This cohort study investigates the administration patterns and results of urine drug screening to assess its feasibility and patient outcomes in a telehealth-based opioid treatment setting.
Key Points
Question
Can routine urine drug testing be adapted to telehealth-based opioid treatment (TBOT) platforms for the treatment of opioid use disorder?
Findings
In this cohort study of 3395 patients, urine drug testing was highly feasible and sustained throughout the duration of treatment, consistent with in-person settings. Rates of unexpected results were low throughout care.
Meaning
Appropriate monitoring of buprenorphine adherence and patterns of drug use can be successfully adapted to TBOT settings.
Abstract
Importance
Amid rapid and widespread adoption of telehealth-based opioid treatment (TBOT), there is an urgent need for rigorous studies exploring the feasibility and characteristics of urine drug screening (UDS).
Objective
To investigate administration patterns and results of UDS to assess feasibility of UDS and patient outcomes in a TBOT setting.
Design
This observational cohort study was conducted between January 1, 2021, and December 6, 2022, and included patients with opioid use disorder treated in Ophelia, a TBOT treatment platform in 14 states. Data analysis was performed from January to March 2023.
Main Outcomes and Measures
Number and percentage of patients with UDS within 30, 90, and 180 days of intake, grouped by adherence to clinical protocols. Associations were assessed between baseline characteristics and UDS completion and opioid positivity in first 30 days using χ2 tests. Baseline and 180-day follow-up UDS results were compared using McNemar tests.
Results
Among 3395 patients (mean [SD] age, 38.2 [9.3] years, mostly male [54.1%], non-Hispanic White [81.5%], urban-residing [80.3%], and cash-pay at intake [74.0%]), 2782 (83.3%) completed a UDS within 30 days (90.0% among protocol-adherent patients, 67.0% among protocol-nonadherent patients). A total of 2750 of 2817 (97.6%) patients retained more than 90 days completed 1 or more UDS, as did 2307 of 2314 (99.7%) patients retained more than 180 days. Younger patients, patients of a racial and ethnic minority group, those living in urban areas, and cash-pay patients were less likely to complete a UDS in the first 30 days. Buprenorphine positivity increased (from 96.9% to 98.4%, P = .004) and opioid positivity declined (from 7.9% to 3.3%, P < .001) over time.
Conclusions and Relevance
In this cohort study of patients with opioid use disorder receiving buprenorphine in a remote care environment, UDS was highly feasible, though early UDS completion rates varied across demographic subgroups. The prevalence of unexpected UDS results was low and declined over time in treatment.
Introduction
Buprenorphine is the most frequently prescribed medication for opioid use disorder (OUD) with upward of 1 million to 2 million individuals treated annually1 among an estimated 7.6 million individuals with OUD.2 It is the only form of opioid agonist treatment prescribed from a general office setting—known as office-based opioid treatment (OBOT)—and is predominantly prescribed among general practice settings rather than behavioral health specialty settings.3,4 There is consensus among the National Academy of Medicine5 and national health agencies6 that buprenorphine is the first-line treatment for OUD, as it is highly effective at reducing opioid use, overdose, and all-cause mortality and improving quality of life.7,8,9,10
Routine urine drug screening (UDS) to test for illicit substance use and adherence to buprenorphine treatment is commonplace in addiction treatment settings, in part due to billing and reimbursement implications.11 However, there is little consensus about testing schedules and how to respond to results,12 leading to great practice variation and inconsistencies even within the same clinical settings.13,14 While UDS collection is typically routine in heavily monitored clinical trial protocols, which may skew published rates of UDS in the medical literature, these standards do not translate to usual care settings.9,15 A large observational study using administrative claims found that only 80% of patients receiving buprenorphine for OUD completed a UDS at any point in 2016,16 highlighting that UDS administration in OBOT settings is far from universal. Despite widespread use, there is surprisingly sparse empirical literature to guide drug testing in OBOT settings.11,17,18,19 Although there is a general premise that UDS is an important and useful part of OBOT delivery, there is not strong evidence that more frequent UDS improves patient outcomes.9 Furthermore, patients often report that they find routine drug testing (especially when observed) as demeaning and an invasion of privacy,20 and clinicians may be concerned that more frequent testing may undermine retention.21 American Society of Addiction Medicine guidelines for appropriate drug testing encourage UDS as a therapeutic rather than punitive tool and that testing frequency be determined on an individualized basis.22 In turn, some state agencies have discouraged excessive drug testing that is not patient centered as part of routine clinical practice.23 The absence of set protocols and a sole reliance on individualized clinical decisions can increase the risk of disparate testing based on factors like race and socioeconomic status, highlighting the need for more formal guidance.17,24,25
The COVID-19 pandemic instigated federal policy changes that allowed for expanded telehealth-based opioid treatment (TBOT).26 Multiple studies have found that TBOT may be associated with improved patient outcomes (eg, increased retention, decreased medically treated overdoses) relative to in-person OBOT.27,28 Two studies documented decreases in UDS frequency concurrent with the COVID-19 pandemic and the corresponding expansion of TBOT,29,30 likely driven by a reactive decision to reduce patients’ exposure to COVID-19 as opposed to a proactive change in protocols.31 Amid the rapid and widespread adoption of TBOT, there is an urgent need for rigorous studies exploring the feasibility and characteristics of UDS in remote care settings.
To address this need and inform practice and policy in this nascent and evolving space, we investigated the administration and results of UDS among a cohort of patients receiving buprenorphine for the treatment of OUD from a virtual-first TBOT platform. The objective of the study was to assess the feasibility of UDS, patterns of UDS administration, and rates of unexpected results (ie, negative for buprenorphine, positive for other opioids).
Methods
Study Setting and Patient Sample
We analyzed data from a cohort of individuals with OUD treated at Ophelia, a virtual-first TBOT platform in 14 states (although the patient sample was mostly located in 2 states; eTable 1 in Supplement 1). Patients were reached directly through online advertisements or word of mouth. The care model, medical visits and protocol, organizational structure, custom electronic health record, and care coordination services were all designed explicitly for remote care without requiring any in-person visits. In-network patients, predominantly Medicaid beneficiaries, used insurance. Patients who were out of network or uninsured paid $195 monthly to participate in synchronous video-based clinical visits, which did not include pharmacy costs associated with filling buprenorphine prescriptions. Eligible patients were prescribed buprenorphine at intake, seen weekly during the stabilization phase, then stepped down to biweekly and then monthly visits under a nurse care manager model. To be eligible for care, patients had to be English-speaking adults and meet Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition) OUD criteria. Patients were considered ineligible if they required a higher level of care, such as for unstable psychiatric conditions (eg, active suicidality or psychosis), physical dependency on high doses of sedative hypnotics, severe alcohol use requiring detoxification, or enrollment in a methadone program with a dose greater than 80 mg per day.
The study sample included all patients who completed an intake visit between January 1, 2021, and June 6, 2022, received their first buprenorphine prescription within 7 days of their intake, and were retained 30 or more days. The study was reviewed and granted a waiver of informed consent by the Western (WCG) IRB owing to use of deidentified data, and reported in accordance with Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.
UDS Protocol
All patients were sent two 16-panel UDS kits. Subsequent UDS kits were sent to patients throughout the care episode such that they always had at least 1 kit on hand and were expected to have a kit with them at every follow-up appointment. Neither patients nor insurance plans were billed for UDS.
Clinical protocols and company culture encouraged the use of UDS to help assess treatment progress and guide the care team in treatment plan adjustments, foster open communication between patient and clinician, and monitor for buprenorphine diversion. If a patient had unexpected UDS results or was unwilling or could not perform a UDS, the prescribing clinician and care team determined appropriate next steps, which could include increasing visit frequency or, for patients who were previously stable and regularly seen by a nurse care manager, escalating the patient back to regular visits with the prescribing clinician. Patients were not discharged solely due to unexpected UDS results.
The clinical protocol recommended that patients complete UDSs accordingly: approximately 1 week postinduction, when advancing from weekly to biweekly visits (typically 4 weeks postinduction), and when advancing from biweekly to monthly visits. Thereafter patients were expected to complete UDSs quarterly and additionally when clinically indicated. Patients self-administered UDSs off screen during visits, and clinicians visually inspected results and discussed them with the patient in real-time over video. Validity of urine specimens was assessed by confirming that temperature, creatinine, nitrite, pH, oxidants, glutaraldehyde, and specific gravity were all within normal ranges. The UDSs were conducted using the Know Drug Test Cup UDS kit (https://cliawaived.com/know-drug-test-cups.html), except during April to June 2022, when the T-Cup Compact UDS kit (https://transmedco.com/t-cup-compact-16-panel-clia-waived-instant-drug-test-cup-with-etg-fyl-k2-tra-25-box/) was used.
Data Collection and Measures
Basic demographic information (date of birth, sex, gender, race and ethnicity, address) was collected via self-report in an online form prior to intake visits, and payment type at intake (ie, insurance vs cash) and for all visits was collected as part of routine billing. Race and ethnicity categories were modeled after those used by the US Census Bureau and grouped as follows: Hispanic/Latino, non-Hispanic African American, non-Hispanic White, and, due to small sample sizes of additional groups (non-Hispanic Asian, non-Hispanic Native American, non-Hispanic Pacific Islander, non-Hispanic multiple races), non-Hispanic other or multiple races. Patient urbanicity (ie, urban or rural) was defined using US Department of Agriculture rural-urban commuting area codes associated with the patient’s zip code, with codes of 1 to 3 denoting urban and 4 to 10 denoting rural locations.32 Dates and results of administered UDSs were collected as part of routine care. For this analysis, we report results for buprenorphine, opioids, methamphetamine, cocaine, benzodiazepines, amphetamines, alcohol, and cannabis. Treatment retention was defined using buprenorphine prescription data with discontinuation defined as a greater than 30-day gap in medication coverage. Patients were considered retained from the date of their first prescription through their last date covered by medication before a gap, or their last date covered by their most recent prescription for patients who had not discontinued within the study period.
Statistical Analysis
We categorized patients into 1 of 3 groups based on UDS results in their first 30 days of care: opioid negative if the patient had at least 1 UDS and no opioid positive results, opioid positive if the patient had at least 1 opioid positive result, and no UDS (“missing”) if the patient had no UDS results. We examined associations between baseline patient characteristics and these groups using χ2 and Fisher exact tests.
We calculated the number and percentage of patients who completed a UDS within 30, 90, and 180 days of starting treatment among patients retained for those minimums. We also calculated the time to first UDS, as well as the number of UDSs completed within the first 180 days of care among patients retained for 180 or more days.
Because our definition of retention allowed for gaps in buprenorphine coverage of up to 30 days, it is possible that patients retained for 30 days were only actively engaged in care for a small portion of that time, which could affect the likelihood of whether a patient completed a UDS early in care. To explore the association between UDS completion in the first 30 days of care and general patient engagement in care and treatment, we grouped patients by whether or not they were adherent to clinical protocols during that time. Patients were considered protocol adherent in the first 30 days if they completed at least 3 follow-up visits and had 7 or fewer days not covered by buprenorphine. We then assessed the number and percentage of patients who completed a UDS within 30 days, grouped by protocol adherence.
To explore variation in UDS completion by clinician and month in care, we summarized clinician-specific rates of completed UDS per patient-month for each month in care, which is described in eMethods in Supplement 1.
To assess UDS results, we defined baseline UDS results as those corresponding to a patient’s first UDS within the first 30 days of treatment, and follow-up UDS results as those corresponding to a patient’s UDS closest to and within 60 days (before or after) of 180 days after starting treatment. We summarized the results of all baseline UDSs. We compared the results of patients’ baseline and follow-up UDSs using McNemar test among a subsample of patients who were retained for at least 180 days and had both a baseline and follow-up UDS. We also conducted sensitivity analyses in which we redefined follow-up UDSs as those closest to and within 30 days (before or after) and 90 days (before or after) of 180 days in care. Patients included in the analysis comparing baseline and 180-day follow-up UDS results were likely not representative of all patients with baseline UDS results. To explore this, we compared baseline UDS results among patients who were included in the baseline/follow-up analysis (ie, who were retained for ≥180 days and had both a baseline and follow-up UDS) and those who were not using χ2 tests. P values were 2-tailed; level of significance was .05. All statistical analyses were conducted using R statistical software, version 4.2.1 (R Project for Statistical Computing).
Results
A total of 3395 patients completed an intake visit during the study period, received a buprenorphine prescription within 7 days, and were retained in treatment for 30 or more days (Table 1). Patients had a mean (SD) age of 38.2 (9.3) years, and were mostly male (54.1%) and non-Hispanic White (81.5%), largely consistent with prior studies of multisite OBOT populations.33,34,35 Most patients lived in urban areas (80.3%) and paid for their intake visit out of pocket (74.0%). A total of 1517 (44.7%) patients were ever in network while in care.
Table 1. Characteristics of Patients Receiving Telehealth-Based Treatment for Opioid Use Disorder With Buprenorphine by Urine Drug Screen Result in First 30 Days (n = 3395).
| Characteristic | Urine drug screen result in first 30 d of care, No. (%) | P valuec | |||
|---|---|---|---|---|---|
| All patientsa | Opioid negativeb | Opioid positiveb | No urine drug screenb | ||
| Total | 3395 | 2494 | 288 | 613 | |
| Age at intake visit, y | |||||
| <30 | 532 (15.7) | 364 (68.4) | 46 (8.6) | 122 (22.9) | .04 |
| 30-39 | 1567 (46.2) | 1151 (73.5) | 140 (8.9) | 276 (17.6) | |
| 40-49 | 864 (25.4) | 645 (74.7) | 70 (8.1) | 149 (17.2) | |
| 50-59 | 319 (9.4) | 239 (74.9) | 28 (8.8) | 52 (16.3) | |
| ≥60 | 108 (3.2) | 90 (83.3) | 4 (3.7) | 14 (13.0) | |
| Gender | |||||
| Female | 1414 (41.6) | 1042 (73.7) | 125 (8.8) | 247 (17.5) | .94 |
| Male | 1835 (54.1) | 1346 (73.4) | 153 (8.3) | 336 (18.3) | |
| Transgender/nonbinary/other | 41 (1.2) | 32 (78.0) | 3 (7.3) | 6 (14.6) | |
| Race and ethnicity | |||||
| Hispanic/Latino | 217 (6.4) | 142 (65.4) | 21 (9.7) | 54 (24.9) | <.001 |
| Non-Hispanic African American | 136 (4.0) | 82 (60.3) | 17 (12.5) | 37 (27.2) | |
| Non-Hispanic White | 2767 (81.5) | 2080 (75.2) | 229 (8.3) | 458 (16.6) | |
| Non-Hispanic other or multiple racesd | 125 (3.7) | 84 (67.2) | 10 (8.0) | 31 (24.8) | |
| Urbanicity | |||||
| Urban | 2727 (80.3) | 1965 (72.1) | 245 (9.0) | 517 (19.0) | <.001 |
| Rural | 660 (19.4) | 524 (79.4) | 42 (6.4) | 94 (14.2) | |
| Payment type at intake visit | |||||
| Cash | 2513 (74.0) | 1821 (72.5) | 198 (7.9) | 494 (19.7) | <.001 |
| Insurance | 882 (26.0) | 673 (76.3) | 90 (10.2) | 119 (13.5) | |
Column percentages.
Row percentages.
P values calculated using χ2 tests, with the exception of the P value for gender, which was calculated using Fisher exact test due to small cell sizes.
Other races include Asian, Native American, and Pacific Islander.
A total of 2494 patients were opioid negative (73.4%), 288 were opioid positive (8.5%), and 613 (18.1%) did not complete a UDS in the first 30 days of care. Younger patients, patients of racial and ethnic minority groups, those living in urban areas, and those who paid out of pocket were less likely to complete a UDS in the first 30 days of care (Table 1).
A total of 2782 of 3395 (81.9%) patients retained for at least 30 days completed a UDS within the first 30 days of care: 1988 of 2210 (90.0%) protocol-adherent patients and 794 of 1185 (67.0%) protocol-nonadherent patients (Table 2). A total of 2750 of 2817 (97.6%) patients retained for at least 90 days completed a UDS, as did 2307 of 2314 (99.7%) patients retained for at least 180 days. Among patients retained for at least 180 days, the median (IQR) number of UDSs was 3 (3-4), and the median (IQR) time to first UDS was 14 (7-21) days.
Table 2. Urine Drug Screen Completion Among Patients Receiving Telehealth-Based Treatment for Opioid Use Disorder With Buprenorphine.
| Measure | Patients, No. (%) |
|---|---|
| Completed urine drug screen within 30 da | 2782 (81.9) |
| Completed urine drug screen within 30 d, protocol-adherent patientsb | 1988 (90.0) |
| Completed urine drug screen within 30 d, protocol-nonadherent patientsc | 794 (67.0) |
| Completed urine drug screen within 90 dd | 2750 (97.6) |
| Completed urine drug screen within 180 de | 2307 (99.7) |
| No. of urine drug screens in first 180 d, median (IQR)e | 3.0 (3.0-4.0) |
Percentage calculated among all 3395 patients.
Percentage calculated among 2210 protocol-adherent patients.
Percentage calculated among 1185 protocol-nonadherent patients.
Percentage calculated among 2817 patients retained for at least 90 days.
Percentage and median calculated among 2314 patients retained for at least 180 days.
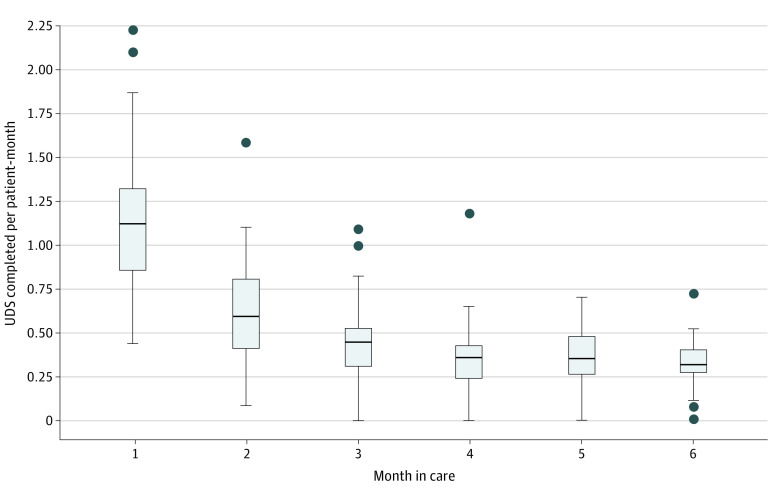
We observed variation in rates of UDS completion across clinicians and months in care, which is presented in the Figure and eTable 2 in Supplement 1.
Figure. Clinician Rates of Completed Urine Drug Screenings (UDS) per Patient-Month, by Month in Care.
The horizontal bar inside the boxes indicates the median, and the lower and upper ends of the boxes are the first and third quartiles. Lower whisker extends to the smallest value no further than 1.5 × IQR from the 25th percentile value, and upper whisker extends to the largest value no further than 1.5 × IQR from the 75th percentile value; shaded circles indicate outlier data points.
Among 2782 patients with a baseline UDS, 96.1% screened positive for buprenorphine, 9.5% for opioids (5.8% for fentanyl, 0.8% for methadone, 2.3% for oxycodone, 0.6% for tramadol, 2.2% for other opioids), 4.9% for methamphetamine, 4.3% for cocaine, 9.2% for benzodiazepines, 10.1% for amphetamines, 12.8% for alcohol, and 39.3% for cannabis (data not shown).
Among 1754 patients retained for 180 days and with both a baseline and follow-up UDS (38% of those with a baseline UDS), there was an increase in the positivity rate for buprenorphine (from 96.9% to 98.4%, P = .004) and a decrease in the positivity rate for opioids (from 7.9% to 3.3%, P < .001), cocaine (from 3.4% to 2.2%, P = .008), and benzodiazepines (from 8.8% to 6.6%, P = .002) (Table 3). Results from sensitivity analyses that defined follow-up UDSs within 30 days (before or after) (eTable 3 in Supplement 1) and 90 days (before or after) (eTable 4 in Supplement 1) of 180 days after starting treatment were largely consistent with the primary definition using 60 days (before or after). Patients included in the baseline/follow-up UDS analysis had a higher baseline positivity rate for buprenorphine and lower baseline positivity rates for opioids, methamphetamine, and cocaine than patients who did not have follow-up UDS data available (eTable 5 in Supplement 1).
Table 3. Urine Drug Screen Results During Baseline (First Urine Drug Screen Within 30 Days of Initiating Treatment) and Follow-Up (Closest Urine Drug Screen to 180 Days After Initiating Treatment, Between 120 and 240 Days After Initiating Treatment) (n = 1754).
| Drug | No. (%) | P valuea | |
|---|---|---|---|
| Baseline | Follow-up | ||
| Buprenorphine | 1699 (96.9) | 1726 (98.4) | .004 |
| Opioids | 138 (7.9) | 58 (3.3) | <.001 |
| Fentanyl | 76 (4.3) | 29 (1.7) | <.001 |
| Methadone | 15 (0.9) | 3 (0.2) | .01 |
| Oxycodone | 36 (2.1) | 20 (1.1) | .02 |
| Tramadol | 8 (0.5) | 6 (0.3) | .77 |
| Other opioids | 33 (1.9) | 10 (0.6) | <.001 |
| Methamphetamine | 66 (3.8) | 54 (3.1) | .14 |
| Cocaine | 60 (3.4) | 38 (2.2) | .008 |
| Benzodiazepines | 155 (8.8) | 116 (6.6) | .002 |
| Amphetamines | 164 (9.4) | 186 (10.6) | .095 |
| Alcohol | 214 (12.2) | 213 (12.1) | 1.00 |
| Cannabis | 680 (38.8) | 654 (37.3) | .17 |
P values calculated using McNemar tests.
Discussion
We found that most patients completed a UDS in the first 30 days of care, and an overwhelming majority completed one within the first 90 days of care, suggesting that drug testing was mostly feasible to conduct over an exclusively telehealth treatment platform without requiring any in-person visits. We also found low rates of opioid use across all time points in care, with additional declines from early to later points in care, which suggests that buprenorphine delivered in TBOT settings is an effective treatment for OUD, and is consistent with favorable retention outcomes observed in this and other TBOT settings.27,28,36,37,38 To our knowledge, this is the first study characterizing UDS administration and results in an exclusively TBOT setting, an important step toward identifying the appropriate role and optimal processes for UDS in remote care settings.
Although TBOT has demonstrated the potential to extend treatment access to underserved areas,38,39 our findings that early UDS completion varied across demographic subgroups suggests that further study of UDS in remote care settings is warranted. As telehealth-based care has expanded in recent years, there have been concerns that the “digital divide” (ie, unequal access to digital technology and Wi-Fi that disproportionately affects marginalized populations) could exacerbate existing disparities related to health care access.40,41 It is thus important for TBOT platforms to consider the complex interplay between clinical suitability for remote care and socioeconomic and digital barriers, aiming to address the latter while not compromising on the former. However, the low rates of unexpected results and declining drug use observed over the first 6 months of care offer further evidence that buprenorphine delivered in TBOT settings is an effective treatment for OUD and suggest that patient-centered drug testing is effective, consistent with prior studies in other settings.42
Limitations
There are several limitations to our analyses that require further study. First, the rate of opioid positivity in our sample was substantially lower than that observed in in-person OBOT settings both early in care and at 6 months,43 which raises questions about how these populations may differ. Also, if patients not completing a UDS were more likely to use opioids (ie, if clinicians were less likely to administer UDS to patients who spoke openly about recent drug use), the rates observed may underestimate the true rates of opioid positivity. Second, patients in TBOT settings and those in our sample in particular may not be representative of all patients with OUD. Specifically, our sample excluded patients requiring a higher level of care and comprised mostly patients residing in New York and Pennsylvania and paying for care out of pocket and thus may not be generalizable to other patient segments. Third, it is possible that remote UDS as described in this study could be more vulnerable to sample tampering than in-person UDS and thus contribute to the low rates of unexpected results. However, we implemented several strategies to mitigate the risk of sample tampering and faked results, including synchronous administration during visits and real-time sample verification and interpretation. Further, the persistence of cannabis positivity, around 38% across time points while in care, replicates prevalence of cannabis use during a similar time period in a prior multisite OBOT study with in-person UDS administration43 and is also theoretically inconsistent with patients regularly procuring substance-negative urine elsewhere to covertly substitute samples. Moreover, while it may seem that in-person UDS samples are largely impervious to the possibility of tampering, 1 study found that 18% of a sample of patients receiving in-person OBOT care submitted urine samples with evidence of tampering.44 This may be true irrespective of treatment setting, and further research is needed to understand whether the reliability of UDS in remote settings is any different than in in-person settings.
Conclusions
There has been a seminal shift in the National Drug Control Strategy and White House prerogatives for responding to the opioid crisis, notably expanding harm reduction services and removing the Drug Enforcement Administration’s X-waiver requirement for buprenorphine prescribers as of January 2023. In the context of ongoing policy debates over responding to the opioid crisis and allowances for telehealth modalities,41,45,46 removing barriers to buprenorphine access via low-threshold or “minimally invasive” care settings,47 aligned with improving equity, has become central.48,49 Largely due to COVID-19 regulatory reforms, the rapid expansion of telehealth for mental health and addiction treatment has bypassed long-standing requirements for initial in-person visits before prescribing controlled substances.50 Typically, TBOT models, similar to emergency department–initiated medication “bridge clinics,” have many fewer requirements—such as group attendance, frequent drug testing, and counseling—than historic standards of care, and yet retention and adherence outcomes appear to be equivalent and possibly superior to those of traditional care settings.27,28,36,37,38 This cohort study’s findings that remotely administered UDS was feasible and associated with low rates of unexpected results that improved with time in care are consistent with the premise that long-standing and stringent requirements (eg, requiring in-person visits, frequent drug testing)51 for being prescribed buprenorphine for OUD can be relaxed without jeopardizing the quality or safety of care for many patients.
eMethods
eTable 1: U.S. state of all patients (n=3,395)
eTable 2: Clinician rates of completed UDS per patient year, by month in care
eTable 3: Urine drug screen results during baseline (first urine drug screen within 30 days of initiating treatment) and follow-up (closest urine drug screen to 180 days after initiating treatment, between 150 and 210 days after initiating treatment) (n=1,178)
eTable 4: Urine drug screen results during baseline (first urine drug screen within 30 days of initiating treatment) and follow-up (closest urine drug screen to 180 days after initiating treatment, between 90 and 270 days after initiating treatment) (n=1,904)
eTable 5: Urine drug screen results during baseline (within 30 days of initiating treatment) by whether or not patient has a urine drug screen during follow-up (between 120 and 240 days after initiating treatment) (n=2,782)
Data Sharing Statement
References
- 1.Olfson M, Zhang VS, Schoenbaum M, King M. Trends in buprenorphine treatment in the United States, 2009-2018. JAMA. 2020;323(3):276-277. doi: 10.1001/jama.2019.18913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keyes KM, Rutherford C, Hamilton A, et al. What is the prevalence of and trend in opioid use disorder in the United States from 2010 to 2019? using multiplier approaches to estimate prevalence for an unknown population size. Drug Alcohol Depend Rep. 2022;3:100052. doi: 10.1016/j.dadr.2022.100052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wen H, Borders TF, Cummings JR. Trends in buprenorphine prescribing by physician specialty. Health Aff (Millwood). 2019;38(1):24-28. doi: 10.1377/hlthaff.2018.05145 [DOI] [PubMed] [Google Scholar]
- 4.Creedon TB, Ali MM, Schuman-Olivier Z. Trends in buprenorphine prescribing for opioid use disorder by psychiatrists in the US from 2003 to 2021. JAMA Health Forum. 2023;4(4):e230221. doi: 10.1001/jamahealthforum.2023.0221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Sciences Policy; Committee on Medication-Assisted Treatment for Opioid Use Disorder; Leshner AI, Mancher M, eds. Medications for Opioid Use Disorder Save Lives. National Academies Press; 2019. [PubMed] [Google Scholar]
- 6.Office of the Secretary, Department of Health and Human Services . Practice guidelines for the administration of buprenorphine for treating opioid use disorder. Published online April 28, 2021. Accessed December 5, 2022. https://www.federalregister.gov/documents/2021/04/28/2021-08961/practice-guidelines-for-the-administration-of-buprenorphine-for-treating-opioid-use-disorder
- 7.Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357:j1550. doi: 10.1136/bmj.j1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santo T Jr, Clark B, Hickman M, et al. Association of opioid agonist treatment with all-cause mortality and specific causes of death among people with opioid dependence: a systematic review and meta-analysis. JAMA Psychiatry. 2021;78(9):979-993. doi: 10.1001/jamapsychiatry.2021.0976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bentzley BS, Barth KS, Back SE, Book SW. Discontinuation of buprenorphine maintenance therapy: perspectives and outcomes. J Subst Abuse Treat. 2015;52:48-57. doi: 10.1016/j.jsat.2014.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larochelle MR, Liebschutz JM, Zhang F, Ross-Degnan D, Wharam JF. Opioid prescribing after nonfatal overdose and association with repeated overdose: a cohort study. Ann Intern Med. 2016;164(1):1-9. doi: 10.7326/M15-0038 [DOI] [PubMed] [Google Scholar]
- 11.Incze MA. Reassessing the role of routine urine drug screening in opioid use disorder treatment. JAMA Intern Med. 2021;181(10):1282-1283. doi: 10.1001/jamainternmed.2021.4109 [DOI] [PubMed] [Google Scholar]
- 12.Bagley SM, Cheng DM, Winter M, et al. Opioid and cocaine use among primary care patients on buprenorphine—self-report and urine drug tests. Drug Alcohol Depend. 2018;192:245-249. doi: 10.1016/j.drugalcdep.2018.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ceasar R, Chang J, Zamora K, et al. Primary care providers’ experiences with urine toxicology tests to manage prescription opioid misuse and substance use among chronic noncancer pain patients in safety net health care settings. Subst Abus. 2016;37(1):154-160. doi: 10.1080/08897077.2015.1132293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gertner AK, Clare HM, Powell BJ, et al. A mixed methods study of provider factors in buprenorphine treatment retention. Int J Drug Policy. 2022;105:103715. doi: 10.1016/j.drugpo.2022.103715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Potter JS, Dreifuss JA, Marino EN, et al. The multi-site prescription opioid addiction treatment study: 18-month outcomes. J Subst Abuse Treat. 2015;48(1):62-69. doi: 10.1016/j.jsat.2014.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sohn M, Talbert JC, Huang Z, Oser C, Freeman PR. Trends in urine drug monitoring among persons receiving long-term opioids and persons with opioid use disorder in the United States. Pain Physician. 2021;24(2):E249-E256. [PMC free article] [PubMed] [Google Scholar]
- 17.Khatri UG, Aronowitz SV. Considering the harms of our habits: the reflexive urine drug screen in opioid use disorder treatment. J Subst Abuse Treat. 2021;123:108258. doi: 10.1016/j.jsat.2020.108258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McEachern J, Adye-White L, Priest KC, et al. Lacking evidence for the association between frequent urine drug screening and health outcomes of persons on opioid agonist therapy. Int J Drug Policy. 2019;64:30-33. doi: 10.1016/j.drugpo.2018.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dupouy J, Mémier V, Catala H, Lavit M, Oustric S, Lapeyre-Mestre M. Does urine drug abuse screening help for managing patients? a systematic review. Drug Alcohol Depend. 2014;136:11-20. doi: 10.1016/j.drugalcdep.2013.12.009 [DOI] [PubMed] [Google Scholar]
- 20.Monwell B, Bülow P, Björn J. The pros and cons of supervised urine tests in opioid replacement therapy: a study of patients’ experiences. Heroin Addict Relat Clin Probl. 2018;20(6):5-15. [Google Scholar]
- 21.Morin KA, Dabous JR, Vojtesek F, Marsh D. Evaluating the association between urine drug screening frequency and retention in opioid agonist treatment in Ontario, Canada: a retrospective cohort study. BMJ Open. 2022;12(10):e060857. doi: 10.1136/bmjopen-2022-060857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jarvis M, Williams J, Hurford M, et al. Appropriate use of drug testing in clinical addiction medicine. J Addict Med. 2017;11(3):163-173. doi: 10.1097/ADM.0000000000000323 [DOI] [PubMed] [Google Scholar]
- 23.New York State Office of Addiction Services and Supports . Guidance on toxicology use in OASAS certified programs. Published online November 24, 2021. Accessed June 25, 2023. https://oasas.ny.gov/system/files/documents/2021/11/oasas-toxicology-guidance_0.pdf
- 24.Becker WC, Starrels JL, Heo M, Li X, Weiner MG, Turner BJ. Racial differences in primary care opioid risk reduction strategies. Ann Fam Med. 2011;9(3):219-225. doi: 10.1370/afm.1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hausmann LRM, Gao S, Lee ES, Kwoh KC. Racial disparities in the monitoring of patients on chronic opioid therapy. Pain. 2013;154(1):46-52. doi: 10.1016/j.pain.2012.07.034 [DOI] [PubMed] [Google Scholar]
- 26.Lin LA, Fernandez AC, Bonar EE. Telehealth for substance-using populations in the age of coronavirus disease 2019: recommendations to enhance adoption. JAMA Psychiatry. 2020;77(12):1209-1210. doi: 10.1001/jamapsychiatry.2020.1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones CM, Shoff C, Hodges K, et al. Receipt of telehealth services, receipt and retention of medications for opioid use disorder, and medically treated overdose among Medicare beneficiaries before and during the COVID-19 pandemic. JAMA Psychiatry. 2022;79(10):981-992. doi: 10.1001/jamapsychiatry.2022.2284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frost MC, Zhang L, Kim HM, Lin LA. Use of and retention on video, telephone, and in-person buprenorphine treatment for opioid use disorder during the COVID-19 pandemic. JAMA Netw Open. 2022;5(10):e2236298. doi: 10.1001/jamanetworkopen.2022.36298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hughes PM, Verrastro G, Fusco CW, Wilson CG, Ostrach B. An examination of telehealth policy impacts on initial rural opioid use disorder treatment patterns during the COVID-19 pandemic. J Rural Health. 2021;37(3):467-472. doi: 10.1111/jrh.12570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huskamp HA, Busch AB, Uscher-Pines L, Barnett ML, Riedel L, Mehrotra A. Treatment of opioid use disorder among commercially insured patients in the context of the COVID-19 pandemic. JAMA. 2020;324(23):2440-2442. doi: 10.1001/jama.2020.21512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uscher-Pines L, Sousa J, Raja P, Mehrotra A, Barnett M, Huskamp HA. Treatment of opioid use disorder during COVID-19: experiences of clinicians transitioning to telemedicine. J Subst Abuse Treat. 2020;118:108124. doi: 10.1016/j.jsat.2020.108124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Economic Research Service, US Dept of Agriculture . Rural-urban continuum codes. Published online December 10, 2020. Accessed December 20, 2022. https://www.ers.usda.gov/data-products/rural-urban-continuum-codes.aspx
- 33.Samples H, Williams AR, Olfson M, Crystal S. Risk factors for discontinuation of buprenorphine treatment for opioid use disorders in a multi-state sample of Medicaid enrollees. J Subst Abuse Treat. 2018;95:9-17. doi: 10.1016/j.jsat.2018.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams AR, Samples H, Crystal S, Olfson M. Acute care, prescription opioid use, and overdose following discontinuation of long-term buprenorphine treatment for opioid use disorder. Am J Psychiatry. 2020;177(2):117-124. doi: 10.1176/appi.ajp.2019.19060612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manhapra A, Agbese E, Leslie DL, Rosenheck RA. Three-year retention in buprenorphine treatment for opioid use disorder among privately insured adults. Psychiatr Serv. 2018;69(7):768-776. doi: 10.1176/appi.ps.201700363 [DOI] [PubMed] [Google Scholar]
- 36.Lin LA, Zhang L, Kim HM, Frost MC. Impact of COVID-19 telehealth policy changes on buprenorphine treatment for opioid use disorder. Am J Psychiatry. 2022;179(10):740-747. doi: 10.1176/appi.ajp.21111141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weintraub E, Greenblatt AD, Chang J, et al. Outcomes for patients receiving telemedicine-delivered medication-based treatment for opioid use disorder: a retrospective chart review. Heroin Addict Relat Clin Probl. 2021;23(2):5-12. [PMC free article] [PubMed] [Google Scholar]
- 38.Williams AR, Aronowitz S, Gallagher R, Behar E, Gray Z, Bisaga A. A virtual-first telehealth treatment model for opioid use disorder. J Gen Intern Med. 2023;38(3):814-816. doi: 10.1007/s11606-022-07955-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weintraub E, Greenblatt AD, Chang J, Himelhoch S, Welsh C. Expanding access to buprenorphine treatment in rural areas with the use of telemedicine. Am J Addict. 2018;27(8):612-617. doi: 10.1111/ajad.12805 [DOI] [PubMed] [Google Scholar]
- 40.Eyrich NW, Andino JJ, Fessell DP. Bridging the digital divide to avoid leaving the most vulnerable behind. JAMA Surg. 2021;156(8):703-704. doi: 10.1001/jamasurg.2021.1143 [DOI] [PubMed] [Google Scholar]
- 41.Uscher-Pines L, Jones M, Sousa J, Predmore Z, Ober A. The doctor will call me maybe: the uncertain future of audio-only visits and why we need them to address disparities. Health Affairs Forefront. Published online March 3, 2021. Accessed June 25, 2023. doi: 10.1377/forefront.20210225.26462 [DOI]
- 42.Sobel HG, Warrington JS, Francis-Fath S, Crocker AM, Berger CA. A descriptive analysis of urine drug screen results in patients with opioid use disorder managed in a primary care setting. Addict Sci Clin Pract. 2021;16(1):59. doi: 10.1186/s13722-021-00264-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams AR, Mauro CM, Feng T, et al. Non-prescribed buprenorphine preceding treatment intake and clinical outcomes for opioid use disorder. J Subst Abuse Treat. 2022;139:108770. doi: 10.1016/j.jsat.2022.108770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Accurso AJ, Lee JD, McNeely J. High prevalence of urine tampering in an office-based opioid treatment practice detected by evaluating the norbuprenorphine to buprenorphine ratio. J Subst Abuse Treat. 2017;83:62-67. doi: 10.1016/j.jsat.2017.10.002 [DOI] [PubMed] [Google Scholar]
- 45.Poorman E. The number needed to prescribe—what would it take to expand access to buprenorphine? N Engl J Med. 2021;384(19):1783-1784. doi: 10.1056/NEJMp2101298 [DOI] [PubMed] [Google Scholar]
- 46.Fiscella K, Wakeman SE, Beletsky L. Buprenorphine deregulation and mainstreaming treatment for opioid use disorder: X the X waiver. JAMA Psychiatry. 2019;76(3):229-230. doi: 10.1001/jamapsychiatry.2018.3685 [DOI] [PubMed] [Google Scholar]
- 47.Englander H, Gregg J, Levander XA. Envisioning minimally disruptive opioid use disorder care. J Gen Intern Med. 2023;38(3):799-803. doi: 10.1007/s11606-022-07939-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hayes BT, Jakubowski A, Fitzsimmons C, Garcia B, Ramirez F, Fox AD. “The doctor says you cannot have [buprenorphine]” autonomy and use of prescribed or non-prescribed buprenorphine. Subst Use Misuse. 2021;56(8):1137-1143. doi: 10.1080/10826084.2021.1908360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lister JJ, Weaver A, Ellis JD, Himle JA, Ledgerwood DM. A systematic review of rural-specific barriers to medication treatment for opioid use disorder in the United States. Am J Drug Alcohol Abuse. 2020;46(3):273-288. doi: 10.1080/00952990.2019.1694536 [DOI] [PubMed] [Google Scholar]
- 50.Alegría M, Frank RG, Hansen HB, Sharfstein JM, Shim RS, Tierney M. Transforming mental health and addiction services. Health Aff (Millwood). 2021;40(2):226-234. doi: 10.1377/hlthaff.2020.01472 [DOI] [PubMed] [Google Scholar]
- 51.Martin SA, Chiodo LM, Bosse JD, Wilson A. The next stage of buprenorphine care for opioid use disorder. Ann Intern Med. 2018;169(9):628-635. doi: 10.7326/M18-1652 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
eTable 1: U.S. state of all patients (n=3,395)
eTable 2: Clinician rates of completed UDS per patient year, by month in care
eTable 3: Urine drug screen results during baseline (first urine drug screen within 30 days of initiating treatment) and follow-up (closest urine drug screen to 180 days after initiating treatment, between 150 and 210 days after initiating treatment) (n=1,178)
eTable 4: Urine drug screen results during baseline (first urine drug screen within 30 days of initiating treatment) and follow-up (closest urine drug screen to 180 days after initiating treatment, between 90 and 270 days after initiating treatment) (n=1,904)
eTable 5: Urine drug screen results during baseline (within 30 days of initiating treatment) by whether or not patient has a urine drug screen during follow-up (between 120 and 240 days after initiating treatment) (n=2,782)
Data Sharing Statement