Abstract
Knee pain, the most common cause of musculoskeletal pain (MSK), constitutes a severe public health burden. Its neurobiological causes, however, remain poorly understood. Among many possible causes, it has been proposed that sleep problems could lead to an increase in chronic pain symptomatology, which may be driven by central nervous system changes. In fact, we previously found that brain cortical thickness mediated the relationship between sleep qualities and pain severity in older adults with MSK. We also demonstrated a significant difference in a machine-learning-derived brain-aging biomarker between participants with low-and high-impact knee pain. Considering this, we examined whether brain aging was associated with self-reported sleep and pain measures, and whether brain aging mediated the relationship between sleep problems and knee pain. Exploratory Spearman and Pearson partial correlations, controlling for age, sex, race and study site, showed a significant association of brain aging with sleep related impairment and self-reported pain measures. Moreover, mediation analysis showed that brain aging significantly mediated the effect of sleep related impairment on clinical pain and physical symptoms. Our findings extend our prior work demonstrating advanced brain aging among individuals with chronic pain and the mediating role of brain-aging on the association between sleep and pain severity. Future longitudinal studies are needed to further understand whether the brain can be a therapeutic target to reverse the possible effect of sleep problems on chronic pain.
Keywords: Aging, Sleep impairment, Chronic pain, Brain age, DeepBrainNet
Introduction
Aging is a complex biological phenomenon with marked changes across multiple physiological systems (e.g., the immune system, musculoskeletal system, nervous system). Also, aging is associated with poor sleep quality and greater chronic pain prevalence [5], [24], [41], [53], with age-related changes in brain structure [8], [28], [30], [29] and function as potential underlying mechanisms [18], [27], [33], [37]. Given that sleep disorders, and chronic pain in older age are both related to brain alterations [38], [43], [44], [55], [62], the interplay between these variables is of considerable interest to researchers and clinicians.
One brain aging biomarker that is increasingly gaining attention is brain predicted age difference, brain-PAD, which is calculated as the difference between brain-predicted age (a measure of the biological brain age predicted from structural, micro-structural, functional, or their combinations based on a machine learning algorithm) and chronological age [15]. Brain aging biomarkers have been proposed as biomarkers of health, risk of mortality, frailty, disease, and cognitive decline [9], [16], [57]. Previous work has characterized brain aging, obtained from structural MRIs, in populations with chronic pain [19], [18], [36], [58]. Overall, increasing evidence suggests that individuals with musculoskeletal pain (MSK), including knee osteoarthritis (OA), have “older” appearing brains compared to pain-free demographically matched controls [19], [35]. However, findings from research addressing the relationship between chronic MSK pain and brain aging have been mixed. For example, one study reported no differences in brain-PAD between a heterogenous chronic pain cohort recruited from multiple pain clinics and pain free controls [58].
Despite the potential impact of sleep on the brain, only one study to date has characterized brain age predictions in participants with sleep disorders. They showed that individuals with sleeping brain activity characteristic of individuals with an older chronological age have a significantly reduced life expectancy compared to those with younger appearing brain activity pattern [47]. Interestingly, Paixao and colleagues [47] derived a different brain aging algorithm, the brain age index (BAI), that is a sleep EEG-based biomarker of the deviation of sleep microstructure from patterns typical of that chronological age. BAI measures the difference between an individual's apparent “brain age”; estimated by comparing EEG features during sleep from an individual with age norms for their chronological age.
Considering the known sleep-pain associations [1], [3], [11], [21], [33], [37], [43], [44], [48], [49], [60], it is important to evaluate how sleep quality and chronic pain are interrelated in the context of brain aging processes. Using a brain aging biomarker to detect brain alterations related to certain pathologies comes with several advantages. Unlike other local morphometric measurements (e.g., cortical thickness) that require multiple independent tests, it is a global measure with contributions from all tissues of the brain. Thus, the aim of the present study was to investigate whether brain aging mediated the relationship between sleep problems and knee pain. In addition, we employed a novel convolutional neural network, DeepBrainNet [6], to predict brain age from structural MRIs, which has shown to be sensitive to pathological conditions, including chronic musculoskeletal pain (Valdes-Hernandez et al., under review). Based on our previous findings that sleep and pain associations were mediated by brain measures, we hypothesized that brain-predicted aging would be associated with self-reported sleep as well as with self-reported pain, and that brain aging would mediate the association between sleep problems and knee pain.
Methods
Participants
The sample for the current analysis included 206 individuals between 45 and 85 years of age who self-identified as non-Hispanic black (NHB) and non-Hispanic white (NHW), and 175 of them reported unilateral or bilateral knee pain, and screened positive for clinical knee OA [54]; the remaining 31 are pain-free controls. Participants were recruited as part of a multi-site observational study which included the University of Florida and University of Alabama at Birmingham. The parent study aims to identify the mechanisms underlying ethic/race differences in pain and functional limitations in persons with knee pain. Participants’ eligibility for study inclusion was determined through a telephone screening. A detailed description of the screening, inclusion, and exclusion criteria has been reported previously [13], [17], [20]. Individuals between a) ages of 45 and 85 who self-identify as non-Hispanic black (NHB) and non-Hispanic white (NHW), b) screening positive for unilateral or bilateral symptomatic osteoarthritis of the knee based on the 1986 American College of Rheumatology (ACR) criteria 4, 5; and also c) qualifies for a magnetic resonance imaging (MRI) of both knees, were included in the study. Participants were excluded for the following conditions: 1) Cognitive impairment; 2) hospitalization within the preceding year for psychiatric illness; 3) history of cardiovascular disease including uncontrolled hypertension (BP > 150/95 mm Hg); 4) prosthetic knee replacement or other clinically significant surgery to the arthritic knee; 5) peripheral neuropathy; 6) systemic diseases including rheumatoid arthritis, systemic lupus erythematosus, gout, and fibromyalgia; 7) neurological diseases such as Parkinson’s, and multiple sclerosis; 8) daily opioid use; 9) pregnant or nursing; or 10) MRI contraindications.
Procedures
At the clinical laboratory, participants provided informed consent and underwent a general health assessment (HAS) as well as a neuroimaging visit session no more than four weeks apart. During the HAS, participants completed sociodemographic, physical health, pain history and sleep questionnaires. A physical exam of the knees assessing current pain, bony enlargement, and crepitus was also conducted by a study rheumatologist. Weight and height measurements for body mass index (BMI) calculation were also obtained. The present study is an ancillary investigation that aimed to determine the relationship of brain aging, knee pain and sleep problems, thus, only measures relevant to the study hypotheses are included and presented below.
Clinical and sleep assessments
-
•
Graded Chronic Pain Scale (GCPS): GCPS is a widely validated and reliable tool for grading chronic pain impact [67]. The instructions were adapted to assess characteristic knee pain intensity and pain-related disability specifically. Participants were asked to rate on a 0 (“no pain”) to 10 (“pain as bad as could be”) numerical rating scale their current knee pain, average knee pain, and worst knee pain in the past six months. These ratings were averaged and multiplied by 10 to yield a 0–100 score, with higher scores indicating more severe pain intensity. Pain-related disability (i.e., how much pain has interfered with daily activities, recreational/social/family activities, and ability to work) on average, over the past six months, was rated on a 0 (“no inference”) to 10 (“unable to carry out activities”) scale and multiplied by 10 to yield a 0–100 score, with higher scores indicating greater disability. Additionally, the GCPS asked respondents “How many days in the last six months have you been kept from your usual activities because of pain?” Disability points were calculated as the sum of the pain-related disability score (i.e., 0–29 = 0 points; 30–49 = 1 point; 50–69 = 2 points; ≥70 = 3 points), and total number of disability days (i.e., 0–6 days = 0 points; 7–14 days = 1 point; 15–30 days = 2 points; 31 days or more = 3 points). Scores from the GCPS characteristic pain intensity scale and disability points were then used to categorize participants according to a pain grade: Grade 0 = no reported pain intensity; Grade 1 = low disability (i.e., less than3 disability points) and low pain intensity (i.e., less than50); Grade 2 = low disability-high intensity pain (i.e., ≥50); Grade 3 = high disability-moderately limiting (i.e., 3–4 Disability Points), regardless of pain intensity; Grade 4 = high disability-severely limiting (i.e., 5–6 Disability Points), regardless of pain intensity. Pain Groups were defined based on pain grade as follows: No chronic pain (i.e., Grade 0), Low impact pain (i.e., Grades 1–2), High impact pain (i.e., Grade 3–4).
-
•
Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC): WOMAC assesses knee OA symptoms in the preceding 48 h, including pain, stiffness, and physical function. Higher scores indicate a greater symptom burden [7].
-
•
Patient-Reported Outcomes Measurement Information System (PROMIS) Sleep short forms. The instruments use a 7-day recall period and a 5-point Likert scale. Raw scores were converted to an interval standardized T score. It consisted of 8 items which assessed self-reported perceptions of alertness, sleepiness, and tiredness during usual waking hours, and the perceived functional impairments during wakefulness associated with sleep problems and impaired alertness. Higher scores indicate greater sleep impairment. [51], [52], [70]
Neuroimaging data
Neuroimaging data were collected at two different sites: 1) the University of Florida’s McKnight Brain Institute on the Advanced Magnetic Resonance Imaging and Spectroscopy (AMRIS) facility’s Philips (Best, the Netherlands) 3-Tesla scanner using a 32-channel radio frequency coil and 2) the University of Alabama at Birmingham with 3-Tesla scanner using an 8-channel radio frequency coil. A high-resolution, T1-weighted (T1w), turbo field echo anatomical scan was collected using the following parameters: repetition time = 7.1 ms, echo time = 3.2 ms, 170 slices acquired in a sagittal orientation, flip angle = 8 deg., resolution = 1 mm isotropic. Head movement was minimized via cushions positioned inside the head coil and instructions to participants.
DeepBrainNet for brain age prediction
DeepBrainNet is a convolutional neural network (a type of machine learning neural network suitable for predictions based on images) that was recently developed by Bashyam et al. [6] to predict brain age [6]. It is built based on the inception-resnet-v2 framework [59] and uses a 2D convolutional architecture, which allows for the use of ImageNet for initialization—ImageNet is a natural scene database with 14 + million hand annotated images. DeepBrainNet was trained using T1w MRI images from 11,729 individuals (ages 3–95 years) from a diverse range of geographic locations, scanners, acquisition protocols, and studies, and tested in an independent sample of 2,739 individuals. Features for the DeepBrainNet are calculated as follows. First, the T1w needs to be skull-stripped. Second, the skull-stripped image has to be spatially normalized to the 1-mm isotropic voxel FSL skull-stripped T1w template using a 12-parameter linear affine transformation. For training, each of the skull-stripped MRI images was divided into 80 2D slices (centered on the z = 0 plane in MNI coordinates) and considered as an independent sample, resulting in a training set of 1 million images. To obtain a final age prediction for a test sample, each of 80 slices of the test scan is input to the trained model independently and the median prediction is calculated as the subject’s predicted brain age. To obtain skull-stripped images in our sample, we used NiPype's smriprep,1 the portion that process the anatomical T1w images in fmriprep [25] as well as visual quality control by one of the co-authors (CLN). The T1w images were corrected for intensity non-uniformity using N4BiasFieldCorrection [65] distributed with ANTs 2.2.0 (Avants et al. 2008, RRID:SCR_004757), and skull-stripped with a Nipype implementation of the antsBrainExtraction.sh workflow from ANTs [4], using OASIS30ANTs as target template. Brain-PAD, was calculated as the difference between brain-predicted age and chronological age [15].
Dealing with bias in the brain age estimations
In regression problems, e.g., brain age predictions, the so-called “regression dilution” bias is very common and has been described and tackled extensively in the brain age prediction literature. The regression dilution bias appears when the features used to predict (the preprocessed MRIs) contain errors. The bias always consists of an overestimation of younger brain ages and an underestimation of older brain ages, yielding a negative slope in the brain-PAD versus age relationship. A well accepted method to deal with this bias is regressing chronological age from the brain age or brain-PAD estimations [22], [50]. In second-level analyses using brain-PAD as the dependent variable, this is equivalent to add chronological age as a covariate.
Statistical methods
We used one-way analysis of variance (ANOVA) for continuous variables, and Chi-square test for categorical variables to compare the clinical and demographic characteristics between subjects with and without pain. Spearman and Pearson partial correlations, controlling by chronological age, sex, race, and study site were used to determine associations of brain-PAD with clinical pain and sleep measures. Note that chronological age must be a covariate of brain-PAD so the above-described regression dilution bias can be accounted for.
A mediation analysis was conducted to test the total indirect effect of sleep on pain through brain-PAD measures. We also controlled for age, sex, race and study site. We used bootstrapping procedures (n = 5,000) to obtain estimates and confidence intervals around the indirect effects to overcome potential problems caused by unmet assumptions in mediation analysis. We used the Hayes PROCESS macro model 4 that provides modern methods for inference about indirect effects including bootstrapped confidence intervals. Data analyses were performed using IBM SPSS 28 software.
Results
Clinical and demographic characteristics
Table 1 presents the demographic and clinical characteristics of the sample, categorized into three groups: those with no pain (31 participants), those with low-impact pain (110 participants), and those with high-impact pain (60 participants). The mean Brain-PAD scores of the participants in each group are listed, with the lowest mean score in the no pain group (-1.78 ± 7.06), and the highest in the high-impact pain group (0.95 ± 6.09). There were significant differences in the ethnicity/race between participants with low-impact and high-impact pain when compared to participants without pain, (p = 0.029).
Table 1.
Demographic and clinical characteristics of the sample (n = 206) for participants with no pain, low-impact pain, and high-impact pain.
| No Pain (n = 31) |
Low- Impact pain (n = 110) | High- Impact pain (n = 60) | P-value | |
|---|---|---|---|---|
| Chronological Age, (Mean ± SD) | 60.13 ± 9.89 | 58.74 ± 8.09 | 56.30 ± 7.24 | 0.073 (ANOVA) |
| Brain-PAD, (Mean ± SD) |
−1.78 ± 7.06 | −1.13 ± 6.47 | 0.95 ± 6.09 | 0.082 (ANOVA) |
| Ethnicity/Race, n(%) | 0.029(χ2) | |||
|
13(41.94%) |
43(39.09%) | 36(60%) | |
|
18(58.06%) | 67(60.90%) | 24(40%) | |
| Gender, n(%) | ||||
| Male | 9(29.39%) | 37 (33.64%) | 23 (38.33%) | |
| Female | 22(70.97%) | 73 (66.36%) | 37 (61.67%) | 0.658 (χ2) |
| BMI, (Mean ± SD) |
1.58 ± 0.99 | 1.56 ± 1.24 | 1.95 ± 1.17 | 0.122 (ANOVA) |
| Site, n(%) | ||||
| UF | 21(67.74%) | 67 (60.90%) | 36 (60%) | |
| UAB | 10(32.26%) | 43 (39.09%) | 24 (40%) | 0.748 (χ2) |
Note: Bold values represent probability less than 0.05. There are 5 missing values. n represents sample size. SD represents standard deviation.
Brain age predictions
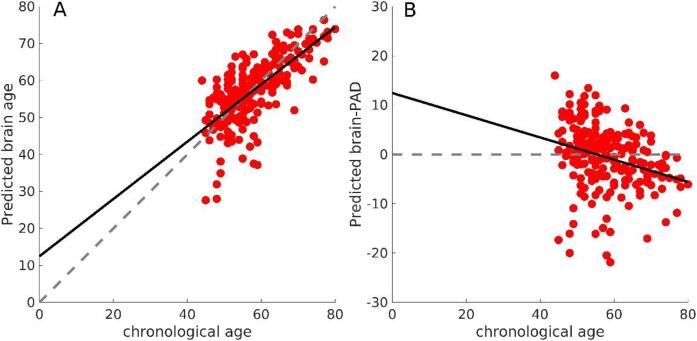
Fig. 1 shows the brain age predictions and the corresponding brain-PAD for our sample. Note that if we extrapolate the predictions to chronological ages younger than those in the sample, brain age estimations would be overestimated. This linear deviation is significant (slope p-value ∼ 1e-5). However, it is rectified when adding chronological age as a covariate in the subsequent analyses.
Fig. 1.
A) Predicted brain age in our sample. The dashed line represents the y = x line, and the solid line represents the linear fit brain age ∼ age. B) A) Predicted brain-PAD. The dashed line represents the y = 0 line, and the solid line represents the linear fit brain-PAD ∼ age. The solid line evidences a linear bias due to lack of a wide distribution of chronological age and/or the well-known “regression dilution” in regression problems.
Brain-PAD is associated with self-reported clinical pain and self-reported sleep measures
In the prediction of brain age, the DeepBrainNet model yielded a mean absolute average (MAE; in years) of 4.98 with 95 %CI [4.43, 5.61] (using 10,000 bootstraps). Also, the correlation between the chronological and predicted brain age was 0.71 and very significant (i.e., with a very small p-value).
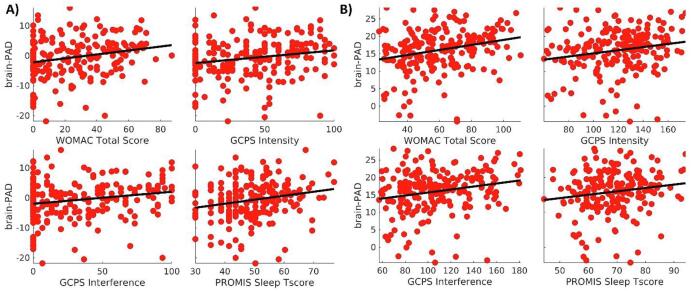
Partial correlations among measures of sleep, pain, and Brain-PAD are presented in Table 2. There is a positive correlation between Brain-PAD and each of the four variables. However, the strength of the correlation varies for each variable, with WOMAC Total Score having the strongest correlation (r = 0.265), and PROMIS Sleep T-Score having the weakest correlation (r = 0.151). The uncorrected p-values, as well as the FDR and Bonferroni corrected across the four measures are also provided. To visualize the relationship between the sleep and pain variables with Brain-PAD, we did a scatter plot. In Fig. 2 the x-axis represents each of the four variables (PROMIS Sleep T-Score, GCPS Intensity, GCPS Interference, and WOMAC Total Score), and the y-axis represents Brain-PAD. Moreover, to provide an additional quantitative characterization of the relationship between brain-PAD and pain and sleep, we also provide the average of the brain-PAD for the participants with extreme values of the measures (i.e., below the 5 percentile and above the 95 percentile). These values are shown in Table 3.
Table 2.
Association between Brain-PAD with clinical and sleep measures.
| PROMIS Sleep T-Score | r = 0.151 p = 0.036* p’= 0.036 p’’=0.144 |
| GCPS Intensity | r = 0.196 p = 0.006*p’=0.008 p’’=0.024 |
| GCPS Interference | r = 0.213 p = 0.003* p’=0.006 p’’=0.012 |
| WOMAC Total Score | r = 0.265 p = 0.001* p’=0.004 p’’=0.004 |
Notes: * Correlation is significant at the 0.05 level (two-tailed); p'= False Discovery Rate (FDR) corrected; p’’= Bonferroni corrected. Bold values represent probability less than 0.05.
Fig. 2.
A) Scatter plots of the brain-PAD versus the pain and sleep measures. B) To illustrate the strength of the partial correlations, the plots are the same as in A) but with the effects of the covariates (i.e., age, sex, race, and site) removed. The line is a linear fit between the corrected variables to represent the sign of the relationship.
Table 3.
Average Brain-PAD (in years) for participants with pain and sleep measures below the 5 percentile and above the 95 percentiles of their distributions.
| Measure | Brain-PAD |
|
|---|---|---|
| Percentiles | 5 | 95 |
| WOMAC Total Score | −2.21 (n = 43) | 3.27 (n = 10) |
| GCPS_Intensity | −1.78 (n = 31) | 0.38 (n = 10) |
| GCPD Interference | −1.68 (n = 56) | 3.24 (n = 12) |
| PROMIS_Sleep_T-score | −2.13 (n = 12) | 3.08 (n = 10) |
Mediation analysis
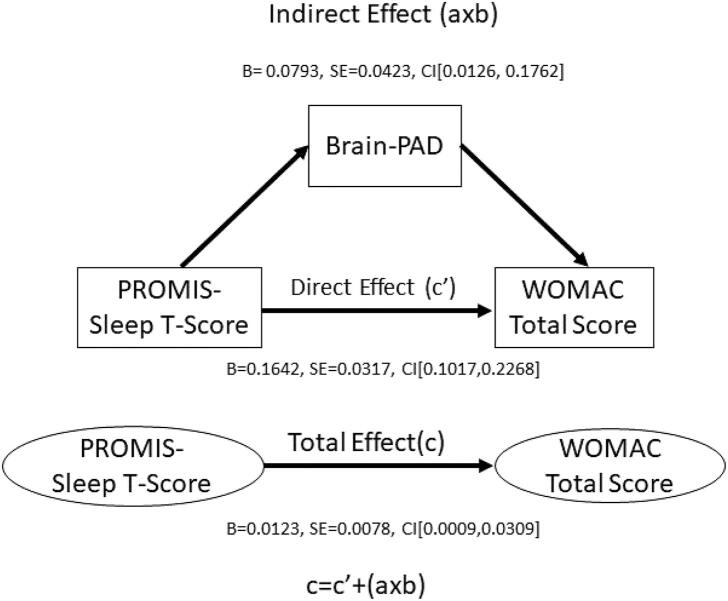
The indirect coefficient (a × b) of PROMIS Sleep T-Score and WOMAC Total Score, was significant (see Fig. 3), while controlling for chronological age, sex, race, and study site. The total effect (c) and the direct effect (c') were also significant but were not of interest in the present study.
Fig. 3.
Brain-PAD mediates the association between PROMIS-Sleep T-Score and WOMAC Total Score.
Discussion
The present study sought to elucidate the associations of sleep quality, self-reported pain and brain aging, and further explore the potential mediating role of brain aging in the association between sleep quality and self-reported pain. To our knowledge, this is the first study examining this mediational association. Brain aging was significantly associated with self-reported pain, and self-reported sleep measures. In addition, brain aging mediated the association between sleep impairment severity and pain severity.
As hypothesized, chronic knee pain and sleep problems were associated with an “older” brain relative to an individual’s chronological age. The older than normal brain, the higher the intensity of pain as well as the higher the severity of sleep impairment. This result is in part consistent with our previous finding in which greater average worst pain intensity was associated with an “older” brain in an older population [19]. Furthermore, our results are in line with the work of Hung et al. [35], where accelerated brain aging is associated with pain characteristics that differ between discrete disorders, for example osteoarthritis [35].
Our second hypothesis was also supported by our results since brain aging mediated the association between sleep impairments and the self-reported pain measures. In general, limited research has investigated brain aging as a mediator in the relation between sleep and pain, however, during the past 10 years, some studies have applied formal tests of mediation to investigate variables on the path between sleep and pain intensity and vice versa [68]. For example, Bonvanie et al [10] used 3 different mediators: 1) symptoms of anxiety and depression, 2) fatigue, and 3) physical inactivity, finding that the sleep problem only had an indirect effect on musculoskeletal pain severity through symptoms of fatigue, and on abdominal pain severity through anxiety and depression. Hamilton et. al. (2012) found that pain helplessness partially mediated the relationship between sleep and pain. In addition, Evans et. al. (2017) reported that positive affect was not identified as a statistically significant mediator while negative affect was a statistically significant partial mediator of the sleep-pain relationship. Goodin et al. (2012) showed that cortisol reactivity totally mediated the relationship between sleep quality (measured by PSQI) and pain (measured by McGill pain questionnaire). O’Brien et al[46] found that negative mood almost fully mediated the relationship between sleep and pain. Interestingly, Valrie et al [66] in a pediatric population found that mood was a statistically significant partial mediator of the influence of pain intensity on sleep and vice versa. Lastly, Nicassio et al [45] reported that depressive symptoms mediated 38% of the total effect of pain on sleep. While not directly related to our results, the mediators identified in these studies (e.g., depression and/or anxiety, attention to pain, pain helplessness, activation of the stress system, fatigue, and physical activity) have all been related to structural changes in the brain [31], with sleep problems [14], [26], [32], [39], [40], [64], and chronic pain [2], [12], [34], [42], [56], [61], [63], [71]. For example, patients with major depressive disorder have a reduction in gray matter in the left anterior cingulate cortex, as well as in the orbital, ventrolateral prefrontal and hippocampal cortices. Moreover, the hypothalamic-pituitary-adrenocortical axis appears overactive with hypersecretion of cortisol [23]. On the other hand, those with mood disorder exhibited volume alterations in structures regulating emotional and cognitive functioning like fronto-limbic cortex, hippocampus and amygdala [69]. In addition, depression and anxiety are associated with increased perception of pain severity, whereas prolonged duration of acute pain leads to increased mood dysregulation [34], [42]. Brain-predicted age biomarkers may capture a more robust picture of the biological consequences of simultaneous pain and sleep disturbances in persons with chronic knee pain.
Limitations
Our study has some limitations. The first is a general limitation of any brain age prediction method that is the regression dilution bias (see Fig. 1). That is why we included chronological age as a covariate [22], [50]. Better preprocessing pipelines and quality control might help improve this problem, but only with generalizable models of the bias we will be able to correct it in individual predictions. Our group is currently working on such models. On top of this, the slope of the linear fit in Fig. 1 could also be affected by the limited range in the chronological age of our sample (44–80 years) since this introduces instability in the estimators of the coefficients of the linear fit (i.e., slop and intercept). Adding participants from much younger chronological ages could lead to less steep slopes of the linear fit. However, the inclusion criteria of this study targeted middle-aged adults or older, for whom knee OA pain is typically prevalent.
Second, the study was cross-sectional, and causality cannot be determined. Future longitudinal studies are needed to understand the clinical significance of predicted brain aging differences, and its ability to predict treatment outcomes in patients with chronic pain and sleep problems. Third, our study focused on people with knee pain, thus generalizability to other types of chronic pain is limited. Future studies, including participants with other specific chronic pain conditions are needed to further elucidate these associations and assist in the development of a brain biomarker specific to each type of chronic pain. Fourth, we observed significant differences in ethnicity/race between the three groups of participants, which is consistent with the existing literature. However, our study was not powered to examine contributions of ethnicity/race to the pain and sleep interactions. Further research is needed to determine whether this relationship differs by ethnicity/race. Finally, evaluation of sleep in this study was based on self-report, and this may differ from findings using objective sleep measures, such as polysomnography or actigraphy, which would provide a more sensitive exploration of the reciprocal relationship between pain and sleep.
It is important to note that DeepBrainNet is a brain age prediction method different from those based on Gaussian Process Regression (GPR) used by our group to find differences in chronic pain groups in previous studies. We also performed our analyses using the GPR-based method but found no significant results. Although the reason for these differences in significance across methods is beyond the scope of the present study, and should be immediately addressed in a future paper, we believe that this could owe to the fact DeepBrainNet was trained on a more diverse and heterogeneous sample, possibly making it more sensitive to seemingly accelerated brain age due to morphometric alterations related to pathologies.
Conclusion
To our knowledge, this is the first investigation implicating brain aging as a mediator of the association between sleep quality and chronic pain in a sample with knee pain. Further mechanistic understanding of the complex, bidirectional relationship between sleep and pain may provide alternative therapeutic targets for treating sleep dysfunction and/or chronic pain conditions. Our findings also suggest that brain predicted age could be a valuable marker of brain aging and a predictor of the risk of mortality in those with chronic pain and sleep disorder.
Funding
This work was supported by NIH/NIA Grants R01AG067757 (YCA); R37AG033906 (RBF). A portion of this work was performed in the McKnight Brain Institute at the National High Magnetic Field Laboratory’s Advanced Magnetic Resonance Imaging and Spectroscopy (AMRIS) Facility, supported by National Science Foundation Cooperative Agreement No. DMR-1157490 and DMR-1644779 and the State of Florida.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
UPLOAD2 participants and study team.
Footnotes
https://www.nipreps.org/smriprep/usage.html.
References
- 1.Alsaadi S.M., McAuley J.H., Hush J.M., Maher C.G. Prevalence of sleep disturbance in patients with low back pain. Eur Spine J. 2011;20:737–743. doi: 10.1007/s00586-010-1661-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alschuler K.N., Theisen-Goodvich M.E., Haig A.J., Geisser M.E. A comparison of the relationship between depression, perceived disability, and physical performance in persons with chronic pain. Eur J Pain. 2008;12:757–764. doi: 10.1016/j.ejpain.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Andersen M.L., Araujo P., Frange C., Tufik S. Sleep Disturbance and Pain: A Tale of Two Common Problems. Chest. 2018;154:1249–1259. doi: 10.1016/j.chest.2018.07.019. [DOI] [PubMed] [Google Scholar]
- 4.Avants B.B., Tustison N., Song G. Advanced Normalization Tools (ANTS) Insight J. 2009:1–35. [Google Scholar]
- 5.Baran T.M., Lin F.V., Geha P. Functional brain mapping in patients with chronic back pain shows age-related differences. Pain. 2022;163:E917–E926. doi: 10.1097/j.pain.0000000000002534. [DOI] [PubMed] [Google Scholar]
- 6.Bashyam V.M., Erus G., Doshi J., Habes M., Nasralah I., Truelove-Hill M., et al. MRI signatures of brain age and disease over the lifespan based on a deep brain network and 14 468 individuals worldwide. Brain. 2020;143:2312–2324. doi: 10.1093/brain/awaa160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellamy N., Buchanan W.W., Goldsmith C.H., Campbell J., Stitt L.W. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 8.Bethlehem R.A.I., Seidlitz J., White S.R., Vogel J.W., Anderson K.M., Adamson C., et al. Brain charts for the human lifespan. Nature. 2022;604:525–533. doi: 10.1038/s41586-022-04554-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biondo F., Jewell A., Pritchard M., Mueller C., Steves C.J., Cole J. Brain‐age predicts subsequent dementia in memory clinic patients: Neuroimaging / Optimal neuroimaging measures for early detection. Alzheimer's Dementia. 2020;16(S5) [Google Scholar]
- 10.Bonvanie I.J., Oldehinkel A.J., Rosmalen J.G.M., Janssens K.A.M. Sleep problems and pain: A longitudinal cohort study in emerging adults. Pain. 2016;157:957–963. doi: 10.1097/j.pain.0000000000000466. [DOI] [PubMed] [Google Scholar]
- 11.Campbell C.M., Buenaver L.F., Finan P., Bounds S.C., Redding M., McCauley L., et al. Sleep, pain catastrophizing, and central sensitization in knee osteoarthritis patients with and without insomnia. Arthritis Care Res. 2015;67:1387–1396. doi: 10.1002/acr.22609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canori A., Amiri A.M., Thapa-Chhetry B., Finley M.A., Schmidt-Read M., Lamboy M.R., et al. Relationship between pain, fatigue, and physical activity levels during a technology-based physical activity intervention. J Spinal Cord Med. 2021;44:549–556. doi: 10.1080/10790268.2020.1766889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cardoso J.S., Riley J.L., Glover T., Sibille K.T., Bartley E.J., Goodin B.R., et al. Experimental pain phenotyping in community-dwelling individuals with knee osteoarthritis. Pain. 2016;157:2104–2114. doi: 10.1097/j.pain.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen C.H., Huang M.C., Chiu Y.H., Chen I.M., Chen C.H., Lu M.L., et al. Stress susceptibility moderates the relationship between eveningness preference and poor sleep quality in non-acute mood disorder patients and healthy controls. Nat Sci Sleep. 2022;14:711–723. doi: 10.2147/NSS.S339898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cole J.H., Franke K. Predicting Age Using Neuroimaging: Innovative Brain Ageing Biomarkers. Trends Neurosci. 2017;40:681–690. doi: 10.1016/j.tins.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Cole J.H., Ritchie S.J., Bastin M.E., Valdés Hernández M.C., Muñoz Maniega S., Royle N., et al. Brain age predicts mortality. Mol Psychiatry. 2018;23:1385–1392. doi: 10.1038/mp.2017.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cruz-Almeida Y., Cardoso J., Riley J.L., Goodin B., King C.D., Petrov M., et al. Physical performance and movement-evoked pain profiles in community-dwelling individuals at risk for knee osteoarthritis. Exp Gerontol. 2017;98:186–191. doi: 10.1016/j.exger.2017.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cruz-Almeida Y., Cole J. Pain, aging, and the brain: new pieces to a complex puzzle. Pain. 2020;161(3):461–463. doi: 10.1097/j.pain.0000000000001757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cruz-Almeida Y., Fillingim R.B., Riley J.L., Woods A.J., Porges E., Cohen R., et al. Chronic pain is associated with a brain aging biomarker in community-dwelling older adults. Pain. 2019;160:1119–1130. doi: 10.1097/j.pain.0000000000001491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cruz-Almeida Y., King C.D., Goodin B.R., Sibille K.T., Glover T.L., Riley J.L., et al. Psychological profiles and pain characteristics of older adults with knee osteoarthritis. Arthritis Care Res. 2013;65:1786–1794. doi: 10.1002/acr.22070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curtis A.F., Williams J.M., McCoy K.J.M., McCrae C.S. Chronic pain, sleep, and cognition in older adults with insomnia: A daily multilevel analysis. J Clin Sleep Med. 2018;14:1765–1772. doi: 10.5664/jcsm.7392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Lange A.M.G., Cole J.H. Commentary: Correction procedures in brain-age prediction. NeuroImage Clin. 2020;26:24–26. doi: 10.1016/j.nicl.2020.102229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drevets W.C., Price J.L., Furey M.L. Brain structural and functional abnormalities in mood disorders: Implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213(1-2):93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eslami V., Zimmerman M.E., Grewal T., Katz M., Lipton R.B. Pain grade and sleep disturbance in older adults: Evaluation the role of pain, and stress for depressed and non-depressed individuals. Int J Geriatr Psychiatry. 2016;31:450–457. doi: 10.1002/gps.4349. [DOI] [PubMed] [Google Scholar]
- 25.Esteban O., Markiewicz C.J., Blair R.W., Moodie C.A., Isik A.I., Erramuzpe A., et al. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat Methods. 2019;16:111–116. doi: 10.1038/s41592-018-0235-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fang H., Tu S., Sheng J., Shao A. Depression in sleep disturbance: A review on a bidirectional relationship, mechanisms and treatment. J Cell Mol Med. 2019;23(4):2324–2332. doi: 10.1111/jcmm.14170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finan P.H., Goodin B.R., Smith M.T. The association of sleep and pain: An update and a path forward. J Pain. 2013;14:1539–1552. doi: 10.1016/j.jpain.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fjell A.M., Sneve M.H., Grydeland H., Storsve A.B., de Lange A.M.G., Amlien I.K., et al. Functional connectivity change across multiple cortical networks relates to episodic memory changes in aging. Neurobiol Aging. 2015;36:3255–3268. doi: 10.1016/j.neurobiolaging.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 29.Fjell A.M., Walhovd K.B. Structural brain changes in aging: courses, causes and cognitive consequences. Rev Neurosci. 2010;21:187–221. doi: 10.1515/revneuro.2010.21.3.187. [DOI] [PubMed] [Google Scholar]
- 30.Fjell A.M., Westlye L.T., Grydeland H., Amlien I., Espeseth T., Reinvang I., et al. Critical ages in the life course of the adult brain: Nonlinear subcortical aging. Neurobiol Aging. 2013;34:2239–2247. doi: 10.1016/j.neurobiolaging.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frangou S. Brain structural changes in mood disorders. Psychiatry. 2009;8(3):105–106. [Google Scholar]
- 32.Goldstein A.N., Walker M.P. The role of sleep in emotional brain function. Annu Rev Clin Psychol. 2014;10(1):679–708. doi: 10.1146/annurev-clinpsy-032813-153716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herrero Babiloni A., De Koninck B.P., Beetz G., De Beaumont L., Martel M.O., Lavigne G.J. Sleep and pain: recent insights, mechanisms, and future directions in the investigation of this relationship. J Neural Transm. 2020;127(4):647–660. doi: 10.1007/s00702-019-02067-z. [DOI] [PubMed] [Google Scholar]
- 34.Humo M., Lu H., Yalcin I. The molecular neurobiology of chronic pain–induced depression. Cell Tissue Res. 2019;377(1):21–43. doi: 10.1007/s00441-019-03003-z. [DOI] [PubMed] [Google Scholar]
- 35.Hung P.-P., Zhang J.Y., Noorani A., Walker M.R., Huang M., Zhang J.W., et al. Differential expression of a brain aging biomarker across discrete chronic pain disorders. Pain Publish Ah. 2022;163(8):1468–1478. doi: 10.1097/j.pain.0000000000002613. [DOI] [PubMed] [Google Scholar]
- 36.Johnson A.J., Cole J., Fillingim R.B., Cruz-Almeida Y. Persistent non-pharmacological pain management and brain-predicted age differences in middle-aged and older adults with chronic knee pain. Front Pain Res. 2022;3:1–8. doi: 10.3389/fpain.2022.868546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krause A.J., Prather A.A., Wager T.D., Lindquist M.A., Walker M.P. The pain of sleep loss: A brain characterization in humans. J Neurosci. 2019;39:2291–2300. doi: 10.1523/JNEUROSCI.2408-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuchinad A., Schweinhardt P., Seminowicz D.A., Wood P.B., Chizh B.A., Bushnell M.C. Accelerated brain gray matter loss in fibromyalgia patients: Premature aging of the brain? J Neurosci. 2007;27:4004–4007. doi: 10.1523/JNEUROSCI.0098-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leung P., Li S.H., Graham B.M. The relationship between repetitive negative thinking, sleep disturbance, and subjective fatigue in women with Generalized Anxiety Disorder. Br J Clin Psychol. 2022;61(3):666–679. doi: 10.1111/bjc.12356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lorenz R.A., Budhathoki C.B., Kalra G.K., Richards K.C. The relationship between sleep and physical function in community-dwelling adults: A pilot study. Fam Community Heal. 2014;37:298–306. doi: 10.1097/FCH.0000000000000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McBeth J., Lacey R.J., Wilkie R. Predictors of new-onset widespread pain in older adults. Arthritis Rheumatol. 2014;66:757–767. doi: 10.1002/art.38284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Michaelides A., Zis P. Depression, anxiety and acute pain: links and management challenges. Postgrad Med. 2019;131(7):438–444. doi: 10.1080/00325481.2019.1663705. [DOI] [PubMed] [Google Scholar]
- 43.Montesino-Goicolea S., Valdes-Hernandez P.A., Cruz-Almeida Y., Hu L.i. Chronic musculoskeletal pain moderates the association between sleep quality and dorsostriatal-sensorimotor resting state functional connectivity in community-dwelling older adults. Pain Res Manag. 2022;2022:1–12. doi: 10.1155/2022/4347759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Montesino-Goicolea S., Valdes-Hernandez P.A., Hoyos L., Woods A.J., Cohen R., Huo Z., et al. Cortical thickness mediates the association between self-reported pain and sleep quality in community-dwelling older adults. J Pain Res. 2020;13:2389–2400. doi: 10.2147/JPR.S260611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nicassio P.M., Ormseth S.R., Kay M., Custodio M., Irwin M.R., Olmstead R., et al. The contribution of pain and depression to self-reported sleep disturbance in patients with rheumatoid arthritis. Pain. 2012;153:107–112. doi: 10.1016/j.pain.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O’Brien E.M., Waxenberg L.B., Atchison J.W., Gremillion H.A., Staud R.M., McCrae C.S., et al. Negative mood mediates the effect of poor sleep on pain among chronic pain patients. Clin J Pain. 2010;26:310–319. doi: 10.1097/AJP.0b013e3181c328e9. [DOI] [PubMed] [Google Scholar]
- 47.Paixao L., Sikka P., Sun H., Jain A., Hogan J., Thomas R., et al. Excess brain age in the sleep electroencephalogram predicts reduced life expectancy. Neurobiol Aging. 2020;88:150–155. doi: 10.1016/j.neurobiolaging.2019.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patel A.R., Patel A.R., Singh S., Singh S., Khawaja I. The Association of Obstructive Sleep Apnea and Hypertension. Cureus. 2019;4–7 doi: 10.7759/cureus.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petrov M.E., Goodin B.R., Cruz-Almeida Y., King C., Glover T.L., Bulls H.W., et al. Disrupted sleep is associated with altered pain processing by sex and ethnicity in knee osteoarthritis. J Pain. 2015;16:478–490. doi: 10.1016/j.jpain.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Popescu S.G., Glocker B., Sharp D.J., Cole J.H. Local brain-age: A U-net model. Front Aging Neurosci. 2021;13:1–17. doi: 10.3389/fnagi.2021.761954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Post M.W. What to Do with “moderate” reliability and validity coefficients? Arch Phys Med Rehabil. 2016;97(7):1051–1052. doi: 10.1016/j.apmr.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 52.PROMIS Health Organization PROMIS Cooperative Group, 2020. Measure Development & Research.
- 53.Ravyts S.G., Dzierzewski J.M., Grah S.C., Buman M.P., Aiken-Morgan A.T., Giacobb P.R., et al. Sleep and Pain in Mid- to Late-Life: An Exploration of Day-to-Day Pain Inconsistency. Clin Gerontol. 2018;41(2):123–129. doi: 10.1080/07317115.2017.1345818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roux C.H., Saraux A., Mazieres B., Pouchot J., Morvan J., Fautrel B., et al. Screening for hip and knee osteoarthritis in the general population: predictive value of a questionnaire and prevalence estimates. Ann Rheum Dis. 2008;67(10):1406–1411. doi: 10.1136/ard.2007.075952. [DOI] [PubMed] [Google Scholar]
- 55.Sabeti S., Al-Darsani Z., Mander B.A., Corrada M.M., Kawas C.H. Sleep, hippocampal volume, and cognition in adults over 90 years old. Aging Clin Exp Res. 2018;30:1307–1318. doi: 10.1007/s40520-018-1030-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Skougaard M., Jørgensen T.S., Rifbjerg-Madsen S., Coates L.C., Egeberg A., Amris K., et al. Relationship between Fatigue and Inflammation, Disease Duration, and Chronic Pain in Psoriatic Arthritis: An Observational DANBIO Registry Study. J Rheumatol. 2020;47:548–552. doi: 10.3899/jrheum.181412. [DOI] [PubMed] [Google Scholar]
- 57.Smith S.M., Elliott L.T., Alfaro-Almagro F., McCarthy P., Nichols T.E., Douaud G., et al. Brain aging comprises many modes of structural and functional change with distinct genetic and biophysical associations. Elife. 2020;9:1–28. doi: 10.7554/eLife.52677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sörös P., Bantel C. Chronic noncancer pain is not associated with accelerated brain aging as assessed by structural magnetic resonance imaging in patients treated in specialized outpatient clinics. Pain. 2020;161:641–650. doi: 10.1097/j.pain.0000000000001756. [DOI] [PubMed] [Google Scholar]
- 59.Szegedy C., Ioffe S., Vanhoucke V., Alemi A.A. Proceedings of the Thirty-First AAAI Conference on Artificial Intelligence, AAAI’17. AAAI Press; 2017. Inception-v4, inception-ResNet and the impact of residual connections on learning; pp. 4278–4284. [Google Scholar]
- 60.Tighe C.A., Youk A., Ibrahim S.A., Weiner D.K., Vina E.R., Kwoh C.K., et al. Pain catastrophizing and arthritis self-efficacy as mediators of sleep disturbance and osteoarthritis symptom severity. Pain Med (United States) 2020;21:501–510. doi: 10.1093/pm/pnz187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Timmers I., Quaedflieg C.W.E.M., Hsu C., Heathcote L.C., Rovnaghi C.R., Simons L.E. The interaction between stress and chronic pain through the lens of threat learning. Neurosci Biobehav Rev. 2019;107:641–655. doi: 10.1016/j.neubiorev.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Torrecillas-Martínez L., Catena A., O’Valle F., Padial-Molina M., Galindo-Moreno P. Does experienced pain affects local brain volumes? Insights from a clinical acute pain model. Int J Clin Heal Psychol. 2019;19:115–123. doi: 10.1016/j.ijchp.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Travaglini L.E., Kuykendall L., Bennett M.E., Abel E.A., Lucksted A. Relationships between chronic pain and mood symptoms among veterans with bipolar disorder. J Affect Disord. 2020;277:765–771. doi: 10.1016/j.jad.2020.08.069. [DOI] [PubMed] [Google Scholar]
- 64.Tsuno N., Besset A., Ritchie K. Sleep and depression. J Clin Psychiatry. 2005;66(10):1254–1269. doi: 10.4088/jcp.v66n1008. [DOI] [PubMed] [Google Scholar]
- 65.Tustison N.J., Avants B.B., Cook P.A., Zheng Y., Egan A., Yushkevich P.A., et al. N4ITK: Improved N3 bias correction. IEEE Trans Med Imaging. 2010;29:1310–1320. doi: 10.1109/TMI.2010.2046908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Valrie C.R., Gil K.M., Redding-Lallinger R., Daeschner C. Brief report: Daily mood as a mediator or moderator of the pain-sleep relationship in children with sickle cell disease. J Pediatr Psychol. 2008;33:317–322. doi: 10.1093/jpepsy/jsm058. [DOI] [PubMed] [Google Scholar]
- 67.Von Korff M., Ormel J., Keefe F.J., Dworkin S.F. Grading the severity of chronic pain. Pain. 1992;50:133–149. doi: 10.1016/0304-3959(92)90154-4. [DOI] [PubMed] [Google Scholar]
- 68.Whibley D., AlKandari N., Kristensen K., Barnish M., Rzewuska M., Druce K.L., et al. Sleep and Pain: A Systematic Review of Studies of Mediation. Clin J Pain. 2019;35(6):544–558. doi: 10.1097/AJP.0000000000000697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wilczyńska K., Simonienko K., Konarzewska B., Szajda S., Waszkiewicz N. Morphological changes of the brain in mood disorders. Psychiatr Pol. 2018;52(5):797–805. doi: 10.12740/PP/89553. [DOI] [PubMed] [Google Scholar]
- 70.Yu L., Buysse D.J., Germain A., Moul D.E., Stover A., Dodds N.E., et al. Development of short forms from the PROMISTM sleep disturbance and sleep-related impairment item banks. Behav Sleep Med. 2011;10:6–24. doi: 10.1080/15402002.2012.636266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zarrabian M.M., Johnson M., Kriellaars D. Relationship between sleep, pain, and disability in patients with spinal pathology. Arch Phys Med Rehabil. 2014;95:1504–1509. doi: 10.1016/j.apmr.2014.03.014. [DOI] [PubMed] [Google Scholar]