Summary
Background
Two randomised controlled trials (RCTs) have previously shown that telemedical monitoring of diabetic foot ulcer (DFU) reduces the number of visits to the outpatient clinic, without losing treatment efficacy or increasing costs. Here we present the results of an open-label, randomised controlled trial designed to investigate whether telemonitoring, provided by an expert nurse (with extensive experience in DFU and trained in remote monitoring), reduces the hospital stay and the associated costs for a patient with DFU (TELEPIED trial).
Methods
Eligible patients (n = 180) were randomly allocated to: (i) a control group, in which they received standard care, and (ii) an intervention group, in which they received asynchronous telemedicine follow-up by the expert nurse. The primary outcome was the cumulative hospital days over 12 months. The main secondary outcomes were (i) direct healthcare costs (estimated in a collective perspective), (ii) wound healing and (iii) amputation rates. ITT (intention-to-treat) population was analysed.
Findings
In the ITT population, cumulative hospital days were significantly higher in the control group (13.4 days [95% CI 9.0–17.8]) than in the intervention group (7.1 days [2.8–11.5]) (p = 0.0458, ANCOVA model). Cumulative direct costs over 12 months were 7185 € (95% CI 5144–9226) in the control group and 3471 € (95% CI 1430–5512) in the intervention group (p = 0.0120). The percentage of wounds healed and amputation rate were not significantly different between groups. Similar results were found with the PP population.
Interpretation
The implementation of a telemedical intervention with an expert nurse could lead to a length of hospitalization and direct costs that were two times lower compared to conventional follow-up. This lower medical and economic burden was obtained without losing effectiveness on the rate of healing, nor increasing the amputation rate. Additional studies are required to confirm these findings.
Funding
This study was designed, funded and conducted by CERITD (Study and Research Centre for Intensification of Diabetes Treatment, Evry, France), Genopole GIP, 20 rue Henri Desbruères, 91030 EVRY Cedex and Laboratoires URGO, 15 Avenue d’Iéna, 75116 Paris Cedex, France. The findings and conclusions in this study are those of the authors and do not necessarily represent the views of the sponsor. The corresponding author (DD) certify that authors were not precluded from accessing data in the study, and they accept responsibility to submit for publication.
Keywords: Clinical trial, Diabetes, Diabetes complications, Diabetic foot ulcer, Health cost, Hospitalization, Expert nurse, Telemedicine, Telehealth
Research in context.
Evidence before this study
Patients with diabetic foot ulcer (DFU) end up with an impaired quality of life, and the medical care of patients with DFU consumes a considerable clinical and economic burden (hospitalizations, amputations, patients’ follow-up, transportation and lost work days. Direct costs of care for diabetic foot complications are comparable to those of cancer in France. Two previous randomised controlled trials (RCTs) showed that telemonitoring makes it possible to reduce outpatient clinic visits, without losing the efficacy of DFU treatment and without increasing costs. Consequently, we conducted an open-label, RCT with a different design, that is, to investigate whether telemonitoring reduces the hospital stay and the cost of a patient with DFU (TELEPIED trial).
Added value of this study
The TELEPIED trial is a mirror image of the RCTs cited above. In those earlier studies, the efficacy and cost of telemonitoring were investigated with a fixed frequency of outpatient clinic visits. In contrast, TELEPIED investigated the efficacy of telemonitoring in reducing hospital stay and associated cost.
Implications of all the available evidence
Telemonitoring associated with a follow-up by an expert nurse represents a promising alternative to standard care for patients with DFUs.
Introduction
Diabetic foot ulcer (DFU) is a frequent, serious and costly complication of diabetes.1, 2, 3, 4, 5, 6, 7, 8, 9 Patients with diabetes have a lifetime incidence of DFU between 19% and 34%.3 Poor glycaemic control, improper foot care, underlying neuropathy and arteriopathy make the feet vulnerable to skin ulcers that can worsen quickly.1,7 A non-healing ulcer causes severe tissue and bone damage and may require amputation (more than 80 percent of lower limb amputations begin with foot ulcers).7 Even when healed, ulcer recurrence occurs at a high rate, with approximately 40% recurrence after one year and 65% recurrence within 5 years.3 The Eurodiale study,8 carried out in 14 European hospitals in ten countries, showed that more than half of the DFU’s become infected, and infection increases the risk of amputation.3 In the United Kingdom, the five year mortality of people with DFU was 46.2%,9 that is, 2.5-fold higher than in the general population.3
For patients with DFU, the high risk of amputation warrants preventive measures and careful treatment. Practical guidelines have been edited by the International Working Group of the Diabetic Foot,10 and by the American Diabetes Association.11 Preventive measures include patient education for proper diabetes management and foot care, and regular medical and nursing examination of the feet, to check for arteriopathy, nerve damage, or other foot problems.1,7,10,11 Treatment measures include surgical ulcer debridement and removal of surrounding callus, dressings to control excess exudation and maintain a moist environment, wound off-loading, vascular and neurological assessment, and infection and glycaemic control.10,12 These practices are best coordinated by a multidisciplinary diabetic foot care team.2,12
Over one year, subjects with a DFU are seen by their outpatient health care provider about 14 times and they are hospitalized about 1.5 times.4 At the very end of the medical journey, the medical care of patients with DFUs has absorbed a considerable amount of clinical and economic resources (hospitalizations, amputations, patients’ follow-up, transportation, and lost work days),6,13 and patients end up with an impaired quality of life.14 It has been suggested that the direct costs for DFU care can exceed the treatment costs of some common cancers, such as colorectal, lung and prostate cancers or leukaemia, by 17–200%.5
A recent overview of eight systematic reviews (including 36 studies with 4357 participants evaluating remote monitoring or management of diabetic foot disease) concluded that effectiveness was unclear, and cost-effectiveness data was limited and unclear.15 A meta-analysis of four controlled studies (including three 3 randomised trials, RCTs) showed that DFU treatment via telemedicine could be at least as effective as face-to-face care.16 An economic evaluation by Jafary et al.17 showed that home care service of DFU was significantly more cost-effective than hospital-based care and a review by Hazenberg et al.18 suggested that telemonitoring of DFUs can reduce clinical burden, without increasing costs.
The above systematic reviews and metanalyses suggested that telemedicine makes it possible to reduce outpatient clinic visits, without losing the efficacy of DFU treatment and without increasing costs. We hypothesized that "bringing the hospital to the patient's home" with an expert nurse (acting in coordination with the principal investigator through a telemedicine platform) could reduce hospital stay and costs. Consequently, we decided to perform an open-label, randomised controlled clinical trial comparing the hospital stay and direct cost of the above telemedicine intervention with that of standard care (the TELEPIED trial).
Methods
Study design and participants
TELEPIED was an open-label (unblinded assessors and patients), single centre, randomised controlled comparative study, conducted at the Department of Diabetes and Endocrinology, South Francilien Hospital Centre (Centre Hospitalier Sud Francilien, CHSF, Corbeil-Essonnes, France). Ethical approval for this study was obtained from the ethics committee of the University Hospital La Pitié Salpétrière (Paris, France). Regulatory approval was obtained from the French National Agency for Medicines and Health Products Safety (ANSM; reference 2016-A01136-45) and from the Committee for the Protection of Persons Ile-de-France VI (reference CPP 71-16). The study was sponsored by the CERITD (Study and Research Centre for Intensification of Diabetes Treatment, Evry, France) and registered under ClinicalTrials.gov number NCT02986256.
Eligible participants were mostly adults with type 1 or type 2 diabetes, who had a DFU or a recurrence of a previously healed ulcer, who had their health care expenses covered by a social security plan, and who had signed an informed consent. Ineligible participants were pregnant women, patients deprived of liberty due to judicial decisions, patients subject to legal protection measures, or patients participating in another clinical trial.
Procedures
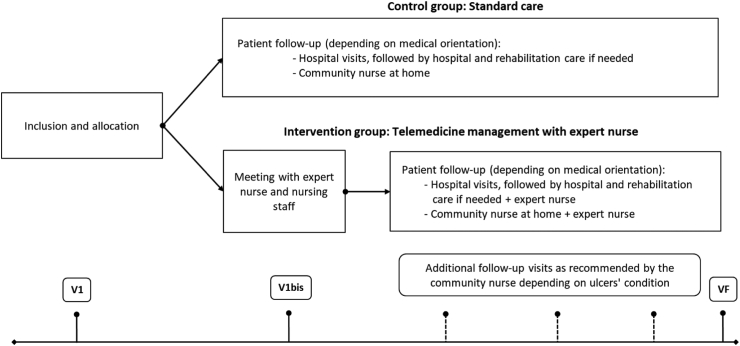
The study protocol has been previously published.19 Briefly, eligible patients were included in the study during one of their regular hospital follow-up visits (principal investigator, DD; Fig. 1). The following information was collected at the inclusion visit (V1): demographic data, general clinical data (diabetes history and complications, HbA1c, blood pressure), description and photo of the DFU, and current treatment. Included patients were asked to fil out the EPICES questionnaire (Evaluation of Deprivation and Inequalities in Health Examination Centres)20 and the AGGIR grid (Autonomy Gerontological Groups Iso-Resources).21 The AGGIR score allows to characterize the level of dependence of an elderly person according to his or her ability to perform daily tasks, while the EPICES score is linked to socio-economic, behavioural and health indicators. Patients were then stratified into three groups according to their ankle-brachial systolic pressure index (ABI, a measure of the severity of arterial disease): ABI ≤ 0.7, 0.7 < ABI ≤ 0.9, and ABI > 0.9 (normal range 0.9–1.3). Such stratification ensured and even distribution of patients suffering from severe arteriopathy between the two groups.
Fig. 1.
Study protocol. Eligible patients were randomly allocated to: (i) a control group, where they received standard follow-up (mixed hospital/ambulatory), and (ii) an intervention group, where they received telemedicine follow-up by an expert nurse. The expert nurse established a personalized telemedicine plan for each patient (intermediate visit, V1bis), based on the availability of the community nurse (who provided home care). The participants were followed up for 12 months.
Randomisation
Randomisation was carried out by Clininfo® (Lyon, France) via an electronic Case Report Form (eCRF; access to eCRF’s was protected by a secured code). Patients were randomly assigned to: (i) a control group, or (ii) an intervention (telemedicine) group. The distribution was made by blocks of predefined size, and stratified according to the ABI measured during the inclusion visit (see above).
Allocation
Patients were allocated to the control group (where they received standard care), and the intervention group (where they received telemedicine follow-up by an expert nurse, Fig. 1). Patients included in the control group were followed at spaced intervals during hospital visits with the investigator assisted by an expert nurse as part of the standard follow-up procedure (dressing changes, ulcer monitoring). Between visits, an independent (or “community”) nurse provided home care on a daily basis. Patients in the intervention group were followed-up by the expert nurse (see Methods, see also Fig. 1): DFU monitoring was performed weekly by the expert nurse using photos taken by herself or by the independent nurse (providing home care and acting under the supervision of the expert nurse) and sent to the expert nurse via telemedicine software. Both groups benefited from regular standard care at home by an independent (or “community”) nurse.
Hospitalization was offered to those patients who presented a worsening of the ulcers (increase in the area and/or depth of the lesion, appearance of signs of local or general infection). Depending on the condition of the ulcer and medical needs, patients of the control group went home to receive daily home care from a community nurse or were hospitalized for care and rehabilitation. The expert nurse established a personalized telemedicine plan for each patient of the intervention group (intermediate visit, V1bis, Fig. 1), based on the availability of the independent nurse.
The participants were followed up for 12 months, even if the DFU had healed before that period. Patients in the control group were followed up regularly by a diabetologist (investigator) from the hospital. The examinations performed and the care provided to each patient were recorded on the corresponding eCRF. The main data collected were: the evolution of the wound (appearance, size, degree of healing), hospitalizations (number of days, cause, treatment, type of follow-up) and the appearance of new wounds.
Patients in the intervention group and/or their community nurse were asked to take photos of the DFUs each week and to send them to the expert nurse for evaluation. The expert nurse analysed the photos and adapted the care plan with the community nurse (in agreement with the investigator diabetologist, if necessary). The expert nurse scheduled regular follow-up visits at the patient's home or hospital. Main data collected was the same as those described above for patients of the control group. In case of ulcer complications, a consultation visit with the principal investigator was made (followed by hospitalization, if required).
The photographs of the ulcers were taken with a camera integrated in the touch tablet provided to the patients in the intervention group. Ulcer dimension was measured using Tracer.exe software developed by the University of Glamorgan (Pontypridd, Wales, UK),22 and a millimeter ruler placed orthogonally no more than 5 cm from the DFU. Rulers indicated the patient number (to respect anonymization) as well as the date the photograph was taken.
A three-member adjudication committee evaluated ulcer healing and determined whether or not therapeutic decisions made by the principal investigator were warranted. Complete healing of the DFU was defined as complete epithelialization of the ulcer, after ruling out the possibility of hyperkeratosis.
At the end of the study, the EPICES questionnaire and the AGGIR grid were filled out again.
Serious adverse events (SAE) included prolonged hospitalizations (worsening of the ulcer, infection, amputation) and events related to diabetes (complications, severe hypoglycaemia, hyperglycaemia with ketoacidosis). SAEs were collected in the eCRF and reported to the sponsor within 24 h.
Outcomes
The primary outcome was the cumulative number of days spent in hospital (due to DFU) over 12 months. Main secondary outcomes included: (i) the cumulative direct costs over 12 months (hospitalisation, transport, nursing, materials and consultations with the investigator), (ii) healing rates and (iii) amputation rates. It should be noted that in France, the health insurance system reimburses the transport costs (ambulance or light medical vehicle) of those patients living with a long-term illness such as diabetes. Other secondary outcomes included: (i) mean duration of DFU-related hospitalization days, (ii) number of DFU per patient, (iii) time course of DFU, including time to observe complete wound healing, time to first DFU improvement, and time to first DFU worsening (improvement or worsening, in terms of overall appearance, size, budding, infection, etc.), and (iv) number of DFU follow-up visits.
Cost calculations
The following costs were calculated for each patient (global cost over a year, €): consultations with the diabetologist, transportation for visits with the diabetologist, visits with the expert nurse at home, and hospitalizations. Then, for each patient, the total cost of direct care was calculated as the sum of all these costs. Of note, in France, all the costs of medical intervention and follow-up of diabetic patients with foot ulcers are covered 100% by a Public Health Insurance System (“l’Assurance Maladie”, https://www.ameli.fr/). In the TELEPIED study: (i) all costs were direct intervention's production costs, under a collective perspective, in accordance with the French HAS (Haute Autorité de Santé—High Authority of Health) Methodological Guidance for Economic Evaluation, and (ii) the expert nurse's fees were not covered by “l'Assurance Maladie”, but by the study sponsor (CERITD).
Outcomes were assessed using intention-to-treat (ITT) and per-protocol (PP) analyses (see below “Protocol modifications section”). The number of DFU follow-up visits and the number of visits from expert nurse were also recorded (only in the intervention group). Finally, EPICES and AGGIR scores were explored in relation to healing score (at least one wound healed versus none). The study adheres to CONSORT and CHEERS Guidelines (Supplementary Appendix, Part 1 and 2, respectively).
Protocol modifications
A change to the statistical analysis of the previously published protocol19 was made on December 5, 2022.19 Instead of a “main evaluable population”, we analysed: (i) the “intention-to-treat” population (ITT, see Consort 2010 guidelines, Moher et al. 2010), and (ii) the per protocol (PP) population.
Statistical analysis
Following data validation and freezing of the eCRF database, the data analysis was carried out by RCTs Company (Lyon, France), using SAS/Windows 7 (version 9.4 or later). As previously published,19 statistical power was calculated using data from a cohort of 185 outpatients registered in our patient database (SD = 28.77). This calculation estimated a sample size of 90 patients in each group to achieve 80% power to detect a mean difference of 9, 10, 11, and 12 days when the standard deviation was 21, 23, 26, 28, respectively.
The primary outcome was assessed with: (i) an ANCOVA model (Analysis of Covariance) and (ii) a GEE model (GEE model with a negative binomial distribution, including treatment as a fixed effect and ABI as a covariate). ANCOVA was used to assess total costs, length of hospitalization, and the frequency of ulceration (baseline, per patient, and throughout the year). A GEE model (Generalized Estimating Equation) was used to assess the recurrence rate of DFU, healing rate, and amputation rate. Statistical comparisons between groups were made using treatment as a fixed effect and the stratification factor (ABI index) as a covariate. Kaplan–Meier survival analysis was used to estimate delays in first wound improvement, first wound worsening, and wound healing (death and amputation were not considered as competitive risks). Correlations between EPICES and GIR scores and healing score were explored using Student’s T-test (at least one wound healed versus none). Data are presented as n (%) or mean (SE) (95% CI or [range]). Statistical significance was accepted at p < 0.05.
Results
The study was conducted from January 4, 2017 (inclusion of the first patient) to May 12, 2020 (last visit of the last patient). A total of 180 patients were included in the study (Fig. 2). Of these, 90 participants were randomly assigned to the control group and another 90 to the intervention group (ITT population). Seventy-two participants of the control group and 67 participants of the intervention group completed the study and were analysed (PP population; Fig. 2).
Fig. 2.
Consort flowchart of the study showing the number of individuals in the intention-to-treat (ITT) analysis (allocation panels) and in the per-protocol analysis (analysis panels). A total of 180 patients were included in the study and randomly allocated to experimental groups. ITT analysis was performed on 90 participants assigned to standard, hospital follow-up (control group) and another 90 assigned to telemedical, outpatient follow-up (intervention group). Per-protocol analysis (PP) was performed on 72 participants assigned to the control group and another 67 assigned to the intervention group.
Baseline characteristics of the participants were comparable between groups (Tables 1 and 2, for ITT and PP population, respectively). In the ITT population, the mean age of the participants was 67.8 (13.7) years, and 70.6% were male patients. The mean BMI was 28.3 (5.5) kg/m2 and glycated haemoglobin was 7.9 (1.8)%. Sixty percent of patients were married and 66.6% had GSCE education level or lower. Diabetes was type 2 in 158 participants (88.8% of the total) and type 1 in 17 participants (9.6%). The mean duration of diabetes was 19.5 years (12.0). Fifty-seven patients (34.7%) were treated with insulin, 46 patients (28%) received oral antidiabetics (OAD), and 61 patients (37.2%) were on insulin and OAD. The number of ulcers at baseline was 1.5 (0.1) and 1.4 (0.1) in the control and intervention groups, respectively (p = 0.72, ANCOVA model).
Table 1.
Baseline characteristics of the ITT population.
| Control group (n = 90) | Intervention group (n = 90) | All (n = 180) | |
|---|---|---|---|
| Age, years | 66.2 (14.3) | 69.3 (13.0) | 67.8 (13.7) |
| Sex | |||
| Male | 66.0 (73.3) | 61.0 (67.8) | 127 (70.6) |
| Female | 24.0 (26.2) | 29.0 (32.2) | 53.0 (29.4) |
| BMI, kg/m2 | 28.2 (5.0) | 28.5 (6.0) | 28.3 (5.5) |
| Marital status, married | 43 (53.1) | 59 (67.0) | 102 (60.4) |
| Education | |||
| Up to GSCE | 48 (68.5) | 54 (65.0) | 102 (66.6) |
| Bachelor/Master degree | 15 (21.5) | 21 (25.3) | 36 (23.6) |
| Higher diploma | 7 (10.0) | 8 (9.6) | 15 (9.8) |
| HbA1ca, % | 8.0 (1.7) | 7.8 (1.9) | 7.9 (1.8) |
| Type of diabetes | |||
| Type 2 | 76 (86.4) | 82 (91.1) | 158 (88.8) |
| Type 1 | 9 (10.2) | 8 (8.9) | 17 (9.6) |
| Secondary | 1 (1.1) | 0 | 1 (0.6) |
| Genetic | 2 (2.3) | 0 | 2 (1.1) |
| Missing | 2 | 0 | 2 |
| Duration of diabetes, years | 19.0 (12.2) | 19.9 (11.9) | 19.5 (12.0) |
| Treatment for diabetes | |||
| Insulin | 30 (37.5) | 27 (32.2) | 57 (34.7) |
| Pharmacological | 26 (32.5) | 20 (23.8) | 46 (28.0) |
| Insulin + Pharmacological | 24 (30.0) | 37 (44.0) | 61 (37.2) |
| ABI, number of patients | |||
| <0.7 | 24 (26.7) | 23 (25.6) | 47 (26.1) |
| 0.7 ≤ ABI ≤ 0.9 | 25 (27.8) | 24 (26.7) | 49 (27.2) |
| >0.9 | 41 (45.6) | 43 (46.7) | 84 (46.1) |
| Number of ulcers | 1.5 (0.1) | 1.4 (0.1) | 1.5 (0.1) |
Data are mean (SD) for continuous variables and n (%) for categorical values. BMI, body mass index.
ABI, ankle-brachial index.
HbA1c values assessed during the previous three months.
Table 2.
Baseline characteristics of the PP population.
| Control group (n = 72) | Intervention group (n = 67) | All (n = 139) | |
|---|---|---|---|
| Age, years | 64.2 (14.1) | 69.8 (12.7) | 66.9 (13.7) |
| Sex | |||
| Male | 56.0 (77.8) | 43.0 (64.2) | 99 (7.2) |
| Female | 16.0 (22.2) | 24.0 (35.8) | 40.0 (28.8) |
| BMI, kg/m2 | 28.4 (5.1) | 28.5 (0.9) | 28.4 (5.5) |
| Marital status, married | 38 (55.9) | 46 (68.7) | 84 (62.2) |
| Education | |||
| Up to GSCE | 39 (65.0) | 42 (65.0) | 81 (65.0) |
| Bachelor/Master degree | 14 (23.0) | 16 (25.0) | 30 (24.0) |
| Higher diploma | 7 (12.0) | 7 (11.0) | 14 (11.0) |
| HbA1ca, % | 8.0 (1.7) | 7.7 (1.9) | 7.9 (1.9) |
| Type of diabetes | |||
| Type 2 | 60 (83.3) | 62 (92.5) | 122 (87.8) |
| Type 1 | 9 (12.5) | 5 (7.5) | 14 (10.1) |
| Secondary | 1 (1.4) | 0 | 1 (0.7) |
| Genetic | 2 (2.8) | 0 | 2 (1.4) |
| Missing | 0 | 0 | 0 |
| Duration of diabetes, years | 18.4 (12.3) | 20.4 (12.4) | 19.4 (12.2) |
| Treatment for diabetes | |||
| Insulin | 25 (38.5) | 20 (31.3) | 45 (34.9) |
| Pharmacological | 21 (32.3) | 14 (21.9) | 35 (27.1) |
| Insulin + Pharmacological | 19 (29.2) | 30 (46.9) | 49 (38.0) |
| ABI, number of patients | |||
| <0.7 | 20 (27.8) | 14 (20.9) | 34 (24.5) |
| 0.7 ≤ ABI ≤ 0.9 | 21 (29.2) | 20 (29.9) | 41 (29.5) |
| >0.9 | 31 (43.1) | 33 (49.3) | 64 (46.0) |
| Number of ulcers | 1.5 (0.8) | 1.5 (1.1) | 1.5 (1.0) |
Data are mean (SD) for continuous variables and n (%) for categorical values.
BMI, body mass index; ABI, ankle-brachial index.
HbA1c values assessed during the previous three months.
Baseline characteristics of the PP population (Table 2) were very similar to those of the ITT population, including ABI stratification (with 28 and 21% of patients with an ABI lower than 0.7, as compared to 27 and 26% in the ITT population, presented in Table 1).
At inclusion, the EPICES score was 40.1 (2.4) in the control group and 36.1 (2.1) in the intervention group (ns). Similarly, the AGGIR scores were similar between groups: 5.7 (0.8) in the control group and 5.6 (0.04) in the intervention group (ns).
Primary outcome
ITT population
Cumulative hospital days over 12 months (primary outcome) were 13.4 days (95% CI 9.0–17.8) in the control group and 7.1 days (95% CI 2.8–11.5) in the intervention group. The adjusted mean difference (6.3 days; 95% CI 0.1–12.4) was statistically significant (ANCOVA model, p = 0.0458) (Table 3). The reduction of hospital stay with telemonitoring was confirmed with a generalized linear model (GEE) (data not shown).
Table 3.
Trial outcomes for the ITT population.
| Control groupa (n = 90) | Intervention groupa (n = 90) | Adjusted mean differencea | Statistical significance | |
|---|---|---|---|---|
| Primary outcome | ||||
| Days spent in hospital (cumulated over 1 year) | 13.4 (9.0–17.8) |
7.1 (2.8–11.5) |
6.3 (0.1–12.4) |
p = 0.0458b |
| Secondary outcomes (main) | ||||
| Direct costs (€, cumulated over 1 year) | 7185 (5144–9226) |
3471 (1430–5512) |
3714 (827–6600) |
p = 0.0120b |
| Healing rate (%) | 52.4 (41.3–61.7) |
62.1 (53.2–71.0) |
−9.7 (−21.1 to 2.7) |
p = 0.1246c {p = 0.0025}d |
| Amputation rate (%) | 15.6 (8.1–23.1) |
12.4 (5.2–19.5) |
3.2 (−7.0 to 13.4) |
p = 0.5356c {p = 0.8637}d |
| Secondary outcomes (others) | ||||
| Mean duration of hospitalization (days) | 4.1 (0.8) 2.5‒5.8 |
3.3 (0.8) 1.7–5.0 |
0.8 −1.5 to 3.2 |
p = 0.4947e {p = 0.3448}d |
| Number of DFU per patients | 2.6 (0.3) 2.0–3.2 |
2.0 (0.3) 1.4–2.5 |
0.58 −0.2 to 1.4 |
p = 0.1543b {p = 0.2344}d |
| Delay to first improvement (days) | 77 58–98 |
21 15–37 |
n.a. | p = 0.0002f n.a. |
| Delay to first aggravation (days) | 213 126–319 |
>365 196–(n.a.) |
n.a. | p = 0.0362f n.a. |
| Delay to healing (days) | 98 70–149 |
85 49–113 |
n.a. | p = 0.1031f n.a. |
| Number of DFU follow-up visitsg | 4.2 (0.4) [2.0; 6.0] | 6.7 (0.5) [3.0; 10.0] | n.a. | p < 0.001h – |
| Number of visits from expert nursed | n.a. | 10.0 (0.6) [6.0; 14.0] | n.a. | n.a. |
Data given as mean (SE, upper line) and 95% CI (lower line; except for IQR, seeg).
ANCOVA model (results confirmed with a GEE model, see text).
GEE model.
Statistical significance of the covariate (ABI).
ANCOVA.
Kaplan–Meier survival time analysis (0.75 survival probability; see text).
IQR, between square brackets.
Statistical significance evaluated with a Wilcoxon test.
PP population
Similar results were obtained in the PP population. ANCOVA analysis showed that cumulative hospital days over 12 months were 14.8 days (95% CI 9.7–19.8) in the control group and 7.1 days (95% CI 1.8–12.3) in the intervention group. The adjusted mean difference (7.7 days; 95% CI 0.4–15.0) was statistically significant (p = 0.0387; see Table 4). This was further confirmed with a GEE model (data not shown; GEE model with a negative binomial distribution, including treatment as a fixed effect and ABI as a covariate).
Table 4.
Trial outcomes for the PP population.
| Control groupa (n = 72) | Intervention groupa (n = 67) | Adjusted mean differencea | Statistical significance | |
|---|---|---|---|---|
| Primary outcome | ||||
| Days spent in hospital (cumulated over 1 year) | 14.8 9.7–19.8 |
7.1 1.8–12.3 |
7.7 0.4–15.0 |
p = 0.0387b |
| Secondary outcomes (main) | ||||
| Direct costs (€, cumulated over 1 year) | 8088 5622–10555 |
3911 1363–6468 |
4177 618–7737 |
p = 0.0218b |
| Healing rateb (%) | 53.7 44.0–63.4 |
66.5 56.9–76.1 |
−12.8 −25.9 to 0.3 |
p = 0.0559c {p = 0.0006}d |
| Amputation rate (%) | 16.7 8.1–25.4 |
13.4 4.8–22.0 |
3.3 −8.5 to 15.2 |
p = 0.5817c {p = 0.6558}d |
| Secondary outcomes (others) | ||||
| Mean duration of hospitalization (days) | 4.0 (0.7) 2.5‒5.4 |
2.8 (0.8) 1.3–4.3 |
1.16 −0.90 to 3.22 |
p = 0.2667b {p = 0.5926}d |
| Number of DFU per patient | 2.4 (0.3) 1.8–3.0 |
2.0 (0.3) 1.4–2.6 |
0.38 −0.5 to 1.2 |
p = 0.3720b {p = 0.0864}d |
| Delay to 1st improvement (days) | 70.0 46.0–89.0 |
18.0 11.0–30.0 |
n.a. | p = 0.0012e n.a. |
| Delay to 1st aggravation (days) | 176.0 98.0–261.0 |
>365 196–(n.a.) |
n.a. | p = 0.0029e n.a. |
| Delay to healing (days) | 98.0 70.0–149.0 |
80.0 43.0–112.0 |
n.a. | p = 0.1302e n.a. |
| Number of DFU follow-up visitsf | 4.6 (0.4) [2.0; 7.0] | 7.4 (0.6) [3.0; 11.0] | n.a. | p < 0.001g – |
| Number of visits from expert nursef | n.a. | 11.7 (0.6) [9.0; 15.0] | n.a. | n.a. |
Data given as mean (SE, upper line) and 95% CI (lower line; except for IQR, seef).
ANCOVA.
GEE model.
Statistical significance of the covariate (ABI).
Kaplan–Meier survival time analysis (0.75 survival probability; see text).
IQR, between square brackets.
Statistical significance evaluated with a Wilcoxon test.
Main secondary outcomes
Direct costs
In the ITT population, cumulative direct costs over 12 months were 7185 € (95% CI 5144–9226) in the control group and 3471 € (95% CI 1430–5512) in the intervention group: the adjusted mean difference (3714 € [95% CI 827–6600]) was statistically significant (p = 0.0120) (Table 3). In the PP population, these figures were 8088 € (95% CI 5622–10,555) in the control group and 3911 € (95% CI 1363–6468) in the intervention group: the adjusted mean difference, 4177 € [95% CI 618–7737], was statistically significant (p = 0.0218) (Table 4). The per item stratification of costs is presented in the Supplementary Appendix, Part 3 (Supplementary Table S1a and S1b, for the ITT and PP populations, respectively).
Healing rate
In the ITT population, over the one-year protocol duration, the healing rate of DFU’s was 52.4 (95% CI 41.3–61.7) in the control group as compared to 62.1 (95% CI 53.2–71.0) in the intervention group; the −9.7% difference was not significant (p = 0.1246, ANCOVA model) (Table 3). Of note, the ABI covariate influence over healing rate was highly significant (p = 0.0025). Results were similar in the PP population, i.e., both favoured intervention (−12.8%, although the difference did not reach significance, p = 0.0559) and the ABI covariate (p = 0.0006; Table 4).
Amputation rate
This outcome was similar in the control and intervention groups, with 15.6% (95% CI 8.1–23.1) amputations in the control group versus 12.4% amputations in the intervention group (95% CI 5.2–19.5), with no control either by the covariate (Table 3). Essentially identical results were obtained with the PP population (Table 4).
Other secondary outcomes
In the ITT population, the mean duration of DFU-related hospitalization days was 4.1 (0.8) and 3.3 (0.8) days in the control and intervention group, respectively (ns; Table 3). Similar results were obtained in the PP population: 4.0 (0.7) and 2.8 (0.8) days, respectively (ns, Table 4).
A total of 341 DFUs were analysed during the 12 months of the study, 184 in the control group and 157 in the intervention group. A large portion of the UPD injuries (66.0%) consisted of a plantar puncture injury.
In the ITT population, the number of ulcers per patient was 2.6 (0.3) and 2.0 (0.3), in the control and intervention groups, respectively. The difference (0.6 ulcer [95% CI −0.2 to 1.4]), was not significant (Table 3). Similar results were obtained in the PP population (Table 4).
In the ITT population, the delay to first wound improvement was significantly longer in the control group (75% estimated survival probability 77.0 days, 95% CI 58.0–98.0) than in the intervention group (21.0 days, 95% CI 15.0–37.0) (p = 0.0002; Table 3). Similarly, in the PP population, this delay was significantly longer in the control group (75% estimated survival probability was 70.0 days, 95% CI 46.0–89.0) than in the intervention group (18.0 days, 95% CI 11.0–30.0) (p = 0.001, Table 4). On the other hand, the delay to first wound aggravation in the intervention group was significantly longer than in the control group: estimated 75% survival time of the control group was 176.0 days (95% CI 98.0–261.0) while the corresponding value for the control group was higher than the duration of the study (365 days, p = 0.003; Table 3). Similar results were obtained with the PP population (Table 4).
The estimated wound healing delay was 98.0 (95% CI 70.0–149.0) and 80.0 (95% CI 43.0–112.0) days in the control and intervention group, respectively (p = 0.130). The percentage of patients with recurrence of at least one ulcer was 13.6% (95% CI 5.8–21.4]) in the control group versus 32.5% (95% CI 21.2–43.7) in the intervention group. The difference (−18.9%; 95% CI −32.6 to −5.2) was statistically significant (p = 0.0070).
During the 12 months of the study, patients of the control group made an average of 5.4 (SD 3.6) visits to the diabetologist, versus 2.6 (SD 2.5) in the intervention group. In the intervention group, patients had 11.7 (SD 4.5) visits from the expert nurse. Overall, a total of 4.6 (SD 3.4) visits for DFU follow-up were made in the control group, versus 7.4 (SD 5.0) in the intervention group (p < 0.01) (Tables 3 and 4).
At the end of the study, no correlation was observed between wound healing and the EPICES score or the AGGIR score.
One-hundred-twenty-four (124) adverse events occurred during the study (59 events in the control group, 65 events in the intervention group). Among them, 102 were serious adverse events (50 in the control group, 52 in the intervention group). Fourteen deaths were reported: 7 deaths each group. None of these adverse events were attributable to the intervention.
Discussion
Hospital stays and direct costs of medical care were twice as low in patients with telemedical follow-up (by a diabetologist assisted by an expert nurse) than in patients with conventional follow-up. This reduction in medical and economic burden was obtained without losing therapeutic efficacy for ulcer healing and without increasing the amputation rate.
The reduced medical burden (hospital stay) confirmed and extended previous (but not all) studies showing the efficacy of telemonitoring to replace outpatient visits, without losing clinical efficacy.16, 17, 18,23,24 The addition of a skilled ulcer care nurse to help community nurses provide standard daily care appears to us to have played a crucial role. Telemedical consultations were carried out between the expert nurse and the physicians at the outpatient clinic (complemented with an image of the ulcer and a detailed written evaluation). No significant differences regarding healing and amputation were found between groups.23 Smith-Strom et al.24 reported that telemonitoring (weekly telemedicine consultations with a healthcare specialist and outpatient clinic visits every 6 weeks), was non-inferior to standard outpatient monitoring (outpatient clinic visits every second week) in healing time. The intervention group had a significantly lower proportion of amputations.24
Fasterholdt et al.25 performed a post-hoc analysis of the RCT by Rasmussen et al.23 and reported that telemonitoring costs were 2039 € less per patient compared to standard monitoring, although this difference was not statistically significant. In our study, the cost was significantly lower with telemonitoring compared to standard monitoring, by 4177 €. This can be explained by the reduced number of days of hospitalization and by the fact that patients did not have to be transported to the hospital for their visits (transport costs are covered by the basic French health insurance), in the intervention group.
In our study, both the delay to first improvement and the delay to worsening of ulcerations were significantly improved with telemonitoring care compared with standard treatment. This can be due to the monthly home visits by the expert nurse, presumably ensuring a better monitoring and diagnosis of the wound evolution.
We have also observed a higher rate of ulcer recurrence in the intervention group. This could be attributed to a more complete examination in the intervention group (through the expert nurse during home visits) and an earlier diagnosis in a minimal stage of wound (therefore, more easily curable and without the need for hospitalization). On the contrary, the diagnosis of recurrence in the control group patients could have occurred later (therefore, at a more serious stage of the wound), thus contributing to a higher rate of hospitalizations.
In the early DFU telemonitoring studies by Clemensen et al.26 patients were offered three consecutive video consultations to substitute three visits to the outpatient clinic.26 The hospital team performed the clinical examinations and made decisions remotely, in close cooperation with the community nurse and the patient.26 In the RCTs by Rasmussen et al.23 and Smith-Strom et al.24 community nurses provided standard care under the remote supervision of an expert nurse. In our study, the expert nurse visited the patient at home approximately once a month (visits occurred every second week during regular follow-up, then once a month after healing). The exact influence of such follow-up modalities on cost of ulcer care and ulcer outcome deserves future investigation.
Conducted in a context of real-life conditions, our study puts forward another interesting aspect: it defines a new wounds management for subjects suffering from DFU’s, based on the intervention of an expert nurse providing remote monitoring instead of a physician. This could prove of particular interest in areas of medical desertification.
Limitations of the study
An important limitation of the study was that DFU recurrence rates were not as well assessed in the control group as they were in the intervention group. This means that only in the intervention group the diabetologist investigator was able to assess DFU recurrence rates at the patient's home (through the expert nurse). Other limitations were: (i) TELEPIED was an open trial (assessors and patients were unblinded to treatment),27 (ii) the patient cohort used to calculate statistical power was not representative of the TELEPIED cohort (in fact, the TELEPIED study was underpowered for the observed effect size), (iii) death and amputation were not considered as competitive risks in the Kaplan–Meier survival analysis; a post-hoc sensitivity analysis could be performed in the near future to determine how model variables are affected by changes in those input variables (death and amputation), (iv) the study did not investigate patient satisfaction or quality of life, and (v) we have not compared the hospital stay and costs before and after the telemedical intervention in the same patient, and it could be that the intervention group had a priori shorter or fewer hospital stays. Future studies are necessary to test this hypothesis.
In conclusion, the telemedical intervention with an expert nurse could lead to a length of hospitalization and direct costs that are two times lower compared to conventional follow-up. This lower medical and economic burden was obtained without losing effectiveness on the rate of healing, nor increasing the amputation rate. Additional studies are required to confirm an refine these findings.
Contributors
DD, GC and AP conceived of the presented idea. DD developed the theory and performed the computations. DD and EB carried out the protocol. GC and AP, LO and SF verified the analytical methods. DD, MB, IX, KLS, ZA and BD supervised the findings of this work. All authors discussed the results and contributed to the final manuscript.
Data sharing statement
Data collected for the study and presented herein will be made available. Data will be organised in a database, and participant data will be de-identified. Related study documents, including the study protocol, informed consent forms, and statistical analysis plan, will also be available. Data requests should be sent by email to the corresponding author (dured.dardari@chsf.fr) who will supply the requested information.
Declaration of interests
DD has received personal compensation for board participation and speaking fees from Sanofi and Urgo. SF has received personal compensation for board participation and speaking fees from Novo Nordisk, Roche Diagnostics, Lifescan, Sanofi, Diabeloop and Eli Lilly, received Research support from MSD and is employed by CERITD and is a shareholder of Diabeloop. GC is employed by CERITD and received personal compensation for board participation, research funding, and speaking fees from Astra-Zeneca, Boehringer, Eli Lilly, Johnson & Johnson, MSD, Novo-Nordisk, Sanofi-Aventis and Voluntis. LO and EB are employed by CERITD.MB has no conflict of interest to declare. IX was employed by CERITD. ZA (Zohra Amrous) is employed by CERITD.KLS has no conflict of interest to declare. BD was employed by Cemka, a consulting team specializing in health economics, epidemiology, and outcomes research. BD also received personal compensation for board participation and speaking fees from MSD, Novo-Nordisk, Sanofi, Eli Lilly, Janssen and Pfizer. AP received personal compensation for board participation and speaking fees from Abbott, Ascencia, Astra-Zeneca, Eli Lilly, Medtronic, MSD, Novartis, Novo Nordisk and Sanofi Aventis.
Acknowledgements
The authors acknowledge Patrick Hannaert (CNRS, INSERM U1313, Poitiers, France) and Ricardo P. Garay (Craven, Villemoisson-sur-Orge, France) for editorial support.
Footnotes
Trial Registration: The TELEPIED trial is registered with ClinicalTrials.govNCT02986256.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanepe.2023.100686.
Appendix A. Supplementary data
References
- 1.Oliver T.I., Mutluoglu M. StatPearls Publishing; 2022. Diabetic foot ulcer.https://www.ncbi.nlm.nih.gov/books/NBK537328/ [PubMed] [Google Scholar]
- 2.Sorber R., Abularrage C.J. Diabetic foot ulcers: epidemiology and the role of multidisciplinary care teams. Semin Vasc Surg. 2021;34(1):47–53. doi: 10.1053/j.semvascsurg.2021.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong D.G., Boulton A.J.M., Bus S.A. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376(24):2367–2375. doi: 10.1056/NEJMra1615439. [DOI] [PubMed] [Google Scholar]
- 4.Margolis D.J., Malay D.S., Hoffstad O.J., et al. Data Points Publication Series; Rockville (MD): 2011. Economic burden of diabetic foot ulcers and amputations: data Points #3. [PubMed] [Google Scholar]
- 5.Armstrong D.G., Swerdlow M.A., Armstrong A.A., Conte M.S., Padula W.V., Bus S.A. Five year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J Foot Ankle Res. 2020;13(1):16. doi: 10.1186/s13047-020-00383-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edmonds M., Manu C., Vas P. The current burden of diabetic foot disease. J Clin Orthop Trauma. 2021;17:88–93. doi: 10.1016/j.jcot.2021.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MC. Mayo Clinic . 2022. Amputation and diabetes: how to protect your feet.https://www.mayoclinic.org/diseases-conditions/diabetes/in-depth/amputation-and-diabetes/art-20048262 [Google Scholar]
- 8.Prompers L., Huijberts M., Apelqvist J., et al. High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe. Baseline results from the Eurodiale study. Diabetologia. 2007;50(1):18–25. doi: 10.1007/s00125-006-0491-1. [DOI] [PubMed] [Google Scholar]
- 9.Walsh J.W., Hoffstad O.J., Sullivan M.O., Margolis D.J. Association of diabetic foot ulcer and death in a population-based cohort from the United Kingdom. Diabet Med. 2016;33(11):1493–1498. doi: 10.1111/dme.13054. [DOI] [PubMed] [Google Scholar]
- 10.Schaper N.C., van Netten J.J., Apelqvist J., et al. Practical guidelines on the prevention and management of diabetic foot disease (IWGDF 2019 update) Diabetes Metab Res Rev. 2020;36(Suppl 1) doi: 10.1002/dmrr.3266. [DOI] [PubMed] [Google Scholar]
- 11.ADA. American Diabetes Association 11. Microvascular complications and foot care: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S135–S151. doi: 10.2337/dc20-S011. [DOI] [PubMed] [Google Scholar]
- 12.Everett E., Mathioudakis N. Update on management of diabetic foot ulcers. Ann N Y Acad Sci. 2018;1411(1):153–165. doi: 10.1111/nyas.13569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DHHS. Department of Health & Human Services . 2011. Agency for health care research and quality. Data points #3: economic burden of diabetic foot ulcers and amputations. Content last reviewed December 2019.https://effectivehealthcare.ahrq.gov/products/diabetes-foot-ulcer-amputation-economics/research [PubMed] [Google Scholar]
- 14.Khunkaew S., Fernandez R., Sim J. Health-related quality of life among adults living with diabetic foot ulcers: a meta-analysis. Qual Life Res. 2019;28(6):1413–1427. doi: 10.1007/s11136-018-2082-2. [DOI] [PubMed] [Google Scholar]
- 15.Drovandi A., Wong S., Seng L., et al. Remotely delivered monitoring and management of diabetes-related foot disease: an overview of systematic reviews. J Diabetes Sci Technol. 2023;17(1):59–69. doi: 10.1177/19322968211012456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yammine K., Estephan M. Telemedicine and diabetic foot ulcer outcomes. A meta-analysis of controlled trials. Foot. 2022;50 doi: 10.1016/j.foot.2021.101872. [DOI] [PubMed] [Google Scholar]
- 17.Jafary M.R., Amini M.R., Sanjari M., et al. Comparison home care service versus hospital-based care in patients with diabetic foot ulcer: an economic evaluation study. J Diabetes Metab Disord. 2020;19(1):445–452. doi: 10.1007/s40200-020-00527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hazenberg C., Aan de Stegge W.B., Van Baal S.G., Moll F.L., Bus S.A. Telehealth and telemedicine applications for the diabetic foot: a systematic review. Diabetes Metab Res Rev. 2020;36(3) doi: 10.1002/dmrr.3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dardari D., Franc S., Charpentier G., et al. Telepied study: a single-centre trial in diabetic subjects comparing total duration of hospitalization over a 1-year period required for complete healing of a foot ulcer using telemedicine management and a referral nurse versus the standard care pathway. Diabetes Ther. 2020;11(6):1419–1427. doi: 10.1007/s13300-020-00821-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Labbe E., Blanquet M., Gerbaud L., et al. A new reliable index to measure individual deprivation: the EPICES score. Eur J Public Health. 2015;25(4):604–609. doi: 10.1093/eurpub/cku231. [DOI] [PubMed] [Google Scholar]
- 21.SNGC. Syndicat National de Gérontologie Clinique AGGIR: guide pratique pour la codification des variables, principaux profils des groupes iso-ressources. Rev Geriatr. 1994;19:249–259. [Google Scholar]
- 22.Plassmann P. Measuring wounds. J Wound Care. 1995;4(6):269–272. doi: 10.12968/jowc.1995.4.6.269. [DOI] [PubMed] [Google Scholar]
- 23.Rasmussen B.S., Froekjaer J., Bjerregaard M.R., et al. A randomized controlled trial comparing telemedical and standard outpatient monitoring of diabetic foot ulcers. Diabetes Care. 2015;38(9):1723–1729. doi: 10.2337/dc15-0332. [DOI] [PubMed] [Google Scholar]
- 24.Smith-Strom H., Igland J., Ostbye T., et al. The effect of telemedicine follow-up care on diabetes-related foot ulcers: a cluster-randomized controlled noninferiority trial. Diabetes Care. 2018;41(1):96–103. doi: 10.2337/dc17-1025. [DOI] [PubMed] [Google Scholar]
- 25.Fasterholdt I., Gerstrom M., Rasmussen B.S.B., Yderstraede K.B., Kidholm K., Pedersen K.M. Cost-effectiveness of telemonitoring of diabetic foot ulcer patients. Health Informatics J. 2018;24(3):245–258. doi: 10.1177/1460458216663026. [DOI] [PubMed] [Google Scholar]
- 26.Clemensen J., Larsen S.B., Kirkevold M., Ejskjaer N. Treatment of diabetic foot ulcers in the home: video consultations as an alternative to outpatient hospital care. Int J Telemed Appl. 2008;2008 doi: 10.1155/2008/132890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Probst P., Grummich K., Heger P., et al. Blinding in randomized controlled trials in general and abdominal surgery: protocol for a systematic review and empirical study. Syst Rev. 2016;5:48. doi: 10.1186/s13643-016-0226-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.