Summary
Cyanobacteria have a long evolutionary history, well documented in marine rocks. They are also abundant and diverse in terrestrial environments; however, although phylogenies suggest that the group colonized land early in its history, paleontological documentation of this remains limited. The Rhynie chert (407 Ma), our best preserved record of early terrestrial ecosystems, provides an opportunity to illuminate aspects of cyanobacterial diversity and ecology as plants began to radiate across the land surface. We used light microscopy and super-resolution confocal laser scanning microscopy to study a new population of Rhynie cyanobacteria; we also reinvestigated previously described specimens that resemble the new fossils. Our study demonstrates that all are part of a single fossil species belonging to the Hapalosiphonaceae (Nostocales). Along with other Rhynie microfossils, these remains show that the accommodation of morphologically complex cyanobacteria to terrestrial ecosystems transformed by embryophytes was well underway more than 400 million years ago.
Subject areas: Earth sciences, Paleontology, Paleobiology
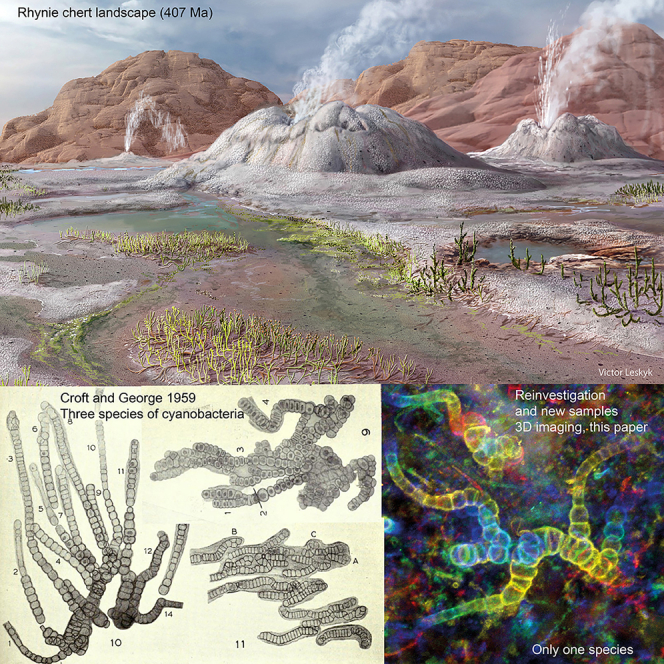
Graphical abstract
Highlights
-
•
New and previously described Devonian cyanobacteria are part of a single species
-
•
Confocal microscopy permits detailed 3D reconstruction of complex cyanobacteria
-
•
Nostocales thrived among early land plants much as their extant relatives do today
Earth sciences; Paleontology; Paleobiology
Introduction
Cyanobacteria, the only group to have evolved oxygenic photosynthesis, originated early in our planet’s history and still accounts for an estimated 25 percent of global primary production.1 Because cyanobacteria were the principal source of dioxygen gas on the early Earth, the Great Oxygenation Event, beginning ca. 2.4 Ga, provides a minimum date for cyanobacterial origins.2 Geochemical signatures of regional, transient oxygenation in older sedimentary rocks suggest a still earlier origin of the clade,3,4 consistent with molecular clocks that point to an Archean emergence of crown group cyanobacteria (e.g.,5,6,7). Although most early cyanobacterial fossils occur in rocks deposited in marine environments, phylogenies indicate that the group colonized non-marine habitats early on.5,6,7 Indeed, fossils of cyanobacteria have been reported from late Mesoproterozoic shales interpreted as non-marine,8,9 although a marine influence has been suggested for at least some of these deposits.10,11 In any event, Gloeobacter, the sister of all other extant cyanobacteria, lives on rock surfaces,12 and a close relative of Gloeobacter, recently identified from metagenomic data, was also discovered in a non-marine environment.13 Moreover, the cyanobacterial endosymbiont that gave rise to photosynthesis in eukaryotes is also inferred to have lived in a low salinity habitat.6,14,15
The preceding paragraph makes it clear that the Paleozoic radiation of embryophytes was not life’s initial colonization of the land surface by phototrophs but rather a much younger event that transformed terrestrial and freshwater ecosytems established billions of years earlier.16 With this in mind, we can view the 407.1 ± 2.2 Ma (40Ar/39Ar age) Rhynie chert17—biostratigraphic age of Pragian-?earliest Emsian18—as one of the Scotland’s geological jewels (e.g.,19,20), not only as a chronicle of early land plant evolution (e.g.,21,22,23,24,25,26), but also as a record of cyanobacterial accommodation to the changing face of terrestrial habitats.
Cyanobacteria are relatively common and diverse elements of the Rhynie biota (e.g.,27,28,29) frequently encountered in ever wet or episodically flooded parts of the local environment.30 Several colony-forming coccoidal taxa have been reported,28,31,32,33,34,35 but filamentous cyanobacteria are more common. Simple filaments, preserved as trichomes or cylindrical sheaths, were first recognized by Kidston and Lang,23 who described Archaeothrix contexta and A. oscillatoriformis, which closely resemble living oscillatorian cyanobacteria. The latter species is either occasionally35 or more commonly27 observed within necrotic lesions in axes of Rhynie plants. Several other described cyanobacteria have also been referred to the Oscillatoriales,34,35,36,37 including one suggested to have developed a symbiotic association with the embryophyte Aglaophyton majus38 and interpreted as an on-again-off-again association linked to episodic flooding. However, whether or not the plant axes were alive when colonized remains an open question.
The Nostocales, distinguished among cyanobacteria by both their morphological complexity and high diversity, have also been observed in the Rhynie cherts. Croft and George39 discovered true-branching cyanobacteria in a single minute chip of chert measuring 1mm in thickness and nearly 5 mm across. They erected two species, Langiella scourfieldii and Kidstoniella fritschii, which they placed within the Stigonematales, as well as a third, Rhyniella vermiformis, which they excluded from this clade because evidence of branching was, at best, incomplete. Traditionally, cyanobacteria with true branching were divided into two classes, the Nostocales and Stigonematales, based on whether filaments were uni- or multiseriate. Molecular phylogenies, however, show that the stigonematalean clade nestles within the Nostocales.40 Recently Krings29 described Rhystigonema obscurum from the Rhynie chert, a multiseriate, true-branching form, placed with confidence within the Stigonemataceae.
Here we describe another population of nostocalean cyanobacteria, discovered in Rhynie chert thin sections from the collections of Sorbonne University, Paris. In order to address the difficulties of reconstructing thallus morphology of these minute organisms, we use light microscopy and confocal laser scanning microscopy (CLSM). Similarities between these new fossils and those described by Croft and George39 prompted a reinvestigation of their original material. Our study shows that the newly discovered fossils and the three species described by Croft and George are part of a single fossil species, belonging to the Hapalosiphonaceae (Nostocales) that thrived in soils, freshwater, and hot springs, much as their extant relatives do today.41
Results
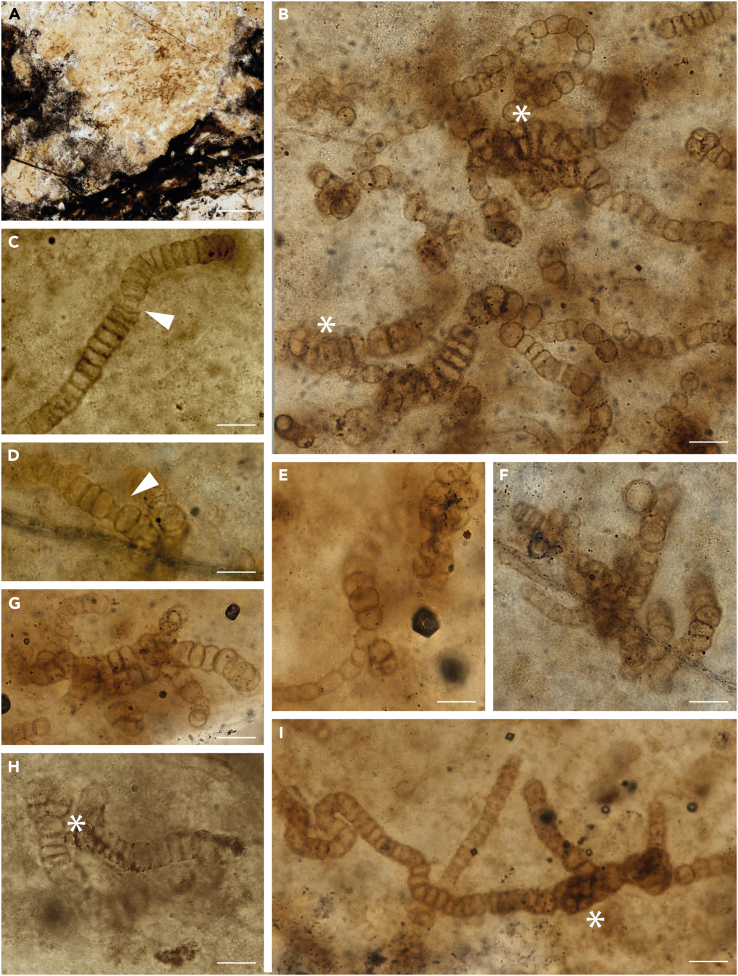
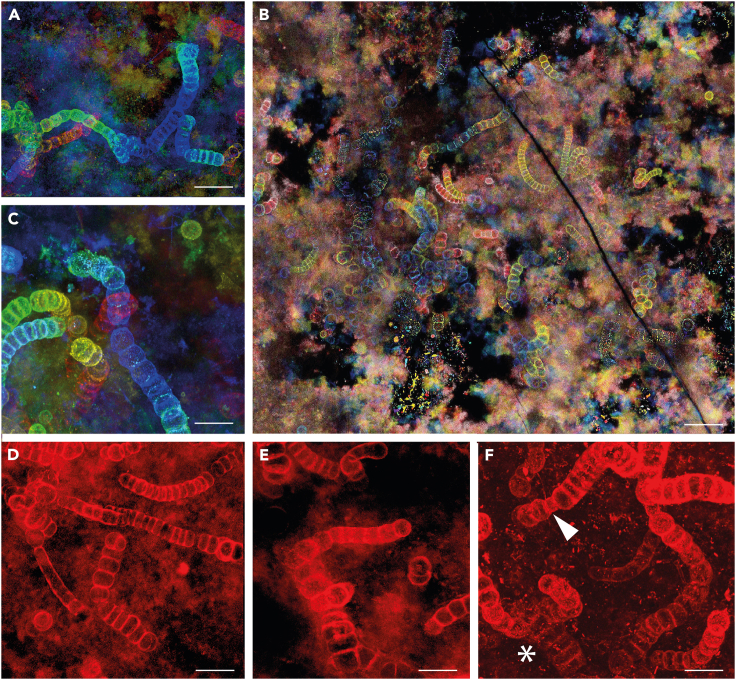
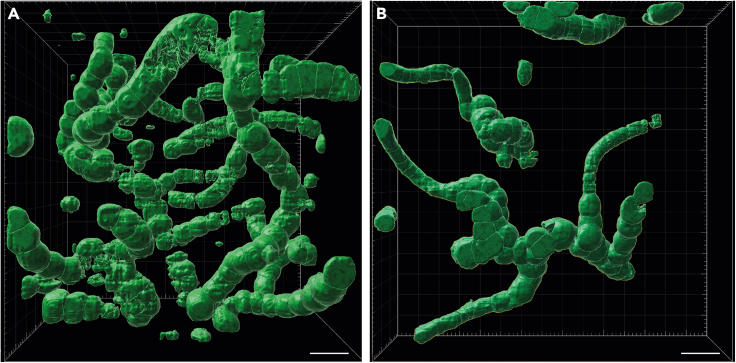
We studied fossils from thin sections of samples collected from the famous Rhynie chert in Scotland. The cyanobacteria reported here occur as part of a ground cover comprising amorphous material (mucilage), small tubular filaments of possible cyanobacterial affinity (possibly an oscillatorian mat) and plant microdebris (Figures 1A, 2B, S1A, and S2B). The newly discovered fossils (Figures 1, 2, 3, 4, S1, and S2) resemble Rhynie cyanobacteria described decades ago by Croft and George39 (Figure 5). Comparison with the three species described by these authors resulted in a revised view of cyanobacterial diversity and taxonomy in the Rhynie chert. The cyanobacteria are represented by uniseriate (Figures 1B–1I, 2, 3, 4, and S2B–S2E) or lightly multiseriate trichomes (asterisks in Figures 1B, 1H, 2F, 2I, and S2F). Associated short uniseriate trichomes resemble hormogonia (i.e., structures specialized for dissemination) produced by some nostocalean cyanobacteria (Figures 1C, 2B, 2D, and S2D). Trichomes show true branching with a differentiation of prostrate and erect portions (Figures 1F, 1G; 2A, 2E, 2F, 3, and 4B). 3D movies obtained from the stack of CSLM images unequivocally show that what is observed in light microscopy corresponds to true branching and not to an overlap of filaments (Figure 4B, Videos S1 and S2). As detailed below, the combination of characters in our new fossils leads us to consider them as part of the same fossil species divided into three by Croft and George and to attribute all of them to Langiella scourfieldii emend.
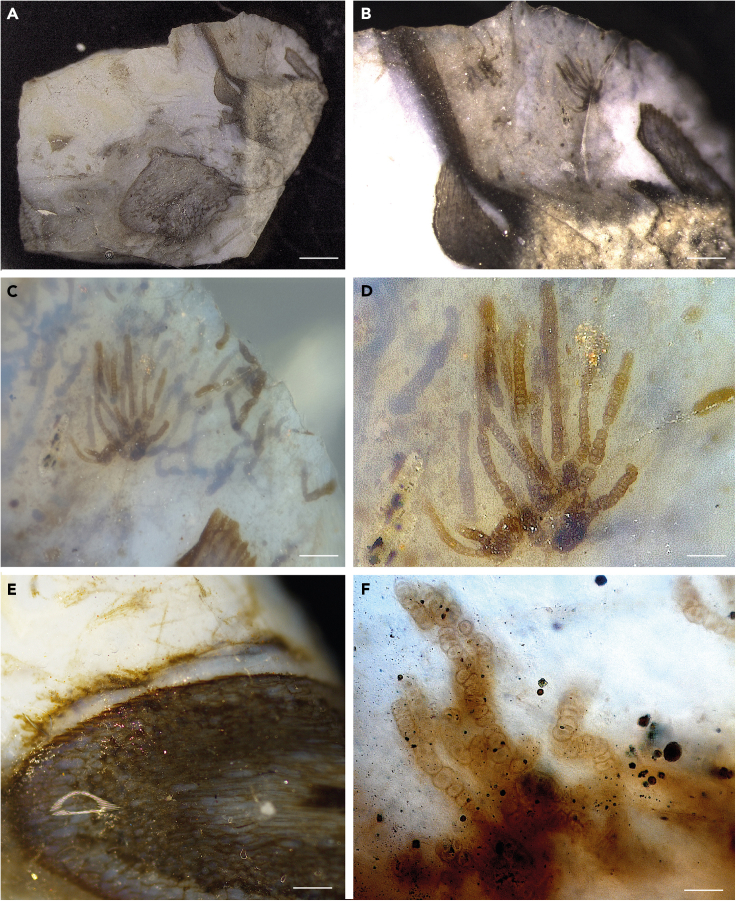
Figure 1.
Light microscopy images of Langiella scourfieldii (Croft and George 1959, emend. Strullu-Derrien and Knoll)
(A) Cyanobacteria as part of a ground cover; dark areas represent plant remains. (B–I) Filaments are mainly uniseriate, sometimes slightly multiseriate (asterisks in B and H). Filaments densely packed and interlaced with one another (B). Short uniseriate trichome resembling hormogonia (C). Necridia visible on some of the filaments (arrow in C, D). A–H: from thin section SU.PB. 2023.0.1.2.8; I: from thin section SU.PB. 2023.0.1.2.9. Scale bars: 0.31mm (A), 20 μm (F, I), 14 μm (C, D, E, H), 18 μm (B, G).
Figure 2.
Confocal Laser Scanning Images of Langiella scourfieldii (Croft and George 1959, emend. Strullu-Derrien and Knoll)
False-colored for z stack depth (A–C). Cyanobacteria as part of the ground cover (B) showing branching (A, C–F). Necridia visible on some of the filaments (F). From thin section SU.PB. 2023.0.1.2.8. Scale bars: 25 μm (A), 50 μm (B),16 μm (C, F), 20 μm (D), 14 μm (E).
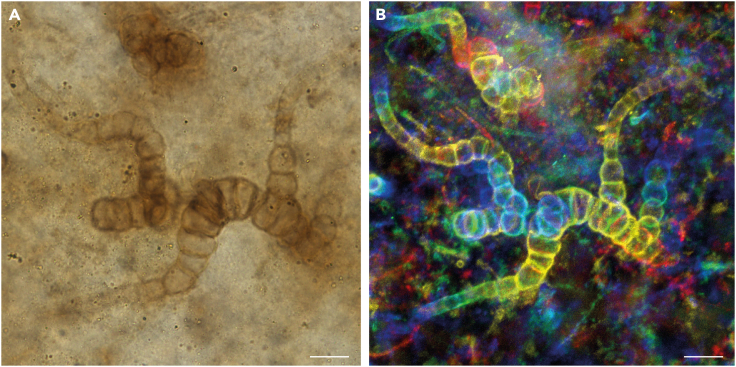
Figure 3.
Light microscopy (A) and confocal laser scanning, false-colored for z stack depth (B) images of heterotrichous thallus of Langiella scourfieldii (Croft and George 1959, emend. Strullu-Derrien and Knoll)
Branches with cells tending to be smaller and barrel-shaped. From thin section SU.PB. 2023.0.1.2.8. Scale bars: 12 μm (A), 14 μm (B).
Figure 4.
Confocal laser scanning images of Langiella scourfieldii (Croft and George 1959, emend. Strullu-Derrien and Knoll) in one plane from movies
(A from Video S1; B from Video S2). From thin section SU.PB. 2023.0.1.2.8. Scale bars: 15 μm (A), 18 μm (B).
Figure 5.
Light microscopy images of cyanobacteria from the chip studied by Croft and George (1959)
(A) Overview of one face of the chip.
(B) Higher magnification of the upper right part of image A.
(C) Langiella scourfieldii (holotype) on the left and Rhyniella vermiformis (holotype) on the right.
(D) Higher magnification of Langiella scourfieldii (holotype).
(E) From the other side of the chip: Kidstoniella fritschii (holotype) close to the plant axis but without physical contact. From specimen NHMUK V32409. Scale bars: 0.55 mm (A); 210 μm (B, E); 87 μm (C); 44 μm (D); 22 μm (F).
From thin section SU.PB. 2023.0.1.2.8 (https://doi.org/10.5281/zenodo.7845413).
From thin section SU.PB. 2023.0.1.2.8 (https://doi.org/10.5281/zenodo.7845413).
Systematics
Description: Filaments up to 175 μm long, densely packed (Figures 1A, 1B, and S1B), and commonly interlaced with one another (Figures 1B, 2B, 4A, and S2C) or occasionally more isolated (Figure S1C). They are mainly uniseriate filaments with true branching (Figures 1F, 1G; 2A, 2E, 2F, 3, and 4B). Main axes can be lightly multiseriate (Figures 1B, 1E, 2D, 2F, and S1E), with rounded cells wider than high, 10 to 12.5 μm in maximum dimension. Branches are uniseriate; constituent cells higher than wide tend to be smaller and barrel-shaped (5 μm in their maximum dimension) (Figures 1E, 3A, 3B, and 4B). No obvious evidence of heterocysts or akinetes, although it is hard to rule out little differentiated heterocysts (yet, no cell wall thickening that often occurs to protect heterocysts from atmospheric oxygen was observed). No sheath has been observed around the filaments. Rarely, filaments seem to show a multiseriate end (Figures 2A and 2D), which could reflect incipient branching. Small rounded cells, 5 μm in their larger dimension, occur along some filaments (Figures 1C, 1D, 2F, 4B, and S2D).
Taxonomy
Division: Cyanobacteria.
Order: Nostocales Borzi42
Family: Hapalosiphonaceae Elenkin43
Genus: Langiella Croft and George,39 emend. Strullu-Derrien and Knoll.
Emended diagnosis: Thallus heterotrichous, with true branching; main axis uniseriate or lightly multiseriate, showing rounded cells; branches generally uniseriate, with smaller, commonly barrel-shaped cells. Smaller rounded cells (necridia) present on some filaments. Short uniseriate trichomes resemble hormogonia.
Type species: Langiella scourfieldii Croft and George,39 emend. Strullu-Derrien and Knoll (Figures 1, 2, 3, and 4).
Species: Langiella scourfieldii Croft and George,39 emend. Strullu-Derrien and Knoll.
Emended diagnosis: As for the genus and rounded cells in main axis 10–12.5 μm in maximum dimension and cells in branches ca. 5 μm in maximum dimension.
Epitype: hic designatus, Pôle Collections scientifiques et patrimoniales, Bibliothèque de Sorbonne Université: assemblage from thin section SU.PB. 2023.0.1.2.8. Figures 1A–1H, 2, 3, and 4.
Holotype: Langiella scourfieldii Croft and George39 (Bulletin of the British Museum (Natural History) Geology 3: 342–343) – Type illustrated in Plate 41, Figure 10. Specimen pro parte NHMUK V32409!
= Kidstoniella fritschii Croft and George39 – Type illustrated in Plate 41, Figure 9. Specimen pro parte NHMUK V32409!
= Rhyniella vermiformis Croft and George39 – Type illustrated in Plate 41, Figure 11. Specimen pro parte NHMUK V32409!
Locality: Rhynie, northwest of Aberdeen (Scotland, UK)
Age: Early Devonian (407.1 ± 2.2 Ma)17
Other materials examined that are attributable to Langiella scourfieldii Croft and George, emend. Strullu-Derrien & Knoll are the specimens considered and illustrated here (Figures 1I and S2) from assemblages in thin section SU.PB. 2023.0.1.2.9, also housed in the same repository as the epitype.
Epitypification is necessary because the holotype is ambiguous: there are no evident sheaths, heterocysts, or akinetes as stated by Croft and George, the size of the filaments is not correct (likely Croft and George included in the measurement what they interpreted as a sheath). The epitype shows the form of the filaments and the connection of filament types more clearly. 3D movies also equivocally show true branching and not overlapping of filaments. Necridia were not reported (these were interpreted as heterocysts by Croft and George).
Our nomenclature follows the Shenzhen Code.44 Since the three species names were simultaneously published, they compete for priority. In accordance with Article 11.5 we, therefore, choose one of these to establish priority. We choose Langiella scourfieldii because it is the first specimen named in the original publication by Croft and George.39 Our higher level classification follows Cavalier-Smith45 and Komárek et al.40
Discussion
Comparison with the three species described by Croft & George39
The newly discovered fossils resemble Langiella scourfieldii Croft and George in most of their characters (Figures 5C and 5D). Croft and George39 reported that the compacted cells of the basal system of Langiella scourfieldii are generally broader and thicker-walled than those composing the erect filaments. Reinvestigation of the original specimens shows that it is impossible to confirm that statement. Croft and George39 (p. 341) themselves wrote that “Examination and illustration of the plants are rendered difficult by the thickness of the chip and the amount of debris contained in it … Moreover, the cell walls often contrast little with the matrix and the outlines and visibility of the cells and of the sheaths vary considerably with the setting of the mirror and the width of the illuminating cone”. Our specimens show variations of size and shape in some of the erect branches (Figures 3 and S2F), similar to a pattern observed by Croft and George in filaments 2 and 5 of their figure 10 of plate 41. We remeasured the specimens illustrated plate 41 figure 10 in Croft and George’s publication (Figures 5C and 5D). The diameter of the prostrate axes is up to 26 μm and not 40 μm; the diameter of the prostrate cells is up to 12.5 μm and not 20 μm; the maximum width of the trichome is 12.5 μm not 16 μm as reported in the publication. These new measurements fit well with measurements of the newly discovered specimens.
Croft and George39 reported the occurrence of a sheath around the trichomes. Our reinvestigation based on light microscopy shows that no sheath is present, although filaments may have a variably thin coat of amorphous, mucilage-like organic matter (Figure 5D). Croft and George39 also identified a limited number of distinctly small cells in their population and interpreted them as heterocysts. Nostocalean cyanobacteria are well known for their capacity to differentiate distinct cell types along filaments. Akinetes, specialized reproductive structures, are commonly larger than surrounding vegetative cells and can be spheroidal or elongated along the axis of the filament or branch. Akinetes also commonly have a distinct granular internal structure that distinguishes them under the light microscope. Heterocysts, cells specialized for nitrogen fixation, may also have distinctive morphology, although generally less pronounced than that of akinetes. Heterocysts can be more rounded, more elongate, slightly larger, or slightly smaller than associated vegetative cells. And, like akinetes, they appear as distinctive structures under the light microscope due to their yellowish color and distinctive polar plugs. Of the distinguishing features of akinetes and heterocysts, only distinctive morphology is likely to be recognizable in the fossil record. To this end, we find diminutive cells like those noted by Croft and George39 in our population (Figures 1C, 1D, and 2F), but suggest that they are necridia (cells whose death, self-organized or by accident, allows division of trichomes46) rather than heterocysts. Indeed, while Komárek et al.47 (Figure 16) noted relatively small heterocysts in extant populations of Hapalosiphon arboreus, we know of no heterocysts among living cyanobacteria with a size as small as that of the Rhynie necridic cells.
Our newly described fossils also bear close similarities to Kidstoniella fritschii (Figures 5E and 5F), differing largely in the absence of internal contents within cells. The postmortem collapse of cell contents within cyanobacteria is well established from both paleontological and experimental studies (e.g.,48), with the complete loss of internal contents a predictable endmember. Therefore, we interpret the differences between cells with and without contents as a taphonomic feature. Kidstoniella was also considered distinct from Langiella on the basis of occasional branching of putatively erect branches, a character also seen in our newly observed specimens (Figures 1B and 2F). Given that the orientation of branches was strongly affected by burial, it is difficult to ascertain whether or not this feature might reflect branching of the main axis. Croft and George39 described Kidstoniella fritschii as epiphytic; however, the cyanobacteria are not in physical contact with the adjacent plant axis, thus providing little evidence for an epiphytic lifestyle.
The newly observed specimens also show features similar to Rhyniella vermiformis (Figures 1B, 1G, 1F, and 1I). Croft and George39 described this single specimen as a distinct species, but it is more parsimoniously interpreted as a fragmented specimen of Langiella scourfieldii. Alternatively, modern hapalosiphacean cyanobacteria commonly reproduce by releasing Oscillatoria-like hormogonia,49 providing another possibility for interpretation. Although Croft and George39 reported a multicellular tip of the Rhyniella trichome, we have not been able to confirm this.
In combination, these observations lead us to consider the newly discovered fossils as part of the same fossil species divided into three by Croft and George39 and to attribute all to Langiella scourfieldii emend. This conclusion is consistent with the close physical proximity of all three taxa, originally described.
Phylogenetic relationships of the fossil cyanobacteria
Croft and George39 assigned two of their three described species to the Stigonemataceae. True branching unambiguously places the augmented microfossil population within the Nostocales, but the uniseriate to modestly multiseriate main axis and multiple uniseriate branches suggest placement with the Hapalosiphonaceae, another family within the order. As noted above, the single simple trichome ascribed by Croft and George39 to Rhyniella, is readily interpreted as a fragmental specimen or hormogonium from the same population.
Several hapalosiphonacean genera are common in non-marine environments, including Fischerella, Hapalosiphon, Westiella, and Westiellopsis, and as Komarek et al.40 stress—as did Geitler50 before them—it can be challenging to differentiate among these genera on the basis of morphology alone, especially in the fossil record, where features such as cell ultrastructure are not preserved. For this reason, we have confidence in the family relationships of Langiella scourfieldii emend but refrain from trying to ally it to any particular extant genus.
Nostocalean cyanobacteria have the capacity to differentiate cells specialized for either nitrogen fixation (heterocysts) or reproduction (akinetes). Our population does not unambiguously exhibit either of these cell types, but it bears mention that such cells occur only rarely in some extant taxa and, if not clearly distinct in terms of morphology, could be hard to distinguish in fossils. This absence does not influence our systematic interpretation, as membership in the Hapalosiphonaceae is demonstrated by their heterotrichous habit and uniseriate branches. In fact, cell differentiation in nostocalean cyanobacteria is induced by environmental triggers and so will not be present in all populations. Akinete differentiation has been related to a number of triggers, including phosphorus deficiency,51,52 and heterocyst development commonly reflects nitrogen starvation.53 We have no independent insights into nutrient status in the Rhynie environment, but the abundance of decaying vegetation is consistent with the hypothesis that local cyanobacteria were not nutrient stressed.
Importance of cyanobacteria in early terrestrial environments
Our new fossils, along with those described by Croft and George,39 clearly document distinctive, morphologically complex Nostocales in wet soils, warm springs and/or episodically inundated soils of the time. And, as noted above, further evidence of Rhynie nostocaleans has been provided by Krings,29 who described clearly multiseriate populations similar to those of extant Stigonema. Loron et al.54 also provide an illustration of a multiseriate cyanobacterial filament from the Rhynie chert. In today’s world, true-branching Nostocalean cyanobacteria largely inhabit non-marine to brackish water habitats (e.g.,50), and so it is not surprising that the oldest currently recognized Hapalosiphonaceae (this paper) and Stigonemataceae29 occur in the Rhynie chert, our earliest clear view of emerging terrestrial ecosystems. That said, possible stigonematacean fossils have been reported from older marine shales,55 perhaps most convincingly multiseriate, true-branching filaments found in ca. one billion-year-old rocks of the Mbuji-Mayi Supergroup, Democratic Republic of the Congo.56 Thus, complex Nostocalean cyanobacteria may well have a much longer, but seldom recorded evolutionary history, as implied by some molecular clock studies (e.g.,5,7). Along with an increasing number of oscillatorian and coccoid taxa (e.g.,28,33,34,35,37), these fossils attest to the importance and diversity of cyanobacteria in early terrestrial ecosystems. Despite the emergence of embryophytes as both competitors for space and nutrients and new structural elements in ecosystems of increasing spatial complexity, cyanobacteria continued to thrive both within Rhynie hot springs and in adjacent wetlands as they do today in comparable terrestrial environments. Able to colonize rapidly, nostocalean cyanobacteria thrived in flooded surfaces with sedimented plant debris.
Limitations of the study
These are induced by the preparation of the original material (chip and thin sections) that cannot be modified because of the risk of damaging or destroying the fossil specimens.
Conclusions
Today, cyanobacteria are important players in extant continental hot springs where they contribute to the development of a favorable environment for further biological establishment. The 407-million-year-old Rhynie chert shows that this was true, as well, 407 million years ago. The Nostocalean cyanobacteria here described allow us to reinterpret three cyanobacterial species previously described and recognize all as part of a single fossil species attributable to the Hapalosiphonaceae. This study adds to our knowledge of photosynthetic prokaryotes in early terrestrial environments and shows something of their diversity. Continuing research should include structural and biogeochemical studies at the nanoscale, as well as investigating the possible role these early cyanobacteria played in Rhynie biomineralization and biogeochemistry of their environment, a role well studied in modern hot springs. For now, Rhynie fossils show that the acclimation of non-marine cyanobacteria to terrestrial ecosystems transformed by embryophytes was well underway more than 400 million years ago.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological samples | ||
| Microfossils from the Lower Devonian Rhynie chert, UK | - Palaeobotany Collection in the Pôle Collections scientifiques et patrimoniales. Bibliothèque de Sorbonne Université, Paris (France) - Palaeobotany Collections. Natural History Museum, London (UK) |
- Thin sections SU.PB. 2023.0.1.2.8 and SU.PB. 2023.0.1.2.9 - Chert chip in cavity slide NHMUK V32409 |
| Deposited data | ||
| Confocal microscopy data | https://www.zenodo.org/ | https://doi.org/10.5281/zenodo.7845413 |
| Software and algorithms | ||
| Three-dimensional reconstruction of the microfossils | - Fiji/ImageJ - Cellpose algorithm-Online platform ZeroCostDL4Mic - Imaris (Bitplane, version 9.9.1) |
https://imagej.nih.gov/ij/download.html https://github.com/HenriquesLab/ZeroCostDL4Mic https://imaris.oxinst.com/ |
Resource availability
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the lead contact, Christine Strullu-Derrien (c.strullu-derrien@nhm.ac.uk)
Materials availability
All specimens are curated in publicly accessible collections. The newly discovered cyanobacteria were observed in thin sections SU.PB. 2023.0.1.2.8 and SU.PB. 2023.0.1.2.9 from the Palaeobotany Collection curated in the Pôle Collections scientifiques et patrimoniales, Bibliothèque de Sorbonne Université, Paris (France). Previously described cyanobacteria and reinvestigated here are located within specimen NHMUK V32409 housed in the Palaeobotany Collections of the Natural History Museum, London (UK).
Experimental model and subject details
The Rhynie chert is a geological site located about 50 km NW of Aberdeen, Scotland. Cherts in this locality were precipitated from a hydrothermal spring system that episodically flooded adjacent floodplains, not unlike modern sinter deposits in Yellowstone Park, USA, and the North Island of New Zealand.57,58 An 40Ar/39Ar date of 407.1 ± 2.2 Ma constrains the age of the hydrothermal system,17 corresponding to the early Devonian Period (Pragian-?earliest Emsian18). The palaeoenvironment is interpreted as a low-energy alluvial plain in which plants grew on sandy substrates or on sinter surfaces close to a river system with associated ephemeral ponds and small lakes.58
Method details
The Rhynie chert block from which thin sections SU.PB. 2023.0.1.2.8 and SU.PB. 2023.0.1.2.9 were cut was collected by palaeobotanist Édouard Boureau (1913–1999) who is known for the landmark synthesis Traité de Paléobotanique published in four volumes.59 The thin sections were prepared by Denise Pons in the 1960-1970s using the petrographic standard method. Sections are ca 30–40 μm in thickness and mounted with glass coverslips.
A Nikon Eclipse Ni-E microscope equipped with a DS-Ri2 camera was used at the Muséum national d’Histoire naturelle (Paris) to examine and photograph the microorganisms under transmitted light. The depth of field of the resultant imagery was enhanced through z-stack montages.
An Axio Zoom V16 (Carl Zeiss) was used to acquire an overview image of the whole slide at the MNHN light microscopy facility (CeMIM, Centre de Microscopie et d’IMagerie numerique, MNHN Paris).
CLSM images were acquired at the MNHN light microscopy facility (CeMIM, Paris) using a Zeiss LSM880 confocal microscope (Carl Zeiss) equipped with Airyscan detectors (Carl Zeiss) and plan Apo objective lenses (20X/0,8 NA - dry or 40X/1.30 NA – oil immersion, Carl Zeiss). The quantum efficiency (QE) of the detector was about 50%. An auto-fluorescence signal was collected with an Airyscan head using a 32 GASP detector array in super resolution mode (0.2 airy unit for each elementary detector of the Airyscan head). Images were recorded with pixel dimensions of 90 nm and 16-bit depth mode. Autofluorescence of the samples was excited with the 561 nm laser line and z-stacks (20-60 μm thick) were acquired with steps of 0.25 μm. Images were then processed (Airyscan processing) and visualized with the Zen software (Carl Zeiss). The fluorescence signal from each z-plane was projected onto a maximum projection image. Color-coded z-projections were used to generate a maximum intensity projection displaying the position in z with a color gradient.
For 3D rendering, brightness attenuation in the z-stacks was compensated using the “bleach correction (histogram matching)” tool in Fiji/ImageJ and a gaussian filter was used to reduce background noise. Then, the Cellpose algorithm60 was applied using the online platform ZeroCostDL4Mic.61 Generated label images were then imported into Imaris (Bitplane, version 9.9.1) and a virtual surface object was created for each detected cyanobacterium. Movies were generated using the “animation” tool in Imaris.
The confocal dataset will be publicly available on https://www.zenodo.org/ (https://doi.org/10.5281/zenodo.7845413).
Cyanobacteria described by Croft and George39 were reinvestigated using sample NHMUK V32409 (a single chip) housed in the collections of the Natural History Museum, London. A Leica 250C stereomicroscope and a Zeiss Axio-Imager M2 were used to observe and photograph the cyanobacteria. The depth of field of the resultant imagery was enhanced through z-stack montages.
Quantification and statistical analysis
No quantification or statistical analyses were used in this study.
Acknowledgments
The authors thank Denise Pons for the preparation of the thin sections, as well as Stéphane Jouve, Maxime Perretta, and the Pôle Collections scientifiques et patrimoniales, Bibliothèque de Sorbonne Université, Paris for the loan of these thin sections. The MNHN light microscopy facility (CeMIM, Center de Microscopie et d’IMagerie numérique, MNHN Paris) is acknowledged for providing access to the Confocal scanning laser microscope. Cyril Willig (CeMIM) is acknowledged for his assistance in using the Zeiss Axio Zoom V.16. The Nikon Eclipse Ni-E microscope was used thanks to the grant ANR-19-CE02-0002 to Florent Martos. CS-D thanks Peta Hayes for her assistance in the collections at the NHM London. Victor Leshyk is acknowledged for his permission to use in the graphical abstract his reconstruction of the Rhynie chert landscape (reprinted from Berbee et al. 2020, https://doi.org/10.1038/s41579-020-0426-8). The Trustees of the Natural History Museum, London are thanked for the reuse of figures 9, 10, 11 (Plate 41) from Croft and George, 1959 (licence CC-BY-NC). CS-D is funded by the Fondation Ars-Cuttoli, Paul Appell/Fondation de France (grant 00103178). FF is funded by the French Agence Nationale de la Recherche (ANR) grant, Project WOLF (ANR-21-CE13-0029).
Author contributions
C.S.-D. and A.H.K. conceptualized the study, C.S-D collected the data in light microcopy, C.S.-D., M.G., and F.F. collected the confocal data; F.F. created the 3D reconstructions and generated the movies. C.S.-D. and A.H.K. analyzed the fossils, interpreted the results and wrote the original draft. P.K. and C.S.-D. fixed the taxonomy/nomenclature. All authors contributed to the final version of the manuscript.
Declaration of interests
The authors declare no competing interests.
Published: July 10, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.107338.
Supplemental information
Data and code availability
CSLM data have been deposited at Zenodo and will be publicly available as of the paper’s publication date. A DOI is reported in the manuscript and listed in the key resources table.
References
- 1.Flombaum P., Gallegos J.L., Gordillo R.A., Rincón J., Zabala L.L., Jiao N., Karl D.M., Li W.K.W., Lomas M.W., Veneziano D., et al. Present and future global distributions of the marine Cyanobacteria Prochlorococcus and Synechococcus. Proc. Natl. Acad. Sci. USA. 2013;110:9824–9829. doi: 10.1073/pnas.1307701110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lyons T.W., Reinhard C.T., Planavsky N.J. The rise of oxygen in Earth’s early ocean and atmosphere. Nature. 2014;506:307–315. doi: 10.1038/nature13068. [DOI] [PubMed] [Google Scholar]
- 3.Buick R. The antiquity of oxygenic photosynthesis: evidence from stromatolites in sulphate-deficient Archaean lakes. Science. 1992;255:74–77. doi: 10.1126/science.11536492. [DOI] [PubMed] [Google Scholar]
- 4.Ostrander C.M., Johnson A.C., Anbar A.D. Earth's first redox revolution. Annu. Rev. Earth Planet Sci. 2021;49:337–366. doi: 10.1146/annurev-earth-072020-055249. [DOI] [Google Scholar]
- 5.Schirrmeister B.E., de Vos J.M., Antonelli A., Bagheri H.C. Evolution of multicellularity coincided with increased diversification of cyanobacteria and the Great Oxidation Event. Proc. Natl. Acad. Sci. USA. 2013;110:1791–1796. doi: 10.1073/pnas.1209927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sánchez-Baracaldo P., Raven J.A., Pisani D., Knoll A.H. Early photosynthetic eukaryotes inhabited low-salinity habitats. Proc. Natl. Acad. Sci. USA. 2017;114:E7737–E7745. doi: 10.1073/pnas.1620089114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fournier G.P., Moore K.R., Rangel L.T., Payette J.G., Momper L., Bosak T. The Archean origin of oxygenic photosynthesis and extant cyanobacterial lineages. Proc. Biol. Sci. 2021;288:20210675. doi: 10.1098/rspb.2021.0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strother P.K., Wellman C.H. The Nonesuch Formation Lagerstätte: a rare window into freshwater life one billion years ago. J. Geol. Soc. London. 2021;178 doi: 10.1144/jgs2020-133. [DOI] [Google Scholar]
- 9.Strother P.K., Wellman C.H. Palaeoecology of a billion-year-old non-marine cyanobacterium from the Torridon Group and Nonesuch Formation. Palaeontology. 2016;59:89–108. doi: 10.1111/pala.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stüeken E.E., Jones S., Raub T.D., Prave A.R., Rose C.V., Linnekogel S., Cloutier J. Geochemical fingerprints of seawater in the Late Mesoproterozoic Midcontinent Rift, North America: Life at the marine-land divide. Chem. Geol. 2020;553:119812. [Google Scholar]
- 11.Jones S., Prave A., Raub T.D., Cloutier J., Stüeken E., Rose C.V., Linnekogel S., Nazarov K. A marine origin for the late Mesoproterozoic Copper Harbor and Nonesuch Formations of the Midcontinent Rift of Laurentia. Precambrian Res. 2020;336:105510. doi: 10.1016/j.precamres.2019.105510. [DOI] [Google Scholar]
- 12.Mareš J., Hrouzek P., Kaňa R., Ventura S., Strunecký O., Komárek J. The primitive thylakoid-less cyanobacterium Gloeobacter is a common rock-dwelling organism. PLoS One. 2013;8:e66323. doi: 10.1371/journal.pone.0066323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grettenberger C.L., Sumner D.Y., Wall K., Brown C.T., Eisen J.A., Mackey T.J., Hawes I., Jospin G., Jungblut A.D. A phylogenetically novel cyanobacterium most closely related to Gloeobacter. ISME J. 2020;14:2142–2152. doi: 10.1038/s41396-020-0668-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ponce-Toledo R.I., Deschamps P., López-García P., Zivanovic Y., Benzerara K., Moreira D. An early-branching freshwater cyanobacterium at the origin of plastids. Curr. Biol. 2017;27:386–391. doi: 10.1016/j.cub.2016.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Vries J., Archibald J.M. Endosymbiosis: did plastids evolve from a freshwater cyanobacterium? Curr. Biol. 2017;27:R103–R105. doi: 10.1016/j.cub.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 16.Wellman C.H., Strother P.K. The terrestrial biota prior to the origin of land plants (embryophytes): a review of the evidence. Palaeontology. 2015;58:601–627. doi: 10.1111/pala.12172. [DOI] [Google Scholar]
- 17.Mark D.F., Rice C.M., Fallick A.E., Trewin N.H., Lee M.R., Boyce A., Lee J.K.W. 40Ar/39Ar dating of hydrothermal activity, biota and gold mineralization in the Rhynie hot-spring system, Aberdeenshire, Scotland. Geochim. Cosmochim. Acta. 2011;75:555–569. [Google Scholar]
- 18.Wellman C.H. Spore assemblages from the Lower Devonian “Lower Old Red Sandstone” deposits of the Rhynie outlier, Scotland. Trans. R. Soc. Edinburgh. 2006;97:167–211. [Google Scholar]
- 19.Edwards D., Kenrick P., Dolan L. History and contemporary significance of the Rhynie cherts – our earliest preserved terrestrial ecosystem. Phil. Trans. R. Soc. B. 2018;373:20160489. doi: 10.1098/rstb.2016.0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strullu-Derrien C., Kenrick P., Knoll A.H. The Rhynie chert. Curr. Biol. 2019;29:R1218–R1223. doi: 10.1016/j.cub.2019.10.030. [DOI] [PubMed] [Google Scholar]
- 21.Kidston R., Lang W.H. On Old Red Sandstone plants showing structure, from the Rhynie Chert bed, Aberdeenshire. Part I. Rhynia gwynne-vaughani Kidston & Lang. Trans. R. Soc. Edinburgh. 1917;51:761–784. [Google Scholar]
- 22.Kidston R., Lang W.H. On Old Red Sandstone plants showing structure, from the Rhynie Chert bed, Aberdeenshire. Part II. Additional notes on Rhynia gwynne-vaughani Kidston & Lang: with descriptions on Rhynia major, n.sp. and Hornea lignieri n.g., n. sp. Trans. R. Soc. Edinburgh. 1921;52:831–854. [Google Scholar]
- 23.Kidston R., Lang W.H. On Old Red sandstone plants showing structure, from the Rhynie Chert bed, Aberdeenshire. Part V. The Thallophyta occurring in the peat bed: the succession of the plants throughout a vertical section of the bed, and the conditions of accumulation and preservation of the deposit. Trans. R. Soc. Edinburgh. 1921;52:855–902. [Google Scholar]
- 24.Lyon A.G., Edwards D. The first zosterophyll from the Lower Devonian Rhynie Chert, Aberdeenshire. Trans. R. Soc. Edinburgh. 1991;82:324–332. doi: 10.1017/S0263593300004193. [DOI] [Google Scholar]
- 25.Powell C.L., Trewin N.H., Edwards D. Palaeoecology and plant succession in a borehole through the Rhynie cherts, Lower Old Red Sandstone, Scotland. Geol. Soc. Spec. Publ. 2000;180:439–457. doi: 10.1144/GSL.SP.2000.180.01.23. [DOI] [Google Scholar]
- 26.Kerp H. Organs and tissues of Rhynie chert plants. Phil. Trans. R. Soc. B. 2018;373:20160495. doi: 10.1098/rstb.2016.0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strullu-Derrien C. Fossil filamentous microorganisms associated with plants in early terrestrial environments. Curr. Opin. Plant Biol. 2018;44:122–128. doi: 10.1016/j.pbi.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Krings M., Sergeev V.N. A coccoid, colony-forming cyanobacterium from the lower Devonian Rhynie chert that resembles Eucapsis (Synechococcales) and Entophysalis (Chroococcales) Rev. Palaeobot. Palynol. 2019;268:65–71. [Google Scholar]
- 29.Krings M. Stigonema (Nostocales, Cyanobacteria) in the Rhynie chert (Lower Devonian, Scotland) Rev. Palaeobot. Palynol. 2021;295:104505. doi: 10.1016/j.revpalbo.2021.104505. [DOI] [Google Scholar]
- 30.Trewin N.H., Kerp H. In: Terrestrial conservation Lagerstätten - windows into the evolution of life on land. Fraser N.C., Sues H.-D., editors. Dunedin Academic Press Ltd; 2017. The Rhynie and Windyfield cherts, Early Devonian, Rhynie, Scotland; pp. 1–38. [Google Scholar]
- 31.Edwards D.S., Lyon A.G. Algae from the Rhynie Chert. Bot. J. Linn. Soc. 1983;86:37–55. [Google Scholar]
- 32.Taylor T.N., Krings M. A colony-forming microorganism with probable affinities to the Chroococcales (Cyanobacteria) from the Lower Devonian Rhynie chert. Rev. Palaeobot. Palynol. 2015;219:147–156. doi: 10.1016/j.revpalbo.2015.04.003. [DOI] [Google Scholar]
- 33.Krings M., Harper C.J. A microfossil resembling Merismopedia (Cyanobacteria) from the 410-million-yr-old Rhynie and Windyfield cherts – Rhyniococcus uniformis revisited. Nova Hedw. 2019;108:17–35. doi: 10.1127/nova_hedwigia/2018/0507. [DOI] [Google Scholar]
- 34.Krings M. Peculiar bundles and a knot of thin filaments in microbial mats from the Lower Devonian Rhynie and Windyfield cherts. Rev. Palaeobot. Palynol. 2021;291:104442. doi: 10.1016/j.revpalbo.2021.104442. [DOI] [Google Scholar]
- 35.Krings M. The Rhynie chert land plant Aglaophyton majus harbored cyanobacteria in necrotic local lesions. N. Jb. Geol. Paläontol. Abh. 2021;300:279–289. [Google Scholar]
- 36.Krings M., Kerp H., Hass H., Taylor T.N., Dotzler N. A filamentous cyanobacterium showing structured colonial growth from the Early Devonian Rhynie chert. Rev. Palaeobot. Palynol. 2007;146:265–276. doi: 10.1016/j.revpalbo.2007.05.002. [DOI] [Google Scholar]
- 37.Krings M. Palaeolyngbya kerpii nov. sp., a large filamentous cyanobacterium with affinities to Oscillatoriaceae from the Lower Devonian Rhynie chert. PalZ. 2019;93:377–386. [Google Scholar]
- 38.Krings M., Hass H., Kerp H., Taylor T.N., Agerer R., Dotzler N. Endophytic cyanobacteria in a 400-million-yr-old land plant: A scenario for the origin of a symbiosis? Rev. Palaeobot. Palynol. 2009;153:62–69. doi: 10.1016/j.revpalbo.2008.06.006. [DOI] [Google Scholar]
- 39.Croft W.N., George E.A. Blue-green algae from the Middle Devonian of Rhynie. Aberdeenshire. Bull. Brit. Mus. Nat. Hist. Geol. 1959;3:339–354. [Google Scholar]
- 40.Komárek J., Kaŝtovskŷ J., Mareš J., Johansen J.R. Taxonomic classification of cyanoprokaryotes (cyanobacterial genera) 2014, using a polyphasic approach. Preslia. 2014;86:295–335. [Google Scholar]
- 41.Ward R.D., Stajich J.E., Johansen J.R., Huntemann M., Clum A., Foster B., Foster B., Roux S., Palaniappan K., Varghese N., et al. Metagenomesequencing to explore phylogenomics of terrestrial cyanobacteria. Microbiol. Resour. Announc. 2021;10:e0025821. doi: 10.1128/MRA.00258-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Borzi A. Studi sulle Mixoficee. I. Cenni generali - Systema Myxophycearum. Nuovo Giornale Botanico Italiano Ser. 1914;2:307–360. [Google Scholar]
- 43.Elenkin A.A. O znachenii nastoyashchago i lozhnago vetvleniya u sinezelenykh vodoroslej v sem. Stigonemataceae. Izviestiia Imperatorskago Sankt-Peterburgskago botanicheskago sada. 1916;16:272–280. [Google Scholar]
- 44.Turland N.J., Wiersema J.H., Barrie F.R., Greuter W., Hawksworth D.L., Herendeen P.S., Knapp S., et al., editors. International code of nomenclature for algae, fungi, and plants (Shenzhen Code) adopted by the Nineteenth International Botanical Congress Shenzhen. Koeltz Botanical Books; China: 2018. [Google Scholar]
- 45.Cavalier-Smith T. The neomuran origin of archaebacteria, the negibacterial root of the universal tree and bacterial megaclassification. Int. J. Syst. Evol. Microbiol. 2002;52:7–76. doi: 10.1099/00207713-52-1-7. [DOI] [PubMed] [Google Scholar]
- 46.Strohl W.R., Larkin J.M. Cell division and trichome breakage in Beggiatoa. Curr. Microbiol. 1978;1:151–155. doi: 10.1007/BF02601668. [DOI] [PubMed] [Google Scholar]
- 47.Komárek J., Komárková J., Ventura S., Kozlíková-Zapomělová E., Rejmánková E. Taxonomic evaluation of Cyanobacterial Microflora from Alkaline Marshes of Northern Belize. 3. Diversity of Heterocytous Genera. Nova Hedw. 2017;105:445–486. [Google Scholar]
- 48.Knoll A.H., Golubic S. Anatomy and taphonomy of a Precambrian algal stromatolite. Precambrian Res. 1979;10:115–151. [Google Scholar]
- 49.Hindák F. Hormogonia in two nostocalean cyanophytes (cyanobacteria) from the genera Hapalosiphon and Fischerella. Biologia. 2012;67:1075–1079. doi: 10.2478/s11756-012-0100-3. [DOI] [Google Scholar]
- 50.Geitler L. Vol. 14. Rabenhorst's Kryptogamen-Flora; Leipzig: 1932. (Cyanophyceae). [Google Scholar]
- 51.Kaplan-Levy R.N., Hadas O., Summers M.L., Rücker J., Sukenik A. In: Dormancy and Resistance in Harsh Environments. Lubzens E., Cerda J., Clark M., editors. Vol. 21. Springer; 2010. Akinetes: dormant cells of cyanobacteria; pp. 5–27. (Topics in Current Genetics). [Google Scholar]
- 52.Sukenik A., Kaplan-Levy R.N., Viner-Mozzini Y., Quesada A., Hadas O. Potassium deficiency triggers the development of dormant cells (akinetes) in Aphanizomenon ovalisporum (Nostocales, Cyanoprokaryota) J. Phycol. 2013;49:580–587. doi: 10.1111/jpy.12069. [DOI] [PubMed] [Google Scholar]
- 53.Zulkefli N.S., Hwang S.-J. Heterocyst development and diazotrophic growth of Anabaena variabilis under different nitrogen availability. Life. 2020;10:279. doi: 10.3390/life10110279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Loron C.C., Rodriguez Dzul E., Orr P.J., Gromov A.V., Fraser N.C., McMahon S. Molecular fingerprints resolve affinities of Rhynie chert organic fossils. Nat. Commun. 2023;14:1387. doi: 10.1038/s41467-023-37047-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Demoulin C.F., Lara Y.J., Cornet L., François C., Baurain D., Wilmotte A., Javaux E.J. Cyanobacteria evolution: insight from the fossil record. Free Radic. Biol. Med. 2019;140:206–223. doi: 10.1016/j.freeradbiomed.2019.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baludikay B.K., Storme J.Y., François C., Baudet D., Javaux E.J. A diverse and exquisitely preserved organic-walled microfossil assemblage from the Meso–Neoproterozoic Mbuji-Mayi Supergroup (Democratic Republic of Congo) and implications for Proterozoic biostratigraphy. Precambrian Res. 2016;281:166–184. [Google Scholar]
- 57.Rice C.M., Ashcroft W.A., Batten D.J., Boyce A.J., Caulfield J.B.D., Fallick A.E., Hole M.J., Jones E., Pearson M.J., Rogers G., et al. A Devonian auriferous hot spring system, Rhynie, Scotland. J. Geol. Soc. London. 1995;152:229–250. [Google Scholar]
- 58.Rice C.M., Trewin N.H., Anderson L.I. Geological setting of the Early Devonian Rhynie cherts, Aberdeenshire, Scotland: an early terrestrial hot spring system. J. Geol. Soc. London. 2002;159:203–214. doi: 10.1144/0016-764900-181. [DOI] [Google Scholar]
- 59.Boureau É. Vols. 1–4. Masson et Cie; Paris: 1964-1975. (Traité de paléobotanique). [Google Scholar]
- 60.Stringer C., Wang T., Michaelos M., Pachitariu M. Cellpose: a generalist algorithm for cellular segmentation. Nat. Methods. 2021;18:100–106. doi: 10.1038/s41592-020-01018-x. [DOI] [PubMed] [Google Scholar]
- 61.von Chamier L., Laine R.F., Jukkala J., Spahn C., Krentzel D., Nehme E., Lerche M., Hernández-Pérez S., Mattila P.K., Karinou E., et al. Democratising deep learning for microscopy with ZeroCostDL4Mic. Nat. Commun. 2021;12:2276. doi: 10.1038/s41467-021-22518-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
From thin section SU.PB. 2023.0.1.2.8 (https://doi.org/10.5281/zenodo.7845413).
From thin section SU.PB. 2023.0.1.2.8 (https://doi.org/10.5281/zenodo.7845413).
Data Availability Statement
CSLM data have been deposited at Zenodo and will be publicly available as of the paper’s publication date. A DOI is reported in the manuscript and listed in the key resources table.