Abstract
Cardiac tumors are rare conditions, typically diagnosed on autopsy, but with the advancement of imaging techniques they are now encountered more frequently in clinical practice. Echocardiography is often the initial method of investigation for cardiac masses and provides a quick and valuable springboard for their characterization. While some cardiac masses can be readily identified by echocardiography alone, several require incorporation of multiple data points to reach diagnostic certainty. Herein, we will provide an overview of the main clinical, diagnostic, and therapeutic characteristics of cardiac masses within the framework of their location.
Keywords: Cardiac computed tomography, Cardiac magnetic resonance, Cardiac masses, Cardiac tumors, Echocardiography, Multimodality imaging, Myxoma, NBTE, Papillary fibroelastoma, Primary tumors
Graphical abstract
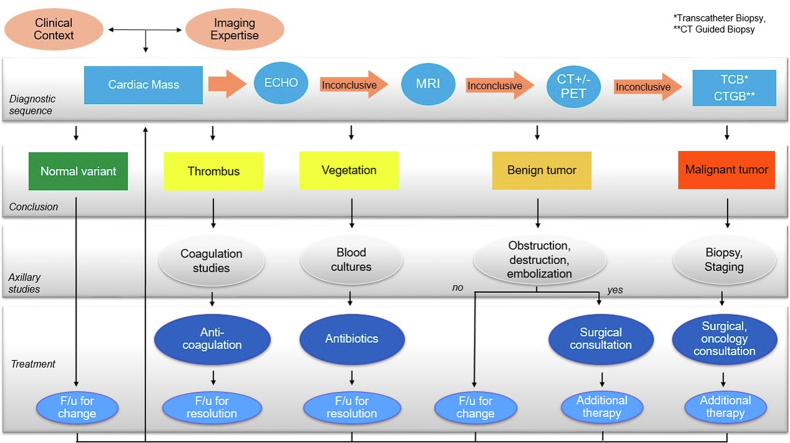
Diagnostic approach to a cardiac mass discovered by echocardiography.
Highlights
-
•
Cardiac masses can have detrimental consequences, even if benign.
-
•
Echocardiography is often the first-line imaging technique to reveal a cardiac mass and to direct further work-up by cardiac MRI, cardiac CT, PET, and/or biopsy.
-
•
Imaging characteristics and location are key aides in the differential diagnosis; papillary fibroelastomas, nonbacterial thrombotic endocarditis, Lambl’s excrescences, and blood cysts are more commonly seen on valves while myxomas, rhabdomyomas, fibromas, and lipomas are more commonly seen in the cardiac chambers.
-
•
A standardized diagnostic pathway provides a solid framework for the best management and outcome scenarios of cardiac masses.
Introduction
It has been estimated that >3 million echocardiography (echo) procedures are performed annually in the United States. Although cardiac masses are uncommon, with better and higher resolution imaging techniques, they are increasingly being recognized in clinical practice. The differential diagnosis of cardiac masses includes infection, thrombus, scar, inflammatory lesions, imaging artifacts, neoplasms (benign and malignant), and foreign bodies.1,2 Thrombi and infections account for the majority. Clinical and imaging features such as location, mobility, tissue characteristics, valve function, and myocardial function are helpful in characterizing masses and narrowing the differential diagnosis. This review aims to take the reader through discovery of an incidental mass found by echo to its ultimate diagnosis.
Clinical Approach to a Mass Discovered by Echocardiography
Echo is often one of the first tests in the evaluation of patients with cardiac symptoms. Both echo modalities, transthoracic echocardiography (TTE) and transesophageal echocardiography (TEE), have advantages for assessing the hemodynamic impact, anatomic location, and tissue characteristics of a mass lesion.1,2
These features are especially important in differentiating between different etiologies. On many occasions, the diagnosis is not straightforward and requires collaboration with colleagues in radiology, interventional cardiology, cardiac surgery, and cardiac pathology. This often leads to further imaging like cardiac magnetic resonance (CMR), cardiac computed tomography (CCT), or fusion imaging (e.g., PET-CT/MRI). Despite great advances in imaging, histopathologic examination of the tissue is necessary for definitive diagnosis.3, 4, 5
Although this review is not meant to be an exhaustive compendium on complimentary imaging, we do provide some guidance on imaging working up in Table 1. Echo is often the first-line cardiac imaging modality for most cardiac patients because of its widespread availability, excellent temporal-spatial resolution, and assessment of hemodynamic impacts.6,7
Table 1.
Relative strength of different imaging modalities in evaluating certain characteristics of cardiac masses
| Modality Strength Tissue |
ECHO Temporal resolution; motion patterns, small structures |
CMR Tissue characterization (edema, fat, infiltration, fibrosis and LGE) |
CT Temporal resolution; fat, calcium, foreign body |
|---|---|---|---|
| Fat | ++ | ++++ | +++ |
| Calcium | +++ | + | ++++ |
| Thrombus | ++ | ++++ | +++ |
| Mobile mass | ++++ | ++ | ++ |
| Foreign body | +++ | + | +++ |
| Extra-cardiac extent | + | +++ | ++++ |
CMR, cardiac magnetic resonance; CT, computed tomography; ECHO, echocardiography; LGE, late gadolinium enhancement.
CMR is often the second-line imaging modality due to excellent tissue characterization with T1- and T2-weighted imaging, diffusion-weighted imaging, and patterns of perfusion and late enhancement. These findings may narrow the differential diagnosis, sometimes lead to a conclusive diagnosis, and be useful for staging.8
CCT scans are also increasingly used for evaluation of a cardiac mass. CCT has very high spatial resolution in a 3-dimensional (3D) volumetric acquisition, and can assess tumor features including calcification, fat content, perfusion, and enhancement. CCT can depict tumor margins, indicating tissue invasion which can point toward malignancy. CCT can define the relationship of the tumor to other structures such as coronary arteries, valves, pericardium, and mediastinal structures to help plan surgical intervention. In addition, nearly all suspected malignant masses will need tumor staging CT of the chest, abdomen, and pelvis—providing an opportunity to simultaneously investigate the cardiac tumor and perform whole body tumor staging in a single visit. CCT images are increasingly used to create 3D printed images which can aid in preprocedural visualization and planning. Hybrid imaging techniques such as combining CCT and positive emission tomography (PET) in cardiac mass work-up can be very impactful.9,10 Additionally, CT and MR images can provide information about extracardiac conditions that can help with the identification and diagnosis of cardiac masses.
Ideally, after imaging, one has confidence to determine if the mass needs further intervention or can be followed longitudinally. The imaging sequence may help to differentiate whether the mass is a neoplasm, thrombus, or vegetation (Figure 1). However, if uncertainty about the diagnosis remains, transcatheter biopsy or CT-guided biopsy or surgical removal may be warranted.11
Figure 1.
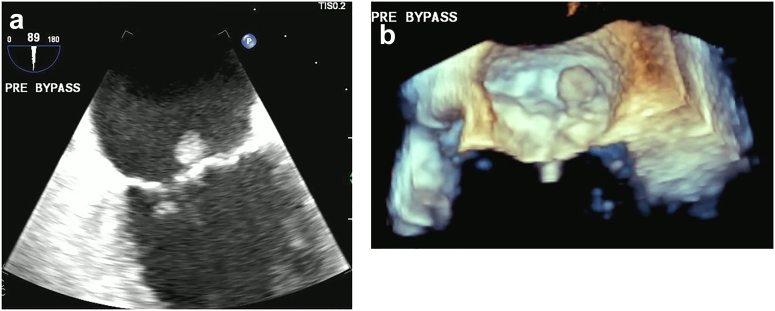
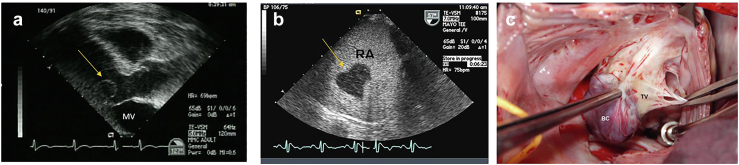
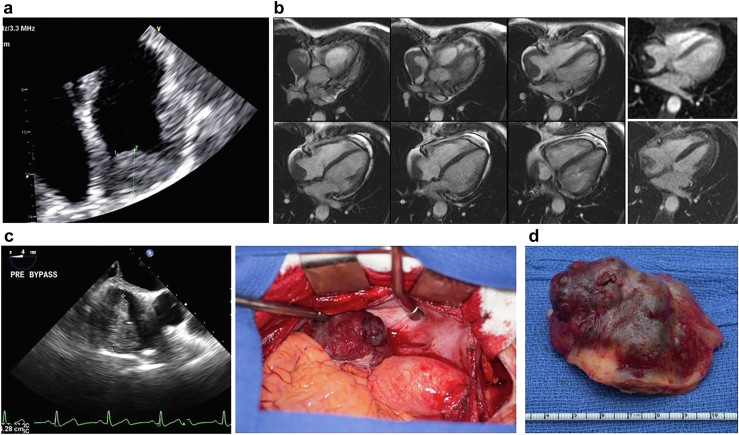
73-year-old man, incidental finding of a PFE on echo done for dyspnea and hypertension. (a) TEE 2D image of a PFE on P2 segment of the posterior leaflet of mitral valve (MV). (b) 3D of PFE measured 10 × 10 mm.
Abbreviations: 2D, 2-dimensional; 3D, 3-dimensional; PFE, papillary fibroelastoma; TEE, transesophageal echocardiography.
This review will take an anatomic approach, reviewing the characteristics which are most helpful to begin the diagnostic algorithm: location, tissue characteristics, tumor behavior, mobility, clinical context, and the hierarchy of imaging sequences (see Graphical Abstract).
Valvular Masses
It is not unusual to observe a mobile echodensity on heart valves; therefore, it is important to understand the clinical context of why the echo is being done. If looking for endocarditis, the mobile echodensity may be a vegetation which is typically accompanied by valvular dysfunction. Endocarditis is beyond the scope of this review but is an important part of the differential diagnosis of echodensities on heart valves and usually demonstrates an element of valve destruction and clinical signs of infection.
Papillary Fibroelastoma (PFE)
Clinical Presentation
PFE, often discovered incidentally (48% to 61%), is the most common primary benign cardiac neoplasm (48% to 61%). 12,13 While many patients with PFE are asymptomatic, embolic events including transient ischemic attack and stroke have been well-documented (24% to 30%). Other signs and symptoms include heart murmur or valvular heart disease (19%), chest pain possibly secondary to emboli to the coronary artery (12%), and arrhythmias (9%). Although PFE has been described on all endocardial surfaces—even a pacemaker lead,12 heart valves are the most common site (aortic valve [AV] > mitral valve [MV] > tricuspid valve [TV] > pulmonic valve). The median age of presentation is in the seventh and eighth decades; however, they have been reported in all ages.12,13 One study reported that PFEs located on the AV and those with increased mobility tend to be associated with an increased risk of stroke13; however, this was not further validated in larger cohorts.12
Pathologic Characteristics
PFE is a tumor consisting of multiple papillary fronds arising from a central stalk. It is often described to be akin to a sea anemone or pom-pom on echo (Figure 1, Supplemental Videos 1-3), a feature best appreciated when the tumor is suspended in an aqueous medium (Figure 2). Histologically (Figure 3), these tumors are avascular, with a single layer of endocardial cells lining every frond, surrounding the internal matrix of proteoglycans, elastic fibers, rare spindle cells and/or smooth muscle cells and fibroblasts.14 Although unusual, dystrophic calcification has been reported.15,16 PFE may be associated with embolic phenomena either from tumor disaggregation or shedding of surface thrombus. Such sequelae have been seen pathologically and retrieved downstream from coronary arteries, accounting for the clinical presentation of acute coronary syndrome. These are slow growing tumors, probably at a rate of approximately 1 mm/y.17
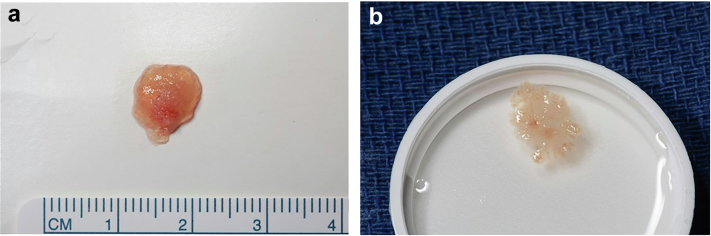
Figure 2.
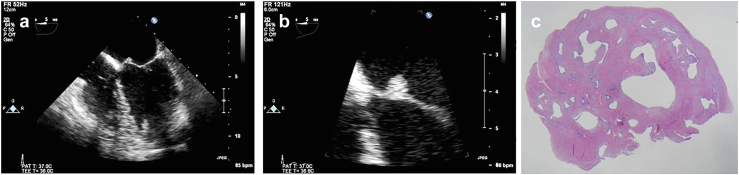
73-year-old man, incidental finding of a PFE on echocardiography done for dyspnea and hypertension. (a) PFE on sterile paper, (b) Surgical specimen suspended in saline which brings out the characteristic fronds classic of PFE.
Abbreviation: PFE, papillary fibroelastoma.
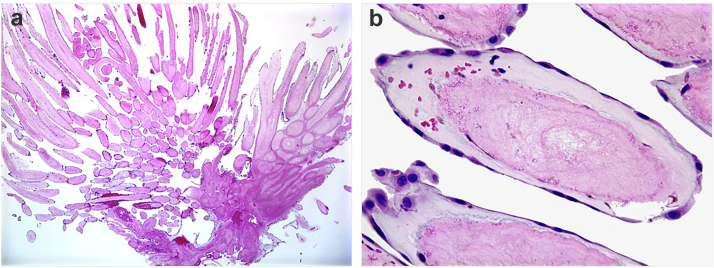
Figure 3.
Microscopic view of PFE. (a) Fibromyxoid frond (H&E, low power). (b) Fibromyxoid frond (H&E, high power) demonstrates the individual fronds surrounded by a single layer of endothelium.
Abbreviation: PFE, papillary fibroelastoma.
Echocardiographic Features and Complementary Imaging
PFEs are identified on echo as small (average size 1 cm, about 0.39 in) mobile masses, attached to an endocardial surface most often via a stalk (95%). They demonstrate an independent motion with a fine shimmering, stippled or fluffy appearing edge at mass-blood interface, representing the ends of the multiple fronds that make up the complex tumor. The border is best observed under high resolution imaging by TEE. PFEs are specifically distinguished from simple strands, fenestrations or Lambl excrescences (LEs), which are single or maybe a few linear echocardiographic densities that do not demonstrate a bulky portion sometimes referred to as the ‘head’ of a PFE (Figure 4, Supplemental Videos 4 and 5). It should be noted that up to 20% of patients can have 2 or more PFE; therefore, careful imaging with meticulous search for other coexisting PFE is warranted12,18 (Supplemental Video 6).
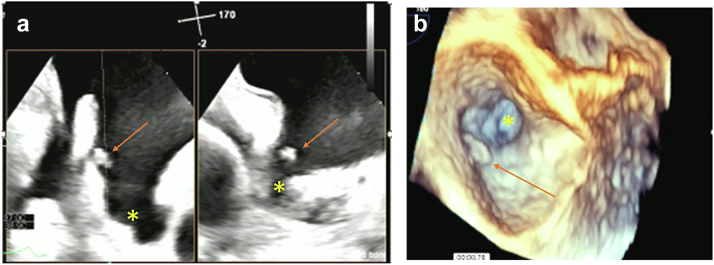
Figure 4.
PFE in a left atrial appendage. (a) 2-dimensional, x-plane view left atrial appendage (∗) with PFE (arrow). (b) 3-dimensional, x-plane view left atrial appendage (∗) with PFE (arrow).
Abbreviation: PFE, papillary fibroelastoma.
Multimodal imaging is proving valuable in some cases, especially when the PFE is found on the AV. Surgical planning may include coronary assessment with computed tomography angiography.19 3D reconstruction may be helpful in finding the aortic cusp and the stalk (Figure 5). However, cardiac MRI is not generally useful in this patient population due to the small size and increased mobility of PFE.
Figure 5.
CCT of PFE. (a) Standard imaging showing a PFE on a stalk attached to the aortic side of the aortic valve. (b) Close up valvular anatomy. (c) 3D reconstruction with 3D printing to plan for surgical removal.
Abbreviations: 3D, 3-dimensional; CCT, cardiac computed tomography; PFE, papillary fibroelastoma.
The differential diagnosis of PFE includes infectious and noninfectious vegetations. Patients with infected vegetations tend to have valve destruction and usually have risk factors for infective endocarditis, fever, night sweats, and positive blood cultures.
On the other hand, nonbacterial thrombotic endocarditis (NBTE) can be due to antiphospholipid syndrome (APS) or other rheumatologic conditions, with systemic lupus erythematosus (SLE) being the most common (see discussion on NBTE below). For that reason, laboratory testing is crucial in differentiating NBTE from infectious endocarditis. Other possibilities for valvular masses include healed vegetation, cardiac myxoma (CM), fibrous/sclerotic or redundant tissue, and very rarely valvular hemangioma. Therefore, attention to details in the mass characteristics is very helpful in distinguishing PFE from other masses, as will be discussed below.
In certain circumstances, serial imaging may be helpful when the diagnosis in doubt. For example, repeating a TEE (in 6-12 months) to see if there is a change in size may be reasonable. PFE growth is about 0.5 ±0.9 mm/y17 whereas myxomas tend to grow at about 1 mm–7 mm/mo (avg. 5 mm/mo).20 In addition, a consideration of therapeutic anticoagulation (with warfarin or direct oral anticoagulant) may be warranted when there is suspicion for thrombus which usually tends to get smaller or resolve completely with treatment.
Management
The management of suspected PFE is focused on preventing embolization, particularly to the central nervous system, retina, or coronary arteries. The mechanism of embolism is two-fold: either from the tumor directly or adherent surface thrombus. Patients with PFE have a 3.5 times risk of stroke compared to age- and sex-matched controls.12 The absolute risk of a neurological event is 6% at 1 year and 13% at 5 years.
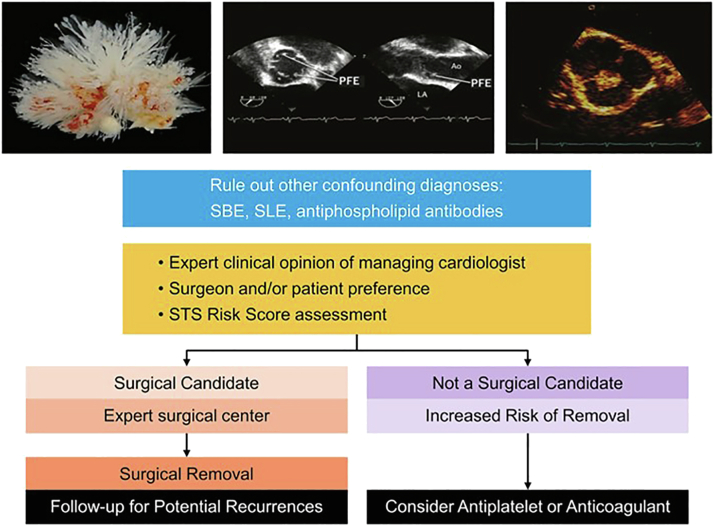
Based on available data, the current treatment of choice is surgical removal of PFE after shared decision-making and careful assessment by the medical and surgical teams (Figure 6).
Figure 6.
Management of patient with clinically high suspicion of PFE.
Abbreviations: PFE, papillary fibroelastoma; SBE, subacute bacterial endocarditis; SLE, systemic lupus erythematosus; STS, Society of Thoracic Surgery.
Typically, a surgical approach is reserved for low-risk surgical candidates (Society of Thoracic Surgeons Risk Score ≤1%). Surgical intervention, in expert hands, has been shown to bring patients back to background risk of embolism.12,18,21 In a single-center study of 294 patients undergoing surgical PFE removal, operative mortality was 0%, the native valve was preserved in 96%, and postoperative stroke occurred in 3 patients (2%).18 In select cases, a robotic approach is possible especially with PFE located on the MV.22,23 Postsurgical recurrence has been reported with a recurrence rate of up to 16%.12,17,18 This could be explained by incomplete PFE removal, unrecognized presence of other PFEs at the time of surgery, or a new PFE forming in a different location.24
When the patient is not a surgical candidate, or declines surgery, then antiplatelet therapy is recommended despite limited evidence regarding its efficacy. There have been reports of recurrent emboli on warfarin.13,20
Lambl Excrescence
Clinical Presentation
LEs are typically found incidentally on echo examinations. They are thin, highly mobile, and filiform strands of echodensities, typically seen on the aortic and/or MV. The prevalence of LEs on native valves is about 46.4% (±2.2%) in asymptomatic patient volunteers aged ≥45 yrs.25 They have been reported in association with rheumatic heart disease, prior endocarditis, and hypertension.26
It has been debated whether they are a cause of stroke, however, contemporary data suggest that these are more likely degenerative and not associated with NE. A community-based study from the SPARC Olmsted County database in which patients were followed for 6 years did not find an association between LEs and NE.26
Pathologic Features
Histologically, they typically are thin, <2 mm, and usually 5 to 10 mm in length, but occasionally have been reported to be as long as 25 mm in length.27 While grossly more simplistic than PFEs, histologically LEs bear resemblance to the PFE and are composed of a fibroelastic, avascular core enclosed by single layer of endothelium (Figure 7).
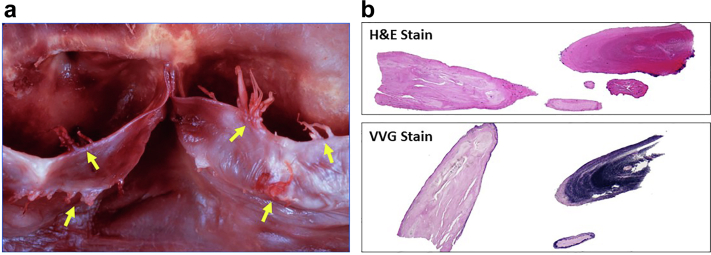
Figure 7.
Pathology of Lambl excrescences. (a) Gross specimen of Lambl excrescences on aortic valve closure margin and leaflet edges, (b) Fibroelastic fronds, surrounded by a single cell layer of endothelium.
Abbreviations: H & E stain, hematoxylin and eosin; VVG stain, Verhoeff–Van Gieson stain.
Echocardiographic Features and Complementary Imaging
LEs are typically single, thin filamentous linear echodensities, although occasionally they can be in a cluster. However, they do not have significant mass to them and are better seen by TEE where they appear as a filamentous structure with independent motion. To our knowledge, there have been no other complementary image studies done to define LEs (Figure 8, Supplemental Video 7).
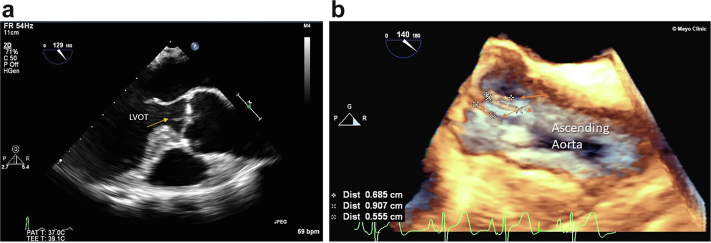
Figure 8.
Lambl excrescences examples on the aortic valve (AV). (a) TEE mid esophageal view at 129 degrees of Lambl (arrow) on LVOT side AV. (b) 3D of the aortic valve showing 3 Lambl excrescences (arrows) on the aortic side of the AV.
Abbreviations: 3D, 3-dimensional; LVOT, left ventricular outflow tract; TEE, transesophageal echocardiography.
Management
Currently there are no definitive guidelines for the management of patients with LE. In our opinion, we typically consider them degenerative changes. The prevalence is quite high, and therefore we do not recommend medical or surgical intervention. The differential diagnosis includes PFE and degenerative strands (see below). PFE will typically have more volume and often a discernible stalk with a rounded head.
Fenestrated AV
Clinical Presentation
Fenestrated AVs are typically associated with degenerative changes. It is rare to have clinical symptomatology except in the setting of underlying valvular pathology such as aortic stenosis or aortic regurgitation which may be coexistent.
Echocardiographic Features and Complementary Imaging
Often there may be single or multiple bright linear echodensities on the aortic side of the AV. Classically, this is noted to be in the setting of bright, sclerotic, and even calcified AV leaflets, plus or minus stenoses of the AV. MRI and CT are generally reserved for challenging patients, or for cases in which imaging the rest of the aorta or coronary tree is of additional use (Figure 9, Supplemental Video 8).
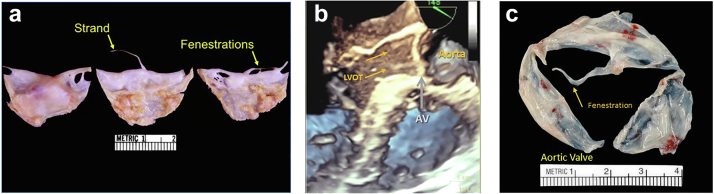
Figure 9.
Pathologic specimens of degenerative strands and fenestrations aortic valve (AV). (a) Pathologic origin of degenerative strands and fenestrations due to the wear and tear of the valve structures. (b) 3D echo of aortic valve (AV) and fenestration (arrows) correlated with pathology. (c) Gross surgical specimen of the fenestrated aortic valve (arrow).
Abbreviation: 3D, 3-dimensional.
Pathologic Features
Fenestrations appear as ovoid defects of the lunular portion of the valve cusp (Figure 9).
Management
Compared to other AV masses, fenestrations are typically echo dense, whereas LEs are typically thinner and more filamentous by echocardiographic criteria, and PFE will typically have a stalk and head with shimmering blood-mass margins.
Since fenestrations have not been shown to be independently associated with embolic phenomena, the management is determined by the presence/absence of underlying valvular pathology.
Nonbacterial Thrombotic Endocarditis
Noninfectious lesions on heart valves have been described in different clinical scenarios and thus given multiple names, such as Libman-Sacks endocarditis in SLE patients, verrucous endocarditis in cardiac imaging, or marantic endocarditis in oncology patients. Currently, NBTE is used as an umbrella term. It was first described by Zeigler28 in 1888 but fully characterized by Libman-Sacks in 1923.28 It describes sterile deposits of fibrin and platelets, usually on the valves. NBTE has been strongly associated with malignancies, and inflammatory or hypercoagulable states. Malignancies associated with NBTE are mostly adenocarcinoma of the lung, breast, and pancreas. Among inflammatory states, NBTE has been associated with SLE, rheumatoid arthritis, Behcet disease, connective tissue diseases, adult-onset Still disease, systemic hyper-eosinophilic syndrome, severe burn cases, HIV infection, and even COVID-19. The most associated hypercoagulable states are APS and disseminated intravascular coagulopathy.29, 30, 31, 32, 33, 34, 35
The pathophysiology of NBTE has not been well-established. It is hypothesized that circulating cytokines lead to local tissue damage and endothelial dysfunction. This is a substrate for fibrin and platelet aggregates mediated by activation of the coagulation or deposition of immune complexes over the damaged endothelium. Active inflammation is rarely seen on pathology.36
Clinical Presentation
NBTE is usually asymptomatic unless the thrombus dislodges and embolizes. Embolization can occur in any vascular bed, including spleen, kidneys, skin, but embolization to the brain and coronaries may present with more devastating symptoms.35,37 Other complications include valvular dysfunction.38
A thorough medical history may uncover clues for an underlying diagnosis. Specifically, a history directed to identify malignancy, autoimmune diseases, or hypercoagulable states is indicated. Family and obstetric history and may be of yield in the search for hypercoagulable states. In addition, it is important to query about any history of malignancy, autoimmune diseases, as well as previous thrombotic or obstetric events.
The first step if NBTE is suspected is to rule out infective endocarditis. Three sets of blood cultures are needed prior to starting therapy. Other microbiological studies, such as blood and urine antigen studies, may also be indicated within the correct clinical scenario. Other testing that should be obtained as first line include: complete blood count with differential to rule out hematologic malignancies and thrombocytopenia, autoimmune disease markers (including antinuclear antibody, extractable nuclear antigens, rheumatoid factor, anti-ds-DNA), and basic coagulation/disseminated intravascular coagulopathy panel. APS needs to be excluded in all patients with lupus anticoagulant, anticardiolipin antibodies, and beta2 glycoprotein (IgG and IgM). Some groups advocate testing for anti-phosphatidyl serine antibodies, but its association with valvular disease has not been well established. There is no consensus on the extension for a workup for malignancy but starting by age-appropriate cancer screening and obtaining imaging studies or biomarkers if the medical history or review of symptoms offer clinical clues or if there is a high index of suspicion.
Pathologic Characteristic
NBTE is characterized by sterile depositions of fibrin and platelets. Occasionally, granulation tissue, red cells, and valvular fibrosis with or without calcification can be encountered. Patients may have one or multiple lesions ranging from submillimeters up to multiple centimeters.36,39 It is important that examination excludes the presence of microorganisms.
Echocardiographic Features and Complementary Imaging
NBTE vegetations are usually present on the left side of the heart, with the mitral and AV most affected. Involvement of right-sided valves is rare. Single valve involvement is most common, and usually occurs on the edge of the valves or areas exposed to high sheer stress.38 Lesions tend to be located along the closure lines of the valve leaflets often referred to as “kissing lesions.” They are typically sessile, mobile, and attached along the closure margin without a stalk (in contradistinction with PFEs, which typically have a stalk). NBTE vegetations can vary in size from very small to impressively large and obstructive in nature (Figures 10 and 11, Supplemental Video 9). They also may be associated with valvular regurgitation. AV NBTE can be hard to distinguish from nodules of Arantius—which will not demonstrate independent mobility (Figure 10). In a patient in whom there is a high suspicion of NBTE with negative/inconclusive TTE, a TEE is indicated and considered the gold standard.
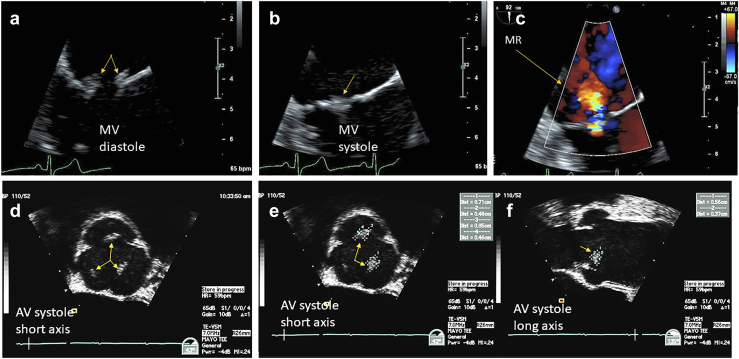
Figure 10.
Nonbacterial thrombotic endocarditis. (a) NBTE arising in the context of antiphospholipid syndrome affecting the mitral valve (MV) in diastolic kissing lesions is well visualized. (b) In systole the MV kissing lesions more difficult. (c) Associated mitral regurgitation (MR). (d) Patient with pancreatic cancer and presenting with a stroke TEE zoom view of the aortic valve (AV) in short axis during systole with thickened midportion of all 3 leaflets (arrows) can be subtle and confused with nodules of Arantius. (e) Showing the AV in systole, short axis view, measurements of the left and right aortic cusp NBTE vegetations (arrows). (f) Showing the AV in systole, long-axis view, measurements of the left and right aortic cusp NBTE vegetations (arrows).
Abbreviations: NBTE, nonbacterial thrombotic endocarditis; TEE, transesophageal echocardiography.
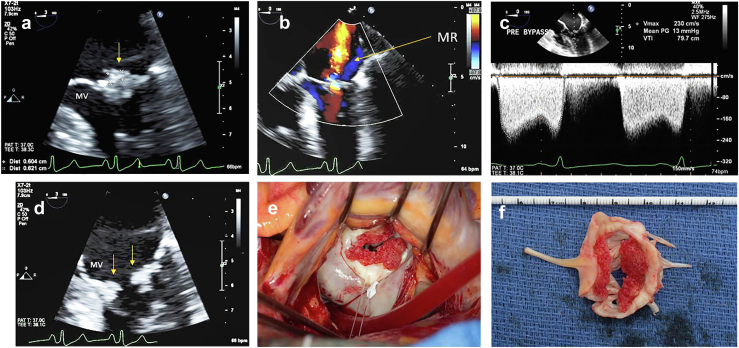
Figure 11.
Nonbacterial thrombotic endocarditis in 61-year-old women with rheumatoid arthritis and progressive dyspnea on exertion echo showed mixed mitral regurgitation and stenosis. (a) TEE of zoom view of the mitral valve (MV) systole with bulky kissing lesions (arrow) well visualized. (b) Color flow of the mitral regurgitation (MR). (c) Diastolic mean gradient 13 mmHg. (d) MV in diastole showing kissing lesions (arrows). (e) Surgeons view showing bulky red thrombotic noninfectious vegetations (V) around MV orifice. (f) Extracted gross pathology of MV with the nonbacterial thrombotic vegetations.
Abbreviation: TEE, transesophageal echocardiography.
A PET could also be considered to rule out culture-negative endocarditis by organisms such as T. whipplei.40,41 Cardiac MRI has been reported to distinguish between thrombus and vegetation42; however, its use is limited given relatively low spatial resolution. An embolic pattern on a head CT and/or brain MRI raises suspicion of NBTE. In one study, diffusion-weighted MRI can help differentiate between infective and noninfective sources for a cardioembolic stroke.36,38 As with PFE, CT may be useful to preoperatively evaluate the coronary arteries noninvasively and may also allow a complementary look at the vegetation and its relationship to the valve.
Management
In addition to addressing the underlying cause, the mainstay of NBTE treatment is anticoagulation to prevent embolization. Agents of choice include unfractionated heparin, low-molecular-weight heparin, and vitamin K antagonist. Although older studies suggest that warfarin may be less effective in the setting of patients with cancer, the treatment plan should be individualized. Our practice is to start with low-molecular-weight heparin and transition to an oral agent after documenting regression or stability of the lesions. There are no clinical trials assessing the efficacy of the direct oral anticoagulants, however, there are several case reports of failure of these agents to prevent embolic events.43, 44, 45 This may be because at least in the subset of APS, they have not been as effective as warfarin to prevent arterial events.46 Surgical management for NBTE is rarely indicated, and is usually reserved for patients that have other indication for cardiac surgery or those with recurrent emboli with cardiac vegetations that are resistant to anticoagulation.47 Recurrence of NBTE has been also described.48 Hence, treating the underlying etiology, if possible, remains a cornerstone of NBTE treatment.
Blood Cyst
Blood cysts (BCs) are a rare type of cardiac tumor with unclear pathogenesis that may present during infancy and adulthood. This entity has been described in more than 64 patients, based on a recent comprehensive review,49 with the largest case series containing 5 patients. It is most reported on the MV, followed by the TV, pulmonary valve and one on the AV.
Clinical Presentation
The tumor has no sex predilection and may be discovered at any age. In most cases, the tumor is an incidental finding. Due to its typical occurrence on the atrioventricular valves (MV > TV), some symptomatic patients present with palpitations, chest discomfort, and exertional dyspnea. Other presentations also include left ventricular outflow tract obstruction, hemiparesis, syncope, stroke, and severe valvular disease.50, 51, 52, 53, 54, 55
Pathologic Characteristic
Grossly, BCs usually appear as uni- or multi-lobular round or oval-shaped masses, either bluish or yellowish, and usually range from a few millimeters up to multiple centimeters.49 Histologically, they consist of endothelium-lined cysts containing blood or blood products.
Echocardiographic Features and Complementary Imaging
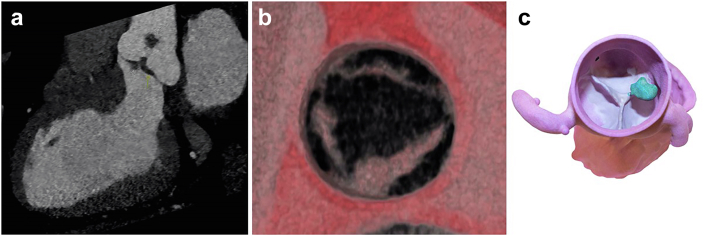
A fluid-filled round cystic structure usually appears on echocardiography; however, it may take multiple irregular shapes.55 The cyst may change size during cardiac cycles, as a lumen may form between cysts and ventricles, allowing for the systolic pressure to be maintained over time.55,56 Contrast can help to highlight the cystic nature and show bubbles within that cyst that are felt to maintain some connection with the chamber preserving the cyst that tend to regress in children, making this finding less common in adults (Figure 12, Supplemental Video 10).
Figure 12.
Blood cyst (BC). (a) Blood cyst (arrow) on the anterior leaflet of the mitral valve (MV) ventricular side; (b) Blood cyst (arrow) highlighted by echo contrast, showing bubbles on the inside of the cystic cavity; (c) Surgical removal of a tricuspid valve (TV) blood cyst.
Management
Treatment is usually surgical; however, echocardiographic surveillance of intracardiac cysts has also been suggested in asymptomatic patients with normal valve function.52,57
Cardiac Hemangioma
Cardiac hemangiomas (CHs) are a rare primary tumor of the heart accounting for less than 5% of primary cardiac neoplasms. A study in 2015 provided a comprehensive literature review of all reported cases of CH, which included 202 patients.58,59 All patients included had pathological confirmation of the diagnosis after surgery or on autopsy.
Clinical Presentation
Patients included spanned a large range of presentations from the 20th week of gestation to 86 years. Most patients with CH are asymptomatic. Clinical manifestations included dyspnea, palpitations, syncope, chest discomfort, or stroke presumably due to embolism58,59 Pericardial effusions, rarely with tamponade physiology, can occur when the pericardium is involved.
Pathologic Characteristics
CH can be in any chamber, on the cardiac valves and in the pericardium. Like their extracardiac counterparts they consist of a benign proliferative endothelial cell lining blood vessels with increasing vascularization (Figure 13). They are classically categorized based on the predominant type of the proliferating vessels: cavernous, capillary, or arteriovenous types. Size varies from 0.5 cm to 14 cm, with most being under 5 cm.58 While they are typically myocardial, they may also involve the valves (Figure 13) or the conduction system.59
Figure 13.
Cardiac hemangioma. (a) Mitral valve (MV) cardiac hemangioma (arrow) near the base of the anterior leaflet. (b) Zoom view of MV. (c) Pathology of cavernous hemangioma forming a 0.7 × 0.5 × 0.5 cm nodule.
Echocardiographic Features and Complementary Imaging
Echocardiography is the mainstay of diagnosis where CH presents as hypoechoic round or irregular mass attached to the cardiac structure, with or without a peduncle (Figure 13). In one study, multiple cases were misclassified preoperatively as CM, owing to the similar shape.58 Cardiac MRI or CT may also be utilized to observe the vascularity and accurate size as well as evaluate for extracardiac involvement of the CHs.60 Several cases involved the use of PET to diagnose CH, some showing resemblance to neuroendocrine tumors due to their hypermetabolic nature.61 Coronary angiography is usually performed in patients with CH to evaluate the presence of tumor blushes, a typical sign of hemangioma.62 CH may involve any cardiac structure, but in the systematic review mentioned above, most patients had their CHs recovered from the right atrium (26%) followed by the left ventricle (23%). Involvement of valves is less frequent than PFEs and NBTE, but valvular involvement is not uncommon.58
Management
Surveillance, medical therapy, and surgical treatment have all been reported for CHs. Specifically, medical therapy with vascular endothelial growth factor antagonists (bevacizumab), beta-blockers, corticosteroids, interferon, and radiotherapy have been reported63, 64, 65 with variable success. Surgical management is commonly, primarily because of risk of embolism, rupture, and other complications. However, the exact approach remains controversial, due to possible surgical complications reported, such as arrhythmia (ventricular tachycardia, varying degrees of heart block) and pericardial effusion.58 In addition, recurrence of symptoms has been described.66
Intracavitary and Myocardial Masses
Cardiac Myxoma
Clinical Presentation
CMs are one of the most common primary cardiac neoplasms (recently determined to be second to PFEs) in adults (mean age 50 years with female predilection of around 70%). The clinical presentation of CM patients can be highly variable and is dependent on tumor size, growth rate, mobility, shape, and location.67 Patients with CM can present with symptoms of heart failure (67%), arrhythmias, or embolization (29%).68 They are isolated, sporadic occurrences in 90% of cases and are identified incidentally on cardiac imaging in 28% of cases. Embolism due to CM has been reported frequently, 30% to 50% of patients have stroke, coronary artery ischemia, and pulmonary embolism as a result of surface thrombi or degeneration of the tumor itself.69
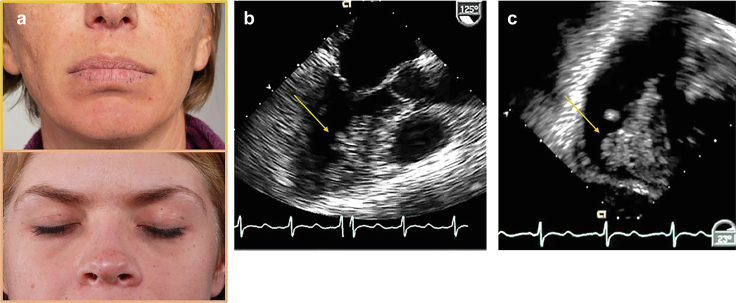
Non-syndromic CMs are mostly located in the left atrium (75%) and tend to form intracavitary masses attached by a stalk to the atrial septum or fossa ovalis.70,71 CMs occurring outside of the atria or in younger patients are more likely to be associated with Carney complex, an autosomal dominant disorder accounting for ∼5% of myxoma patients. Patients will have pigmented skin lesions, lentigines (freckles on the vermilion border of the lips and eyelids) known as the Carney facies (Figure 14), and blue nevi. Patients with Carney complex are at increased risk for developing neoplasms in other tissues, particularly endocrine tissue. The myxomas occurring in Carney complex72 are more likely to recur in unique locations than non-syndromic CMs which typically only recur after an incomplete resection.
Figure 14.
Cardiac myxoma pathology. (a) Carney faces: typical lentigines along the vermilion border of lips (top panel) and eye lids (bottom panel) in 2 patients with Carney complex. (b and c) Transesophageal echo images from a patient with Carney complex with 2 cardiac myxoma (CM). (b) Mid-esophageal view in long axis showing myxoma along the anterior septum left ventricle (arrow). (c) Transgastric view of myxoma on right ventricular septum (arrow).
Morphologically, they can be polypoid or papillary. Polypoid tumors are usually larger in size and present with obstructive symptoms, and the classic “tumor plop” on physical exam.73 Patients may have constitutional symptoms such as anemia, leukocytosis, and an elevated erythrocyte sedimentation rate which is thought to be secondary to release of cytokines by the tumor itself.74 The papillary type is more frequently associated with embolic symptoms.
Pathology
Cross-sectional evaluation shows gelatinous, firm masses with focal-to-diffuse hemorrhage and/or cavitation with a short, narrow stalk. The myxoma cell has a bland ovoid nucleus with amphophilic cytoplasm and may occur singly or in clusters, particularly around small capillary channels. They reside in a myxoid background.
Echocardiographic Features and Complementary Imaging
Identification of a mobile mass with a stalk attached to the atrial septum allows confident diagnosis whether seen with echocardiography, CCT, or CMRI and therefore biopsy usually does not have a role in diagnosis (Figure 15). In cases where a narrow stalk is not present or when the mass does not arise from the atrial septum (Figure 14 Carney syndrome left, Figure 16 nonsyndromic CM in atypical location), it is important to differentiate a malignant neoplasm (either metastasis or sarcoma) from benign neoplasms (in particular, hemangioma). Well-circumscribed borders and mass effect with displacement of adjacent structures without invasion on imaging suggest a benign tumor. On the other hand, irregular margins and invasion of adjacent structures suggest a malignant neoplasm. On CCT, the lesions typically demonstrate smooth and rounded margins and water attenuation. CMR can add improved tissue characterization with myxoid tissue demonstrating hypointensity on T1-weighted images and hyperintensity on T2-weighted images, and little to no first pass perfusion or late enhancement. CMR is most useful to distinguish CM from thrombus.
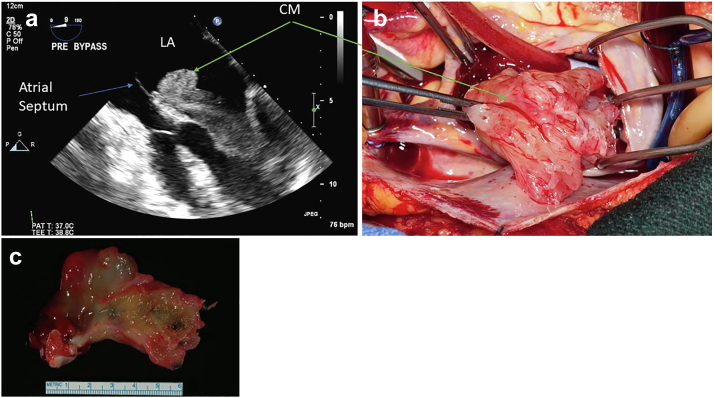
Figure 15.
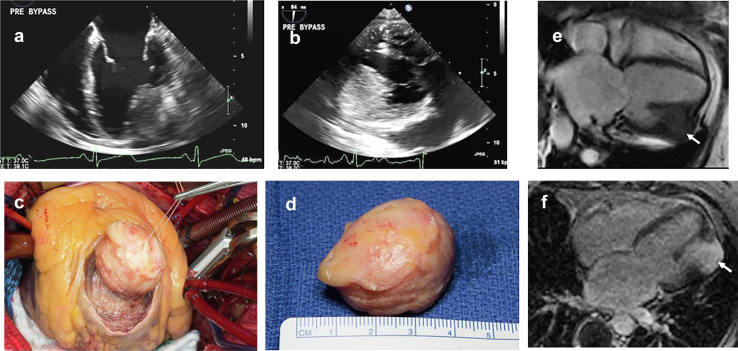
Typical sporadic cardiac myxoma left atrial (LA) cardiac myxoma (CM), papillary morphology in a 55-year-old woman who presented with cough and dyspnea. Transesophageal echo (a) LA CM (green arrow) attached to the atrial septum (blue arrow) demonstrating prolapse through mitral valve into left ventricle during diastole. (b) Surgical view of the LA myxoma. (c) Gross pathology of CM.
Figure 16.
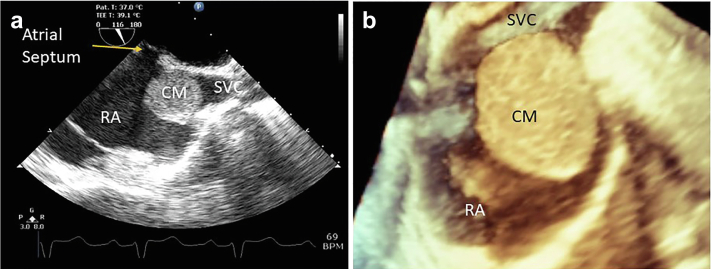
Atypically located cardiac myxoma. (a) Intraoperative 2D TEE bicaval view of cardiac myxoma (CM) at the junction of the superior vena cava (SVC) and right atrium (RA) smooth morphology. (b) 3D zoom demonstration of smooth morphological CM.
Abbreviations: 2D, 2-dimensional; 3D, 3-dimensional; TEE, transesophageal echocardiography.
Management
Surgical resection of CM is the only effective therapeutic option for patients who experience embolic complications, and potentially those at risk for embolic complications 67,75 or obstruction of valves and/or chambers. Growth rates of CM are also variable, and reported from stable behavior to over 1 cm in a month—on average 0.49 cm/mo.76 Recurrence rate is low in cases where complete resection is achieved; however, additional tumor development is common in the setting of Carney complex. Annual transthoracic echocardiogram is recommended in syndromic patients to monitor for such recurrence.77 In cases where Carney complex is suspected, testing of the tumor for PRKAR1A expression and peripheral blood to evaluate for a germline PRKAR1A mutation are recommended.78,79 However, the extent to which surveillance is indicated warrants further investigation.80,81
Rhabdomyoma
Clinical Presentation
Rhabdomyomas account for 75% of childhood primary cardiac tumors. It is often identified in utero and is rarely diagnosed after the age of 10 years. Presenting symptoms are often related to obstruction or arrhythmias. Rhabdomyoma is strongly associated with tuberous sclerosis as over 75% of patients with these tumors have a clinical, radiological, or a family history of tuberous sclerosis.82 They are typically found on echo in multiples.
Pathology
Morphologically, they are hamartomatous lesions that arise from cardiac myocytes and are grossly described as homogenous and well-circumscribed pale tan masses.83 Most commonly located in the ventricles, these tumors can result in arrhythmias or ventricular outflow obstruction.59
Echocardiographic Features and Complementary Imaging
The differential diagnosis is very limited due to the unique presentation and imaging features of rhabdomyoma. They are usually identified on TTE as left or right ventricular (rarely atrial) homogenously echogenic masses with more echogenicity than the surrounding myocardium84 (Figure 17, Supplemental Video 11). On MRI, they are well-defined masses that are T1 isointense and mildly T2 hyperintense to adjacent myocardium.
Figure 17.
Rhabdomyoma in a one-year-old boy. (a) Parasternal long-axis view, rhabdomyoma (arrows) in the left atrium (LA) and left ventricular outflow tract (LVOT). (b) Left ventricular (LV) apical four-chamber view congenital format (apex down) showing left ventricular lateral wall rhabdomyoma (arrow).
Management
Asymptomatic patients are managed conservatively especially since many of these lesions spontaneously regress with time. Patients with large rhabdomyomas and those resulting in ventricular outflow obstruction should be referred for surgical evaluation.85, 86, 87 Noteworthy, mTOR pathway inhibitors (for example everolimus) have been found to hasten tumor regression in symptomatic cases, thus preventing surgery in most patients.88, 89, 90
Fibroma
Clinical Presentation
Fibroma is a benign tumor that is considered the second most common primary cardiac neoplasm of childhood and has been associated with Gorlin syndrome (PTCH1 mutation, bifid ribs, and nevoid basal cell carcinomas).59 Around 90% occur in children and 30% of these tumors occur in children under the age of 191 although they have been reported in adults.92 Sudden cardiac death can be the initial presentation in 25% of patients.93 Other presentations include heart failure, syncope, and arrhythmias. They almost always manifest as single lesions, which help to distinguish them from rhabdomyoma.
Pathology
Grossly, these tumors present as well-circumscribed, whirled, tan masses (Figure 18). Despite gross circumscription, histologically they interdigitate into nearby myocardium and may show punctate calcification. They arise from benign fibroblasts, which may account for their arrhythmogenic presentation.94
Figure 18.
Fibroma: 63-year-old incidentally found to have LV mass when she presented with atrial fibrillation. (a) Mid-esophageal 4-chamber view with large anterolateral wall intramyocardial mass (arrow) (b) Mass as seen well in the transgastric long axis view inferior-lateral (posterior) wall. (c) Surgeons view from an incision made in the apex of the left ventricle. (d) A golf ball-sized tumor was noted in the posterolateral wall of the left ventricle. The tumor was completely excised without entering the left ventricular cavity, 3.7 × 3.2 × 2.8 cm firm white solid mass diagnosed as a fibroma. (e) 4-ch Fiesta sequence showing 3.7 cm mass in the anterolateral wall. (f) There is intense late gadolinium enhancement of the mass.
Abbreviation: LV, left ventricle.
Echocardiographic Features and Complementary Imaging
They are most commonly located within the ventricular septum, followed by the left/right ventricular free wall and less commonly in the left or right atria91,95 (Figure 18). In one case series, most patients had a suspected cardiac fibroma based on imaging findings and clinical context. Most (68%) were confirmed with histopathology after resection. The other patients who were imaging-diagnosed did not undergo confirmatory biopsy or resection. This suggests that combined imaging modalities are sensitive in diagnosis, and in those with stable masses and symptoms, an invasive procedure, such as biopsy and resection, may not be necessary.
Cardiac MR is more specific in differentiating fibroma showing isointense T1 and hypointense T2 images. Fibromas will display intense and homogeneous delayed gadolinium enhancement92 (Figure 18). On CT, they may demonstrate areas of calcification.
Management
Spontaneous regression has been reported, although much less frequently than rhabdomyomas.96 Calcifications are common in fibromas, helping to distinguish them from rhabdomyomas. Management of patients with cardiac fibroma depends on clinical presentation. In one study, 43% of the monitored group did not develop cardiopulmonary symptoms over a prolonged follow-up period. Therefore, in adult patients who are not severely symptomatic, clinical monitoring may be the best management option and in patients that present with arrhythmias, a trial of anti-arrhythmic therapy or implantable cardioverter defibrillator implantation. However, in certain cases, surgical resection may be necessary to control the arrhythmias or obstruction/compression of chambers. Typically, cardiac fibromas do not recur after surgical resection.92
Lipoma
Clinical Presentation, Pathology, Management, and Imaging
Lipomas are typically solitary tumors, and patients are generally asymptomatic. Therefore, they are incidentally discovered, and the clinical challenge is to differentiate these masses as a benign lipoma from something more sinister.97 Lipomas are well circumscribed, encapsulated mesenchymal masses of mature adipocytes. They are usually located on epicardial surfaces but have also been reported on endocardial and intramural regions.97 They are very slow growing tumors and generally must attain large sizes before causing arrhythmias or obstruction to blood flow when located intramurally or in the endocardium.98 There is no sex or age predilection. Lipomas located in the pericardial/epicardial space appear as hypoechoic on TTE, while those in the cardiac chambers are visualized as hyperechoic structures19,99,100 (Figure 19, Supplemental Video 12). Both MRI and CT are excellent modalities for definitive characterization of macroscopic fat throughout the lesion. These lesions also demonstrate no enhancement, and on MRI will demonstrate a characteristic “india ink” artifact on specific pulse sequences. (Figure 20).
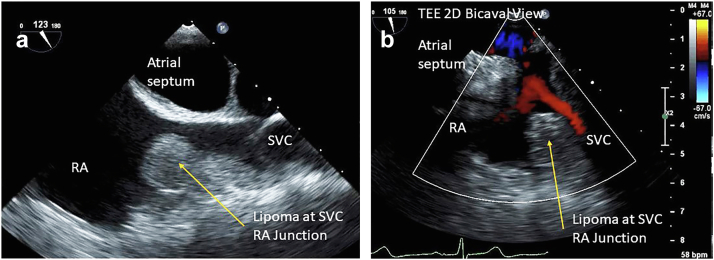
Figure 19.
74-year-old woman with history of breast cancer TEE 2D bicaval view right atrial (RA) superior vena cava (SVC) junction cardiac lipoma on echo. (a) Shows a 1.8 × 1.5 cm homogeneous, well circumscribed rounded mass, in a posterior-superior position within the RA, arising from the mouth of the SVC as it enters the right atrium. (b) Subtle color Doppler velocity acceleration suggesting mild obstruction to the SVC by the RA.
Abbreviations: 2D, 2-dimensional; TEE, transesophageal echocardiography.
Figure 20.
74-year-old woman with history of breastcancermass was differentiated as a lipoma by CMR. (a) T1-weighted image (fat is bright). (b) Is a T1 fat suppression series where fat gets dark. (c) T1-weighted spoiled gradient echo pulse LAVA—water only. (d) T1-weighted spoiled gradient echo pulse liver acquistion with volume acquisition)—fat only.
Abbreviation: CMR, cardiac magnetic resonance.
Management
Resection for lipoma is only indicated in patients that are severely symptomatic, which is rare.6
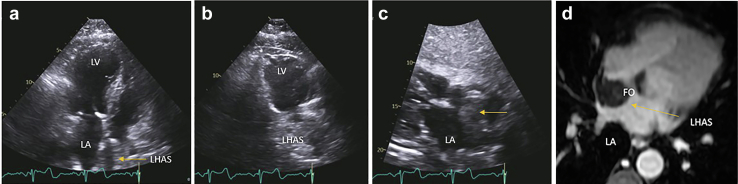
Lipomatous Hypertrophy of the Atrial Septum (LHAS)
Clinical Presentation, Pathology, Management, and Imaging
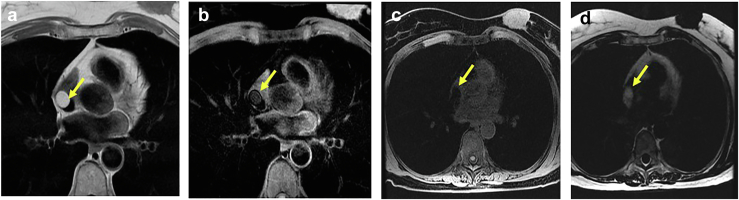
LHAS is characterized by unencapsulated fatty thickening of the atrial septum, most commonly within the posterior limbus of the fossa oval, sparing the valvular portion.101 Generally, it thickens the atrial septum to 1 to 2 cm. LHAS most commonly arises in patients older than 60 years and tends to be associated with obesity.102 Most patients are asymptomatic but can also present with various arrhythmias and obstructive symptoms, such as heart failure of the vena cava are obstructed.103 Transthoracic or transesophageal echocardiography is usually sufficient to diagnose LHAS due to the distinctive dumbbell shape imparted on the atrial septum. Multimodality imaging can be helpful in unclear clinical scenarios. Cardiac MRI and sequences such as CINE balanced steady-state free precession are excellent at detecting fatty attenuation, and thereby distinguishing between adipose and muscular tissue while delineating the complex architecture associated with LHAS104 (Figure 21, Supplemental Video 13). In addition, due to the metabolically active nature of brown fat content in LHAS, increased uptake of fluorodeoxyglucose on PET scan can be helpful in differentiating LHAS, which is a benign entity, from malignancy.105
Figure 21.
64-year-old woman with mental status changes TTE and MRI in a patient with lipomatous hypertrophy of atrial septum (LHAS). (a) Apical 4-chamber view left ventricle (LV), left atrium (LA) showing massive LHAS particularly notable in the superior limbus. (b) In the 2 chambers view, the LHAS shows extensive mass. (c) In the subcostal view, the clearing of the fossa ovalis (FO) sometimes referred to as the ‘dumbbell’ atrial septal configuration and makes a strong case for the LHAS. (d) Cardiac MRI confirmed echo suspicion of massive LHAS sparing the fossa ovalis in T1-weighted spoiled gradient echo pulse LAVA—water only sequence.
Abbreviations: MRI, magnetic resonance imaging; TTE, transthoracic echocardiography.
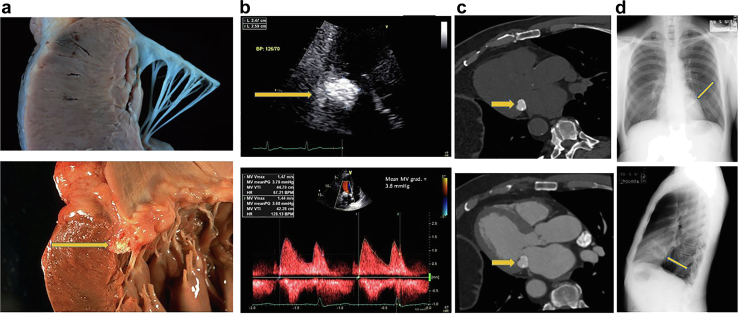
Calcified Amorphous Tumor
Clinical Presentation
Calcified amorphous tumor (CAT) appears on echo as a calcified intracavitary mass in any heart chamber, valvular anulus, or heart valve. Most are found incidentally in asymptomatic patients; however, symptoms related to obstruction or embolization can occur. CATs embolic potential is mostly associated with its location, mobility, and size.106 CATs can grow rapidly, and therefore follow-up is advised. Surgical excision is recommended if the tumor is large, if malignancy has to be excluded, or if the patient develops symptoms.107 Surgery should be curative, although recurrence of CAT is documented, if resection was incomplete.108
The scientific data about CAT are still mostly from case reports and systematic review; publications with large series are lacking. Differential diagnosis of CAT is calcified myxoma, fibroma, osteosarcoma, and other calcified structures such as old vegetations.
Risk factors include age, female sex, abnormal calcium metabolism, and renal impairment especially renal failure.109 Although a benign condition and generally incidentally found, it requires one to be certain it is not something more sinister such as myocardial abscess, other tumors, or thrombi. CAT has been reportedly associated with peripheral embolization.110
Pathology
Cardiac CATs are non-neoplastic masses of degenerating calcium within a cardiac cavity in any heart chamber, valve, or subvalvular structure. The pathological compositions are consistent with a calcium core involving a mixture of fatty acids, cholesterol, and calcium surrounded by amorphous and fibrinous material (Figure 22). The pathogenesis of CAT is thought to occur owing to a collection of thrombus or fibrin debris within a cardiac chamber/cavity that most probably mummifies (calcifies) over time. In addition, certain subgroups of CATs are echogenic spindle-shaped masses that arise from the MV and are frequently related to bulky mitral annular calcification (MAC). This is most commonly in the form of annular calcification with central softening (so called “caseous” or cheese like degeneration of the mitral annulus) which is an unusual variant of MAC. It is typically asymptomatic but may be mistaken for a neoplasm or abscess in certain situations.
Figure 22.
Calcified amorphous tumor 3 different examples of imaging modalities. (a) Upper panel shows a normal mitral annulus and lower panel shows a calcified amorphous tumor (CAT) with the caseous liquefaction material just under the valve leaflets (arrow). (b) TTE upper panel shows CAT (arrow), lower panel showing increased gradient across the mitral inflow mean gradient of nearly 4 mmHg. (c) Upper panel shows the CAT on a cardiac CT scan without IV contrast and lower panel shows the CAT with IV contrast. (d) CAT as visualized on a chest x-ray upper panel frontal view, lower panel lateral view with arrows pointing out the calcium in the MV annulus.
Abbreviations: CT, computed tomography; IV, intravenous; MV, mitral valve; TTE, transthoracic echocardiography.
Echocardiographic Features and Complementary Imaging
Echocardiographic differentiation between CAT and MAC can often be hard, as there is a certain overlap. Annular calcification can fissure and extrude the toothpaste-like material into the cavity—where it would then be identified as a CAT. CAT can be quite large, echodense with areas of echo lucency and protrude into the left ventricular and left atrial cavity posteriorly and laterally greater than medially or anteriorly. The mass could appear quite bright compared to the adjacent myocardium with distinct border. There may be mobile components in some cases, especially if the caseous material has extruded into the cavity110 (Figure 22, Supplemental Video 14). Complimentary imaging with CCT and cardiac MRI can complement echocardiographic imaging in usually lead to a definitive diagnosis and differentiation of other, more malign causes.111 In general, obtaining a CT without and with IV contrast allows for precise delineation of the calcification and relationship to the adjacent valvular apparatus and myocardium, as well as the degree of caseous degeneration.
Management
Incidental CAT should be monitored without intervention. However, extensive growth, neurological, or obstructive symptoms might demand surgical intervention. Furthermore, exclusion of other sinister diagnosis, especially calcified malignant tumors, is recommended by further imaging such as CCT and/or MRI, or via PET fusion imaging.
Pericardial Masses
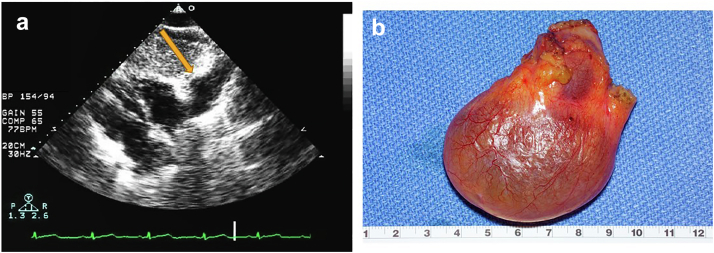
Pericardial Cyst
Clinical Presentation
Pericardial cysts are rare and benign mediastinal tumors with an incidence of about 1 in every 100,000 persons. Most pericardial cysts are congenital in origin but can also be rarely caused by inflammatory processes such as rheumatic pericarditis, tuberculosis and echinococcosis infections or acquired by trauma and post-cardiothoracic surgery. The trick is to differentiate a relatively benign cyst from something more sinister such as a sarcoma which is also often on the right side of the heart.
They are mostly asymptomatic (∼60% of cases) and thus usually found incidentally on imaging. Symptoms may arise due to compression of a nearby structure and patients can present with chest pain, dyspnea, and cough. Rarely serious complications such as cardiac tamponade and sudden death may occur.
The most common locations are the right cardiophrenic angle in 51% to 70% of the cases followed by the left cardiophrenic angle in 28% to 38%.112,113
Pathologic Characteristics
Pericardial cysts can be simple or complex. Histologically, they consist of a fibrous wall with a single layer of mesothelial lining.112
Echocardiographic Features and Complementary Imaging Techniques
Pericardial cyst may present in the echo lab as a hypoechoic, echo lucent mass often along the cardiac border, most often right side. Echo contrast can be used to confirm clinical suspicion, since contrast will not enter the cyst and thereby confirming a loculated pericardial fluid collection.114 Most pericardial cysts are initially found incidentally as a mass along the right heart border on chest X-rays in an asymptomatic patient (Figure 23, Supplemental Video 15).
Figure 23.
Pericardial cyst in a 54-year-old woman with chest positional chest tightness and dyspnea. (a) Subcostal view showing an echolucent space adjacent to the right cardiac border (arrow). (b) Gross pathology right cardiophrenic pericardial cyst, 6.6 × 5.7 × 2.5 cm, uninoculated and serous fluid filled the benign mass.
Once a pericardial cyst is suspected on a chest radiograph or echocardiogram, the American Society of Echocardiography recommends obtaining a CT or a CMR to confirm the diagnosis. On CT, a pericardial cyst will typically appear as a non-enhancing cyst with homogenous water attenuation. MRI is also frequently used to evaluate pericardial cysts. A cyst will typically produce a hypointense signal on T1-weighted images and a hyperintense signal on T2-weighted images,115 no internal enhancement or diffusion restriction, and at most thin smooth peripheral enhancement.
Management
Asymptomatic cases are conservatively monitored with serial echocardiography in anticipation of potential cyst rupture, internal hemorrhage or compression of an adjacent vascular or cardiac structure.
For symptomatic cases, surgical excision of the pericardial cyst is recommended. Minimally invasive video-assisted thoracoscopic surgery is preferred over open thoracotomy procedures as it is associated with better wound healing and less blood loss. An alternative to surgical resection is percutaneous aspiration of the cyst and is usually recommended as the first-line procedure for symptomatic patients by the European Society of Cardiology.116,117
Cardiac Sarcoma
Clinical Presentation
Primary cardiac sarcomas are rare malignant neoplasms of the heart with a prevalence of 0.0017% on autopsy. Angiosarcomas (37% of the cases) and undifferentiated sarcomas account for most cases of cardiac sarcomas.118
Angiosarcomas are typically found along the right heart border, atrioventricular groove, and in the right atrium. Occurrence tends to be between the third and fifth decades of life with a male to female predilection of 2 to one. They typically present late with dyspnea, chest pain, malaise, and fever mainly due to right heart failure and pericardial effusion and tamponade. Cytology of pericardial fluid is typically negative.119 Metastases develop in 66% to 89% of cases, most commonly to the lungs.84,120 Undifferentiated pleomorphic sarcomas, which include undifferentiated sarcomas and malignant fibrous histiocytoma, are more commonly left-sided, often arising in the left atrium.19
Pathologic Characteristics
Macroscopically, angiosarcomas are red-brown hemorrhagic lesions with poorly defined borders. They tend to occur at the right atrioventricular groove with extension into contiguous structures such as the vena cava, TV, and the pericardium. They are characterized as hemorrhagic neoplasms with pleiomorphic and atypical cells in vasoformative arrangements. Immunohistochemical staining may be positive for factor VIII, von Willebrand factor, CD34, FLI-1, ERG, and CD31.118
Echocardiographic Features and Complementary Imaging Techniques
Echocardiography is the imaging modality of choice for cardiac tumors. On echo, angiosarcomas appear as broad-based nonmobile masses with myocardial extension121 (Figure 24). CT imaging depicts an irregular mass with a variable enhancement pattern with intravenous iodinated contrast. Angiosarcomas usually exhibit significant but heterogeneous early/arterial phase enhancement due to the vascular nature. CT will also show pericardial effusion and nodular thickening when invasion of the pericardium occurs. On MRI, the tumor will have a cauliflower appearance and will show immediate enhancement on gadolinium perfusion due to high vascularity. However, due to peripheral fibrosis, delayed contrast imaging might show heterogenous enhancement with areas of increased signal intensity and regions of focal hypointensity.19,120 Myxoid sarcomas demonstrate T2 hyperintense signal, and undifferentiated pleomorphic sarcomas usually demonstrate less avid and intense enhancement.
Figure 24.
Angiosarcoma (AS) in a 60-year-old woman that presented with tamponade, and a right atrial (RA) mass. (a) TTE of RA with 2.8 × 1.8 cm mass in the dome and around the wall atrial chamber. (b) CMR heterogenous, nonobstructive right atrial mass which extends along the posterior and posterolateral wall and fills the right atrial appendage. Top right most image shows mild perfusion and bottom right shows moderate patchy late gadolinium enhancement. The location along with these features suggests angiosarcoma as the diagnosis. (c) Surgical view of the tumor upon opening of the RA. (d) Gross pathology of the surgical specimen with rim of the RA.
Abbreviations: CMR, cardiac magnetic resonance; TTE, transthoracic echocardiography.
Management
Complete excision of the tumor is the preferred treatment strategy in most cases; however, this is not always possible due to the aggressive behavior of these tumors and the extent of local invasion and metastases. Most sarcomas are associated with very poor prognosis. Treatment choices are limited and not well studied for advanced disease. Short-term outcomes might be improved with surgical debulking in addition to neoadjuvant chemotherapy administration.122,123
Conclusions
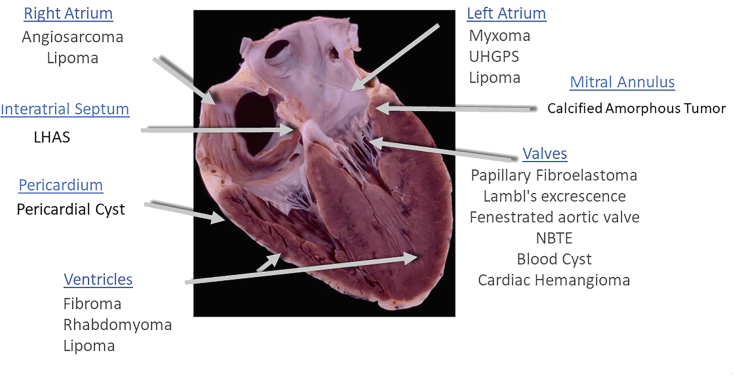
Given the advent of improved cardiac imaging with echocardiography, incidental cardiac masses will be encountered more frequently. The location of the mass on echocardiography is highly informative in narrowing the differential diagnosis of the type of cardiac mass we are dealing with (Figure 25). Table 2 gives salient imaging characteristics of the four most common adult and pediatric benign neoplasms.
Figure 25.
The location of the mass is highly informative on echo as to first understanding of the type of cardiac mass.
Abbreviations: LHAS, lipomatous hypertrophy of the atrial septum; NBTE, nonbacterial thrombotic endocarditis; UHGPS, undifferentiated high-grade pleomorphic sarcoma.
Table 2.
Typical imaging findings of different benign cardiac masses on multimodality imaging
| Imaging modality | Myxoma | Fibroma | Lipoma | Papillary fibroelastoma | Rhabdomyoma |
|---|---|---|---|---|---|
| Echocardiography | -Hyperechoic -Well defined mobile mass attached to endocardial surface by a stalk |
-Distinct and well demarcated -Hyperechoic -Homogenous -Myocardially embedded, noncontractile -Calcified flecks centrally |
-Hyperechoic within cardiac chambers (if located pericardial space = hypoechoic) -Broad-base, usually immobile |
-TEE more sensitive -Usually mobile -Smaller in size, stippled borders due to vibration at blood-tumor attachment interface |
-Small, well circumscribed solid hyperechoic masses -can mimic diffuse myocardial thickening |
| Cardiac Computed Tomography | -Decreased attenuation -Calcified attenuating areas (40-100 HU), more common in right sided mass -Intracavitary |
-Variable attenuation depending on contrast timing (usually low attenuation) -Homogenous -Intramurally located -Calcified -Infiltrative/sharp demarcation -Non contrast enhancing |
-Homogeneous -Hypoattenuation, resembling surrounding mediastinal adipose tissue (∼-100 HU) |
-Hypoattenuating mass with irregular borders | -Intramural lesion -Variable attenuation -No calcification -Mild contrast enhancement |
| Cardiac Magnetic Resonance Imaging | -Heterogenous LGE update -T1: Isointense -T2: Hyperintense ∗T1/T2 can heterogenous due to varying amounts of myxoid, hemorrhagic, ossific, and necrotic tissue |
-Hypointense in areas of calcium -Minimal/no LGE -T1: Isointense -T2: Hypointense |
-No LGE -T1: Hyperintense -T2: Hyperintense |
-LGE: Variable, usually absent -T1: Isointense -T2: Variable (can be hypointense if high fibrous content) |
-No/minimal LGE -T1: Isointense -T2: Iso/hyper-intense |
LGE, late gadolinium enhancement; TEE, transesophageal echocardiography.
These masses may be artifacts, normal structures, vegetations, neoplasms (benign or malignant), or primary/secondary manifestations of cardiac and noncardiac etiologies. Cardiac tumors are rare but can have devastating clinical consequences. In general, rapid tumor growth, hemodynamic or neurological symptoms dictate the urgency of surgical intervention. Some masses may be appropriately followed over time. Therefore, establishing diagnostic certainty is important in treatment, estimation of prognosis, and follow-up recommendations. Often, the diagnosis of cardiac masses requires a multidisciplinary approach combined with multimodality imaging and regular clinical and cardiac imaging follow-up. As newer imaging technologies emerge, they will improve detection and characterization of cardiac masses and assist in clinical decision-making.
Funding
The authors have no funding to report.
Disclosure Statement
The authors report no conflict of interest.
Acknowledgments
Tessa Flies—reference and formatting assistance.
Mark Zangs—video and image editing assistance.
Footnotes
Supplemental data for this article can be accessed on the publisher’s website.
Supplementary Material
-373-year-old man with PFE found incidentally on the mitral valve. 1: Zoomed in view of mitral valve showing PFE attached to the posterior mitral valve leaflet. It shows independent motion and a very short stalk. 2: stalk is more apparent in 2D color side-by-side images. Classically, PFE will not cause significant valvular dysfunction as demonstrated by color-flow and no significant regurgitation. 3: three-dimensional view of the PFE attached to the P2 segment. 2D, 2-dimensional; PFE, papillary fibroelastoma.
to 570-year-old man with TIA who was found to have a left atrial appendage mass. He was treated with anticoagulation for 3 months and then repeat transesophageal echocardiogram was performed. 4: the left atrial appendage PFE shows independent motion. 5: 7-8 PFE was removed by robotic technique. The intraoperative three-dimensional transesophageal view shows the PFE attached to the left atrial appendage wall. PFE, papillary fibroelastoma; TIA, transient ischemic attack.
Zoomed in view of the aortic valve with three-dimensional technique showing multiple PFE on all 3 aortic valve cusps. PFE, papillary fibroelastoma.
Zoomed in view of the aortic valve with three-dimensional technique showing multiple Lambl’s excrescences on the aortic side of the aortic valve. Notice none of these long filamentous strands have a head that is classically associated with PFE. PFE, papillary fibroelastoma.
Two-dimensional and three-dimensional imaging of the aortic valve showing a degenerative strand.
NBTE: Zoomed in view of the mitral valve apparatus showing “kissing lesions” along the closing margin of the anterior and posterior leaflets of the mitral valve. Broad-based attachment to the valve often associated with regurgitation. NBTE, nonbacterial thrombotic endocarditis.
Example of a blood cyst on a 55-year-old man with severe tricuspid regurgitation, presumably due to the mass effect on the valve due to the cyst.
Child with 2 rhabdomyomas; 1 on the LVOT side of the anterior mitral leaflet and 1 on the left atrial side of the anterior mitral leaflet.
Midesophageal bicaval view of right atrial myxoma at the junction between the right atrium and the superior vena cava.
Lipomatous hypertrophy of the atrial septum which appears like a large mass but is distinguished from other masses or invasive masses because the fossa ovalis is free of any lipomatous infiltration. Some shadowing due to the mitral annular calcification noted.
Apical 4-chamber view (Mayo format with left ventricle on the left side and right ventricle on the right side) demonstrating significant massive calcified amorphous tumor of the mitral valve annulus.
Subcostal view showing a pericardial cyst next to the right atrium.
References
- 1.Bruce C.J. Cardiac tumours: diagnosis and management. Heart. 2011;97:151–160. doi: 10.1136/hrt.2009.186320. [DOI] [PubMed] [Google Scholar]
- 2.Yusuf S.W., Bathina J.D., Qureshi S., et al. Cardiac tumors in a tertiary care cancer hospital: clinical features, echocardiographic findings, treatment and outcomes. Heart Int. 2012;7:e4. doi: 10.4081/hi.2012.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Mamgani A., Baartman L., Baaijens M., de Pree I., Incrocci L., Levendag P.C. Cardiac metastases. Int J Clin Oncol. 2008;13:369–372. doi: 10.1007/s10147-007-0749-8. [DOI] [PubMed] [Google Scholar]
- 4.Reynen K., Kockeritz U., Strasser R.H. Metastases to the heart. Ann Oncol. 2004;15:375–381. doi: 10.1093/annonc/mdh086. [DOI] [PubMed] [Google Scholar]
- 5.Bussani R., De-Giorgio F., Abbate A., Silvestri F. Cardiac metastases. J Clin Pathol. 2007;60:27–34. doi: 10.1136/jcp.2005.035105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mankad R., Herrmann J. Cardiac tumors: echo assessment. Echo Res Pract. 2016;3:R65–R77. doi: 10.1530/ERP-16-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nomoto N., Tani T., Konda T., et al. Primary and metastatic cardiac tumors: echocardiographic diagnosis, treatment and prognosis in a 15-years single center study. J Cardiothorac Surg. 2017;12:103. doi: 10.1186/s13019-017-0672-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shenoy C., Grizzard J.D., Shah D.J., et al. Cardiovascular magnetic resonance imaging in suspected cardiac tumour: a multicentre outcomes study. Eur Heart J. 2021;43:71–80. doi: 10.1093/eurheartj/ehab635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Angelo E.C., Paolisso P., Vitale G., et al. Diagnostic accuracy of cardiac computed tomography and 18-F Fluorodeoxyglucose positron emission tomography in cardiac masses. JACC Cardiovasc Imaging. 2020;13:2400–2411. doi: 10.1016/j.jcmg.2020.03.021. [DOI] [PubMed] [Google Scholar]
- 10.Anavekar N.S., Bonnichsen C.R., Foley T.A., et al. Computed tomography of cardiac pseudotumors and neoplasms. Radiol Clin North Am. 2010;48:799–816. doi: 10.1016/j.rcl.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Reddy G., Maor E., Bois M.C., et al. Percutaneous transcatheter biopsy for intracardiac mass diagnosis. EuroIntervention. 2017;13:e1436–e1443. doi: 10.4244/EIJ-D-17-00707. [DOI] [PubMed] [Google Scholar]
- 12.Tamin S.S., Maleszewski J.J., Scott C.G., et al. Prognostic and bioepidemiologic implications of papillary fibroelastomas. J Am Coll Cardiol. 2015;65:2420–2429. doi: 10.1016/j.jacc.2015.03.569. [DOI] [PubMed] [Google Scholar]
- 13.Gowda R.M., Khan I.A., Nair C.K., Mehta N.J., Vasavada B.C., Sacchi T.J. Cardiac papillary fibroelastoma: a comprehensive analysis of 725 cases. Am Heart J. 2003;146:404–410. doi: 10.1016/S0002-8703(03)00249-7. [DOI] [PubMed] [Google Scholar]
- 14.Maleszewski J.J., Bois M.C., Bois J.P., Young P.M., Stulak J.M., Klarich K.W. Neoplasia and the heart: pathological review of effects with clinical and radiological correlation. J Am Coll Cardiol. 2018;72:202–227. doi: 10.1016/j.jacc.2018.05.026. [DOI] [PubMed] [Google Scholar]
- 15.Paelinck B., Vermeersch P., Kockx M. Calcified papillary fibroelastoma of the tricuspid valve. Acta Cardiol. 1998;53:165–167. [PubMed] [Google Scholar]
- 16.Fine N.M., Foley D.A., Breen J.F., Maleszewski J.J. Multimodality imaging of a giant aortic valve papillary fibroelastoma. Case Rep Med. 2013;2013:705101. doi: 10.1155/2013/705101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurmann R.D., El-Am E.A., Sorour A.A., et al. Papillary fibroelastoma growth: a retrospective follow-up study of patients with pathology-proven papillary fibroelastoma. J Am Coll Cardiol. 2021;77:2154–2155. doi: 10.1016/j.jacc.2021.02.027. [DOI] [PubMed] [Google Scholar]
- 18.Mazur P., Kurmann R., Klarich K.W., et al. Operative management of cardiac papillary fibroelastomas. J Thorac Cardiovasc Surg. 2022 doi: 10.1016/j.jtcvs.2022.06.022. pii: S0022-5223(22)00744-9. [DOI] [PubMed] [Google Scholar]
- 19.Maleszewski J.J., Anavekar N.S., Moynihan T.J., Klarich K.W. Pathology, imaging, and treatment of cardiac tumours. Nat Rev Cardiol. 2017;14:536–549. doi: 10.1038/nrcardio.2017.47. [DOI] [PubMed] [Google Scholar]
- 20.Klarich K.W., Enriquez-Sarano M., Gura G.M., Edwards W.D., Tajik A.J., Seward J.B. Papillary fibroelastoma: echocardiographic characteristics for diagnosis and pathologic correlation. J Am Coll Cardiol. 1997;30:784–790. doi: 10.1016/s0735-1097(97)00211-8. [DOI] [PubMed] [Google Scholar]
- 21.Ngaage D.L., Mullany C.J., Daly R.C., et al. Surgical treatment of cardiac papillary fibroelastoma: a single center experience with eighty-eight patients. Ann Thorac Surg. 2005;80:1712–1718. doi: 10.1016/j.athoracsur.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 22.Nisivaco S., Henry M., Ward R.P., Balkhy H.H. Totally endoscopic robotic-assisted excision of right ventricular papillary fibroelastoma. J Robot Surg. 2019;13:779–782. doi: 10.1007/s11701-018-00913-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonnichsen C., Burkhart H., Klarich K., Suri R. Surgical resection of mitral valve papillary fibroelastoma: a robot-assisted, minimally invasive approach with three-dimensional transesophageal echocardiography imaging. World J Cardiovasc Surg. 2012;02 [Google Scholar]
- 24.Sorour A.A., Kurmann R.D., El-Am E.A., et al. Recurrence of pathologically proven papillary fibroelastoma. Ann Thorac Surg. 2022;113:1208–1214. doi: 10.1016/j.athoracsur.2021.03.114. [DOI] [PubMed] [Google Scholar]
- 25.Meissner I., Whisnant J.P., Khandheria B.K., et al. Prevalence of potential risk factors for stroke assessed by transesophageal echocardiography and Carotid ultrasonography: the SPARC study. Mayo Clin Proc. 1999;74:862–869. doi: 10.4065/74.9.862. [DOI] [PubMed] [Google Scholar]
- 26.Roldan C.A., Schevchuck O., Tolstrup K., et al. Lambl's excrescences: association with Cerebrovascular disease and pathogenesis. Cerebrovasc Dis. 2015;40:18–27. doi: 10.1159/000381906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fitzgerald D., Gaffney P., Dervan P., Doyle C.T., Horgan J., Nelligan M. Giant Lambl's excrescence presenting as a peripheral embolus. Chest. 1982;81:516–517. doi: 10.1378/chest.81.4.516. [DOI] [PubMed] [Google Scholar]
- 28.LIBMAN E. Characterization of various forms of endocarditis. JAMA. 1923;80:813–818. [Google Scholar]
- 29.Mazokopakis E.E., Syros P.K., Starakis I.K. Nonbacterial thrombotic endocarditis (marantic endocarditis) in cancer patients. Cardiovasc Hematol Disord Drug Targets. 2010;10:84–86. doi: 10.2174/187152910791292484. [DOI] [PubMed] [Google Scholar]
- 30.Deppisch L.M., Olusegun Fayemi A. Non-bacterial thrombotic endocarditis: clinicopathologic correlations. Am Heart J. 1976;92:723–729. doi: 10.1016/s0002-8703(76)80008-7. [DOI] [PubMed] [Google Scholar]
- 31.el-Shami K., Griffiths E., Streiff M. Nonbacterial thrombotic endocarditis in cancer patients: pathogenesis, diagnosis, and treatment. Oncologist. 2007;12:518–523. doi: 10.1634/theoncologist.12-5-518. [DOI] [PubMed] [Google Scholar]
- 32.Hughson M.D., Nadasdy T., McCarty G.A., Sholer C., Min K.W., Silva F. Renal thrombotic microangiopathy in patients with systemic lupus erythematosus and the antiphospholipid syndrome. Am J Kidney Dis. 1992;20:150–158. doi: 10.1016/s0272-6386(12)80543-9. [DOI] [PubMed] [Google Scholar]
- 33.Sharma S., Mayberry J.C., Deloughery T.G., Mullins R.J. Fatal Cerebroembolism from nonbacterial thrombotic endocarditis in a trauma patient: case report and review. Mil Med. 2000;165:83–85. [PubMed] [Google Scholar]
- 34.Balata D., Mellergard J., Ekqvist D., et al. Non-bacterial thrombotic endocarditis: a presentation of COVID-19. Eur J Case Rep Intern Med. 2020;7:001811. doi: 10.12890/2020_001811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel M.J., Elzweig J. Non-bacterial thrombotic endocarditis: a rare presentation and literature review. BMJ Case Rep. 2020;13:e238585. doi: 10.1136/bcr-2020-238585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eiken P.W., Edwards W.D., Tazelaar H.D., McBane R.D., Zehr K.J. Surgical pathology of nonbacterial thrombotic endocarditis in 30 patients, 1985–2000. Mayo Clin Proc. 2001;76:1204–1212. doi: 10.4065/76.12.1204. [DOI] [PubMed] [Google Scholar]
- 37.Oueida Z., Scola M. Ovarian Clear Cell Carcinoma Presenting as Non-bacterial Thrombotic Endocarditis and Systemic Embolization. World J Oncol. 2011;2:270–274. doi: 10.4021/wjon367e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quintero-Martinez J.A., Hindy J.R., El Zein S., et al. Contemporary Demographics, Diagnostics and Outcomes in Non-bacterial Thrombotic Endocarditis. Heart. 2022 doi: 10.1136/heartjnl-2022-320970. [DOI] [PubMed] [Google Scholar]
- 39.Llenas-García J., Guerra-Vales J.M., Montes-Moreno S., López-Ríos F., Castelbón-Fernández F.J., Chimeno-García J. Nonbacterial thrombotic endocarditis: clinicopathologic study of a Necropsy series. Rev Esp Cardiol. 2007;60:493–500. [PubMed] [Google Scholar]
- 40.Buteau J.P., Morais J., Keu K.V. 18F-FDG PET/CT uptake of a nonbacterial thrombotic endocarditis. J Nucl Cardiol. 2016;23:1501–1503. doi: 10.1007/s12350-016-0459-6. [DOI] [PubMed] [Google Scholar]
- 41.Sánchez-Enrique C., Vilacosta I., Moreno H.G., et al. Infected Marantic Endocarditis With Leukemoid Reaction. Circ J. 2014;78:2325–2327. doi: 10.1253/circj.cj-14-0079. [DOI] [PubMed] [Google Scholar]
- 42.El ouazzani J., Jandou I., Thuaire C. Thrombus or vegetation?Importance of cardiac MRI as a diagnostic tool based on case report and literature review. Ann Med Surg (Lond) 2020;60:690–694. doi: 10.1016/j.amsu.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shoji M.K., Kim J.-H., Bakshi S., Govea N., Marukian N., Wang S.J. Nonbacterial thrombotic endocarditis due to primary Gallbladder malignancy with recurrent stroke despite anticoagulation: case report and literature review. J Gen Intern Med. 2019;34:1934–1940. doi: 10.1007/s11606-019-05166-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tamura Y., Sakata K., Terada K., Usui S., Kawashiri M.-A., Takamura M. Treatment with a direct oral anticoagulant for nonbacterial thrombotic endocarditis. Intern Med. 2021;60:1881–1885. doi: 10.2169/internalmedicine.6368-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mantovani F., Navazio A., Barbieri A., Boriani G. A first described case of cancer-associated non-bacterial thrombotic endocarditis in the era of direct oral anticoagulants. Thromb Res. 2017;149:45–47. doi: 10.1016/j.thromres.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 46.Pengo V., Denas G., Zoppellaro G., et al. Rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome. Blood. 2018;132:1365–1371. doi: 10.1182/blood-2018-04-848333. [DOI] [PubMed] [Google Scholar]
- 47.Yamashita G., Kanemitsu N., Nakashima Y., et al. Hypertrophic obstructive cardiomyopathy and mitral regurgitation in Libman–Sacks endocarditis. Gen Thorac Cardiovasc Surg. 2020;68:181–184. doi: 10.1007/s11748-018-1042-7. [DOI] [PubMed] [Google Scholar]
- 48.Muramatsu K., Kawada N., Naganuma H., Ishiwari K., Amagaya S. Recurrent nonbacterial thrombotic endocarditis the day after mitral valve replacement. J Cardiol Cases. 2022;25:119–122. doi: 10.1016/j.jccase.2021.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bortolotti U., Vendramin I., Lechiancole A., et al. Blood cysts of the cardiac valves in adults: review and analysis of published cases. J Card Surg. 2021;36:4690–4698. doi: 10.1111/jocs.15992. [DOI] [PubMed] [Google Scholar]
- 50.Arnold I.R., Hubner P.J., Firmin R.K. Blood filled cyst of the papillary muscle of the mitral valve producing severe left ventricular outflow tract obstruction. Br Heart J. 1990;63:132. doi: 10.1136/hrt.63.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hauser A.M., Rathod K., McGill J., Rosenberg B.F., Gordon S., Timmis G.C. Blood cyst of the papillary muscle: clinical, echocardiographic and anatomic observations. Am J Cardiol. 1983;51:612–613. doi: 10.1016/s0002-9149(83)80109-x. [DOI] [PubMed] [Google Scholar]
- 52.Lodha A., Patel J., Haran M., Sadiq A., Shani J. Blood cyst of the mitral valve: a rare cause of stroke. Echocardiography. 2009;26:736–738. doi: 10.1111/j.1540-8175.2008.00879.x. [DOI] [PubMed] [Google Scholar]
- 53.Ludhwani D., Sheikh B., Sheikh Y. Evaluation of mitral apparatus blood cyst: a case report and review of literature. Cureus. 2019;11:e5812. doi: 10.7759/cureus.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Halim J., van Schaagen F.R.N., Riezebos R.K., Lalezari S. Giant intracardiac blood cyst: assessing the relationship between its formation and previous cardiac surgery. Neth Heart J. 2015;23:392–394. doi: 10.1007/s12471-015-0707-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Michelena H.I., Mulvagh S.L., Schaff H.V., Enriquez-Sarano M.L., Klarich K.W. A heart-shaped mass inside a heart: echocardiographic diagnosis, pathology, and surgical repair of a flail tricuspid valve caused by a large blood-filled cyst. J Am Soc Echocardiogr. 2007;20:771.e3–771.e6. doi: 10.1016/j.echo.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 56.Timperley J., Forfar C., Becher H., Pillai R. Images in cardiovascular medicine. Heart within a heart: contrast 3-dimensional echocardiography imaging of a tricuspid valve blood cyst. Circulation. 2004;110:e324–e325. doi: 10.1161/01.CIR.0000143101.78864.60. [DOI] [PubMed] [Google Scholar]
- 57.Nkomo V.T., Miller F.A. Eustachian valve cyst. J Am Soc Echocardiogr. 2001;14:1224–1226. doi: 10.1067/mje.2001.115390. [DOI] [PubMed] [Google Scholar]
- 58.Li W., Teng P., Xu H., Ma L., Ni Y. Cardiac hemangioma: a comprehensive analysis of 200 cases. Ann Thorac Surg. 2015;99:2246–2252. doi: 10.1016/j.athoracsur.2015.02.064. [DOI] [PubMed] [Google Scholar]
- 59.WHO Classification of Tumours. 5th ed. World Health Organization; Lyon, France: 2021. [Google Scholar]
- 60.Alsaileek A., Tepe S.M., Alveraz L., Miller D.V., Tajik J., Breen J. Diagnostic features of cardiac hemangioma on cardiovascular magnetic resonance, a case report. Int J Cardiovasc Imaging. 2006;22:699–702. doi: 10.1007/s10554-006-9083-x. [DOI] [PubMed] [Google Scholar]
- 61.Shah A., Binkovitz L., Layman A.J., Bois M.C., Nguyen B. Cardiac hemangioma mimicking a neuroendocrine tumor on 68Ga Dotatate PET/CT. J Card Surg. 2022;37:2849–2851. doi: 10.1111/jocs.16578. [DOI] [PubMed] [Google Scholar]
- 62.Marok R., Klein L.W. Tumor blush in primary cardiac tumors. J Invasive Cardiol. 2012;24:139–140. [PubMed] [Google Scholar]
- 63.Yoshikawa M., Hayashi T., Sato T., Akiba T., Watarai J., Nakamura C. A case of pericardial hemangioma with consumption coagulopathy cured by radiotherapy. Pediatr Radiol. 1987;17:149–150. doi: 10.1007/BF02388095. [DOI] [PubMed] [Google Scholar]
- 64.Zanati S.G., Hueb J.C., Cogni A.L., et al. Cardiac hemangioma of the right atrium. Eur J Echocardiogr. 2008;9:52–53. doi: 10.1016/j.euje.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 65.Chen X., Lodge A.J., Dibernardo L.R., Milano C.A. Surgical treatment of a cavernous haemangioma of the heart. Eur J Cardiothorac Surg. 2012;41:1182–1183. doi: 10.1093/ejcts/ezr153. [DOI] [PubMed] [Google Scholar]
- 66.Colli A., Budillon A.M., DeCicco G., et al. Recurrence of a right ventricular hemangioma. J Thorac Cardiovasc Surg. 2003;126:881–883. doi: 10.1016/s0022-5223(03)00720-7. [DOI] [PubMed] [Google Scholar]
- 67.Keeling I.M., Oberwalder P., Anelli-Monti M., et al. Cardiac myxomas: 24 years of experience in 49 patients. Eur J Cardio Thorac Surg. 2002;22:971–977. doi: 10.1016/s1010-7940(02)00592-4. [DOI] [PubMed] [Google Scholar]
- 68.Pinede L., Duhaut P., Loire R. Clinical presentation of left atrial cardiac myxoma. A series of 112 consecutive cases. Medicine (Baltimore) 2001;80:159–172. doi: 10.1097/00005792-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 69.Orlandi A., Ciucci A., Ferlosio A., Pellegrino A., Chiariello L., Spagnoli L.G. Increased expression and activity of matrix metalloproteinases characterize embolic cardiac myxomas. Am J Pathol. 2005;166:1619–1628. doi: 10.1016/S0002-9440(10)62472-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Turkmen N., Eren B., Fedakar R., Comunoglu N. An unusual cause of sudden death: cardiac myxoma. Adv Ther. 2007;24:529–532. doi: 10.1007/BF02848775. [DOI] [PubMed] [Google Scholar]
- 71.Freedom R.M., Lee K.J., MacDonald C., Taylor G. Selected aspects of cardiac tumors in infancy and childhood. Pediatr Cardiol. 2000;21:299–316. doi: 10.1007/s002460010070. [DOI] [PubMed] [Google Scholar]
- 72.Carney J.A., Gordon H., Carpenter P.C., Shenoy B.V., Go V.L. The complex of myxomas, spotty pigmentation, and endocrine overactivity. Medicine (Baltimore) 1985;64:270–283. doi: 10.1097/00005792-198507000-00007. [DOI] [PubMed] [Google Scholar]
- 73.Tyebally S., Chen D., Bhattacharyya S., et al. Cardiac tumors: JACC CardioOncology state-of-the-Art review. JACC CardioOncol. 2020;2:293–311. doi: 10.1016/j.jaccao.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Endo A., Ohtahara A., Kinugawa T., Ogino K., Hisatome I., Shigemasa C. Characteristics of cardiac myxoma with constitutional signs: a multicenter study in Japan. Clin Cardiol. 2002;25:367–370. doi: 10.1002/clc.4950250805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Basso C., Rizzo S., Valente M., Thiene G. Cardiac masses and tumours. Heart. 2016;102:1230–1245. doi: 10.1136/heartjnl-2014-306364. [DOI] [PubMed] [Google Scholar]
- 76.Walpot J., Shivalkar B., Rodrigus I., Pasteuning W.H., Hokken R. Atrial myxomas grow faster than we think. Echocardiography. 2010;27:E128–E131. doi: 10.1111/j.1540-8175.2010.01186.x. [DOI] [PubMed] [Google Scholar]
- 77.Jain S., Maleszewski J.J., Stephenson C.R., Klarich K.W. Current diagnosis and management of cardiac myxomas. Expert Rev Cardiovasc Ther. 2015;13:369–375. doi: 10.1586/14779072.2015.1024108. [DOI] [PubMed] [Google Scholar]
- 78.Maleszewski J.J., Larsen B.T., Kip N.S., et al. PRKAR1A in the development of cardiac myxoma: a study of 110 cases including isolated and syndromic tumors. Am J Surg Pathol. 2014;38:1079–1087. doi: 10.1097/PAS.0000000000000202. [DOI] [PubMed] [Google Scholar]
- 79.Stratakis C.A., Kirschner L.S., Carney J.A. Clinical and molecular features of the Carney complex: diagnostic criteria and recommendations for patient evaluation. J Clin Endocrinol Metab. 2001;86:4041–4046. doi: 10.1210/jcem.86.9.7903. [DOI] [PubMed] [Google Scholar]
- 80.Seidman J.D., Berman J.J., Hitchcock C.L., et al. DNA analysis of cardiac myxomas: flow cytometry and image analysis. Hum Pathol. 1991;22:494–500. doi: 10.1016/0046-8177(91)90137-e. [DOI] [PubMed] [Google Scholar]
- 81.Symbas P.N., Hatcher C.R., Jr., Gravanis M.B. Myxoma of the heart: clinical and experimental observations. Ann Surg. 1976;183:470–475. doi: 10.1097/00000658-197605000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tworetzky W., McElhinney D.B., Margossian R., et al. Association between cardiac tumors and tuberous sclerosis in the fetus and neonate. Am J Cardiol. 2003;92:487–489. doi: 10.1016/s0002-9149(03)00677-5. [DOI] [PubMed] [Google Scholar]
- 83.Groves A.M., Fagg N.L., Cook A.C., Allan L.D. Cardiac tumours in intrauterine life. Arch Dis Child. 1992;67:1189–1192. doi: 10.1136/adc.67.10_spec_no.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Butany J., Nair V., Naseemuddin A., Nair G.M., Catton C., Yau T. Cardiac tumours: diagnosis and management. Lancet Oncol. 2005;6:219–228. doi: 10.1016/S1470-2045(05)70093-0. [DOI] [PubMed] [Google Scholar]
- 85.Stiller B., Hetzer R., Meyer R., et al. Primary cardiac tumours: when is surgery necessary? Eur J Cardio Thorac Surg. 2001;20:1002–1006. doi: 10.1016/s1010-7940(01)00951-4. [DOI] [PubMed] [Google Scholar]
- 86.Thomas-de-Montpreville V., Nottin R., Dulmet E., Serraf A. Heart tumors in children and adults: clinicopathological study of 59 patients from a surgical center. Cardiovasc Pathol. 2007;16:22–28. doi: 10.1016/j.carpath.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 87.Tiberio D., Franz D.N., Phillips J.R. Regression of a cardiac rhabdomyoma in a patient receiving everolimus. Pediatrics. 2011;127:e1335–e1337. doi: 10.1542/peds.2010-2910. [DOI] [PubMed] [Google Scholar]
- 88.Martinez-Garcia A., Michel-Macias C., Cordero-Gonzalez G., et al. Giant left ventricular rhabdomyoma treated successfully with everolimus: case report and review of literature. Cardiol Young. 2018;28:903–909. doi: 10.1017/S1047951118000598. [DOI] [PubMed] [Google Scholar]
- 89.Nespoli L.F., Albani E., Corti C., et al. Efficacy of everolimus low-Dose treatment for cardiac rhabdomyomas in Neonatal tuberous sclerosis: case report and literature review. Pediatr Rep. 2021;13:104–112. doi: 10.3390/pediatric13010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sugalska M., Tomik A., Jozwiak S., Werner B. Treatment of cardiac rhabdomyomas with mTOR inhibitors in children with tuberous sclerosis complex-A systematic review. Int J Environ Res Public Health. 2021;18:4907. doi: 10.3390/ijerph18094907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Torimitsu S., Nemoto T., Wakayama M., et al. Literature survey on epidemiology and pathology of cardiac fibroma. Eur J Med Res. 2012;17:5. doi: 10.1186/2047-783X-17-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Covington M.K., Young P.M., Bois M.C., et al. Clinical impact of cardiac fibromas. Am J Cardiol. 2022;182:95–103. doi: 10.1016/j.amjcard.2022.06.062. [DOI] [PubMed] [Google Scholar]
- 93.Cronin B., Lynch M.J., Parsons S. Cardiac fibroma presenting as sudden unexpected death in an adolescent. Forensic Sci Med Pathol. 2014;10:647–650. doi: 10.1007/s12024-014-9582-3. [DOI] [PubMed] [Google Scholar]
- 94.Patel J., Sheppard M.N. Pathological study of primary cardiac and pericardial tumours in a specialist UK Centre: surgical and autopsy series. Cardiovasc Pathol. 2010;19:343–352. doi: 10.1016/j.carpath.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 95.Burke A.P., Rosado-de-Christenson M., Templeton P.A., Virmani R. Cardiac fibroma: clinicopathologic correlates and surgical treatment. J Thorac Cardiovasc Surg. 1994;108:862–870. [PubMed] [Google Scholar]
- 96.Filiatrault M., Beland M.J., Neilson K.A., Paquet M. Cardiac fibroma presenting with clinically significant arrhythmias in infancy. Pediatr Cardiol. 1991;12:118–120. doi: 10.1007/BF02238417. [DOI] [PubMed] [Google Scholar]
- 97.Shu S., Wang J., Zheng C. From pathogenesis to treatment, a systemic review of cardiac lipoma. J Cardiothorac Surg. 2021;16:1. doi: 10.1186/s13019-020-01379-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Burton C., Johnston J. Multiple cerebral aneurysms and cardiac myxoma. N Engl J Med. 1970;282:35–36. doi: 10.1056/NEJM197001012820109. [DOI] [PubMed] [Google Scholar]
- 99.King S.J., Smallhorn J.F., Burrows P.E. Epicardial lipoma: imaging findings. AJR Am J Roentgenol. 1993;160:261–262. doi: 10.2214/ajr.160.2.8424329. [DOI] [PubMed] [Google Scholar]
- 100.Mullen J.C., Schipper S.A., Sett S.S., Trusler G.A. Right atrial lipoma. Ann Thorac Surg. 1995;59:1239–1241. doi: 10.1016/0003-4975(94)00890-j. [DOI] [PubMed] [Google Scholar]
- 101.McAllister H.A., Fenoglio J.J. Armed Forces Institute of Pathology; Washington: 1978. Tumors of the Cardiovascular System. [Google Scholar]
- 102.Reyes C.V., Jablokow V.R. Lipomatous hypertrophy of the cardiac interatrial septum. A report of 38 cases and review of the literature. Am J Clin Pathol. 1979;72:785–788. doi: 10.1093/ajcp/72.5.785. [DOI] [PubMed] [Google Scholar]
- 103.Isner J.M., Swan C.S., 2nd, Mikus J.P., Carter B.L. Lipomatous hypertrophy of the interatrial septum: in vivo diagnosis. Circulation. 1982;66:470–473. doi: 10.1161/01.cir.66.2.470. [DOI] [PubMed] [Google Scholar]
- 104.Aquaro G.D., Todiere G., Strata E., Barison A., Di Bella G., Lombardi M. Usefulness of India ink artifact in steady-state free precession pulse sequences for detection and quantification of intramyocardial fat. J Magn Reson Imaging. 2014;40:126–132. doi: 10.1002/jmri.24335. [DOI] [PubMed] [Google Scholar]
- 105.Fan C.M., Fischman A.J., Kwek B.H., Abbara S., Aquino S.L. Lipomatous hypertrophy of the interatrial septum: increased uptake on FDG PET. AJR Am J Roentgenol. 2005;184:339–342. doi: 10.2214/ajr.184.1.01840339. [DOI] [PubMed] [Google Scholar]
- 106.de Hemptinne Q., de Canniere D., Vandenbossche J.L., Unger P. Cardiac calcified amorphous tumor: a systematic review of the literature. Int J Cardiol Heart Vasc. 2015;7:1–5. doi: 10.1016/j.ijcha.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kubota H., Fujioka Y., Yoshino H., et al. Cardiac swinging calcified amorphous tumors in end-stage renal failure patients. Ann Thorac Surg. 2010;90:1692–1694. doi: 10.1016/j.athoracsur.2010.04.097. [DOI] [PubMed] [Google Scholar]
- 108.Fealey M.E., Edwards W.D., Reynolds C.A., Pellikka P.A., Dearani J.A. Recurrent cardiac calcific amorphous tumor: the CAT had a kitten. Cardiovasc Pathol. 2007;16:115–118. doi: 10.1016/j.carpath.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 109.Deluca G., Correale M., Ieva R., Del Salvatore B., Gramenzi S., Di Biase M. The incidence and clinical course of caseous calcification of the mitral annulus: a prospective echocardiographic study. J Am Soc Echocardiogr. 2008;21:828–833. doi: 10.1016/j.echo.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 110.Higashi H., Ohara T., Nakatani S., et al. A case of caseous calcification of the mitral annulus: a potential source of embolic stroke. J Cardiol Cases. 2010;2:e141–e143. doi: 10.1016/j.jccase.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Di Bella G., Carerj S., Andò G., et al. Cardiac imaging in the evaluation of mitral annulus caseous calcification. Int J Cardiol. 2006;113:E30–E31. doi: 10.1016/j.ijcard.2006.07.084. [DOI] [PubMed] [Google Scholar]
- 112.Khayata M., Alkharabsheh S., Shah N.P., Klein A.L. Pericardial cysts: a contemporary comprehensive review. Curr Cardiol Rep. 2019;21:64. doi: 10.1007/s11886-019-1153-5. [DOI] [PubMed] [Google Scholar]
- 113.Sorour A.A., Maleszewski J.J., Schaff H.V., Klarich K.W. A symptomatic calcified pericardial cyst. Mayo Clin Proc. 2019;94:367–369. doi: 10.1016/j.mayocp.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 114.Patel J., Park C., Michaels J., Rosen S., Kort S. Pericardial cyst: case reports and a literature review. Echocardiography. 2004;21:269–272. doi: 10.1111/j.0742-2822.2004.03097.x. [DOI] [PubMed] [Google Scholar]
- 115.Alkharabsheh S., Gentry J.L., Iii, Khayata M., et al. Clinical features, natural history, and management of pericardial cysts. Am J Cardiol. 2019;123:159–163. doi: 10.1016/j.amjcard.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 116.Kar S.K., Ganguly T. Current concepts of diagnosis and management of pericardial cysts. Indian Heart J. 2017;69:364–370. doi: 10.1016/j.ihj.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Maisch B., Seferović P.M., Ristić A.D., et al. Guidelines on the diagnosis and management of pericardial diseases executive summary: the task Force on the diagnosis and management of pericardial diseases of the European society of cardiology. Eur Heart J. 2004;25:587–610. doi: 10.1016/j.ehj.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 118.Orlandi A., Ferlosio A., Roselli M., Chiariello L., Spagnoli L.G. Cardiac sarcomas: an update. J Thorac Oncol. 2010;5:1483–1489. doi: 10.1097/JTO.0b013e3181e59a91. [DOI] [PubMed] [Google Scholar]
- 119.Kupsky D.F., Newman D.B., Kumar G., Maleszewski J.J., Edwards W.D., Klarich K.W. Echocardiographic features of cardiac angiosarcomas: the mayo clinic experience (1976–2013) Echocardiography. 2016;33:186–192. doi: 10.1111/echo.13060. [DOI] [PubMed] [Google Scholar]
- 120.Shanmugam G. Primary cardiac sarcoma. Eur J Cardio Thorac Surg. 2006;29:925–932. doi: 10.1016/j.ejcts.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 121.Gupta A. Primary cardiac sarcomas. Expert Rev Cardiovasc Ther. 2008;6:1295–1297. doi: 10.1586/14779072.6.10.1295. [DOI] [PubMed] [Google Scholar]
- 122.Ramlawi B., Leja M.J., Abu Saleh W.K., et al. Surgical treatment of primary cardiac sarcomas: review of a single-institution experience. Ann Thorac Surg. 2016;101:698–702. doi: 10.1016/j.athoracsur.2015.07.087. [DOI] [PubMed] [Google Scholar]
- 123.Tan N.Y., Najam M., Lyle M.A., Maleszewski J.J., Collins J.D., Klarich K.W. Dramatic presentation of cardiac pleomorphic liposarcoma. Circ Cardiovasc Imaging. 2021;14:e012620. doi: 10.1161/CIRCIMAGING.121.012620. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
-373-year-old man with PFE found incidentally on the mitral valve. 1: Zoomed in view of mitral valve showing PFE attached to the posterior mitral valve leaflet. It shows independent motion and a very short stalk. 2: stalk is more apparent in 2D color side-by-side images. Classically, PFE will not cause significant valvular dysfunction as demonstrated by color-flow and no significant regurgitation. 3: three-dimensional view of the PFE attached to the P2 segment. 2D, 2-dimensional; PFE, papillary fibroelastoma.
to 570-year-old man with TIA who was found to have a left atrial appendage mass. He was treated with anticoagulation for 3 months and then repeat transesophageal echocardiogram was performed. 4: the left atrial appendage PFE shows independent motion. 5: 7-8 PFE was removed by robotic technique. The intraoperative three-dimensional transesophageal view shows the PFE attached to the left atrial appendage wall. PFE, papillary fibroelastoma; TIA, transient ischemic attack.
Zoomed in view of the aortic valve with three-dimensional technique showing multiple PFE on all 3 aortic valve cusps. PFE, papillary fibroelastoma.
Zoomed in view of the aortic valve with three-dimensional technique showing multiple Lambl’s excrescences on the aortic side of the aortic valve. Notice none of these long filamentous strands have a head that is classically associated with PFE. PFE, papillary fibroelastoma.
Two-dimensional and three-dimensional imaging of the aortic valve showing a degenerative strand.
NBTE: Zoomed in view of the mitral valve apparatus showing “kissing lesions” along the closing margin of the anterior and posterior leaflets of the mitral valve. Broad-based attachment to the valve often associated with regurgitation. NBTE, nonbacterial thrombotic endocarditis.
Example of a blood cyst on a 55-year-old man with severe tricuspid regurgitation, presumably due to the mass effect on the valve due to the cyst.
Child with 2 rhabdomyomas; 1 on the LVOT side of the anterior mitral leaflet and 1 on the left atrial side of the anterior mitral leaflet.
Midesophageal bicaval view of right atrial myxoma at the junction between the right atrium and the superior vena cava.
Lipomatous hypertrophy of the atrial septum which appears like a large mass but is distinguished from other masses or invasive masses because the fossa ovalis is free of any lipomatous infiltration. Some shadowing due to the mitral annular calcification noted.
Apical 4-chamber view (Mayo format with left ventricle on the left side and right ventricle on the right side) demonstrating significant massive calcified amorphous tumor of the mitral valve annulus.
Subcostal view showing a pericardial cyst next to the right atrium.