Abstract
Glaucomatous optic neuropathy (GON) is the major cause of irreversible visual loss worldwide and can result from a range of disease etiologies. The defining features of GON are retinal ganglion cell (RGC) degeneration and characteristic cupping of the optic nerve head (ONH) due to tissue remodeling, while intraocular pressure remains the only modifiable GON risk factor currently targeted by approved clinical treatment strategies. Efforts to understand the mechanisms that allow species such as the zebrafish to regenerate their retinal cells have greatly increased our understanding of regenerative signaling pathways. However, proper integration within the retina and projection to the brain by the newly regenerated neuronal cells remain major hurdles. Meanwhile, a range of methods for in vitro differentiation have been developed to derive retinal cells from a variety of cell sources, including embryonic and induced pluripotent stem cells. More recently, there has been growing interest in the implantation of glial cells as well as cell-derived products, including neurotrophins, microRNA, and extracellular vesicles, to provide functional support to vulnerable structures such as RGC axons and the ONH. These approaches offer the advantage of not relying upon the replacement of degenerated cells and potentially targeting earlier stages of disease pathogenesis. In order to translate these techniques into clinical practice, appropriate cell sourcing, robust differentiation protocols, and accurate implantation methods are crucial to the success of cell-based therapy in glaucoma.
Translational Relevance: Cell-based therapies for glaucoma currently under active development include the induction of endogenous regeneration, implantation of exogenously derived retinal cells, and utilization of cell-derived products to provide functional support.
Keywords: glial cell, glaucoma, stem cell
Glaucoma Pathogenesis
Glaucoma is the major cause of irreversible visual loss worldwide, second only to the reversible causes of uncorrected refractive error and cataract.1 A variety of clinical presentations and disease etiologies can ultimately lead to glaucomatous optic neuropathy (GON), characterized by degeneration of retinal ganglion cells (RGCs) and optic nerve head (ONH) remodeling. In addition to these two disease-defining processes, intraocular pressure (IOP) is the major modifiable risk factor in the management of glaucoma, although a significant proportion of patients with glaucoma present with IOP values within the normal range.2 Understanding the causal relationships between IOP, ONH remodeling, and RGC degeneration in the etiology of glaucoma is key to the formulation of successful treatment strategies.
Cross-sectional studies have demonstrated IOP within the general population to follow an approximately Gaussian distribution with an exaggerated central peak and modest skew toward higher pressures.3,4 The original suggestion that glaucoma can be defined simply by an IOP of 21 mm Hg or higher has been fundamentally refuted.5 In modern glaucoma research, there is agreement across different study cohorts that using IOP values alone to diagnose glaucoma offers poor sensitivity and specificity.3,6 This diagnostic challenge is further compounded by the finding that glaucoma prevalence varies significantly between different regions and ethnic groups.7 Nevertheless, medical, laser, and surgical approaches to reduce IOP are currently the only approved clinical treatment strategies in the treatment of glaucoma.8
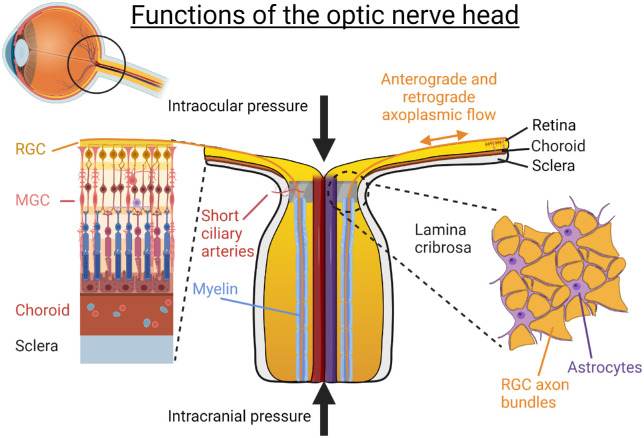
Among the pathogenic features of glaucoma, characteristic cupping of the ONH as a result of tissue remodeling appears to be the most specific. In a healthy retina, over 1 million RGC axons converge at the ONH before turning sharply to exit the globe via the optic nerve. Prior to passing through the lamina cribrosa, RGC axons have very high energy requirements, as evidenced by the elevated concentration of cytochrome C oxidase and mitochondria seen in the prelaminar and lamina cribrosa regions.9,10 This decreases dramatically as the RGC axons acquire myelin sheaths after passing through the lamina cribrosa (Fig. 1).
Figure 1.
Normal physiologic function within the ONH. Complex biomechanical forces converge on the ONH, which results in a pressure gradient of around 3.5 mm Hg per 100 µm across the lamina cribrosa. The appropriate distribution of these forces, as well as structural and metabolic support from the astrocytes, allows for adequate flow within the short ciliary arteries and retinal ganglion cell axons.
The ONH is constantly subject to static and dynamic biomechanical forces, in part determined by IOP in its anterior aspect and intracranial pressure (ICP) in its posterior aspect.11 In a healthy individual, this creates a translaminar pressure gradient of around 3.5 mm Hg per 100 µm, which appears to cause a congestive effect to RGC axons even during physiologic conditions.12 The translaminar pressure gradient is particularly sensitive to postural changes, diurnal variation, and disease states where IOP may be increased or ICP decreased.13 Significant increases in the translaminar pressure gradient are associated with GON, as it compromises axonal transport through the lamina cribrosa.
The lamina cribrosa is a specialized porous collagenous plate, which provides structural integrity to the ONH. Situated between RGC axons and the lamina cribrosa are astrocytes, the major glial cell type found in this region, which provide structural and metabolic support to the RGCs.14,15 Astrocytes are intimately coupled to adjacent RGCs, blood vessels, and extracellular matrices to facilitate the distribution of key molecules such as neurotrophic factors16 and assist in the normal turnover and migration of mitochondria.12,17 There is also evidence that astrocytes are capable of remodeling the collagenous lamina cribrosa over time.18,19
At times of excess pressure-induced stress, studies have demonstrated ONH astrocytes to become reactive and decrease their expression of glial fibrillary acidic protein, a finding that consistently coincides with morphologic changes, axonal transport delay, and astrocyte swelling.20 A detailed electron microscopy study of the optic nerve after experimental induction of raised intraocular pressure demonstrated early dissociation between the astrocytes and their adjacent nerve fibers, as well as focal areas of axoplasmic holdup with potential deprivation of neurometabolic support to these highly energy-dependent nonmyelinated axons.20 This unfavorable sequence of events ultimately results in longer term antero- and retrograde degeneration, which forms the basis of the “energy theory” of glaucomatous damage.21
Another important aspect of the ONH structure is the blood supply to this region. Indeed, impaired blood flow is currently thought to be an important pathogenic factor for normal-tension glaucoma (NTG).22 This may be due to the observation that short ciliary arteries that supply the ONH appear to be particularly vulnerable to changes in ocular perfusion pressure, defined as the difference between systemic blood pressure and IOP,23 when compared to the central retinal artery. It should be noted that IOP reduction remains an effective treatment strategy in a large proportion of patients with NTG.
Maintenance of healthy RGCs requires the availability of essential metabolic substrates, specific trophic factors, and prompt removal of environment stressors.24 In particular, the neurotrophin family of proteins is crucial in promoting the survival of RGCs, while the BCL-2 family of genes exerts antiapoptotic effects.25 In glaucoma models, significant disruption of these pathways causes downstream activation of effector caspases and results in RGC death via apoptosis. Moreover, studies have increasingly revealed important roles played by other constituents of the neuroretina, including Müller glial cells (MGCs), microglia, and the complement cascade.26,27
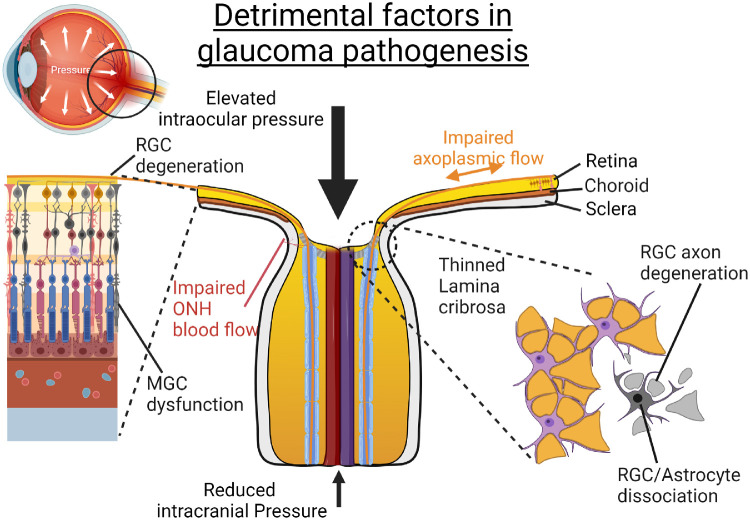
In summary, potential sources of axonal injury are counterbalanced by a range of protective mechanisms within the healthy individual. However, eyes of individuals who are particularly susceptible to GON can become overwhelmed in the presence of significant detrimental factors (Fig. 2). While the contribution of the various pathogenic mechanisms outlined in this section may depend on the glaucoma subtype and patient factors, the pathways ultimately converge to cause degeneration of RGC axons and cell bodies and therefore irreversible loss of vision.
Figure 2.
Detrimental factors in glaucoma pathogenesis. An increased pressure gradient across the lamina cribrosa can result from elevated intraocular pressure, reduced intracranial pressure, or abnormal tissue hysteresis. Over time, a characteristic “cupped” optic disc appearance can develop, which can cause thinning and bowing of the lamina cribrosa, as well as dissociation of astrocytes. In turn, these changes can lead to degeneration of RGC axons and glial cell dysfunction.
Development of Cell-Based Therapies
The retina is particularly well suited for the development of cell-based therapies for several reasons. First, most cell types within the retina are derived from a common neuronal lineage; second, the retina is arranged into well-defined layers that contain distinct cell types28; and third, in vivo visualization of the retina and its sublayers, up to a cellular resolution, is possible throughout any therapeutic period.29 A multitude of cell-based approaches have been employed with the goal of preventing and treating ocular diseases. Broadly speaking, these approaches can be divided into the induction of endogenous regeneration, replacement therapy using exogenous sources of cells, and application of cell-derived products for neuroprotection (Figs. 3 and 4).
Figure 3.

Stem cell–derived products for glaucoma therapy. Embryonic and induced pluripotent stem cells have been demonstrated to be capable of differentiating into Müller cells, astrocytes, and retinal ganglion cells in vitro. Animal experiments have demonstrated beneficial effects resulting from the direct implantation of these cells, while other recent studies have explored the potential therapeutic effects of products such as miRNAs and extracellular vesicles derived from various sources.
Figure 4.
Treatment strategies for cell-based therapies in glaucoma. Cell replacement strategies aim to insert stem cell–derived glial cells and RGCs into their normal anatomic locations, whereas the implantation of glial cells into locations adjacent to RGCs has been shown to promote visual benefit. Furthermore, the injection of cell-derived products such as neurotrophic factors, miRNAs, and extracellular vesicles is a more recently developed modality of neuroprotection.
Endogenous Regeneration
Regeneration of the retina is a phenomenon that has been well described in certain species such as the zebrafish, where cell lineage tracing attributed the source of the regenerated cells to MGCs present in the inner nuclear layer of the central retina.30 Unfortunately, while a population of MGCs with neural stem cell characteristics has been identified in the human retina,31 retinal regeneration has not been observed to occur naturally in mammals. As such, considerable research has focused on identifying the fundamental mechanisms that allow for the repopulation of retinal cells.
A host of differentially expressed factors by zebrafish MGCs following injury has been found by both genetic and proteomic methods.32,33 Of particular importance are the presence of factors such as stat3, ascl1, and its downstream target lin-28 in allowing the reentry of MGCs back into the cell cycle.34 Activation of these intracellular pathways is thought to be due, at least in part, to the paracrine release of cytokines, including tumor necrosis factor–α (TNF-α) and transforming growth factor–β (TGF-β),35 as well as growth factors such as epidermal growth factor (EGF) 1 and fibroblast growth factor (FGF).36
The detailed interactions of these pathways during the regenerative process have been extensively reviewed elsewhere.37 Although investigations in this area have substantially advanced our knowledge of intracellular mechanisms during retinal injury in different species, endogenous retinal regeneration has not yet been achieved in mammals. A major hurdle preventing the development of RGC replacement therapy is the sheer distance covered by the axons of RGCs during their projection to the brain. A typical RGC axon measures 50 mm in length, which is some 10,000 times the width of its cell body.38 In other words, if the diameter of an RGC axon (0.5–1 µm) was scaled up to the width of an average road, its projection would proportionally be 200 km. As such, precise methods of axonal guidance must be developed in order for such therapies to restore functional visual circuits between the retina and the brain.
Cell Replacement Therapies
An alternative approach to treat degenerative retinal conditions is to prepare functional cells in vitro, followed by implantation into the damaged tissue. Although current technologies can derive therapeutic cells to closely match damaged cells, designing a method of delivery that promotes integration within the retinal circuit has proven to be exceptionally difficult.39 This is further complicated by the finding that evidence previously thought to demonstrate structural integration was in fact due to cytoplasmic material exchange.40–42 Nevertheless, several studies have demonstrated functional recovery following treatment despite a lack of integration of transplanted cells.43 Currently, a variety of approaches are being developed to achieve functional cell replacement. These can broadly be divided according to the type of source cell used (Fig. 3).
Embryonic Stem Cells and Induced Pluripotent Stem Cells
Embryonic stem cells (ESCs) are derived from the inner cell mass of the blastocyst and have the ability to differentiate to any cell type from the three germ layers, mesoderm, endoderm, and ectoderm.44 Discovered more recently, induced pluripotent stem cells (iPSCs) can be generated from adult cells by the overexpression of “Yamanaka factors,” which include Oct3/4, Sox2, Klf4, and cMyc.45,46 Various human fibroblasts, keratinocytes, and hematopoietic cells have been used to generate stable iPSC cell lines that show characteristics similar to ESCs.47,48
The use of human ESCs (hESCs) has been explored for use in a wide range of retinal degenerative conditions. Established protocols include the differentiation of RPE (retinal pigment epithelium) for AMD (age-related macular degeneration)-based therapies49 and the generation of retinal progenitors that express markers for differentiated rod and cone photoreceptors following injection to the rodent eye.50 More recently, studies have shown that ESCs can be differentiated to generate RGCs using either chemically defined or CRISPR-engineered protocols.51–54 Using a reporter stem cell line, RGCs expressing BRN3B and BRN3C can be FACS (fluorescence-activated cell sorting) sorted from adherent hESC-differentiated retinal tissue and grown on scaffolds to guide axonal outgrowth.51 These cells survive in culture and could be generated rapidly within 4 weeks, exhibited axonal outgrowth, and were viable when transplanted in adult rat retina.52
Both hESCs and human iPSCs (hiPSCs) have been used to generate three-dimensional retinal organoids that are shown to closely mimic in vivo development.55–57 This differentiation protocol developed by Nakano et al.55 has provided an easy-to-follow method for reliably generating laminated neuroretinal tissues. Adapted by many other laboratories,56,58–60 this novel strategy has paved the way for modern retinal research, including development, disease pathogenesis, and regenerative medicine. Retinal organoids are physiologically and metabolically functional, where photoreceptors61 and RGCs62 have been demonstrated to produce electrophysiologic responses to light activity. RNA sequencing analysis of retinal organoids has identified multiple RGC subtypes, which highlights their cellular diversity during in vivo development.53 One recent study demonstrated that RGCs derived from retinal organoids formed by hiPSCs survived for up to a month and improved function following injection into the vitreous of mice with optic neuropathy.63
Differentiation of photoreceptor precursors has also been increased in retinal organoids by the addition of COCO, an antagonist of the Wnt, Notch, TGF-β, and BMP pathways.64,65 The generation of RGCs using this method has proved more challenging, as there is a short competence window for the differentiation of RGCs during early retinal development. Furthermore, the number of RGCs decreases over time in retinal organoid cultures,66 while photoreceptor numbers increase.56 There is some in vivo evidence that the addition of microRNAs such as miR‐125b, miR‐9, and Let‐7 can increase the production of RGCs, in addition to promoting progenitor cell competence.67 Additionally, it may also be possible to regulate RGC differentiation by mitophagy-dependent metabolic reprogramming via glycolysis.68 Regulation of differentiation pathways such as Shh, TGFβ, Notch, and Wnt signaling has also shown to control RGC differentiation in mammalian retinas,69–71 while the addition of netrin-1 to retinal organoid culture medium has shown to enhance RGC neurite outgrowth.72 An in vitro approach using retinal organoids provides the perfect platform to discover and refine RGC differentiation techniques; as such, a robust protocol may be available in the not too distant future.
In some cases, methods to isolate cells from stem cell–derived retinal organoids may be more advantageous than trying to develop protocols to promote direct differentiation. RGCs are especially well suited for this, as they can be produced more rapidly due to being one of the first cell types to develop in retinal organoids.72 In summary, ESC- and iPSC-based methodologies are ideal for the development of cell-based disease models and therapies for glaucoma and other retinal diseases.
Mesenchymal Stem Cells
Mesenchymal stem cells (MSCs) are adult stem cells that can be derived from the bone marrow or umbilical cord. They are considered multipotent and have the capacity to differentiate toward cells of mesodermal lineage such as osteocytes, adipocytes, and chondrocytes.73 More recently, some studies have also shown that MSCs have the capacity to differentiate to neural lineages by the activation of Wnt/β-catenin, Notch, and Shh pathways74–78 and addition of growth factors, including EGF, bFGF (basic fibroblast growth factor), and HGF (hepatocyte growth factor).79–81 The secretome of MSCs comprise of a wide range of cytokines and growth factors, including interleukin (IL) 6, IL-8, BDNF (brain-derived neurotrophic factor), CNTF (ciliary neurotrophic factor), NGF (nerve growth factor), PDGF (Platelet-derived growth factor), LIF (leukemia inhibitory factor), NT-3 (eurotrophin-3), TGF-β2, and FGF2,82–84 and inflammatory factors, including TNF-α and IL-1β.85,86
MSCs have been extensively explored for their therapeutic use in the treatment of glaucoma. Intravitreal injections of MSCs in glaucoma-induced rodent eyes align along the ILM (Internal limiting membrane) and can survive for several weeks, resulting in increased RGC survival.87 A study compared the injection of human MSCs with their MSC-derived extracellular vesicles (EVs) into a rat optic nerve crush model, and the results showed sustained neuroprotection of RGCs by whole-cell treatment as compared to EVs alone, suggesting cellular-based therapies might confer better sustained neurotrophic support to the retina.88 MSCs have also been engineered to produce high levels of BDNF, and transplantation studies into hypertensive rat eyes demonstrated significant neuronal protection.89 Presently, few MSC-based therapies have reached clinical trial stages. Unfortunately, a recent study showed no significant changes in visual function as measured by ERG in two patients with open-angle glaucoma, and one patient was removed due to retinal detachment complications,90 which suggests that additional refinement is required for this type of cell therapy. Two further clinical trials using bone marrow–derived stem cells to treat optic nerve damage are currently in the recruitment phase (NCT01920867, NCT03011541).
Müller Glial Cells
MGCs are the main glial cell type in the retina, as they span radially across all neuroretinal layers to form contacts with all cells of the neurovascular unit. The main functions of MGCs are to provide structural and metabolic support to retinal neurons similar to astrocytes within the ONH. MGCs become reactive in response to retinal injury, which can lead to the regeneration of lost neural cells in some species. Although this regenerative ability is not replicated in mammalian retina, MGCs with stem cell characteristics have been identified in the adult human eye. These cells can be isolated from postmortem tissues and spontaneously immortalize in vitro.91,92 Isolated human MGCs express characteristic stem cell markers such as SOX2, PAX6, βIII-tubulin, and notch 1 and, depending on culture supplementation, can be induced to become photoreceptor or retinal ganglion cell precursors.93,94
Extensive research has been conducted on the potential for MGCs in the treatment of glaucoma. In a rodent model of NMDA-induced retinal ganglion cell depletion, transplantation of human MGC-derived RGC precursors into the vitreous space resulted in a partial recovery of RGC function as judged by the negative scotopic threshold response of the electroretinogram.93 These cells align along the inner limiting membrane, but little to no integration is observed.93 Other studies have shown that rodent primary RGCs and progenitor-derived RGCs survive transplantation and become orientated along the host axons toward the ONH,95 but there has been limited evidence for substantial RGC integration and extension of axons through the ONH. One study found that following ablation of RGCs by intravitreal injection of NMDA to the feline eye, transplantation of MGCs into the intravitreal space resulted in partial recovery visual function.43 However, transplanted MGCs did not attach directly to the retina as seen in rodent studies but instead formed aggregates, suggesting that the vitreous may constitute a barrier for cell attachment onto the retina in the larger eye. As highlighted previously, RGC integration and axon projection remain major hurdles for this type of therapy.
Isolation of MGCs has been performed successfully from hiPSC-derived retinal organoids in vitro. Subsequent intravitreal transplantation of these MGCs into a rat model of RGC depletion has been demonstrated to induce a partial recovery of visual function.60 This study validates MGC implantation as a method of providing neuroprotection, and allogenic transplant studies are currently under way. There are important risks to consider when applying these cells to human therapies, however, such as the need for traceability, elimination of potential pathogens, and histocompatibility. Nevertheless, it is evident that MGCs are highly specialized to function within the retina while being a versatile source of cells for a variety of potential therapeutic applications.96
Cell-Derived Products
As retinal regeneration has proven to be an immensely difficult goal, attention in recent years has shifted somewhat into strategies of neuroprotection. As we gain deeper understanding of the pathogenesis of glaucoma, various experimental approaches have emerged that aim to inhibit detrimental pathways or enhance endogenous protective mechanism to prevent GON.97 Although there is a large body of data to support the neuroprotective effects of neurotrophins and antioxidants, effective delivery into the eye remains a significant hurdle preventing the translation of our current knowledge into clinical practice.25
Neurotrophins
The dependence of RGCs on neurotrophic factors is evident during optic nerve development,98 as well as in the adult tissue, where RGC survival is reliant upon the retrograde delivery of neurotrophic signals from targets within the central nervous system.99 In rodent models, adeno‐associated viral delivery of bFGF and BDNF can promote RGC survival following glutamate insult,100 while CNTF treatment significantly reduced RGC loss.101 There is also compelling evidence for RGC neuroprotection by the neurotrophin NGF.102,103
Despite these promising data, there remain concerns over the duration of any therapeutic effects from direct delivery of these neurotrophins, whereas a whole-cell transplant-based strategy may offer a longer-lasting solution. In support for this hypothesis, rat and human bone marrow–derived MSCs induced to secrete high levels of BDNF, GDNF (glial cell-derived neurotrophic factor), and VEGF (vascular endothelial growth factor) were found to exert a marked neuroprotective effect in a rodent optic nerve transection model following intravitreal injection.104 An alternative approach has incorporated the use of a polymeric capsule containing immortalized pigment epithelial cells transfected with the human CNTF gene.105 These devices have been demonstrated to be capable of sustained CNTF secretion and delivery to the posterior chamber for up to 1.5 years following implantation.106,107 Randomized phase I and II clinical trials are currently ongoing to evaluate the safety and efficacy of these devices in patients with primary open-angle glaucoma (NCT01408472, NCT02862938).
In order to realize the potential of neurotrophic factors in the future management of glaucoma, a safe, stable, and continuous system for their delivery remains a major challenge. Furthermore, despite deprivation of neurotrophic factors being characteristic of the glaucomatous retina, prolonged delivery of BDNF and TrkB receptor to axotomized optic nerve was unable to maintain long-term RGC survival.108 It is likely, therefore, that neurotrophin secretion constitutes a part but not all of the efficacy of whole-cell transplantation, as the roles of additional paracrine signals continue to be elucidated.
MicroRNA and Extracellular Vesicles
MicroRNAs (miRNAs) are endogenous, small, noncoding RNAs. Their primary function is the posttranscriptional regulation of protein-coding gene expression by binding to the targeted messenger RNA (mRNA), which leads to the inhibition of translation or mRNA degradation. Through this system of interference, miRNAs have been demonstrated to play pivotal roles in cell proliferation, differentiation, and apoptosis.109,110 Several miRNAs have shown potential for RGC neuroprotection. These include miR-141-3p, which indirectly inhibits proapoptotic Bax and caspase 3 signaling pathways via targeting of docking protein 5 (DOK5)111; miR-93-5p, which was found to protect RGCs in culture from NMDA-induced cell death112; and miR-200a, which was found to preserve the thickness of the nerve fiber layer in a mouse model of glaucoma.113
In recent years, significant research interest in neuroprotective strategies has been focused upon the phosphatase and tensin homolog (PTEN) gene, the master inhibitor of the proregenerative mTOR/PI3K/Akt pathway.114 In nervous tissue, this pathway is responsible for the regulation of axon formation and extension during development, as well as the regeneration of peripheral nerve axons.115 Numerous miRNAs have been found to target PTEN and subsequently activate the mTOR (mammalian target of rapamycin) pathway, including miR-214,116 miR-1908,117 miR-494,118 and miR-21.119 In a model of glaucoma, inhibitors of miR-149 were shown to be neuroprotective of RGCs along with an associated upregulation of the PI3K/Akt pathway.120
Over the past decade, secreted EVs comprising cytosol enclosed in a lipid bilayer membrane have been identified as a major mode of intercellular communication,121 and some populations of EVs were found to be highly enriched in nucleic acids, particularly mRNAs and miRNAs.122 In the context of glaucoma, a recent study reported evidence of RGC axon regeneration using a heterogenous EV population isolated from the L-cell fibroblast line in an optic nerve crush model.123 Various MSC-derived EVs have also been found to significantly enhance survival of RGC and regenerate their axons, while partially preventing RGC axonal loss in rodent models.124–127 Furthermore, delivery of miRNAs via Schwann cell–derived exosomes into cultured neurons were found to promote neuritogenesis.128
Since EVs are capable of delivering their cargo directly into target cells, EVs derived from healthy cells may potentially have a comparable therapeutic potential as the cells themselves. Cell-derived EVs also contain a range of miRNAs to potentially activate multiple antiapoptotic and prosurvival pathways at once. As evidence accumulates for their neuroprotective efficacy, low immunogenicity, and high stability, EVs appear to be promising candidates as an adjunctive therapy to IOP-lowering medications and thus a potential future treatment for glaucoma.
Conclusion and Future Directions
In recent years, there has been tremendous progress in the development of cell-based therapies for glaucoma. In addition to established strategies for endogenous regeneration and cell replacement, there is growing interest in more novel approaches using cell-derived products such as EVs. Appropriate cell sourcing, robust differentiation protocols, and accurate implantation techniques are all crucial to the development of successful cell-based therapy in glaucoma. In this rapidly progressing field, the increasing diversity of approaches and incremental refinements of existing techniques are bringing the prospect of clinical application ever closer to reality.
Acknowledgments
Funding was provided by Moorfields Eye Charity, Fight for Sight and the NIHR Biomedical Centre at Moorfields Eye Hospital, UCL Institute of Ophthalmology.
Figures in this review were created with BioRender.com.
Disclosure: J. Luis, None; K. Eastlake, None; W.D.B. Lamb, None; G.A. Limb, None; H. Jayaram, None; P.T. Khaw, None
References
- 1. Quigley HA, Broman AT.. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006; 90: 262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weinreb RN, Khaw PT.. Primary open-angle glaucoma. Lancet. 2004; 363: 1711–1720. [DOI] [PubMed] [Google Scholar]
- 3. Chan MPY, Broadway DC, Khawaja AP, et al.. Glaucoma and intraocular pressure in EPIC-Norfolk Eye Study: cross sectional study. BMJ. 2017; 358: j3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bonomi L, Marchini G, Marraffa M, et al.. Prevalence of glaucoma and intraocular pressure distribution in a defined population. The Egna-Neumarkt Study. Ophthalmology. 1998; 105: 209–215. [DOI] [PubMed] [Google Scholar]
- 5. Leydhecker W, Akiyama K, Neumann HG.. Intraocular pressure in normal human eyes [in German]. Klin Monbl Augenheilkd Augenarztl Fortbild. 1958; 133: 662–670. [PubMed] [Google Scholar]
- 6. Tielsch JM, Katz J, Singh K, et al.. A population-based evaluation of glaucoma screening: the Baltimore Eye Survey. Am J Epidemiol. 1991; 134: 1102–1110. [DOI] [PubMed] [Google Scholar]
- 7. Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY.. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014; 121: 2081–2090. [DOI] [PubMed] [Google Scholar]
- 8. Kass MA, Heuer DK, Higginbotham EJ, et al.. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002; 120: 701–713; discussion 729-730. [DOI] [PubMed] [Google Scholar]
- 9. Balaratnasingam C, Pham D, Morgan WH, Bass L, Cringle SJ, Yu DY.. Mitochondrial cytochrome c oxidase expression in the central nervous system is elevated at sites of pressure gradient elevation but not absolute pressure increase. J Neurosci Res. 2009; 87: 2973–2982. [DOI] [PubMed] [Google Scholar]
- 10. Yu-Wai-Man P, Griffiths PG, Chinnery PF.. Mitochondrial optic neuropathies—disease mechanisms and therapeutic strategies. Prog Retin Eye Res. 2011; 30: 81–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baneke AJ, Aubry J, Viswanathan AC, Plant GT. The role of intracranial pressure in glaucoma and therapeutic implications. Eye (Lond). 2020; 34: 178–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hollander H, Makarov F, Stefani FH, Stone J.. Evidence of constriction of optic nerve axons at the lamina cribrosa in the normotensive eye in humans and other mammals. Ophthalmic Res. 1995; 27: 296–309. [DOI] [PubMed] [Google Scholar]
- 13. Ren R, Jonas JB, Tian G, et al.. Cerebrospinal fluid pressure in glaucoma: a prospective study. Ophthalmology. 2010; 117: 259–266. [DOI] [PubMed] [Google Scholar]
- 14. Morgan JE. Optic nerve head structure in glaucoma: astrocytes as mediators of axonal damage. Eye (Lond). 2000; 14(pt 3B): 437–444. [DOI] [PubMed] [Google Scholar]
- 15. Burgoyne CF, Downs JC, Bellezza AJ, Suh JK, Hart RT.. The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog Retin Eye Res. 2005; 24: 39–73. [DOI] [PubMed] [Google Scholar]
- 16. Quigley HA, McKinnon SJ, Zack DJ, et al.. Retrograde axonal transport of BDNF in retinal ganglion cells is blocked by acute IOP elevation in rats. Invest Ophthalmol Vis Sci. 2000; 41: 3460–3466. [PubMed] [Google Scholar]
- 17. Davis CH, Kim KY, Bushong EA, et al.. Transcellular degradation of axonal mitochondria. Proc Natl Acad Sci USA. 2014; 111: 9633–9638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hernandez MR. The optic nerve head in glaucoma: role of astrocytes in tissue remodeling. Prog Retin Eye Res. 2000; 19: 297–321. [DOI] [PubMed] [Google Scholar]
- 19. Wang B, Lucy KA, Schuman JS, et al.. Tortuous pore path through the glaucomatous lamina cribrosa. Sci Rep. 2018; 8: 7281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Balaratnasingam C, Morgan WH, Bass L, et al.. Elevated pressure induced astrocyte damage in the optic nerve. Brain Res. 2008; 1244: 142–154. [DOI] [PubMed] [Google Scholar]
- 21. Li Y, Li D, Ying X, Khaw PT, Raisman G.. An energy theory of glaucoma. Glia. 2015; 63: 1537–1552. [DOI] [PubMed] [Google Scholar]
- 22. Trivli A, Koliarakis I, Terzidou C, et al.. Normal-tension glaucoma: pathogenesis and genetics. Exp Ther Med. 2019; 17: 563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Choi J, Kim KH, Jeong J, Cho HS, Lee CH, Kook MS.. Circadian fluctuation of mean ocular perfusion pressure is a consistent risk factor for normal-tension glaucoma. Invest Ophthalmol Vis Sci. 2007; 48: 104–111. [DOI] [PubMed] [Google Scholar]
- 24. Levkovitch-Verbin H. Retinal ganglion cell apoptotic pathway in glaucoma: initiating and downstream mechanisms. Prog Brain Res. 2015; 220: 37–57. [DOI] [PubMed] [Google Scholar]
- 25. Eastlake K, Luis J, Limb GA.. Potential of Muller glia for retina neuroprotection. Curr Eye Res. 2020; 45: 339–348. [DOI] [PubMed] [Google Scholar]
- 26. Almasieh M, Wilson AM, Morquette B, Cueva Vargas JL, Di Polo A. The molecular basis of retinal ganglion cell death in glaucoma. Prog Retin Eye Res. 2012; 31: 152–181. [DOI] [PubMed] [Google Scholar]
- 27. Nguyen JV, Soto I, Kim KY, et al.. Myelination transition zone astrocytes are constitutively phagocytic and have synuclein dependent reactivity in glaucoma. Proc Natl Acad Sci USA. 2011; 108: 1176–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Heavner W, Pevny L.. Eye development and retinogenesis. Cold Spring Harb Perspect Biol. 2012; 4: a008391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Burns SA, Elsner AE, Sapoznik KA, Warner RL, Gast TJ.. Adaptive optics imaging of the human retina. Prog Retin Eye Res. 2019; 68: 1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Raymond PA, Barthel LK, Bernardos RL, Perkowski JJ.. Molecular characterization of retinal stem cells and their niches in adult zebrafish. BMC Dev Biol. 2006; 6: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bhatia B, Singhal S, Lawrence JM, Khaw PT, Limb GA.. Distribution of Muller stem cells within the neural retina: evidence for the existence of a ciliary margin-like zone in the adult human eye. Exp Eye Res. 2009; 89: 373–382. [DOI] [PubMed] [Google Scholar]
- 32. Qin Z, Barthel LK, Raymond PA.. Genetic evidence for shared mechanisms of epimorphic regeneration in zebrafish. Proc Natl Acad Sci USA. 2009; 106: 9310–9315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Eastlake K, Heywood WE, Banerjee P, et al.. Comparative proteomic analysis of normal and gliotic PVR retina and contribution of Muller glia to this profile. Exp Eye Res. 2018; 177: 197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nelson CM, Gorsuch RA, Bailey TJ, Ackerman KM, Kassen SC, Hyde DR.. Stat3 defines three populations of Muller glia and is required for initiating maximal muller glia proliferation in the regenerating zebrafish retina. J Comp Neurol. 2012; 520: 4294–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yafai Y, Iandiev I, Lange J, et al.. Muller glial cells inhibit proliferation of retinal endothelial cells via TGF-beta2 and Smad signaling. Glia. 2014; 62: 1476–1485. [DOI] [PubMed] [Google Scholar]
- 36. Wan J, Zhao XF, Vojtek A, Goldman D.. Retinal injury, growth factors, and cytokines converge on beta-catenin and pStat3 signaling to stimulate retina regeneration. Cell Rep. 2014; 9: 285–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Goldman D. Muller glial cell reprogramming and retina regeneration. Nat Rev Neurosci. 2014; 15: 431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yu DY, Cringle SJ, Balaratnasingam C, Morgan WH, Yu PK, Su EN.. Retinal ganglion cells: energetics, compartmentation, axonal transport, cytoskeletons and vulnerability. Prog Retin Eye Res. 2013; 36: 217–246. [DOI] [PubMed] [Google Scholar]
- 39. Stout JT, Francis PJ.. Surgical approaches to gene and stem cell therapy for retinal disease. Hum Gene Ther. 2011; 22: 531–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. MacLaren RE, Pearson RA, MacNeil A, et al.. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006; 444: 203–207. [DOI] [PubMed] [Google Scholar]
- 41. Santos-Ferreira T, Llonch S, Borsch O, Postel K, Haas J, Ader M.. Retinal transplantation of photoreceptors results in donor-host cytoplasmic exchange. Nat Commun. 2016; 7: 13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pearson RA, Gonzalez-Cordero A, West EL, et al.. Donor and host photoreceptors engage in material transfer following transplantation of post-mitotic photoreceptor precursors. Nat Commun. 2016; 7: 13029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Becker S, Eastlake K, Jayaram H, et al.. Allogeneic transplantation of Muller-derived retinal ganglion cells improves retinal function in a feline model of ganglion cell depletion. Stem Cells Transl Med. 2016; 5: 192–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yamanaka S, Li J, Kania G, et al.. Pluripotency of embryonic stem cells. Cell Tissue Res. 2008; 331: 5–22. [DOI] [PubMed] [Google Scholar]
- 45. Takahashi K, Okita K, Nakagawa M, Yamanaka S.. Induction of pluripotent stem cells from fibroblast cultures. Nat Protoc. 2007; 2: 3081–3089. [DOI] [PubMed] [Google Scholar]
- 46. Takahashi K, Tanabe K, Ohnuki M, et al.. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007; 131: 861–872. [DOI] [PubMed] [Google Scholar]
- 47. Wernig M, Meissner A, Foreman R, et al.. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007; 448: 318–324. [DOI] [PubMed] [Google Scholar]
- 48. Guenther MG, Frampton GM, Soldner F, et al.. Chromatin structure and gene expression programs of human embryonic and induced pluripotent stem cells. Cell Stem Cell. 2010; 7: 249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. da Cruz L, Fynes K, Georgiadis O, et al.. Phase 1 clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration. Nat Biotechnol. 2018; 36: 328–337. [DOI] [PubMed] [Google Scholar]
- 50. Zhou S, Flamier A, Abdouh M, et al.. Differentiation of human embryonic stem cells into cone photoreceptors through simultaneous inhibition of BMP, TGFbeta and Wnt signaling. Development. 2015; 142: 3294–3306. [DOI] [PubMed] [Google Scholar]
- 51. Sluch VM, Davis CH, Ranganathan V, et al.. Differentiation of human ESCs to retinal ganglion cells using a CRISPR engineered reporter cell line. Sci Rep. 2015; 5: 16595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang X, Tenerelli K, Wu S, et al.. Cell transplantation of retinal ganglion cells derived from hESCs. Restor Neurol Neurosci. 2020; 38: 131–140. [DOI] [PubMed] [Google Scholar]
- 53. Langer KB, Ohlemacher SK, Phillips MJ, et al.. Retinal ganglion cell diversity and subtype specification from human pluripotent stem cells. Stem Cell Reports. 2018; 10: 1282–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kobayashi W, Onishi A, Tu HY, et al.. Culture systems of dissociated mouse and human pluripotent stem cell-derived retinal ganglion cells purified by two-step immunopanning. Invest Ophthalmol Vis Sci. 2018; 59: 776–787. [DOI] [PubMed] [Google Scholar]
- 55. Nakano T, Ando S, Takata N, et al.. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell. 2012; 10: 771–785. [DOI] [PubMed] [Google Scholar]
- 56. Volkner M, Zschatzsch M, Rostovskaya M, et al.. Retinal organoids from pluripotent stem cells efficiently recapitulate retinogenesis. Stem Cell Reports. 2016; 6: 525–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cui Z, Guo Y, Zhou Y, et al.. Transcriptomic analysis of the developmental similarities and differences between the native retina and retinal organoids. Invest Ophthalmol Vis Sci. 2020; 61: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lane A, Jovanovic K, Shortall C, et al.. Modeling and rescue of RP2 retinitis pigmentosa using iPSC-derived retinal organoids. Stem Cell Reports. 2020; 15: 67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sridhar A, Hoshino A, Finkbeiner CR, et al.. Single-cell transcriptomic comparison of human fetal retina, hPSC-derived retinal organoids, and long-term retinal cultures. Cell Rep. 2020; 30: 1644–1659.e1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Eastlake K, Wang W, Jayaram H, et al.. Phenotypic and functional characterization of Muller glia isolated from induced pluripotent stem cell-derived retinal organoids: improvement of retinal ganglion cell function upon transplantation. Stem Cells Transl Med. 2019; 8: 775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kim S, Lowe A, Dharmat R, et al.. Generation, transcriptome profiling, and functional validation of cone-rich human retinal organoids. Proc Natl Acad Sci USA. 2019; 116: 10824–10833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hallam D, Hilgen G, Dorgau B, et al.. Human-induced pluripotent stem cells generate light responsive retinal organoids with variable and nutrient-dependent efficiency. Stem Cells. 2018; 36: 1535–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rabesandratana O, Chaffiol A, Mialot A, et al.. Generation of a transplantable population of human iPSC-derived retinal ganglion cells. Front Cell Dev Biol. 2020; 8: 585675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pan D, Xia XX, Zhou H, et al.. COCO enhances the efficiency of photoreceptor precursor differentiation in early human embryonic stem cell-derived retinal organoids. Stem Cell Res Ther. 2020; 11: 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kruczek K, Gonzalez-Cordero A, Goh D, et al.. Differentiation and transplantation of embryonic stem cell-derived cone photoreceptors into a mouse model of end-stage retinal degeneration. Stem Cell Rep. 2017; 8: 1659–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Aparicio JG, Hopp H, Choi A, et al.. Temporal expression of CD184(CXCR4) and CD171(L1CAM) identifies distinct early developmental stages of human retinal ganglion cells in embryonic stem cell derived retina. Exp Eye Res. 2017; 154: 177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. La Torre A, Lamba DA, Jayabalu A, Reh TA. Production and transplantation of retinal cells from human and mouse embryonic stem cells. Methods Mol Biol. 2012; 884: 229–246. [DOI] [PubMed] [Google Scholar]
- 68. Esteban-Martinez L, Boya P.. BNIP3L/NIX-dependent mitophagy regulates cell differentiation via metabolic reprogramming. Autophagy. 2018; 14: 915–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Walshe TE, Leach LL, D'Amore PA. TGF-beta signaling is required for maintenance of retinal ganglion cell differentiation and survival. Neuroscience. 2011; 189: 123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Silva AO, Ercole CE, McLoon SC.. Regulation of ganglion cell production by Notch signaling during retinal development. J Neurobiol. 2003; 54: 511–524. [DOI] [PubMed] [Google Scholar]
- 71. Zhang XM, Yang XJ.. Regulation of retinal ganglion cell production by Sonic hedgehog. Development. 2001; 128: 943–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Fligor CM, Langer KB, Sridhar A, et al.. Three-dimensional retinal organoids facilitate the investigation of retinal ganglion cell development, organization and neurite outgrowth from human pluripotent stem cells. Sci Rep. 2018; 8: 14520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Pittenger MF, Mackay AM, Beck SC, et al.. Multilineage potential of adult human mesenchymal stem cells. Science. 1999; 284: 143–147. [DOI] [PubMed] [Google Scholar]
- 74. Kondo T, Matsuoka AJ, Shimomura A, et al.. Wnt signaling promotes neuronal differentiation from mesenchymal stem cells through activation of Tlx3. Stem Cells. 2011; 29: 836–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Leite C, Silva NT, Mendes S, et al.. Differentiation of human umbilical cord matrix mesenchymal stem cells into neural-like progenitor cells and maturation into an oligodendroglial-like lineage. PLoS One. 2014; 9: e111059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Yu Q, Liu L, Duan Y, et al.. Wnt/beta-catenin signaling regulates neuronal differentiation of mesenchymal stem cells. Biochem Biophys Res Commun. 2013; 439: 297–302. [DOI] [PubMed] [Google Scholar]
- 77. Long Q, Luo Q, Wang K, Bates A, Shetty AK.. Mash1-dependent Notch signaling pathway regulates GABAergic neuron-like differentiation from bone marrow-derived mesenchymal stem cells. Aging Dis. 2017; 8: 301–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Huang JG, Shen CB, Wu WB, et al.. Primary cilia mediate sonic hedgehog signaling to regulate neuronal-like differentiation of bone mesenchymal stem cells for resveratrol induction in vitro. J Neurosci Res. 2014; 92: 587–596. [DOI] [PubMed] [Google Scholar]
- 79. Li M, Zhao W, Gao Y, et al.. Differentiation of bone marrow mesenchymal stem cells into neural lineage cells induced by bFGF-chitosan controlled release system. Biomed Res Int. 2019; 2019: 5086297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Huat TJ, Khan AA, Pati S, Mustafa Z, Abdullah JM, Jaafar H.. IGF-1 enhances cell proliferation and survival during early differentiation of mesenchymal stem cells to neural progenitor-like cells. BMC Neurosci. 2014; 15: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bae KS, Park JB, Kim HS, Kim DS, Park DJ, Kang SJ.. Neuron-like differentiation of bone marrow-derived mesenchymal stem cells. Yonsei Med J. 2011; 52: 401–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Baberg F, Geyh S, Waldera-Lupa D, et al.. Secretome analysis of human bone marrow derived mesenchymal stromal cells. Biochim Biophys Acta Proteins Proteom. 2019; 1867: 434–441. [DOI] [PubMed] [Google Scholar]
- 83. Petrenko Y, Vackova I, Kekulova K, et al.. A comparative analysis of multipotent mesenchymal stromal cells derived from different sources, with a focus on neuroregenerative potential. Sci Rep. 2020; 10: 4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Teixeira FG, Carvalho MM, Panchalingam KM, et al.. Impact of the secretome of human mesenchymal stem cells on brain structure and animal behavior in a rat model of Parkinson's disease. Stem Cells Transl Med. 2017; 6: 634–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Martire A, Bedada FB, Uchida S, et al.. Mesenchymal stem cells attenuate inflammatory processes in the heart and lung via inhibition of TNF signaling. Basic Res Cardiol. 2016; 111: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Guo J, Lin GS, Bao CY, Hu ZM, Hu MY.. Anti-inflammation role for mesenchymal stem cells transplantation in myocardial infarction. Inflammation. 2007; 30: 97–104. [DOI] [PubMed] [Google Scholar]
- 87. Johnson TV, Bull ND, Hunt DP, Marina N, Tomarev SI, Martin KR.. Neuroprotective effects of intravitreal mesenchymal stem cell transplantation in experimental glaucoma. Invest Ophthalmol Vis Sci. 2010; 51: 2051–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. da Silva-Junior AJ, Mesentier-Louro LA, Nascimento-Dos-Santos G, et al.. Human mesenchymal stem cell therapy promotes retinal ganglion cell survival and target reconnection after optic nerve crush in adult rats. Stem Cell Res Ther. 2021; 12: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Harper MM, Grozdanic SD, Blits B, et al.. Transplantation of BDNF-secreting mesenchymal stem cells provides neuroprotection in chronically hypertensive rat eyes. Invest Ophthalmol Vis Sci. 2011; 52: 4506–4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Vilela CAP, Messias A, Calado RT, et al.. Retinal function after intravitreal injection of autologous bone marrow-derived mesenchymal stromal cells in advanced glaucoma. Doc Ophthalmol. 2021; 143: 33–38. [DOI] [PubMed] [Google Scholar]
- 91. Limb GA, Salt TE, Munro PM, Moss SE, Khaw PT.. In vitro characterization of a spontaneously immortalized human Muller cell line (MIO-M1). Invest Ophthalmol Vis Sci. 2002; 43: 864–869. [PubMed] [Google Scholar]
- 92. Lawrence JM, Singhal S, Bhatia B, et al.. MIO-M1 cells and similar muller glial cell lines derived from adult human retina exhibit neural stem cell characteristics. Stem Cells. 2007; 25: 2033–2043. [DOI] [PubMed] [Google Scholar]
- 93. Singhal S, Bhatia B, Jayaram H, et al.. Human Muller glia with stem cell characteristics differentiate into retinal ganglion cell (RGC) precursors in vitro and partially restore RGC function in vivo following transplantation. Stem Cells Transl Med. 2012; 1: 188–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Jayaram H, Jones MF, Eastlake K, et al.. Transplantation of photoreceptors derived from human Muller glia restore rod function in the P23H rat. Stem Cells Transl Med. 2014; 3: 323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hertz J, Qu B, Hu Y, Patel RD, Valenzuela DA, Goldberg JL.. Survival and integration of developing and progenitor-derived retinal ganglion cells following transplantation. Cell Transplant. 2014; 23: 855–872. [DOI] [PubMed] [Google Scholar]
- 96. Eastlake K, Lamb WDB, Luis J, Khaw PT, Jayaram H, Limb GA.. Prospects for the application of Muller glia and their derivatives in retinal regenerative therapies. Prog Retin Eye Res. 2021; 85: 100970. [DOI] [PubMed] [Google Scholar]
- 97. Pardue MT, Allen RS.. Neuroprotective strategies for retinal disease. Prog Retin Eye Res. 2018; 65: 50–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Meyer-Franke A, Kaplan MR, Pfrieger FW, Barres BA.. Characterization of the signaling interactions that promote the survival and growth of developing retinal ganglion cells in culture. Neuron. 1995; 15: 805–819. [DOI] [PubMed] [Google Scholar]
- 99. Iwabe S, Moreno-Mendoza NA, Trigo-Tavera F, Crowder C, Garcia-Sanchez GA.. Retrograde axonal transport obstruction of brain-derived neurotrophic factor (BDNF) and its TrkB receptor in the retina and optic nerve of American Cocker Spaniel dogs with spontaneous glaucoma. Vet Ophthalmol. 2007; 10(suppl 1): 12–19. [DOI] [PubMed] [Google Scholar]
- 100. Schuettauf F, Vorwerk C, Naskar R, et al.. Adeno-associated viruses containing bFGF or BDNF are neuroprotective against excitotoxicity. Curr Eye Res. 2004; 29: 379–386. [DOI] [PubMed] [Google Scholar]
- 101. Ji JZ, Elyaman W, Yip HK, et al.. CNTF promotes survival of retinal ganglion cells after induction of ocular hypertension in rats: the possible involvement of STAT3 pathway. Eur J Neurosci. 2004; 19: 265–272. [DOI] [PubMed] [Google Scholar]
- 102. Chen Q, Wang H, Liao S, et al.. Nerve growth factor protects retinal ganglion cells against injury induced by retinal ischemia-reperfusion in rats. Growth Factors. 2015; 33: 149–159. [DOI] [PubMed] [Google Scholar]
- 103. Lambiase A, Coassin M, Tirassa P, Mantelli F, Aloe L.. Nerve growth factor eye drops improve visual acuity and electrofunctional activity in age-related macular degeneration: a case report. Ann Ist Super Sanita. 2009; 45: 439–442. [DOI] [PubMed] [Google Scholar]
- 104. Levkovitch-Verbin H, Sadan O, Vander S, et al.. Intravitreal injections of neurotrophic factors secreting mesenchymal stem cells are neuroprotective in rat eyes following optic nerve transection. Invest Ophthalmol Vis Sci. 2010; 51: 6394–6400. [DOI] [PubMed] [Google Scholar]
- 105. Thanos CG, Bell WJ, O'Rourke P, et al.. Sustained secretion of ciliary neurotrophic factor to the vitreous, using the encapsulated cell therapy-based NT-501 intraocular device. Tissue Eng. 2004; 10: 1617–1622. [DOI] [PubMed] [Google Scholar]
- 106. Sieving PA, Caruso RC, Tao W, et al.. Ciliary neurotrophic factor (CNTF) for human retinal degeneration: phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proc Natl Acad Sci USA. 2006; 103: 3896–3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Zhang K, Hopkins JJ, Heier JS, et al.. Ciliary neurotrophic factor delivered by encapsulated cell intraocular implants for treatment of geographic atrophy in age-related macular degeneration. Proc Natl Acad Sci USA. 2011; 108: 6241–6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Di Polo A, Aigner LJ, Dunn RJ, Bray GM, Aguayo AJ.. Prolonged delivery of brain-derived neurotrophic factor by adenovirus-infected Muller cells temporarily rescues injured retinal ganglion cells. Proc Natl Acad Sci USA. 1998; 95: 3978–3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Cai J, Liu X, Cheng J, et al.. MicroRNA-200 is commonly repressed in conjunctival MALT lymphoma, and targets cyclin E2. Graefes Arch Clin Exp Ophthalmol. 2012; 250: 523–531. [DOI] [PubMed] [Google Scholar]
- 110. Zhang Z, Luo X, Ding S, et al.. MicroRNA-451 regulates p38 MAPK signaling by targeting of Ywhaz and suppresses the mesangial hypertrophy in early diabetic nephropathy. FEBS Lett. 2012; 586: 20–26. [DOI] [PubMed] [Google Scholar]
- 111. Zhang LQ, Cui H, Yu YB, Shi HQ, Zhou Y, Liu MJ.. MicroRNA-141-3p inhibits retinal neovascularization and retinal ganglion cell apoptosis in glaucoma mice through the inactivation of Docking protein 5-dependent mitogen-activated protein kinase signaling pathway. J Cell Physiol. 2019; 234: 8873–8887. [DOI] [PubMed] [Google Scholar]
- 112. Li R, Jin Y, Li Q, Sun X, Zhu H, Cui H.. MiR-93-5p targeting PTEN regulates the NMDA-induced autophagy of retinal ganglion cells via AKT/mTOR pathway in glaucoma. Biomed Pharmacother. 2018; 100: 1–7. [DOI] [PubMed] [Google Scholar]
- 113. Peng H, Sun YB, Hao JL, Lu CW, Bi MC, Song E.. Neuroprotective effects of overexpressed microRNA-200a on activation of glaucoma-related retinal glial cells and apoptosis of ganglion cells via downregulating FGF7-mediated MAPK signaling pathway. Cell Signal. 2019; 54: 179–190. [DOI] [PubMed] [Google Scholar]
- 114. Park KK, Liu K, Hu Y, et al.. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008; 322: 963–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Saijilafu Hur EM, Liu CM, Jiao Z, Xu WL, Zhou FQ. PI3K-GSK3 signalling regulates mammalian axon regeneration by inducing the expression of Smad1. Nat Commun. 2013; 4: 2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Bera A, Das F, Ghosh-Choudhury N, Mariappan MM, Kasinath BS, Ghosh Choudhury G. Reciprocal regulation of miR-214 and PTEN by high glucose regulates renal glomerular mesangial and proximal tubular epithelial cell hypertrophy and matrix expansion. Am J Physiol Cell Physiol. 2017; 313: C430–C447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Xia X, Li Y, Wang W, et al.. MicroRNA-1908 functions as a glioblastoma oncogene by suppressing PTEN tumor suppressor pathway. Mol Cancer. 2015; 14: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Wang X, Zhang X, Ren XP, et al.. MicroRNA-494 targeting both proapoptotic and antiapoptotic proteins protects against ischemia/reperfusion-induced cardiac injury. Circulation. 2010; 122: 1308–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Sayed D, He M, Hong C, et al.. MicroRNA-21 is a downstream effector of AKT that mediates its antiapoptotic effects via suppression of Fas ligand. J Biol Chem. 2010; 285: 20281–20290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Nie XG, Fan DS, Huang YX, He YY, Dong BL, Gao F.. Downregulation of microRNA-149 in retinal ganglion cells suppresses apoptosis through activation of the PI3K/Akt signaling pathway in mice with glaucoma. Am J Physiol Cell Physiol. 2018; 315: C839–C849. [DOI] [PubMed] [Google Scholar]
- 121. Klingeborn M, Dismuke WM, Bowes Rickman C, Stamer WD.. Roles of exosomes in the normal and diseased eye. Prog Retin Eye Res. 2017; 59: 158–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Nolte-'t Hoen E, Cremer T, Gallo RC, Margolis LB.. Extracellular vesicles and viruses: are they close relatives? Proc Natl Acad Sci USA. 2016; 113: 9155–9161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Tassew NG, Charish J, Shabanzadeh AP, et al.. Exosomes mediate mobilization of autocrine Wnt10b to promote axonal regeneration in the injured CNS. Cell Rep. 2017; 20: 99–111. [DOI] [PubMed] [Google Scholar]
- 124. Mead B, Tomarev S.. Bone marrow-derived mesenchymal stem cells-derived exosomes promote survival of retinal ganglion cells through miRNA-dependent mechanisms. Stem Cells Transl Med. 2017; 6: 1273–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Pan D, Chang X, Xu M, et al.. UMSC-derived exosomes promote retinal ganglion cells survival in a rat model of optic nerve crush. J Chem Neuroanat. 2019; 96: 134–139. [DOI] [PubMed] [Google Scholar]
- 126. Mead B, Ahmed Z, Tomarev S.. Mesenchymal stem cell-derived small extracellular vesicles promote neuroprotection in a genetic DBA/2J mouse model of glaucoma. Invest Ophthalmol Vis Sci. 2018; 59: 5473–5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Mead B, Amaral J, Tomarev S.. Mesenchymal stem cell-derived small extracellular vesicles promote neuroprotection in rodent models of glaucoma. Invest Ophthalmol Vis Sci. 2018; 59: 702–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Ching RC, Wiberg M, Kingham PJ.. Schwann cell-like differentiated adipose stem cells promote neurite outgrowth via secreted exosomes and RNA transfer. Stem Cell Res Ther. 2018; 9: 266. [DOI] [PMC free article] [PubMed] [Google Scholar]