Abstract
The conventional means of studying Epstein-Barr virus (EBV)-induced cytotoxic T-lymphocyte (CTL) memory, by in vitro stimulation with the latently infected autologous lymphoblastoid cell line (LCL), has important limitations. First, it gives no information on memory to lytic cycle antigens; second, it preferentially amplifies the dominant components of latent antigen-specific memory at the expense of key subdominant reactivities. Here we describe an alternative approach, based on in vitro stimulation with epitope peptide-loaded dendritic cells (DCs), which allows one to probe the CTL repertoire for any individual reactivity of choice; this method proved significantly more efficient than stimulation with peptide alone. Using this approach we first show that reactivities to the immunodominant and subdominant lytic cycle epitopes identified by T cells during primary EBV infection are regularly detectable in the CTL memory of virus carriers; this implies that in such carriers chronic virus replication remains under direct T-cell control. We further show that subdominant latent cycle reactivities to epitopes in the latent membrane protein LMP2, though rarely undetectable in LCL-stimulated populations, can be reactivated by DC stimulation and selectively expanded as polyclonal CTL lines; the adoptive transfer of such preparations may be of value in targeting certain EBV-positive malignancies.
Cytotoxic T lymphocytes (CTLs) of CD8+ type recognize peptides derived from the intracellular breakdown of foreign antigens and presented at the cell surface as a complex with major histocompatibility complex (MHC) class I molecules (58). Such CTLs appear to play an important role in controlling virus infection. Even with genetically complex viruses, however, the virus-induced CTL response tends to focus on a few immunodominant peptide epitopes whose identities are specific for the particular MHC type of the host. The present study concerns the CTL response to Epstein-Barr virus (EBV), a human herpesvirus with cell growth transforming ability which is linked to several malignancies and yet is carried by most individuals as an asymptomatic lifelong infection. Virus carriage is characterized by latent infection of the B lymphocyte pool and by chronic shedding of infectious virus from productively infected cells within the oropharynx (37).
The repertoire of EBV-specific T cells in virus carriers is conventionally studied by challenging peripheral blood lymphocytes in vitro with cells of the autologous EBV-transformed B lymphoblastoid cell line (LCL) (38). However, this approach has at least two important limitations. First, because LCLs are largely composed of latently infected cells, LCL stimulation tells us very little about CTL responses to antigens of the virus lytic cycle. In consequence, the latter issue was largely, if not completely (8), ignored until recently when analysis of the in vivo-activated primary response to EBV in infectious mononucleosis patients showed that lytic-cycle-specific reactivities not only existed but were unusually strong and could be mapped to defined epitopes within immediate-early/early-lytic proteins (14, 49). It is important now to develop methods of probing T cell memory to determine whether such reactivities persist in the longer term. A second potential limitation of the LCL stimulation protocol stems from the marked skewing of the latent antigen-specific response towards a particular subset of latent proteins. Thus, while LCL cells express all six EBV-coded nuclear antigens EBNA1, -2, -3A, -3B, -3C, and -LP and the latent membrane proteins 1 and 2 (LMP1 and LMP2), LCL-stimulated CTL preparations are very often dominated by reactivities to peptide epitopes from the EBNA3A, -3B, and -3C proteins (22, 33). Usually it is only through single-cell cloning that CTLs recognizing one or another of the subdominant latent antigens, most commonly LMP2, are detected (10, 22, 24). In adoptive therapy the above polyclonal CTLs have proven effective against at least one EBV-associated malignancy, immunoblastic lymphoma of immunosuppressed subjects (42), where the tumor cells express the immunodominant EBNA3 proteins (19, 51). However, such CTLs are unlikely to be as effective in the context of other malignancies, such as nasopharyngeal carcinoma, where viral antigen expression is limited to EBNA1, LMP2, and in some cases LMP1 (37). Since endogenously expressed EBNA1 is protected from presentation to CD8+ T cells by virtue of its Gly-Ala repeat domain (7, 25, 26), the LMPs (and particularly LMP2) constitute potentially important targets for immune recognition if polyclonal CTL preparations enriched for such reactivities could be produced.
Against this background, we sought to develop an efficient protocol for the selective reactivation of memory CTLs specific for defined lytic and subdominant latent cycle epitopes. Dendritic cells (DC) are the most attractive vehicles for this purpose since they have proved most effective as antigen-presenting cells (APCs) in a variety of in vivo and in vitro systems (5, 21, 28, 29, 31). In vivo, immature DCs develop from hematopoietic progenitors and are located strategically at body surfaces, where they play a sentinel role in capturing and processing antigens. Following antigen exposure, DCs migrate to lymphoid organs and acquire potent antigen-presenting function with cell surface upregulation of adhesion molecules such as CD54 (ICAM1) and of costimulatory molecules such as CD80 and CD86 (5). Human DCs can be generated in vitro either from rare CD34+ cell precursors in peripheral blood (15, 40) or, more commonly, from monocytes by culturing in medium supplemented with interleukin 4 (IL-4) and granulocyte-macrophage colony-stimulating factor GM-CSF followed by maturation stimuli (6, 41, 43). Following reports of the successful use of peptide-loaded DCs to induce epitope-specific CTL responses to melanoma-associated antigens (34, 52, 53), we have used here a parallel approach in an attempt to selectively reactivate responses to defined EBV epitope peptides.
MATERIALS AND METHODS
Media and reagents.
The medium used throughout was RPMI 1640 (Gibco Laboratories, Grand Island, N.Y.) supplemented with 2 mM l-glutamine, 100 IU of penicillin per ml, 100 μg of streptomycin per ml, and 10% (vol/vol) fetal calf serum (RPMI–10% FCS). Human recombinant IL-7 and β2-microglobulin were obtained from Sigma. Human recombinant IL-2 was kindly provided by Glaxo-Wellcome (Stevenage, United Kingdom), and human recombinant IL-4 and GM-CSF was provided by Schering-Plough (Dardilly, France). Monoclonal antibody (MAb) to CD83 (59) was kindly provided by T. Tedder (Duke University, Durham, N.C.); MAbs Bu74 to CD54 and Bu63 to CD86 were obtained from D. Hardie (Department of Immunology, University of Birmingham, Birmingham, United Kingdom), and MAb L307.4 to CD80 was obtained from Becton Dickinson (Immunocytometry Systems, Lincoln Park, N.J.).
Preparation of stimulator and target cells.
To prepare DCs, peripheral blood mononuclear cells (PBMCs) were isolated by centrifugation on Lymphoprep (Nycomed Pharma, Oslo, Norway), resuspended in RPMI–10% FCS at 5 × 106 cells/ml, and seeded into 6-well plates (Costar Corp., Cambridge, Mass.) at 107 cells/well. After 2 h at 37°C, nonadherent cells were removed, and the adherent cell population was cultured in RPMI–10% FCS supplemented with 50 ng of GM-CSF and 1,000 U of IL-4 per ml. The cultures were refed on days 2 and 4 by replacing half of the medium with fresh medium as described above; on day 6 the culture medium was changed to fresh RPMI–10% FCS with GM-CSF and IL-4 as described above, but now supplemented with 25% (vol/vol) macrophage-conditioned medium (see below) as a maturation stimulus. Nonadherent cells were harvested 2 days later and used as a source of DCs; the quality of the DC preparation was checked in each case by immunofluorescence staining for surface markers CD54, CD80, CD83, and CD86 by using specific MAbs. Macrophage-conditioned medium was produced by culturing adherent PBMCs (isolated by adherence to human immunoglobulin-coated plates [40]) for 24 h at 37°C in RPMI–10% FCS and then harvesting the supernatant medium, followed by filtration through a 0.2-μm (pore size) membrane (Acrodisc; Gelman Sciences) and storage at −20°C for up to 8 weeks before use.
As described elsewhere (50), EBV-transformed LCLs (carrying the B95.8 or the BL74 virus strain) and PHA blast targets were established and maintained as a source of stimulation and/or target cells.
Peptides.
The EBV epitope peptides used in this work are listed in Table 1, which gives their EBV antigen location, amino acid sequence, and human lymphocyte antigen (HLA) class I restriction element. All peptides were synthesized by 9-fluorenylmethoxycarbonyl chemistry (Alta Bioscience, University of Birmingham, Birmingham, United Kingdom) dissolved in dimethylsulfoxide (DMSO), and their concentrations were determined by biuret assay. Peptides were used at a concentration of 50 μg/ml for loading onto DC stimulators and at 5 μg/ml for presensitization of LCL and PHA blast target cells.
TABLE 1.
Epitope peptides used in DC stimulation
| Epitope type | Designation | Location
|
Sequence | HLA class I restriction | Reference | |
|---|---|---|---|---|---|---|
| Antigen | Amino acid site | |||||
| Immunodominant latent-cycle epitope | QAK | EBNA3A | 158–166 | QAKWRLQTL | B8 | 38 |
| FLR | EBNA3A | 325–333 | FLRGRAYGL | B8 | 2 | |
| IVT | EBNA3B | 416–424 | IVTDFSVIK | A11.01 | 18 | |
| AVF | EBNA3B | 399–408 | AVFDRKSDAK | A11.01 | 18 | |
| RRI | EBNA3C | 258–266 | RRIYDLIEL | B27.02/27.05 | 9 | |
| RRA | EBNA3B | 244–254 | RRARSLSAERY | B27.02 | 10 | |
| Subdominant latent-cycle epitope | LLW | LMP2 | 329–337 | LLWTLVVLL | A2.01 | 24 |
| CLG | LMP2 | 426–434 | CLGGLLTMV | A2.01 | 24 | |
| IED | LMP2 | 200–208 | IEDPPFNSL | B40.01 | 24 | |
| SSC | LMP2 | 340–350 | SSCSSCPLSKI | A11.01 | 24 | |
| TYG | LMP2 | 419–427 | TYGPVFMCL | A24.02 | 24 | |
| Lytic-cycle epitope | APE | BZLF1 | 82–90 | APENAYQAY | B35.01 | Unpublished |
| RAK | BZLF1 | 190–197 | RAKFKQLL | B8 | 8 | |
| GLC | BMLF1 | 280–288 | GLCTLVAML | A2.01 | 49 | |
| TLD | BMRF1 | 208–216 | TLDYKPLSV | A2.01 | Unpublished | |
T-cell stimulation protocols.
Donors used in these experiments were healthy adults of known HLA type and of known EBV antibody status as determined by the standard immunofluorescence assay for antiviral capsid antigen reactivity (37). DC stimulators were first preexposed for 2 h at 37°C to peptides in serum-free RPMI 1640 supplemented with human β2-microglobulin at 3 μg/ml and then washed and seeded at 105 cells/2-ml well in RPMI–10% FCS supplemented with recombinant IL-7 (rIL-7) at 5 ng/ml. Responder PBMCs were added at 2 × 106/well to give a responder/stimulator ratio of 20:1. The cultures were restimulated on days 14 and 21 with autologous peptide-loaded cells (DCs or freshly prepared adherent monocytes) now in RPMI–10% FCS supplemented with rIL2 at 20 U/ml. The cultures were expanded into additional wells on day 14, if necessary, and on day 21.
Stimulation with peptide alone followed a protocol based on that of Plebanski et al. (36) which has been shown to be able to reactivate memory CTLs against immunodominant EBV latent cycle epitopes (23). Briefly, peptide was added to PBMC cultures (2 × 106 cells/2-ml well) at 50 μg/ml, and the cells were then maintained in RPMI–10% FCS supplemented with rIL-7 at 20 ng/ml from day 0 and with rIL-2 at 20 U/ml from day 3.
Stimulation with LCL followed the standard protocol (33). Briefly, PBMCs were cocultured with γ-irradiated autologous LCL cells in 2-ml wells in RPMI–10% FCS to give a responder/stimulator ratio of 20:1, followed by restimulations on days 14 and 21 in medium supplemented with rIL-2 at 20 U/ml.
Cytotoxicity assays.
Polyclonal T-cell populations produced by the above stimulation protocols were harvested in parallel and used as effectors in standard 5-h chromium release assays at known effector/target ratios. Autologous PHA blasts and LCL cells were used as targets following a 2-h exposure to epitope peptides (or DMSO solvent as a control) and extensive washing. In some cases the LCL targets were also tested after overnight infection with recombinant vaccinia viruses encoding individual EBV proteins; the control recombinant vTK− and the recombinants expressing EBNA3B, EBNA3C, LMP2, BMLF1, and BMRF1 have been described, as has their use in cytotoxicity assays (33, 49).
RESULTS
Pilot experiments with immunodominant latent cycle epitopes.
Throughout this study DC preparations were generated from PBMCs by 7-day culture in IL-4 and GM-CSF followed by a 2-day maturation step in macrophage-conditioned medium. These preparations were monitored by immunofluorescence staining and contained >50% cells with surface expression of the mature DC marker CD83 (59), as well as CD54, CD80, and CD86 (data not shown). In the first series of experiments we aimed to assess the in vitro stimulatory capacity of peptide-loaded DCs by using defined epitope peptides from the immunodominant EBNA3A, -3B, and -3C subset of latent cycle proteins. Peripheral blood responder cells from selected donors were challenged in vitro with peptide-loaded autologous DC stimulators, and the resulting effector population was assayed against autologous targets (PHA blast and LCL) with or without their preexposure to the relevant peptide. Preliminary experiments showed that peptide-specific reactivities were detectable at low levels in DC-stimulated cultures on day 14 but against a background of nonspecific lysis; however, two further stimulations on days 14 and 21 in the presence of IL-2 selectively amplified the peptide-specific CTL population. Data in the present study are from such restimulated populations; in each experiment, parallel effectors were generated from the same donor by conventional LCL stimulation on day 0 followed by LCL restimulation and IL-2 addition on days 14 and 21.
Figure 1A presents typical results from an HLA-B27.02-positive EBV-immune donor, LY, already known to possess memory CTLs to two immunodominant B27.02-restricted latent cycle epitopes, the EBNA3C-derived epitope RRIYDLIEL (designated RRI) and the EBNA3B-derived epitope RRARSLSAERY (designated RRA) (9, 10). Conventional LCL stimulation generated a polyclonal CTL population containing both RRI-specific and RRA-specific effectors; these could be detected both by using peptide-loaded PHA blast or peptide-loaded LCL targets (Fig. 1A, LCL data). In contrast, effector CTLs generated by peptide-loaded DC stimulators were preferentially directed against the stimulator peptide, either RRI or RRA (Fig. 1A, RRI/DC and RRA/DC data). Note that RRI-stimulated effectors showed higher levels of lysis of the untreated autologous LCL target than did RRA-stimulated effectors, though in both cases levels of killing were enhanced by exogenous peptide loading. These differences in lysis of the naturally infected LCL targets resemble those already noted with epitope-specific clones derived from donor LY by conventional LCL stimulation (9).
FIG. 1.
Cytotoxic activities of polyclonal effector T cells induced by peptide-loaded autologous DC stimulation (peptide/DC) or by autologous LCL stimulation (LCL). (A) EBV-immune donor LY (HLA-A1, A24.02, B27.02, B35.02) responses to the immunodominant B27.02-restricted latent-cycle epitopes RRI and RRA versus the response to B95.8 virus-transformed LCL. (B) EBV-immune donor AR (HLA-A1, B8, B57) and nonimmune donor LS (HLA-A1, B8) responses to the immunodominant B8-restricted latent cycle epitopes QAK and FLR versus the response to BL74 virus-transformed LCL. Effector T cells were tested at an effector/target (E/T) ratio of 10:1 on autologous PHA blast targets and on autologous B95.8 virus-transformed LCL targets either untreated (Cont) or following target cell exposure to 5 μg of peptides per ml. Results are expressed as percent specific cytotoxicity observed in a 5-h chromium release assay.
Figure 1B (left) presents parallel data from an HLA-B8-positive EBV-immune donor AR known to possess memory CTLs to two B8-restricted latent cycle epitopes in EBNA3A: QAKWRLQTL (designated QAK) and FLRGRAYGL (designated FLR). As expected, stimulation with autologous LCL (carrying a viral strain BL74 encoding both epitopes [2]) reactivated both components of virus-specific memory. Strong reactivation was also induced by using peptide-loaded DCs but was selective for the specific epitope peptide used. Note that QAK-stimulated effectors (QAK/DC) did recognize naturally infected LCL target cells even without peptide loading, whereas the FLR-stimulated effectors (FLR/DC) did not; this reflects the fact that in these particular assays the autologous LCL target carried a viral strain (B95.8) with a mutation in the FLR epitope (2). Similar data were obtained from a second HLA-B8-positive EBV-immune donor, MR, who also mounted strong epitope-specific responses to QAK- and to FLR-loaded DCs (data not shown). Using the same protocol, we then studied two HLA-B8-positive donors, LS and IA, who were nonimmune as reflected by EBV antibody negativity in serological assays. Both individuals were tested on two successive occasions by using peptide-loaded DC stimulators and autologous LCL stimulators but gave no evidence of any epitope-specific response. Figure 1B (right) shows the data from one such experiment with donor LS. Although all three types of coculture generated T-cell proliferation which was sustained in rIL-2, no cytotoxicity was detectable against either autologous PHA blast or LCL targets with or without peptide loading. Similar results were obtained from a second EBV nonimmune donor, IA, with QAK-loaded or FLR-loaded DC stimulators; in this case LCL stimulations produced broad-ranging cytotoxicity detectable on various target lines but no EBV epitope-specific killing (data not shown).
In subsequent experiments we tested the above DC-based stimulation protocol against an alternative published method for the in vitro reactivation of virus-specific CTL memory (23); this method involves the addition of peptide to mononuclear cell cultures in the presence of IL-7, a cytokine found to improve peptide-induced responses in vitro (36), and expansion of reactive cells by IL-2 supplementation from day 3 onwards. In assays on a range of EBV-immune donors, we found that the DC-based protocol induced stronger and more consistent epitope-specific responses and lower background reactivity. This is illustrated by the representative results in Fig. 2 obtained from an HLA-A11, B8-positive donor, CMc, known to possess memory CTLs to two A11-restricted epitopes in EBNA3B, IVTDFSVIK (designated IVT) and AVFDRKSDAK (designated AVF), and to the B8-restricted epitope QAK. The non-DC stimulation protocol produced detectable responses to the IVT and QAK epitopes but not to AVF (Fig. 2, left). In contrast, all three peptides presented on autologous DCs induced polyclonal effector populations with a strong epitope-specific component (Fig. 2, right). The latter was detectable on peptide-loaded PHA blast targets and, as incremental lysis, on LCL targets either preloaded with the relevant peptide or expressing increased levels of the relevant target antigen (EBNA3A or EBNA3B) from a recombinant vaccinia virus vector.
FIG. 2.
Cytotoxic activities of polyclonal effector T cells induced by stimulation with peptide alone or with peptide-loaded autologous DCs (peptide/DC). Responses are shown from EBV-immune donor CMc (HLA-A2.01, A11.01, B8, B44) to the immunodominant A11-restricted latent cycle epitopes IVT and AVF (both from EBNA3B) and to the immunodominant B8-restricted epitope QAK (from EBNA3A). Effector T cells were tested (E/T, 10:1) on autologous PHA blast and LCL targets either untreated or peptide-loaded as in Fig. 1 or on LCL targets infected with recombinant vaccinia viruses vTK−, vE3B, (expressing EBNA3B), and vE3A (expressing EBNA3A).
Responses to defined lytic cycle epitopes.
Following the identification of a number of EBV lytic antigen-derived epitopes recognized by components of the primary virus-induced CTL response in IM patients (49), here we used stimulation with peptide-loaded DCs to probe the T-cell pool of long-term virus carriers for evidence of such reactivities in CTL memory.
Two such epitopes, restricted through the HLA A2.01 allele, are the peptides GLCTLVAML (designated GLC) and TLDYKPLSV (designated TLD), derived from the EBV early lytic cycle antigens BMLF1 and BMRF1, respectively. We first screened two HLA-A2.01-positive EBV carriers, JS and SL, for CTL reactivity to these epitopes and in both individuals detected specific responses to both epitopes in at least two independent in vitro reactivations. Representative results from one experiment with donor JS are illustrated in Fig. 3. Stimulation with GLC-loaded or with TLD-loaded DCs produced effector populations which specifically recognized PHA blasts preloaded with the relevant peptide. Note here that baseline levels of LCL lysis are low because LCL populations contain very few lytically infected cells; however, LCLs could be sensitized to lysis either by exogenous peptide or by infection with a recombinant vaccinia virus expressing the relevant lytic cycle protein: BMLF1 in the case of GLC-specific T cells and BMRF1 in the case of TLD-specific T cells (Fig. 3, GLC/DC and TLD/DC data). In the same experiment, LCL stimulation produced latent antigen-specific effectors capable of recognizing the LCL target but did not induce any detectable response to the lytic cycle epitopes (Fig. 3, LCL data). In a similar way it was possible to detect memory CTL responses to other defined lytic cycle epitopes from EBV-immune donors with relevant HLA types. For instance, responses to the B8-restricted epitope RAKFKQLL (designated RAK) from the immediate-early lytic cycle protein BZLF1 were seen in all three B8-positive individuals tested (AR, MR, and CMc), and responses to the B35.01-restricted epitope APENAYQAY, also from BZLF1 were seen in the B35.01-positive virus carrier RT (data not shown).
FIG. 3.
Cytotoxic activities of polyclonal effector T cells induced by peptide-loaded autologous DC stimulation (peptide/DC) or by autologous B95.8 virus-transformed LCL stimulation (LCL). Responses are shown from EBV-immune donor JS (HLA-A2.01, B27.05, B44) to the A2.01-restricted lytic-cycle epitopes GLC (from BMLF1) and TLD (from BMRF1). Effector T cells were tested (E/T, 10:1) on autologous PHA blast and LCL targets either untreated or peptide-loaded as in Fig. 1 or on LCL targets infected with recombinant vaccinia viruses vTK−, vBMLF1 (expressing BMLF1), and vBMRF1 (expressing BMRF1).
The strength of the GLC-induced and RAK-induced CTL responses in the above experiments led us to reexamine EBV-immune versus nonimmune individuals in their responses to DC stimulation with these particular epitope peptides. Figure 4 shows representative data from such experiments. Both of the HLA-A2.01-positive EBV-immune donors, DA and SL, generated polyclonal T-cell populations with strong epitope-specific reactivity following stimulation with GLC-loaded DCs, whereas the A2.01-positive nonimmune donors CD and LD gave no detectable reactivity (Fig. 4A). A similar pattern of results were obtained by using the B8-restricted RAK epitope peptide. Strong responses were detected in polyclonal cultures from the two EBV-immune donors AR and MR but were not detectable in the parallel cultures established from nonimmune donors IA and LS (Fig. 4B).
FIG. 4.
Cytotoxic activities of polyclonal effector T cells induced by peptide-loaded autologous DC stimulation. (A) Response of two EBV-immune donors, DA (A2.01, A11.01; B7, B44) and SL (A1, A2.01; B16, B40.01), and of two nonimmune donors, CD (A1, A2.01; B37, B62) and LD (A2.01: B51, B63), to the A2.01-restricted lytic cycle epitope GLC. (B) Response of two EBV-immune donors, AR (A1; B8, B57) and MR (A2.01, A29; B8, B40.01) and of two nonimmune donors, IA (A1; B7, B8) and LS (A1; B8), to the B8-restricted lytic cycle epitope RAK. Effector T cells were tested (E/T, 10:1) on autologous PHA blast and LCL targets either untreated or peptide-loaded as in Fig. 1.
Responses to epitopes from the subdominant latent cycle protein LMP2.
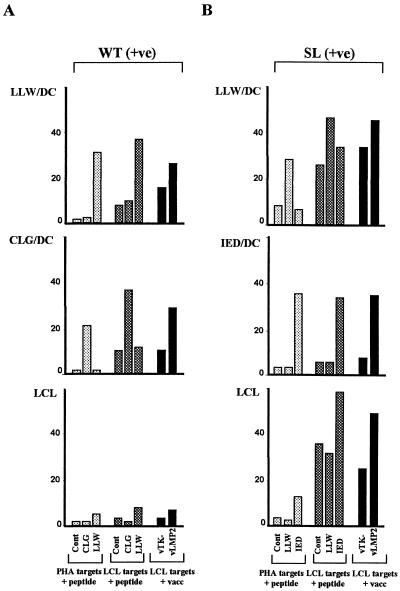
We next asked whether DC stimulation was capable of accessing low-frequency components of CTL memory, in particular CTLs specific for defined epitopes in the subdominant latent cycle protein LMP2. We first chose two EBV-immune donors whose polyclonal CTL response to conventional LCL stimulation is known to contain a small but detectable LMP2-specific component. Donor WT (A2.01-positive) gives an unusually weak response to LCL stimulation, the only detectable EBV latent antigen-specific reactivity being to an A2.01-restricted epitope LLWTLVVLL (designated LLW) in LMP2. This is illustrated in Fig. 5A (LCL data), where LCL-stimulated effectors from this donor not only failed to kill the LCL but also contained barely detectable reactivity against LLW peptide-loaded targets and no detectable reactivity to a second A2.01-restricted epitope within LMP2, CLGGLLTMV (designated CLG). However, in vitro stimulation with these peptides presented on DCs was able to selectively induce responses both to LLW and to CLG; these effectors recognized peptide-loaded targets and also targets endogenously expressing LMP2 (Fig. 5A, LLW/DC and CLG/DC data). In a second donor, SL (A2.01, B40.01-positive), LCL stimulation generated an LMP2-specific response which mapped entirely to the B40.01-restricted epitope, IEDPPFNSL (designated IED), with no detectable reactivity to either of the above A2.01-restricted epitopes (Fig. 5B, LCL data, and data not shown). In this case, stimulation with peptide-loaded DCs was able to reactivate not just the IED-specific reactivity but also a significant response to one of the A2.01-restricted epitopes, LLW, though not to the other epitope CLG (Fig. 5B and data not shown).
FIG. 5.
Cytotoxic activities of polyclonal effector T cells induced by peptide-loaded autologous DC stimulation (peptide/DC) or by autologous B95.8 virus-transformed LCL stimulation (LCL). (A) EBV-immune donor WT (HLA-A2.01; B14, B15) responses to the subdominant A2.01-restricted latent cycle epitopes LLW and CLG (both from LMP2). (B) EBV-immune donor SL (HLA-A1, A2.01, B16, B40.01) responses to the subdominant A2.01-restricted epitope LLW and to the B40.01-restricted epitope IED (both from LMP2). Effector T cells were tested (E/T, 10:1) on autologous PHA blast and LCL targets infected with recombinant vaccinia viruses vTK− and vLMP2 (expressing LMP2).
The experiments were then extended to donors whose polyclonal CTL response to LCL stimulation lacks a detectable LMP2-specific component and is dominated by reactivities against epitopes from EBNA3A, -3B, and -3C proteins. Figure 6A presents the results from one such individual, RT, whose major component of CTL memory to latent cycle antigens is directed against the B27.05-restricted CTL epitope RRI from EBNA3C (see Fig. 6A, LCL data). As expected, therefore, stimulation with RRI peptide-loaded DCs was able to induce a strong epitope-specific response (Fig. 6A, RRI/DC). However, donor RT is also positive for HLA A24.02, a potential restricting element for a defined epitope TYGPVFMCL (designated TYG) in LMP2. We found that stimulation with TYG-loaded DCs did indeed selectively induce a specific response from this donor that was capable of recognizing both exogenously loaded epitope and endogenously expressed LMP2 protein (Fig. 6A, TYG/DC), even though such effectors were never detected in LCL-stimulated populations. Analogous results came from another donor, DM (HLA-A11-positive), whose polyclonal response to LCL stimulation is largely A11-restricted and focused on the IVT epitope peptide derived from EBNA3B (see Fig. 6B, LCL data). Such effectors are also efficiently reactivated by stimulation with IVT-loaded DCs (Fig. 6B, IVT/DC); however, we were interested to know whether such a donor might respond to a subdominant A11-restricted epitope SSCSSCPLSKI (designated SSC) in LMP2. In a previous study (24) responses to this epitope were only identified in A11-positive individuals who lack dominant EBNA3B-specific responses because their resident virus carries an epitope-loss mutation in the EBNA3B protein (18). We in fact observed that SSC-specific effector CTLs, active against both the peptide and endogenously expressed LMP2 protein, could be generated from donor DM by stimulation with SSC-loaded DCs (Fig. 6B, SSC/DC); such effectors have never been detected in polyclonal LCL-stimulated effectors from this individual.
FIG. 6.
Cytotoxic activities of polyclonal effector T cells induced by peptide-loaded autologous DC stimulation (peptide/DC) or by autologous B95.8 virus-transformed LCL stimulation (LCL). (A) EBV-immune donor RT (HLA-A2.01, A24.02; B27.05, B35.01) responses to the subdominant A24.02-restricted latent-cycle epitope TYG (from LMP2) and to the immunodominant B27.05-restricted latent cycle epitope RRI (from EBNA3C). (B) EBV-immune donor DM (HLA-A1, A11.01; B8, B57) responses to two A11-restricted latent cycle epitopes, the subdominant epitope SSC (from LMP2) and the immunodominant epitope IVT (from EBNA3B). Effector T cells were tested (E/T, 10:1) on autologous PHA blast and LCL targets either untreated or peptide loaded as in Fig. 1 or on LCL targets infected with recombinant vaccinia viruses vTK−, vE3B (expressing EBNA3B), vE3C (expressing EBNA3C), or vLMP2 (expressing LMP2).
DISCUSSION
The present study sought to avoid LCL stimulation and to develop an alternative means of probing EBV-specific CTL memory that was efficient and that allowed even rare components of the memory pool to be accessed without laborious cell cloning. Experiments in the influenza virus system had first shown that individual epitope specificities could be reactivated in vitro by stimulation with epitope peptides and expanded thereafter as bulk effector populations in IL-2 (20, 30). However, those studies, which used PBMCs as a source both of peptide-loaded stimulator and of responder cells, were limited to immunodominant epitopes. In the EBV system also, such a protocol can reactivate CTL memory to immunodominant latent cycle epitopes (13, 23), but in our experience it has been much more difficult to access low-frequency subdominant components of CTL memory as bulk effector populations in this way (23a). It is only as rare clones in limiting dilution assays that such subdominant components become detectable after conventional peptide stimulation (50) and, even then, recent evidence suggests that the limiting dilution approach may not be accessing all memory cells with the relevant peptide specificity (14).
We therefore set out to determine whether the efficiency of EBV epitope peptide stimulation could be improved by using DC preparations as the source of stimulator cells. This was prompted by the mounting evidence on the potency of DCs as APCs both in vivo and in vitro (reviewed reference 5). Thus CD8+ CTL responses can be elicited in a variety of mouse model systems by DCs expressing the antigen endogenously from viral or plasmid vectors (12, 17, 44, 47, 48, 54), or preexposed to exogenous antigen in such a way as to encourage its entry into the MHC class I pathway (1, 3, 11, 35, 46, 57), or preloaded with epitope peptides (16, 27, 31). Studies to date in human systems are more limited and most in vitro work has focused on DC presentation of melanoma-associated target antigens or of epitope peptides, usually HLA-A2.01 restricted, derived from such antigens (4, 34, 52, 53, 55). Here a number of groups have shown that an initial stimulus with peptide-loaded DCs followed by several repeat stimulations, usually involving adherent monocytes as presenting cells, can generate bulk T-cell populations which contain epitope-specific reactivity and which frequently also recognize melanoma cell lines endogenously expressing the relevant antigen.
Our study used a DC-based in vitro stimulation protocol which is essentially similar in design to those used in the melanoma studies. We first sought to validate this protocol by testing the capacity of DCs to reactivate CTL responses against immunodominant EBV latent cycle epitopes. Using virus-immune donors known to possess the relevant reactivities, we found that peptide-loaded DCs are quantitatively at least as efficient at reactivating these immunodominant responses as is LCL stimulation itself (Fig. 1). Furthermore the peptide-DC protocol allows reactivities that are codominant in CTL memory to be accessed individually; such selective access can only be achieved by LCL stimulation in rare cases: for instance, where LCLs are available carrying an EBV isolate with a natural mutation in one or more of the immunodominant epitope sequences (2, 18). These initial experiments also showed that DCs were more efficient than PBMCs as stimulators of peptide-induced responses. As illustrated in Fig. 2 with three epitope peptides relevant to donor CMc, only the two most abundant components of latent-antigen-specific memory (to the QAK and IVT epitopes) could be accessed as bulk effectors by PBMC stimulators; by comparison, DC stimulation not only produced more potent effector populations specific for these epitopes but also generated a significant response from memory CTLs to a third epitope, AVF. Throughout these initial experiments, it is important to note that the effectors induced by peptide-loaded DCs were capable of recognizing naturally processed antigen as well as epitope peptide. Thus, these CTLs killed LCL targets, expressing the relevant EBV latent cycle target antigen, as efficiently as did LCL-stimulated effectors and displayed incremental lysis when the level of that target antigen was selectively increased by expression from a recombinant vaccinia vector (Fig. 1 and 2).
Such pilot experiments allowed us to optimize a stimulation protocol with which to probe EBV-induced CTL memory for reactivities that would not be efficiently accessed by LCL stimulation. A first important question in this regard concerned memory to lytic cycle antigens, since recent work on IM patients has revealed that primary EBV infection is accompanied by unusually strong responses of this kind (14, 49); this implies that such reactivities may be present, albeit undetected, in long-term virus carriers. Here we focused on two epitope-HLA combinations (GLC/A2.01 and RAK/B8) known to be immunodominant targets of the primary CTL response in IM patients of the relevant HLA type (14, 49) and on two other combinations (TLD/A2.01 and APE/B35.01) which appear to be subdominant targets in that the relevant reactivities are only detectable in IM effector populations after extensive screening of in vitro-derived clones (1a). Stimulation with peptide-loaded DCs revealed, for each of these four epitopes, the existence of a specific memory CTL response in healthy virus carriers with the appropriate HLA type (Fig. 3 and 4). This work adds significantly to the original study of Bogedain et al. (8) who used in vitro stimulation with pooled peptides from the sequence of the immediate-early lytic cycle protein BZLF1 and, without including DCs in the protocol, elicited a memory response from a B8-positive donor which was subsequently mapped to the RAK epitope. We have indeed confirmed that RAK- (and also GLC-) specific memory responses can be reactivated by peptide stimulation alone (1a), a fact we presume to reflect the unusual strength of these particular responses (14). These two studies, and recent evidence from T cells cloned from the synovium of EBV-carrying rheumatoid arthritis patients (45), make it clear that lytic-antigen-specific responses are not confined to primary infection but are maintained throughout life. This strongly suggests that virus replicative lesions, whose persistence in the oropharynx is a feature of the asymptomatic carrier state (37), remain under direct T-cell control.
It is worth noting, however, that these conclusions are only valid if the responses observed in vitro are being generated from antigen-experienced rather than naive T cells in the repertoire. This is a particularly important issue to resolve because there are reports that peptide-loaded DCs can initiate primary responses in vitro not just in murine systems but also occasionally, with human immunodeficiency virus peptides, in humans (28, 32). Furthermore, in vitro CTL responses to melanoma-associated epitope peptides are frequently reported even from healthy donors (39, 56), and it is not known whether this reflects a primary response or some preexisting T-cell immunity to “self” peptides from lineage-restricted cellular proteins. Our studies with EBV-seronegative donors by using immunodominant latent-cycle (FLR/B8 and QAK/B8) and lytic-cycle (GLC/A2.01 and RAK/B8) epitopes suggest that primary responses are not detectable when using our particular DC stimulation protocol (Fig. 1 and 4). Where primary human CTL responses to microbial peptides have been elicited in vitro, with or without DCs as stimulator cells, the protocols have involved either multiple restimulation of purified CD8+ T-cell responders (32) or extensive screening of the PBMC response by cloning (36). In contrast, our protocol involves a limited number of stimulations of the entire PBMC population and analysis of the reactive cells in bulk.
A second important application of the peptide-DC stimulation protocol is in the selective reactivation of responses to subdominant latent cycle proteins, in particular proteins which constitute potentially useful targets for the immune therapy of virus-associated tumours (38). Of the few EBV latent-cycle proteins constitutively expressed in EBV-positive malignancies, such as NPC, LMP2 is arguably the best candidate target antigen (24), and yet CTLs reactive to this protein are almost always minor components of the memory response induced by virus infection (22, 33). Here we show that DCs loaded with LMP2 epitope peptides are capable of eliciting epitope-specific CTL responses in vitro from donors for whom the conventional LCL-induced response contains little if any of the relevant LMP2 reactivity. We tested five different LMP2 epitope-HLA class I combinations in this way and for each we obtained positive responses in most (but not all) of the individuals tested with the relevant HLA type. It is encouraging to note that the range of restriction elements presenting LMP2 epitopes includes some alleles (HLA-A11.01, -A24.02, and -B40.01) which are common in the Southeast Asian population, where NPC is seen in particularly high frequency (24). This opens up the possibility of using epitope peptide-loaded DCs therapeutically as stimulators of memory T cells with the capacity to recognize and destroy tumor cells.
ACKNOWLEDGMENTS
This work was supported by the Cancer Research Campaign, London, United Kingdom.
We are grateful to Deborah Williams for excellent secretarial help.
REFERENCES
- 1.Albert M L, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 1a.Annels, N., and A. B. Rickinson. Unpublished data.
- 2.Apolloni A, Moss D, Stumm R, Burrows S, Suhrbier A, Misko I, Schmidt C, Sculley T. Sequence variation of cytotoxic T cell epitopes in different isolates of Epstein-Barr virus. Eur J Immunol. 1992;22:183–189. doi: 10.1002/eji.1830220127. [DOI] [PubMed] [Google Scholar]
- 3.Bachmann M F, Lutz M B, Layton G T, Harris S J, Fehr T, Rescigno M, Ricciardicastagnoli P. Dendritic cells process exogenous viral proteins and virus-like particles for class I presentation to CD8(+) cytotoxic T lymphocytes. Eur J Immunol. 1996;26:2595–2600. doi: 10.1002/eji.1830261109. [DOI] [PubMed] [Google Scholar]
- 4.Bakker A B H, Marland G, Deboer A J, Huijbens R J F, Danen E H J, Adema G J, Figdor C G. Generation of anti-melanoma cytotoxic T lymphocytes from healthy donors after presentation of melanoma-associated antigen-derived epitopes by dendritic cells in vitro. Cancer Res. 1995;55:5330–5334. [PubMed] [Google Scholar]
- 5.Banchereau J, Steinman R M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 6.Bender A, Sapp M, Schuler G, Steinman R M, Bhardwaj N. Improved methods for the generation of dendritic cells from non-proliferating progenitors in human blood. J Immunol Methods. 1996;196:121–135. doi: 10.1016/0022-1759(96)00079-8. [DOI] [PubMed] [Google Scholar]
- 7.Blake N, Lee S P, Redchenko I, Thomas W A, Steven N, Leese A, Steigerwald-Mullen P M, Kurilla M G, Frappier L, Rickinson A B. Human CD8(+) T cell responses to EBV EBNA1: HLA class I presentation of the (Gly-Ala)-containing protein requires exogenous processing. Immunity. 1997;7:791–802. doi: 10.1016/s1074-7613(00)80397-0. [DOI] [PubMed] [Google Scholar]
- 8.Bogedain C, Wolf H, Modrow S, Stuber G, Jilg W. Specific cytotoxic T-lymphocytes recognize the immediate-early transactivator ZTA of Epstein-Barr virus. J Virol. 1995;69:4872–4879. doi: 10.1128/jvi.69.8.4872-4879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brooks, J. M., R. A. Colbert, J. P. Mear, A. Leese, and A. B. Rickinson. HLA-B27 subtype polymorphism and CTL epitope choice: studies with EBV peptides link immunogenicity with stability of the B27:peptide complex. J. Immunol., in press. [PubMed]
- 10.Brooks J M, Murray R J, Thomas W A, Kurilla M G, Rickinson A B. Different HLA-B27 subtypes present the same immunodominant Epstein-Barr virus peptide. J Exp Med. 1993;178:879–887. doi: 10.1084/jem.178.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brossart P, Bevan M J. Presentation of exogenous protein antigens on major histocompatability complex class I molecules by dendritic cells: pathway of presentation and regulation by cytokines. Blood. 1997;90:1594–1599. [PMC free article] [PubMed] [Google Scholar]
- 12.Brossart P, Goldrath A W, Butz E A, Martin S, Bevan M J. Virus-mediated delivery of antigenic epitopes into dendritic cells as a means to induce CTL. J Immunol. 1997;158:3270–3276. [PubMed] [Google Scholar]
- 13.Burrows S R, Silins S L, Moss D J, Khanna R, Misko I S, Argaet V P. T cell receptor repertoire for a viral epitope in humans is diversified by tolerance to a background major histocompatibility complex antigen. J Exp Med. 1995;182:1703–1715. doi: 10.1084/jem.182.6.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Callan M F C, Tan L, Annels N, Ogg G S, Wilson J D K, O’Callaghan C A, Steven N, McMichael A J, Rickinson A B. Direct visualization of antigen-specific CD8(+) T cells during the primary immune response to Epstein-Barr virus in vivo. J Exp Med. 1998;187:1395–1402. doi: 10.1084/jem.187.9.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caux C, Dezutterdambuyant C, Schmitt D, Banchereau J. GM-CSF and TNF-Alpha co-operate in the generation of dendritic Langerhans cells. Nature. 1992;360:258–261. doi: 10.1038/360258a0. [DOI] [PubMed] [Google Scholar]
- 16.Celluzzi C, Mayordomo J I, Storkus W J, Lotze M T, Falo L D. Peptide-pulsed dendritic cells induce antigen-specific, CTL-mediated protective tumor-immunity. J Exp Med. 1996;183:283–287. doi: 10.1084/jem.183.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Condon C, Watkins S C, Celluzzi C, Thompson K, Falo L D. DNA-based immunisation by in vivo transfection of dendritic cells. Nat Med. 1996;2:1122–1128. doi: 10.1038/nm1096-1122. [DOI] [PubMed] [Google Scholar]
- 18.de Campos-Lima P-O, Levitsky V, Brooks J, Lee S P, Hu L F, Rickinson A B, Masucci M G. T cell responses and virus evolution: Loss of HLA A11-restricted CTL epitopes in Epstein-Barr virus isolates from high A11-positive populations by selective mutation of anchor residues. J Exp Med. 1994;179:1297–1305. doi: 10.1084/jem.179.4.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gratama J W, Zutter M M, Minarovits J, Oosterveer M A P, Thomas E D, Klein G, Ernberg I. Expression of Epstein-Barr virus growth-transformation-associated proteins in lymphoproliferations of bone-marrow transplant recipients. Int J Cancer. 1991;47:188–192. doi: 10.1002/ijc.2910470205. [DOI] [PubMed] [Google Scholar]
- 20.Hogan K T, Shimojo N, Walk S F, Engelhard V H, Maloy W L, Coligan J E. Mutations in the alpha-2 helix of HLA-A2 affect presentation but do not inhibit binding of influenza-virus matrix peptide. J Exp Med. 1988;168:725–736. doi: 10.1084/jem.168.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inaba K, Inaba M, Naito M, Steinman R M. Dendritic cell progenitors phagocytose particulates, including bacillus-calmette-guerin organisms, and sensitise mice to mycobacterial antigens in vivo. J Exp Med. 1993;178:479–488. doi: 10.1084/jem.178.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khanna R, Burrows S R, Kurilla M G, Jacob C A, Misko I S, Sculley T B, Kieff E, Moss D J. Localisation of Epstein-Barr virus cytotoxic T cell epitopes using recombinant vaccinia: implications for vaccine development. J Exp Med. 1992;176:169–178. doi: 10.1084/jem.176.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee S P, Morgan S, Skinner J, Thomas W A, Jones S R, Sutton J, Khanna R, Whittle H C, Rickinson A B. Epstein-Barr virus isolates with the major HLA B35.01-restricted cytotoxic T-lymphocyte epitope are prevalent in a highly B35.01-positive African population. Eur J Immunol. 1995;25:102–110. doi: 10.1002/eji.1830250119. [DOI] [PubMed] [Google Scholar]
- 23a.Lee, S. P., and A. B. Rickinson. Unpublished observations.
- 24.Lee S P, Tierney R J, Thomas W A, Brooks J M, Rickinson A B. Conserved cytotoxic T lymphocyte (CTL) epitopes within Epstein-Barr virus (EBV) latent membrane protein 2, a potential target for CTL-based tumour therapy. J Immunol. 1997;158:3325–3334. [PubMed] [Google Scholar]
- 25.Levitskaya J, Coram M, Levitsky V, Imreh S, Steigerwald-Mullen P M, Klein G, Kurilla M G, Masucci M G. Inhibition of antigen processing by the internal repeat region of the Epstein-Barr virus nuclear antigen 1. Nature. 1995;375:685–688. doi: 10.1038/375685a0. [DOI] [PubMed] [Google Scholar]
- 26.Levitskaya J, Sharipo A, Leonchiks A, Ciechanover A, Masucci M G. Inhibition of ubiquitin/proteasome-dependent protein degradation by the Gly-Ala repeat domain of the Epstein-Barr virus nuclear antigen 1. Proc Nat Acad Sci USA. 1997;94:12616–12621. doi: 10.1073/pnas.94.23.12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ludewig B, Ehl S, Karrer U, Odermatt B, Hengartner H, Zinkernagel R M. Dendritic cells efficiently induce protective antiviral immunity. J Virol. 1998;72:3812–3818. doi: 10.1128/jvi.72.5.3812-3818.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macatonia S E, Patterson S, Knight S C. Primary proliferative and cytotoxic T cell responses to HIV induced in vitro by human dendritic cells. Immunology. 1991;74:399–406. [PMC free article] [PubMed] [Google Scholar]
- 29.Macatonia S E, Taylor P M, Knight S C, Askonas B A. Primary stimulation by dendritic cells induces antiviral proliferative and cytotoxic T cell responses in vitro. J Exp Med. 1989;169:1255–1264. doi: 10.1084/jem.169.4.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martinon F, Gomard E, Hannoun C, Levy J P. In vitro human cytotoxic T cell responses against influenza A virus can be induced and selected by synthetic peptides. Eur J Immunol. 1990;20:2171–2176. doi: 10.1002/eji.1830201004. [DOI] [PubMed] [Google Scholar]
- 31.Mayordomo J I, Zorina T, Storkus W J, Zitvogel L, Celluzzi C, Falo L D. Bone marrow-derived dendritic cells pulsed with synthetic tumor peptides elicit protective and therapeutic anti-tumor immunity. Nat Med. 1995;1:1297–1302. doi: 10.1038/nm1295-1297. [DOI] [PubMed] [Google Scholar]
- 32.Mehtadamani A, Markowicz S, Engleman E G. Generation of antigen-specific CD8+ CTLs from naive precursors. J Immunol. 1994;153:996–1003. [PubMed] [Google Scholar]
- 33.Murray R J, Kurilla M G, Brooks J M, Thomas W A, Rowe M, Kieff E, Rickinson A B. Identification of target antigens for the human cytotoxic T cell response to Epstein-Barr virus (EBV): implications for the immune control of EBV-positive malignancies. J Exp Med. 1992;176:157–168. doi: 10.1084/jem.176.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nestle F O, Alijagic S, Gilliet M, Sun Y S, Grabbe S, Dummer R, Burg G, Schadendorf D. Vaccination of melanoma patients with peptide- or tumour lysate-pulsed dendritic cells. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 35.Paglia P, Chiodoni C, Rodolfo M, Colombo M P. Murine dendritic cells loaded in vitro with soluble protein prime cytotoxic T lymphocytes against tumor antigen in vivo. J Exp Med. 1996;183:317–322. doi: 10.1084/jem.183.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plebanski M, Allsopp C E M, Aidoo M, Reyburn H, Hill A V S. Induction of peptide-specific primary cytotoxic T-lymphocyte responses from human peipheral blood. Eur J Immunol. 1995;25:1783–1787. doi: 10.1002/eji.1830250645. [DOI] [PubMed] [Google Scholar]
- 37.Rickinson A B, Kieff E. Epstein-Barr Virus. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2397–2446. [Google Scholar]
- 38.Rickinson A B, Moss D J. Human cytotoxic T lymphocyte responses to Epstein-Barr virus infection. Annu Rev Immunol. 1997;15:405–431. doi: 10.1146/annurev.immunol.15.1.405. [DOI] [PubMed] [Google Scholar]
- 39.Rivoltini L, Kawakami Y, Sakaguchi K, Southwood S, Sette A, Robbins P F, Marincola F M, Salgaller M L, Yannelli Y R, Appella E, Rosenberg S A. Induction of tumor-reactive CTL from peripheral blood and tumor-infiltrating lymphocytes of melanoma patients by in vitro stimulation. J Immunol. 1995;154:2257–2265. [PubMed] [Google Scholar]
- 40.Romani N, Gruner S, Brang D, Kampgen E, Lenz A, Trockenbacher B, Konwalinka G, Fritsch P O, Steinman R M, Schuler G. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180:83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Romani N, Reider D, Heuer M, Ebner S, Kampgen E, Eibl B, Niederwieser D, Schuler G. Generation of mature dendritic cells from human blood: an improved method with special regard to clinical applicability. J Immunol Methods. 1996;196:137–151. doi: 10.1016/0022-1759(96)00078-6. [DOI] [PubMed] [Google Scholar]
- 42.Rooney C M, Smith C A, Ng C Y C, Loftin S, Li C, Krance R A, Brenner M K, Heslop H E. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr virus-related lymphoproliferation. Lancet. 1995;345:9–13. doi: 10.1016/s0140-6736(95)91150-2. [DOI] [PubMed] [Google Scholar]
- 43.Sallusto F, Lanzavecchi A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte-macrophage colony stimulating factor plus interleukin-4 and down-regulated by tumor-necrosis-factor-alpha. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schuler G, Steinman R M. Dendritic cells as adjuvants for immune-mediated resistance to tumors. J Exp Med. 1997;186:1183–1187. doi: 10.1084/jem.186.8.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scotet E, David-Ameline J, Peyrat M-A, Moreau-Aubry A, Pinczon D, Lim A, Even J, Semana G, Berthelot J M, Breathnach R, Bonneville M, Houssaint E. T cell response to Epstein-Barr virus transactivators in chronic rheumatoid arthritis. J Exp Med. 1996;184:1791–1800. doi: 10.1084/jem.184.5.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen Z H, Reznikoff G, Dranoff G, Rock K L. Cloned dendritic cells can present exogenous antigens on both MHC class I and class II molecules. J Immunol. 1997;158:2723–2730. [PubMed] [Google Scholar]
- 47.Song W, Kong H L, Carpenter H, Torii H, Granstein R, Rafii S, Moore M A S, Crystal R G. Dendritic cells genetically modified with an adenovirus vector encoding the cDNA for a model antigen induce protective and therapeutic anti-tumor immunity. J Exp Med. 1997;186:1247–1256. doi: 10.1084/jem.186.8.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Specht J M, Wang G, Do M T, Lam J S, Royal R E, Reeves M E, Rosenberg S A, Hwu P. Dendritic cells retrovirally transduced with a model antigen gene are therapeutically effective against established pulmonary metastases. J Exp Med. 1997;186:1213–1221. doi: 10.1084/jem.186.8.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steven N M, Annels N, Kumar A, Leese A, Kurilla M G, Rickinson A B. Immediate early and early lytic cycle proteins are frequent targets of the Epstein-Barr virus-induced cytotoxic T cell response. J Exp Med. 1997;185:1605–1617. doi: 10.1084/jem.185.9.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steven N M, Leese A M, Annels N, Lee S, Rickinson A B. Epitope focusing in the primary cytotoxic T-cell response to Epstein-Barr virus and its relationship to T-cell memory. J Exp Med. 1996;184:1801–1813. doi: 10.1084/jem.184.5.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thomas J A, Hotchin N, Allday M J, Yacoub M, Crawford D H. Immunohistology of Epstein-Barr virus associated antigens in B cell disorders from immunocompromised individuals. Transplantation. 1990;49:944–953. doi: 10.1097/00007890-199005000-00022. [DOI] [PubMed] [Google Scholar]
- 52.Tjandrawan T, Martin D M, Maeurer M J, Castelli C, Lotze M T, Storkus W J. Autologous human dendriphages pulsed with synthetic or natural tumor peptides elicit tumor-specific CTLs in vitro. J Immunother. 1998;21:149–157. doi: 10.1097/00002371-199803000-00009. [DOI] [PubMed] [Google Scholar]
- 53.Tsai V, Southwood S, Sidney J, Sakaguchi K, Kawakami Y, Appella E, Sette A, Celis E. Identification of subdominant CTL epitopes of the GP100 melanoma-associated tumor antigen by primary in vitro. J Immunol. 1997;158:1796–1802. [PubMed] [Google Scholar]
- 54.Tuting T, DeLeo A B, Lotze M T, Storkus W J. Genetically modified bone marrow-derived dendritic cells expressing tumor-associated viral or “self” antigens induce anti-tumor immunity in vivo. Eur J Immunol. 1997;27:2702–2707. doi: 10.1002/eji.1830271033. [DOI] [PubMed] [Google Scholar]
- 55.Vanderbruggen P, Bastin J, Gajewki T, Coulie P G, Boel P, Desmet C, Traversari C, Townsend A, Boon T. A peptide encoded by human gene MAGE-3 and presented by HLA-A2 induces cytolytic T lymphocytes that recognise tumor cells expressing MAGE-3. Eur J Immunol. 1994;24:3038–3043. doi: 10.1002/eji.1830241218. [DOI] [PubMed] [Google Scholar]
- 56.Visseren M J W, Vanelsas A, Vandervoort E I H, Ressing M E, Kast W M, Schrier P I, Melief C J M. CTL-specific for the tyrosinase auto-antigen can be induced from healthy donor blood to lyse melanoma cells. J Immunol. 1995;154:3991–3998. [PubMed] [Google Scholar]
- 57.Yang S X, Darrow T L, Vervaert C E, Seigler H F. Immunotherapeutic potential of tumor antigen-pulsed and unpulsed dendritic cells generated from murine bone marrow. Cell Immunol. 1997;179:84–95. doi: 10.1006/cimm.1997.1151. [DOI] [PubMed] [Google Scholar]
- 58.York I A, Rock K I. Antigen-processing and presentation by the class I major histocompatibility complex. Annu Rev Immunol. 1996;14:369–396. doi: 10.1146/annurev.immunol.14.1.369. [DOI] [PubMed] [Google Scholar]
- 59.Zhou L J, Schwarting R, Smith H M, Tedder T F. A novel cell surface molecule expressed by human interdigitating reticulum cells, Langerhans cells, and activated lymphocytes is a new member of the Ig superfamily. J Immunol. 1992;149:735–742. [PubMed] [Google Scholar]