Abstract
In primary human diploid fibroblasts, infection with an unpurified stock of human cytomegalovirus induced accumulation of the CC chemokine MCP-1 in the cell culture medium. By 24 h postinfection, the level of MCP-1 returned to that in uninfected cultures. When cells were infected with UV-inactivated human cytomegalovirus, the induction of MCP-1 was still observed, but no reduction was seen by 24 h postinfection or later. This effect was the result of a decrease in the level of MCP-1 mRNA present within the infected cell. Infection with purified virus revealed that the induction of MCP-1 was due to an activity found in the medium of infected cells; purified virions did not induce the expression of MCP-1. However, infection with purified virions repressed the level of MCP-1 mRNA below that found in uninfected cells. Additionally, infection with human cytomegalovirus prevented the induction of MCP-1 expression by tumor necrosis factor alpha and interleukin-1β. The CC chemokine receptor encoded by the human cytomegalovirus US28 open reading frame (ORF) did not appear to play a role in this process, since a mutant virus in which the US28 ORF had been deleted downregulated MCP-1 in the same manner.
Human cytomegalovirus (HCMV) is a ubiquitous human pathogen. HCMV infection is associated with several clinical manifestations, including CMV mononucleosis, birth defects when infection occurs in pregnant women, and a variety of clinical syndromes in immunocompromised and immunosuppressed individuals (for a review, see reference 6). HCMV has been shown to exert a variety of effects on the gene expression of the infected host cell, including the induction of cellular transcription factors by viral attachment to the cell surface (37), modulation of the cell cycle with characteristic changes in gene expression (5, 9, 18), and direct effects on host cell promoters by viral gene products (16, 19). Some of the genes that have been shown to be induced by HCMV are likely to be involved in the host response to viral infection. These include several members of the cytokine family and several members of a subclass of cytokines, the chemokines.
The chemokines are a group of cytokines that exhibit chemotactic activity for a variety of leukocytes (for reviews, see references 2 and 28). They are divided into four classes, CXC, CC, C, and CX3C, based on the arrangement of conserved cysteine residues near the N terminus of the protein. Chemokines are expressed by a variety of cell types, including monocytes, lymphocytes, epithelial cells, and fibroblasts. Expression of the chemokines has been shown to be induced by a variety of stimuli, including other cytokines, bacterial endotoxins, and viral infection. Because of their chemoattractant activity for leukocytes, it is believed that the chemokines are mediators of the inflammatory process and therefore play an important role in the resolution of viral infection. Consistent with this view, a mouse with a homozygous deletion of the gene for the CC chemokine MIP-1α has been shown to exhibit a dramatically reduced inflammatory response to coxsackievirus and influenza virus and to experience delayed viral clearance (8). Additionally, these mice show reduced natural killer (NK) cell-mediated inflammation in the liver during murine CMV infection (29).
Several lines of evidence suggest that chemokines may play a role in HCMV infection. HCMV has been shown to induce the expression of the CXC chemokine interleukin-8 (IL-8) and the CC chemokine RANTES (10, 22, 23). In addition, elevated levels of the CC chemokine MCP-1 have been detected in the cerebrospinal fluid of human immunodeficiency virus-infected patients with CMV encephalitis (4). MCP-1 has been demonstrated to be induced by tumor necrosis factor alpha (TNF-α) and IL-1β, cytokines that have been shown to be stimulated by HCMV (10, 27, 32). Furthermore, the HCMV open reading frame (ORF) US28 encodes a putative seven-transmembrane-domain G-protein-coupled receptor that has been shown to be a functional receptor for MCP-1, RANTES, MIP-1α, and MIP-1β (12, 15, 24).
In this report, we examine the effect of HCMV on the expression of the CC chemokine MCP-1. We have found that an unpurified stock of HCMV strongly induces MCP-1 mRNA and protein expression. This induction is not a direct effect of the virus but appears to be due to a factor that is secreted into the cell culture medium by HCMV-infected cells. Additionally, HCMV acts to inhibit MCP-1 expression at the level of transcription during infection. This transcriptional repression occurs at early times postinfection (p.i.) and requires viral gene expression.
MATERIALS AND METHODS
Cells and viruses.
Primary human foreskin fibroblasts (HFFs) were maintained in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum. HFFs were used between passages 7 and 14 and infected with HCMV at a multiplicity of infection (MOI) of 3 PFU/cell 3 to 4 days after reaching confluence. To avoid cell stimulation by fresh serum, cells were returned after infection to the culture medium in which they were previously maintained.
Supernatants of infected cells for use as viral stocks were obtained by infecting HFFs at an MOI of 0.1 with HCMV laboratory strain AD169. Five to seven days after cells showed 100% cytopathic effect, the medium was harvested and cellular debris was removed by centrifugation at 8,000 × g for 20 min at 4°C. Stock titers were determined by plaque assay.
Viral particles were purified by velocity centrifugation in a d-sorbitol gradient as previously described (31). Briefly, 30 ml of an infected-cell supernatant prepared as described above was underlayed with a 7-ml sorbitol cushion containing 20% d-sorbitol, 50 mM Tris-HCl (pH 7.2), 1 mM MgCl2, and 100 μg of bacitracin/ml in an Ultra-Clear centrifuge tube (Beckman). Virions were pelleted by centrifugation at 55,000 × g for 1 h at room temperature. Pellets were resuspended in DMEM to produce a purified virus stock.
To separate the MCP-1-inducing activity of an unpurified viral stock from the virus, the stock was filtered through a 100-kDa-cutoff membrane in a stirred cell concentrator (model 8200; Amicon, Inc.).
Virus stocks were UV inactivated by placing 2 to 5 ml of an unpurified stock or a purified stock resuspended in 2 to 5 ml of serum-free DMEM in a 15-cm-diameter dish and irradiating with UV light for 15 min at 2 J/m2 per s as previously described (38).
Northern blotting.
Total RNA was isolated from HFFs by using TRIzol reagent (Gibco BRL), subjected to electrophoresis in formaldehyde-containing agarose gels, and transferred to a nylon membrane as described previously (1). Four micrograms of membrane-bound RNA was probed with cDNA probes labeled by random priming in the presence of 32P. The MCP-1 cDNA probe was obtained from the I.M.A.G.E. Consortium (Genome Systems). The RANTES cDNA was obtained by reverse transcription of RNA obtained from 12-O-tetradecanoylphorbol13-acetate (TPA)-differentiated THP-1 cells with RANTES-specific oligonucleotide primers. A cDNA for one of the cytosolic phospholipases A2, cPL2 (39), which is not transcriptionally induced during HCMV infection (38), was used as a loading control for all Northern blots. Where indicated, TNF-α (R&D Systems) was added to cells at a final concentration of 10 ng/ml. IL-1β (R&D Systems) was added to a final concentration of 1 ng/ml. To inhibit viral DNA replication, phosphonoacetic acid (PAA) was added to a final concentration of 100 μg/ml 1 h after infection. To block viral attachment, heparin was added to cells at 10 μg/ml before the addition of virus (7).
ELISA and immunoprecipitations.
Medium was collected from infected HFF cultures, and the supernatant was assayed by enzyme-linked immunosorbent assay (ELISA), using a Quantikine plate specific for human MCP-1 (R&D Systems) in accordance with the manufacturer’s protocol.
For immunoprecipitations, confluent 10-cm-diameter dishes of HFFs were labeled with [35S]methionine for 1 h and lysed in 0.5 ml of lysis buffer (50 mM Tris-Cl [pH 8.0], 150 mM NaCl, 0.1% sodium dodecyl sulfate, 1.0% Nonidet P-40, 0.5% sodium deoxycholate, 100 μg/ml phenylmethylsulfonyl-fluoride, complete protease inhibitor cocktail tablet [Boehringer Mannheim Biochemicals] [1 tablet/50 ml of buffer]). Radioactivity in 5-μl samples of lysates was quantified in a Beckman scintillation counter (model LS5000TD). Equal amounts of radioactivity were used in a total volume of 0.25 ml for each immunoprecipitation. Immunoprecipitations were carried out with anti-MCP-1 monoclonal antibody 5D3-F7 (Pharmingen) (26), and complexes were captured with protein A-Sepharose beads. Precipitates were washed in lysis buffer, and proteins were separated on a 10% polyacrylamide gel containing sodium dodecyl sulfate. Proteins were visualized by autoradiography.
Nuclear run-on assays.
Approximately 4 × 107 HFFs were used for each determination. Where indicated, cells were infected at an MOI of 3 PFU/cell and treated with IL-1β at a final concentration of 1 ng/ml. Cells were harvested at 24 h p.i., which was 1.5 h after IL-1β addition. Nuclei were prepared and run-on transcription was performed as described elsewhere (1).
Construction of ADsubUS28 virus.
Cosmid pCM1035 (11) was digested with EcoRI and ClaI, and the 3.5-kb fragment corresponding to HCMV strain AD169 nucleotides 218438 to 221952 was isolated and cloned into pSP72 (Promega). This fragment contains the US28 ORF (nucleotides 219200 to 220256) and approximately 1 kb of flanking sequence on either side. This plasmid was digested with SacII and StuI, which cut within the US28 ORF (at positions 219222 and 219629, respectively), and the US28 sequences were replaced by the enhanced green fluorescent protein (EGFP) ORF (from plasmid EGFP-N1 [Clontech]), regulated by the simian virus 40 (SV40) early promoter and containing an SV40 poly(A) site, to create psubUS28. The pSP72 background sequences were removed from psubUS28, and 5 μg of the fragment containing the HCMV and GFP sequences was transfected with 2 μg of AD169 DNA and 2 μg of the pp71 expression plasmid pCMV-pp71 (3) into HFFs. Green fluorescent plaques were isolated, and the substituted virus was propagated and plaque purified. DNA was isolated from infected cells, digested with BamHI, and analyzed by Southern blotting as described elsewhere (1) to confirm the structure of the mutant virus, ADsubUS28.
RESULTS
Induction and subsequent repression of MCP-1 protein levels in cells infected with crude, unpurified HCMV stocks.
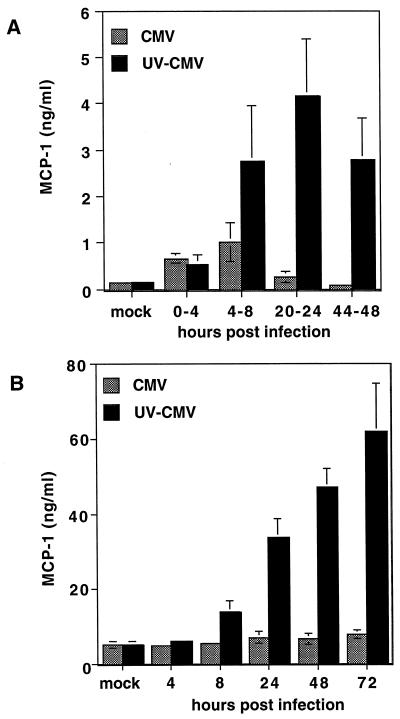
Initially, we examined the effect of HCMV infection on cellular expression of MCP-1. Primary HFFs were infected with an unpurified stock of HCMV at an MOI of 3 PFU/cell. The cells were washed at various times and fed with fresh, serum-free medium. At the end of a 4-h interval, the medium was collected and the MCP-1 protein concentration was measured by a sandwich ELISA (Fig. 1A). During the 0- to 4-h interval, approximately fourfold more MCP-1 accumulated in the culture medium of infected cells than in that of uninfected cells. Between 4 and 8 h p.i., infected cells continued to secrete more MCP-1 than did uninfected controls, but at 20 to 24 h and 44 to 48 h p.i., little MCP-1 was secreted by HCMV-infected cells.
FIG. 1.
MCP-1 concentration in the culture medium of infected fibroblasts. (A) MCP-1 secreted into the culture medium during 4-h intervals. HFFs were infected with active (CMV) or UV-inactivated (UV-CMV) HCMV at an MOI of 3 PFU/cell. At the indicated times, fresh, serum-free DMEM was added, and supernatants were collected 4 h later. MCP-1 concentration in the medium was measured by ELISA. (B) Total MCP-1 secreted into the culture medium during infection. Supernatant was collected at the indicated times p.i., and the MCP-1 concentration was measured by ELISA. All values shown are the averages of data from three independent experiments. mock, supernatant from mock-infected cells.
When the infectivity of the viral stock was destroyed by exposure to UV light prior to infection, induction of MCP-1 similar to that seen with active virus was observed between 0 and 4 h (Fig. 1A). However, during the 4- to 8-h time interval, significantly more MCP-1 was secreted by cells treated with inactivated virus than by those treated with active virus. Furthermore, between 20 and 24 h p.i. and between 44 and 48 h p.i., cells treated with the UV-inactivated virus secreted levels of MCP-1 that were approximately 25-fold higher than those secreted by uninfected or HCMV-infected cells.
We also measured the total MCP-1 that accumulated in the medium over the course of infection. HFFs were infected as described above, and medium was harvested at various time points. Before infection, the MCP-1 concentration in the medium was approximately 5 ng/ml. In cells infected with active HCMV, the increase in MCP-1 above this background level was insignificant (Fig. 1B). In contrast, infection of cells with UV-inactivated HCMV resulted in a dramatic increase in the MCP-1 concentration in the culture medium. This increase was clearly observed by 24 h p.i. By 48 h p.i., MCP-1 levels were approximately 10-fold higher in cultures infected with inactivated virus than in uninfected cultures or in the medium of HCMV-infected cells (Fig. 1B).
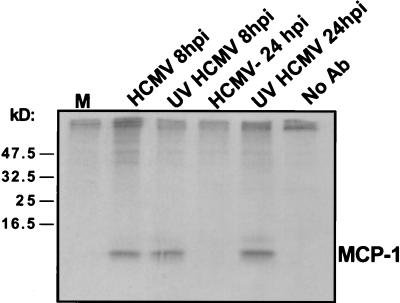
It has been reported previously that RANTES accumulates within cells late in infection but is not secreted into the medium (22). To determine if MCP-1 protein is sequestered within infected cells, HFFs were infected with HCMV or UV-inactivated HCMV and labeled with [35S]methionine, and MCP-1 was immunoprecipitated from cellular extracts by using an anti-MCP-1 monoclonal antibody. As shown in Fig. 2, at 8 h p.i., the antibody precipitated an approximately 10-kDa protein, consistent with the reported size of the unglycosylated precursor form of MCP-1. Similar levels of MCP-1 were present in cells infected with HCMV and UV-inactivated HCMV at this time point. At 24 h p.i., however, MCP-1 was detected only in cells treated with UV-inactivated HCMV, indicating that MCP-1 protein is not sequestered within the cells infected with the active virus but rather is almost completely absent by 24 h p.i.
FIG. 2.
MCP-1 within infected cells. HFFs were infected with HCMV or UV-inactivated HCMV (UV HCMV). 35S-labeled proteins were immunoprecipitated by an antibody (Ab) specific to MCP-1, and the immunoprecipitate was analyzed by electrophoresis and autoradiography. The positions to which marker proteins migrated are indicated on the left (in kilodaltons), and bands corresponding to the chemokine are labeled MCP-1. M, mock-infected cells.
MCP-1 mRNA levels change in concert with protein levels.
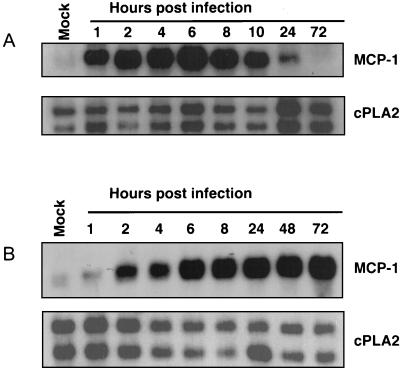
We next examined the effect of HCMV infection on the level of MCP-1 mRNA. HFFs were infected at an MOI of 3 PFU/cell, and RNA was prepared at various times after infection and analyzed by Northern blotting. The level of MCP-1 mRNA initially rose after infection, reaching a peak by approximately 6 h p.i., and declined during later time periods (Fig. 3A). When cells were exposed to the UV-inactivated virus stock, MCP-1 mRNA was induced with similar kinetics but remained elevated through 72 h p.i. (Fig. 3B). Because MCP-1 protein levels change in concert with the levels of mRNA, we believe that the regulation of MCP-1 by HCMV occurs primarily at the RNA level.
FIG. 3.
MCP-1 mRNA accumulation during infection. HFFs were infected by HCMV (A) or UV-inactivated HCMV (B). RNA was collected at the indicated times and analyzed by Northern blotting with an MCP-1-specific cDNA probe. A cDNA probe for one of the cellular cPLA2 mRNAs was used as a loading control. Mock, mock-infected cells.
Induction of MCP-1 mRNA is due to a factor present in the medium of infected cells.
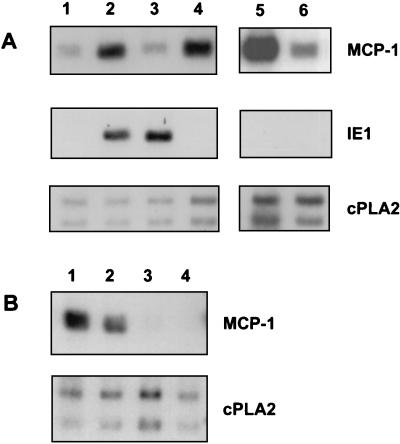
The experiments described above used an unpurified virus stock, i.e., an infected-cell supernatant, as the source of virus. Such a virus stock almost certainly contains various cytokines and other signaling molecules that have been induced during the course of viral infection. These factors in the virus stock may be responsible for, or contribute to, the observed induction of MCP-1 mRNA. To differentiate between direct induction of MCP-1 by the virus and induction by a contaminating factor, the crude virus stock was purified by high-speed centrifugation of virions through a sorbitol cushion, separating the particles from low-molecular-weight contaminants. After determination of its titer, the purified virus stock was used to infect HFFs at an MOI of 3 PFU/cell; this MOI was the same as that used for infection with the unpurified virus stock. As seen in Fig. 4 (compare lanes 1 and 3), the purified virus did not induce MCP-1 mRNA levels at 8 h p.i., even though it expressed the virus-coded IE1 mRNA. To further characterize the inducing activity, the unpurified virus stock was filtered through a 100-kDa-cutoff membrane and the flowthrough was added to HFFs (Fig. 4A, lane 4). The filter effectively removed virus from the medium, as measured by Northern blotting of the virally encoded IE1 mRNA and by plaque assay, and the filtered medium retained the ability to induce MCP-1. In addition, blocking viral attachment to the cells by the addition of heparin to the crude viral stock did not interfere with induction of MCP-1 mRNA (Fig. 4, lane 5). Failure to induce expression of IE1 mRNA confirmed that heparin successfully blocked the infection. The above-described experiments demonstrated that the observed induction of MCP-1 RNA during HCMV infection is due to a factor that accumulates in the medium of infected cells and is not mediated directly by the viral particle. The inducing factor is present only in the medium of infected cells, not in that of uninfected cells.
FIG. 4.
MCP-1 is induced by a factor present in the medium of infected cells. (A) HFFs were infected and/or treated as described in the text, and RNA was collected 8 h later and analyzed by Northern blotting. Lanes: 1, mock-infected cells; 2, crude HCMV stock, MOI = 3; 3, purified HCMV stock, MOI = 3; 4, flowthrough fraction of crude virus stock filtered through a 100-kDa-cutoff filter; 5, crude HCMV stock plus heparin (10 μg/ml); 6, mock-infected cells treated with heparin (10 μg/ml). (B) Purified HCMV stock represses MCP-1 mRNA levels. Lanes: 1, mock-infected cells; 2, HCMV, 6 h p.i.; 3, HCMV, 24 h p.i.; 4, HCMV, 48 h p.i. The autoradiograph was exposed for a longer time than in other panels so that the MCP-1 mRNA in the mock lane would be clearly visible.
In different experiments we found that the basal level of MCP-1 mRNA relative to the amount of control mRNAs varied to some extent. We have not been able to identify the cause of this variation. The effect of viral infection on the level of MCP-1 mRNA was entirely reproducible in repeated experiments.
After infection with purified virions, MCP-1 mRNA levels were reduced compared to mock-infection levels. The level of MCP-1 mRNA was reduced below the levels found in uninfected controls at 24 h p.i. and later times (Fig. 4B; compare lane 1 with lanes 3 and 4).
HCMV infection interferes with the ability of TNF-α and IL-1β to induce MCP-1 mRNA.
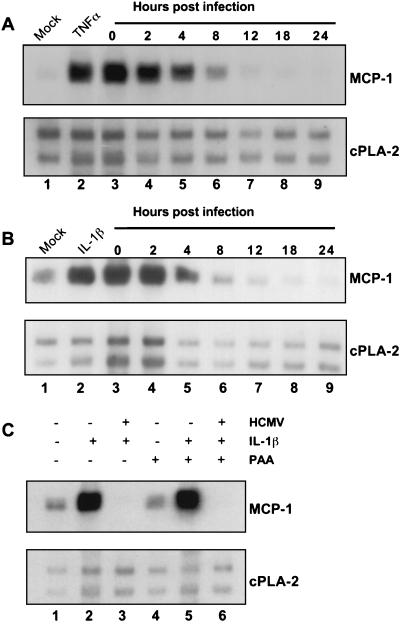
We next determined whether purified HCMV could block the induction of MCP-1 mRNA by two known inducers, TNF-α and IL-1β. We observed a marked increase in MCP-1 mRNA at 2 h after treatment of HFFs with either TNF-α (Fig. 5A; compare lanes 1 and 2) or IL-1β (Fig. 5B; compare lanes 1 and 2). To observe the effect of HCMV on this induction, cells were treated with TNF-α or IL-1β for a 2-h interval at various time points after infection with purified virus. Infection with HCMV at the time of TNF-α treatment (Fig. 5A, lane 3) did block the induction of MCP-1-specific RNA by TNF-α. TNF-α could detectably induce MCP-1-specific RNA through 4 h p.i., but by 8 h p.i., induction was significantly reduced. By 12 h p.i. and later (Fig. 5A, lanes 7 to 9), TNF-α could no longer detectably induce MCP-1 RNA expression. Similar results were obtained for IL-1β (Fig. 5B), showing that HCMV does not solely block TNF-α-mediated effects on MCP-1. The inhibition of IL-1β-mediated activation of MCP-1 does not appear to require the secreted IL-1β receptor antagonist (IL-1ra), a cellular factor whose expression has been shown to be increased by HCMV infection (13). When the medium of infected cells was replaced with fresh medium before the addition of IL-1β, inhibition of MCP-1 was still observed (data not shown), suggesting that inhibitory factors in the culture medium are not responsible for the inability of IL-1β to upregulate MCP-1 in infected cells.
FIG. 5.
HCMV infection prevents the induction of MCP-1 mRNA accumulation by TNF-α and IL-1β. RNA was collected at the indicated times p.i. and analyzed by Northern blotting with an MCP-1-specific cDNA probe. A cDNA probe for one of the cellular cPLA2 mRNAs was used as a control. (A) TNF-α treatment. Where indicated, HFFs were treated with TNF-α (10 ng/ml) and infected with HCMV. RNA was collected 2 h after addition of TNF-α. Lanes: 1, mock-infected cells; 2, TNF-α only; lanes 3 to 9, TNF-α added at the indicated times p.i. (B) IL-1β treatment. IL-1β was added (1 ng/ml) at the indicated times p.i. (lanes 3 to 9) or alone (lane 2). (C) PAA treatment. HFFs were infected with HCMV, treated (+) or not treated (−) with IL-1β or PAA (100 μg/ml, 1 h p.i.) as indicated. RNA was collected at 24 h p.i., 2 h after IL-1β addition.
The inhibition of IL-1β induction of MCP-1 mRNA occurs in the presence of the HCMV DNA replication inhibitor PAA (Fig. 5C). Therefore, this phenomenon does not require viral late gene expression.
HCMV prevents enhancement of the rate of transcription of MCP-1 mRNA by IL-1β.
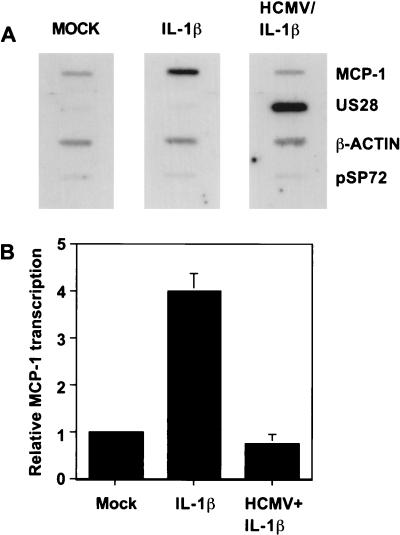
To determine if the block to MCP-1 mRNA accumulation occurs at the level of transcription, we performed a nuclear run-on assay. The addition of IL-1β to HFFs resulted in a fourfold increase in the rate of transcription (Fig. 6). When cells that had been infected with purified HCMV were treated with IL-1β at 24 h p.i., when inhibition of MCP-1 RNA induction was clearly observed, there was no increase in the rate of transcription (Fig. 6). This result demonstrates that HCMV blocks the induction of MCP-1 mRNA accumulation at the level of transcription. Similar results were obtained with TNF-α (data not shown).
FIG. 6.
HCMV blocks the transcriptional induction of MCP-1 by IL-1β. (A) Nuclear run-on assay of mock-infected HFFs, mock-infected HFFs treated with IL-1β (1 ng/ml, 1.5 h), and HFFs infected with HCMV (24 h) and treated with IL-1β (1 ng/ml, 1.5 h). Newly transcribed, labeled RNAs for MCP-1, β-actin, and the viral gene encoding US28 were hybridized to specific cDNAs immobilized on a nitrocellulose filter. pSP72 is a plasmid lacking a virus-specific insert that was included to monitor nonspecific binding. (B) Relative rate of MCP-1 transcription after various HFF treatments. The bands in panel A were quantitated with a phosphorimager, and values are normalized to β-actin. Mock, mock-infected cells; IL-1β, treated with IL-1β only; HCMV + IL-1β, HCMV infected and treated with IL-1β.
The US28 ORF is not involved in the regulation of MCP-1 expression.
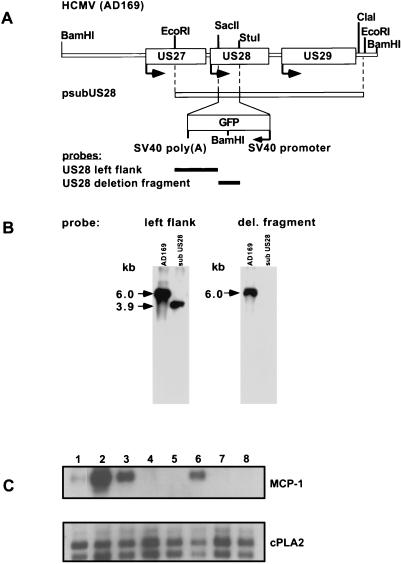
It has been shown that the product of the HCMV US28 ORF is a functional receptor for several CC chemokines, including MCP-1 (12, 15, 24). Because this receptor has been shown to be capable of transducing an intracellular signal in response to ligand binding, it is conceivable that this receptor is involved in preventing the induction of MCP-1, perhaps through a negative feedback loop. To determine whether the US28-encoded chemokine receptor is involved in the observed regulation of MCP-1 mRNA expression, we constructed a mutant virus that could not express the US28 product (AdsubUS28). As shown in Fig. 7A, a plasmid construct containing viral sequences that flank the US28 ORF positioned on either side of a GFP marker (psubUS28) was used to delete the US28 ORF by homologous recombination. After several rounds of plaque purification, the substitution mutant was found to be free of wild-type sequences, as determined by Southern blot assay (Fig. 7B). A probe corresponding to the genomic region to the left of the US28 ORF detected a 6.0-kb fragment in a Southern blot of BamHI-digested AD169 DNA. After correct recombination of psubUS28 into the viral genome, this probe detected a 3.9-kb fragment in BamHI-digested viral DNA. The US28 gene product is not essential for virus growth in tissue culture; the titers of the mutant and wild-type virus stocks produced by infection of HFFs at the same input multiplicity were identical (data not shown).
FIG. 7.
The US28 gene product is not required for the inhibition of MCP-1 mRNA induction. (A) Schematic representation of the US27-US29 region of the HCMV genome, the plasmid construct used to delete the US28 ORF, and probes used to characterize the mutant virus. (B) Southern blot of AD169 and ADsubUS28 viral DNA. Viral DNA was digested with BamHI, resulting in a 6.0-kb fragment containing the US28 ORF for the wild-type virus and in 3.9- and 2.8-kb fragments for the mutant, due to a BamHI site within the GFP marker. (C) Northern blot of RNA isolated from cells infected with AD169 or the AdsubUS28 mutant and treated with IL-1β (1 ng/ml). RNA was collected at various times p.i. and 2 h after IL-1β addition. An MCP-1-specific cDNA probe and a cDNA probe for a cellular cPLA2 RNA (control) were used. Lanes: 1, mock infection; 2, IL-1β only; 3, IL-1β and HCMV (AD169), 4 h p.i.; 4, IL-1β and HCMV (AD169), 24 h p.i.; 5, IL-1β and HCMV (AD169), 48 h p.i.; 6, IL-1β and HCMV (ADsubUS28), 4 h p.i.; 7, IL-1β and HCMV (ADsubUS28), 24 h p.i.; 8, IL-1β and HCMV (ADsubUS28), 48 h p.i.
HFFs were infected with AdsubUS28 and treated with IL-1β at various time points (Fig. 7C). AdsubUS28 prevented the induction of MCP-1 mRNA in a manner identical to that of the wild-type virus, demonstrating that the US28 ORF is not required for MCP-1 regulation during infection.
Regulation of RANTES mRNA expression occurs in a manner distinct from MCP-1 mRNA regulation.
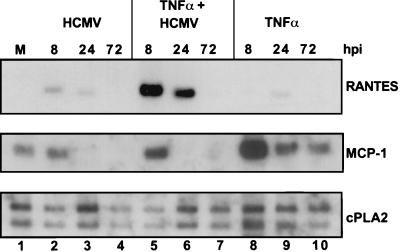
As mentioned above, the CC chemokine RANTES has previously been shown to be induced by HCMV infection in a manner independent of exogenous virally induced cytokines (22). We detected the induction of RANTES mRNA by Northern blotting after infection of HFFs with purified HCMV (Fig. 8, lanes 1 to 4), in agreement with the previous result. We also found that TNF-α modestly induced RANTES RNA expression (Fig. 8, lanes 8 to 10). Interestingly, TNF-α and HCMV appeared to synergize to transiently induce RANTES expression to a much greater extent (Fig. 8, lanes 5 to 7), and these elevated levels of mRNA were clearly detected at 8 and 24 h p.i. For comparison, MCP-1 mRNA levels in the same preparations as those analyzed in the RANTES blot are shown (middle panel), demonstrating the antagonistic effect of HCMV on TNF-α-mediated MCP-1 induction. Again, similar results were obtained when IL-1β was used in place of TNF-α (data not shown). Thus, HCMV induces RANTES mRNA accumulation while inhibiting MCP-1 transcription.
FIG. 8.
HCMV and TNF-α synergize to induce RANTES mRNA accumulation. HFFs were infected with HCMV (MOI = 3) and/or treated with TNF-α (10 ng/ml). RNA was collected at the indicated times p.i. Northern blots were probed with cDNA specific for RANTES, MCP-1, or cPLA2. M, mock-infected cells.
DISCUSSION
Infection of primary human diploid fibroblasts with HCMV results in the induction of a secreted activity that can induce MCP-1 mRNA expression (Fig. 3). We have not yet identified the activity responsible for this induction, but it might include TNF-α and/or IL-1β, both of which are induced during HCMV infection and are known to induce MCP-1 (10, 27, 32). During the course of infection, however, the virus prevents induction of MCP-1 mRNA by this activity (Fig. 3). The prevention of MCP-1 mRNA induction requires viral gene expression (Fig. 3) and occurs within 8 h after infection. Elevated levels of MCP-1 protein were secreted into the cell culture medium during all time intervals when elevated mRNA levels were present (Fig. 1), and MCP-1 protein is not sequestered within the infected cell (Fig. 2), as is seen with RANTES, another CC chemokine (22). The virus can be purified away from the MCP-1-inducing activity (Fig. 4), and the purified virus retains the ability to prevent induction of MCP-1 by TNF-α and IL-1β (Fig. 5). This effect occurs at the level of transcription (Fig. 6). The US28 ORF, which has been shown to encode a functional receptor for MCP-1 as well as other CC chemokines, does not contribute to the observed effects on MCP-1 expression (Fig. 7).
How does HCMV block expression of the MCP-1 gene? The prevention of MCP-1 accumulation occurs at the level of transcription (Fig. 6) and clearly requires viral gene expression (Fig. 1 and 3). Additionally, TNF-α and IL-1β are prevented from inducing MCP-1 transcription by 8 h p.i. (Fig. 3), and inhibition of DNA replication does not prevent this inhibition (Fig. 5C). Therefore, we believe that it is likely that one or more HCMV gene products produced relatively soon after infection block the induction of MCP-1 transcription. As yet, the identity of this putative virus-coded inhibitor(s) is unknown. It might specifically target MCP-1 transcription or interfere at an earlier point in the signal transduction pathways of TNF-α and IL-1β.
The MCP-1 promoter has been shown to contain binding sites for the transcription factors NF-κB and Sp1 (33, 34). NF-κB activation has been shown to be crucial for activation of this promoter by TNF-α. Interestingly, it has been shown that HCMV infection induces the activity of both of these transcription factors (14, 37). However, infection with a purified stock of HCMV does not stimulate transcription of MCP-1 (Fig. 4A and B), even though NF-κB and Sp1 are upregulated within 30 min following infection. Of note is the fact that proinflammatory factors such as RANTES and cyclooxygenase-2 (Cox-2) have also been shown to be induced by NF-κB (21, 25, 30), and RANTES and Cox-2 are induced during HCMV infection (22, 38a). It is possible that the inhibitor postulated above is contained within the viral tegument and introduced immediately upon infection, blocking the induction of MCP-1, while other NF-κB-responsive genes are induced. If this is the case, the initial level of this inhibitor within the infected cell must be insufficient to prevent MCP-1 induction by TNF-α or IL-1β (and the unidentified activity within the crude viral stock) because prevention of the induction of MCP-1 by these cytokines is not observed until 8 h p.i.
High MCP-1 levels have been reported in the cerebrospinal fluid of AIDS patients with HCMV encephalitis (4). If our results with fibroblasts hold true for other cell types, then we would not expect MCP-1 to be produced by HCMV-infected cells; rather, it would be produced by cells in the vicinity that would likely respond to TNF-α and IL-1β produced by neighboring infected cells.
It has been shown that MCP-1 is capable of attracting monocytes and NK cells in vitro and in vivo (17, 20, 35, 36). These cells play a major role in the inflammatory response to viral infection. It is not clear why it might be beneficial to the virus to block MCP-1 expression and secretion in infected cells but not in neighboring cells that may respond to infection-induced TNF-α and IL-1β by producing MCP-1. Perhaps monocytes and NK cells discriminate MCP-1-producing cells from cells that do not secrete this chemokine. Further, HCMV can infect monocytes and undergo latency there. Conceivably, the production of MCP-1 by neighboring cells might serve to lure monocytes to the site of infection, where they can be infected by HCMV, facilitating the maintenance and spread of the virus.
ACKNOWLEDGMENTS
We thank W. Bresnahan, F. Ferrari, C. Patterson, and B. Wing for commenting on the manuscript and H. Zhu for helpful discussions.
A.J.H. was supported in part by an American Heart Association predoctoral fellowship (95-FS-05). T.S. is an American Cancer Society Professor and an Investigator of the Howard Hughes Medical Institute.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley and Sons, Inc.; 1997. [Google Scholar]
- 2.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines—CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 3.Baldick C J, Jr, Marchini A, Patterson C E, Shenk T. Human cytomegalovirus tegument protein pp71 (ppUL82) enhances the infectivity of viral DNA and accelerates the infectious cycle. J Virol. 1997;71:4400–4408. doi: 10.1128/jvi.71.6.4400-4408.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernasconi S, Cinque P, Peri G, Sozzani S, Crociati A, Torri W, Vicenzi E, Vago L, Lazzarin A, Poli G, Mantovani A. Selective elevation of monocyte chemotactic protein-1 in the cerebrospinal fluid of AIDS patients with cytomegalovirus encephalitis. J Infect Dis. 1996;174:1098–1101. doi: 10.1093/infdis/174.5.1098. [DOI] [PubMed] [Google Scholar]
- 5.Bresnahan W A, Boldogh I, Thompson E A, Albrecht T. Human cytomegalovirus inhibits cellular DNA synthesis and arrests productively infected cells in late G1. Virology. 1996;224:150–160. doi: 10.1006/viro.1996.0516. [DOI] [PubMed] [Google Scholar]
- 6.Britt W J, Alford C A. Cytomegalovirus. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2493–2523. [Google Scholar]
- 7.Compton T, Nowlin D M, Cooper N R. Initiation of human cytomegalovirus infection requires initial interaction with cell surface heparan sulfate. Virology. 1993;193:834–841. doi: 10.1006/viro.1993.1192. [DOI] [PubMed] [Google Scholar]
- 8.Cook D N, Beck M A, Coffman T M, Kirby S L, Sheridan J F, Pragnell I B, Smithies O. Requirement of MIP-1α for an inflammatory response to viral infection. Science. 1995;269:1583–1585. doi: 10.1126/science.7667639. [DOI] [PubMed] [Google Scholar]
- 9.Dittmer D, Mocarski E S. Human cytomegalovirus infection inhibits G1/S transition. J Virol. 1997;71:1629–1634. doi: 10.1128/jvi.71.2.1629-1634.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dudding L, Haskill S, Clark B D, Auron P E, Sporn S, Huang E S. Cytomegalovirus infection stimulates expression of monocyte-associated mediator genes. J Immunol. 1989;143:3343–3352. [PubMed] [Google Scholar]
- 11.Fleckenstein B, Muller I, Collins J. Cloning of the complete human cytomegalovirus genome in cosmids. Gene. 1982;18:39–46. doi: 10.1016/0378-1119(82)90054-3. [DOI] [PubMed] [Google Scholar]
- 12.Gao J-L, Murphy P M. Human cytomegalovirus open reading frame US28 encodes a functional β-chemokine receptor. J Biol Chem. 1994;269:28539–28542. [PubMed] [Google Scholar]
- 13.Kline J N, Geist L J, Monick M M, Stinski M F, Hunninghake G W. Regulation of expression of the IL-1 receptor antagonist (IL-1ra) gene by products of the human cytomegalovirus immediate early genes. J Immunol. 1994;152:2351–2357. [PubMed] [Google Scholar]
- 14.Kowalik T F, Wing B, Haskill J S, Azizkhan J C, Baldwin A S, Jr, Huang E S. Multiple mechanisms are implicated in the regulation of NF-κB activity during human cytomegalovirus infection. Proc Natl Acad Sci USA. 1993;90:1107–1111. doi: 10.1073/pnas.90.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuhn D E, Beall C J, Kolattukudy P E. The cytomegalovirus US28 protein binds multiple CC chemokines with high affinity. Biochem Biophys Res Commun. 1995;211:325–330. doi: 10.1006/bbrc.1995.1814. [DOI] [PubMed] [Google Scholar]
- 16.Liu B, Stinski M F. Human cytomegalovirus contains a tegument protein that enhances transcription from promoters with upstream ATF and AP-1 cis-acting elements. J Virol. 1992;66:4434–4444. doi: 10.1128/jvi.66.7.4434-4444.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu B, Rutledge B J, Gu L, Fiorillo J, Lukacs N W, Kunkel S L, North R, Gerard C, Rollins B J. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J Exp Med. 1998;187:601–608. doi: 10.1084/jem.187.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu M, Shenk T. Human cytomegalovirus infection inhibits cell cycle progression at multiple points, including the transition from G1 to S. J Virol. 1996;70:8850–8857. doi: 10.1128/jvi.70.12.8850-8857.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lukac D M, Manuppello J R, Alwine J C. Transcriptional activation by the human cytomegalovirus immediate-early proteins: requirements for simple promoter structures and interactions with multiple components of the transcription complex. J Virol. 1994;68:5184–5193. doi: 10.1128/jvi.68.8.5184-5193.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maghazachi A A, al-Aoukaty A, Schall T J. C-C chemokines induce the chemotaxis of NK and IL-2-activated NK cells. Role for G proteins. J Immunol. 1994;153:4969–4977. [PubMed] [Google Scholar]
- 21.Manni A, Kleimberg J, Ackerman V, Bellini A, Patalano F, Mattoli S. Inducibility of RANTES mRNA by IL-1β in human bronchial epithelial cells is associated with increased NF-κB DNA binding activity. Biochem Biophys Res Commun. 1996;220:120–124. doi: 10.1006/bbrc.1996.0367. [DOI] [PubMed] [Google Scholar]
- 22.Michelson S, Dal Monte P, Zipeto D, Bodaghi B, Laurent L, Oberlin E, Arenzana-Seisdedos F, Virelizier J-L, Landini M P. Modulation of RANTES production by human cytomegalovirus infection of fibroblasts. J Virol. 1997;71:6495–6500. doi: 10.1128/jvi.71.9.6495-6500.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murayama T, Ohara Y, Obuchi M, Khabar K S A, Higashi H, Mukaida N, Matsushima K. Human cytomegalovirus induces interleukin-8 production by a human monocytic cell line, THP-1, through acting concurrently on AP-1- and NF-κB-binding sites of the interleukin-8 gene. J Virol. 1997;71:5692–5695. doi: 10.1128/jvi.71.7.5692-5695.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neote K, DiGregorio D, Mak J Y, Horuk R, Schall T J. Molecular cloning, functional expression, and signaling characteristics of a C-C chemokine receptor. Cell. 1993;72:415–425. doi: 10.1016/0092-8674(93)90118-a. [DOI] [PubMed] [Google Scholar]
- 25.Newton R, Kuitert L M, Bergmann M, Adcock I M, Barnes P J. Evidence for involvement of NF-κB in the transcriptional control of COX-2 gene expression by IL-1β. Biochem Biophys Res Commun. 1997;237:28–32. doi: 10.1006/bbrc.1997.7064. [DOI] [PubMed] [Google Scholar]
- 26.Peri G, Milanese C, Matteucci C, Ruco L, Zhou D, Sozzani S, Coletta I, Mantovani A. A new monoclonal antibody (5D3-F7) which recognizes human monocyte-chemotactic protein-1 but not related chemokines. Development of a sandwich ELISA and in situ detection of producing cells. J Immunol Methods. 1994;174:249–257. doi: 10.1016/0022-1759(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 27.Rolfe M W, Kunkel S L, Standiford T J, Orringer M B, Phan S H, Evanoff H L, Burdick M D, Strieter R M. Expression and regulation of human pulmonary fibroblast-derived monocyte chemotactic peptide-1. Am J Physiol. 1992;263:L536–L545. doi: 10.1152/ajplung.1992.263.5.L536. [DOI] [PubMed] [Google Scholar]
- 28.Rollins B J. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- 29.Salazar-Mather T P, Orange J S, Biron C A. Early murine cytomegalovirus (MCMV) infection induces liver natural killer (NK) cell inflammation and protection through macrophage inflammatory protein 1α (MIP-1α)-dependent pathways. J Exp Med. 1998;187:1–14. doi: 10.1084/jem.187.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmedtje J F, Jr, Ji Y S, Liu W L, DuBois R N, Runge M S. Hypoxia induces cyclooxygenase-2 via the NF-κB p65 transcription factor in human vascular endothelial cells. J Biol Chem. 1997;272:601–608. doi: 10.1074/jbc.272.1.601. [DOI] [PubMed] [Google Scholar]
- 31.Stinski M F. Human cytomegalovirus: glycoproteins associated with virions and dense bodies. J Virol. 1976;19:594–609. doi: 10.1128/jvi.19.2.594-609.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strieter R M, Wiggins R, Phan S H, Wharram B L, Showell H J, Remick D G, Chensue S W, Kunkel S L. Monocyte chemotactic protein gene expression by cytokine-treated human fibroblasts and endothelial cells. Biochem Biophys Res Commun. 1989;162:694–700. doi: 10.1016/0006-291x(89)92366-8. [DOI] [PubMed] [Google Scholar]
- 33.Ueda A, Ishigatsubo Y, Okubo T, Yoshimura T. Transcriptional regulation of the human monocyte chemoattractant protein-1 gene. Cooperation of two NF-κB sites and NF-κB/Rel subunit specificity. J Biol Chem. 1997;272:31092–31099. doi: 10.1074/jbc.272.49.31092. [DOI] [PubMed] [Google Scholar]
- 34.Ueda A, Okuda K, Ohno S, Shirai A, Igarashi T, Matsunaga K, Fukushima J, Kawamoto S, Ishigatsubo Y, Okubo T. NF-κB and Sp1 regulate transcription of the human monocyte chemoattractant protein-1 gene. J Immunol. 1994;153:2052–2063. [PubMed] [Google Scholar]
- 35.Yoshimura T, Robinson E A, Tanaka S, Appella E, Kuratsu J, Leonard E J. Purification and amino acid analysis of two human glioma-derived monocyte chemoattractants. J Exp Med. 1989;169:1449–1459. doi: 10.1084/jem.169.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshimura T, Robinson E A, Tanaka S, Appella E, Leonard E J. Purification and amino acid analysis of two human monocyte chemoattractants produced by phytohemagglutinin-stimulated human blood mononuclear leukocytes. J Immunol. 1989;142:1956–1962. [PubMed] [Google Scholar]
- 37.Yurochko A D, Hwang E-S, Rasmussen L, Keay S, Pereira L, Huang E-S. The human cytomegalovirus UL55 (gB) and UL75 (gH) glycoprotein ligands initiate the rapid activation of Sp1 and NF-κB during infection. J Virol. 1997;71:5051–5059. doi: 10.1128/jvi.71.7.5051-5059.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu H, Cong J P, Shenk T. Use of differential display analysis to assess the effect of human cytomegalovirus infection on the accumulation of cellular RNAs: induction of interferon-responsive RNAs. Proc Natl Acad Sci USA. 1997;94:13985–13990. doi: 10.1073/pnas.94.25.13985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38a.Zhu, H., D. Yu, and T. Shenk. Unpublished data.
- 39.Zupan L A, Steffens D L, Berry C A, Landt M, Gross R W. Cloning and expression of a human 14-3-3 protein mediating phospholipolysis. Identification of an arachidonoyl-enzyme intermediate during catalysis. J Biol Chem. 1992;267:8707–8710. [PubMed] [Google Scholar]