Abstract
Breast cancer stem cells (BCSCs) are a small subpopulation of breast cancer cells, capable of metastasis, recurrence, and drug resistance in breast cancer patients. Therefore, targeting BCSCs appears to be a promising strategy for the treatment and prevention of breast cancer metastasis. Mounting evidence supports the fact that carnitine, a potent antioxidant, modulates various mechanisms by enhancing cellular respiration, inducing apoptosis, and reducing proliferation and inflammatory responses in tumor cells. The objective of this study was to investigate the impact of L-carnitine (LC) on the rate of proliferation and induction of apoptosis in CD44+ CSCs. To achieve this, the CD44+ cells were enriched using the Magnetic-activated cell sorting (MACS) isolation method, followed by treatment with LC at various concentrations. Flow cytometry analysis was used to determine cell apoptosis and proliferation, and western blotting was performed to detect the expression levels of proteins. Treatment with LC resulted in a significant decrease in the levels of p-JAK2, p-STAT3, Leptin receptor, and components of the leptin pathway. Moreover, CD44+ CSCs-treated cells with LC exhibited a reduction in the proliferation rate, accompanied by an increase in the percentage of apoptotic cells. Hence, it was concluded that LC could potentially influence the proliferation and apoptosis of CD44+ CSC by modulating the expression levels of specific protein.
Keywords: Cancer stem cells, L-carnitine, Proliferation, Apoptosis, Signaling pathways
1. Introduction
A growing body of evidence indicates the presence of highly tumorigenic cells within tumor microenvironments of various cancers, including breast, ovarian, etc. These populations exhibit stem cell characteristics such as multipotency, self-renewal, and stem cell markers [1].
Notably, these stem cells exist in cell lines of cancer previously believed to be homogeneous [2]. This rare population possesses unique features of a highly tumorigenic nature, self-renewal, multi-lineage differentiation, and clonogenicity known as Cancer stem cells (CSCs) [3]. Specific cell surface markers, including CD133, CD44, and CD1172, can be used to isolate and characterize CSCs. Furthermore, growing evidence has demonstrated that CSCs and embryonic stem cells maintain common stemness factors such as Nanog, SOX2, and Oct4 [4]. Several studies declare that CSCs contribute to tumor initiation, metastasis, relapse, and chemotherapy resistance [1]. It is well established that CSCs play a crucial role in tumor progression and resistance to therapy; however, targeting and destroying these cells is still a clinical challenge. Therefore, it is imperative to understand better the molecular pathways involved in maintaining CSC characteristics. It is well documented that metabolic regulation is critical in cancer progression and drug resistance [5]. Indeed, cancer cells reprogrammed their metabolic pathways towards glycolytic metabolism to fulfill biosynthesis demands for synthesizing macromolecules and biomass to support survival and unrestrained proliferation during tumor growth. On the other hand, proliferating cancer cells ferment substantially more glucose into ATP despite abundant oxygen. In other words, cancer cells bypass the mitochondrial respiratory chain for synthesizing ATP even in an abundance of oxygen. This phenomenon is known as the Warburg effect [6]. L-carnitine (LC) is the biologically active form of carnitine that is endogenously synthesized from lysine and methionine.
LC is responsible for transporting the long-chain acyl groups of fatty acids from the cytosol to the mitochondrial matrix, thus allowing β-oxidation to acetyl-CoA and entering the Citric acid cycle (CAC) to produce energy [7]. Relying on these concepts, LC is an essential compound for generating metabolic energy in living cells. It has been found that the rapidly growing tumor cells maintain a high glycolytic rate (200 times higher) than found in normal tissues of origin. Warburg effect is challenging due to its complexity; however, it remains the most widely evident for the metabolic phenotype exhibited by tumors. In other words, the Warburg effect is caused partly by dysfunctions of respiratory metabolism [8]. In light of this theory that the citric cycle is detrimental to most cancer cells, we hypothesize that LC would result in disruption of cellular metabolism in CSCs. Most cancer cells avoid CAC due to its occurrence in the mitochondria; since the mitochondria are associated with apoptotic events through cytochrome c and caspase-dependent cascades [9]. As a result, we hypothesize that LC may disturb the cellular metabolism of CSCs.
Considering these explanations, the purpose of this study was to elucidate the influence of LC on proliferation rate and apoptosis activity in CD44+ CSCs. To accomplish this, CD44+ CSCs were incubated in the presence and absence of LC, and were analyzed by Annexin/PI staining and Ki/caspase-3 assessment by flow cytometric method. The expression level of some components of JAK-2, and STAT3 proteins was determined, too.
2. Materials and methods
Cell culture plates and other materials were purchased from SPL Life Sciences (Pocheon, South Korea) and Gibco Co. (UK), respectively. Besides, a list of other compounds used in this study were provided in the text. Cells were divided into three experimental groups, including control group I: CD44+ CSCs cells with no treatment, group II and group III CD44+ CSCs treated with a concentration of 2.5 mM and 5 mM of LC, respectively.
2.1. Culture of MDA-MB-231 cell line
In this study, the MDA-MB-231 cell line was selected as a model of triple-negative breast cancer (TNBC). The cells were provided by Pasteur Institute in Iran and cultured in RPMI 1640 medium supplemented with 10% Fetal bovine serum (FBS) and 100 U/ml penicillin/streptomycin. The cells were cultivated at a density of 15 × 104 cells/cm2, incubated in an air humidified atmosphere at 37 °C with 5% CO2, and passaged twice a week to obtain logarithmic growth phase [10].
2.2. CD44+ CSCs enrichment by MACS technique
Magnetic-activated cell sorting (MACS) technique was used to isolate and enrich CD44+ cells. In brief, the cells were harvested using 0.25% trypsin-EDTA (Gibco, USA). The detached cells were washed and re-suspended in PBS buffer. In the following, 1 × 107 cells were labeled with 20 μl of CD44 microbeads (Miltenyi Biotec., Germany) for 20 min at 4 °C on a rotator. Next, the cells were washed with PBS containing FBS (0.5%) and enriched with LS column (Miltenyi Biotec., Germany) attached to a Midi-MACS magnet (Miltenyi Biotec., Germany). At the end of separation, CD44+ cells were harvested as primary CSCs for subsequent analysis [11].
2.3. Flow cytometric analysis for purity assessment of isolated CD44+ CSCS
CD44 intensity was detected by an anti-PE-CD44 antibody (Miltenyi Biotec, Germany) as a cell surface marker, according to the manufacturer's instructions. For this purpose, enriched CD44+ CSCs were incubated in the presence of 10 μl CD44 antibody for 30 min at room temperature in a dark place and were characterized using a flow cytometer (FACS Calibur, BD Bioscience, USA). Flowjo Software was utilized to analyze the obtained data [12]. An appropriate isotype-matched antibody identified nonspecific protein labeling.
2.4. Cell viability determination test
An MTT-based colorimetric was used to elucidate cell viability on LC-treated cells. The basis of the test is the reduction of the tetrazolium dye MTT 3-(4, 5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (Sigma-Aldrich, USA) to a water-insoluble formazan crystal. 1 × 104/well were seeded onto a 96-well plate containing culture medium supplemented with FBS for 24 h. Next, 20 μl of media containing MTT dye solution 5 mg/ml was added to each well, and incubation was continued for a further 4 h. Finally, the formazan crystals were formed and dissolved by adding 200 μl DMSO solution after removing the culture media. The optical density was evaluated by a microplate reader (Biotek Instruments, USA) at 630 nm. Each reagent's IC50 value was determined separately by calculating the slope and intercept concentrations. The experiment was conducted in triplicate for three times [13].
2.5. Flow cytometric detection of apoptosis using Annexin V/PI
To investigate whether the treatment of LC (2.5 and 5 Mm) induces apoptosis, staining for AnnexinV/PI was carried out in all experimental groups. Following the treatment, 20 × 104 cells/wells were harvested, followed by blocking with FBS and washing with PBS. Afterward, the cells were gently re-suspended in the 1X binding buffer (ref no.: 00-0055-56, ebioscience) and stored at 4 °C for 20 min in a dark condition. Following this, the cells were stained with 100 μL of binding buffer to which 5 μL of FITC conjugated Annexin V (ref no.: 11-8005-74, e-bioscience) was added and kept for 15 min at 25 °C. Then, cells were washed and re-suspended in 100 μL of binding buffer containing 5 μL of PI and incubated for 15 min in a dark place at room temperature. The fluorescence was measured using FACSCalibur (BD Bioscience), and the obtained data were analyzed using FlowJo software ver. X.0.7.49 [14].
2.6. Proliferation and apoptosis determination using Ki-67 and caspase-3
The ratio of Ki-67 (a marker of proliferation) and Caspase-3 (crucial mediator of apoptosis) were evaluated in all experimental groups to determine apoptosis vs. cell proliferation. For ki67 staining, treated cells and untreated cells were washed with PBS followed by incubation with 0.2% Triton X-100 for 15 min. Afterward, the cells were stained with 5 μl of Ki-67 antibody (Cat No: 12-5699-42) for 30 min, followed by washing and re-suspending in staining buffer for flow cytometry analysis. To evaluate caspase-3 enzymatic activity, harvested cells were washed twice with washing buffer (PBS containing 5% FBS) and immediately fixed by FCM fixation buffer for 30 min (sc-3622, Santa Cruz, CA) and washed twice with PBS buffer. The cells were permeabilized by incubating with FCM permeabilization buffer for 5 min at RT (sc-3623, Santa Cruz, CA). After washing the cells, a PE-conjugated anti-caspase antibody (BD Bioscience, Germany) was added to the cells and incubated for 30 min to analyze with the flow cytometry method [15].
2.7. Western blotting analysis for determination of protein expression
The impact of LC on the protein levels of JAK2, p-JAK2, STAT3, p-STAT3, Leptin, and Leptin receptor were evaluated in tissue homogenates in all experimental groups using a western-blotting assay. For this purpose, 1.5 × 106 cells of all three groups were gently lysed in a protein extraction buffer containing 1% Triton X-100, 0.1 m NaCl, 25 mm HEPES, 2 mm EDTA, 25 mm NaF, and 1 mm sodium orthovanadate supplemented with 1% protease inhibitor (Roche, Basel, Switzerland) for 30 min on ic. Then lysates were centrifuged at 12000×g for 20 min, and protein concentration was measured in the supernatant using a picodrop (Picodrop LTD, Cambridge, UK). Following this, ∼100 μg of each protein sample was loaded and separated on 12% % SDS- acrylamide gel and transferred to the PVDF membrane. The membranes were blocked with skim milk (5% in PBS). To prevent nonspecific interaction of proteins in the membrane. Next, the membranes were incubated with primary antibodies (1:1000) against targeted proteins at 4 °C for overnight incubation as follows: JAK2, p-JAK2, STAT3, p-STAT3, Leptin, Leptin receptor, and β-actin as an internal control. After washing the membranes, the bindings of primary antibodies were detected by HRP-conjugated antibody as a secondary antibody (Santa Cruz Biotechnology, Santa Cruz, USA) at 1:1000 dilution at room temperature for 1 h. Finally, protein bands were visualized using an ECL reagent, and the density of each band was quantified by ImageJ software [16].
2.8. Statistical analysis
The results were analyzed using the software program Graph Pad Prism version 6.01. We used one-way, and two-way ANOVA followed by Dunnett's and Sidak post hoc test to determine the significant difference among groups, respectively.
3. Results
3.1. Identification and characterization of CD44+ CSCs
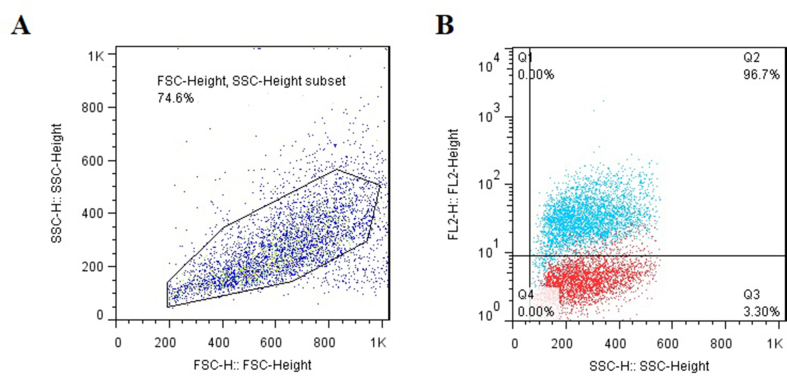
CD44+ CSCs cells enriched by the MACS method and to determine the purity of cells they were subjected to flow cytometry analysis. The results revealed that the expression of the CD44 marker was about 96.7% in the MDA-MB-231 cell line (Fig. 1).
Fig. 1.
Flow cytometric characterization of enriched CD44+ CSCs. (A) A total population of cells for CD44+ CSCs evaluation; (B) Flow cytometry showed that 96.7% of cells positive for CD44 marker, Data are expressed as mean ± SD.
3.2. Effect of LC on the CD44+ CSCs proliferation
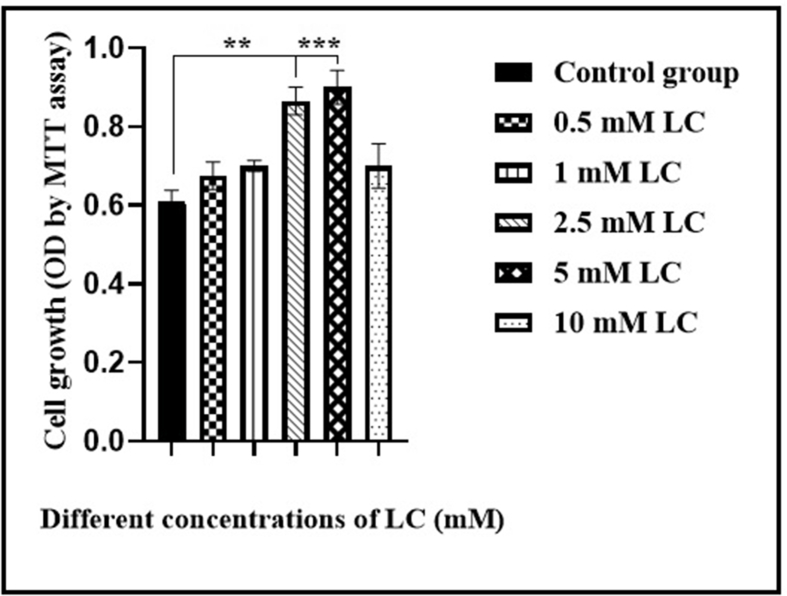
MTT assay was performed to find the optimal LC concentration and quantify cell viability. In this regard, CD44+ CSCs were treated with various concentrations of LC (0.5, 1, 2.5, 5, and 10 mM) for 24 h, and then the proliferation was assessed by MTT assay, according to the method of Fathi et al. (2020) [17]. No remarkable changes were found in the cell viability of CD44+ CSC treated with the 0.5, 1 and 10 mM LC. LC treatment at a dose of 2.5 and 5 mM of resulted in significant changes in CD44+ CSC proliferation, compared to the control group (∗∗P < 0.01, ∗∗∗P < 0.001, respectively). Notably, after 24 h of treatment of CD44+ CSCs with 2.5 and 5 mM, substantial rapid growth was detected when compared to those cells treated with the 10 mM LC (∗∗P < 0.01) (Fig. 2).
Fig. 2.
Proliferation rates of isolated CD44+ CSCs following treatment of LC for 24 h. MTT assay was conducted to determine the growth/proliferation of CD44+ CSCs treated with different concentrations of LC (0.5, 1, 2.5, 5, and 10). ∗∗P<0.01 and ∗∗∗P<0.001 in comparison with the control group.
3.3. Quantitative analysis of apoptosis induction using Annexin V/PI staining assay
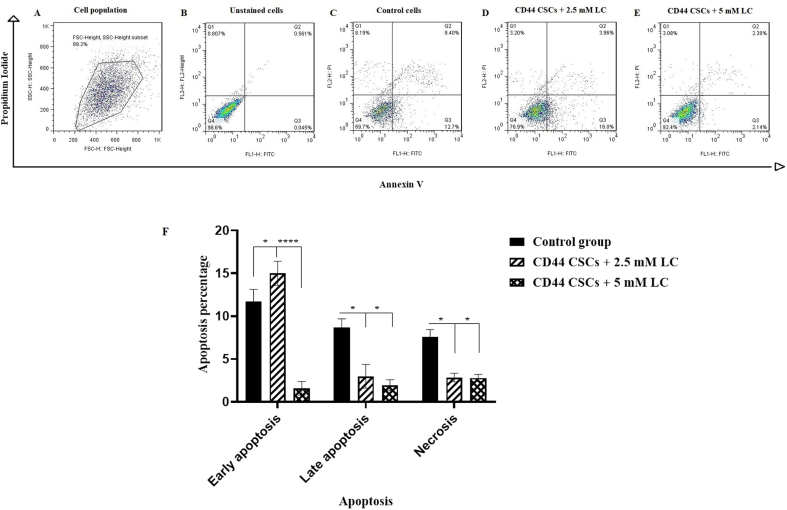
To determine the apoptosis rate of CD44+ CSCs, Annexin V/PI double staining assay was conducted and analyzed by flow cytometry. As depicted in Fig. 3, following LC treatment, the percentage of early apoptosis (Annexin+, PI−) was about 16% in group II (CD44+ CSCs treated with 2.5 mM LC) (∗P < 0.05). However, almost 3.96% and 2.38% of the cells group II and III were in late apoptotic stage (Annexin+, PI+), respectively. The late apoptotic percentage in group I (control group) was 9.40% and it was interpreted that 2.5 and 5 mM LC cause to reduce the late apoptosis of CD44+ CSCs. In the other words, it was seen that LC just in 2.5 mM is able to induce apoptosis of CD44+ CSCs.
Fig. 3.
CD44+ CSCs-treated with LC at a dose of 2.5 and 5 mM enhanced apoptosis rate. After 24 h of treatment, CD44+ CSCs were stained with Annexin V/PI assay followed by flow cytometry analysis. A shift from bottom-right quadrant panel (early apoptosis) to top-right quadrant panel (late apoptosis) and top-left quadrant panel (necrosis) was observed. (A) A total population of CD44+ CSCs; (B) Unstained cells; (C) CD44+ CSCs in the absence of LC; (D) CD44+ CSCs treated with 2.5 mM LC; (E) CD44+ CSCs treated with 5 mM LC. The quantification of apoptosis was shown in part F. Values are mean ± SD from independent experiments (∗P < 0.05 and ∗∗∗∗P < 0.0001, n = 3).
3.4. Flow cytometry analysis for quantification of Ki-67 and caspase-3 expression
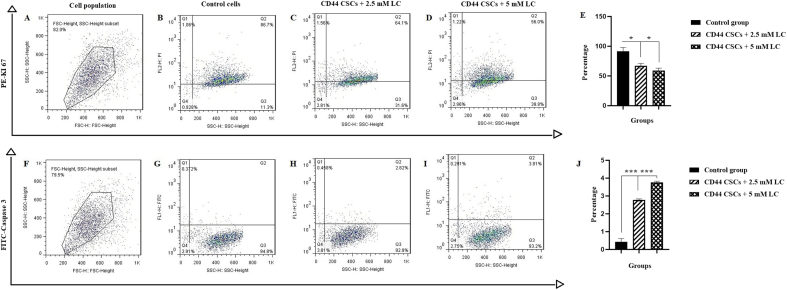
The effect of LC on inhibition of CD44+ CSCs proliferation was detected by Ki-67 (proliferative surface marker) expression. It was shown that LC could down-regulate the expression level of Ki-67 in CD44+ CSCs. At the end of the treatment period (24 h), the percentage of Ki-67 in group II (2.5 mM LC-treated CD44+ CSCs) and group III (5 mM LC-treated CD44+ CSCs) reached 64.1% and 56% when compared to non-treated cells with proliferation rate of 86.7% (Fig. 4A–E), (∗P < 0.05). Also, apoptosis of CD44+ CSCs was assessed following treatment with 2.5 and 5 mM LC by caspase-3 investigation. As shown in Fig. 4F–J, the level of caspase-3 went up to 9.4 and 12.7-fold in group II (2.5 mM LC-treated CD44+ CSCs) and III (5 mM LC-treated CD44+ CSCs), respectively (∗∗∗P < 0.001).
Fig. 4.
Representative proliferation assay of CD44+ CSCs-treated with LC at a dose of 2.5 and 5 mM. After 24 h of treatment, CD44+ CSCs were stained by antibodies and evaluated by flow cytometry as follows: Ki-67 (A–E) and caspase-3 (F–J). In this panel, A and F revealed selected cell population, B and G represent non-treated cells (control), C and H represent CD44+ CSCs exposed to 2.5 mM LC, D and I are CD44+ CSCs exposed to 5 mM LC, and E and J are the statistical analysis. All values are shown as mean ± SD from three independent experiments (∗P < 0.05 and ∗∗∗P < 0.001).
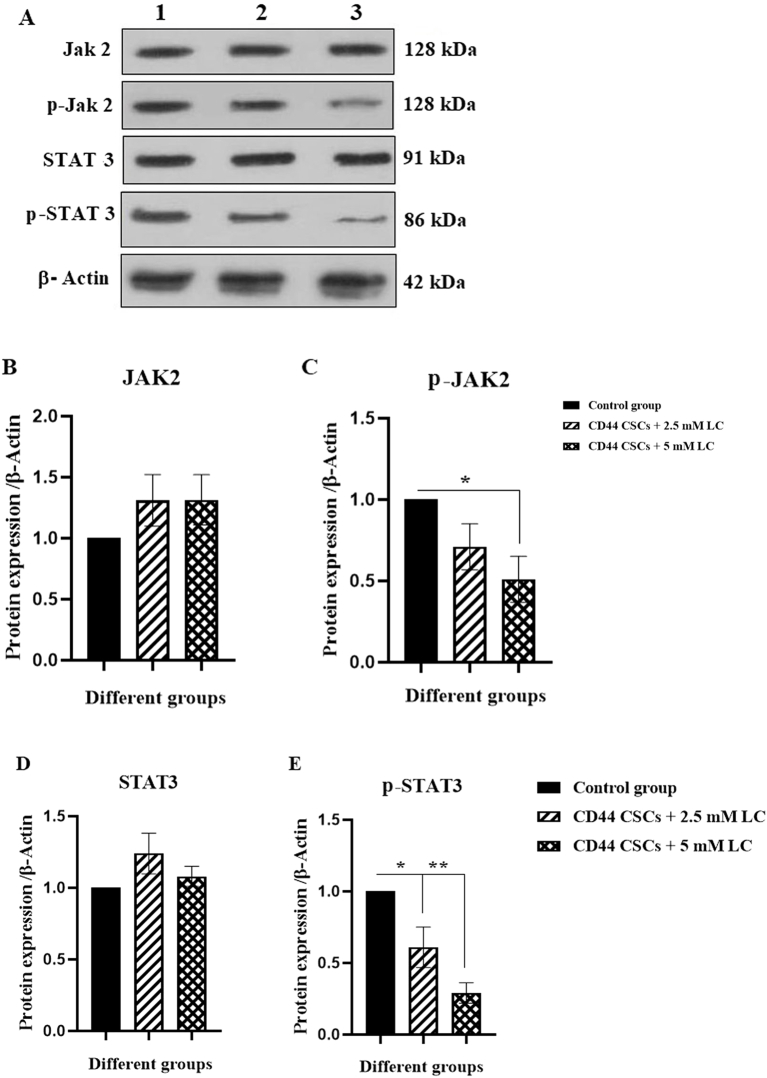
3.5. LC induced an alteration in the protein level of JAK2, STAT3, LEPR, and leptin signaling pathways
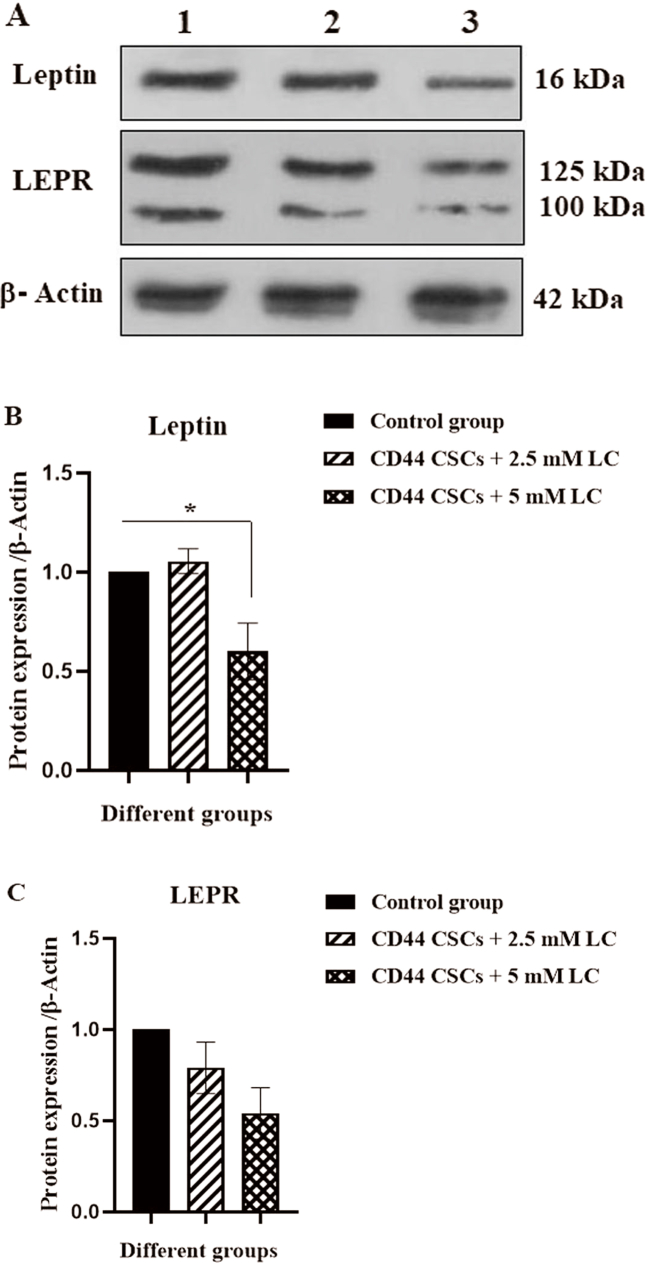
To determine the effective signaling pathways of CD44+ CSCs the expression level of JAK2/p-JAK2, STAT3/p-STAT3, leptin, and LEPR were measured using western blotting analysis. As revealed, there was a reduction in p-JAK2 protein level by about 0.5-fold in CD44+ CSCs-treated with 5 mM LC (∗p < 0.05). Group II (2.5 mM LC-treated CD44+ CSCs) and III (5 mM LC-treated CD44+ CSCs) revealed a reduction in p-STAT3 protein level by about 0.6-fold and 0.29-fold, respectively (∗p < 0.05 and ∗∗p < 0.01) (Fig. 5). The ratios of p-JAK2 to total JAK2 expression was significantly decreased in the group III (5 mM LC-treated CD44+ CSCs) (∗p < 0.05) (Fig. 5F). Also, the ratios of p-STAT3 to total STAT3 expression was significantly decreased in the Group II (2.5 mM LC-treated CD44+ CSCs) and III (5 mM LC-treated CD44+ CSCs) (∗p < 0.05) (Fig. 5G). As compared to non-treated CD44+ CSCs, LC treatment with a dose of 5 mM could reduce the protein level of leptin by 0.6-fold (∗p < 0.05) (Fig. 6).
Fig. 5.
Western blotting analysis for JAK2, p-JAK, STAT3, and p-STAT3 protein levels in CD44+ CSCs treated with LC. After 24 h of treatment, proteins were extracted from all experimental groups (I, II, and III) and subjected to western blotting to determine protein expressions. (A) The band intensity of JAK2, p-JAK, STAT3, and p-STAT3 was shown. (B–E) The statistical analysis of protein expressions was depicted. Values are shown as mean ± SD from three independent experiments (∗P < 0.05 and ∗∗P < 0.01). The ratio of p-JAK2/total JAK2 and p-STAT3/STAT3 levels were shown as F and G, respectively. Lane 1: Control group, lane 2: CD44+ CSCs +2.5 mM LC, and Lane 3: CD44+ CSCs +5 mM LC.
Fig. 6.
Western blotting analysis for Leptin, and LEPR protein levels in CD44+ CSCs treated with LC. After 24 h of treatment, proteins were isolated from all experimental groups (I, II, and III) and subjected to western blotting to determine protein expressions. (A) The protein expression of leptin, and LEPR was revealed. (B–C). The statistical analysis of protein expressions was depicted. All values are shown as mean ± SD from three independent experiments (∗P < 0.05). Lane 1: Control group, lane2: CD44+ CSCs 2.5 + mM LC, and lane 3: CD44+ CSCs +5 mM LC.
4. Discussion
Some recent studies have explored the impact of carnitine on cancer cell growth, metastasis, and invasion [18]. Previous studies have examined the interplay between carnitine and various factors affecting apoptosis induction in cancer cell lines [19,20]. Based on the literature review, carnitine positively impacts apoptosis and DNA fragmentation in cancer cells. It can be due to an increased rate of lactate and pyruvate flux to mitochondria that increased the availability of these substrates to form acetyl-CoA. As a result of these events, superoxide anion (O2−) production in mitochondria is accelerated, resulting in apoptosis induction [21]. Growing evidences have declared that chemotherapy resistance is still an important obstacle in cancer treatment. As noted above CSCs exhibited high tumorigenicity and resistance to therapeutic agents [22]. Despite the established roles of CSCs in tumorigenic behavior, targeting and destroying these cells is still a challenging endeavor in the clinic. With this background, understanding the molecular pathways underlying the maintenance of CSC characteristics is vital. As clearly evidenced, chemotherapy and radiotherapy are required procedures for cancer therapy. It is estimated that 60% of all cancer patients receive radiotherapy every year, whether as a single treatment or in conjunction with chemotherapy or surgery, both of which have disadvantages. Consequently, finding more effective cytotoxic compounds with less side effects is in demand to combat cancer. In this context, vitamins and natural antioxidants have drawn many researchers’ attention to cancer treatment. Given the potential antioxidant and apoptotic activity of LC, this study was conducted to investigate the possible effect of LC in cancer cells. Indeed, this study aimed to provide new insights into LC anti-tumor activity on CD44+ breast CSCs. For this objective, CSCs were isolated using cell-specific surface marker CD44. Several studies have suggested that CD24, and CD44 markers are the most well-known markers for isolating and identifying breast CSCs [23]. The results of the present work confirmed previous studies and found that 96.7% of enriched cells with MACS technique were CD44-positive. Following this, we evaluated how LC affected CD44+ CSC apoptosis rate. Fathi et al. (2021) provided evidence that early apoptosis significantly increased bone marrow-derived CD34+ hematopoietic stem cells in an animal model [24]. In a study by Jiang et al. (2016), it was found that the rate of apoptosis in hepatocyte cancer cells increased in the LC treated group when compared to the non-treated group [25]. The induction of apoptosis could be due to the overexpression of key proteins involved in the extrinsic apoptosis pathway, including FasL, TNF-α, and caspase-8. Besides, low expression of the level of BCL-2 protein can cause apoptosis. In hepatocyte cancer cells, increasing apoptosis by LC-mediated activity could result from the down-regulation of BCL-2 levels concurrent with overexpression of FasL [26]. In addition, Vescovo et al. (2002) found that cancer cells had lower levels of free LC than normal cells [27].
According to Qi et al. (2006), LC treatment abolished the effect of GP7, as an anti-cancer agent. Gp7 promotes caspase-3-induced apoptosis and its cleavage accompanied by internucleosomal DNA fragmentation in Raji cells. Interestingly, LC reversed all the mentioned apoptotic events; thereby, LC could be regarded as a potent anti-apoptotic compound [28].
The finding of the current study is in line with other reports with the fact that LC promotes the percentage of early apoptosis in CD44+ CSCs. Furthermore, there was an increment in the level of caspase-3 in CD44+ CSCs treated with 2.5 and 5 mM LC, (2.82 and 3.81-fold), respectively.
As evidenced, multiple signaling cascades play a pivotal role in cancer biology. It was widely accepted that one of the main regulators of lipid metabolism is JAK2/STAT3 pathway, which enhances the breast CSCs and chemo resistance to chemotherapy [23]. In a recently published in vivo report, blocking the JAK2/STAT3 signaling pathways impeded self-renewal and proliferation of cancer cells, indicating that JAK2/STAT3 is a potential target for the treatment of solid tumors [29]. It has also been revealed that STAT3 promotes glycolytic profile by supporting the transcription of proliferative breast cancer cells [30]. Consistent with these reports concerning the involvement of signaling pathways, our findings reflect the crucial contributory role of the JAK2/STAT3 pathway in the CD44+ CSCs in breast cancer. Based on obtained data, LC-treated CD44+ CSCs with 2.5 mM and 5 mM remarkably reduced the expression of p-JAK2 and p-STAT3 when compared to non-treated ones. Besides, a decrease was observed in the protein level of leptin and LEPR following LC treatment. Since the leptin and LEPR expression is strongly associated with chemo resistance and self-renewal capacity of breast CSCs [23].
In agreement with previous studies, we found that LC in concentrations of 2.5 and 5 mM decreased leptin and LEPR expression. Our findings indicate that LC induces apoptosis in CD44+ CSCs, which is associated with caspase-3 up-regulation. A further consequence of these effects is the reduction of p-JAK2 and p-STAT3 expression, which play a critical role in breast CSCs along with cancer chemo resistance.
Funding
This project was financially funded by a research grant from the Tabriz University of Medical Sciences, Tabriz, Iran (Pazhoohan ID: 65757).
Availability of data
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethical approval
Ethical consent was approved by an ethics committee at Tabriz University of Medical Sciences, Tabriz, Iran (Ethic Code No: IR. TBZMED.VCR.REC.1399.193).
Author contributions
R. F as the executive of the project, had the main contribution to conception and design, the performance of experiments, data analysis, and manuscript writing; E. Z and S. M involved in the performance of manuscript writing and supervised the manuscript preparation; Z. S and A.A. M involved in the performance of experiments; all authors approved the final version of the article.
Declaration of competing interest
The authors declare no conflict of interest, financial or otherwise.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Contributor Information
Ezzatollah Fathi, Email: ez.fathi@tabrizu.ac.ir.
Soheila Montazersaheb, Email: smontazersaheb@gmail.com.
References
- 1.Long H., Xiang T., Qi W., Huang J., Chen J., He L., et al. CD133+ ovarian cancer stem-like cells promote non-stem cancer cell metastasis via CCL5 induced epithelial-mesenchymal transition. Oncotarget. 2015;6(8):5846–5859. doi: 10.18632/oncotarget.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Setoguchi T., Taga T., Kondo T. Cancer stem cells persist in many cancer cell lines. Cell Cycle. 2004;3(4):414–415. doi: 10.4161/cc.3.4.799. [DOI] [PubMed] [Google Scholar]
- 3.Sheng X., Li Z., Wang D.L., Li W.B., Luo Z., Chen K.H., et al. Isolation and enrichment of PC-3 prostate cancer stem-like cells using MACS and serum-free medium. Oncol Lett. 2013;5(3):787–792. doi: 10.3892/ol.2012.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ezeh U.I., Turek P.J., Reijo R.A., Clark A.T. Human embryonic stem cell genes OCT4, NANOG, STELLAR, and GDF3 are expressed in both seminoma and breast carcinoma. Cancer. 2005;104(10):2255–2265. doi: 10.1002/cncr.21432. [DOI] [PubMed] [Google Scholar]
- 5.Camarda R., Zhou A.Y., Kohnz R.A. Inhibition of fatty acid oxidation as a therapy for MYC-overexpressing triple-negative breast cancer. Nature medicine. 2016;vol. 22(4):427–432. doi: 10.1038/nm.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vander Heiden M.G., Cantley L.C., Thompson C.B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faye A., Esnous C., Price N.T., Onfray M.A., Girard J., Prip-Buus C. Rat liver carnitine palmitoyltransferase 1 forms an oligomeric complex within the outer mitochondrial membrane. J Biol Chem. 2007;282(37):26908–26916. doi: 10.1074/jbc.M705418200. [DOI] [PubMed] [Google Scholar]
- 8.DeBerardinis R.J. Is cancer a disease of abnormal cellular metabolism? New angles on an old idea. Genet Med. 2008;10(11):767–777. doi: 10.1097/GIM.0b013e31818b0d9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonora E., Porcelli A.M., Gasparre G., Biondi A., Ghelli A., Carelli V., et al. Defective oxidative phosphorylation in thyroid oncocytic carcinoma is associated with pathogenic mitochondrial DNA mutations affecting complexes I and III. Cancer Res. 2006;66(12):6087–6096. doi: 10.1158/0008-5472.CAN-06-0171. [DOI] [PubMed] [Google Scholar]
- 10.Jafari S., Dabiri S., Mehdizadeh Aghdam E., Fathi E., Saeedi N., Montazersaheb S., et al. Synergistic effect of chrysin and radiotherapy against triple-negative breast cancer (TNBC) cell lines. Cli Trans Oncol. 2023:1–10. doi: 10.1007/s12094-023-03141-5. [DOI] [PubMed] [Google Scholar]
- 11.Farahzadi R., Fathi E., Mesbah-Namin S.A., Vietor I. Granulocyte differentiation of rat bone marrow resident C-kit+ hematopoietic stem cells induced by mesenchymal stem cells could be considered as new option in cell-based therapy. Regen Therapy. 2023;23:94–101. doi: 10.1016/j.reth.2023.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fathi E., Valipour B., Farahzadi R. Targeting the proliferation inhibition of chronic myeloid leukemia cells by bone marrow derived-mesenchymal stem cells via ERK pathway as a therapeutic strategy. Acta Med Iran. 2020:199–206. [Google Scholar]
- 13.Adibkia K., Ehsani A., Jodaei A., Fathi E., Farahzadi R., Barzegar-Jalali M. Silver nanoparticles induce the cardiomyogenic differentiation of bone marrow derived mesenchymal stem cells via telomere length extension. Beilstein J Nanotechnol. 2021;12(1):786–797. doi: 10.3762/bjnano.12.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fathi E., Vandghanooni S., Montazersaheb S., Farahzadi R. Mesenchymal stem cells promote caspase-3 expression of SH-SY5Y neuroblastoma cells via reducing telomerase activity and telomere length. Iran J Basic Med Sci. 2021;24(11):1583–1589. doi: 10.22038/IJBMS.2021.59400.13187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farahzadi R., Fathi E., Vietor I. Mesenchymal stem cells could be considered as a candidate for further studies in cell-based therapy of Alzheimer's disease via targeting the signaling pathways. ACS Chem Neurosci. 2020;11(10):1424–1435. doi: 10.1021/acschemneuro.0c00052. [DOI] [PubMed] [Google Scholar]
- 16.Bagheri Y., Barati A., Nouraei S., Namini N.J., Bakhshi M., Fathi E., et al. Comparative study of gavage and intraperitoneal administration of gamma-oryzanol in alleviation/attenuation in a rat animal model of renal ischemia/reperfusion-induced injury. Iran J Basic Med Sci. 2021;24(2):175. doi: 10.22038/IJBMS.2020.51276.11642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fathi E., Farahzadi R., Vietor I., Javanmardi S. Cardiac differentiation of bone-marrow-resident c-kit+ stem cells by L-carnitine increases through secretion of VEGF, IL6, IGF-1, and TGF-β as clinical agents in cardiac regeneration. J Biosci. 2020;45(1):1–11. [PubMed] [Google Scholar]
- 18.Engle D.B., Belisle J.A., Gubbels J.A., Petrie S.E., Hutson P.R., Kushner D.M., et al. Effect of acetyl-l-carnitine on ovarian cancer cells’ proliferation, nerve growth factor receptor (Trk-A and p75) expression, and the cytotoxic potential of paclitaxel and carboplatin. Gynecol Oncol. 2009;112(3):631–636. doi: 10.1016/j.ygyno.2008.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roy M.J., Dionne S., Marx G., Qureshi I., Sarma D., Levy E., et al. In vitro studies on the inhibition of colon cancer by butyrate and carnitine. Nutrition. 2009;25(11–12):1193–1201. doi: 10.1016/j.nut.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Radkhouy F., Soltanieh S., Solgi S., Ansari M., Abbasi B. 2018. The effect of L-carnitine on colorectal cancer: A review on current evidence. [Google Scholar]
- 21.Wenzel U., Nickel A., Daniel H. Increased mitochondrial palmitoylcarnitine/carnitine countertransport by flavone causes oxidative stress and apoptosis in colon cancer cells. Cell Mol Life Sci. 2005;62(24):3100–3105. doi: 10.1007/s00018-005-5378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bose D., et al. Chemoresistant colorectal cancer cells and cancer stem cells mediate growth and survival of bystander cells. Br J Cancer. 2011;105(11):1759–1767. doi: 10.1038/bjc.2011.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang T., et al. JAK/STAT3-regulated fatty acid β-oxidation is critical for breast cancer stem cell self-renewal and chemoresistance. Cell Metabol. 2018;27(1):136–150. doi: 10.1016/j.cmet.2017.11.001. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fathi E., et al. L-carnitine in a certain concentration increases expression of cell surface marker CD34 and apoptosis in the rat bone marrow CD34+ hematopoietic stem cells. Iran J Vet Res. 2021;22(4):264. doi: 10.22099/ijvr.2021.39045.5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang F., et al. L-carnitine ameliorates the liver inflammatory response by regulating carnitine palmitoyltransferase I-dependent PPARγ signaling. Mol Med Rep. 2016;13(2):1320–1328. doi: 10.3892/mmr.2015.4639. [DOI] [PubMed] [Google Scholar]
- 26.Fan J.P., Kim H.S., Han G.D. Induction of apoptosis by L-carnitine through regulation of two main pathways in Hepa1c1c 7 cells. Amino Acids. 2009;36(2):365. doi: 10.1007/s00726-008-0093-y. [DOI] [PubMed] [Google Scholar]
- 27.Vescovo G., L-Carnitine, et al. A potential treatment for blocking apoptosis and preventing skeletal muscle myopathy in heart failure. Am J Physiol Cell Physiol. 2002;283(3):C802–C810. doi: 10.1152/ajpcell.00046.2002. [DOI] [PubMed] [Google Scholar]
- 28.Qi S.N., et al. L-carnitine inhibits apoptotic DNA fragmentation induced by a new spin-labeled derivative of podophyllotoxin via caspase-3 in Raji cells. Oncol Rep. 2006;15(1):119–122. [PubMed] [Google Scholar]
- 29.Herrmann A., et al. TLR9 is critical for glioma stem cell maintenance and targeting. Cancer Res. 2014;74(18):5218–5228. doi: 10.1158/0008-5472.CAN-14-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Demaria M., et al. A STAT3-mediated metabolic switch is involved in tumour transformation and STAT3 addiction. Aging (Albany NY) 2010;2(11):823–842. doi: 10.18632/aging.100232. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.