Abstract
Background
In microsurgical tissue transfer, skin flap transplantation is frequently used to heal the surface of a wound. Effective microcirculation surveillance of the skin flap is crucial. However, with traditional monitoring methods—that is, clinical observation—vascular crisis can still occur, thereby impairing postoperative recovery. A smartphone application is required to assist health care professionals in the standardized collection of flap perfusion parameters for flap management.
Methods
The Vascular Crisis Prewarning Application was created using a design science research methodology that prioritizes users and problems. The system usability scale was used to assess the application's usability among medical practitioners. The application was used at the clinic from December 2020 to September 2022. The unplanned return to the operating room, time to diagnose vascular crisis, and flap survival rate were compared with and without the application.
Results
The application consisted of 5 modules: patient addition and basic information entry, flap labeling, flap observation, crisis warning, and case archiving. The average rating for the application's usability among medical practitioners was 97.95 score (SD 2.36). With the application, the time to detect vascular crisis reduced from 26.71 to 16.26 h (P < 0.001), the unplanned return to the operation room increased from 8.18% to 10.24% (P = 0.587), and the flap survival rate went from 94.55% to 99.21% (P = 0.083).
Conclusions
An easy-to-use flap perfusion monitoring and prewarning application for medical practitioners was produced using a user-centered development method. The application provided a more standardized and accurate platform for data collection in flap management and reduced the time to detect vascular crisis. Larger cohort studies are required in the future to better assess the full potential of the application.
Keywords: Flap transplantation, Mobile applications, Nurse managers, Prewarning, Vascular crisis
Introduction
Flap transplantation has been widely applied to repair wound surfaces and restore the recipient site shape and physiological function.1, 2 However, microvascular tissue transfer takes a long time to operate, and flap failures cannot be avoided, thereby putting patients at risk of re-transplantation or even amputation.3 Survival rates for compromised flaps are inversely related to the time interval between the onset of vascular occlusion and exploration.4 The success of surgical revascularization depends on both high-quality perfusions of the flap and rapid identification and salvaging of the failing flap.5 Therefore, effective postsurgical nurse monitoring and prewarning of transplanted flap ischemia or congestion is an important aspect of perioperative care, thereby placing demands on clinical care.6
Clinical bedside observation by experienced nurses has long served as the standard for assessing postoperative flap perfusion,7,8 which is highly subjective and depends on the experience of the nurse.9 The aspects of observation mainly include flap color, temperature, capillary refill, turgor, and bleeding characteristics.10, 11, 12 Without a good unified system to guide postoperative monitoring and prewarning, the quality and work efficiency of nurses are likely to be hampered. There have been numerous advances in flap monitoring techniques over the last 3 decades. These techniques can be classified—among other attributes—according to their monitoring mechanism (measurement of vascular flow or tissue ischemia), the timing of use (intraoperative or postoperative), and invasiveness (noninvasive or invasive). Despite their effectiveness, these techniques—such as laser doppler flowmetry, near-infrared spectroscopy, and tissue oximetry—are costly and require specialized training to use. Therefore, they are rarely applied in resource-limited settings, and most surgeons prefer “conventional techniques.”4,13 In 2015, Kidakorn developed an Android mobile application called SlipaRamanitor for postoperative free flap monitoring. Photographs of the transplanted free flap are taken and compared for color difference.14 The clinical efficacy of SlipaRamanitor suggests a way forward for finding a medical solution through mobile phone technology. However, being limited to the small area of the skin that is monitored, the application is unable to assess the viability of every part of the tissue in the flap, which reduces the accuracy of this method.15
The increased use of smartphones and mobile technology has ushered in a new era of flap care monitoring techniques.5 In addition, there is an emerging trend to combine traditional flap monitoring techniques—such as tissue oximetry—with mobile alert systems to provide real-time feedback, thereby increasing objectivity and reducing the time taken to detect flap failure.16 However, there remain deficiencies in continuous care monitoring and the management of flap vascular crisis. A clinically reliable and easy-to-use postoperative flap monitoring and prewarning mobile application is of great value and needs to be designed and developed.
In this study, we developed the Vascular Crisis Prewarning Application (VCPWApp) and decomposed the management process of vascular crisis for prewarning and management after flap transplantation. We aimed to share the design and development process of the VCPWApp, its structure, functions, current implementation status, and the results of the evaluation of its use, with the objective of providing a reference point for other medical practitioners.
Methods
Study Design
This study included 2 phases—application development and evaluation. The first phase involved the design and development of the VCPWApp. The second phase assessed the sustainability and effectiveness of the VCPWApp.
All doctors and nurses from the Department of Hand and Microsurgery at XX hospital were selected as co-creators for the duration of our project. This department has the highest number of patients with flap transplant in our hospital. We began with the identification of requirements, followed by the iterative development and evaluation phases.
User Participation Design Framework
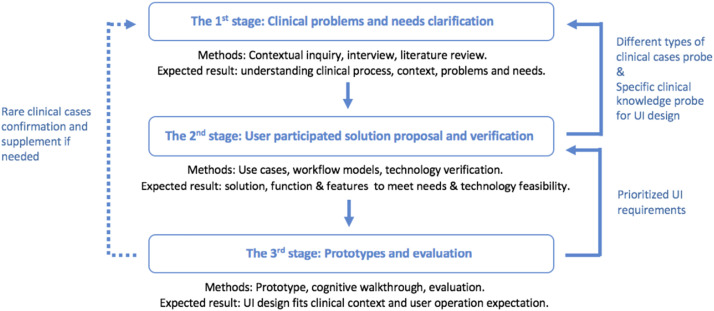
Similar to applications in a specific domain, there is inevitably a large knowledge and cognitive gap between target users, technologists, and designers in the field of smart health care. How to effectively engage target users—that is, doctors and nurses—so that the final design output can be tailored to clinical practice and context was an important issue to address at the beginning of this research. The user-centered design framework of “explore, design, and evaluate” clarifies user requirements and design solutions for technological feasibility and usability. In addition, it enables the successful involvement of target users, technologists, and key stakeholders in the product design and development process.17,18 However, as a design case for smart medical care involves highly specialized clinical knowledge and complicated clinical use cases, a more rapid and iterative “explore, design, and evaluate” user-centered design and development process is required (see Figure 1).
Figure 1.
User participated in the design process customized for the clinical application case.
On the basis of the customized design framework, the aim of this research was to clarify the current clinical issues and needs in the postoperative flap monitoring and prewarning process. In addition, an attempt was made to process an innovative flap postsurgical prewarning mobile application solution. Various main use cases, functions, user interface features, and workflow models in the solution were enriched to fit into clinical practice through an iterative loop among contextual probes, design, and review. Then, an attempt was made to design and prioritize the user interface flow, layout, and visuals through a cognitive walkthrough of doctors and nurses on prototypes to meet the user's operational expectations. Finally, confirmation and additions were made of certain special medical cases in clinical practice for a version update of the application.
Identification of Requirements
The entire process was initiated with the identification of needs. In-depth interviews with medical professionals were conducted to obtain a better understanding of the workflow, challenges, and requirements of the existing skin flap management procedure. The interviews focused on the following 3 aspects: a) current workflows for standard postoperative skin flap care; b) current workflows for prewarning of vascular crisis; and c) participants’ needs with regard to the design of a mobile application for prewarning of vascular crisis.
Development Process
The iterative user-centered development process was initiated as a result of the first need.19 Mock-ups were created at the beginning of the development process, including the specifications identified in the previous cycle.20 The project team discussed the mock-ups to confirm them. Thereafter, the functional prototype was created by utilizing the verified evolutions, which were then put to the test through functional evaluations. The ability of the application to meet the defined end-user needs was then determined by comparing the actual and anticipated outcomes.
Evaluations
Usability of the VCPWApp
The system usability scale (SUS),21 a straightforward five-point Likert scale used to create an overview of a system's usability,22 was used to measure the usability of the application. Higher ratings imply better usability. SUS scores range from 0 to 100. In-depth analyses of the SUS by Bangor et al. found that a system must score over 70 to be deemed adequate, at least. Better systems will likely score in the high 70s and high 80s, and scores above 90 indicate a truly superior system.23 The authors also made the case that any system that scores below 70 needs more usability testing and ongoing development. In addition, the users should have used the application for more than a month. Qualified users were invited to participate in the scale. Perceived usage during the study and general comments were also collected.
Clinical Trial: Comparative Study
A convenience sampling method was used to select patients who were admitted to the Hand Microsurgery Department from March 2018 to September 2022. Patients with flap transplant from December 2020 to September 2022 were managed using the VCPWApp for flap care, whereas patients with flap transplant from March 2018 to November 2020 were managed using the traditional method of clinical observation (observing temperature, swelling, discoloration, and capillary filling every hour for the first 3 days postoperatively and every 4 hours for 4 to 7 days postoperatively). Inclusion criteria: identified for skin flap grafting. The exclusion criteria were as follows: (i) combined with serious complications, such as cardiac disfunction, malignancy, etc.; (ii) missing important information; and (iii) transferred to another hospital midway after surgery or discharged automatically.24
Unplanned return to the operating room, time to detect vascular crisis, and flap survival rates were used as the quality and performance indicators of the VCPWApp. Unplanned return to the operating room was defined as any return to the operating room that was not anticipated at the time of the primary operative procedure. This excludes patients with planned return to the operating room for reasons such as delayed reconstruction or flap revisions.25 The time to detect vascular crisis refers to the time taken to detect a flap vascular crisis after microsurgical reconstruction. The flap survival rate refers to the number of patients with active flaps as a percentage of all patients with flap transplant. A graft flap that heals on its own or whose blood perfusion is well managed by timely treatment is considered to be alive.
Statistical Analysis
Descriptive data for the SUS score, unplanned returns to the operating room, time to detect vascular crisis, and flap survival rate were prospectively collected. Data were analyzed using SPSS version 22 (IBM, USA). Differences in the distribution of patient demographics and outcome measures were calculated using t-tests or chi-square tests. Statistical significance was set at P value of ≤0.05.
Results
Identification of Requirements
To provide a reference for predicting vascular crisis, postsurgical flap monitoring images need to be captured at the same angle and distance as the images captured by doctors to enable consistent comparison. In addition, a dynamic monitor parameter curve graph is required for an overview of the flap status and the access to images. In addition, video recording, uploading, and analysis are required to review capillary refill and skin turgor.
Mobile Application Solution Design
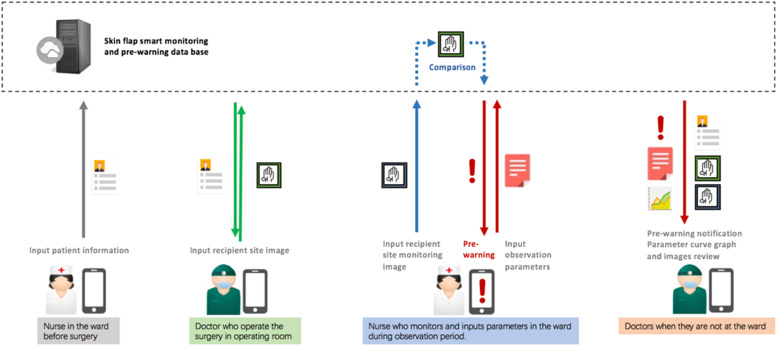
On the basis of the abovementioned clinical requirements, the main flap use cases that are to be covered in this mobile application are defined and decided. Its targets include regular and irregular flap shapes of different sizes. By involving doctors, nurses, and technologies, the postsurgical monitoring and prewarning mobile application solution for the skin flap is proposed (see Figure 2).
Figure 2.
Skin flap postsurgical prewarning mobile solution.
The User Interface Function and Features Design
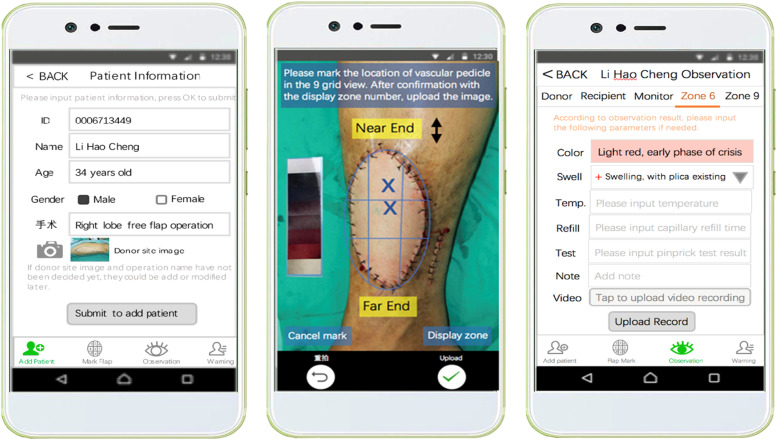
On the basis of the user-participated mobile application solution, the user interface functions, main user interface screens, and user interface features of regular and irregular skin flap use cases were created (see Figure 3). Through reviews of the design from doctors and nurses, more detailed clinical requirements for user interface features and flow were clarified with relevant clinical knowledge applied in the user interface design. In addition, using the iterative loop among probe, design, and review, the feature list and the flow model of the user interface are enriched to fit clinical practice. Subsequently, this feature list was reviewed by developers for software development feasibility.
Figure 3.
Part of the key user interface screens.
The User Interface Design Prototypes and Cognitive Walkthrough
We created a blueprint specification based on the confirmed functions, feature lists, key user interface screens, and workflow models. Subsequently, we conducted an iterative cognitive walkthrough of the blueprint specification by doctors and nurses to confirm and prioritize the specification. The functional modules and workflow were finally clarified in the following manner. (i) Patient addition and basic information entry module: once the patient is confirmed for skin flap transplantation, the nurse first enters the patient's basic information in the VCPWApp, including inpatient number, name, age, gender, bed number, admission time, charge nurse, charge doctor, name of surgery, date of surgery, etc. The information related to the surgery is usually added by the doctor one day before the surgery. (ii) Flap labeling module: after the surgery, the doctor captures the recipient site of this patient at a distance of 35–50 cm parallel to the flap by referring to the ideal capture hint in the viewfinder. The doctor labels the image with the point of penetration and the location of the proximal and distal end of the vessel tip, thereby providing an original reference for postoperative perfusion monitoring and identification of vascular crisis. (iii) Flap observation module: after the patient returns to the ward from the operating room, the nurse monitors and records the perfusion of the flap in the form of data, pictures, and video, including temperature, color, skin turgor, and capillary reflux at a frequency consistent with traditional clinical observation. During this period, the nurse determines whether the flap is in a vascular crisis based on the observation records and decides whether or not to notify the doctor. (iv) Crisis warning module: the doctor receives an alert message from the nurse on the VCPWApp, checks the perfusion and previous observation records of the flap, and prepares medical prescriptions. Thereafter, the nurse reviews the doctor's diagnosis and prescriptions for the flap, performs the appropriate operation, and records. (v) Case archiving module: when the nurse is done observing the patient's condition, the VCPWApp automatically archives the patient's information and generates relevant data forms in the background.
Evaluation
Usability
The application was provided to medical practitioners on institutional smartphones. A total of 28 doctors and nurses participated in the survey (see Table 1). Table 2 presents the results of all participants for the SUS items. For all participants, the total mean score for the SUS scale was 97.95 (SD 2.36), which would, according to Bangor et al.,23 qualify as a successful system. Each item can have a score contribution between 0 and 4. All the items scored above 3.
Table 1.
Medical professionals’ demographic and clinical characteristics (n = 28).
| Characteristics | Statistics |
|---|---|
| Sexuality | |
| Male (%) | 13 (46.4) |
| Female (%) | 15 (53.6) |
| Age (years) | |
| 21–30 | 8 (28.6) |
| 31–40 | 14 (50.0) |
| 41–50 | 6 (21.4) |
Table 2.
The system usability scale score of the VCPWApp (n = 28).
| SUSa analysis item | Value per 5-point Likert scale responseb, n (%) |
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| I think that I would like to use this system frequently. | 0 | 0 | 0 | 1 (3.6) | 27 (96.4) |
| I found the system unnecessarily complex. | 26 (92.9) | 1 (3.6) | 1 (3.6) | 0 | 0 |
| I thought the system was easy to use. | 0 | 0 | 0 | 1 (3.6) | 27 (96.4) |
| I think that I would need the support of a technical person to be able to use this system. | 26 (92.9) | 2 (7.1) | 0 | 0 | 0 |
| I found the various functions in this system were well integrated. | 0 | 0 | 1 (3.6) | 1 (3.6) | 26 (92.9) |
| I thought there was too much inconsistency in this system. | 26 (92.9) | 1 (3.6) | 1 | 0 | 0 |
| I would imagine that most people would learn to use this system very quickly. | 0 | 0 | 0 | 1 (3.6) | 27 (96.4) |
| I found the system very cumbersome to use. | 25 (89.3) | 2 (7.1) | 1 (3.6) | 0 | 0 |
| I felt very confident using the system. | 0 | 0 | 1 (3.0) | 1 (3.6) | 26 (92.9) |
| I needed to learn a lot of things before I could get going with this system. | 26 (92.9) | 2 (7.1) | 0 | 0 | 0 |
SUS: System Usability Scale.
From 1 (“Do not agree at all”) to 5 (“Completely agree”).
Functionality
Without the VCPWApp, 110 patients were enrolled, of whom 92 (83.6%) were male and 18 (16.4%) were female. Their mean age was 35.3 ± 6.4 years (range, 13–65 years). The reconstructive sites included the upper limbs in 13 cases (11.82%), the lower limbs in 93 cases (84.55%), and the trunk in 4 cases (3.64%). The types of surgery were free flap transplantation in 88 cases (80.0%) and pediculated flap transplantation in 22 cases (20.0%).
With the VCPWApp, 127 patients were enrolled, of whom 106 (83.5%) were male and 21 (16.5%) were female (16.5%). Their mean age was 36.2 ± 7.8 years (range 11–68 years). The reconstructive sites included the head and neck in 2 cases (1.57%), the upper limbs in 25 cases (19.69%), lower limbs in 97 cases (76.38%), and trunk in 3 cases (2.36%). The types of surgery were free flap transplantation in 104 patients (81.9%) and pediculated flap transplantation in 23 patients (18.1%). There were no significant differences in gender, age, reconstructive sites, and surgical type between the 2 groups (P > 0.05).
From among the 237 flaps evaluated, 22 flaps (9.28%) required an unplanned return to the operating room. There was no statistically significant difference in the rate of unplanned return to the operating room (P > 0.05). In addition, the time to detect vascular crisis was shorter in cases where the VCPWApp was used. The flap survival rate was higher in the with VCPWApp group than that in the group without VCPWApp, but this was not significantly different (P > 0.05). Detailed results are reported in Table 3.
Table 3.
Comparison of the clinical indicators between the 2 groups (case [percent, %]).
| Group | Cases | Unplanned return to operation room (case [percent, %]) | Time to detect vascular crisis (h) | Success (case [percent, %]) |
|---|---|---|---|---|
| Without VCPW | 110 | 9 (8.18) | 26.71 | 104 (94.55) |
| With VCPW | 127 | 13 (10.24) | 16.26 | 126 (99.21) |
| P | 0.587 | <0.001 | 0.083 |
Discussion
To date, the VCPWApp is the first application and a postsurgical prewarning system that is specially designed to be employed in real-life settings for the nursing management of patients with flap transplants. The application underwent several phases of development. This innovative, iterative, and user-centric approach is the result of a rather close and fruitful collaboration among medical practitioners, user interface designers, and software developers.
Guided by clinical needs, the VCPWApp specified and specialized its function—for example, flap color, temperature, capillary refill, turgor, and other bleeding characteristics were concentrated on the flap observation and recording interface, which avoided the omission and blindness of work, improved the initiative and predictability of clinical work, and helped nurses to perform systematic, dynamic, and continuous nursing and guidance for postoperative patients. The data collected from the SUS measure provided useful information regarding the acceptance of VCPWApp by medical professionals. The willingness to use the VCPWApp again appears to suggest that this instrument is useful for postoperative monitoring of flap transplantation and also for research studies that requires longer participation periods. The resulting SUS measure of the VCPWApp was 97.95, thereby revealing that the functional modules and workflow are suitable for postoperative flap nursing management. Among all the items of the SUS, “I found the system very cumbersome to use” scored the lowest. A series of measures can be taken to improve the ease of use, including but not limited to simplifying unnecessary operations steps; adding more informative tips to the operation steps (e.g., highlighting the prompt at the time, if necessary); recording an actual demonstration video; redesigning the user interface; and creating and distributing questionnaires to understand the specific needs of users.
With regard to the clinical trial, the VCPWApp group showed a significant functional decrease (P < 0.001) in the time to detect vascular crisis, thereby suggesting that the VCPWApp can help doctors and nurses to reduce the time taken to identify flap vascular crisis. Because the time of detection of flap compromise is significantly associated with the salvage of the flap, early identification is crucial.4
Traditional observation methods have limitations in terms of the complete recording of flap perfusion information and may lead to biased recall by the nursing staff. The VCPWApp fully utilizes the advantages of the digital platform to archive all perfusion information of the skin flap in the form of data, pictures and videos, thereby making flap information more objective and reliable and reducing the uncertainty in the judgment of a vascular crisis when observing the flap. In addition, the application can generate data curves, thereby facilitating dynamic observation and avoiding the bias in nurse recall. Moreover, the platform enables remote monitoring and, thus, facilitates health care practitioners to understand flap information and make decisions quickly,26 thereby making the application an important tool for perioperative flap management.
In addition, the VCPWApp establishes a database of flap perfusion in the background and creates new possibilities for the analysis of flap vascular crisis data, which is conducive to further understanding the epidemiological characteristics of vascular crisis and establishing scientific standards for the prevention of vascular crisis after flap transplantation.
Limitations
Health care settings, needs, and workflow can differ considerably; however, this study was conducted at a single institution, and the application is currently only available in Chinese. Therefore, generalizability to other health care settings may be limited. The results of this study are preliminary and are the first step of a larger cohort study that aims to test the effectiveness of the VCPWApp. As such, these results cannot be considered definitive, as the sample size is rather limited.
Conclusions
The assessment of flap perfusion is of great importance to the survival of grafts and flaps. In this study, we developed a novel interactive application (VCPWApp) for the management of skin flap vascular crisis. The application contains modules for patient addition and basic information entry, flap labeling, flap observation, crisis warning, and case archiving. We found that the application significantly reduced the time to detect vascular crisis and showed a good usability profile. In the future, the team will continue to study a larger sample size to verify the long-term effects of the VCPWApp on the management of flap vascular crisis, summarize the change trajectory, and influencing factors of flap perfusion, and lay the foundation for building an intelligent early warning model for vascular crisis after flap transplantation.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
Acknowledgments
Thanks to colleagues in the Department of Hand and Micorsurgery who gave so much of their valuable time and thoughts in developing the VCPWApp.
Authors’ contributions
Study design: PLL
Workflow Analysis and APP Design: PLL, HWH, WSH, WPF, HNT, TJY
System Testing and Application: PLL, XLY, LQQ, WPF
Data Collection and Analysis: XLY, LQQ
Study Supervision: TJY.
Funding
This study was supported by the National Natural Science Foundation of China [82102177].
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding authors on reasonable request.
Ethics approval and consent to participate
This study was approved by the institutional ethics board of Central South University (protocol number: 202103629).
Contributor Information
Ling-li Peng, Email: pll98124@126.com.
Lai-yu Xu, Email: 13820300115@163.com.
Shi-hui Wang, Email: sophiawang1005@qq.com.
Wei-hong Huang, Email: whuangcn@qq.com.
Qing-qing Liu, Email: 1145972628@qq.com.
Nv-tong Huang, Email: 305326801@qq.com.
Pan-feng Wu, Email: 56696995@qq.com.
Ju-yu Tang, Email: tangjuyu@csu.edu.cn.
References
- 1.Wang C, Yang W, Zhang F, Lineaweaver WC, Wen G, Chai Y. Superficial Peroneal Neurocutaneous Flap for Coverage of Donor Site Defect After the Combined Transfer of Toe and Dorsal Foot Flap. Ann Plast Surg. 2021;86(4):440–443. doi: 10.1097/SAP.0000000000002520. [DOI] [PubMed] [Google Scholar]
- 2.Xiao N, Zhang L, Peng X, Mao C, Zhang J, Cai ZG. Non-vascularised fibular bone graft after vascular crisis: compensation for the failure of vascularised fibular free flaps. Br J Oral Maxillofac Surg. 2018;56(8):667–670. doi: 10.1016/j.bjoms.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 3.Starkman SJ, Williams CT, Sherris DA. Flap Basics I: Rotation and Transposition Flaps. Facial Plast Surg Clin North Am. 2017;25(3):313–321. doi: 10.1016/j.fsc.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Karinja SJ, Lee BT. Advances in flap monitoring and impact of enhanced recovery protocols. J Surg Oncol. 2018;118(5):758–767. doi: 10.1002/jso.25179. [DOI] [PubMed] [Google Scholar]
- 5.Suchyta M, Mardini S. Innovations and Future Directions in Head and Neck Microsurgical Reconstruction. Clin Plast Surg. 2020;47(4):573–593. doi: 10.1016/j.cps.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 6.Tan S, Pan L, Zhao H, Hu J, Chen H. Perioperative nursing for immediate breast reconstruction with deep inferior epigastric perforator flap after breast cancer resection. J Thorac Dis. 2018;10(7):4017–4022. doi: 10.21037/jtd.2018.07.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindelauf AAMA, Saelmans AG, van Kuijk SMJ, van der Hulst RRWJ, Schols RM. Near-Infrared Spectroscopy (NIRS) versus Hyperspectral Imaging (HSI) to Detect Flap Failure in Reconstructive Surgery: A Systematic Review. Life (Basel) 2022;12(1):65. doi: 10.3390/life12010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown E, Suh HP, Han HH, Pak CJ, Hong JP. Best New Flaps and Tips for Success in Microsurgery. Plast Reconstr Surg. 2020;146(6):796e–807e. doi: 10.1097/PRS.0000000000007331. [DOI] [PubMed] [Google Scholar]
- 9.Zhao L, Yang J, Liu W. Application and effect evaluation of nursing quality target management in free flap transplantation for hand injury. PLoS One. 2021;16(1) doi: 10.1371/journal.pone.0245097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haddock NT, Gobble RM, Levine JP. More consistent postoperative care and monitoring can reduce costs following microvascular free flap reconstruction. J Reconstr Microsurg. 2010;26(7):435–439. doi: 10.1055/s-0030-1254232. [DOI] [PubMed] [Google Scholar]
- 11.Smit JM, Zeebregts CJ, Acosta R, Werker PMN. Advancements in free flap monitoring in the last decade: a critical review. Plast Reconstr Surg. 2010;125(1):177–185. doi: 10.1097/PRS.0b013e3181c49580. [DOI] [PubMed] [Google Scholar]
- 12.Salgado CJ, Moran SL, Mardini S. Flap monitoring and patient management. Plast Reconstr Surg. 2009;124(6 Suppl):e295–e302. doi: 10.1097/PRS.0b013e3181bcf07b. [DOI] [PubMed] [Google Scholar]
- 13.Engel H, Huang JJ, Tsao CK, Lin CY, Chou PY, Brey EM, Henry SL, Cheng MH. Remote real-time monitoring of free flaps via smartphone photography and 3G wireless Internet: a prospective study evidencing diagnostic accuracy. Microsurgery. 2011;31(8):589–595. doi: 10.1002/micr.20921. [DOI] [PubMed] [Google Scholar]
- 14.Kiranantawat K, Sitpahul N, Taeprasartsit P, Constantinides J, Kruavit A, Srimuninnimit V, Punyahotra N, Chatdokmaiprai C, Numhom S. The first Smartphone application for microsurgery monitoring: SilpaRamanitor. Plast Reconstr Surg. 2014;134(1):130–139. doi: 10.1097/PRS.0000000000000276. [DOI] [PubMed] [Google Scholar]
- 15.Armstrong KA, Coyte PC, Semple JL. The first smartphone application for microsurgery monitoring: SilpaRamanitor. Plast Reconstr Surg. 2015 Feb;135(2):458e. doi: 10.1097/PRS.0000000000000896. [DOI] [PubMed] [Google Scholar]
- 16.Ricci JA, Vargas CR, Lin SJ, Tobias AM, Taghinia AH, Lee BT. A Novel Free Flap Monitoring System Using Tissue Oximetry with Text Message Alerts. J Reconstr Microsurg. 2016;32(5):415–420. doi: 10.1055/s-0036-1582264. [DOI] [PubMed] [Google Scholar]
- 17.Sharmil H, Kelly J, Bowden M, Galletly C, Cairney I, Wilson C, Hahn L, Liu D, Elliot P, Else J, Warrior T, Wanganeen T, Taylor R, Wanganeen F, Madrid J, Warner L, Brown M, de Crespigny C. Participatory Action Research-Dadirri-Ganma, using Yarning: methodology co-design with Aboriginal community members. Int J Equity Health. 2021;20(1):160. doi: 10.1186/s12939-021-01493-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeffery AD, Novak LL, Kennedy B, Dietrich MS, Mion LC. Participatory design of probability-based decision support tools for in-hospital nurses. J Am Med Inform Assoc. 2017;24(6):1102–1110. doi: 10.1093/jamia/ocx060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Witteman HO, Dansokho SC, Colquhoun H, Coulter A, Dugas M, Fagerlin A, Giguere AM, Glouberman S, Haslett L, Hoffman A, Ivers N, Légaré F, Légaré J, Levin C, Lopez K, Montori VM, Provencher T, Renaud JS, Sparling K, Stacey D, Vaisson G, Volk RJ, Witteman W. User-centered design and the development of patient decision aids: protocol for a systematic review. Syst Rev. 2015;4(1):11. doi: 10.1186/2046-4053-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Vito Dabbs A, Myers BA, Mc Curry KR, Dunbar-Jacob J, Hawkins RP, Begey A, Dew MA. User-centered design and interactive health technologies for patients. Comput Inform Nurs. 2009;27(3):175–183. doi: 10.1097/NCN.0b013e31819f7c7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brooke J. In: Usability evaluation in industry. Jordan PW, Thomas B, Mcclelland IL, Weerdmeester B, editors. Taylor & Francis; London: 1996. SUS-a quick and dirty usability scale. [Google Scholar]
- 22.Maramba I, Chatterjee A, Newman C. Methods of usability testing in the development of eHealth applications: A scoping review. Int J Med Inform. 2019;126:95–104. doi: 10.1016/j.ijmedinf.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 23.Bangor A, Kortum PT, Miller JT. An Empirical Evaluation of the System Usability Scale. Int J Hum Comput Interact. 2008;24(6):574–594. doi: 10.1080/10447310802205776. [DOI] [Google Scholar]
- 24.Tam S, Weber RS, Liu J, Ting J, Hanson S, Lewis CM. Evaluating Unplanned Returns to the Operating Room in Head and Neck Free Flap Patients. Ann Surg Oncol. 2020;27(2):440–448. doi: 10.1245/s10434-019-07675-3. [DOI] [PubMed] [Google Scholar]
- 25.Cruz-Segura A, Cruz-Domínguez MP, Jara LJ, Miliar-García Á, Hernández-Soler A, Grajeda-López P, Martínez-Bencomo MA, Montes-Cortés DH. Early Detection of Vascular Obstruction in Microvascular Flaps Using a Thermographic Camera. J Reconstr Microsurg. 2019;35(7):541–548. doi: 10.1055/s-0039-1688749. [DOI] [PubMed] [Google Scholar]
- 26.Liu K, Xie Z, Or CK. Effectiveness of Mobile App-Assisted Self-Care Interventions for Improving Patient Outcomes in Type 2 Diabetes and/or Hypertension: Systematic Review and Meta-Analysis of Randomized Controlled Trials. JMIR Mhealth Uhealth. 2020;8(8):e15779. doi: 10.2196/15779. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding authors on reasonable request.