performed under local anesthesia.66 The procedure involves releasing the points of compression from the ulnar nerve without mobilizing it anterior to the medial epicondyle and performing a soft tissue stabilization procedure.
Figure 13.
Intraoperative photograph of in situ decompression of the ulnar nerve in a patient with CuTS. The arrow indicates preservation of the medial antebrachial cutaneous nerve.
In a recent survey of American Society for Surgery of the Hand members, in situ decompression was the treatment of choice for the majority of members in patients without ulnar nerve subluxation.4 This mirrors the trend observed in other studies that have found an overall rise in in situ release but a decrease in transposition over time,5,67 both in the United States and abroad.68 Explanations for the rise of in situ release are likely multifactorial. However, improved understanding of disease etiology, increased ability to detect CuTS earlier in its course, faster surgical time, decreased morbidity, equivalent reimbursement, and surgeon preference all likely play a role.69
Both open and endoscopic approaches have been described and have demonstrated equivalent efficacy for postoperative clinical improvement and similar complication rates.70,71 Patient satisfaction has been reported to be higher (79%) for endoscopic versus open (60%) release.72 In addition, a smaller incision of visualization through a smaller (2 cm vs 4 cm) has been reported.73 Despite lower compensation and higher procedural cost than open in situ release, the frequency of endoscopic cubital tunnel release appears to be increasing.74
Revision surgery following in situ decompression has been reported to be as high as 19%.75 Reasons for persistent or recurrent symptoms are often secondary to incomplete release, persistent tension on the nerve during elbow flexion, and nerve instability.76 Patients with prior elbow fracture or dislocation (OR 7.1) as well as patients requesting surgery for mild clinically graded disease (OR 3.2), have a higher rate of revision surgery. Young patients with a sharp-angled ulnar nerve groove have previously been found to be at a higher risk of anterior dislocation of the ulnar nerve after simple decompression.77,78 Interestingly, some studies found that age, sex, body mass index, tobacco use, and diabetes status are not associated with a greater likelihood of revision surgery following in situ release.75
Transposition
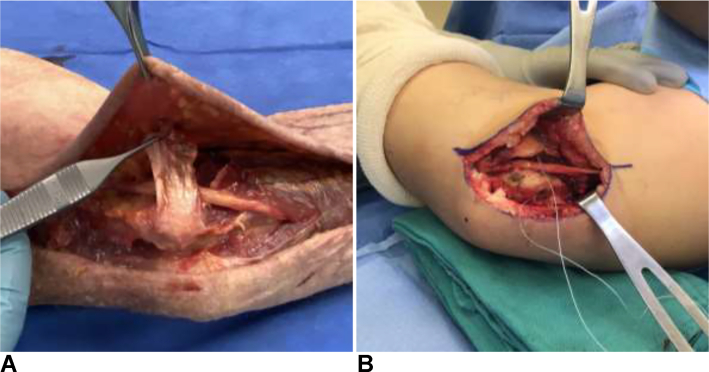
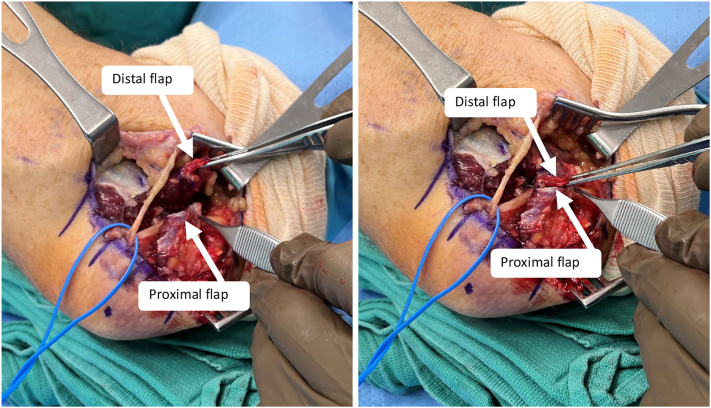
Transposition has historically been the most widely used method of treatment with the final position of the nerve located subcutaneous,79 intramuscular, 80,81 or submuscular (Fig. 14).82 This technique appears the most logical as it treats both the compression and traction components of the disease.83 Subcutaneous transposition was first described by Curtis79 in 1898 and allows for immediate mobilization.69 However, concerns with neuritis and recurrent subluxation posterior to the medial epicondyle later led Learmonth84 in 1942 to modify the transposition technique to include excising the medial intermuscular septum and placing the ulnar nerve next to the median nerve beneath the flexor-pronator mass. Although this modification provided nerve stability, the requisite postoperative immobilization to protect the flexor-pronator mass repair to the medial epicondyle was associated with significant stiffness, fibrosis, and decreased nerve gliding.69 Several modifications of the technique have since been developed to help facilitate earlier range of motion, such as musculofascial lengthening of the flexor-pronator mass.85 For subcutaneous transposition, the use of a fasciodermal sling86 has been described to prevent posterior subluxation, as well as adipose flaps to decrease perineural scarring and neuritis (Fig. 15).87,88
Figure 14.
Ulnar nerve transposition surgery. A Anterior subcutaneous transposition. B Submuscular ulnar nerve transposition.
Figure 15.
Submuscular transposition of the ulnar nerve demonstrating both the musculofascial lengthening of the flexor-pronator mass and the distal and proximal flaps of the fasciodermal sling.
Randomized controlled trials and meta-analyses have yet to elucidate a superior treatment outcome between subcutaneous and submuscular transposition89 as well as transposition versus in situ release.56 It remains unclear which preoperative factors predict positive outcomes for anterior transposition.90 Although some studies support transposition for unstable ulnar nerves,91 others have found that older age, weak preoperative grip strength, and abnormal 2-point discrimination are associated with unfavorable outcomes at 2 years after surgery regardless of treatment.91 Currently, history or examination consistent with ulnar nerve instability, recurrent disease, and muscle atrophy are the most common indicators for transposition.30 Submuscular transposition is most commonly performed in thin patients but has greater wound complications and peri-incisional numbness compared with subcutaneous transposition.1 Subcutaneous transposition is the preferred technique for throwing athletes and has demonstrated good clinical results.92
Other areas in which transposition of the ulnar nerve can be beneficial include traumatic elbow reconstruction and total elbow arthroplasty. McKee et al93 previously reported neurolysis and transposition during reconstruction of failed elbow fixations that resulted in functional improvements, return of strength, and patient satisfaction among patients with varying levels of ulnar nerve injury. However, in the primary care setting, a recent multicenter randomized controlled trial failed to show any significant difference between prophylactic in situ decompression and transposition for distal humerus fractures treated with bicolumnar fixation.94 For primary total elbow arthroplasty, ulnar nerve transposition is recommended only if preoperative elbow flexion is markedly limited or abnormal tracking or increased nerve tension is identified intraoperatively after insertion of the prosthesis.95 Otherwise, an in situ release is sufficient to minimize ulnar nerve complications after total elbow arthroplasty, which occurs approximately 3% of the time and can have devastating consequences.95
Regardless of the final position of the nerve, the success of any ulnar nerve transposition procedure requires creation of a completely untethered path for the ulnar nerve without kinking or tension.96 In addition to proximal excision of the medial intermuscular septum, structures distal to the medial epicondyle must also be considered. These structures include but are not limited to the medial antebrachial cutaneous nerve (whose branches cross the ulnar nerve approximately 2.0, 3.7, and 7.7 cm distal to the medial epicondyle96), Osborne’s fascia, FCU nerve branches of the ulnar nerve, crossing vascular branches from the ulnar artery, distal intermuscular septum between FCU/FDS, flexor-pronator origin, and investing fascia of the FDS over the ulnar nerve.96
Medial epicondylectomy
Medial epicondylectomy was first described by King and Morgan97 in 1959 and involves resection of the medial epicondyle of the humerus to decrease both compression and tension on the ulnar nerve without creating secondary nerve instability. Although this procedure was effective in improving ulnar nerve symptoms, medial elbow pain and iatrogenic elbow instability limited its widespread adoption.76 Following O’Driscoll’s98 discovery that only 20% of the width of medial epicondyle could be removed without risking injury to the origin of the medial collateral ligament (MCL), a modified MCL-sparing technique was subsequently developed utilizing an oblique osteotomy halfway between the coronal and sagittal planes.99 Outcomes of this technique have been favorable, with 93% of patients reporting good or excellent results with no subsequent elbow instability.99 Furthermore, a prospective randomized study reported superior outcomes and patient satisfaction following medial epicondylectomy compared with transposition.100 Current indications for medial epicondylectomy include patients with hypermobile nerves, pre-existing vascular disease where transposition could potentially worsen ischemia, and thin patients.1,76 A 13% revision rate has been reported in the literature with younger age, associated workers’ compensation claims, mild disease, and preoperative narcotic use being identified as risk factors for reoperation.101
Revision cubital tunnel surgery
Evaluation and treatment of recurrent and/or persistent symptoms after cubital tunnel release is challenging.102 Recurrent CuTS has been attributed to inaccurate preoperative diagnosis, incomplete nerve decompression, iatrogenic injury, postsurgical perineural adhesions, irreversible nerve pathology, or conditions associated with secondary nerve compression.103 In addition to evaluating for medial antebrachial cutaneous nerve neuroma and more proximal sites of nerve compression in the neck and thoracic outlet, psychiatric comorbidities and psychological coping skills must also be assessed.102
It is estimated that as many as 25% of patients treated for CuTS will experience recurrence.104 Younger age at presentation (<50 years)101 as well as greater static 2-point discrimination and history of diabetes have been associated with a greater number of revision surgeries.105 In a large systematic review of patients undergoing revision cubital tunnel surgery, transposition surgery was the most common procedure for primary surgery (51%), perineural scarring was the most common intraoperative finding at revision surgery (79%), and the medial intermuscular septum was the most frequent entrapment site (33%).106 In their study, Novak and Mackinnon107 examined a series of 100 patients who underwent reoperation following cubital tunnel surgery. The authors found that the most common operative findings included a medial antebrachial cutaneous nerve neuroma (n = 73) and a distal kink of the ulnar nerve (n = 57) caused by fascial flaps or tendinous bands.107 Outcomes following revision surgery are inferior to those following primary surgery, with only 75% to 80% of revision patients reporting symptomatic improvement, and worse outcomes reported on all measured standardized questionnaires.108,109 In addition, patients over the age of 50 years, electromyographic evidence of denervation, and previous submuscular transposition have been associated with poor outcomes after revision surgery.109
External neurolysis,110 anterior submuscular111 and subcutaneous112 transposition, and medial epicondylectomy have been described as revision treatment options. The most common revision surgery is submuscular transposition of the ulnar nerve (75%).106 Table 3 provides a comprehenseive approach to the optimization of the treatment of CuTS. More recently, vein,113 collagen nerve wraps,104 and porcine extracellular matrix wraps114 have been used as adjuvants to help prevent perineural scarring with promising results. However, randomized prospective studies and long-term results are currently lacking to support the use of such expensive options.
Table 3.
Clinical Guidelines to Optimize Surgical Treatment for CuTS
| Preoperative | Intraoperative | Postoperative |
|---|---|---|
| In patients that show clinical symptoms of CuTS but a negative EDX, we recommend US of the ulnar nerve given the high false-negative rate of EDX. This will provide the best treatment results when CuTS is diagnosed early. Assess for proximal (cervical) and distal (Guyon’s canal) sites of compression when evaluating CuTS (double crush syndrome). For concomitant cervical radiculopathy and CuTS of ulnar nerve compression, treat CuTS first. For concomitant CuTS and Guyon’s canal compression, treat both at index procedure. Nerve transfers are best used in patients with viable motor endplates and should not be used when atrophy and sever clawing are present. Success is dependent on appropriate indications. |
Infiltration with 1% lidocaine with epinephrine prior to incision around cubital tunnel can help with hemostasis and avoid the need for a tourniquet. Identification of the ulnar nerve in between the 2 heads of FCU is fast and reliable, especially in obese patients. Maintain vascularity to ulnar nerve by minimizing dissection posterior to the nerve in an in situ release. Retrograde dissection of the ulnar nerve proximal to the medial epicondyle to the arcade of Struthers is safe as there are no nerve branches between these anatomic sites During anterior submuscular transposition, a blunt hemostat passed beneath flexor-pronator mass can help protect the underlying ulnar collateral ligament during flap dissection. |
For posttransition patients, a short arm volar-based wrist splint is better tolerated than a long arm posterior-based splint and effectively protects the flexor-pronator mass. We encourage the use of a neoprene sleeve postoperatively to aid with swelling and incisional discomfort. In the revision setting, we recommend anterior submuscular transposition. |
Nerve transfers
For severe primary or recurrent CuTS, nerve transfers have been described as a technique to “supercharge” the ulnar nerve, and thus facilitate faster and more complete recovery with minimal morbidity (Fig. 16).15,115, 116, 117 Patient selection is guided by preoperative EDX: the best candidate demonstrates decreased CMAP amplitudes that reflect a lower number of axons crossing the compression site, and the presence of fibrillations/positive sharp waves indicating that at least some motor endplates remain available and receptive to reinnervation.118 The terminal branch of the anterior interosseus nerve (AIN) innervates the pronator quadratus and provides an expendable donor in the forearm with approximately 900 axons.119 Recently, Doherty et al115 found that more than three quarters of patients with advanced CuTS had partial or complete resolution of clawing or muscle wasting following end-to-side AIN to ulnar nerve motor transfer. Improvement in function within the first 2 to 3 months is thought to be related to remyelination, 4 to 5 months for axonal regeneration, and approximately 6 to 7 months after supercharge nerve transfer.118 The mean time for observing nascent units was 8.5 months in some studies; however, continued recovery has been observed at 12 and 24 months at the level of endplate and as a result of neuroplasticity.120 Additional procedures that may augment recovery include concomitant Guyon’s release, side-to-side profundus tenodesis, and cross-palm nerve grafts from the median to ulnar sensory nerve.118,121
Figure 16.
Intraoperative photograph of anterior interosseous nerve (AIN)-to-ulnar motor nerve transfer for CuTS. Anterior interosseous nerve (arrow) as it is transferred to the ulnar nerve.
Another adjunct treatment for severe CuTS is postsurgical electrical stimulation (PES). Recently, Power et al122 reported on PES enhanced muscle reinnervation and functional recovery following surgery for severe CuTS. In their randomized, double-blind, placebo-controlled study, patients underwent cubital tunnel release followed by either a sham stimulation or a 1-hour treatment of 20 Hz PES. At the 3-year follow-up, the motor unit number estimation increased and key pinch strength was 3-fold higher in the cohort that underwent PES.122 Although the ideal PES timing and intensity has yet to be determined, PES is an emerging technology likely to be used more often in the future.
Conclusions
The reason for the current lack of standardized algorithm for diagnosing and treating CuTS is multifactorial. However, controversies regarding the diagnostic tools, treatment, and outcomes at research level all play a role. Diagnostic challenges include heterogenous patient population that often present symptoms at a later stage of the disease when treatment outcomes are less reliable. In early disease, when CuTS is most reliably treated, EDX are less dependable 36 and history and physical examination can be equivocal. Therefore, it is not surprising that additional imaging modalities are being studied to assist in accurately identifying CuTS prior to demyelination, axon loss, or before further irrecoverable changes occur. This would decrease time to surgery in patients who fail conservative treatment and increase their likelihood of favorable results with a less invasive surgical approach.
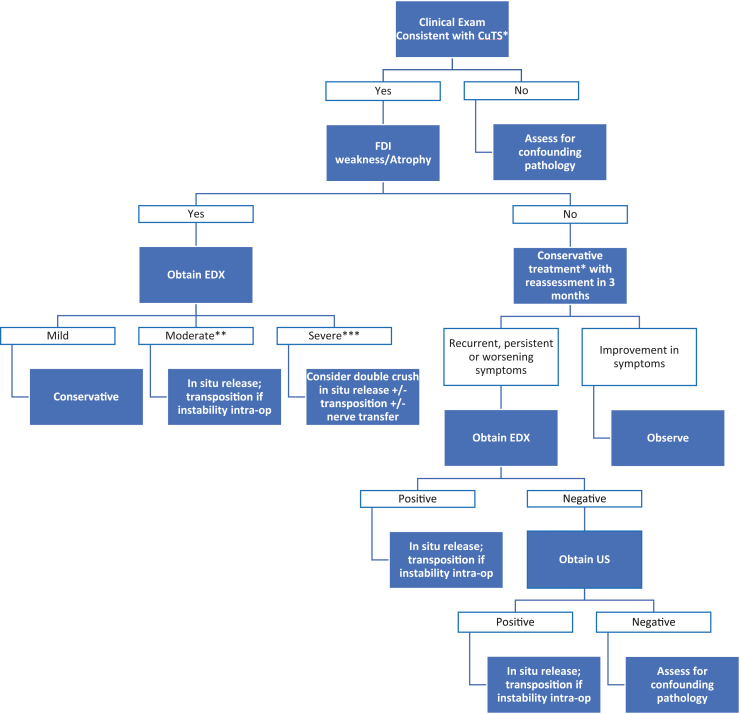
Treatment controversies stem from a lack of prospective, randomized, controlled trials of patients with a similar diagnosis being treated with different techniques using standard objective and subjective outcomes measures. In addition, the presence of preoperative nerve instability in many studies, including systematic reviews, has been shown to influence treatment options and present an important selection bias123. It is also important to develop new techniques and consistently use reliable and reproducible outcome measures that are validated in the CuTS patient population.124 A previous systematic review has identified 45 unique outcomes and 31 postoperative outcome measures using 101 published studies on the surgical treatment of CuTS.125 This heterogeneity limits the interpretation of current studies and represents a barrier we must overcome in future studies moving forward. The included treatment algorithm acknowledges the limitation of the evidence supporting each technique with the aim to provide clarity about the challenges for treating this complex patient population (Fig. 17).
Figure 17.
Algorithm for CuTS diagnosis and treatment. ∗Clinical examination consistent with CuTS is defined as having paresthesias in the distribution of the ulnar nerve, symptoms caused by elbow flexion, a positive Tinel sign at the medial elbow, and/or widened 2-point discrimination in ulnar nerve distribution. ∗∗Moderate findings of EDX include decreased conduction velocity. ∗∗∗Severe findings of EDX include decreased CMAP with/without abnormal electromyography findings.
Footnotes
Declaration of interests: No benefits in any form have been received or will be received related directly or indirectly to the subject of this article.
References
- 1.Staples J.R., Calfee R. Cubital tunnel syndrome: current concepts. J Am Acad Orthop Surg. 2017;25(10):e215–e224. doi: 10.5435/JAAOS-D-15-00261. [DOI] [PubMed] [Google Scholar]
- 2.An T.W., Evanoff B.A., Boyer M.I., Osei D.A. The prevalence of cubital tunnel syndrome: a cross-sectional study in a U.S. metropolitan cohort. J Bone Joint Surg Am. 2017;99(5):408–416. doi: 10.2106/JBJS.15.01162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naran S., Imbriglia J.E., Bilonick R.A., Taieb A., Wollstein R. A demographic analysis of cubital tunnel syndrome. Ann Plast Surg. 2010;64(2):177–179. doi: 10.1097/SAP.0b013e3181a2c63e. [DOI] [PubMed] [Google Scholar]
- 4.Yahya A., Malarkey A.R., Eschbaugh R.L., Bamberger H.B. Trends in the surgical treatment for cubital tunnel syndrome: a survey of members of the American Society for Surgery of the Hand. Hand (N Y) 2018;13(5):516–521. doi: 10.1177/1558944717725377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soltani A.M., Best M.J., Francis C.S., Allan B.J., Panthaki Z.J. Trends in the surgical treatment of cubital tunnel syndrome: an analysis of the national survey of ambulatory surgery database. J Hand Surg. 2013;38(8):1551–1556. doi: 10.1016/j.jhsa.2013.04.044. [DOI] [PubMed] [Google Scholar]
- 6.Burahee A, Fullilove S, Power D. Survey of the current practice of management of cubital tunnel syndrome among surgeons in the UK. J Hand Surg Eur Vol. Published online December 21, 2021. https://doi.org/10.1177/17531934211064688 [DOI] [PubMed]
- 7.Kim N., Stehr R., Matloub H.S., Sanger J.R. Anconeus epitrochlearis muscle associated with cubital tunnel syndrome: a case series. Hand (N Y) 2019;14(4):477–482. doi: 10.1177/1558944718762566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suwannakhan A., Chaiyamoon A., Yammine K., et al. The prevalence of anconeus epitrochlearis muscle and Osborne’s ligament in cubital tunnel syndrome patients and healthy individuals: an anatomical study with meta-analysis. Surg J R Coll Surg Edinb Irel. 2021;19(6):e402–e411. doi: 10.1016/j.surge.2020.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Maslow J.I., Johnson D.J., Block J.J., Lee D.H., Desai M.J. Prevalence and clinical manifestations of the anconeus epitrochlearis and cubital tunnel syndrome. Hand (N Y) 2020;15(1):69–74. doi: 10.1177/1558944718789412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gelberman R.H., Yamaguchi K., Hollstien S.B., et al. Changes in interstitial pressure and cross-sectional area of the cubital tunnel and of the ulnar nerve with flexion of the elbow. an experimental study in human cadavera. J Bone Joint Surg Am. 1998;80(4):492–501. doi: 10.2106/00004623-199804000-00005. [DOI] [PubMed] [Google Scholar]
- 11.James J., Sutton L.G., Werner F.W., Basu N., Allison M.A., Palmer A.K. Morphology of the cubital tunnel: an anatomical and biomechanical study with implications for treatment of ulnar nerve compression. J Hand Surg. 2011;36(12):1988–1995. doi: 10.1016/j.jhsa.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 12.Wright T.W., Glowczewskie F., Cowin D., Wheeler D.L. Ulnar nerve excursion and strain at the elbow and wrist associated with upper extremity motion. J Hand Surg. 2001;26(4):655–662. doi: 10.1053/jhsu.2001.26140. [DOI] [PubMed] [Google Scholar]
- 13.Watchmaker G.P., Lee G., Mackinnon S.E. Intraneural topography of the ulnar nerve in the cubital tunnel facilitates anterior transposition. J Hand Surg. 1994;19(6):915–922. doi: 10.1016/0363-5023(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 14.Lee S.K., Hwang S.Y., Choy W.S. Validity of computed tomographic measurements and morphological comparison of cubital tunnel in idiopathic cubital tunnel syndrome. BMC Musculoskelet Disord. 2020;21(1):76. doi: 10.1186/s12891-020-3108-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dengler J., Dolen U., Patterson J.M.M., et al. Supercharge end-to-side anterior interosseous-to-ulnar motor nerve transfer restores intrinsic function in cubital tunnel syndrome. Plast Reconstr Surg. 2020;146(4):808–818. doi: 10.1097/PRS.0000000000007167. [DOI] [PubMed] [Google Scholar]
- 16.Maroukis B.L., Ogawa T., Rehim S.A., Chung K.C. Guyon canal: the evolution of clinical anatomy. J Hand Surg. 2015;40(3):560–565. doi: 10.1016/j.jhsa.2014.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li M.X.L., He Q., Hu Z.L., et al. Applied anatomical study of the vascularized ulnar nerve and its blood supply for cubital tunnel syndrome at the elbow region. Neural Regen Res. 2015;10(1):141–145. doi: 10.4103/1673-5374.150723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sugawara M. Experimental and clinical studies of the vascularized anterior transposition of the ulnar nerve for cubital tunnel syndrome. Nihon Seikeigeka Gakkai Zasshi. 1988;62(8):755–766. [PubMed] [Google Scholar]
- 19.Ogata K., Shimon S., Owen J., Manske P.R. Effects of compression and devascularisation on ulnar nerve function. A quantitative study of regional blood flow and nerve conduction in monkeys. J Hand Surg Br. 1991;16(1):104–108. doi: 10.1016/0266-7681(91)90143-c. [DOI] [PubMed] [Google Scholar]
- 20.Mallette P., Zhao M., Zurakowski D., Ring D. Muscle atrophy at diagnosis of carpal and cubital tunnel syndrome. J Hand Surg. 2007;32(6):855–858. doi: 10.1016/j.jhsa.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 21.Zhang D, Earp BE, Homer SH, Blazar P. Factors associated with severity of cubital tunnel syndrome at presentation. Hand (N Y). Published online December 7, 2021. https://doi.org/10.1177/15589447211058821 [DOI] [PMC free article] [PubMed]
- 22.Andrews K., Rowland A., Pranjal A., Ebraheim N. Cubital tunnel syndrome: anatomy, clinical presentation, and management. J Orthop. 2018;15(3):832–836. doi: 10.1016/j.jor.2018.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drake M.L., Hensley D.T., Chen W.C., Taylor K.F. Muscle atrophy at presentation of cubital tunnel syndrome: demographics and duration of symptoms. Hand (N Y) 2017;12(1):64–67. doi: 10.1177/1558944716643096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Folberg C.R., Weiss A.P., Akelman E. Cubital tunnel syndrome. Part I: presentation and diagnosis. Orthop Rev. 1994;23(2):136–144. [PubMed] [Google Scholar]
- 25.Novak C.B., Lee G.W., Mackinnon S.E., Lay L. Provocative testing for cubital tunnel syndrome. J Hand Surg. 1994;19(5):817–820. doi: 10.1016/0363-5023(94)90193-7. [DOI] [PubMed] [Google Scholar]
- 26.Nakashian M.N., Ireland D., Kane P.M. Cubital tunnel syndrome: current concepts. Curr Rev Musculoskelet Med. 2020;13(4):520–524. doi: 10.1007/s12178-020-09650-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ochi K., Horiuchi Y., Tanabe A., Waseda M., Kaneko Y., Koyanagi T. Shoulder internal rotation elbow flexion test for diagnosing cubital tunnel syndrome. J Shoulder Elbow Surg. 2012;21(6):777–781. doi: 10.1016/j.jse.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 28.Cheng C.J., Mackinnon-Patterson B., Beck J.L., Mackinnon S.E. Scratch collapse test for evaluation of carpal and cubital tunnel syndrome. J Hand Surg. 2008;33(9):1518–1524. doi: 10.1016/j.jhsa.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 29.Montgomery K., Wolff G., Boyd K.U. Evaluation of the scratch collapse test for carpal and cubital tunnel syndrome-a prospective, blinded Study. J Hand Surg. 2020;45(6):512–517. doi: 10.1016/j.jhsa.2020.02.016. [DOI] [PubMed] [Google Scholar]
- 30.DeGeorge B.R., Kakar S. Decision-making factors for ulnar nerve transposition in cubital tunnel surgery. J Wrist Surg. 2019;8(2):168–174. doi: 10.1055/s-0038-1665548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calfee R.P., Manske P.R., Gelberman R.H., Van Steyn M.O., Steffen J., Goldfarb C.A. Clinical assessment of the ulnar nerve at the elbow: reliability of instability testing and the association of hypermobility with clinical symptoms. J Bone Joint Surg Am. 2010;92(17):2801–2808. doi: 10.2106/JBJS.J.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rutter M., Grandizio L.C., Malone W.J., Klena J.C. The use of preoperative dynamic ultrasound to predict ulnar nerve stability following in situ decompression for cubital tunnel syndrome. J Hand Surg. 2019;44(1):35–38. doi: 10.1016/j.jhsa.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 33.Dy C.J., Mackinnon S.E. Ulnar neuropathy: evaluation and management. Curr Rev Musculoskelet Med. 2016;9(2):178–184. doi: 10.1007/s12178-016-9327-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.American Association of Electrodiagnostic Medicine, Campbell W.W. Guidelines in electrodiagnostic medicine. Practice parameter for electrodiagnostic studies in ulnar neuropathy at the elbow. Muscle Nerve Suppl. 1999;8:S171–S205. [PubMed] [Google Scholar]
- 35.Landau M.E., Campbell W.W. Clinical features and electrodiagnosis of ulnar neuropathies. Phys Med Rehabil Clin N Am. 2013;24(1):49–66. doi: 10.1016/j.pmr.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 36.Shubert D.J., Prud’homme J., Sraj S. Nerve conduction studies in surgical cubital tunnel syndrome patients. Hand (N Y) 2021;16(2):170–173. doi: 10.1177/1558944719840750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Friedrich J.M., Robinson L.R. Prognostic indicators from electrodiagnostic studies for ulnar neuropathy at the elbow. Muscle Nerve. 2011;43(4):596–600. doi: 10.1002/mus.21925. [DOI] [PubMed] [Google Scholar]
- 38.Power H.A., Sharma K., El-Haj M., Moore A.M., Patterson M.M., Mackinnon S.E. Compound muscle action potential amplitude predicts the severity of cubital tunnel syndrome. J Bone Joint Surg Am. 2019;101(8):730–738. doi: 10.2106/JBJS.18.00554. [DOI] [PubMed] [Google Scholar]
- 39.Chiou H.J., Chou Y.H., Cheng S.P., et al. Cubital tunnel syndrome: diagnosis by high-resolution ultrasonography. J Ultrasound Med. 1998;17(10):643–648. doi: 10.7863/jum.1998.17.10.643. [DOI] [PubMed] [Google Scholar]
- 40.Babusiaux D., Laulan J., Bouilleau L., et al. Contribution of static and dynamic ultrasound in cubital tunnel syndrome. Orthop Traumatol Surg Res. 2014;100(4 Suppl):S209–S212. doi: 10.1016/j.otsr.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 41.Volpe A., Rossato G., Bottanelli M., et al. Ultrasound evaluation of ulnar neuropathy at the elbow: correlation with electrophysiological studies. Rheumatology (Oxford) 2009;48(9):1098–1101. doi: 10.1093/rheumatology/kep167. [DOI] [PubMed] [Google Scholar]
- 42.Lee G.J., Park D. Ultrasonographic findings of the ulnar nerve following elbow flexion in patients with cubital tunnel syndrome. Pain Med Malden Mass. 2020;21(11):2684–2691. doi: 10.1093/pm/pnaa169. [DOI] [PubMed] [Google Scholar]
- 43.Yoon J.S., Walker F.O., Cartwright M.S. Ulnar neuropathy with normal electrodiagnosis and abnormal nerve ultrasound. Arch Phys Med Rehabil. 2010;91(2):318–320. doi: 10.1016/j.apmr.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vosbikian M.M., Tarity T.D., Nazarian L.N., Ilyas A.M. Does the ulnar nerve enlarge after surgical transposition? J Ultrasound Med. 2014;33(9):1647–1652. doi: 10.7863/ultra.33.9.1647. [DOI] [PubMed] [Google Scholar]
- 45.Gruber H., Baur E.M., Plaikner M., Loizides A. The ulnar nerve after surgical transposition: can sonography define the reason of persisting neuropathy? ROFO. 2015;187(11):998–1002. doi: 10.1055/s-0035-1553221. [DOI] [PubMed] [Google Scholar]
- 46.Omejec G., Žgur T., Podnar S. Diagnostic accuracy of ultrasonographic and nerve conduction studies in ulnar neuropathy at the elbow. Clin Neurophysiol. 2015;126(9):1797–1804. doi: 10.1016/j.clinph.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 47.Vucic S., Cordato D.J., Yiannikas C., Schwartz R.S., Shnier R.C. Utility of magnetic resonance imaging in diagnosing ulnar neuropathy at the elbow. Clin Neurophysiol. 2006;117(3):590–595. doi: 10.1016/j.clinph.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 48.Bäumer P., Dombert T., Staub F., et al. Ulnar neuropathy at the elbow: MR neurography--nerve T2 signal increase and caliber. Radiology. 2011;260(1):199–206. doi: 10.1148/radiol.11102357. [DOI] [PubMed] [Google Scholar]
- 49.Padua L., Aprile I., Caliandro P., Foschini M., Mazza S., Tonali P. Natural history of ulnar entrapment at elbow. Clin Neurophysiol. 2002;113(12):1980–1984. doi: 10.1016/s1388-2457(02)00295-x. [DOI] [PubMed] [Google Scholar]
- 50.Shah C.M., Calfee R.P., Gelberman R.H., Goldfarb C.A. Outcomes of rigid night splinting and activity modification in the treatment of cubital tunnel syndrome. J Hand Surg. 2013;38(6):1125–1130.e1. doi: 10.1016/j.jhsa.2013.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stutz C.M., Calfee R.P., Steffen J.A., Goldfarb C.A. Surgical and nonsurgical treatment of cubital tunnel syndrome in pediatric and adolescent patients. J Hand Surg. 2012;37(4):657–662. doi: 10.1016/j.jhsa.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 52.Kooner S., Cinats D., Kwong C., Matthewson G., Dhaliwal G. Conservative treatment of cubital tunnel syndrome: a systematic review. Orthop Rev. 2019;11(2):7955. doi: 10.4081/or.2019.7955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Svernlöv B., Larsson M., Rehn K., Adolfsson L. Conservative treatment of the cubital tunnel syndrome. J Hand Surg Eur Vol. 2009;34(2):201–207. doi: 10.1177/1753193408098480. [DOI] [PubMed] [Google Scholar]
- 54.Altay T., Yamak K., Koyuncu Ş., Kayali C., Sözkesen S. Comparison of simple decompression and anterior subcutaneous transposition of the ulnar nerve for the treatment of cubital tunnel syndrome. Ortop Traumatol Rehabil. 2018;20(6):475–481. doi: 10.5604/01.3001.0012.8394. [DOI] [PubMed] [Google Scholar]
- 55.Zlowodzki M., Chan S., Bhandari M., Kalliainen L., Schubert W. Anterior transposition compared with simple decompression for treatment of cubital tunnel syndrome. A meta-analysis of randomized, controlled trials. J Bone Joint Surg Am. 2007;89(12):2591–2598. doi: 10.2106/JBJS.G.00183. [DOI] [PubMed] [Google Scholar]
- 56.Said J., Van Nest D., Foltz C., Ilyas A.M. Ulnar nerve in situ secompression versus transposition for idiopathic cubital tunnel syndrome: an updated meta-analysis. J Hand Microsurg. 2019;11(1):18–27. doi: 10.1055/s-0038-1670928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Macadam S.A., Gandhi R., Bezuhly M., Lefaivre K.A. Simple decompression versus anterior subcutaneous and submuscular transposition of the ulnar nerve for cubital tunnel syndrome: a meta-analysis. J Hand Surg. 2008;33(8) doi: 10.1016/j.jhsa.2008.03.006. 1314.e1‒12. [DOI] [PubMed] [Google Scholar]
- 58.Matzon J.L., Lutsky K.F., Hoffler C.E., Kim N., Maltenfort M., Beredjiklian P.K. Risk factors for ulnar nerve instability resulting in transposition in patients with cubital tunnel syndrome. J Hand Surg. 2016;41(2):180–183. doi: 10.1016/j.jhsa.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 59.Hutchinson D.T., Sullivan R., Sinclair M.K. Long-term reoperation rate for cubital tunnel syndrome: subcutaneous transposition versus in situ decompression. Hand (N Y) 2021;16(4):447–452. doi: 10.1177/1558944719873153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kilinc B.E., Celik H., Oc Y., Unlu R., Keskinoz E.N., Yilmaz B. Analysis of subcutaneous anterior transposition versus in-situ decompression of ulnar nerve with force transducer in cadaver specimen. Turk Neurosurg. 2020;30(1):99–103. doi: 10.5137/1019-5149.JTN.27190-19.2. [DOI] [PubMed] [Google Scholar]
- 61.Foran I., Vaz K., Sikora-Klak J., Ward S.R., Hentzen E.R., Shah S.B. Regional ulnar nerve strain following decompression and anterior subcutaneous transposition in patients with cubital tunnel syndrome. J Hand Surg. 2016;41(10):e343–e350. doi: 10.1016/j.jhsa.2016.07.095. [DOI] [PubMed] [Google Scholar]
- 62.Wade R.G., Griffiths T.T., Flather R., Burr N.E., Teo M., Bourke G. Safety and outcomes of different surgical techniques for cubital tunnel decompression: s systematic review and network meta-analysis. JAMA Netw Open. 2020;3(11) doi: 10.1001/jamanetworkopen.2020.24352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Buzzard E.F. Some varieties of traumatic and toxic ulnar neuritis: (A paper read before the Section of Neurology of the Royal Society of Medicine on Feb. 9th, 1922.) Lancet. 1922;199(5138):317–319. [Google Scholar]
- 64.Osborne G. The surgical treatment of tardy ulnar neuritis: proceedings and reports of councils and associations. J Bone Joint Surg. 1957;39(B):782. [Google Scholar]
- 65.Kazmers N.H., Lazaris E.L., Allen C.M., Presson A.P., Tyser A.R. Comparison of surgical encounter direct costs for three methods of cubital tunnel decompression. Plast Reconstr Surg. 2019;143(2):503–510. doi: 10.1097/PRS.0000000000005196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huq N.S., Ahmed N., Razeghi M. Cubital tunnel release using local anesthesia. Clin Plast Surg. 2013;40(4):557–565. doi: 10.1016/j.cps.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 67.Adkinson J.M., Zhong L., Aliu O., Chung K.C. Surgical treatment of cubital tunnel syndrome: trends and the influence of patient and surgeon characteristics. J Hand Surg. 2015;40(9):1824–1831. doi: 10.1016/j.jhsa.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.O’Grady E., Power D., Tan S. Current attitudes regarding surgical treatment of cubital tunnel syndrome in the UK. J Hand Surg Eur Vol. 2017;42(9):959–960. doi: 10.1177/1753193417714399. [DOI] [PubMed] [Google Scholar]
- 69.Eberlin K.R., Marjoua Y., Jupiter J.B. Compressive neuropathy of the ulnar nerve: a perspective on history and current controversies. J Hand Surg. 2017;42(6):464–469. doi: 10.1016/j.jhsa.2017.03.027. [DOI] [PubMed] [Google Scholar]
- 70.Ren Y.M., Zhou X.H., Qiao H.Y., et al. Open versus endoscopic in situ decompression in cubital tunnel syndrome: a systematic review and meta-analysis. Int J Surg. 2016;35:104–110. doi: 10.1016/j.ijsu.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 71.Byvaltsev V.A., Stepanov I.A., Kerimbayev T.T. A systematic review and meta-analysis comparing open versus endoscopic in situ decompression for the treatment of cubital tunnel syndrome. Acta Neurol Belg. 2020;120(1):1–8. doi: 10.1007/s13760-019-01149-9. [DOI] [PubMed] [Google Scholar]
- 72.Watts A.C., Bain G.I. Patient-rated outcome of ulnar nerve decompression: a comparison of endoscopic and open in situ decompression. J Hand Surg. 2009;34(8):1492–1498. doi: 10.1016/j.jhsa.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 73.Said J., Frizzell K., Heimur J., Kachooei A., Beredjiklian P., Rivlin M. Visualization during endoscopic versus open cubital tunnel decompression: a cadaveric study. J Hand Surg. 2019;44(8):697.e1–697.e6. doi: 10.1016/j.jhsa.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 74.Law T.Y., Hubbard Z.S., Chieng L.O., Chim H.W. Trends in open and endoscopic cubital tunnel release in the Medicare patient population. Hand (N Y) 2017;12(4):408–412. doi: 10.1177/1558944716679610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Krogue J.D., Aleem A.W., Osei D.A., Goldfarb C.A., Calfee R.P. Predictors of surgical revision after in situ decompression of the ulnar nerve. J Shoulder Elbow Surg. 2015;24(4):634–639. doi: 10.1016/j.jse.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 76.Boone S., Gelberman R.H., Calfee R.P. The management of cubital tunnel syndrome. J Hand Surg. 2015;40(9):1897–1904. doi: 10.1016/j.jhsa.2015.03.011. quiz 1904. [DOI] [PubMed] [Google Scholar]
- 77.Murata K., Omokawa S., Shimizu T., et al. Risk factors for dislocation of the ulnar nerve after simple decompression for cubital tunnel syndrome. Hand Surg. 2014;19(1):13–18. doi: 10.1142/S0218810414500038. [DOI] [PubMed] [Google Scholar]
- 78.Kong L., Bai J., Yu K., Zhang B., Zhang J., Tian D. Predictors of surgical outcomes after in situ ulnar nerve decompression for cubital tunnel syndrome. Ther Clin Risk Manag. 2018;14:69–74. doi: 10.2147/TCRM.S155284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Curtis B.F. Traumatic ulnar neuritis: transplantation of the nerve. J Nerv Ment Dis. 1898;25:480–481. [Google Scholar]
- 80.Wang T.H., Shih J.T. Functional results after intramuscular ulnar nerve anterior transposition for young adults patients. J Hand Surg Asian Pac Vol. 2019;24(4):400–404. doi: 10.1142/S2424835519500504. [DOI] [PubMed] [Google Scholar]
- 81.Frantz L.M., Adams J.M., Granberry G.S., Johnson S.M., Hearon B.F. Outcomes of ulnar nerve anterior transmuscular transposition and significance of ulnar nerve instability in cubital tunnel syndrome. J Shoulder Elbow Surg. 2019;28(6):1120–1129. doi: 10.1016/j.jse.2018.11.054. [DOI] [PubMed] [Google Scholar]
- 82.Henry M. Modified intramuscular transposition of the ulnar nerve. J Hand Surg. 2006;31(9):1535–1542. doi: 10.1016/j.jhsa.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 83.Kleinman W.B. Cubital tunnel syndrome: anterior transposition as a logical approach to complete nerve decompression. J Hand Surg. 1999;24(5):886–897. doi: 10.1053/jhsu.1999.0886. [DOI] [PubMed] [Google Scholar]
- 84.Learmonth J.R. A technique for transplanting the ulnar nerve. Surg Gynecol Obstet. 1942;75:792–793. [Google Scholar]
- 85.Dellon A.L., Coert J.H. Results of the musculofascial lengthening technique for submuscular transposition of the ulnar nerve at the elbow. J Bone Joint Surg Am. 2004;86‒A(Suppl 1Pt 2):169–179. doi: 10.2106/00004623-200409001-00007. [DOI] [PubMed] [Google Scholar]
- 86.Eaton R.G., Crowe J.F., Parkes J.C., III Anterior transposition of the ulnar nerve using a non-compressing fasciodermal sling. J Bone Joint Surg Am. 1980;62(5):820–825. [PubMed] [Google Scholar]
- 87.Danoff J.R., Lombardi J.M., Rosenwasser M.P. Use of a pedicled adipose flap as a sling for anterior subcutaneous transposition of the ulnar nerve. J Hand Surg. 2014;39(3):552–555. doi: 10.1016/j.jhsa.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 88.Verveld C.J., Danoff J.R., Lombardi J.M., Rosenwasser M.P. Adipose flap versus fascial sling for anterior subcutaneous transposition of the ulnar nerve. Am J Orthop (Belle Mead NJ) 2016;45(2):89–94. [PubMed] [Google Scholar]
- 89.Liu C.H., Wu S.Q., Ke X.B., et al. Subcutaneous versus submuscular anterior transposition of the ulnar nerve for cubital tunnel syndrome: a systematic review and meta-analysis of randomized controlled trials and observational studies. Medicine (Baltimore) 2015;94(29) doi: 10.1097/MD.0000000000001207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shi Q., MacDermid J.C., Santaguida P.L., Kyu H.H. Predictors of surgical outcomes following anterior transposition of ulnar nerve for cubital tunnel syndrome: a systematic review. J Hand Surg. 2011;36(12):1996–2001.e1‒6. doi: 10.1016/j.jhsa.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 91.Kang H.J., Oh W.T., Koh I.H., Kim S., Choi Y.R. Factors influencing outcomes after ulnar nerve stability-based surgery for cubital tunnel syndrome: a prospective cohort study. Yonsei Med J. 2016;57(2):455–460. doi: 10.3349/ymj.2016.57.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nicholson G.P., Rao A.J., Naylor A.J., et al. Return to sporting activity after ulnar nerve transposition for isolated neuritis in competitive overhead athletes. J Shoulder Elbow Surg. 2020;29(7):1401–1405. doi: 10.1016/j.jse.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 93.McKee M.D., Jupiter J.B., Bosse G., Goodman L. Outcome of ulnar neurolysis during post-traumatic reconstruction of the elbow. J Bone Joint Surg Br. 1998;80(1):100–105. doi: 10.1302/0301-620x.80b1.7822. [DOI] [PubMed] [Google Scholar]
- 94.Dehghan N., Nauth A., Hall J., et al. In situ placement versus anterior transposition of the ulnar nerve for distal humerus fractures treated with plate fixation: a multicenter randomized controlled trial. J Orthop Trauma. 2021;35(9):465–471. doi: 10.1097/BOT.0000000000002066. [DOI] [PubMed] [Google Scholar]
- 95.Dachs R.P., Vrettos B.C., Chivers D.A., Du Plessis J.P., Roche S.J. Outcomes after ulnar nerve in situ release during total elbow arthroplasty. J Hand Surg. 2015;40(9):1832–1837. doi: 10.1016/j.jhsa.2015.06.107. [DOI] [PubMed] [Google Scholar]
- 96.Felder J.M., Mackinnon S.E., Patterson M.M. The 7 structures distal to the elbow that are critical to successful anterior transposition of the ulnar nerve. Hand (N Y) 2019;14(6):776–781. doi: 10.1177/1558944718771390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.King T., Morgan F.P. Late results of removing the medial humeral epicondyle for traumatic ulnar neuritis. J Bone Joint Surg Br. 1959;41‒B(1):51–55. doi: 10.1302/0301-620X.41B1.51. [DOI] [PubMed] [Google Scholar]
- 98.O’Driscoll S.W., Jaloszynski R., Morrey B.F., An K.N. Origin of the medial ulnar collateral ligament. J Hand Surg. 1992;17(1):164–168. doi: 10.1016/0363-5023(92)90135-c. [DOI] [PubMed] [Google Scholar]
- 99.Osei D.A., Padegimas E.M., Calfee R.P., Gelberman R.H. Outcomes following modified oblique medial epicondylectomy for treatment of cubital tunnel syndrome. J Hand Surg. 2013;38(2):336–343. doi: 10.1016/j.jhsa.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 100.Geutjens G.G., Langstaff R.J., Smith N.J., Jefferson D., Howell C.J., Barton N.J. Medial epicondylectomy or ulnar-nerve transposition for ulnar neuropathy at the elbow? J Bone Joint Surg Br. 1996;78(5):777–779. [PubMed] [Google Scholar]
- 101.Gaspar M.P., Kane P.M., Putthiwara D., Jacoby S.M., Osterman A.L. Predicting revision following in situ ulnar nerve decompression for patients with idiopathic cubital tunnel syndrome. J Hand Surg. 2016;41(3):427–435. doi: 10.1016/j.jhsa.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 102.Grandizio L.C., Maschke S., Evans P.J. The management of persistent and recurrent cubital tunnel syndrome. J Hand Surg. 2018;43(10):933–940. doi: 10.1016/j.jhsa.2018.03.057. [DOI] [PubMed] [Google Scholar]
- 103.Lauder A., Chen C., Bolson R.M., Leversedge F.J. Management of recalcitrant cubital tunnel syndrome. J Am Acad Orthop Surg. 2021;29(15):635–647. doi: 10.5435/JAAOS-D-20-01381. [DOI] [PubMed] [Google Scholar]
- 104.Soltani A.M., Allan B.J., Best M.J., Mir H.S., Panthaki Z.J. Revision decompression and collagen nerve wrap for recurrent and persistent compression neuropathies of the upper extremity. Ann Plast Surg. 2014;72(5):572–578. doi: 10.1097/SAP.0b013e3182956475. [DOI] [PubMed] [Google Scholar]
- 105.Izadpanah A., Maldonado A.A., Bishop A.T., Spinner R.J., Shin A.Y. Risk factors for revision cubital tunnel surgery. J Plast Reconstr Aesthetic Surg. 2020;73(5):959–964. doi: 10.1016/j.bjps.2019.12.008. [DOI] [PubMed] [Google Scholar]
- 106.Kholinne E., Alsharidah M.M., Almutair O., et al. Revision surgery for refractory cubital tunnel syndrome: a systematic review. Orthop Traumatol Surg Res. 2019;105(5):867–876. doi: 10.1016/j.otsr.2019.03.020. [DOI] [PubMed] [Google Scholar]
- 107.Mackinnon S.E., Novak C.B. Operative findings in reoperation of patients with cubital tunnel syndrome. Hand (N Y) 2007;2(3):137–143. doi: 10.1007/s11552-007-9037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Aleem A.W., Krogue J.D., Calfee R.P. Outcomes of revision surgery for cubital tunnel syndrome. J Hand Surg. 2014;39(11):2141–2149. doi: 10.1016/j.jhsa.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 109.Gabel G.T., Amadio P.C. Reoperation for failed decompression of the ulnar nerve in the region of the elbow. J Bone Joint Surg Am. 1990;72(2):213–219. [PubMed] [Google Scholar]
- 110.Dagregorio G., Saint-Cast Y. Simple neurolysis for failed anterior submuscular transposition of the ulnar nerve at the elbow. Int Orthop. 2004;28(6):342–346. doi: 10.1007/s00264-004-0589-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rogers M.R., Bergfield T.G., Aulicino P.L. The failed ulnar nerve transposition. Etiology and treatment. Clin Orthop. 1991;269:193–200. [PubMed] [Google Scholar]
- 112.Caputo A.E., Watson H.K. Subcutaneous anterior transposition of the ulnar nerve for failed decompression of cubital tunnel syndrome. J Hand Surg. 2000;25(3):544–551. doi: 10.1053/jhsu.2000.6005. [DOI] [PubMed] [Google Scholar]
- 113.Varitimidis S.E., Vardakas D.G., Goebel F., Sotereanos D.G. Treatment of recurrent compressive neuropathy of peripheral nerves in the upper extremity with an autologous vein insulator. J Hand Surg. 2001;26(2):296–302. doi: 10.1053/jhsu.2001.22528. [DOI] [PubMed] [Google Scholar]
- 114.Papatheodorou L.K., Williams B.G., Sotereanos D.G. Preliminary results of recurrent cubital tunnel syndrome treated with neurolysis and porcine extracellular matrix nerve wrap. J Hand Surg. 2015;40(5):987–992. doi: 10.1016/j.jhsa.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 115.Doherty C.D., Miller T.A., Larocerie-Salgado J., Byers B.A., Ross D.C. Reverse end-to-side anterior interosseous nerve-to-ulnar motor transfer for severe ulnar neuropathy. Plast Reconstr Surg. 2020;146(3):306e–313e. doi: 10.1097/PRS.0000000000007059. [DOI] [PubMed] [Google Scholar]
- 116.Dunn J.C., Gonzalez G.A., Fernandez I., Orr J.D., Polfer E.M., Nesti L.J. Supercharge end-to-side nerve transfer: systematic review. Hand (N Y) 2021;16(2):151–156. doi: 10.1177/1558944719836213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Davidge K.M., Yee A., Moore A.M., Mackinnon S.E. The supercharge end-to-side anterior interosseous-to-ulnar motor nerve transfer for restoring intrinsic function: clinical experience. Plast Reconstr Surg. 2015;136(3):344e–352e. doi: 10.1097/PRS.0000000000001514. [DOI] [PubMed] [Google Scholar]
- 118.Power H.A., Kahn L.C., Patterson M.M., Yee A., Moore A.M., Mackinnon S.E. Refining indications for the supercharge end-to-side anterior interosseous to ulnar motor nerve transfer in cubital tunnel syndrome. Plast Reconstr Surg. 2020;145(1):106e–116e. doi: 10.1097/PRS.0000000000006399. [DOI] [PubMed] [Google Scholar]
- 119.Üstün M.E., Ögün T.C., Büyükmumcu M., Salbacak A. Selective restoration of motor function in the ulnar nerve by transfer of the anterior interosseous nerve: an anatomical feasibility study. J Bone Joint Surg Am. 2001;83(4):549. doi: 10.2106/00004623-200104000-00009. [DOI] [PubMed] [Google Scholar]
- 120.Nyman E., Nyman T., Rubensson C., Thordstein M. Neuroplasticity following nerve transfer of the anterior interosseous nerve for proximal ulnar nerve injuries. Plast Reconstr Surg Glob Open. 2021;9(7) doi: 10.1097/GOX.0000000000003684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Felder J.M., Hill E.J.R., Power H.A., Hasak J., Mackinnon S.E. Cross-palm nerve grafts to enhance sensory recovery in severe ulnar neuropathy. Hand (N Y) 2020;15(4):526–533. doi: 10.1177/1558944718822851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Power H.A., Morhart M.J., Olson J.L., Chan K.M. Postsurgical electrical stimulation enhances recovery following surgery for severe cubital tunnel syndrome: a double-blind randomized controlled trial. Neurosurgery. 2020;86(6):769–777. doi: 10.1093/neuros/nyz322. [DOI] [PubMed] [Google Scholar]
- 123.Clark D.M., Piscoya A.S., Dunn J.C., Nesti L.J. The impact of pre-existing ulnar nerve instability on the surgical treatment of cubital tunnel syndrome: a systematic review. J Shoulder Elbow Surg. 2020;29(11):2339–2346. doi: 10.1016/j.jse.2020.05.028. [DOI] [PubMed] [Google Scholar]
- 124.Macadam S.A., Bezuhly M., Lefaivre K.A. Outcomes measures used to assess results after surgery for cubital tunnel syndrome: a systematic review of the literature. J Hand Surg. 2009;34(8):1482–1491.e5. doi: 10.1016/j.jhsa.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 125.Gallo L., Gallo M., Murphy J., et al. Reporting outcomes and outcome measures in cubital tunnel syndrome: a systematic review. J Hand Surg. 2020;45(8):707‒728–e9. doi: 10.1016/j.jhsa.2020.04.001. [DOI] [PubMed] [Google Scholar]
- 126.Tubbs R.S., Rizk E., Shoja M., Loukas M., Barbaro N., Spinner R. Nerves and Nerve Injuries. Vol2: Pain, Treatment, Injury, Disease and Future Directions. Academic Press; 2015. https://www.elsevier.com/books/nerves-and-nerve-injuries/tubbs/978-0-12-802653-3?country=US&format=print&utm_source=google_ads&utm_medium=paid_search&utm_campaign=usashopping&gclid=CjwKCAjw46CVBhB1EiwAgy6M4iSVuYUXQn6kGAIBpFlOUMSttGCVDm3Rz5oVaiMSLlWP4oVXDwl-FRoCC0AQAvD_BwE&gclsrc=aw.ds