Abstract
Background
CDK4/6 inhibitors combined with endocrine therapy have significantly improved treatment outcomes for metastatic hormone receptor-positive (HR+) breast cancer patients. However, the impact of low HER2 expression on treatment response and progression-free survival (PFS) remains unclear.
Methods
This multicenter retrospective study included 204 HR+ breast cancer patients treated with a combination of CDK4/6 inhibitor and endocrine therapy. HER2-zero disease was detected in 138 (68%) and HER2-low disease in 66 (32%) patients. Treatment-related characteristics and clinical outcomes were analyzed, with a median follow-up of 22 months.
Results
The objective response rate (ORR) was 72.7% in the HER2 low group and 66.6% in the HER2 zero group (p = 0.54). Median PFS was not significantly different between the HER2-low and HER2 zero groups (19 months vs.18 months, p = 0.89), although there was a trend toward longer PFS in the HER2-low group for first-line treatment (24 months progression-free survival rate 63% vs 49%). In recurrent disease, the median PFS was 25 months in the HER2-low group and 12 months in the HER2-zero group (p = 0.08), while in de novo metastatic disease, the median PFS was 18 months in the HER2-low group and 27 months in the HER2-zero group (p = 0.16). The order of CDK4/6 inhibitor use and the presence of visceral metastasis were identified as independent variables affecting PFS.
Conclusion
Low HER2 expression did not significantly impact treatment response or PFS in HR+ breast cancer patients treated with a CDK4/6 inhibitor and endocrine therapy. Because of the conflicting results in the literature, further prospective studies are needed to evaluate the clinical significance of HER2 expression in HR+ breast cancer.
Keywords: HER2-Low, ribociclib, palbociclib, hormone positive, breast cancer
Highlights
-
•
Combination therapy with CDK4/6 inhibitors and endocrine therapy achieves impressive objective response rates in metastatic hormone-positive breast cancer patients regardless of HER2 status.
-
•
Low HER2 expression did not significantly affect survival in hormone-positive metastatic breast cancer patients receiving CDK4/6 inhibitors and endocrine therapy.
-
•
The order of CDK4/6 inhibitor use and the presence of visceral metastasis were identified as independent factors influencing progression-free survival.
1. Introduction
According to Globocan 2022, breast cancer is responsible for 31% of cancers in women and 15% of cancer-related deaths [1]. Although there have been groundbreaking advances in breast cancer treatment in recent years, metastatic breast cancer remains a major health problem. Breast cancer is a highly heterogeneous disease with different prognoses due to different genomic and molecular characteristics[2]. Since genomic profiling is not always possible, the treatment decision is mainly based on hormone receptor and human epidermal growth factor receptor 2 (HER2) status [3]. HER2 positivity is seen in approximately 15–20% of all breast cancer cases and is considered a poor prognostic factor. In addition, HER2 positivity is also considered a predictive factor due to the advances in HER2-targeted therapies, resulting in good outcomes in HER2-positive patients.
According to the American Society of Clinical Oncology and College of American Pathologists (ASCO/CAP) 2018 guidelines, HER2-negative breast cancer includes those with IHC 0, IHC 1+, and IHC 2+/negative in situ hybridization (ISH) based on immunohistochemical (IHC) staining [4]. Within this group, defined as HER2-negative, the IHC1+ and IHC 2+/ISH-negative group, observed at a rate of 45–55%, is now considered under the title of HER2-low [5]. Until the last few years, the number of studies in which HER2-targeted therapy was tried for this group was very limited, and no benefit could be demonstrated with targeted therapies [6,7]. However, after the breakthrough findings of the DESTINY 04 Breast study of trastuzumab deruxtecan, an antibody-drug conjugate (ADC), in HER2 low metastatic breast cancer patients, interest in HER2-low breast cancer has increased [8]. These encouraging results have raised the question of whether HER2-low breast cancer is a tumor with different clinical and biological characteristics, leading to an increase in studies in this field. However, the results of studies on this patient group's clinical and biological characteristics are controversial. There are conflicting findings that vary according to the stage of breast cancer, hormone receptor status, and treatment received [[9], [10], [11], [12]].
Despite the impressive results of ADCs in HER2-low tumors, the current recommended treatment of hormone receptor-positive metastatic breast cancer is the combination of cyclin-dependent kinase 4 and 6 inhibitors (CDK4/6i) and endocrine therapy (ET) [13]. The impressive survival results with CDK4/6 inhibitor and endocrine therapy combinations have led to a paradigm shift in the treatment of metastatic hormone-positive breast cancer. However, despite these practice-changing successes, the search for biomarkers indicating resistance or sensitivity to CDK4/6 inhibitors continues [14,15]. From a biological point of view, it is known that there is a bidirectional crosstalk between HER2 and hormone receptor (HR) pathways, which can modulate HER2 expression even in the absence of gene amplification [16,17]. It has been shown that the expression of HER2 protein may increase after endocrine therapies [18], chemotherapy [19], and radiotherapy [20]. Considering the interplay between HER2 and HR pathways, the question arises whether HER2 low status may affect the efficacy of the CDK4/6 inhibitor-endocrine therapy combination. There are very few studies in the literature, and the results are contradictory.
This study aimed to investigate the effect of HER2 expression status on treatment responses and progression-free survival in hormone-positive metastatic breast cancer patients treated with CDK4/6i + ET.
2. .Materials and methods
2.1. Study population
This is a multicenter, retrospective cohort study, approved by the Ethics Committee of Dokuz Eylul University (2023/03–09). A total of 246 patients with metastatic hormone receptor-positive breast cancer treated with CDK4/6i + ET were retrospectively screened across four different oncology clinics between March 2019 and December 2022. The study included patients who met the following criteria: being 18 years of age or older, having metastatic hormone-positive breast cancer confirmed radiologically, and having received treatment with CDK4/6 inhibitor (either ribociclib or palbociclib according to physician's choice) in combination with endocrine therapy. Patients with a follow-up duration less than 6 months, incomplete pathological data, or inadequate evaluation of treatment response were excluded from the study. Clinicopathologic and treatment-related characteristics were reviewed from patients' files and electronic records of each tertiary cancer center for a total of 204 patients, including age at diagnosis, menopausal status, tumor histology and differentiation, HR and HER2 expression status, Ki67 index, de novo metastatic/recurrent disease status, site of metastasis, and progression date. The use of CDK4/6 inhibitors and concomitant endocrine therapy (aromatase inhibitor or fulvestrant), as well as the order in which they were used, were also recorded. All patients underwent PET-CT (positron emission tomography-computed tomography) imaging for metastasis evaluation, and other imaging modalities such as cranial MRI (magnetic resonance imaging) and abdominal MRI were utilized as necessary.
2.1.1. Pathologic evaluation
The estrogen receptor (ER), progesterone receptor (PR), and HER2 status of pathology specimens were evaluated through immunohistochemistry (IHC) by accredited pathology laboratories at each center, no central evaluation was performed. In patients with HER2 status 2+ due to IHC evaluations, HER2 amplification was examined through the in situ hybridization (ISH) test. Pathology evaluations were conducted using biopsy materials from both primary tumors and metastases, when possible. Pathologic biomarker detection, interpretation, and reporting were performed based on the current ASCO-CAP guideline applicable during the assessment year. ER and PR status were considered positive when stained >10% through IHC. HER2 low status was defined as 1+ on IHC or 2+ on IHC but no amplification on ISH. If HER2 low expression was detected pathologically in either the primary tumor or metastasis, the patient was included in the HER2 low group.
2.2. Treatment response evaluation
The response of the patients to treatment was monitored using PET-CT or CT according to the physician's choice. For patients who underwent CT imaging, response evaluation was performed according to RECIST (Response Evaluation Criteria in Solid ) version 1.1 [21]. On the other hand, for patients who underwent PET-CT imaging, response evaluation was conducted using PERCIST criteria [22].
2.3. Statistical analysis
The patients' clinicopathological and treatment-related features were compared in the HER2-low and HER2-zero groups. The categorical variables were compared using Pearson's chi-squared test or Fisher's exact test. For descriptive analysis, percentages were used for categorical variables, and medians and ranges were used for continuous variables. PFS was the time from the beginning of treatment until disease progression. OS was defined as the time from treatment start to death from any cause. Survival was calculated using the Kaplan–Meier method and was compared between the groups using the log-rank test. The median follow-up period was evaluated by the reverse Kaplan-Meier method. Subgroup analyses were performed according to the order of CDK4/6 inhibitor use and metastasis status (de novo metastatic or recurrent disease). Unadjusted hazard ratios (HRs) for progression-free survival (PFS) were determined using Cox proportional hazard regression models. To account for potential confounding factors, adjusted HRs were calculated using multivariate regression analysis. Factors that showed statistical significance in the univariate analyses (p < 0.05) were included in the multivariate analysis.
All statistical analyses were performed with the SPSS Statistics 25.0 for iOS software program (SPSS, Inc., Chicago, IL, USA), and P < 0.05 was considered statistically significant.
3. Results
A total of 204 patients were included in the study. HER2-zero disease was detected in 138 (68%) and HER2-low disease in 66 (32%) patients. The median age at CDK4/6 inhibitor initiation was 58 years (47–67 years), and approximately 27% of patients were older than 65. The histologic subtype was invasive ductal carcinoma (IDC) in 61% of patients, invasive lobular carcinoma (ILC) in 16%, and ILC + IDC mixed type in 9%. Those with a Ki-67 proliferation index below 20% constituted approximately one-third of the entire population. Regarding tumor grade, 90% of the patients had grade 2 and 3 tumors. De novo metastatic patients (52.5%) and recurrent metastatic patients (47.5%) were almost equally distributed. The presence of visceral metastasis was detected in 62% of patients. There was no statistically significant difference between HER2 zero and HER2 low groups regarding all these clinicopathologic features (Table 1).
Table 1.
Clinicopathologic and treatment-related characteristics of study population and patients group according to HER2 status.
| All population (n = 204) | HER2-zero (n = 138) | HER2-low (n = 66) | P value | |
|---|---|---|---|---|
| Age | ||||
| Median (IQR) | 58 (47–67) | 58 (48–67) | 57 (46–66) | 0.88 |
| <65 | 147 (72.1) | 99 (71.7) | 48 (72.7) | |
| >65 | 57 (27.9) | 39 (28.3) | 18 (27.3) | |
| Menapause status (n (%)) | ||||
| Premenaposal | 76 (38) | 49 (36) | 27 (42.2) | 0.40 |
| Postmenaposal | 124 (62) | 87 (64) | 37 (57.8) | |
| Histology (n (%)) | ||||
| IDC | 114 (61) | 78 (63.4) | 36 (56.3) | 0.29 |
| ILC | 31 (16.6) | 22 (17.9) | 9 (14.1) | |
| IDC + ILC | 16 (8.6) | 10 (8.1) | 6 (9.4) | |
| Others | 26 (13.9 | 13 (10.6) | 13 (20.3) | |
| Ki67 proliferation index (n (%)) | ||||
| <20 | 59 (34.7) | 42 (37.2) | 17 (29.8) | 0.39 |
| >20 | 111 (65.3) | 71 (62.8) | 40 (70.2) | |
| Grade (n (%)) | ||||
| 1 | 16 (9.6) | 12 (10.6) | 4 (7.4) | 0.49 |
| 2 | 116 (69.5) | 80 (70.8) | 36 (66.7) | |
| 3 | 35 (21) | 21 (18.6) | 14 (25.9) | |
| ER status (n (%)) | ||||
| ER ≤%10 | 6 (3) | 4 (3) | 2 (3.1) | 0.96 |
| ER>%10 | 198 (97) | 129 (97) | 62 (96.9) | |
| Disease status (n (%)) | ||||
| De novo metastatic | 107 (52.5) | 76 (55.1) | 31 (47) | 0.29 |
| Recurren metastatic | 97 (47.5) | 62 (44.9) | 35 (53) | |
| Site of metastatic disease (n (%)) | ||||
| Visceral metastasis | 127 (62.3) | 90 (65.2) | 37 (56.1) | 0.2 |
| Non-visceral metastasis | 77 (37.7) | 48 (34.8) | 29 (43.9) | |
| Treatment line (n (%)) | ||||
| First line | 99 (48.5) | 74 (53.6) | 25 (37.9) | 0.03 |
| Second line | 59 (28.9) | 32 (23.2) | 27 (40.9) | |
| Third and more line | 46 (22.5) | 32 (23.2) | 14 (22.2) | |
| CDK4/6 inhibitors (n (%)) | ||||
| Palbociclib | 79 (38.7) | 53 (38.4) | 26 (39.4) | 0.89 |
| Ribociclib | 125 (61.3) | 85 (61.6) | 40 (60.6) | |
| Endocrine therapy (n (%)) | ||||
| Aromatase inhibitor | 115 (56.4) | 83 (60.1) | 32 (48.5) | 0.11 |
| Fulvestrant | 89 (43.6) | 55 (39.9) | 34 (51.5) | |
Among 105 patients in whom HER2 status was evaluated both in primary tumor and in a metastatic lesion. A shift in HER2 status was observed in 26 (24%)patients. Specifically, 17 (16%) tumors changed from HER2 zero to HER2-low, while 6 (8%) tumors shifted from HER2-low to HER2-zero.
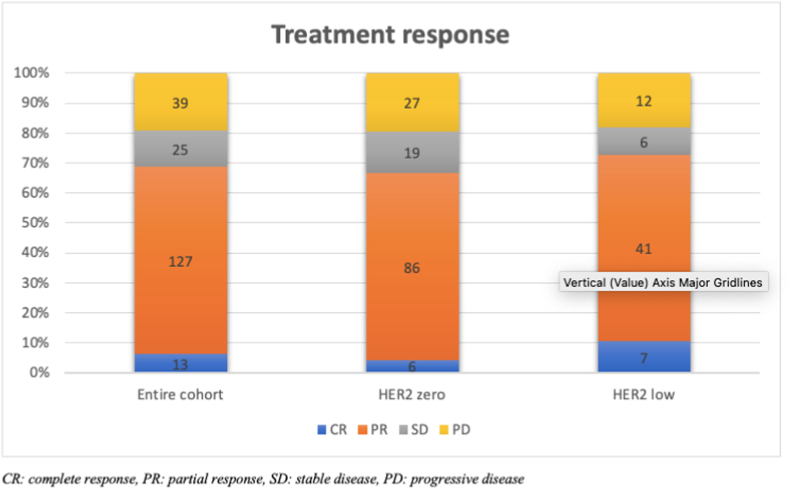
Considering the treatment-related characteristics of the study population, it was shown that ribociclib is preferred as the CDK 4/6 inhibitor in 61% of the patients, and there was no difference between the groups. Endocrine therapy accompanying CDK4/6 inhibitor was aromatase inhibitor in 56.4% of patients and fulvestrant in 43.3% of patients. While 48% of the entire population received CDK 4/6 inhibitor-endocrine combination as the first line of treatment, this rate was 53.6% in the HER2-zero group and 37.9% in HER2 low group (p = 0.03). The objective response rate (ORR) was 72.7% in the HER2 low group and 66.6% in the HER2 zero group, with no statistically significant difference (Fig. 1).
Fig. 1.
Treatment responses according to HER2 status
CR: complete response, PR: partial response, SD: stable disease, PD: progressive disease.
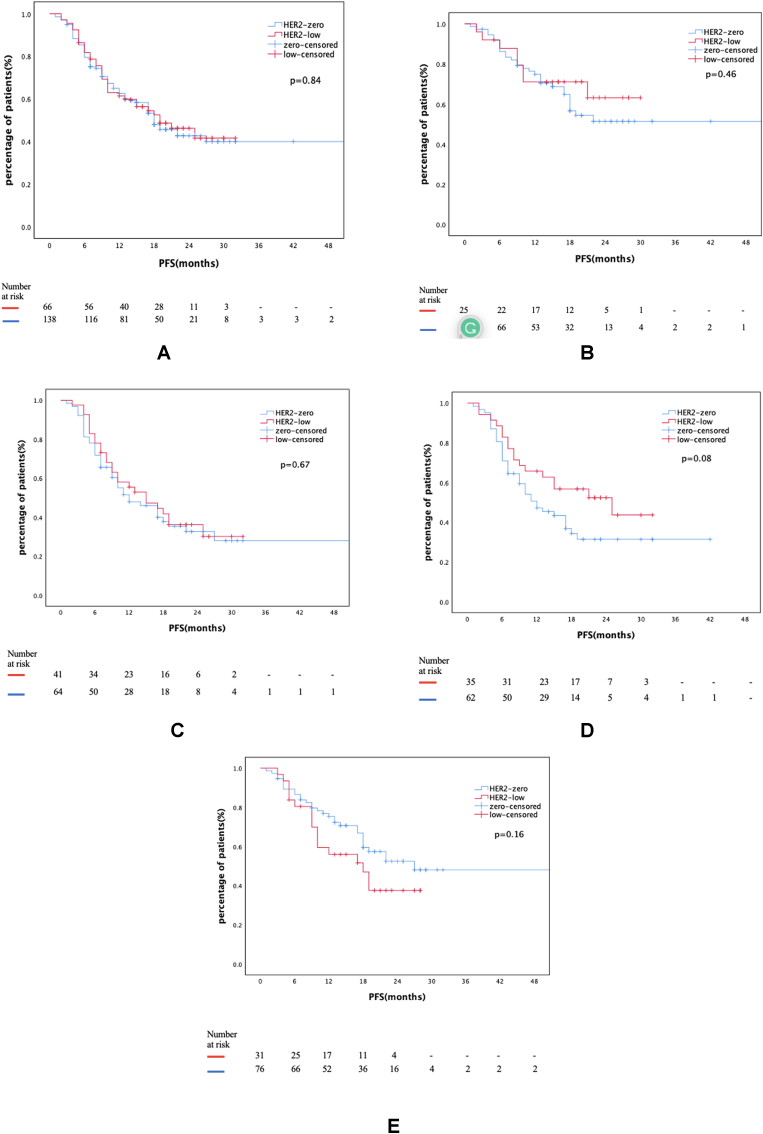
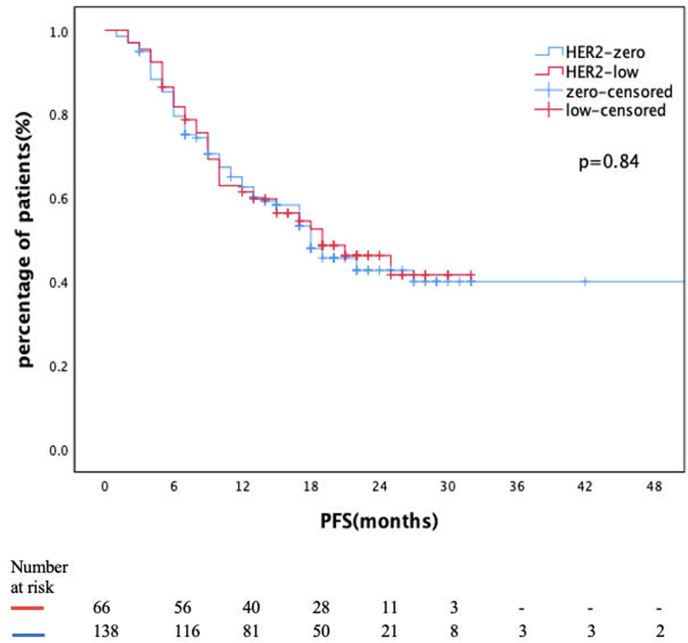
With a data cut-off date of March 2023, the median follow-up was 22 (CI 20.6–23.3) months. One hundred-five patients (51.5%) experienced disease progression during this time, with a median PFS of 18 months (CI 14.2–21.7) for the entire cohort. There was no statistically significant difference in median PFS between HER2 low and HER2 zero groups (19 months vs. 18 months, p = 0.89, Fig. 2a). Median PFS was not reached in patients receiving first-line CDK4/6i + ET combination. However, at 24 months, 63% of patients in the HER2-low group had progression-free survival compared to 49% in the HER2-zero group (p = 0.64) (Fig. 2b). In the group receiving CDK4/6i + ET combination as a subsequent-line treatment, the rate of progression-free survival at 24 months was 27% in both groups (Fig. 2c).
Fig. 2.
Fig. 2a: PFS of entire cohort according to HER2 status
Fig. 2b: PFS of patients receiving CDK4/6i + ET as a treatment
Fig. 2c: PFS of patients receiving CDK4/6i + ET as a treatment
Fig. 2d: PFS of patients with recurrent disease
Fig. 2e: PFS of patients with de novo metastatic disease.
In recurrent metastatic disease, mPFS was 25 months (CI 8.7–41.2) in the HER2 low group and 12 months (CI 7.2–16.7) in the HER2 zero group (p = 0.08) (Fig. 2d). In de novo metastatic disease, mPFS was 18 months (CI 10.7–25.2) in HER2 low group and 27 months (CI 11.3–42.6) in HER2 zero group (p = 0.16) (Fig. 2e).
In the univariate analysis, the factors affecting PFS were determined as grade, metastasic status, presence of visceral metastasis, and the order of CDK 4/6 inhibitor use. In multivariate Cox regression analysis, the order of CDK4/6i + ET combination and the presence of visceral metastasis were identified as independent variables affecting PFS (Table 2).
Table 2.
Prognostic factors for PFS in univariate and multivariate analysis.
| Univariate Analysis HR (%95CI) p-value | Multivariate Analysis HR (%95 CI) p- value | |||
|---|---|---|---|---|
| HER2-0 vs HER2 low | 1.04 (0.69–1.56) | 0.85 | ||
| Ki67<%20 vs Ki67>%20 | 1.35 (0.86–2.11) | 0.19 | ||
| Grade 1–2 vs Grade 3 | 1.80 (1.12–2.88) | 0.014 | 1.49 (0.92–2.41) | 0.10 |
| De novo vs recurren disease | 0.68 (.46–1.01) | 0.057 | ||
| Visceral met vs non visceral met | 3.20 (1.98–5.18) | <0.001 | 2.70 (1.62–4.52) | <0.001 |
| First-line treatment vs subsequent lines treatment | 0.50 (0.33–.74) | 0.001 | 1.76 (1.10–2.82) | 0.017 |
| AI vs Fulvestrant | 1.70 (1.16–2.51) | 0.007 | 1.47 (0.92–2.33) | 0.09 |
HR:hazard ratio, CI:confidence interval.
During follow-up, 46 patients (22.5%) died. No median OS value was reached in the study. While 79% of patients were alive at 24 months in the HER2 low group, this rate was 67% in the HER2 zero group (Fig. 3).
Fig. 3.
OS of entire cohort according to HER2 status.
4. Discussion
The combination of CDK4/6 inhibitor and endocrine therapy is the standard of care in the first-line treatment of metastatic HR+ breast cancer and in subsequent lines in patients who have not previously received this therapy [[23], [24], [25], [26], [27]]. Despite the impressive results of these agents in clinical trials and real-life data, at some point, progression occurs. The search for biomarkers to predict clinical outcomes continues. Especially after the revolutionary results of trastuzumab deruxtecan in tumors with HR+ HER2 low expression, the curiosity of whether HER2 low expression status without amplification can be a predictive and prognostic factor in breast cancer has emerged [8].
HER2-low breast cancer is a relatively new concept. HER2 expression level is determined according to ASCO/CAP guidelines, and there have been significant changes in these guidelines recently. While before 2013, 30% or more IHC staining was considered +1 positive, this rate was reduced to 10% in 2013 guidelines [4,28]. With this change in definition, the prevalence of HER2-low varies according to the year of biopsy evaluation. There is a large interobserver variation in evaluating this new concept of HER2-low as IHC. It has been even mentioned that interobserver variability is higher with the current ASCO/CAP guideline, and therefore HER2 expression should be evaluated with a more reliable method [29]. In one study, it was suggested that RT-PCR should perform HER2 expression evaluation at the mRNA level [30]. In our study, we observed that the HER2-low group was smaller than in other studies. There may be several reasons for this. One of them is that a central pathology evaluation was not performed, and the analysis was conducted based on the accredited laboratory results of the participating centers. Since not all recurrent metastatic patients underwent re-biopsy, the HER2-zero rate may be higher in the group with biopsies before 2013 due to the guideline change.
Although there are conflicting results in studies on the prognostic effect of HER2 status in early-stage breast cancer, a meta-analysis of 23 studies revealed that the HER2-low group had better DFS and OS regardless of hormone status [31]. Mutai et al. emphasized that HER2-low group may affect prognosis according to genomic risk in HR+ early-stage breast cancer. As a result of their genomic risk assessment with Oncotype Dx, they demonstrated that the HER2-low group had better survival results than HER2zero in patients with high genomic risk, and this difference was not observed in the low genomic risk group [32]. In advanced breast cancer, a study by Jiang et al. involving approximately 26,000 HR+ de novo metastatic breast cancer patients showed statistically better overall survival results in the HER2-low group, but since this difference was only one month, its clinical significance is controversial [33]. The Australian metastatic breast cancer group also found no difference between HER2-low and HER2-zero groups in both HR+ patients and triple-negative patients [34].
The question of whether HER2 low expression status predicts treatment response was specifically examined in breast cancer patients receiving neoadjuvant chemotherapy. In a pooled data analysis of four neoadjuvant chemotherapy clinical trials, pathologic complete response (pCR) was statistically significantly lower in the HER2-low group than in the HER2-zero group in HR-positive patients (17.5% vs. 23.6%). However, there was no difference in DFS and OS between the groups [10]. In the study by Kang et al. no correlation was shown between HER2 expression and pCR in HR+ breast cancer patients [35]. In the intrinsic group analysis of HER2 low expressing tumors with PAM50, it was observed that HER2-low tumors were in the luminal subtype subgroup more than HER2-zero in HR + patients. HER2 enriched subtype was observed at a very low rate of approximately 3% in both groups. The basal subtype was found to be 9% in the HER2-zero group and 1.8% in the HER2-low group9.
The number of studies investigating the effect of HER2 low status on treatment efficacy in metastatic hormone-positive breast cancer patients treated with CDK 4/6i + ET combination is quite limited. Bao et al. conducted a study in 106 metastatic HR + breast cancer patients treated with palbociclib/ribociclib and found that the mPFS of the HER2-low group was 8.9 months and the mPFS of the HER2-zero group was 18.8 months [36]. In this study, the results should be interpreted with caution as the preferred CDKi was palbociclib with 85% of the patients and patients had a high rate of low HER2 expression (77%) compared to the literature. A multicentric study evaluating metastatic HR+ breast cancer patients receiving palbociclib + endocrine therapy combination as a first-line treatment showed that HER2 low status did not affect survival data [37]. The 2022 San Antonia Breast Cancer Symposium also included a study presentation showing that in 436 patients treated with a combination of CDK 4/6i and ET, the HER2 low status was independently associated with worse PFS and OS [38]. In our study, HER2 low expression status was shown to have no effect on treatment response and PFS. The same comparison was made between first-line and subsequent use in subgroup analyses, and no difference was found. When subgroup analysis was performed according to the preferred CDK4/6 inhibitor, it was shown that HER2 low expression did not affect PFS in both palbociclib or ribociclib subgroups (Supplementary Figs. 1a and 1b). However, in de novo metastatic disease, a statistically insignificant mPFS favoring HER2-zero group was obtained (18 m vs. 27 m). This result may be related to the fact that in de novo metastatic disease, patients in the HER2-zero group used CDK4/6 inhibitors more as a first-line treatment and had a lower metastatic tumor burden (Supplementary Table 1). The observed discrepancies across studies may arise from variations in patient population, including differences in patient- and tumor-related characteristics. Additionally, diverse methods of assessing HER2 status could contribute to these discrepancies. The use of different types of backbone ET or CDK4/6i combination and order of treatment may further impact the results. Lastly, the presence of patients with different tumor burden may also be one of the reason for the conflicting results. Therefore, prospective and long follow-up studies are needed to interpret these conflicting results to determine treatment strategies for metastatic HR + breast cancer in the ADCs era.
The retrospective nature of our study has limitations, including selection bias, confounding variables and recall bias. The most significant limitation is that HER2 expression evaluation was not centralized, which may have introduced variability in the results. Another limitation is that biopsy samples from metastases were not available for all patients, as this could have impacted the accuracy of the HER2 status assessment. Despite the retrospective study design and short follow-up period, the current study provides valuable insights into the effect of HER2 status on treatment outcomes in patients treated with CDK4/6i + ET combinations. This study is unique in that it includes subgroup analyses and evaluates the effect of HER2 status on treatment response in patients receiving ribociclib, whereas most studies in the literature focus on palbociclib.
5. .Conclusions
In conclusion, our study suggests that HER2 expression status does not significantly affect treatment response and progression-free survival outcomes in metastatic HR+ breast cancer patients treated with CDK4/6 inhibitor and endocrine therapy combination. However, in the subgroup of patients with recurrent metastatic disease, there was a trend towards longer PFS in favor of HER2 low status. Determining the impact of HER2 status on patient outcomes will be crucial for treatment sequencing in HR+ metastatic breast cancer, considering the emergence of new treatment options. To confirm these findings and provide more conclusive evidence, prospective studies with standardized HER2 assessment methods should be conducted, specifically targeting subgroups.
Ethics Committee approval
The study was approved by Ethics Board of Dokuz Eylul University (Decision number 2023/03–09).
Declaration of competing interest
None.
Acknowledgments
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2023.06.006.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. 2022. [DOI] [PubMed] [Google Scholar]
- 2.Sørlie T., Perou C.M., Tibshirani R., Aas T., Geisler S., Johnsen H., et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gennari A., André F., Barrios C.H., Cortés J., de Azambuja E., DeMichele A., et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol. 2021;32:1475–1495. doi: 10.1016/j.annonc.2021.09.019. [DOI] [PubMed] [Google Scholar]
- 4.Wolff A.C., Hammond M.E.H., Allison K.H., Harvey B.E., Mangu P.B., Bartlett J.M.S., et al. Human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American Pathologists clinical practice guideline focused update. Arch Pathol Lab Med. 2018;142:1364–1382. doi: 10.5858/arpa.2018-0902-SA. [DOI] [PubMed] [Google Scholar]
- 5.Tarantino P., Hamilton E., Tolaney S.M., Cortes J., Morganti S., Ferraro E., et al. HER2-Low breast cancer: pathological and clinical landscape. J Clin Oncol. 2020;38:1951–1962. doi: 10.1200/JCO.19.02488. [DOI] [PubMed] [Google Scholar]
- 6.Fehrenbacher L., Cecchini R.S., Geyer C.E., Jr., Rastogi P., Costantino J.P., Atkins J.N., et al. NSABP B-47/NRG oncology phase III randomized trial comparing adjuvant chemotherapy with or without trastuzumab in high-risk invasive breast cancer negative for HER2 by FISH and with IHC 1+ or 2. J Clin Oncol. 2020;38:444–453. doi: 10.1200/JCO.19.01455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gianni L., Lladó A., Bianchi G., Cortes J., Kellokumpu-Lehtinen P.L., Cameron D.A., et al. Open-label, phase II, multicenter, randomized study of the efficacy and safety of two dose levels of Pertuzumab, a human epidermal growth factor receptor 2 dimerization inhibitor, in patients with human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2010;28:1131–1137. doi: 10.1200/JCO.2009.24.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Modi S., Jacot W., Yamashita T., Sohn J., Vidal M., Tokunaga E., et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022;387:9–20. doi: 10.1056/NEJMoa2203690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schettini F., Chic N., Brasó-Maristany F., Paré L., Pascual T., Conte B., et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer. 2021;7:1. doi: 10.1038/s41523-020-00208-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denkert C., Seither F., Schneeweiss A., Link T., Ju Blohmer, Just M., et al. Clinical and molecular characteristics of HER2-low-positive breast cancer: pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol. 2021;22:1151–1161. doi: 10.1016/S1470-2045(21)00301-6. [DOI] [PubMed] [Google Scholar]
- 11.Ergun Y., Ucar G., Akagunduz B. Comparison of HER2-zero and HER2-low in terms of clinicopathological factors and survival in early-stage breast cancer: a systematic review and meta-analysis. Cancer Treat Rev. 2023;115 doi: 10.1016/j.ctrv.2023.102538. [DOI] [PubMed] [Google Scholar]
- 12.Baez-Navarro X., van Bockstal M.R., Andrinopoulou E.R., van Deurzen C.H.M. HER2-Low breast cancer: incidence, clinicopathologic features, and survival outcomes from real-world data of a large nationwide cohort. Mod Pathol. 2023;36 doi: 10.1016/j.modpat.2022.100087. [DOI] [PubMed] [Google Scholar]
- 13.Burstein H.J., Somerfield M.R., Barton D.L., Dorris A., Fallowfield L.J., Jain D., et al. Endocrine treatment and targeted therapy for hormone receptor–positive, human epidermal growth factor receptor 2–negative metastatic breast cancer: ASCO guideline update. J Clin Oncol. 2021;39:3959–3977. doi: 10.1200/JCO.21.01392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finn R.S., Liu Y., Zhu Z., Martin M., Rugo H.S., Diéras V., et al. Biomarker analyses of response to cyclin-dependent kinase 4/6 inhibition and endocrine therapy in women with treatment-naïve metastatic breast cancer. Clin Cancer Res. 2020;26:110–121. doi: 10.1158/1078-0432.CCR-19-0751. [DOI] [PubMed] [Google Scholar]
- 15.Prat A., Chaudhury A., Solovieff N., Paré L., Martinez D., Chic N., et al. Correlative biomarker analysis of intrinsic subtypes and efficacy across the MONALEESA phase III studies. J Clin Oncol. 2021;39:1458–1467. doi: 10.1200/JCO.20.02977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giuliano M., Trivedi M.V., Schiff R. Bidirectional crosstalk between the estrogen receptor and human epidermal growth factor receptor 2 signaling pathways in breast cancer: molecular basis and clinical implications. Breast Care. 2013;8:256–262. doi: 10.1159/000354253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osborne C.K., Shou J., Massarweh S., Schiff R. Crosstalk between estrogen receptor and growth factor receptor pathways as a cause for endocrine therapy resistance in breast cancer. Clin Cancer Res. 2005;11 865s-70s. [PubMed] [Google Scholar]
- 18.Wright C., Nicholson S., Angus B., Sainsbury J.R., Farndon J., Cairns J., et al. Relationship between c-erbB-2 protein product expression and response to endocrine therapy in advanced breast cancer. Br J Cancer. 1992;65:118–121. doi: 10.1038/bjc.1992.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kan S., Koido S., Okamoto M., Hayashi K., Ito M., Kamata Y., et al. Gemcitabine treatment enhances HER2 expression in low HER2-expressing breast cancer cells and enhances the antitumor effects of trastuzumab emtansine. Oncol Rep. 2015;34:504–510. doi: 10.3892/or.2015.3974. [DOI] [PubMed] [Google Scholar]
- 20.Cao N., Li S., Wang Z., Ahmed K.M., Degnan M.E., Fan M., et al. NF-kappaB-mediated HER2 overexpression in radiation-adaptive resistance. Radiat Res. 2009;171:9–21. doi: 10.1667/RR1472.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eisenhauer E.A., Therasse P., Bogaerts J., Schwartz L.H., Sargent D., Ford R., et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 22.Wahl R.L., Jacene H., Kasamon Y., Lodge M.A. From RECIST to PERCIST: evolving Considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl 1) doi: 10.2967/jnumed.108.057307. 122s-50s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finn R.S., Martin M., Rugo H.S., Jones S., Im S.-A., Gelmon K., et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375:1925–1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 24.Hortobagyi G.N., Stemmer S.M., Burris H.A., Yap Y.-S., Sonke G.S., Hart L., et al. Overall survival with ribociclib plus letrozole in advanced breast cancer. N Engl J Med. 2022;386:942–950. doi: 10.1056/NEJMoa2114663. [DOI] [PubMed] [Google Scholar]
- 25.Goetz M.P., Toi M., Campone M., Sohn J., Paluch-Shimon S., Huober J., et al. Monarch 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35:3638–3646. doi: 10.1200/JCO.2017.75.6155. [DOI] [PubMed] [Google Scholar]
- 26.Slamon D.J., Neven P., Chia S., Fasching P.A., De Laurentiis M., Im S.-A., et al. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N Engl J Med. 2019;382:514–524. doi: 10.1056/NEJMoa1911149. [DOI] [PubMed] [Google Scholar]
- 27.Turner N.C., Slamon D.J., Ro J., Bondarenko I., Im S.-A., Masuda N., et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. 2018;379:1926–1936. doi: 10.1056/NEJMoa1810527. [DOI] [PubMed] [Google Scholar]
- 28.Wolff A.C., Hammond M.E.H., Hicks D.G., Dowsett M., McShane L.M., Allison K.H., et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 29.Baez-Navarro X., van Bockstal M.R., Nawawi D., Broeckx G., Colpaert C., Doebar S.C., et al. Interobserver variation in the assessment of immunohistochemistry expression levels in HER2-negative breast cancer: can we improve the identification of low levels of HER2 expression by adjusting the criteria? An international interobserver study. Mod Pathol. 2023;36 doi: 10.1016/j.modpat.2022.100009. [DOI] [PubMed] [Google Scholar]
- 30.Shu L., Tong Y., Li Z., Chen X., Shen K. Can HER2 1+ breast cancer Be considered as HER2-low tumor? A comparison of clinicopathological features, quantitative HER2 mRNA levels, and prognosis among HER2-negative breast cancer. Cancers. 2022;14:4250. doi: 10.3390/cancers14174250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ergun Y., Ucar G., Akagunduz B. Comparison of HER2-zero and HER2-low in terms of clinicopathological factors and survival in early-stage breast cancer: a systematic review and meta-analysis. Cancer Treat Rev. 2023;115 doi: 10.1016/j.ctrv.2023.102538. [DOI] [PubMed] [Google Scholar]
- 32.Mutai R., Barkan T., Moore A., Sarfaty M., Shochat T., Yerushalmi R., et al. Prognostic impact of HER2-low expression in hormone receptor positive early breast cancer. Breast. 2021;60:62–69. doi: 10.1016/j.breast.2021.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang C., Perimbeti S., Deng L., Shapiro C.L., Gandhi S. Clinical outcomes of de novo metastatic HER2-low breast cancer: a National Cancer Database Analysis. npj Breast Cancer. 2022;8:135. doi: 10.1038/s41523-022-00498-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gampenrieder S.P., Rinnerthaler G., Tinchon C., Petzer A., Balic M., Heibl S., et al. Landscape of HER2-low metastatic breast cancer (MBC): results from the Austrian AGMT_MBC-Registry. Breast Cancer Res. 2021;23:112. doi: 10.1186/s13058-021-01492-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang S., Lee S.H., Lee H.J., Jeong H., Jeong J.H., Kim J.E., et al. Pathological complete response, long-term outcomes, and recurrence patterns in HER2-low versus HER2-zero breast cancer after neoadjuvant chemotherapy. European Journal of Cancer. 2022;176:30–40. doi: 10.1016/j.ejca.2022.08.031. [DOI] [PubMed] [Google Scholar]
- 36.Bao K.K.H., Sutanto L., Tse S.S.W., Man Cheung K., Chan J.C.H. The association of ERBB2-low expression with the efficacy of cyclin-dependent kinase 4/6 inhibitor in hormone receptor–positive, ERBB2-negative metastatic breast cancer. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.33132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carlino F., Diana A., Ventriglia A., Piccolo A., Mocerino C., Riccardi F., et al. HER2-Low status does not affect survival outcomes of patients with metastatic breast cancer (MBC) undergoing first-line treatment with endocrine therapy plus palbociclib: results of a multicenter, retrospective cohort study. Cancers. 2022;14 doi: 10.3390/cancers14204981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zattarin E., Sposetti C., Leporati R., Mariani L., Menichetti A., Corti C., et al. Abstract HER2-02: HER2-02 HER2-low status is associated with worse clinical outcomes in hormone receptor-positive, HER2-negative advanced breast cancer patients treated with first-line cyclin-dependent kinase 4/6 inhibitors plus endocrine therapy. Cancer Res. 2023;83 HER2-02-HER2- [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.