Abstract
Retinal diseases are one of the leading causes of blindness globally. The mainstay treatments for these blinding diseases are laser photocoagulation, vitrectomy, and repeated intravitreal injections of anti-vascular endothelial growth factor (VEGF) or steroids. Unfortunately, these therapies are associated with ocular complications like inflammation, elevated intraocular pressure, retinal detachment, endophthalmitis, and vitreous hemorrhage. Recent advances in nanomedicine seek to curtail these limitations, overcoming ocular barriers by developing non-invasive or minimally invasive delivery modalities. These modalities include delivering therapeutics to specific cellular targets in the retina, providing sustained delivery of drugs to avoid repeated intravitreal injections, and acting as a scaffold for neural tissue regeneration. These next-generation nanomedicine approaches could potentially revolutionize the treatment landscape of retinal diseases. This review describes the availability and limitations of current treatment strategies and highlights insights into the advancement of future approaches using next-generation nanomedicines to manage retinal diseases.
Keywords: nanomedicines, retina, nanoparticles, nanostructured scaffolds, nanodevices, nanodelivery
1. Introduction
The retina is a multi-layered neural tissue with a complex cellular organization at the back of the eye. It senses light and converts it into electrical signals, which the brain perceives for vision. Any dysfunction or abnormality of the retina can give rise to temporary or irreversible vision loss [1]. Therefore, globally, retinal diseases significantly cause ocular morbidity and visual impairment. The prevalence of retinal diseases ranges from 5.35% to 21.02% at age 40 years and above [2]. In developed countries, retinal diseases are the most common cause of irreversible blindness. The major retinal diseases are age-related macular degeneration (AMD), diabetic retinopathy (DR), ischemic retinopathy, proliferative vitreoretinopathy (PVR), and inherited retinal disorders (IRDs) [3]. Current treatment strategies for these retinal disorders are invasive and often associated with side effects such as raised intraocular pressure, corneal and crystalline lens irregularities, vitreous hemorrhage, or damage to retinal cells.
Nanomedicine presents a new perspective, embodying nanotechnology, nanodevices, and nanomaterials for tissue repair and drug delivery for the management of retinal diseases. It utilizes materials with a 1–100 nm dimension to function at the cellular and molecular level [4]. In recent years, nanomedicine has evolved dramatically for managing ocular diseases, providing tools for tissue engineering, implants, and drug delivery. This review highlights the recent advances in nanomedicine for managing vision-impairing retinal diseases.
2. Retinal Diseases and Current Treatment Strategies
Retinal diseases are the leading cause of blindness and low vision worldwide, resulting in a considerable economic burden and poor quality of life [5,6]. Common retinal diseases, current treatment strategies, and their limitations are described in Table 1.
Table 1.
Retinal diseases, current treatment strategies, and their limitations.
| Retinal Diseases | Current Treatment Strategies | Limitations |
|---|---|---|
| Diabetic retinopathy (DR) and ischemic retinopathy (IR) |
Intravitreal injection of anti-VEGF * | Complexities and treatment burden (monthly visits) associated with multiple intravitreal injections and treatment non-responders [7,8,9] |
| Laser photocoagulation | Causes permanent damage to the retinal cells, can leave scotomas/blind spots [7,10] | |
| Vitrectomy | Surgical and anesthesia risks, post-operative infection/inflammation or retinal detachment [7,11] | |
| Age-related macular degeneration (AMD) | Lutein + zeaxanthin | Daily administration may lead to adherence problems, cost can be a concern [12] |
| Intravitreal injection of anti-VEGF | Risk and treatment burden associated with multiple intravitreal injections [9] | |
| Photodynamic therapy | Limited efficacy [13] | |
| Retinopathy of prematurity (ROP) and proliferative vitreoretinopathy (PVR) |
Cryotherapy and laser photocoagulation | Cornea, iris, and lens burns, hyphema [14] |
| Intravitreal injection of anti-VEGF | Complexities associated with multiple intravitreal injections and retinal detachment [9,14,15] | |
| Vitrectomy | Vitreous or subretinal hemorrhage [16] | |
| Retinopathy of prematurity (ROP) and inherited retinal diseases (IRDs) |
Pre-retinal membrane removal or retinal reattachment surgery | Reoccurrence of retinal fibrosis [17] |
| No treatment available, gene therapy and stem cell therapy are under investigation | Issues related to viral gene therapy. Direct administration of stem cells may have difficulty with integration [18] |
* VEGF—Vascular endothelial growth factor.
DR is a common cause of vision loss in people with prolonged diabetes due to neurovascular complications. In current treatment strategies, neurodegeneration is often overlooked. Treatment options for DR include anti-VEGF injections into the eye, topical corticosteroid eye drops, laser photocoagulation, or vitreous surgery [7]. These intravitreal injections may require repeated doses, resulting in several ocular complications. Additionally, several patients may not respond to anti-VEGF therapy [7,8]. The financial burden and poor patient compliance also limit the effective use of intravitreal injections. Laser photocoagulation may also be performed to shrink problematic blood vessels or vitrectomy surgery. However, this invasive treatment strategy may result in discomfort, and multiple procedures may be required to protect against vision loss [7]. Furthermore, laser photocoagulation is a destructive procedure that causes permanent damage to the retina while preserving functional vision and may result in reduced night vision and field of view [10].
AMD is the leading cause of central vision loss in the aged population. Macular damage can result in poor contrast, blurry central vision, or the perception of wavy lines. The Age-Related Eye Disease Study 2 (AREDS 2) found that Lutein + zeaxanthin and omega-3 fatty acid supplementation may help slow the progression of early-stage AMD. However, these supplements can interfere with other medications and should be carefully discussed with a medical professional before consumption [12]. Anti-VEGF injections and photodynamic therapy (PDT) are other options for treating late-stage AMD. However, unlike DR, anti-VEGF injections require multiple doses due to their short durability, and PDT is infrequently used alone due to its inadequacy and unpredictability [13].
Ischemic retinopathy (IR) occurs due to an inadequate blood supply to the retina. IR leads to alterations in retinal metabolic functions and can result in vision loss and blindness. Common causes of retinal ischemia include central and branch artery or vein occlusion and DR [19]. The treatment options available for ischemic retinopathies are anti-VEGF injections, laser treatment, and vitrectomy [20]. Another ischemic disease is retinopathy of prematurity (ROP), a condition caused by the underdevelopment of retinal vessels in prematurely born infants. ROP is characterized by the proliferation of abnormal fibrovascular tissue and vessels at the border of the vascularized and non-vascularized retina. ROP treatment strategies include cryotherapy, laser photocoagulation, and anti-VEGF injections [14]. Unfortunately, these treatments have been associated with undesirable complications, such as increased intraocular pressure, vitreous hemorrhage, choroidal or retinal detachment, and the formation of choroidal neovascular membranes [21,22].
PVR is the proliferative growth of contractile membranes in the vitreous or retina that may result in tractional retinal detachment. Surgical intervention is the only management option for PVR. Vitrectomy and pre-retinal membrane removal are commonly performed procedures for PVR treatment. Also, intravitreal injections of corticosteroids or anti-proliferative agents may prevent PVR [16]. However, one of the major problems after retinal surgery is the re-occurrence of retinal fibrosis. Pharmacological intervention to inhibit vitreoretinal scarring, either preceding or following retinal reattachment surgery, promises to reduce PVR and enhance the surgical success rate and visual outcomes [17]. Nevertheless, surgery remains the primary treatment option for PVR, and post-operative complications, including ocular hypertension, subretinal hemorrhage, corneal or lens opacification, and recurrent retinal detachment, may still occur.
RP is a genetic disorder characterized by the degeneration of retinal photoreceptors, resulting in night blindness and progressive loss of vision. There is currently no treatment for RP [18]. Management of RP involves the use of low vision aids to maximize existing vision and vitamin A supplementation to slow the progression of the disorder [23]. However, there are various forms of RP, with no universal treatment targeting all forms of RP. Gene therapy trials are ongoing, using modified viral vectors to target specific retinal cell types [24]. However, the dosage, efficacy, specificity, and long-term effects of this therapy are yet to be determined. Stem cell therapy has also been explored to replace photoreceptors by growing them in vitro and implanting them into the subretinal space [18]. However, stem cell therapies for RP are still in the early stages of development.
Next-generation nanomedicines are under development to overcome several limitations associated with the currently available treatment strategies. Nanomedicines have immense potential to inhibit neurodegeneration and prevent vascular complications. They can also act as scavengers for reactive oxygen species, enhance the specificity and durability of current therapeutics, or even act as a drug delivery system to deliver a sustained release of individual or combinatory drugs.
3. Nanomedicine Approaches for Retinal Diseases
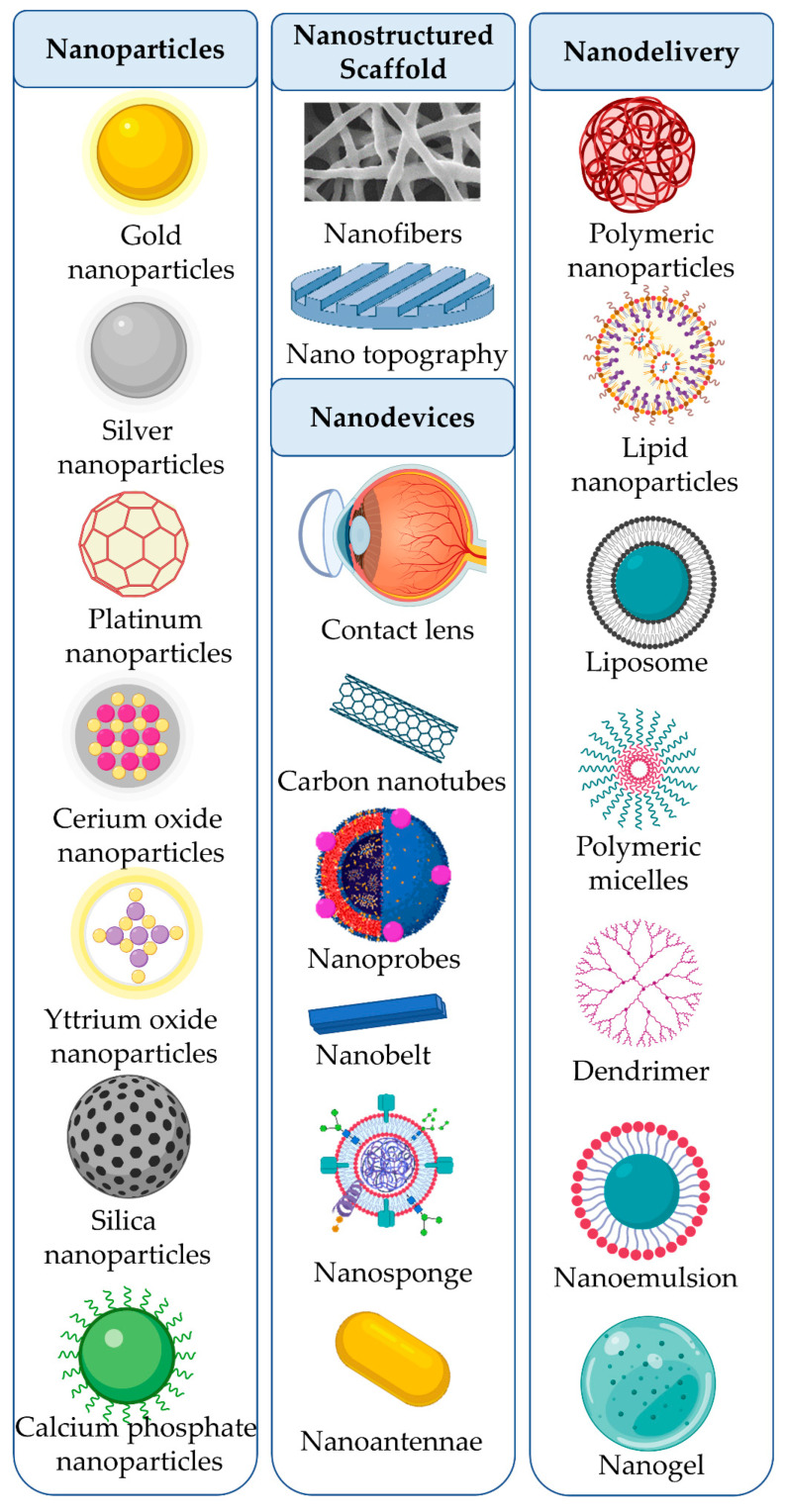
Nanomedicine is an emerging field of medical sciences that has been used for imaging, diagnosis, biosensors, tissue engineering, and drug delivery. Nanomedicine can be classified into (i) nanoparticles, (ii) nanostructured scaffolds, (iii) nanodevices, and (iv) nanodelivery systems. The diversity of the materials used to synthesize nanomedicines allows for manipulating the shape, size, surface properties, and ultimately their use as next-generation therapeutics for retinal diseases. A significant challenge for drug absorption lies with the limited time the drug stays on the ocular surface. Many factors contribute to this problem, including rapid tear turnover, blinking, nasolacrimal drainage, and systemic absorption. As a result, the amount of bioavailable drug within the eye is <5%, and penetration into intraocular tissues is <0.001%. In the following section, current advancements in nanomedicines for the treatment of retinal diseases are described. Table 2 and Figure 1 describe the next-generation nanomedicines available for managing retinal diseases.
Table 2.
Next-generation nanomedicines and their potential applications in retinal diseases.
| Nanomedicines | Applications | Refs. |
|---|---|---|
| Gold nanoparticles |
|
[25,26,27,28] |
| Silver nanoparticles |
|
[29,30] |
| Platinum, cerium oxide and yttrium oxide nanoparticles |
|
[31,32,33] |
| Iron oxide nanoparticles |
|
[34,35,36] |
| Silica nanoparticles, carbon nanospheres, calcium phosphate nanoparticles |
|
[37,38,39,40] |
| Nanofibers |
|
[4,41,42,43] |
| Nano topography |
|
[42,44] |
| Contact lens |
|
[45] |
| Carbon nanotubes |
|
[46,47] |
| Nanoprobes |
|
[48] |
| Selenium nanobelts |
|
[49] |
| Nanosponge |
|
[50,51] |
| Nanoantennae |
|
[52] |
| Polymeric nanoparticles Dendrimers |
|
[8,53,54,55,56,57,58,59,60,61,62,63] |
| Lipid nanoparticles Liposomes |
|
[64,65,66,67,68,69,70,71,72,73] |
| Polymeric micelles |
|
[74,75,76,77,78,79] |
| Nanoemulsion Nanogels |
|
[80,81,82,83,84,85,86] |
* BRB—Blood–retinal barrier.
Figure 1.
Schematic diagram depicting next-generation nanomedicine for the management of retinal diseases.
3.1. Nanoparticles
Nanoparticles range from 1–100 nm in size and can be classified as metallic or non-metallic [4]. These nanoparticles can act as therapeutic agents by targeting systems or carriers for multiple ligands such as DNA, antibodies, peptides, molecular sensors, therapeutic molecules, and probes for retinal diseases.
3.1.1. Metallic Nanoparticles
Metallic nanoparticles, such as gold, silver, platinum, cerium oxide, and yttrium oxide, have been explored as therapeutic agents for retinal diseases. Other metals, such as iron, cobalt, and nickel-based nanoparticles, have also been studied for their magnetic properties to target specific cells or to overcome the blood–retinal barrier [34,35,36].
Gold nanoparticles are highly suited for treating retinal diseases, as they can pass through the anatomical and physical barriers of the eye, such as the ocular surface, cornea, conjunctiva, sclera, choroid, and blood–retinal barriers. Additionally, when applied as eye drops, they can pass through dynamic barriers such as tear fluid, lymphatic blood vessels, and conjunctival and choroidal blood vessels [25]. The administration of these nano-invasive drugs can overcome the complexities associated with repeated intravitreal injections, such as increased intraocular pressure, endophthalmitis, cataracts, retinal toxicity, inflammation, poor localization, and retinal detachment [25,87]. Furthermore, intravenous injection of 20-nm gold nanoparticles can cross the blood–retinal barrier without causing inflammation or toxicity [26,88]. Gold nanoparticles have also been reported to inhibit retinal neovascularization in a mouse model of ROP by blocking VEGF-induced auto-phosphorylation of VEGFR-2 to inhibit ERK 1/2 activation [27]. Additionally, gold nanoparticles are able to inhibit vascular permeability and VEGF-induced cell migration [28].
Silver nanoparticles can also inhibit VEGF-induced endothelial cell migration and proliferation by acting on the PI3/AKT signaling pathway [29]. Additionally, they can inhibit VEGF and IL-1β-induced vascular permeability by acting on the Src signaling pathway [30]. These findings are essential, as many chronic retinal diseases, such as DR and AMD, are associated with vascular permeability and abnormal angiogenesis. Furthermore, other metallic nanoparticles, such as platinum, cerium oxide, and yttrium oxide, display antioxidant properties and inhibit oxidative stress-induced apoptosis in retinal cells [31,32,33]. Thus, these nanoparticles may effectively regulate the progression of oxidative stress-induced cell death in macular degeneration, RP, ischemic retinopathy, retinal vascular occlusion, DR, and other blinding diseases.
Iron oxide superparamagnetic nanoparticles can magnetize stem cells and can be delivered to the inner and outer retina using a magnetic field after intravitreal or intravenous injection [34]. This method allows for targeted drug delivery of particular cell types, such as those found in the retinal pigment epithelium (RPE) [35]. Furthermore, a transient, reversible enhancement of blood–retinal barrier breakdown using magnetic nanoparticles can enhance drug bioavailability in the retina when applied systemically. Ferritin-modified iron oxide magnetic nanoparticles have been explored to enhance drug bioavailability during induced transient hyperthermia [36]. However, dosage optimization to avoid neuronal toxicity is crucial. Nevertheless, administering drugs to the retina less invasively might be a promising strategy.
3.1.2. Non-Metallic Nanoparticles
Non-metallic nanoparticles, like mesoporous silica, carbon nanospheres/nanodiamonds, and calcium phosphate, have recently been explored for drug delivery due to their biocompatible and bioresorbable properties [37,89,90,91]. Mesoporous silica nanoparticles have a large surface area, uniform pore size, high pore volume, and excellent ocular biocompatibility for retinal drug delivery. Immunomodulatory drugs like tacrolimus, antiangiogenic drugs like bevacizumab, and anticancer drugs like topotecan can be encapsulated for targeting the retina using non-metallic nanoparticles [38,39,89]. Conjugating these nanoparticles with target moieties on the surface of mesoporous silica nanoparticles further enhances cellular uptake, thereby increasing the specificity and bioavailability of the drug. For example, folic acid-modified mesoporous silica nanoparticles significantly enhanced cellular uptake by retinoblastoma cells and exhibited superior anticancer efficacy [39]. Surface modification like PEGylation and pullulan modification with ultrasound can also enhance drug retention in the blood circulation and the tumor microenvironment [37,90]. Additionally, biodegradable calcium phosphate nanoparticles loaded with hypotensive agents like 7-hydroxy-2-dipropyl-aminotetralin and lisinopril showed long-term anti-glaucoma effects by reducing the intraocular pressure in experimental animal models [40,91].
3.2. Nanostructured Scaffolds
Neurodegeneration is a significant consequence in the progression of retinal diseases such as AMD, DR, RP, and Stargardt’s disease. Currently, no established treatment strategies exist to stop or reverse the degenerative process completely, or to reinstate regular retinal function to restore vision [92]. Cell therapy seems to be a promising strategy to overcome this problem [93]. However, the success of retinal transplantation by injecting cell suspensions has limited cell survival and lacks cellular integration [92]. Nanostructured scaffolds such as nanofibers, nanoporous gels, nanotopography, and 3D printing can support cell adhesion, survival, proliferation, migration, differentiation, and integration, and improve visual function [92,93,94,95,96].
3.2.1. Nanofibers
Nanofibers have been used as scaffolds for retinal tissue engineering and as carriers for the controlled delivery of drugs, proteins, and DNA to the retina. These nanofibers can be fabricated through electrospinning, self-assembly, and phase separation [4,97]. Nanofibers fabricated from natural and synthetic polymers have been explored for achieving cellular viability, differentiation, and integration in the retina. Transplantation in the RPE is a promising strategy for the management of AMD and Stargardt’s disease. However, the AMD-affected Bruch’s membrane may not be sufficient to support the growth of transplanted RPE cells. Ultrathin electrospun 3D nanofibrous membranes from collagen type I and poly(lactic-co-glycolic) acid (PLGA), mimicking Bruch’s membrane, have been explored to grow the RPE monolayer. Interestingly, human RPE cells grown on nanofibrous membranes exhibited correct orientation, polygonal cell shape, microvilli on their apical surfaces, and tight junctions, similar to the native human RPE. Furthermore, the cells expressed RPE65 protein, which is crucial for RPE maintenance [41]. Nanofibers from natural materials like pectin-modified polyhydroxy butyrate or soy protein have similar effects on human RPE cells, ARPE-19 cells, and stem cell-derived RPE cells, demonstrating that natural matrigels can be explored for RPE tissue engineering [43,93]. Additionally, electrospun polypyrrole–graphene-modified PLGA nanofibers have shown promising results for optic nerve regeneration in vitro [42]. However, in vivo transplantation of these cells/scaffolds has yet to be performed in the retina.
3.2.2. Nanotopography
Optic neuropathies, such as glaucoma and Leber’s hereditary optic neuropathy, can result in retinal ganglion cell (RGC) death. The major challenges for RGC transplantation are maintaining cell survival and controling axon orientation to target the optic nerve head. Axonal growth of retinal cells can be guided by micro/nanopattern topography on a crafted material surface. Topography plays a vital role in neurite growth and orientation. Electrospinning and 3D printing are suitable methods for generating nanotopograhy [42,44,95]. In a previous study, RGCs were seeded on randomly arranged polypyrrole–graphene-modified PLGA nanofibers to obtain neurite growth of 80–100 µm. Additionally, the authors showed that the aligned fibers encouraged neurite growth extension up to 140 µm, along with the orientation of the fibers [42]. Gallium phosphide nanowires of different geometries can also support the long-term survival of retinal cells and the elongation of neuronal outgrowth. Cells clustered on long nanowires significantly increased neurite outgrowth compared to those clustered on short nanowires [44]. These nanowires also support synapse formation significantly better than a flat surface, suggesting the importance of topography in retinal tissue engineering [44]. In another study, poly(ethylene-co-vinyl acetate) was designed with parallel straight grooves using 3D imprinting lithography [95]. Human-induced pluripotent stem cell (iPSC)-derived RGCs grown on this scaffolding displayed organized axons, which projected axially. These studies highlight the importance of topography on neurite growth and axon guidance. However, further in vivo evaluation is still required to determine its functionality and integration into the eye.
3.3. Nanodevices
Nanodevices have great potential for treating retinal diseases. In recent years, nanodevices have been developed for sustained drug delivery, retinal tissue engineering, and as interface materials for retinal recording, biosensors, photosensors, neutralization of pore-forming toxins, and infrared vision. The nanodevices that have been explored for retinal use include contact lenses, carbon nanotubes, nanoprobes, nanobelts, nanosponges, and nanoantennae.
Drug delivery to the retina using contact lenses is an innovative strategy that overcomes the toxicity issues associated with intravitreal injection. It also has enhanced bioavailability compared to topical eye drops, as it overcomes tear dilution, reflex blinking, and rapid fluid drainage that collectively reduces the drug’s retention time on the ocular surface. Dexamethasone–PLGA contact lenses have shown sustained delivery of the drug to the retina and successfully inhibited retinal vascular leakage induced by intravitreal injection of VEGF in a rabbit model. The safety and biocompatibility of these contact lenses were found to be suitable for four weeks [45].
Carbon nanotubes are an attractive option for retinal tissue engineering and as an interface material for retinal function. Human RGCs grown on carbon nanotubes showed enhanced growth, physiological synaptic activity, and biocompatibility [98]. In addition, vertically aligned carbon nanotubes showed increased cell viability, proliferation, neurite outgrowth, biocompatibility, and mechanical integrity [46,47]. However, a critical review of the biological mechanisms of carbon nanotubes reported the possibility for toxicity [99]. As such, further studies are required to determine if carbon nanotubes are safe for use. One intriguing use of nanoprobes is the development of an alizarin red aluminum(III) complex conjugated to graphene oxide, which was successful in detecting intracellular lysine residues. This study provided an excellent model for monitoring lysine in retinal diseases using fluorescence spectroscopy [48]. Similarly, single-crystal selenium nanobelts with a large surface area, fine photoconductivity, and biocompatibility showed immense potential for developing implantable artificial retinas or rapid photon detector/stimulator for optogenetics. However, in vivo evaluation is still required [49].
During ocular infection, microorganisms secrete pore-forming toxins that cause damage to retinal function. Biomimetic nanosponges derived from erythrocytes showed promising neutralizing effects on the cytolysin secreted by Enterococcus faecalis and pore-forming toxins produced from Staphylococcus aureus, Enterococcus faecalis, Streptococcus pneumoniae, and Bacillus cereus, encouraging the use of nanosponges as adjunctive therapies for the treatment of ocular infections [50,51]. One of the most interesting findings was the development of nanoantennae to visualize near-infrared light. The nanoantennae consisted of photoreceptor-binding upconversion nanoparticles that acted as near-infrared light transducers. Mice injected with nanoantennae could differentiate sophisticated near-infrared shape patterns and showed no potential toxicity [52]. These developments demonstrate potential therapeutic opportunities for emerging bio-integrated nanodevice designs and applications.
3.4. Nanostructure-Based Drug Delivery Systems
One of the most critical advances in nanomedicine is the development of nanoparticle-based drug delivery systems for retinal diseases. These newer delivery systems are anticipated to prevent the drawbacks of conventional drug administration. Nanostructure-based drug delivery systems will (i) provide a non-invasive drug delivery system that can be applied via eye drops, which cross the ocular barriers to reach the retina for enhanced bioavailability; (ii) provide targeted systemic delivery to overcome the blood–retinal barriers, thereby reducing the drug dosage and overcoming systemic barriers; (iii) increase the half-life of the drug to prevent any complexities associated with repeated intraocular injections; (iv) aid in sustained drug delivery for the long durations required to treat chronic diseases like DR and AMD; (v) enhance the versatility of the drug delivery system to allow for the delivery of hydrophilic and hydrophobic drugs, proteins, peptides, DNA or RNA, and aptamers; and (vi) help modulate stimulus-responsive drug delivery systems. In this context, the nanostructure-mediated drug delivery systems that have been explored are polymeric nanoparticles, lipid nanoparticles, liposomes, polymeric micelles, dendrimers, nanoemulsions, and nanogels.
3.4.1. Polymeric Nanoparticles
Polymeric nanoparticles are used for the retinal delivery of drugs ranging from 10–1000 nm in size [4]. Polymeric nanoparticles show excellent bioavailability, biocompatibility, and biodegradability in the retina. There are different types of polymeric nanoparticles, such as nanocapsules or nanospheres, in which a drug can be entrapped/encapsulated or dissolved in a matrix. The ability of drug-loaded nanoparticles to overcome physiological barriers and redirect drugs to the site of action through passive diffusion or targeted mechanisms has become an adaptive method for researchers in designing polymeric nanoparticulate delivery systems [100,101]. Polymeric nanoparticulate drug carriers offer various advantages, such as imparting better stability than lipidic carriers, preventing entrapped drugs from degradation, releasing drugs sustainably, and allowing better penetration through biological barriers. However, despite numerous advantages, polymeric nanoparticles have limitations, such as toxicity and slow clearance [102,103].
Biodegradable polymers play an essential role in the formulation of polymeric nanoparticles, and particle size (nanoscale) is critical for ophthalmic formulations to avoid patient discomfort. After applying nanoparticles as eyedrops, the nanoparticles need to be retained in the ocular cul-de-sac to allow for adequate retention and achieve sustained drug release. Hence, designing nanoparticles with biomaterials is vital to retain the drug appropriately. Many biodegradable polymers have been developed to improve ocular drug delivery, including chitosan, hyaluronic acid (HA), PLGA, polyacrylamides, polylactic acid (PLA), poly ε-caprolactone, and albumin [104,105,106]. Chitosan, a mucoadhesive polymer, has been used to improve the retention time of nanoparticles. This improved retention time has been attributed to chitosan’s ability to develop strong molecular attractions between the negatively charged sialic acid residues of mucin and chitosan’s positive amino acid groups [107]. The various polymers utilized in the preparation of polymeric nanoparticles for the management of retinal diseases are described in Table 3.
Table 3.
Polymers used for preparing polymeric nanoparticles and their potential applications in retinal diseases.
| Polymers | Method of the Preparation | Characterization | Applications | Refs. |
|---|---|---|---|---|
| N-isopropylacrylamide and acrylamide | Radical polymerization |
|
|
[108] |
| PLGA-PEG | Double emulsion solvent evaporation |
|
|
[109] |
| Chitosan-coated PLGA | Oil-in-water emulsion |
|
|
[110] |
| Chitosan functionalized PLGA | Nanoprecipitation |
|
|
[111] |
| Bovine serum albumin, hyaluronic acid. | Desolvation |
|
|
[112] |
| PLGA-hyaluronic acid | Solvent displacement |
|
|
[113] |
| Polydopamine | Classical Stober method |
|
|
[114] |
| Thiolated and methylated chitosan carboxymethyl dextran | Coacervation |
|
|
[115] |
Polymer nanoparticles can be fabricated using various methods, such as solvent evaporation, desolvation, emulsification, and ionotropic gelation. Several polymers have been utilized to deliver chemical entities, such as drugs, peptides, genes, and proteins, to the target tissues. However, selecting appropriate polymers with a good biocompatibility and biodegradability has been critical for nanoparticle design [105]. Physicochemical properties, such as size, surface charge, hydrophobicity, surface functionality, and mucoadhesive properties, can be controlled to make the nanoparticles more suitable for retinal drug delivery [116,117]. Polymeric nanoparticles with hydrophobic cores and hydrophilic mucoadhesive shells have been delivered to the retina. One example demonstrated that applying triamcinolone acetonide-loaded nanoparticles as eye drops significantly inhibited inflammation, neurodegeneration, and angiogenesis in a DR rat model [8]. Furthermore, a dual drug delivery system developed by the core–shell nanoparticle system showed superior anti-inflammatory and antibacterial properties in vitro, demonstrating a promising strategy for developing non-invasive therapies for endophthalmitis [21]. In addition, a single intravenous injection of PLGA nanoparticles modified with arginine–glycine–aspartate (RGD) peptide significantly reduced angiogenesis and fibrosis in primates and murine models of choroidal neovascularization [53]. This study provided a proof-of-concept for the targeted intravenous delivery of nanoparticles with an increased half-life. In another study, doxorubicin-conjugated polyethylene glycol and poly(sebacic acid) nanoformulation (DXR-PSA-PEG3) successfully delivered a drug to the eye, with detectable levels in the aqueous and vitreous humor for at least 105 days. The study also suggested significant inhibition in choroidal neovascularization for 35 days in a rabbit choroidal neovascularization (CNV) model [56]. The versatility in the application of polymeric nanoparticles for retinal diseases was further demonstrated in several studies in which PLGA nanoparticles were reported to (i) encapsulate hydrophobic drug-like celecoxib for the inhibition of oxidative stress in a DR rat model, (ii) work as hydrophilic dye-like rhodamine 123 for retinal imaging, (iii) bind protein/antibody-like bevacizumab for long-term inhibition of choroidal neovascularization, and (iv) deliver siRNA and plasmid DNA in vivo to the retina and choroid to prevent neovascularization [53,57,58,59,60]. Recent developments have led to stimulus-like ultrasound and light-responsive nanoparticles as next-generation nanomedicines for retinal diseases. Intravitreal injection of ultrasound-sensitive monomethoxypoly(ethylene glycol)-PLGA-poly-L-lysine nanoparticles has successfully delivered siRNA to the neural retina. In this study, ultrasound significantly down-regulated the mRNA and protein expression of PDGF-BB [60]. Additionally, blue light exposure cleaved the 7-(diethylamino)-4-(hydroxymethyl)-coumarin (DEACM) photocleavable group attached to the cell-penetrating peptide (CPP)-modified PEG-PLA nanoparticles delivered intravenously and caused a significant reduction in neovascular lesions in mice [118].
3.4.2. Lipid Nanoparticles
Currently, the lipid nanoparticles used for retinal drug delivery vary from 10–1000 nm. Lipid nanoparticles are comprised of solid lipid nanoparticles, nanostructured lipid carriers, and lipid drug conjugates. These nanoparticles are biocompatible and biodegradable, with enhanced drug solubility and increased bioavailability in the retina. They can also be produced on a large scale [65]. Out of the three categories mentioned above, solid lipid nanoparticles are most effectively used for carrying the payload. In an aqueous dispersion, a layer of surfactants surrounds the solid lipid matrix. Solid lipid nanoparticles are mainly produced by applying solidified nanoemulsion technologies in which high-pressure homogenization, hot-melt emulsification, and ultrasonication are most widely used. Other preparation methods include solvent-based techniques, micro-emulsification, coacervation methods, and membrane hydration [119]. In ocular drug delivery, liposomes have advantages, such as achieving high encapsulation efficiency and permeation, increasing corneal permeability and retention time, controlling release of drugs, having no toxicity, and enhancing bioavailability and biodistribution. Lipid nanoparticles also maintain adequate drug levels in the posterior segment of the eye (aqueous/vitreous humor and retina). These advantages make them ideal candidates for delivering molecules to the posterior segment of the eye. Lipid nanoparticles have some disadvantages, such as an initial burst release of drugs, and reduced drug loading capacity, and the formulation can subject to polymorphic changes upon longer storage. Additionally, the toxicity of lipid nanoparticles to retinal cells is not fully understood [120,121].
Nanostructured lipid carriers (NLCs) have a solid lipid core comprising a combination of solid–liquid and liquid–liquid interfaces with unique characteristic features. The matrix obtained from the solid–lipid and liquid–lipid mixture has a lower melting point than the original solid lipid. Various types of NLCs (imperfect, amorphous, and multiple) can be prepared depending on the lipid composition and production methods. A small change in the oil can affect the lipid crystallization, changing it into an imperfect type. Upon mixing some unique lipids, the amorphous type of lipids can be obtained, wherein the lipid matrix is solid and not crystalline. In multiple types, to improve the encapsulation of the payload into the lipid matrix and prevent compound leakage during storage, the lipid’s nanostructure can be tuned by adding more oil, followed by mixing with a solid–lipid [119].
The administration of solid–lipid nanoparticles as eye drops has been reported to reach the posterior part of the eye [64,122]. Intravenous injection of solid–lipid nanoparticles loaded with tobramycin showed higher drug bioavailability in the retina compared to topical eye drops. However, it is interesting that it can cross the ocular blood barriers in the retina [64]. Moreover, solid–lipid nanoparticles are good tools for delivering genes to the retina through intravitreal or intravenous injections [66,123]. Due to their structural advantages, solid–lipid nanoparticles have been loaded with hydrophilic drugs, hydrophobic drugs, DNA, siRNA, and miRNA for retinal diseases [65,66,67,68]. Furthermore, a recent study demonstrated the successful delivery of a switchable lipid nanoparticle based on dual drug delivery of miRNA-181 and melphalan, which significantly reduced retinoblastoma in an in vivo rat model [68]. The various types of lipids, preparation methods, and their potential applications in retinal diseases are summarized in Table 4.
Table 4.
Lipid nanoparticle preparation methods and their potential applications in retina diseases.
| Lipid Nanoparticle | Method of the Preparation | Characterization | Applications | Refs. |
|---|---|---|---|---|
| Dioctadecyl dimethyl ammonium bromide, glyceryl monostearate, soy phosphatidylcholine, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine (DSPE)- PEG2000-maleimide | Film-ultrasonic |
|
|
[124] |
| 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), hydrogenated soy phosphocholine, methoxy PEG2000-DSPE | Thin-film hydration |
|
|
[125] |
| Chitosan, glyceryl monostearate, linoleoyl polyoxyl-6-glycerides (Labrafil M 2125 CS) | Melt emulsification–ultrasonication |
|
|
[126] |
| Medium-chain triglycerides, dioctadecyl-3,3,3,3 tetramethylindodicarbocyanine (DiO), polyethylene glycol (15)-hydroxy stearate, hydrogenated phosphatidylcholine | Phase inversion |
|
|
[127] |
| Poloxamers 407/188, glycerol tripalmitate (GTP), soybean lecithin, stearic acid, PLGA | Emulsification |
|
|
[128] |
| Glyceryl di behenate (Compritol 888), behenoyl polyoxyl-8 glycerides (Compritol HD5 ATO) | Microemulsion |
|
|
[129] |
| Di Stearoyl phosphatidylcholine (DSPC), sterols, 1,2-dimyristoyl-rac-glycero-3-methoxy polyethylene glycol (DMG-PEG) | Rapid microfluidic mixing |
|
|
[130] |
| Lauroyl PEG -32 glycerides, propylene glycol monocaprylate, hydrogenated coco glycerides | Hot-melt emulsification and ultrasonication |
|
|
[131] |
| DSPE-PEG, PLGA, soybean lecithin | Membrane hydration and high-power ultrasonic method |
|
|
[132] |
3.4.3. Liposomes
Liposomes are phospholipids containing an aqueous core and bilayer phospholipids capable of loading hydrophilic and hydrophobic drugs. The unique arrangement of liposomal structures allows for hydrophilic drugs to be loaded into the aqueous core through passive loading methods. In contrast, hydrophobic drugs are encapsulated in a phospholipid bilayer through passive or postinsertion methods. Liposomes have been tried as eye drops for drug and gene delivery in the retina. Commonly used liposomes range from 10–10,000 nm. Liposomes, upon disintegration, can be readily metabolized due to the presence of phospholipids, and they show low toxicity and immunogenicity. Liposomes have been widely used as a potential solution for overcoming challenges in treating retinal diseases. They can be loaded with multiple therapeutics, and their surface can be functionalized for targeting several retina receptors. Liposomes also offer intracellular drug release, and they bypass the ocular barriers [133]. Their properties are greatly affected by their chemical composition and preparation methods. The hydrophobic tail length of liposomes is influenced by properties such as the thickness and rigidity of the phospholipid bilayer. Bilayer rigidity is influenced by critical factors, including encapsulation of non-phospholipid molecules, liposome diameter, and the ratio of the aqueous core to the phospholipid bilayer [134]. The major advantages of liposomes as nanocarriers lie in their ability to enhance the permeation of poorly absorbed drugs by attaching to the corneal surface and improving the corneal residence time. Other advantages of liposomes include biodegradability, biocompatibility, the ability to encapsulate both hydrophilic and hydrophobic drugs, an enhanced pharmacokinetic profile, and low toxicity at higher concentrations. Liposomes have some limitations related to stability and increased particle size upon storage, susceptibility to being engulfed by phagocytes, and limited retention time in the retina [135,136].
One studies has suggested that liposomes can be retained in the retina for 180 min [70]. A edaravone/berberine hydrochloride-loaded liposome eye drop formulation significantly protected the retina against photo-induced damage [71,72]. Successful gene transfer using liposomal eye drops has also been reported in the retina, though it has not been evaluated in retinal disease models [73]. In a previous study, intravitreal injection of a siRNA-entrapped hyaluronic acid-coated lipoplex demonstrated significant neuroprotective effects in a retinal light damage model, without evidence of retinal toxicity [137]. Systemic administration of a rosiglitazone-loaded liposomal formulation resulted in neuroprotection in the retina of a rotenone-insult Parkinson’s disease rodent model [138]. One major drawback of liposomal delivery is the retention time in the retina, which is usually less than 24 h. [137]. However, due to their structural advantage, liposomes can encapsulate hydrophobic and hydrophilic drugs, DNA, RNA, and polypeptides or proteins, making them attractive options for treating retinal diseases [71,73,137,139]. Therefore, liposomal preparation may be adequate for the short-term delivery of drugs, but unsuitable for chronic conditions where repeated administration may be needed. Recent advances in the application of liposomes in retinal diseases are summarized in Table 5.
Table 5.
Liposome preparation methods and potential applications in retinal diseases.
| Liposomes | Method of the Preparation | Characterization | Applications | Refs. |
|---|---|---|---|---|
| 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocoline (POPC), DSPE-mPEG2000, DSPE-N-[maleimide (PEG)2000], cholesterol, monocarboxylate | Thin-film hydration |
|
|
[140] |
| DSPE-PEG2000-NHS, DSPE-PEG2000-TAT, egg phosphatidylcholine, cholesterol | Thin-film evaporation |
|
|
[141] |
| 1,2-oleoyl-3-trimethylammonium-propane (DOTAP), 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), cholesterol, LipoTrust Ex Oligo (short oligonucleotides) | Thin-film evaporation |
|
|
[142] |
| DSPC, DOPC, DSPE-PEG2000, 1,2-distearoyl-sn-glycero-3-phosphoglycerol (DSPG) | Thin-film hydration |
|
|
[143] |
| Soybean phosphatidylcholine, diacetyl phosphate, cholesterol, chitosan | Hydration |
|
|
[144] |
| DSPC,DSPE-PEG2000, DSPE-PEG-Mal, cholesterol | Reverse-phase evaporation technique |
|
|
[145] |
| DSPE-PEG2000, HSPC (hydrogenated soy phosphatidylcholine), DMPC, DOTAP |
Thin-film hydration |
|
|
[146] |
3.4.4. Polymeric Micelles
Polymeric micelles consist of self-assembled units in a nanoscale structure with a hydrophobic core and a hydrophilic corona ranging from 10–200 nm. These micelles are biodegradable and biocompatible, making them suited for retinal drug delivery. The self-assembly of the block polymers or amphiphilic molecules takes place in an aqueous environment at a concentration more than the critical micellar concentration (CMC) [147]. Therapeutic outcomes can be improved by using polymeric micelle’s prolonged retention, as they have the potential to solubilize, stabilize, and encapsulate hydrophobic compounds [148]. The premature degeneration release of therapeutic cargo from the micelles can be prevented by crosslinking their core/corona through covalent bonding, hydrogen bonding, or π–π stacking [149]. After topical administration, the polymeric micelles penetrate through the cornea due to their small size via the conjunctival–scleral pathway [150]. Polymeric micelles reach the posterior segment of the eye through lateral diffusion from the broader conjunctival surface area. After the micelles reach the posterior segment, the RPE cells, through endocytosis, can engulf them, resulting in higher therapeutic concentrations.
Nevertheless, the surface charge and particle size of micelles define their cellular uptake and tissue absorption [151]. Polymeric micelles offer high drug loading capacity, enhanced stability, small particle size, high water solubility, and tunable surface modification. They are mainly preferred to improve the delivery of hydrophobic drugs, enhancing their therapeutic efficiency. However, polymeric micelles lack adequate retention times, are susceptible to accumulation at the target site, and are difficult to produce on a large scale [152,153].
For drug delivery, most polymeric micelles comprise different types of polymers, such as amphiphilic di-block (hydrophilic-hydrophobic) polymers, tri-block (hydrophilic-hydrophobic-hydrophilic) polymers, graft, and ionic (hydrophilic-ionic) co-polymers. Hydrophilic polymers that are primarily used in the preparation of polymeric micelles include polyethylene glycol (PEG), hydroxy propyl methyl acrylamide (HPMA), polyethylene oxide (PEO), and poly(vinyl pyrrolidine). The hydrophobic core of the polymeric micelles can be comprised of poly(L-amino acids) such as poly(L-aspartate) and poly(L-glutamate), polyesters such as polyglycolic acid and poly(D-lactic acid), and co-polymers such as PLGA and polycaprolactone [152] Polymeric micelles can be prepared using various methods such as direct dissolution, dialysis, oil/water emulsion, thin-film hydration, solvent evaporation, and freeze-drying methods [152].
The administration of polymeric nanomicellar eye drops containing rapamycin has been shown to increase the availability of rapamycin in the rabbit retina and choroidal tissues, suggesting that the sequestration of rapamycin in lipoidal retinal tissues via a non-corneal route (conjunctiva–sclera–choroid–retina) might be the preferred method for delivery of micellar drugs to the retina [74]. In another study, intravenous administration of polymeric nanomicelles encapsulating plasmid DNA of yellow fluorescent protein (pYFP) was found to be localized in the choroidal vascularization area in a CNV mouse model. Additionally, CNV was significantly reduced in psFlt-1-encapsulated polymeric nanomicelles compared to the control group. These results suggest the usefulness of polymeric micelles for non-viral gene therapy for CNV [75]. Additionally, when hexadecyloxypropyl-cidofovir micelle formulation was administered via intravitreal injection in a rabbit retinitis model, the therapeutic level of the drug was detected for up to nine weeks. Micellar formulation halted active HSV-1 retinitis in 80% of the infected eyes. In contrast, the non-micellar formulation failed to suppress active HSV-1 retinitis, indicating that lipid-derived nucleoside analogs could be formulated as micelles for intravitreal injection for sustained release of drugs in chronic retinal diseases [76]. As polymeric micelles encapsulate hydrophobic drugs more efficiently due to their hydrophobic core and hydrophilic shell, they have also been explored for siRNA delivery to the RPE and gene therapy for the retina [79].
3.4.5. Dendrimers
Dendrimers are highly branched, spherical dendritic nanometers ranging from 3 to 20 nm. Polyamidoamine (PAMAM) dendrimers have mostly been explored for their water-soluble and non-immunogenic properties. The biocompatibility of PAMAM can also be increased through PEGylation [61]. Furthermore, penetrating modified PEGylated PAMAM dendrimer eye drops result in greater distribution throughout the cornea and the retina. Further modification can extend retention times for more than 12 h in the retina [61]. In an in vitro study, the PEGylated PAMAM dendrimer has been explored for siRNA delivery to retinal microvascular endothelial cells to inhibit angiogenesis. However, in vivo, its efficacy has not been evaluated [154]. Another study showed systemic and intravitreal administration of fluorescently labeled dendrimers selectively co-localized with activated microglia and astrocytes for 21 days in the retina. However, the study also suggested that a thirty-times higher dosage was required for the intravenous administration of dendrimers compared to intravitreous administration to reach the retina [62]. Furthermore, intravitreal injection of a dendrimer drug conjugate significantly inhibited neuroinflammation in a rat model of retinal degeneration [63]. Intraocular injection of anti-VEGF oligonucleotide conjugate has also been reported to significantly alleviate CNV for four months compared to the control group without toxicity, indicating the long-term safety of dendrimers in retinal neovascular diseases [54]. In comparison to other drug delivery systems, dendrimers have some unique advantages, such as a controllable structure and size, the potential for more surface modifications, the feasibility of conjugating drugs and other therapeutic molecules to the surface of active terminal groups, a lower molecular volume compared to the other linear polymers of similar molecular weights, and controlled drug release with effective targeting. Despite these advantages, dendrimers have some limitations, including a high cost for synthesis, elimination, and metabolism depending on their generation and materials used. Additionally, their cellular toxicity has not been adequately assessed [155,156].
3.4.6. Nanoemulsions
Nanoemulsions are nanometer droplets made through the heterogeneous dispersions of two immiscible liquids (oil-in-water or water-in-oil), ranging from 10–1000 nm. Nanoemulsions are biocompatible, with adequate ocular tolerance [80]. Moreover, nanoemulsions can solubilize hydrophobic and hydrophilic drugs to enhance drug stability [4]. Previous studies have suggested that the administration of nanoemulsions as eye drops can follow corneal and scleral pathways to the retina [80,157]. In another study, fenofibrate-loaded nanoemulsion eye drops reduced retinal inflammation and vascular leakage in rodent models of DR and laser-induced CNV [81]. Furthermore, when applied as eye drops or through intravitreal injection, cationic nanoemulsions containing antisense oligonucleotides showed a significant reduction in neovascularization compared to the untreated control group. Cationic nanoemulsion can thus be considered a promising potential therapeutic tool for the management of retinal neovascularization [82]. Nanoemulsions have a high surface area and a small droplet size, enhancing drug absorption. They are also physically stable, non-irritating, and non-toxic to the eye. The major drawback of nanoemulsions is the requirement for many surfactants, and they have a low stability in acidic conditions. Nanoemulsions with particle sizes of more than 100 nm are known to cause blurred vision due to changes in the formulation properties. Due to the use of highly concentrated surfactants, the formulation has reduced ocular tolerance [158,159].
3.4.7. Nanogels
Nanogels are cross-linked polymer networks ranging from 1–1000 nm. Nanogels are biocompatible, flexible to design, stable in aqueous systems, and are responsive to external stimuli, with a high drug loading capacity and controlled release [84]. However, there are few reports using nanogels for drug delivery to the retina. In one study, a penetration-complexed redox-responsive hyaluronic acid-based nanogel was used to release and deliver a drug (visual chromophore analog, 9-cis-retinal) to the posterior part of the eye via eye drops. Interestingly, the nanogels released the drug in a reducing environment, similar to the cytoplasm. The nanogel treatment resulted in partial recovery of photoreceptor function in treated eyes compared to the untreated controls [85]. In an in vitro study, a chitosan-based nanogel was significantly internalized by human retinal pigment epithelial cells (ARPE-19), cells with no cytotoxic or inflammatory response. However, in vivo studies on nanogels targeting the RPE have not been reported. In another study, acetazolamide-loaded nanovesicles embedded in chitosan–sodium tripolyphosphate nanogels significantly reduced ocular pressure in rabbits compared to oral tablets. Furthermore, the nanogel system was suggested to result in decreased irritation compared to free acetazolamide, indicating that nanogels can be an attractive strategy for treating ocular diseases [86].
Nanogels are known to prolong retention times in the eye and enhance bioavailability. Nanogels consist of long polymer chains containing many hydrophilic groups such as -OH, -COOH, and -CONH2. Due to the presence of these groups, nanogels absorb water and retain large amounts of fluids. Nanogels are highly biocompatible and protect the payload from degradation and elimination. In addition to these properties, nanogels offer a stimulus-responsive release of the payload. Nanogel bases are prepared using semi-solids, and due to the presence of refractive indices in tears, they can cause blurred vision and result in imprecise dosage [160,161].
4. The Future of Nanomedicines in the Management of Retinal Diseases
The challenges and exciting future of nanomedicines for managing retinal diseases are discussed below.
4.1. Overcoming Blood–Ocular Barriers
Developing therapeutic nanoparticles incorporated into eye drops is one of the most crucial challenges for overcoming the invasiveness of current therapeutic strategies. The challenges for treating chronic retinal diseases with nanomedicines will be creating nanoparticles that can cross static and dynamic blood–ocular barriers and provide therapeutic effects for sustained durations. Designing appropriate non-invasive or minimally invasive drug delivery systems for the retina and controlling the physicochemical properties of delivery systems are essential [116,117]. Furthermore, integrating hydrogels with polymeric nanoparticles, liposomes, micelles, dendrimers, and cyclo-dextrins can aid in drug delivery and release [162,163,164,165]. Pharmacokinetics and drug loading capacity can also be controlled by optimizing the size and concentration of the loaded nanomedicines. Additionally, cross-linking of hydrogels can alter pharmacokinetics, allowing for fine-tuning and optimization of drug release to the retina.
4.2. Cell-Specific Delivery in the Retina
Cell-specific targeted delivery of therapeutics to the retina is desirable. Developing targeted drug delivery systems to activate microglia in the retina may be advantageous for inhibiting gliosis and neuroinflammation. In a study examining PLGA nanoparticles conjugated with CD47, a blood–brain barrier peptide loaded with the microglia-modulating agent Nec-1s specifically targeted microglia and converted them from a pathological to a beneficial state [166]. Another study on glucocorticoid-loaded inorganic–organic hybrid nanoparticles found that they were preferentially engulfed by macrophages. The study showed promising results in preventing neuroinflammatory diseases [167], suggesting the benefits of the targeted delivery of nanomedicines.
Astrocytes are macroglia that contribute to neurodegenerative retinal diseases [168]. Interestingly, astrocytes can be targeted by dendrimers in the brain [169]. This opens new opportunities for similar technology to be applied to retinal astrocytes, which can be further extrapolated for treating neurovascular complications in the retina. Müller glia are another macroglia cell type that plays a critical role in the regulation of retinal homeostasis. Activation of Müller glia has been reported in several chronic retinal diseases. Targeting Müller glia using lipid nanoparticles has shown some promise in regulating retinal homeostasis [170]. Furthermore, targeted delivery of overexpressed adhesion molecules in retinal endothelial cells may aid in managing retinal vascular diseases [171]. Several reports have discussed RPE-targeting nanoparticles [35,172,173], encouraging future treatments against AMD and other RPE-associated retinal diseases. Additionally, intravitreal injection of PAMAM–PVL–PEG unimolecular micelle nanoparticles has been reported to accumulate in RGCs and has been shown to possess sustained viability for 14 days [174]. This shines promising light on the possibility of developing effective drug delivery systems for retinal diseases. Lastly, compact DNA nanoparticles have shown significant gene transfer abilities in photoreceptors after subretinal injection, setting the premise for potential treatments for rescuing photoreceptor degeneration [175].
4.3. Safety and Efficacy
Successful nanomedicines for the retina should be sterile, scalable, efficient, and safe. Sterilization and scaleup are significant problems faced in translating drug delivery systems. The critical parameters for successful translation are the size, shape, morphology, targetability, and functionality of the developed nanomedicines. Though methods such as membrane extrusion, supercritical fluid technology, and microfluidizer technology can scale up production, the large-scale generation of functional nanoparticles is still problematic [176]. Furthermore, producing therapeutic agents that remain biologically active after their formulation is still challenging, especially for protein/polypeptide-based therapeutic agents, where structural confirmation is crucial for their function. Nanomedicine architecture is thus a crucial factor during development. Additionally, it is essential to reduce toxicity and enhance efficacy.
Advanced nanotechnology and tissue engineering should be employed to develop drug delivery systems with long-term stability and low toxicity to eliminate the need for repeated applications. Extensive, detailed, and long-term investigations should focus on the biochemical, immunological, and ophthalmological aspects of ocular toxicity and biocompatibility. Controlled in vitro and in vivo toxicological evaluation and human clinical trials should also be conducted before entry into the market.
4.4. Drug Delivery Systems
Drug delivery systems activated via non-invasive stimuli are in the early stages of development. For example, pH and ultrasound-responsive lipid nanoparticles have been reported as effective treatments for RPE and cancer cells [69,177]. However, in vivo evaluation of these nanoparticles in the retina is warranted for further validation. In addition, different pH and ultrasound-responsive liposomes, micelles, and nanoemulsions reported for cancer have not yet been explored for retinal diseases [77,78,83,178]. Testing these responsive nanoparticles in the eye may provide new insights into their viability as a therapeutic agent. Reactive oxygen species (ROS)-responsive dendrimers might also be an attractive option for managing retinal diseases such as DR, AMD, and ROP [55]. Additionally, since the eye is sensitive to different light sources, photo-responsive nanomedicines may be a promising future drug delivery system that can be easily controlled. A recent study examining nanoparticles with peptides that contain photocleavable bonds demonstrated favorable effects in a laser CNV model [118]. Further development in stimulus-induced drug delivery systems could be the next generation of nanomedicines.
4.5. Gene Therapy
Gene therapy has evolved rapidly, with various non-viral vectors being explored for their cytocompatibility and reduced immunogenicity [179]. Non-viral vectors that form nanoparticle complexes can deliver genes into specific cell systems. One such example is the use of polyethyleneimine (PEI). PEI is efficient in gene transfer, without affecting corneal cell viability or morphology. Furthermore, the delivery of decorin gene therapy using PEI nanoparticles in our previous study reduced TGFβ-induced fibrosis in an in vitro model of equine corneal fibrosis [180]. This approach can open new doors for delivering gene therapies or other therapeutic agents into different parts of the eye [37,181]. Our recent study reported that polyethylene amine-conjugated gold nanoparticles showed substantial BMP7 gene delivery into rabbit keratocytes in vivo. Additionally, it inhibited fibrosis, suggesting its versatility in therapeutic applications [182], which can be further explored for treating retinal fibrosis.
4.6. Precision Nanomedicine
Precision nanomedicine will be the next step forward for the therapeutic development of drugs. Retinal diseases such as DR, IR, ROP, AMD, and PVR increase vascular permeability [183,184]. Delivery systems that detect fluorescent nanoparticles can be designed to distinguish the permeation effect in the retina for diagnostic purposes, as reported in cancer treatments [185]. Precision nanomedicine can be designed based on the degree of permeability by controlling drug loading, release, and pharmacokinetics. For example, the amount of drug can be controlled in a nanowafer, an ultra-thin transparent lens prepared by filling drug solutions into the dissoluble PVA template and adjusting the well size [186]. Although precision nanomedicine is still in early stages of development, future nanotools and nanotechniques will better design therapeutic drugs and molecules to manage retinal diseases.
5. Conclusions
Over the past decade, nanomedicine research has made several potential advancements in diagnosing, monitoring, and managing retinal diseases. In addition, recent studies have suggested that nanomedicines may act as next-generation therapeutic agents for neovascular complications, enhance the half-life of therapeutic agents, provide an alternative for the sustained delivery of therapeutic drugs, and act as a scaffold for tissue engineering in the retina. Nanomedicine has immense potential to revolutionize the current treatment strategies by overcoming the limitations associated with invasive therapies, providing targeted drug delivery, offering sustained-release formulations, and promoting tissue regeneration for managing retinal diseases. Overall, nanomedicine holds promise for improving patient outcomes and quality of life.
Acknowledgments
We would like to acknowledge administrative and technical support provided by the Eye Institute and Department of Ophthalmology and Visual Sciences department.
Author Contributions
Conceptualization and manuscript design, S.S.C.; writing—original draft preparation, B.M., S.W.Y.L. and S.C.; writing—review and editing, U.R.A., B.A., T.B.C., R.R.M., S.B. and S.S.C.; supervision, S.S.C.; funding acquisition, S.S.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This research was funded by National Eye Institute, NIH, Bethesda, MD, grant numbers R01EY029795 and R01EY030077 to SSC. This investigation was conducted in part in a facility constructed with support from the Research Facilities Improvement Program, grant number C06RR016511 from the National Center for Research Resources, NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. SB was supported by the research funds provided by the Indian Council of Medical Research (ICMR), File No.: 20218945. SC is thankful to ICMR for providing senior research fellowship (45/64/2020-Nan/BMS).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Duvvuri S., Majumdar S., Mitra A.K. Drug delivery to the retina: Challenges and opportunities. Expert Opin. Biol. Ther. 2003;3:45–56. doi: 10.1517/14712598.3.1.45. [DOI] [PubMed] [Google Scholar]
- 2.Thapa R., Khanal S., Tan H.S., Thapa S.S., van Rens G.H.M.B. Prevalence, Pattern and Risk Factors of Retinal Diseases Among an Elderly Population in Nepal: The Bhaktapur Retina Study. Clin. Ophthalmol. 2020;14:2109. doi: 10.2147/OPTH.S262131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahaling B., Low S.W.Y., Beck M., Kumar D., Ahmed S., Connor T.B., Ahmad B., Chaurasia S.S. Damage-Associated Molecular Patterns (DAMPs) in Retinal Disorders. Int. J. Mol. Sci. 2022;23:2591. doi: 10.3390/ijms23052591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaurasia S.S., Lim R.R., Lakshminarayanan R., Mohan R.R. Nanomedicine Approaches for Corneal Diseases. J. Funct. Biomater. 2015;6:277. doi: 10.3390/jfb6020277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bourne R.R.A., Steinmetz J.D., Saylan M., Mersha A.M., Weldemariam A.H., Wondmeneh T.G., Sreeramareddy C.T., Pinheiro M., Yaseri M., Yu C., et al. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: The Right to Sight: An analysis for the Global Burden of Disease Study. Lancet Glob. Health. 2021;9:e144–e160. doi: 10.1016/S2214-109X(20)30489-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenblatt T.R., Vail D., Saroj N., Boucher N., Moshfeghi D.M., Moshfeghi A.A. Increasing Incidence and Prevalence of Common Retinal Diseases in Retina Practices Across the United States. Ophthalmic Surg. Lasers Imaging Retin. 2021;52:29–36. doi: 10.3928/23258160-20201223-06. [DOI] [PubMed] [Google Scholar]
- 7.Wang W., Lo A.C.Y. Diabetic retinopathy: Pathophysiology and treatments. Int. J. Mol. Sci. 2018;19:1816. doi: 10.3390/ijms19061816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahaling B., Srinivasarao D.A., Raghu G., Kasam R.K., Bhanuprakash Reddy G., Katti D.S. A non-invasive nanoparticle mediated delivery of triamcinolone acetonide ameliorates diabetic retinopathy in rats. Nanoscale. 2018;10:16485–16498. doi: 10.1039/C8NR00058A. [DOI] [PubMed] [Google Scholar]
- 9.Falavarjani K.G., Nguyen Q.D. Adverse events and complications associated with intravitreal injection of anti-VEGF agents: A review of literature. Eye. 2013;27:787–794. doi: 10.1038/eye.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fong D.S., Girach A., Boney A. Visual side effects of successful scatter laser photocoagulation surgery for proliferative diabetic retinopathy: A literature review. Retina. 2007;27:816–824. doi: 10.1097/IAE.0b013e318042d32c. [DOI] [PubMed] [Google Scholar]
- 11.Schachat A.P., Oyakawa R.T., Michels R.G., Rice T.A. Complications of vitreous surgery for diabetic retinopathy. II. Postoperative complications. Ophthalmology. 1983;90:522–530. doi: 10.1016/S0161-6420(83)34540-1. [DOI] [PubMed] [Google Scholar]
- 12.Chew E.Y., Clemons T.E., SanGiovanni J.P., Danis R., Ferris F.L., Elman M., Antoszyk A., Ruby A., Orth D., Bressler S., et al. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: The Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309:2005–2015. doi: 10.1001/JAMA.2013.4997. [DOI] [PubMed] [Google Scholar]
- 13.Al-Zamil W.M., Yassin S.A. Recent developments in age-related macular degeneration: A review. Clin. Interv. Aging. 2017;12:1313. doi: 10.2147/CIA.S143508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark D., Mandal K. Treatment of retinopathy of prematurity. Early Hum. Dev. 2008;84:95–99. doi: 10.1016/j.earlhumdev.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Honda S., Hirabayashi H., Tsukahara Y., Negi A. Acute contraction of the proliferative membrane after an intravitreal injection of bevacizumab for advanced retinopathy of prematurity. Graefes Arch. Clin. Exp. Ophthalmol. 2008;246:1061–1063. doi: 10.1007/s00417-008-0786-7. [DOI] [PubMed] [Google Scholar]
- 16.Idrees S., Sridhar J., Kuriyan A.E. Proliferative Vitreoretinopathy: A Review. Int. Ophthalmol. Clin. 2019;59:221. doi: 10.1097/IIO.0000000000000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wubben T.J., Besirli C.G., Zacks D.N. Pharmacotherapies for Retinal Detachment. Ophthalmology. 2016;123:1553–1562. doi: 10.1016/j.ophtha.2016.02.040. [DOI] [PubMed] [Google Scholar]
- 18.Fahim A. Retinitis pigmentosa: Recent advances and future directions in diagnosis and management. Curr. Opin. Pediatr. 2018;30:725–733. doi: 10.1097/MOP.0000000000000690. [DOI] [PubMed] [Google Scholar]
- 19.Minhas G., Morishita R., Anand A. Preclinical models to investigate retinal ischemia: Advances and drawbacks. Front. Neurol. 2012;3:75. doi: 10.3389/fneur.2012.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomita Y., Lee D., Tsubota K., Negishi K., Kurihara T. Updates on the Current Treatments for Diabetic Retinopathy and Possibility of Future Oral Therapy. J. Clin. Med. 2021;10:4666. doi: 10.3390/jcm10204666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahaling B., Baruah N., Ahamad N., Maisha N., Lavik E., Katti D.S. A non-invasive nanoparticle-based sustained dual-drug delivery system as an eyedrop for endophthalmitis. Int. J. Pharm. 2021;606:120900. doi: 10.1016/j.ijpharm.2021.120900. [DOI] [PubMed] [Google Scholar]
- 22.Mester U., Volker B., Kroll P., Berg P. Complications of prophylactic argon laser treatment of retinal breaks and degenerations in 2000 eyes. Ophthalmic Surg. 1988;19:482–484. doi: 10.3928/1542-8877-19880701-08. [DOI] [PubMed] [Google Scholar]
- 23.Retinitis Pigmentosa|National Eye Institute. [(accessed on 14 March 2022)]; Available online: https://www.nei.nih.gov/learn-about-eye-health/eye-conditions-and-diseases/retinitis-pigmentosa.
- 24.Cehajic-Kapetanovic J., Xue K., Martinez-Fernandez de la Camara C., Nanda A., Davies A., Wood L.J., Salvetti A.P., Fischer M.D., Aylward J.W., Barnard A.R., et al. Initial results from a first-in-human gene therapy trial on X-linked retinitis pigmentosa caused by mutations in RPGR. Nat. Med. 2020;26:354–359. doi: 10.1038/s41591-020-0763-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laradji A., Karakocak B.B., Kolesnikov A.V., Kefalov V.J., Ravi N. Hyaluronic Acid-Based Gold Nanoparticles for the Topical Delivery of Therapeutics to the Retina and the Retinal Pigment Epithelium. Polymers. 2021;13:3324. doi: 10.3390/polym13193324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim J.H., Kim J.H., Kim K.W., Kim M.H., Yu Y.S. Intravenously administered gold nanoparticles pass through the blood-retinal barrier depending on the particle size, and induce no retinal toxicity. Nanotechnology. 2009;20:505101. doi: 10.1088/0957-4484/20/50/505101. [DOI] [PubMed] [Google Scholar]
- 27.Kim J.H., Kim M.H., Jo D.H., Yu Y.S., Lee T.G., Kim J.H. The inhibition of retinal neovascularization by gold nanoparticles via suppression of VEGFR-2 activation. Biomaterials. 2011;32:1865–1871. doi: 10.1016/j.biomaterials.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 28.Chan C.M., Hsiao C.Y., Li H.J., Fang J.Y., Chang D.C., Hung C.F. The Inhibitory Effects of Gold Nanoparticles on VEGF-A-Induced Cell Migration in Choroid-Retina Endothelial Cells. Int. J. Mol. Sci. 2019;21:109. doi: 10.3390/ijms21010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalishwaralal K., Banumathi E., Pandian S.B.R.K., Deepak V., Muniyandi J., Eom S.H., Gurunathan S. Silver nanoparticles inhibit VEGF induced cell proliferation and migration in bovine retinal endothelial cells. Colloids Surf. B Biointerfaces. 2009;73:51–57. doi: 10.1016/j.colsurfb.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 30.Sheikpranbabu S., Kalishwaralal K., Venkataraman D., Eom S.H., Park J., Gurunathan S. Silver nanoparticles inhibit VEGF-and IL-1β-induced vascular permeability via Src dependent pathway in porcine retinal endothelial cells. J. Nanobiotechnol. 2009;7:8. doi: 10.1186/1477-3155-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clark A., Zhu A., Sun K., Petty H.R. Cerium oxide and platinum nanoparticles protect cells from oxidant-mediated apoptosis. J. Nanopart. Res. 2011;13:5547. doi: 10.1007/s11051-011-0544-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen J., Patil S., Seal S., McGinnis J.F. Rare earth nanoparticles prevent retinal degeneration induced by intracellular peroxides. Nat. Nanotechnol. 2006;1:142–150. doi: 10.1038/nnano.2006.91. [DOI] [PubMed] [Google Scholar]
- 33.Mitra R.N., Merwin M.J., Han Z., Conley S.M., Al-Ubaidi M.R., Naash M.I. Yttrium oxide nanoparticles prevent photoreceptor death in a light-damage model of retinal degeneration. Free Radic. Biol. Med. 2014;75:140–148. doi: 10.1016/j.freeradbiomed.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yanai A., Häfeli U.O., Metcalfe A.L., Soema P., Addo L., Gregory-Evans C.Y., Po K., Shan X., Moritz O.L., Gregory-Evans K. Focused magnetic stem cell targeting to the retina using superparamagnetic iron oxide nanoparticles. Cell Transplant. 2012;21:1137–1148. doi: 10.3727/096368911X627435. [DOI] [PubMed] [Google Scholar]
- 35.Giannaccini M., Giannini M., Calatayud M.P., Goya G.F., Cuschieri A., Dente L., Raffa V. Magnetic nanoparticles as intraocular drug delivery system to target retinal pigmented epithelium (RPE) Int. J. Mol. Sci. 2014;15:1590–1605. doi: 10.3390/ijms15011590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tabatabaei S.N., Tabatabaei M.S., Girouard H., Martel S. Hyperthermia of magnetic nanoparticles allows passage of sodium fluorescein and Evans blue dye across the blood-retinal barrier. Int. J. Hyperth. 2016;32:657–665. doi: 10.1080/02656736.2016.1193903. [DOI] [PubMed] [Google Scholar]
- 37.Li H., Ma M., Zhang J., Hou W., Chen H., Zeng D., Wang Z. Ultrasound-Enhanced Delivery of Doxorubicin-Loaded Nanodiamonds from Pullulan-all-trans-Retinal Nanoparticles for Effective Cancer Therapy. ACS Appl. Mater. Interfaces. 2019;11:20341–20349. doi: 10.1021/acsami.9b03559. [DOI] [PubMed] [Google Scholar]
- 38.Paiva M.R.B., Andrade G.F., Dourado L.F.N., Castro B.F.M., Fialho S.L., Sousa E.M.B., Silva-Cunha A. Surface functionalized mesoporous silica nanoparticles for intravitreal application of tacrolimus. J. Biomater. Appl. 2021;35:1019–1033. doi: 10.1177/0885328220977605. [DOI] [PubMed] [Google Scholar]
- 39.Qu W., Meng B., Yu Y., Wang S. Folic acid-conjugated mesoporous silica nanoparticles for enhanced therapeutic efficacy of topotecan in retina cancers. Int. J. Nanomed. 2018;13:4379–4389. doi: 10.2147/IJN.S142668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shimanovskaya E.V., Nikol’skaya I.I., Binevskii P.V., Beznos O.V., Klyachko N.L., Pavlenko T.A., Chesnokova N.B., Kost O.A. Lisinopril in the composition of calcium phosphate nanoparticles as a promising antiglaucoma agent. Nanotechnol. Russ. 2014;9:219–226. doi: 10.1134/S1995078014020141. [DOI] [Google Scholar]
- 41.Warnke P.H., Alamein M., Skabo S., Stephens S., Bourke R., Heiner P., Liu Q. Primordium of an artificial Bruch’s membrane made of nanofibers for engineering of retinal pigment epithelium cell monolayers. Acta Biomater. 2013;9:9414–9422. doi: 10.1016/j.actbio.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 42.Yan L., Zhao B., Liu X., Li X., Zeng C., Shi H., Xu X., Lin T., Dai L., Liu Y. Aligned Nanofibers from Polypyrrole/Graphene as Electrodes for Regeneration of Optic Nerve via Electrical Stimulation. ACS Appl. Mater. Interfaces. 2016;8:6834–6840. doi: 10.1021/acsami.5b12843. [DOI] [PubMed] [Google Scholar]
- 43.Phelan M.A., Kruczek K., Wilson J.H., Brooks M.J., Drinnan C.T., Regent F., Gerstenhaber J.A., Swaroop A., Lelkes P.I., Li T. Soy Protein Nanofiber Scaffolds for Uniform Maturation of Human Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelium. Tissue Eng. Part C Methods. 2020;26:433–446. doi: 10.1089/ten.tec.2020.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Piret G., Perez M.T., Prinz C.N. Neurite outgrowth and synaptophysin expression of postnatal CNS neurons on GaP nanowire arrays in long-term retinal cell culture. Biomaterials. 2013;34:875–887. doi: 10.1016/j.biomaterials.2012.10.042. [DOI] [PubMed] [Google Scholar]
- 45.Ross A.E., Bengani L.C., Tulsan R., Maidana D.E., Salvador-Culla B., Kobashi H., Kolovou P.E., Zhai H., Taghizadeh K., Kuang L., et al. Topical sustained drug delivery to the retina with a drug-eluting contact lens. Biomaterials. 2019;217:119285. doi: 10.1016/j.biomaterials.2019.119285. [DOI] [PubMed] [Google Scholar]
- 46.Johnen S., Meißner F., Krug M., Baltz T., Endler I., Mokwa W., Walter P. Properties of Retinal Precursor Cells Grown on Vertically Aligned Multiwalled Carbon Nanotubes Generated for the Modification of Retinal Implant-Embedded Microelectrode Arrays. J. Ophthalmol. 2016;2016:2371021. doi: 10.1155/2016/2371021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watterson W.J., Moslehi S., Rowland C., Zappitelli K.M., Smith J.H., Miller D., Chouinard J.E., Golledge S.L., Taylor R.P., Perez M.T., et al. The Roles of an Aluminum Underlayer in the Biocompatibility and Mechanical Integrity of Vertically Aligned Carbon Nanotubes for Interfacing with Retinal Neurons. Micromachines. 2020;11:546. doi: 10.3390/mi11060546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheng R., Peng Y., Ge C., Bu Y., Liu H., Huang H., Ou S., Xue Y., Dai L. A turn-on fluorescent lysine nanoprobe based on the use of the Alizarin Red aluminum(III) complex conjugated to graphene oxide, and its application to cellular imaging of lysine. Microchim. Acta. 2017;184:3521–3528. doi: 10.1007/s00604-017-2375-0. [DOI] [Google Scholar]
- 49.Niu Y., Qin A., Song W., Wang M., Gu X., Zhang Y., Yu M., Zhao X., Dai M., Yan L., et al. Biocompatible single-crystal selenium nanobelt based nanodevice as a temperature-tunable photosensor. J. Nanomater. 2012;2012:384671. doi: 10.1155/2012/384671. [DOI] [Google Scholar]
- 50.Coburn P.S., Miller F.C., LaGrow A.L., Land C., Mursalin H., Livingston E., Amayem O., Chen Y., Gao W., Zhang L., et al. Disarming Pore-Forming Toxins with Biomimetic Nanosponges in Intraocular Infections. mSphere. 2019;4:e00262-19. doi: 10.1128/mSphere.00262-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.LaGrow A.L., Coburn P.S., Miller F.C., Land C., Parkunan S.M., Luk B.T., Gao W., Zhang L., Callegan M.C. A Novel Biomimetic Nanosponge Protects the Retina from the Enterococcus faecalis Cytolysin. mSphere. 2017;2:e00335-17. doi: 10.1128/mSphere.00335-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ma Y., Bao J., Zhang Y., Li Z., Zhou X., Wan C., Huang L., Zhao Y., Han G., Xue T. Mammalian Near-Infrared Image Vision through Injectable and Self-Powered Retinal Nanoantennae. Cell. 2019;177:243–255.e15. doi: 10.1016/j.cell.2019.01.038. [DOI] [PubMed] [Google Scholar]
- 53.Luo L., Zhang X., Hirano Y., Tyagi P., Barabás P., Uehara H., Miya T.R., Singh N., Archer B., Qazi Y., et al. Targeted intraceptor nanoparticle therapy reduces angiogenesis and fibrosis in primate and murine macular degeneration. ACS Nano. 2013;7:3264–3275. doi: 10.1021/nn305958y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marano R.J., Toth I., Wimmer N., Brankov M., Rakoczy P.E. Dendrimer delivery of an anti-VEGF oligonucleotide into the eye: A long-term study into inhibition of laser-induced CNV, distribution, uptake and toxicity. Gene Ther. 2005;12:1544–1550. doi: 10.1038/sj.gt.3302579. [DOI] [PubMed] [Google Scholar]
- 55.Patil N.G., Augustine R., Zhang Y., Hong S.C., Kim I. Synthesis of Stimuli-Responsive Heterofunctional Dendrimer by Passerini Multicomponent Reaction. ACS Omega. 2019;4:6660–6668. doi: 10.1021/acsomega.9b00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iwase T., Fu J., Yoshida T., Muramatsu D., Miki A., Hashida N., Lu L., Oveson B., Lima E Silva R., Seidel C., et al. Sustained delivery of a HIF-1 antagonist for ocular neovascularization. J. Control. Release. 2013;172:625–633. doi: 10.1016/j.jconrel.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ayalasomayajula S.P., Kompella U.B. Subconjunctivally administered celecoxib-PLGA microparticles sustain retinal drug levels and alleviate diabetes-induced oxidative stress in a rat model. Eur. J. Pharmacol. 2005;511:191–198. doi: 10.1016/j.ejphar.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 58.Zhang E., Zhukova V., Semyonkin A., Osipova N., Malinovskaya Y., Maksimenko O., Chernikov V., Sokolov M., Grigartzik L., Sabel B.A., et al. Release kinetics of fluorescent dyes from PLGA nanoparticles in retinal blood vessels: In vivo monitoring and ex vivo localization. Eur. J. Pharm. Biopharm. 2020;150:131–142. doi: 10.1016/j.ejpb.2020.03.006. [DOI] [PubMed] [Google Scholar]
- 59.Pan C.K., Durairaj C., Kompella U.B., Agwu O., Oliver S.C.N., Quiroz-Mercado H., Mandava N., Olson J.L. Comparison of Long-Acting Bevacizumab Formulations in the Treatment of Choroidal Neovascularization in a Rat Model. J. Ocul. Pharmacol. Ther. 2011;27:219. doi: 10.1089/jop.2010.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Du J., Sun Y., Li F.H., Du L.F., Duan Y.R. Enhanced delivery of biodegradable mPEG-PLGA-PLL nanoparticles loading Cy3-labelled PDGF-BB siRNA by UTMD to rat retina. J. Biosci. 2017;42:299–309. doi: 10.1007/s12038-017-9677-6. [DOI] [PubMed] [Google Scholar]
- 61.Yang X., Wang L., Li L., Han M., Tang S., Wang T., Han J., He X., He X., Wang A., et al. A novel dendrimer-based complex co-modified with cyclic RGD hexapeptide and penetratin for noninvasive targeting and penetration of the ocular posterior segment. Drug Deliv. 2019;26:989. doi: 10.1080/10717544.2019.1667455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kambhampati S.P., Clunies-Ross A.J.M., Bhutto I., Mishra M.K., Edwards M., McLeod D.S., Kannan R.M., Lutty G. Systemic and Intravitreal Delivery of Dendrimers to Activated Microglia/Macrophage in Ischemia/Reperfusion Mouse Retina. Investig. Ophthalmol. Vis. Sci. 2015;56:4413–4424. doi: 10.1167/iovs.14-16250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Iezzi R., Guru B.R., Glybina I.V., Mishra M.K., Kennedy A., Kannan R.M. Dendrimer-based targeted intravitreal therapy for sustained attenuation of neuroinflammation in retinal degeneration. Biomaterials. 2012;33:979–988. doi: 10.1016/j.biomaterials.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 64.Chetoni P., Burgalassi S., Monti D., Tampucci S., Tullio V., Cuffini A.M., Muntoni E., Spagnolo R., Zara G.P., Cavalli R. Solid lipid nanoparticles as promising tool for intraocular tobramycin delivery: Pharmacokinetic studies on rabbits. Eur. J. Pharm. Biopharm. 2016;109:214–223. doi: 10.1016/j.ejpb.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 65.Del Pozo-Rodríguez A., Delgado D., Gascón A.R., Solinís M.Á. Lipid nanoparticles as drug/gene delivery systems to the retina. J. Ocul. Pharmacol. Ther. 2013;29:173–188. doi: 10.1089/jop.2012.0128. [DOI] [PubMed] [Google Scholar]
- 66.Apaolaza P.S., del Pozo-Rodríguez A., Solinís M.A., Rodríguez J.M., Friedrich U., Torrecilla J., Weber B.H.F., Rodríguez-Gascón A. Structural recovery of the retina in a retinoschisin-deficient mouse after gene replacement therapy by solid lipid nanoparticles. Biomaterials. 2016;90:40–49. doi: 10.1016/j.biomaterials.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 67.Amadio M., Pascale A., Cupri S., Pignatello R., Osera C., D’Agata V., D’Amico A.G., Leggio G.M., Ruozi B., Govoni S., et al. Nanosystems based on siRNA silencing HuR expression counteract diabetic retinopathy in rat. Pharmacol. Res. 2016;111:713–720. doi: 10.1016/j.phrs.2016.07.042. [DOI] [PubMed] [Google Scholar]
- 68.Tabatabaei S.N., Derbali R.M., Yang C., Superstein R., Hamel P., Chain J.L., Hardy P. Co-delivery of miR-181a and melphalan by lipid nanoparticles for treatment of seeded retinoblastoma. J. Control. Release. 2019;298:177–185. doi: 10.1016/j.jconrel.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 69.Nahire R., Haldar M.K., Paul S., Mergoum A., Ambre A.H., Katti K.S., Gange K.N., Srivastava D.K., Sarkar K., Mallik S. Polymer-coated echogenic lipid nanoparticles with dual release triggers. Biomacromolecules. 2013;14:841–853. doi: 10.1021/bm301894z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hironaka K., Inokuchi Y., Tozuka Y., Shimazawa M., Hara H., Takeuchi H. Design and evaluation of a liposomal delivery system targeting the posterior segment of the eye. J. Control. Release. 2009;136:247–253. doi: 10.1016/j.jconrel.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 71.Shimazaki H., Hironaka K., Fujisawa T., Tsuruma K., Tozuka Y., Shimazawa M., Takeuchi H., Hara H. Edaravone-Loaded Liposome Eyedrops Protect against Light-Induced Retinal Damage in Mice. Investig. Ophthalmol. Vis. Sci. 2011;52:7289–7297. doi: 10.1167/iovs.11-7983. [DOI] [PubMed] [Google Scholar]
- 72.Lai S., Wei Y., Wu Q., Zhou K., Liu T., Zhang Y., Jiang N., Xiao W., Chen J., Liu Q., et al. Liposomes for effective drug delivery to the ocular posterior chamber. J. Nanobiotechnol. 2019;17:1–12. doi: 10.1186/s12951-019-0498-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Matsuo T., Masuda I., Yasuda T., Matsuo N. Gene transfer to the retina of rat by liposome eye drops. Biochem. Biophys. Res. Commun. 1996;219:947–950. doi: 10.1006/bbrc.1996.0326. [DOI] [PubMed] [Google Scholar]
- 74.Cholkar K., Gunda S., Earla R., Pal D., Mitra A.K. Nanomicellar Topical Aqueous Drop Formulation of Rapamycin for Back-of-the-Eye Delivery. AAPS PharmSciTech. 2015;16:610. doi: 10.1208/s12249-014-0244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Iriyama A., Oba M., Ishii T., Nishiyama N., Kataoka K., Tamaki Y., Yanagi Y. Gene Transfer Using Micellar Nanovectors Inhibits Choroidal Neovascularization In Vivo. PLoS ONE. 2011;6:28560. doi: 10.1371/journal.pone.0028560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ma F., Nan K., Lee S., Beadle J.R., Hou H., Freeman W.R., Hostetler K.Y., Cheng L. Micelle formulation of hexadecyloxypropyl-cidofovir (HDP-CDV) as an intravitreal long-lasting delivery system. Eur. J. Pharm. Biopharm. 2015;89:271–279. doi: 10.1016/j.ejpb.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu P., Jia Y., Qu F., Sun Y., Wang P., Zhang K., Xu C., Liu Q., Wang X. Ultrasound-Responsive Polymeric Micelles for Sonoporation-Assisted Site-Specific Therapeutic Action. ACS Appl. Mater. Interfaces. 2017;9:25706–25716. doi: 10.1021/acsami.7b05469. [DOI] [PubMed] [Google Scholar]
- 78.Zhang Y., Li P., Pan H., Liu L., Ji M., Sheng N., Wang C., Cai L., Ma Y. Retinal-conjugated pH-sensitive micelles induce tumor senescence for boosting breast cancer chemotherapy. Biomaterials. 2016;83:219–232. doi: 10.1016/j.biomaterials.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 79.Osipova O., Sharoyko V., Zashikhina N., Zakharova N., Tennikova T., Urtti A., Korzhikova-Vlakh E. Amphiphilic Polypeptides for VEGF siRNA Delivery into Retinal Epithelial Cells. Pharmaceutics. 2020;12:39. doi: 10.3390/pharmaceutics12010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nayak K., Misra M. Triamcinolone Acetonide-Loaded PEGylated Microemulsion for the Posterior Segment of Eye. ACS Omega. 2020;5:7928–7939. doi: 10.1021/acsomega.9b04244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang L., Liang W., Zhou K., Wassel R.A., Ridge Z.D., Ma J.X., Wang B. Therapeutic Effects of Fenofibrate Nano-Emulsion Eye Drops on Retinal Vascular Leakage and Neovascularization. Biology. 2021;10:1328. doi: 10.3390/biology10121328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hagigit T., Abdulrazik M., Valamanesh F., Behar-Cohen F., Benita S. Ocular antisense oligonucleotide delivery by cationic nanoemulsion for improved treatment of ocular neovascularization: An in-vivo study in rats and mice. J. Control. Release. 2012;160:225–231. doi: 10.1016/j.jconrel.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 83.Liu F., Lin S., Zhang Z., Hu J., Liu G., Tu Y., Yang Y., Zou H., Mo Y., Miao L. pH-responsive nanoemulsions for controlled drug release. Biomacromolecules. 2014;15:968–977. doi: 10.1021/bm4018484. [DOI] [PubMed] [Google Scholar]
- 84.Sivaram A.J., Rajitha P., Maya S., Jayakumar R., Sabitha M. Nanogels for delivery, imaging and therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015;7:509–533. doi: 10.1002/wnan.1328. [DOI] [PubMed] [Google Scholar]
- 85.Laradji A.M., Kolesnikov A.V., Karakoçak B.B., Kefalov V.J., Ravi N. Redox-Responsive Hyaluronic Acid-Based Nanogels for the Topical Delivery of the Visual Chromophore to Retinal Photoreceptors. ACS Omega. 2021;6:6172–6184. doi: 10.1021/acsomega.0c05535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Abdel-Rashid R.S., Helal D.A., Omar M.M., El Sisi A.M. Nanogel loaded with surfactant based nanovesicles for enhanced ocular delivery of acetazolamide. Int. J. Nanomed. 2019;14:2973–2983. doi: 10.2147/IJN.S201891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Patel D., Patel S.N., Chaudhary V., Garg S.J. Complications of intravitreal injections: 2022. Curr. Opin. Ophthalmol. 2022;33:137–146. doi: 10.1097/ICU.0000000000000850. [DOI] [PubMed] [Google Scholar]
- 88.Sharma A., Tandon A., Tovey J.C.K., Gupta R., Robertson J.D., Fortune J.A., Klibanov A.M., Cowden J.W., Rieger F.G., Mohan R.R. Polyethylenimine-Conjugated Gold Nanoparticles: Gene Transfer Potential and Low Toxicity in the Cornea. Pharm. Fac. Artic. Res. 2011;7:505–513. doi: 10.1016/j.nano.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sun J.G., Jiang Q., Zhang X.P., Shan K., Liu B.H., Zhao C., Yan B. Mesoporous silica nanoparticles as a delivery system for improving antiangiogenic therapy. Int. J. Nanomed. 2019;14:1489–1501. doi: 10.2147/IJN.S195504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mahaling B., Verma M., Mishra G., Chaudhuri S., Dutta D., Sivakumar S. Fate of GdF3 nanoparticles-loaded PEGylated carbon capsules inside mice model: A step toward clinical application. Nanotoxicology. 2020;14:577–594. doi: 10.1080/17435390.2019.1708494. [DOI] [PubMed] [Google Scholar]
- 91.Chu T.C., He Q., Potter D.E. Biodegradable calcium phosphate nanoparticles as a new vehicle for delivery of a potential ocular hypotensive agent. J. Ocul. Pharmacol. Ther. 2002;18:507–514. doi: 10.1089/108076802321021054. [DOI] [PubMed] [Google Scholar]
- 92.Rajendran Nair D.S., Seiler M.J., Patel K.H., Thomas V., Camarillo J.C.M., Humayun M.S., Thomas B.B. Tissue Engineering Strategies for Retina Regeneration. Appl. Sci. 2021;11:2154. doi: 10.3390/app11052154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chan S.Y., Chan B.Q.Y., Liu Z., Parikh B.H., Zhang K., Lin Q., Su X., Kai D., Choo W.S., Young D.J., et al. Electrospun pectin-polyhydroxybutyrate nanofibers for retinal tissue engineering. ACS Omega. 2017;2:8959–8968. doi: 10.1021/acsomega.7b01604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang P., Li X., Zhu W., Zhong Z., Moran A., Wang W., Zhang K., Chen S. 3D bioprinting of hydrogels for retina cell culturing. Bioprinting. 2018;11:e00029. doi: 10.1016/j.bprint.2018.e00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang T.C., Chuang J.H., Buddhakosai W., Wu W.J., Lee C.J., Chen W.S., Yang Y.P., Li M.C., Peng C.H., Chen S.J. Elongation of Axon Extension for Human iPSC-Derived Retinal Ganglion Cells by a Nano-Imprinted Scaffold. Int. J. Mol. Sci. 2017;18:2013. doi: 10.3390/ijms18092013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang D., Luo M., Huang B., Gao W., Jiang Y., Li Q., Nan K., Lin S. Localized co-delivery of CNTF and FK506 using a thermosensitive hydrogel for retina ganglion cells protection after traumatic optic nerve injury. Drug Deliv. 2020;27:556. doi: 10.1080/10717544.2020.1748759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mahaling B., Katti D.S. Fabrication of micro-structures of poly [(R)-3-hydroxybutyric acid] by electro-spraying/-spinning: Understanding the influence of polymer concentration and solvent type. J. Mater. Sci. 2014;49:4246–4260. doi: 10.1007/s10853-014-8120-8. [DOI] [Google Scholar]
- 98.Cellot G., La Monica S., Scaini D., Rauti R., Bosi S., Prato M., Gandolfi S., Ballerini L. Successful regrowth of retinal neurons when cultured interfaced to carbon nanotube platforms. J. Biomed. Nanotechnol. 2017;13:559–565. doi: 10.1166/jbn.2017.2364. [DOI] [Google Scholar]
- 99.Johnston H.J., Hutchison G.R., Christensen F.M., Peters S., Hankin S., Aschberger K., Stone V. A critical review of the biological mechanisms underlying the in vivo and in vitro toxicity of carbon nanotubes: The contribution of physico-chemical characteristics. Nanotoxicology. 2010;4:207–246. doi: 10.3109/17435390903569639. [DOI] [PubMed] [Google Scholar]
- 100.Hamidi M., Azadi A., Rafiei P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008;60:1638–1649. doi: 10.1016/j.addr.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 101.Sahoo S.K., Dilnawaz F., Krishnakumar S. Nanotechnology in ocular drug delivery. Drug Discov. Today. 2008;13:144–151. doi: 10.1016/j.drudis.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 102.Kahraman E., Güngör S., Özsoy Y. Potential enhancement and targeting strategies of polymeric and lipid-based nanocarriers in dermal drug delivery. Ther. Deliv. 2017;8:967–985. doi: 10.4155/tde-2017-0075. [DOI] [PubMed] [Google Scholar]
- 103.Zielinska A., Carreiró F., Oliveira A.M., Neves A., Pires B., Nagasamy Venkatesh D., Durazzo A., Lucarini M., Eder P., Silva A.M., et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules. 2020;25:3731. doi: 10.3390/molecules25163731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lang J.C. Ocular drug delivery conventional ocular formulations. Adv. Drug Deliv. Rev. 1995;16:39–43. doi: 10.1016/0169-409X(95)00012-V. [DOI] [Google Scholar]
- 105.Nagarwal R.C., Kant S., Singh P.N., Maiti P., Pandit J.K. Polymeric nanoparticulate system: A potential approach for ocular drug delivery. J. Control. Release. 2009;136:2–13. doi: 10.1016/j.jconrel.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 106.Barbu E., Verestiuc L., Nevell T.G., Tsibouklis J. Polymeric materials for ophthalmic drug delivery: Trends and perspectives. J. Mater. Chem. 2006;16:3439–3443. doi: 10.1039/b605640g. [DOI] [Google Scholar]
- 107.Ludwig A. The use of mucoadhesive polymers in ocular drug delivery. Adv. Drug Deliv. Rev. 2005;57:1595–1639. doi: 10.1016/j.addr.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 108.Colucci P., Giannaccini M., Baggiani M., Kennedy B.N., Dente L., Raffa V., Gabellini C. Neuroprotective Nanoparticles Targeting the Retina: A Polymeric Platform for Ocular Drug Delivery Applications. Pharmaceutics. 2023;15:1096. doi: 10.3390/pharmaceutics15041096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sánchez-López E., Egea M.A., Davis B.M., Guo L., Espina M., Silva A.M., Calpena A.C., Souto E.M.B., Ravindran N., Ettcheto M., et al. Memantine-Loaded PEGylated Biodegradable Nanoparticles for the Treatment of Glaucoma. Small. 2018;14:1701808. doi: 10.1002/smll.201701808. [DOI] [PubMed] [Google Scholar]
- 110.Dandamudi M., McLoughlin P., Behl G., Rani S., Coffey L., Chauhan A., Kent D., Fitzhenry L. Chitosan-Coated PLGA Nanoparticles Encapsulating Triamcinolone Acetonide as a Potential Candidate for Sustained Ocular Drug Delivery. Pharmaceutics. 2021;13:1590. doi: 10.3390/pharmaceutics13101590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Suri R., Nag T.C., Mehra N., Neupane Y.R., Shafi S., Sharma D., Sharma K., Sultana Y., Kohli K. Sirolimus Loaded Chitosan Functionalized PLGA Nanoparticles Protect Against Sodium Iodate-Induced Retinal Degeneration. J. Drug Deliv. Sci. Technol. 2023;82:104369. doi: 10.1016/j.jddst.2023.104369. [DOI] [Google Scholar]
- 112.Radwan S.E.S., El-Kamel A., Zaki E.I., Burgalassi S., Zucchetti E., El-Moslemany R.M. Hyaluronic-Coated Albumin Nanoparticles for the Non-Invasive Delivery of Apatinib in Diabetic Retinopathy. Int. J. Nanomed. 2021;16:4481–4494. doi: 10.2147/IJN.S316564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chittasupho C., Kengtrong K., Chalermnithiwong S., Sarisuta N. Anti-angiogenesis by dual action of R5K peptide conjugated itraconazole nanoparticles. AAPS PharmSciTech. 2020;21:74. doi: 10.1208/s12249-019-1568-8. [DOI] [PubMed] [Google Scholar]
- 114.Lou X., Hu Y., Zhang H., Liu J., Zhao Y. Polydopamine nanoparticles attenuate retina ganglion cell degeneration and restore visual function after optic nerve injury. J. Nanobiotechnol. 2021;19:1–15. doi: 10.1186/s12951-021-01199-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Delrish E., Ghassemi F., Jabbarvand M., Lashay A., Atyabi F., Soleimani M., Dinarvand R. Biodistribution of Cy5-labeled Thiolated and Methylated Chitosan-Carboxymethyl Dextran Nanoparticles in an Animal Model of Retinoblastoma. J. Ophthalmic Vis. Res. 2022;17:58–68. doi: 10.18502/JOVR.V17I1.10171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mahaling B., Katti D.S. Understanding the influence of surface properties of nanoparticles and penetration enhancers for improving bioavailability in eye tissues in vivo. Int. J. Pharm. 2016;501:1–9. doi: 10.1016/j.ijpharm.2016.01.053. [DOI] [PubMed] [Google Scholar]
- 117.Mahaling B., Katti D.S. Physicochemical properties of core-shell type nanoparticles govern their spatiotemporal biodistribution in the eye. Nanomed. Nanotechnol. Biol. Med. 2016;12:2149–2160. doi: 10.1016/j.nano.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 118.Wang Y., Liu C.H., Ji T., Mehta M., Wang W., Marino E., Chen J., Kohane D.S. Intravenous treatment of choroidal neovascularization by photo-targeted nanoparticles. Nat. Commun. 2019;10:1–9. doi: 10.1038/s41467-019-08690-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Battaglia L., Gallarate M. Lipid nanoparticles: State of the art, new preparation methods and challenges in drug delivery. Expert Opin. Drug Deliv. 2012;9:497–508. doi: 10.1517/17425247.2012.673278. [DOI] [PubMed] [Google Scholar]
- 120.Ghasemiyeh P., Mohammadi-Samani S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: Applications, advantages and disadvantages. Res. Pharm. Sci. 2018;13:288–303. doi: 10.4103/1735-5362.235156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xu L., Wang X., Liu Y., Yang G., Falconer R.J., Zhao C.-X. Lipid Nanoparticles for Drug Delivery. Adv. NanoBiomed. Res. 2022;2:2100109. doi: 10.1002/anbr.202100109. [DOI] [Google Scholar]
- 122.Yadav M., Schiavone N., Guzman-Aranguez A., Giansanti F., Papucci L., Perez de Lara M.J., Singh M., Kaur I.P. Correction to: Atorvastatin-loaded solid lipid nanoparticles as eye drops: Proposed treatment option for age-related macular degeneration (AMD) Drug Deliv. Transl. Res. 2020;10:1531. doi: 10.1007/s13346-020-00777-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.del Pozo-Rodríguez A., Delgado D., Solinís M.Á., Pedraz J.L., Echevarría E., Rodríguez J.M., Gascón A.R. Solid lipid nanoparticles as potential tools for gene therapy: In vivo protein expression after intravenous administration. Int. J. Pharm. 2010;385:157–162. doi: 10.1016/j.ijpharm.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 124.Li W., Chen L., Gu Z., Chen Z., Li H., Cheng Z., Li H., Zou L. Co-delivery of microRNA-150 and quercetin by lipid nanoparticles (LNPs) for the targeted treatment of age-related macular degeneration (AMD) J. Control. Release. 2023;355:358–370. doi: 10.1016/j.jconrel.2023.01.080. [DOI] [PubMed] [Google Scholar]
- 125.Supe S., Upadhya A., Tripathi S., Dighe V., Singh K. Liposome-polyethylenimine complexes for the effective delivery of HuR siRNA in the treatment of diabetic retinopathy. Drug Deliv. Transl. Res. 2023;13:1675–1698. doi: 10.1007/s13346-022-01281-9. [DOI] [PubMed] [Google Scholar]
- 126.Sharma D.S., Wadhwa S., Gulati M., Kumar B., Chitranshi N., Gupta V.K., Alrouji M., Alhajlah S., AlOmeir O., Vishwas S., et al. Chitosan modified 5-fluorouracil nanostructured lipid carriers for treatment of diabetic retinopathy in rats: A new dimension to an anticancer drug. Int. J. Biol. Macromol. 2023;224:810–830. doi: 10.1016/j.ijbiomac.2022.10.168. [DOI] [PubMed] [Google Scholar]
- 127.Christensen G., Urimi D., Lorenzo-Soler L., Schipper N., Paquet-Durand F. Ocular permeability, intraocular biodistribution of lipid nanocapsule formulation intended for retinal drug delivery. Eur. J. Pharm. Biopharm. 2023;187:175–183. doi: 10.1016/j.ejpb.2023.04.012. [DOI] [PubMed] [Google Scholar]
- 128.Huang L., Himawan E., Belhadj S., Pérez García R.O., Paquet Durand F., Schipper N., Buzgo M., Simaite A., Marigo V. Efficient Delivery of Hydrophilic Small Molecules to Retinal Cell Lines Using Gel Core-Containing Solid Lipid Nanoparticles. Pharmaceutics. 2021;14:74. doi: 10.3390/pharmaceutics14010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cheng Z., Li Y., Wang K., Zhu X., Tharkar P., Shu W., Zhang T., Zeng S., Zhu L., Murray M., et al. Compritol solid lipid nanoparticle formulations enhance the protective effect of betulinic acid derivatives in human Müller cells against oxidative injury. Exp. Eye Res. 2022;215:108906. doi: 10.1016/j.exer.2021.108906. [DOI] [PubMed] [Google Scholar]
- 130.Gautam M., Ryals R.C., Sahay G. Novel lipid nanoparticle variants for in-vivo mRNA delivery to photoreceptors. Investig. Ophthalmol. Vis. Sci. 2022;63:69-A0042. [Google Scholar]
- 131.Zingale E., Rizzo S., Bonaccorso A., Consoli V., Vanella L., Musumeci T., Spadaro A., Pignatello R. Optimization of Lipid Nanoparticles by Response Surface Methodology to Improve the Ocular Delivery of Diosmin: Characterization and In-Vitro Anti-Inflammatory Assessment. Pharmaceutics. 2022;14:1961. doi: 10.3390/pharmaceutics14091961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Huang Y., Zhu Y., Cai D., Guo Q., Wang J., Lei L., Li X., Shi S. Penetrating-peptide-mediated non-invasive Axitinib delivery for anti-neovascularisation. J. Control. Release. 2022;347:449–459. doi: 10.1016/j.jconrel.2022.05.009. [DOI] [PubMed] [Google Scholar]
- 133.Urquhart A.J., Eriksen A.Z. Recent developments in liposomal drug delivery systems for the treatment of retinal diseases. Drug Discov. Today. 2019;24:1660–1668. doi: 10.1016/j.drudis.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 134.Bulbake U., Doppalapudi S., Kommineni N., Khan W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics. 2017;9:12. doi: 10.3390/pharmaceutics9020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mishra G.P., Bagui M., Tamboli V., Mitra A.K. Recent applications of liposomes in ophthalmic drug delivery. J. Drug Deliv. 2011;2011:1–14. doi: 10.1155/2011/863734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Agarwal R., Iezhitsa I., Agarwal P., Abdul Nasir N.A., Razali N., Alyautdin R., Ismail N.M. Liposomes in topical ophthalmic drug delivery: An update. Drug Deliv. 2016;23:1075–1091. doi: 10.3109/10717544.2014.943336. [DOI] [PubMed] [Google Scholar]
- 137.Ribeiro M.C.S., de Miranda M.C., Cunha P.d.S., Andrade G.F., Fulgêncio G.d.O., Gomes D.A., Fialho S.L., Pittella F., Charrueau C., Escriou V., et al. Neuroprotective effect of siRNA entrapped in hyaluronic acid-coated lipoplexes by intravitreal administration. Pharmaceutics. 2021;13:845. doi: 10.3390/pharmaceutics13060845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Normando E.M., Davis B., Brenton J., Guo L., Cordeiro M.F. Retinal Neuroprotective Effect of a Liposomal Formulation of Rosiglitazone in a Rat Model of Parkinson’s Disease. Investig. Ophthalmol. Vis. Sci. 2016;57:4406. [Google Scholar]
- 139.Fujisawa T., Miyai H., Hironaka K., Tsukamoto T., Tahara K., Tozuka Y., Ito M., Takeuchi H. Liposomal diclofenac eye drop formulations targeting the retina: Formulation stability improvement using surface modification of liposomes. Int. J. Pharm. 2012;436:564–567. doi: 10.1016/j.ijpharm.2012.07.024. [DOI] [PubMed] [Google Scholar]
- 140.Christensen G., Chen Y., Urimi D., Zizmare L., Trautwein C., Schipper N., Paquet-Durand F. Pyruvate-conjugation of PEGylated liposomes for targeted drug delivery to retinal photoreceptors. Biomed. Pharmacother. 2023;163:114717. doi: 10.1016/j.biopha.2023.114717. [DOI] [PubMed] [Google Scholar]
- 141.Li Z., Yu H., Liu C., Wang C., Zeng X., Yan J., Sun Y. Efficiency co-delivery of ellagic acid and oxygen by a non-invasive liposome for ameliorating diabetic retinopathy. Int. J. Pharm. 2023;641:122987. doi: 10.1016/j.ijpharm.2023.122987. [DOI] [PubMed] [Google Scholar]
- 142.Nishida S., Takashima Y., Udagawa R., Ibaraki H., Seta Y., Ishihara H. A Multifunctional Hybrid Nanocarrier for Non-Invasive siRNA Delivery to the Retina. Pharmaceutics. 2023;15:611. doi: 10.3390/pharmaceutics15020611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tavakoli S., Puranen J., Bahrpeyma S., Lautala V.E., Karumo S., Lajunen T., del Amo E.M., Ruponen M., Urtti A. Liposomal sunitinib for ocular drug delivery: A potential treatment for choroidal neovascularization. Int. J. Pharm. 2022;620:121725. doi: 10.1016/j.ijpharm.2022.121725. [DOI] [PubMed] [Google Scholar]
- 144.Cheng T., Li J., Cheng Y., Zhang X., Qu Y. Triamcinolone acetonide-chitosan coated liposomes efficiently treated retinal edema as eye drops. Exp. Eye Res. 2019;188:107805. doi: 10.1016/j.exer.2019.107805. [DOI] [PubMed] [Google Scholar]
- 145.Asasutjarit R., Managit C., Phanaksri T., Treesuppharat W., Fuongfuchat A. Formulation development and in vitro evaluation of transferrin-conjugated liposomes as a carrier of ganciclovir targeting the retina. Int. J. Pharm. 2020;577:119084. doi: 10.1016/j.ijpharm.2020.119084. [DOI] [PubMed] [Google Scholar]
- 146.Lee J., Goh U., Lee H.J., Kim J., Jeong M., Park J.H. Effective retinal penetration of lipophilic and lipid-conjugated hydrophilic agents delivered by engineered liposomes. Mol. Pharm. 2017;14:423–430. doi: 10.1021/acs.molpharmaceut.6b00864. [DOI] [PubMed] [Google Scholar]
- 147.Lu Y., Park K. Polymeric micelles and alternative nanonized delivery vehicles for poorly soluble drugs. Int. J. Pharm. 2013;453:198–214. doi: 10.1016/j.ijpharm.2012.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Arnarson T., Elworthy P.H. Effects of structural variations of non-ionic surfactants on micellar properties and solubilization: Surfactants based on erucyl and behenyl (C22) alcohols. J. Pharm. Pharmacol. 2011;32:381–385. doi: 10.1111/j.2042-7158.1980.tb12947.x. [DOI] [PubMed] [Google Scholar]
- 149.Talelli M., Barz M., Rijcken C.J.F., Kiessling F., Hennink W.E., Lammers T. Core-Crosslinked Polymeric Micelles: Principles, Preparation, Biomedical Applications and Clinical Translation. Nano Today. 2015;10:93–117. doi: 10.1016/j.nantod.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hughes P.M., Olejnik O., Chang-Lin J.E., Wilson C.G. Topical and systemic drug delivery to the posterior segments. Adv. Drug Deliv. Rev. 2005;57:2010–2032. doi: 10.1016/j.addr.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 151.Kabanov A.V., Kabanov V.A. DNA Complexes with Polycations for the Delivery of Genetic Material into Cells. Bioconjug. Chem. 1995;6:7–20. doi: 10.1021/bc00031a002. [DOI] [PubMed] [Google Scholar]
- 152.Mandal A., Bisht R., Rupenthal I.D., Mitra A.K. Polymeric micelles for ocular drug delivery: From structural frameworks to recent preclinical studies. J. Control. Release. 2017;248:96–116. doi: 10.1016/j.jconrel.2017.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Li Z., Liu M., Ke L., Wang L.J., Wu C., Li C., Li Z., Wu Y.L. Flexible polymeric nanosized micelles for ophthalmic drug delivery: Research progress in the last three years. Nanoscale Adv. 2021;3:5240–5254. doi: 10.1039/D1NA00596K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Xu L., Shen W., Wang B., Wang X., Liu G., Tao Y., Qi R. Efficient siRNA Delivery Using PEG-conjugated PAMAM Dendrimers Targeting Vascular Endothelial Growth Factor in a CoCl2-induced Neovascularization Model in Retinal Endothelial Cells. Curr. Drug Deliv. 2016;13:590–599. doi: 10.2174/1567201812666150817123049. [DOI] [PubMed] [Google Scholar]
- 155.Aurelia Chis A., Dobrea C., Morgovan C., Arseniu A.M., Rus L.L., Butuca A., Juncan A.M., Totan M., Vonica-Tincu A.L., Cormos G., et al. Applications and Limitations of Dendrimers in Biomedicine. Molecules. 2020;25:3982. doi: 10.3390/MOLECULES25173982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yavuz B., Bozdaǧ Pehlivan S., Ünlü N. Dendrimeric Systems and Their Applications in Ocular Drug Delivery. Sci. World J. 2013;2013:13. doi: 10.1155/2013/732340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nayak K., Misra M. PEGylated microemulsion for dexamethasone delivery to posterior segment of eye. J. Biomater. Sci. Polym. Ed. 2020;31:1071–1090. doi: 10.1080/09205063.2020.1740964. [DOI] [PubMed] [Google Scholar]
- 158.Ahmed S., Amin M.M., Sayed S. Ocular Drug Delivery: A Comprehensive Review. AAPS PharmSciTech. 2023;24:66. doi: 10.1208/s12249-023-02516-9. [DOI] [PubMed] [Google Scholar]
- 159.Dhahir R.K., Al-Nima A.M., Al-Bazzaz F.Y. Nanoemulsions as Ophthalmic Drug Delivery Systems. Turkish J. Pharm. Sci. 2021;18:652. doi: 10.4274/tjps.galenos.2020.59319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wu Y., Tao Q., Xie J., Lu L., Xie X., Zhang Y., Jin Y. Advances in Nanogels for Topical Drug Delivery in Ocular Diseases. Gels. 2023;9:292. doi: 10.3390/gels9040292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Soni K.S., Desale S.S., Bronich T.K. Nanogels: An overview of properties, biomedical applications and obstacles to clinical translation. J. Control. Release. 2016;240:109–126. doi: 10.1016/j.jconrel.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yang H., Tyagi P., Kadam R.S., Holden C.A., Kompella U.B. Hybrid dendrimer hydrogel/PLGA nanoparticle platform sustains drug delivery for one week and antiglaucoma effects for four days following one-time topical administration. ACS Nano. 2012;6:7595–7606. doi: 10.1021/nn301873v. [DOI] [PubMed] [Google Scholar]
- 163.Yu Y., Huang Y., Feng W., Yang M., Shao B., Li J., Ye F. NIR-triggered upconversion nanoparticles@thermo-sensitive liposome hybrid theranostic nanoplatform for controlled drug delivery. RSC Adv. 2021;11:29065–29072. doi: 10.1039/D1RA04431A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ju C., Sun J., Zi P., Jin X., Zhang C. Thermosensitive micelles-hydrogel hybrid system based on poloxamer 407 for localized delivery of paclitaxel. J. Pharm. Sci. 2013;102:2707–2717. doi: 10.1002/jps.23649. [DOI] [PubMed] [Google Scholar]
- 165.Ito T., Yoshida C., Murakami Y. Design of novel sheet-shaped chitosan hydrogel for wound healing: A hybrid biomaterial consisting of both PEG-grafted chitosan and crosslinkable polymeric micelles acting as drug containers. Mater. Sci. Eng. C. 2013;33:3697–3703. doi: 10.1016/j.msec.2013.04.056. [DOI] [PubMed] [Google Scholar]
- 166.Zhang L., Liu X.G., Liu D.Q., Yu X.L., Zhang L.X., Zhu J., Lu S., Liu R.T. A Conditionally Releasable “Do not Eat Me” CD47 Signal Facilitates Microglia-Targeted Drug Delivery for the Treatment of Alzheimer’s Disease. Adv. Funct. Mater. 2020;30:1910691. doi: 10.1002/adfm.201910691. [DOI] [Google Scholar]
- 167.Montes-Cobos E., Ring S., Fischer H.J., Heck J., Strauß J., Schwaninger M., Reichardt S.D., Feldmann C., Lühder F., Reichardt H.M. Targeted delivery of glucocorticoids to macrophages in a mouse model of multiple sclerosis using inorganic-organic hybrid nanoparticles. J. Control. Release. 2017;245:157–169. doi: 10.1016/j.jconrel.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 168.Fernández-Sánchez L., Lax P., Campello L., Pinilla I., Cuenca N. Astrocytes and Müller cell alterations during retinal degeneration in a transgenic rat model of retinitis pigmentosa. Front. Cell. Neurosci. 2015;9:484. doi: 10.3389/fncel.2015.00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jimnez J.L., Clemente M.I., Weber N.D., Sanchez J., Ortega P., De La Mata F.J., Gmez R., Garca D., Lpez-Fernndez L.A., Muoz-Fernndez M.N. Carbosilane dendrimers to transfect human astrocytes with small interfering RNA targeting human immunodeficiency virus. BioDrugs. 2010;24:331–343. doi: 10.2165/11538400-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 170.Patel S., Ryals R.C., Weller K.K., Pennesi M.E., Sahay G. Lipid nanoparticles for delivery of messenger RNA to the back of the eye. J. Control. Release. 2019;303:91–100. doi: 10.1016/j.jconrel.2019.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Glassman P.M., Myerson J.W., Ferguson L.T., Kiseleva R.Y., Shuvaev V.V., Brenner J.S., Muzykantov V.R. Targeting drug delivery in the vascular system: Focus on endothelium. Adv. Drug Deliv. Rev. 2020;157:96–117. doi: 10.1016/j.addr.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bourges J.L., Gautier S.E., Delie F., Bejjani R.A., Jeanny J.C., Gurny R., BenEzra D., Behar-Cohen F.F. Ocular drug delivery targeting the retina and retinal pigment epithelium using polylactide nanoparticles. Investig. Ophthalmol. Vis. Sci. 2003;44:3562–3569. doi: 10.1167/iovs.02-1068. [DOI] [PubMed] [Google Scholar]
- 173.Tuovinen L., Ruhanen E., Kinnarinen T., Rönkkö S., Pelkonen J., Urtti A., Peltonen S., Järvinen K. Starch acetate microparticles for drug delivery into retinal pigment epithelium—In vitro study. J. Control. Release. 2004;98:407–413. doi: 10.1016/j.jconrel.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 174.Zhao L., Chen G., Li J., Fu Y., Mavlyutov T.A., Yao A., Nickells R.W., Gong S., Guo L.W. An intraocular drug delivery system using targeted nanocarriers attenuates retinal ganglion cell degeneration. J. Control. Release. 2017;247:153–166. doi: 10.1016/j.jconrel.2016.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Farjo R., Skaggs J., Quiambao A.B., Cooper M.J., Naash M.I. Efficient non-viral ocular gene transfer with compacted DNA nanoparticles. PLoS ONE. 2006;1:e38. doi: 10.1371/journal.pone.0000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Patel D.M., Patel N.N., Patel J.K. Emerging Technologies for Nanoparticle Manufacturing. Springer International Publishing; Cham, Switzerland: 2021. Nanomedicine scale-up technologies: Feasibilities and challenges; pp. 511–539. [DOI] [Google Scholar]
- 177.Sun D., Schur R.M., Sears A.E., Gao S.Q., Sun W., Naderi A., Kern T., Palczewski K., Lu Z.R. Stable Retinoid Analogue Targeted Dual pH-Sensitive Smart Lipid ECO/ pDNA Nanoparticles for Specific Gene Delivery in the Retinal Pigment Epithelium. ACS Appl. Bio Mater. 2020;3:3078–3086. doi: 10.1021/acsabm.0c00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Obata Y., Tajima S., Takeoka S. Evaluation of pH-responsive liposomes containing amino acid-based zwitterionic lipids for improving intracellular drug delivery in vitro and in vivo. J. Control. Release. 2010;142:267–276. doi: 10.1016/j.jconrel.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 179.Mohan R.R., Tovey J.C.K., Sharma A., Tandon A. Gene therapy in the Cornea: 2005–present. Prog. Retin. Eye Res. 2012;31:43–64. doi: 10.1016/j.preteyeres.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Donnelly K.S., Giuliano E.A., Sharma A., Tandon A., Rodier J.T., Mohan R.R. Decorin-PEI nanoconstruct attenuates equine corneal fibroblast differentiation. Vet. Ophthalmol. 2014;17:162–169. doi: 10.1111/vop.12060. [DOI] [PubMed] [Google Scholar]
- 181.Liao H.W., Yau K.W. In vivo gene delivery in the retina using polyethylenimine. Biotechniques. 2007;42:285–288. doi: 10.2144/000112404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tandon A., Sharma A., Rodier J.T., Klibanov A.M., Rieger F.G., Mohan R.R. BMP7 Gene Transfer via Gold Nanoparticles into Stroma Inhibits Corneal Fibrosis In Vivo. PLoS ONE. 2013;8:e66434. doi: 10.1371/journal.pone.0066434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Erickson K.K., Sundstrom J.M., Antonetti D.A. Vascular permeability in ocular disease and the role of tight junctions. Angiogenesis. 2007;10:103–117. doi: 10.1007/s10456-007-9067-z. [DOI] [PubMed] [Google Scholar]
- 184.Ando N., Sen H.A., Berkowitz B.A., Wilson C.A., Juan E. Localization and quantitation of blood-retinal barrier breakdown in experimental proliferative vitreoretinopathy. Arch. Ophthalmol. 1994;112:117–122. doi: 10.1001/archopht.1994.01090130127029. [DOI] [PubMed] [Google Scholar]
- 185.Wang Y., Wang Z., Xu C., Tian H., Chen X. A disassembling strategy overcomes the EPR effect and renal clearance dilemma of the multifunctional theranostic nanoparticles for cancer therapy. Biomaterials. 2019;197:284–293. doi: 10.1016/j.biomaterials.2019.01.025. [DOI] [PubMed] [Google Scholar]
- 186.Yuan X., Marcano D.C., Shin C.S., Hua X., Isenhart L.C., Pflugfelder S.C., Acharya G. Ocular drug delivery nanowafer with enhanced therapeutic efficacy. ACS Nano. 2015;9:1749–1758. doi: 10.1021/nn506599f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.