Abstract
Herpes simplex virus (HSV) and varicella-zoster virus (VZV) are two pathogenic human alphaherpesviruses whose intracellular assembly is thought to follow different pathways. VZV presumably acquires its envelope in the trans-Golgi network (TGN), and it has recently been shown that its major envelope glycoprotein, VZV-gE, accumulates in this compartment when expressed alone. In contrast, the envelopment of HSV has been proposed to occur at the inner nuclear membrane, although to which compartment the gE homolog (HSV-gE) is transported is unknown. For this reason, we have studied the intracellular traffic of HSV-gE and have found that this glycoprotein accumulates at steady state in the TGN, both when expressed from cloned cDNA and in HSV-infected cells. In addition, HSV-gE cycles between the TGN and the cell surface and requires a conserved tyrosine-containing motif within its cytoplasmic tail for proper trafficking. These results show that VZV-gE and HSV-gE have similar intracellular trafficking pathways, probably reflecting the presence of similar sorting signals in the cytoplasmic domains of both molecules, and suggest that the respective viruses, VZV and HSV, could use the same subcellular organelle, the TGN, for their envelopment.
Varicella-zoster virus (VZV) and herpes simplex virus (HSV) are two human pathogens that belong to the alpha subfamily of herpesviruses. Both viruses possess a lipidic envelope in which different glycoproteins of viral origin are embedded (27, 62). However, the pathways taken by both viruses during their intracellular maturation have remained controversial. It is clear that nucleocapsids assemble in the nucleus and that they become enveloped during budding from the inner nuclear membrane into the periplasmic space, both for HSV (62) and for VZV (14, 23, 24). In the case of VZV, observation of VZV-infected cells at the ultrastructural level has led to the proposal that VZV virions, after reaching the periplasmic space, lose their primary envelope by fusing with the outer nuclear membrane and that they become subsequently wrapped by the membrane of the trans-Golgi network (TGN) (24, 84). A similar pathway has been proposed for a related herpesvirus—the pseudorabies virus (PRV) (26, 81). This pathway could be referred to as “cytoplasmic envelopment.” In support of this model, it has been shown the VZV-gE, the most abundant envelope glycoprotein of the VZV envelope, is transported to the TGN, in both VZV-gE-transfected cells (1, 85, 86) and in VZV-infected cells (1). Another glycoprotein of the VZV envelope, gI, is also transported to the TGN in VZV-infected cells and in VZV-gI-transfected cells when VZV-gE is simultaneously expressed (1).
The transport of the VZV-gE glycoprotein to the TGN has been studied in detail and has been shown to require at least two different types of signals within its cytoplasmic domain: two tyrosine-based signals and an acidic stretch containing phosphorylatable amino acids (2, 86). Tyrosine-containing sorting motifs usually consist of a tetrapeptide bearing the sequence YXXØ (Y is tyrosine, X is any amino acid, and Ø represents any hydrophobic residue) (42, 66, 76). They are thought to perform their sorting function by directly interacting with the medium chains of the assembly or adapter proteins AP-1 and AP-2—two heterotetrameric protein complexes associated, respectively, with the TGN or the plasma membrane (55)—and AP-3, a new adapter-related protein complex of unknown function (16, 68). The assembly proteins AP-1 and AP-2 can be recruited onto the TGN and the plasma membrane, respectively, where they interact with transmembrane proteins containing tyrosine-based motifs (57, 67). Thus, tyrosine-based motifs are able to function in many different transport steps (42). In contrast, only a limited number of examples have illustrated the role of acidic stretches of amino acids in regulating the intracellular traffic of membrane proteins. For example, the TGN localization of furin requires the presence of a casein kinase II phosphorylation site in its cytoplasmic domain (33). A similar site in the mannose 6-phosphate receptors’ cytoplasmic tails determines high-affinity binding of AP-1 (44). In the case of VZV-gE, the concerted action of tyrosine-containing motifs and casein kinase II phosphorylation sites also determines (i) its localization at steady state in the TGN; (ii) its cycling between this compartment, endosomes, and the cell surface; and (iii) its ability to promote the translocation of the Golgi-specific assembly proteins (AP-1) onto the TGN membrane (2, 54, 85, 86).
An alternative model of intracellular assembly, referred to here as “nuclear envelopment,” has been proposed for HSV-1. According to this model, the HSV capsids would also acquire their envelope by budding into the periplasmic space of the nuclear envelope, but this is thought to be the final envelope containing the full set of viral glycoproteins (75). These glycoproteins are presumed to mature while the virus is transported along the secretory pathway (8). This model implies that all envelope glycoproteins are targeted to the inner membrane of the nuclear envelope.
In the present study, we addressed the question of whether the proposed differences in the biogenesis of VZV and HSV-1 correlate with differential intracellular trafficking of their respective envelope glycoproteins. We have expressed the glycoprotein gE from HSV-1 (HSV-gE) in HeLa cells and shown that it localizes at steady state in the TGN, like in HSV-infected cells. In addition, gE cycles between this compartment and the cell surface. This TGN localization is determined by a tyrosine-based motif and a stretch of negatively charged amino acids in the gE cytoplasmic tail that are conserved among the members of the Alphaherpesvirinae subfamily. Our data support the notion that HSV envelopment occurs at the TGN rather than at the inner nuclear membrane.
MATERIALS AND METHODS
Materials.
The HSV-gE full-length open reading frame in the pCMV3 vector was provided by Harvey Friedman (University of Pennsylvania, Philadelphia). The VZV-gE expression construct has been previously described (2). Monoclonal antibodies (MAbs) 7520 and 3114/109 specific for HSV-gE were provided by Peter Marsden (MRC Virology Unit, Glasgow, United Kingdom). MAb SG1 specific for VZV-gE was provided by Viro Research, Inc. (Rockford, Ill.). The human melanoma cell line MeWo was provided by J. Piette (University of Liège, Liège, Belgium).
Recombinant DNA procedures.
To clone the full-length HSV-gE into the mammalian expression vector pSFFV6 (10), a 1.8-kb fragment containing the complete HSV-gE open reading frame was excised from the pCMV3gE-1 construct (provided by Harvey Friedman) by using XbaI and cloned in the same site in the pSFFV6 vector. The sequence of the cloned HSV-gE was found to be identical to the published sequence derived from strain 17 (47).
The HSV-gEΔ1, -Δ2, -Δ3, and -Δ4 truncation mutants were obtained by PCR with the pCMV3gE-1 construct as a template and Pfu DNA polymerase (Stratagene, Heidelberg, Germany). The forward primer (5′-GCTCTAGAATGGATCGCGGGGCGGTGGTGGGG-3′) contained an XbaI site followed by the sequence corresponding to the first eight amino acids of HSV-gE. Different reverse primers were used that consisted of the sequence encoding the last 10 amino acids of the HSV-gE cytoplasmic tail to be included followed by a stop codon and an XbaI site. The resulting fragments were digested with XbaI and cloned in the same site in the pSFFV6 vector.
The Y463A point mutation in the pSFFV-HSV-gE construct was introduced by a PCR-based site-directed mutagenesis (19) with the wild-type HSV-gE as a template and synthetic oligonucleotides that contained the desired mutations within their sequence to produce two fragments overlapping the mutagenic site. These fragments were reamplified by a second round of PCR with external primers to generate the whole HSV-gE open reading frame bearing the desired mutation.
All constructs were verified by sequencing by the dideoxy chain termination method.
Antibody production.
The antibody 1667 against the full-length gE was obtained by cloning a cDNA fragment coding for the mature VZV-gE open reading frame with a hexahistidine tag at the C terminus into the NcoI-BamHI sites of the pET15b vector (Novagen, Wiesbaden, Germany). The protein was expressed in BL21 cells, and the insoluble fraction (containing most of the recombinant gE) was solubilized in 8 M urea and loaded on a Talon metal affinity column (Clontech, Heidelberg, Germany). After extensive washing, the bound protein was eluted with sodium dodecyl sulfate loading buffer, and approximately 50 μg was loaded on a preparative sodium dodecyl sulfate–7.5% polyacrylamide gel. The part of the gel containing the recombinant protein was excised, homogenized with a Teflon-glass homogenizer, mixed with either Freund’s complete adjuvant or incomplete adjuvant, and used to immunize rabbits according to standard procedures. The rabbit serum can be used in immunofluorescence experiments to detect VZV-gE in fixed cells, as well as in immunoprecipitation experiments to precipitate both the immature and mature forms of VZV-gE (1).
Multiple sequence alignment.
The sequences of the gE homologs from HSV-1 (47), equid herpesvirus 1 (HEV-1) (72), bovine herpesvirus (BHV-1) (36), VZV (15), simian varicella virus (SVV) (21), and PRV (56) were retrieved from the SwissProt database, and the sequence of the gE gene from feline herpesvirus 1 (FHV-1) (48) was obtained from the GenBank database. The extent of the cytoplasmic domain of each protein was estimated by using the PredictProtein PHDhtm program (63). The different cytoplasmic domains were aligned by using the PILEUP program from the Wisconsin package, followed by slight manual adjustment.
Transient and stable transfections.
Transient transfections were carried out by the calcium phosphate precipitate procedure. For this purpose, a precipitate was prepared by mixing under constant agitation equal volumes of 2 × HBS (50 mM HEPES, 280 mM NaCl, 1.43 mM NaH2PO4 [pH 7.07]) and a 25 mM CaCl2 solution containing 40 ng of each of the DNAs per μl and incubating the mixture for 30 min at room temperature. One hundred microliters of the precipitate was added per well containing 1 ml of medium. After 24 h, the cells were extensively washed with phosphate-buffered saline and incubated for another 16 h with fresh medium. For stable transfections, a similar procedure was used, except that the MeWo cells were plated in 10-cm-diameter dishes and transfected with 150 μg of pSRα-STVSVG, a plasmid containing the VSVG-tagged sialyltransferase (ST-VSVG) (58) and the neomycin resistance gene (71). Forty-eight hours after transfection, G418 was added to the culture medium to a final concentration of 0.6 mg/ml. Colonies were picked and tested for expression of ST-VSVG by immunofluorescence with the P5D4 monoclonal antibody specific for the VSVG epitope.
HSV-2 infections.
HeLa cells transiently transfected with the indicated TGN markers or stable clones of MeWo cells expressing ST-VSVG were seeded on coverslips (11 mm in diameter) and incubated for 2 h with 200 μl of a tissue culture supernatant of HSV-2-infected MRC-5 cells showing 100% cytopathic effect. After removal of the medium containing the virus, cells were washed two times with warm minimal essential medium (α-MEM), incubated for the indicated times with fresh medium, and directly processed for immunofluorescence. HSV-2-gE shows 73% identity at the amino acid level with HSV-1-gE. Furthermore, both gE homologs can be labeled with the same MAb in immunofluorescence experiments, suggesting that they show a high degree of structural conservation.
Immunofluorescence.
Cells were fixed by incubation with 4% paraformaldehyde for 15 min at room temperature. The excess paraformaldehyde was quenched with a 50 mM NH4Cl solution, and the cells were permeabilized with 0.1% Triton X-100 for 5 min. After being quenched with 10% goat serum for 30 min, the coverslips were layered on a drop of goat serum in which the primary antibodies (or the mixture of them) had been added at the proper dilution and incubated for 30 min. After three washes of 10 min each with phosphate-buffered saline, the coverslips were incubated in a way similar to that used before with the secondary antibodies, washed again three times, and mounted on microscopy slides by using Mowiol and DABCO (Sigma). The cells were visualized with a Zeiss Axioscop and a ×63 (N.A. = 1.4) oil immersion Zeiss lens. Pictures were collected on 400 ASA black and white Kodak Tmax film by using a photographic camera attached to the microscope. The photographic negatives were digitized at 3,000 dots per mm using a ScanMaker III scanner with a transparency adapter. The digitalized images were then stored and mounted by using the image processing software Adobe Photoshop 3.0 running on a PowerMacintosh 9500 computer.
To verify the quality of the antibodies, pilot experiments were performed in which cells were single transfected with HSV-gE and stained with the anti-VZV-gE antibody. This experiment revealed no antigenic cross-reactivity between HSV-gE and VZV-gE. In addition, the localization experiments (see Fig. 1) were also performed with two different MAbs directed against HSV-gE and with two different antibodies directed against the VSVG epitope, when a tagged version of HSV-gE in which the VSVG epitope had been inserted in the luminal domain of HSV-gE was used. All of these antibodies gave identical results, indicating that the antibody used throughout the whole study was not just recognizing immature or partially processed forms of HSV-gE.
FIG. 1.
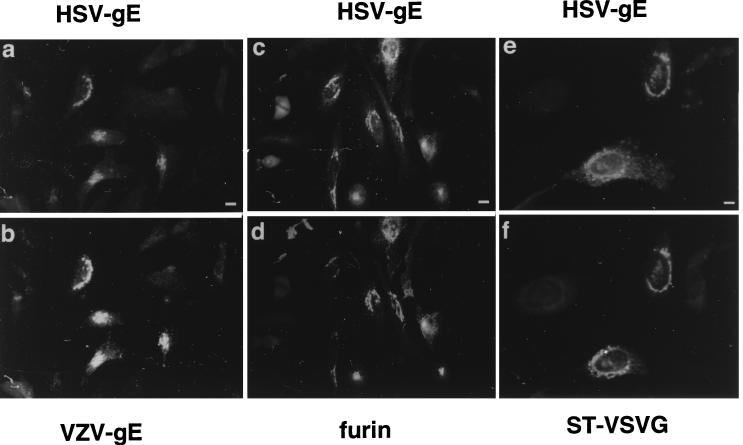
Colocalization of HSV-gE with TGN proteins. HeLa cells double transfected with mammalian expression vectors encoding HSV-gE and either VZV-gE (a and b), furin (c and d), or ST-VSVG (e and f) were visualized with the 7520 MAb against HSV-gE (a, c, and e) and the 1667 polyclonal antiserum against VZV-gE (b), a polyclonal serum against furin (d), and a polyclonal antiserum against the VSVG epitope (f), followed by fluorescein isothiocyanate- or rhodamine-coupled antirabbit and antimouse secondary antibodies. Bar, 5 μm.
Antibody uptake experiments.
For the internalization assays, a continuous uptake was performed in which transfected cells seeded on coverslips were washed with prewarmed α-MEM medium and overlaid with 200 μl of complete α-MEM in which the antibodies had been diluted as specified in the figure legends. After 1 h of incubation, the internalization medium was removed, and the cells were immediately fixed and processed for immunofluorescence with fluorescein- or rhodamine-coupled secondary antibodies.
RESULTS
HSV-1-gE is located at steady state in the TGN.
We first compared the intracellular distributions of HSV-1-gE and VZV-gE by cotransfecting HeLa cells with eukaryotic expression vectors encoding both proteins. By double indirect immunofluorescence microscopy, VZV-gE was exclusively located in the perinuclear region of the cell as shown previously (Fig. 1b) (2). The signal corresponding to HSV-gE was also present in the perinuclear region (Fig. 1a), largely colocalizing with VZV-gE. It has been proposed that overexpression of VZV-gE in HeLa cells leads to the formation of VZV-gE homodimers (53). Therefore, in principle, it would be possible that the intracellular retention of HSV-gE could result from its heterodimerization with VZV-gE. To exclude this possibility, the intracellular distribution of HSV-gE was compared to that of two other unrelated TGN markers in the absence of VZV-gE expression: the convertase furin (49, 64, 70, 80) and a VSVG epitope-tagged version of sialyltransferase (ST-VSVG) (59). In both cases, an almost identical perinuclear colocalization of the molecules considered could be observed (Fig. 1c and d and e and f). In every transfection experiment, around 10% of the HSV-gE-expressing cells showed some degree of cell surface staining (not shown). A similar behavior was observed for the cotransfected TGN markers in the same cells. These cells were assumed to express the transfected genes at high levels and, therefore, were not considered further in the subsequent transfection experiments.
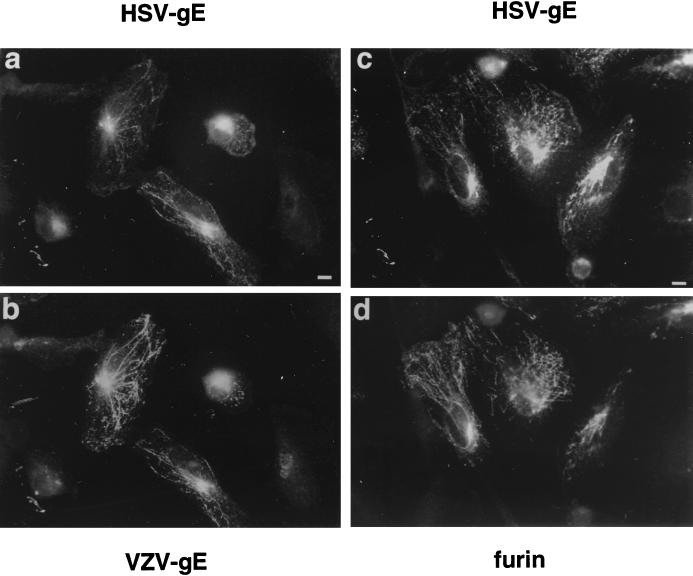
The cells expressing HSV-gE were also treated for 5 min with 10 μg of brefeldin A (BFA) per ml, a drug known to induce a rapid tubularization of the TGN and endosomes (38, 60). When this treatment was applied to cells that had been double transfected with either VZV-gE and HSV-gE or furin and HSV-gE, both proteins were redistributed into the same tubular structures (Fig. 2).
FIG. 2.
Effect of BFA treatment on the subcellular distribution of HSV-gE. HeLa cells were double transfected with pSFFV-HSV-gE and either pSFFV-VZV-gE (a and b) or pSG5-furin (c and d) and treated for 5 min with 10 μg of BFA per ml. After fixation, the coverslips were processed for indirect immunofluorescence to detect HSV-gE (7520 MAb) (a and c) and VZV-gE (1667 polyclonal antiserum) (b) or furin (d). Bar, 5 μm.
These results showed that in the absence of additionally encoded viral factors, HSV-gE, like VZV-gE, localizes to the TGN at steady state. This localization does not reflect a saturation of the export process, because the same localization was observed when the cells were pretreated with 10 μg of cycloheximide per ml for 1 h prior to fixation (not shown).
Distribution of HSV-gE in HSV-2-infected cells.
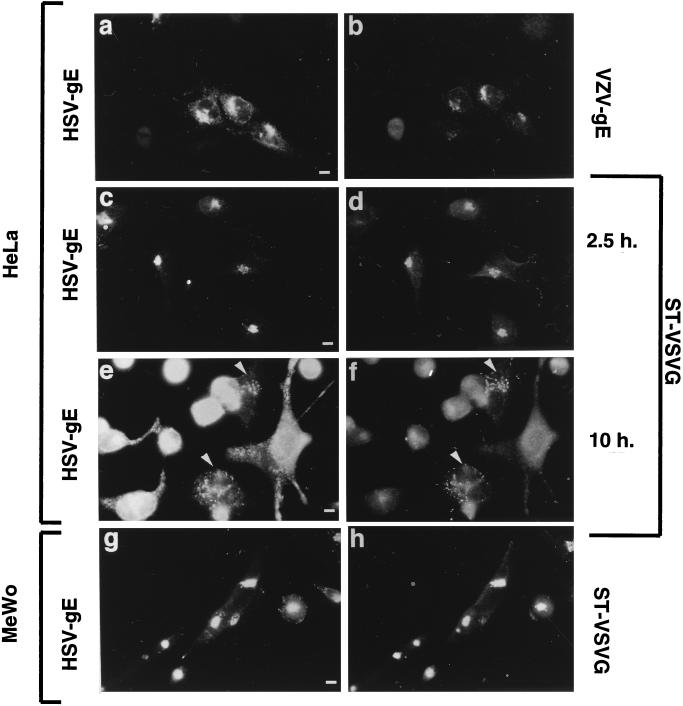
Does HSV-gE show the same localization in HSV-infected cells? To address this question, HeLa cells were transiently transfected with either VZV-gE or ST-VSVG expression constructs and subsequently infected with HSV-2 as described in Materials and Methods. The distribution of HSV-gE and of the transfected proteins was determined by immunofluorescence microscopy with the 7520 antibody, which recognizes both HSV-1- and HSV-2-gE. At early time points after infection (2.5 to 5 h postinfection) HSV-gE was found in the perinuclear region of the infected cells, colocalizing with the two transfected TGN proteins, VZV-gE (Fig. 3a and b) and ST-VSVG (Fig. 3c and d). When the cells were fixed 30 min after addition of the virus, no labelling of the cells could be detected with the anti-HSV-gE antibody (not shown), most likely due to the small amount of viruses added to the cell monolayer. These data suggest that the signal observed in the TGN after 2.5 h of infection corresponds to the newly synthesized HSV-gE rather than to the protein delivered to the cell by the incoming virus. In cells infected for longer times (10 to 20 h postinfection), there was a strong cytopathic effect. However, in those few cells that showed reduced cytopathic effect, HSV-gE labeling was predominantly found in cytoplasmic vesicles. These vesicles were also labeled with antibodies against ST-VSVG (Fig. 3e and f) or VZV-gE (data not shown). HSV infection is known to lead to a fragmentation of the Golgi complex, most likely as a secondary effect resulting from the disassembly of the microtubule network (3, 7). Thus, the cytoplasmic vesicles containing HSV-gE and ST-VSVG most likely represent the fragmented Golgi complex.
FIG. 3.
Intracellular distribution of HSV-gE in HSV-2-infected cells. (a to f) HeLa cells grown on coverslips were transfected with plasmids encoding VZV-gE (a and b) or ST-VSVG (c to f) and, 40 h after transfection, exposed to a tissue culture supernatant of MRC-5 cells infected with HSV-2 for 2 h, and then the inoculum was removed and further incubated for another 2.5 h (a to d) or 10 h (e and f). Cells were fixed and processed for indirect immunofluorescence by using the 7520 anti-HSV-gE MAb (a, c, and e) and either the 1667 polyclonal antiserum against VZV-gE (b) or a polyclonal antiserum against the VSVG epitope (d and f). (g and h) A clone of MeWo cells stably expressing ST-VSVG was exposed to a tissue culture supernatant of MRC-5 cells infected with HSV-2 for 5 h, fixed, and stained with the 7520 anti-HSV-gE MAb (g) and a polyclonal antiserum against the VSVG epitope (h). Bar, 5 μm.
In addition, we have generated cloned MeWo cells that stably express ST-VSVG. When these cells were infected with HSV-2, already at 2.5 to 5 h postinfection, most of the MeWo cells showed a clear signal for HSV-gE in the perinuclear region of the cell, colocalizing with ST-VSVG (Fig. 3g and h). In contrast to the HeLa cells, no apparent disassembly of the TGN was observed in MeWo cells, even after long infection times (data not shown). This is consistent with results from other studies showing that the HSV-induced Golgi fragmentation only occurs in some cell types (3, 7). The colocalization of HSV-2-gE with TGN markers, both in the intact TGN and after fragmentation of this organelle, indicates that gE accumulates in the TGN as well in HSV-infected cells.
HSV-1-gE cycles between the TGN and the cell surface.
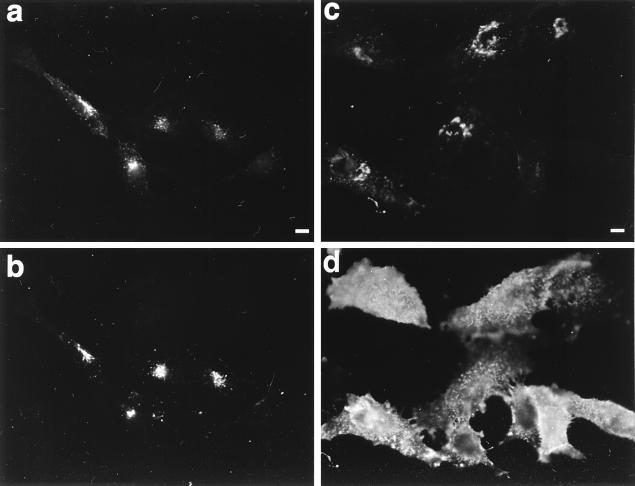
Since the accumulation of VZV-gE at the TGN relies, at least partially, on its fast retrieval from the cell surface (2, 86), we investigated whether this was also the case for HSV-gE. HeLa cells were double transfected with VZV-gE and HSV-gE expression plasmids and incubated at 37°C with a mixture of antibodies against the luminal domains of both proteins. After fixation, the localization of the exogenously added antibodies was examined by indirect immunofluorescence microscopy. The antibodies against both proteins were colocalized in the perinuclear region of the cell (Fig. 4a and b), suggesting that both glycoproteins can reach the plasma membrane, where they can bind exogenously added antibody molecules and then drag them into an intracellular compartment, presumably the TGN. These data also suggest that although the bulk of HSV-gE is found at steady state in the TGN, a minor and undetectable fraction of the protein must be present in the plasma membrane and/or in endocytic compartments.
FIG. 4.
Recycling of HSV-gE through the plasma membrane. HeLa cells were double transfected with plasmids encoding VZV-gE and either HSV-gE (a and b) or HSV-gEΔ1 (c and d) and 48 h after transfection were incubated for 1 h at 37°C in culture medium containing 1:200 dilutions of the 1667 polyclonal rabbit antiserum against VZV-gE and the 7520 MAb against HSV-gE. Cells were fixed and processed for immunofluorescence by using a mixture of fluorescein isothiocyanate-coupled antirabbit IgG (to detect anti-VZV-gE [a and c]) and rhodamine (tetramethyl rhodamine isothiocyanate)-coupled antimouse IgG (to detect anti-HSV-gE [b and d]). Bar, 5 μm.
Since neither HSV-gE nor VZV-gE shows binding activity toward murine Fc, the trivial possibility that the anti-HSV-gE had been internalized by being bound to the luminal domain of VZV-gE can be excluded. However, the possibility remained that the anti-VZV-gE antibodies had been internalized by being bound to the luminal domain of HSV-gE. If this were happening, it would not be possible to conclude that after internalization, both glycoproteins follow the same pathway and reach the same compartment. To exclude this possibility, an analogous experiment was performed with cells expressing VZV-gE and an HSV-gE mutant lacking the cytoplasmic domain (HSV-gEΔ1). Under these conditions, all of the anti-VZV-gE antibody was internalized as efficiently as before (Fig. 4c), whereas the anti-HSV-gE antibody remained associated with the plasma membrane (Fig. 4d). Since no anti-VZV-gE antibody was detected at the cell surface, this result indicates that internalization of anti-VZV-gE was exclusively mediated by its binding to VZV-gE and not through the Fc binding activity of HSV-gE. Therefore, both HSV-gE and VZV-gE accumulate in the same intracellular compartment after internalization.
The cytoplasmic tail of HSV-gE contains potential TGN-sorting information conserved in the gE homologs from other Herpesviridae.
All currently known herpesviruses, although initially ascribed to this viral family according to morphological and biological criteria (61), are now thought to be derived from a common ancestor (45). Therefore, we reasoned that if HSV-gE and VZV-gE follow similar trafficking pathways, their sorting signals could have also been conserved during evolution. Since all of the sorting information in the sequence of VZV-gE resides in its cytoplasmic tail (2, 86), we compared the degrees of conservation within the cytoplasmic tails of HSV-gE and those of the different gE homologs.
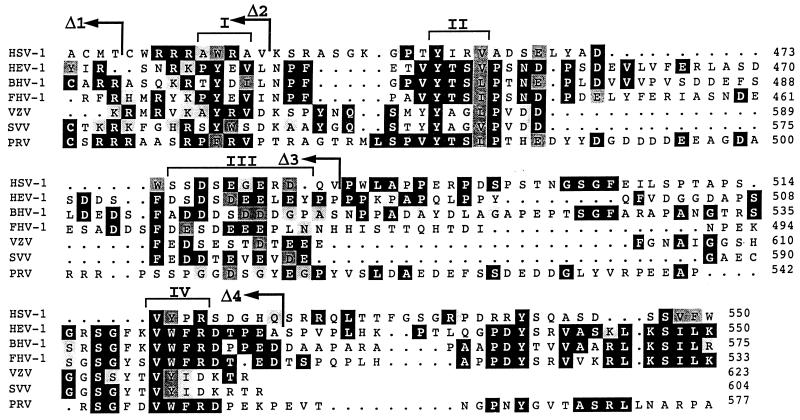
Figure 5 shows the multiple sequence alignment of the cytoplasmic tails of the gE homologs from HSV-1, HEV-1, BHV, FHV-1, VZV, SVV, and PRV. Four regions with high degrees of similarity could be distinguished (regions I to IV in Fig. 5). Interestingly, two of these highly conserved regions (II and III in Fig. 5) were previously shown to contain sorting information for the VZV-gE homolog, namely the tyrosine-containing tetrapeptide (region II) (2) and the cluster of acidic residues containing phosphorylatable amino acids (region III) (2, 86). In the tyrosine motif, both the essential tyrosine and the hydrophobic residue at position +3 are conserved within all of the proteins considered (Fig. 5).
FIG. 5.
Multiple sequence alignment of the cytoplasmic tails of the gE homologs forms seven different members of the Alphaherpesvirinae subfamily. The sequences shown include those of HSV-1 (47), HEV-1 (72), BHV-1 (36), FHV-1 (48), VZV (15), SVV (21), and PRV (56). The sequences of the gE homologs from HSV-1, HEV-1, BHV-1, VZV, SVV, and PRV are under SwissProt accession no. vgle_hsv11, vgle_hsveb, vgle_hsvbs, vgle_vzvd, vgle_svvd, and vgl_prvri, respectively, and FHV-1 gE is under GenBank accession no. X98449. Identical amino acids found in at least three of the seven sequences are shown as white characters on a black background, and conserved substitutions are shown on a grey background. Brackets on top of the alignment indicate the conserved regions discussed in the text. The amino acid positions for every sequence (considering the full-length precursor proteins) at the end of each block in the alignment are shown in the right-hand margin. The last amino acids of the different HSV-gE truncation mutants (Δ1, Δ2, Δ3, and Δ4) are indicated by arrows.
In addition, all of the sequences contain one or more serine or threonine residues within the cluster of acidic amino acids that are potential casein kinase II phosphorylation sites (Fig. 5). In HSV-gE, the tyrosine-based motif consists of the tetrapeptide YIRV, and within the acidic cluster, which is reduced in length compared to that from VZV, there are two serine residues with aspartic acid at position +3 (consensus site for casein kinase II phosphorylation [Fig. 5]). Two additional regions also possess a high degree of similarity among all of the sequences considered: a tyrosine-containing tetrapeptide (region I), which has also been shown to be involved in determining the intracellular sorting of VZV-gE (86); and a stretch containing a conserved aromatic residue surrounded by two hydrophobic amino acids (region IV). Interestingly, another consensus tyrosine-based signal can be found in the sequences of HEV-1, BHV-1, and FHV-1, and a putative dileucine motif is present at positions −2 and −3 (from the C terminus) of the sequences of HEV-1, BHV-1, and FHV-1.
Sorting determinants of the HSV-gE cytoplasmic tail necessary for TGN localization.
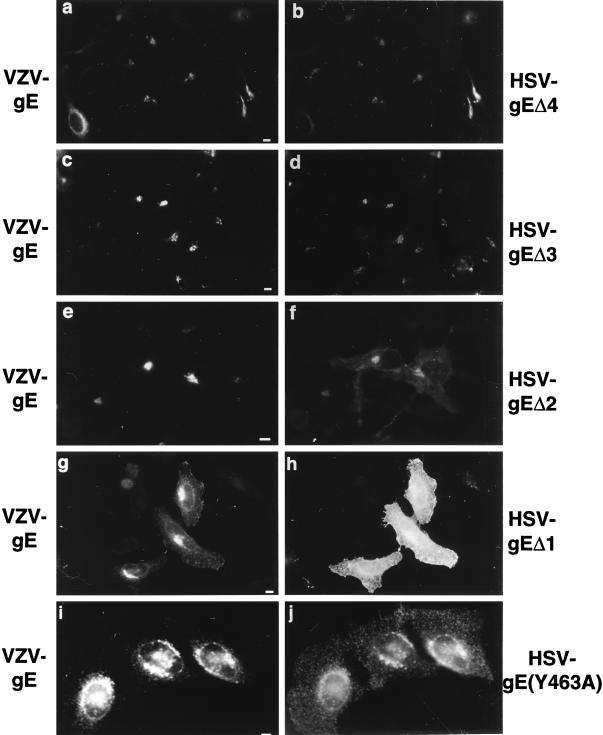
Based on the homologies found among the gE homologs, we introduced truncations in the HSV-gE cytoplasmic tail in order to progressively remove the four conserved regions (Fig. 5) and investigated by indirect immunofluorescence the steady-state distribution of different mutants. A mutant lacking the last 28 amino acids of the tail (HSV-gEΔ4) or interrupted right after the acid-rich region (HSV-gEΔ3) showed steady-state distributions identical to those of the wild-type protein (Fig. 6a to d). Removal of the conserved acid-rich region (HSV-gEΔ2) led to a partial mislocalization of the mutated HSV-gE to the cell surface (Fig. 6 e and f), whereas a completely truncated form of HSV-gE (HSV-gEΔ1) was exclusively found at the cell surface (Fig. 6 g and h). Therefore, two sorting signals within the HSV-gE cytoplasmic tail seem to be required for the proper TGN localization of this molecule: one found within the acidic stretch (region III in Fig. 5) and a second one located within the first 28 amino acids of HSV-gE tail containing two potential tyrosine-based sorting motifs (regions I and II in Fig. 5). The tyrosine at position 463 fits into the consensus sequence for tyrosine-based sorting signals and is conserved among the gE homologs considered. Replacement of this tyrosine by an alanine (HSV-gE Y463A) resulted in a partial but substantial mislocalization of HSV-gE (Fig. 6i and j).
FIG. 6.
Intracellular distribution of HSV-gE mutants in the cytoplasmic domain. HeLa cells were double transfected with a plasmid encoding VZV-gE and either HSV-gEΔ4 (a and b), the HSV-gEΔ3 (c and d), the HSV-gEΔ2 (e and f), or the HSV-gEΔ1 (g and h) truncation mutants or the HSV-gE(Y463A) (i and j) point mutant and stained with a mixture of the 1667 polyclonal antiserum against VZV-gE (a, c, e, g, and i) and the 7520 MAb against HSV-gE (b, d, f, h, and j). Bar, 5 μm.
DISCUSSION
We present here evidence that the HSV glycoprotein gE accumulates at steady state in the TGN, both in cells expressing HSV-gE from cloned cDNA and in HSV-infected cells. HSV-gE gains access to the cell surface, from where it can be rapidly endocytosed. HSV-gE trafficking requires both an intact tyrosine-based motif and an acidic stretch of amino acids in its cytoplasmic tail. Thus, HSV-gE and VZV-gE exhibit an identical behavior that might also be shared with the gE homologs from the different alphaherpesviruses.
The subcellular localization of HSV-gE in transfected and HSV-infected cells.
In this paper, we provide different evidence suggesting that HSV-gE can be found in the TGN of cells that are either transfected with an HSV-gE expression construct or infected with HSV. First, HSV-gE colocalizes in the perinuclear region of the cell with three different TGN markers. Second, HSV-gE redistributes into cytoplasmic tubules in response to BFA treatment. And third, in HSV-infected cells, HSV-gE and ST-VSVG still colocalize to the cytoplasmic vesicles that result from the fragmentation of the TGN. The last finding can be considered additional evidence for the colocalization of both proteins in normal cells, since it often occurs that proteins found in closely apposed but distinct compartments separate into different vesicle populations when the organization of the microtubule cytoskeleton is lost (65).
Similar localization experiments performed with VZV-gE-transfected cells (2, 85, 86) and VZV-infected cells (1) have provided similar conclusions with regard to the VZV-gE homolog. In the case of cells infected with PRV, the gE glycoprotein has also been detected in the perinuclear region of the cell (74). However, several studies have now reported the presence of HSV-gE in the surface of HSV-gE-transfected cells by using either an immunoglobulin G (IgG)-coated erythrocyte binding assay (28) or by immunofluorescence and flow cytometry (4). In addition, VZV-gE has been detected in the surface of transfected cells (39), as well in VZV-infected cells (40, 50).
The most likely explanation for such discrepancies is that the localization of the herpesvirus gE glycoprotein might depend on its expression levels. We have previously shown that the localization of these glycoproteins involves its recycling from the cell surface back to the TGN (Fig. 4) (2). At low levels of expression, most of the cells will contain the protein exclusively in the TGN. However, if the expression levels increase, the endocytic machinery responsible for the recycling of these glycoproteins will become saturated, leading to their accumulation at the cell surface. In fact, most of the studies conducted so far that have reported the appearance of VZV-gE or HSV-gE in the cell surface have been carried out with expression systems that confer very high expression levels (vaccinia virus T7 and cytomegalovirus promoters and recombinant adenovirus) (4, 28, 40). A similar behavior has also been reported for other unrelated TGN markers that also rely on endocytic recycling for proper localization (43).
The obvious question that comes up is whether the expression levels reached in herpesvirus-infected cells are high enough to determine the cell surface appearance of the gE glycoproteins. Our previous study of VZV-gE (1), together with data from other groups (40, 50), and the results presented in the present paper for HSV-gE show that some of the HSV- and VZV-infected cells display a clear surface staining of the gE glycoproteins. However, we have also detected both HSV- and VZV-infected cells in which gE is exclusively found in the perinuclear region (1) (Fig. 3). Whether these cells represent an intermediate stage in the biogenesis of the respective viruses or whether they correspond to cells undergoing an abortive infective process remains to be established. However, the increasing evidence that has accumulated that different herpesviruses could use the TGN as a membrane donor for its lipidic envelope (24, 26, 35, 69, 81) suggests that the transient localization of the envelope glycoproteins in the TGN could be an intermediate stage in the maturation process of the virus required for its proper envelopment. This is further supported by the fact that the appearance of gE in the TGN of herpesvirus-infected cells seems to be restricted to the earlier stages of infection (Fig. 3) (1).
Role of tyrosine-based motifs in the intracellular traffic of gE and other viral glycoproteins.
The HSV-gE cytoplasmic domain contains one putative, highly conserved tyrosine-based sorting signal (region II). The mutation of the key tyrosine leads to the partial mislocalization of the protein to the cell surface. Among the gE homologs, there is an additional tyrosine-containing internalization motif (region I) that in VZV-gE (the AYRVD sequence), has been shown to contribute to some extent to the endocytosis of this protein (86). In the HSV-gE tail, however, this tyrosine is replaced by a tryptophan, giving rise to the AWRAV sequence. The ability of a tryptophan to substitute for a tyrosine within endocytic signals does not appear to be a general rule (76). Therefore, mutagenesis of the AWRA signal in the HSV-gE tail will be required to determine whether it has been evolutionarily preserved as a functional internalization motif. In addition to the conserved tyrosine-containing motif, the deletion mutagenesis analysis indicates the existence of an additional sorting determinant in the cytoplasmic tail of HSV-gE. This signal could correspond to the conserved acidic stretch containing potential casein kinase II phosphorylation sites (region III). Serine and threonine residues within this region in the tail of VZV-gE have been shown to be phosphorylated both in vitro by casein kinase II and in vivo (83). In addition, substitution by alanine of the phosphorylatable residues within this region leads to a partial mislocalization of VZV-gE (2, 86). Evidence for a role of phosphorylation in determining the intracellular transport of other unrelated TGN markers has been provided in the case of the prohormone convertase furin (33, 70). It remains to be established whether the homologous region in the tail of HSV-gE could also be phosphorylated and whether this is required for its proper intracellular transport. Experiments performed with PRV have shown that its gE homolog is also found in a perinuclear region of the cell in infected cells (74). In addition, internalization of PRV-gE glycoprotein from the surface of PRV-infected cells is inhibited at 6 h postinfection (73). This result is in agreement with the increased surface staining observed in our experiments with HSV-infected cells at 10 h postinfection. It is tempting to speculate that improper functioning of the endocytic machinery in infected cells might be a general mechanism used by most herpesviruses during the last stages of infection to increase accumulation of their envelope glycoproteins at the cell surface.
Mutations in the tyrosine-based sorting signal in the HSV-gE tail lead to its partial mislocalization. Since HSV-gE cycles between the TGN and the cell surface, the effect of the tyrosine mutation could result from a defect in the rapid endocytosis of the protein. A similar situation has been reported for two other TGN proteins (VZV-gE and TGN38) which also rely on tyrosine motifs for their efficient internalization (2, 5, 29, 54, 82). We have recently shown that overexpression of VZV-gE promotes the recruitment of AP-1 onto the TGN membranes and that this process requires the cytoplasmic tail of VZV-gE (2). Therefore, it is also conceivable that HSV-gE would act in a similar manner.
Evolutionary conservation of the sorting motifs in the gE homologs.
The multiple sequence alignment of the cytoplasmic tails of the gE homologs from different herpesviruses indicates that the two sorting signals previously identified in the tail of VZV-gE (the YXX0 motif and the acidic stretch with phosphorylatable residues) (2) are well conserved in other members of the family. Although the Alphaherpesvirinae subfamily has undergone extensive evolutionary divergence (45), the conservation of the sorting signals in every member of the family suggests that functional constraints have prevented them from disappearing during evolution and that, therefore, they must play an essential role in viral biology.
Based on phylogenetic analysis of the sequences of the gB homologs from different alphaherpesviruses (46), HSV-1 has been proposed to be the most divergent member of different herpesviruses considered in Fig. 5. Nevertheless, our data indicate that, indeed, HSV-gE, as happens with VZV-gE, is found in the TGN at steady state and cycles between this compartment and the cell surface. These findings suggest that in other viruses of the family, which are still more closely related to VZV than HSV-1, the corresponding gE homologs will most likely follow similar behavior. Given the high degree of conservation of the different sorting signals in all of the alphaherpesvirus gE homologs, it is likely that all of these molecules traffic inside the cell following identical signal-mediated transport processes. However, most of the alphaherpesviruses are released from infected cells, whereas VZV remains associated with intracellular compartments of an endocytic nature (22, 24). Our results would therefore suggest that this different behavior reflects differences in late steps of virus biogenesis.
HSV-gE localization and its role in the viral life cycle of HSV.
How can we reconcile the data presented herein about the intracellular traffic of gE with the two different models that have been put forward to explain herpesvirus biogenesis? The so-called “cytoplasmic envelopment” model has been mainly derived from studies performed with VZV (24, 34), PRV (26, 81), and Epstein-Barr virus, a gammaherpesvirus (25). However, different lines of evidence suggest that HSV and related herpesviruses could follow an analogous pathway. For instance, ultrastructural studies performed with HSV-1 (32, 35) and a related species (69) have shown that viral nucleocapsids undergo sequential envelopment by the inner nuclear membrane, followed by release of naked nucleocapsids to the cytoplasm, from which they are enveloped again by a Golgi-derived membrane. In addition, when HSV- and PRV-infected cells are exposed to BFA, naked nucleocapsids accumulate in the cytosol (11, 81), whereas the nuclear envelopment model would predict that under these conditions, enveloped viruses should accumulate in the periplasmic space. In addition, Browne and coworkers (6) have constructed a recombinant HSV in which the glycoprotein gH has been modified to introduce a KKXX endoplasmic reticulum (ER) retention motif. When cells are infected with the recombinant HSV, the amount of viruses released into the medium is the same as that in cells infected with the wild-type virus. However, these viruses are completely devoid of gH and have a 100-fold-lower infectivity than cells infected with the wild-type virus. This result suggests that the ER nuclear membrane does not act as a donor for the viral envelope, but rather that virions acquire their final envelope in a post-ER compartment, from which the modified gH is absent because of the ER retention motif. Further evidence in favor of a cytoplasmic envelopment model for the biogenesis of HSV derives from the work of van Gederen and coworkers (79). These authors have shown that the lipid composition of isolated HSV envelopes is very different from that of nuclear membranes, suggesting that the nuclear membrane does not act as a membrane donor for the viral envelope.
While we cannot formally exclude the presence of small amounts of HSV-gE in the inner nuclear membrane, the accumulation of HSV-gE in the TGN in HSV-infected cells provides additional support in favor of HSV following the cytoplasmic envelopment model during its biogenesis. Whether other HSV envelope glycoproteins can also be transported to the TGN remains to be characterized. This could occur either by using so-far-uncharacterized sorting signals within their sequences or, more likely, because they associate with other glycoproteins that carry the sorting information. In fact, the latter situation seems to hold true in the case of VZV, whose gI glycoprotein cannot be transported to the TGN when expressed alone, but is efficiently transported to the TGN when gE is simultaneously expressed (1). In the case of PRV, experiments performed with mutant PRV strains have shown that its gI glycoprotein cannot be internalized from the surface of PRV-infected cells, unless a full-length gE is simultaneously expressed (73). A similar mechanism might be used as well by HSV to colocalize gE and gI, since these two molecules can also be found as a complex in HSV-infected cells (31).
The gE and gI homologs from HSV, PRV, and BHV-1 are not required for viral infection of cultured cells (20, 52, 77). However, gI and gE mutants of most alphaherpesviruses produce much smaller plaques when tested on cultured cells (17, 30, 87) and show a reduced pathogenicity to host animals (9, 51, 77, 78). Both phenotypes could be due to an impairment of the spread of the mutant viruses via direct cell-to-cell contact, as has been shown to occur in gE-negative HSV-1 mutants, both in cultured monolayers (17) and in synaptically linked neurons (18). In addition to direct cell-to-cell transmission, infectious HSVs are released into the medium from infected cells. These viruses can bind and penetrate new cells. This process does not seem to require the gE-gI complex (20, 52, 77), but rather requires gD, another envelope glycoprotein (37). Therefore, it is tempting to speculate that in the case of VZV, which relies exclusively on cell-to-cell spread to infect new cells (13, 22, 23), a functional gE-gI complex will be absolutely required for the viability of the virus. In contrast, deletion of the gE and/or the gI genes in other herpesviruses that can also propagate via the extracellular medium will result in a much less severe growth defect. The facts that gE is the major envelope glycoprotein in VZV (27), that there is no gD homolog in the VZV genome (15), and that gE-deficient VZVs have not been isolated so far all suggest that the function of this protein is essential for the viability of VZV. Two different groups have reported the isolation of gI-deficient VZV. Although this protein is not essential for VZV viability, gI-negative viruses show a small-plaque phenotype (12, 41), suggesting that also in the case of VZV, the gE-gI complex might be for direct cell-to-cell contact. However, the fact that in both VZV and HSV, gE can be detected in the TGN in the early stages of the infection suggests that this protein, together with gI, may play a role in herpesvirus biogenesis different from mediating cell-to-cell spread.
ACKNOWLEDGMENTS
We acknowledge Peter Marsden (MRC Virology Unit, Glasgow, United Kingdom) and Harvey Friedman (University of Pennsylvania, Philadelphia) for providing the 7520 and 3114/109 anti-gE MAbs and the pCMV3-gE construct, respectively. The expert technical assistance of M. S. Ebersold is gratefully acknowledged. We are indebted to R. LeBorgne for critical reading of the manuscript, to J. Dubuisson (IBL, Lille, France) for advice on herpesvirus culture, and to G. Duverlie (IBL) for providing the HSV-2 strain.
Work in the author’s laboratory was partially supported by funds from the European Community and “Vaincre les Maladies Lysosomales.” B.S. was supported by a National Institutes of Health grant to Ari Helenius (AI18599). A.A. was supported by a CNRS fellowship.
REFERENCES
- 1.Alconada A, Bauer U, Baudoux L, Piette J, Hoflack B. Intracellular transport of the glycoproteins gE and gI of the varicella-zoster virus: gE accelerates the maturation of gI and determines its accumulation in the trans-Golgi network. J Biol Chem. 1998;273:13430–13436. doi: 10.1074/jbc.273.22.13430. [DOI] [PubMed] [Google Scholar]
- 2.Alconada A, Bauer U, Hoflack B. A tyrosine-based motif and a casein kinase II phosphorylation site regulate the intracellular trafficking of the varicella-zoster virus glycoprotein I, a protein localized in the trans-Golgi network. EMBO J. 1996;15:6096–6110. [PMC free article] [PubMed] [Google Scholar]
- 3.Avitabile E, Di Gaeta S, Torrisi M R, Ward P L, Roizman B, Campadelli-Fiume G. Redistribution of microtubules and Golgi apparatus in herpes simplex virus-infected cells and their role in viral exocytosis. J Virol. 1995;69:7472–7482. doi: 10.1128/jvi.69.12.7472-7482.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basu S, Dubin G, Basu M, Nguyen V, Friedman H M. Characterization of regions of herpes simplex virus type 1 glycoprotein E involved in binding the Fc domain of monomeric IgG and in forming a complex with glycoprotein I. J Immunol. 1995;154:260–267. [PubMed] [Google Scholar]
- 5.Bos K, Wraight C, Stanley K. TGN38 is maintained in the trans-Golgi network by a tyrosine-containing motif in the cytoplasmic domain. EMBO J. 1993;12:2219–2228. doi: 10.1002/j.1460-2075.1993.tb05870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Browne H, Bell S, Minson T, Wilson D W. An endoplasmic reticulum-retained herpes simplex virus glycoprotein H is absent from secreted virions: evidence for reenvelopment during egress. J Virol. 1996;70:4311–4316. doi: 10.1128/jvi.70.7.4311-4316.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campadelli G, Brandimarti R, Di Lazzaro C, Ward P L, Roizman B, Torrisi M R. Fragmentation and dispersal of Golgi proteins and redistribution of glycoproteins and glycolipids processed through the Golgi apparatus after infection with herpes simplex virus 1. Proc Natl Acad Sci USA. 1993;90:2798–2802. doi: 10.1073/pnas.90.7.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campadelli-Fume G, Farabegoli F, Di Gaeta S, Roizman B. Origin of unenveloped capsids in the cytoplasm of cells infected with herpes simplex virus 1. J Virol. 1991;65:1589–1595. doi: 10.1128/jvi.65.3.1589-1595.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Card J P, Enquist L W. The neurovirulence of pseudorabies virus. Crit Rev Neurobiol. 1995;9:137–162. [PubMed] [Google Scholar]
- 10.Chen H J, Remmler J, Delaney J C, Messner D J, Lobel P. Mutational analysis of the cation-independent mannose 6-phosphate/insulin-like growth factor II receptor: a consensus casein kinase II site followed by 2 leucines near the carboxyl terminus is important for intracellular targeting of lysosomal enzymes. J Biol Chem. 1993;268:22338–22346. [PubMed] [Google Scholar]
- 11.Cheung P, Banfield B W, Tufaro F. Brefeldin A arrests the maturation and egress of herpes simplex virus particles during infection. J Virol. 1991;65:1893–1904. doi: 10.1128/jvi.65.4.1893-1904.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen J I, Nguyen H. Varicella-zoster virus glycoprotein I is essential for growth of virus in Vero cells. J Virol. 1997;71:6913–6920. doi: 10.1128/jvi.71.9.6913-6920.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cook M L, Stevens J G. Labile coat: reason for noninfectious cell-free varicella-zoster virus in culture. J Virol. 1968;2:1458–1464. doi: 10.1128/jvi.2.12.1458-1464.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cook M L, Stevens J G. Replication of varicella-zoster virus in cell cultures. An ultrastructural study. J Ultrastruct Res. 1970;32:334–350. doi: 10.1016/s0022-5320(70)80014-4. [DOI] [PubMed] [Google Scholar]
- 15.Davidson A J, Scott J E. The complete DNA sequence of the varicella-zoster virus. J Gen Virol. 1986;67:1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- 16.Dell’Angelica E C, Ohno H, Ooi C E, Rabinovich E, Roche K W, Bonifacino J S. An adaptor-like protein complex with ubiquitous expression. EMBO J. 1997;16:917–928. doi: 10.1093/emboj/16.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dingwell K S, Brunetti C R, Hendricks R L, Tang Q, Tang M, Rainbow A J, Johnson D C. Herpes simplex virus glycoproteins E and I facilitate cell-to-cell spread in vivo and across junctions of cultured cells. J Virol. 1994;68:834–845. doi: 10.1128/jvi.68.2.834-845.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dingwell K S, Doering L C, Johnson D C. Glycoproteins E and I facilitate neuron-to-neuron spread of herpes simplex virus. J Virol. 1995;69:7087–7098. doi: 10.1128/jvi.69.11.7087-7098.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dulau L, Cheyrou A, Aigle M. Directed mutagenesis using PCR. Nucleic Acids Res. 1989;17:2873. doi: 10.1093/nar/17.7.2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Enquist L W, Dubin J, Whealy M E, Card J P. Complementation analysis of pseudorabies virus gE and gI mutants in retinal ganglion cell neurotropism. J Virol. 1994;68:5275–5279. doi: 10.1128/jvi.68.8.5275-5279.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fletcher T M I, Gray W L. DNA sequence and genetic organization of the unique short (US) region of the simian varicella virus genome. Virology. 1993;193:762–773. doi: 10.1006/viro.1993.1185. [DOI] [PubMed] [Google Scholar]
- 22.Gabel C A, Dubey L, Steinberg S P, Sherman D, Gershon M D, Gershon A A. Varicella-zoster virus glycoprotein oligosaccharides are phosphorylated during posttranslational maturation. J Virol. 1989;63:4264–4276. doi: 10.1128/jvi.63.10.4264-4276.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gershon A, Cosio L, Brunell P A. Observations on the growth of varicella-zoster virus in human diploid cells. J Gen Virol. 1973;18:21–31. doi: 10.1099/0022-1317-18-1-21. [DOI] [PubMed] [Google Scholar]
- 24.Gershon A A, Sherman D L, Zhu Z, Gabel C A, Ambron R T, Gershon M D. Intracellular transport of newly synthesized varicella-zoster virus: final envelopment in the trans-Golgi network. J Virol. 1994;68:6372–6390. doi: 10.1128/jvi.68.10.6372-6390.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gong M, Kieff E. Intracellular trafficking of two major Epstein-Barr virus glycoproteins, gp350/220 and gp110. J Virol. 1990;64:1507–1516. doi: 10.1128/jvi.64.4.1507-1516.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Granzow H, Weiland F, Jöns A, Klupp B G, Karger A, Mettenleiter T C. Ultrastructural analysis of the replication cycle of pseudorabies virus in cell culture: a reassessment. J Virol. 1997;71:2072–2082. doi: 10.1128/jvi.71.3.2072-2082.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grose C. Glycoproteins encoded by varicella-zoster virus: biosynthesis, phosphorylation, and intracellular trafficking. Annu Rev Microbiol. 1990;44:59–80. doi: 10.1146/annurev.mi.44.100190.000423. [DOI] [PubMed] [Google Scholar]
- 28.Hanke T, Graham F L, Lulitanond V, Johnson D C. Herpes simplex virus IgG Fc receptors induced using recombinant adenovirus vectors expressing glycoproteins E and I. Virology. 1990;177:437–444. doi: 10.1016/0042-6822(90)90507-n. [DOI] [PubMed] [Google Scholar]
- 29.Humphrey J S, Peters P J, Yuan L C, Bonifacino J S. Localization of TGN38 to the trans-Golgi network: involvement of a cytoplasmic tyrosine-containing sequence. J Cell Biol. 1993;120:1123–1135. doi: 10.1083/jcb.120.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobs L. Glycoprotein E of pseudorabies virus and homologous proteins in other Alphaherpesvirinae. Arch Virol. 1994;137:209–228. doi: 10.1007/BF01309470. [DOI] [PubMed] [Google Scholar]
- 31.Johnson D C, Feenstra V. Identification of a novel herpes simplex virus type 1-induced glycoprotein which complexes with gE and binds immunoglobulin. J Virol. 1987;61:2208–2216. doi: 10.1128/jvi.61.7.2208-2216.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson D C, Spear P G. Monensin inhibits the processing of herpes virus glycoproteins, their transport to the cell surface, and the egress of virions from infected cells. J Virol. 1982;43:1102–1112. doi: 10.1128/jvi.43.3.1102-1112.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones B G, Thomas L, Molloy S S, Thulin C D, Fry M D, Walsh K A, Thomas G. Intracellular trafficking of furin is modulated by the phosphorylation state of a casein kinase II site in its cytoplasmic tail. EMBO J. 1995;14:5869–5883. doi: 10.1002/j.1460-2075.1995.tb00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones F, Grose C. Role of cytoplasmic vacuoles in varicella-zoster virus glycoprotein trafficking and virion envelopment. J Virol. 1988;62:2701–2711. doi: 10.1128/jvi.62.8.2701-2711.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Komuro M, Tajima M, Kato K. Transformation of Golgi membrane into the envelope of herpes simplex virus in rat anterior pituitary cells. Eur J Cell Biol. 1989;50:398–406. [PubMed] [Google Scholar]
- 36.Leung-Tack P, Audonnet J F, Riviere M. The complete DNA sequence and the genetic organization of the short unique region (US) of the bovine herpesvirus type 1 (ST strain) Virology. 1994;199:409–421. doi: 10.1006/viro.1994.1139. [DOI] [PubMed] [Google Scholar]
- 37.Ligas M W, Johnson D C. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by β-galactosidase sequences binds to but is unable to penetrate into cells. J Virol. 1988;62:1486–1494. doi: 10.1128/jvi.62.5.1486-1494.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lippincott-Schwartz J, Yuan L, Tipper C, Amherdt M, Orci L, Klausner R D. Brefeldin A’s effects on endosomes, lysosomes, and the TGN suggest a general mechanism for regulating organelle structure and membrane traffic. Cell. 1991;67:601–616. doi: 10.1016/0092-8674(91)90534-6. [DOI] [PubMed] [Google Scholar]
- 39.Litwin V, Jackson W, Grose C. Receptor properties of two varicella-zoster virus glycoproteins, gpI and gpIV, homologous to herpes simplex virus gE and gI. J Virol. 1992;66:3643–3651. doi: 10.1128/jvi.66.6.3643-3651.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Litwin V, Sandor M, Grose C. Cell surface expression of varicella-zoster virus glycoproteins and Fc receptor. Virology. 1990;78:263–272. doi: 10.1016/0042-6822(90)90402-d. [DOI] [PubMed] [Google Scholar]
- 41.Mallory S, Sommer M, Arvin A M. Mutational analysis of the role of glycoprotein I in varicella-zoster virus replication and its effect on glycoprotein E conformation and trafficking. J Virol. 1997;71:8279–8288. doi: 10.1128/jvi.71.11.8279-8288.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marks M, Ohno H, Kirchhausen T, Bonifacino J S. Protein sorting by tyrosine-based signals: adapting to the Ys and wherefores. Trends Cell Biol. 1997;7:124–128. doi: 10.1016/S0962-8924(96)10057-X. [DOI] [PubMed] [Google Scholar]
- 43.Marks M S, Woodruf L, Ohno H, Bonifacino J S. Protein targeting by tyrosine and di-leucine-based signals: evidence for distinct saturable components. J Cell Biol. 1996;135:341–354. doi: 10.1083/jcb.135.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mauxion F, Le Borgne R, Munier-Lehmann H, Hoflack B. A casein kinase II phosphorylation site in the cytoplasmic domain of the cation-dependent mannose 6-phosphate receptors determines the high affinity interaction of the AP-1 Golgi assembly proteins with membranes. J Biol Chem. 1996;271:2171–2178. doi: 10.1074/jbc.271.4.2171. [DOI] [PubMed] [Google Scholar]
- 45.McGeoch D J. Molecular evolution of large DNA viruses of eukaryotics. Semin Virol. 1992;3:399–408. [Google Scholar]
- 46.McGeoch D J, Cook S. Molecular phylogeny of the alphaherpesvirinae subfamily and a proposed evolutionary timescale. J Mol Biol. 1994;238:9–22. doi: 10.1006/jmbi.1994.1264. [DOI] [PubMed] [Google Scholar]
- 47.McGeoch D J, Dolan A, Donald S, Rixon F J. Sequence determination and genetic content of the short unique region in the genome of herpes simplex virus type 1. J Mol Biol. 1985;181:1–13. doi: 10.1016/0022-2836(85)90320-1. [DOI] [PubMed] [Google Scholar]
- 48.Mijnes J D F, van der Horst L M, van Anken E, Horzinek M C, Rottier P J M, de Groot R J. Biosynthesis of glycoproteins E and I of feline herpesvirus: gE-gI interaction is required for intracellular transport. J Virol. 1996;70:5466–5475. doi: 10.1128/jvi.70.8.5466-5475.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Molloy S S, Thomas L, VanSlyke J K, Stenberg P E, Thomas G. Intracellular trafficking and activation of the furin proprotein convertase: localization to the TGN and recycling from the cell surface. EMBO J. 1994;13:18–33. doi: 10.1002/j.1460-2075.1994.tb06231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Montalvo E A, Parmley R T, Grose C. Structural analysis of the varicella-zoster virus gp98-gp62 complex: posttranslational addition of N-linked and O-linked oligosaccharide moieties. J Virol. 1985;53:761–770. doi: 10.1128/jvi.53.3.761-770.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mulder W A M, Jacobs L, Priem J, Kok G L, Wagenaar F, Kimman T G, Pol J M A. Glycoprotein gE-negative pseudorabies virus has a reduced capability to infect second- and third-order neurons of the olfactory and trigeminal routes in the porcine central nervous system. J Gen Virol. 1994;75:3095–3106. doi: 10.1099/0022-1317-75-11-3095. [DOI] [PubMed] [Google Scholar]
- 52.Neidhardt H, Schröder C H, Kaerner H C. Herpes simplex virus type 1 glycoprotein E is not indispensable for viral infectivity. J Virol. 1987;61:600–603. doi: 10.1128/jvi.61.2.600-603.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Olson J K, Bishop G A, Grose C. Varicella-zoster virus Fc receptor gE glycoprotein: serine/threonine and tyrosine phosphorylation of monomeric and dimeric forms. J Virol. 1997;71:110–119. doi: 10.1128/jvi.71.1.110-119.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Olson J K, Grose C. Endocytosis and recycling of varicella-zoster virus Fc receptor glycoprotein gE: internalization mediated by a YXXL motif in the cytoplasmic tail. J Virol. 1997;71:4042–4054. doi: 10.1128/jvi.71.5.4042-4054.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pearse B M F, Robinson M S. Clathrin, adaptors, and sorting. Annu Rev Cell Biol. 1990;6:151–171. doi: 10.1146/annurev.cb.06.110190.001055. [DOI] [PubMed] [Google Scholar]
- 56.Petrovskis E A, Timmins J G, Post L E. Use of λgt11 to isolate genes for two pseudorabies virus glycoproteins with homology to herpes simplex virus and varicella-zoster virus glycoproteins. J Virol. 1986;60:185–193. doi: 10.1128/jvi.60.1.185-193.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pley U, Parham P. Clathrin: its role in receptor-mediated vesicular transport and specialized functions in neurons. Crit Rev Biochem Mol Biol. 1993;28:431–464. doi: 10.3109/10409239309078441. [DOI] [PubMed] [Google Scholar]
- 58.Rabouille C, Hui N, Hunte F, Kieckbusch R, Berger E G, Warren G, Nilsson T. Mapping the distribution of Golgi enzymes involved in the construction of complex oligosaccharides. J Cell Sci. 1995;108:1617–1627. doi: 10.1242/jcs.108.4.1617. [DOI] [PubMed] [Google Scholar]
- 59.Rabouille C, Misteli T, Watson R, Warren G. Reassembly of Golgi stacks from mitotic Golgi fragments in a cell free system. J Cell Biol. 1995;129:605–618. doi: 10.1083/jcb.129.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reaves B, Banting G. Perturbation of the morphology of the trans-Golgi network following brefeldin A treatment: redistribution of a TGN-specific membrane protein TGN38. J Cell Biol. 1992;116:85–94. doi: 10.1083/jcb.116.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roizman B, Desrosiers R C, Fleckenstein B, Lopez C, Minson A C, Studdert M J. The family Herpesviridae: an update. Arch Virol. 1992;123:425–449. doi: 10.1007/BF01317276. [DOI] [PubMed] [Google Scholar]
- 62.Roizman B, Sears A E. Herpes simplex viruses and their replication. In: Roizman B, Whitley R J, Lopez C, editors. The human herpesviruses. New York, N.Y: Raven Press; 1993. pp. 11–68. [Google Scholar]
- 63.Rost B, Casadio R, Fariselli P, Sander C. Prediction of helical transmembrane segments at 85% accuracy. Protein Sci. 1995;4:521–533. doi: 10.1002/pro.5560040318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schäfer W, Stroh A, Berghöfer S, Seiler J, Vey M, Kruse M-L, Kern H F, Klenk H-D, Garten W. Two independent targeting signals in the cytoplasmic domain determine the trans-Golgi network localization and endosomal trafficking of the proprotein convertase furin. EMBO J. 1995;14:2424–2435. doi: 10.1002/j.1460-2075.1995.tb07240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scheel J, Matteoni R, Ludwig T, Hoflack B, Kreis T E. Microtubule depolymerization inhibits transport of cathepsin D from the Golgi apparatus to lysosomes. J Cell Sci. 1990;96:711–720. doi: 10.1242/jcs.96.4.711. [DOI] [PubMed] [Google Scholar]
- 66.Schekman R, Orci L. Coat proteins and vesicle budding. Science. 1996;271:1526–1533. doi: 10.1126/science.271.5255.1526. [DOI] [PubMed] [Google Scholar]
- 67.Schmid S L, Damke H. Coated vesicles: a diversity of form and function. FASEB J. 1995;9:1445–1453. doi: 10.1096/fasebj.9.14.7589986. [DOI] [PubMed] [Google Scholar]
- 68.Simpson F, Peden A A, Christopoulou L, Robinson M S. Characterization of the adaptor-related protein complex AP-3. J Cell Biol. 1997;137:835–845. doi: 10.1083/jcb.137.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stackpole C W. Herpes-type virus of the frog renal adenocarcinoma. I. Virus development in tumor transplants maintained at low temperature. J Virol. 1969;4:75–93. doi: 10.1128/jvi.4.1.75-93.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Takahashi S, Nakagawa T, Banno T, Watanabe T, Murakami K, Nakayama K. Localization of furin to the trans-Golgi network and recycling from the cell surface involves Ser and Tyr residues within the cytoplasmic domain. J Biol Chem. 1995;270:28397–28401. doi: 10.1074/jbc.270.47.28397. [DOI] [PubMed] [Google Scholar]
- 71.Takebe Y, Seiki M, Fujisawa J-I, Hoy P, Yokota K, Arai K-I, Yoshida M, Arai N. SRα promoter: an efficient and versatile mammalian cDNA system composed of the simian virus 40 early promoter and the R-U5 segment of human T-cell leukemia virus type 1 long terminal repeat. Mol Cell Biol. 1988;8:466–472. doi: 10.1128/mcb.8.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Telford E A R, Watson M S, McBride K, Davison A J. The DNA sequence of equine herpes virus 1. Virology. 1992;189:304–316. doi: 10.1016/0042-6822(92)90706-u. [DOI] [PubMed] [Google Scholar]
- 73.Tirabassi R S, Enquist L W. Role of envelope protein gE endocytosis in the pseudorabies virus life cycle. J Virol. 1998;72:4571–4579. doi: 10.1128/jvi.72.6.4571-4579.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tirabassi R S, Townley R A, Eldridge M G, Enquist L W. Characterization of pseudorabies virus mutants expressing carboxy-terminal truncations of gE: evidence for envelope incorporation, virulence, and neurotropism domains. J Virol. 1997;71:6455–6464. doi: 10.1128/jvi.71.9.6455-6464.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Torrisi M R, Di Lazzaro C, Pavan A, Pereira L, Campadelli-Fiume G. Herpes simplex virus envelopment and maturation studied by fracture label. J Virol. 1992;66:554–561. doi: 10.1128/jvi.66.1.554-561.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Trowbridge I S, Collawn J F, Hopkins C R. Signal-dependent membrane protein trafficking in the endocytic pathway. Annu Rev Cell Biol. 1993;9:129–161. doi: 10.1146/annurev.cb.09.110193.001021. [DOI] [PubMed] [Google Scholar]
- 77.van Engelenburg F A C, Kaashoek M J, Rijsewijk F A M, van den Burg L, Moerman A, Gielkens A L J, van Oirschot J T. A glycoprotein E deletion mutant of bovine herpesvirus 1 is avirulent in calves. J Gen Virol. 1994;75:2311–2318. doi: 10.1099/0022-1317-75-9-2311. [DOI] [PubMed] [Google Scholar]
- 78.van Engelenburg F A C, Kaashoek M J, van Oirschot J T, Rijsewijk F A M. A glycoprotein E deletion mutant of bovine herpesvirus 1 infect the same limited number of tissues in calves as wild-type virus, but for a shorter period. J Gen Virol. 1995;76:2387–2392. doi: 10.1099/0022-1317-76-9-2387. [DOI] [PubMed] [Google Scholar]
- 79.van Gederen I L, Brandimarti R, Torrisi M R, Campadelli G, van Meer G. The phospholipid composition of extracellular herpes simplex virions differs from that of host cell nuclei. Virology. 1994;200:831–836. doi: 10.1006/viro.1994.1252. [DOI] [PubMed] [Google Scholar]
- 80.Voorhoes P, Deignan E, van Donselaar E, Humphrey J, Marks M, Peters P J, Bonifacino J S. An acidic sequence within the cytoplasmic domain of furin functions as a determinant of trans-Golgi network localization and internalization from the cell surface. EMBO J. 1995;14:4961–4975. doi: 10.1002/j.1460-2075.1995.tb00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Whealy M E, Card J P, Meade R P, Robbins A K, Enquist L W. Effect of brefeldin A on alphaherpesvirus membrane protein glycosylation and virus egress. J Virol. 1991;65:1066–1081. doi: 10.1128/jvi.65.3.1066-1081.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wong S H, Hong W. The SXYQRL sequence in the cytoplasmic domain of TGN38 plays a major role in trans-Golgi network localization. J Biol Chem. 1993;268:22853–22862. [PubMed] [Google Scholar]
- 83.Yao Z, Jackson W, Grose C. Identification of the phosphorylation sequence in the cytoplasmic tail of the varicella-zoster virus Fc receptor glycoprotein gpI. J Virol. 1993;67:4464–4473. doi: 10.1128/jvi.67.8.4464-4473.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhu Z, Gershon M D, Gabel C, Sherman D, Ambron R, Gershon A. Entry and egress of varicella-zoster virus: role of mannose 6-phosphate, heparan sulphate proteoglycan, and signal sequences in targeting virions and viral glycoproteins. Neurology. 1995;45:S15–S17. doi: 10.1212/wnl.45.12_suppl_8.s15. [DOI] [PubMed] [Google Scholar]
- 85.Zhu Z, Gershon M D, Hao Y, Ambron R T, Gabel C A, Gershon A A. Envelopment of varicella-zoster virus: targeting of viral glycoproteins to the trans-Golgi network. J Virol. 1995;69:7951–7959. doi: 10.1128/jvi.69.12.7951-7959.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhu Z, Hao Y, Gershon M D, Ambron R T, Gershon A A. Targeting of glycoprotein I (gE) of varicella-zoster virus to the trans-Golgi network by an AYRV sequence and an acidic amino acid-rich patch in the cytosolic domain of the molecule. J Virol. 1996;70:6563–6575. doi: 10.1128/jvi.70.10.6563-6575.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zsak L, Zuckermann F, Sugg N, Ben-Porat T. Glycoprotein gI of pseudorabies virus promotes cell fusion and virus spread via direct cell-to-cell transmission. J Virol. 1992;66:2316–2325. doi: 10.1128/jvi.66.4.2316-2325.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]