Abstract
Several vaccines have been investigated experimentally in the herpes simplex virus type 2 (HSV-2) model system. While it is believed that CD4+-T-cell responses are important for protection in general, the correlates of protection from HSV-2 infection are still under investigation. Recently, the use of molecular adjuvants to drive vaccine responses induced by DNA vaccines has been reported in a number of experimental systems. We sought to take advantage of this immunization model to gain insight into the correlates of immune protection in the HSV-2 mouse model system and to further explore DNA vaccine technology. To investigate whether the Th1- or Th2-type immune responses are more important for protection from HSV-2 infection, we codelivered the DNA expression construct encoding the HSV-2 gD protein with the gene plasmids encoding the Th1-type (interleukin-2 [IL-2], IL-12, IL-15, and IL-18) and Th2-type (IL-4 and IL-10) cytokines in an effort to drive immunity induced by vaccination. We then analyzed the modulatory effects of the vaccine on the resulting immune phenotype and on the mortality and the morbidity of the immunized animals following a lethal challenge with HSV-2. We observed that Th1 cytokine gene coadministration not only enhanced the survival rate but also reduced the frequency and severity of herpetic lesions following intravaginal HSV challenge. On the other hand, coinjection with Th2 cytokine genes increased the rate of mortality and morbidity of the challenged mice. Moreover, of the Th1-type cytokine genes tested, IL-12 was a particularly potent adjuvant for the gD DNA vaccination.
Herpes simplex virus (HSV) is the causative agent of a spectrum of human diseases including cold sores, ocular infections, encephalitis, and genital infections (41). A variety of traditional vaccine strategies have been explored against HSV. Live, attenuated, and killed viruses have been shown to provide protective immunity against HSV infection in an animal model system (4, 26). In addition, immunization with Freund’s adjuvant-emulsified gD protein of either HSV-1 or HSV-2 has been reported to provide protective immunity against infection with both types of HSV in animals (34). On the other hand, subunit vaccines analyzed in clinical trials recently failed to protect humans from recurrent HSV infection (58). One significant challenge in the development of a vaccine for HSV is the uncertainty about the exact immune correlates of protection. It remains controversial if protective immune responses can be provided by either the humoral arm or the cellular arm of the immune system or both (49, 50).
DNA vaccination is a novel vaccination and immunotherapeutic technique which delivers DNA expression constructs encoding specific immunogens into host cells. These expression cassettes transfect the host cells, which become the in vivo protein source for the production of antigen. This antigen then is the focus of the resulting immune response. This vaccination technique is being explored as an immunization strategy against a variety of viral pathogens including HSV (2, 29, 30, 32, 33, 36, 56, 61–63, 68). In addition to the ability of DNA vaccines to induce both antigen-specific cellular and humoral immune responses, this technique has the potential to drive immune responses in a particular direction through the codelivery of genes for immunologically important molecules such as cytokines and costimulatory molecules (21, 23–25, 60). The effects of such codelivery have not been investigated in the herpesvirus model; they should be particularly useful as a test of the ability of such a technology to modulate in vivo immunity and correlate this modulation with infectious status.
In this study, we used a DNA vaccine model to investigate whether driving an HSV-2 immunogen toward a Th1 or Th2-phenotypic immune response could affect the protection against HSV-2 challenge in a defined mouse model system. To investigate the modulation of immune responses and protective immunity, we codelivered a DNA expression construct encoding HSV-2 gD protein with the gene plasmids encoding Th1-type (interleukin-2 [IL-2], IL-12, IL-15, and IL-18) and Th2-type (IL-4 and IL-10) cytokines. We then analyzed their modulatory effects in immunity and protection. Our focus was to determine the effects of the cytokine gene codelivery on the mortality and the morbidity in the immunized animals following HSV challenge. We observed that significant immune system modulation could be achieved through the use of codelivered cytokine genes and that the use of these gene-delivered adjuvants (especially IL-12) could be important in crafting more efficacious vaccines for HSV.
MATERIALS AND METHODS
Mice.
Female 4- to 6-week-old BALB/c mice were purchased from Harlan Sprague-Dawley (Indianapolis, Ind.). They were cared for under the guidelines of the National Institutes of Health (Bethesda, Md.) and the University of Pennsylvania IACUC (Philadelphia, Pa.).
Reagents.
HSV-2 strain 186 (a kind gift from P. Schaffer, University of Pennsylvania, Philadelphia, Pa.) was propagated in the Vero cell line (American Type Culture Collection, Rockville, Md.). The DNA vaccine, pAPL-gD2 encoding HSV-2 gD protein, was previously described (46). The expression vectors, pCDNA3-IL-2, pCDNA3-IL-4, pCDNA3-IL-10, pCDNA3-IL-12, pCDNA3-IL-15, and pCDNA3-IL-18, were previously constructed in our laboratory (24, 25). Plasmid DNA was produced in bacteria by using double banded CsCl preparations. Recombinant HSV-2 gD proteins, a generous gift from G. H. Cohen and R. J. Eisenberg, University of Pennsylvania, Philadelphia, Pa., were used as recombinant antigens in these studies.
DNA inoculation of mice.
The quadriceps muscles of BALB/c mice were injected with 60 μg of gD DNA constructs, formulated in a final volume of 100 μl of phosphate-buffered saline–0.25% bupivacaine-HCl (Sigma, St. Louis, Mo.), via a 28-gauge needle (Becton Dickinson, Franklin Lakes, N.J.). Samples (40 μg) of various cytokine gene expression cassettes were mixed with pgD plasmid solution before the injection.
ELISA.
Enzyme-linked immunosorbent assay (ELISA) was performed as previously described (56). In particular, HSV-2 gD protein (0.75 μg/ml in phosphate-buffered saline) was used as a coating antigen. For the determination of relative levels of gD-specific immunoglobulin G (IgG) subclasses, anti-murine IgG1, IgG2a, IgG2b, or IgG3 conjugated with horseradish peroxidase (Zymed, San Francisco, Calif.) was substituted for anti-murine IgG-horseradish peroxidase. To determine ELISA titers, sera pooled in an equal volume from 10 mice per group were twofold serially diluted and reacted with gD protein. The titers were defined as the reverse of the highest sera dilution showing the same optical density as sera of naive mice.
Th-cell proliferation assay.
The Th-cell proliferation assay was performed as previously described (24).
Intravaginal HSV-2 challenge.
Three weeks after the last DNA injection, mice were challenged intravaginally with HSV-2 strain 186 as previously described with some modifications (39, 40). Before inoculating the virus, the intravaginal area was swabbed with a cotton-tipped applicator (Hardwood Products Company, Guiford, Maine) soaked with 0.1 M NaOH solution and then cleaned with dried cotton applicators. Mice were then examined daily to evaluate pathological conditions and survival rates.
Statistical analysis.
Statistical analysis was done by the paired Student t test and analysis of variance (ANOVA). Values for gD DNA vaccines alone were compared with values for cytokine coinjection groups. P < 0.05 was considered significant.
RESULTS
IL-12 coadministration inhibits the systemic IgG response.
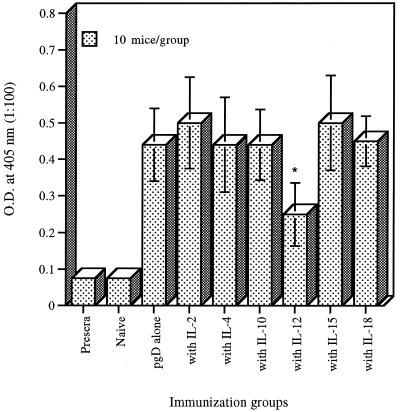
To modulate gD-specific IgG levels in gD DNA vaccination, animals were coimmunized twice with gD DNA construct and a collection of Th1 and Th2-type cytokine genes (IL-2, IL-4, IL-10, IL-12, IL-15, and IL-18). Co-injection with IL-4, IL-10, or IL-18 cytokine genes showed similar levels of antibody responses to that of gD vaccination alone. Coadministration with IL-2 or IL-15 genes resulted in a moderate but not significant enhancement of gD-specific IgG antibodies (Fig. 1). In contrast, coinjection with the IL-12 gene displayed a significant inhibition of gD-specific IgG levels. ELISA titers of equal pools of sera collected 2 weeks after the second immunization were also determined as 12,800 (IL-2), 6,400 (IL-4), 6,400 (IL-10), 1,600 (IL-12), 12,800 (IL-15), 6,400 (IL-18), and 6,400 (gD DNA vaccine alone). This type of suppressive effect of IL-12 on antigen-specific antibody responses has been previously reported for other viral antigens (24). However, granulocyte-macrophage colony-stimulating factor (GM-CSF) coinjection used as a positive control significantly enhanced systemic IgG responses (data not shown) and displayed a twofold-increased ELISA titer of 25,600.
FIG. 1.
Levels of systemic gD-specific IgG in mice immunized with DNA vectors. Each group of mice (n = 10) was immunized with gD DNA vaccines (60 μg per mouse) and/or cytokine genes (40 μg per mouse) at 0 and 2 weeks. The mice were bled 2 weeks after the last immunization, and then sera were diluted to 1:100 for reaction with gD. The optical density (O.D.) was measured at 405 nm. Values and bars represent the mean and standard deviation, respectively. ∗, statistically significant at P < 0.05 by Student’s t test compared to gD DNA vaccine alone.
Cytokine coimmunization shifts IgG subclasses to Th1 or Th2 isotypes.
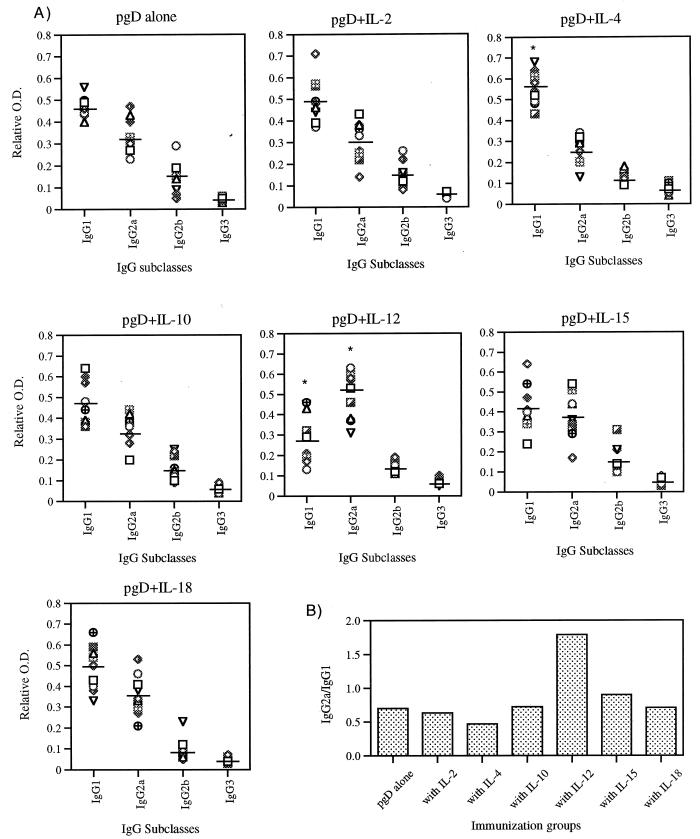
IgG subclasses give an indication of the Th1-versus-Th2 nature of the induced responses. Since we believe that the cytokine genes should drive the resulting immune responses in different directions, we analyzed the IgG subclasses induced by the coinjections. IgG isotypes induced by each immunization group are shown in Fig. 2A, and the relative ratios of IgG2a to IgG1 (Th1 to Th2) are shown in Fig. 2B. The pgD-immunized group had an IgG2a-to-IgG1 ratio of 0.7. Coinjection with IL-2, IL-10, and IL-18 genes produced an IgG subtype pattern similar to that for pgD vaccination. On the other hand, coinjection with IL-4 decreased the relative ratio of gD-specific IgG2a to IgG1 (to 0.5), whereas coimmunization with Th1 cytokine genes (IL-12 and IL-15) increased the relative ratio of IgG2a to IgG1. In particular, the group immunized with pgD plus IL-12 had the highest ratio (1.8), indicating a dramatic shift to Th1 phenotype.
FIG. 2.
(A) Levels of IgG subclasses in mice immunized with DNA vectors. Each group of mice (n = 10) was immunized with gD DNA vaccines (60 μg per mouse) and/or cytokine genes (40 μg per mouse) at 0 and 2 weeks. The mice were bled 2 weeks after the last immunization, and then sera were diluted to 1:100 for reaction with gD. Optical density (O.D.) was measured at 405 nm. The relative optical density was calculated as optical density of each IgG subclass/total optical density. Line bars represent the mean of the relative optical densities of each mouse IgG subclass. (B) Relative ratio of IgG2a to IgG1. The mean (n = 10) IgG2a level was divided by the mean IgG1 level in each immunization group. ∗, statistically significant at P < 0.05 by Student’s t test compared to each corresponding isotype of gD DNA vaccine alone.
Th1 cytokine coinjection enhances Th-cell proliferative responses.
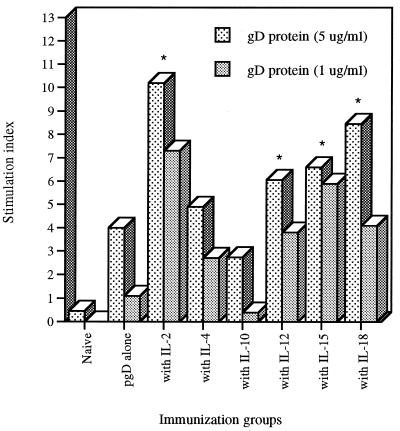
Th-cell proliferation is a standard parameter used to evaluate the potency of cell-mediated immunity. We measured Th-cell proliferative responses following coimmunization with cytokine genes by stimulating splenocytes from immunized animals in vitro with gD protein. As shown in Fig. 3, pgD DNA vaccination alone resulted in gD-specific Th-cell proliferative responses. We also observed the enhancement of Th-cell proliferative responses over that of gD DNA vaccine alone by coinjection with Th1 cytokine genes (IL-2, IL-12, IL-15, and IL-18). In contrast, coimmunization with IL-4 and IL-10 genes appeared to have minimal effects on the levels of Th-cell proliferative responses. However, cytokine coinjection showed no effects on phytohemagglutinin (PHA)-induced non-specific Th cell proliferative responses (stimulation index, 30 to 50).
FIG. 3.
Th-cell proliferation levels of splenocytes after in vitro gD stimulation. Each group of mice (n = 2) was immunized with gD DNA vaccines (60 μg per mouse) and/or cytokine genes (40 μg per mouse) at 0 and 2 weeks. Two weeks after the last DNA injection, two mice were sacrificed and their spleen cells were pooled for the proliferation assay. Splenocytes were stimulated with 5 and 1 μg of gD-2 proteins per ml and 5 μg of PHA per ml as a positive control. After 3 days of stimulation, the cells were harvested and the cpm was counted. Samples were assayed in triplicate. The figure shows the results of one of three separate experiments with similar results. The PHA control sample showed a stimulation index of 30 to 50. ∗, statistically significant at P < 0.05 by Student’s t test compared to gD DNA vaccine alone.
Th1 cytokine coadministration improves the survival rate following intravaginal HSV challenge.
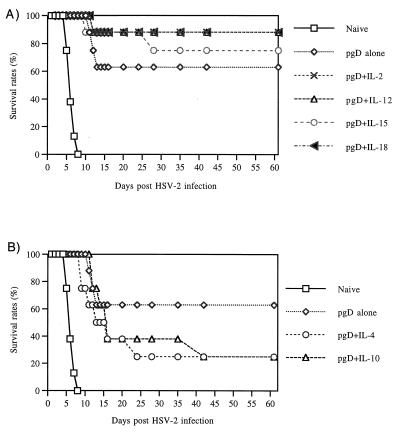
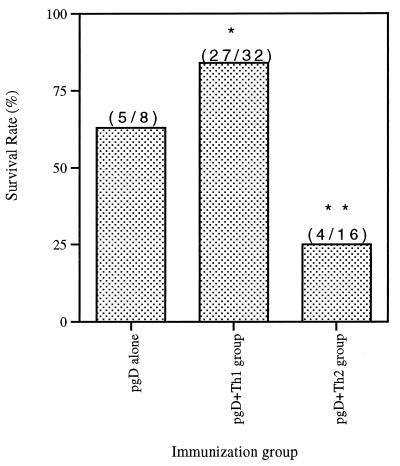
To determine the 50% lethal dose (LD)50 for infectivity studies, naive mice were challenged intravaginally with different doses of HSV-2 strain 186. Table 1 shows the survival rates of naive and immunized mice after different doses of HSV challenge. In our preliminary challenge studies, we observed that coimmunization with Th1 cytokines appeared to improve survival while coimmunization with Th2 cytokines appeared to decrease survival (data not shown). Accordingly, a comparative study was initiated. Eight immunized mice per group immunized with gD plus cytokines were challenged with 200 LD50 of HSV-2 (7 × 105 PFU). The high lethal dose was used to investigate differences in survival rates among vaccinated animals. As shown in Fig. 4, all naive mice died within 8 days after an intravaginal challenge with 200 LD50 of HSV-2. The group immunized with gD DNA vaccine alone had a 63% survival rate after the challenge. In contrast, coinjection with Th1 cytokine genes (IL-2, IL-12, and IL-18) increased the survival rate to 88% (Fig. 4A), while IL-15 coinjection also increased survival but only moderately. Coimmunization with Th2 cytokine genes (IL-4 and IL-10), however, resulted in a significant reduction of the survival rates to 25% (Fig. 4B). As shown in Fig. 5, 84% survival rates in a Th1 group (27 of 32 animals) were noted, compared to 25% survival rates in a Th2 group (4 of 16) and 63% in a group receiving gD DNA vaccines alone (5 of 8). These data support the notion that the type of immunity induced by a vaccine is particularly important in this challenge model.
TABLE 1.
Protection against intravaginal challenges with a different dose of HSV-2 in mice immunized with gD DNA vaccinesa
| Immunogen | Challenge dose (PFU) | No. of mice that survived/no. tested (%) |
|---|---|---|
| None | 1.4 × 102 | 6/6 (100) |
| None | 1.4 × 103 | 4/6 (67) |
| None | 1.4 × 104 | 0/6 (0) |
| None | 1.4 × 105 | 0/6 (0) |
| None | 1.4 × 106 | 0/6 (0) |
| gD | 1.4 × 104 (4 LD50) | 8/8 (100) |
| gD | 1.4 × 105 (40 LD50) | 8/8 (100) |
| gD | 7 × 105 (200 LD50) | 5/8 (63) |
| gD | 1.4 × 106 (400 LD50) | 3/8 (38) |
Mice were immunized intramuscularly with gD DNA vaccines (60 μg) at 0 and 2 weeks. Three weeks after the second immunization, naive and immunized mice were challenged intravaginally with different doses of HSV-2 186 strain. The number of surviving mice was scored 30 days after HSV-2 challenge.
FIG. 4.
Survival rates of mice immunized with gD DNA vaccines plus Th1-type cytokine genes (A) or Th2 type cytokine genes (B) by lethal virus challenges. Each group of mice (n = 8) was immunized with gD DNA vaccines (60 μg per mouse) and/or cytokine genes (40 μg per mouse) at 0 and 2 weeks. Three weeks after the second immunization, mice were challenged intravaginally with 200 LD50 of HSV-2 strain 186 (7 × 105 PFU).
FIG. 5.
Difference in protection rates between Th1 and Th2 cytokine groups. Numbers in parentheses are the number of surviving animals/number tested in group. ∗, statistically significant at P < 0.05 by ANOVA compared to the pgD-plus-Th2 cytokine coinjection group. ∗∗, statistically significant at P < 0.05 by ANOVA compared to the gD DNA vaccine alone.
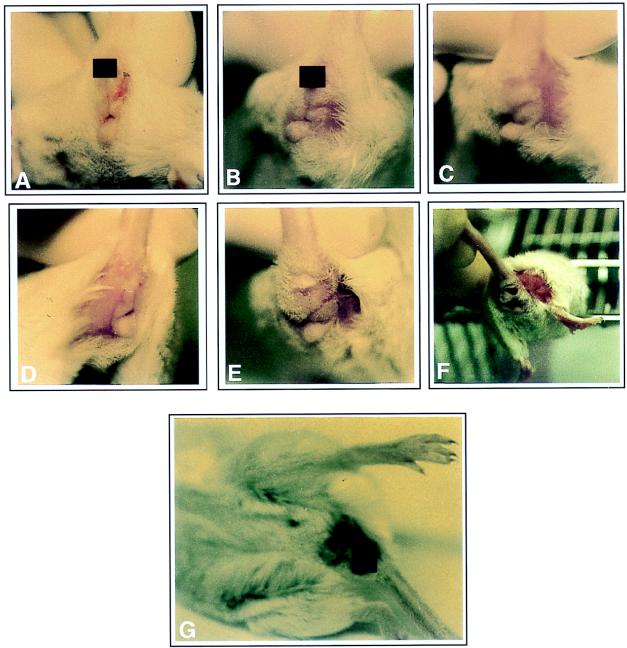
Th1 coadministration reduces the frequency and severity of herpetic lesions following intravaginal HSV challenge.
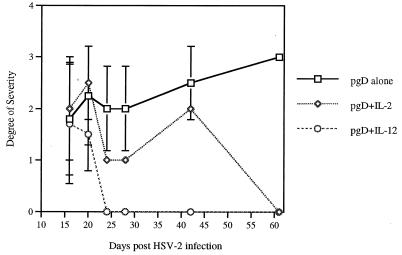
Mice challenged with 200 LD50 of HSV-2 were observed for herpetic lesions for 2 months. Naive mice infected with HSV-2 started to show pathological signs, such as lethargy, abnormal gaits, and ruffling fur 2 to 3 days after virus infection; they died starting 5 days after infection, and they had all died by 8 days of infection. Figure 6 displays representative herpetic lesions (Fig. 6A to F) of mice infected with HSV-2 and deceased mice (Fig. 6G) showing necrosis around the abdominal area. As shown in Table 2, the groups coinjected with Th1 cytokine genes (IL-2, IL-12, IL-15, and IL-18) contained a smaller number of mice exhibiting herpetic lesions on the vaginal area than the group immunized with gD DNA vaccine alone. Rather dramatically, not only did the pgD-plus-IL-12-immunized group contain the fewest number of mice with herpetic lesions, but also 100% of the mice had recovered completely from the lesions at 24 days after viral challenge. Coinjection with IL-2 genes also led ultimately to complete healing of herpetic lesions, but at a later time (61 days after viral challenge). In contrast, this effect was not detected in mice coinjected with IL-15, and IL-18 genes. This study demonstrates two distinct advantages of such a vaccination scheme: one on survival and one on actual disease. We scored the herpetic lesions 1 to 3 based upon their severity (3 being the highest level of severity). As shown in Fig. 7, the gD-plus-IL-2 and gD-plus-IL-12 coimmunized groups showed a lower degree of severity in herpetic lesions than did the group immunized with gD DNA vaccine. However, coinjections with IL-15 and IL-18 genes displayed a similar level of lesion severity to those of gD DNA vaccine alone (data not shown).
FIG. 6.
Photographs showing scoring of deceased mice and those with herpetic lesions after HSV-2 infection. Each group of mice (n = 8) was immunized with gD DNA vaccines (60 μg per mouse) and/or cytokine genes (40 μg per mouse) at 0 and 2 weeks. Three weeks after the last DNA injection, the mice were challenged intravaginally with lethal doses of HSV-2. The mice were checked every day after the viral challenges to observe the pathological symptoms (A to F). The severity of herpetic lesions was recorded as tiny lesions (A and B), mild to less severe lesions (C and D), and severe lesions (E and F). (G) Deceased mouse.
TABLE 2.
Number of mice showing herpetic lesions after HSV-2 infectiona
| Immunization group | No. of mice with herpetic lesion/no. that survived (%) on postinfection day:
|
|||||
|---|---|---|---|---|---|---|
| 16 | 20 | 24 | 28 | 42 | 61 | |
| gD alone | 5/5 (100) | 4/5 (80) | 4/5 (80) | 4/5 (80) | 2/5 (40) | 2/5 (40) |
| gD + IL-2 | 3/7 (43) | 2/7 (29) | 2/7 (29) | 1/7 (14) | 1/7 (14) | 0/7 (0) |
| gD + IL-12 | 3/7 (43) | 2/7 (29) | 0/7 (0) | 0/7 (0) | 0/7 (0) | 0/7 (0) |
| gD + IL-15 | 5/7 (71) | 6/7 (86) | 3/6 (50) | 2/6 (33) | 2/6 (33) | 1/6 (17) |
| gD + IL-18 | 3/7 (43) | 4/7 (57) | 2/7 (29) | 2/7 (29) | 2/7 (14) | 1/7 (14) |
Each group of mice (n = 8) was immunized with gD DNA vaccines (60 μg per mouse) and/or cytokine genes (40 μg per mouse) at 0 and 2 weeks. Three weeks after the last DNA injection, the mice were challenged intravaginally with 200 LD50 of HSV-2 strain 186 (7 × 105 PFU). The mice were checked every day after viral challenges to observe the pathological symptoms.
FIG. 7.
Severity of herpetic lesions in mice surviving after HSV-2 infection. Each group of mice (n = 8) was immunized with gD DNA vaccines (60 μg per mouse) and/or cytokine genes (40 μg per mouse) at 0 and 2 weeks. Three weeks after the last DNA injection, the mice were challenged intravaginally with 200 LD50 of HSV-2 strain 186 (7 × 105 PFU). The mice were checked every day after viral challenges to observe the pathological symptoms. The degrees of severity of herpetic lesions were recorded as tiny lesions (1+), mild to less severe lesions (2+), and severe lesions (3+). Values and bars represent the mean degree of severity and the standard deviation, respectively.
DISCUSSION
HSV infection is a major cause of morbidity in humans (41). HSV-2 infects mucocutaneously and causes genital infection (41). After infection, the virus maintains itself in the sensory ganglia, some with recurrent HSV infection (49). In animal models, immunization of some HSV glycoproteins or DNA constructs expressing the viral components provides complete or partial protection against viral challenge (2, 29, 34, 37, 38, 48). Several HSV proteins have been analyzed as potential immunization targets. Immunization with cDNA encoding gC, ICP27, or gD induces antigen-specific immune responses and protection against in vivo challenge with HSV in animals (2, 29, 37, 38). The gD protein is a glycoprotein which is involved in the binding and entry of HSV into the host cells (66). This protein is highly conserved and antigenically cross-reactive between HSV-1 and HSV-2 (42). This increased the belief that gD could function as a preventive vaccine against both types of HSV infection. Recently, clinical trials with a subunit vaccine failed to protect from recurrent HSV infection, supporting that additional insight is needed to design a more effective approach for this pathogen (58).
An important feature of the DNA approach is the potential to manipulate the immune responses generated by DNA vaccination through the codelivery of immunologically important molecules, effectively driving immune responses in a particular direction. In this study, we sought to take advantage of this feature to drive immune responses in the HSV-2 system and then analyze if such driving could affect morbidity or mortality in this small-animal model. We focused the HSV DNA immunization model to investigate the effects of Th1 and Th2 cytokine genes on the induction and regulation of immune responses. Recently, several groups including ours have reported that specific immune responses generated by DNA vaccine could be modulated with the coinjection of gene expression cassettes encoding cytokines and costimulatory molecules (6, 21, 23–25, 56, 60, 67). When GM-CSF cDNA was used for coimmunization with a DNA vaccine, protective immune responses against rabies virus and encephalomyocarditis virus K were enhanced (56, 67). More recently, we investigated the induction and regulation of immune responses to the codelivery of Th1, and Th2 cytokines, as well as proinflammatory molecules (25). None of the above studies compared directionally driven immune responses in these models, nor has there been an examination of this phenomenon in the HSV system.
In this study, we investigated the effect of cytokine gene codelivery in HSV immunization because cytokines play important roles in immune and inflammatory responses as the initiators and regulators of the immune system network. For HSV-2 specifically, it has been suggested that cytotoxic T cells or B cells or perhaps both are critical to protection in this model, but the nature of the protective responses is still under investigation. The Th1-type cytokines (IL-2, IL-12, IL-15, and IL-18) and the Th2-type cytokines (IL-4, IL-5, and IL-10) predominantly induce the cellular and humoral arms of the immune responses, respectively. IL-2, a cytokine secreted mainly from activated Th cells, can activate NK cells and cytotoxic T cells (10, 17), induce gamma interferon (IFN-γ) production from T cells (22), and stimulate B cells (65). IL-12 has been found to induce Th1-type immune responses by eliciting the differentiation of Th1 cells from uncommitted Th0 cells, to promote NK activity, and to activate cytotoxic T lymphocytes (1, 13, 14, 27). IL-12, a heterodimeric cytokine consisting of p35 and p40 chains, is produced mainly from macrophages and B cells and is a potent inducer of cytotoxic cells (1, 13, 14, 51). IL-15, a recently identified analogue of IL-2, is reported to have T-cell-stimulatory effects similar to those of IL-2 (3, 15). IL-18, previously known as the IFN-γ inducing factor, induces IFN-γ production and NK-cell activity and inhibits IL-10 production from activated peripheral blood mononuclear cells (45). As a prototypical Th2-type cytokine, IL-4 stimulates B-cell growth and plays a major role in suppressing cell-mediated immune responses by inhibiting Th1 cytokine production from T cells (35, 53). IL-4 also stimulates the production of IgE- and IgG1-type antibodies (47). IL-10, a cytokine secreted mainly from Th2 cells, as well as B cells and monocytes (9, 12), inhibits cell-mediated immune responses by interfering with the activation of macrophages and NK cells, as well as IL-2- and IFN-γ-producing Th1 cells (19).
We investigated the in vivo effects of Th1 and Th2 type cytokine genes on the induction of protective immunity against HSV-2 infection by coinjecting them along with gD DNA vaccine constructs. We observed that the groups coimmunized with IL-2 and IL-15 cytokine genes had slightly higher IgG responses than did the gD-immunized group. However, we previously observed that coinjection with both Th1 and Th2 type cytokine genes enhanced systemic IgG production in HIV DNA vaccine studies (25). Only coinjection with GM-CSF cDNAs induced significantly higher production of IgG in gD DNA vaccines (data not shown). This discrepancy might be due to a difference in the nature of the antigens tested. This is based upon our observation that gD DNA vaccines alone could induce an ELISA titer of 6,400 but HIV DNA vaccines alone induced significantly lower ELISA titers under similar immunization conditions. In contrast, IL-12 gene coinjection resulted in a significant inhibition of gD-specific IgG responses. In this case, this activity cannot be ascribed to backbone CpG motifs, since mixing of gD plasmids with pCDNA3 vector did not result in similar immune system modulatory function or change the outcome of the challenge (data not shown). This finding is compatible with previous findings that IL-12 gene coadministration inhibits antigen-specific humoral immune responses in human immunodeficiency virus DNA vaccination studies (24).
It is known that production of IgG1 isotype is induced by Th2-type cytokines whereas the IgG2a isotype production is influenced by Th1-type cytokines (7, 11). This has also been used as an indicator of whether immune responses are under control of the Th1 or Th2 type. In this study, modulation of antigen-specific IgG isotype responses has been achieved by using cytokine genes as a molecular adjuvant. We have observed that Th1 cytokine genes (IL-12 and IL-15), more significantly IL-12 genes, increased the relative ratio of gD-specific IgG2a to IgG1. However, coinjection with IL-4 genes induced more favorable production of IgG1 than of IgG2a. Thus, these results strongly imply that the shift in humoral immune responses to either Th1 or Th2 could be achieved by using the Th1 or Th2 cytokine genes as adjuvants in DNA vaccination. Interestingly the IL-12-coimmunized group had a lower total IgG level but had better protection. In terms of both mortality and morbidity, the Th1 shift in IgG isotype seems to be an important marker for protection.
In vitro immune system parameters, such as Th-cell proliferative and cytotoxic T-lymphocyte responses have been used to evaluate the potency of cell-mediated immunity. We observed that coinjection with Th1-type cytokine genes (IL-2, IL-12, IL-15, and IL-18) induced higher Th-cell proliferation. On the other hand, we did not see significant enhancement of Th-cell proliferation responses with Th2-type cytokine gene coadministration (IL-4 and IL-10). However, we previously observed that coinjection with HIV DNA vaccines plus IL-10 slightly enhanced Th-cell proliferation (25). It is not unlikely that different antigens may behave uniquely in this regard. These results support the conclusion that Th1-type cytokines may be more important in the induction of antigen-specific cell-mediated immune responses, and they extend the validity of this model for testing the driving of immune responses in vivo.
It has been reported that humoral or cellular immune responses or both could be responsible for protective immunity against HSV infection (49, 50). During viral infection, neutralizing antibodies can inactivate free viral particles but are not able to inhibit intracellular HSV infection (44). It appears that antibody-dependent complement-mediated and antibody-dependent cell-mediated cytotoxicity are insufficient to control HSV infection (28, 43, 44, 55, 59). Therefore, it has been suggested that HSV-specific cell-mediated immunity may play a more major effector role in eradicating HSV-infected cells and controlling HSV infection (44, 54, 70, 71).
To emulate the natural route of HSV infection, we chose a well-tested intravaginal HSV-2 challenge model for this study (39, 40). We found that gD genetic vaccination conferred complete protection against infection with 4 LD50 of HSV-2 strain 186. However, gD vaccination alone resulted in 63% survival rates at the challenge inoculum of 200 LD50 of HSV-2. By coinjecting Th1 cytokine genes (IL-2, IL-12, IL-15, and IL-18), better survival rates (88%) were achieved. In contrast, codelivery of Th2 cytokine genes (IL-4 and IL-10) reduced the rate of survival of challenged mice to 25%, more than a 50% reduction in overall survival from that due to the gD vaccine alone. These observations are particularly striking if one considers the entire group of Th1 versus that of Th2 cytokines (survival rates of Th1 types, 27 of 32 [84%]; survival rates of Th2 types, 4 of 16 [25%]). Although Th-cell proliferation levels and gD-specific antibody levels in mice coinjected with Th2 cytokine genes were no worse than those for gD DNA vaccination alone, Th2-type cytokine-mediated susceptibility to HSV-2 infection was observed in these animals. This implies that polarization of gD-specific immune responses to Th2 types by coinjection with IL-4 and IL-10 genes results in increased susceptibility of animals to HSV-2 infection. This appears to be a different finding from that of a previous study, which found that enteric immunization with incompetent HSV expressing IL-4 or IFN-γ showed better protection against intravaginal HSV challenge (31). However, the vaccination method used was very different, suggesting that the type as well as the route of immunization is irrelevant here. Furthermore, our data is compatible with previous in vivo findings that UV irradiation of mice suppressed IL-2 and IFN-γ production but enhanced IL-4 production, resulting in more susceptible infection with HSV (69). It was also suggested that a Th1-like cytokine response might be responsible for resistance from UV-induced herpetic infection in humans (57). Moreover, Th1-cell-mediated ocular inflammatory disease due to HSV infection was suppressed by tropical administration of plasmid expressing IL-10 but not by intramuscular immunization whereas the disease (inflammation in the eye) was exacerbated by IL-2 cDNA inoculation (8). This finding in this alternative disease model is similar to our findings that Th1 cytokines induced strong cellular responses. Furthermore, our findings appear to be similar to those with the Th1/Th2 parasitic infection model in which shifts from Th1 cellular immune types to Th2 humoral immune types are correlated with pathogenic progression in murine leishmaniasis (16, 52). Thus, Th1-type cytokine-mediated cellular immune responses in the context of this type of immunization seem to play more important roles in preventing mice from lethal infection by HSV-2. More specifically, these results suggest that HSV-2 infection is more effectively controlled by the modulation of antigen-specific cellular immune environment.
In the case of morbidity, we also observed that coinjection with IL-2 and, most impressively, IL-12 resulted in reduction of the number of mice with herpetic lesions, compared to injection of gD DNA vaccine alone. Compared with IL-15, an analogue of IL-2, IL-2 coinjections seem to be more effective in reducing morbidity. This might result partially from antiviral effects of IFN-γ induced by IL-2 coinjections. This is based upon our observation that compared to IL-15, IL-2 coinjections induced higher production of IFN-γ from splenocytes after stimulation in vitro with gD protein (data not shown). Similarly, recombinant IL-2 as an adjuvant has been also found to enhance protective efficacy against mortality, morbidity, and recurrent infection by HSV (18, 64). Furthermore, coinjection with IL-12 genes resulted in much faster recovery from the onset of lesions. These results further suggest that Th1-type cytokine (most significantly IL-12)-mediated cellular immune responses play a critical role in reducing the emergence of herpetic lesions and in shortening the recovery time from the lesions. This is supported by our earlier reports that codelivery of IL-12 genes induces potent T-helper cell proliferation and CTL activity but inhibits humoral immunity in human immunodeficiency virus DNA vaccine studies (24). This is also in line with the previous finding that recurrence of latent herpesvirus is linked to decreased cellular responses in the guinea pig model (20). Of interest, a regimen of IL-12 protein injection has been reported to induce protective immunity against lethal intraperitoneal infection with HSV-2 (5). One could extrapolate on our data to hypothesize that one problematic feature of subunit vaccines with regard to HSV infection may be their polarization toward Th2-type immunity. This should be examined in additional studies.
In conclusion, the data presented here demonstrates that Th1-type immune responses lead to better protective immunity against herpetic infection whereas Th2-type immune responses worsen disease, at least with this type of vaccine (Table 3). Moreover, among the Th1-type cytokine genes tested, IL-2 to some extent but IL-12 in particular is a superior molecular adjuvant for gD DNA vaccination, strongly suggesting that IL-12 should be investigated as an adjuvant for HSV-2 DNA vaccine studies. These studies further support the conclusion that molecular adjuvants can increase both the potency and focus of DNA vaccine preparations, suggesting that additional studies of such multicomponent preparations for both vaccine and immune therapeutic applications should be performed.
TABLE 3.
Summary of the effects of Th1 and Th2 cytokine coinjection on IgG levels, the ratio of IgG2a to IgG1, Th-cell proliferation responses, mortality, and morbiditya
| Cytokine | IgG level | IgG2a/IgG1 | Th-cell response | Mortality | Morbidity |
|---|---|---|---|---|---|
| Th1 types | |||||
| IL-2 | ↑ | — | ↑ | ↓ | ↓ |
| IL-12 | ↓ | ↑ | ↑ | ↓ | ↓ |
| IL-15 | ↑ | ↑ | ↑ | ↓ | ↓ |
| IL-18 | — | — | ↑ | ↓ | ↓ |
| Th2 types | |||||
| IL-4 | — | ↓ | ↑ | ↑ | ND |
| IL-10 | — | — | ↓ | ↑ | ND |
↑ and ↓, increase and decrease of the responses, respectively, compared to that without cytokine coinjections; —, no change; ND, not determined.
ACKNOWLEDGMENTS
We thank G. H. Cohen and R. J. Eisenberg for providing HSV-1, 2 gD(306t). We also thank P. Schaffer and R. Jordan for providing a stock of HSV-2(186) for this study. J. I. Sin thanks M. Merva for helpful technical assistance and S. Specter for advice on this study.
REFERENCES
- 1.Bloom E T, Horvath J A. Cellular and molecular mechanisms of the IL-12-induced increase in allospecific murine cytolytic T cell activity: implications for the age-related decline in CTL. J Immunol. 1994;152:4242–4254. [PubMed] [Google Scholar]
- 2.Bourne N, Stanberry L R, Bernstein D I, Lew D. DNA immunization against experimental genital herpes simplex virus infection. J Infect Dis. 1996;173:800–807. doi: 10.1093/infdis/173.4.800. [DOI] [PubMed] [Google Scholar]
- 3.Burton J D, Bramford R N, Peters C, Grant A J, Kurys G, Goldman C K, Brennan J, Roessler E, Waldmann T A. A lymphokine, provisionally designated interleukin T and produced by a human adult T-cell leukemia line, stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proc Natl Acad Sci USA. 1994;91:4935–4939. doi: 10.1073/pnas.91.11.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cappel R, Sprecher S, Rickaert F, de Cuer F. Immune response to a DNA free herpes simplex vaccine in man. Arch Virol. 1982;73:61–67. doi: 10.1007/BF01341728. [DOI] [PubMed] [Google Scholar]
- 5.Carr J A, Rogerson J, Mulqueen M J, Roberts N A, Booth R F G. Interleukin-12 exhibits potent antiviral activity in experimental herpesvirus infections. J Virol. 1997;71:7799–7803. doi: 10.1128/jvi.71.10.7799-7803.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chow Y-H, Huang W-L, Chi W-K, Chu Y-D, Tao M-H. Improvement of hepatitis B virus DNA vaccines by plasmids coexpressing hepatitis B surface antigen and interleukin-2. J Virol. 1997;71:169–178. doi: 10.1128/jvi.71.1.169-178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coffman R L, Seymour B W P, Lebman D A, Hiraki D D, Christiansen J A, Shrader B, Cherwinski H M, Savelkoul H F J, Finkelman F D, Bond M W, Mosmann T R. The role of helper T cell products in mouse B cell differentiation and isotype regulation. Immunol Rev. 1988;102:5–28. doi: 10.1111/j.1600-065x.1988.tb00739.x. [DOI] [PubMed] [Google Scholar]
- 8.Daheshia M, Kuklin N, Kanangat S, Manickan E, Rouse B T. Suppression of ongoing ocular inflammatory disease by tropical administration of plasmid DNA encoding IL-10. J Immunol. 1997;159:1945–1952. [PubMed] [Google Scholar]
- 9.de Waal-Malefyt R, Abrams J, Bennett B, Figdor C G, de Vries J. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farrar W L, Johnson H M, Farrar J J. Regulation of the production of immune interferon and cytotoxic T lymphocytes by interleukin-2. J Immunol. 1981;126:1120–1125. [PubMed] [Google Scholar]
- 11.Finkelman F D, Holmes J, Katona I M, Urban J F, Beckmann M P, Park L S, Schooley K A, Coffman R L, Mosmann T R, Paul W E. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 12.Fiorentino D F, Bond M W, Mosmann T R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gately M K, Wolitzky A G, Quinn P M, Chizzonite R. Regulation of human cytolytic lymphocyte responses by interleukin-12. Cell Immunol. 1992;143:127–142. doi: 10.1016/0008-8749(92)90011-d. [DOI] [PubMed] [Google Scholar]
- 14.Germann T, Gately M K, Schoenhaut D S, Lohoff M, Mattner F, Fischer S, Jin S-C, Schmitt E, Rude E. Interleukin-12/T cell stimulating factor, a cytokine with multiple effects on T helper type 1 (Th1) but not on Th2 cells. Eur J Immunol. 1993;23:1762–1770. doi: 10.1002/eji.1830230805. [DOI] [PubMed] [Google Scholar]
- 15.Grabstein K H, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V, Beers C, Richardson J, Schoenborn M A, Ahdieh M, Johnson L, Alderson M R, Watson J D, Anderson D M, Giri J G. Cloning of a T cell growth factor that interacts with the β chain of the interleukin-2 receptor. Science. 1994;264:965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 16.Heinzel F P, Sadick M D, Holaday B J, Coffman R L, Locksley R M. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989;169:59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henney C S, Kuribayashi K, Kern D E, Gillis S. Interleukin-2 augments natural killer cell activity. Nature. 1981;291:335–338. doi: 10.1038/291335a0. [DOI] [PubMed] [Google Scholar]
- 18.Ho R J Y, Burke R L, Merigan T C. Liposome-formulated interleukin-2 as an adjuvant of recombinant HSV glycoprotein gD for the treatment of recurrent genital HSV-2 in guinia pigs. Vaccine. 1992;4:209–213. doi: 10.1016/0264-410x(92)90153-b. [DOI] [PubMed] [Google Scholar]
- 19.Hsu D H, Moore K W, Spits H. Differential effects of interleukin-4 and -10 on interleukin-2 induced interferon-gamma synthesis and lymphokine-activated killer activity. Int Immunol. 1992;4:563–569. doi: 10.1093/intimm/4.5.563. [DOI] [PubMed] [Google Scholar]
- 20.Iwasaka T, Sheridan J F, Aurelian L. Immunity to herpes simplex virus type 2: recurrent lesions are associated with the induction of suppressor cells and soluble suppressor factors. Infect Immun. 1983;42:955–964. doi: 10.1128/iai.42.3.955-964.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwasaki A, Stiernholm B J, Chan A K, Berstein N L, Barber B H. Enhanced CTL responses mediated by plasmid DNA immunogens encoding costimulatory molecules and cytokines. J Immunol. 1997;158:4591–4601. [PubMed] [Google Scholar]
- 22.Kawase I, Brooks C G, Kuribayashi K, Olabuenaga S, Newman W, Gillis S, Henney C S. Interleukin 2 induces gamma-interferon production: participation of macrophages and NK-like cells. J Immunol. 1983;131:288–292. [PubMed] [Google Scholar]
- 23.Kim J J, Bagarazzi M L, Trivedi N, Hu Y, Chattergoon M A, Dang K, Mahalingam S, Agadjanyan M G, Boyer J D, Wang B, Weiner D B. Engineering of in vivo immune responses to DNA immunization via co-delivery of costimulatory molecule genes. Nat Biotechnol. 1997;15:641–645. doi: 10.1038/nbt0797-641. [DOI] [PubMed] [Google Scholar]
- 24.Kim J J, Ayyavoo V, Bagarazzi M L, Chattergoon M A, Dang K, Wang B, Boyer J D, Weiner D B. In vivo engineering of a cellular immune response by co-administration of IL-12 expression vector with a DNA immunogen. J Immunol. 1997;158:816–826. [PubMed] [Google Scholar]
- 25.Kim J J, Trivedi N N, Nottingham L, Morrison L, Tsai A, Hu Y, Mahalingam S, Dang K, Ahn L, Doyle N K, Wilson D M, Chattergoon M A, Chalian A A, Boyer J D, Agadjanyan M G, Weiner D B. Modulation of amplitude and direction of in vivo immune responses by co-administration of cytokine gene expression cassettes with DNA immunogens. Eur J Immunol. 1998;28:1089–1103. doi: 10.1002/(SICI)1521-4141(199803)28:03<1089::AID-IMMU1089>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 26.Klein R E, Buivovici-Klein E, Moser H, Moucha R, Hilfenhaus J. Efficacy of a virion envelope herpes simplex vaccine against experimental skin infections in hairless mice. Arch Virol. 1981;68:73–80. doi: 10.1007/BF01314437. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi M, Fitz L, Ryan M, Hewick R M, Clark S C, Chan S, Loudon R, Sherman F, Perussia B, Trinchieri G. Identification and purification of natural killer cell stimulator factor (NKSF): a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989;170:827–845. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kohl S, Starr S E, Oleske J M, Shore S L, Ashman R B, Nahmias A J. Human monocyte-macrophage-mediated antibody dependent cytotoxicity to herpes simplex virus-infected cells. J Immunol. 1977;118:729–735. [PubMed] [Google Scholar]
- 29.Kriesel J D, Spruance S L, Daynes R A, Araneo B A. Nucleic acid vaccine encoding gD2 protects mice from herpes simplex virus type 2 disease. J Infect Dis. 1996;173:536–541. doi: 10.1093/infdis/173.3.536. [DOI] [PubMed] [Google Scholar]
- 30.Kuhober A, Pudollek H-P, Reifenberg K, Chisari F V, Schlicht H-J, Reimann J, Schirmbeck R. DNA immunization induces antibody and cytotoxic T cell responses to hepatitis B core antigen in H-2b mice. J Immunol. 1996;156:3687–3695. [PubMed] [Google Scholar]
- 31.Kuklin N A, Daheshia M, Marconi P C, Krisky D M, Rouse R J D, Glorioso J C, Manican E, Rouse B. Modulation of mucosal and systemic immunity by enteric administration of nonreplicating herpes simplex virus expressing cytokines. Virology. 1998;240:245–253. doi: 10.1006/viro.1997.8926. [DOI] [PubMed] [Google Scholar]
- 32.Lagging L M, Meyer K, Hoft D, Houghton M, Belshe R B, Ray R. Immune responses to plasmid DNA encoding the hepatitis C virus core protein. J Virol. 1995;69:5859–5863. doi: 10.1128/jvi.69.9.5859-5863.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levy M Y, Barron L G, Meyer K B, Szoka F C., Jr Characterization of plasmid DNA transfer into mouse skeletal muscle: evaluation of uptake mechanism, expression and secretion of gene products into blood. Gene Ther. 1996;3:201–211. [PubMed] [Google Scholar]
- 34.Long D, Madara T J, de Leon M P, Cohen G H, Montgomery P C, Eisenberg R J. Glycoprotein D protects mice against lethal challenge with herpes simplex virus types 1 and 2. Infect Immun. 1984;43:761–764. doi: 10.1128/iai.43.2.761-764.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maggi E, Parronchi P, Manetti R, Simonelli C, Piccinni M-P, Rugiu F S, Carli M D, Ricci M, Romagnani S. Reciprocal regulatory effects of IFN-gamma and IL-4 on the in vitro development of human Th1 and Th2 clones. J Immunol. 1992;148:2142–2147. [PubMed] [Google Scholar]
- 36.Major M E, Vitvitski L, Mink M A, Schleef M, Whalen R G, Trepo C, Inchauspe G. DNA-based immunization with chimeric vectors for the induction of immune responses against the hepatitis C virus nucleocapsid. J Virol. 1995;69:5798–5805. doi: 10.1128/jvi.69.9.5798-5805.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manickan E, Rouse R J D, Yu Z, Wire W S, Rouse B T. Genetic immunization against herpes simplex virus. Protection is mediated by CD4+ T lymphocytes. J Immunol. 1995;155:259–265. [PubMed] [Google Scholar]
- 38.Manickan E, Yu Z, Rouse R J D, Wire W S, Rouse B T. Induction of protective immunity against herpes simplex virus with DNA encoding the immediate early protein ICP 27. Viral Immunol. 1995;8:53–61. doi: 10.1089/vim.1995.8.53. [DOI] [PubMed] [Google Scholar]
- 39.McDermott M R, Smiley J R, Leslie P, Brais J, Rudzroga H E, Bienenstock J. Immunity in the female genital tract after intravaginal vaccination of mice with an attenuated strain of herpes simlex virus type 2. J Virol. 1984;51:747–753. doi: 10.1128/jvi.51.3.747-753.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Milligan G N, Bernstein D I. Generation of humoral immune responses against herpes simplex virus type 2 in the murine female genital tract. Virology. 1995;206:234–241. doi: 10.1016/s0042-6822(95)80038-7. [DOI] [PubMed] [Google Scholar]
- 41.Nahmias A J, Dannenbarger J, Wickliffe C, Muther J. Clinical aspects of infection with herpes simplex virus 1 and 2. New York, N.Y: Elsevier Science Publishing, Inc.; 1980. [Google Scholar]
- 42.Nicola A V, Willis S H, Naidoo N N, Rosenberg R J, Cohen G H. Structure-function analysis of soluble forms of herpes simplex virus glycoprotein D. J Virol. 1996;70:3815–3822. doi: 10.1128/jvi.70.6.3815-3822.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Norrild B, Shore S L, Nahmias A J. Herpes simplex virus glycoproteins: participation of individual herpes simplex virus type 1 glycoprotein antigens in immunocytosis and their correlation with previously identified glycopolypeptides. J Virol. 1979;32:741–748. doi: 10.1128/jvi.32.3.741-748.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Notkins A L. Immune mechanisms by which the spread of viral infections is stopped. Cell Immunol. 1974;11:478–483. doi: 10.1016/0008-8749(74)90045-8. [DOI] [PubMed] [Google Scholar]
- 45.Okamura H, Tsutsui H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K, Akita K, Namba M, Tanabe F, Konishi K, Fukuda S, Kurimoto M. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 46.Pachuk C J, Arnold R, Herold K, Ciccarelli R B, Higgins T J. Humoral and cellular immune responses to herpes simplex virus-2 glycoprotein D generated by facilitated DNA immunization of mice. Curr Top Microbiol Immunol. 1998;226:79–89. doi: 10.1007/978-3-642-80475-5_6. [DOI] [PubMed] [Google Scholar]
- 47.Paul W. Interleukin-4: a prototypic immunoregulatory lymphokine. Blood. 1991;77:1859–1870. [PubMed] [Google Scholar]
- 48.Peng T, Ponce-de-Leon M, Jiang H, Dubin G, Lubinski J M, Eisenberg R J, Cohen G H. The gH-gL complex of herpes simplex virus (HSV) stimulates neutralizing antibody and protects mice against HSV type 1 challenge. J Virol. 1998;72:65–72. doi: 10.1128/jvi.72.1.65-72.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Price R W, Walz M A, Wohlenberg C, Notkins A L. Latent infection of sensory ganglia with herpes simplex virus: efficacy of immunization. Science. 1975;188:938–940. doi: 10.1126/science.166432. [DOI] [PubMed] [Google Scholar]
- 50.Rager-Zisman B, Allison A C. Mechanism of immunologic resistance to herpes simplex virus (HSV-1) infection. J Immunol. 1976;116:35–40. [PubMed] [Google Scholar]
- 51.Robertson M J, Soiffer R J, Wolf S F, Manley T J, Donahue C, Young D, Hermann S H, Ritz J. Responses of human natural killer (NK) cells to NK cell stimulatory factor (NKSF): cytolytic activity and proliferation of NK cells are differentially regulated by NKSF. J Exp Med. 1992;175:779–788. doi: 10.1084/jem.175.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scott P, Natovitz P, Coffman R L, Pearce E, Sher A. Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J Exp Med. 1988;168:1675–1684. doi: 10.1084/jem.168.5.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seder R A, Paul W E. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu Rev Immunol. 1994;12:635–673. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- 54.Sethi K K, Omata Y, Schneweis K E. Protection of mice from lethal herpes simplex virus type 1 infection by adoptive transfer of cloned virus-specific and H-2-restricted cytotoxic T lymphocytes. J Gen Virol. 1983;64:443–447. doi: 10.1099/0022-1317-64-2-443. [DOI] [PubMed] [Google Scholar]
- 55.Shore S L, Cromeans T L, Norrild B. Early damage of herpes infected cells by antibody-dependent cellular cytotoxicity: relative roles of virus-specific cell surface antigens and input virus. J Immunol. 1979;123:2239–2244. [PubMed] [Google Scholar]
- 56.Sin J I, Sung J H, Suh Y S, Lee A H, Chung J H, Sung Y C. Protective immunity against heterologous challenge with encephalomyocarditis virus by VP1 DNA vaccination: effect of co-injection with a granulocyte, macrophage-colony stimulating factor gene. Vaccine. 1997;15:1827–1833. doi: 10.1016/s0264-410x(97)88856-1. [DOI] [PubMed] [Google Scholar]
- 57.Spruance S L, Evans T G, McKeough M B, Thai L, Araneo B A, Daynes R A, Mishkin E M, Abramovitz A S. Th1/Th2-like immunity and resistance to herpes simplex labialis. Antiviral Res. 1995;28:39–55. doi: 10.1016/0166-3542(95)00037-m. [DOI] [PubMed] [Google Scholar]
- 58.Straus S E, Wald A, Kost R G, McKenzie R, Langenberg A G, Hohman P, Lekstrom J, Cox E, Nakamura M, Sekulovich R, Izu A, Dekker C, Corey L. Immunotherapy of recurrent genital herpes with recombinant herpes simplex virus type 2 glycoproteins D and B: results of a placebo-controlled vaccine trial. J Infect Dis. 1997;176:1129–1134. doi: 10.1086/514103. [DOI] [PubMed] [Google Scholar]
- 59.Tada M, Hinuma S, Abo T, Kumagi K. Murine antibody-dependent cell-mediated cytotoxicity: failure to detect effector cells equivalent to human K cells. J Immunol. 1980;124:1929–1936. [PubMed] [Google Scholar]
- 60.Tsuji T, Hamajima K, Ishii N, Aoki I, Fukushima J, Xin K Q, Kawamoto S, Sasaki S, Matsunaga K, Ishigatsubo Y, Tani K, Okubo T, Okuda K. Immunomodulatory effects of a plasmid expressing B7-2 on human immunodeficiency virus-1-specific cell-mediated immunity induced by a plasmid encoding the viral antigen. Eur J Immunol. 1997;27:782–787. doi: 10.1002/eji.1830270329. [DOI] [PubMed] [Google Scholar]
- 61.Ulmer J B, Donnelly J, Parker S E, Rhodes G H, Felgner P L, Dwarki V L, Gromkowski S H, Deck R, DeVitt C M, Friedman A, Hawe L A, Leander K R, Marinez D, Perry H, Shiver J W, Montgomery D, Liu M A. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 62.Wang B, Boyer J D, Srikantan V, Coney L, Carrano R, Phan C, Merva M, Dang K, Agadjanyan M G, Ugen K E, Williams W V, Weiner D B. DNA inoculation induces neutralizing immune responses against human immunodeficiency virus type 1 in mice and non-human primates. DNA Cell Biol. 1993;12:799–805. doi: 10.1089/dna.1993.12.799. [DOI] [PubMed] [Google Scholar]
- 63.Wang B, Ugen K E, Srikantan V, Agadjanyan M G, Dang K, Refaeli Y, Sato A, Boyer J, Williams W V, Weiner D B. Gene inoculation generates immune responses against human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1993;90:4156–4160. doi: 10.1073/pnas.90.9.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weinberg A, Merigan T C. Recombinant interleukin 2 as an adjuvant for vaccine-induced protection: immunization of guinea pigs with herpes simplex virus subunit vaccines. J Immunol. 1988;140:294–299. [PubMed] [Google Scholar]
- 65.Weyand C M, Goronzy J, Dallman M J, Fathman C G. Interleukin-2 in vivo also induces a polyclonal IgM response. J Exp Med. 1986;163:1607–1612. doi: 10.1084/jem.163.6.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Whitbeck J C, Peng C, Lou H, Xu R, Willis S H, Ponce de Leon M, Peng T, Nicola A V, Montgomery R I, Warner M S, Soulika A M, Spruce L A, Moore W T, Lambris J D, Spear P G, Cohen G H, Eisenberg R J. Glycoprotein D of herpes simplex virus (HSV) binds directly to HVEM, a member of the tumor necrosis factor receptor superfamily and a mediator of HSV entry. J Virol. 1997;71:6083–6093. doi: 10.1128/jvi.71.8.6083-6093.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xiang Z, Ertl H C. Manipulation of the immune response to a plasmid-encoded viral antigen by coinoculation with plasmids expressing cytokines. Immunity. 1995;2:129–135. doi: 10.1016/s1074-7613(95)80001-8. [DOI] [PubMed] [Google Scholar]
- 68.Xiang Z Q, Spitalnik S, Tran M, Wunner W H, Cheng J, Ertl H C J. Vaccination with a plasmid vector carrying the rabies virus glycoprotein gene induces protective immunity against rabies virus. Virology. 1994;199:132–140. doi: 10.1006/viro.1994.1105. [DOI] [PubMed] [Google Scholar]
- 69.Yasumoto S, Moroi Y, Koga T, Kawamura I, Mitsuyama M, Hori Y. Ultraviolet-B irradiation alters cytokine production by immune lymphocytes in herpes simplex virus-infected mice. J Dermatol Sci. 1994;8:218–223. doi: 10.1016/0923-1811(94)90058-2. [DOI] [PubMed] [Google Scholar]
- 70.Zinkernagel R M, Althage A. Antiviral protection by virus-immune cytotoxic T cells; Infected target cells are lysed before infectious virus progeny is assembled. J Exp Med. 1977;145:644–651. doi: 10.1084/jem.145.3.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zinkernagel R M, Doherty P C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:52–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]