Abstract
Plasma human immunodeficiency virus type 1 (HIV-1) turnover and kinetics were studied in children aged 15 days to 2 years following the initiation of a triple antiretroviral drug regimen consisting of zidovudine, lamivudine, and nevirapine. HIV-1 turnover was at least as rapid as that previously described in adults; turnover rates were more rapid in infants and children aged 3 months to 2 years than in infants less than 3 months of age. These data confirm the central role of HIV-1 replication in the pathogenesis of vertical HIV-1 infection and reinforce the importance of early, potent combination therapies for the long-term control of HIV-1 replication.
Improvements in assays to detect and quantify human immunodeficiency virus type 1 (HIV-1) RNA in peripheral blood and tissues have allowed the appreciation of the central role of HIV-1 replication in HIV-1 pathogenesis (5–7). By using molecular techniques, HIV-1 can be detected in peripheral blood plasma and mononuclear cells shortly after infection (16, 24, 25). Within weeks of infection, plasma HIV-1 RNA copy numbers ranging from 105 to 107 per ml of plasma have been documented.
During primary infection in adults, peak plasma HIV-1 RNA levels fall by 100- to 1,000-fold within 1 to 2 months after the onset of symptoms (8, 24). This decline is observed even in the absence of antiretroviral therapy; host immune responses (1, 2, 8) or exhaustion of permissive host cells (20) is believed to contribute to this phenomenon. By 4 to 6 months after primary infection, a steady-state plasma HIV-1 RNA level is reached (13, 24); this steady-state plasma HIV-1 RNA level has been found to be predictive of the rate of subsequent disease progression and survival independent of other parameters such as CD4 lymphocyte count. The analysis of changes in plasma HIV-1 RNA levels following the initiation of potent antiretroviral therapies to perturb the virus-host steady state has allowed an improved understanding of the dynamics of HIV-1 replication in vivo (3, 4, 6, 7, 18, 19, 27). From such studies, it has been calculated that an average of 1010 HIV-1 virions are produced daily in adults with established disease. These studies have formed the basis for a model of HIV-1 replication in which the majority of plasma virions (>93 to 99%) are produced by productively infected CD4 T lymphocytes, while smaller contributions (<10%) to the plasma virion pool are made by populations of long-lived cells (e.g., macrophages) and latently infected lymphocytes (18).
Rapid increases in the plasma HIV-1 RNA copy numbers to 105 to 107 copies per ml of plasma have also been documented in vertically infected infants during the first weeks of life (15, 17, 25). In contrast to the natural history of HIV-1 RNA levels following primary infection in adults, plasma HIV-1 RNA levels remain high (mean 105 copies per ml of plasma) over the first 2 years of life. After the first 1 to 2 years of life, a reduction in plasma HIV-1 RNA (mean, −0.2 to −0.3 log decline per year) has been observed in vertically infected children that continues through 5 to 6 years of age (12, 14, 16). As observed in infected adults, higher plasma HIV-1 RNA levels are independently associated with increased risk of progression to AIDS or death in older children (14, 16, 26, 30). Additionally, antiretroviral therapy-induced reductions in plasma HIV-1 RNA have been associated with clinical benefit in both children (16) and adults (23).
Little information regarding the kinetics of HIV-1 replication in vertically infected infants and children is available. We therefore undertook a study in which potent antiretroviral therapies were used to probe the kinetics of HIV-1 replication in infants and children. In this study, a triple antiretroviral drug regimen consisting of zidovudine (ZDV), lamivudine (3TC), and nevirapine (NVP) was administered to infants and children aged 15 days to 2 years with limited or no prior antiretroviral therapy. Frequent blood sampling for plasma HIV-1 RNA copy number following the initiation of antiretroviral therapy allowed the estimation of HIV-1 kinetic parameters. The implications of these studies regarding the pathogenesis and therapy of vertical HIV-1 infection are discussed.
MATERIALS AND METHODS
Study design.
This open-label, phase I/II study was conducted at 13 Pediatric AIDS Clinical Trials Group sites, including Bellevue Hospital—New York University, Boston Children’s Hospital—Boston City Hospital, University of Massachusetts—Baystate Medical Center, Children’s Hospital—Philadelphia, Duke University, State University of New York Health Science Center at Syracuse, University of Mississippi, University of California—Los Angeles, University of California—San Francisco, Medical University of South Carolina, and Tulane University. The full details of this study will be described separately (unpublished data).
This study was approved by the human subject committees at the participating sites, and written informed consent was obtained from the children’s legal guardians. The guidelines of the U.S. Department of Health and Human Services governing experimentation in humans were followed.
Study medications.
Infants 15 to 29 days old at the time of enrollment received the following doses of study drugs: ZDV (Retrovir; Glaxo Wellcome), 4 mg/kg three times daily through 29 days of age, then 160 mg per m2 of body surface area three times a day beginning at 30 days of age; 3TC (Epivir; Glaxo Wellcome), 2 mg/kg every 12 h through 29 days of age, then 4 mg/kg every 12 h beginning at 30 days of age; NVP (Viramune; Boehringer-Ingelheim), 5 mg/kg once daily for 14 days, then 120 mg per m2 of body surface area for 14 days, and then 200 mg per m2 every 12 h. Infants who were 30 days or older at the time of enrollment received the following doses of study drugs: ZDV, 160 mg per m2 of body surface area three times a day; 3TC, 4 mg/kg every 12 h; and NVP, 120 mg per m2 of body surface area for 14 days and then 200 mg per m2 every 12 h. All medications were administered as a syrup or a suspension, at concentrations of 10 mg/ml.
Quantification of plasma HIV-1 RNA copy number by reverse transcriptase PCR.
HIV-1 RNA was quantified in 200 μl of EDTA-anticoagulated plasma (stored at −70°C within 6 hours after phlebotomy) by PCR after reverse transcription (Amplicor; Roche). The lower detection limit of the assay is 400 copies of HIV-1 RNA per ml of plasma. All assays were performed in a single laboratory that participates in an ongoing quality certification program for HIV-1 RNA quantitation sponsored by the National Institutes of Health. Sequential samples through 12 weeks of therapy from individual patients were assayed in batches to avoid variability between assays.
RESULTS
Patient population.
Sixteen infants aged 15 days to 2 years enrolled in this study, and therapy was initiated with the triple antiretroviral drug regimen of ZDV, 3TC, and NVP. Subjects were stratified into two age cohorts (seven infants of ≤3 months of age and nine infants or children of >3 months). The stratification was performed because it was hypothesized that viral kinetics of infants who were experiencing primary infection (≤3 months of age) and of those who were past the period of primary infection (>3 months) might differ.
All 16 infants received a minimum of 12 weeks of therapy. A minimum 2-log reduction (range, 2.1 to 3.87 log) of virus in plasma was maintained through 12 weeks in 12 (75%) of the 16 infants (5 infants aged ≤3 months and 7 children aged >3 months to 2 years). In the remaining four infants, initial reductions in plasma HIV-1 RNA copy numbers were not maintained. Potential explanations for the observed rebounds in plasma HIV-1 load in these four children include incomplete adherence to the prescribed medication regimens and the selection of drug-resistant variants. Since these factors could affect the calculated viral clearance rates, these children were excluded from analysis. The calculation of HIV-1 kinetic parameters for the 12 children (Table 1) who had sustained reductions in plasma HIV-1 RNA copy numbers form the basis of this report.
TABLE 1.
Summary of patient characteristics and viral kinetic parameters
| Cohort and patient no. | Age at entry | Prior therapy | Baseline % CD4 | Baseline RNA (copies/ml) | δa | T1b (days) | R1c (%) | μd | T2e | R2f (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Cohort 1 | ||||||||||
| 1 | 15 days | ZDV, 2 wk | 34 | 4.93 | 0.56 | 1.23 | 89.3 | 0.04 | 15.38 | 10.7 |
| 2 | 1.1 mo | ZDV, 4 wk | 47 | 5.90 | 0.26 | 2.69 | 98.3 | 0.02 | 33.26 | 1.7 |
| 3 | 2.7 mo | ZDV, 10 wk | 35 | 4.81 | 1.03 | 0.67 | 98.6 | 0.03 | 23.13 | 1.4 |
| 4 | 2.8 mo | ZDV, 6 wk | 14 | 5.92 | 0.32 | 2.18 | 95.7 | 0.04 | 16.51 | 4.3 |
| 5 | 3.0 mo | None | 24 | 6.82 | 0.59 | 1.17 | 98.2 | 0.04 | 17.17 | 1.8 |
| Range (median) | 14–47 (34) | 4.93–6.82 (5.90) | 0.26–1.03 (0.56) | 0.67–2.69 (1.23) | 89.3–98.6 (98.2) | 0.02–0.04 (0.04) | 15.38–33.26 (17.17) | 1.8–10.7 (1.8) | ||
| Cohort 2 | ||||||||||
| 6 | 3.6 mo | None | 45 | 6.38 | 1.04 | 0.66 | 99.8 | 0.06 | 11.48 | 0.2 |
| 7 | 4.0 mo | None | 48 | 5.70 | 1.22 | 0.57 | 99.2 | 0.06 | 11.58 | 0.8 |
| 8 | 7.7 mo | None | 41 | 6.47 | 1.20 | 0.58 | 99.5 | 0.06 | 11.27 | 0.5 |
| 9 | 10.4 mo | None | 41 | 5.03 | 0.97 | 0.71 | 95.5 | 0.11 | 6.31 | 4.5 |
| 10 | 13.8 mo | None | 14 | 6.29 | 1.14 | 0.61 | 99.3 | 0.06 | 12.26 | 0.7 |
| 11 | 19.2 mo | None | 24 | 5.22 | 1.04 | 0.66 | 99.0 | 0.12 | 5.97 | 1.0 |
| 12 | 24.3 mo | None | 38 | 4.70 | 2.02 | 0.34 | 99.6 | 0.15 | 4.68 | 0.4 |
| Range (median) | 14–48 (41) | 4.70–6.47 (5.70) | 0.97–2.02 (1.14) | 0.34–0.71 (0.61) | 95.5–99.8 (99.3) | 0.06–0.15 (0.06) | 4.68–12.26 (11.27) | 0.2–4.5 (0.7) |
δ = first-phase viral decay rate. Cohort 1 versus cohort 2, P = 0.0051 (Wilcoxon rank sum test).
T1 = first-phase viral half-life (days).
R1 = percentage of plasma virus cleared during first phase.
μ = second-phase viral decay rate. Cohort 1 versus Cohort 2, P = 0.0025 (Wilcoxon rank sum test).
T2 = second-phase viral half-life (days).
R2 = percentage of plasma virus cleared during the second phase.
All 12 infants were of Centers for Disease Control and Prevention clinical category N, A, or B, and the percentage of peripheral blood CD4 T cells ranged from 14 to 48 at the time of entry into the study. Four study participants (all <3 months of age) had received prior antiretroviral therapy, consisting of ZDV alone, ranging in duration from 15 days to 10 weeks. One infant received ZDV until the time of study enrollment; for the three remaining infants who had received prior ZDV therapy, ZDV was discontinued 3 days, 9 days, and 5 weeks, respectively, prior to the time of study enrollment.
Antiretroviral activity of ZDV-3TC-NVP.
Baseline plasma HIV-1 RNA copy numbers were defined as the arithmetic mean of the plasma HIV-1 RNA measured just prior to the initiation of study therapy and at least one other measurement obtained within the prior 2 weeks. Baseline plasma HIV-1 RNA copy numbers ranged from 104.70 to 106.82 (median, 105.8 per ml) (Table 1). These plasma HIV-1 RNA copy numbers are of similar magnitude to those reported by others for the age range studied (15, 16, 25). Pretreatment plasma HIV-1 RNA levels were similar for the two age cohorts (for ≤3 months, range of 104.81 to 106.82 and median of 105.9; for >3 months, range of 104.70 to 106.47 and median of 105.70), even though four of the five infants of ≤3 months of age had received ZDV therapy prior to study enrollment.
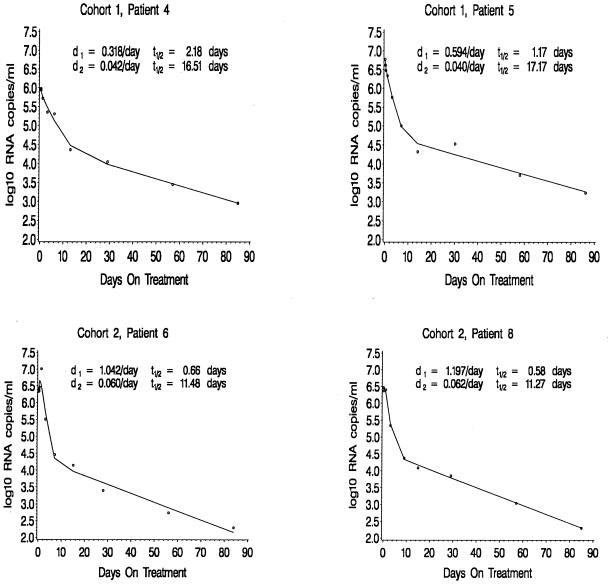
Blood samples for the measurement of plasma HIV-1 RNA levels were drawn just prior to therapy and at 3 and 8 h; at 1, 3, 7, 14, and 28 days; and then every 28 days after the initiation of therapy. Little change (<0.5 log) in plasma HIV-1 RNA level was observed over the first 24 h of therapy. Thereafter, a biphasic reduction in plasma HIV-1 RNA copy numbers was observed (Fig. 1). The initial decline in plasma HIV-1 RNA copy number was exponential, rapid, and profound and was followed by a slower, exponential second-phase decline. Over the first week of therapy, plasma HIV-1 RNA copy numbers fell 0.61 (76% reduction) to 2.45 (99.6% reduction) logs, with a median decrease of 1.85 logs (98.69% reduction). By the end of the second week of therapy, plasma HIV-1 RNA copy numbers had fallen 1.22 (94% reduction) to 2.62 (99.76% reduction) logs, with a median reduction of 2.12 logs (99.3% reduction). After 12 weeks of therapy, plasma HIV-1 RNA copy numbers had fallen 2.21 (99.4% reduction) to 3.87 (99.99% reduction) logs, with a median reduction of 2.93 logs (99.85% reduction). The magnitude and rapidity of the observed reductions in plasma HIV-1 copy numbers after the initiation of therapy attest to the antiviral potency of the regimen.
FIG. 1.
Plasma HIV-1 RNA copy numbers (log 10 scale) for four patients. The circles represent the observed data, and the solid lines are the fitted curves. Under the perfect treatment assumption, d1 = δ, d2 = μ, and t1/2 represents the corresponding half-lives.
Kinetics of plasma HIV-1 decay following the initiation of ZDV-3TC-NVP therapy.
The biphasic decay observed in this study is similar to that described by Perelson and colleagues following the initiation of potent combination antiretroviral therapies in adults (18). We therefore used a model similar to that of Perelson et al. to calculate plasma viral load decay rates during each phase of viral load reduction. As in previous studies (18, 19), if the treatment is assumed to completely block new viral replication, the first phase decay rate (δ) represents the death rate of productively infected cells and the second phase decay rate (μ) represents the death rate of long-lived or latently infected cells. In our calculations, we considered the long-lived and latently infected cells as one cellular compartment since they cannot be distinguished by using plasma HIV-1 RNA measurements alone.
The following equation was used to calculate the first (δ) and second (μ) phase decay constants:
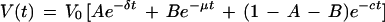 |
1 |
where V(t) is the measured number of RNA copies in the plasma at time t, V0 is the measured number of RNA copies in the plasma prior to the initiation of therapy, and e=2.718. The nonlinear least squares method was used to fit the above model to each individual’s viral load data.
Because of limitations in the amount of blood that could be drawn, particularly from young infants, we were unable to obtain adequate numbers of specimens to estimate the clearance rate (c) of free virions. Simulation studies (3a) using a c of 3 to 100 suggest little variability in the estimate of δ and μ when c is >5. We used a c of 3 per day to allow comparison of our data with those from adult studies by Perelson and colleagues (18).
Since a majority of the infants and children were studied close to the time of the acquisition of infection, their viral load and CD4 T-cell numbers were not likely to be in a steady state prior to the initiation of therapy. We therefore used a model not restricted by the steady-state condition in which the terms A and B in equation 1 are treated as arbitrary constants. Further discussion regarding the evaluation of the non-steady-state condition can be found in the work of Wu and Ding (29).
Both phases of viral load reduction appeared to be more rapid in the older group of infants (>3 months to 2 years) than in the younger group of infants (≤3 months). Overall, first-phase viral decay rates ranged from 0.26 to 2.02 (median, 1.03), which corresponds to calculated half-lives ranging from 0.34 to 2.69 (median, 0.66) days. First-phase viral decay rates were notably lower in the younger group of infants (range, 0.26 to 1.03; median, 0.56) than in the older group of infants (range, 0.97 to 2.02; median, 1.14). These values correspond to calculated half-lives (i.e., the time necessary for reduction of the plasma virus level by one-half) ranging from 0.67 to 2.69 days (median, 1.23 days) in the younger group and 0.34 to 0.71 days (median, 0.61 days) in the older group. The difference in first-phase viral decay rates between the younger and older children was examined by using the Wilcoxon rank sum test and was found to be highly significant (P = 0.0051) (Table 1). No apparent correlations were observed between the first-phase decay rate (δ) and any of the following: baseline plasma RNA levels, peripheral blood CD4 T-cell counts (absolute numbers or percentages), the percentage of peripheral blood CD4 T cells coexpressing CD45RA (naive CD4 T cells) or CD45RO (memory CD4 T cells), peripheral blood CD8 T-cell counts (absolute numbers or percentages), or the percentage of peripheral blood CD8 T cells coexpressing the activation antigen DR (data not shown).
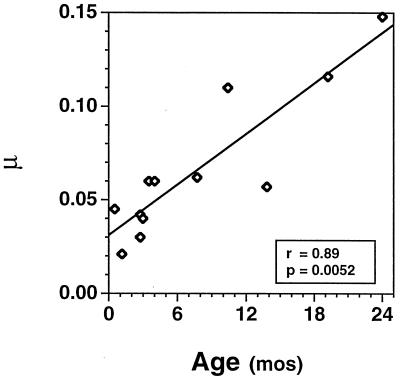
The transition time between first- and second-phase decay ranged from 3 to 17 days, with a median of 5 days. Second-phase viral decay rates ranged from 0.02 to 0.15 (median, 0.06), which correspond to calculated half-lives ranging from 4.68 to 33.26 days (median, 9.3 days). Again, the second-phase decay rates were notably lower in the younger group (range, 0.02 to 0.04; median, 0.04) than in the older group (range, 0.06 to 0.15; median, 0.06). These values correspond to calculated half-lives ranging from 15.38 to 33.26 days (median, 17.17 days) in the younger group and 4.68 to 12.26 days (median, 11.27 days) in the older group. The difference in second-phase viral decay rates between the younger and older children was also examined by using the Wilcoxon rank sum test and was found to be highly significant (P = 0.0025). In addition, a highly significant positive linear correlation was found between age at initiation of therapy and the second-phase decay rate (P = 0.0052) (Fig. 2). No apparent correlations were observed between the second-phase decay constant (μ) and any of the following: baseline plasma RNA levels, peripheral blood CD4 T-cell counts (absolute numbers or percentages), the percentage of peripheral blood CD4 T cells coexpressing CD45RA (naive CD4 T cells) or CD45RO (memory CD4 T cells), peripheral blood CD8 T-cell counts (absolute numbers or percentages), or the percentage of peripheral blood CD8 T cells coexpressing the activation antigen DR (data not shown).
FIG. 2.
Correlation between the second-phase viral decay rate (μ) and the age of patients at the time of study entry.
DISCUSSION
In this study, potent antiretroviral therapies were used to probe the kinetics of HIV-1 replication in infants and children aged 15 days to 2 years with limited prior antiretroviral therapy. Frequent blood sampling to measure plasma HIV-1 RNA copy number following the initiation of potent antiretroviral therapy allowed the calculation of the first detailed evaluation of viral kinetics in young, HIV-1-infected infants and children.
The clearance of HIV-1 virions in plasma following the initiation of therapy was biphasic. The majority (>90%) of virus in plasma was cleared during an initial rapid, exponential decline. A slower, exponential second-phase decline was then observed. This pattern of virion clearance is similar to that previously described following the initiation of potent combination antiretroviral therapy in cohorts of adults experiencing primary infection (9) and in adults with established disease (18). The consistency in this pattern is striking given the diversity in age, viral load, disease stage, and CD4 counts of the patient populations studied, in addition to the various regimens used.
As suggested by Ho, Perelson, and coworkers (6, 18, 19), the observed biphasic pattern of viral clearance likely represents two distinct cellular sources for plasma virions: short-lived, productively infected cells (CD4 T cells; first phase) and long-lived cells with stably integrated HIV-1 provirus (tissue macrophages, dendritic cells, or latently infected CD4 T cells undergoing activation; second phase). Alternatively, the biphasic decay following the initiation of therapy could represent an exponential decay of viral production by a single cellular source with a decreasing exponent over time due to a reduction in either the number of virus-producing cells or the ability of the cells to produce virus (e.g., an increased number of cells moving from the activated state to a resting state).
Viral decay rates were used to calculate half-lives for viral turnover. During the first phase of viral decay (representing >90% of plasma virus), half of the plasma virus turned over approximately every 30 h on average in the younger group and approximately every 14 h on average in the older group. These estimates are likely a composite of the clearance of free virions and short-lived, productively infected cells. Due to limitations in the blood volumes that we were able to obtain from these young children, we were unable to obtain samples at a sufficient frequency to distinguish the separate contributions of the free virions and the short-lived productively infected cells. Data from adult studies suggest that δ primarily reflects the clearance rate of short-lived productively infected cells; the calculated first-phase half-lives measured in the young infants are comparable to the half-lives of productively infected CD4 T cells reported in similar studies of adults. Overall, these data suggest rates of HIV-1 production and turnover that are at least as rapid as those previously reported for adults. Interestingly, the half-lives in the older infants and children are approximately half those reported in adults, suggesting even more rapid production and turnover of HIV-1 in plasma in these children.
Several hypotheses could be raised to explain the observed age-related differences in clearance rates. The slower clearance of HIV-1 from the younger infants might reflect a reduced activity of the regimen in those infants. Plasma NVP levels were measured at several time points during the study; plasma NVP levels in the younger infants were similar to or exceeded those measured in the older infants (data not shown). Differences in NVP absorption and metabolism are thus unlikely explanations for the observed age-related differences in clearance rates.
Alternatively, the slower clearance rates observed in the younger infants could reflect a lesser activity of the regimen due to the effects of prior therapy (e.g., preexisting resistance mutations). In this regard, it must be noted that while none of the older children had received antiretroviral therapy prior to study entry, the majority of the young infants had received ZDV therapy prior to their enrollment in the study. Sequencing of the reverse transcriptase gene from viral isolates obtained prior to the initiation of therapy is under way. This should allow the assessment of whether mutations associated with resistance to any of the reverse transcriptase inhibitors used in this study might have influenced the response to therapy.
A difference in the proportion of virus produced by different cellular sources and a differential susceptibility of these sources to the effects of antiretroviral therapies could also explain the observed age-related differences in viral kinetics. Likewise, age-related differences in the number of cells capable of supporting productive infection (particularly activated CD4 T cells) might explain the observed age-related differences. Finally, less vigorous or delayed development of HIV-1-specific immune responses (most notably antibody-dependent cellular cytotoxicity and cytotoxic T-lymphocyte responses [11, 21, 22]) have been described in young infants and could contribute to the less rapid clearance of HIV-1 following the initiation of antiretroviral therapy. The analysis of additional cohorts of infants who have begun therapy with quadruple-therapy regimens (ZDV-3TC-NVP-abacavir or stavudine-3TC-NVP-nelfinavir) is currently under way and should allow us to distinguish between these possibilities.
Based on their initial studies with adults, Perelson et al. (19) suggested that the use of potent combination therapies that arrest HIV-1 replication might allow the eradication of HIV-1 infection over time; a minimum of 2 to 3 years of continuous therapy was estimated to be necessary to clear HIV-1 from the two major cellular compartments (productively infected T cells and the long-lived cell population). In the present study, extrapolation of the first- and second-order viral decay rates in infants suggest a time to viral extinction of 10 to 74 days (median, 20 days) in the first compartment (productively infected T cells) and from 102 to 721 days (median, 303 days) in the second compartment (long-lived infected cells). As we (10) and others (5, 6, 28) have noted, however, none of the regimens studied to date are capable of eradicating an integrated HIV-1 genome from infected cells. These cells, then, represent a small but potentially significant barrier to the eradication of infection from an individual. Sufficient blood samples to estimate the size of this potentially long-lived pool of infected cells in infants and children were not obtained in this study but are currently being collected for cohorts of infants who have begun therapy with quadruple-therapy regimens (ZDV-3TC-NVP-abacavir or stavudine-3TC-NVP-nelfinavir).
In summary, HIV-1 turnover and kinetics in plasma were studied in children aged 15 days to 2 years. Calculated rates of HIV-1 turnover were at least as rapid as those previously described in adults; turnover rates were more rapid in older infants and children than in younger infants. These data confirm the central role of HIV-1 replication in the pathogenesis of vertical HIV-1 infection and reinforce the importance of early, potent combination therapies for the long-term control of HIV-1 replication.
ACKNOWLEDGMENTS
We thank the children who participated in this study and their guardians. We also thank Meg Gwynne and Barbara Wells for support in protocol development and implementation; Linda Lambrecht, Randy Huelsman, John Latino, Kevin Byron, and Dena Giokas for technical support; Bobbie Graham for data management; A. Adam Ding, Jane Lindsey, and Peter Gaccione for assistance in analysis of the data; and Melinda Engel for preparation of the manuscript.
This study was supported by the Pediatric AIDS Clinical Trials Group of the National Institutes of Health, by NIH grants AI-32907/97PICL02/97PVCL09 and AI-43220, and by Boehringer-Ingelheim Pharmaceuticals. Katherine Luzuriaga is an Elizabeth Glaser Scientist of the Elizabeth Glaser Pediatric AIDS Foundation.
REFERENCES
- 1.Borrow P, Lewicki H, Hahn B H, Shaw G M, Oldstone M B. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borrow P, Lewicki H, Wei X, Horwitz M, Peffer N, Meyers H, Nelson J, Gairin J, Hahn B, Oldstone M, Shaw G. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat Med. 1997;3:205–211. doi: 10.1038/nm0297-205. [DOI] [PubMed] [Google Scholar]
- 3.Coffin J M. HIV population dynamics in vivo: implications for genetic variation, pathogenesis and therapy. Science. 1995;267:483–489. doi: 10.1126/science.7824947. [DOI] [PubMed] [Google Scholar]
- 3a.Ding, A. A., and H. Wu. Unpublished data.
- 4.Finzi D, Hermankova M, Pierson T, Carruth L M, Buck C, Chaisson R E, Quinn T C, Chadwick K, Margolick J, Brookmeyer R, Gallant J, Markowitz M, Ho D D, Richman D D, Siliciano R F. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 5.Finzi D, Silicano R F. Viral dynamics in HIV-1 infection. Cell. 1998;93:665–672. doi: 10.1016/s0092-8674(00)81427-0. [DOI] [PubMed] [Google Scholar]
- 6.Ho D D. Dynamics of HIV-1 replication in vivo. J Clin Invest. 1997;99:2565–2567. doi: 10.1172/JCI119443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ho D D, Neumann A U, Perelson A S, Chen W, Leonard J M, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 8.Koup R A, Safrit J T, Cao Y, Andrews C A, McLeod G, Borkowsky W, Farthing C, Ho D D. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Little S, Havlir D, Richman D, Spina C, McLean A. Presented at the Fifth Conference on Retroviruses and Opportunistic Infections, 1–5 February 1998, Chicago, Ill. 1998. Viral population dynamics during acute HIV infection. [Google Scholar]
- 10.Luzuriaga K, Bryson Y, Krogstad P, Robinson J, Stechenberg B, Lamson M, Cort S, Sullivan J L. Combination treatment with zidovudine, didanosine, and nevirapine in infants with human immunodeficiency virus type 1 infection. N Engl J Med. 1997;336:1343–1349. doi: 10.1056/NEJM199705083361902. [DOI] [PubMed] [Google Scholar]
- 11.Luzuriaga K, Holmes D, Hereema A, Wong J, Panicali D L, Sullivan J L. HIV-1-specific cytotoxic T lymphocyte responses in the first year of life. J Immunol. 1995;154:433–443. [PubMed] [Google Scholar]
- 12.McIntosh K, Shevitz A, Zaknun D, Kornegay J, Chatis P, Karthas N, Burchett S K. Age- and time-related changes in extracellular viral load in children vertically infected by human immunodeficiency virus. Pediatr Infect Dis J. 1996;15:1087–1091. doi: 10.1097/00006454-199612000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Mellors J W, Rinaldo C R, Gupta P, White R M, Todd J A, Kingsley L A. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272:1167–1170. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- 14.Mofenson L M, Korelitz J, Meyer W A, Bethel J, Rich K, Pahwa S, Moye J, Nugent R, Read J. The relationship between serum human immunodeficiency virus type 1 (HIV-1) RNA level, CD4 lymphocyte percent, and long-term mortality risk in HIV-1-infected children. J Infect Dis. 1997;175:1029–1038. doi: 10.1086/516441. [DOI] [PubMed] [Google Scholar]
- 15.Palumbo P E, Kwok S, Waters S, Wesley Y, Lewis D, McKinney N, Bardeguez A, Connor E M, Oleske J. Viral measurement by polymerase chain reaction-based assays in human immunodeficiency virus-infected infants. J Pediatr. 1995;126:592–595. doi: 10.1016/s0022-3476(95)70357-8. [DOI] [PubMed] [Google Scholar]
- 16.Palumbo P E, Raskino C, Fiscus S, Pahwa S, Fowler M G, Spector S A, Englund J A, Baker C J. Predictive value of quantitative plasma HIV RNA and CD4+ lymphocyte count in HIV-infected infants and children. JAMA. 1998;279:756–761. doi: 10.1001/jama.279.10.756. [DOI] [PubMed] [Google Scholar]
- 17.Pediatric European Network for the Treatment of AIDS. HIV-1 viral load and CD4 cell count in untreated children with vertically acquired asymptomatic or mild disease. AIDS. 1998;12:F1–F8. [PubMed] [Google Scholar]
- 18.Perelson A S, Essunger P, Cao Y, Vesanen M, Hurley A, Saksela K, Markowitz M, Ho D D. Decay characteristics of HIV-1 infected compartments during combination therapy. Nature. 1997;387:188–191. doi: 10.1038/387188a0. [DOI] [PubMed] [Google Scholar]
- 19.Perelson A S, Neumann A U, Markowitz M, Leonard J M, Ho D D. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science. 1996;271:1582–1586. doi: 10.1126/science.271.5255.1582. [DOI] [PubMed] [Google Scholar]
- 20.Phillips A N. Reduction of HIV concentration during acute infection: independence from a specific immune response. Science. 1996;271:497–499. doi: 10.1126/science.271.5248.497. [DOI] [PubMed] [Google Scholar]
- 21.Pikora C A, Sullivan J L, Panicali D, Luzuriaga K. Early HIV-1 envelope-specific cytotoxic T lymphocyte responses in vertically infected infants. J Exp Med. 1997;185:1153–1161. doi: 10.1084/jem.185.7.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pugatch D, Sullivan J, Pikora C, Luzuriaga K. Delayed generation of antibodies mediating HIV-1 specific antibody-dependent cellular cytotoxicity in vertically-infected infants. J Infect Dis. 1997;176:643–648. doi: 10.1086/514085. [DOI] [PubMed] [Google Scholar]
- 23.Saag M S, Holodniy M, Kuritzkes D R, O’Brien W A, Coombs R, Poscher M E, Jacobsen D M, Shaw G M, Richman D D, Volberding P A. HIV viral load markers in clinical practice. Nat Med. 1996;2:625–631. doi: 10.1038/nm0696-625. [DOI] [PubMed] [Google Scholar]
- 24.Schacker T W, Hughes J P, Shea T, Coombs R W, Corey L. Biological and virologic characteristics of primary HIV infection. Ann Intern Med. 1998;128:613–620. doi: 10.7326/0003-4819-128-8-199804150-00001. [DOI] [PubMed] [Google Scholar]
- 25.Shearer W T, Quinn T C, LaRussa P, Lew J F, Mofenson L, Almy S, Rich K, Handelsman E, Diaz C, Pagano M, Smeriglio V, Kalish L. Viral load and disease progression in infants infected with human immunodeficiency virus type 1. N Engl J Med. 1997;336:1137–1349. doi: 10.1056/NEJM199705083361901. [DOI] [PubMed] [Google Scholar]
- 26.Valentine M E, Jackson C R, Vavro C. Evaluation of surrogate markers and clinical outcomes in 2-year follow-up of 86 HIV-infected pediatric patients. Pediatr Infect Dis. 1998;17:18–23. doi: 10.1097/00006454-199801000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Wei X, Ghosh S K, Taylor M E, Johnson V A, Emini E A, Deutsch P, Lifson J D, Bonhoeffer S, Nowak M A, Hahn B H, Saag M S, Shaw G M. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995;373:117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- 28.Wong J K, Ignacio C C, Torriani F, Havlir D, Fitch N J, Richman D D. In vivo compartmentalization of human immunodeficiency virus: evidence from the examination of pol sequences from autopsy tissues. J Virol. 1997;71:2059–2071. doi: 10.1128/jvi.71.3.2059-2071.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu, H., and A. A. Ding. Population HIV-1 dynamics in vivo: applicable models and inferential tools for virological data from AIDS clinical trials. Biometrics, in press. [DOI] [PubMed]
- 30.Zaknun D, Orav J, Kornegay J. Correlation of RNA PCR, ICD p24 antigen and neopterin with progression of disease. J Pediatr. 1997;130:898–905. doi: 10.1016/s0022-3476(97)70275-0. [DOI] [PubMed] [Google Scholar]