Abstract
Astrocytes infected with human immunodeficiency virus type 1 (HIV-1) produce only minimal quantities of virus. The molecular events that limit acute-phase HIV-1 infection of astrocytes were examined after inducing acute-phase replication by transfection with the pNL4-3 proviral plasmid. The levels of HIV-1 mRNA were similarly high in both astrocytes and HeLa cells, but astrocytes produced approximately 50-fold less supernatant p24 than HeLa cells. We found that diminished HIV-1 production in astrocytes resulted from inefficient translation of gag, env, and nef mRNAs that were efficiently transported to the cytoplasm. Tat- or Rev-dependent reporter constructs showed no defect in Tat or Rev function in astrocytes compared with HeLa cells. HIV-1 mRNAs were correctly spliced, but only Rev and Tat proteins were efficiently translated from their native mRNAs. Pulse-chase labelling and immunoblot experiments revealed no defect in protein processing, but levels of Gag, Env, or Nef protein expressed were dramatically reduced in astrocytes compared to HeLa cells. These results demonstrate that inefficient translation of HIV-1 structural proteins underlies the restricted infection of astrocytes. The efficient expression of functional Tat and Rev by astrocytes may contribute to HIV-1 neuropathogenesis.
Human immunodeficiency virus type 1 (HIV-1) infection of the central nervous system occurs early in the course of HIV-1 disease. HIV-1 productively infects brain macrophages and microglial cells (3, 7, 18, 24, 43). A subpopulation of astrocytes is also consistently infected (3, 41, 43), but astrocyte infection yields little progeny virus (6, 14, 21, 25, 36, 49, 50). However, nonproductively infected astrocytes may be activated to produce virus that can be transferred to other susceptible cells (6, 19, 50). Thus, infected astrocytes might serve as a reservoir of HIV-1 in the brain and could be a sanctuary site for HIV-1 that might thwart virus eradication by current therapeutic regimens. Astrocytes are terminally differentiated in adult brain tissue and are crucial for neuronal function and survival. Therefore, elimination of HIV-1-infected astrocytes by therapeutic or immunological means would be detrimental. A preferable approach would permanently lock HIV-1 into a dormant state in astrocytes. However, to achieve this, the precise mechanisms that initiate and maintain the latent infection of astrocytes must be understood. Our present study examined the initial events during acute-phase viral replication that drive HIV-1 infection of astrocytes toward a dormant state.
Most previous studies on HIV-1 replication in astrocytes have focused on the transcriptional control of stably integrated HIV-1 in long-term- or latently infected cells. During this dormant phase that follows the initial infection, restricted expression of virus results from low-level basal long terminal repeat (LTR) activity which can be modestly induced by cytokines or other chemical stimuli (13, 32, 40, 42). In contrast, during acute-phase virus replication in astrocytes, low-level virus production appears to be controlled posttranscriptionally, since high levels of HIV-1 mRNA are synthesized after transfection with a proviral plasmid (19, 49). These observations suggest that there may be two phases of entry into the dormant state, one operating initially to suppress virion production despite high-level RNA synthesis and another that eventually suppresses RNA transcription. Transcriptional repression in long-term-infected cells is not unique to astrocytes (10, 16, 17), but the action of a novel central nervous system-derived molecule that promotes TAR-independent transcription in the presence of Tat indicates that unique transcriptional regulatory mechanisms may exist in astrocytes (28, 45–47, 53).
Earlier studies using a persistently HIV-1-infected astrocyte cell line, TH4-7-5, demonstrated that inefficient HIV-1 Rev function prevented the nucleocytoplasmic export and subsequent expression of gag and env RNAs bearing the Rev-responsive element (RRE) (31). Here, we examined acute-phase HIV-1 replication in astrocytes following transfection of an infectious molecular clone to examine whether an inactive Rev-RRE regulatory axis leads to the restricted expression of virus during the initial infection. We showed that astrocytes acutely infected by HIV-1 produce high levels of viral mRNA, which is correctly spliced and efficiently translocated to the cytoplasm. Efficient RNA expression in astrocytes is associated with normal expression and function of Rev and Tat proteins. However, the major HIV-1 structural and Nef proteins are expressed at low levels in astrocytes during the acute infection phase, despite high levels of available mRNA and the efficient translation of a coexpressed, non-HIV-1 reporter. We showed that diminished expression of these proteins in astrocytes results from an HIV-1-specific restriction in the translation of gag, env, and nef mRNAs to proteins. This severely reduces the synthesis of progeny virions and initiates the nonproductive or latent infection. The efficient translation of Tat and Rev proteins by HIV-1-infected astrocytes may contribute to AIDS neuropathogenesis.
MATERIALS AND METHODS
Cell culture.
The astrocytoma cell line U251MG (5) was obtained from J. Kort, Department of Medicine, Albany Medical College, Albany, N.Y., and was maintained in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% (vol/vol) fetal calf serum, penicillin (100 U/ml), streptomycin (100 μg/ml), and glutamine (25 μg/ml) (DMEM-10). Primary fetal astrocytes (PFA), obtained from fetal abortuses and judged to be >99% pure by positivity to the astrocyte-specific marker glial fibrillary acidic protein (GFAP), were cultured in DMEM-10 for up to 10 weeks postisolation. HeLa cells (39) were also cultured in DMEM-10.
Plasmids and cell transfection.
The HIV-1 proviral plasmids pNL4-3 (1) and pNL4-3Tat−, which has two premature stop codons introduced into the tat open reading frame (23), were obtained from M. Martin (Laboratory of Molecular Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Md.). pNL4-3Rev− contains an ACG point mutation at the initiating Met codon of the rev gene and was prepared via site-directed mutagenesis by using the pALTER system (Promega, Madison, Wis.). The Rev reporter plasmid, pDM128, expresses the chloramphenicol acetyltransferase (CAT) gene from an intron bearing the RRE in the presence of HIV-1 Rev protein (22). pEGFP-N1 (Clontech, Palo Alto, Calif.) produces green fluorescent protein (GFP) under the control of a cytomegalovirus promoter. pLTR-EGFP, in which the AseI-to-HindIII cytomegalovirus promoter fragment of pEGFP-N1 is replaced with the XhoI-to-HindIII 3′-LTR fragment from pNL4-3, expresses high levels of GFP in the presence of HIV-1 Tat. pRSV-Rev produces Rev protein under the direction of a Rous sarcoma virus promoter (22). In pCMV-hGH, the GFP fragment of pEGFP-N1 was replaced with the human growth hormone (hGH) gene. Unless indicated otherwise, cells were cotransfected with 20 μg of proviral plasmid plus 5 μg of GFP-expressing plasmid and 2 μg of pCMV-hGH plasmid by the calcium phosphate method as previously described (19).
Antibodies.
BB10 (a gift from E. Dax, National HIV Reference Laboratory, Fairfield, Victoria, Australia) is pooled human HIV-1-immune serum that recognizes the major HIV-1 structural proteins p24, p55, and gp120. Sheep anti-Nef15–27 (provided by A. Greenway) is an affinity-purified polyclonal antibody raised against a peptide corresponding to amino acids 15 to 27 of HIV-1NL Nef (20).
hGH and HIV-1 p24 assays.
HIV-1 p24 antigen production was measured by enzyme immunoassay in accordance with the manufacturer’s instructions (Organon Teknika, Durham, N.C.). hGH production was measured from 100 μl of culture supernatant by radioimmunoassay (Nichols Institute Diagnostics, San Juan Capistrano, Calif.).
Analysis of HIV-1 mRNA.
Total cell RNA was extracted by using TRIzol reagent in accordance with the manufacturer’s protocol (BRL Life Technologies, Gaithersburg, Md.). Cytoplasmic RNA extracts were prepared from 106 transfected cells that were washed three times in phosphate-buffered saline (PBS) and then hypotonically swollen in 3 ml of a solution consisting of 10 mM KCl, 1.5 mM MgCl2 and 10 mM HEPES (pH 7.9) for 10 min on ice. Cells were lysed in 200 μl of ice-cold cytoplasmic extraction buffer (150 mM NaCl; 10 mM Tris-HCl, pH 8.0; 2 mM MgCl2; 0.5% [vol/vol] Nonidet P-40 [NP-40]; 10 mM ribonucleoside complexes), and nondisruption of cell nuclei was confirmed by light microscopy. Cell lysates were vortexed for 10 s and centrifuged at 1,700 × g for 5 min to pellet intact nuclei. The supernatant was collected and centrifuged again to remove any residual nuclei. One hundred eighty microliters of supernatant was added to 1 ml of TRIzol reagent, the mixture was gently inverted, and RNA was purified as above. Northern blot detection of the three major HIV-1 mRNA classes in total-cell and cytoplasmic RNA extracts was performed with a 3′ HIV-1 LTR region PCR product labelled with [α-32P]dCTP as previously described (19).
First-strand cDNA was synthesized from total RNA extracts with avian myeloblastosis virus reverse transcriptase, using a cDNA cycle kit and an oligo(dT) primer (Invitrogen, Carlsbad, Calif.). PCR for spliced HIV-1 mRNA species was performed by a method described previously (34), with the following modifications: for PCR analysis of cDNA from the 4.0-kb mRNA class, the oligonucleotide primers Odp.045 (5′-CTGAGCCTGGGAGCTCTCTG-3′, positions 477 to 499 of HIV-1NL) and Odp.084 (5′-TCATTGCCACTGTCTTCTGCTCT-3′, positions 6202 to 6225 of HIV-1NL) were used. Amplification products were radiolabelled by performing a single round of PCR, replacing dCTP with 10 μCi of [α-32P]dCTP, and then analyzed by electrophoresis on a 6% polyacrylamide-urea gel and autoradiographed. Individual HIV-1 mRNA species were named according to the nomenclature of Purcell and Martin (34).
CAT assays.
Cells were trypsinized 72 h after cotransfection, washed twice in PBS, resuspended in 120 μl of 250 mM Tris-HCl (pH 7.8) with 0.5% (vol/vol) NP-40, and then subjected to three rapid freeze-rapid thaw cycles to induce lysis. The tubes were vortexed for 10 s between each freeze-thaw cycle. Lysates were centrifuged at 15,300 × g for 5 min at 4°C, supernatants were heat inactivated at 65°C for 10 min and then centrifuged at 15,300 × g for 5 min at 4°C, and the resultant supernatants were harvested for CAT assay. To control for transfection efficiency, the amount of cell lysate assayed was adjusted according to hGH production by dilution with 1 M Tris-HCl (pH 7.8), and then 25 μl of this supernatant was added to 25 μl of 1 M Tris-HCl (pH 7.8) and reacted with 10 μl of acetyl coenzyme A (Boehringer, Mannheim, Germany; 3.5 mg/ml) and 5 μl of d-threo[dichloracetyl-1,2-14C]chloramphenicol (NEN, Boston, Mass.; 57 mCi/mmol) at 37°C for 6 to 24 h, depending on the experiment. Incubation times were varied to measure CAT activity in the linear range (<30% conversion). Reaction mixtures were extracted with ethyl acetate and analyzed by thin-layer chromatography. Acetylation by CAT protein was assessed by autoradiography and quantified with a model FLA-2000 phosphorimager (Fuji, Tokyo, Japan).
Assessment of GFP fluorescence.
Cells transfected with pEGFP-N1 or pLTR-EGFP were fixed in 1% (vol/vol) formaldehyde (ultrapure electron microscopy grade; Polysciences, Warrington, Pa.) and analyzed for the percentage and mean fluorescence of GFP-expressing cells on a FACSCalibur flow cytometer (Becton Dickinson, San Jose, Calif.) at 48 or 72 h posttransfection. To account for both the number and intensity of fluorescent cells in experiments using the pLTR-EGFP reporter, the amount of fluorescence was determined from the fluorescence-activated cell sorter (FACS) profiles by multiplying the percentage of positive cells by the mean channel fluorescence.
Immunoblotting.
At 72 h after transfection, cells (106) were washed twice in PBS and resuspended in 200 μl of ice-cold lysis buffer (0.5% [vol/vol] NP-40; 0.5% [wt/vol] sodium deoxycholate; 50 mM NaCl; 25 mM Tris-HCl, pH 8.0; 10 mM EDTA; 5 mM benzamidine HCl; 10 mM phenylmethylsulfonyl fluoride) for 10 min, and then cellular debris was removed by centrifugation at 15,300 × g for 10 min. Lysate concentrations were standardized for transfection efficiency against hGH production, and 20 μl of lysate was added to 5 μl of 5× sodium dodecyl sulfate (SDS) gel loading buffer (10% [wt/vol] SDS; 500 mM dithiothreitol; 300 mM Tris-HCl, pH 6.8; 0.001% [wt/vol] bromophenol blue). Samples were boiled for 3 min and then electrophoresed on SDS–13% polyacrylamide gels, and proteins were transferred to Hybond-C nitrocellulose membranes (Amersham, Buckinghamshire, England). Nonspecific binding of membranes was blocked with Tris-buffered saline containing 3% (wt/vol) casein and 0.3% (vol/vol) Tween 20 for 16 h. After being washed four times with Tris-buffered saline containing 0.3% (vol/vol) Tween 20, the membranes were probed with either polyclonal HIV-1 antiserum BB10 (1:5,000) or sheep anti-Nef15–27 (1:500). After being subjected to four more washing steps as described above, the membrane was incubated with a horseradish peroxidase-conjugated antibody to human or sheep immunoglobulin G (1:5,000) for 1 h and then washed seven times as described above, and immunoreactive proteins were detected by enhanced chemiluminescence (ECL; Amersham).
Pulse-chase labelling and immunoprecipitation.
Cells were transfected with 20 μg of pNL4-3 and 5 μg pEGFP-N1 and cultured until 24 h before the peak of p24 production (48 h for HeLa cell transfectants or 72 h for U251MG transfectants). Transfected (or mock-transfected) cells (6 × 106) were washed twice in PBS and starved in 6 ml of DMEM lacking methionine and cysteine (ICN, Costa Mesa, Calif.) but containing 2% dialyzed fetal calf serum for 30 min at 37°C. Cells were pulse-labelled for 1 h by adding 500 μCi of 35S-labelled methionine-cysteine (NEN) after suspension in 600 μl of starvation medium. Labelled cells were washed twice in PBS, resuspended in 6 ml of DMEM-10 medium, and divided into six tubes (106 cells each). Cells were incubated at 37°C and harvested at 0, 1, 2, 3, 4, and 6 h post-pulse-labelling. Preparation of cell and virion lysates and immunoprecipitation of HIV-1 structural proteins with pooled HIV-1-immune serum were performed as previously described (51). Immunoprecipitation for Rev protein was performed with a sheep antiserum to glutathione S-transferase–Rev (ICN) from lysates of 6 × 106 transfected HeLa or U251MG cells that were radiolabelled with 500 μCi of 35S-labelled methionine-cysteine for 16 h.
RESULTS
Inefficient expression of p24 antigen in PFA and U251MG astrocytoma cells acutely replicating HIV-1.
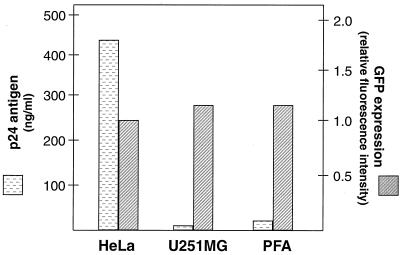
In vitro infection of astrocytes with cell-free HIV-1 is highly inefficient at initiating measurable virus replication. To ensure sufficient synchronous expression of HIV-1, we cotransfected either PFA or the U251MG astrocytoma cell line with the pNL4-3 HIV-1 proviral plasmid, as well as with pEGFP-N1 to monitor the transfection efficiency. When compared to control HeLa cell transfections, the primary astrocytes and U251MG astrocytoma cells synthesized 22- and 30-fold less p24 antigen, respectively, despite comparable GFP expression (Fig. 1). Hence, since HIV-1 production was similarly restricted in both primary and transformed astrocytes during acute-phase viral replication, the U251MG astrocytoma cell line was chosen to investigate the reduced expression of virus.
FIG. 1.
Restricted expression of HIV-1 p24 during acute-phase viral replication in primary and transformed astrocytes compared with HeLa cells. The expression of HIV-1 p24 or the GFP reporter is shown from HeLa, U251MG astrocytoma, or PFA cells that were cotransfected with the pNL4-3 proviral plasmid and pEGFP-N1. Cells and supernatant were tested at their peak of expression, 48 h for HeLa cells and 72 h for both astrocytic cell types.
Efficient transcription and nucleocytoplasmic transport of Rev-dependent HIV-1 mRNAs in astrocytes.
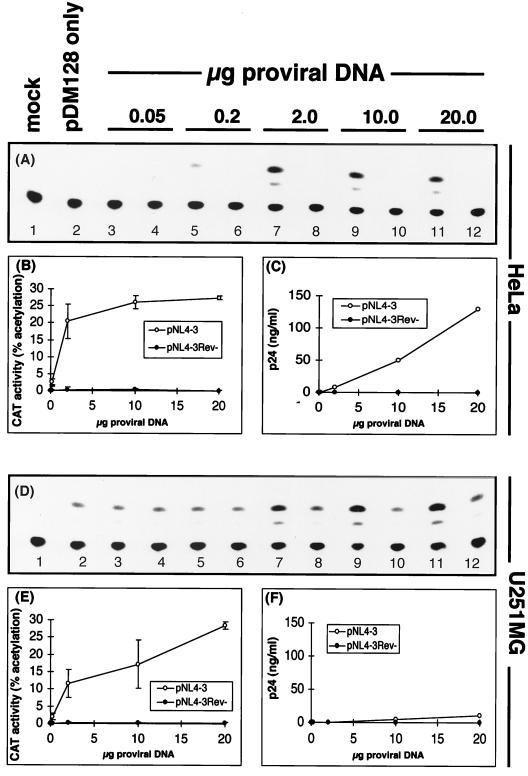
To study the molecular mechanisms restricting HIV-1 expression during an acute-phase infection, we first assessed the HIV-1 mRNAs by Northern blot analysis. HIV-1-transfected U251MG astrocytes and HeLa cells contained all three HIV-1 mRNA classes (Fig. 2A). The synthesis of 9-kb genomic and 4-kb env HIV-1 mRNAs was comparable in transfected astrocytes and HeLa cells. The multiply spliced 2-kb mRNA was expressed at even higher levels in astrocytes than in HeLa cells and represented a greater proportion of the total HIV-1 mRNA, as previously reported (19, 25, 49). We performed Northern blot experiments with astrocytes infected by cell-free HIV-1 in vitro but could not detect HIV-1 RNA (data not shown). Despite high levels of HIV-1 mRNA, the level of soluble p24 antigen detected in these transfected astrocytes (12 ng/ml) was severely reduced compared to the level in HeLa cells (540 ng/ml) in this experiment. We considered that restricted virus production in acutely infected astrocytes might result from a block in Rev-mediated nucleocytoplasmic transport of Rev-dependent mRNA as was previously shown for a persistently infected astrocyte cell line (31).
FIG. 2.
Efficient transcription and nucleocytoplasmic transport of HIV-1 mRNA in astrocytes. Northern blotting, using a 3′ HIV-1 LTR region probe designed to detect all HIV-1 mRNAs, was performed with total cellular RNA from HeLa (lanes 1 and 2) and U251MG cells (lanes 3 and 4) transfected with pNL4-3 (lanes 2 and 4) or mock transfected (lanes 1 and 3) (A) or with cytoplasmic (C) or total (T) RNA extracted from HeLa (lanes 1 to 4) and U251MG (lanes 5 to 8) cells transfected with pNL4-3 (lanes 3, 4, 7, and 8) or mock transfected (lanes 1, 2, 5, and 6) (B) or with cytoplasmic or total RNA extracted from HeLa (lanes 1 to 4) and U251MG (lanes 5 to 8) cells transfected with pNL4-3 Rev− (lanes 3, 4, 7, and 8) or mock transfected (lanes 1, 2, 5, and 6) (C).
Rev function in astrocytes transfected with pNL4-3 was first assessed from the cytoplasmic accumulation of Rev-dependent HIV-1 mRNA. Northern blot analyses comparing the relative ratios of HIV-1 mRNAs in total-cell and cytoplasmic extracts from transfected astrocytes and HeLa cells revealed no defect in the nucleocytoplasmic transport of Rev-dependent HIV-1 mRNAs in astrocytes (Fig. 2B). By densitometry, it was determined that astrocytes and HeLa cells translocated similar proportions of the 9-kb mRNA into the cytoplasm (Table 1), indicating that this function of Rev was not compromised in astrocyte transfections. Aside from this finding, densitometry also showed that the multiply spliced 2-kb mRNA accumulated in the cytoplasmic fraction with a fivefold higher efficiency in astrocytes (Fig. 2B, lanes 7 and 8). Transfection of astrocytes and HeLa cells with a Rev-defective provirus control, pNL4-3Rev−, resulted in the 9- and 4-kb Rev-responsive mRNAs being retained almost exclusively in the nucleus, with efficient cytoplasmic accumulation of 2-kb mRNA (Fig. 2C and Table 1).
TABLE 1.
Nuclear export of 9-kb Rev-dependent HIV-1 mRNA
| Cell line | Plasmid transfected | Cytoplasmic mRNA (% of total)a |
|---|---|---|
| HeLa | pNL4-3 | 26 |
| U251MG | pNL4-3 | 24 |
| HeLa | pNL4-3Rev− | 0.8 |
| U251MG | pNL4-3Rev− | 0.5 |
The percent accumulation of 9-kb HIV-1 mRNA detected in the cytoplasmic fraction relative to that in the total-cell RNA of astrocytes and HeLa cells transfected with pNL4-3 and pNL4-3Rev− (Fig. 2B and C) was determined from densitometric analysis of Northern blot pNL4-3Rev− assays.
HIV-1 mRNA is correctly spliced in astrocytes.
Since adequate levels of HIV-1 mRNA were available in transfected astrocytes for viral protein production, the pattern of alternatively spliced mRNA was assessed to determine whether the restriction in virus production occurred due to incorrect processing. The multiply spliced 2-kb mRNA species were examined by semiquantitative reverse transcription (RT)-PCR (Fig. 3A). The full array of multiply spliced mRNA species could be observed in both HeLa and U251MG astrocytoma cells transfected with the pNL4-3 proviral plasmid. A reduced synthesis of Vpr1 (1/3E/7) mRNA (34) by U251MG astrocytes was observed. Vpr expression is unlikely to account for the observed differences in viral production, since pNL4-3 wild type and Vpr mutants expressed similar levels of p24 and reverse transcriptase in HeLa cells. Similarly, cotransfection of Vpr expression constructs with pNL4-3 in U251MG astrocytes did not increase the level of p24 or reverse transcriptase (data not shown). For the 4-kb species of mRNA, the two cell types showed similar patterns of spliced RNA, with the primary env transcript, Env1 (1/5E), predominating (Fig. 3B). Thus, RNA was spliced similarly in HeLa and U251MG astrocytoma cells, and RNA splicing does not account for the differences in the expression of HIV-1 particles from these two cell types.
FIG. 3.
Astrocytes correctly splice HIV-1 mRNAs. RT-PCR analysis of multiply spliced 2.0-kb (A) and singly spliced 4.0-kb (B) HIV-1 mRNAs from total-cell RNA extracted from HeLa (lane 1) or U251MG (lane 2) cells transfected with pNL4-3. cDNAs were amplified by PCR, labelled, and analyzed on a 6% acrylamide-urea gel. Nonspecific amplification was not observed in reactions lacking reverse transcriptase (data not shown).
Rev protein is expressed and functional in astrocytes.
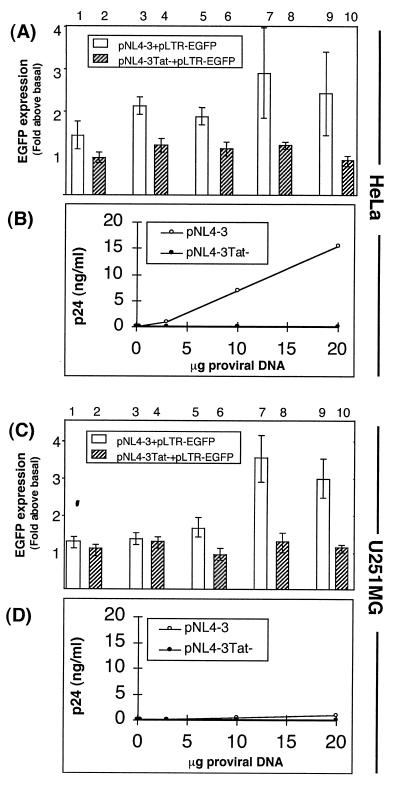
The pDM128 reporter plasmid requires Rev for the expression of CAT (22). Cotransfection of pDM128 with a control Rev expression plasmid, pRSV-Rev, showed comparable Rev function in the U251MG astrocytoma and HeLa cell lines (Fig. 4). Increased expression of Rev from pRSV-Rev in HeLa cells led to dose-dependent CAT activity, indicating increasing Rev function (Fig. 4A and B). A very similar dose dependency was observed following cotransfection of pRSV-Rev in the U251MG astrocytoma cells (Fig. 4C and D). Rev-dependent regulation of pDM128 expression was not as stringent in U251MG astrocytes as in HeLa cells, since low-level acetylation was observed in astrocytes when pDM128 was transfected without Rev (Fig. 4C, lane 2). Thus, Rev protein functions efficiently in U251MG astrocytes when expressed from a Rev expression plasmid.
FIG. 4.
Rev-dependent expression of a CAT reporter in astrocytes. Acetylation of chloramphenicol was measured in extracts of HeLa (A and B) or U251MG (C and D) cells that were transfected with 5 μg of the Rev-dependent CAT reporter pDM128 alone (lane 2) or together with increasing amounts of Rev from the pRSV-Rev expression plasmid (0.05, 0.2, 2.0, or 5.0 μg [lanes 3, 4, 5, and 6, respectively]). Cells were cotransfected with 2 μg of pCMV-hGH to control for transfection efficiency. Mock transfections received pCMV-hGH alone (lane 1). CAT assays were performed on cell lysates that were normalized for hGH production. Acetylation was quantitated by phosphorimaging, with basal acetylation defined as CAT activity by pDM128 without Rev (lane 2), and means and standard errors of the means of the CAT activity for data from three experiments were determined after subtracting basal acetylation (B and D).
To measure Rev expression from the native rev mRNA, the pDM128 plasmid was cotransfected with increasing amounts of the pNL4-3 proviral plasmid or a Rev-defective control, pNL4-3Rev−. Again, similar Rev activities were observed in U251MG astrocytoma and HeLa cells (Fig. 5). No CAT activity was observed when the pNL4-3Rev− proviral plasmid was transfected into HeLa cells (Fig. 5A), but low-level acetylation similar to background levels was observed when that plasmid was transfected into U251MG astrocytoma cells (Fig. 5D). Despite finding similar Rev activities in the two cell types, p24 antigen was expressed in amounts up to 27-fold higher in HeLa cells (compare Fig. 5C and F). These results indicate that expression of functional Rev protein from the native mRNA, but not p24, is equally efficient in U251MG astrocytes and HeLa cells. Thus, Rev function does not restrict the acute-phase replication of HIV-1 in astrocytes as demonstrated by the limited expression of p24.
FIG. 5.
Efficient expression of Rev from the native rev mRNA in astrocytes. Acetylation of chloramphenicol was measured in extracts of HeLa (A and B) or U251MG (D and E) cells that were transfected with 5 μg of pDM128 without HIV-1 provirus (lane 2) or together with increasing amounts of pNL4-3 (lanes 3, 5, 7, 9, and 11) or pNL4-3Rev− (lanes 4, 6, 8, 10, and 12). Cells were transfected with 2 μg of pCMV-hGH to control for transfection efficiency. Mock transfections received pCMV-hGH alone (lane 1). CAT assays were performed on cell lysates that were normalized for hGH production. Acetylation was quantitated by phosphorimaging, with basal acetylation defined as CAT activity by pDM128 without Rev (lane 2), and means and standard errors of the means of the CAT activity for data from three experiments were determined after subtracting basal acetylation (B and E). Soluble p24 antigen was measured in culture supernatant samples collected prior to cell harvesting and normalized for hGH production (C and F).
Efficient Tat expression and function in astrocytes.
The Tat-dependent reporter plasmid pLTR-EGFP was used to measure Tat function by fluorescence via flow cytometry. When Tat was expressed from the pNL4-3 proviral plasmid, similar Tat activities were measured in HeLa and U251MG cells (Fig. 6). Basal LTR activity was obtained when a Tat-mutated proviral plasmid, pNL4-3Tat−, was used. Despite having similar Tat function, the synthesis of p24 antigen was up to 23-fold higher in HeLa cell supernatants that produced equivalent levels of hGH (compare Fig. 6B and D). This shows that restricted virus production in U251MG cells was not due to inefficient Tat protein function. These results indicate that Tat protein is efficiently synthesized from its native mRNA in U251MG astrocytes and is fully functional for transactivating transcription from the HIV-1 LTR.
FIG. 6.
Efficient expression of Tat from the native tat mRNA in astrocytes. The fluorescence of cells expressing GFP was measured by flow cytometry of HeLa cells (A) or U251MG cells (C) that were transfected with 5 μg of pLTR-EGFP together with increasing amounts of pNL4-3 (0.05, 0.2, 2.0, 10.0, or 20.0 μg [lanes 1, 3, 5, 7, and 9, respectively]) or pNL4-3Tat− (0.05, 0.2, 2.0, 10.0, or 20.0 μg [lanes 2, 4, 6, 8, and 10, respectively]). Transfections included 2 μg of pCMV-hGH to control for transfection efficiency. Fluorescence (see Materials and Methods) was expressed as the ratio of basal fluorescence obtained from transfections of pLTR-EGFP without Tat and normalized for hGH production. Shown are mean values and standard errors of the means of data from four experiments. Soluble p24 antigen was measured in HeLa (B) or U251MG (D) culture supernatant samples collected prior to cell harvesting and normalized for hGH production.
The restricted acute-phase infection of astrocytes results from a specific downmodulation in the translation of HIV-1 structural and Nef proteins.
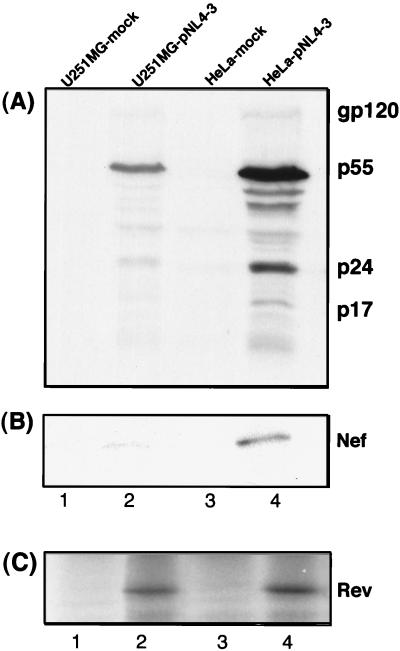
The preceding experiments showed that astrocytes express and process HIV-1 RNA in the same way as HeLa cells. We therefore measured the production of HIV-1 proteins after transfection with pNL4-3, using immunoblotting or pulse-chase labelling and immunoprecipitation. Immunoblotting revealed very low-level synthesis of HIV-1 proteins in pNL4-3-transfected astrocytes compared to HeLa cells (Fig. 7A). Similarly, there were fivefold lower levels of Nef in astrocytes than in HeLa cells (Fig. 7B), despite the existence of fivefold higher levels of nef mRNA (Fig. 2A and 3A). The ratio of intracellular p24 (measured by enzyme-linked immunosorbent assay of cell lysates) to hGH (measured from transfected-cell supernatant) in astrocytes (0.073 ng/ng of hGH) was 15-fold lower than that in HeLa cells (1.1 ng/ng of hGH). In contrast, Rev was expressed with similar efficiencies by HeLa and U251MG astrocytes, since equivalent amounts of Rev were immunoprecipitated from the respective cell lysates after normalization for trichloroacetic acid (TCA)-precipitable counts (Fig. 7C, compare lanes 2 and 4).
FIG. 7.
Low levels of HIV-1 structural and Nef protein expression compared to Rev protein expression by astrocytes. Immunoblotting or immunoprecipitation of viral antigens expressed in U251MG (lanes 1 and 2) and HeLa (lanes 3 and 4) cells after cotransfection with the HIV-1 proviral plasmid pNL4-3 and pCMV-hGH (lanes 2 and 4) or pCMV-hGH transfection efficiency reporter alone (lanes 1 and 3). Cell lysates were normalized for hGH production, and viral proteins were detected with polyclonal HIV-immune serum (BB10; 1:5,000) (A) or anti-Nef15–27 (1:500) (B). (C) Rev protein detected by immunoprecipitation with sheep antiserum to HIV-1 Rev (1:500) from HeLa and U251MG cells labelled with [35S]methionine-cysteine for 16 h, 48 and 72 h after transfection, respectively.
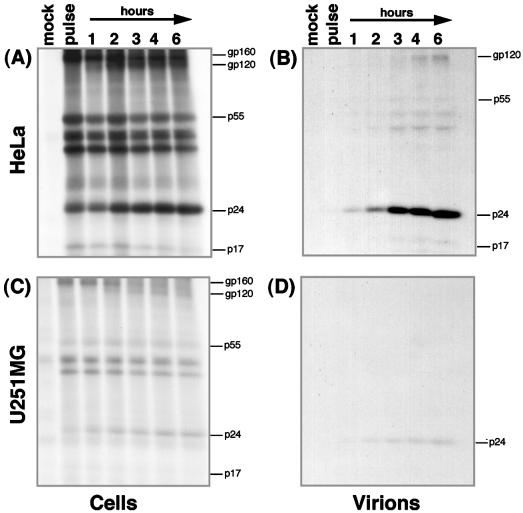
To determine whether the reduced levels of HIV-1 structural proteins expressed by astrocytes resulted from abnormal protein processing or from a specific block in translation of these mRNAs, pulse-chase labelling and immunoprecipitation were performed with equal numbers of U251MG and HeLa cells previously cotransfected with pEGFP-N1 and pNL4-3 plasmids. Approximately 10% more U251MG cells were transfected than HeLa cells, but the two cell types expressed GFP with equivalent mean intensities (Fig. 8). Despite a higher overall level of expression of GFP by astrocytes, they synthesized 25-fold less pulse-labelled HIV-1 protein than did HeLa cells after normalization for TCA-precipitable counts in the respective cell lysates (compare Fig. 9A and C). This indicates that astrocytes have a specific block in expression of HIV-1 structural proteins. However, HIV-1 Gag and Env protein processing was identical in the two cell types, because the processing of Env precursor gp160 to gp120-SU and gp41-TM and that of Gag Pr55 to p24-CA and p17-MA were comparable at all chase time points in lysates from both cell types (Fig. 9A and C) and their progeny virions (Fig. 9B and D).
FIG. 8.
Efficient translation of a non-HIV-1 fluorescence reporter by astrocytes. Flow cytometry profiles showing the transfection-translation efficiency of GFP (light shading) by U251MG astrocytes (A and C) and HeLa cells (B and D) transfected with 5 μg of pEGFP-N1 alone (A and B) or together with 20 μg of the HIV-1 proviral plasmid pNL4-3 (C and D). FACS analysis was performed at the peak of expression posttransfection (72 h for U251MG and 48 h for HeLa cells). Mock transfections (dark shading) contained either no DNA (A and B) or pNL4-3 alone (C and D). The region R1 shows the percentage and the mean channel fluorescence (MCF) of cells expressing GFP above the mock-transfection values.
FIG. 9.
Inefficient translation but correct processing of HIV-1 structural proteins by astrocytes. HeLa (A) and U251MG (C) cells were pulse-labelled with [35S]methionine for 1 h at 48 h (HeLa) or 72 h (U251MG) after cotransfection with pEGFP-N1 alone (mock) or together with 20 μg of HIV-1 proviral plasmid pNL4-3 (pulse). Labelled cells were then incubated at 37°C for 1, 2, 3, 4, and 6 h in complete medium, and HIV-1 proteins were detected by immunoprecipitation from cell lysate volumes (standardized by total TCA-precipitable 35S-labelled proteins) with pooled HIV-1 immune serum (BB10). Virions pelleted from the supernatant of HeLa (B) or U251MG (D) transfections were lysed and subjected to immunoprecipitation for analysis of HIV-1 structural proteins.
Thus, the restricted virus production from astrocytes acutely replicating HIV-1 results from inefficient translation of structural protein from high levels of correctly processed HIV-1 mRNA.
DISCUSSION
We examined the mechanisms restricting acute-phase HIV-1 production in astrocytes by using a transfection system to optimize expression during early viral replication. Despite normal transcription, splicing, and transport of HIV-1 mRNA, immunoblot and radioimmunoprecipitation experiments showed that the translation of a subgroup of HIV-1 mRNAs is highly inefficient during acute-phase viral replication in the U251MG astrocyte cell line. The cytoplasmic gag, env, and nef RNAs were poor substrates for protein expression, producing 25-fold less protein in astrocytes than in HeLa cells. Pulse-chase labelling showed that reduced accumulation of HIV-1 structural proteins during acute-phase viral replication was due to their inefficient synthesis in the astrocyte cell line and was not due to any abnormal processing or targeted degradation of these proteins.
All HIV-1 mRNA classes were expressed at high levels in U251MG cells (Fig. 2A) and were correctly spliced (Fig. 3) but yielded 50-fold lower p24 levels than HeLa cells expressing comparable levels of HIV-1 mRNA. Previous studies of a persistently HIV-1-infected astrocyte cell line, TH4-7-5, demonstrated that in that model, restricted infection resulted from an astrocyte-specific block in HIV-1 Rev function, causing inefficient nucleocytoplasmic transport of unspliced and singly spliced HIV-1 mRNA species (31). In our acute-phase replication model, this mechanism did not account for the reduced expression of virus because the Rev-dependent mRNA classes were readily detected in cytoplasmic RNA extracts of astrocytes (Fig. 2B), showing that Rev-dependent mRNAs were efficiently translocated to the cytoplasm. Control transfections with a Rev-deficient proviral plasmid, pNL4-3Rev−, demonstrated almost complete nuclear retention of Rev-dependent mRNA species in astrocytes (Fig. 2C), confirming that Rev is required in U251MG astrocytes for cytoplasmic translocation of HIV-1 RNAs containing the RRE.
Further confirmation of competent Rev expression and function in astrocytes was obtained biochemically by immunoprecipitation and functionally by using a Rev-dependent CAT reporter plasmid. Rev expressed from either the native rev RNA or a Rous sarcoma virus-Rev expression plasmid confirmed that Rev is translated and functions with equivalent efficiency in U251MG astrocytes and HeLa cells. An earlier study of the persistently infected TH4-7-5 cell line that attributed the low production of virus to a defect in Rev function used a Rev-dependent reporter that expressed Gag (31). Our studies clearly show that translation of Gag protein is inefficient in U251MG astrocytes and raise the possibility that the Gag reporter for Rev function used in those studies might also be restricted for Gag translation. Our use of the pDM128 Rev reporter that expresses CAT protein might explain the differences in Rev function determined in our study and in that of Neumann et al. (31). Thus, we conclude that expression and function of Rev during acute-phase replication of HIV-1 in U251MG astrocytes appear to be typical.
Latent HIV-1 infection of the T-cell line ACH2 and a promyelomonocytic cell line, U1, results from mutations in Tat or its RNA binding site, TAR, respectively (2, 8, 12). For astrocytes, several studies have demonstrated efficient Tat transactivation of the HIV-1 LTR in the absence of TAR (42, 44–47), demonstrating a unique transcriptional control mechanism in astrocytes. Our results showed efficient expression of functional Tat from the native tat mRNA, confirming earlier studies using other Tat expression constructs. This suggests that the early events leading to nonproductive HIV-1 infection of astrocytes involve suppressed translation of HIV-1 structural proteins, as opposed to the transcriptional repression that underpins the latent infection of T cells and monocytes/macrophages. The suppressed translation of HIV-1 structural proteins in the face of efficient HIV-1 mRNA transcription may explain the discrepancies in measurements of the numbers of HIV-1-infected astrocytes in vivo. Measurements of HIV-1 structural proteins may significantly underrepresent the distribution and transcriptional activity of HIV-1-infected astrocytes in vivo compared to RNA or DNA detection (3, 7, 18, 41, 43). Furthermore, efficient expression of the neurotoxic Tat protein by astrocytes in vivo may contribute to neuropathogenesis in AIDS patients (30, 37).
Our studies showed that Nef protein is expressed at fivefold lower levels in U251MG cells than in HeLa cells, despite the fivefold higher level of nef mRNA synthesis in the astrocytes. This reduced Nef synthesis contrasts with the high levels measured in the TH4-7-5 cell line that was selected for Nef expression (6) and may indicate that changes in the native nef mRNA alleviate the inefficient translation. The high-level expression of Nef in postmortem astrocytes from pediatric and adult AIDS patients (35, 38, 48) might demonstrate the existence of in vivo mechanisms that overcome the inefficient translation of Nef, possibly modulated by local cytokines. However, the suppressed translation of nef mRNA observed in our study is in agreement with other reports showing low-level Nef synthesis that could be relieved by coculture with monocytes/macrophages (15) or tumor necrosis factor alpha stimulation (49).
The mechanism underlying the inefficient translation of the gag, env, and nef mRNAs is unclear. The strength of the Kozak signal surrounding the initiating AUG codon does not correlate with the translation efficiency of these mRNAs, because HIV-1NL tat, gag, env, and nef mRNAs all have strong Kozak signals whereas rev mRNA has a weak Kozak signal (26, 27). The translational control mechanism accounting for our results must accommodate (i) the selective repression of HIV-1 structural protein synthesis over that of the other reporter proteins used here (hGH and GFP), (ii) suppression mediated by 5′ HIV-1 noncoding RNA proximal to TAR, and (iii) a relief of translational suppression for tat and rev mRNAs that is mediated by a short stretch of unique 5′ noncoding RNA.
Several reports have shown that the translation of HIV-1 proteins may be downmodulated by the alpha-interferon-inducible double-stranded RNA-activated protein kinase R (PKR) (4, 33). It is possible that astrocytes exhibit high-level constitutive expression of activated PKR that might inhibit engagement of initiator tRNAMet at the 7-methyl-Gppp cap structure at the 5′ end of the RNA (reviewed by Clemens [9]). However, PKR-mediated translational control has not been reported to discriminate between mRNAs translated by typical ribosome scanning. The existence of polypyrimidine tracts proximal to the initiating AUG of tat and rev mRNAs (34) raises the possibility that the translation of these mRNAs escapes a cap-dependent translational suppression imposed on the other HIV-1 RNAs by direct interactions of the 40S ribosomal subunit with polypyrimidine tract binding proteins, in a manner resembling the activity of the internal ribosome entry sites of some viruses, e.g., picornavirus (11).
The efficient expression of Tat protein in astrocytes raises the possibility that this viral protein contributes to the translation control observed in these cells. Recently, the second coding exon of HIV-1 Tat was shown to directly interact with a protein of the human translation machinery, translation elongation factor 1 delta (52). This interaction caused downmodulated translation of CAT protein in vitro and of CD4 protein in transfected HeLa cells. Interestingly, unlike CD4 cells, the translation of HIV-1 structural proteins was not diminished in HeLA cells cotransfected with CD4 and HIV-1 proviral plasmids (52). This discriminatory modulation of translation in HeLa cells mediated by the second coding exon of Tat raises the possibility that efficient expression of HIV-1 Tat in astrocytes contributes to the observed downmodulated translation of Gag, Env, and Nef. Another role for Tat might result from the reported direct interaction of the first coding exon of Tat (Tat1–72) with PKR causing an inhibition of autophosphorylation and activation of PKR (29). In addition, the second coding exon of Tat (Tat73–86) is itself a substrate for phosphorylation by PKR (29). An accumulation of Tat in astrocytes might result in interactions with PKR that preferentially restrict translation of HIV-1 structural proteins. Further experiments to examine the translational control mechanisms of astrocytes that cause the diminished expression of HIV-1 are under way. What is clear from our present results is that the translational control mechanism of astrocytes discriminates between two subgroups of HIV-1 mRNAs.
With regard to identifying the relevant differences between the HIV-1 mRNAs, it is of interest that the pDM128 reporter, which has the CAT gene inserted at the translation initiation site for Env, efficiently expresses CAT protein in astrocytes, whereas Env is inefficiently expressed from the native env mRNA. The significant differences in the 5′ untranslated regions (UTRs) of the pDM128 and the native env mRNAs (22) provide insight into the RNA elements that might increase the translation efficiency of the CAT mRNA as well as rev and tat mRNA. The 5′ end of the pDM128 mRNA has up to 428 bases of the 5′ UTR from simian virus 40 large T antigen joined to the bulge of the HIV-1 TAR region. The HIV-1 sequence extends to the end of the U5 region but excludes 102 bases upstream of the major 5′ splice site and includes noncoding mRNA 3′ of the seventh codon of Tat. In pDM128, the start site for Rev has been mutated and the CAT gene has been inserted at the Env initiation codon. Thus, pDM128 includes up to 125 extra bases of the 5′ UTRs from rev and tat mRNAs that are not in the predominant env mRNA. Thus, unique RNA elements from the rev and tat mRNAs, such as the polypyrimidine tracts of the splice acceptor sites for rev and env, are included into the 5′ UTR of pDM128. These RNA elements are spliced from the native env and nef mRNAs and might confer a more-efficient translation to some mRNAs in astrocytes. However, translation modulation by sequences in the Env coding frame cannot be excluded.
In the adult brain, astrocytes are unrenewable and crucial for neurological function. The elimination of astrocytes latently infected by HIV-1 may be neurologically detrimental. However, this dormant infection provides a sanctuary for HIV-1 that may thwart viral eradication and serve as a source for renewed systemic infection. Inducing permanent viral dormancy may be a strategy to control HIV-1. Our results show that the dormant infection of astrocytes originates from a block in the translation of gag, env, and nef mRNAs. Translation of the rev and tat mRNAs was unaffected. Understanding the mechanisms modulating the translation of HIV-1 structural proteins may identify strategies to sustain the suppression of HIV-1 in viral sanctuaries such as brain astrocytes.
ACKNOWLEDGMENTS
This work was supported by the National Health and Medical Research Council (D.F.J.P., J.L.H., and J.L.A.; Reg Key 970558), the National Centre for HIV Virology Research (D.A.M., M.J.C., A.C., and D.A.), RMIT University (P.R.G.), and the Research Fund of the Macfarlane Burnet Centre for Medical Research (P.R.G., J.L.H., M.J.C., J.L.A., D.A.M., and D.F.J.P.).
We thank J. Mills, A. Jaworowski, and D. Gabuzda for review of the manuscript, G. Paukovics and A. Meikle (Flow Cytometry Unit, Macfarlane Burnet Centre for Medical Research) for FACS analysis, and B. Rumble (Department of Medical Laboratory Science, RMIT University) for advice and support in this project.
REFERENCES
- 1.Adachi A, Gendelman H E, Koenig S, Folks T, Willey R, Rabson A, Martin M A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986;59:284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams M, Sharmeen L, Kimpton J, Romeo J M, Garcia J V, Peterlin B M, Groudine M, Emerman M. Cellular latency in human immunodeficiency virus-infected individuals with high CD4 levels can be detected by the presence of promoter-proximal transcripts. Proc Natl Acad Sci USA. 1994;91:3862–3866. doi: 10.1073/pnas.91.9.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagasra O, Lavi E, Bobroski L, Khalili K, Pestaner J P, Tawadros R, Pomerantz R J. Cellular reservoirs of HIV-1 in the central nervous system of infected individuals: identification by the combination of in situ polymerase chain reaction and immunohistochemistry. AIDS. 1996;10:573–585. doi: 10.1097/00002030-199606000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Benkirane M, Neuveut C, Chun R F, Smith S M, Samuel C E, Gatignol A, Jeang K-T. Oncogenic potential of TAR RNA binding protein TRBP and its regulatory interaction with RNA-dependent protein kinase PKR. EMBO J. 1997;16:611–624. doi: 10.1093/emboj/16.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bigner D D, Bigner S H, Ponten J, Westermark B, Mahaley M S, Ruoslahti E, Herschman H, Eng L F, Wilkstrand C J. Heterogeneity of genotypic and phenotypic characteristics of fifteen permanent cell lines derived from human gliomas. J Neuropathol Exp Neurol. 1981;40:201–229. doi: 10.1097/00005072-198105000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Brack-Werner R, Kleinschmidt A, Ludvigsen A, Mellert W, Neumann M, Herrmann R, Khim M C L, Burny A, Muller-Lantzsch N, Stavrou D, Erfle V. Infection of human brain cells by HIV-1: restricted virus production in chronically infected human glial cell lines. AIDS. 1992;6:273–285. [PubMed] [Google Scholar]
- 7.Brew B J, Rosenblum M, Cronin K, Price R W. AIDS dementia complex and HIV-1 brain infection: clinical-virological correlations. Ann Neurol. 1995;38:563–570. doi: 10.1002/ana.410380404. [DOI] [PubMed] [Google Scholar]
- 8.Cannon P, Kim S-H, Ulich C, Kim S. Analysis of Tat function in human immunodeficiency virus type 1-infected low-level-expression cell lines U1 and ACH-2. J Virol. 1994;68:1993–1997. doi: 10.1128/jvi.68.3.1993-1997.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clemens M J. Protein kinases that phosphorylate eIF2 and eIF2B, and their role in eukaryotic cell translational control. In: Hershey J W B, Mathews M B, Sonenberg N, editors. Translational control. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 139–172. [Google Scholar]
- 10.Clouse K A, Powell D, Washington I, Poli G, Strebel K, Farrar W, Barstad P, Kovacs J, Fauci A S, Folks T M. Monokine regulation of human immunodeficiency virus-1 expression in a chronically infected T cell clone. J Immunol. 1989;142:431–438. [PubMed] [Google Scholar]
- 11.Ehrenfeld E. Initiation of translation by picornavirus RNAs. In: Hershey J W B, Mathews M B, Sonenberg N, editors. Translational control. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 549–573. [Google Scholar]
- 12.Emiliani S, Van Lint C, Fischle W, Paris P, Jr, Ott M, Brady J, Verdin E. A point mutation in the HIV-1 Tat responsive element is associated with postintegration latency. Proc Natl Acad Sci USA. 1996;93:6377–6381. doi: 10.1073/pnas.93.13.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ensoli F, Wang H, Fiorelli V, Zeichner S L, De Cristofaro M R, Luzi G, Thiele C J. HIV-1 infection and the developing nervous system: lineage-specific regulation of viral gene expression and replication in distinct neuronal precursors. J Neurovirol. 1997;3:290–298. doi: 10.3109/13550289709029470. [DOI] [PubMed] [Google Scholar]
- 14.Erfle V, Stoeckbauer P, Kleinschmidt A, Kohleisen B, Mellert W, Stavrou D, Brack-Werner R. Target cells for HIV in the central nervous system: macrophages or glial cells? Res Virol. 1991;142:139–144. doi: 10.1016/0923-2516(91)90050-d. [DOI] [PubMed] [Google Scholar]
- 15.Fiala M, Rhodes R H, Shapshak P, Nagano I, Martinez-Maza O, Diagne A, Baldwin G, Graves M. Regulation of HIV-1 infection in astrocytes: expression of Nef, TNF-α and IL-6 is enhanced in coculture of astrocytes with macrophages. J Neurovirol. 1996;2:158–166. doi: 10.3109/13550289609146878. [DOI] [PubMed] [Google Scholar]
- 16.Folks T, Powell D M, Lightfoote M M, Benn S, Martin M A, Fauci A S. Induction of HTLV-III/LAV from a nonvirus-producing T-cell line: implications for latency. Science. 1986;231:600–602. doi: 10.1126/science.3003906. [DOI] [PubMed] [Google Scholar]
- 17.Folks T M, Justement J, Kinter A, Dinarello C A, Fauci A S. Cytokine-induced expression of HIV-1 in a chronically infected promonocyte cell line. Science. 1987;238:800–802. doi: 10.1126/science.3313729. [DOI] [PubMed] [Google Scholar]
- 18.Glass J D, Fedor H, Wesselingh S L, McArthur J C. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann Neurol. 1995;38:755–762. doi: 10.1002/ana.410380510. [DOI] [PubMed] [Google Scholar]
- 19.Gorry P, Purcell D, Howard J, McPhee D. Restricted HIV-1 infection of human astrocytes: potential role of nef in the regulation of virus replication. J Neurovirol. 1998;4:377–386. doi: 10.3109/13550289809114536. [DOI] [PubMed] [Google Scholar]
- 20.Greenway A L, McPhee D A, Grgacic E, Hewish D, Lucantoni A, Macreadie I, Azad A. Nef 27, but not Nef 25 isoform of human immunodeficiency virus-type 1 pNL4.3 down-regulates surface CD4 and IL-2R expression in peripheral blood mononuclear cells and transformed T cells. Virology. 1994;198:245–256. doi: 10.1006/viro.1994.1027. [DOI] [PubMed] [Google Scholar]
- 21.Hatch W C, Pousada E, Losev L, Rashbaum W K, Lyman W D. Neural cell targets of human immunodeficiency virus type 1 in human fetal organotypic cultures. AIDS Res Hum Retroviruses. 1994;10:1597–1607. doi: 10.1089/aid.1994.10.1597. [DOI] [PubMed] [Google Scholar]
- 22.Hope T J, Huang X, McDonald D, Parslow T G. Steroid-receptor fusion of the human immunodeficiency virus type 1 Rev transactivator: mapping cryptic functions of the arginine-rich motif. Proc Natl Acad Sci USA. 1990;87:7787–7791. doi: 10.1073/pnas.87.19.7787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang L, Joshi A, Willey R, Ornstein J, Jeang K T. Human immunodeficiency viruses regulated by alternative trans-activators: genetic evidence for a novel non-transcriptional function of Tat in virion infectivity. EMBO J. 1994;13:2886–2896. doi: 10.1002/j.1460-2075.1994.tb06583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson R T, Glass J D, McArthur J C, Chesebro B W. Quantitation of human immunodeficiency virus in brains of demented and nondemented patients with acquired immunodeficiency syndrome. Ann Neurol. 1996;39:392–395. doi: 10.1002/ana.410390319. [DOI] [PubMed] [Google Scholar]
- 25.Kleinschmidt A, Neumann M, Moller C, Erfle V, Brack-Werner R. Restricted expression of HIV-1 in human astrocytes: molecular basis for viral persistence in the CNS. Res Virol. 1994;145:147–153. doi: 10.1016/s0923-2516(07)80016-1. [DOI] [PubMed] [Google Scholar]
- 26.Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986;44:283–293. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- 27.Kozak M. The scanning model for translation: an update. J Cell Biol. 1989;108:229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurth J, Buzy J M, Lindstrom L, Clements J E. In vivo transcriptional regulation of the human immunodeficiency virus in the central nervous system in transgenic mice. J Virol. 1996;70:7686–7694. doi: 10.1128/jvi.70.11.7686-7694.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McMillan N A, Chun R F, Siderovski D P, Galabru J, Toone W M, Samuel C E, Mak T W, Hovanessian A G, Jeang K T, Williams B R. HIV-1 Tat directly interacts with the interferon-induced, double-stranded RNA-dependent kinase, PKR. Virology. 1995;213:413–424. doi: 10.1006/viro.1995.0014. [DOI] [PubMed] [Google Scholar]
- 30.Nath A, Psooy K, Martin C, Knudsen B, Magnuson D S K, Haughey N, Geiger J D. Identification of a human immunodeficiency virus type 1 Tat epitope that is neuroexcitatory and neurotoxic. J Virol. 1996;70:1475–1480. doi: 10.1128/jvi.70.3.1475-1480.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neumann M, Felber B K, Kleinschmidt A, Froese B, Erfle V, Pavlakis G N, Brack-Werner R. Restriction of human immunodeficiency virus type 1 production in a human astrocytoma cell line is associated with a cellular block in Rev function. J Virol. 1995;69:2159–2167. doi: 10.1128/jvi.69.4.2159-2167.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niikura M, Dornadula G, Zhang H, Mukhtar M, Lingxun D, Khalili K, Bagasra O, Pomerantz R. Mechanisms of transcriptional transactivation and restriction of human immunodeficiency virus type 1 replication in an astrocytic glial cell. Oncogene. 1996;13:313–322. [PubMed] [Google Scholar]
- 33.Park H, Davies M V, Langland J O, Chang H-W, Nam Y-S, Tartaglia J, Paoletti E, Jacobs B L, Kaufman R J, Venkatesan S. TAR RNA-binding protein is an inhibitor of the interferon induced protein kinase, PKR. Proc Natl Acad Sci USA. 1994;91:4713–4717. doi: 10.1073/pnas.91.11.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Purcell D F J, Martin M A. Alternative splicing of human immunodeficiency virus type 1 mRNA modulates viral protein expression, replication, and infectivity. J Virol. 1993;67:6365–6378. doi: 10.1128/jvi.67.11.6365-6378.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ranki A, Nyberg M, Ovod V, Haltia M, Elovaara I, Raininko R, Haapaasalo H, Krohn K. Abundant expression of HIV Nef and Rev proteins in brain astrocytes in vivo is associated with dementia. AIDS. 1995;9:1001–1008. doi: 10.1097/00002030-199509000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Rytik P G, Eremin V F, Kvacheva Z B, Poleschuk N N, Popov S A, Schroder H C, Bachmann M, Weiler B E, Muller W E G. Susceptibility of primary human glial fibrillary acidic protein-positive brain cells to human immunodeficiency virus infection in vitro: anti-HIV activity of memantine. AIDS Res Hum Retroviruses. 1991;7:89–95. doi: 10.1089/aid.1991.7.89. [DOI] [PubMed] [Google Scholar]
- 37.Sabatier J-M, Vives E, Mabrouk K, Benjouad A, Rochat H, Duval A, Hue B, Bahraoui E. Evidence for neurotoxic activity of tat from human immunodeficiency virus type 1. J Virol. 1991;65:961–967. doi: 10.1128/jvi.65.2.961-967.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saito Y, Sharer L R, Epstein L G, Michaels J, Mintz M, Louder M, Golding K, Cvetkocitch T A, Blumberg B M. Overexpression of nef as a marker for restricted HIV-1 infection of astrocytes in postmortem pediatric central nervous tissue. Neurology. 1994;44:474–481. doi: 10.1212/wnl.44.3_part_1.474. [DOI] [PubMed] [Google Scholar]
- 39.Scherer W F, Syverton J T, Gey G O. Viral multiplication in a stable strain of human malignant epithelial cells (strain HeLa) derived from an epidermoid carcinoma of the cervix. J Exp Med. 1953;97:695–710. doi: 10.1084/jem.97.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shahabuddin M, Volsky B, Kim H, Sakai K, Volsky D J. Regulated expression of human immunodeficiency virus type 1 in human glial cells: induction of dormant virus. Pathobiology. 1992;60:195–205. doi: 10.1159/000163723. [DOI] [PubMed] [Google Scholar]
- 41.Sharer L R, Saito Y, De Cunha A, Padiwath D, Gelbard H A, Epstein L G, Blumberg B M. In situ amplification and detection of HIV-1 DNA in fixed pediatric AIDS brain tissue. Hum Pathol. 1996;27:614–617. doi: 10.1016/s0046-8177(96)90172-0. [DOI] [PubMed] [Google Scholar]
- 42.Swingler S, Easton A, Morris A. Cytokine augmentation of HIV-1 LTR-driven gene expression in neural cells. AIDS Res Hum Retroviruses. 1992;8:487–493. doi: 10.1089/aid.1992.8.487. [DOI] [PubMed] [Google Scholar]
- 43.Takahashi K, Wesselingh S L, Griffin D E, McArthur J C, Johnson R T, Glass J D. Localization of HIV-1 in human brain using polymerase chain reaction/in situ hybridization and immunocytochemistry. Ann Neurol. 1996;39:705–711. doi: 10.1002/ana.410390606. [DOI] [PubMed] [Google Scholar]
- 44.Taylor J P, Kundu M, Khalili K. TAR-independent activation of HIV-1 requires the activation domain but not the RNA-binding domain of Tat. Virology. 1993;195:780–785. doi: 10.1006/viro.1993.1430. [DOI] [PubMed] [Google Scholar]
- 45.Taylor J P, Pomerantz R, Bagasra O, Chowdhury M, Rappaport J, Khalili K, Amini S. TAR-independent transactivation by Tat in cells derived from the CNS: a novel mechanism of HIV-1 gene regulation. EMBO J. 1992;11:3395–3403. doi: 10.1002/j.1460-2075.1992.tb05418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taylor J P, Pomerantz R J, Oakes J W, Khalili K, Amini S. A CNS-enriched factor that binds to NF-κB and is required for interaction with HIV-1 Tat. Oncogene. 1995;10:395–400. [PubMed] [Google Scholar]
- 47.Taylor J P, Pomerantz R J, Raj G V, Kashanchi F, Brady J N, Amini S, Khalili K. Central nervous system-derived cells express a κB-binding activity that enhances human immunodeficiency virus type 1 transcription in vitro and facilitates TAR-independent transactivation by Tat. J Virol. 1994;68:3971–3981. doi: 10.1128/jvi.68.6.3971-3981.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tornatore C, Chandra R, Berger J R, Major E O. HIV-1 infection of subcortical astrocytes in the pediatric central nervous system. Neurology. 1994;44:481–487. doi: 10.1212/wnl.44.3_part_1.481. [DOI] [PubMed] [Google Scholar]
- 49.Tornatore C, Meyers K, Atwood W, Conant K, Major E. Temporal patterns of human immunodeficiency virus type 1 transcripts in human fetal astrocytes. J Virol. 1994;68:93–102. doi: 10.1128/jvi.68.1.93-102.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tornatore C, Nath A, Amemiya K, Major E O. Persistent human immunodeficiency virus type 1 infection in human fetal glial cells reactivated by T-cell factor(s) or by the cytokines tumor necrosis factor alpha and interleukin-1 beta. J Virol. 1991;65:6094–6100. doi: 10.1128/jvi.65.11.6094-6100.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Willey R L, Klimkait T, Frucht D M, Bonifacino J S, Martin M A. Mutations within the human immunodeficiency virus type 1 gp160 envelope glycoprotein alter its intracellular transport and processing. Virology. 1991;184:319–329. doi: 10.1016/0042-6822(91)90848-6. [DOI] [PubMed] [Google Scholar]
- 52.Xiao H, Neuveut C, Benkirane M, Jeang K T. Interaction of the second coding exon of Tat with human EF-1δ delineates a mechanism for HIV-1-mediated shut-off of host mRNA translation. Biochem Biophys Res Comm. 1998;244:384–389. doi: 10.1006/bbrc.1998.8274. [DOI] [PubMed] [Google Scholar]
- 53.Yang L, Morris G F, Lockyer J M, Lu M, Wang Z, Morris C B. Distinct transcriptional pathways of TAR-dependent and TAR-independent human immunodeficiency virus type-1 transactivation by Tat. Virology. 1997;235:48–64. doi: 10.1006/viro.1997.8672. [DOI] [PubMed] [Google Scholar]