Abstract
Gram-negative Azospirillum brasilense accumulates approximately 80% of polyhydroxybutyrate (PHB) as dry cell weight. For this reason, this bacterium has been characterized as one of the main microorganisms that produce PHB. PHB is synthesized inside bacteria by the polymerization of 3-hydroxybutyrate monomers. In this review, we are focusing on the analysis of the PHB production by A. brasilense in order to understand the metabolism during PHB accumulation. First, the carbon and nitrogen sources used to improve PHB accumulation are discussed. A. brasilense accumulates more PHB when it is grown on a minimal medium containing a high C/N ratio, mainly from malate and ammonia chloride, respectively. The metabolic pathways to accumulate and mobilize PHB in A. brasilense are mentioned and compared with those of other microorganisms. Next, we summarize the available information to understand the role of the genes involved in the regulation of PHB metabolism as well as the role of PHB in the physiology of Azospirillum. Finally, we made a comparison between the properties of PHB and polypropylene, and we discussed some applications of PHB in biomedical and commercial areas.
Keywords: polyhydroxybutyrate, Azospirillum brasilense, PHB genes, PHB regulation, PHB metabolism
1. Introduction
Gram-negative Azospirillum brasilense belongs to the α-proteobacteria class. It is a motile, vibrio-shaped bacterium of 2.0–4.0 μm length [1]. Azospirillum promotes plant growth. Also, it produces high quantities of bioplastic called poly-β-hydroxybutyrate (PHB) [2]. PHB is part of a cluster of bioplastics called polyhydroxyalkanoates (PHA). There are more than 150 different PHAs discovered. The two PHAs most studied are polyhydroxyvalerate (PHV) and PHB [3].
PHB is a biodegradable and biocompatible plastic characterized to have a methyl radical in the β-position of the carbon skeleton of PHA [4]. It has been shown that A. brasilense produces only 3-hydroxybutyrate monomers [5,6]. Fourier-transform infrared spectroscopy (FTIR) analyses have shown an ester band v(C=O) at 1727 cm−1, which is compatible with PHB [7]. This review aims to summarize the most important factors to consider for understanding PHB metabolism in A. brasilense. Throughout the text, we discuss the best carbon and nitrogen sources for improving PHB production. The regulation of PHB metabolism and the functions of PHB are analyzed. Finally, a comparison between the characteristics of PHB and polypropylene (PP) is reviewed, and some examples of uses of PHB in the medical industry are described.
2. The Role of the Carbon Source in PHB Production by A. brasilense
Previously, it was demonstrated that A. brasilense accumulates large quantities of PHB when it grows on a medium supplemented with high concentrations of carbon with minimal quantities of nitrogen (high C/N ratio) [2,8,9]. Azospirillum can use a wide range of carbon and nitrogen sources. In terms of carbon, it grows well in fructose, malate, succinate, oxaloacetate, pyruvate, glycerol, lactate, and β-hydroxybutyric acid, among others [1,10,11]. Amino acids are poorly used as carbon, and glucose cannot support the growth of A. brasilense [12,13]. N2, amino acids, NH3, NH4, and NO3− have been reported as good nitrogen sources for this bacterium [11,14,15]. Azospirillum can use a wide spectrum of carbon and nitrogen sources because in this bacterium occur tricarboxylic acid (TCA), glyoxylate, and Entner–Duodoroff cycles, but it lacks Embden–Meyerhof–Parnas and hexose monophosphate pathways [12,13,16].
To improve PHB synthesis, several carbon and nitrogen sources have been evaluated. The highest quantities of PHB were produced when malic acid and ammonia chloride were used as carbon and nitrogen sources, respectively [5,9,17]. When A. brasilense grows on malate and ammonia chloride, it accumulates up to 88% of dry cell weight (DCW) as PHB. The carbon source, malate, enters the TCA cycle to produce both primary and secondary metabolites.
On fructose or lactate, Azospirillum reaches 40 and 50% of PHB as dry cell weight, respectively [5]. Another nitrogen source that allowed high PHB accumulation was sodium nitrate [18]. A. brasilense fixes nitrogen when the nitrogen source is depleted. Under nitrogen-fixing conditions, it accumulates from 30 to 75% of PHB as dry cell weight [8,18,19]. Oxygen is also important for PHB synthesis. Data showed that the use of malate and ammonium chloride in addition to high oxygen levels inhibits PHB accumulation by A. brasilense [8]. However, low oxygen availability leads Azospirillum to accumulate more than 70% of dry cell weight as PHB [5,9]. Previous studies found the highest accumulation of PHB when a 70–140 C/N ratio was used [2,8].
Another Rhodospirillaceae, Rhodospirillum rubrum, uses acetate for PHB synthesis and prefers anaerobic conditions to improve it [20]. Pseudomonads turn acetate, ethanol, fructose, glucose, gluconate, and glycerol into acetyl-CoA for PHA synthesis. In this bacterium, PHA metabolites are obtained through β-oxidation and de novo fatty acid synthesis pathways [21]. Other carbon sources used by bacteria to produce PHB are methane for Methylobacterium strains [22], mannitol for Bradirhizobium diazoefficiens [23], and glucose for R. eutropha. The latter accumulates up to 90% of PHB (Table 1) [24]. R. eutropha, Azotobacter spp., Bacillus, Pseudomonas, and Azospirillum spp. are the most studied microorganisms in terms of PHB production [2,24].
Table 1.
PHB accumulation by common strains.
| Strain | Carbon Source | %PHB/DCW | Reference |
|---|---|---|---|
| A. brasilense Sp7 | Malate, fructose, pyruvate | 70–88% | [2,8,9,18] |
| R. eutropha | Glucose | 80–90% | [25] |
| R. rubrum | Acetate | [20] | |
| Pseudomonas extremaustralis | Octanoate, fructose, glucose, glycerol | 70–80% | [21,26,27] |
| Methylocystis hirsuta | Methanol:ethanol, methane | 73–85% | [28] |
| Bradyrhizobium diazoefficiens | Mannitol, glucose, and glycerol | 68% | [29] |
| A. vinelandii | Sucrose | 85% | [30] |
| Bacillus subtilis | Various sources | 60% | [31] |
| Rhizobium nepotum | Pyruvate | 62% | [28] |
3. PHB Synthesis and Degradation by A. brasilense
Biopolymer synthesis by A. brasilense involves three enzymatic reactions. The first is catalyzed by β-ketothiolase (coded by the phbA gene), which condenses two acetyl-CoA molecules and synthesizes acetoacetyl-CoA. Afterward, it is reduced to β-hydroxybutyryl-CoA by an NAD(P)-dependent acetoacetyl-CoA reductase (coded by the phbB gene). Finally, the β-hydroxybutyryl-CoA is polymerized into PHB by the PHB polymerase coded by the phbC gene (Figure 1) [32,33]. This pathway occurs similarly in A. beijerinckii, R. eutropha, and Sinorhizobium meliloti, among others [34,35,36]. Other microorganisms such as P. putida can synthesize PHB and PHV and copolymers, for example, PHB-co-PHV. In PHA synthesis by P. putida, the roles of PhaJ, epimerase, and FabG have been described. The PhaJ oxidizes acyl-CoAs into enoyl-CoA. The latter is converted into 3-hydroxyacyl-CoA by an epimerase. Then, 3-hydroxyacyl-CoA is reduced by FabG to form (R)-3-ketoacyl-CoA. Finally, a PhaC polymerizes (R)-3-ketoacyl-CoA into PHA. Another pathway to synthesize PHA in P. putida begins with the transacylation of malonyl-CoA and acetyl-CoA with the acyl carrier protein (ACP). The resulting malonyl-ACP and acyl-ACP are condensed into ketoacyl-ACP. Afterward, it is reduced to (R)-3-hydroxyacyl-ACP. Next, PhaG elongates (R)-3-hydroxyacyl-ACP with two carbon units into PHA monomers. To finish, a PhaC polymerizes monomers into PHA [21].
Figure 1.
The metabolic pathway for PHB synthesis and degradation. The enzymes involved in PHB synthesis are PhbA (β-ketothiolase), PhbB (Acetoacetyl-CoA reductase), and PhbC (PHB synthase). The enzymes involved in PHB degradation are PhbZ (PHB depolymerase), Bdh (β-hydroxybutyrate dehydrogenase), Acs (Acetyl-CoA synthetase), and PhbA (β-ketothiolase) (Created with data previously reported [32,37,38]).
PHB (and PHA in general) is a hydrophobic material that needs to be stabilized in the cytoplasm. The proteins involved in stabilizing it are known as granule-associated proteins (GAPs). A single PHB granule (carbonosome) contains 98% polymer and 2% GAPs [39,40,41,42]. GAPs include PHB synthases, PHB depolymerases, regulators, and phasins (Figure 2) [43]. PHB synthase and PHB depolymerase initiate PHB synthesis or degradation, respectively [32,38]. Phasins coat and stabilize PHB chains inside bacteria and control the size of the PHB granules [2,43,44,45]. Finally, regulator proteins regulate the expression level of phasins when PHB is synthesized or degraded [46].
Figure 2.
PHB granules in bacteria. Granules contain growing PHB chains at the core and are surrounded by GAPs (Created with data previously reported [39,43,47]).
PHB degradation occurs when bacteria enter a state of starvation, and the exogenous carbon source is depleted. The resulting products can support bacterial growth, serving as a carbon and energy source [5,38]. PHB mobilization in A. brasilense involves a PHB depolymerase (PhaZ) that cuts PHB into β-hydroxybutyrate monomers. Then, an NAD(P)-dependent β-hydroxybutyrate dehydrogenase oxidizes β-hydroxybutyrate monomers to acetoacetate. The subsequent step is to convert acetoacetate into acetoacetyl-CoA by an acetoacetyl-CoA synthetase, and, finally, the acetoacetyl-CoA is hydrolyzed by a β-ketothiolase that releases two acetyl-CoA molecules (Figure 1) [18,37,48]. Acetyl-CoA can enter the TCA cycle, β-oxidation, or glyoxylate pathways, among others, and be used to produce metabolic intermediates and energy to sustain the growth of the bacterium [2,8,29]. S. meliloti, R. eutropha, and other microorganisms share the same mobilization pathway as Azospirillum [36]. In R. eutropha, PHB is poorly degraded in the absence of nitrogen [49]. In contrast, nitrogen-fixing bacteria such as A. brasilense can mobilize PHB when the nitrogen source is depleted, due to the nitrogenase complex. Previous studies suggest PHB mobilization provides enough energy to sustain nitrogen fixation and two binary fissions [50]. Most PHA-producer microorganisms code for several depolymerase isoenzymes [49]. Since A. brasilense can use β-hydroxybutyric acid for growth, this bacterium may have extracellular and intracellular depolymerases. However, more studies are needed to provide us with more information [38].
PHB is accumulated at the middle and the end of the logarithmic growth phases. At the stationary phase, when the carbon source is depleted, PHB begins to be degraded, and it is used to sustain bacterial growth [5,8]. Martínez-Martínez et al. [2] observed that PHB was mainly accumulated after 72 h of growth when a high C/N ratio and microaerophilic conditions were used.
4. Studies on PHB Synthesis and Degradation Genes
In A. brasilense Sp7, some genes involved in PHB synthesis and degradation have been analyzed. It was shown that a phbC mutant strain was unable to accumulate PHB granules after 48 h of growth [32]. On the contrary, a phbZ mutant strain accumulated the highest quantities of biopolymer and was incapable of using it [37]. Until now, there are no studies evaluating the effect of deleting the phbA gene on PHB synthesis; it may probably be because PhbA has other functions than polymer synthesis. However, a phbB mutant strain was reported to be unable to produce PHB. PhbB is the only enzyme implicated in PHB synthesis, and it uses NADH and NAD(P)H as coenzymes [51]. The enzyme β-hydroxybutyrate dehydrogenase has been reported to function as an NAD-dependent tetramer formed by four similar subunits [17]. It was found that NADH, NADPH, pyruvate, and acetyl-CoA inhibit β-hydroxybutyrate dehydrogenase activity [17,37].
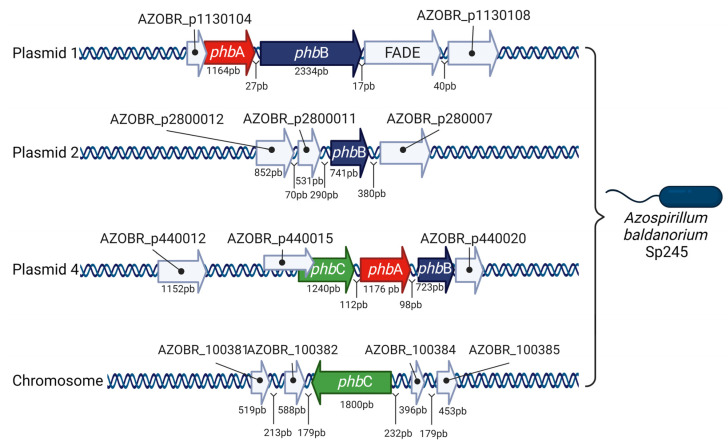
Kadouri et al. [32] were the first to report the genetic sequence of genes involved in PHB synthesis by A. brasilense. The phaA and phaB genes were co-transcribed, whereas the phbC gene was located in the complementary strand. Recently, the whole available genomic sequence of A. baldaniorum Sp245 (formerly named A. brasilense Sp245) has shown several copies of PHB genes. In the A. baldaniorum chromosome, a copy of the phbC gene was located. The phbCAB operon was in plasmid 4. A phbA homolog was found in plasmid 1, whereas copies of phbB were in plasmids 1 and 2 (Figure 3) [52]. In most microorganisms, genes encoding PhbA, PhbB, and PhbC are commonly clustered in an operon [36]. R. eutropha and A. brasilense contain the phbCAB operon, whereas Azotobacter vinelandii contains the phbBAC operon [36,52,53]. In P. putida, the PHA cluster is organized into two operons, phaC1ZC2D and phaIF [21]. However, there are bacteria with several copies of homologous genes randomly distributed throughout bacterial chromosomes and plasmids [52,54].
Figure 3.
Genes involved in PHB synthesis in A. baldaniorum Sp245 (Created with data modified from reference [52]).
Martínez-Martínez et al. [2] analyzed phasin content in the A. brasilense Sp7 genome by looking for a phasin_2 domain (PF09361). It was found that this bacterium contains six genes that encode for phasins. The genes were named phaP1 (AMK58_RS17065), phaP2 (AMK58_RS04265), phaP3 (AMK58_RS04270), phaP4 (AMK58_RS07520), phaP5 (AMK58_RS13850), and phaP6 (AMK58_RS20955). Deletion of the phaP1 gene showed a phenotype compatible with phasins in other microorganisms. The phaP1 mutant strain accumulated fewer PHB granules of a higher size in comparison with the wild-type strain, which accumulated more PHB granules of a lower size.
5. PHB Metabolism Regulation in A. brasilense Sp7
The PHB pathway is closely related to the TCA cycle because acetyl-CoA molecules are metabolic intermediates. Depending on bacterial needs, acetyl-CoA may be used for one or another [55]. When bacteria are grown on a medium with a high C/N ratio, acetyl-CoA molecules are turned towards the TCA cycle to synthesize the metabolic intermediates required to produce macromolecules such as carbohydrates, proteins, nucleic acids, and lipids. As the TCA cycle is active, high quantities of citrate are synthesized and inhibit the enzymatic activity of citrate synthase. Then, acetyl-CoA molecules are condensed into acetoacetyl-CoA by β-ketothiolase, beginning PHB synthesis [9,54]. The increment of CoA-SH released from acetyl-CoA condensation inhibits β-ketothiolase activity [9]. Excessive acetoacetyl-CoA also inhibits PhbA activity, the key enzyme for PHB biosynthesis [9,56].
Another factor involved in switching on/off PHB synthesis is the redox state of bacteria [39]. Large quantities of NAD(P)H are produced mainly during the TCA cycle and cell respiration. The increase of NAD(P)H is eliminated by acetoacetyl-CoA reductase, which requires NAD(P)H to reduce acetoacetyl-CoA into β-hydroxybutyryl-CoA monomers [39,57]. Then, PHB synthesis leads to an increase in NAD(P) levels. The latter favors the activity of PhaZ that starts the PHB degradation [9,17,21,34,37,39,55,58,59]. Azotobacter beijerinckii controls PHB accumulation and mobilization in a similar way as Azospirillum [34].
The enzymes β-ketothiolase, acetoacetyl-CoA reductase, PHB synthase, β-hydroxybutyrate dehydrogenase, and acetoacetyl-CoA synthetase are constitutively expressed in A. brasilense [9,37]. It seems to be that phasins and regulator proteins are also constitutive [2,60]. For PHB accumulation, A. brasilense Sp7 prefers growth in a medium with low oxygen availability. Microaerophilic conditions protect the nitrogenase complex [60]. A high C/N ratio environment allows bacterial growth even if nitrogen is depleted from the medium, due to the nitrogenase complex. Studies by Tal et al. [9] demonstrated that enzymatic activities of β-ketothiolase, acetoacetyl-CoA reductase, and β-hydroxybutyrate dehydrogenase were higher in Azospirilla grown in a medium with oxygen limitation.
Although A. brasilense Sp7 grows well in ammonia chloride, it has been demonstrated that high ammonia chloride decreases the citrate synthase, isocitrate dehydrogenase, and succinate dehydrogenase activities of Azospirillum lipoferum. The effect increases with lower dissolved oxygen (DO). Then, carbon metabolism is restricted to PHB synthesis [61].
6. Nitrogen’s Role in PHB Metabolism
Azospirillum grows in a medium with a low ammonium concentration. Ammonium assimilation by Azospirilla occurs through glutamate dehydrogenase activity that converts glutamate into α-ketoglutarate and ammonia [61]. After ammonium depletion, this bacterium continues growing because it fixes atmospheric nitrogen [62,63]. This process expends a lot of energy, supported by PHB catabolism [50]. The presence of exogenous nitrogen, such as ammonium, nitrate, and nitrite, represses nitrogen fixation [64].
The regulation of nitrogen metabolism in A. brasilense Sp7 includes the well-known proteins: GlnD, GlnB, GlnZ, NtrB, and NtrC and other genes involved in the nitrogen fixation process. In the PII-Pz sensing nitrogen system, glnB encodes for the PII protein, whereas the Pz protein is coded by glnZ. GlnD transfers uridyl groups to GlnB and GlnZ in a medium with nitrogen deficiency [65,66,67]. Under non-nitrogen-limiting conditions, GlnD removes uridyl groups of GlnB and GlnZ. When nitrogen is absent in the growth medium, uridylated GlnB cannot interact with NtrB. Then, NtrB phosphorylates NtrC. NtrC, phosphorylated, induces transcription of genes involved in sensing nitrogen-alternative sources [66,67].
Sun et al. [65] evaluated PHB accumulation by an A. brasilense Sp7 glnD mutant strain. This mutant was unable to sense nitrogen cell status. Under no nitrogen-limiting conditions, the PII-Pz system in the glnD mutant strain was not uridylated/deuridylated. Furthermore, the glnD mutant strain accumulated higher amounts of PHB in comparison with the wild-type strain. The A. brasilense Sp7 glnB–glnZ double mutant strain also synthesized higher amounts of PHB than the wild-type strain [65]. In both the glnD single mutant strain and the glnB–glnZ double mutant strain, PHB was accumulated during the logarithmic phase of growth.
Sun et al. [58] analyzed the PHB production of A. brasilense Sp7 ntrB and ntrC single mutant strains when ammonium chloride was used as a nitrogen source. The ntrB and ntrC mutant strains were unable to sense the nitrogen levels when a medium with a low C/N ratio was used for growth, in comparison with the A. brasilense Sp7 wild type. As a result, the mutant strains accumulated up to 45% of PHB as dry cell weight, whereas the wild-type strain produced only 10% of PHB under the same conditions. The ntrC mutant of Herbaspirillum seropedicae accumulated more PHB than the wild-type strain. Also, it was more resistant to oxidative stress [68].
Kukolj et al. [66] analyzed the gene expression profile of A. brasilense grown in a medium with low and high nitrogen availability. Bacteria grown under nitrogen-limiting conditions increased the expression of proteins involved in energy production and conversion, signal transduction, and amino acid metabolism. However, when there was no nitrogen limiting, the bacterium increased the expression of proteins related to signal transduction, cell wall biogenesis, coenzyme metabolism, and energy metabolism.
7. Flocculation and Cyst Involvement in PHB Production, the Role of Oxygen
Apart from inducing PHB accumulation, media with a high C/N ratio can also stimulate the flocculation of bacteria [11,69,70,71,72]. However, the flocculation phenomenon is present mainly under high oxygen concentrations [11,69,70,73]. For generating energy and fixing nitrogen, the appropriate oxygen concentration for A. brasilense is 3–5 μM [62,74]. Under elevated DO A. brasilense Sp7 tends to clump. In clumping, motile cells interact between them in response to an elevated aeration rate. Clumped bacteria keep a microaerophilic environment to protect them from excessive oxygen [75]. When high aeration is maintained, clumped cells form irreversible macromolecular aggregates known as floccules, but when high aeration decreases, clumped cells return to a vegetative state [11,55,60,69].
Floccules are macroscopic bacterial aggregates that are clumped by a high accumulation of exopolysaccharides (EPS). EPS form a fibrilar matrix surrounding bacteria. Floccules protect A. brasilense Sp7 against the stresses caused by high temperatures (up to 40 °C), desiccation, oxygen availability, and pH [8,11,72,75,76,77,78,79]. A. brasilense floccules contain PHB granules with high biopolymer levels [80,81,82,83].
The EPS of A. brasilense Sp7 contain glucose during the exponential growth phase. However, in the stationary phase growth, they contain mainly arabinose [70,71,84]. EPS, whether glucose-containing or arabinose-rich, served as a carbon source when exogenous carbon was depleted. Interestingly, A. brasilense Sp7 cannot grow in media with arabinose or glucose as unique exogenous carbon sources, but it uses its arabinose or glucose as a carbon source [81].
Low nitrogen conditions and a high aeration rate led to fclA overexpression. The fclA gene controls the morphological transition from vegetative to cystic in response to environmental changes. Also, fclA may control nitrogen assimilation by downregulating glutamine synthetase, which synthesizes glutamine from glutamate and ammonia. GlnA (citrate synthase) was also implicated in EPS production. It seems that fclA promotes sugar assimilation by synthesizing carbohydrates for EPS. Also, it was noted that acetyl-CoA was mainly addressed to PHB synthesis rather than the TCA cycle in the A. brasilense fclA mutant strain due to the phbA gene being overexpressed in an fclA mutant strain [55].
Bible et al. [60] analyzed protein expression during growth under clumping conditions in A. brasilense cheY1 and the cheB1–cheR1 mutants. The A. brasilense cheY1 mutant strain clumped more than the wild-type strain. On the contrary, the A. brasilense cheB1–cheR1 double mutant strain was unable to clump. Both cheY1 and the cheB1–cheR1 mutants showed an increase in the expression of genes involved in PHB metabolism, such as the acetoacetyl-CoA reductase gene and the phaP1, phaP2, and phaP6 genes.
Malinich and Bauer [85] analyzed the transcription profile of encysted A. brasilense. Cysts repress genes involved in amino acid biosynthesis, ribosomal biogenesis, and translation. In A. brasilense cysts, the phaR (AMK58_RS26785) and 3-hydroxyacyl-CoA dehydrogenase (AMK58_19430) genes were also repressed. Genes required for nitrogen metabolism, such as NasT, a response regulator required for growth under nitrate, nitrite/nitrite transporter (AMK58_21400, AMK58_21405, and AMK58_21410), and nitrate reductase (AMK58_21395) were upregulated as well as genes involved in nitrogen fixation [85].
8. PHB and Biofilm in A. brasilense
Depending on the growth conditions, A. brasilense produces a biofilm [86]. Vieruega et al. [87] have reported that the Azospirillum biofilm contains proteins such as polar flagellin and OmaA (outer membrane protein), extracellular DNA, and EPS. According to Tugarova et al. [88] and Shelud’ko et al. [89], the Azospirillum biofilm also contains high quantities of PHB. PHB would allow bacterial stabilization during biofilm formation.
9. Functions of PHB in Azospirillum brasilense
PHB accumulation and utilization by bacteria allow its establishment and survival in competitive environments. In such conditions, PHB functions as a carbon and energy source to sustain bacterial growth [18,32,38,84,90]. Also, it was demonstrated that bacteria with high PHB content colonize the rhizosphere to exert beneficial effects on plant growth and crop yield [38,72,78]. The use of inoculants based on Azospirillum with a high PHB content can prolong their useful life [38,72,83,90,91,92]. Previous studies have reported that the inability to synthesize or degrade PHB affects the resistance of A. brasilense to osmotic and oxidant stresses, as well as its resistance to UV radiation and high temperatures [23]. In Pseudomonas extremaustralis, better UV resistance was observed in agreement with a higher PHB accumulation [93]. PHB provides enough energy to sustain growth under nitrogen-fixing conditions. Previous studies have suggested PHB degradation can supply enough energy to sustain nitrogen fixation and spore development [94]. Similarly, PHB synthesis controls the redox state of bacteria, serving as an electron sink [95].
10. PHB Properties and Applications
A comparison between the characteristics of PHB and polypropylene (PP) is listed in Table 2. PHB shares similar properties with PP, in areas such as tensile modulus, tensile strength, and melting temperature. However, PHB is biodegradable and biocompatible, and its degradation does not release toxic products, which does not occur with PP [47]. The above characteristics would make PHB suitable to replace the use of PP in the industry. However, its high production costs, in addition to low thermal stability, a high degree of crystallinity, hydrophobicity, and brittleness, make PHB less suitable for being used in commercial applications [96,97]. To improve the quality of PHB, it has been combined with other materials, which has enhanced its mechanical and physical properties. Some examples of PHB blending are polylactic acid (PLA), hyaluronic acid (HA), polycaprolactone (PCL), polyethylene glycol (PEG), chitosan, and cellulose pectin, among others [97]. Functionalization of PHB by adding epoxy, hydroxyl, carbonyl, phenyl groups, and halogen atoms improves PHB properties [97,98]. It makes it possible to expand the uses of PHB, for example, in drug delivery.
Table 2.
| Parameter | PP | PHB |
|---|---|---|
| Tensile modulus (GPa) | 1.95 | 3–3.5 |
| Tensile strength (Mpa) | 31–45 | 20–40 |
| Elongation at break (%) | 50–400 | 5–10 |
| Crystallinity (%) | 42.6–70 | 50–60 |
| Melting temperature (°C) | 160–176 | 165–180 |
| Glass transition (°C) | −20–−5 | 2–9 |
| Density (g/cm2) | 0.905 | 1.25 |
| UV resistance | Poor | Good |
| Biodegradability | No | Yes |
| Biocompatibility | No | Yes |
Drug delivery has been one of the most important uses of PHB. Previous studies have shown that PHB can be implanted in the human body, where it causes a mild inflammatory reaction that does not lead to fibrosis or necrosis [101]. Macrophage-mediated inflammation results in an exposition of PHB to extracellular liquids and cells, which results in a slow biopolymer degradation into 3-hydroxybutytyrate monomers and oligomers [102]. These properties suggest PHB is a good candidate for drug delivery [103]. Pandian et al. [104] loaded ursolic acid (an inhibitory agent against the proliferation of tumors) into PHB nanoparticles and evaluated the delivery, availability, and activity of the ursolic acid released from PHB nanoparticles against HeLa cells. In agreement with the number of dead tumor cells, it was concluded that the PHB nanoparticles had released ursolic acid, and they were more effective at 96 h. Another example of drug delivery was reported by Parsian et al. [105], who designed PHB-coated magnetic nanoparticles loaded with gemcitabine (GEM-PHB-MNPs) in order to release gemcitabine for treating breast cancer. Results showed that gemcitabine was released in an acidic microenvironment, like tumors. It was also shown that PHB-MNPs were not cytotoxic to cells. PHB/chitosan blends have been impregnated with ketoprofen [106]. PHB nanospheres and PHB microspheres loaded with extended-spectrum antibiotics have been used to prevent post-surgery infections. Sulbactam ampicillin/cefoperazone, and gentamicin have been loaded into PHB-co-PHV for drug delivery [107]. Other studies on drug release from PHB can be reviewed [97,108,109,110].
PHB can be used for tissue engineering. Deng et al. [111] developed an extracellular matrix of rabbit chondrocytes grown on PHB-co-PHH (polyhybroxybutyrate-co-hydroxyhexanoate) scaffolds. The results showed better seeding on PHB-co-PHH scaffolds than on single PHB scaffolds. It was also shown that more collagen was produced on PHB-co-PHH than on PHB. Temporary stents, bone plates, patches, and screws have been fabricated [111,112]. PHB-based composites were suitable for wound dressing and ocular implants [113]. Also, several artificial tissues, such as retinal, bone, tendon, cartilage, and muscle, have been developed [114,115]. Biopolymers can support cell growth [111,116]. PHB blends can be used as scaffolds and bone implants [97,117].
To be used for packaging, PHB must be stable, flexible, and highly resistant. A good biopolymer must provide a barrier against water vapor, oxygen, and carbon dioxide [118]. PHB blends are promising materials for packaging because they exhibit good barrier properties, and their degradation products are non-toxic for the environment [119]. PHB blends are used in bottles, jars, films, et cetera [120]. Composites of PHB prepared with coconut fibers showed good thermal stability and better tensile properties, making them suitable to be used as plastic bags for recovered seeds and planted for agriculture [121].
11. PHB Biodegradability
PHB biodegradability occurs in soils, water, and aerobic and anaerobic environments. It occurs in microorganisms that have extracellular depolymerases. PHB is degraded upon exposure to soil, compost, or marine sediment. It is supposed that there are approximately 10% of PHB-degrading microbes. PHB can be degraded in aerobic and anaerobic environments. In aerobic environments, PHB degradation results in CO2 and H2O, whereas methane is released in anaerobic environments. PHB degradation depends on microbial activity, moisture, temperature, pH, molecular weight of PHB, et cetera. PHB degradation units can be processed throughout the β-oxidation and TCA cycles [122].
12. Future Outlooks
The metabolic abilities of A. brasilense are extensive. It can grow in minimal to rich media and in a wide range of carbon and nitrogen sources. A. brasilense accumulates up to 80% of its dry cell weight as PHB, a biopolymer characterized as biodegradable and biocompatible. In bacteria, PHB serves as carbon and energy reserves. Also, it provides resistance to several stressful conditions. Understanding PHB metabolism and regulation in A. brasilense may help exploit PHB properties to improve quality of life. In this review were summarized the requirements to improve PHB accumulation by A. brasilense. Studies have shown this bacterium accumulates more PHB when it is grown at a high C/N ratio, ranging from 70 to 90. Also, the best carbon and nitrogen sources were malate and ammonia chloride, respectively. A microaerophilic environment is important too.
Although there are some studies on the genes involved in PHB metabolism, these genes have not been fully understood. Further studies are needed to identify other genes implicated in PHB synthesis and degradation. Now that the A. brasilense genome is available, it will be possible to analyze other genes that may be involved in PHB metabolism. The National Center for Biotechnology Information (NCBI), the Kyoto Encyclopedia of Genes and Genomes (KEGG), and the Clusters of Orthologous Genes (COG) databases showed that A. brasilense contains several copies of genes involved in PHB synthesis and degradation. It would be interesting to know when these genes are expressed and the interactions that occur between each gene, as well as the transcription factors involved in regulating genetic expression. Bioinformatics and in vitro analyses will help us improve our understanding.
PHB is a biodegradable and biocompatible plastic with potential utility for medical and environmental purposes. However, recovering PHB from bacterial cultures is highly expensive. Given that A. brasilense is one of the most important microorganisms that produce PHB, it is necessary to develop environmentally friendly techniques that allow us to increase the recovery and purity of the polymer. Also, it is important to make more efforts toward functionalizing PHB in order to expand its uses and to decrease the use of polypropylene.
Acknowledgments
The authors gratefully acknowledge financial support from the VIEP-BUAP projects: MAML-NAT17-1, SOUL-NAT17-1. The authors are very grateful to Cristina Guadalupe Aguilera Chapital for helping with the English language.
Abbreviations
PHB: poly-β-hydroxybutyrate; PHA: polyhydroxyalkanoates; PHV: polyhydroxyvalerate; FTIR: Fourier-transform infrared spectroscopy; PP: polypropylene; C/N: carbon to nitrogen ratio; N2: nitrogen gas; NH3: ammonia; NH4: ammonium; NO3−: ammonium nitrate; TCA: tricarboxylic acid; DCW: dry cell weight; NAD(P: nicotinamide adenine dinucleotide (phosphate); ACP: acyl carrier protein; GAP: granule-associated protein; h:hours; DO: dissolved oxygen; PHB-co-PHH: Polyhydroxybutyrate-co-hydroxyhexanoate; NCBI: National Center for Biotechnology Information; KEGG: Kyoto Encyclopedia of Genes and Genomes; COG: Clusters of Orthologous Genes.
Author Contributions
Conceptualization, L.J.M.M., L.S.U. and M.d.l.Á.M.M.; methodology, M.d.l.Á.M.M. and Y.A.C.; software, Y.A.C., M.d.l.Á.M.M. and M.B.R.; validation, L.S.U. and M.d.l.Á.M.M.; formal analysis, L.J.M.M. and L.S.U.; investigation, M.d.l.Á.M.M., Y.A.C., M.B.R. and L.S.U.; resources, L.J.M.M. and L.S.U.; data curation, L.J.M.M. and L.S.U.; writing—original draft preparation, L.J.M.M. and L.S.U.; writing—review and editing, M.d.l.Á.M.M. and L.S.U.; visualization, M.d.l.Á.M.M., Y.A.C. and M.B.R.; supervision, L.S.U.; project administration, L.J.M.M.; funding acquisition, L.J.M.M. and L.S.U. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This research was funded by: Vicerrectoría de Investigación y Estudios de Posgrado, Benemérita Universidad Autónoma de Puebla (VIEP-BUAP), grant number: 00243. The APC was funded by VIEP-BUAP.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Pereg L. Azospirillum Cell Aggregation, Attachment, and Plant Interaction. In: Cassán F.D., Okon Y., Creus C.M., editors. Handbook for Azospirillum Technical Issues and Protocols. 1st ed. Volume 1. Springer International Publishing; Cham, Switzerland: 2015. pp. 181–197. [DOI] [Google Scholar]
- 2.Martínez-Martínez M.D.L.A., González-Pedrajo B., Dreyfus G., Soto-Urzúa L., Martínez-Morales L.J. Phasin PhaP1 is involved in polyhydroxybutyrate granules morphology and in controlling early biopolymer accumulation in Azospirillum brasilense Sp7. AMB Express. 2019;9:1–15. doi: 10.1186/s13568-019-0876-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volova T.G., Boyandin A.N., Vasiliev A.D., Karpov V.A., Prudnikova S.V., Mishukova O.V., Boyarskikh U.A., Filipenko M.L., Rudnev V.P., Xuân B.B., et al. Biodegradation of polyhydroxyalkanoates (PHAs) in tropical coastal waters and identification of PHA-degrading bacteria. Polym. Degrad. Stab. 2010;95:2350–2359. doi: 10.1016/j.polymdegradstab.2010.08.023. [DOI] [Google Scholar]
- 4.Halevas E.G., Pantazaki A.A. Polyhydroxyalkanoates: Chemical structure. In: Williams H., Kelly P., editors. Polyhydroxyalkanoates: Biosynthesis, Chemical Structure and Applications. 1st ed. Volume 1. Nova Science Publishers Inc.; New York, NY, USA: 2018. pp. 133–166. [Google Scholar]
- 5.Itzigsohn R., Yarden O., Okon Y. Polyhydroxyalkanoate analysis in Azospirillum brasilense. Can. J. Micrcobiol. 1995;41:73–76. doi: 10.1139/m95-171. [DOI] [Google Scholar]
- 6.Patiño I.M.E., Soto U.L., Orea F.M.L., López V.D., Martínez-Morales L.J. Extraction and NMR determinantion of PHB from Azospirillum brasilense Sp7. JCBPS Spec. Issue. 2014;4:26–32. [Google Scholar]
- 7.Kamnev A.A., Antonyuk L.P., Tugarova A.V., Tarantilis P.A., Polissiou M.G., Gardiner P.H.E. Fourier transform infrared spectroscopic characterisation of heavy metal-induced metabolic changes in the plant-associated soil bacterium Azospirillum brasilense Sp7. J. Mol. Struct. 2002;610:127–131. doi: 10.1016/S0022-2860(02)00021-2. [DOI] [Google Scholar]
- 8.Tal S., Okon Y. Production of the reserve material poly-β-hydroxybutyrate and its function in Azospirillum brasilense Cd. Can. J. Microbiol. 1985;31:608–613. doi: 10.1139/m85-115. [DOI] [Google Scholar]
- 9.Tal S., Smirnoff P., Okon Y. The regulation of poly-β-hydroxybutyrate metabolism in Azospirillum brasilense during balanced growth and starvation. Microbiology. 1990;136:1191–1196. doi: 10.1099/00221287-136-7-1191. [DOI] [Google Scholar]
- 10.Westby C.A., Cutshall D.S., Vigil G.V. Metabolism of various carbon sources by Azospirillum brasilense. J. Bacteriol. 1983;156:1369–1372. doi: 10.1128/jb.156.3.1369-1372.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sadasivan L., Neyra C.A. Flocculation in Azospirillum brasilense and Azospirillum lipoferum: Exopolysaccharides and cyst formation. J. Bacteriol. 1985;163:716–723. doi: 10.1128/jb.163.2.716-723.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loh W.H., Randles C.I., Sharp W.R., Miller R.H. Intermediary carbon metabolism of Azospirillum brasilense. J. Bacteriol. 1984;158:264–268. doi: 10.1128/jb.158.1.264-268.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexandre G., Greer S.E., Zhulin I.B. Energy taxis is the dominant behavior in Azospirillum brasilense. J. Bacteriol. 2000;182:6042–6048. doi: 10.1128/JB.182.21.6042-6048.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okon Y., Albrecht S.L., Burris R.H. Factors affecting growth and nitrogen fixation of Spirillum lipoferum. J. Bacteriol. 1976;127:1248–1254. doi: 10.1128/jb.127.3.1248-1254.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neyra C.A., Döbereiner J., Lalande R., Knowles R. Denitrification by N2-fixing Spirillum lipoferum. Can. J. Microbiol. 1977;23:300–305. doi: 10.1139/m77-044. [DOI] [PubMed] [Google Scholar]
- 16.Martinez-Drets G., Del Gallo M., Burpee C., Burris R.H. Catabolism of carbohydrates and organic acids and expression of nitrogenase by Azospirilla. J. Bacteriol. 1984;159:80–85. doi: 10.1128/jb.159.1.80-85.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tal S., Smirnoff P., Okon Y. Purification and characterization of d-β-hydroxybutyrate dehydrogenase from Azospirillum brasilense Cd. Microbiology. 1990;136:645–649. doi: 10.1099/00221287-136-4-645. [DOI] [Google Scholar]
- 18.Okon Y., Itzigsohn R. Poly-β-hydroxybutyrate metabolism in Azospirillum brasilense and the ecological role of PHB in the rhizosphere. FEMS Microbiol. 1992;9:131–139. doi: 10.1016/0378-1097(92)90302-5. [DOI] [Google Scholar]
- 19.Manna A., Pal S., Paul A.K. Occurrence of poly-3-hydroxybutyrate in Azospirillum sp. Folia Microbiol. 1997;42:629–634. doi: 10.1007/BF02815477. [DOI] [Google Scholar]
- 20.Narancic T., Scollica E., Kenny S.T., Gibbons H., Carr E., Brennan L., Cafney G., Wynne K., Murphy C., Raberg M., et al. Understanding the physiological roles of polyhydroxybutyrate (PHB) in Rhodospirillum rubrum S1 under aerobic chemoheterotrophic conditions. Appl. Microbiol. Biotechnol. 2016;100:8901–8912. doi: 10.1007/s00253-016-7711-5. [DOI] [PubMed] [Google Scholar]
- 21.Prieto A., Escapa I.F., Martínez V., Dinjaski N., Herencias C., de la Peña F., Tarazona N., Revelles O. A holistic view of polyhydroxyalkanoate metabolism in Pseudomonas putida. Environ. Microbiol. 2016;18:341–357. doi: 10.1111/1462-2920.12760. [DOI] [PubMed] [Google Scholar]
- 22.Ochsner A.M., Sonntag F., Buchhaupt M., Schrader J., Vorholt J.J. Methylobacterium extorquens: Methylotrophy and biotechnological applications. Appl. Microbiol. Biotechnol. 2015;99:517–534. doi: 10.1007/s00253-014-6240-3. [DOI] [PubMed] [Google Scholar]
- 23.Quelas J.I., Mesa S., Mongiardini E.J., Jendrossek D., Lodeiro A.R. Regulation of polyhydroxybutyrate synthesis in the soil bacterium Bradyrhizobium diazoefficiens. Appl. Environ. Microbiol. 2016;82:4299–4308. doi: 10.1128/AEM.00757-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sreedevi S., Unni K.N., Sajith S., Priji P., Josh M.S., Benjamin S. Bioplastics: Advances in polyhydroxybutyrate research. In: Abe A., Albertsson A.C., Coates G.W., Genzer J., Kobayashi S., Lee K.S., Leibler L., Long T.E., Möller M., Okay O., et al., editors. Advances in Polymer Science. Volume 1. Springer; Berlin/Heidelberg, Germany: 2016. pp. 1–30. [DOI] [Google Scholar]
- 25.Soto L.R., Byrne E., Van Niel E.W., Sayed M., Villanueva C.C., Hatti-Kaul R. Hydrogen and polyhydroxybutyrate production from wheat straw hydrolysate using Caldicellulosiruptor species and Ralstonia eutropha in a coupled process. Bioresour. Technol. 2009;272:259–266. doi: 10.1016/j.biortech.2018.09.142. [DOI] [PubMed] [Google Scholar]
- 26.Ayub N.D., Pettinari M.J., Ruiz J.A., López N.I. A polyhydroxybutyrate-producing Pseudomonas sp. isolated from Antarctic environments with high stress resistance. Curr. Microbiol. 2004;49:170–174. doi: 10.1007/s00284-004-4254-2. [DOI] [PubMed] [Google Scholar]
- 27.Ayub N.D., Pettinari M.J., Méndez B.S., López N.I. Impaired polyhydroxybutyrate biosynthesis from glucose in Pseudomonas sp. 14-3 is due to a defective beta-ketothiolase gene. FEMS Microbiol. Lett. 2006;264:125–131. doi: 10.1111/j.1574-6968.2006.00446.x. [DOI] [PubMed] [Google Scholar]
- 28.Ghoddosi F., Golzar H., Yazdian F., Khosravi-Darani K., Vasheghani-Farahani E. Effect of carbon sources for PHB production in bubble column bioreactor: Emphasis on improvement of methane uptake. J. Environ. Chem. Eng. 2019;7:102978. doi: 10.1016/j.jece.2019.102978. [DOI] [Google Scholar]
- 29.Manju J., Prabakaran P. Effect of carbon sources in the production of polyhydroxybutyrate (PHB) by Bradyrhizobium and Rhizobium sp. from Aeschynomene indica. Int. J. Res. Anal. Rev. 2019;6:823–827. [Google Scholar]
- 30.Torres-Pedraza A.J., Salgado-Lugo H., Segura D., Díaz-Barrera A., Peña C. Composition control of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) copolymerization by oxygen transfer rate (OTR) in Azotobacter vinelandii OPNA. J. Chem. Technol. Biotechnol. 2021;96:2782–2791. doi: 10.1002/jctb.6825. [DOI] [Google Scholar]
- 31.Hassan M.A., Bakhiet E.K., Hussein H.R., Ali S.G. Statistical optimization studies for polyhydroxybutyrate (PHB) production by novel Bacillus subtilis using agricultural and industrial wastes. Int. J. Environ. Sci. Technol. 2019;16:3497–3512. doi: 10.1007/s13762-018-1900-y. [DOI] [Google Scholar]
- 32.Kadouri D., Burdman S., Jurkevitch E., Okon Y. Identification and isolation of genes involved in poly (β-hydroxybutyrate) biosynthesis in Azospirillum brasilense and characterization of a phbC mutant. Appl. Environ. Microbiol. 2002;68:2943–2949. doi: 10.1128/AEM.68.6.2943-2949.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kadouri D., Jurkevitch E., Okon Y., Castro-Sowinski S. Ecological and Agricultural Significance of Bacterial Polyhydroxyalkanoates. Crit. Rev. Microbiol. 2005;31:55–67. doi: 10.1080/10408410590899228. [DOI] [PubMed] [Google Scholar]
- 34.Senior P.J., Dawes E.A. The regulation of poly-β-hydroxybutyrate metabolism in Azotobacter beijerinckii. Biochem. J. 1973;134:225–238. doi: 10.1042/bj1340225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Volova T.G., Kalacheva G.S., Gorbunova O.V., Zhila N.O. Dynamics of Activity of the Key Enzymes of Polyhydroxyalkanoate Metabolism in Ralstonia eutropha. Appl. Biochem. Microbiol. 2004;40:170–177. doi: 10.1023/B:ABIM.0000018921.04863.d5. [DOI] [PubMed] [Google Scholar]
- 36.Trainer M.A., Charles T.C. The role of PHB metabolism in the symbiosis of rhizobia with legumes. Appl. Microbiol. Biotechnol. 2006;71:377–386. doi: 10.1007/s00253-006-0354-1. [DOI] [PubMed] [Google Scholar]
- 37.Edelshtein Z., Kadouri D., Jurkevitch E., Vande Broek A., Vanderleyden J., Okon Y. Characterization of genes involved in poly-β-hydroxybutyrate metabolism in Azospirillum brasilense. Symbiosis. 2003;34:157–170. [Google Scholar]
- 38.Kadouri D., Jurkevitch E., Okon Y. Involvement of the reserve material poly-β-hydroxybutyrate in Azospirillum brasilense stress endurance and root colonization. Appl. Environ. Microbiol. 2003;69:3244–3250. doi: 10.1128/AEM.69.6.3244-3250.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Müller-Santos M., Maltempi de Souza E., de Oliveira-Pedrosa F., Chubatsu L.S. Polyhydroxybutyrate in Azospirillum brasilense. In: Cassán F.D., Okon Y., Creus C.M., editors. Handbook for Azospirillum Technical Issues and Protocols. 1st ed. Volume 1. Springer International Publising; Cham, Switzerland: 2015. pp. 241–250. [DOI] [Google Scholar]
- 40.Jendrossek D. Polyhydroxyalkanoate granules are complex subcellular organelles (carbonosomes) J. Bacteriol. 2009;191:3195–3202. doi: 10.1128/JB.01723-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bresan S., Sznajder A., Hauf W., Forchhammer K., Pfeiffer D., Jendrossek D. Polyhydroxyalkanoate (PHA) granules have no phospholipids. Sci. Rep. 2016;6:1–13. doi: 10.1038/srep26612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bresan S., Jendrossek D. New Insights into PhaM-PhaC-Mediated Localization of Polyhydroxybutyrate Granules in Ralstonia eutropha H16. Appl. Environ. Microbiol. 2017;83:e00505–17. doi: 10.1128/AEM.00505-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jurasek L., Marchessault R.H. The role of phasins in the morphogenesis of poly (3-hydroxybutyrate) granules. Biomacromolecules. 2002;3:256–261. doi: 10.1021/bm010145d. [DOI] [PubMed] [Google Scholar]
- 44.Pötter M., Steinbüchel A. Poly (3-hydroxybutyrate) granule-associated proteins: Impacts on poly (3-hydroxybutyrate) synthesis and degradation. Biomacromolecules. 2005;6:552–560. doi: 10.1021/bm049401n. [DOI] [PubMed] [Google Scholar]
- 45.Tirapelle E.F., Müller-Santos M., Tadra-Sfeir M.Z., Kadowaki M.A.S., Steffens M.B.R., Monteiro R.A., Souza E.M., Pedrosa F.O., Chubatsu L.S. Identification of proteins associated with polyhydroxybutyrate granules from Herbaspirillum seropedicae SmR1-old partners, new players. PLoS ONE. 2013;8:e75066. doi: 10.1371/journal.pone.0075066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maehara A., Doi Y., Nishiyama T., Takagi Y., Ueda S., Nakano H., Yamane T. PhaR, a protein of unknown function conserved among short-chain-length polyhydroxyalkanoic acids producing bacteria, is a DNA-binding protein and represses Paracoccus denitrificans phaP expression in vitro. FEMS Microbiol. Lett. 2001;200:9–15. doi: 10.1111/j.1574-6968.2001.tb10685.x. [DOI] [PubMed] [Google Scholar]
- 47.Chai J.M., Amelia T.S.M., Mouriya G.K., Bhubalan K., Amirul A.A.A., Vigneswari S., Ramakrishna S. Surface-modified highly biocompatible bacterial-poly (3-hydroxybutyrate-co-4-hydroxybutyrate): A review on the promising next-generation biomaterial. Polymers. 2020;13:51. doi: 10.3390/polym13010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aneja P., Charles T.C. Poly-3-hydroxybutyrate degradation in Rhizobium (Sinorhizobium) meliloti: Isolation and characterization of a gene encoding 3-hydroxybutyrate dehydrogenase. J. Bacteriol. 1999;181:849–857. doi: 10.1128/JB.181.3.849-857.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Handrick R., Reinhardt S., Jendrossek D. Mobilization of poly (3-hydroxybutyrate) in Ralstonia eutropha. J. Bacteriol. 2000;182:5916–5918. doi: 10.1128/JB.182.20.5916-5918.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bashan Y., Holguin G., De-Bashan L.E. Azospirillum-plant relationships: Physiological, molecular, agricultural, and environmental advances (1997–2003) Can. J. Microbiol. 2004;50:521–577. doi: 10.1139/w04-035. [DOI] [PubMed] [Google Scholar]
- 51.Vieille M., Elmerich C. Characterization of an Azospirillum brasilense Sp7 gene homologous to Alcaligenes eutrophus phbB and Rhizobium meliloti nodG. Mol. Gen. Genet. 1992;231:375–384. doi: 10.1007/BF00292706. [DOI] [PubMed] [Google Scholar]
- 52.Aguilar G.G. Master’s Thesis. Benemérita Universidad Autónoma de Puebla; Puebla, México: 2016. Análisis del Efecto de la Co-Transcripción de los Genes phbABC Sobre la Producción de PHB de Azospirillum brasilense Sp245. [Google Scholar]
- 53.Peralta-Gil M., Segura D., Guzman J., Servin-Gonzalez L., Espin G. Expression of the Azotobacter vinelandii poly-3-hydroxybutyrate biosynthetic phbBAC operon is driven by two overlapping promoters and is dependent on the transcriptional activator PhbR. J. Bacteriol. 2002;184:5672–5677. doi: 10.1128/JB.184.20.5672-5677.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aneja P., Dai M., Lacorre D.A., Pillon B., Charles T.C. Heterologous complementation of the exopolysaccharide synthesis and carbon utilization phenotypes of Sinorhizobium meliloti Rm1021 polyhydroxyalkanoate synthesis mutants. FEMS Microbiol. Lett. 2004;239:277–283. doi: 10.1016/j.femsle.2004.08.045. [DOI] [PubMed] [Google Scholar]
- 55.Hou X., McMillan M., Coumans J.V., Poljak A., Raftery M.J., Pereg L. Cellular responses during morphological transformation in Azospirillum brasilense and its flcA knockout mutant. PLoS ONE. 2014;9:e114435. doi: 10.1371/journal.pone.0114435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Akhlaq S., Singh D., Mittal N., Srivastava G., Siddiqui S., Faridi S.A., Siddiqui M.H. Polyhydroxybutyrate biosynthesis from different waste materials, degradation, and analytic methods: A short review. Polym. Bull. 2023;80:5965–5997. doi: 10.1007/s00289-022-04406-9. [DOI] [Google Scholar]
- 57.Paul E., Mulard D., Blanc P., Fages J., Goma G., Pareilleux A. Effects of partial O2 pressure, partial CO2 pressure, and agitation on growth kinetics of Azospirillum lipoferum under fermentor conditions. Appl. Environ. Microbiol. 1990;56:3235–3239. doi: 10.1128/aem.56.11.3235-3239.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Eugenio L.I., Escapa I.F., Morales V., Dinjaski N., Galán B., García J.L., Prieto M.A. The turno-ver of medium-chain-length polyhydroxyalkanoates in Pseudomonas putida KT2442 and the fundamental role of PhaZ depolymerase for the metabolic balance. Environ. Microbiol. 2010;12:207–221. doi: 10.1111/j.1462-2920.2009.02061.x. [DOI] [PubMed] [Google Scholar]
- 59.Hauf W., Schlebusch M., Hüge J., Kopka J., Hagemann M., Forchhammer K. Metabolic changes in Synechocystis PCC6803 upon nitrogen starvation: Excess NADPH sustains polyhydroxybutyrate accumulation. Metabolites. 2013;3:101–118. doi: 10.3390/metabo3010101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bible A.N., Khalsa-Moyers G.K., Mukherjee T., Green C.S., Mishra P., Purcell A., Aksenova A., Hurst G.B., Alexandre G. Metabolic adaptations of Azospirillum brasilense to oxygen stress by cell-to-cell clumping and flocculation. Appl. Environ. Microbiol. 2015;81:8346–8357. doi: 10.1128/AEM.02782-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kefalogianni I., Aggelis G. Metabolic activities in Azospirillum lipoferum grown in the presence of NH4. Appl. Microbiol. Biotechnol. 2003;62:574–578. doi: 10.1007/s00253-003-1349. [DOI] [PubMed] [Google Scholar]
- 62.Kefalogianni I., Aggelis G. Modeling growth and biochemical activities of Azospirillum spp. Appl. Microbiol. Biotechnol. 2002;58:352–357. doi: 10.1007/s00253-001-0890-7. [DOI] [PubMed] [Google Scholar]
- 63.Kamnev A.A., Sadovnikova J.N., Tarantilis P.A., Polissiou M.G., Antonyuk L.P. Responses of Azospirillum brasilense to nitrogen deficiency and to wheat lectin: A diffuse reflectance infrared Fourier transform (DRIFT) spectroscopic study. Microb. Ecol. 2008;56:615–624. doi: 10.1007/s00248-008-9381-z. [DOI] [PubMed] [Google Scholar]
- 64.Gallori E., Bazzicalupo M. Effect of nitrogen compounds on nitrogenase activity in Azospirillum brasilense. FEMS Microbiol. Lett. 1985. 28:35–38. doi: 10.1111/j.1574-6968.1985.tb00759.x. [DOI] [Google Scholar]
- 65.Sun J., Van Dommelen A., Van Impe J., Vanderleyden J. Involvement of glnB, glnZ, and glnD genes in the regulation of poly-3-hydroxybutyrate biosynthesis by ammonia in Azospirillum brasilense Sp7. Appl. Environ. Microbiol. 2002;68:985–988. doi: 10.1128/AEM.68.2.985-988.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kukolj C., Pedrosa F.O., de Souza G.A., Sumner L.W., Lei Z., Sumner B., Lei Z., Summer B., do Amaral F.P., Juexin W., et al. Proteomic and metabolomic analysis of Azospirillum brasilense ntrC mutant under high and low nitrogen conditions. J. Proteome Res. 2020;19:92–105. doi: 10.1021/acs.jproteome.9b00397. [DOI] [PubMed] [Google Scholar]
- 67.Sun J., Peng X., Van Impe J., Vanderleyden J. The ntrB and ntrC genes are involved in the regulation of poly-3-hydroxybutyrate biosynthesis by ammonia in Azospirillum brasilense Sp7. Appl. Environ. Microbiol. 2000;66:113–117. doi: 10.1128/AEM.66.1.113-117.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sacomboio E.N.M., Kim E.Y.S., Ruchaud-Correa H.L., Bonato P., de Oliveira-Pedrosa F., de Souza E.M., Chubatsu L.S., Müller-Santos M. The transcriptional regulator NtrC controls glucose-6-phosphate dehydrogenase expression and polyhydroxybutyrate synthesis through NADPH availability in Herbaspirillum seropedicae. Sci. Rep. 2017;7:13546. doi: 10.1038/s41598-017-12649-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Burdman S., Jurkevitch E., Schwartsburd B., Hampel M., Okon Y. Aggregation in Azospirillum brasilense: Effects of chemical and physical factors and involvement of extracellular components. Microbiology. 1998;144:1989–1999. doi: 10.1099/00221287-144-7-1989. [DOI] [PubMed] [Google Scholar]
- 70.Burdman S., Jurketvitch E., Soria-Diaz M.E., Gil-Serrano A.M., Okon Y. Extracellular polysaccharide composition of Azospirillum brasilense and its relation with cell aggregation. FEMS Microbiol. Lett. 2000;489:259–264. doi: 10.1111/j.1574-6968.2000.tb09240.x. [DOI] [PubMed] [Google Scholar]
- 71.Fischer S.E., Marioli J.M., Mori G. Effect of root exudates on the exopolysaccharide composition and the lipopolysaccharide profile of Azospirillum brasilense Cd under saline stress. FEMS Microbiol. Lett. 2003;219:53–62. doi: 10.1016/S0378-1097(02)01194-1. [DOI] [PubMed] [Google Scholar]
- 72.Oliveira A.L., Santos O.J., Marcelino P.R., Milani K.M., Zuluaga M.Y., Zucareli C., Gonçalves L.S. Maize inoculation with Azospirillum brasilense Ab-V5 cells enriched with exopolysaccharides and polyhydroxybutyrate results in high productivity under low N fertilizer input. Front. Microbiol. 2017;8:1873. doi: 10.3389/fmicb.2017.01873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Joe M., Karthikeyan M.B., Sekar C., Deiveekasundaram M. Optimization of biofloc production in Azospirillum brasilense (MTCC-125) and evaluation of its adherence with the roots of certain crops. Indian J. Microbiol. 2010;50:21–25. doi: 10.1007/s12088-010-0050-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhulin I.B., Bespalov V.A., Johnson M.S., Taylor B.L. Oxygen taxis and proton motive force in Azospirillum brasilense. J. Bacteriol. 1996;178:5199–5204. doi: 10.1128/jb.178.17.5199-5204.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Konnova S.A., Makarov O.E., Skvortsov I.M., Ignatov V.V. Isolation, fractionation and some properties of polysaccharides produced in a bound form by Azospirillum brasilense and their possible involvement in Azospirillum–wheat root interactions. FEMS Microbiol. Lett. 1994;118:93–99. doi: 10.1111/j.1574-6968.1994.tb06809.x. [DOI] [Google Scholar]
- 76.Konnova S.A., Brykova O.S., Sachkova O.A., Egorenkova I.V., Ignatov V.V. Protective role of the polysaccharide-containing capsular components of Azospirillum brasilense. Microbiology. 2001;70:436–440. doi: 10.1023/A:1010434227671. [DOI] [PubMed] [Google Scholar]
- 77.Vendan R.T., Thangaraju M. Development and standardization of cyst based liquid formulation of Azospirillum bioinoculant. Acta Microbiol. Immunol. Hung. 2007;54:167–177. doi: 10.1556/amicr.54.2007.2.7. [DOI] [PubMed] [Google Scholar]
- 78.Fadel-Picheth C.M.T., Souza E.M., Rigo L.U., Yates M.G., Pedrosa F.O. Regulation of Azospirillum brasilense nifA gene expression by ammonium and oxygen. FEMS Microbiol. Lett. 1999;179:281–288. doi: 10.1111/j.1574-6968.1999.tb08739.x. [DOI] [PubMed] [Google Scholar]
- 79.Hartmann A., Burris R.H. Regulation of nitrogenase activity by oxygen in Azospirillum brasilense and Azospirillum lipoferum. J. Bacteriol. 1987;169:944–948. doi: 10.1128/jb.169.3.944-948.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bleakley B.H., Gaskins M.H., Hubbell D.H., Zam S.G. Floc formation by Azospirillum lipoferum grown on poly-β-hydroxybutyrate. Appl. Environ. Microbiol. 1988;54:2986–2995. doi: 10.1128/aem.54.12.2986-2995.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sadasivan L., Neyra C.A. Cyst production and brown pigment formation in aging cultures of Azospirillum brasilense ATCC 29145. J. Bacteriol. 1987;169:1670–1677. doi: 10.1128/jb.169.4.1670-1677.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li H., Cui Y., Wu L., Tu R., Chen S. cDNA-AFLP analysis of differential gene expression related to cell chemotactic and encystment of Azospirillum brasilense. Microbiol. Res. 2011;166:595–605. doi: 10.1016/j.micres.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 83.Santos M.S., Hungria M., Nogueira M.A. Production of polyhydroxybutyrate (PHB) and biofilm by Azospirillum brasilense aiming at the development of liquid inoculants with high performance. Afr. J. Bitechnol. 2017;16:1855–1862. doi: 10.5897/AJB2017.16162. [DOI] [Google Scholar]
- 84.Bahat-Samet E., Castro-Sowinski S., Okon Y. Arabinose content of extracellular polysaccharide plays a role in cell aggregation of Azospirillum brasilense. FEMS Microbiol. Lett. 2004;237:195–203. doi: 10.1111/j.1574-6968.2004.tb09696.x. [DOI] [PubMed] [Google Scholar]
- 85.Malinich E.A., Bauer C.E. Transcriptome analysis of Azospirillum brasilense vegetative and cyst states reveals large-scale alterations in metabolic and replicative gene expression. Microb. Genom. 2018;4 doi: 10.1099/mgen.0.000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ramírez-Mata A., López-Lara L.I., Xiqui-Vázquez M.L., Jijón-Moreno S., Romero-Osorio A., Baca B.E. The cyclic-di-GMP diguanylate cyclase CdgA has a role in biofilm formation and exopolysaccharide production in Azospirillum brasilense. Res. Microbiol. 2016;167:190–201. doi: 10.1016/j.resmic.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 87.Viruega-Góngora V.I., Acatitla-Jácome I.S., Zamorano-Sánchez D., Reyes-Carmona S.R., Xiqui-Vázquez M.L., Baca B.E., Ramírez-Mata A. The GGDEF-EAL protein CdgB from Azospirillum baldaniorum Sp245, is a dual function enzyme with potential polar localization. PLoS ONE. 2022;17:e0278036. doi: 10.1371/journal.pone.0278036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tugarova A.V., Scheludko A.V., Dyatlova Y.A., Filip’echeva Y.A., Kamnev A.A. FTIR spectroscopic study of biofilms formed by the rhizobacterium Azospirillum brasilense Sp245 and its mutant Azospirillum brasilense Sp245. 1610. J. Mol. Struct. 2017;1140:142–147. doi: 10.1016/j.molstruc.2016.12.063. [DOI] [Google Scholar]
- 89.Shelud’ko A.V., Mokeev D.I., Evstigneeva S.S., Filip’echeva Y.A., Burov A.M., Petrova L.P., Ponomareva E.G., Katsy E.I. Cell Ultrastructure in Azospirillum brasilense Biofilms. Microbiology. 2020;89:50–63. doi: 10.1134/S0026261720010142. [DOI] [Google Scholar]
- 90.Sivasakthivelan P., Saranraj P., Al-Tawaha A.R., Amala K., Al Tawaha A.R., Thangadurai D., Sangeetha J., Rauf A., Khalid S., Alsultan W., et al. Adaptation of Azospirillum to stress conditions: A review. Adv. Environ. Biol. 2021;15:1–5. doi: 10.22587/aeb.2021.15.3.1. [DOI] [Google Scholar]
- 91.Fibach-Paldi S., Burdman S., Okon Y. Key physiological properties contributing to rhizosphere adaptation and plant growth promotion abilities of Azospirillum brasilense. FEMS Microbiol. Lett. 2012;326:99–108. doi: 10.1111/j.1574-6968.2011.02407.x. [DOI] [PubMed] [Google Scholar]
- 92.Carrasco-Espinosa K., García-Cabrera R.I., Bedoya-López A., Trujillo-Roldán M.A., Valdez-Cruz N.A. Positive effect of reduced aeration rate on growth and stereospecificity of DL-malic acid consumption by Azospirillum brasilense: Improving the shelf life of a liquid inoculant formulation. J. Biotechnol. 2015;195:74–81. doi: 10.1016/j.jbiotec.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 93.Tribelli P.M., Pezzoni M., Brito M.G., Montesinos N.V., Costa C.S., López N.I. Response to lethal UVA radiation in the Antarctic bacterium Pseudomonas extremaustralis: Polyhydroxybutyrate and cold adaptation as protective factors. Extremophiles. 2020;24:265–275. doi: 10.1007/s00792-019-01152-1. [DOI] [PubMed] [Google Scholar]
- 94.López J.A., Naranjo J.M., Higuita J.C., Cubitto M.A., Cardona C.A., Villar M.A. Biosynthesis of PHB from a new isolated Bacillus megaterium strain: Outlook on future developments with endospore forming bacteria. Biotechnol. Bioprocess Eng. 2012;17:250–258. doi: 10.1007/s12257-011-0448-1. [DOI] [Google Scholar]
- 95.Koch M., Forchhammer K. Polyhydroxybutyrate: A useful product of chlorotic cyanobacteria. Microb. Physiol. 2021;31:67–77. doi: 10.1159/000515617. [DOI] [PubMed] [Google Scholar]
- 96.Majerczak K., Wadkin-Snaith D., Magueijo V., Mulheran P., Liggat J., Johnston K. Polyhydroxybutyrate: A review of experimental and simulation studies of the effect of fillers on crystallinity and mechanical properties. Polym. Int. 2022;71:1398–1408. doi: 10.1002/pi.6402. [DOI] [Google Scholar]
- 97.Raza Z.A., Khalil S., Abid S. Recent progress in development and chemical modification of poly (hydroxybutyrate)-based blends for potential medical applications. Int. J. Biol. Macromol. 2020;160:77–100. doi: 10.1016/j.ijbiomac.2020.05.114. [DOI] [PubMed] [Google Scholar]
- 98.McAdam B., Brennan F.M., McDonald P., Mojicevic M. Production of polyhydroxybutyrate (PHB) and factors impacting its chemical and mechanical characteristics. Polymer. 2020;12:2908. doi: 10.3390/polym12122908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Verlinden R.A., Hill D.J., Kenward M., Williams C.D., Radecka I. Bacterial synthesis of biodegradable polyhydroxyalkanoates. J. Appl. Microbiol. 2007;102:1437–1449. doi: 10.1111/j.1365-2672.2007.03335.x. [DOI] [PubMed] [Google Scholar]
- 100.Hankermeyer C.R., Tjeerdema R.S. Polyhydroxybutyrate: Plastic made and degraded by microorganisms. Rev. Environ. Contam. Toxicol. 1999;159:1–24. doi: 10.1007/978-1-4612-1496-0_1. [DOI] [PubMed] [Google Scholar]
- 101.Nair L.S., Laurencin C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007;32:762–798. doi: 10.1016/j.progpolymsci.2007.05.017. [DOI] [Google Scholar]
- 102.Bonartsev A.P., Bonartseva G.A., Reshetov I.V., Kirpichnikov M.P., Shaitan K.V. Application of polyhydroxyalkanoates in medicine and the biological activity of natural poly (3-hydroxybutyrate) Acta Nat. 2019;11:4–16. doi: 10.32607/20758251-2019-11-2-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shishatskaya E.I., Voinova O.N., Goreva A.V., Mogilnaya O.A., Volova T.G. Biocompatibility of polyhydroxybutyrate microspheres: In vitro and in vivo evaluation. J. Mat. Sci. 2008;19:2493–2502. doi: 10.1007/s10856-007-3345-6. [DOI] [PubMed] [Google Scholar]
- 104.Pandian S.R.K., Kunjiappan S., Pavadai P., Sundarapandian V., Chandramohan V., Sundar K. Delivery of Ursolic Acid by Polyhydroxybutyrate Nanoparticles for Cancer Therapy: In silico and in vitro Studies. Drug Res. 2022;72:72–81. doi: 10.1055/a-1640-0009. [DOI] [PubMed] [Google Scholar]
- 105.Parsian M., Mutlu P., Yalcin S., Gunduz U. Characterization of gemcitabine loaded polyhydroxybutyrate coated magnetic nanoparticles for targeted drug delivery. Anticancer Agents Med. Chem. 2020;20:1233–1240. doi: 10.2174/1871520620666200310091026. [DOI] [PubMed] [Google Scholar]
- 106.Lins L.C., Bazzo G.C., Barreto P.L., Pires A.T. Composite PHB/chitosan microparticles obtained by spray drying: Effect of chitosan concentration and crosslinking agents on drug relesase. J. Braz. Chem. Soc. 2014;25:1462–1471. doi: 10.5935/0103-5053.20140129. [DOI] [Google Scholar]
- 107.Yagmurlu M.F., Korkusuz F., Gürsel I., Korkusuz P., Örs Ü., Hasirci V. Sulbactam-cefoperazone polyhydroxybutyrate-co-hydroxyvalerate (PHBV) local antibiotic delivery system: In vivo effectiveness and biocompatibility in the treatment of implant-related experimental osteomyelitis. J. Biomed. Mater. Res. 1999;46:494–503. doi: 10.1002/(SICI)1097-4636(19990915)46:4<494::AID-JBM7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 108.Shrivastav A., Kim H.Y., Kim Y.R. Advances in the applications of polyhydroxyalkanoate nanoparticles for novel drug delivery system. Biomed. Res. Int. 2013;2013:581684. doi: 10.1155/2013/581684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Barouti G., Jaffredo C.G., Guillaume S.M. Advances in drug delivery systems based on synthetic poly (hydroxybutyrate)(co) polymers. Prog. Polym. Sci. 2017;73:1–31. doi: 10.1016/j.progpolymsci.2017.05.002. [DOI] [Google Scholar]
- 110.Prakash P., Lee W.H., Loo C.Y., Wong H.S.J., Parumasivam T. Advances in polyhydroxyalkanoate nanocarriers for effective drug delivery: An overview and challenges. Nanomaterial. 2022;12:175. doi: 10.3390/nano12010175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Deng Y., Lin X.S., Zheng Z., Deng J.G., Chen J.C., Ma H., Chen G.Q. Poly (hydroxybutyrate-co-hydroxyhexanoate) promoted production of extracellular matrix of articular cartilage chondrocytes in vitro. Nanomaterial. 2003;24:4273–4281. doi: 10.1016/S0142-9612(03)00367-3. [DOI] [PubMed] [Google Scholar]
- 112.Lootz D., Behrend D., Kramer S., Freier T., Haubold A., Benkiesser G., Schmitz K.P., Becher B. Laser cutting: Influence on morphological and physicochemical properties of polyhydroxybutyrate. Nanomaterial. 2001;22:2447–2452. doi: 10.1016/S0142-9612(00)00245-3. [DOI] [PubMed] [Google Scholar]
- 113.Sharma N. Polyhydroxybutyrate (PHB) production by bacteria and its application as biodegradable plastic in various industries. Acad. J. Polym. Sci. 2019;2:001–003. doi: 10.19080/AJOP.2019.02.555587. [DOI] [Google Scholar]
- 114.Lee C.W., Horiike M., Masutani K., Kimura Y. Characteristic cell adhesion behaviors on various derivatives of poly (3-hydroxybutyrate) (PHB) and a block copolymer of poly (3-[RS]-hydroxybutyrate) and poly (oxyethylene) Polym. Degrad. Stab. 2015;111:194–202. doi: 10.1016/j.polymdegradstab.2014.11.014. [DOI] [Google Scholar]
- 115.Garcia-Garcia D., Ferri J.M., Boronat T., López-Martínez J., Balart R. Processing and characterization of binary poly (hydroxybutyrate)(PHB) and poly (caprolactone)(PCL) blends with improved impact properties. Polym. Bull. 2016;73:3333–3350. doi: 10.1007/s00289-016-1659-6. [DOI] [Google Scholar]
- 116.Chu C.F., Lu A., Liszkowski M., Sipehia R. Enhanced growth of animal and human endothelial cells on biodegradable polymers. Biochim. Biophys. Acta. 1999;1472:479–485. doi: 10.1016/S0304-4165(99)00151-8. [DOI] [PubMed] [Google Scholar]
- 117.Sanhueza C., Acevedo F., Rocha S., Villegas P., Seeger M., Navia R. Polyhydroxyalkanoates as biomaterial for electrospun scaffolds. Int. J. Biol. Macromol. 2019;124:102–110. doi: 10.1016/j.ijbiomac.2018.11.068. [DOI] [PubMed] [Google Scholar]
- 118.Rhim J.W., Park H.M., Ha C.S. Bio-nanocomposites for food packaging applications. Prog. Polym Sci. 2013;38:1629–1652. doi: 10.1016/j.progpolymsci.2013.05.008. [DOI] [Google Scholar]
- 119.Popa M.S., Frone A.N., Panaitescu D.M. Polyhydroxybutyrate blends: A solution for biodegradable packaging? Int. J. Biol. Macromol. 2022;207:263–277. doi: 10.1016/j.ijbiomac.2022.02.185. [DOI] [PubMed] [Google Scholar]
- 120.Atta O.M., Manan S., Shahzad A., Ul-Islam M., Ullah M.W., Yang G. Biobased materials for active food packaging: A review. Food Hydrocoll. 2022;125:107419. doi: 10.1016/j.foodhyd.2021.107419. [DOI] [Google Scholar]
- 121.Hosokawa M.N., Darros A.B., Moris V.A.D., Paiva J.M.F.D. Polyhydroxybutyrate composites with random mats of sisal and coconut fibers. Mater. Res. 2016;20:279–290. doi: 10.1590/1980-5373-mr-2016-0254. [DOI] [Google Scholar]
- 122.Rajan K.P., Thomas S.P., Gopanna A., Chavali M. Polyhydroxybutyrate (PHB): A standout biopolymer for environmental sustainability. In: Torres-Martínez L.M., Vasilievna-Kharissova O., Ildusovich-Kharisov B., editors. Handbook of Ecomaterials. 1st ed. Springer; Cham, Switzerland: 2020. pp. 2803–2825. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.