Abstract
Nerve growth factor β subunit (β-NGF) transgene delivery and expression by herpes simplex virus type 1 (HSV-1) vectors was examined in a cell culture model of neuroprotection from hydrogen peroxide toxicity. Replication-competent (tk− K mutant background) and replication-defective (ICP4−;tk− S mutant background) vectors were engineered to contain the murine β-NGF cDNA under transcriptional control of either the human cytomegalovirus immediate-early gene promoter (HCMV IEp) (e.g., KHN and SHN) or the latency-active promoter 2 (LAP2) (e.g., KLN and SLN) within the viral thymidine kinase (tk) locus. Infection of rat B103 and mouse N2A neuronal cell lines, 9L rat glioma cells, and Vero cells with the KHN or SHN vectors resulted in the production of β-NGF-specific transcripts and β-NGF protein reaching a maximum at 3 days postinfection (p.i.). NGF protein was released into the culture media in amounts ranging from 10.83 to 352.86 ng/ml, with the highest levels being achieved in B103 cells, and was capable of inducing neurite sprouting of PC-12 cells. The same vectors produced high levels of NGF in primary dorsal root ganglion (DRG) cultures at 3 days. In contrast to HCMV IEp-mediated expression, the LAP2-NGF vectors showed robust expression in primary DRG neurons at 14 days. The neuroprotective effect of vector produced NGF was assessed by its ability to inhibit hydrogen peroxide-induced neuron toxicity in primary DRG cultures. Consistent with the kinetics of vector-mediated NGF expression, HCMV-NGF vectors were effective in abrogating the toxic effects of peroxide at 3 but not 14 days p.i. whereas LAP2-NGF vector transduction inhibited apoptosis in DRG neurons at 14 days p.i. but was ineffective at 3 days p.i. Similar kinetics of NGF expression were observed with the KHN and KLN vectors in latently infected mouse trigeminal ganglia, where high levels of β-NGF protein expression were detected at 4 wks p.i. only from the LAP2; HCMV-NGF-driven expression peaked at 3 days but could not be detected during HSV latency at 4 weeks. Together, these results indicate that (i) NGF vector-infected cells produce and secrete mature, biologically active β-NGF; (ii) vector-synthesized NGF was capable of blocking peroxide-induced apoptosis in primary DRG cultures; and (iii) the HCMV-IEp functioned to produce high levels of NGF for several days; but (iv) only the native LAP2 was capable of long-term expression of a therapeutic gene product in latently infected neurons in vivo.
Nerve growth factor (NGF) is a potent neurotrophic factor originally identified by its ability to promote the survival of sensory and sympathetic neurons during development. The natural protein is composed of two α and two β subunits, but the β subunit, synthesized as a precursor, is proteolytically cleaved to yield the mature polypeptide (22, 97), which has full biological activity residing in a dimerized carboxy-terminal 118-amino-acid peptide (13, 22, 98). In addition to promoting survival during development, NGF has been shown to induce the differentiation of neuronal precursor cells, accelerate the sprouting of neurites, increase the survival of cholinergic neurons of the septohippocampal pathway following axotomy, and promote the survival of sensory and sympathetic neurons following a variety of insults including treatment with calcium ionophores, suramin, hydrogen peroxide, and excitatory amino acids (42, 44, 55, 59, 60, 111, 120).
The neuroprotective effects of NGF suggest its potential utility for treatment of neurodegenerative diseases of the central nervous system (CNS) such as Alzheimer’s disease, and Parkinson’s disease, stroke, and peripheral nervous system (PNS) disorders (2, 7, 12, 32, 45, 54, 76, 95, 104). However, administration of β-NGF protein is limited by its short half-life, lack of bioavailability following oral administration (54), and undesirable side effects acruing from systemic delivery (9, 94, 110). In addition, the blood-brain barrier prevents NGF from reaching the brain parenchyma (43). This last problem can be partially circumvented through the use of anti-transferrin receptor antibody-conjugated β-NGF to promote delivery to the CNS in levels sufficient to enhance the survival of septal implants and to rescue cholinergic interneurons in the striatum following quinolinic acid-induced lesion formation (28, 37, 53), but these conjugates are difficult to synthesize and may be immunogenic.
Gene transfer has obvious advantages for the delivery of trophic factors such as NGF. An ex vivo approach involving transplantation of cells transduced with a β-NGF-expressing retrovirus has been demonstrated to be effective in transiently rescuing neurons from physical or chemical lesions (29, 31, 54, 62, 69, 79, 84, 85, 92, 99, 114, 115). The development of vectors for the treatment of chronic neurodegenerative conditions has generally been impeded by the limited time course of transgene expression. With most combinations of vectors and promoter elements tested, expression is maximal shortly after transfection and declines by 1 order of magnitude or more over a few weeks to months. Direct gene transfer to the brain has also encountered difficulties in either efficient infection or lack of target cell specificity, an important issue for this complex organ.
Many features of the natural biology of herpes simplex virus type 1 (HSV-1) support its development as a vector to deliver and express neurotropins in the nervous system. The HSV-1 genome is large (152 kb), and the deletion of toxic gene functions not only substantially reduces the cytotoxic nature of the vector (66, 86, 88, 128) but also can provide ample space (>40 kb) in which to insert one or more gene expression cassettes. Of its 84 genes, approximately half may be individually deleted without preventing virus replication in vitro (83), although elimination of some of these genes can reduce virus production by 10- to 100-fold. Deletion of the essential genes prevents virus replication without complementation, and deletion of multiple immediate-early (IE) genes in various combinations can reduce or eliminate virus cytotoxicity (17, 57, 66, 87, 88, 128). HSV-1 efficiently infects neurons, where lytic gene expression can be curtailed, and a lifelong latent state can be established (107). The latent viral genome persists in the nucleus as an episome bound by nucleosomes (18, 23, 73, 82). The virus possesses a natural promoter system which remains active during latency (16, 103, 108), producing a family of nonpolyadenylated latency-associated transcripts (LATs). Two latency-active promoter (LAP) elements (LAP1 and LAP2) that may be used to express transgenes within cells of the nervous system (11, 21, 36, 64, 126) have been identified (5, 11, 21, 36, 75). LAP1 is primarily responsible for LAT expression during latency in animal models, while LAP2 is primarily responsible for LAT expression during lytic infection in cell culture (11, 21, 75). Nevertheless, LAP2 has been shown to function independently in expressing a reporter gene during latency, demonstrating that this promoter is functionally active in an otherwise quiescent viral genome (36). Although wild-type HSV can reactivate from latency to cause clinical disease, virus mutants that fail to reactivate have been identified, and even highly defective viruses can be maintained in neurons in a latent state where the LAT promoter system remains active (24). The ability of HSV to provide a platform for transgene expression from the nervous system suggests its utility for gene therapy applications involving neurons of the CNS and PNS.
To test the potential utility of a genomic HSV vector to support prolonged expression of a biologically active transgene product in the PNS, we constructed replication-conditional and replication-defective genomic HSV-1-based vectors carrying the gene encoding murine β-NGF. Studies carried out in vitro and in vivo demonstrated that vectors containing expression cassettes with the strong human cytomegalovirus immediate-early promoter (HCMV IEp) (109, 112) driving the transcription of β-NGF achieved transient high-level bioactive NGF production that promoted neurite growth and protected neurons in vitro from the cytotoxic effects of hydrogen peroxide. Conversely, vectors utilizing the HSV-1 LAP2 to express NGF resulted in delayed but high-level protective NGF expression in vitro and similar kinetics during viral latency in vivo. These results suggest that LAP2 is promising for the achievement of long-term expression of biologically active transgenes in the PNS.
MATERIALS AND METHODS
Cells.
African green monkey kidney (Vero) cells (ATCC CCL-81) and the ICP4-complementing cell line derivative of Vero cells, E5 (17), as well as mouse N2A neuroblastoma (ATCC CCL-131), rat B103 neuroma (105), and rat 9L glioma (118) cells, were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (containing 4.5 g of glucose per liter; Life Technologies, Inc., Gaithersburg, Md.) supplemented with fetal bovine serum (10% final concentration; Life Technologies, Inc.), l-glutamine (2 mM; Life Technologies, Inc.), and penicillin-streptomycin (100 U/ml and 100 μg/ml, respectively; Life Technologies, Inc.). Dorsal root ganglia (DRG) were prepared by following established protocols (50, 52, 122, 123). Briefly, DRG were isolated from day 16 embryos and dissociated for 1 h in Leibowitz-15 medium (L-15; 10% fetal bovine serum [FBS], 20,000 U of penicillin and streptomycin [Life Technologies, Inc.]) plus 3 mg of collagenase A (Boehringer-Mannheim, Indianapolis, Ind.) per ml. After being washed four times in fresh L-15–10% FBS and triturated 10 to 20 times with a fire-polished narrow-bore Pasteur pipette (0.5 to 1 mm), the cells were plated on rat tail collagen-coated 12-well plates or 8-well slides (Falcon/Becton Dickinson, Franklin Lakes, N.J.) and maintained in medium (DMEM high glucose plus sodium pyruvate; Life Technologies, Inc.) supplemented with 10% FBS, 100 μg of NGF (Harlan Bioproducts, Indianapolis, Ind.) per ml, plus 10 μM uridine (Sigma) and 10 μM fluorodeoxyuridine (Sigma) (added to inhibit the growth of nonneuronal cells). Nondissociated DRG cultures were established from individual DRG isolated from the day 16 rat embryos as described above; however, they were not treated with collagenase or triturated before being plated.
Viruses.
Two different thymidine kinase (tk) mutants of HSV-1 and a recombinant with a mutation in the Us3 protein kinase gene were used to construct the β-NGF gene transfer vectors. A plasmid containing the HSV-1 KOS BamHI P fragment with a P1 phage lox recombination site in the coding sequence of the tk gene at the unique SnaBI site had previously been recombined into both wild-type KOS and the ICP4− d120 HSV to yield Klox and Slox, respectively (Fig. 1A and E) (74, 81). The ICP4 deletion virus (d120) and the complementing cell line E5 were kindly provided by Neal DeLuca (University of Pittsburgh) (17). Insertion of the lox site rendered tk nonfunctional, enabling selection and purification of the recombinant viruses in the presence of 100 μg of thymine-β-d-arabinofuranoside (ara-T) per ml (Sigma). The HSV-1 KOS BamHI N fragment with a lox site inserted into Us3 at the PstI-SalI (+466 to +539) sites (pUs3::lox) was recombined into the genome of the Us3::pgC-lacZ virus (26, 117) so as to eliminate the lacZ expression cassette, enabling the purification of the Ulox recombinant (Fig. 1I) through the identification of clear plaques (81).
FIG. 1.
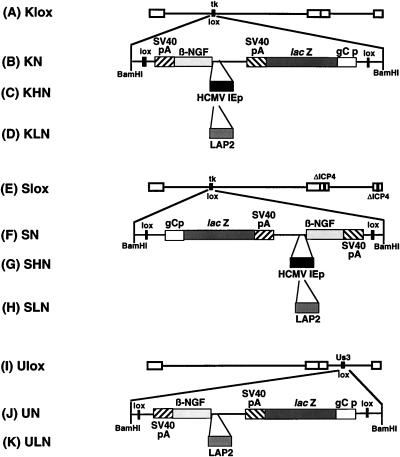
Diagram of recombinant HSV-1 β-NGF gene transfer vectors. Three HSV-1 backbones were used in the construction of β-NGF expression vectors. Wild-type KOS virus and the ICP4 deletion mutant d120 (17) each were engineered to contain a lox recombination site inserted into the tk structural gene (74, 81) so as to disrupt the synthesis of functional tk to produce Klox and Slox, respectively (A and E). A lox site was also inserted into the Us3 recombinant (26), disrupting Us3 and eliminating the lacZ expression cassette present within the Us3::pgC-lacZ virus to create Ulox (I). A lox-containing plasmid, constructed with the murine β-NGF cDNA lacking a promoter and the lacZ gene cassette under the control of the HSV-1 glycoprotein C promoter (gCp), was recombined by using the site-specific P1 phage recombinase Cre into each backbone to yield three β-NGF containing control viruses, KN (B), SN (F), and UN (J). Since these recombinants lack a promoter upstream of the β-NGF cDNA, they should act as NGF expression controls. The HCMV IEp was cloned into the lox plasmid immediately upstream the murine β-NGF cDNA to achieve high levels of expression. This promoter-containing plasmid was recombined into the viral backbones by Cre-lox recombination to create the expression vectors KHN (C) and SHN (G). The sequences comprising the LAP2 promoter (−597 to +42) were cloned into the lox plasmid containing the murine β-NGF cDNA in an attempt to achieve long-term expression. This promoter-containing plasmid was recombined into three mutant viral backbones by Cre-lox recombination to create the expression vectors KLN (D), SLN (H), and ULN (K).
Three promoterless control viruses containing only the murine β-NGF cDNA were created by using the lox recombinant viruses. A lox-containing plasmid (pNGF-lox) with the β-NGF cDNA (kindly provided by William Rutter, Chiron) (93) present in a 959-bp SmaI-PstI fragment fused to the simian virus 40 (SV40) late poly(A) (671-bp PstI-BamHI fragment) was inserted into the lox viruses Klox, Slox, and Ulox (Fig. 1A, E, and I) by Cre-lox recombination to produce the NGF expression vectors KN, SN, and UN (Fig. 1B, F, and J), respectively. pNGF-lox also contained the HSV-1 glycoprotein C late-gene promoter driving lacZ, which allowed identification of positive recombinants by 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) staining (26, 35, 81). In KN, NGF is parallel to tk, while in the d120 backbone (SN), NGF is antiparallel to tk. For test viruses, the HCMV IEp (−760 to +3) (109, 112), present on a 874-bp ClaI fragment, was inserted into pNGF-lox at a unique SalI site upstream of the β-NGF cDNA and the resulting plasmid was recombined into the replication-competent and -defective viral backbones (Fig. 1A and D) to yield the KHN and SHN expression vectors (Fig. 1C and G). A 641-bp PstI-BamHI fragment (−597 to +42) containing the LAP2 promoter (36) was made blunt with Klenow fragment, cloned into the unique SalI site of the lox plasmid containing the murine β-NGF cDNA (pNGF-lox), and recombined into the three mutant viral backbones Klox, Slox, and Ulox (Fig. 1A, E, and I) by Cre-lox recombination to create the KLN, SLN, and ULN (Fig. 1D, H, and K) expression vectors, respectively. Positive-staining isolates were further purified through three rounds of limiting dilution (35), and the presence of the recombinant expression cassette was confirmed by Southern blot (102) analysis.
In vitro analysis of transgene expression.
Vero, E5 (17), N2A mouse neuroblastoma, B103 rat neuroma, or 9L rat glioma cells were either mock infected or infected at a multiplicity of infection (MOI) of 10 with Klox, Slox, KN, SN, KHN, and SHN. At 2, 6, and 16 h postinfection (p.i.), cell culture supernatants were collected and centrifuged at 48,400 × g to remove cells and virus. A sample of clarified supernatant was used to measure NGF protein by enzyme-linked immunosorbent assay (ELISA), while the remainder was placed on PC-12 cells to determine bioactivity. The remaining adherent cells were used to isolate total cell RNA or for immunocytochemical analysis.
RNA analysis.
Total-cell RNA was isolated by the RNAzol B method (Biotecx, Houston, Tex.). RNA (10 μg) was fractionated on 1.5% agarose–2.2 M formaldehyde gels and blotted onto Nytran membrane (Schleicher & Schuell, Keene, N.H.). The blotted membrane was briefly rinsed with 5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), UV cross-linked using the Stratalinker UV cross-linker (Stratagene, La Jolla, Calif.), prehybridized in 50% formamide (Life Technologies, Inc.)–5× Denhardt’s reagent–5× SSC–0.1% sodium dodecyl sulfate (SDS)–100 μg of denatured salmon sperm DNA per ml–100 μg of denatured tRNA (Sigma) per ml at 42°C for 2 h, and hybridized overnight at 42°C. An 803-bp StyI DNA probe containing the NGF coding sequence and the SV40 polyadenylation site was random primer labeled (Boehringer Mannheim) by [α-32P]dCTP (3,000 Ci/mmol; DuPont-NEN, Wilmington, Del.) incorporation. The blots were washed at room temperature with 2× SSC–0.1% SDS three times for 15 min each and then once at 50°C with 0.2× SSC–0.05% SDS for 5 min. X-ray film (XAR5; Kodak, Rochester, N.Y.) was exposed to the blots for various times at −80°C with intensifying screens.
Immunohistochemistry.
The cells were fixed with 4% paraformaldehyde (Sigma) for 15 min, washed three times with 1× phosphate-buffered saline (PBS), and blocked with 20% horse serum (Life Technologies, Inc.) in 1× PBS for 1 h at room temperature. Primary rabbit anti–β-NGF polyclonal antibody (Chemichon Int., Inc., Temecula, Calif.) diluted 1:5,000 in 2× PBS–0.3% Triton X-100 (Sigma) containing 0.02% sodium azide was added, and the plates were incubated at room temperature for 24 h and washed three times with 1× PBS–0.1% Tween (Sigma) for 15 min at room temperature. Goat anti-rabbit immunoglobulin G secondary antibody (Sigma) diluted 1:10,000 in 2× PBS–0.3% Triton X-100 (Sigma) containing 0.02% sodium azide was added, and the plates were incubated for 2 h at room temperature followed by another three washes with 1× PBS–0.1% Tween. The reaction product was detected with the VECTASTAIN ABC Elite kit (Vector Labs, Burlingame, Calif.) as specified by the manufacturer.
ELISA.
Medium from infected cell cultures was centrifuged at 48,400 × g to remove cell debris and virus, and the supernatant was assayed in triplicate for β-NGF. Tissue samples were sonicated on ice in 10 volumes of extraction buffer (0.1 M Tris-HCl, 0.4 M NaCl, 2% albumin, 0.05 M sodium azide, 0.0001 M phenylmethylsulfonyl fluoride, 0.001 M aprotinin, 0.004 M EDTA [pH 7.0]) and centrifuged at 17,000 × g at 4°C for 60 min, and 100-μl samples were assayed in triplicate. Polystyrene microtiter plates (MicroWell; Nunc Inc., Naperville, Ill.) coated at 100 μl/well with 50 mM sodium carbonate-bicarbonate buffer (pH 9.6) containing 0.5 μg of mouse anti-NGF monoclonal antibody (Boehringer Mannheim) per ml were incubated for 2 h at 37°C and washed three times with 50 mM Tris-HCl–200 mM NaCl–10 mM CaCl2–0.1% Triton X-100–0.1% sodium azide (pH 7.0), and NGF standards (0.031 to 1.0 ng/ml) and samples were added. After incubation overnight at 4°C, the plates were washed three times with the same buffer, an anti-NGF monoclonal antibody conjugated to β-galactosidase (4 U/ml) was added (100 μl/well, at a 1:30 dilution in 50 mM Tris-HCl–200 mM NaCl–10 mM CaCl2–1% albumin–0.1% Triton X-100–0.1% sodium azide [pH 7.0]), and the plates were incubated for 4 h at 37°C. After three washes with buffer, 200 μl of substrate solution (2 mg of chlorophenol red–β-d-galactopyranoside [CPRG] in 100 mM HEPES–150 mM NaCl–2 mM MgCl2–0.1% sodium azide–1% albumin [pH 7.0]) was added to each well; the plates were incubated for 60 min at 37°C and then read at 570 nm on an MR700 microplate reader (Dynatech Laboratories, Chantilly, Va.) at 15-min intervals. The total protein concentration for the tissue samples was determined using the Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, Calif.) by the method of Lowry et al. (65), and all values of NGF levels in tissue were expressed as nanograms per milligram of protein.
PC-12 bioassay.
PC-12 cells grown in DMEM supplemented with horse serum (10% final concentration), fetal bovine serum (5% final concentration), l-glutamine (2 mM), and penicillin-streptomycin (100 U/ml and 100 μg/ml, respectively) covered with freshly prepared rat tail collagen were used to assess bioactivity (39). At 48 h after replacement of the culture medium with the test medium or supernatant, neurite formation in PC-12 cells was assessed and photographed with a Diaphot (Nikon Inc., Melville, N.Y.) inverted microscope. As a positive control, DMEM containing 50 ng of purified mouse β-NGF (2.5S; Sigma) per ml was added to the PC-12 cultures.
Infection of primary DRG cultures.
For infection with the replication-conditional viruses, the primary DRG cultures were treated with 100 μM acyloguanosine (ACV) (Sigma) for 24 h and then infected with 5 × 104 PFU of virus in a total volume of 200 μl/well. The ACV treatment was continued for 7 days. ACV treatment was not required for the replication-defective vectors. NGF (100 ng/ml) was present in the media up to the time of infection but was not present following infection for both replication-conditional and -defective vectors.
Immunofluorescence detection of vector-mediated β-NGF.
Dissociated primary DRG cultures were fixed in 100% cold methanol for 2 min, air dried, washed once with 1× PBS, and incubated for 1 h in 1× PBS–horse serum (10% final concentration). After being rinsed in 1× PBS, the cultures were incubated with anti-NGF polyclonal serum (1:2,000; Chemichon) in 1× PBS–1% horse serum for 2 h, rinsed three times with 1× PBS, and incubated in biotin-labeled goat anti-rabbit secondary antibody (1:250; Sigma) in 1× PBS/1% horse serum for 1 h. Following three washes in 1× PBS, the samples were incubated with extra-avidin-fluorescein isothiocyanate (FITC) (1:500, Sigma) for 1 h, washed three times in 1× PBS for 10 min, and photographed with a Diaphot inverted microscope.
Hydrogen peroxide treatment of vector-infected primary DRG cultures.
Nondissociated primary DRG neuronal cultures isolated from E16 rat embryos were infected at a MOI of 10 with SHN, SLN, or the SN control viruses. Cultures were maintained in growth medium without NGF, except for the mock-infected control, which was supplemented with 100 μg of NGF per ml. At 3 or 14 days p.i., the cells were placed for 30 min in serum-free medium containing 1 mM hydrogen peroxide. After the peroxide treatment, the cells were washed extensively with normal medium, returned to normal medium plus serum for 24 to 72 h, and fixed with 100% cold methanol, and immunofluorescence was performed with either an NGF-specific polyclonal primary antibody (Chemichon) or a neurofilament (NF)-specific monoclonal primary antibody (Boehringer Mannheim) as above. NGF was detected with a biotin-conjugated goat anti-rabbit secondary antibody and extra-avidin-conjugated FITC as described above, while NF was detected with a Cy3-conjugated sheep anti-mouse secondary antibody (1:250; Sigma). Neutral red-stained samples were examined microscopically for the toxic effects of peroxide-induced neurite degeneration by determining the number of processes exceeding 0.25 mm in length.
In situ determination of apoptosis in hydrogen peroxide-treated, vector-infected primary DRG cultures.
Vector- or mock-infected peroxide-treated primary DRG cultures were examined for the number of apoptotic neuronal cell nuclei by terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) analysis with the fluorescein in situ death detection kit (Boehringer Mannheim). The cultures were fixed for 30 min in 4% paraformaldehyde and washed once in 1× PBS, and the cells and nuclei were permeabilized in 0.1% Triton X-100 (Boehringer Mannheim)–0.1% sodium citrate for 2 min on ice. Following two washes with 1× PBS, 50 μl of TUNEL reaction mixture was added to each well and the plates were incubated for 1 h at 37°C in a humidified chamber stored in the dark. The samples were then washed extensively (five times with 1× PBS), and the number of apoptotic nuclei per whole ganglion was counted under a Diaphot inverted microscope equipped with a fluorescent light source and filters for FITC.
Enzyme assays for catalase, SOD, and GSH-Px in hydrogen peroxide-treated vector-infected primary DRG cultures.
The levels of cellular enzymes involved in antioxidant injury were determined on cell lysates from either mock-infected (+100 μg of NGF per ml) or vector-infected primary DRG cultures following hydrogen peroxide treatment. Superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) activities were measured with the superoxide dismutase and cellular glutathione peroxidase assay kits (Calbiochem, San Diego, Calif.) under the conditions specified by the supplier. Catalase activity was determined by the assay procedure of Jackson et al. (47, 49) and compared to a calibration curve determined with mouse liver catalase (Sigma) as a standard. All enzymatic assays were performed in triplicate, and the numbers represent the mean of two experiments (n = 4).
In vivo analysis of HSV-1 vector-mediated NGF expression.
Female Swiss-Webster mice (6 weeks old; Harlan Sprague Dawley, Indianapolis, Ind.) were anesthetized with Metofane (Pitman-Moore, Mundelein, Ill.) and infected with 5 × 106 PFU of the various vectors following corneal scarification of both eyes. At the times p.i. corresponding to the lytic (3 days) and latent (28 days) states, the animals were sacrificed, the trigeminal ganglia (TG) were removed by microdissection, and samples from three animals were pooled and assayed in triplicate in an antigen capture ELISA for the presence of β-NGF. Separate TG samples were embedded in Cryo-Gel (Instrumedics, Inc., Hackensack, N.J.) and snap-frozen in dry ice-acetone, and 10-μm sections fixed in ice-cold acetone for 2 min were examined by immunocytochemistry with an anti-NGF polyclonal antiserum (1/2,000; Chemichon). NGF was detected with a biotin-conjugated goat anti-rabbit secondary antibody (1/250: Sigma) and extra-avidin-conjugated Cy3 (1/500: Sigma) as described above.
RESULTS
HSV-1 vector β-NGF transcription and protein production in vitro.
We constructed both replication-competent and -defective HSV-1 vectors expressing β-NGF (Fig. 1). The replication-competent vectors had either the tk (Fig. 1A to D) or Us3 (Fig. 1I to K) accessory genes deleted. The replication-defective vectors, with both tk and the essential IE gene ICP4 (Fig. 1E to H) deleted, were propagated on ICP4-complementing E5 cells (17). The recombinants contained β-NGF cDNA driven by either the strong HCMV IEp (Fig. 1C and G) or the HSV-1 LAP2 (Fig. 1H and K).
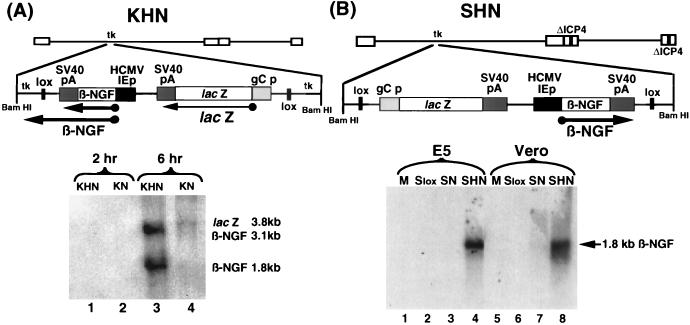
The HCMV IEp-NGF vector KHN produced NGF-specific mRNA after Northern blot analyses of RNA collected from infected cells with an NGF-specific probe; no signal was observed at 2 h after infection with either KHN or KN (Fig. 2A, lanes 1 and 2), but by 6 h p.i. a 1.8-kb β-NGF-specific mRNA was found in KHN-infected cells, along with a larger, 3.1-kb β-NGF-specific RNA (lane 3). The 3.1-kb mRNA represents a readthrough mRNA using the tk polyadenylation and cleavage signal (data not shown). The promoterless recombinant, KN, did not produce an NGF-specific mRNA (lane 4), but both viruses expressed low levels of lacZ mRNA (3.8 kb) at 6 h p.i., which was also detected on this blot because the gCp-lacZ cassette contained the SV40 poly(A) signal present within the NGF probe.
FIG. 2.
Expression of HSV-1 vector-mediated β-NGF transcripts in vitro. (A) Genomic organization of replication-competent virus recombinant KHN and expression in Vero cells. Vero cells were infected with either KHN or KN (MOI = 10). RNA was recovered from the infected cells at 2 and 6 h p.i. and subjected to Northern blot hybridization with a radiolabeled probe specific for β-NGF and SV40 poly(A) sequences. The locations of the 1.8- and 3.1-kb β-NGF-specific mRNAs, as well as the 3.8-kb lacZ-specific mRNA, are shown. (B) Structure of replication-defective SHN recombinant vector and vector-mediated expression in Vero cells. Vero cells or the ICP4-complementing cell line E5 (17) were either mock infected (M) or infected with Slox, SN, or SHN at MOI = 10. RNA was recovered at 2, 6, and 16 h p.i. and subjected to Northern blot analysis with a radiolabeled probe specific for β-NGF sequences. The location of the 1.8-kb β-NGF-specific mRNA is depicted.
Infection with the replication-defective SHN vector produced no detectable β-NGF transcript at 2 and 6 h p.i. (data not shown), but by 16 h p.i. a single band of 1.8 kb was observed in infected Vero and E5 cells (Fig. 2B, lanes 4 and 8), while the promoterless construct (SN) failed to express a β-NGF specific RNA (lanes 3 and 7). The amount of β-NGF RNA produced from infected E5 cells compared to infected Vero cells reflects the increased number of templates resulting from replication of viral DNA in the complementing cell line. Expression of NGF-specific mRNA was not dependent on viral replication since a similar band was detected in infected noncomplementing Vero cells.
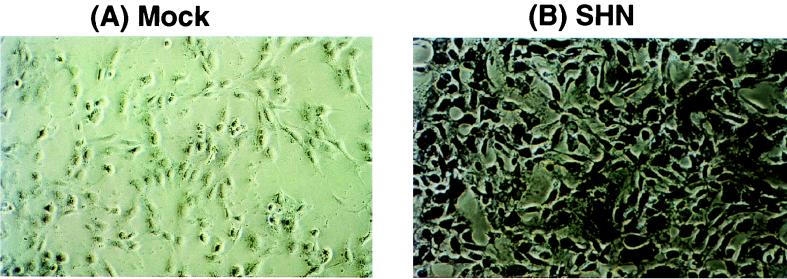
NGF was also detected by immunocytochemistry after infection of Vero cells at an MOI of 10. The replication-defective SHN vector expressed high levels of β-NGF product in most cells within the population (Fig. 3B), although the amount of NGF per cell varied substantially. Mock-infected cells (Fig. 3A) or cells infected with the promoterless vectors (SN and KN) failed to show expression of the transgene product. These results corroborated the Northern blot analysis of RNA expression (Fig. 2).
FIG. 3.
Expression of HSV-1 vector-mediated β-NGF immunoreactive protein in vitro. Vero cells were either mock infected (A) or infected (MOI = 10) with the SHN replication-defective expression vector (B). At various times p.i., cell monolayers were fixed with 4% paraformaldehyde, examined for β-NGF expression by immunohistochemistry with a polyclonal antibody specific for the protein (Chemichon), and incubated overnight at 25°C with alkaline phosphatase-labeled secondary antibody. The localization of the alkaline phosphatase product was detected with 5-bromo-4-chloro-3-indolylphosphate–nitroblue tetrazolium. This result displays β-NGF immunoreactive protein product within the cytoplasm at 16 h p.i. Magnification, ×40.
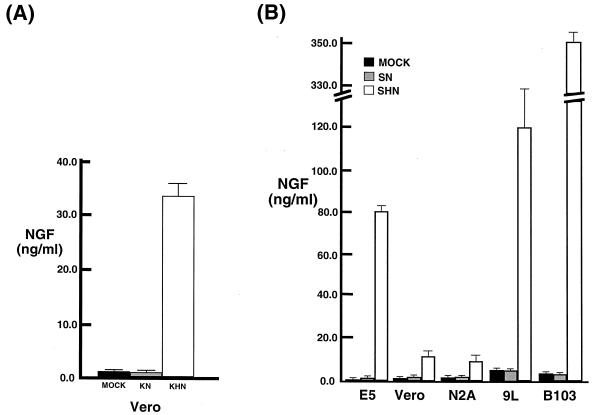
Antigen capture ELISA confirmed that β-NGF was secreted from the vector-transduced cells. Little β-NGF protein was detected at 2 and 6 h p.i. (data not shown), but 33.42 ng of β-NGF per ml was produced by KHN-infected Vero cells by 16 h p.i. (Fig. 4A), 79.09 ng of β-NGF per ml was produced by SHN-infected from ICP4-complementing E5 (17) cells (Fig. 4B), and 10.83 ng/ml was produced by SHN-infected Vero cells. The lower level of NGF produced by SHN infection of Vero cells was in agreement with the level of NGF-specific mRNA detected by Northern blot analysis (Fig. 2B). The promoterless recombinants failed to express NGF. Infection of rat neuroma (B103) and glioma (9L) cell lines with the SHN replication-defective vector resulted in the release of substantially more NGF into the medium (352.86 and 119.22 ng/ml, respectively). A similar pattern of NGF expression was detected in KHN-infected cells (data not shown). The amount of β-NGF mRNA detected in these cells was not appreciably greater than that found in SHN-infected Vero cells (data not shown), suggesting that enhanced release of the synthesized peptide occurred in these cell lines.
FIG. 4.
Level of HSV-1 vector-mediated β-NGF immunoreactive protein expression in vitro. Cell culture conditioned media from 106 Vero cells infected with the replication-competent NGF expression vectors (MOI = 10) (A) and 106 E5, Vero, mouse N2A neuroblastoma, rat 9L glioma, and rat B103 neuroma cells infected with the replication-defective NGF expression vectors (MOI = 10) (B) were collected at various times p.i. and centrifuged at 48,400 × g to remove cells and virus. The resulting supernatants were assayed in triplicate for β-NGF by an antigen capture ELISA (Boehringer Mannheim). The results (n = 3 to 7) are plotted as NGF concentration for mock-infected (MOCK), promoterless NGF vector (KN and SN)-infected, and NGF expression vector (KHN and SHN)-infected cell supernatants.
Bioactivity of vector-produced β-NGF.
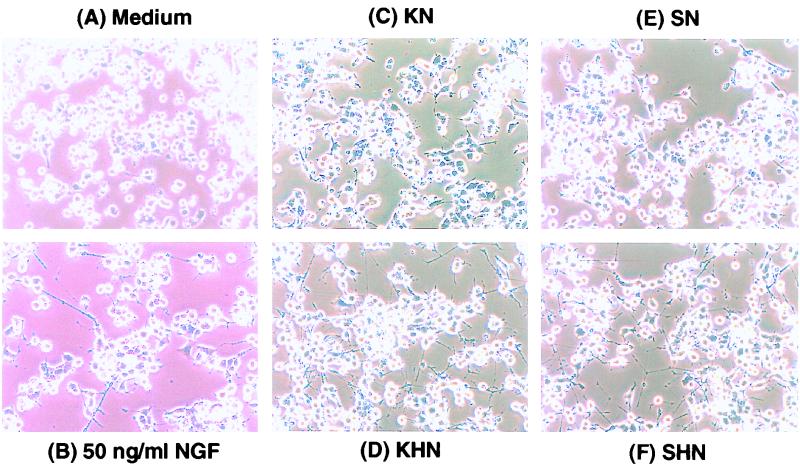
The biological activity of vector-mediated NGF production was assayed on PC-12 cells, which differentiate, sprout neurites, and assume a neuronal morphology in response to NGF (38–40). In agreement with the kinetics of NGF detection by ELISA, supernatants collected from KHN-infected or SHN-infected Vero cells at 16 h p.i. (Fig. 5D and F), but not 6 h p.i. (data not shown respectively), induced elaborate neurite formation. Supernatants from the KN-infected and SN-infected expression control vectors failed to induce neurite formation (Fig. 5C and E).
FIG. 5.
Detection of HSV-1 vector-mediated bioactive β-NGF with PC-12 cells. Biologically active NGF induces terminal differentiation of rat pheochromocytoma (PC-12) cells into neural cells, as shown by morphological changes and the development of extensive neurite sprouting. Cell culture conditioned media from Vero cells infected with the β-NGF expression vectors (MOI = 10) were collected at various times p.i. and centrifuged at 48,400 × g to remove cells and virus. The resulting supernatants were overlaid onto PC-12 cells. After 48 h, the PC-12 cells were observed for neurite formation. PC-12 cells treated for 48 h with supernatants from Vero cells infected with SHN (F) and KHN (D) harvested at 16 h p.i. display extensive neurite outgrowth. The effect of treatment of PC-12 cells with 50 ng of commercially available β-NGF per ml is also shown (B). The negative controls include supernatants from SN (E), KN (C), and PC-12 cells in normal medium (A) consisting of 10% horse serum and 5% FBS in high-glucose (4.5 g/liter) DMEM. Magnification, ×40.
HSV-1 vector-mediated NGF expression during in vitro latency.
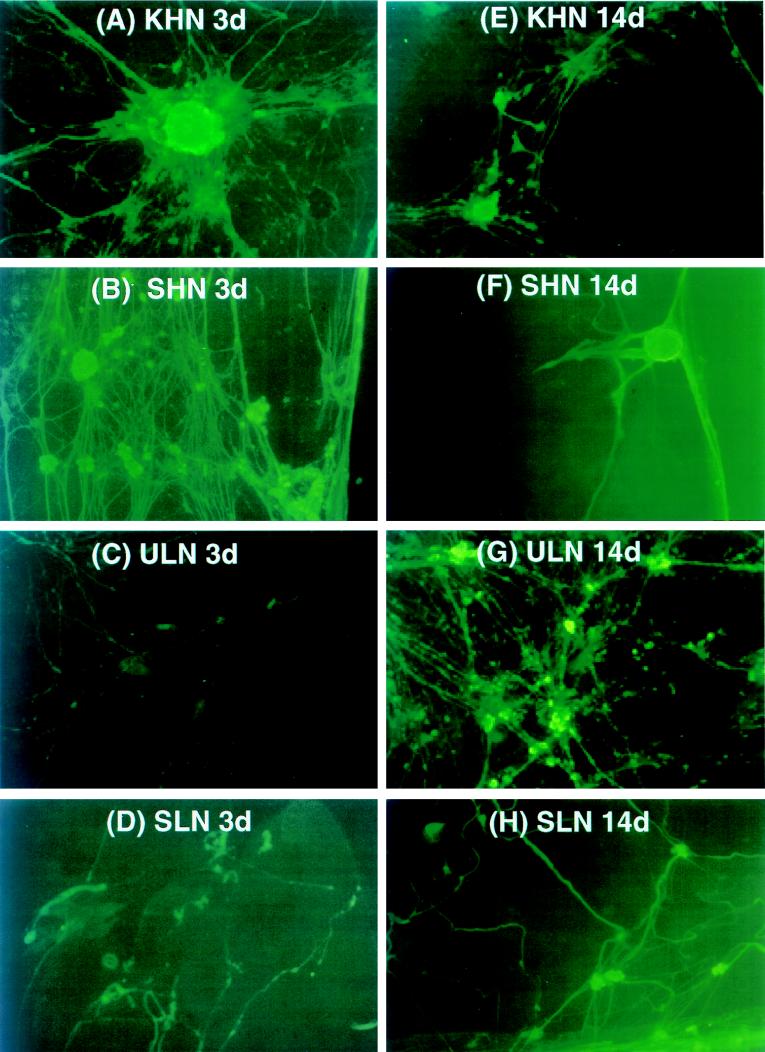
To assay the ability of the vectors to express NGF from the latent viral genome, we used an in vitro latency model established by Wilcox and Johnson (122, 123). Dissociated primary DRG cultures were infected with the various NGF expression vectors. Replication-conditional vector-infected DRG cultures were maintained in the presence of ACV for the first 7 days following infection, after which time ACV was removed and the medium was replaced every 2 days. At 3 and 14 days p.i., the cultures were fixed and NGF expression was determined by immunocytochemistry. KHN and SHN recombinants expressed high levels of NGF at 3 days p.i. in primary DRG cultures (Fig. 6A and B), in agreement with the results obtained following infection of Vero or E5 cells. By 14 days p.i., however, the amount of NGF produced was much lower and detectable in many fewer cells (Fig. 6E and F). In contrast, the ULN recombinant, in which LAP2 drives NGF expression, produced barely detectable amounts of NGF at 3 days p.i. (Fig. 6C), but high levels of NGF were detected at 14 days p.i. (Fig. 6G). The replication-defective recombinant SLN (Fig. 6D and H) displayed a similar expression pattern to ULN, although LAP2 appeared more active in expressing NGF at 3 days p.i. than did the same promoter in the Us3 locus in ULN. The promoterless vectors (SN and UN) failed to synthesize any immunodetectable product at either time point (data not shown), in agreement with the previous assays of NGF production for these control vectors.
FIG. 6.
Immunofluorescence detection of HSV-1 vector-mediated β-NGF immunoreactive protein in primary DRG cultures in vitro. Primary dissociated DRG cultures isolated from E16 rat embryos were either mock infected or infected (MOI = 10) with KHN (A and E) or ULN (C and G) in the presence of acyclovir (ACV) or with SHN (B and F) or SLN (D and H). At 3 days (A to D) and 14 days (E to H) p.i., cell monolayers on collagen-coated 12-well plates or 8-well glass slides were fixed and examined for β-NGF expression by immunofluorescence with a polyclonal antibody specific for the protein (Chemichon) and FITC-labeled secondary antibody. KHN (A) and SHN (B) expressed high levels of β-NGF at 3 days p.i., yet continued to express low levels even at 14 days p.i. (E and F). LAP2 (ULN and SLN) was effective in driving NGF expression during in vitro latency at 14 days p.i. (G and H). Magnification, ×40 for panels B to E, G, and H, and ×100 for panels A and F.
HSV-1 vector-mediated NGF expression protects DRG neurons from hydrogen peroxide-induced toxicity in culture.
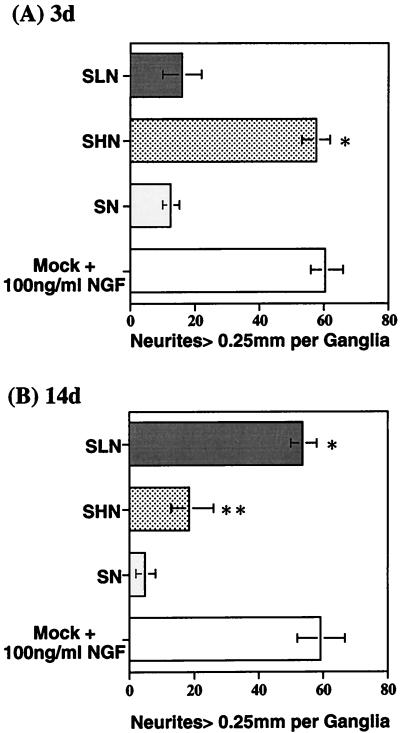
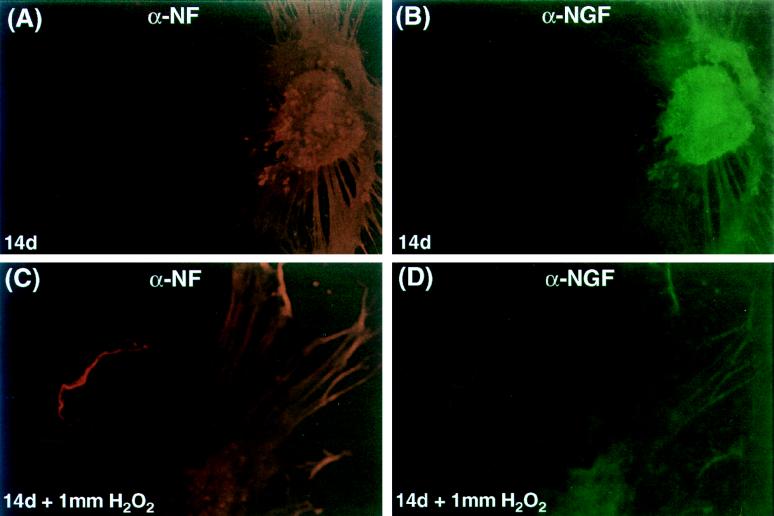
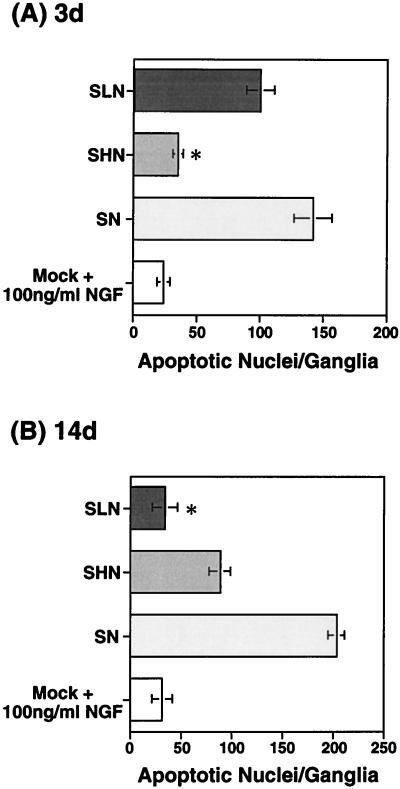
Hydrogen peroxide has previously been shown to induce apoptosis in PC-12 (47, 49, 51, 68, 89, 90) and neuroblastoma (70) cell lines through the production of reactive oxygen species. The ability of our NGF expression vectors to protect neurons from hydrogen peroxide excitotoxicity was evaluated in the primary DRG model. In these experiments, the replication-defective ICP4− mutant virus recombinants were used due to their reduced toxicity compared with the replication-competent KOS vectors. SHN, SLN, or SN vector-infected (MOI = 10) or mock-infected, NGF-supplemented (100 μg/ml) nondissociated primary DRG cultures were treated with 1 mM hydrogen peroxide for 30 min at either 3 or 14 days p.i. At 48 h posttreatment, the cultures were fixed with methanol and stained with neutral red or examined by immunocytochemistry with anti-NF antibody. The number of intact processes exceeding 0.25 mm in length per ganglion was used to quantitate the toxic effect of peroxide treatment, which is readily observable in nondissociated cultures. Infection with the HCMV IEp-NGF vector (SHN) resulted in an increased number of intact processes at 3 days (57.7 ± 4.2) compared to infection by the SN control vector (12.5 ± 2.5); the effect was similar to that observed with mock-infected NGF supplemented cultures (60.2 ± 5.7) (Fig. 7A). The vector expressing NGF from the viral latency promoter (SLN) did not protect DRG neurites from the toxic effects of hydrogen peroxide insult at 3 days p.i. (16.0 ± 6.0) (Fig. 7A) but was protective at 14 days p.i. (53.8 ± 4.2) compared to the SN control vector-infected neurons (5.0 ± 3.0) (Fig. 7B). However, the SHN vector failed to promote survival of the neuronal processes at 14 days (18.7 ± 7.3), in contrast to the mock-infected NGF-supplemented control (59.2 ± 7.6). Both NGF and NF were detected in SLN-infected primary DRG cultures at 14 days p.i. (Fig. 8), with extensive NF expression being detected in the DRG processes. Even after hydrogen peroxide treatment (1 mM for 30 min), both markers were visualized in the DRG neurons (Fig. 8), although the levels were decreased in degenerating neurites. Similar results were obtained in SHN vector-infected DRG cultures at 3 days p.i. or mock-infected NGF supplemented cultures at either 3 or 14 days p.i. (data not shown).
FIG. 7.
HSV-1 vector-mediated β-NGF protects primary DRG cultures from peroxide toxicity. Nondissociated primary DRG neuronal cultures isolated from E16 rat embryos were either mock infected or infected with SN, SHN, or SLN (MOI = 10). At 3 (A) and 14 (B) days p.i., wells containing intact DRGs were treated for 30 min with medium containing 1 mM hydrogen peroxide. Then the wells were washed extensively with normal medium and the cultures were placed in normal medium for 24 to 72 h. At this point, the cultures were fixed with 100% methanol, stained with neutral red, and examined microscopically for the toxic effects of peroxide-induced degeneration. The number of processes exceeding 0.25 mm in length per ganglion was determined, and the graphed data represent the mean and standard error of the mean of two experiments (n = 4 to 8). The SHN vector-mediated NGF protected the peroxide-treated DRG axonal processes at 3 days p.i. compared to the SN control vector (∗, P < 0.001 by Student’s t test). SLN vector-mediated NGF protected the peroxide-treated DRG axonal processes during in vitro latency at 14 days p.i., compared to the SN control vector (∗, P < 0.001, t test), which failed to express NGF. Some protection was seen in the SHN-infected cultures compared to the SN control (∗∗, P < 0.01, t test); however, the level of neurodegeneration was greater than that observed with SLN (∗∗∗, P < 0.005, t test).
FIG. 8.
Expression of β-NGF from the SLN vector correlates with protection of primary DRG cultures from peroxide toxicity. Nondissociated primary DRG neuronal cultures isolated from E16 rat embryos were either mock infected or infected (MOI = 10) with SLN. At 14 days p.i., wells containing intact DRGs were treated for 30 min with medium containing 1 mM hydrogen peroxide. Following the peroxide treatment, the wells were washed extensively with normal medium and placed in normal medium for 24 to 72 h. At that time, the cultures were fixed with methanol and examined for β-NGF expression by immunofluorescence with a rabbit polyclonal antibody specific for the protein (Chemichon) and FITC-labeled goat anti-rabbit secondary antibody and for NF expression with a mouse monoclonal antibody (Promega) and a Cy3-labeled donkey anti-mouse secondary antibody. The ability of these DRG neurons to express NF was determined to assess the viability of the cells during peroxide treatment. SLN vector-mediated NGF expression maintained the integrity of most DRG axonal processes; however, some are beginning to degenerate in the peroxide-treated cultures. Magnification, ×200.
We examined nondissociated, vector-infected, peroxide-treated DRG cultures for the number of apoptotic neuronal cell nuclei per whole ganglion by TUNEL analysis with the fluorescein in situ death detection kit. The HCMV IEp-NGF vector (SHN) protected the peroxide-treated DRG neurons from apoptosis at 3 days (35 ± 2 apoptotic nuclei/ganglion) compared to control vector-infected cells (142 ± 11 apoptotic nuclei/ganglion) or the SLN vector-infected cells (100 ± 6 apoptotic nuclei/ganglion), at levels similar to that observed in mock-infected neurons supplemented with NGF (24 ± 3 apoptotic nuclei/ganglion) (Fig. 9A). NGF expressed from the LAP2 vector (SLN) inhibited peroxide-induced DRG cell death at 14 days (34 ± 7 apoptotic nuclei/ganglion) compared to the control (SN) vector (204 ± 6 apoptotic nuclei/ganglion) (Fig. 9B). The level of protection achieved with the LAP2-NGF vector (SLN) was similar to that observed in the mock-infected, NGF-supplemented controls (31 ± 6 apoptotic nuclei/ganglion). The SHN vector displayed an intermediate level of protection (89 ± 5 apoptotic nuclei/ganglion), which may correlate with the low-level activity of the HCMV IEp observed in the DRG neuronal cultures at 14 days p.i. (Fig. 6E and F).
FIG. 9.
HSV vector-mediated NGF expression protects primary DRG neuronal cultures from peroxide-induced apoptotic cell death. Nondissociated primary DRG neuronal cultures isolated from E16 rat embryos were either mock infected or infected with SN, SHN, or SLN (MOI = 10). At 3 (A) and 14 (B) days p.i., wells containing intact DRGs were treated for 30 min with medium containing 1 mM hydrogen peroxide. Following the peroxide treatment, the wells were washed extensively with normal medium, and the cultures were placed in normal media for 24 to 72 h. Apoptotic cells were detected by a TUNEL assay with the in situ cell death detection kit. The number of FITC-labeled apoptotic neuronal cell nuclei per ganglion were determined, and the graphed data represent the mean and standard error of the mean of two experiments (n = n to 8). The SHN vector protected the peroxide-treated DRG from entering apoptosis at 3 days compared to the SN control vector (∗, P < 0.001, Student’s t test), similar to that observed in mock-infected neurons supplemented with 100 μg of NGF per ml. NGF expressed from the SLN vector blocked peroxide-induced DRG cell death compared to the SN control vector (∗, P < 0.001, t test), at 14 days. The level of protection achieved with the SLN vector was similar to that observed in the mock-infected, NGF-supplemented controls.
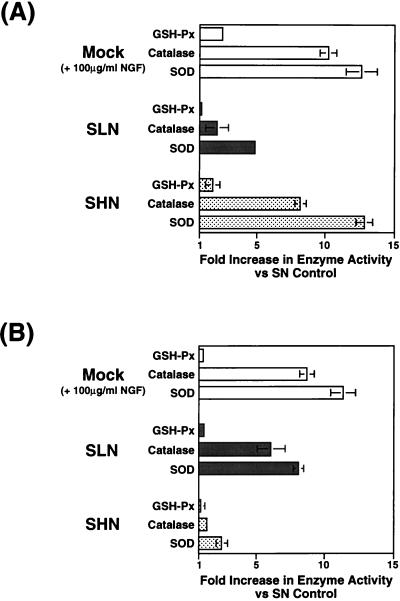
A number of antioxidant enzymes such as catalase, SOD, GSH-Px, and glutathione reductase (GSH-R), are generated in response to reactive oxygen species, and NGF has been shown to increase the levels of some or all of these enzymes in PC-12 cells (47–49, 51, 89, 90). We analyzed the levels of cellular enzymes involved in antioxidant injury from cell lysates of either mock-infected or vector-infected nondissociated primary DRG neuronal cultures following hydrogen peroxide treatment. SOD and GSH-Px activities were measured with the superoxide dismutase and cellular glutathione peroxidase assay kits, and catalase activity was determined by the assay procedure of Jackson et al. (47, 49). Expression of NGF from the HCMV IEp vector SHN produced a 12.8-fold increase in SOD and an 8.2-fold increase in catalase compared to the results with the control vector at 3 days p.i.; the GSH-Px levels increased only 1.8-fold (Fig. 10A). These results were similar to those observed in mock-infected, NGF-supplemented cultures at 3 days (SOD, 12.6-fold; catalase, 10.2-fold; GSH-Px, 2.5-fold) (Fig. 10A). LAP2-driven NGF expression from SLN resulted in only minimal induction of antioxidant enzyme levels at 3 days (SOD, 4.9-fold; catalase, 2.1-fold; GSH-Px, 1.0-fold), in agreement with the level of protection seen from this vector at the same time point (Fig. 7A and 9A) and the activity of LAP2 at this time point (Fig. 6C and D). LAP2-driven NGF expression resulted in an 8.1-fold induction of SOD and a 6.1-fold induction of catalase compared to that in the control vector-infected DRG neurons at 14 days (Fig. 10B) and was similar to that found in the NGF-supplemented controls (SOD, 11.3-fold; catalase, 8.7-fold). The levels of GSH-Px enzyme activity did not increase in either vector-infected or mock-infected NGF-supplemented cultures at 14 days in response to peroxide insult. These results support the notion that HSV vector-mediated NGF expression inhibits apoptosis in DRG neurons at least in part by stimulation of antioxidant enzymes involved in the scavenging of free radicals and other reactive oxygen species.
FIG. 10.
HSV vector-mediated NGF expression increases the levels of antioxidant enzymes in primary DRG neuronal cultures following peroxide treatment. Dissociated primary DRG neuronal cultures isolated from E16 rat embryos were either mock infected or infected with SN, SHN, or SLN (MOI = 10). At 3 and 14 days p.i., cultures were treated for 30 min with medium containing 1 mM hydrogen peroxide. Following the peroxide treatment, the wells were washed extensively with normal medium, and the cultures were placed in normal medium for 24 to 72 h. Cell lysates harvested from the wells were subjected to enzymatic assays for SOD, catalase, and GSH-Px, as described in Materials and Methods, and the fold increase in enzyme activity (units per milligram of protein) for each antioxidant enzyme with respect to control vector (SN)-infected DRG cultures is plotted. NGF expression from the SHN vector at 3 days or the SLN vector at 14 days resulted in increased levels of SOD and catalase.
HSV-1 vector-mediated NGF expression in mouse PNS in vivo.
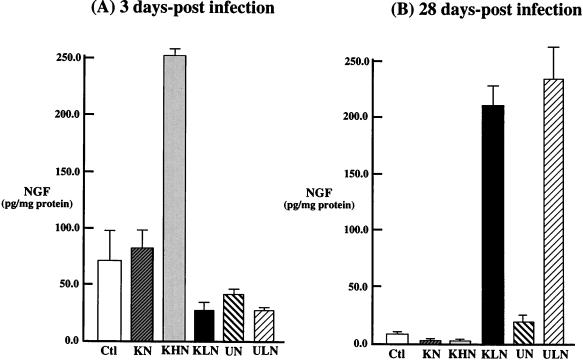
The ability of replication-competent HSV-1 vectors to express β-NGF was examined in vivo. Mice were infected with the β-NGF recombinants by topical corneal scarification. At 3 and 28 days p.i., the TG were removed and the level of β-NGF was determined by ELISA. Infection with the HCMV IE-NGF vector (KHN) produced high levels (252 pg/mg) of β-NGF in TG at 3 days p.i. (Fig. 11A), but NGF was barely detectable (3.1 pg/mg) at 28 days p.i., a time consistent with viral latency (Fig. 11B). This result was consistent with results of previous studies of the HCMV promoter in a variety of HSV-1 vectors (25, 34). In contrast, infection with the LAP-NGF vectors and KLN resulted in continued levels of NGF in TG at 3 days p.i., but substantial (ULN, 236 pg/mg; KLN, 208 pg/mg) amounts of NGF at 28 days p.i. (Fig. 11B). As with the in vitro assays, recombinants containing promoterless β-NGF cassettes (KN and UN) failed to synthesize measurable levels of β-NGF at either 3 days p.i. (83.5 and 43.0 pg/mg, respectively) or 28 days p.i. (4.0 and 17.0 pg/mg, respectively) compared to the control animals (70.0 pg/mg at 3 days p.i. and 7.2 pg at 28 days p.i.), which had an equal volume (5 μl) of media containing 10% FBS added to each eye following corneal scarification.
FIG. 11.
Levels of HSV vector-mediated expression of β-NGF immunoreactive protein in vivo. Following topical corneal inoculation of mice with 5 × 106 PFU of the β-NGF expression vectors containing either the strong HCMV IEp (KHN) or HSV-1 LAP2 (KLN and ULN) driving NGF, animals were sacrificed at 3 (A) and 28 (B) days p.i., and the TG were removed by microdissection and analyzed in triplicate for β-NGF by ELISA. The results (n = 3) are plotted as picograms of NGF per milligram of total protein for both the expression vectors (HCMV IEp [KHN] and LAP2 [KLN and ULN]) and mock-infected (MOCK) animals and the promoterless control vectors (KN and UN).
DISCUSSION
The studies presented in this report document that recombinant genomic HSV-1 β-NGF expression vectors produced biologically active NGF. In vitro, transgene expression was demonstrated at the level of RNA by Northern blotting and at the level of protein by immunocytochemistry and ELISA. The biological activity of the transgene product was demonstrated by the standard assay of neurite extension in PC-12 cells and by the ability of transduced cells to survive hydrogen peroxide insult measured by neurite survival, prevention of apoptosis, and induction of SOD and catalase activity in primary DRG neurons in culture. The HCMV IEp-NGF expression cassette produced NGF expression in Vero and E5 cells, whether placed in the context of a replication-competent or a replication-defective vector. However, in primary DRG neurons in culture, expression driven by this promoter was high at 3 days but fell to low levels by 14 days. In contrast, the LAP2-NGF expression cassette produced only low levels of NGF expression at 3 days but induced robust transgene expression at 14 days in vitro. The ability of NGF to abrogate peroxide-induced toxicity reflected the same time course as the other measures of NGF synthesis. Similar results were obtained in vivo following infection of TG by corneal scarification, where the HCMV IEp-NGF vectors produced NGF at 3 but not 28 days p.i. whereas the LAP2-NGF vectors produced NGF at 28 but not 3 days p.i. The differential time course of expression by these two promoters is consistent with the HCMV promoter functioning during the pre-latent phase (3 days) of infection and LAP2 functioning during latency (28 days).
Release of NGF from infected cells.
The amount of NGF produced in vitro by our vectors was comparable to that reported following transduction with retroviral (30, 62, 84, 85, 92, 98, 115), adenovirus (6), or HSV (33, 116) vectors, although the amount of sprouting induced by exposure to media from HCMV IEp-NGF-infected vector cells was greater than that previously reported with either HSV amplicon (33) or genomic (116) vectors, reflecting the strength of this promoter. Rat neuroma (B103) and glioma (9L) cells infected by SHN released substantially more NGF into the medium than did similarly infected Vero cells, although the amount of β-NGF-specific mRNA was similar in all three infected cell lines. Native NGF is secreted in a regulated pathway, by sorting into secretory organelles that are targeted to specific cellular destinations. A family of acidic proteins known as secretogramins act as chaperones to sort proteins into the regulated (as opposed to constitutive) secretory pathway. Two members of the secretogranin family, chromogranin B and secretogranin II, have been shown to affect NGF secretion in AkT-20 neuroendocrine cells (96). It is likely that these or related secretogranins are responsible for the increased level of secretion in vector-infected 9L and B103 cells, compared to Vero cells.
NGF and HSV.
Although NGF is a neurotrophic factor essential for the development and survival of sensory and sympathetic neurons, it has been demonstrated to play a role in HSV latency both in vitro and in vivo. NGF is required to establish and maintain in vitro latency in both PC-12 cells (8) or primary DRG or SCG neurons (100, 121–124), and disruption of the NGF-TrkA signaling pathway with anti-NGF antibodies results in virus reactivation (100). These antibodies have been shown to have a similar effect in vivo in both mouse (58) and rabbit (46) latency models. We have been not able to determine the effects of vector-mediated NGF expression on maintenance of latency by using our recombinant vectors, since either the replication-defective mutants or the Us3 and tk replication-competent recombinants are incapable of reactivation (15, 26, 56, 72, 117). Administration of NGF via an Alzat pump to rats receiving stereotactic inoculation of either wild-type HSV (77) or HSV amplicon vector (78) resulted in reduced toxicity from the virus yet did not affect the number of infected neurons. This suggests that NGF may have neuroprotective effects against HSV toxicity, which we have observed in the DRG neuronal culture experiments and are now pursuing in other analyses.
NGF expression has a repressive effect on HSV lytic gene expression in neurons (14) which may in part be due to the upregulation of the Oct-2 transcription factor in DRG neuronal cultures (127) that has been proposed to down-regulate HSV IE gene expression (61, 119). In contrast, NGF expression has a positive effect on LAT expression in PC-12 cells (27), and an NGF responsive element has been mapped to a specific region of LAP1. Since our current promoter expression constructs lack LAP1, the potential role of vector-mediated NGF in stimulating its own synthesis has not been explored.
LAP2-driven transgene expression.
The most important finding of this study related to the kinetics of transgene expression in neurons by the HCMV IEp and the LAP2 promoters. The most attractive reasons for development of HSV vectors for nervous system applications concerns the ability of the wild-type virus to establish a lifelong latent state in neurons, in which the genome is transcriptionally silent except for the production of a family of LATs. We have previously shown that in vivo the HCMV IEp is only transiently active in neurons of either the CNS or PNS (25, 34), with expression peaking between 2 and 4 days p.i. A similar time course of expression is found with HSV lytic-cycle gene promoters, other viral promoters, or mammalian promoter elements placed in the HSV genome to drive transgene expression. In contrast, we have previously shown that the LAP2 sequence alone is capable of driving reporter-gene (lacZ) expression in TG neurons in vivo up to 300 days p.i. (36). This finding is surprising considering that LAP2 can be deleted from the virus genome without substantially compromising LAT expression during latency (11). Moreover, LAP2 in the absence of LAP1 can express LAT from the native locus but in substantially reduced amounts (11). In contrast to these observations, LAP1 alone, either in the native LAT loci (21, 67, 126) or replaced ectopically in the HSV gC locus (63), fails to produce prolonged transgene expression, and the reintroduction of LAP2 sequences to the LAP1 transgene cassette restores long-term transgene expression (64). These findings suggest that LAP1 in the native locus behaves differently and does not require LAP2 sequences. The results of the present study confirm that LAP2 alone, in an ectopic locus, functions effectively to drive transgene expression with a time course consistent with viral latency. This appears to be true whether the virus is capable or incapable of active replication. Delayed but prolonged transgene expression may be useful for therapeutic applications, particularly for diseases of the PNS.
Summary.
Together, our results demonstrate that replication-defective genomic HSV vectors are capable of either transient high-level NGF expression or long-term expression in neurons, depending on the promoter used to drive transgene expression. In addition, HSV vector-mediated NGF expression can protect neurons from apoptotic cell death, demonstrating the therapeutic efficacy of these vectors. NGF administration is neuroprotective to peripheral neurons in diabetic neuropathy (1, 2, 19, 45, 113), cisplatinin-induced neuropathy (3, 41, 91, 125), diabetic cystopathy (20, 106), and following sciatic nerve injury (80, 101). The ability of vector-transduced NGF production to affect neuronal survival in vivo following injury remains to be determined. We are currently testing these vectors in a variety of models of peripheral nerve injury. Although clinical trials with neurotropins (NGF and CNTF) have failed to be effective in preventing neuronal cell loss (4, 10, 71), the failure involved the inability of the neurotropin to be efficiently delivered to the cells which require it and from the unwanted side effects of expression in cells that are not the target. HSV vectors such as those described in this study could be used to specifically express the neurotropin in the target cells in the PNS for the treatment of peripheral neurodegenerative disease. For some applications, such as neuronal injury resulting from trauma, transient expression of the neurotropin from promoters such as the strong HCMV IEp might be required, but for applications requiring continuous long-term expression, prolonged expression driven by the LAP2 may prove useful.
ACKNOWLEDGMENTS
We thank William Rutter for providing the NGF cDNA and Mark Stinski for the HCMV IE promoter clone; Neal DeLuca for the ICP4 mutant and complementing cell line; Michelle Pike-Cavalcoli, Johnny Huard, and Ted Kaplan for excellent technical assistance; and Steve Wilson, Steve Phillips, Johnny Huard, Tom Holland, and Darren Wolfe for helpful discussions.
This work was supported by Public Health Service grants GM34534 (to J.C.G.), AG0947001 (to J.C.G. and D.J.F.), and NS19608 (to J.C.G.).
REFERENCES
- 1.Anand P, Terenghi G, Warner G, Kopelman P, Williams-Chestnut R E, Sinicropi D V. The role of endogenous nerve growth factor in human diabetic neuropathy. Nat Med. 1996;2:703–707. doi: 10.1038/nm0696-703. [DOI] [PubMed] [Google Scholar]
- 2.Apfel S C, Arezzo J C, Brownlee M, Federoff H, Kessler J A. Nerve growth factor administration protects against experimental diabetic sensory neuropathy. Brain Res. 1994;634:7–12. doi: 10.1016/0006-8993(94)90252-6. [DOI] [PubMed] [Google Scholar]
- 3.Apfel S C, Arezzo J C, Lipson L, Kessler J A. Nerve growth factor prevents experimental cisplatin neuropathy. Ann Neurol. 1992;31:76–80. doi: 10.1002/ana.410310114. [DOI] [PubMed] [Google Scholar]
- 4.Apfel S C, Kessler J A. Neurotrophic factors in the therapy of peripheral neuropathy. Baillieres Clin Neurol. 1995;4:593–606. [PubMed] [Google Scholar]
- 5.Batchelor A H, O’Hare P O. Regulation and cell-type-specific activity of a promoter located upstream of the latency-associated transcript of herpes simplex virus type 1. J Virol. 1990;64:3269–3279. doi: 10.1128/jvi.64.7.3269-3279.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baumgartner B, Shine H. Targeted transduction of CNS neurons with adenoviral vectors carrying neurotrophic factor genes confers neuroprotection that exceeds the transduced population. J Neurosci. 1997;17:6504–6511. doi: 10.1523/JNEUROSCI.17-17-06504.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bilang-Bleuel A, Revah F, Colin P, Locquet I, Robert J-J, Mallet J, Horellou P. Intrastriatal injection of an adenoviral vector expressing glial-cell-line-derived neurotrophic factor prevents dopaminergic neuron degeneration and behavioral impairment in a rat model of Parkinson disease. Proc Natl Acad Sci USA. 1997;94:8818–8823. doi: 10.1073/pnas.94.16.8818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Block T, Barney S M, Maggioncalda J, J, Valyi-Nagy T, Fraser N W. Long term herpes simplex virus type 1 infection of nerve growth factor-treated PC12 cells. J Gen Virol. 1994;75:2481–2487. doi: 10.1099/0022-1317-75-9-2481. [DOI] [PubMed] [Google Scholar]
- 9.Butcher L L, Woolf N J. Neurotrophic agents may exacerbate the pathologic cascade of Alzheimer’s disease. Neurobiol Aging. 1989;10:557–570. doi: 10.1016/0197-4580(89)90130-9. [DOI] [PubMed] [Google Scholar]
- 10.Cedarbaum J M, Stambler N. Performance of the amyotrophic lateral sclerosis functional rating scale (ALSFRS) in multicenter clinical trials. J Neurol Sci. 1997;152:S1–S9. doi: 10.1016/s0022-510x(97)00237-2. [DOI] [PubMed] [Google Scholar]
- 11.Chen X, Schmidt M C, Goins W F, Glorioso J C. Two herpes simplex virus type-1 latency active promoters differ in their contribution to latency-associated transcript expression during lytic and latent infection. J Virol. 1995;69:7899–7908. doi: 10.1128/jvi.69.12.7899-7908.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi-Lundberg D, Lin Q, Chang Y-N, Chiang Y-L, Hay C, Mohajeri H, Davidson B, Bohn M. Dopaminergic neurons protected from degeneration by GDNF gene therapy. Science. 1996;275:838–841. doi: 10.1126/science.275.5301.838. [DOI] [PubMed] [Google Scholar]
- 13.Clegg D O. Characterization of a beta-nerve growth factor expression vector for mammalian cells. Gene. 1993;25:291–296. doi: 10.1016/0378-1119(93)90434-5. [DOI] [PubMed] [Google Scholar]
- 14.Clements G B, Kennedy P G E. Modulation of herpes simplex virus (HSV) infection of cultured neuronal cells by nerve growth factor and antibody to HSV. Brain. 1989;112:1277–1294. doi: 10.1093/brain/112.5.1277. [DOI] [PubMed] [Google Scholar]
- 15.Coen D M, Kosz-Venchak M, Jacobson J G, Leib D A, Bogard C L, Schaffer P A, Tyler K L, Knipe D M. Thymidine kinase-negative herpes simplex virus mutants establish latency in mouse trigeminal ganglia but do not reactivate. Proc Natl Acad Sci USA. 1989;86:4736–4740. doi: 10.1073/pnas.86.12.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Croen K D, Ostrove J M, Dragovic L J, Smialek J E, Straus S E. Latent herpes simplex virus in human trigeminal ganlia. Detection of an immediate early gene “anti-sense” transcript by in situ hybridization. N Engl J Med. 1987;317:1427–1432. doi: 10.1056/NEJM198712033172302. [DOI] [PubMed] [Google Scholar]
- 17.DeLuca N A, McCarthy A M, Schaffer P A. Isolation and characterization of deletion mutants of herpes simplex virus type 1 in the gene encoding immediate-early regulatory protein ICP4. J Virol. 1985;56:558–570. doi: 10.1128/jvi.56.2.558-570.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deshmane S L, Fraser N W. During latency, herpes simplex virus type 1 DNA is associated with nucleosomes in a chromatin structure. J Virol. 1989;63:943–947. doi: 10.1128/jvi.63.2.943-947.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diemel L T, Brewster W J, Fernyhough P, Tomlinson D R. Expression of neuropeptides in experimental diabetes: effects of treatment with nerve growth factor or brain-derived neurotrophic factor. Brain Res Mol Brain Res. 1994;21:171–175. doi: 10.1016/0169-328x(94)90391-3. [DOI] [PubMed] [Google Scholar]
- 20.Dmitrieva N, Shelton D, Rice A S, McMahon S B. The role of nerve growth factor in a model of visceral inflammation. Neuroscience. 1997;78:449–459. doi: 10.1016/s0306-4522(96)00575-1. [DOI] [PubMed] [Google Scholar]
- 21.Dobson A T, Sederati F, Devi-Rao G, Flanagan W M, Farrell M J, Stevens J G, Wagner E K, Feldman L T. Identification of the latency-associated transcript promoter by expression of rabbit β-globin mRNA in mouse sensory nerve ganglia latently infected with a recombinant herpes simplex virus. J Virol. 1989;63:3844–3851. doi: 10.1128/jvi.63.9.3844-3851.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edwards R H, Selby M J, Garcia P D, Rutter W J. Processing of the native nerve growth factor precursor to form biologically active nerve growth factor. J Biol Chem. 1988;263:6810–6815. [PubMed] [Google Scholar]
- 23.Efstathiou S, Minson A, Field H, Anderson J, Wildy P. Detection of herpes simplex virus-specific DNA sequences in latently infected mice and in human. J Virol. 1986;57:446–455. doi: 10.1128/jvi.57.2.446-455.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fink D, DeLuca N, Goins W, Glorioso J. Gene transfer to neurons using herpes simplex virus-based vectors. Annu Rev Neurosci. 1996a;19:265–287. doi: 10.1146/annurev.ne.19.030196.001405. [DOI] [PubMed] [Google Scholar]
- 25.Fink D J, Ramakrishnan R, Marconi P, Goins W F, Holland T C, Glorioso J C. Advances in the development of herpes simplex virus-based gene transfer vectors for the nervous system. Clin Neurosci. 1996b;3:1–8. [PubMed] [Google Scholar]
- 26.Fink D J, Sternberg L R, Weber P C, Mata M, Goins W F, Glorioso J C. In vivo expression of β-galactosidase in hippocampal neurons by HSV-mediated gene transfer. Hum Gene Ther. 1992;3:11–19. doi: 10.1089/hum.1992.3.1-11. [DOI] [PubMed] [Google Scholar]
- 27.Frazier D, Cox D, Godshalk E, Schaffer P. Identification of cis-acting sequences in the promoter of the herpes simplex virus type 1 latency-associated transcripts required for activation by nerve growth factor and sodium butyrate in PC12 cells. J Virol. 1996;70:7433–7444. doi: 10.1128/jvi.70.11.7433-7444.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friden P M, Walus L R, Watson P, Doctrow S R, Kozarich J W, Backman C, Bergman H, Hoffer B, Bloom F, Granholm A-C. Blood-brain barrier penetration and in vivo activity of NGF conjugate. Science. 1993;259:373–377. doi: 10.1126/science.8420006. [DOI] [PubMed] [Google Scholar]
- 29.Frim D M, Short M P, Rosenberg W S, Simpson J, Breakefield X O, Isacson O. Local protective effects of nerve growth factor-secreting fibroblasts against excitotoxic lesions in the rat striatum. J Neurosurg. 1993;78:267–273. doi: 10.3171/jns.1993.78.2.0267. [DOI] [PubMed] [Google Scholar]
- 30.Frim D M, Simpson J, Uhler T A, Short M P, Bossi S R, Breakefield X O, Isacson O. Striatal degeneration induced by mitochondrial blockade is prevented by biologically delivered NGF. J Neurosci Res. 1993;35:452–458. doi: 10.1002/jnr.490350413. [DOI] [PubMed] [Google Scholar]
- 31.Frim D M, Uhler T A, Short M P, Ezzedine Z D, Klagsbrun M, Breakefield X O, Isacson O. Effects of biologically delivered NGF, BDNF and bFGF on striatal excitotoxic lesions. NeuroReport. 1993;4:367–370. doi: 10.1097/00001756-199304000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Gage F H, Wolff J A, Rosenberg M B, Xu L, Yee J-K, Shults C, Firedmann T. Grafting genetically modified cells to the brain: possibilities for the future. Neuroscience. 1987;23:795–807. doi: 10.1016/0306-4522(87)90159-x. [DOI] [PubMed] [Google Scholar]
- 33.Geschwind M, Kessler J, Geller A, Federoff H. Transfer of the nerve growth factor gene into cell lines and cultured neurons using a defective herpes simplex virus vector: transfer to the NGF gene into cells by a HSV-1 vector. Brain Res. 1994;24:327–335. doi: 10.1016/0169-328x(94)90146-5. [DOI] [PubMed] [Google Scholar]
- 34.Glorioso J C, DeLuca N A, Fink D J. Development and application of herpes simplex virus vectors for human gene therapy. Annu Rev Microbiol. 1995;49:675–710. doi: 10.1146/annurev.mi.49.100195.003331. [DOI] [PubMed] [Google Scholar]
- 35.Goins W F, Marconi P, Krisky D, Ramakrishnan R, Fink D J, Glorioso J C. Construction of replication defective herpes simplex virus vectors. Curr Protocols Hum Genet. 1996;12:2.1–2.29. doi: 10.1002/0471142905.hg1211s33. [DOI] [PubMed] [Google Scholar]
- 36.Goins W F, Sternberg L R, Croen K D, Krause P R, Hendricks R L, Fink D J, Straus S E, Levine M, Glorioso J C. A novel latency-active promoter is contained within the herpes simplex virus type 1 UL flanking repeats. J Virol. 1994;68:2239–2252. doi: 10.1128/jvi.68.4.2239-2252.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Granholm A-C, Backman C, Bloom F, Ebendal T, Gerhardt G A, Hoffer B, Mackerlova L, Olson L, Soderstrom S, Walus L R, Friden P M. NGF and anti-transferrin receptor antibody conjugate: short- and long-term effects on survival of cholinergic neurons in intraocular septal transplants. J Pharmacol Exp Ther. 1994;268:448–459. [PubMed] [Google Scholar]
- 38.Greene L A. Nerve growth factor prevents the death and stimulates the neuronal differentiation of clonal PC12 pheochromocytoma cells in serum-free medium. J Cell Biol. 1978;78:747–755. doi: 10.1083/jcb.78.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greene L A, Aletta J M, Rukenstein A, Green S H. PC12 pheochromocytoma cells: culture, nerve growth factor treatment, and experimental exploitation. Methods Enzymol. 1987;147:207–216. doi: 10.1016/0076-6879(87)47111-5. [DOI] [PubMed] [Google Scholar]
- 40.Greene L A, Tischler A S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci USA. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hayakawa K, Sobue G, Itoh T, Mitsuma T. Nerve growth factor prevents neurotoxic effects of cisplatin, vincristine and taxol, on adult rat sympathetic ganglion explants in vitro. Life Sci. 1994;55:519–525. doi: 10.1016/0024-3205(94)00744-6. [DOI] [PubMed] [Google Scholar]
- 42.Hefti F, Hartikka J, Knusel B. Function of neurotrophic factors in the adult and aging brain and their possible use in the treatment of neurodegenerative diseases. Neurobiol Aging. 1989;10:515–533. doi: 10.1016/0197-4580(89)90118-8. [DOI] [PubMed] [Google Scholar]
- 43.Hefti F, Schneider L S. Nerve growth factor and Alzheimer’s disease. Clin Neuropharmacol. 1991;14:S62–S76. doi: 10.1097/00002826-199114001-00008. [DOI] [PubMed] [Google Scholar]
- 44.Hefti F, Weiner W J. Nerve growth factor and Alzheimer’s disease. Ann Neurol. 1986;20:275–281. doi: 10.1002/ana.410200302. [DOI] [PubMed] [Google Scholar]
- 45.Hellweg R, Jockers-Scherubl M. Neurotrophic factors in memory disorders. Life Sci. 1994;55:2165–2169. doi: 10.1016/0024-3205(94)00397-1. [DOI] [PubMed] [Google Scholar]
- 46.Hill J M, Garza Jr H H, Helmy M F, Cook S D, Osborne P A, Johnson E M, Jr, Thompson H W, Green L C, O’Callaghan R J, Gebhardt B M. Nerve growth factor antibody stimulates reactivation of ocular herpes simplex virus type 1 in latently infected rabbits. J Neurovirol. 1997;3:206–211. doi: 10.3109/13550289709018295. [DOI] [PubMed] [Google Scholar]
- 47.Jackson G R, Apffel L, Werrbach-Perez K, Perez-Polo J R. Role of nerve growth factor in oxidant-antioxidant balance and neuronal injury. I. Stimulation of hydrogen peroxide resistance. J Neurosci Res. 1990;25:360–368. doi: 10.1002/jnr.490250313. [DOI] [PubMed] [Google Scholar]
- 48.Jackson G R, Sampath D, Werrbach-Perez K, Perez-Polo J R. Effects of nerve growth factor on catalase and glutathione peroxidase in a hydrogen peroxide-resistant pheochromocytoma subclone. Brain Res. 1994;634:69–76. doi: 10.1016/0006-8993(94)90259-3. [DOI] [PubMed] [Google Scholar]
- 49.Jackson G R, Werrbach-Perez K, Perez-Polo J R. Role of nerve growth factor in oxidant-antioxidant balance and neuronal injury. II. A conditioning lesion paradigm. J Neurosci Res. 1990;25:369–374. doi: 10.1002/jnr.490250314. [DOI] [PubMed] [Google Scholar]
- 50.Johnson M, Argiro V. Techniques in the tissue culture of rat sympathetic neurons. Methods Enzymol. 1983;103:334–347. doi: 10.1016/s0076-6879(83)03022-0. [DOI] [PubMed] [Google Scholar]
- 51.Kamata H, Tanaka C, Yagisawa H, Hirata H. Nerve growth factor and forskolin prevent H2O2-induced apoptosis in PC12 cells by glutathione independent mechanism. Neurosci Lett. 1996;212:179–182. doi: 10.1016/0304-3940(96)12806-8. [DOI] [PubMed] [Google Scholar]
- 52.Kleitman N, Woof P, Bunge R. Tissue culture methods for the study of myelination. Cambridge, Mass: MIT Press; 1992. [Google Scholar]
- 53.Kordower J H, Charles V, Bayer R, Bartus R T, Putney S, Walus L R, Friden P M. Intravenous administration of a transferrin receptor antibody-nerve growth factor conjugate prevents the degeneration of cholinergic striatal neurons in a model of Huntington disease. Proc Natl Acad Sci USA. 1994;91:9077–9080. doi: 10.1073/pnas.91.19.9077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kordower J H, Mufson E J, Granholm A-C, Hoffer B, Friden P M. Delivery of trophic factors to the primate brain. Exp Neurol. 1993;124:21–30. doi: 10.1006/exnr.1993.1169. [DOI] [PubMed] [Google Scholar]
- 55.Korsching S, Heumann R, Thoenen H, Hefti F. Cholinergic denervation of the rat hippocampus by fimbrial transection leads to a transient accumulation of nerve growth factor (NGF) without change in NGF mRNA content. Neurosci Lett. 1986;15:175–180. doi: 10.1016/0304-3940(86)90186-2. [DOI] [PubMed] [Google Scholar]
- 56.Kosz-Vnenchak M, Coen D, Knipe D. Restricted expression of herpes simplex virus lytic genes during establishment of latent infection by thymidine kinase-negative mutant viruses. J Virol. 1990;64:5396–5402. doi: 10.1128/jvi.64.11.5396-5402.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Krisky, D. M., D. Wolfe, W. F. Goins, P. C. Marconi, R. Ramakrishnan, M. Mata, R. J. D. Rouse, D. J. Fink, and J. C. Glorioso. Deletion of multiple immediate early genes from herpes simplex virus reduces cytotoxicity and permits long-term gene expression in neurons. Gene Ther., in press. [DOI] [PubMed]
- 58.Laycock K A, Brady R H, Lee S F, Osborne P A, Johnson E M, Pepose J S. The role of nerve growth factor in modulating herpes simplex virus reactivation in vivo. Graefe’s Arch Clin Exp Ophthalmol. 1994;232:421–425. doi: 10.1007/BF00186584. [DOI] [PubMed] [Google Scholar]
- 59.Levi-Montalcini R, Aloe L, Mugnaini E, Oesch F, Thoenen H. Nerve growth factor induces volume increase and enhances tyrosine hydroxylase synthesis in chemically axotomized sympathetic ganglia of newborn rats. Proc Natl Acad Sci USA. 1975;72:595–599. doi: 10.1073/pnas.72.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Levi-Montalcini R, Caramia F, Luse S A, Angeletti P U. In vitro effects of the nerve growth factor on the fine structure of the sensory nerve cells. Brain Res. 1968;8:347–362. doi: 10.1016/0006-8993(68)90054-1. [DOI] [PubMed] [Google Scholar]
- 61.Lillycrop K A, Dent C L, Wheatley S C, Beech M N, Ninkina N N, Wood J N, Latchman D S. The octamer-binding protein Oct-2 represses HSV immediate-early genes in cell lines derived from latently infectable sensory neurons. Neuron. 1991;7:381–390. doi: 10.1016/0896-6273(91)90290-g. [DOI] [PubMed] [Google Scholar]
- 62.Lin Q, Cunningham L A, Epstein L G, Pechan P A, Short M P, Fleet C, Bohn M C. Human fetal astrocytes as an ex vivo gene therapy vehicle for delivering biologically active nerve growth factor. Hum Gene Ther. 1997;8:331–339. doi: 10.1089/hum.1997.8.3-331. [DOI] [PubMed] [Google Scholar]
- 63.Lokensgard J R, Bloom D C, Dobson A T, Feldman L T. Long-term promoter activity during herpes simplex virus latency. J Virol. 1994;68:7148–7158. doi: 10.1128/jvi.68.11.7148-7158.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lokensgard J R, Feldman L T, Berthomme H. The latency-associated promoter of herpes simplex virus type 1 requires a region downstream of the transcription start site for long-term expression during latency. J Virol. 1997;71:6714–6719. doi: 10.1128/jvi.71.9.6714-6719.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lowry O H, Rosenbrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 66.Marconi P, Krisky D, Oligino T, Poliani P L, Ramakrishnan R, Goins W F, Fink D J, Glorioso J C. Replication-defective HSV vectors for gene transfer in vivo. Proc Natl Acad Sci USA. 1996;93:11319–11320. doi: 10.1073/pnas.93.21.11319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Margolis T P, Bloom D C, Dobson A T, Feldman L T, Stevens J G. Decreased reporter gene expression during latent infection with HSV LAT promoter constructs. Virology. 1993;197:585–592. doi: 10.1006/viro.1993.1632. [DOI] [PubMed] [Google Scholar]
- 68.Maroto R, Perez-Polo J R. BCL-2-related protein expression in apoptosis: oxidative stress versus serum deprivation in PC12 cells. J Neurochem. 1997;69:514–523. doi: 10.1046/j.1471-4159.1997.69020514.x. [DOI] [PubMed] [Google Scholar]
- 69.Martinez-Serrano A, Fischer W, Soderstrom S, Ebendal T, Bjorklund A. Long-term functional recovery from age-induced spatial memory impairments by nerve growth factor gene transfer to the rat basal forebrain. Proc Natl Acad Sci USA. 1996;93:6355–6360. doi: 10.1073/pnas.93.13.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mattson M P, Cheng B, Smith-Swintosky V L. Mechanisms of neurotrophic factor protection against calcium- and free radical-mediated excitotoxic injury: implications for treating neurodegenerative disorders. Exp Neurol. 1993;124:89–95. doi: 10.1006/exnr.1993.1178. [DOI] [PubMed] [Google Scholar]
- 71.McGuire D, Ross M A, Petajan J H, Parry G J, Miller R. A brief quality-of-life measure for ALS clinical trials based on a subset of items from the sickness impact profile. The Syntex-Synergen ALS/CNTF Study Group. J Neurol Sci. 1997;152:S18–S22. doi: 10.1016/s0022-510x(97)00239-6. [DOI] [PubMed] [Google Scholar]
- 72.Meignier B, Longnecker R, Mavromara-Nazos P, Sears A, Roizman B. Virulence of and establishment of latency by genetically engineered deletion mutants of herpes simplex virus type 1. Virology. 1988;162:251–254. doi: 10.1016/0042-6822(88)90417-5. [DOI] [PubMed] [Google Scholar]
- 73.Mellerick D M, Fraser N. Physical state of the latent herpes simplex virus genome in a mouse model system: evidence suggesting an episomal state. Virology. 1987;158:265–275. doi: 10.1016/0042-6822(87)90198-x. [DOI] [PubMed] [Google Scholar]
- 74.Mester J C, Pitha P, Glorioso J C. Anti-viral activity of herpes simplex virus vectors expressing alpha-interferon. Gene Ther. 1995;3:187–196. [PubMed] [Google Scholar]
- 75.Nicosia M, Deshmane S L, Zabolotny J M, Valyi-Nagy T, Fraser N W. Herpes simplex virus type 1 latency-associated transcript (LAT) promoter deletion mutants can express a 2-kilobase transcript mapping to the LAT region. J Virol. 1993;67:7276–7283. doi: 10.1128/jvi.67.12.7276-7283.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Olson L. NGF and the treatment of Alzheimer’s disease. Exp Neurol. 1993;124:5–15. doi: 10.1006/exnr.1993.1167. [DOI] [PubMed] [Google Scholar]
- 77.Pakzaban P, Chiocca E A. Nerve growth factor protects against herpes simplex virus type 1 neurotoxicity in the rat striatum. NeuroReport. 1994;5:993–996. doi: 10.1097/00001756-199404000-00035. [DOI] [PubMed] [Google Scholar]
- 78.Pakzaban P, Geller A I, Isacson O. Effect of exogenous nerve growth factor on neurotoxicity of and neuronal gene delivery by a herpes simplex amplicon vector in the rat brain. Hum Gene Ther. 1994;5:987–995. doi: 10.1089/hum.1994.5.8-987. [DOI] [PubMed] [Google Scholar]
- 79.Pechan P A, Yoshida T, Panahian N, Moskowitz M A, Breakefield X O. Genetically modified fibroblasts producing NGF protect hippocampal neurons after ischemia in the rat. NeuroReport. 1995;6:669–672. doi: 10.1097/00001756-199503000-00021. [DOI] [PubMed] [Google Scholar]
- 80.Raivich G, Hellweg R, Kreutzberg G W. NGF receptor-mediated reduction in axonal NGF uptake and retrograde transport following sciatic nerve injury and during regeneration. Neuron. 1991;7:151–164. doi: 10.1016/0896-6273(91)90083-c. [DOI] [PubMed] [Google Scholar]
- 81.Rasty S, Goins W F, Glorioso J C. Site-specific integration of multigenic shuttle plasmids into the herpes simplex virus type 1 (HSV-1) genome using a cell-free Cre-lox recombination system. New York, N.Y: Academic Press, Inc.; 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rock D, Fraser N. Latent herpes simplex virus type 1 DNA contains two copies of the virion DNA joint region. J Virol. 1985;55:849–852. doi: 10.1128/jvi.55.3.849-852.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Roizman B, Sears A. Herpes simplex viruses and their replication. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Hirsch M S, Melnick J L, Monath T P, Roizman B, editors. Fields virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2231–2295. [Google Scholar]
- 84.Rosenberg M B, Friedmann T, Robertson R C, Tuszynski M, Wolff J A, Breakefield X O, Gage F H. Grafting genetically modified cells to the damaged brain: restorative effects of NGF expression. Science. 1998;242:1575–1578. doi: 10.1126/science.3201248. [DOI] [PubMed] [Google Scholar]
- 85.Rossner S, Yu J, Pizzo D, Werrbach-Perez K, Schliebs R, Bigl V, Perez-Polo J R. Effects of intraventricular transplantation of NGF-secreting cells on cholinergic basal forebrain neurons after partial immunolesion. J Neurosci Res. 1996;45:40–56. doi: 10.1002/(SICI)1097-4547(19960701)45:1<40::AID-JNR4>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 86.Samaniego L, Webb A, DeLuca N. Functional interaction between herpes simplex virus immediate-early proteins during infection: gene expression as a consequence of ICP27 and different domains of ICP4. J Virol. 1995;69:5705–5715. doi: 10.1128/jvi.69.9.5705-5715.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Samaniego L A, Neiderhiser L, DeLuca N A. Persistence and expression of the herpes simplex virus genome in the absence of immediate-early proteins. J Virol. 1998;72:3307–3320. doi: 10.1128/jvi.72.4.3307-3320.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Samaniego L A, Wu N, DeLuca N A. The herpes simplex virus immediate-early protein ICP0 affects transcription from the viral genome and infected-cell survival in the absence of ICP4 and ICP27. J Virol. 1997;71:4614–4625. doi: 10.1128/jvi.71.6.4614-4625.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sampath D, Perez-Polo R. Regulation of antioxidant enzyme expression by NGF. Neurochem Res. 1997;22:351–362. doi: 10.1023/a:1027387105882. [DOI] [PubMed] [Google Scholar]
- 90.Satoh T, Sakai N, Enokiodo Y, Uchiyama Y, Hatanaka H. Free radical-independent protection by nerve growth factor and Bcl-2 of PC12 cells from hydrogen peroxide-triggered apoptosis. J Biochem. 1996;120:540–546. doi: 10.1093/oxfordjournals.jbchem.a021447. [DOI] [PubMed] [Google Scholar]
- 91.Schmidt Y, Unger J W, Bartke I, Reiter R. Effect of nerve growth factor on peptide neurons in dorsal root ganglia after taxol or cisplatin treatment and in diabetic (db/db) mice. Exp Neurol. 1995;132:16–23. doi: 10.1016/0014-4886(95)90054-3. [DOI] [PubMed] [Google Scholar]
- 92.Schumacher J, Short M, Hyman B, Breakefield X, Isacson O. Intracerebral implantation of nerve growth factor-producing fibroblasts protects striatum against neurotoxic levels of excitatory amino acids. Neuroscience. 1991;45:561–570. doi: 10.1016/0306-4522(91)90271-o. [DOI] [PubMed] [Google Scholar]
- 93.Scott J, Selby M, Urdea M, Quiroga M, Bell G I, Rutter W J. Isolation and nucleotide sequence of a cDNA encoding the precursor of mouse nerve growth factor. Nature. 1983;302:538–540. doi: 10.1038/302538a0. [DOI] [PubMed] [Google Scholar]
- 94.Scott S A, Crutcher K A. Nerve growth factor and Alzheimer’s disease. Rev Neurosci. 1994;5:179–211. doi: 10.1515/revneuro.1994.5.3.179. [DOI] [PubMed] [Google Scholar]
- 95.Seeburger J L, Springer J E. Experimental rationale for the therapeutic use of neurotrophins in amyotrophic lateral sclerosis. Exp Neurol. 1993;124:64–72. doi: 10.1006/exnr.1993.1176. [DOI] [PubMed] [Google Scholar]
- 96.Seidah N G, Benjannet S, Pareek S, Savaria D, Hamelin J, Goulet B, Laliberte J, Lazure C, Chretien M, Murphy R A. Cellular processing of the nerve growth factor precursor by the mammalian pro-protein convertases. Biochem J. 1996;314:951–960. doi: 10.1042/bj3140951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Selby M J, Edwards R, Sharp F, Rutter W J. Mouse nerve growth factor gene: structure and expression. Mol Cell Biol. 1987;7:3057–3064. doi: 10.1128/mcb.7.9.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Short M P, Choi B C, Lee J K, Malick A, Breakefield X O, Martuza R L. Gene delivery to glioma cells in rat brain by grafting of a retrovirus packaging cell line. J Neurosci Res. 1990;27:427–439. doi: 10.1002/jnr.490270322. [DOI] [PubMed] [Google Scholar]
- 99.Short M P, Rosenberg M B, Ezzedine Z D, Gage F H, Friedmann T, Breakefield X O. Autocrine differentiation of PC12 cells mediated by retroviral vectors. Dev Neurosci. 1990;12:34–45. doi: 10.1159/000111833. [DOI] [PubMed] [Google Scholar]
- 100.Smith R L, Pizer L I, Johnson E M J, Wilcox C L. Activation of second-messenger pathways reactivates latent herpes simplex virus in neuronal cultures. Virology. 1992;188:311–318. doi: 10.1016/0042-6822(92)90760-m. [DOI] [PubMed] [Google Scholar]
- 101.Sorenson E J, Windebank A J. Relative importance of basement membrane and soluble growth factors in delayed and immediate regeneration of rat scietic nerve. J Neuropathol Exp Neurol. 1993;52:216–222. doi: 10.1097/00005072-199305000-00005. [DOI] [PubMed] [Google Scholar]
- 102.Southern E M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 103.Spivack J G, Fraser N W. Detection of herpes simplex virus type 1 transcripts during latent infection in mice. J Virol. 1987;61:3841–3847. doi: 10.1128/jvi.61.12.3841-3847.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Springer J E. Experimental evidence for growth factor treatment and function in certain neurological disorders. Exp Neurol. 1993;124:2–4. doi: 10.1006/exnr.1993.1166. [DOI] [PubMed] [Google Scholar]
- 105.Stallcup W B, Cohn M. Correlation of surface antigens and cell type in cloned cell lines from the rat central nervous system. Exp Cell Res. 1976;98:285–297. doi: 10.1016/0014-4827(76)90440-7. [DOI] [PubMed] [Google Scholar]
- 106.Steers W D, Creedon D J, Tuttle J B. Immunity to nerve growth factor prevents afferent plasticity following urinary bladder hypertrophy. J Urol. 1996;155:379–385. [PubMed] [Google Scholar]
- 107.Stevens J G. Human herpesviruses: a consideration of the latent state. Microbiol Rev. 1989;53:318–332. doi: 10.1128/mr.53.3.318-332.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stevens J G, Wagner E K, Devi-Rao G B, Cook M L, Feldman L T. RNA complementary to a herpesviruses α gene mRNA is prominent in latently infected neurons. Science. 1987;255:1056–1059. doi: 10.1126/science.2434993. [DOI] [PubMed] [Google Scholar]
- 109.Stinski M F, Roehr T J. Activation of the major immediate early gene of human cytomegalovirus by cis-acting elements in the promoter-regulatory sequence and by virus-specific trans-acting components. J Virol. 1985;55:431–441. doi: 10.1128/jvi.55.2.431-441.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Taglialatela G, Foreman P J, Perez-Polo J R. Effect of long-term nerve growth factor treatment on body weight, blood pressure, and serum corticosterone in rats. Int J Dev Neurosci. 1997;15:703–710. doi: 10.1016/s0736-5748(97)00032-4. [DOI] [PubMed] [Google Scholar]
- 111.Thoenen H, Barde Y A. Physiology of nerve growth factor. Physiol Rev. 1980;60:1284–1335. doi: 10.1152/physrev.1980.60.4.1284. [DOI] [PubMed] [Google Scholar]
- 112.Thomsen D R, Sternberg R M, Goins W F, Stinski M F. Promoter regulatory region of the major immediate early gene of human cytomegalovirus. Proc Natl Acad Sci USA. 1984;81:659–663. doi: 10.1073/pnas.81.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tomlinson D R, Fernyhough P, Diemel L T. Neurotrophins and peripheral neuropathy. Philos Trans R Soc London Ser B. 1996;351:455–462. doi: 10.1098/rstb.1996.0042. [DOI] [PubMed] [Google Scholar]
- 114.Tuszynski M H, Gage F H. Bridging grafts and transient nerve growth factor infusions promote long-term central nervous system neuronal rescue and partial functional recovery. Proc Natl Acad Sci USA. 1995;92:4621–4625. doi: 10.1073/pnas.92.10.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tuszynski M H, Roberts J, Senut M-C, U H-S, Gage F H. Gene therapy in the adult primate brain: intraparenchymal grafts of cells genetically modified to produce nerve growth factor prevent cholinergic neuronal degeneration. Gene Ther. 1996;3:305–314. [PubMed] [Google Scholar]
- 116.Wang M, Friedmann T, Johnson P. Differentiation of PC12 cells by infection with an HSV-1 vector expression nerve growth factor. Gene Ther. 1995;2:323–335. [PubMed] [Google Scholar]
- 117.Weber P C, Levine M, Glorioso J C. Rapid identification of nonessential genes of herpes simplex virus type 1 by Tn5 mutagenesis. Science. 1987;236:576–579. doi: 10.1126/science.3033824. [DOI] [PubMed] [Google Scholar]
- 118.Weizsaecker M, Deen D F, Rosenblum M L, Hoshino T, Gutin P H, Barker M. The 9L rat brain tumor: description and application of an animal model. J Neurol. 1981;224:183–192. doi: 10.1007/BF00313280. [DOI] [PubMed] [Google Scholar]
- 119.Wheatley S C, L. M. K, Wood J N, Latchman D S. Cell lines derived from dorsal root ganglion neurons are nonpermissive for HSV and express only the latency-associated transcript following infection. Exp Cell Res. 1990;190:243–246. doi: 10.1016/0014-4827(90)90192-d. [DOI] [PubMed] [Google Scholar]
- 120.Whittemore S R, Larkfors L, Ebendal T, Holets V R, Ericsson A, Persson H. Increased beta-nerve growth factor messenger RNA and protein levels in neonatal rat hippocampus following specific cholinergic lesions. J Neurosci. 1987;7:244–251. doi: 10.1523/JNEUROSCI.07-01-00244.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wilcox C, Crnic L, Pizer L. Replication, latent infection, and reactivation in neuronal culture with a herpes simplex virus thymidine kinase-negative mutant. Virology. 1992;187:348–352. doi: 10.1016/0042-6822(92)90326-k. [DOI] [PubMed] [Google Scholar]
- 122.Wilcox C, Johnson E. Characterization of nerve growth factor-dependent herpes simplex latency in neurons in vitro. J Virol. 1988;62:393–399. doi: 10.1128/jvi.62.2.393-399.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wilcox C, Johnson E. Nerve growth factor deprivation results in the reactivation of latent herpes simplex virus in vitro. J Virol. 1987;61:2311–2315. doi: 10.1128/jvi.61.7.2311-2315.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wilcox C, Smith R, Freed C, Johnson E. Nerve growth factor-dependence of herpes simplex virus latency in peripheral sympathetic and sensory neurons in vitro. J Neurosci. 1990;10:1268–1275. doi: 10.1523/JNEUROSCI.10-04-01268.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Windebank A J, Smith A G, Russel J W. The effect of nerve growth factor, ciliary neurotrophic factor, and ACTH analogs on cisplatin neurotoxicity in vitro. Neurology. 1994;44:488–494. doi: 10.1212/wnl.44.3_part_1.488. [DOI] [PubMed] [Google Scholar]
- 126.Wolfe J H, Deshmane S L, Fraser N W. Herpesvirus vector gene transfer and expression of β-glucuronidase in the central nervous system of MPS VII mice. Nat Genet. 1992;1:379–384. doi: 10.1038/ng0892-379. [DOI] [PubMed] [Google Scholar]
- 127.Wood J N, Lillycrop K A, Dent C L, Ninkina N N, Beech M N, Willoughby J J, Winter J, Latchman D S. Regulation of expression of the neuronal POU protein oct-2 by nerve growth factor. J Biol Chem. 1992;267:17787–17791. [PubMed] [Google Scholar]
- 128.Wu N, Watkins S C, Schaffer P A, DeLuca N A. Prolonged gene expression and cell survival after infection by a herpes simplex virus mutant defective in the immediate-early genes encoding ICP4, ICP27, and ICP22. J Virol. 1996;70:6358–6368. doi: 10.1128/jvi.70.9.6358-6369.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]