Abstract
Background & Aims
Alcohol abuse and metabolic disorders are leading causes of hepatocellular carcinoma (HCC) worldwide. Alcohol-related aetiology is associated with a worse prognosis compared with viral agents, because of the lower percentage of patients diagnosed with HCC under routine surveillance and a higher burden of comorbidity in alcohol abusers. This study aimed to describe the evolving clinical scenario of alcohol-related HCC over 15 years (2006–2020) in Italy.
Methods
Data from the Italian Liver Cancer (ITA.LI.CA) registry were used: 1,391 patients were allocated to three groups based on the year of HCC diagnosis (2006–2010; 2011–2015; 2016–2020). Patient characteristics, HCC treatment, and overall survival were compared among groups. Survival predictors were also investigated.
Results
Approximately 80% of alcohol-related HCCs were classified as cases of metabolic dysfunction-associated fatty liver disease. Throughout the quinquennia, <50% of HCCs were detected by surveillance programmes. The tumour burden at diagnosis was slightly reduced but not enough to change the distribution of the ITA.LI.CA cancer stages. Intra-arterial and targeted systemic therapies increased across quinquennia. A modest improvement in survival was observed in the last quinquennia, particularly after 12 months of patient observation. Cancer stage, HCC treatment, and presence of oesophageal varices were independent predictors of survival.
Conclusions
In the past 15 years, modest improvements have been obtained in outcomes of alcohol-related HCC, attributed mainly to underuse of surveillance programmes and the consequent low amenability to curative treatments. Metabolic dysfunction-associated fatty liver disease is a widespread condition in alcohol abusers, but its presence did not show a pivotal prognostic role once HCC had developed. Instead, the presence of oesophageal varices, an independent poor prognosticator, should be considered in patient management and refining of prognostic systems.
Impact and Implications
Alcohol abuse is a leading and growing cause of hepatocellular carcinoma (HCC) worldwide and is associated with a worse prognosis compared with other aetiologies. We assessed the evolutionary landscape of alcohol-related HCC over 15 years in Italy. A high cumulative prevalence (78%) of metabolic dysfunction-associated fatty liver disease, with signs of metabolic dysfunction, was observed in HCC patients with unhealthy excessive alcohol consumption. The alcohol + metabolic dysfunction-associated fatty liver disease condition tended to progressively increase over time. A modest improvement in survival occurred over the study period, likely because of the persistent underuse of surveillance programmes and, consequently, the lack of improvement in the cancer stage at diagnosis and the patients’ eligibility for curative treatments. Alongside the known prognostic factors for HCC (cancer stage and treatment), the presence of oesophageal varices was an independent predictor of poor survival, suggesting that this clinical feature should be carefully considered in patient management and should be included in prognostic systems/scores for HCC to improve their performance.
Keywords: Hepatocellular carcinoma, Alcohol abuse, ITA.LI.CA staging system, Metabolic dysfunction-associated fatty liver disease, Surveillance programs, Oesophageal varices
Graphical abstract
Highlights
-
•
Alcohol-related HCC is increasing in incidence and has a poor prognosis.
-
•
Signs of metabolic dysfunction are present in most patients with HCC.
-
•
The rate of alcohol-related HCC diagnosed by ultrasound surveillance is <50%.
-
•
Only a modest survival improvement has been achieved in the last 15 years.
-
•
Cancer stage, oesophageal varices, and treatment were predictors of survival.
Introduction
Hepatocellular carcinoma (HCC) is the second leading cause of death from cancer worldwide.1 Almost 90% of HCCs develop in the setting of chronic liver disease, usually within the context of advanced fibrosis or cirrhosis.2,3
The incidence of this tumour varies markedly across geographic regions according to the prevalence of its risk factors.4 Chronic viral hepatitis and alcohol-related liver disease are the main risk factors; however, in high-income countries, the rising prevalence of metabolic disorders has led to an increasing prevalence of HCCs associated with non-alcoholic fatty liver disease (NAFLD).5,6
Approximately 30% of HCC cases worldwide are attributable to alcohol abuse,7 ranging from 20% in Southern European countries (e.g. Italy and Spain) to 63% in Eastern countries.8 Heavy alcohol consumption is associated with a relative risk of 2.07 for the development of this tumour compared with non-consumers, and the annual incidence of HCC in patients with alcohol-related cirrhosis is approximately 3%.8,9 It is expected that the relative and absolute causative roles of alcohol will increase in the future, as alcohol consumption is rising in many countries,10 whereas the frequency of potentially causative HBV and HCV viral infections is declining because of the decreased incidence of new infections and the efficacy of antiviral treatments.11 This changing aetiological scenario has been recently confirmed by a retrospective analysis of the Italian Liver Cancer (ITA.LI.CA) database, showing that the proportion of non-viral HCCs, and particularly of ‘metabolic’ or ‘metabolic + alcohol-related’ tumours, is rapidly growing and is challenging that of HCV-related HCCs, which is still the largest aetiological subgroup in Italy.12
The prognosis of patients with alcohol-related HCC is worse than that of non-alcohol-related cases because of more frequent delayed detection rates rather than greater cancer aggressiveness or poorer treatment outcomes.13,14 Indeed, in alcohol abusers a HCC diagnosis is more often made outside surveillance programmes and in the setting of more advanced cirrhosis.8,15,16 This combination entails a more advanced cancer stage at the time of diagnosis, with a lower amenability to curative treatments, and hence, worse survival.13 Moreover, a more conservative therapeutic approach to alcohol-related HCCs may be motivated by the higher prevalence of extrahepatic comorbidities and poorer social conditions of patients, limiting access to a cure. This assumption is supported by the results of a multicentre prospective study conducted in patients with alcohol-related cirrhosis who were periodically screened for HCC; only 56% of these patients underwent curative treatments, despite 77% of the cases having been diagnosed with a tumour within the Milan criteria.9 Therefore, the above-mentioned unfavourable characteristics of alcohol abusers could have prevented or limited any prognostic improvement reported in an unselected case series of patients with HCC.17 Despite the growing importance of alcohol abuse in HCC development and its alarming features, this topic has received less attention than other causes of HCC, with research interest mainly focused on the challenging problem of liver damage associated with metabolic disorders.
Recently, a new taxonomy of liver diseases has been proposed that includes the setting of metabolic syndrome, named metabolic dysfunction-associated fatty liver disease (MAFLD).18 Unlike NAFLD, which was conceived as a diagnosis of exclusion,19 this new entity includes patients with metabolic dysfunction and liver disease, regardless of the concurrent presence of other causative factors. In Italy, MAFLD accounts for >50% of patients with HCC, and this condition is expected to be detectable in the vast majority of cases of HCC in the forthcoming years.20 However, the prevalence of MAFLD and its prognostic impact in patients with alcohol-related HCC remains unclear.
This study aimed to describe the evolving landscape of alcohol-related HCC over 15 years (calendar years: 2006–2020) in Italy, where approximately 15% of HCC have been attributed to alcohol abuse.12
Patients and methods
Patients
Data were extracted from the ITA.LI.CA registry which currently includes 9,379 patients with HCC consecutively evaluated from January 1986 to December 2020 in 24 medical institutions in Italy. Data were collected prospectively and updated every 2 years. After a biennial control of data quality by the coordinating centre (Semeiotics Unit, Bologna University), the database was available for use for new studies. The participating ITA.LI.CA centres collected patient data at the time of HCC diagnosis (baseline) and during follow-up. For those patients initially managed outside the ITA.LI.CA network, baseline and follow-up data until the first access to the ITA.LI.CA network were retrospectively obtained. To avoid selection bias, no patient was excluded from the registry as a result of incomplete data (‘all comers’ recruitment). The management of the ITA.LI.CA database conforms to the current Italian legislation on privacy. According to Italian law, patient consent is not required for retrospective data analysis. Nonetheless, all patients provided written informed consent to undergo each diagnostic and therapeutic procedure and for having their clinical data recorded anonymously in the ITA.LI.CA database. The database used for scientific research was approved by the Institutional Review Board of the ITA.LI.CA Coordinating Center (approval number 99/2012/O/Oss) and the study was conducted following the ethical guidelines of the 1975 Declaration of Helsinki.
For this study, a total of 1,391 consecutive patients with alcohol-related HCC diagnosed from 1 January 2006 to 31 December 2020 were selected. The patients were allocated to three groups according to the year of cancer diagnosis:
-
•
G1 = 2006–2010: n = 308 (22.1%) patients
-
•
G2 = 2011–2015: n = 558 (40.1%) patients
-
•
G3 = 2016–2020: n = 525 (37.8%) patients.
We analysed the following variables: age, sex, alcohol consumption (expressed as alcohol units [AU] per week), drinking habit at the time of HCC diagnosis (categorised as past [abstainers at the cancer diagnosis] or current drinkers), smoking habit (categorised as never, past [had quit smoking before the HCC diagnosis], or current smokers at the time of HCC detection), presence of MAFLD, type 2 diabetes (diabetes or active glucose-lowering therapy) and cirrhosis, Charlson Comorbidity Index, modality of HCC diagnosis (under surveillance, incidental, symptomatic), surveillance interval (categorised as ≤6 months and others), Child-Pugh (C–P) class, albumin/bilirubin (ALBI) grade, Aspartate aminotransferase–Platelet Ratio Index (APRI), Model for End-stage Liver Disease (MELD) score, ECOG Performance Status (PS, categorised as 0, 1, and ≥2), presence of oesophageal varices, platelet count (threshold ≤100,000/μl, below which the presence of clinically significant portal hypertension can be suspected in liver cirrhosis), tumour gross pathology (monofocal, multifocal, ‘spreading mass’ infiltrative or massive), number of lesions (categorised as 1, 2–3 and >3), size of the greatest lesion (categorised as ≤2, 2.1–5 and >5 cm), presence of portal or caval macrovascular invasion (MVI) and its site (categorised as segmental, main portal branches and portal trunk/caval vein), presence of metastases, serum alpha-foetoprotein (AFP) levels (categorised as ≤10, 11–200, and >200 ng/ml), ITA.LI.CA21 tumour stage, HCC main treatment (the administered treatment with highest efficacy), causes of death, and patient survival. All these variables were available for >80% of the cases, except for weekly alcohol consumption and surveillance interval.
Aetiology of liver disease
In all patients, the diagnosed liver disease was classified as ‘alcohol-related’ if the daily ethanol intake was >20 g for women and >30 g for men, for at least 10 years, and no other causes of liver injury, except for features of metabolic syndrome, were found. Such a choice allowed us to enrol patients with both pure alcohol-related liver disease and alcohol-related/metabolic liver damage.
As in our previous study,20 MAFLD was defined as the presence of type 2 diabetes and/or previous or current overweight or obesity (defined as BMI >25 kg/m2), or at least 2 of the following hallmarks of metabolic dysfunction:
-
-
impaired fasting glucose (fasting glucose between 100 and 125 mg/dl) or receiving antidiabetic therapy;
-
-
triglycerides >150 mg/dl or receiving specific therapy;
-
-
HDL cholesterol <50 mg/dl in women or <40 mg/dl in men or specific lipid-lowering therapy;
-
-
current or past hypertension (defined as blood pressure >130/85 mmHg) or current antihypertensive treatment.
A subanalysis of the baseline characteristics of the patients was also performed according to the presence or absence of MAFLD.
The presence of cirrhosis was confirmed by histology in 59 (4.3%) patients and by laparotomy or laparoscopy in nine (0.7%) patients; in the remaining cases, the diagnosis was made according to clinical, laboratory, endoscopic, and imaging findings.
If a recent description of oesophageal varices was lacking at the time of HCC diagnosis, endoscopy was considered mandatory. Moreover, when a previously unreported portal vein thrombosis was detected on imaging, the endoscopic examination was repeated regardless of the date of the previous examination. Varices were graded and managed according to the Baveno guidelines available at the time of HCC diagnosis.
Diagnosis and staging
The diagnosis of HCC was classified as follows:
-
•
under surveillance, if HCC was detected during an ultrasound (US)-based surveillance programme (±AFP determination) started at least 1 year before HCC diagnosis. Patients were subgrouped according to the surveillance interval (≤6 months/others). To minimise the effect of length bias, patients under surveillance were maintained in their original group even if the occurrence of symptoms anticipated the scheduled US.
-
•
incidental, when the diagnosis was made during investigations of other diseases or a general check-up outside of regular surveillance.
-
•
symptomatic, if HCC was detected through investigations motivated by the appearance of cancer-related symptoms in patients outside regular surveillance.
A subanalysis of the main characteristics of the patient according to the type of diagnosis (surveillance or no surveillance) was performed. Moreover, the evolution of the modality of HCC diagnosis through the 3 quinquennia was reported for NAFLD + cryptogenic and viral aetiologies.
The diagnosis of HCC was based on typical features of one or more imaging techniques (dynamic computed tomography [CT], magnetic resonance imaging [MRI], and contrast-enhanced US [CEUS]) and/or histological findings according to the European and American guidelines available at the time of patient recruitment.
Cancer burden was assessed using liver CT and/or MRI, and further investigations were systematically performed aimed at detecting extrahepatic involvement for patients with advanced HCC or for those eligible for liver transplant (LT). In addition, these explorations were also repeated when clinically indicated.
HCC was staged according to the ITA.LI.CA staging system.21
Treatment
If patients were subjected to multiple treatments, they were classified considering the most effective treatment (named ‘main’), following this hierarchical order: LT, hepatic resection, radiofrequency ablation (RF), percutaneous ethanol injection ablation (PEI), intra-arterial treatment ([IAT], including transarterial chemoembolisation [TACE], transarterial embolisation [TAE], and transarterial radioembolisation [TARE]), systemic therapy, or other therapies and best supportive care (BSC).22
Lead-time estimation
For each period, patients diagnosed with HCC during surveillance or incidentally were compared with those with a symptomatic diagnosis for lead-time estimation.23 The mean ± standard deviation (SD) of lead times for the surveyed patients or those diagnosed incidentally were as follows:
-
•
8.6 ± 2.1 months and 4.6 ± 1.1 months in G1
-
•
8.2 ± 2.7 months and 4.4 ± 1.3 months in G2
-
•
7.7 ± 2.6 months and 4.3 ± 1.3 months in G3.
Statistical analysis
Continuous variables are expressed as median and 25th–75th percentiles and discrete variables as absolute and relative frequencies. Kruskal–Wallis and Mann–Whitney U tests were used to compare continuous variables among the three periods or between two periods. The χ2 test or Fisher’s exact test was used to compare discrete variables, as appropriate. Data missing from covariates included in the ITA.LI.CA staging system were estimated using the maximum likelihood estimation method24 to obtain a sample size large enough to be representative of the original patient population and not to limit the number of cases included in the multivariate analysis. The lack of significant data distortion as a result of imputation was confirmed by comparing the results obtained with raw and imputed data (Table S1).
Overall survival (OS) was defined as the time elapsed between HCC diagnosis and the patient's death, the last follow-up evaluation, or data censoring (31 December 2020), whichever occurred first, and was reported as the median and 95% CI. OS was calculated according to the Kaplan–Meier method and was compared using the Log-rank test. If the potential for nonproportionality of hazards was evident, piecewise hazard ratios (HRs) were calculated over distinct periods using time-varying Cox regression analysis.
The ‘lead-time adjusted’ OS (a-OS) was calculated by subtracting the relevant lead-time from the OS obtained from surveyed or incidentally diagnosed patients. Survival rates at 1, 2, 3, 4, and 5 years were also reported, and the proportions of patients alive at any time in the 3 quinquennia were compared using the χ2 test.
To provide a more comprehensive picture of the changing HCC scenario in the 3 quinquennia, a subanalysis of the evolution of main treatment, causes of death, and a-OS in patients with NAFLD + cryptogenetic and viral HCC was also performed.
Baseline characteristics (except for alcohol consumption and surveillance interval) were tested using univariate and multivariate Cox analyses to identify their associations with a-OS.
Variables associated with a-OS in the univariate analysis (p <0.10) were entered into the multivariate models. Adjusted HR and 95% CI were reported for each prognostic factor. To avoid collinearity between covariates, the components of the ITA.LI.CA staging system (PS, tumour burden, AFP, and liver function tests) were excluded from the model that included this system. Moreover, we created two additional models to test the prognostic significance of the C–P classes and ALBI grades. The three models tested were as follows:
Model 1 (C–P-based) included age, MAFLD, cirrhosis, modality of HCC diagnosis, C–P class, Eastern Cooperative Oncology Group-Performance Status (ECOG PS), platelet count, oesophageal varices, AFP levels, gross tumour pathology, size of the greatest lesion, metastases, MVI, and the main treatment.
Model 2 (ALBI-based) included age, MAFLD, cirrhosis, modality of HCC diagnosis, ALBI grade, ECOG PS, platelet count, oesophageal varices, AFP levels, gross tumour pathology, size of the greatest lesion, metastases, MVI, and the main treatment.
Model 3 (ITA.LI.CA-based) included age, MAFLD, cirrhosis, modality of HCC diagnosis, platelet count, oesophageal varices, ITA.LI.CA cancer stage, and the main treatment.
A two-tailed p value <0.05 was considered statistically significant. All statistical analyses were performed with SPSS v28.0 (Apache Software Foundation, Chicago, IL, USA).
Details of the deposited data, software, and corresponding methods are reported in the CTAT Table.
Results
Demographic and clinical characteristics
We observed an increase in the median age at HCC diagnosis, from 67 years in G1–G2 to 69 years in G3 (p = 0.028), whereas the large predominance of males (≥92%) did not change over time (Table 1).
Table 1.
Demographic and clinical characteristics of patients with alcohol-related hepatocellular carcinoma in the 3 quinquennia.
| Variables | Available |
2006–2010 |
2011–2015 |
2016–2020 |
p value |
|---|---|---|---|---|---|
| N (%) | n (%) 308 (22.1) |
n (%) 558 (40.1) |
n (%) 525 (37.7) |
||
| Demographic and clinical features | |||||
| Age, years Median (25th–75th) |
1,391 (100) |
67.5 (60–73) |
67 (63–73) |
69 (63–75) |
0.028 |
| Sex (M/F) | 1,391 (100) | 284/24 (92.2/7.8) | 523/35 (93.7/6.3) | 486/39 (92.6/7.4) | 0.641 |
| Alcohol consumption (AU/week) | 808 (58.1) | 178 (57.8) | 310 (55.6) | 320 (61.0) | 0.313 |
| Median (25th–75th) | 56 (40–56) | 46 (30–60) | 50 (30–70) | ||
| Drinking habit | 1,391 (100) | 308 (22.2) | 558 (40.1) | 525 (37.7) | <0.001 |
| Past | 98 (31.8) | 298 (53.4) | 240 (45.7) | ||
| Current | 210 (68.2) | 260 (46.6) | 285 (54.3) | ||
| Smoking habit | 1,149 (82.6) | 233 (74.9) | 465 (83.3) | 451 (85.9) | <0.001 |
| Never | 132 (56.7) | 258 (55.5) | 215 (47.7) | ||
| Past | 29 (12.4) | 83 (17.8) | 121 (26.8) | ||
| Current | 72 (30.9) | 124 (26.7) | 115 (25.5) | ||
| MAFLD | 1,369 (98.4) | 308 (100) | 550 (98.6) | 511 (97.3) | 0.301 |
| Yes | 234 (76.0) | 437 (79.5) | 411 (80.0) | ||
| Type 2 diabetes | 1,391 (100) | 308 (100) | 558 (100) | 525 (100) | 0.208 |
| Yes | 116 (37.7) | 241 (43.2) | 228 (43.4) | ||
| Cirrhosis | 1,369 (98.4) | 305 (99.0) | 550 (98.6) | 514 (97.9) | 0.049 |
| Yes | 293 (96.1) | 504 (91.6) | 477 (92.8) | ||
| Charlson Comorbidity Index | 1,391 (100) | 308 (100) | 558 (100) | 525 (100) | 0.204 |
| Median (25th–75th) | 3 (2–4) | 3 (2–4) | 3 (2–4) | ||
| Modality of HCC diagnosis | 1,270 (91.3) | 282 (91.6) | 502 (90.0) | 486 (92.6) | 0.250 |
| Under surveillance | 107 (37.9) | 228 (45.4) | 221 (45.5) | ||
| Incidental | 121 (42.9) | 192 (38.2) | 190 (39.1) | ||
| Symptomatic | 54 (19.1) | 82 (16.3) | 75 (15.4) | ||
| Surveillance interval | 392 (70.5)a | 65 (60.7) | 153 (67.1) | 174 (78.7) | 0.010 |
| ≤6 months | 41 (63.1) | 119 (77.8) | 142 (81.6) | ||
| Others | 24 (36.9) | 34 (22.2) | 32 (18.4) | ||
| Child–Pugh class | 1,391 (100.0) | 308 (100.0) | 558 (100.0) | 525 (100.0) | 0.567 |
| A | 175 (56.8) | 298 (53.4) | 277 (52.8) | ||
| B | 107 (34.7) | 218 (39.1) | 212 (40.4) | ||
| C | 26 (8.4) | 42 (7.5) | 36 (6.9) | ||
| ALBI grade | 1,391 (100.0) | 308 (100.0) | 558 (100.0) | 525 (100.0) | 0.270 |
| Grade 1, ≤−2.60 | 52 (16.9) | 105 (18.8) | 108 (20.6) | ||
| Grade 2, >−2.60 ≤−1.39 | 212 (68.8) | 397 (71.1) | 352 (67.2) | ||
| Grade 3, >−1.39 | 44 (14.3) | 56 (10.0) | 64 (12.2) | ||
| APRI | 1,155 (83.0) | 261 (84.7) | 456 (81.7) | 438 (83.4) | 0.603 |
| First tertile | 75 (28.7) | 158 (34.6) | 146 (33.3) | ||
| Second tertile | 94 (36.0) | 149 (32.7) | 147 (33.6) | ||
| Third tertile | 92 (35.2) | 149 (32.7) | 145 (33.1) | ||
| MELD score | 1,391 (100.0) | 308 (100.0) | 558 (100.0) | 525 (100.0) | 0.156 |
| Median (25th–75th) | 11.0 (8.4–14.0) | 10.3 (8.2–14.0) | 10.2 (8.3–13.4) | ||
| ECOG PS | 1,391 (100.0) | 308 (100.0) | 558 (100.0) | 525 (100.0) | 0.113 |
| 0 | 196 (63.6) | 370 (66.3) | 376 (71.6) | ||
| 1 | 77 (25.0) | 132 (23.7) | 97 (18.5) | ||
| ≥2 | 35 (11.4) | 56 (10.0) | 52 (9.9) | ||
| Oesophageal varices | 1,391 (100.0) | 308 (100.0) | 558 (100.0) | 525 (100.0) | 0.238 |
| Yes | 140 (45.5) | 255 (45.7) | 264 (50.3) | ||
| Platelet count | 1,391 (100.0) | 308 (100.0) | 558 (100.0) | 525 (100.0) | 0.075 |
| ≤100,000/μl | 102 (33.1) | 195 (34.9) | 211 (40.2) | ||
| Tumour burden and stage | |||||
| Gross pathology | 1,391 (100.0) | 308 (100.0) | 558 (100.0) | 525 (100.0) | 0.684 |
| Monofocal | 135 (43.8) | 279 (50.0) | 255 (48.6) | ||
| Multifocal | 151 (49.0) | 241 (43.2) | 229 (43.6) | ||
| Infiltrative | 14 (4.2) | 20 (3.6) | 22 (4.2) | ||
| Massive | 9 (2.9) | 18 (3.2) | 19 (4.2) | ||
| Number of lesions | 1,290 (92.7) | 286 (92.9) | 520 (93.2) | 484 (92.2) | 0.012 |
| 1 | 135 (47.2) | 279 (53.7) | 255 (52.7) | ||
| 2 or 3 | 82 (28.7) | 162 (31.2) | 156 (32.2) | ||
| >3 | 69 (24.1) | 79 (15.2) | 73 (15.1) | ||
| Size of the greatest lesion | 1,376 (98.9) | 307 (99.7) | 553 (99.1) | 516 (98.3) | 0.091 |
| ≤2 cm | 71 (23.1) | 150 (27.1) | 156 (30.2) | ||
| 2.1–5.0 cm | 154 (50.2) | 253 (45.8) | 248 (48.1) | ||
| >5 cm | 82 (26.7) | 150 (27.1) | 112 (21.7) | ||
| MVI | 1,391 (100.0) | 308 (100.0) | 558 (100.0) | 525 (100.0) | 0.717 |
| Yes | 42 (13.6) | 87 (15.6) | 81 (15.4) | ||
| MVI site | 210 (100.0) | 42 (100.0) | 87 (100.0) | 81 (100.0) | 0.004 |
| Segmental | 1 (2.4) | 6 (6.9) | 7 (8.6) | ||
| Main portal branches | 6 (14.3) | 39 (44.8) | 29 (35.8) | ||
| Portal trunk/caval vein | 35 (83.3) | 42 (48.3) | 45 (55.6) | ||
| Metastases | 1,391 (100.0) | 308 (100.0) | 558 (100.0) | 525 (100.0) | 0.028 |
| Yes | 10 (3.2) | 33 (5.9) | 15 (2.9) | ||
| Alpha-foetoprotein levels | 1,391 (100.0) | 308 (100.0) | 558 (100.0) | 525 (100.0) | 0.025 |
| ≤10 ng/ml | 162 (52.6) | 305 (54.7) | 321 (61.1) | ||
| 11–200 ng/ml | 101 (32.8) | 151 (27.1) | 128 (24.4) | ||
| >200 ng/ml | 45 (14.6) | 102 (18.3) | 76 (14.5) | ||
| ITA.LI.CA tumour stage | 1,391 (100) | 308 (100.0) | 558 (100.0) | 525 (100.0) | 0.136 |
| 0 | 52 (16.9) | 115 (20.6) | 120 (22.9) | ||
| A | 71 (23.1) | 129 (23.1) | 58 (11.0) | ||
| B1 | 42 (13.6) | 74 (13.3) | 93 (17.7) | ||
| B2 | 44 (14.3) | 51 (9.1) | 53 (10.1) | ||
| B3 | 12 (3.9) | 39 (7.0) | 26 (5.0) | ||
| C | 36 (11.7) | 69 (12.4) | 50 (9.5) | ||
| D | 51 (16.6) | 81 (14.5) | 86 (16.4) | ||
Levels of significance: <0.05 (Kruskal–Wallis test and χ2 test).
Among patients under surveillance, the interval was specified in 392 cases, representing the 70.5% of these patients.
ALBI, albumin–bilirubin; APRI, AST to Platelet Ratio Index; ECOG-PS, Eastern Cooperative Oncology Group Performance Status; HCC, hepatocellular carcinoma; ITA.LI.CA, Italian Liver Cancer; MAFLD, metabolic dysfunction associated fatty liver disease; MELD, model for end-stage liver disease; MVI, macrovascular Invasion.
The distribution of the weekly median AU did not change over time (p = 0.313), with 56 (40–56), 46 (30–60), and 50 (30–70) in G1, G2, and G3, respectively. However, the proportion of current drinkers was significantly higher in G1 (p <0.001).
Smoking habits varied (p <0.001), showing a reduction in never-smokers (from 56.7% in G1 to 47.7% in G3) and current smokers (from 30.9% to 25.5%), and an increase in the proportion of people who had quit smoking before HCC diagnosis (from 12.4% to 26.8%).
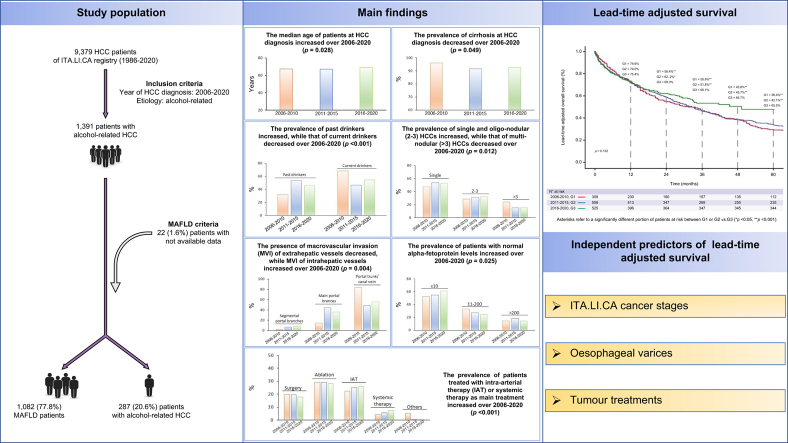
The overall prevalence of patients fulfilling MAFLD criteria was very high (77.8%) (Fig. 1), and tended to increase across quinquennia (76.0%, 79.5%, and 80.0%). The comparison of baseline characteristics between patients with MAFLD and ‘non-MAFLD’ alcohol-related HCC is reported in Table S2. In patients with MAFLD, the prevalence of type 2 diabetes and comorbidities was significantly higher, whereas the prevalence of cirrhosis and the median MELD score were lower than those observed in the counterpart (Table S2).
Fig. 1.
Metabolic dysfunction-associated fatty liver disease (MAFLD) prevalence among patients with hepatocellular carcinoma originally considered as merely alcohol-related.
Considering the entire population (Table 1), after the first calendar period the proportion of patients with cirrhosis decreased from 96.1% to 92.8% (p = 0.049).
Comorbidity burden, measured using the Charlson Comorbidity Index, maintained a median score of 3 during the study period.
Overall, the severity of liver disease, as assessed by the C–P class distribution, MELD score, and ALBI grade, and the prevalence of clinically significant portal hypertension (highlighted by the presence of oesophageal varices) remained unchanged over time. Similarly, the ECOG PS did not change significantly, despite an increasing trend in the proportion of PS 0 (63.6%, 66.3%, and 71.6%).
Regarding the modality of HCC diagnosis, both symptomatic (19.1%, 16.3%, and 15.4%) and incidental diagnoses (42.9%, 38.2%, and 39.1%) tended to decrease after the first quinquennium, favouring diagnoses made under surveillance (37.9%, 45.4%, and 45.5%); however, these trends did not reach statistical significance (p = 0.250). Among the patients surveyed, the predominant surveillance interval was always ≤6 months, and the prevalence of HCC detected under this surveillance programme progressively increased (63.1%, 77.8%, and 81.6%; p = 0.010).
Overall, the surveyed patients showed a significantly better distribution of baseline C–P classes, ECOG PS, tumour burden, AFP levels and ITA.LI.CA stages, so that they were more frequently suited for curative treatments (56.1% vs. 38.8%), and their median a-OS was longer (34.7 vs. 28.4 months) than their counterpart (Table S3).
Table S4 shows the evolution of diagnostic modalities in contemporary patients with non-alcohol-related HCC. In the NAFLD + cryptogenic population (n = 628), the proportion of HCC detected under surveillance was similar to that observed in alcohol-related disease, and did not significantly change across quinquennia (41.5%, 47.9%, and 44.9%). Instead, in viral patients (n = 4,296), the proportion of HCC detected under surveillance was always >60%, showing its nadir (61.2%) in the last quinquennium.
Tumour burden
The prevalence of patients with monofocal HCC tended to be higher (albeit not significantly) after the first quinquennium (from 43.8% to approximately 50% in G2 and G3) at the expense of multinodular tumours (particularly of those with more than three nodules), whereas the proportion of infiltrative or massive HCC remained very low over time (Table 1).
Concomitantly, a tendency toward a more favourable distribution of cancer size with a progressive increase in tiny tumours (≤2 cm) was observed (from 23.1% to 27.1% and 30.2% (p = 0.091).
The proportion of patients with MVI remained stable at approximately 15% throughout the study. Nevertheless, among patients with MVI, there was a significant (p = 0.004) shift in the site of vascular invasion from extrahepatic vessels (portal trunk and caval vein, 83.3%, 48.3%, and 55.6%) to the main portal branches (14.3%, 44.8%, and 35.8%) through the 3 quinquennia.
The prevalence of extrahepatic spread was low and stable, with its nadir in G3 (2.9%).
The distribution of AFP levels favourably changed (p = 0.025), as the proportion of patients with a normal value (≤10 ng/ml) progressively increased (52.6%, 54.7%, and 61.1%) at the expense of those with values ranging from to 11–200 ng/ml (32.8%, 27.1%, and 24.4%).
The distribution of ITA.LI.CA stages did not change significantly over time (p = 0.136).
Treatment
The distribution of the main HCC treatments received by the patients changed significantly with time (Table 2). Namely, the proportion of curative treatments (surgery or ablation) and BSC did not change, whereas the proportion of both IAT (from 22.4% to 26.0%) and systemic treatment (from 4.2% to 7.3%) progressively increased (p <0.001). Notably, when sorafenib became available in clinical practice, it became the main systemic treatment, with other agents almost completely disappearing. As a result, in the last quinquennium, approximately 18% of patients underwent surgical interventions, 28% percutaneous ablation, 26% IAT, and 7% received systemic therapy with TKI.
Table 2.
Main treatments performed for hepatocellular carcinoma and causes of death.
| Available |
2006–2010 |
2011–2015 |
2016–2020 |
p value | |
|---|---|---|---|---|---|
| N (%) | n (%) | n (%) | n (%) | ||
| Main treatment | 1,385 (99.6) | 308 (100.0) | 558 (100.0) | 519 (98.9) | <0.001 |
| Liver transplant | 12 (3.9) | 25 (4.5) | 21 (4.6) | ||
| Resection | 49 (15.9) | 83 (14.9) | 69 (13.3) | ||
| Ablation | 90 (29.2) | 162 (29.0) | 147 (28.3) | ||
| IAT | 69 (22.4) | 141 (25.3) | 135 (26.0) | ||
| Systemic therapy | 13 (4.2) | 32 (5.7) | 38 (7.3) | ||
| Others | 16 (5.2) | 1 (0.2) | 2 (0.4) | ||
| BSC | 52 (16.9) | 95 (17.0) | 91 (17.5) | ||
| Not known | 7 (2.3) | 19 (3.4) | 16 (3.1) | ||
| Causes of death | 771 (100.0) | 239 (31.0) | 350 (45.4) | 182 (23.6) | <0.001 |
| Tumour progression | 109 (45.6) | 155 (44.3) | 72 (39.6) | ||
| Liver failure | 39 (16.3) | 75 (21.4) | 47 (25.8) | ||
| Haemorrhage | 5 (2.1) | 10 (2.9) | 7 (3.8) | ||
| Kidney failure | 4 (1.7) | 10 (2.9) | 1 (0.5) | ||
| Infection | 1 (0.4) | 10 (2.9) | 15 (8.2) | ||
| Embolism | 1 (0.4) | 0 (0.0) | 4 (2.2) | ||
| Other | 70 (29.3) | 58 (16.6) | 22 (12.6) | ||
| Not known | 10 (4.2) | 32 (9.1) | 13 (7.1) |
Levels of significance: <0.05 (χ2 test).
BSC, best supportive care; IAT, intra-arterial therapy.
The treatment allocation of patients with NAFLD + cryptogenic and viral HCC across the quinquennia is reported in Table S5.
Causes of death and survival
During a median follow-up of 24.3 months (25th–75th, 8.1–47.1), 771 patients died (Table 2). The main causes of death were tumour progression (43.2%) and liver failure (21.2%). No differences in the causes of death were observed between patients with MAFLD and those non-MAFLD (Table S2). Their distribution changed across quinquennia (p <0.001), with an increasing death rate attributable to liver failure, bleeding, infections, and embolism; conversely, mortality from tumour progression, kidney failure, and other causes declined (Table 2).
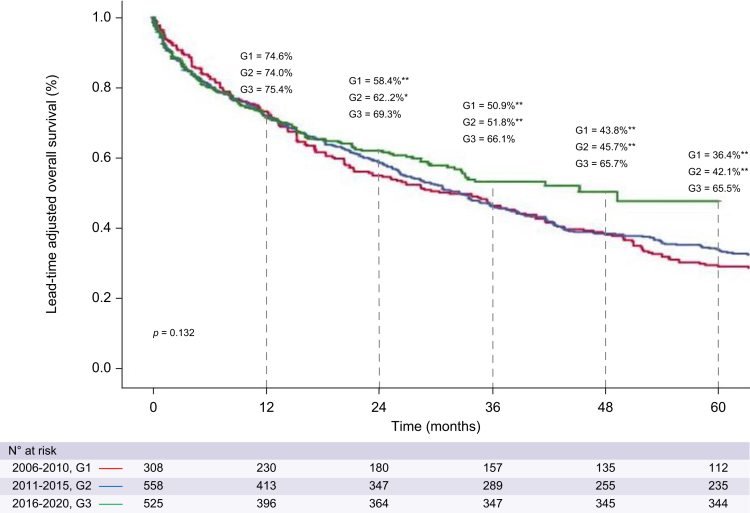
The median a-OS improved in the last quinquennium, being 31.4 months (95% CI 22.6–40.2) in G1, 32.5 months (95% CI 27.6–37.4) in G2, and 49.2 months (n.d.) in G3, although this change did not reach statistical significance (p = 0.132) (Fig. 2). The a-OS rates across quinquennia were: 74.6%, 74.0%, and 75.4% at 1 year, 58.4%, 62.2% and 69.3% at 2 years, and 36.4%, 42.1% and 65.5% at 5 years, respectively. The a-OS in the last quinquennium was compared with the other time periods using a piecewise analysis (using as the starting point 1 year after HCC detection). This analysis showed a significant a-OS improvement (HR, 0.51; 95% CI 0.33–0.78; p = 0.002, compared with the first quinquennium, and HR, 0.63; 95% CI 0.43–0.94; p = 0.023, compared with the second quinquennium).
Fig. 2.
Lead-time adjusted overall survival according to quinquennia. Levels of significance: p <0.05 (log-rank test). Asterisks refer to a significantly different proportion of at-risk patients between G1 or G2 vs. G3: ∗p <0.05, ∗∗p <0.001.
Sensitivity analyses performed in the entire population showed that both the ITA.LI.CA staging system and ALBI grades could efficiently stratify patients according to a-OS (Fig. S1).
Fig. S2 describes the changes over time in a-OS observed in patients with HCC originating from NAFLD + cryptogenic or viral liver disease.
Uni- and multivariate analyses
In the univariate analysis, the variables associated with a-OS were age, the mode of diagnosis of HCC, the presence of MAFLD and cirrhosis, C–P class, MELD score, ALBI grade, ECOG PS, Charlson Comorbidity Index, presence of oesophageal varices, platelet count, AFP levels, gross pathology, number of lesions, size of the largest lesion, presence of MVI or metastases, ITA.LI.CA stage, and the main treatment for HCC (Table 3).
Table 3.
Univariate Cox proportional hazard regression analysis of factors affecting the lead time-adjusted survival.
| Univariate analysis | ||||
|---|---|---|---|---|
| Variables | Exp(B) | 95% CI | p value | |
| Age (years) | 1.013 | 1.005–1.022 | 0.002 | |
| Sex | ||||
| F | Ref. Cat. | — | — | |
| M | 1.017 | 0.773–1.339 | 0.902 | |
| Drinking habit | ||||
| Past | Ref. Cat. | — | — | |
| Current | 0.946 | 0.821–1.090 | 0.444 | |
| Smoking habit | ||||
| Never | Ref. Cat. | — | — | |
| Past | 1.065 | 0.867–1.307 | 0.549 | |
| Current | 0.958 | 0.795–1.154 | 0.651 | |
| Modality of HCC diagnosis | ||||
| Surveillance | Ref. Cat. | — | — | |
| Incidental | 0.975 | 0.826–1.150 | 0.760 | |
| Symptomatic | 1.780 | 1.460–2.169 | <0.001 | |
| Type 2 diabetes | 0.975 | 0.846–1.125 | 0.731 | |
| MAFLD | 0.806 | 0.681–0.954 | 0.012 | |
| Cirrhosis | 1.390 | 1.028–1.878 | 0.032 | |
| Child–Pugh class | ||||
| A | Ref. Cat. | — | — | |
| B | 1.705 | 1.467–1.983 | <0.001 | |
| C | 4.343 | 3.414–5.523 | <0.001 | |
| MELD score | 1.062 | 1.048–1.076 | <0.001 | |
| ALBI grade | ||||
| Grade 1, ≤-2.60 | Ref. Cat. | — | — | |
| Grade 2, >-2.60 ≤-1.39 | 1.580 | 1.292–1.932 | <0.001 | |
| Grade 3, >-1.39 | 3.791 | 2.941–4.888 | <0.001 | |
| APRI | ||||
| First tertile | Ref. Cat. | — | — | |
| Second tertile | 1.118 | 0.921–1.358 | 0.259 | |
| Third tertile | 1.627 | 1.346–1.966 | <0.001 | |
| ECOG PS | ||||
| 0 | Ref. Cat. | — | — | |
| 1 | 1.933 | 1.635–2.285 | <0.001 | |
| ≥2 | 4.566 | 3.722–5.601 | <0.001 | |
| Charlson Comorbidity Index | 1.058 | 1.016–1.102 | 0.006 | |
| Oesophageal varices | 1.496 | 1.298–1.724 | <0.001 | |
| Platelet count | ||||
| >100,000/μl | Ref. Cat. | — | — | |
| ≤100,000/μl | 1.174 | 1.015–1.359 | 0.031 | |
| Alpha-foetoprotein levels | ||||
| ≤10 ng/ml | Ref. Cat. | — | — | |
| 11–200 ng/ml | 1.377 | 1.169–1.620 | <0.001 | |
| >200 ng/ml | 1.973 | 1.632–2.386 | <0.001 | |
| Tumour gross pathology | ||||
| Single | Ref. Cat. | — | — | |
| Multifocal | 1.636 | 1.408–1.900 | <0.001 | |
| Infiltrative | 4.818 | 3.491–6.651 | <0.001 | |
| Massive | 4.016 | 2.780–5.800 | <0.001 | |
| Number of lesions | ||||
| 1 | Ref. Cat. | — | — | |
| 2–3 lesions | 1.408 | 1.187–1.671 | <0.001 | |
| >3 lesions | 2.189 | 1.804–2.657 | <0.001 | |
| Size of the greatest lesion | ||||
| ≤2.0 cm | Ref. Cat. | — | — | |
| 2.1–5.0 cm | 1.214 | 1.012–1.457 | 0.037 | |
| >5 cm | 2.283 | 1.873–2.782 | <0.001 | |
| MVI | ||||
| No | Ref. Cat. | — | — | |
| Segmental | 2.186 | 1.131–4.226 | 0.020 | |
| Main portal branches | 2.282 | 1.714–3.039 | <0.001 | |
| Portal trunk/caval vein | 2.960 | 2.362–3.709 | <0.001 | |
| Metastases | 3.075 | 2.286–4.138 | <0.001 | |
| ITA.LI.CA tumour stage | ||||
| 0 | Ref. Cat. | — | — | |
| A | 1.103 | 0.865–1.408 | 0.429 | |
| B1 | 1.567 | 1.192–2.062 | 0.001 | |
| B2 | 1.670 | 1.264–2.205 | <0.001 | |
| B3 | 2.289 | 1.618–3.239 | <0.001 | |
| C | 3.559 | 2.719–4.658 | <0.001 | |
| D | 4.426 | 3.479–5.629 | <0.001 | |
| Main treatment | ||||
| BSC | Ref. Cat. | — | — | |
| Liver transplant | 0.024 | 0.012–0.048 | <0.001 | |
| Resection | 0.086 | 0.066–0.113 | <0.001 | |
| Ablation | 0.118 | 0.095–0.147 | <0.001 | |
| IAT | 0.211 | 0.172–0.259 | <0.001 | |
| Systemic therapy | 0.457 | 0.339–0.616 | <0.001 | |
| Others | 0.799 | 0.479–1.331 | 0.389 | |
Levels of significance: <0.05 (Cox regression model).
ALBI, albumin–bilirubin; APRI, AST to Platelet Ratio Index; BSC, best supportive care; ECOG-PS, Eastern Cooperative Oncology Group Performance Status; HCC, hepatocellular carcinoma; IAT, intra-arterial therapy; ITA.LI.CA, Italian Liver Cancer; MAFLD, metabolic dysfunction associated fatty liver disease; MELD, model for end-stage liver disease; MVI, macrovascular Invasion; Ref. Cat., reference category.
The independent prognostic factors identified by the three models of the multivariate analysis summarised in Table 4 (each including at least 88% of the initial cases) were as follows:
Table 4.
Multivariate analyses of factors affecting the lead time-adjusted survival, based on three different models.
| Multivariate models | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Model 1 n = 1,222 (87.9%) |
Model 2 n = 1,222 (87.9%) |
Model 3 n = 1,235 (88.8%) |
|||||||
| Exp(B) | 95% CI | p value | Exp(B) | 95% CI | p values | Exp(B) | 95% CI | p value | ||
| Age | 1.010 | 1.000–1.020 | 0.044 | 1.007 | 0.998–1.017 | 0.126 | 1.006 | 0.997–1.016 | 0.187 | |
| MAFLD | 0.928 | 0.770–1.119 | 0.434 | 0.919 | 0.764–1.106 | 0.371 | 0.910 | 0.760–1.090 | 0.307 | |
| Cirrhosis | 0.960 | 0.688–1.340 | 0.811 | 0.940 | 0.671–1.317 | 0.720 | 1.007 | 0.723–1.402 | 0.968 | |
| Modality of HCC diagnosis | ||||||||||
| Surveillance/Incidental | Ref. Cat. | |||||||||
| Symptomatic | 0.980 | 0.800–1.200 | 0.841 | 0.955 | 0.778–1.173 | 0.663 | 1.112 | 0.912–1.355 | 0.293 | |
| Child–Pugh class | NA | NA | NA | NA | NA | NA | ||||
| A | Ref. Cat. | |||||||||
| B | 1.407 | 1.179–1.679 | <0.001 | |||||||
| C | 1.766 | 1.292–2.414 | <0.001 | |||||||
| ALBI grade | NA | NA | NA | NA | NA | NA | ||||
| Grade 1, ≤-2.60 | Ref. Cat. | |||||||||
| Grade 2, >-2.60 ≤-1.39 | 1.349 | 1.082-1.682 | 0.008 | |||||||
| Grade 3, >-1.39 | 1.820 | 1.346–2.460 | <0.001 | |||||||
| ECOG PS | NA | NA | NA | |||||||
| 0 | Ref. Cat. | |||||||||
| 1 | 1.102 | 0.909–1.336 | 0.325 | 1.119 | 0.924–1.356 | 0.249 | ||||
| ≥2 | 1.643 | 1.265–2.133 | <0.001 | 1.712 | 1.326–2.211 | <0.001 | ||||
| Platelet count | ||||||||||
| >100,000/μl | Ref. Cat. | |||||||||
| ≤100,000/μl | 1.225 | 1.035–1.450 | 0.019 | 1.214 | 1.025–1.439 | 0.025 | 1.177 | 0.998–1.387 | 0.053 | |
| Oesophageal varices | 1.242 | 1.052–1.465 | 0.010 | 1.261 | 1.070–1.486 | 0.006 | 1.254 | 1.066–1.475 | 0.006 | |
| Alpha–foetoprotein levels | NA | NA | NA | |||||||
| ≤10 ng/ml | Ref. Cat. | |||||||||
| 11–200 ng/ml | 1.054 | 0.878–1.266 | 0.574 | 1.068 | 0.890–1.281 | 0.479 | ||||
| >200 ng/ml | 1.325 | 1.053–1.667 | 0.016 | 1.315 | 1.044–1.656 | 0.020 | ||||
| Tumour gross pathology | NA | NA | NA | |||||||
| Single | Ref. Cat. | |||||||||
| Multifocal | 1.145 | 0.965–1.360 | 0.122 | 1.150 | 0.969–1.366 | 0.109 | ||||
| Infiltrative | 1.971 | 1.281–2.869 | 0.002 | 1.938 | 1.296–2.896 | 0.001 | ||||
| Massive | 1.842 | 1.165–2.912 | 0.009 | 1.931 | 1.218–3.005 | 0.005 | ||||
| Size of the greatest lesion | NA | NA | NA | |||||||
| ≤2.0 cm | Ref. Cat. | |||||||||
| 2.1–5.0 cm | 1.031 | 0.845–1.257 | 0.764 | 1.025 | 0.840–1.250 | 0.808 | ||||
| >5 cm | 1.439 | 1.133–1.827 | 0.003 | 1.445 | 1.138–1.835 | 0.002 | ||||
| Metastases | 0.991 | 0.698–1.408 | 0.960 | 0.888 | 0.627–1.260 | 0.506 | NA | NA | NA | |
| MVI | NA | NA | NA | |||||||
| No | Ref. Cat. | |||||||||
| Segmental | 1.221 | 0.620–2.405 | 0.563 | 1.273 | 0.648–2.501 | 0.483 | ||||
| Main portal branches | 0.865 | 0.613–1.220 | 0.409 | 0.888 | 0.631–1.250 | 0.496 | ||||
| Portal trunk/caval | 1.327 | 1.016–1.734 | 0.038 | 1.351 | 1.036–1.762 | 0.026 | ||||
| ITA.LI.CA tumour stage | NA | NA | NA | NA | NA | NA | ||||
| 0 | Ref. Cat. | |||||||||
| A | 1.146 | 0.885–1.483 | 0.303 | |||||||
| B1 | 1.451 | 1.070–1.969 | 0.017 | |||||||
| B2 | 1.389 | 1.025–1.881 | 0.034 | |||||||
| B3 | 1.452 | 0.994–2.121 | 0.054 | |||||||
| C | 1.988 | 1.454–2.718 | <0.001 | |||||||
| D | 2.243 | 1.692–2.973 | <0.001 | |||||||
| Main treatment | ||||||||||
| BSC | Ref. Cat. | |||||||||
| Liver transplant | 0.039 | 0.019–0.077 | <0.001 | 0.038 | 0.019–0.077 | <0.001 | 0.035 | 0.017–0.069 | <0.001 | |
| Resection | 0.176 | 0.126–0.244 | <0.001 | 0.166 | 0.120–0.230 | <0.001 | 0.144 | 0.105–0.198 | <0.001 | |
| Ablation | 0.216 | 0.164–0.284 | <0.001 | 0.204 | 0.156–0.267 | <0.001 | 0.180 | 0.139–0.233 | <0.001 | |
| IAT | 0.343 | 0.267–0.440 | <0.001 | 0.328 | 0.256–0.420 | <0.001 | 0.286 | 0.227–0.362 | <0.001 | |
| Systemic therapy | 0.732 | 0.517–1.036 | 0.079 | 0.690 | 0.489–0.975 | 0.036 | 0.577 | 0.412–0.807 | 0.001 | |
| Others | 0.830 | 0.454–1.517 | 0.545 | 0.766 | 0.422–1.392 | 0.382 | 0.886 | 0.487–1.614 | 0.693 | |
Model 1 includes: age, MAFLD, cirrhosis, modality of HCC diagnosis, Child–Pugh class, ECOG PS, platelet count, oesophageal varices, alpha–foetoprotein levels, tumour gross pathology, size of the greatest lesion, metastasis, MVI and main treatment.
Model 2 includes: age, MAFLD, cirrhosis, modality of HCC diagnosis, ALBI grade, ECOG PS, platelet count, oesophageal varices, alpha-foetoprotein levels, tumour gross pathology, size of the greatest lesion, metastasis, MVI and main treatment.
Model 3 includes: age, MAFLD, cirrhosis, modality of HCC diagnosis, platelet count, oesophageal varices, ITA.LI.CA tumour stage and main treatment. Levels of significance: p <0.05 (Cox regression model).
ALBI, albumin–bilirubin; BSC, best supportive care; HCC, hepatocellular carcinoma; IAT, Intra-arterial therapy; ITA.LI.CA, Italian Liver Cancer; MAFLD, metabolic associated fatty liver disease; MVI, macrovascular invasion; NA, not assessed; Ref. Cat., reference category.
Model 1 (C–P-based): age, C–P class, ECOG PS ≥2, oesophageal varices, platelet count, AFP levels, gross tumour pathology, size of the greatest lesion, MVI, and main treatment.
Model 2 (ALBI-based): ALBI grade, ECOG PS ≥2, oesophageal varices, platelet count, AFP levels, gross tumour pathology, size of the largest lesion, MVI, and main treatment.
Model 3 (ITA.LI.CA-based): oesophageal varices, ITA.LI.CA tumour stage, and main treatment.
Notably, oesophageal varices and the main treatment were independent prognostic factors in all models. Variables related to tumour burden and liver function were independent prognosticators in Models 1 and 2 and, as components of the ITA.LI.CA stage, even in Model 3.
Discussion
This multicentre study, including a large cohort of patients managed in the ‘real world’ setting of clinical practice, provides a dynamic picture spanning 15 years of the clinical landscape of alcohol-related HCC in Italy. The ‘all comers’ strategy adopted for the inclusion of patients in the ITA.L.ICA network did not change over time and therefore the observed variations confidently reflect the evolving clinical scenario that occurred in this population rather than changes as a result of patient referral. Instead, these patients, as well as those with other aetiologies of HCC, could benefit from improvements in the management of HCC and cirrhosis during the data collection period.
All patients reported weekly alcohol intake far beyond the amount considered tolerable, although no healthy amount of alcohol intake exists;10 indeed, the alcohol-related risk for malignancies starts at a daily dose as low as 10 g.25 Moreover, as alcohol synergises with other liver carcinogens (infectious and metabolic),26 its negative effects should be considered when defining a ‘tolerable’ amount of alcohol intake.
Taking these considerations into account, novel information provided by our study suggests that with the advent of the MAFLD definition, most patients with HCC previously classified as having a ‘merely’ alcohol-related disease (according to their alcohol abuse and the apparent absence of other causes of liver injury) migrated to this new taxonomic group (Fig. 1), and their prevalence tended to increase over time. The ‘epidemic’ expansion of metabolic traits among patients with HCC is not sparing other aetiologies.20 Moreover, as mobility restrictions and social stress caused by the COVID-19 pandemic has led to increased alcohol consumption and increased incidence of metabolic disorders in the general population of many countries, a further increase in the prevalence of patients with chronic liver disease characterised by both offending factors can be anticipated. Our study indicates that dysmetabolic traits are present in most patients with dangerously unhealthy alcohol use, and the lower prevalence of cirrhosis in patients with ‘MAFLD’ compared with merely alcohol-related cases (Table S2) would suggest that these traits boost hepatocarcinogenesis, increasing the risk of HCC development in pre-cirrhotic stages, as in patients with NAFLD.19 Unexpectedly, MAFLD (i.e. the presence of features of metabolic syndrome) did not independently affect the prognosis of our patients, in whom the fundamental prognosticators remained those usually reported for HCC, such as PS, liver function tests, portal hypertension signs (oesophageal varices and low platelet counts), tumour burden, AFP levels, and HCC treatment. Thus, it can be inferred that once HCC appears in the context of alcohol-related liver disease, the co-presence of features of metabolic disorders does not seem to significantly affect the risk of death. The similar distribution of the causes of death between patients with alcohol-related and alcohol/MAFLD-related HCC supports this hypothesis.
Another meaningful finding of this study is that the proportion of alcohol-related HCC detected by regular surveillance remained below 50% across all 3 quinquennia evaluated. This figure was comparable to that observed in NAFLD + cryptogenic patients during the same study period and was considerably lower than that observed in patients with viral diseases (Table S4). This difference has various well-known causes, including lower awareness of underlying liver disease by patients and/or providers and poorer adherence to surveillance programmes among alcohol abusers.15 As expected, HCC diagnoses made outside surveillance programmes were associated with worse oncologic profiles, lower amenability to curative treatments, and poorer survival compared with patients diagnosed within surveillance programmes (Table S3). Indeed, the protective role of periodic screening relies on an anticipated cancer diagnosis and increased amenability to curative/effective HCC treatments, as evidenced by the disappearance of the positive prognostic impact of surveillance when challenged by cancer stage in multivariate analysis. The persistent underuse of surveillance led to three sequentially associated key findings of our investigation: (i) modest improvement in cancer burden at the time of diagnosis, (ii) steady use of curative treatments for HCC, and (iii) modest improvement in patient survival. The lack of a significant overall prognostic improvement may rely on the stability of several prognostic determinants over time, such as liver function, comorbidity burden, and ITA.LI.CA cancer stage, despite some, albeit modest, improvements in relieving tumour burden.
Nevertheless, it should not be overlooked that in the last quinquennium, the a-OS of patients significantly improved when the survival was calculated using as starting point 12 months after HCC diagnosis (Fig. 2). This "delayed" improvement could have several determinants: (a) better management of cirrhosis complications; (b) the observed decrease in tumours with more than three nodules, MVI of extrahepatic veins, and abnormal AFP levels; (c) the increased use of IAT (and its improved outcome);27 and (d) the abandonment of systemic therapies with unproven efficacy in favour of sorafenib, and the improved results obtained with this therapy over time.28
In our study, all HCC treatments produced a benefit compared with BSC, with a clear hierarchical classification, confirming the utility of detecting HCCs in stages that allow to obtain the most effective responses to treatments. Moreover, HCC therapy was an independent prognostic factor in all multivariate models, even when challenged by the cancer stage. Such a finding lends support to the management of patients with HCC according to the ‘therapeutic hierarchy’ approach, that is treating every patient with the most effective treatment usable according to liver function, PS, tumour burden, and comorbidities, rather than according to a ‘stage-dictated’ strategy.22,29
Our study also showed that the ITA.LI.CA staging system and ALBI grade, both derived from a population of patients with HCC with different aetiologies, provide reliable prognostic information even for the subset of patients with alcohol-related HCC, and hence, can be confidently used in this setting.
Finally, as previously reported,30 the presence of oesophageal varices is another independent prognostic factor. This is not surprising as varices, which reveal the presence of clinically significant portal hypertension, indicate a more advanced stage of liver disease and a lower amenability to HCC treatments compared with patients without varices. The prognostic significance of oesophageal varices emphasises two important aspects: first, their presence cannot be disregarded in the management of patients with HCC, as pointed out by a recent expert opinion article;31 second, this variable should be included in prognostic scores for HCC to improve their performance.
Our study has several limitations. First, owing to its retrospective design, it was vulnerable to the effects of unintended biases and, therefore, its results should be scrutinised in this light. Second, an unavoidable drawback of multicentre databases established in clinical practice is the presence of missing data, which can be managed using data imputation methods. However, these methods need to be carefully applied to avoid potential distortion of results, a risk which increases with the increasing percentage of missing data. Thus, we only applied imputation methods to covariates with <25% of missing data. Consequently, the BMI was excluded from this manipulation, making it impossible to determine its independent prognostic value. Third, analyses of clinical databases provide results that are vulnerable to selection bias, which does not disturb the reviews of population-based registries. Therefore, despite our investigation relying on a large, multicentre, and broadly distributed HCC population managed in both academic and non-academic hospitals, the results cannot be generalised to the entire HCC population. Indeed, the Italian population registry currently estimates a 5-year survival rate of approximately 20% for patients with primary liver cancer,32 a figure much lower than that we observed in the last quinquennium. Fourth, some transient differences between the periods, although statistically significant, may not be clinically meaningful. Conversely, for variables showing a unidirectional trend that eventually reached statistical significance, it can be expected that this behaviour is predictive of forthcoming changes in outcomes of alcohol-related HCC. Fifth, data on alcohol intake after HCC diagnosis were not available and, although only one-fourth of patients were active consumers of alcohol at the time of cancer detection, this drawback prohibited the possibility of testing another potentially important predictor of outcome. Lastly, since the survival of patients with HCC is generally the composite result of a sequence of treatments, particularly in patients diagnosed at early or intermediate stages, the result achieved by any ‘main’ treatment should be interpreted in this light.
In conclusion, in patients with alcohol-related HCC, any improvement in cancer burden at diagnosis was limited by persistent underuse of surveillance programmes. Consequently, the amenability to curative treatments did not increase, and survival showed only modest improvement. Therefore, achieving marked prognostic improvement relies on the implementation of health policies that can expand the prescription of surveillance strategies and increase patient adherence to these programmes. Moreover, as the presence of oesophageal varices represents an independent negative prognostic factor, this clinical aspect should be carefully considered in the prognosis and management of patients with HCC. Lastly, the clinical features of metabolic disorders are extremely common in patients with alcohol-related liver disease; however, once HCC appears, these may not significantly affect the prognosis of these patients.
Financial support
Roche supported the data collection for this study.
Authors’ contributions
Concept of the study: FT, LB, NR, VS. Statistical analysis: LB. Drafting of the manuscript: FT, LB, NR, VS. Data collection, revision for important intellectual content and approval of the final manuscript: all authors. Guarantors of the article: NR, FT.
Data availability statement
The data of this study were supplied by the ITA.LI.CA Consortium and confidential patient level data are available from this Consortium upon motivated request to the guarantor(s) (FT).
Conflicts of interest
CC (speaker fees, advisory board): Eisai, Ipsen and MSD; EGG (advisory board, consulting fees): Astra Zeneca, Eisai, MSD, and Roche; FGF (advisory board, consulting fees): AbbVie, Bayer, Eisai, Gilead, MSD, and Intercept; FP (consulting or lecture fees): Astra Zeneca, Bayer, Bracco, EISAI, ESAOTE, Exact Sciences, IPSEN, MSD, Roche, Samsung, and Tiziana Life Sciences; GR (lectures, advisory board, consulting fees): Gilead, Alfa Wasserman, Intercept, and Eisai; FT (research grant, advisory board, consulting fees): Astra Zeneca, AbbVie, Bayer, Eisai, Gilead, MSD, and Roche.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
We thank Editage (www.editage.com) for English language editing.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2023.100784.
Appendix
Other members of the Italian Liver Cancer (ITA.LI.CA) group :
Unit of Semeiotics, Liver and Alcohol diseases, IRCCS Azienda Ospedaliero-Universitaria di Bologna: Maurizio Biselli, Paolo Caraceni, Annagiulia Gramenzi.
Division of Internal Medicine, Hepatobiliary Diseases and Immunoallergology, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy Azienda Ospedaliero-Universitaria S. Orsola-Malpighi, Internal Medicine–Piscaglia Unit, Bologna: Francesca Benevento, Alessandro Granito, Luca Muratori, Fabio Piscaglia, Francesco Tovoli.
Division of Gastroenterology and Hepatology, Fondazione IRCCS Ca’ Granda Ospedale maggiore Policlinico and C.R.C. “A.M. & A. Migliavacca Center for Liver Disease”, Milano: Gloria Allegrini.
Department of Health Promotion, Mother & Child Care, Internal Medicine & Medical Specialties, PROMISE, Section of Gastroenterology & Hepatology, University of Palermo, Palermo: Calogero Cammà, Giuseppe Cabibbo, Carmelo Marco Giacchetto, Paolo Giuffrida, Maria Vittoria Grassini, Mauro Grova, Gabriele Rancatore, Caterina Stornello.
Department of Experimental and Clinical Medicine, Internal Medicine and Hepatology Unit, University of Firenze, Firenze: Valentina Adotti, Tancredi Li Cavoli, Fabio Marra, Martina Rosi.
Department of Emergency – Internal Medicine and Cardiology – IRCCS-IRST Meldola, AUSL Romagna, and Ospedale per gli Infermi di Faenza, Faenza: Vittoria Bevilacqua, Alberto Borghi, Lucia Napoli, Fabio Conti, GL Frassineti, Maria Teresa Migliano.
Liver Injury and Transplant Unit and Obesity Center, Polytechnic University of Marche, Ancona, Italy: Gloria Allegrini.
Internal Medicine and Gastroenterology, Fondazione Policlinico Universitario Agostino Gemelli, IRCCS, Roma – Università Cattolica del Sacro Cuore, Roma: Nicoletta de Matthaeis, Francesca Romana Ponziani.
Department of Medicine and Surgery, Infectious Diseases and Hepatology Unit, University of Parma and Azienda Ospedaliero-Universitaria of Parma, Parma: Gabriele Missale, Andrea Olivani.
Department of Clinical Medicine and Surgery, Departmental Program "Diseases of the liver and biliary system", University of Napoli "Federico II": Mario Capasso, Valentina Cossiga, Maria Guarino. Gastroenterology and Digestive Endoscopy Unit, Foggia University Hospital, Foggia: Ester Marina Cela, Antonio Facciorusso.
Gastroenterology Unit, Belcolle Hospital, Viterbo: Camilla Graziosi, Valentina Lauria, Giorgio Pelecca.
Medical Oncology Unit, Belcolle Hospital, Viterbo: Marta Schirripa.
Vascular and Interventional Radiology Unit, Belcolle Hospital, Viterbo: Fabrizio Chegai, Armando Raso, Alessio Bozzi.
Department of Clinical and Experimental Medicine, Division of Medicine and Hepatology, University of Messina, Messina, Italy: Maria Stella Franzè, Carlo Saitta.
Department of Medical, Surgical and Experimental Sciences, Azienda Ospedaliero-Universitaria of Sassari, Sassari: Assunta Sauchella.
Division of Gastroenterology, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna: Elton Dajti, Federico Ravaioli.
Department of Internal Medicine, Gastroenterology Unit, University of Genova, IRCCS Ospedale Policlinico San Martino, Genova: Maria Corina Plaz Torres, Giulia Pieri.
Department of Clinical and Experimental Medicine, Hepatology and Liver Physiopathology Laboratory, University Hospital of Pisa, Pisa: Filippo Oliveri, Gabriele Ricco, Veronica Romagnoli.
Gastroenterology Unit, IRCCS Sacro Cuore Don Calabria Hospital, Negrar: Alessandro Inno, Fabiana Marchetti.
Department of Clinical Medicine and Surgery, Hepato-Gastroenterology Unit, University of Napoli "Federico II", Napoli: Pietro Coccoli, Antonio Malerba.
Radiology Unit Golfieri, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy: Alberta Cappelli, Rita Golfieri, Cristina Mosconi, Matteo Renzulli.
Contributor Information
Franco Trevisani, Email: franco.trevisani@unibo.it.
for the Italian Liver Cancer (ITA.LI.CA) group:
Maurizio Biselli, Paolo Caraceni, Annagiulia Gramenzi, Francesca Benevento, Alessandro Granito, Luca Muratori, Fabio Piscaglia, Francesco Tovoli, Gloria Allegrini, Calogero Cammà, Giuseppe Cabibbo, Carmelo Marco Giacchetto, Paolo Giuffrida, Maria Vittoria Grassini, Mauro Grova, Gabriele Rancatore, Caterina Stornello, Valentina Adotti, Tancredi Li Cavoli, Fabio Marra, Martina Rosi, Vittoria Bevilacqua, Alberto Borghi, Lucia Napoli, Fabio Conti, G.L. Frassineti, Maria Teresa Migliano, Gloria Allegrini, Nicoletta de Matthaeis, Francesca Romana Ponziani, Gabriele Missale, Andrea Olivani, Mario Capasso, Valentina Cossiga, Maria Guarino, Ester Marina Cela, Antonio Facciorusso, Camilla Graziosi, Valentina Lauria, Giorgio Pelecca, Marta Schirripa, Fabrizio Chegai, Armando Raso, Alessio Bozzi, Maria Stella Franzè, Carlo Saitta, Assunta Sauchella, Elton Dajti, Federico Ravaioli, Maria Corina Plaz Torres, Giulia Pieri, Filippo Oliveri, Gabriele Ricco, Veronica Romagnoli, Alessandro Inno, Fabiana Marchetti, Pietro Coccoli, Antonio Malerba, Alberta Cappelli, Rita Golfieri, Cristina Mosconi, and Matteo Renzulli
Supplementary data
The following are the supplementary data to this article:
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Trevisani F., Frigerio M., Santi V., Grignaschi A., Bernardi M. Hepatocellular carcinoma in non-cirrhotic liver: a reappraisal. Dig Liver Dis. 2010;42:341–347. doi: 10.1016/j.dld.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y., Wang C., Xu H., Xiao P., Gao Y. Hepatocellular carcinoma in the noncirrhotic liver: a literature review. Eur J Gastroenterol Hepatol. 2019;31:743–748. doi: 10.1097/MEG.0000000000001419. [DOI] [PubMed] [Google Scholar]
- 4.Forner A., Reig M., Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–1314. doi: 10.1016/S0140-6736(18)30010-2. [DOI] [PubMed] [Google Scholar]
- 5.Siegel A.B., Zhu A.X. Metabolic syndrome and hepatocellular carcinoma: two growing epidemics with a potential link. Cancer. 2009;115:5651–5661. doi: 10.1002/cncr.24687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anstee Q.M., Reeves H.L., Kotsiliti E., Govaere O., Heikenwalder M. From NASH to HCC: current concepts and future challenges. Nat Rev Gastroenterol Hepatol. 2019;16:411–428. doi: 10.1038/s41575-019-0145-7. [DOI] [PubMed] [Google Scholar]
- 7.Akinyemiju T., Abera S., Ahmed M., Alam N., Alemayohu M.A., Allen C., et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the Global Burden of Disease Study 2015. JAMA Oncol. 2017;3:1683–1691. doi: 10.1001/jamaoncol.2017.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ganne-Carrié N., Nahon P. Hepatocellular carcinoma in the setting of alcohol-related liver disease. J Hepatol. 2019;70:284–293. doi: 10.1016/j.jhep.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 9.Ganne-Carrié N., Chaffaut C., Bourcier V., Archambeaud I., Perarnau J.-M., Oberti F., et al. Estimate of hepatocellular carcinoma incidence in patients with alcoholic cirrhosis. J Hepatol. 2018;69:1274–1283. doi: 10.1016/j.jhep.2018.07.022. [DOI] [PubMed] [Google Scholar]
- 10.Mehta G., Sheron N. No safe level of alcohol consumption-Implications for global health. J Hepatol. 2019;70:587–589. doi: 10.1016/j.jhep.2018.12.021. [DOI] [PubMed] [Google Scholar]
- 11.Kulik L., El-Serag H.B. Epidemiology and management of hepatocellular carcinoma. Gastroenterology. 2019;156:477–491.e1. doi: 10.1053/j.gastro.2018.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garuti F., Neri A., Avanzato F., Gramenzi A., Rampoldi D., Rucci P., et al. The changing scenario of hepatocellular carcinoma in Italy: an update. Liver Int. 2021;41:585–597. doi: 10.1111/liv.14735. [DOI] [PubMed] [Google Scholar]
- 13.Bucci L., Garuti F., Camelli V., Lenzi B., Farinati F., Giannini E.G., et al. Comparison between alcohol- and hepatitis C virus-related hepatocellular carcinoma: clinical presentation, treatment and outcome. Aliment Pharmacol Ther. 2016;43:385–399. doi: 10.1111/apt.13485. [DOI] [PubMed] [Google Scholar]
- 14.Costentin C.E., Mourad A., Lahmek P., Causse X., Pariente A., Hagège H., et al. Hepatocellular carcinoma is diagnosed at a later stage in alcoholic patients: results of a prospective, nationwide study. Cancer. 2018;124:1964–1972. doi: 10.1002/cncr.31215. [DOI] [PubMed] [Google Scholar]
- 15.Singal A.G., Yopp A.C., Gupta S., Skinner C.S., Halm E.A., Okolo E., et al. Failure rates in the hepatocellular carcinoma surveillance process. Cancer Prev Res. 2012;5:1124–1130. doi: 10.1158/1940-6207.CAPR-12-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schoenberger H., Chong N., Fetzer D.T., Rich N.E., Yokoo T., Khatri G., et al. Dynamic changes in ultrasound quality for hepatocellular carcinoma screening in patients with cirrhosis. Clin Gastroenterol Hepatol. 2022 Jul;20(7):1561–1569.e4. doi: 10.1016/j.cgh.2021.06.012. Epub 2021 Jun 10. PMID: 34119640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Llovet J.M., Kelley R.K., Villanueva A., Singal A.G., Pikarsky E., Roayaie S., et al. Hepatocellular carcinoma. Nat Rev Dis Primer. 2021;7:6. doi: 10.1038/s41572-020-00240-3. [DOI] [PubMed] [Google Scholar]
- 18.Eslam M., Newsome P.N., Sarin S.K., Anstee Q.M., Targher G., Romero-Gomez M., et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73:202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 19.Eslam M., Sanyal A.J., George J., International Consensus Panel MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158:1999–2014.e1. doi: 10.1053/j.gastro.2019.11.312. [DOI] [PubMed] [Google Scholar]
- 20.Vitale A., Svegliati-Baroni G., Ortolani A., Cucco M., Riva G.V.D., Giannini E.G., et al. Epidemiological trends and trajectories of MAFLD-associated hepatocellular carcinoma 2002–2033: the ITA.LI.CA database. Gut. 2023 Jan;72(1):141–152. doi: 10.1136/gutjnl-2021-324915. Epub 2021 Dec 21. [DOI] [PubMed] [Google Scholar]
- 21.Farinati F., Vitale A., Spolverato G., Pawlik T.M., Huo T., Lee Y.-H., et al. Development and validation of a new prognostic system for patients with hepatocellular carcinoma. Plos Med. 2016;13 doi: 10.1371/journal.pmed.1002006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vitale A., Trevisani F., Farinati F., Cillo U. Treatment of hepatocellular carcinoma in the precision medicine era: from treatment stage migration to therapeutic hierarchy. Hepatology. 2020;72:2206–2218. doi: 10.1002/hep.31187. [DOI] [PubMed] [Google Scholar]
- 23.Cucchetti A., Trevisani F., Pecorelli A., Erroi V., Farinati F., Ciccarese F., et al. Estimation of lead-time bias and its impact on the outcome of surveillance for the early diagnosis of hepatocellular carcinoma. J Hepatol. 2014;61:333–341. doi: 10.1016/j.jhep.2014.03.037. [DOI] [PubMed] [Google Scholar]
- 24.Baraldi A.N., Enders C.K. An introduction to modern missing data analyses. J Sch Psychol. 2010;48:5–37. doi: 10.1016/j.jsp.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 25.GBD 2016 Alcohol Collaborators Alcohol use and burden for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018;392:1015–1035. doi: 10.1016/S0140-6736(18)31310-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Decraecker M., Dutartre D., Hiriart J.-B., Irles-Depé M., Marraud des Grottes H., Chermak F., et al. Long-term prognosis of patients with alcohol-related liver disease or non-alcoholic fatty liver disease according to metabolic syndrome or alcohol use. Liver Int. 2022;42:350–362. doi: 10.1111/liv.15081. [DOI] [PubMed] [Google Scholar]
- 27.Trevisani F., Golfieri R. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: where are we now? Hepatology. 2016;64:23–25. doi: 10.1002/hep.28554. [DOI] [PubMed] [Google Scholar]
- 28.Tovoli F., Ielasi L., Casadei-Gardini A., Granito A., Foschi F.G., Rovesti G., et al. Management of adverse events with tailored sorafenib dosing prolongs survival of hepatocellular carcinoma patients. J Hepatol. 2019;71:1175–1183. doi: 10.1016/j.jhep.2019.08.015. [DOI] [PubMed] [Google Scholar]
- 29.Vitale A., Finotti M., Trevisani F., Farinati F., Giannini E.G. Treatment allocation in patients with hepatocellular carcinoma: need for a paradigm shift? Liver Cancer Int. 2022;3:34–36. [Google Scholar]
- 30.Giannini E.G., Risso D., Testa R., Trevisani F., Di Nolfo M.A., Del Poggio P., et al. Prevalence and prognostic significance of the presence of esophageal varices in patients with hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2006;4:1378–1384. doi: 10.1016/j.cgh.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 31.Thabut D., Kudo M. Treatment of portal hypertension in patients with HCC at the era of Baveno VII. J Hepatol. 2023;78:658–662. doi: 10.1016/j.jhep.2022.11.019. [DOI] [PubMed] [Google Scholar]
- 32.I numeri del cancro in Italia 2020 | Associazione Italiana Registri Tumori n.d. https://www.registri-tumori.it/cms/pubblicazioni/i-numeri-del-cancro-italia-2020 (accessed May 31, 2022).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data of this study were supplied by the ITA.LI.CA Consortium and confidential patient level data are available from this Consortium upon motivated request to the guarantor(s) (FT).