Abstract
DNA vaccination can induce humoral and cellular immune response to viral antigens and confer protection to virus infection. In woodchucks, we tested the protective efficacy of immune response to woodchuck hepatitis core antigen (WHcAg) and surface antigen (WHsAg) of woodchuck hepatitis virus (WHV) elicited by DNA-based vaccination. Plasmids pWHcIm and pWHsIm containing WHV c- or pre-s2/s genes expressed WHcAg and WHsAg in transient transfection assays. Pilot experiments in mice revealed that a single intramuscular injection of 100 μg of plasmid pWHcIm DNA induced an anti-WHcAg titer over 1:300 that was enhanced by boost injections. However, two injections of 100 μg of pWHcIm did not induce detectable anti-WHcAg in woodchucks. With an increase in the dose to 1 mg of pWHcIm per injection, transient anti-WHcAg response and WHcAg-specific proliferation of peripheral mononuclear blood cells (PMBCs) appeared in woodchucks after repeated immunizations. Four woodchucks vaccinated with pWHcIm were challenged with 104 or 105 of the WHV 50% infective dose. They remained negative for markers of WHV replication (WHV DNA and WHsAg) in peripheral blood and developed anti-WHs in week 5 after challenge. In contrast, woodchucks not immunized or immunized with the control vector pcDNA3 developed acute WHV infection. Two woodchucks immunized with 1 mg of pWHsIm developed WHsAg-specific proliferative response of PBMCs but no measurable anti-WHsAg response. A rapid anti-WHsAg response developed during week 2 after virus challenge. Neither woodchuck developed any signs of WHV infection. These data indicate that DNA-based vaccination with WHcAg and WHsAg can elicit immunity to WHV infection.
Hepatitis B virus (HBV) causes acute self-limiting and chronic infection in humans (24). A chronic HBV infection leads to a high risk for the development of liver cirrhosis and hepatocellular carcinoma (30, 54). The current strategy for preventing HBV infection is vaccination with hepatitis B surface antigen (HBsAg), which induces virus-neutralizing anti-HBsAg antibodies (28). Though HBsAg is a potent immunogen and induces protective immunity in the majority of vaccines, 5 to 10% of persons who receive the HBsAg vaccine failed to develop anti-HBsAg antibodies. In addition, HBV variants carrying mutations within the HBsAg can escape the neutralization of vaccine-induced anti-HBsAg and establish acute or chronic infection (3, 4, 6, 25, 29, 43). Therefore, a new vaccine strategy would be desirable to induce a multiple immune response consisting of HBV-specific T helper (Th), cytotoxic T cells (CTLs), and anti-HBsAg antibodies. HBV-specific Th and CTL responses play a pivotal role for the clearance of virus in a primary HBV infection and may control HBV persisting in unknown reservoirs in patients whose disease is resolved (1, 7, 16, 18, 26, 27, 35, 41, 42, 44, 45). The induction of HBV-specific humoral and cellular immune response by a single vaccine may overcome the nonresponsiveness of individuals to conventional HBsAg vaccines and control immune escape variants of HBV with mutations within HBsAg.
DNA vaccination is a powerful method to induce antigen-specific humoral and cellular immune response (14, 56). DNA-induced immune response provides protective immunity to various viruses in animal models (2, 5, 13, 19, 22, 31, 34, 36, 51, 55, 57). Genetic vaccination to HBsAg, HBV core antigen (HBcAg), and HBV e antigen (HBeAg) was evaluated in different animal models. In mice, a single intramuscular injection of plasmids expressing HBsAg is sufficient to induce a long-lasting humoral response to HBsAg and CTL response (10, 12, 40, 50). A plasmid vaccination of chimpanzees led to the production of low anti-HBsAg antibody titers (11, 47). Recently, Triyatni et al. reported that vaccination of ducks with plasmid expressing duck hepatitis B virus (DHBV) surface antigens (DHBsAg) induced antibodies to DHBsAg (55). Anti-DHBsAg antibodies induced by DNA vaccination were able to neutralize virus in vitro. DHBV was removed more rapidly from the bloodstreams of vaccinated ducks after a challenge. Infection of hepatocytes by DHBV was limited or prevented in vaccinated ducks. Therefore, the genetic vaccination was effective to prime an anti-HBsAg antibody response in this model. The vaccination of mice with HBcAg or HBeAg was also effective for inducing specific CTL responses (33).
The woodchuck (Marmota monax) model is useful to study immune response to hepadnavirus and to perform vaccination trials (8, 9, 23, 39, 48, 49, 52). Woodchuck hepatitis virus (WHV) causes acute self-limiting and chronic infection, like HBV in humans (53). The humoral immune responses to woodchuck hepatitis surface antigen (WHsAg) and core antigen (WHcAg) in acute and chronic WHV infection have the same features as those of HBV infection. Anti-WHcAg develops in woodchucks during the early phase of a primary WHV infection and persists lifelong. Anti-WHsAgs, like anti-HBsAgs, increase at the end of the viremic phase and may provide immunity to a secondary WHV infection. Recently, T-cell response to WHsAg and WHcAg in woodchucks during acute and chronic WHV infection was investigated by an in vitro assay to measure the antigen-specific proliferation of peripheral blood mononuclear cells (PBMCs) (8, 32, 38, 39). Multispecific Th response to WHcAg and WHsAg was present during acute WHV infection but absent in woodchucks with chronic WHV infection (39). Thus, the Th response to WHV in woodchucks closely resembles the HBV-specific Th response in humans (52).
The woodchuck model is informative in the study of immune response induced by vaccines and virus challenge. Immunization of woodchucks with WHsAg-induced anti-WHsAg antibodies provided protection against a subsequent challenge with WHV (9). Interestingly, woodchucks immunized with WHcAg were protected against WHV challenge even though anti-WHcAg antibodies do not possess the ability to neutralize WHV (49, 52). Apparently, WHcAg induced a specific T-cell response which conferred protective immunity (39). We demonstrated that immunization with a peptide containing a T-cell epitope derived from WHcAg leads to the protection of woodchucks against WHV infection. These results emphasize the significance of T-cell response to the core antigen for control of hepadnavirus infection (16, 18).
In the present study, we wanted to determine whether vaccination of woodchucks with plasmids expressing WHV proteins can induce a protective immune response to WHV. We vaccinated mice and woodchucks with plasmids expressing WHcAg and WHsAg and investigated the humoral and cellular immune response to WHcAg and WHsAg in woodchucks. The protective efficacy of plasmid vaccination was demonstrated in woodchucks in subsequent challenge experiments.
MATERIALS AND METHODS
Woodchucks.
Adult WHV-negative woodchucks trapped in the state of New York were purchased from North Eastern Wildlife (Ithaca, N.Y.). Previous exposure to WHV of these woodchucks was excluded by testing for anti-WHcAg, anti-WHsAg, and WHsAg.
Construction of plasmids pWHcIm and pWHsIm for DNA vaccination.
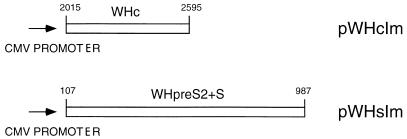
The core gene of WHV8 was amplified by PCR with primers wc1 (nucleotides [nt] 2015 to 2038, 5′-TGGGGCCATGGACATAGATCCTTA-3′) and wc2 (nt 2595 to 2570, 5′-CATTGAATTCAGCAGTTGGCAGATGG-3′) according to the sequence described by Girones et al. (21). The PCR products were cloned into pCRII vectors (Invitrogen, San Diego, Calif.) according to the manufacturer’s instructions. A clone, pWHc, was selected by sequencing to verify the correct nucleotide sequence of the PCR product. The fragment containing the WHV core gene was isolated by digestion with EcoRI and inserted into the EcoRI site of the pcDNA3 vector (Invitrogen). A generated plasmid, pWHcIm, contains the WHV core gene under the control of the cytomegalovirus (CMV) promoter (Fig. 1). The pre-S2-S region of WHV8 (nt 107 to 987) was amplified by PCR with primers whpres2 (nt 107 to 129, 5′-CACTTAACTATGAAAAATCAGAC-3′) and whs2 (nt 987 to 968, 5′-CCACCATTTTGTTTTATTAA-3′). This PCR fragment was cloned into pCRII vector and recloned into the EcoRI site of pcDNA3 to generate plasmid pWHsIm by a procedure similar to that described above (Fig. 1). The integrity of the clones was verified by sequencing.
FIG. 1.
Construction of pWHcIm and pWHsIm. Both pWHcIm and pWHsIm were constructed on the basis of pcDNA3 (Invitrogen). The fragments from the WHV genome comprising the complete reading frame of WHcAg and WHsAg (including pre-S2) were cloned into pcDNA3 as described in Materials and Methods. The expression of WHcAg and WHsAg is under the control of the CMV promoter of the vector. The cloned fragments of the WHV genome are numbered according to Girones et al. (21).
Purification of plasmids for immunization.
Plasmids pWHcIm and pWHsIm for immunization were prepared by using the Giga plasmid purification kit (Qiagen, Hilden, Germany). Plasmids were dissolved in phosphate-buffered saline (PBS) at a concentration of 1 mg/ml. The amount of bacterial protein contaminants in these preparations was in the range of 11 ng/ml, as determined by using microbicinchoninic acid (BCA) protein assay reagent (Pierce, Oud Beijerland, The Netherlands).
Transient expression of WHcAg and WHsAg by transfection of pWHcIm and pWHsIm into a baby hamster kidney (BHK) cell line and a woodchuck liver cell line.
A BHK cell line and a woodchuck liver cell line WH12/6 (kindly provided by P. Banasch, Deutsche Krebsforschungszentrum, Heidelberg, Germany) were used for transfection experiments. Transfection of liver cells was performed with Lipofectamine (Gibco BRL, Eggenstein-Leopoldshafen, Germany). Plasmid (4 μg) was incubated with 10 μg of lipofectamine in 100 μl of media for 45 min and incubated further with cells in 1 ml of Opti-Media (Gibco BRL) for 6 h at 37°C, 5% CO2. Transfected cells were maintained for 48 h at 37°C in 5% CO2 and fixed with acetone-methanol (1:1). The expressed WHcAg and WHsAg were detected by indirect immunofluorescence staining with rabbit antisera to respective WHV proteins.
Immunization of mice and woodchucks by intramuscular injection of pWHcIm and pWHsIm.
Immunization of mice was performed by the procedure described by Schirmbeck et al. (50). Briefly, mice were pretreated by intramuscular injection of 50 μl of cardiotoxin (10 μM) into musculus tibialis anterior. After a week, 50 μg of plasmid (1 mg/ml) was injected into each site in the same muscle. The plasmid injection was repeated twice at 3-week intervals. Mice were sacrificed 3 weeks after the last immunization. This immunization protocol was modified for woodchucks. A week prior to the injection of plasmids, 500 μl of cardiotoxin (10 μM in PBS) was injected into the M. tibialis cranialis of woodchucks. Woodchucks were vaccinated three times by intramuscular injection of 50 or 500 μl of plasmid (1 mg/ml in PBS) into each M. tibialis cranialis at 4- or 5-week intervals. Four weeks after the last vaccination, woodchucks were challenged with an inoculum containing 104 or 105 WHV genome equivalents.
Serology and detection of WHV DNA.
Anti-WHcAg, anti-WHsAg, and WHsAg were determined by enzyme-linked immunosorbent assay (ELISA) as described previously (49, 52). The sensitivity of ELISA was determined by tests of serially diluted positive sera of woodchucks experimentally infected with WHV. The ELISA was able to detect anti-WHcAg in woodchuck sera at a dilution of 10−3 to 10−6. Anti-WHsAg titers of post-acute phase sera were positive, ranging between 10−3 and 10−4. The ELISA for WHsAg detected WHsAg in sera of chronic WHV-infected woodchucks in dilutions of up to 10−4. The dot blot technique was routinely performed to detect WHV DNA in woodchuck sera. For PCR detection of WHV DNA in woodchuck sera, nucleic acids were isolated from sera by proteinase K digestion and phenol extraction. PCR for amplification of the WHV core gene was run with primers wc1 (nt 2015 to 2038, 5′-TGGGGCCATGGACATAGATCCTTA-3′) and wc2 (nt 2595 to 2570, 5′-CATTGAATTCAGCAGTTGGCAGATGG-3′). In testing serial dilutions of a cloned WHV core fragment, 10 copies of specific templates were sufficient to give a positive result by PCR. Therefore, a virus DNA titer of 500 copies per ml of serum could be detected.
Measurement of WHV antigen-specific proliferation of woodchuck PBMC.
Antigen-specific proliferation of woodchuck PBMCs was determined by 2[3H]adenine assay described previously (32). Briefly, woodchuck PBMCs were separated by Ficoll-Paque (Pharmacia, Freiburg, Germany) density gradient centrifugation and suspended in 0.9% NaCl. Triplicates of 5 × 104 PBMCs were cultured in flat-bottom 96-well microtiter plates (Falcon, Becton Dickinson, N.J.) at 37°C in a humidified atmosphere containing 5% CO2. AIM-V medium (200 μl; Gibco BRL) supplemented with 2% 0.2 M l-glutamine (Sigma), 1% 0.125 M gentamicin sulfate (Sigma), and 10% fetal calf serum (Gibco BRL) was added to each well. PBMC proliferation in response to WHcAg, WHsAg, or peptides was measured at an antigen concentration of 1 μg/ml. Peptides of the WHcAg were described previously by Menne et al. (39). Nonoverlapping peptides of WHsAg, including the pre-S1 region as listed in Table 1, were purchased from Genosys (Cambridge, United Kingdom). After a 5-day incubation, cells were labeled with 1 μCi of 2[3H]adenine (Amersham, Braunschweig, Germany) for 20 h and collected with a cell harvester (Skatron).
TABLE 1.
Peptides of WHsAg for stimulation of woodchuck PBMC
| Peptidea | Amino acid sequence |
|---|---|
| S1-15 | MGNNIKVTFNPDKIA |
| S16-36 | AWWPAVGTYYTTTYPQNQSVF |
| S37-44 | QPGIYQTT |
| S45-59 | SLINPKNQQELDSVL |
| S60-81 | INRYKQIDWNTWQGFPVDQKF |
| S82-101 | SLVSRDPPPKPYINQSAQTF |
| S102-121 | EIKPGPIIVPGIRDIPRGLV |
| S122-141 | PPQTPTNRDQGRKPTPPTPP |
| S142-161 | LRDTHPHLTMKNQTFHLQGF |
| S162-181 | VDGLRDLTTTERQHNAYRDP |
| S182-203 | FTTLSPAVPTVSTILSPPSTTG |
| S204-226 | DPALSPEMSPSSLLGLLAGLQVV |
| S227-234 | YFLWTKIL |
| S235-243 | TIAQNLDWW |
| S244-261 | CTSLSFPGGIPECTGQNS |
| S262-281 | QFQTCKHLPTSCPPTCNGFR |
| S282-291 | WMYLRRFIIY |
| S302-321 | LLVLLDWKGLIPVCPLQPTT |
| S322-341 | ETTVNCRQCTISAQDMYTPP |
| S342-361 | YCCCLKPTAGNCTCWPIPSS |
| S362-371 | WALGNYLWEW |
| S372-381 | ALARLSWLNL |
| S382-401 | LVPLLQWLGGISLIAWFLLI |
| S402-411 | WMIWFWGPAL |
| S412-432 | LSILPPFIPIFVLFFLIWVYI |
Peptides were synthesized according to the deduced amino acid sequence of the large surface antigen (LWHsAg) of WHV8. Peptides are designated according to their position within LWHsAg. The peptides have different lengths because of the limitations of the synthesis procedure.
Results for triplicate cultures are presented as mean stimulation index (SI [mean total absorption for stimulated PBMCs divided by the mean total absorption for control]). The standard deviations of the means were less than 30% of the mean (range, 15 to 50%). An SI of ≥3.1 was considered significant, to distinguish the specific stimulation and possible variation within an assay as described previously (39).
RESULTS
Construction of plasmids expressing WHV core and pre-S2-S protein.
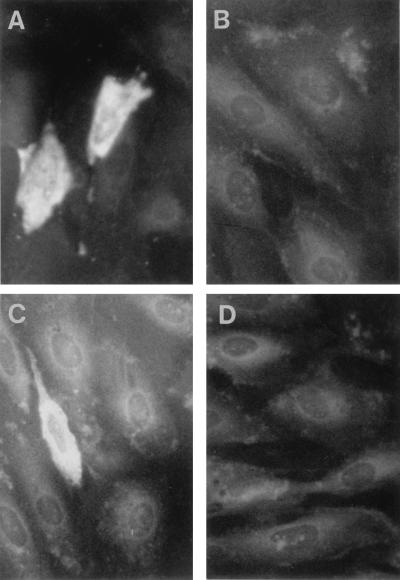
The WHV core region in pWHcIm and the pre-S2-S region in pWHsIm were cloned into pcDNA3 and placed under the control of the CMV promoter (Fig. 1). To test the expression of WHV proteins by the plasmids, BHK cells and woodchuck liver cells (line WH12/6) were transiently transfected with pWHcIm and pWHsIm. WHcAg and WHsAg expressed in transfected WH12/6 cells were detected by indirect immunofluorescence staining with polyclonal rabbit anti-WHsAg antibody or mouse anti-WHcAg antibody, respectively (Fig. 2). The expression of WHV proteins did not show an obvious difference in BHK cells (data not shown). No specific immunofluorescence staining was seen in cells transfected with control plasmid pcDNA3. WHsAgs produced in transfected BHK cells were released into medium and detectable by ELISA.
FIG. 2.
Expression of WHsAg and WHcAg in woodchuck liver cells (WH12/6) after transfection of pWHsIm and pWHcIm, respectively. WH12/6 cells were transfected with 4 μg of plasmids. After 48 h, transfected cells were fixed with acetone-methanol (1:1). The expressed WHcAg and WHsAg were detected by immunofluorescence staining with rabbit antisera to respective WHV antigens. (A) Transfection with pWHcIm and staining with anti-WHcAg antibody. (B) Transfection with pcDNA3 and staining with anti-WHcAg antibody. (C) Transfection with pWHsIm and staining with anti-WHsAg antibody. (D) Transfection with pcDNA3 and staining with anti-WHsAg antibody.
Induction of WHcAg-specific immune response by vaccination of mice and woodchucks with pWHcIm.
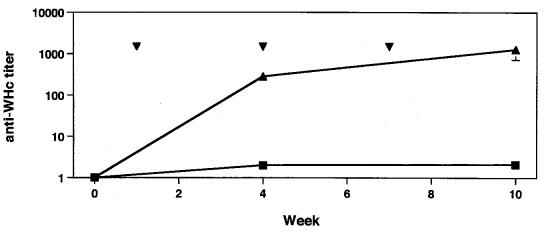
Eight mice were immunized with pWHcIm to test its ability to induce an WHcAg-specific immune response. Immunized mice developed an anti-WHcAg titer of about 1:300 after one injection of 100 μg of pWHcIm (Fig. 3). The titer of anti-WHcAg in these mice increased to 1:500 and 1:1,800 after three immunizations with pWHcIm. Immunizations with control plasmids which do not express WHcAg did not lead to production of anti-WHcAg in mice.
FIG. 3.
Anti-WHcAg response in pWHcIm-immunized mice. Mice were immunized thrice with 100 μg of plasmid pWHcIm (▴) or control (■) at 3-week intervals. Vaccinations with plasmid are indicated by ▾. Anti-WHcAgs were tested with sera from mice collected at weeks 0, 3, and 12. The mean titer of anti-WHcAg is shown.
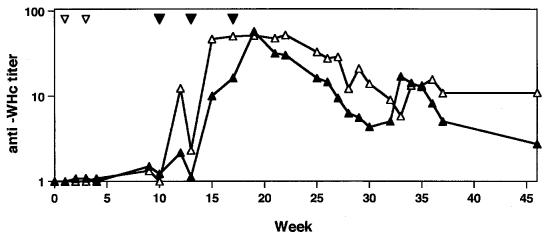
Though a single injection with 100 μg of pWHcIm induced an anti-WHc response in mice, no anti-WHcAg was measurable in woodchuck WH282 or woodchuck WH564 after two vaccinations at weeks 1 and 3 with the same dose of pWHcIm. Both woodchucks remained negative for anti-WHcAg until week 10 (Fig. 4). The dose of 100 μg of plasmids was not sufficient to induce anti-WHcAg in woodchucks. Therefore, both woodchucks received three additional immunizations with 1 mg of pWHcIm at weeks 10, 13, and 17. Woodchucks WH282 and WH564 developed anti-WHcAg after two additional vaccinations at 10 and 13 weeks. The titer of anti-WHcAg increased transiently and dropped gradually from week 20 to week 46 (Fig. 4). In WH282, the anti-WHcAg titer even dropped under 1:10. Control animals either unvaccinated or vaccinated with control plasmid pcDNA3 remained anti-WHcAg negative (see below).
FIG. 4.
Anti-WHcAg response in woodchucks WH282 and WH564 after immunization with pWHcIm. Vaccinations with plasmids are indicated with ▿ (100 μg) and ▾ (1 mg). Sera from woodchucks were used for titration of anti-WHcAg (WH282 [▴]; WH564 [▵]) by serial dilution.
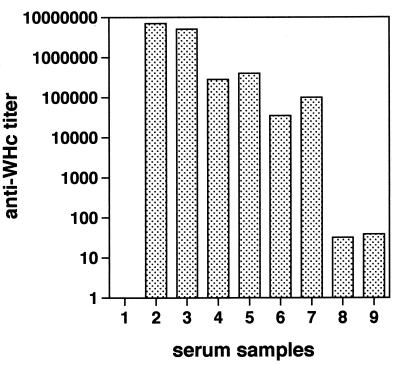
Titers of anti-WHcAg in sera from pWHcIm-vaccinated woodchucks were compared with antibody titers in woodchucks with acute or chronic WHV infection and in woodchucks immunized with recombinant WHcAg (Fig. 5). Titers of anti-WHcAg in sera from WHV-infected woodchucks and WHcAg-vaccinated woodchucks ranged between 1:104 and 1:106. In comparison, woodchucks WH282 and WH564 developed a rather low anti-WHcAg titer under 1:100 after immunization with pWHcIm.
FIG. 5.
Comparison of anti-WHc titer in sera of pWHcIm-immunized, WHV-infected and recombinant WHcAg-immunized woodchucks. Sera from seven woodchucks of different status were tested for anti-WHcAg. Samples: 1, woodchuck without WHV infection; 2 and 3, two woodchucks with chronic WHV infection; 4 and 5, two woodchucks after three immunizations with 50 μg of recombinant WHcAg; 6 and 7, two woodchucks whose WHV infection was resolved. Samples 4 to 7 were taken from woodchucks included in experiments described by Menne et al. (39). Samples 8 and 9, WH282 and WH564 after the last immunization with pWHcIm.
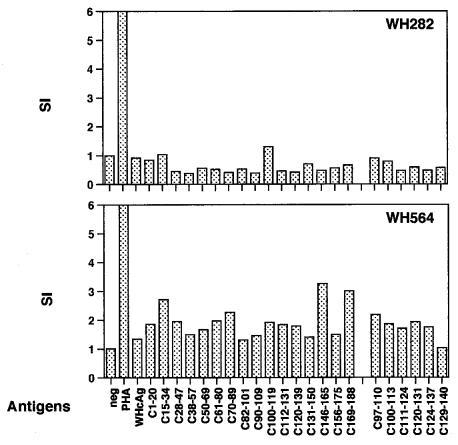
The proliferative response to WHcAg of PBMCs from WH282 and WH564 was determined at each blood drawing indicated in Fig. 4 by in vitro assay. Proliferation of PBMCs from both animals to WHcAg and WHcAg-derived peptides by in vitro assays was low. Figure 6 shows the PBMC proliferation to WHcAg measured at week 21 after three immunizations with 1 mg of pWHcIm. A PBMC proliferation with an SI of ≥3 was seen in only WH574 in response to two WHcAg-derived peptides, C156-175 and C169-188. No stimulation of PBMC proliferation by WHcAg or peptides was measurable in WH282.
FIG. 6.
The proliferative response to recombinant WHcAg and peptides of WHcAg of PBMC from woodchucks WH282 and WH564 after immunizations with pWHcIm at week 21. neg, no WHV antigen was added; PHA, phytohemagglutinin.
Challenge of pWHcIm-vaccinated woodchucks with WHV.
To test whether DNA immunization confers protection against WHV infection, four woodchucks, WH290, WH291, WH8902, and WH8904, were challenged with WHV after three immunizations with 1 mg of pWHcIm. As a control, two untreated woodchucks, WH880 and WH882, and two woodchucks which received plasmid pcDNA3, WH281 and WH574, were challenged with WHV (Table 2).
TABLE 2.
DNA immunization of woodchucks with plasmids pcDNA3, pWHcIm, and pWHsIm
| Woodchuck | Immunogena | Interval (no. of weeks) | WHV ID50 used for challenge |
|---|---|---|---|
| WH282 | pWHcIm | ||
| WH564 | pWHcIm | ||
| WH880 | None | 105 | |
| WH882 | None | 105 | |
| WH281 | pcDNA3 | 4 | 104 |
| WH574 | pcDNA3 | 4 | 104 |
| WH290 | pWHcIm | 4 | 104 |
| WH291 | pWHcIm | 4 | 104 |
| WH8902 | pWHcIm | 3 | 105 |
| WH8904 | pWHcIm | 3 | 105 |
| WH8899 | pWHsIm | 3 | 105 |
| WH8897 | pWHsIm | 3 | 105 |
WH282 and WH564 were immunized twice with 100 μg of pWHcIm and then three times with 1 mg of pWHcIm but not challenged (see text).
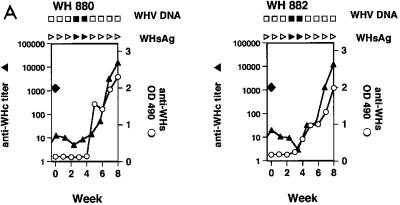
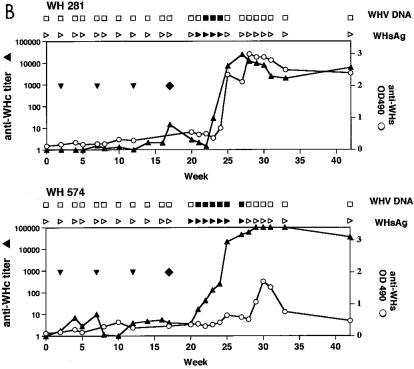
Two naive woodchucks, WH880 and WH882, received 105 WHV at a 50% infective dose (ID50). They were viremic during weeks 3 and 4 week postinfection (p.i.) and positive for anti-WHs at 5 weeks p.i. (Fig. 7A). The peak level of WHV titers ranged from 106 to 108, as estimated by dot blot hybridization. WH281 and WH576, which received three injections of pcDNA3, did not show antibody response or PBMC proliferation in response to WHV proteins. After the challenge with 104 WHV ID50, both woodchucks were positive for WHsAg and WHV DNA at 5 and 4 weeks p.i., respectively (Fig. 7B) and had a maximum WHV titer of 106 to 108. Immediately after the viremic phase, anti-WHsAg developed in WH281 and WH574 at weeks 8 and 11 p.i., respectively.
FIG. 7.
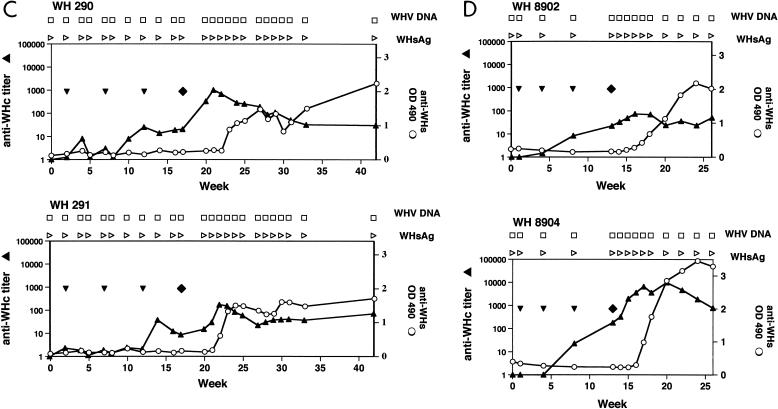
Immunization of woodchucks with pWHcIm and challenge with WHV. (A) Unimmunized control animals WH880 and WH882. (B) Immunization with pcDNA3. (C) Immunization with pWHcIm. Vaccinations with plasmids are indicated with ▾. ⧫, challenge with WHV; ▴, anti-WHcAg; ○, anti-WHs; □, WHV DNA negative; ■, WHV DNA positive; ▹, WHsAg negative; ▸, WHsAg positive. PCR was used to prove the absence of WHV DNA in sera from WH290, WH291, WH8902, and WH8904. OD, optical density.
In all four woodchucks receiving pWHcIm, a seroconversion to anti-WHc occurred after three injections (Fig. 7C and D). Like WH282 and WH564, these animals developed only a low anti-WHcAg titer. The anti-WHcAg in WH290, WH291, and WH8902 ranged between 1:10 and 1:100 after three immunizations. WH8904 showed an anti-WHc titer of 1:179 at week 13 after challenge. Two pWHcIm-vaccinated woodchucks, WH290 and WH291, were challenged with 104 WHV ID50 at week 5 after the last DNA injection (Fig. 7C). Two woodchucks, WH8902 and WH8904, were vaccinated with 1 mg of pWHcIm three times at 4-week intervals and challenged with 105 WHV ID50 at week 4 after the last DNA injection (Fig. 7D). All sera from these four animals were negative for WHsAg and WHV DNA in a follow-up period of 14 weeks. Anti-WHcAg titer was transiently increased in all woodchucks, presumably due to limited viral replication and production of viral proteins. In WH8904, anti-WHcAg titer reached nearly 1:10,000 at the peak level at week 17 but decreased continuously thereafter. Unlike in control animals, anti-WHsAg developed independently on the virus titer of challenge at 5 weeks p.i. Thus, transient WHV replication could take place in pWHcIm-vaccinated woodchucks and was sufficient to induce an anti-WHsAg response.
Similar to previous experiments, PBMCs from woodchucks showed no or weak WHcAg- or peptide-specific proliferation after immunization with pWHcIm. After challenge, there was no significant increase in PBMC proliferation to WHV core and surface proteins in these animals (data not shown). These results indicate that T-cell response to WHV proteins, if present, was local and did not spread, consistent with the previous observation that T-cell response to WHV proteins was measurable in the periphery only during the acute viremic phase of WHV infection (37).
Immunization of woodchucks with pWHsIm and challenge.
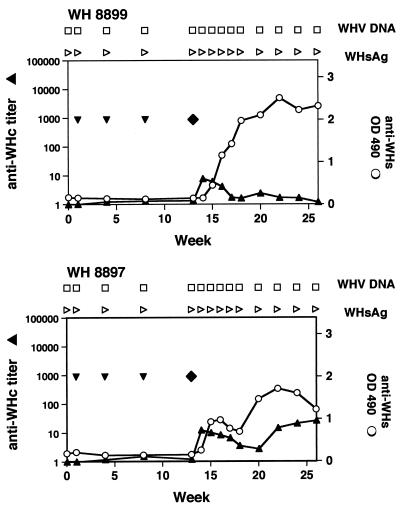
Two woodchucks, WH8897 and WH8899, received three intramuscular injections of 1 mg of pWHsIm each, in parallel to other immunizations described above (Table 2 and Fig. 8). Anti-WHsAg antibody was not detectable after three immunizations but developed 2 weeks after challenge with 105 WHV ID50. Thus, anti-WHsAg antibody response of pWHsIm-vaccinated woodchucks resembled a rather anamnestic response. WHsAg and WHV DNA were not detected in peripheral blood in the follow-up period of 14 weeks. Anti-WHcAg was only transiently detectable in WH8899, mainly due to carryover of antibodies in the inoculum. WH8897 developed a low titer of anti-WHcAg (1:26) at week 20.
FIG. 8.
Immunization with pWHsIm. Vaccinations with plasmids are indicated with ▾. ⧫, challenges with WHV; ▴ anti-WHcAg; ○, anti-WHs; □, WHV DNA negative; ▹, WHsAg negative; PCR was used to prove the absence of WHV DNA in sera from WH8897 and WH8899.
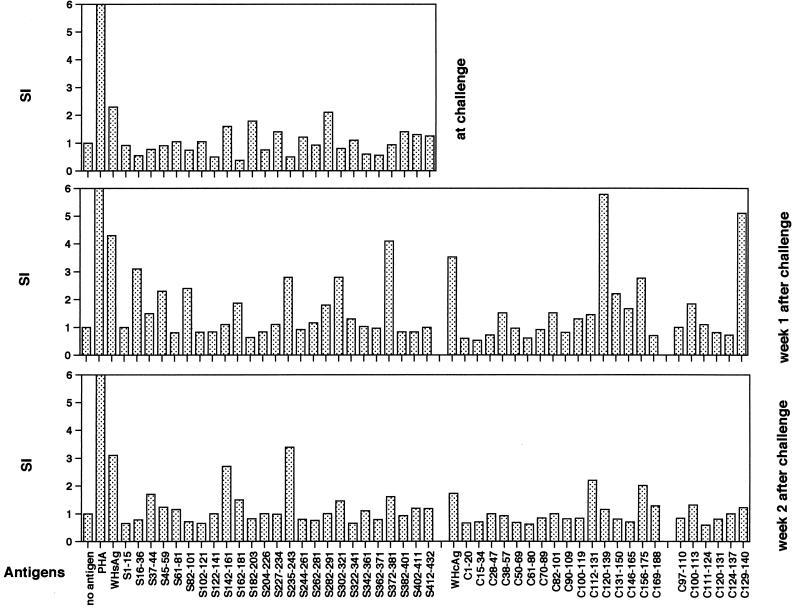
Vaccination with pWHsIm did not induce a significant proliferative response to WHsAg and WHcAg of PBMCs from WH8897 and WH8899. One week after challenge, WHsAg- and WHcAg-specific PBMC proliferation were detected in WH8899 (Fig. 9). PBMC proliferation with an SI of >3 was measured to WHsAg-derived peptides S16-36, S235-243, and S372-381 and to two overlapping WHcAg-derived peptides, C120-139 and C129-140. In the second week, PBMCs from WH8899 responded to WHsAg-derived peptides S142-161 and S235-243. The proliferative response of PBMCs to WHcAg and peptides decreased to an SI of under 2. No significant increase of PBMC proliferation to WHsAg and WHcAg was seen in WH8897.
FIG. 9.
The proliferative response to WHsAg and WHcAg of PBMCs from WH8899. WH8899 was vaccinated with pWHsIm three times and challenged with 105 WHV ID50 at week 5 after the last vaccination. The proliferation of PBMCs in response to stimulation with WHsAg, recombinant WHcAg and peptides at week 13, 14, and 15 is shown. PHA, phytohemagglutinin.
DISCUSSION
In the present study, we have demonstrated that vaccination of woodchucks with plasmids expressing WHcAg and WHsAg induced immune response and controlled a subsequent WHV infection.
The level of antibody response induced by plasmid vaccination appears to be dependent on the relation between the doses of DNA vaccines and the body weight of the animals. After immunization with 100 μg of pWHcIm, an anti-WHcAg titer of 1:300 developed in mice. However, the same dose of plasmids was not effective to induce a measurable anti-WHcAg response in woodchucks. Woodchucks transiently developed anti-WHcAg of low titer after receiving 10-fold doses of plasmids. Considering that woodchucks weigh 4 kg on average, a dose of 1 mg of plasmids per injection is rather low compared with doses used for mice (100 μg of plasmids for 20 g of body weight). Our finding is concordant with the published results of Davis et al. that the effect of plasmid immunization of chimpanzees with an HBsAg-expressing plasmid was also dependent on the amount of plasmids (11).
The challenge experiments showed that a WHV infection could be controlled by vaccination with pWHcIm. These results are consistent with previous experiments showing that immunization with WHcAg confers protection against WHV infection (17, 28, 49). Anti-WHcAg antibody does not possess the activity to neutralize infectious virions of WHV. Therefore, a minimal WHV infection of hepatocytes obviously took place in vaccinated woodchucks and induced a transient increase of anti-WHcAg titer and in the production of anti-WHsAg. The release of WHsAg and WHV virions into the periphery was apparently limited by the cellular branch of the immune system which was primed by vaccination. WHcAg-specific PBMC proliferation was at least measurable in some vaccinated woodchucks. It appears that the plasmid vaccination primed a localized immune response, and the number of WHV antigen-specific T cells in peripheral blood was rather low. These results are very similar to a previous immunization experiment with peptides containing a T-cell epitope. Immunization of woodchucks with C91-110 of WHcAg, though it did not induce measurable PBMC proliferation, protected woodchucks from WHV infection (39). An increase of peptide-specific PBMC proliferation occurred after a subsequent challenge. Anti-WHsAgs appeared 5 weeks after challenge and may contribute to virus clearance.
Three vaccinations of woodchucks with 1 mg of pWHsIm each did not induce a measurable anti-WHsAg response in woodchucks; this may be explained by the following reasons. Whereas WHcAg is a potent immunogen and leads to a high level of anti-WHc antibody in WHV-infected or WHcAg-vaccinated woodchucks, the anti-WHsAg antibody response is usually lower and can be absent in WHsAg-vaccinated woodchucks (nonresponders). The difference between anti-WHcAg and anti-WHsAg may also be partly biased by ELISAs used for follow-up of WHV infection. Since plasmid vaccination in woodchucks induced only a low humoral response, as shown for anti-WHcAg, the anti-WHsAg response induced by pWHsIm might be below the detection limit of the ELISA. Nevertheless, the rapid appearance of anti-WHsAg 2 weeks after challenge demonstrated clearly that priming of WHsAg-specific B-cell response took place as a result of plasmid vaccination. Similar to results for the vaccination with pWHcIm, WHsAg, and WHV DNA, the periphery remained below the detection limit in both woodchucks. Our results are in concordance with results for vaccination of chimpanzees with plasmids expressing HBsAg (11, 47). In general, a transient low-level anti-HBsAg response could be measured in vaccinated chimpanzees. Upon an additional vaccination with HBsAg (11) or a challenge with HBV (47), an anamnestic anti-HBsAg developed in these chimpanzees. The primed anti-WHsAg B-cell response seems unable to block the infection of hepatocytes by input virus. A minimal anti-WHc response was measured in WH8897. A WHcAg-specific PBMC proliferation was detected in WH8899 in the first 2 weeks after challenge before the appearance of anti-WHsAg. These facts indicate that WHcAg was synthesized at a minimal level in pWHsIm-vaccinated woodchucks. The protection conferred by the plasmid vaccination may be improved by different WHsAg expression vectors or by coadministration of cytokine-expressing plasmids (55). In ducks, two DHBsAg-expressing plasmids showed very different protection efficacies for preventing infection of hepatocytes, though both induced high titers of anti-DHBs (55).
Successful genetic vaccination may depend on an appropriate delivery of plasmid DNA. In an early experiment, three woodchucks were vaccinated three times intramuscularly with 1 mg of WHsAg-expressing plasmid at many randomly chosen sites. Two woodchucks developed viremia after challenge, and only one woodchuck remained negative for viral marker (13a). Therefore, M. tibialis cranialis in woodchuck was chosen for DNA vaccination because of its small size and easy location. Pretreatment with cardiotoxin may induce a local inflammatory response and thereby enhance the antigen-specific immune response. Experiments are under way to compare different delivery protocols for their efficacy for inducing immunity to subsequent WHV infections. Intradermal application of plasmids by gene gun was reported to be especially effective for inducing Th2-dominant response and would be useful for achieving an enhanced humoral immune response to surface antigens (15, 46). DNA vaccination against hepadnavirus infection provides new opportunities for the immunotherapy of chronic hepatitis B. Unlike conventional vaccines, DNA vaccines may be modified and enhanced by adding other relevant genes or applied through different routes (15, 20, 46). With an understanding of the mechanisms of viral persistence, effective therapeutic vaccines may be developed to overcome these mechanisms, leading to unresponsiveness of the immune system to hepatitis B proteins in chronically infected patients.
ACKNOWLEDGMENTS
We thank K.-H. Heermann for the preparation of WHsAg and P. Banasch for providing woodchuck cell lines.
This work was supported by the German BMBF (project 01GE96125).
REFERENCES
- 1.Bertoletti A, Ferrari C, Fiaccadori F, Penna A, Margolskee R, Schlicht H J, Fowler P, Guilhot S, Chisari F V. HLA class I-restricted human cytotoxic T cells recognize endogenously synthesized hepatitis B virus nucleocapsid antigen. Proc Natl Acad Sci USA. 1991;88:10445–10449. doi: 10.1073/pnas.88.23.10445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyer J D, Ugen K E, Wang B, Agadjanyan M, Gilbert L, Bagarazzi M L, Chattergoon M, Frost P, Javadian A, Williams W V, Refaeli Y, Ciccarelli R B, McCallus D, Coney L, Weiner D B. Protection of chimpanzees from high-dose heterologous HIV-1 challenge by DNA vaccination. Nat Med. 1997;3:526–532. doi: 10.1038/nm0597-526. [DOI] [PubMed] [Google Scholar]
- 3.Carman, W. F. 1997. The clinical significance of surface antigen variants of hepatitis B virus. J. Virol. Hepat. 4(Suppl. 1):11–20. [DOI] [PubMed]
- 4.Carman W F, Zanetti A R, Karayiannis P, Waters J, Manzillo G, Tanzi E, Zuckerman A J, Thomas H C. Vaccine-induced escape mutant of hepatitis B virus. Lancet. 1990;336:325–329. doi: 10.1016/0140-6736(90)91874-a. [DOI] [PubMed] [Google Scholar]
- 5.Casares S, Brumeanu T D, Bot A, Bona C A. Protective immunity elicited by vaccination with DNA encoding for a B cell and a T cell epitope of the A/PR/8/34 influenza virus. Viral Immunol. 1997;10:129–136. doi: 10.1089/vim.1997.10.129. [DOI] [PubMed] [Google Scholar]
- 6.Chiou H L, Lee T S, Kuo J, Mau Y C, Ho M S. Altered antigenicity of ‘a’ determinant variants of hepatitis B virus. J Gen Virol. 1997;78:2639–2645. doi: 10.1099/0022-1317-78-10-2639. [DOI] [PubMed] [Google Scholar]
- 7.Chisari F V, Ferrari C. Hepatitis B virus immunopathogenesis. Annu Rev Immunol. 1995;13:29–60. doi: 10.1146/annurev.iy.13.040195.000333. [DOI] [PubMed] [Google Scholar]
- 8.Cote P J, Gerin J L. In vitro activation of woodchuck lymphocytes measured by radiopurine incorporation and interleukin-2 production: implications for modeling immunity and therapy in hepatitis B virus infection. Hepatology. 1995;22:687–699. [PubMed] [Google Scholar]
- 9.Cote P J, Shapiro M, Engle R E, Popper H, Purcell R H, Gerin J L. Protection of chimpanzees from type B hepatitis by immunization with woodchuck hepatitis virus surface antigen. J Virol. 1986;60:895–901. doi: 10.1128/jvi.60.3.895-901.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis H L, Mancini M, Michel M L, Whalen R G. DNA-mediated immunization to hepatitis B surface antigen: longevity of primary response and effect of boost. Vaccine. 1996;14:910–915. doi: 10.1016/0264-410x(95)00255-y. [DOI] [PubMed] [Google Scholar]
- 11.Davis H L, McCluskie M J, Gerin J L, Purcell R H. DNA vaccine for hepatitis B: evidence for immunogenicity in chimpanzees and comparison with other vaccines. Proc Natl Acad Sci USA. 1996;93:7213–7218. doi: 10.1073/pnas.93.14.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis H L, Schirmbeck R, Reimann J, Whalen R G. DNA-mediated immunization in mice induces a potent MHC class I-restricted cytotoxic T lymphocyte response to the hepatitis B envelope protein. Hum Gene Ther. 1995;6:1447–1456. doi: 10.1089/hum.1995.6.11-1447. [DOI] [PubMed] [Google Scholar]
- 13.Deck R R, DeWitt C M, Donnelly J J, Liu M A, Ulmer J B. Characterization of humoral immune responses induced by an influenza hemagglutinin DNA vaccine. Vaccine. 1997;15:71–78. doi: 10.1016/s0264-410x(96)00101-6. [DOI] [PubMed] [Google Scholar]
- 13a.Deka, J., and M. Roggendorf. Unpublished results.
- 14.Donnelly J J, Ulmer J B, Shiver J W, Liu M A. DNA vaccines. Annu Rev Immunol. 1997;15:617–648. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]
- 15.Feltquate D M, Heaney S, Webster R G, Robinson H L. Different T helper cell types and antibody isotypes generated by saline and gene gun DNA immunization. J Immunol. 1997;158:2278–2284. [PubMed] [Google Scholar]
- 16.Ferrari C, Bertoletti A, Penna A, Cavalli A, Valli A, Missale G, Pilli M, Fowler P, Giuberti T, Chisari F V. Identification of immunodominant T cell epitopes of the hepatitis B virus nucleocapsid antigen. J Clin Investig. 1991;88:214–222. doi: 10.1172/JCI115280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrari C, Mondelli M U, Penna A, Fiaccadori F, Chisari F V. Functional characterization of cloned intrahepatic, hepatitis B virus nucleoprotein-specific helper T cell lines. J Immunol. 1987;139:539–544. [PubMed] [Google Scholar]
- 18.Ferrari C, Penna A, Bertoletti A, Valli A, Antoni A D, Giuberti T, Cavalli A, Petit M A, Fiaccadori F. Cellular immune response to hepatitis B virus-encoded antigens in acute and chronic hepatitis B virus infection. J Immunol. 1990;145:3442–3449. [PubMed] [Google Scholar]
- 19.Fynan E F, Webster R G, Fuller D H, Haynes J R, Santoro J C, Robinson H L. DNA vaccines: protective immunizations by parenteral, mucosal, and gene-gun inoculations. Proc Natl Acad Sci USA. 1993;90:11478–11482. doi: 10.1073/pnas.90.24.11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geissler M, Schirmbeck R, Reimann J, Blum H E, Wands J R. Cytokine and hepatitis B virus DNA co-immunizations enhance cellular and humoral immune responses to the middle but not to the large hepatitis B virus surface antigen in mice. Hepatology. 1998;28:202–210. doi: 10.1002/hep.510280126. [DOI] [PubMed] [Google Scholar]
- 21.Girones R, Cote P J, Hornbuckle W E, Tennant B C, Gerin J L, Purcell R H, Miller R H. Complete nucleotide sequence of a molecular clone of woodchuck hepatitis virus that is infectious in the natural host. Proc Natl Acad Sci USA. 1989;86:1846–1849. doi: 10.1073/pnas.86.6.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hariharan M J, Driver D A, Townsend K, Brumm D, Polo J M, Belli B A, Catton D J, Hsu D, Mittelstaedt D, McCormack J E, Karavodin L, Dubensky T W, Chang S M, Banks T A. DNA immunization against herpes simplex virus: enhanced efficacy using a Sindbis virus-based vector. J Virol. 1998;72:950–958. doi: 10.1128/jvi.72.2.950-958.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hervas-Stubbs S, Lasarte J J, Sarobe P, Prieto J, Cullen J, Roggendorf M, Borras-Cuesta F. Therapeutic vaccination of woodchucks against chronic woodchuck hepatitis virus infection. J Hepatol. 1997;27:726–737. doi: 10.1016/s0168-8278(97)80090-6. [DOI] [PubMed] [Google Scholar]
- 24.Hollinger F B. Hepatitis B virus. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. p. 2739. [Google Scholar]
- 25.Hsu H Y, Chang M H, Ni Y H, Lin H H, Wang S M, Chen D S. Surface gene mutants of hepatitis B virus in infants who develop acute or chronic infections despite immunoprophylaxis. Hepatology. 1997;26:786–791. doi: 10.1002/hep.510260336. [DOI] [PubMed] [Google Scholar]
- 26.Jung M C, Diepolder H M, Spengler U, Wierenga E A, Zachoval R, Hoffmann R M, Eichenlaub D, Frosner G, Will H, Pape G R. Activation of a heterogeneous hepatitis B (HB) core and e antigen-specific CD4+ T-cell population during seroconversion to anti-HBe and anti-HBs in hepatitis B virus infection. J Virol. 1995;69:3358–3368. doi: 10.1128/jvi.69.6.3358-3368.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung M C, Spengler U, Schraut W, Hoffmann R, Zachoval R, Eisenburg J, Eichenlaub D, Riethmuller G, Paumgartner G, Ziegler-Heitbrock H W. Hepatitis B virus antigen-specific T-cell activation in patients with acute and chronic hepatitis B. J Hepatol. 1991;13:310–317. doi: 10.1016/0168-8278(91)90074-l. [DOI] [PubMed] [Google Scholar]
- 28.Kane M A. Global status of hepatitis B immunization. Lancet. 1996;348:696. doi: 10.1016/S0140-6736(05)65598-5. [DOI] [PubMed] [Google Scholar]
- 29.Karthigesu V D, Allison L M, Fortuin M, Mendy M, Whittle H C, Howard C R. A novel hepatitis B virus variant in the sera of immunized children. J Gen Virol. 1994;75:443–448. doi: 10.1099/0022-1317-75-2-443. [DOI] [PubMed] [Google Scholar]
- 30.Kew M C. The development of hepatocellular cancer in humans. Cancer Surv. 1986;5:719–739. [PubMed] [Google Scholar]
- 31.Kodihalli S, Haynes J R, Robinson H L, Webster R G. Cross-protection among lethal H5N2 influenza viruses induced by DNA vaccine to the hemagglutinin. J Virol. 1997;71:3391–3396. doi: 10.1128/jvi.71.5.3391-3396.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kreuzfelder E, Menne S, Ferencik S, Roggendorf M, Grosse-Wilde H. Assessment of peripheral blood mononuclear cell proliferation by [2-3H]adenine uptake in the woodchuck model. Clin Immunol Immunopathol. 1996;78:223–227. doi: 10.1006/clin.1996.0033. [DOI] [PubMed] [Google Scholar]
- 33.Kuhrober A, Wild J, Pudollek H P, Chisari F V, Reimann J. DNA vaccination with plasmids encoding the intracellular (HBcAg) or secreted (HBeAg) form of the core protein of hepatitis B virus primes T cell responses to two overlapping Kb- and Kd-restricted epitopes. Int Immunol. 1997;9:1203–1212. doi: 10.1093/intimm/9.8.1203. [DOI] [PubMed] [Google Scholar]
- 34.Lin Y L, Chen L K, Liao C L, Yeh C T, Ma S H, Chen J L, Huang Y L, Chen S S, Chiang H Y. DNA immunization with Japanese encephalitis virus nonstructural protein NS1 elicits protective immunity in mice. J Virol. 1998;72:191–200. doi: 10.1128/jvi.72.1.191-200.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lohr H F, Weber W, Schlaak J, Goergen B, Meyer zum Buschenfelde K H, Gerken G. Proliferative response of CD4+ T cells and hepatitis B virus clearance in chronic hepatitis with or without hepatitis B e-minus hepatitis B virus mutants. Hepatology. 1995;22:61–68. doi: 10.1002/hep.1840220110. [DOI] [PubMed] [Google Scholar]
- 36.McClements W L, Armstrong M E, Keys R D, Liu M A. Immunization with DNA vaccines encoding glycoprotein D or glycoprotein B, alone or in combination, induces protective immunity in animal models of herpes simplex virus-2 disease. Proc Natl Acad Sci USA. 1996;93:11414–11420. doi: 10.1073/pnas.93.21.11414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Menne S, Maschke J, Lu M, Grosse-Wilde H, Roggendorf M. T-cell response to woodchuck hepatitis virus (WHV) antigens during acute self-limited WHV infection and convalescence and after viral challenge. J Virol. 1998;72:6083–6091. doi: 10.1128/jvi.72.7.6083-6091.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Menne S, Maschke J, Tolle T, Kreuzfelder E, Grosse-Wilde H, Roggendorf M. Determination of peripheral blood mononuclear cell responses to mitogens and woodchuck hepatitis virus core antigen in woodchucks by 5-bromo-2′-deoxyuridine or 2[3H]adenine incorporation. Arch Virol. 1997;142:511–521. doi: 10.1007/s007050050097. [DOI] [PubMed] [Google Scholar]
- 39.Menne S, Maschke J, Tolle T K, Lu M, Roggendorf M. Characterization of T-cell response to woodchuck hepatitis virus core protein and protection of woodchucks from infection by immunization with peptides containing a T-cell epitope. J Virol. 1997;71:65–74. doi: 10.1128/jvi.71.1.65-74.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Michel M L, Davis H L, Schleef M, Mancini M, Tiollais P, Whalen R G. DNA-mediated immunization to the hepatitis B surface antigen in mice: aspects of the humoral response mimic hepatitis B viral infection in humans. Proc Natl Acad Sci USA. 1995;92:5307–5311. doi: 10.1073/pnas.92.12.5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Missale G, Redeker A, Person J, Fowler P, Guilhot S, Schlicht H J, Ferrari C, Chisari F V. HLA-A31- and HLA-Aw68-restricted cytotoxic T cell responses to a single hepatitis B virus nucleocapsid epitope during acute viral hepatitis. J Exp Med. 1993;177:751–762. doi: 10.1084/jem.177.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nayersina R, Fowler P, Guilhot S, Missale G, Cerny A, Schlicht H J, Vitiello A, Chesnut R, Person J L, Redeker A G. HLA A2 restricted cytotoxic T lymphocyte responses to multiple hepatitis B surface antigen epitopes during hepatitis B virus infection. J Immunol. 1993;150:4659–4671. [PubMed] [Google Scholar]
- 43.Ni F, Fang D, Gan R, Li Z, Duan S, Xu Z. A new immune escape mutant of hepatitis B virus with an Asp to Ala substitution in aa144 of the envelope major protein. Res Virol. 1995;146:397–407. doi: 10.1016/0923-2516(96)80899-5. [DOI] [PubMed] [Google Scholar]
- 44.Penna A, Chisari F V, Bertoletti A, Missale G, Fowler P, Giuberti T, Fiaccadori F, Ferrari C. Cytotoxic T lymphocytes recognize an HLA-A2-restricted epitope within the hepatitis B virus nucleocapsid antigen. J Exp Med. 1991;174:1565–1570. doi: 10.1084/jem.174.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Penna A, Fowler P, Bertoletti A, Guilhot S, Moss B, Margolskee R F, Cavalli A, Valli A, Fiaccadori F, Chisari F V. Hepatitis B virus (HBV)-specific cytotoxic T-cell (CTL) response in humans: characterization of HLA class II-restricted CTLs that recognize endogenously synthesized HBV envelope antigens. J Virol. 1992;66:1193–1198. doi: 10.1128/jvi.66.2.1193-1198.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pertmer T M, Roberts T R, Haynes J R. Influenza virus nucleoprotein-specific immunoglobulin G subclass and cytokine responses elicited by DNA vaccination are dependent on the route of vector DNA delivery. J Virol. 1996;70:6119–6125. doi: 10.1128/jvi.70.9.6119-6125.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prince A M, Whalen R, Brotman B. Successful nucleic acid based immunization of newborn chimpanzees against hepatitis B virus. Vaccine. 1997;15:916–919. doi: 10.1016/s0264-410x(96)00248-4. [DOI] [PubMed] [Google Scholar]
- 48.Roggendorf M, Tolle T K. The woodchuck: an animal model for hepatitis B virus infection in man. Intervirology. 1995;38:100–112. doi: 10.1159/000150418. [DOI] [PubMed] [Google Scholar]
- 49.Roos S, Fuchs K, Roggendorf M. Protection of woodchucks from infection with woodchuck hepatitis virus by immunization with recombinant core protein. J Gen Virol. 1989;70:2087–2095. doi: 10.1099/0022-1317-70-8-2087. [DOI] [PubMed] [Google Scholar]
- 50.Schirmbeck R, Bohm W, Ando K, Chisari F V, Reimann J. Nucleic acid vaccination primes hepatitis B virus surface antigen-specific cytotoxic T lymphocytes in nonresponder mice. J Virol. 1995;69:5929–5934. doi: 10.1128/jvi.69.10.5929-5934.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmaljohn C, Vanderzanden L, Bray M, Custer D, Meyer B, Li D, Rossi C, Fuller D, Fuller J, Haynes J, Huggins J. Naked DNA vaccines expressing the prM and E genes of Russian spring summer encephalitis virus and Central European encephalitis virus protect mice from homologous and heterologous challenge. J Virol. 1997;71:9563–9569. doi: 10.1128/jvi.71.12.9563-9569.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schodel F, Neckermann G, Peterson D, Fuchs K, Fuller S, Will H, Roggendorf M. Immunization with recombinant woodchuck hepatitis virus nucleocapsid antigen or hepatitis B virus nucleocapsid antigen protects woodchucks from woodchuck hepatitis virus infection. Vaccine. 1993;11:624–628. doi: 10.1016/0264-410x(93)90307-j. [DOI] [PubMed] [Google Scholar]
- 53.Summers J, Smolec J M, Snyder R. A virus similar to human hepatitis B virus associated with hepatitis and hepatoma in woodchucks. Proc Natl Acad Sci USA. 1978;75:4533–4537. doi: 10.1073/pnas.75.9.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Szmuness W. Hepatocellular carcinoma and the hepatitis B virus: evidence for a causal association. Prog Med Virol. 1978;24:40–69. [PubMed] [Google Scholar]
- 55.Triyatni M, Jilbert A R, Qiao M, Miller D S, Burrell C J. Protective efficacy of DNA vaccines against duck hepatitis B virus infection. J Virol. 1998;72:84–94. doi: 10.1128/jvi.72.1.84-94.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ulmer J B, Sadoff J C, Liu M A. DNA vaccines. Curr Opin Immunol. 1996;8:531–536. doi: 10.1016/s0952-7915(96)80042-2. [DOI] [PubMed] [Google Scholar]
- 57.Xu L, Sanchez A, Yang Z, Zaki S R, Nabel E G, Nichol S T, Nabel G J. Immunization for Ebola virus infection. Nat Med. 1998;4:37–42. doi: 10.1038/nm0198-037. [DOI] [PubMed] [Google Scholar]