Abstract
Background and Objectives: The increase in antimicrobial resistance (AMR) across countries has seriously impacted the effective management of infectious diseases, with subsequent impact on morbidity, mortality and costs. This includes Pakistan. Antimicrobial surveillance activities should be mandatory to continually assess the extent of multidrug-resistant bacteria and the implications for future empiric prescribing. The objective of this retrospective observational study was to monitor the susceptibility pattern of microbes in Pakistan. Materials and Methods: Clinical samples from seven laboratories in Punjab, Pakistan were collected between January 2018 and April 2019, with Punjab being the most populous province in Pakistan. The isolates were identified and their antimicrobial susceptibility was tested using the Kirby-Bauer disc diffusion assay and micro broth dilution methods. The antibiotics assessed were those typically prescribed in Pakistan. Results: In total, 2523 bacterial cultural reports were studied. The most frequently isolated pathogens were Staphylococcus aureus (866, 34.3%), followed by Escherichia coli (814, 32.2%), Pseudomonas aeruginosa (454, 18.0%) and Klebsiella pneumoniae (269, 10.7%). Most pathogens were isolated from pus (1464, 58.0%), followed by urine (718, 28.5%), blood (164, 6.5%) and sputum (81, 3.2%). Conclusions: The findings suggest that current antimicrobial options are severally restricted in Pakistan due to the emergence of multidrug-resistant pathogens. This calls for urgent actions including initiating antimicrobial stewardship programs to enhance prudent prescribing of antibiotics. This includes agreeing on appropriate empiric therapy as part of agreed guidelines, in line with the WHO EML and AWaRe book, whilst awaiting culture reports. This is alongside other measures to reduce inappropriate antimicrobial prescribing and reverse the threat of rising AMR.
Keywords: Pakistan, culture and sensitivity, surveillance, national action plans, antimicrobial stewardship programs, audits, AWaRe classification
1. Introduction
Antimicrobial resistance (AMR) has become a considerable threat across countries increasing morbidity, mortality and costs, especially among low- and middle-income countries (LMICs) including Pakistan [1,2,3,4,5,6,7,8]. Globally, in 2019, there were 1.27 million deaths directly attributable to AMR, with rates expected to continue rising unless proactively addressed [3]. However, despite affecting all the parameters of healthcare systems, and being increasingly seen as the next pandemic, concerns regarding AMR have remained largely ignored among a number of key stakeholder groups [9,10,11,12,13]. Many drivers contribute to the development of AMR in countries [14,15,16,17]. Key drivers include irrational prescribing and dispensing of antibiotics, including for self-limiting conditions, and, in addition, the absence of fully functioning surveillance systems to monitor the consumption of antibiotics alongside bacterial resistance patterns. In many LMICs, the lack of infection, prevention and control (IPC) groups in hospitals, concerns with sanitation and healthcare infrastructures, lack of knowledge regarding AMR and antibiotics among physicians, as well as their overuse in farming and agriculture, exacerbate AMR [14,15,18,19,20,21,22,23,24,25,26]. Addressing these multiple factors is a challenge to healthcare authorities across different countries, especially among LMICs with more limited resources in terms of available finances, personnel and infrastructure, to undertake routine surveillance of AMR patterns across all sectors of care. One output to coordinate activities within countries has been the development of the Global Action Plan by the World Health Organization (WHO), including the preparedness of countries to tackle AMR [27,28,29]. This has resulted in the development of country-specific National Action Plans (NAPs) including Pakistan [30,31,32,33].
The WHO Global Action Plan (GAP) on AMR declared AMR surveillance as a ‘cornerstone’ to assess the burden of the AMR [34]. The objective of the GAP is to monitor the resistance of specific bacterial pathogens to a number of antibiotics, especially where the irrational use of antimicrobials is appreciably higher compared to other regions [35]. Currently, there are appreciable gaps in knowledge regarding current antimicrobial utilization patterns across sectors as well as resistance rates at a local, regional and national level in a number of countries [32,35]. This is a concern that needs to be addressed, as a lack of knowledge hamper efforts to produce a clear picture of the overall AMR scenario to instigate appropriate actions to adequately address rising concerns with AMR within countries [36]. These concerns are particularly prevalent among LMICs with their resource and personnel issues [32,34,35]. The Global Antimicrobial Resistance Surveillance System (GLASS), developed in response to the WHO Global Action Plan, relies on the NAPs of individual countries [37,38,39]. This surveillance system combines the patient’s epidemiological and laboratory data to understand the extent and impact of AMR on populations, which is increasingly important in Pakistan given rising rates of AMR [40]. However, there are serious concerns with implementing the NAP in Pakistan including resource, knowledge and infrastructure issues [41].
Surveillance to monitor resistance patterns among common human bacterial pathogens is an essential first step to strengthening the AMR evidence base [38]. However, there are concerns with available resources, expertise and infrastructures to fully undertake surveillance in a number of LMICs [11,39,41,42]. This includes Pakistan, where there are appreciable concerns with the current surveillance to fully document AMR patterns across the country, which is currently exacerbated by a lack of requests in hospitals for culture and sensitivity testing; however, progress is being made [6,41,43,44,45]. This is illustrated by very high rates of prescribing of antibiotics in Pakistan among patients admitted to hospitals with COVID-19 during the first five waves at 89.7% of patients, with ‘Watch’ antibiotics being prescribed on 93.4% of occasions adding to AMR [46]. This was despite only a limited number of patients in these tertiary care/teaching hospitals having proven bacterial co-infections (1.14% of admitted patients) or secondary infections (3.14% of patients) [46]. This endorses the need for accurate diagnostic and sensitivity data, even among tertiary hospitals in Pakistan, to improve future antibiotic prescribing and reduce AMR.
One challenge generally among LMICs is the lack of diagnostic facilities and antibiotic sensitivity data, which reduces appropriate prescribing of antibiotics, potentially increasing AMR [47]. This is a concern as the susceptibility patterns of microbes should be increasingly monitored among primary, secondary and tertiary hospitals as well as nationally within LMICs to improve empiric prescribing. This is important given concerns with rising AMR rates in Pakistan, as well as high rates of antimicrobial prescribing among hospitals in Pakistan, often without culture and sensitivity testing [43,44,46,48,49,50], as well as the current absence of national prescribing guidance.
Existing studies performed in Pakistan already show high resistance rates in Escherichia coli, Staphylococcus aureus, Acinetobacter baumannii and Klebsiella pneumoniae against cotrimoxazole, third-generation cephalosporins, fluoroquinolones and methicillin [6,44,45]. Consequently, the objective of this study was to build on these findings and describe the susceptibility patterns of bacteria in Pakistan against a range of antibiotics. The findings can subsequently be used to suggest future activities, including introducing additional antimicrobial stewardship programs (ASPs) in hospitals, given increasing concerns about the inappropriate prescribing of antibiotics in hospitals in Pakistan, including high rates of prescribing of ‘Watch’ and ‘Reserve’ antibiotics and their impact on AMR [46,49,50,51,52,53,54,55,56]. This is important since whilst hospitals in Pakistan are currently putting in place the building blocks for ASPs, there are still an appreciable number of activities that are needed before ASPs can be fully implemented in the country [55,57]. The introduction of ASPs in Pakistan, though, is currently hampered by concerns with available resources, as well as the ability of patients to cover the costs of culture and sensitivity testing (CST) themselves, together with treatment costs within public hospitals [50,51,58,59]. The latter must be tackled by the authorities in Pakistan for CST to become routine and for inappropriate prescribing to reduce. Adequate resources must form part of the NAP going forward since it is recognized that ASPs are more difficult to undertake in LMICs due to available personnel and financial issues [41,43,60].
In the first instance, we describe the results of a passive surveillance system from seven public and private sector microbiological laboratories across different cities of Pakistan and the changing antibiotic susceptibility profiles observed across these hospitals. As mentioned, the findings can subsequently be used to guide successful empiric antibiotic treatment in hospitals in Pakistan in the near future as part of planned ASPs to meet the NAP goals. The findings can also be used to push for increased resources among the authorities for increased funding in hospitals to cover the costs of routine CST testing and associated antibiograms.
2. Materials and Methods
2.1. Study Design and Population
This was a retrospective observational study conducted to observe the patterns of AMR among a range of bacterial isolates. An overview of the methodology is provided in Supplementary Figure S1. The data were collected from both public and private sector microbiological laboratories in different cities across Punjab, Pakistan.
Punjab was chosen for this research project because it is one of the four provinces of Pakistan located in the central-eastern region. It is also the largest province by population, accounting for more than half of the population of Pakistan, as well as the second-largest province of Pakistan by land area [46,49,52,53]. Consequently, if there are considerable issues with AMR in the Punjab province, this will have serious implications for the whole of Pakistan.
A total of eleven pathology and diagnostic laboratories in different areas of Punjab were initially visited. From these, seven laboratories subsequently took part in this study as these laboratories fulfilled the high standards of precision and technique set by the Clinical and Laboratory Standard Institute (CLSI). The consensus-driven medical laboratory standards of CLSI are widely acknowledged as the go-to resources for improving testing quality, safety, and efficiency on an ongoing basis, internationally [61]. The samples were subsequently collected through a non-probability purposive sampling approach in order to select only common pathogens.
All clinical samples arriving at the laboratories between January 2018 and April 2019 were included. These samples included pus (n = 1464), urine (n = 718), blood (n = 164), sputum (n = 81), tissue (n = 53) and body fluids (n = 43), which were collected using aseptic techniques and subsequently transported to the designated microbiology laboratories for analysis.
2.2. Inclusion and Exclusion Criteria
The bacterial pathogens were only considered if they were isolated more than 50 times. Patient culture reports with only one particular resistant isolate was included. Patient culture reports with more than one resistant bacterial isolate were excluded from the study because it would interfere with the resistance pattern of each other.
A comprehensive data collection form was developed, keeping in mind the objectives of the study. The following information was included in the data collection form: (a) source of a clinical sample, (b) isolated pathogen and (c) antimicrobial susceptibility pattern.
In the final analysis, four Gram-negative microbes including Acinetobacter baumannii, Escherechia coli, Klebsiella pneumoniae, Proteus mirabilis and Pseudomonas aeruginosa, and one Gram-positive pathogen, Staphylococcus aureus, were included in this study. The data collection form was based on previous studies coupled with the considerable experience of the co-authors, which is similar to other studies conducted by the co-authors [62,63,64,65].
2.3. Processing of the Samples and Susceptibility Testing
For blood cultures, 1–3 mL of blood was drawn from children and 5–10 mL of blood was collected from adult patients. The blood culture bottles were placed in an automatic BACTEC 9120 system for up to 5 days to be declared negative. The positive blood samples were subsequently sub-cultured on blood, MacConkey and chocolate agar. The plates were incubated at 35c overnight aerobically.
After overnight incubation, the isolates were preliminary identified based on colonial morphology, culture characteristic, Gram stain and biochemical profile. The Gram-negative isolates were biologically confirmed by API 20 E and 20NE. The Gram-positive bacteria were biochemically confirmed by catalase, coagulase and DNAse test.
The isolates were further analyzed for antimicrobial susceptibility testing by Kirby-Bauer disc diffusion assay as per clinical laboratory standard institute (CLSI) 2020 guidelines. The MRSA was confirmed as per CLSI guidelines. In short, the 0.5McFarland bacterial suspension was swabbed on the Mueller Hinton Agar plate, a cefoxitin disc was placed, and the plate was incubated overnight at 37 °C. The zone of inhibition was measured as described by the CLSI guidelines. MIC test was performed for the Polymyxin B and Colistin by micro broth dilution methods as per CLSI guidelines.
The following antibiotics were assessed and broken down by their ATC classification [66], as they are typically prescribed in Pakistan: doxycycline, tetracycline, minocycline, tigecycline, ampicillin, amoxicillin, penicillin, co-amoxiclav, ampicillin–sulbactam, Piperacillin–tazobactam, oxacillin, piperacillin, cephalexin, cephazolin, cephradine, cefoxitin, cefuroxime, cefaclor, cefotaxime, ceftazidime, ceftriaxone, cefixime, cefoperazone, cefoperazone–sulbactam, cefepime, aztreonam, meropenem, ertapenem, imipenem, erythromycin, clarithromycin, azithromycin, clindamycin, tobramycin, gentamycin, amikacin, ofloxacin, ciprofloxacin, norfloxacin, levofloxacin, moxifloxacin, nalidixic acid, pipemedic acid, vancomycin, teicoplanin, colistin, nitrofurantoin, fosfomycin, linezolid, polymixin-B, streptomycin, rifampin, and co-trimoxazole. The antibiotics included in our study were selected based on the internal policy of the hospitals where the tests were performed. These antibiotics were routinely tested for susceptibility in clinical laboratories as part of their standard practice. Therefore, we included these specific antibiotics in our study to reflect the real-world scenario and provide insights into the susceptibility patterns of the bacteria isolated from the clinical samples. In addition, further analysis using the WHO AWaRe classification [67,68,69,70] and the sample source were conducted. Only the sensitivity of the pathogens against pertinent antibiotics was recorded, with a number of antibiotics inappropriate for the given organism.
2.4. Statistical Analysis
SPSS (Statistical Package for the Social Sciences) was used to analyze the data by cross tab which gave selective information about individual susceptibility pattern microbes against various antibiotics.
2.5. Ethical Consideration and Patient’s Consent
The study was approved by the Human Ethics Committee of the College of Pharmacy, Bahauddin Zakariya University, Multan, Pakistan (BZU- DEPP-22-830-1005It), with subsequent approval among participating hospitals. The study adheres to the guidelines set forth in the Declaration of Helsinki [71], good clinical practice (GCP) and relevant regulatory requirements.
3. Results
3.1. Identification of Clinical Pathogens
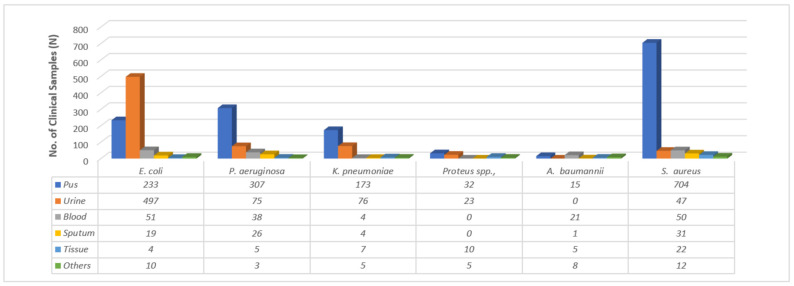
A total of 2523 cultural reports were studied. Of the 2523 reports, 65.4% were for Gram-negative bacteria and 34.3% were for Gram-positive bacteria. Among Gram-positive bacteria, the most frequently isolated pathogens were Staphylococcus aureus (866, 34.3%), while among Gram-negative bacteria, Escherichia coli 814, 32.2%), Pseudomonas aeruginosa (454, 18.0%) and Klebsiella pneumoniae (269, 10.7%) were the most common. Most of the pathogens were isolated from pus (1464, 58.0%), followed by urine (718, 28.5%), blood (164, 6.5%) and sputum (81, 3.2%) (Figure 1). Microbes such as E. coli (497, 61.10%) were most frequently isolated from urine, whereas S. aureus (704, 81.30%) and P. aeruginosa (307, 67.60%) were mostly isolated from pus.
Figure 1.
Prevalence of pathogens in different clinical samples.
3.2. Antimicrobial Resistance
Twelve classes of antibiotics were considered for this analysis. These 12 classes included the tetracyclines, penicillins, cephalosporins, carbapenems/monobactams, macrolides, aminoglycosides, fluoroquinolones, glycopeptides, polymixins, nitrofurans, phosphonic antibiotics and oxazolidinones. They were chosen because they constitute the principal antibiotic classes currently being prescribed in hospitals in Pakistan. Out of these 12 classes, if a microbe was resistant to 3 or more classes, it was subsequently considered multidrug-resistant (MDR). If a microbe was resistant to 7 or more classes, it was subsequently considered extensively drug-resistant (XDR).
Table 1 and Figure 2 provide the prevalence of MDR and XDR clinical isolates for the various organisms. Out of 814 isolates of E. coli, 74.44% (606) were MDR and 0.24% (2) were XDR. Among 454 isolates of P. aeruginosa, 68.2% (310) were MDR and 1.6% (5) were XDR. Out of 269 isolates of K. pneumoniae, 71.375% (192) were MDR and 0.37% (1) were XDR, and among 866 isolates of S. aureus, 51.38% (445) were MDR. In total, there were 2523 isolates analyzed, with 65.43% (1651) identified as MDR and 0.31% (8) identified as XDR. We did not identify any extensively drug-resistant (PDR) bacteria in our study.
Table 1.
Resistance of isolates to antibiotic classes.
| Isolates | Resistant to 1 Antibiotic Class n (%) |
Resistant to 2 Antibiotic Classes n (%) |
Resistant to 3 Antibiotic Classes n (%) |
Resistant to 4 Antibiotic Classes n (%) |
Resistant to 5 Antibiotic Classes n (%) |
Resistant to 6 Antibiotic Classes n (%) |
Resistant to 7 Antibiotic Classes n (%) |
Total No. of Isolates |
|---|---|---|---|---|---|---|---|---|
| E. coli | 81 (9.95) | 108 (13.26) | 221 (27.14) | 174 (21.37) | 127 (15.60) | 61 (7.49) | 6 (0.73) | 814 |
| P. aeruginosa | 42 (9.25) | 76 (16.74) | 122 (26.87) | 86 (18.94) | 57 (12.55) | 23 (5.06) | 6 (1.32) | 454 |
| K. pneumoniae | 30 (11.15) | 38 (14.12) | 72 (26.76) | 51 (18.95) | 39 (14.49) | 23 (8.55) | 5 (1.85) | 269 |
| Proteus spp. | 6 (8.57) | 5 (7.14) | 21 (30) | 16 (22.85) | 12 (17.14) | 6 (8.57) | 0 (0) | 70 |
| A. baumannii | 3 (6) | 6 (12) | 8 (16) | 9 (18) | 8 (16) | 16 (32) | 0 (0) | 50 |
| S. aureus | 180 (20.78) | 202 (23.32) | 190 (21.93) | 115 (13.27) | 50 (5.77) | 40 (4.61) | 7 (0.80) | 866 |
| Total | 342 | 435 | 634 | 451 | 293 | 169 | 24 | 2523 |
The principal 7 classes of antibiotics were considered for this analysis. These classes included tetracyclines, penicillins, cephalosporins, carbapenems/monobactams, macrolides, aminoglycosides and fluoroquinolones.
Figure 2.
Prevalence of MDR and XDR clinical isolates.
3.3. Antimicrobial Susceptibility Profile
The five most sensitive antibiotics against E. coli were fosfomycin (255/277, 92.1), polymyxin-B (80/87, 92.0%), tigecycline (86/95, 90.5%), imipenem (373/427, 87.4%) and meropenem (542/639, 84.8%). For Klebsiella pneumoniae, the five most sensitive antibiotics were polymyxin-B (48/48, 100.0%), tigecycline (16/16, 100.0%), colistin (94/99, 94.9%), imipenem (92/108, 85.2%) and fosfomycin (25/32, 78.1%). For Pseudomonas aeruginosa, five highly sensitive antibiotics were polymyxin-B (102/108, 94.4%), colistin (179/209, 85.6%), minocycline (54/70, 77.1%), imipenem (105/149, 70.5%) and tigecycline (18/26, 69.2%). For Acinetobacter, the most sensitive antibiotics were polymyxin-B (27/28, 96.4%), colistin (28/35, 80.0%), tigecycline (19/26, 73.1%) and minocycline (21/30, 70.0%). For S. aureus, five highly sensitive antibiotics were tigecycline (60/64, 93.8%), minocycline (228/248, 91.9%), linezolid (497/575, 86.4%), fosfomycin (25/29, 86.2%) and amikacin (319/386, 82.6%).
Overall, the most potent antibiotics were polymyxin-B (274/298, 91.9%), tigecycline (208/236, 88.1%), fosfomycin (339/395, 85.8%), minocycline (345/431, 80.0%), imipenem (703/891, 78.9%) and colistin (503/642, 78.3%) (Table 2). Table S1 (Supplementary Materials) contains details on some of the published papers from Pakistan regarding susceptibility profiles of antibiotics to offer further guidance to all key stakeholders in Pakistan to enhance the appropriate prescribing of antibiotics.
Table 2.
Frequency (%) of antimicrobial sensitivity of the clinical isolates.
| Antibiotics | ATC Classification 1 | AWaRe Classification 2 | A. baumannii | E. coli | K. pneumoniae | Proteus spp. | P. aeruginosa | S. aureus | Total |
|---|---|---|---|---|---|---|---|---|---|
| Penicillins | |||||||||
| Penicillin | J01CE01 | Access | NA * | NA | NA | NA | NA | 153/511 (29.9) | 153/579 (26.39%) |
| Ampicillin | J01CA01 | Access | NA | 54/543 (9.9%) | NA | 5/53 (9.4) | NA | NA | 129/1112 (11.61%) |
| Oxacillin | J01CF04 | Access | NA | NA | NA | NA | NA | 23/53 (43.4) | 23/53 (43.4) |
| Beta-lactam inhibitors | |||||||||
| Amoxicillin-clavulanate | J01CR02 | Access | NA | 51/198 (25.8) | 23/81 (28.4) | 14/44 (31.8) | NA | 27/48 (56.3) | 115/371 (31.00%) |
| Ampicillin–Sulbactam | J01CR01 | Access | NA | 31/93 (33.3) | 4/16 (25.0) | 0/1 (0) | NA | 14/38 (36.8) | 49/148 (33.11%) |
| Piperacillin–Tazobactam | J01CR05 | Watch | 10/28 (35.7) | 415/573 (72.4) | 173/231 (74.9) | 54/60 (90.0) | 241/360 (66.9) | 25/47 (53.2) | 918/1299 (70.70%) |
| Cephalosporins | |||||||||
| Cephalexin | J01DB01 | Access | NA | 7/145 (4.8) | 3/37 (8.1) | 0/3 (0.0) | NA | 60/138 (43.5) | 70/323 (21.67%) |
| Cefazolin | J01DB04 | Access | NA | 3/44 (6.8) | 2/19 (10.5) | 1/2 (50.0) | NA | 35/67 (52.2) | 41/132 (31.06%) |
| Cephradine | J01DB09 | Access | NA | NA | NA | NA | NA | 14/42 (33.3) | 14/42 (33.3) |
| Cefoxitin | J01DC01 | Watch | NA | NA | NA | NA | NA | 243/336 (72.3) | 279/400 (69.75%) |
| Cefuroxime | J01DC02 | Watch | NA | 74/474 (15.6) | 50/201 (24.9) | 10/53 (18.9) | NA | 69/134 (51.4) | 203/862 (23.56%) |
| Cefaclor | J01DC04 | Watch | NA | 31/222 (14) | 32/91 (35.2) | 1/14 (7.1) | NA | 35/85 (41.2) | 99/412 (24.03%) |
| Cefotaxime | J01DD01 | Watch | 0/1 (0.0) | 38/171 (22.2) | 9/34 (26.5) | 0/1 (0.0) | NA | 23/72 (31.9) | 70/279 (25.09%) |
| Ceftazidime | J01DD02 | Watch | 0/22 (0.0) | 122/336 (36.3) | 67/156 (42.9) | 14/25 (56) | 122/271 (45.0) | 28/86 (32.6) | 353/896 (39.42%) |
| Ceftriaxone | J01DD04 | Watch | 1/38 (2.6) | 95/412 (23.1) | 20/86 (23.3) | 9/34 (26.5) | NA | 111/210 (52.9) | 236/780 (30.26%) |
| Cefixime | J01DD08 | Watch | NA | 94/437 (21.5) | 56/169 (33.1) | 11/38 (28.9) | NA | 30/86 (34.9) | 191/730 (26.16%) |
| Cefoprazone | J01DD12 | Watch | NA | NA | NA | NA | NA | 40/64 (62.5) | 40/64 (62.5) |
| Cefoperazone–Sulbactam | J01DD62 | Watch | NA | NA | NA | NA | NA | 12/29 (41.4) | 31/62 (50.0%) |
| Cefepime | J01DE01 | Watch | 4/34 (11.8) | 56/244 (23.0) | 22/56 (39.3) | 8/19 (42.1) | 48/107 (44.9) | 20/69 (29.0) | 37/117 (31.7) |
| Carbapenems | |||||||||
| Imipenem | J01DH51 | Watch | 10/28 (35.7) | 373/427 (87.4) | 92/108 (85.2) | 25/36 (69.4) | 105/149 (70.5) | 98/143 (68.5) | 703/891 (78.9) |
| Meropenem | J01DH02 | Watch | 17/48 (35.4) | 542/639 (84.8) | 142/203 (70) | 48/57 (84.2) | 228/346 (65.9) | 148/244 (60.7) | 1125/1537 (73.12%) |
| Etrapenem | J01DH03 | Watch | NA | 96/123 (78.0) | 27/42 (64.3) | 14/19 (73.7) | NA | 2/4 (50.0) | 156/225 (69.3) |
| Macrolides | |||||||||
| Erythromycin | J01FA01 | Watch | NA | NA | NA | NA | NA | 296/559 (53) | 296/559 (53) |
| Clarithromycin | J01FA09 | Watch | NA | NA | NA | NA | NA | 122/255 (47.8) | 122/255 (47.8) |
| Azithromycin | J01FA10 | Watch | NA | NA | NA | NA | NA | 36/128 (28.1) | 36/128 (28.1) |
| Clindamycin | J01FF01 | Access | NA | NA | NA | NA | NA | 412/665 (62.0) | 412/665 (62.0) |
| Aminoglycosides | |||||||||
| Gentamicin | J01GB03 | Access | 7/38 (18.4) | 291/518 (56.2) | 75/149 (50.3) | 22/48 (45.8) | 120/225 (53.3) | 331/495 (66.9) | 846/1473 (57.4) |
| Amikacin | J01GB06 | Access | 16/41 (39) | 498/631(78.9) | 141/211 (66.8) | 40/58 (69) | 247/356 (69.4) | NA | 942/1297 (72.63%) |
| Tobramycin | J01GB01 | Watch | 8/23 (34.8) | 107/233 (45.9) | 61/111 (55) | 14/23 (60) | 137/227 (60) | NA | 327/617 (52.97%) |
| Fluoroquinolones | |||||||||
| Ciprofloxacin | J01MA02 | Watch | 7/32 (21.9) | 164/555 (29.5) | 81/164 (49.4) | 27/47 (57.4) | 150/288 (52.1) | 250/580 (43.1) | 679/1666 (40.8) |
| Levofloxacin | J01MA12 | Watch | 11/23 (47.8) | 143/374 (38.2) | 59/152 (38.8) | 20/39 (51.3) | 117/215 (54.4) | 134/245 (54.7) | 484/1048 (46.2) |
| Norfloxacin | J01MA06 | Watch | NA | 90/344 (26.2) | 46/118 (39.0) | 11/23 (47.8) | 73/182 (40.1) | 33/116 (28.4) | 253/783 (32.36%) |
| Ofloxacin | J01MA01 | Watch | NA | 62/256 (24.2) | 50/83 (60.2) | 1/9 (11.1) | 56/133 (42.1) | 25/68 (36.8) | 194/549 (35.34%) |
| Moxifloxacin | J01MA14 | Watch | NA | NA | 6/34 (17.6) | NA | NA | 56/107 (52.3) | 62/141 (44.0%) |
| Nalidixic acid | J01MB02 | NA | NA | 48/294 (16.3) | 24/104 (23.1) | 2/20 (10.0) | NA | NA | 74/418 (17.70%) |
| Tetracyclines | |||||||||
| Doxycycline | J01AA02 | Access | 1/11 (9.1) | 82/191 (42.9) | 9/20 (45.0) | 3/5 (60.0) | NA | 69/126 (54.8) | 164/353 (46.45) |
| Tetracycline | J01AA07 | Access | 2/16 (12.5) | 77/258 (29.8) | 57/120 (47.5) | NA | NA | 194/399 (48.6) | 339/825 (36.63) |
| Minocycline | J01AA08 | Watch | 21/30 (70.0) | 25/52 (48.1) | 12/19 (63.2) | 5/12 (41.7) | NA | 228/248 (91.9) | 291/361 (80.61%) |
| Tigecycline | J01AA12 | Reserve | NA | 86/95 (90.5) | NA | NA | NA | 60/64 (93.8) | 146/159 (91.82%) |
| Carb–Monobactams | |||||||||
| Aztreonam | J01DF01 | Reserve | NA | 35/100 (35) | 1/3 (33.3) | NA | 14/42 (33.3) | 0/6 (0.0) | 50/151 (33.1) |
| Glycopeptide | |||||||||
| Vancomycin | A07AA09 | Watch | NA | NA | NA | NA | NA | 513/737 (69.6) | 513/737 (69.6) |
| Tecoplanin | J01XA02 | Watch | NA | NA | NA | NA | NA | 73/125 (58.4) | 73/125 (58.4) |
| Polymixin | |||||||||
| Colistin | A07AA10 | Reserve | 28/35 (80.0) | 177/231 (76.6) | 94/99 (94.9) | NA | 179/209 (85.6) | 489/601 (81.36%) | |
| Polymixin-B | A07AA05 | Reserve | 27/28 (96.4) | 80/87 (92.0) | 48/48 (100.0) | NA | 102/108 (94.4) | NA | 265/288 (92.01%) |
| Phosphonic | |||||||||
| Fosfomycin | J01XX01 | Watch | NA | 255/277 (92.1) | 25/32 (78.1) | NA | NA | NA | 280/309 (90.61%) |
| Nitrofuran | |||||||||
| Nitrofurantoin | J01XE01 | Access | 3/5 (60.0) | 171/205 (83.4) | 14/18 (77.8) | NA | 25/34 (73.5) | 222/283 (78.4) | |
| Oxazolidinones | |||||||||
| Linezolid | J01XX08 | Reserve | NA | NA | NA | NA | NA | 497/575 (86.4) | 497/575 (86.4) |
| Sulphonamide | |||||||||
| Trimethoprim–sulfamethoxazole | J01EE01 | Access | 1/32 (3.1) | 68/359 (18.9) | 28/92 (30.4) | 4/25 (16.0) | NA | 78/327 (23.8) | 179/835 (21.44%) |
4. Discussion
We believe this is one of the first studies in Pakistan to comprehensively review antibiotic susceptibility patterns against a range of Gram-positive and Gram-negative pathogens to assist with future antibiotic prescribing. As mentioned, such activities are increasingly important in Pakistan given the current high empiric prescribing across a range of hospitals and ages in Pakistan exacerbated by high patient co-payments including for sensitivity testing [49,50,53,58]. In addition, currently considerable prescribing of ‘Watch’ and ‘Reserve’ antibiotics, reaching 100% of all antibiotics prescribed in some hospitals during the recent pandemic. An appreciable proportion of these will not be appropriate, especially with the WHO target of 60% of prescriptions in hospitals should ideally be ‘Access’ antibiotics [46,49,50,51,52,53,69,72] Alongside this, concerns generally with adherence to recommended guidelines [46,49,50,51,52].
Regular reviews of susceptibility patterns of pathogens are essential to inform empiric antibiotic guidelines at an institutional level and reduce AMR. In the present study, E. coli was the most common pathogen isolated, which was resistant to more than one antibiotic, similar to other studies [73,74]. The present data showed that E. coli was highly resistant to penicillins, cephalosporins, macrolide and quinolone antibiotics. The resistance of this uropathogenic against quinolone is a concern given the appreciable prescribing or dispensing in Pakistan for this indication [75]. The dispensing of antibiotics without a prescription in most community-acquired infections could be a potential reason for this high resistance, with high rates of dispensing of ‘Watch’ and ‘Reserve’ antibiotics in Pakistan without a prescription [76,77]. Other published studies have also shown increasing resistance against antibiotics with the passage of time [78,79]. In a study involving private hospitals in Lahore, Pakistan, the authors found that out of 93 Escherichia coli isolates, 82% were resistant to beta-lactam antibiotics with many resistant to fluoroquinolones and trimethoprim–sulfamethoxazole, which was attributable to antibiotic overuse [80].
Antibiotic sensitivity in commensal E. coli has been monitored among healthy Bolivian children. The researchers found that resistance to earlier discovered antibiotics including penicillin and ampicillin was greater than to the relatively newly discovered antibiotics including fluoroquinolones [81]. This has implications for Pakistan.
Staphylococcus aureus was the second most common pathogen isolated in our study, highly dominant in pus where they cause skin infections, and is also a major cause of bloodstream infections (BSI) [82,83]. The current data in our study indicated that penicillins, cephalosporins, macrolide and quinolone antibiotics are no longer active against multidrug-resistant pathogens. Even now, these pathogens have developed resistance against linezolid and glycopeptides which also needs addressing going forward [84,85,86].
Similarly, the penicillin class of antibiotics was the first line of defense in the treatment of infections caused by the genus Staphylococcus in Pakistan. However, with the passage of time, increased resistance has been acquired by the microbes resulting in an increased percentage of methicillin-resistant Staphylococcus aureus (MRSA) pathogens [87]. Recently acquired resistances of B-lactamases have made treatment difficult for the Gram-negative bacilli and Gram-positive cocci [88]. However, sensitivity patterns are closely related to the person’s age, sex and anti-microbial therapy background. Consequently, physicians should keep these factors in mind when prescribing antibiotics, guided by regularly updated antibiograms in hospitals.
After E. coli and Staphylococcus aureus, the third most common isolate was Pseudomonas aeruginosa, and the resistance pattern of this pathogen was similar. The published data also highlighted the resistance pattern of this microbe against pneumonia [89]. Klebsiella pneumoniae is another multidrug-resistant microbe, which is a major cause of community-acquired pneumonia [90,91]. The studies indicated an increase in resistance patterns of this strain, especially against carbapenem, which is another concern going forward [92,93]. However, the present data showed that carbapenems are still active against Klebsiella pneumoniae, which is encouraging.
Colistin remains the last resort of antibiotics for life-threatening infections due to carbapenem-resistant pathogens [94] and should be treated as such [68,69]. Similar to other studies, Enterococci were also showing resistance to the quinolones [95], whereas penicillins were showing relatively better sensitivities. Moreover, linezolid and glycopeptides were showing full response against Enterococcus faecalis. Contrary to other studies, encouragingly no vancomycin-resistant enterococci (VRE) was isolated in this current study [96]. According to a recent study from Pakistan, E.coli and Klebsiella were the most common pathogens causing urinary tract infections, and aminoglycosides, quinolones and 2nd- and 3rd-generation cephalosporins are the antibiotics of choice for their treatment [97]. However, AMR was prevalent among K. pneumoniae, and E. coli carried in the clients attending outpatient clinics in Uganda [98], which is a future concern for Pakistan unless addressed.
AMR has also appreciably impacted the management of healthcare-associated infections (HAIs), which have been the major causes of morbidity and mortality in hospitals across countries [99]. Surveillance of HAIs and AMR are key activities in the management of infection control programs in hospitals globally [100]. Surveillance activities also inform the responsible authorities as well as hospitals to design appropriate protocols for empiric therapy [101]. Consequently, surveillance activities should be regularly performed in hospitals in Pakistan to monitor the extent of multidrug-resistant bacteria in hospitals as well as in the food chain as part of a One Health approach [93,102]. Surveillance activities can be part of future ASPs. However, as mentioned, there have been concerns that ASPs were difficult to implement in LMICs [60]. This is now changing with ASPs being successfully instigated among a range of LMICs [59,103,104,105,106]. We will continue to monitor the situation in Pakistan given the urgency of the situation, especially surrounding the appreciable prescribing of ‘Watch’ antibiotics and their impact on AMR, as well as the ongoing issues with implementing the NAP in the country [41,107].
We are aware of a number of limitations with the study. Firstly, the study design was retrospective and observational, which has inherent limitations, including the inability to establish causality or control for potential confounding variables. This may affect the generalizability and interpretation of the findings. Secondly, this initial study focused on selected cities in the province of Punjab in Pakistan for the reasons stated. Consequently, the findings may not be representative of the entire country. Such studies need to be urgently performed in other provinces of Pakistan to gain a more widespread picture. Thirdly, the study relied on bacterial cultural reports for microbial identification and susceptibility testing. This method may have limitations in terms of sensitivity, specificity and the ability to detect specific resistance mechanisms, particularly in the absence of molecular diagnostic tools. The specific criteria used for selecting the bacterial cultural reports may also introduce potential bias as this method may not capture the full spectrum of microbial isolates and susceptibility patterns in the study population. The absence of molecular diagnostic tools for bacterial detection in the study is also a limitation. These tools can provide more accurate identification and characterization of bacterial strains and resistance genes, which would enhance the understanding of antimicrobial resistance patterns. Despite these limitations, we believe our findings are robust, providing direction to the province to review recent recommendations for the treatment of infectious diseases in the newly launched AWaRe book and adapt accordingly [76].
5. Conclusions
In conclusion, based on the findings, the availability of effective antimicrobial treatments is currently appreciably limited in Pakistan due to the rise of multidrug-resistant microorganisms. Consequently, it is increasingly imperative that hospitals in Pakistan implement appropriate ASPs to enhance the appropriate prescribing of antibiotics and reduce AMR. This entails adhering to suitable empiric therapy based on established guidelines aligned with recommendations from the WHO Essential Medicines List (EML) and the AWaRe book, while also including local antibiograms. It is also increasingly essential for clinicians to await culture reports before prescribing antibiotics; however, routine testing requires these costs to be covered by hospitals and not by patients. Alongside this, implementing other measures to enhance appropriate antimicrobial usage to counteract the escalating threat of AMR. We will be following this up in future research projects.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/medicina59071215/s1, Supplementary Table S1: Published paper from Pakistan on susceptibility profiles of antibiotics. This contains additional references [97,108,109,110,111,112].
Author Contributions
Conceptualization: Z.S., A.H., B.G. Methodology: Z.S., S.S.A.A., C.E.M., S.H.K., M.U.Q., G.P., S.A., A.A. (Aisha Azmat), J.C.M., I.U.R., M.U.N., A.A. (Aisha Azmat). Validation: Z.S., A.H., S.S.A.A., M.U.Q., F.R., A.A. (Aisha Azmat), F.K.H., I.U.R., M.U.N., A.A. (Afreenish Amir). Formal Analysis: Z.S., A.H., S.S.A.A., C.E.M., M.U.Q., G.P., F.R., A.A. (Aisha Azmat), F.K.H., J.C.M., I.A.S., I.U.R., M.U.N., A.A. (Afreenish Amir). Writing—Original Draft Preparation: Z.S., A.S., A.A. (Afreenish Amir). Review and Editing: A.H., S.S.A.A., C.E.M., M.U.Q., S.H.K., A.A. (Aisha Azmat), G.P., F.R., S.A., J.C.M., I.U.R., M.U.N., A.A. (Afreenish Amir). Data Curation: C.E.M., J.C.M., I.A.S., M.U.N., A.A. (Aisha Azmat), A.A. (Afreenish Amir). Visualization: C.E.M., J.C.M., G.P. Project Administration: Z.S., B.G. Resources: A.A. (Aisha Azmat), A.S., A.A. (Afreenish Amir). Investigation: S.A., M.U.Q., F.R. Design of the Work: I.A.S. Supervision: F.K.H., B.G. Final Approval & Agreement: Z.S., A.H., S.S.A.A., C.E.M., S.H.K., M.U.Q., A.A. (Aisha Azmat), G.P., F.R., S.A., J.C.M., I.U.R., M.U.N., A.A. (Afreenish Amir), B.G. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was approved by the Human Ethics Committee of the College of Pharmacy, Bahauddin Zakariya University, Multan, Pakistan (BZU- DEPP-22-830-1005It).
Informed Consent Statement
No patient consent was deemed necessary for this study as no patients were identified. This is similar to numerous point prevalence surveys undertaken by the co-authors [46,47,50,113,114,115].
Data Availability Statement
Additional data is available upon reasonable request from the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Algammal A., Hetta H.F., Mabrok M., Behzadi P. Emerging multidrug-resistant bacterial pathogens “superbugs”: A rising public health threat. Front. Microbiol. 2023;14:1135614. doi: 10.3389/fmicb.2023.1135614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saleem Z., Hassali M.A. Travellers take heed: Outbreak of extensively drug resistant (XDR) typhoid fever in Pakistan and a warning from the US CDC. Travel Med. Infect. Dis. 2019;27:127. doi: 10.1016/j.tmaid.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 3.Antimicrobial Resistance Collaborators Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet. 2022;399:629–655. doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hofer U. The cost of antimicrobial resistance. Nat. Rev. Microbiol. 2019;17:3. doi: 10.1038/s41579-018-0125-x. [DOI] [PubMed] [Google Scholar]
- 5.Cassini A., Diaz Högberg L., Plachouras D., Quattrocchi A., Hoxha A., Simonsen G., Colomb-Cotinat M., Kretzschmar M., Devleesschauwer B., Cecchini M., et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modeling analysis. Lancet Infect. Dis. 2019;19:56–66. doi: 10.1016/S1473-3099(18)30605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saeed D.K., Farooqi J., Shakoor S., Hasan R. Antimicrobial resistance among GLASS priority pathogens from Pakistan: 2006–2018. BMC Infect. Dis. 2021;21:1231. doi: 10.1186/s12879-021-06795-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shrestha P., Cooper B.S., Coast J., Oppong R., Do Thi Thuy N., Phodha T., Celhay O., Guerin P.J., Wertheim H., Lubell Y. Enumerating the economic cost of antimicrobial resistance per antibiotic consumed to inform the evaluation of interventions affecting their use. Antimicrob. Resist. Infect. Control. 2018;7:98. doi: 10.1186/s13756-018-0384-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dadgostar P. Antimicrobial Resistance: Implications and Costs. Infect. Drug Resist. 2019;12:3903–3910. doi: 10.2147/IDR.S234610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Will C.M. The problem and the productivity of ignorance: Public health campaigns on antibiotic stewardship. Sociol. Rev. 2020;68:55–76. doi: 10.1177/0038026119887330. [DOI] [Google Scholar]
- 10.Gautam A. Antimicrobial Resistance: The Next Probable Pandemic. JNMA. 2022;60:225–228. doi: 10.31729/jnma.7174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou N., Cheng Z., Zhang X., Lv C., Guo C., Liu H., Dong K., Zhang Y., Liu C., Chang Y.-F., et al. Global antimicrobial resistance: A system-wide comprehensive investigation using the Global One Health Index. Infect. Dis. Poverty. 2022;11:92. doi: 10.1186/s40249-022-01016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Almangour T.A., Ghonem L., Aljabri A., Alruwaili A., Al Musawa M., Damfu N., Almalki M.S., Alattas M., Abed H., Naeem D., et al. Ceftazidime-avibactam versus colistin for the treatment of infections due to carbapenem-resistant Enterobacterales: A multicenter cohort study. Infect. Drug Resist. 2022;15:211. doi: 10.2147/IDR.S349004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Almangour T.A., Alenazi B., Ghonem L., Alhifany A.A., Aldakheel B.A., Alruwaili A. Inhaled colistin for the treatment of nosocomial pneumonia due to multidrug-resistant Gram-negative bacteria: A real-life experience in tertiary care hospitals in Saudi Arabia. Saudi Pharm. J. 2020;28:1009–1013. doi: 10.1016/j.jsps.2020.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collignon P., Beggs J.J., Walsh T.R., Gandra S., Laxminarayan R. Anthropological and socioeconomic factors contributing to global antimicrobial resistance: A univariate and multivariable analysis. Lancet Planet. Health. 2018;2:e398–e405. doi: 10.1016/S2542-5196(18)30186-4. [DOI] [PubMed] [Google Scholar]
- 15.Sartelli M., Hardcastle T.C., Catena F., Chichom-Mefire A., Coccolini F., Dhingra S., Haque M., Hodonou A., Iskandar K., Labricciosa F.M., et al. Antibiotic Use in Low and Middle-Income Countries and the Challenges of Antimicrobial Resistance in Surgery. Antibiotics. 2020;9:497. doi: 10.3390/antibiotics9080497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alghamdi M., Alotaibi F., Ahmed H., Alharbi F., Bukhari O., Youssef A.-R. Effect of medical education on the knowledge, attitude and compliance regarding infection control measures among dental students in Makkah. J. Umm Al-Qura Univ. Med. Sci. 2021;7:14–17. doi: 10.54940/ms31191227. [DOI] [Google Scholar]
- 17.Almeleebia T.M., Alhifany A.A., Almutairi F., Alshibani M., Alhossan A.M. Regulating antimicrobial sales in Saudi Arabia: Achievements and challenges. Int. J. Clin. Pract. 2021;75:e13833. doi: 10.1111/ijcp.13833. [DOI] [PubMed] [Google Scholar]
- 18.Khonsari M.S., Behzadi P., Foroohi F. The prevalence of type 3 fimbriae in Uropathogenic Escherichia coli isolated from clinical urine samples. Meta Gene. 2021;28:100881. doi: 10.1016/j.mgene.2021.100881. [DOI] [Google Scholar]
- 19.Saleem Z., Hassali M.A., Godman B., Hashmi F.K., Saleem F. Antimicrobial prescribing and determinants of antimicrobial resistance: A qualitative study among physicians in Pakistan. Int. J. Clin. Pharm. 2019;41:1348–1358. doi: 10.1007/s11096-019-00875-7. [DOI] [PubMed] [Google Scholar]
- 20.Karampatakis T., Tsergouli K., Behzadi P. Carbapenem-resistant Klebsiella pneumoniae: Virulence factors, molecular epidemiology and latest updates in treatment options. Antibiotics. 2023;12:234. doi: 10.3390/antibiotics12020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Njoga E.O., Ogugua A.J., Nwankwo I.O., Awoyomi O.J., Okoli C.E., Buba D.M., Oyeleye F.A., Ajibo F.E., Azor N., Ogunniran T.M., et al. Antimicrobial drug usage pattern in poultry farms in Nigeria: Implications for food safety, public health and poultry disease management. Vet. Ital. 2021;57:5–12. doi: 10.12834/VetIt.2117.11956.1. [DOI] [PubMed] [Google Scholar]
- 22.Gajdács M., Paulik E., Szabó A. Knowledge, attitude and practice of community pharmacists regarding antibiotic use and infectious diseases: A cross-sectional survey in Hungary (KAPPhA-HU) Antibiotics. 2020;21:41. doi: 10.3390/antibiotics9020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Godman B., Egwuenu A., Haque M., Malande O.O., Schellack N., Kumar S., Saleem Z., Sneddon J., Hoxha I., Islam S., et al. Strategies to Improve Antimicrobial Utilization with a Special Focus on Developing Countries. Life. 2021;11:528. doi: 10.3390/life11060528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bell B.G., Schellevis F., Stobberingh E., Goossens H., Pringle M. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect. Dis. 2014;14:13. doi: 10.1186/1471-2334-14-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohsin M., Van Boeckel T.P., Saleemi M.K., Umair M., Naseem M.N., He C., Khan A., Laxminarayan R. Excessive use of medically important antimicrobials in food animals in Pakistan: A five-year surveillance survey. Glob. Health Action. 2019;12((Suppl. S1)):1697541. doi: 10.1080/16549716.2019.1697541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hadi M.A., Karami N.A., Al-Muwalid A.S., Al-Otabi A., Al-Subahi E., Bamomen A., Mohamed M.M.A., Elrggal M.E. Community pharmacists’ knowledge, attitude, and practices towards dispensing antibiotics without prescription (DAwP): A cross-sectional survey in Makkah Province, Saudi Arabia. Int. J. Infect. Dis. 2016;47:95–100. doi: 10.1016/j.ijid.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Elton L., Thomason M.J., Tembo J., Velavan T.P., Pallerla S.R., Arruda L.B., Vairo F., Montaldo C., Ntoumi F., Abdel Hamid M.M., et al. Antimicrobial resistance preparedness in sub-Saharan African countries. Antimicrob. Resist. Infect. Control. 2020;9:145. doi: 10.1186/s13756-020-00800-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WHO Global Action Plan on Antimicrobial Resistance. 2015. [(accessed on 27 March 2023)]. Available online: https://apps.who.int/iris/bitstream/handle/10665/193736/9789241509763_eng.pdf?sequence=1.
- 29.Inoue H. Strategic approach for combating antimicrobial resistance (AMR) Glob. Health Med. 2019;1:61–64. doi: 10.35772/ghm.2019.01026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harant A. Assessing transparency and accountability of national action plans on antimicrobial resistance in 15 African countries. Antimicrob. Resist. Infect. Control. 2022;11:15. doi: 10.1186/s13756-021-01040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.WHO Implementation Handbook for National Action Plans on Antimicrobial Resistance: Guidance for the Human Health Sector. 2022. [(accessed on 27 March 2023)]. Available online: https://www.who.int/publications/i/item/9789240041981.
- 32.Godman B., Egwuenu A., Wesangula E., Schellack N., Kalungia A.C., Tiroyakgosi C., Kgatlwane J., Mwita J.C., Patrick O., Niba L.L., et al. Tackling antimicrobial resistance across sub-Saharan Africa: Current challenges and implications for the future. Expert Opin. Drug Saf. 2022;21:1089–1111. doi: 10.1080/14740338.2022.2106368. [DOI] [PubMed] [Google Scholar]
- 33.Ministry of National Health Services Regulations & Coordination Government of Pakistan National AMR Action Plan for Pakistan. [(accessed on 29 March 2023)];2017 Available online: https://www.nih.org.pk/wp-content/uploads/2018/08/AMR-National-Action-Plan-Pakistan.pdf.
- 34.Mendelson M., Matsoso M.P. The World Health Organization global action plan for antimicrobial resistance. SAMJ S. Afr. Med. J. 2015;105:325. doi: 10.7196/SAMJ.9644. [DOI] [PubMed] [Google Scholar]
- 35.Munkholm L., Rubin O. The global governance of antimicrobial resistance: A cross-country study of alignment between the global action plan and national action plans. Glob. Health. 2020;16:1–11. doi: 10.1186/s12992-020-00639-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Furuya-Kanamori L., Yakob L. Filling the gaps in global antimicrobial resistance research/surveillance. BMC Infect. Dis. 2020;20:39. doi: 10.1186/s12879-019-4708-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.WHO Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report. 2021. [(accessed on 21 March 2023)]. Available online: https://www.who.int/publications/i/item/9789240027336.
- 38.Tornimbene B., Eremin S., Abednego R., Abualas E.O., Boutiba I., Egwuenu A., Fuller W., Gahimbare L., Githii S., Kasambara W., et al. Global Antimicrobial Resistance and Use Surveillance System on the African continent: Early implementation 2017–2019. Afr. J. Lab. Med. 2022;11:1594. doi: 10.4102/ajlm.v11i1.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seale A.C., Gordon N.C., Islam J., Peacock S.J., Scott J.A.G. AMR Surveillance in low and middle-income settings—A roadmap for participation in the Global Antimicrobial Surveillance System (GLASS) Wellcome Open Res. 2017;2:92. doi: 10.12688/wellcomeopenres.12527.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Global Antimicrobial Resistance Surveillance System (GLASS) Report: Early Implementation 2017–2018. WHO; Geneva, Switzerland: 2018. [Google Scholar]
- 41.Saleem Z., Godman B., Azhar F., Kalungia A.C., Fadare J., Opanga S., Markovic-Pekovic V., Hoxha I., Saeed A., Al-Gethamy M., et al. Progress on the national action plan of Pakistan on antimicrobial resistance (AMR): A narrative review and the implications. Expert Rev. Anti-Infect. Ther. 2022;20:71–93. doi: 10.1080/14787210.2021.1935238. [DOI] [PubMed] [Google Scholar]
- 42.Seale A.C., Hutchison C., Fernandes S., Stoesser N., Kelly H., Lowe B., Turner P., Hanson K., Chandler C.I.R., Goodman C., et al. Supporting surveillance capacity for antimicrobial resistance: Laboratory capacity strengthening for drug resistant infections in low and middle income countries. Wellcome Open Res. 2017;2:91. doi: 10.12688/wellcomeopenres.12523.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saleem Z., Hassali M.A., Hashmi F.K. Pakistan’s national action plan for antimicrobial resistance: Translating ideas into reality. Lancet Infect. Dis. 2018;18:1066–1067. doi: 10.1016/S1473-3099(18)30516-4. [DOI] [PubMed] [Google Scholar]
- 44.Bilal H., Khan M.N., Rehman T., Hameed M.F., Yang X. Antibiotic resistance in Pakistan: A systematic review of past decade. BMC Infect. Dis. 2021;21:244. doi: 10.1186/s12879-021-05906-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jabeen F., Khan Z., Sohail M., Tahir A., Tipu I., Murtaza Saleem H.G. Antibiotic Resistance Pattern Of Acinetobacter Baumannii Isolated From Bacteremia Patients In Pakistan. J. Ayub Med. Coll. Abbottabad. 2022;34:95–100. doi: 10.55519/JAMC-01-9105. [DOI] [PubMed] [Google Scholar]
- 46.Shaikh Q., Sarfaraz S., Rahim A., Hussain A., Behram S., Kazi A.S., Hussain M., Salahuddin N. WHO Point Prevalence Survey to Describe the Use of Antimicrobials at a Tertiary Care Center in Pakistan: A Situation Analysis for Establishing an Antimicrobial Stewardship Program. Antibiotics. 2022;11:1555. doi: 10.3390/antibiotics11111555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vernet G., Mary C., Altmann D.M., Doumbo O., Morpeth S., Bhutta Z.A., Klugman K.P. Surveillance for antimicrobial drug resistance in under-resourced countries. Emerg. Infect. Dis. 2014;20:434. doi: 10.3201/EID2003.121157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Atif M., Azeem M., Saqib A., Scahill S. Investigation of antimicrobial use at a tertiary care hospital in Southern Punjab, Pakistan using WHO methodology. Antimicrob. Resist. Infect. Control. 2017;6:41. doi: 10.1186/s13756-017-0199-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saleem Z., Hassali M.A., Versporten A., Godman B., Hashmi F.K., Goossens H., Saleem F. A multicenter point prevalence survey of antibiotic use in Punjab, Pakistan: Findings and implications. Expert Rev. Anti Infect. Ther. 2019;17:285–293. doi: 10.1080/14787210.2019.1581063. [DOI] [PubMed] [Google Scholar]
- 50.Saleem Z., Haseeb A., Godman B., Batool N., Altaf U., Ahsan U., Mustafa Z.U., Nadeem M.U., Farrukh M.J., Mugheera M., et al. Point Prevalence Survey of Antimicrobial Use during the COVID-19 Pandemic among Different Hospitals in Pakistan: Findings and Implications. Antibiotics. 2023;12:70. doi: 10.3390/antibiotics12010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saleem Z., Saeed H., Hassali M.A., Godman B., Asif U., Yousaf M., Ahmed Z., Riaz H., Raza S.A. Pattern of inappropriate antibiotic use among hospitalized patients in Pakistan: A longitudinal surveillance and implications. Antimicrob. Resist. Infect. Control. 2019;8:188. doi: 10.1186/s13756-019-0649-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Akhtar H., Akhtar S., Rahman F.U., Afridi M., Khalid S., Ali S., Akhtar N., Khader Y.S., Ahmad H., Khan M.M. An overview of the treatment options used for the management of COVID-19 in Pakistan: Retrospective observational study. JMIR Public Health Surveill. 2021;7:e28594. doi: 10.2196/28594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arif S., Sadeeqa S., Saleem Z. Patterns of antimicrobial use in hospitalized children: A repeated point prevalence survey from Pakistan. J. Pediatr. Infect. Dis. Soc. 2021;10:970–974. doi: 10.1093/jpids/piab026. [DOI] [PubMed] [Google Scholar]
- 54.Nathwani D., Varghese D., Stephens J., Ansari W., Martin S., Charbonneau C. Value of hospital antimicrobial stewardship programs [ASPs]: A systematic review. Antimicrob. Resist. Infect. Control. 2019;8:35. doi: 10.1186/s13756-019-0471-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saleem Z., Hassali M.A., Hashmi F.K., Godman B., Ahmed Z. Snapshot of antimicrobial stewardship programs in the hospitals of Pakistan: Findings and implications. Heliyon. 2019;5:e02159. doi: 10.1016/j.heliyon.2019.e02159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hayat K., Rosenthal M., Gillani A.H., Chang J., Ji W., Yang C., Jiang M., Zhao M., Fang Y. Perspective of Key Healthcare Professionals on Antimicrobial Resistance and Stewardship Programs: A Multicenter Cross-Sectional Study From Pakistan. Front. Pharmacol. 2019;10:1520. doi: 10.3389/fphar.2019.01520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mubarak N., Khan A.S., Zahid T., Ijaz U.E.B., Aziz M.M., Khan R., Mahmood K., Saif-ur-Rehman N., Zin C.S. Assessment of Adherence to the Core Elements of Hospital Antibiotic Stewardship Programs: A Survey of the Tertiary Care Hospitals in Punjab, Pakistan. Antibiotics. 2021;10:906. doi: 10.3390/antibiotics10080906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Atif M., Zia R., Malik I., Ahmad N., Sarwar S. Treatment outcomes, antibiotic use and its resistance pattern among neonatal sepsis patients attending Bahawal Victoria Hospital, Pakistan. PLoS ONE. 2021;16:e0244866. doi: 10.1371/journal.pone.0244866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haseeb A.S.Z., Altaf U., Batool N., Godman B., Ahsan U., Ashiq M., Razzaq M., Hanif R., E-Huma Z., Amir A., et al. Impact of Positive Culture Reports of E. coli or MSSA on De-Escalation of Antibiotic Use in a Teaching Hospital in Pakistan and the Implications. Infect. Drug Resist. 2023;16:77–86. doi: 10.2147/IDR.S391295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cox J.A., Vlieghe E., Mendelson M., Wertheim H., Ndegwa L., Villegas M.V., Gould I., Levy Hara G. Antibiotic stewardship in low- and middle-income countries: The same but different? Clin. Microbiol. Infect. 2017;23:812–818. doi: 10.1016/j.cmi.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 61.Hindler J.F., Stelling J. Analysis and presentation of cumulative antibiograms: A new consensus guideline from the Clinical and Laboratory Standards Institute. Clin. Infect. Dis. 2007;44:867–873. doi: 10.1086/511864. [DOI] [PubMed] [Google Scholar]
- 62.Guma S.P., Godman B., Campbell S.M., Mahomed O. Determinants of the Empiric Use of Antibiotics by General practitioners in South Africa: Observational, Analytic, Cross-Sectional Study. Antibiotics. 2022;11:1423. doi: 10.3390/antibiotics11101423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Godman B., Haque M., McKimm J., Abu Bakar M., Sneddon J., Wale J., Campbell S., Martin A.P., Hoxha I., Abilova V., et al. Ongoing strategies to improve the management of upper respiratory tract infections and reduce inappropriate antibiotic use particularly among lower and middle-income countries: Findings and implications for the future. Curr. Med. Res. Opin. 2020;36:301–327. doi: 10.1080/03007995.2019.1700947. [DOI] [PubMed] [Google Scholar]
- 64.Saleem Z., Hassali M.A., Godman B., Hashmi F.K., Saleem F. A multicenter point prevalence survey of healthcare-associated infections in Pakistan: Findings and implications. Am. J. Infect. Control. 2019;47:421–424. doi: 10.1016/j.ajic.2018.09.025. [DOI] [PubMed] [Google Scholar]
- 65.Saleem Z., Hassali M.A., Hashmi F.K., Godman B., Saleem F. Antimicrobial dispensing practices and determinants of antimicrobial resistance: A qualitative study among community pharmacists in Pakistan. Fam. Med. Community Health. 2019;7:e000138. doi: 10.1136/fmch-2019-000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.WHO Anatomical Therapeutic Chemical (ATC) Classification. 2021. [(accessed on 27 March 2023)]. Available online: https://www.who.int/tools/atc-ddd-toolkit/atc-classification.
- 67.Sharland M., Pulcini C., Harbarth S., Zeng M., Gandra S., Mathur S., Magrini N. Classifying antibiotics in the WHO Essential Medicines List for optimal use-be AWaRe. Lancet Infect. Dis. 2018;18:18–20. doi: 10.1016/S1473-3099(17)30724-7. [DOI] [PubMed] [Google Scholar]
- 68.Sharland M., Gandra S., Huttner B., Moja L., Pulcini C., Zeng M., Mendelson M., Cappello B., Cooke G., Magrini N. Encouraging AWaRe-ness and discouraging inappropriate antibiotic use—The new 2019 Essential Medicines List becomes a global antibiotic stewardship tool. Lancet Infect. Dis. 2019;19:1278–1280. doi: 10.1016/S1473-3099(19)30532-8. [DOI] [PubMed] [Google Scholar]
- 69.Zanichelli V., Sharland M., Cappello B., Moja L., Getahun H., Pessoa-Silva C., Sati H., van Weezenbeek C., Balkhy H., Simão M., et al. The WHO AWaRe (Access, Watch, Reserve) antibiotic book and prevention of antimicrobial resistance. Bull. World Health Organ. 2023;101:290–296. doi: 10.2471/BLT.22.288614. [DOI] [Google Scholar]
- 70.Hsia Y., Lee B.R., Versporten A., Yang Y., Bielicki J., Jackson C., Newland J., Goossens H., Magrini N., Sharland M. Use of the WHO Access, Watch, and Reserve classification to define patterns of hospital antibiotic use (AWaRe): An analysis of paediatric survey data from 56 countries. Lancet Glob. Health. 2019;7:e861–e871. doi: 10.1016/S2214-109X(19)30071-3. [DOI] [PubMed] [Google Scholar]
- 71.Latif S., Anwar M.S., Ahmad I. Bacterial pathogens responsible for blood stream infection (BSI) and pattern of drug resistance in a tertiary care hospital of Lahore. Biomedica. 2009;25:101–105. [Google Scholar]
- 72.Zeshan B., Karobari M.I., Afzal N., Siddiq A., Basha S., Basheer S.N., Peeran S.W., Mustafa M., Daud N.H., Ahmed N., et al. The usage of antibiotics by COVID-19 patients with comorbidities: The risk of increased antimicrobial resistance. Antibiotics. 2021;11:35. doi: 10.3390/antibiotics11010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Senbayrak S., Boz E.S., Cevan S., Inan A., Engin D.O., Dosoglu N., Cobanoglu N., Dagli O., Davarci I., Aksaray S. Antibiotic Resistance Trends and The ESBL Prevalence of Escherichia coli and Klebsiella spp. Urinary Isolates in In-and Outpatients in a Tertiary Care Hospital in Istanbul, 2004–2012. Jundishapur J. Microbiol. 2017;10:e13098. doi: 10.5812/jjm.13098. [DOI] [Google Scholar]
- 74.Córdoba G., Holm A., Hansen F., Hammerum A.M., Bjerrum L. Prevalence of antimicrobial resistant Escherichia coli from patients with suspected urinary tract infection in primary care, Denmark. BMC Infect. Dis. 2017;17:670. doi: 10.1186/s12879-017-2785-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sakran W., Smolkin V., Odetalla A., Halevy R., Koren A. Community-acquired urinary tract infection in hospitalized children: Etiology and antimicrobial resistance. A comparison between first episode and recurrent infection. Clin. Pediatr. 2015;54:479–483. doi: 10.1177/0009922814555974. [DOI] [PubMed] [Google Scholar]
- 76.Arslan H., Azap Ö.K., Ergönül Ö., Timurkaynak F. Risk factors for ciprofloxacin resistance among Escherichia coli strains isolated from community-acquired urinary tract infections in Turkey. J. Antimicrob. Chemother. 2005;56:914–918. doi: 10.1093/jac/dki344. [DOI] [PubMed] [Google Scholar]
- 77.Saleem Z., Hassali M.A., Godman B., Fatima M., Ahmad Z., Sajid A., Rehman I.U., Nadeem M.U., Javaid Z., Malik M., et al. Sale of WHO AWaRe groups antibiotics without a prescription in Pakistan: A simulated client study. J. Pharm. Policy Pract. 2020;13:26. doi: 10.1186/s40545-020-00233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yamaji R., Rubin J., Thys E., Friedman C.R., Riley L.W. Persistent Pandemic Lineages of Uropathogenic Escherichia coli in a College Community from 1999 to 2017. J. Clin. Microbiol. 2018;56:e01834-17. doi: 10.1128/JCM.01834-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Elsayed T.I., Ismail H.A., Elgamal S.A., Gad A.H. The Occurrence of Multidrug Resistant E. coli which Produce ESBL and Cause Urinary Tract Infections. J. Appl. Microbiol. Biochem. 2017;1:8. doi: 10.21767/2576-1412.100008. [DOI] [Google Scholar]
- 80.Cheema S., Cheema S.U.R. Prevalence of Antibiotic Resistance among Patients with Escherichia Coli Urinary Tract Infection in a Private Hospital at Lahore-Pakistan. Pak. J. Med. Health Sci. 2016;10:364–367. [Google Scholar]
- 81.Bartoloni A., Pallecchi L., Riccobono E., Mantella A., Magnelli D., Di Maggio T., Villagran A.L., Lara Y., Saavedra C., Strohmeyer M., et al. Relentless increase of resistance to fluoroquinolones and expanded-spectrum cephalosporins in Escherichia coli: 20 years of surveillance in resource-limited settings from Latin America. Clin. Microbiol. Infect. 2013;19:356–361. doi: 10.1111/j.1469-0691.2012.03807.x. [DOI] [PubMed] [Google Scholar]
- 82.Seybold U., Kourbatova E.V., Johnson J.G., Halvosa S.J., Wang Y.F., King M.D., Ray S.M., Blumberg H.M. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 genotype as a major cause of health care—Associated bloodstream infections. Clin. Infect. Dis. 2006;42:647–656. doi: 10.1086/499815. [DOI] [PubMed] [Google Scholar]
- 83.Brumfitt W., Hamilton-Miller J. Methicillin-Resistant Staphylococcus aureus. New Engl. J. Med. 1989;320:1188–1196. doi: 10.1056/NEJM198905043201806. [DOI] [PubMed] [Google Scholar]
- 84.Stryjewski M.E., Corey G.R. Methicillin-resistant Staphylococcus aureus: An evolving pathogen. Clin. Infect. Dis. 2014;58((Suppl. S1)):S10–S19. doi: 10.1093/cid/cit613. [DOI] [PubMed] [Google Scholar]
- 85.Roy P.C., Shaheduzzaman M., Sultana N., Jahid I.K. Comparative Antibiotic Sensitivity Pattern of Hospital and Community Acquired Staphylococcus aureus Isolates of Jessore, Bangladesh. J. Biosci. Med. 2015;3:17. [Google Scholar]
- 86.Wilson P., Andrews J.A., Charlesworth R., Walesby R., Singer M., Farrell D.J., Robbins M. Linezolid resistance in clinical isolates of Staphylococcus aureus. J. Antimicrob. Chemother. 2003;51:186–188. doi: 10.1093/jac/dkg104. [DOI] [PubMed] [Google Scholar]
- 87.Hafiz S., Hafiz A., Ali L., Chughtai A., Memon B., Ahmed A., Hussain S., Sarwar G., Mughal T., Awan A., et al. Methicillin resistant Staphylococcus aureus: A multicentre study. J. Pak. Med. Assoc. 2002;52:312–314. [PubMed] [Google Scholar]
- 88.Wong C.K.M., Kung K., Au-Doung P.L.W., Ip M., Lee N., Fung A., Wong S.Y.S. Antibiotic resistance rates and physician antibiotic prescription patterns of uncomplicated urinary tract infections in southern Chinese primary care. PLoS ONE. 2017;12:e0177266. doi: 10.1371/journal.pone.0177266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yayan J., Ghebremedhin B., Rasche K. Antibiotic resistance of Pseudomonas aeruginosa in pneumonia at a single university hospital center in Germany over a 10-year period. PLoS ONE. 2015;10:e0139836. doi: 10.1371/journal.pone.0139836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Navon-Venezia S., Kondratyeva K., Carattoli A. Klebsiella pneumoniae: A major worldwide source and shuttle for antibiotic resistance. FEMS Microbiol. Rev. 2017;41:252–275. doi: 10.1093/femsre/fux013. [DOI] [PubMed] [Google Scholar]
- 91.Villafuerte D., Reyes L., Faverio P., Aliberti S., Restrepo M. prevalence and Risk Factors for Enterobacteriaceae (eb) and Multidrug-resistant Eb in Community-acquired Pneumonia. Chest. 2017;152:A156. doi: 10.1016/j.chest.2017.08.187. [DOI] [Google Scholar]
- 92.Agodi A., Barchitta M., Quattrocchi A., Maugeri A., Aldisio E., Marchese A.E., Mattaliano A.R., Tsakris A. Antibiotic trends of Klebsiella pneumoniae and Acinetobacter baumannii resistance indicators in an intensive care unit of Southern Italy, 2008–2013. Antimicrob. Resist. Infect. Control. 2015;4:43. doi: 10.1186/s13756-015-0087-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.WHO . Antimicrobial Resistance: Global Report on Surveillance. World Health Organization; Geneva, Switzerland: 2014. [Google Scholar]
- 94.Karaaslan A., Çağan E., Kadayifci E.K., Atıcı S., Akkoç G., Yakut N., Öcal Demir S., Soysal A., Bakır M. Intravenous Colistin Use for Multidrug-Resistant Gram-Negative Infections in Pediatric Patients. Balk. Med. J. 2016;33:627. doi: 10.5152/balkanmedj.2016.16210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Billington E.O., Phang S.H., Gregson D.B., Pitout J.D.D., Ross T., Church D.L., Laupland K.B., Parkins M.D. Incidence, risk factors, and outcomes for Enterococcus spp. blood stream infections: A population-based study. Int. J. Infect. Dis. 2014;26:76–82. doi: 10.1016/j.ijid.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 96.Monteserin N., Larson E. Temporal trends and risk factors for healthcare-associated vancomycin-resistant enterococci in adults. J. Hosp. Infect. 2016;94:236–241. doi: 10.1016/j.jhin.2016.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zafar H., Lakhnana N.K., Tauseef K., Zahid M., Kazmi A., Usman A., Ali A. Antibiotic Susceptibility Pattern of Various Isolates in Urine Specimen at a Tertiary Care Hospital of Islamabad. Br. J. Pharm. Res. 2016;10:1–9. doi: 10.9734/BJPR/2016/23905. [DOI] [Google Scholar]
- 98.Najjuka C.F., Kateete D.P., Kajumbula H.M., Joloba M.L., Essack S.Y. Antimicrobial susceptibility profiles of Escherichia coli and Klebsiella pneumoniae isolated from outpatients in urban and rural districts of Uganda. BMC Res. Notes. 2016;9:235. doi: 10.1186/s13104-016-2049-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Allegranzi B., Nejad S.B., Combescure C., Graafmans W., Attar H., Donaldson L., Pittet D. Burden of endemic health-care-associated infection in developing countries: Systematic review and meta-analysis. Lancet. 2011;377:228–241. doi: 10.1016/S0140-6736(10)61458-4. [DOI] [PubMed] [Google Scholar]
- 100.Núñez-Núñez M., Navarro M.D., Palomo V., Rajendran N.B., del Toro M.D., Voss A., Sharland M., Sifakis F., Tacconelli E., Rodríguez-Baño J. The methodology of surveillance for antimicrobial resistance and healthcare-associated infections in Europe (SUSPIRE): A systematic review of publicly available information. Clin. Microbiol. Infect. 2017;24:105–109. doi: 10.1016/j.cmi.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 101.Rehman I.U., Khan T.M., Ali I., Bukhsh A. Antimicrobial susceptibility in a tertiary care hospital in Pakistan. Can. J. Infect. Control. 2016;31:178–181. [Google Scholar]
- 102.Barlow R., Gobius K. Pilot Survey for Antimicrobial Resistant (AMR) Bacteria in Australian Food. CSIRO; Cannon Hill, Australia: 2008. [Google Scholar]
- 103.Akpan M.R., Isemin N.U., Udoh A.E., Ashiru-Oredope D. Implementation of antimicrobial stewardship programmes in African countries: A systematic literature review. J. Glob. Antimicrob. Resist. 2020;22:317–324. doi: 10.1016/j.jgar.2020.03.009. [DOI] [PubMed] [Google Scholar]
- 104.Siachalinga L., Mufwambi W., Lee I.H. Impact of antimicrobial stewardship interventions to improve antibiotic prescribing for hospital inpatients in Africa: A systematic review and meta-analysis. J. Hosp. Infect. 2022;129:124–143. doi: 10.1016/j.jhin.2022.07.031. [DOI] [PubMed] [Google Scholar]
- 105.Otieno P.A., Campbell S., Maley S., Obinju Arunga T., Otieno Okumu M. A Systematic Review of Pharmacist-Led Antimicrobial Stewardship Programs in Sub-Saharan Africa. Int. J. Clin. Pract. 2022;2022:3639943. doi: 10.1155/2022/3639943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Haseeb A., Saleem Z., Maqadmi A.F., Allehyani R.A., Mahrous A.J., Elrggal M.E., Kamran S.H., AlGethamy M., Naji A.S., AlQarni A., et al. Ongoing Strategies to Improve Antimicrobial Utilization in Hospitals across the Middle East and North Africa (MENA): Findings and Implications. Antibiotics. 2023;12:827. doi: 10.3390/antibiotics12050827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sulis G., Sayood S., Katukoori S., Bollam N., George I., Yaeger L.H., Chavez M.A., Tetteh E., Yarrabelli S., Pulcini C., et al. Exposure to World Health Organization’s AWaRe antibiotics and isolation of multidrug-resistant bacteria: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2022;28:1193–1202. doi: 10.1016/j.cmi.2022.03.014. [DOI] [PubMed] [Google Scholar]
- 108.Samad A., Ahmed T., Rahim A., Khalil A., Ali I. Antimicrobial susceptibility patterns of clinical isolates of Pseudomonas aeruginosa isolated from patients of respiratory tract infections in a Tertiary Care Hospital, Peshawar. Pak. J. Med. Sci. 2017;33:670. doi: 10.12669/pjms.333.12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mansoor T., Musani M.A., Khalid G., Kamal M. Pseudomonas aeruginosa in chronic suppurative otitis media: Sensitivity spectrum against various antibiotics in Karachi. J. Ayub Med. Coll. Abbottabad. 2009;21:120–123. [PubMed] [Google Scholar]
- 110.Khan M., Siddiqui S., Haider S., Zafar A., Zafar F., Khan R., Afshan K., Jabeen A., Khan M.S., Hasan R. Infection control education: Impact on ventilator-associated pneumonia rates in a public sector intensive care unit in Pakistan. Trans. R. Soc. Trop. Med. Hyg. 2009;103:807–811. doi: 10.1016/j.trstmh.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 111.Masood R., Aman A., Karim F. Microbial Etiology of Pneumonia: Epidemiology, Diagnosis and Resistance Pattern. Pak. J. Med. Health Sci. 2022;16:846. doi: 10.53350/pjmhs22168846. [DOI] [Google Scholar]
- 112.Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. WMA; Ferney-Voltaire, France: 2009. [PubMed] [Google Scholar]
- 113.Chowdhury K., Haque M., Nusrat N., Adnan N., Islam S., Lutfor A.B., Begum D., Rabbany A., Karim E., Malek A., et al. Management of Children Admitted to Hospitals across Bangladesh with Suspected or Confirmed COVID-19 and the Implications for the Future: A Nationwide Cross-Sectional Study. Antibiotics. 2022;11:105. doi: 10.3390/antibiotics11010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Skosana P.P., Schellack N., Godman B., Kurdi A., Bennie M., Kruger D., Meyer J.C. A point prevalence survey of antimicrobial utilisation patterns and quality indices amongst hospitals in South Africa; findings and implications. Expert Rev. Anti Infect. Ther. 2021;19:1353–1366. doi: 10.1080/14787210.2021.1898946. [DOI] [PubMed] [Google Scholar]
- 115.Kurdi A., Hasan A.J., Baker K.I., Seaton R.A., Ramzi Z.S., Sneddon J., Godman B. A multicentre point prevalence survey of hospital antibiotic prescribing and quality indices in the Kurdistan regional government of Northern Iraq: The need for urgent action. Expert Rev. Anti Infect. Ther. 2021;19:805–814. doi: 10.1080/14787210.2021.1834852. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Additional data is available upon reasonable request from the corresponding authors.