Abstract
Atrial fibrillation (AF) has a recognized association with not only stroke, but also neurocognitive impairment and both vascular and Alzheimer's dementia. Effective management of AF can reduce the risk of such complications. In this narrative review article, we discuss the pathophysiological links between AF and dementia, as well as the benefits of adherence to the guideline-recommended ‘ABC’ pathway.
Keywords: Atrial fibrillation, Neurocognitive, Neurological, Dementia, Cognition, ABC pathway
Introduction
Atrial fibrillation (AF), the most common cardiac arrhythmia, has a well-established association with stroke. Perhaps less well recognized is the link between AF, cognitive impairment, and dementia.1–3
Defining terms such as ‘cognitive impairment’ and ‘dementia’ is challenging. As noted in previous meta-analyses, different definitions are often used in studies.1,2 Cognitive impairment, in particular, may be defined in many ways. Generally, this term refers to a decline in the ability to think, remember, reason, or judge. The point when cognitive impairment becomes dementia is difficult to determine.
The World Health Organization defines dementia as ‘a syndrome in which there is deterioration in cognitive function beyond what might be expected from the usual consequences of biological ageing’.4 There is no single test that can diagnose dementia; the diagnosis relies on evidence of cognitive impairment—using validated cognitive screening tools—along with evidence that this is adversely affecting a person's normal daily functioning. To further complicate this, there are multiple types of dementia, including Alzheimer's, vascular, Lewy body, and others.
Although there is overlap between risk factors for both AF and dementia, AF independently predicts cognitive decline and incident dementia.5 The risk increases further with advancing age, hypertension, dyslipidaemia, depression, smoking, diabetes, and prior stroke.6,7
Dementia has a significant impact on quality of life for both the patient and their loved ones. Additionally, the global healthcare economic impact of dementia is substantial—estimated at over $900 billion US dollars in 2016.8 Hence, there are multiple reasons to prevent this disease, where possible, by addressing risk factors. In this narrative review article, we will discuss the neurocognitive impact of AF, the effect of treatments—such as anticoagulation and rhythm control—and the impact of the Atrial Fibrillation Better Care (ABC) pathway on such outcomes.
The neurocognitive impact of atrial fibrillation
Whilst the evidence for neurocognitive impairment relating to AF is strong (Table 1), the pathophysiological links are complex. Numerous mechanisms have been proposed, including microbleeds, cerebral hypoperfusion, neuroinflammation, genetic predisposition, and microembolism resulting in asymptomatic white matter lesions detected on magnetic resonance imaging (MRI)—so-called silent cerebral lesions (SCLs).9 SCLs are more often seen in patients with AF, regardless of AF type, compared to those in sinus rhythm; being seen in 22% of patients in studies where computed tomography was deployed, and as many as 40% where MRI was used.10 The rates are similar in Asian and non-Asian individuals.11 Cha et al. reported a higher risk of symptomatic stroke in those with SCLs compared to those without (5.6% vs. 2.7% per year, P = 0.022).6
Table 1.
Meta-analyses studying the relationship between atrial fibrillation and cognitive decline
| Effects of atrial fibrillation on cognition and dementia risk | |||
|---|---|---|---|
| Study | Participants | Summary of findings | Heterogeneity |
| Santangeli et al. (2012)77 | 77 668 | AF was independently associated with development of dementia (HR 1.42, 95% CI 1.17–1.72; P < 0.001) | Moderate |
| Stefanidis et al. (2018)3 | 50 544 | AF was significantly associated with cognitive decline and dementia (HR 1.26, 95% CI 1.12–1.43; P < 0.001) | Low |
| Lipnicki et al. (2019)7 | 48 522 | AF was not independently associated with cognitive decline; however, duration of AF and OAC usage were not available | High |
| Proietti et al. (2019)17 | 56 370 | AF was significantly associated with development of Alzheimer's dementia (HR 1.30, 1.01–1.59) | Moderate |
| Kokkinidis et al. (2020)1 | 14 360 | In patients with prior stroke, there was an association between AF and cognitive impairment or dementia (OR 2.26, 95% CI 1.61–3.19) | Moderate |
| Papanastasiou et al. (2021)2 | 3549 569 | AF was significantly associated with cognitive impairment and dementia (OR 1.54, 95% CI 1.35–1.75; P < 0.001) | Moderate |
| Zuin et al. (2021)78 | 3559 349 | AF was significantly associated with development of dementia, both in <10 year follow-up (aHR 1.59, 95% CI 1.51–1.67, P < 0.001) and longer ≥10 year follow-up (aHR 1.37, 95% CI 1.21–1.55; P < 0.001) | High |
| Koh et al. (2022)12 | 2822 974 | AF was associated with a significantly increased risk of cognitive impairment (HR 1.39; 95% CI 1.25–1.53; P < 0.001) | High |
| Giannone et al. (2022)23 | ∼1600 000 | AF was associated with an increased risk of early onset dementia (RR 1.50, 95% CI 1.00–2.26) with more pronounced effects at older age | High |
AF, atrial fibrillation; aHR, adjusted hazard ratio; CI, confidence interval; HR, hazard ratio; OR, odds ratio; and RR, risk ratio.
Cerebral microbleeds are also more frequently seen in those with AF compared to those in sinus rhythm. A large meta-analysis found microbleeds in 30.1% of AF patients compared with 26.5% of those without AF.12 Furthermore, the presence of microbleeds in this analysis was associated with a significant 1.7-fold increased risk of mortality and stroke, although heterogeneity was moderate, and confidence intervals were wide. Another meta-analysis found microbleeds to be associated with an increased risk of intracranial haemorrhage, as well as ischaemic stroke.13
Additionally, Barber et al. found higher levels of thrombotic biomarkers in those with AF and dementia compared to those with AF without dementia,14 suggesting that the prothrombotic state associated with AF may not be entirely responsible for neurocognitive impairment. Two studies have also shown that left atrial dysfunction, but not size, correlated significantly with risk of dementia15 and ischaemic cerebrovascular events.16 This suggests a link between AF, dementia, and underlying atrial cardiomyopathy—itself caused by established cardiovascular risk factors.
Logic would dictate that the resultant type of dementia would be vascular; however, there is evidence that Alzheimer's dementia also associates with AF.2,17 Indeed, the risk of Alzheimer's dementia in those with AF may be increased by up to 30%.17 Proposed mechanisms include abnormal cerebral blood flow resulting in damage to the blood–brain barrier, hypoperfusion relating to irregular cardiac rhythm, atherosclerosis as a consequence of cerebral adaptation to AF, and consequent amyloid-β plaque deposition.17–19 Hippocampal atrophy has also been noted in those with AF.20 Heart rates below 50 b.p.m. or above 90 b.p.m. during AF have been associated with increased risk.21 The risk increases with CHA2DS2VASc score, reflective of the added burden of cardiovascular co-morbidities.22
Importantly, the duration of AF has been proposed as a mechanism, with those exposed to AF at a younger age at higher risk of cognitive decline, with potentially earlier onset.17,23 Such long-term outcomes are not measured in rhythm control trials, and as such, the benefit of early rhythm control may be underappreciated. Furthermore, AF patients with cognitive impairment may have a worse long-term prognosis than those without.24
The obvious question, then, is whether or not we can reduce the risk of neurocognitive impairment by treating AF with a combination of anticoagulation, rate or rhythm control, and risk factor modification. These elements make up the guideline-recommended ABC pathway25 and are discussed in detail below.
A—avoiding stroke with anticoagulation
Anticoagulation to prevent stroke forms the ‘A’ component of the ABC pathway.25 It is well known that anticoagulation with either vitamin K antagonists (VKAs) or direct oral anticoagulants (DOACs) reduces the risk of stroke in AF patients. Given the premise that cognitive impairment in AF may, at least in part, relate to microemboli and silent infarctions, anticoagulation should theoretically reduce the number of SCLs and the risk of cognitive decline.
Kühne et al. recently described new infarcts on MRI at a 2-year follow-up in 5.5% of AF patients, with a direct association with dementia.26 Over 85% of these lesions were clinically silent and occurred despite anticoagulation. On multivariable regression, anticoagulation trended towards a reduced risk of new SCLs but did not meet statistical significance. Three meta-analyses have, however, found a significant reduction in cognitive impairment with anticoagulation in AF patients, though heterogeneity was high,27–29 and prospective studies are needed (Table 2). Unfortunately, as mentioned above, cognitive decline and dementia are very long-term outcomes, which makes longitudinal analysis challenging.
Table 2.
Meta analyses studying the effects of oral anticoagulation on cognitive decline in atrial fibrillation
| Effects of anticoagulation on AF-related cognitive impairment and dementia | |||
|---|---|---|---|
| Study | Participants | Summary of findings | Heterogeneity |
| Cheng et al. (2018)27 | 471 057 | Cognitive impairment in AF was significantly reduced by OAC use (HR 0.71, 95% CI 0.69–0.74; P < 0.001) | Low |
| Mongkhon et al. (2019)29 | 452 878 | OAC therapy (RR 0.79, 95% CI 0.67–0.93; P = 0.005) and higher TTR (RR 0.38, 95% CI 0.22–0.64; P < 0.001) reduced the risk of dementia in patients with AF | Moderate to high |
| Zeng et al. (2019)35 | 454 273 | OAC was associated with significant reductions in cognitive impairment in patients with AF (RR 0.72, 95% CI 0.69–0.75) but was not superior to antiplatelet therapy (RR 1.01, 95% CI 0.68–1.50) | Low to moderate |
| Lin et al. (2021)28 | 613 920 | OAC therapy reduced the risk of dementia in patients with AF (RR 0.72, 95% CI 0.60–0.86, P < 0.001) | High |
AF, atrial fibrillation; OAC, oral anticoagulation; RR, risk ratio; TTR, time in therapeutic range (for vitamin K antagonists).
Risk reduction may be achieved with either VKAs or DOACs. When utilizing VKAs, higher time in therapeutic range is associated with a lower risk.27,29,30 There is also evidence of superiority for DOACs over VKAs in this setting,31–33 including in one meta-analysis [hazard ratio (HR) 0.51, 95% confidence interval (CI) 0.37–0.71; P < 0.00001].27
In contrast to stroke prevention, oral anticoagulants have not been found to outperform antiplatelets in terms of cognitive decline in the general population.34,35 Given shared cardiovascular co-morbidities may result in arterial atherosclerosis, cerebral emboli may arise from non-cardiac sites such as the carotid arteries. This is treated with antiplatelets rather than anticoagulants, which may explain this finding. In clinical practice, this is of debatable relevance—AF patients meeting guideline-based recommendations should be anticoagulated for stroke prevention.25 Antiplatelets, meanwhile, are indicated for secondary prevention in those with established atherosclerotic disease. The effect of these treatments upon incident dementia does not, therefore, necessarily alter management. A reduction in neurocognitive risk, however, provides further incentive to ensure appropriate patients are initiated on such therapies.
A further question is whether or not left atrial appendage occlusion (LAAO)—utilized in those who are unsuitable for long-term anticoagulation—can reduce the risk of dementia in a similar fashion. The evidence is sparse, although Mohanty et al. studied this in 98 patients assigned to LAAO or OAC36 following AF ablation. They found that cognitive scores decreased by a small but statistically significant amount in the OAC group and were non-significantly different to baseline in the LAAO group. Given the low patient number and the fact that rhythm control with ablation was performed in all patients, these results should be interpreted with caution, and further studies are needed.
The potential benefit of anticoagulation on cognitive function may be an important factor for patients when deciding if they wish to take such medication. Risk aversion is common, and both patients37,38 and physicians39 may under or overestimate stroke and bleeding risk. When faced with the potential to reduce the risk of long-term irreversible cognitive decline, decisions may be swayed in favour of anticoagulation.
Since anticoagulation appears to reduce the risk of neurocognitive impairment via thromboembolic means, the next question is whether restoration of sinus rhythm can reduce the risk via improvement in cerebral blood flow. The clinical studies relating to rhythm control described here are summarized in Table 3.
Table 3.
Studies assessing the effects of rhythm control on cognitive decline in atrial fibrillation
| Effects of rhythm control on AF-related cognitive impairment and dementia | |||
|---|---|---|---|
| Study | Participants | Study type | Summary of findings |
| AFFIRM52 | 4060 | Interventional RCT | Cognitive performance was similar at all timepoints between rhythm and rate control groups. |
| Bunch et al. (2011)41 | 37 908 | Retrospective observational | Patients treated with AF catheter ablation had similar rates of dementia to a control group without AF, and significantly lower rates than AF patients who did not undergo ablation. |
| Damanti et al. (2018)42 | 1082 | Retrospective observational | Cognitive performance was highest in those who underwent rhythm control for AF compared with rate control or no therapy (rhythm control adjusted OR 0.56 95% CI 0.40–0.79; P = −0.001). |
| Jin et al. (2019)45 | 308 | Prospective observational | After 12 month follow-up, MoCA score significantly improved in the ablation cohort, even after propensity score matching, but not in the unablated cohort. |
| Tischer et al. (2019)50 | 90 | Prospective observational | No difference in prevalence or progression of cognitive impairment was detected between ablation and medically treated groups. |
| Hsieh et al. (2020)44 | 2344 | Retrospective observational | Over mean 9 year follow-up, the incidence of dementia was lower in the AF group who received catheter ablation compared with AF without ablation (aHR 0.44; P = 0.005). |
| Kim et al. (2020)46 | 194 928 | Retrospective observational | Over median 52 month follow-up, ablation for AF was associated with reduced risk of dementia compared with medical therapy (HR 0.73, 95% CI 0.58–0.93). |
| EAST-AFNET 4 (2020)40 | 2789 | Interventional RCT | Early rhythm control (mostly with antiarrhythmic drugs) was not associated with significant cognitive change after median 5 years of follow-up (MoCA score treatment effect −0.14 (95% CI −0.39 to +0.12). |
| Wang et al. (2021)51 | 139 | Prospective observational | Cognitive scores were significantly improved in the catheter ablation group (with no difference by ablation modality) compared with medically treated patients. |
| Kim et al. (2022)54 | 41 135 | Retrospective observational | Rhythm control was associated with significantly reduced risk of dementia (sHR 0.89, 95% CI 0.82–0.97). |
| Bodagh et al. (2022)55 | 15 886 | Meta-analysis | Catheter ablation was associated with a significantly lower risk of dementia compared with medical therapy (HR 0.60, 95% CI 0.42–0.88; P < 0.05). |
AF, atrial fibrillation; aHR, adjusted hazard ratio; MoCA, Montreal Cognitive Assessment; RCT, randomised controlled trial; and sHR, subdistribution hazard ratio.
B—better symptom control—rate and rhythm control
The ‘B’ component of the ABC pathway represents ‘Better symptom control’. A major element of this involves a decision on whether to pursue rate or rhythm control. The benefits of each approach remain a subject of debate. Symptomatic benefit is the biggest contributing factor; those with highly symptomatic—ideally paroxysmal—AF clearly benefit most from restoration of sinus rhythm. There is emerging evidence of a potential prognostic benefit to early rhythm control,40 though further studies are needed. It is also widely accepted that catheter ablation is superior to antiarrhythmic medication in maintaining sinus rhythm, whilst also avoiding the potentially toxic side effects of such drugs.
The question of whether rhythm control reduces dementia risk is complex due to the multifactorial mechanisms underlying neurocognitive decline in AF, as discussed earlier. Not all of these will be altered by restoring sinus rhythm. Equally, dementia may take many years to manifest—longer than most clinical trials can reasonably be funded for—and subclinical cognitive decline may go unnoticed. Furthermore, many studies do not adjust for all factors associated with cognitive decline—in particular, socioeconomic circumstances and mental health.
Despite a limited follow-up duration of around 3 years, one population-based study of over 37 000 patients found that AF catheter ablation was associated with a significantly lower incidence of dementia, as well as heart failure, stroke, and mortality41—in fact, those who underwent ablation had similar rates of dementia as a control group without AF. This study was limited by a lack of data around anticoagulation, and the authors noted that anticoagulation adherence may be stronger in the ablation group due to closer follow-up. Other studies have shown that the benefits of rhythm control appear independent of anticoagulation status.42,43
An observational, propensity-score matched study with very long follow-up (mean 9 years) found a striking reduction in dementia following ablation vs. AF patients who were not ablated [adjusted hazard ratio (aHR) 0.44, 95% CI 0.25–0.78; P = 0.005].44 Whilst limited by methodological constraints, this adds to the evidence that ablation may be of benefit in improving cognitive outcomes.
A further study even found that AF ablation was associated with an increase in cognitive performance at the 12-month follow-up, compared with controls, who showed a slight decrease.45 This study should be interpreted with caution, however, due to its small sample size, small effect size, and potential selection bias. In keeping with these findings, patients with recurrent AF despite ablation had no significant cognitive improvement, which is similar to another study where dementia was reduced post-ablation, but there was no benefit where ablation was unsuccessful.46
A recent, small, prospective observational study assessed cerebral blood flow following catheter ablation for AF.47 The authors described significant improvements in blood flow and cerebral perfusion, measured by MRI, in the ablation group compared with the medically treated group. This fits with the evidence described earlier—that AF may result in impaired cerebral blood flow and thus may contribute to neurocognitive risk. Non-paroxysmal AF was a significant predictor of improved cerebral blood flow on multivariate analysis—and specifically in those who did not suffer recurrence of AF—suggesting these patients may stand to gain the most benefit.
Two studies have reported on the effects of electrical cardioversion on cerebral perfusion. Gardarsdottir and colleagues studied 27 patients using MRI, showing improvements in cerebral perfusion and grey matter perfusion after successful restoration of sinus rhythm.48 Saglietto et al. utilized near-infrared spectroscopy (NIRS) to analyse beat-to-beat perfusion. The authors found that both microcirculatory hypertensive and hypotensive events were reduced by restoration of sinus rhythm.49 This fits with the pathophysiological evidence described earlier: Although not conclusively proven, a logical hypothesis is that abnormal cerebral blood flow results in microbleeds and infarcts due to intermittent micro-hypertensive and micro-hypotensive events, coupled with attempts by the microcirculation to adapt to this constantly changing environment.
Other data comparing ablation to medical therapy are conflicting. One retrospective study found no difference in cognitive measures between those treated with catheter ablation and medical therapy.50 This study was significantly limited, however, due to underpowering (only 45 patients) and short follow-up (6 months). Furthermore, the drug arm was inappropriately homogenized; this group could be treated with either rate or rhythm control, but no stratification was performed.
Conversely, a prospective study of 139 patients found significant improvements in cognitive scores with catheter ablation compared to medical therapy.51 Again, this study allowed rate or rhythm control in the drug arm and did not state the proportion taking each.
Two landmark trials—AFFIRM and EAST-AFNET 4—assessed rate vs. rhythm control strategies for AF.40,52 AFFIRM was performed entirely using drug therapy. EAST-AFNET 4 allowed catheter ablation; however, the vast majority were managed with antiarrhythmics. It is important to note that oral anticoagulation rates were far higher in the more contemporary EAST-AFNET 4 trial than the older AFFIRM trial. Neither trial demonstrated a significant difference in cognitive performance at 3- and 2-year follow-ups, respectively.40,53
Conversely, a recent large retrospective study by Kim et al. found significantly reduced dementia risk in those managed by rhythm control (mostly with drugs) compared with rate control (sHR 0.86, CI 0.80–0.93).54 A recently published systematic review found a similar reduction (HR 0.60, CI 0.42–0.88), but noted that there is conflicting evidence and called for more studies.55
Interestingly, a study of 358 AF patients found that poor ventricular rate control (defined as <50 b.p.m. or >90 b.p.m.) was associated with dementia.21 This points to potential confounding in some studies. Simply assigning patients to rate control does not mean that their heart rate was adequately controlled. Equally, medical rhythm control leaves open the possibility of drug toxicity and polypharmacy, which might adversely affect cognition. Pacemaker implant to prevent bradycardia may be of benefit and is recommended by expert consensus.56
It is worth noting that AF catheter ablation itself may cause SCL formation.57,58 An in-depth discussion is beyond the scope of this review, but suffice it to say that recent studies suggest these ablation-related SCLs mostly resolve and do not cause long-term cognitive impairment.59
Overall, the data suggest that rhythm control—particularly with catheter ablation—may improve the risk of neurocognitive decline in patients with AF. However, many of these studies are retrospective, allowing for potential selection bias—for example, those referred for catheter ablation may be healthier than those assigned to drug therapy. Prospective randomized controlled trials would be ideal but may be impossible due to the long-term nature of the outcome measures. The benefits of antiarrhythmic drugs alone are less certain, especially in the long term.
It is important to recognize that a rhythm control strategy may restore sinus rhythm, but this does not address the underlying risk factors, such as hypertension, obesity, diabetes, and sleep apnoea. Resultant atrial cardiomyopathy may impair LA function, and this may remain impaired despite successful rhythm control.60 Nevertheless, it is plausible that dysregulated blood flow due to AF may play a causative role in cognitive impairment, and restoration of sinus rhythm may therefore reduce the risk.
There appears to be minimal evidence relating to the ‘pace-and-ablate’ strategy (atrioventricular nodal ablation with pacemaker implantation). One small study did report a significant improvement in brain perfusion and neurocognitive performance utilizing this approach in 17 patients with medication-refractory high ventricular rates.61
C—cardiovascular risk modification—the importance of lifestyle
Addressing cardiovascular risk factors, largely through lifestyle modification, comprises the ‘C’ component of the ABC pathway. The underlying mechanisms relating these conditions to AF are complex, multifactorial, and incompletely understood, but involve a proinflammatory state with resulting endothelial dysfunction and atrial cardiomyopathy.62
Managing such risk factors has established benefits in the treatment of AF itself,63 so improvements in cognition would be logically expected to follow. Indeed, large retrospective studies have found that smoking cessation, alcohol abstinence, and regular exercise significantly reduce risk of dementia in those with AF.64–66
Management of hypertension in AF presents a particular challenge. It is recognized that controlling hypertension reduces the risk of developing dementia in the general population.67 However, there is evidence that aggressive blood pressure management in those with AF may actually increase the risk of dementia.68 This might relate to the irregular blood flow state created by AF, with antihypertensives resulting in further cerebral hypoperfusion episodes; however, this is speculative. Kim et al. found that the risk was ‘U-shaped’, with an increase or decrease of blood pressure from an optimal target of 120/80 being associated with an increased risk of dementia in those with AF.69 Interestingly, in this study, vascular dementia increased with increasing blood pressure, but Alzheimer's increased with reducing blood pressure. Prescription of antihypertensives—specifically thiazides, beta-blockers, calcium channel blockers, and renin–angiotensin–aldosterone system blockers—has been shown to decrease the risk of dementia in this population,70 but clearly, our understanding of this complex patient group requires further study.
Obesity is unfortunately common in the modern world, and a body mass index >30 is associated with an approximately doubled risk of developing AF.71 Obesity increases the risk of obstructive sleep apnoea (OSA), which itself has a recognized association with multiple cardiovascular risk factors, such as hypertension, type 2 diabetes mellitus, and ischaemic heart disease. Both obesity and OSA further increase the risk of dementia, independently of AF.72,73 Investigating and managing sleep apnoea—especially with weight loss strategies—may therefore aid in treating AF-related symptoms and reducing the risk of neurocognitive decline.
Table 4 summarizes the evidence for risk factor modification on neurocognitive decline in patients with AF.
Table 4.
Studies analysing the effects of risk factor modification on cognitive decline in atrial fibrillation
| Effects of cardiovascular risk factor management on AF-related cognitive impairment and dementia | |||
|---|---|---|---|
| Study | Participants | Study type | Summary of findings |
| Wändell et al. (2018)70 | 12 096 | Retrospective observational | Prescription of antihypertensives, alone or in combination, significantly reduced the risk of dementia in those with AF. |
| Kim et al. (2020)69 | 171 228 | Retrospective observational | A U-shaped relationship with blood pressure and dementia risk in AF was noted, with a target of 120/80 being optimal for dementia prevention. |
| Park et al. (2022)64 | 199 952 | Retrospective observational | Alcohol abstinence, no current smoking and regular exercise were associated with reduced risk of dementia in newly diagnosed AF patients (HR 0.62, 95% CI 0.57–0.68 for all three behaviours vs. none). |
| Lee et al. (2022)65 | 126 252 | Retrospective observational | Smoking cessation, compared with continued smoking, was significantly associated with a reduced risk of dementia in newly diagnosed AF patients (aHR 0.83, 95% CI 0.72–0.95). |
| Jiang et al. (2022)68 | 9361 | Subgroup analysis of RCT (SPRINT) | Intensive blood pressure control in those with AF was associated with an increased risk of developing dementia (HR 2.22, 95% CI 1.03–4.80; P for interaction = 0.009). |
Medical therapy cannot replace lifestyle modification. Unfortunately, lifestyle modification is difficult; so many patients are unable to sustain such measures. Nevertheless, we should continue to encourage improvements in lifestyle, even alongside therapies to mitigate the effects of cardiovascular risk factors. This benefit particularly applies to AF in the context of this review; however, it is noteworthy that healthy lifestyle habits are associated with a reduced risk of developing dementia outside of the setting of AF as well.74,75
The benefits of adherence to the ABC pathway
The ABC pathway provides a logical framework for the management of AF patients. It follows that, for all the reasons given in the sections above, adherence to the ABC pathway should reduce the risk of neurocognitive impairment amongst AF patients.
This was demonstrated in a study of over 200 000 Korean AF patients.76 Adherence to the principles of the ABC pathway significantly reduced the incidence of dementia (HR 0.80; 95% CI 0.73–0.87). Though limited by its retrospective nature, this study provides compelling evidence to support regular implementation of the pathway into clinical practice.
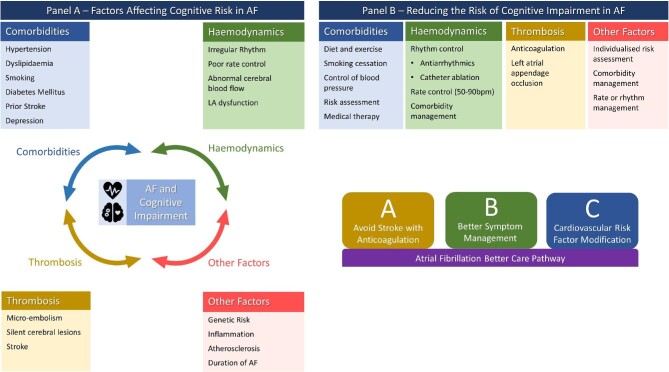
A summary of the relationship between AF and cognitive impairment is shown in Figure 1, alongside the benefits of the ABC pathway.
Figure 1.
Summary of factors affecting cognitive risk in AF and strategies to reduce the risk. AF, atrial fibrillation; b.p.m., beats per minute; LA, left atrium.
Conclusion
AF is associated with the development of SCLs and microbleeds, and an increased risk of not only stroke, but also vascular and Alzheimer's dementia. The risk of neurocognitive decline in AF patients may be reduced by adherence to the ABC pathway, which is recommended by expert consensus.56 Specifically, we should ensure that our AF patients are appropriately anticoagulated, have effective rhythm or rate control as appropriate to the individual, and are educated about improving lifestyle to manage cardiovascular risk factors.
Contributor Information
Peter Calvert, Department of Cardiology, Liverpool Centre for Cardiovascular Science and Liverpool Heart & Chest Hospital NHS Foundation Trust, Thomas Drive, Liverpool, L14 3PE, UK.
Dhiraj Gupta, Department of Cardiology, Liverpool Centre for Cardiovascular Science and Liverpool Heart & Chest Hospital NHS Foundation Trust, Thomas Drive, Liverpool, L14 3PE, UK.
Gregory Y H Lip, Department of Cardiology, Liverpool Centre for Cardiovascular Science and Liverpool Heart & Chest Hospital NHS Foundation Trust, Thomas Drive, Liverpool, L14 3PE, UK.
Funding
No funding was received towards this work.
Conflicts of interest: P.C. reports no conflicts of interest. D.G. reports institutional research grants from Boston Scientific and Medtronic, and speaker fees from Boston Scientific. G.Y.H.L. reports consultant and speaker for BMS/Pfizer, Boehringer Ingelheim, and Daiichi Sankyo. No fees are received personally.
Data availability
No new data were generated as part of this work.
References
- 1. Kokkinidis DG, Zareifopoulos N, Theochari CA, Arfaras-Melainis A, Papanastasiou CA, Uppal D, Giannakoulas G, Kalogeropoulos AP, Fontes JDT. Association between atrial fibrillation and cognitive impairment in individuals with prior stroke: a meta-analysis and meta-regression analysis. Stroke 2020;51:1662–1666. http://www.ncbi.nlm.nih.gov/pubmed/32312222 [DOI] [PubMed] [Google Scholar]
- 2. Papanastasiou CA, Theochari CA, Zareifopoulos N, Arfaras-Melainis A, Giannakoulas G, Karamitsos TD, Palaiodimos L, Ntaios G, Avgerinos KI, Kapogiannis D, Kokkinidis DG. Atrial fibrillation is associated with cognitive impairment, all-cause dementia, vascular dementia, and Alzheimer's disease: a systematic review and meta-analysis. J Gen Intern Med 2021;36:3122–3135. http://www.ncbi.nlm.nih.gov/pubmed/34244959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stefanidis KB, Askew CD, Greaves K, Summers MJ. The effect of non-stroke cardiovascular disease states on risk for cognitive decline and dementia: a systematic and meta-analytic review. Neuropsychol Rev 2018;28:1–15. http://www.ncbi.nlm.nih.gov/pubmed/28856507 [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization . Dementia fact sheet. 2023https://www.who.int/news-room/fact-sheets/detail/dementia (15 March 2023).
- 5. Kim D, Yang P-S, Lip GYH, Joung B. Atrial fibrillation increases the risk of early-onset dementia in the general population: data from a population-based cohort. J Clin Med 2020;9:3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cha M-J, Park HE, Lee M-H, Cho Y, Choi E-K, Oh S. Prevalence of and risk factors for silent ischemic stroke in patients with atrial fibrillation as determined by brain magnetic resonance imaging. Am J Cardiol 2014;113:655–661. http://www.ncbi.nlm.nih.gov/pubmed/24360776 [DOI] [PubMed] [Google Scholar]
- 7. Lipnicki DM, Makkar SR, Crawford JD, Thalamuthu A, Kochan NA, Lima-Costa MF, Castro-Costa E, Ferri CP, Brayne C, Stephan B, Llibre-Rodriguez JJ, Llibre-Guerra JJ, Valhuerdi-Cepero AJ, Lipton RB, Katz MJ, Derby CA, Ritchie K, Ancelin M-L, Carrière I, Scarmeas N, Yannakoulia M, Hadjigeorgiou GM, Lam L, Chan W-C, Fung A, Guaita A, Vaccaro R, Davin A, Kim KW, Han JW, Suh SW, Riedel-Heller SG, Roehr S, Pabst A, Van Boxtel M, Köhler S, Deckers K, Ganguli M, Jacobsen EP, Hughes TF, Anstey KJ, Cherbuin N, Haan MN, Aiello AE, Dang K, Kumagai S, Chen T, Narazaki K, Ng TP, Gao Q, Nyunt MSZ, Scazufca M, Brodaty H, Numbers K, Trollor JN, Meguro K, Yamaguchi S, Ishii H, Lobo A, Lopez-Anton R, Santabárbara J, Leung Y, Lo JW, Popovic G, Sachdev PS. Determinants of cognitive performance and decline in 20 diverse ethno-regional groups: a COSMIC collaboration cohort study. PLoS Med 2019;16:e1002853. http://www.ncbi.nlm.nih.gov/pubmed/31335910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xu J, Zhang Y, Qiu C, Cheng F. Global and regional economic costs of dementia: a systematic review. Lancet North Am Ed 2017;390:S47. [Google Scholar]
- 9. Gallinoro E, D'Elia S, Prozzo D, Lioncino M, Natale F, Golino Pet al. . Cognitive function and atrial fibrillation: from the strength of relationship to the dark side of prevention. Is there a contribution from sinus rhythm restoration and maintenance? Medicina (Kaunas) 2019;55:589. http://www.ncbi.nlm.nih.gov/pubmed/31540311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kalantarian S, Ay H, Gollub RL, Lee H, Retzepi K, Mansour M, Ruskin JN. Association between atrial fibrillation and silent cerebral infarctions: a systematic review and meta-analysis. Ann Intern Med 2014;161:650–658. http://www.ncbi.nlm.nih.gov/pubmed/25364886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Senoo K, Kondo Y, Kobayashi Y, Lip GY. Silent cerebral infarction in East Asian vs. non-Asian atrial fibrillation patients––meta-analysis. Circ J 2018;82:672–676. [DOI] [PubMed] [Google Scholar]
- 12. Koh YH, Lew LZW, Franke KB, Elliott AD, Lau DH, Thiyagarajah A, Linz D, Arstall M, Tully PJ, Baune BT, Munawar DA, Mahajan R. Predictive role of atrial fibrillation in cognitive decline: a systematic review and meta-analysis of 2.8 million individuals. Europace 2022;24:1229–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Corica B, Romiti GF, Raparelli V, Cangemi R, Basili S, Proietti M. Epidemiology of cerebral microbleeds and risk of adverse outcomes in atrial fibrillation: a systematic review and meta-analysis. EP Europace 2022;24:1395–1403. [DOI] [PubMed] [Google Scholar]
- 14. Barber M, Tait RC, Scott J, Rumley A, Lowe GDO, Stott DJ. Dementia in subjects with atrial fibrillation: hemostatic function and the role of anticoagulation. J Thromb Haemost 2004;2:1873–1878. http://www.ncbi.nlm.nih.gov/pubmed/15550013 [DOI] [PubMed] [Google Scholar]
- 15. Wang W, Zhang MJ, Inciardi RM, Norby FL, Johansen MC, Parikh R, Van't Hof JR, Alonso A, Soliman EZ, Mosley TH, Gottesman RF, Shah AM, Solomon SD, Chen LY. Association of echocardiographic measures of left atrial function and size with incident dementia. JAMA 2022;327:1138–1148. http://www.ncbi.nlm.nih.gov/pubmed/35315884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Habibi M, Zareian M, Ambale Venkatesh B, Samiei S, Imai M, Wu C, Launer LJ, Shea S, Gottesman RF, Heckbert SR, Bluemke DA, Lima JAC. Left atrial mechanical function and incident ischemic cerebrovascular events independent of AF: insights from the MESA study. JACC Cardiovasc Imaging 2019;12:2417–2427. http://www.ncbi.nlm.nih.gov/pubmed/31005519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Proietti R, Alturki A, Vio R, Licchelli L, Rivezzi F, Marafi M, Russo V, Potpara TS, Kalman JM, De Villers-Sidani E, Bunch TJ. The association between atrial fibrillation and Alzheimer's disease: fact or fallacy? A systematic review and meta-analysis. J Cardiovasc Med (Hagerstown) 2020;21:106–112. http://www.ncbi.nlm.nih.gov/pubmed/31815852 [DOI] [PubMed] [Google Scholar]
- 18. Benenati S, Canale C, de Marzo V, della Bona R, Rosa GM, Porto I. Atrial fibrillation and Alzheimer's disease: a conundrum. Eur J Clin Invest 2021;51:e13451. http://www.ncbi.nlm.nih.gov/pubmed/33219514 [DOI] [PubMed] [Google Scholar]
- 19. Ihara M, Washida K. Linking atrial fibrillation with Alzheimer's disease: epidemiological, pathological, and mechanistic evidence. J Alzheimers Dis 2018;62:61–72. http://www.ncbi.nlm.nih.gov/pubmed/29439352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Knecht S, Oelschlager C, Duning T, Lohmann H, Albers J, Stehling C, Heindel W, Breithardt G, Berger K, Ringelstein EB, Kirchhof P, Wersching H. Atrial fibrillation in stroke-free patients is associated with memory impairment and hippocampal atrophy. Eur Heart J 2008;29:2125–2132. http://www.ncbi.nlm.nih.gov/pubmed/18667399 [DOI] [PubMed] [Google Scholar]
- 21. Cacciatore F, Testa G, Langellotto A, Galizia G, Della-Morte D, Gargiulo G, Bevilacqua A, Del Genio MT, Canonico V, Rengo F, Abete P. Role of ventricular rate response on dementia in cognitively impaired elderly subjects with atrial fibrillation: a 10-year study. Dement Geriatr Cogn Disord 2012;34:143–148. http://www.ncbi.nlm.nih.gov/pubmed/22986752 [DOI] [PubMed] [Google Scholar]
- 22. Kim D, Yang P-S, Yu HT, Kim T-H, Jang E, Sung J-H, Pak H-N, Lee M-Y, Lee M-H, Lip GYH, Joung B. Risk of dementia in stroke-free patients diagnosed with atrial fibrillation: data from a population-based cohort. Eur Heart J 2019;40:2313–2323. http://www.ncbi.nlm.nih.gov/pubmed/31212315 [DOI] [PubMed] [Google Scholar]
- 23. Giannone ME, Filippini T, Whelton PK, Chiari A, Vitolo M, Boriani G, Vinceti M. Atrial fibrillation and the risk of early-onset dementia: a systematic review and meta-analysis. J Am Heart Assoc 2022;11:e025653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Malavasi VL, Zoccali C, Brandi MC, Micali G, Vitolo M, Imberti JF, Mussi C, Schnabel RB, Freedman B, Boriani G. Cognitive impairment in patients with atrial fibrillation: implications for outcome in a cohort study. Int J Cardiol 2021;323:83–89. [DOI] [PubMed] [Google Scholar]
- 25. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan G-A, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau J-P, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL, Kirchhof P, Kühne M, Aboyans V, Ahlsson A, Balsam P, Bauersachs J, Benussi S, Brandes A, Braunschweig F, Camm AJ, Capodanno D, Casadei B, Conen D, Crijns HJGM, Delgado V, Dobrev D, Drexel H, Eckardt L, Fitzsimons D, Folliguet T, Gale CP, Gorenek B, Haeusler KG, Heidbuchel H, Iung B, Katus HA, Kotecha D, Landmesser U, Leclercq C, Lewis BS, Mascherbauer J, Merino JL, Merkely B, Mont L, Mueller C, Nagy KV, Oldgren J, Pavlović N, Pedretti RFE, Petersen SE, Piccini JP, Popescu BA, Pürerfellner H, Richter DJ, Roffi M, Rubboli A, Scherr D, Schnabel RB, Simpson IA, Shlyakhto E, Sinner MF, Steffel J, Sousa-Uva M, Suwalski P, Svetlosak M, Touyz RM, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan G-A, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau J-P, Lettino M, Lip GYH, Pinto FJ, Neil Thomas G, Valgimigli M, Van Gelder IC, Watkins CL, Delassi T, Sisakian HS, Scherr D, Chasnoits A, Pauw MD, Smajić E, Shalganov T, Avraamides P, Kautzner J, Gerdes C, Alaziz AA, Kampus P, Raatikainen P, Boveda S, Papiashvili G, Eckardt L, Vassilikos V, Csanádi Z, Arnar DO, Galvin J, Barsheshet A, Caldarola P, Rakisheva A, Bytyçi I, Kerimkulova A, Kalejs O, Njeim M, Puodziukynas A, Groben L, Sammut MA, Grosu A, Boskovic A, Moustaghfir A, Groot ND, Poposka L, Anfinsen O-G, Mitkowski PP, Cavaco DM, Siliste C, Mikhaylov EN, Bertelli L, Kojic D, Hatala R, Fras Z, Arribas F, Juhlin T, Sticherling C, Abid L, Atar I, Sychov O, Bates MGD, Zakirov NU. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the Europe. Eur Heart J 2021;42:373–498. https://academic.oup.com/eurheartj/article-pdf/42/5/373/36414945/ehaa612.pdf [DOI] [PubMed] [Google Scholar]
- 26. Kühne M, Krisai P, Coslovsky M, Rodondi N, Müller A, Beer JH, Ammann P, Auricchio A, Moschovitis G, Hayoz D, Kobza R, Shah D, Stephan FP, Schläpfer J, Di Valentino M, Aeschbacher S, Ehret G, Eken C, Monsch A, Roten L, Schwenkglenks M, Springer A, Sticherling C, Reichlin T, Zuern CS, Meyre PB, Blum S, Sinnecker T, Würfel J, Bonati LH, Conen D, Osswald S. Silent brain infarcts impact on cognitive function in atrial fibrillation. Eur Heart J 2022; http://www.ncbi.nlm.nih.gov/pubmed/35171989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cheng W, Liu W, Li B, Li D. Relationship of anticoagulant therapy with cognitive impairment among patients with atrial fibrillation: a meta-analysis and systematic review. J Cardiovasc Pharmacol 2018;71:380–387. http://www.ncbi.nlm.nih.gov/pubmed/29528873 [DOI] [PubMed] [Google Scholar]
- 28. Lin M, Han W, Zhong J, Wu L. A systematic review and meta-analysis to determine the effect of oral anticoagulants on incidence of dementia in patients with atrial fibrillation. Int J Clin Pract 2021;75:e14269. http://www.ncbi.nlm.nih.gov/pubmed/33894031 [DOI] [PubMed] [Google Scholar]
- 29. Mongkhon P, Naser AY, Fanning L, Tse G, Lau WCY, Wong ICK, Kongkaew C. Oral anticoagulants and risk of dementia: a systematic review and meta-analysis of observational studies and randomized controlled trials. Neurosci Biobehav Rev 2019;96:1–9. http://www.ncbi.nlm.nih.gov/pubmed/30391408 [DOI] [PubMed] [Google Scholar]
- 30. Jacobs V, Woller SC, Stevens S, May HT, Bair TL, Anderson JL, Crandall BG, Day JD, Johanning K, Long Y, Mallender C, Olson JL, Osborn JS, Weiss JP, Bunch TJ. Time outside of therapeutic range in atrial fibrillation patients is associated with long-term risk of dementia. Heart Rhythm 2014;11:2206–2213. http://www.ncbi.nlm.nih.gov/pubmed/25111326 [DOI] [PubMed] [Google Scholar]
- 31. Lee S-R, Choi E-K, Park S-H, Jung J-H, Han K-D, Oh S, Lip GYH. Comparing warfarin and 4 direct oral anticoagulants for the risk of dementia in patients with atrial fibrillation. Stroke 2021;52:3459–3468. [DOI] [PubMed] [Google Scholar]
- 32. Hsu J-Y, Liu PP-S, Liu A-B, Lin S-M, Huang H-K, Loh C-H. Lower risk of dementia in patients with atrial fibrillation taking non-vitamin K antagonist oral anticoagulants: a nationwide population-based cohort study. J Am Heart Assoc 2021;10:e016437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bezabhe WM, Bereznicki LR, Radford J, Wimmer BC, Salahudeen MS, Garrahy E, Bindoff I, Peterson GM. Oral anticoagulant treatment and the risk of dementia in patients with atrial fibrillation: a population-based cohort study. J Am Heart Assoc 2022;11:e023098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mavaddat N, Roalfe A, Fletcher K, Lip GYH, Hobbs FDR, Fitzmaurice D, Mant J. Warfarin versus aspirin for prevention of cognitive decline in atrial fibrillation: randomized controlled trial (Birmingham Atrial Fibrillation Treatment of the Aged Study). Stroke 2014;45:1381–1386. http://www.ncbi.nlm.nih.gov/pubmed/24692475 [DOI] [PubMed] [Google Scholar]
- 35. Zeng D, Jiang C, Su C, Tan Y, Wu J. Anticoagulation in atrial fibrillation and cognitive decline: a systematic review and meta-analysis. Medicine 2019;98:e14499. http://www.ncbi.nlm.nih.gov/pubmed/30762777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mohanty S, Mohanty P, Trivedi C, Assadourian J, Mayedo AQ, Macdonald B, Della Rocca DG, Gianni C, Horton R, Al‐Ahmad A, Bassiouny M, Burkhardt JD, Di Biase L, Gurol ME, Natale A. Impact of oral anticoagulation therapy versus left atrial appendage occlusion on cognitive function and quality of life in patients with atrial fibrillation. J Am Heart Assoc 2021;10:e019664. http://www.ncbi.nlm.nih.gov/pubmed/33870705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bamgbade BA, Mcmanus DD, Helm R, Mehawej J, Gurwitz JH, Mailhot T, Abu HO, Goldberg R, Wang Z, Tisminetzky M, Pierre‐Louis IC, Saczynski JS. Differences in perceived and predicted bleeding risk in older adults with atrial fibrillation: the SAGE-AF study. J Am Heart Assoc 2021;10:e019979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hijazi M, Aljohani S, Alqahtani F, Chaker Z, Al Hajji M, Al Hallak A, Alkhouli M. Perception of the risk of stroke and the risks and benefits of oral anticoagulation for stroke prevention in patients with atrial fibrillation: a cross-sectional study. Mayo Clin Proc 2019;94:1015–1023. [DOI] [PubMed] [Google Scholar]
- 39. Raptis S, Chen JN, Saposnik F, Pelyavskyy R, Liuni A, Saposnik G. Aversion to ambiguity and willingness to take risks affect therapeutic decisions in managing atrial fibrillation for stroke prevention: results of a pilot study in family physicians. Patient Prefer Adherence 2017;11:1533–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan A, Fetsch T, Van Gelder IC, Haase D, Haegeli LM, Hamann F, Heidbüchel H, Hindricks G, Kautzner J, Kuck K-H, Mont L, Ng GA, Rekosz J, Schoen N, Schotten U, Suling A, Taggeselle J, Themistoclakis S, Vettorazzi E, Vardas P, Wegscheider K, Willems S, Crijns HJGM, Breithardt G. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med 2020;383:1305–1316. [DOI] [PubMed] [Google Scholar]
- 41. Bunch TJ, Crandall BG, Weiss JP, May HT, Bair TL, Osborn JS, Anderson JL, Muhlestein JB, Horne BD, Lappe DL, Day JD. Patients treated with catheter ablation for atrial fibrillation have long-term rates of death, stroke, and dementia similar to patients without atrial fibrillation. J Cardiovasc Electrophysiol 2011;22:839–845. https://onlinelibrary.wiley.com/doi/10.1111/j.1540-8167.2011.02035.x [DOI] [PubMed] [Google Scholar]
- 42. Damanti S, Pasina L, Cortesi L, Rossi PD, Cesari M. Atrial fibrillation: possible influences of rate and rhythm control strategy on cognitive performance. J Am Geriatr Soc 2018;66:2178–2182. https://onlinelibrary.wiley.com/doi/10.1111/jgs.15568 [DOI] [PubMed] [Google Scholar]
- 43. Gaita F, Sardi D, Battaglia A, Gallo C, Toso E, Michielon A, Caponi D, Garberoglio L, Castagno D, Scaglione M. Incidence of cerebral thromboembolic events during long-term follow-up in patients treated with transcatheter ablation for atrial fibrillation. Europace 2014;16:980–986. https://academic.oup.com/europace/article-lookup/doi/10.1093/europace/eut406 [DOI] [PubMed] [Google Scholar]
- 44. Hsieh Y-C, Chen Y-Y, Chien K-L, Chung F-P, Lo L-W, Chang S-L, Chao T-F, Hu Y-F, Lin C-Y, Tuan T-C, Liao J-N, Lin Y-J, Chen S-A. Catheter ablation of atrial fibrillation reduces the risk of dementia and hospitalization during a very long-term follow-up. Int J Cardiol 2020;304:75–81. http://www.ncbi.nlm.nih.gov/pubmed/31884008 [DOI] [PubMed] [Google Scholar]
- 45. Jin M-N, Kim T-H, Kang K-W, Yu HT, Uhm J-S, Joung B, Lee M-H, Kim E, Pak H-N. Atrial fibrillation catheter ablation improves 1-year follow-up cognitive function, especially in patients with impaired cognitive function. Circ Arrhythm Electrophysiol 2019;12:e007197. http://www.ncbi.nlm.nih.gov/pubmed/31442075 [DOI] [PubMed] [Google Scholar]
- 46. Kim D, Yang P-S, Sung J-H, Jang E, Yu HT, Kim T-H, Uhm J-S, Kim J-Y, Pak H-N, Lee M-H, Lip GYH, Joung B. Less dementia after catheter ablation for atrial fibrillation: a nationwide cohort study. Eur Heart J 2020;41:4483–4493. http://www.ncbi.nlm.nih.gov/pubmed/33022705 [DOI] [PubMed] [Google Scholar]
- 47. Takahashi Y, Yamamoto T, Oyama J, Sugihara G, Shirai Y, Tao S, Takigawa M, Sato H, Sasaki M, Hirakawa A, Takahashi H, Goya M, Sasano T. Increase in cerebral blood flow after catheter ablation of atrial fibrillation. JACC Clin Electrophysiol 2022;8:1369–1377. [DOI] [PubMed] [Google Scholar]
- 48. Gardarsdottir M, Sigurdsson S, Aspelund T, Gardarsdottir VA, Forsberg L, Gudnason V, Arnar DO. Improved brain perfusion after electrical cardioversion of atrial fibrillation. EP Europace 2020;22:530–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Saglietto A, Scarsoglio S, Canova D, Roatta S, Gianotto N, Piccotti A, Franzin S, Gaita F, De Ferrari GM, Ridolfi L, Anselmino M. Increased beat-to-beat variability of cerebral microcirculatory perfusion during atrial fibrillation: a near-infrared spectroscopy study. Europace 2021;23:1219–1226. [DOI] [PubMed] [Google Scholar]
- 50. Tischer TS, Nitschke D, Krause I, Kundt G, Öner A, D'Ancona Get al. . Prevalence and progression of cognitive impairment in atrial fibrillation patients after treatment with catheter ablation or drug therapy. Cardiol Res Pract 2019;2019:7216598. http://www.ncbi.nlm.nih.gov/pubmed/31915546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang X, Wang Z, Yan X, Huang M, Wu Y. Radiofrequency and cryoballoon ablation improve cognitive function in patients with atrial fibrillation. Medicine 2021;100:e26914. http://www.ncbi.nlm.nih.gov/pubmed/34397930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EBet al. . A comparison of rate control and rhythm control in patients with atrial fibrillation. New Engl J Med 2002;347:1825–1833. http://www.nejm.org/doi/abs/10.1056/NEJMoa021328 [DOI] [PubMed] [Google Scholar]
- 53. Chung MK, Shemanski L, Sherman DG, Greene HL, Hogan DB, Kellen JC, Kim SG, Martin LW, Rosenberg Y, Wyse DG. Functional status in rate- versus rhythm-control strategies for atrial fibrillation: results of the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Functional Status substudy. J Am Coll Cardiol 2005;46:1891–1899. http://www.ncbi.nlm.nih.gov/pubmed/16286177 [DOI] [PubMed] [Google Scholar]
- 54. Kim D, Yang PS, You SC, Sung JH, Jang E, Yu HTet al. . Association of rhythm control with incident dementia among patients with atrial fibrillation: a nationwide population-based cohort study. Age Ageing 2022;51:afab248. http://www.ncbi.nlm.nih.gov/pubmed/35061873 [DOI] [PubMed] [Google Scholar]
- 55. Bodagh N, Yap R, Kotadia I, Sim I, Bhalla A, Somerville P, O'neill M, Williams SE. Impact of catheter ablation versus medical therapy on cognitive function in atrial fibrillation: a systematic review. J Interv Card Electrophysiol 2022; 65:271–286.http://www.ncbi.nlm.nih.gov/pubmed/35380337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dagres N, Chao T-F, Fenelon G, Aguinaga L, Benhayon D, Benjamin EJ, Bunch TJ, Chen LY, Chen S-A, Darrieux F, De Paola A, Fauchier L, Goette A, Kalman J, Kalra L, Kim Y-H, Lane DA, Lip GYH, Lubitz SA, Márquez MF, Potpara T, Pozzer DL, Ruskin JN, Savelieva I, Teo WS, Tse H-F, Verma A, Zhang S, Chung MK, Bautista-Vargas W-F, Chiang C-E, Cuesta A, Dan G-A, Frankel DS, Guo Y, Hatala R, Lee YS, Murakawa Y, Pellegrini CN, Pinho C, Milan DJ, Morin DP, Nadalin E, Ntaios G, Prabhu MA, Proietti M, Rivard L, Valentino M, Shantsila A. European Heart Rhythm Association (EHRA)/Heart Rhythm Society (HRS)/Asia Pacific Heart Rhythm Society (APHRS)/Latin American Heart Rhythm Society (LAHRS) expert consensus on arrhythmias and cognitive function: what is the best practice? EP Europace 2018;20:1399–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gaita F, Caponi D, Pianelli M, Scaglione M, Toso E, Cesarani F, Boffano C, Gandini G, Valentini MC, De Ponti R, Halimi F, Leclercq JF. Radiofrequency catheter ablation of atrial fibrillation: a cause of silent thromboembolism? Magnetic resonance imaging assessment of cerebral thromboembolism in patients undergoing ablation of atrial fibrillation. Circulation 2010;122:1667–1673. http://www.ncbi.nlm.nih.gov/pubmed/20937975 [DOI] [PubMed] [Google Scholar]
- 58. Martinek M, Sigmund E, Lemes C, Derndorfer M, Aichinger J, Winter S, Jauker W, Gschwendtner M, Nesser H-J, Pürerfellner H. Asymptomatic cerebral lesions during pulmonary vein isolation under uninterrupted oral anticoagulation. Europace 2013;15:325–331. http://www.ncbi.nlm.nih.gov/pubmed/23097222 [DOI] [PubMed] [Google Scholar]
- 59. Haeusler KG, Eichner FA, Heuschmann PU, Fiebach JB, Engelhorn T, Blank B, Callans D, Elvan A, Grimaldi M, Hansen J, Hindricks G, Al-Khalidi HR, Mont L, Nielsen JC, Piccini JP, Schotten U, Themistoclakis S, Vijgen J, Di Biase L, Kirchhof P. MRI-detected brain lesions and cognitive function in atrial fibrillation patients undergoing left atrial catheter ablation in the randomized AXAFA-AFNET 5 trial. Circulation 2022;145:906–915. http://www.ncbi.nlm.nih.gov/pubmed/35135308 [DOI] [PubMed] [Google Scholar]
- 60. Wang YC, Lin JL, Hwang JJ, Lin MS, Tseng CD, Huang SKSet al. . Left atrial dysfunction in patients with atrial fibrillation after successful rhythm control for >3 months. Chest 2005;128:2551–2556. https://linkinghub.elsevier.com/retrieve/pii/S0012369216507910 [DOI] [PubMed] [Google Scholar]
- 61. Efimova I, Efimova N, Chernov V, Popov S, Lishmanov Y. Ablation and pacing: improving brain perfusion and cognitive function in patients with atrial fibrillation and uncontrolled ventricular rates. Pacing Clin Electrophysiol 2012;35:320–326. [DOI] [PubMed] [Google Scholar]
- 62. Alturki A, Maj JB, Marafi M, Donato F, Vescovo G, Russo V, Proietti R. The role of cardiovascular and metabolic comorbidities in the link between atrial fibrillation and cognitive impairment: an appraisal of current scientific evidence. Medicina (B Aires) 2019;55:767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Middeldorp ME, Ariyaratnam J, Lau D, Sanders P. Lifestyle modifications for treatment of atrial fibrillation. Heart 2020;106:325–332. [DOI] [PubMed] [Google Scholar]
- 64. Park S-H, Lee S-R, Choi E-K, Lee H, Chung J, Choi J, Han M, Ahn H-J, Kwon S, Lee S-W, Han K-D, Oh S, Lip GYH. Low risk of dementia in patients with newly diagnosed atrial fibrillation and a clustering of healthy lifestyle behaviors: a nationwide population-based cohort study. J Am Heart Assoc 2022;11:e023739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lee H-J, Lee S-R, Choi E-K, Park S-H, Chung J-W, Choi J-M, Han M-J, Jung J-H, Han K-D, Oh S, Lip GYH. Risk of dementia after smoking cessation in patients with newly diagnosed atrial fibrillation. JAMA Netw Open 2022;5:e2217132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lim J, Lee S-R, Choi E-K, Han K-D, Jung J-H, Ahn H-J, Yun JP, Kwon S, Oh S, Lip GYH. Exercise and the risk of dementia in patients with newly diagnosed atrial fibrillation: a nationwide population-based study. J Clin Med 2021;10:3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Jung H, Yang P-S, Kim D, Jang E, Yu HT, Kim T-H, Sung J-H, Pak H-N, Lee M-H, Lip GYH, Joung B. Associations of hypertension burden on subsequent dementia: a population-based cohort study. Sci Rep 2021;11:12291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jiang C, Lai Y, Du X, Wang Y, Li S, He L, Hu R, Lv Q, Wu J, Feng L, Ning M, Ruan Y, Li X, Jia C, Dai W, Guo X, Jiang C, Tang R, Sang C, Long D, Arima H, Dong J, Anderson CS, Ma C. Effects of intensive blood pressure control on cardiovascular and cognitive outcomes in patients with atrial fibrillation: insights from the SPRINT trial. EP Europace 2022;24:1560–1568. [DOI] [PubMed] [Google Scholar]
- 69. Kim D, Yang P-S, Jang E, Tae Yu H, Kim T-H, Uhm J-S, Kim J-Y, Sung J-H, Pak H-N, Lee M-H, Lip GYH, Joung B. Blood pressure control and dementia risk in midlife patients with atrial fibrillation. Hypertension 2020;75:1296–1304. [DOI] [PubMed] [Google Scholar]
- 70. Wändell P, Carlsson AC, Sundquist J, Sundquist K. Antihypertensive drugs and relevant cardiovascular pharmacotherapies and the risk of incident dementia in patients with atrial fibrillation. Int J Cardiol 2018;272:149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Frost L, Hune LJ, Vestergaard P. Overweight and obesity as risk factors for atrial fibrillation or flutter: the Danish Diet, Cancer, and Health Study. Am J Med 2005;118:489–495. [DOI] [PubMed] [Google Scholar]
- 72. Barletta P, Abreu AR, Ramos AR, Dib SI, Torre C, Chediak AD. Role of obstructive sleep apnea in cognitive impairment. Int J Head Neck Surg 2019;10:57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Culebras A, Anwar S. Sleep apnea is a risk factor for stroke and vascular dementia. Curr Neurol Neurosci Rep 2018;18:53. [DOI] [PubMed] [Google Scholar]
- 74. Dominguez LJ, Veronese N, Vernuccio L, Catanese G, Inzerillo F, Salemi G, Barbagallo M. Nutrition, physical activity, and other lifestyle factors in the prevention of cognitive decline and dementia. Nutrients 2021;13:4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kivipelto M, Mangialasche F, Ngandu T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat Rev Neurol 2018;14:653–666. [DOI] [PubMed] [Google Scholar]
- 76. Yang P-S, Sung J-H, Jang E, Yu HT, Kim T-H, Uhm J-S, Kim J-Y, Pak H-N, Lee M-H, Lip GYH, Joung B. The effect of integrated care management on dementia in atrial fibrillation. J Clin Med 2020;9:1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Santangeli P, di Biase L, Bai R, Mohanty S, Pump A, Cereceda Brantes Met al. . Atrial fibrillation and the risk of incident dementia: a meta-analysis. Heart Rhythm 2012;9:1761–1768. http://www.ncbi.nlm.nih.gov/pubmed/22863685 [DOI] [PubMed] [Google Scholar]
- 78. Zuin M, Roncon L, Passaro A, Bosi C, Cervellati C, Zuliani G. Risk of dementia in patients with atrial fibrillation: short versus long follow-up. A systematic review and meta-analysis. Int J Geriatr Psychiatry 2021;36:1488–1500. http://www.ncbi.nlm.nih.gov/pubmed/34043846 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated as part of this work.