Abstract
Lentivirus vectors can transduce dividing and nondividing cells. Using three-plasmid transient transfections, high-titer (>109 IU/ml) recombinant lentivirus vectors pseudotyped with vesicular stomatitis virus G (VSV-G) protein can be generated (T. Kafri et al., Nat. Genet. 17:314–317, 1997; H. Miyoshi et al., Proc. Natl. Acad. Sci. USA 94:10319–10323, 1997; L. Naldini et al., Science 272:263–267, 1996). The recombinant lentiviruses can efficiently infect brain, liver, muscle, and retinal tissue in vivo. Furthermore, the transduced tissues demonstrated long-term expression of reporter genes in immunocompetent rodents. We now report the generation of a tetracycline-inducible VSV-G pseudotyped lentivirus packaging cell line which can generate virus particles at titers greater than 106 IU/ml for at least 3 to 4 days. The vector produced by the inducible cell line can be concentrated to titers of 109 IU/ml and can efficiently transduce nondividing cells in vitro and in vivo. The availability of a lentivirus packaging cell line will significantly facilitate the production of high-titer lentivirus vectors for gene therapy and study of human immunodeficiency virus biology.
Retrovirus vectors have been used extensively for gene therapy (23). However, currently available recombinant retroviral vectors are not suitable for in vivo gene delivery because they can transduce dividing cells only. The advent of lentivirus vectors has overcome this obstacle and can efficiently transduce a variety of tissues in vivo, including brain, liver, muscle, retina, and hematopoietic cells. Sustained expression of the transgene in the transduced tissues of immunocompetent mice or rats further boosts their utility as a desirable vector for gene therapy.
Human immunodeficiency virus (HIV)-based vectors have been generated by transient transfection of (i) the packaging construct plasmid encoding Gag-Pol, Tat, Rev, Nef, Vpr, Vpu, and Vif proteins; (ii) a plasmid encoding vesicular stomatitis virus G (VSV-G) protein; and (iii) the vector containing the HIV long terminal repeats (LTRs), packaging signal, Rev response element, and foreign promoter driving the transgene (10, 13–15). Recombinant viruses with titers of 3 × 106 IU/ml were generated which could be concentrated by centrifugation to titers of 1 × 109 to 3 × 109 IU/ml (10, 12, 13). To facilitate vector production for in vivo experiments in larger animals we need to develop a stable VSV-G pseudotyped lentivirus vector packaging cell line. An earlier report on the development of a tetracycline-inducible VSV-G pseudotyped murine leukemia virus vector packaging cell line encouraged us to adopt a similar approach (16). Lentivirus vector packaging cells have previously been described but the vector titers were quite low (1, 3, 6, 18, 19, 25). Additionally, some of the viral accessory proteins in these cell lines were either missing or nonfunctional (25). In some of these cell lines, the gene encoding the envelope protein and the genes encoding the packaging proteins were transcribed from the same expression cassette, thereby increasing the probability of generation of helper viruses. Furthermore, none of the earlier cell lines expressed the VSV-G envelope protein that allows vector concentration and expands the range of target tissues. A major hurdle in the construction of a packaging cell line generating recombinant lentiviruses was that VSV-G and some HIV proteins, including protease and Vpr, have been reported to be cytotoxic (11, 17, 20). Here we describe the generation of an inducible lentivirus vector packaging cell line that was constructed in 293 cells and that constitutively expresses a tetracycline-regulated transactivator (tTA) (8, 9). All HIV type 1 (HIV-1) genes (excluding the HIV-1 envelope gp 120) are transcribed in the cell line from a single expression cassette, which is regulated by tetracycline (8). In addition, the cell line expresses the VSV-G envelope protein and the green fluorescent protein (GFP) from a bidirectionl tetracycline-regulated promoter. This gives the advantage not only of pseudotyping the lentivirus vectors with the VSV-G envelope but also of monitoring the induction process. A rapid and high level of gene induction can be obtained by the addition of sodium butyrate. Following transduction by lentivirus vectors, the novel packaging cell line can generate vector particles at titers greater than 106 IU/ml for at least 3 to 4 days. The vectors produced by the inducible cell line can be concentrated and can transduce growth-arrested cells in culture and in terminally differentiated neurons in immunocompetent rats.
MATERIALS AND METHODS
Plasmid construction.
pSKVG was constructed by cloning the EcoRI VSV-G (Indiana serotype) from pMDG (15) into the EcoRI site of Bluescript SK+. The GFP coding fragment was excised from pEGFP-N1 by SacI-NotI digestion and ligated to the SacI-NotI fragment of Bluescript SK+ to create pSKGFP. The PstI fragment containing the GFP coding region from pSKGFP was ligated to the PstI site in pBI (Clontech 6152-1) to create pBIGF. The NheI-EcoRV fragment containing the VSV-G coding region from pSKVG was ligated to the XbaI-PvuII sites in pBIGF to create pBIGFVS. The BamHI-BglII fragment containing a minimal cytomegalovirus (CMV) immediate-early gene promoter linked to seven tandem copies of the tetR-binding site replaced the CMV promoter (BglII-BamHI fragment) in pcDNAneo to create phCMVn. The plasmid pPTK was constructed by ligation of the BglI-SacII fragment bearing the genes encoding all the HIV-1 proteins from pΔR8.2 (14) to the BglI (partial)-SacII fragment from phCMVn. The XhoI fragment containing the neomycin resistance gene was deleted from ptTet-Off (Clontech K1620-A) to create ptTAΔn, from which a fusion protein containing the carboxy terminus of the tetracycline repressor and the herpes simplex virus VP16 transactivation domain is expressed under the control of the CMV promoter. The pCLBFP was constructed by ligation of the BamHI-EcoRI fragment containing the blue fluorescent protein (BFP) gene from pEBFP (Clontech 6068-1) into the BamHI-EcoRI fragment of pCL. The HindIII-NotI fragment containing the BFP coding region was inserted into the HindIII-NotI fragment of pcDNA3.1/hygro (Invitrogen V870-20) to create pcDNABFP.
Vector production.
All the cell lines in this study were maintained in Dulbecco’s modified Eagle’s medium containing tetracycline-free 10% fetal calf serum (Clontech 8630-1). To generate the stable cell line SODk0 that expresses the fusion protein tetracycline repressor-VP16 transactivation domain, human 293 embryonic kidney cells were cotransfected with 20 μg of ptTAΔn and 1 μg of pSRαBSR that expresses the blastocydine resistance gene by the calcium phosphate precipitation method (5). Individual cell colonies were selected with 20 μg of blastocydine/ml. The lentivirus vector stable packaging cell line SODk1 was generated by transfection of SODk0 cells with 10 μg of pPTK and 10 μg of pBIGFVS. The transfected cells were selected for neomycin resistance (400 μg/ml) in the presence of 0.7 μg of doxycycline/ml. Individual colonies were screened for HIV-1 p24 and GFP production and cell fusion in the presence or absence of doxycycline as follows. Cells from a confluent 10-cm plate were split by a ratio of 1:4 into PolyLysine precoated plates. Induced cells were cultured in the absence of doxycycline. Cell media were changed daily. Control cells were cultured in the presence of 0.7 μg of doxycycline/ml. On day 4 postinduction, the levels of HIV-1 p24 in conditioned media were measured by enzyme-linked immunosorbent assay (ELISA) (DuPont). Cell fusion was used as a marker for VSV-G production, and GFP production was determined by fluorescence microscopy. The colony that was found to be negative for p24 and GFP production in the presence of doxycycline and yet showed the highest levels of p24 production (>800 ng/ml) with more than 85% green cells upon induction was chosen as the packaging cell line for the lentivirus vector. The lentivirus vector producer cell line SODk1CGFI was generated by transducing SODk1 cells with HRcmvGFP lentivirus vector using a multiplicity of infection (MOI) of 2.
SODk1CGFI cells were split from a confluent 10-cm plate into a precoated PolyLysine plate at a ratio of 1:4 in the absence of doxycycline. Twenty-four hours after the split, the cells were washed twice with phosphate-buffered saline (PBS) and refed with doxycycline-free media that contained 5 mM sodium butyrate. The media were replenished daily. Induced SODk1CGFI-conditioned media were filtered through a 0.45-μm-pore-size filter and assayed daily for vector titers and p24 concentration by serial dilutions on 293 cells and by p24 ELISA, respectively. To further concentrate the vector, conditioned media were collected 3 days after addition of sodium butyrate filtered as described above and ultracentrifuged at 50,000 × g for 2 h. The pellet was resuspended and incubated for 2 h at 37°C in Tris-buffered saline (TBS) containing 10 mM MgCl2, four dNTPs (0.1 mM each), 3 mM spermine, and 0.3 mM spermidine. After a second ultracentrifugation at 50,000 × g for 2 h, the vector was resuspended in TBS with 2 μg of Polybrene/ml. The concentrated vector was assayed for p24 concentration and titrated on 293 cells as described above.
Protein analysis.
Induced (3 days after adding sodium butyrate) and uninduced (cultured in the presence of doxycycline) cells were lysed, and proteins were denatured by boiling for 10 min in a buffer containing 68 mM Tris (pH 6.8), 50 mM NaCl, 0.5 mM EDTA, 20 μg of aprotinin/ml, 50 μg of phenylmethylsulfonyl fluoride/ml, 1.5% sodium dodecyl sulfate (SDS), 5% glycerol, and 5% β-mercaptoethanol. Twenty micrograms of the denatured protein was separated on a 12.5% polyacrylamide gel containing SDS and blotted onto an Immobilon-P membrane (Millipore). After blocking with 5% nonfat milk in PBS–0.2% Tween 20 for 15 min, the membranes were incubated with mouse monoclonal anti-VSV-G (Sigma V-5507) or with rabbit HIV-1 Vpr (National Institute of Allergy and Infectious Diseases, AIDS Research and Reference Reagent Program, reagent 3252) and then with goat anti-mouse immunoglobulin horseradish peroxidase (Pierce) or donkey anti-rabbit horseradish peroxidase (Amersham), respectively. The protein bands were detected with an ECL kit (Amersham).
Transduction of nondividing cells.
Serial dilution of induced SODkCGFVGI-conditioned media was used to transduce either HeLa cells arrested by culturing in the presence of 15 μg of aphidicolin/ml for 12 h or human embryo fibroblasts arrested by culturing for 48 h in 0.1% fetal calf serum. Titers were scored 48 h posttransduction by dividing the number of GFP-positive foci by the dilution factor.
Assays for replication-competent virus.
We used three independent methods to assay for replication-competent virus.
(i) tat transfer assay.
This assay is based on a reporter HeLa P4.2 cell line which expresses CD4 and contains an integrated lacZ reporter gene driven by the HIV LTR. Since the HIV LTR is not active in naive HeLa cells, the expression of the lacZ gene in P4.2 cells can serve as a sensitive indicator for the presence of HIV tat (4). The sensitivity of the assay was of 20 tat-transducing units per ml of test medium. The use of the P4.2 cell line as an indicator of HIV-1 helper activity in lentivirus vector stocks was described extensively in the past (15). In our assay, 105 P4.2 cells were transduced with 106 IU of the vector. The transduced cells were serially passaged for 3 weeks (about five passages), after which they were scored for HIV-1 tat activity by X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) staining. Using this method, no transduction of tat was detected.
(ii) HIV gag transfer assay.
This method is based on measuring p24gag in conditioned media obtained from vector-transduced cells. The use of this method to assay for HIV-1 helper activity in lentivirus vector stocks and its sensitivity was previously described (15). In our assay, 105 p4.2 cells were transduced by vector particles at an MOI of 10. Following five passages in culture, the concentration of p24gag in conditioned media obtained from these cells was determined by ELISA (p24 ELISA kit; NEN Life Science Products). The detection limit of this method is ≥1 pg/ml, which is about 1 to 2 IU/ml. By this method, vector preparation is considered helper negative when p24 concentrations are below detection levels.
(iii) Marker rescue assay.
A total of 105 p4.2 cells transduced with HR′CMVGFP lentivirus vector (p4.2G.) were plated on a 10-cm-diameter plate and transduced with 106 IU of the tester vector stock. The transduced cells were cultured for 3 weeks, after which conditioned medium was harvested and filtered through a 0.45-μm-pore-size filter. A total of 105 293T cells were transduced in a 10-cm plate with 10 ml of the medium in the presence of 4 μg of Polybrene/ml. Seventy-two hours posttransduction, the cells were scored for GFP expression. Vector stock was considered helper free when no green cells were detected.
FACS analysis.
Trypsinized cells were resuspended in 5 ml of culture medium and washed in PBS minus Ca2+ and Mg2+. The cells were fixed in 3 ml of 5% paraformaldehyde, washed twice in PBS, and diluted in PBS to a concentration of 106 cells/ml. Fluorescence-activated cell sorter (FACS) analysis for cellular GFP and BFP was performed by FACScan analysis (Becton Dickinson) with the CellQuest program (version 3.0.1f; Becton Dickinson).
RNA analysis.
Total cellular RNA was isolated by using a Qiagen RNeasy kit. RNA (8 mg) was electrophoresed on a 1.2% agarose-formaldehyde gel, transferred to Hybond-N+ (Amersham) membrane, and hybridized at 68°C to a 32P-labelled DNA probe. The membrane was washed twice for 15 min in buffer containing 0.1% SDS–2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) at 68°C and once in buffer containing 0.1% SDS–0.1× SSC at 68°C for 15 min. The washed membrane was exposed to Kodak X-Omat Blue XB-1 film.
Transduction of brain of immunocompetent rat.
Adult female Fisher 344 rats (n = 6) were anesthetized (80 mg of ketamine, 0.75 mg of acepromazine, and 4 mg of xylazine per ml/per kg of body weight), and 2 μl of the viral-vector concentrated stock (1 × 109 IU/ml) was injected into the left striatum (anterior-posterior, +1; medial lateral, 3.5; dorsal-ventral, 4) with a 10-μl Hamilton syringe. After 4 weeks, the rats were deeply anesthetized and perfused intracardially with 100 ml of saline followed by 200 ml of 4% paraformaldehyde. Brains were postfixed for 24 h and stored in 30% phosphate-buffered sucrose at 4°C until sectioning. Brains were frozen and sectioned on a sliding microtome into 40-μm slices. Sections were washed and blocked in TBS with 3% donkey serum and 0.3% Triton X-100 (TBS plus). Primary antibodies raised in two different species were pooled in TBS-plus and incubated for 48 h at 4°C. The antibody for Neu-N (mouse monoclonal antibody [a generous gift of R. J. Mullin]; 1:20) was combined with antibody for GFAP (glialfibrillary acidic protein) (guinea pig polyclonal antibody [Advanced Immunochemicals]; 1:500). Corresponding secondary antibodies (donkey anti-mouse Texas red and donkey anti-guinea pig Cy5; 1:250) were pooled, and sections were incubated for 4 h at room temperature following washing in TBS plus. Every 12th section was mounted and coverslipped with DABCO-PVA. Sections were analyzed by confocal scanning laser microscopy (Bio-Rad). To determine the nature of transduced cells, double-labelled sections were scanned with a confocal laser microscope, and a representative sample of 100 transduced cells was examined for colocalization of GFP with either NeuN or GFAP.
RESULTS
SODk0: a tTA cell line.
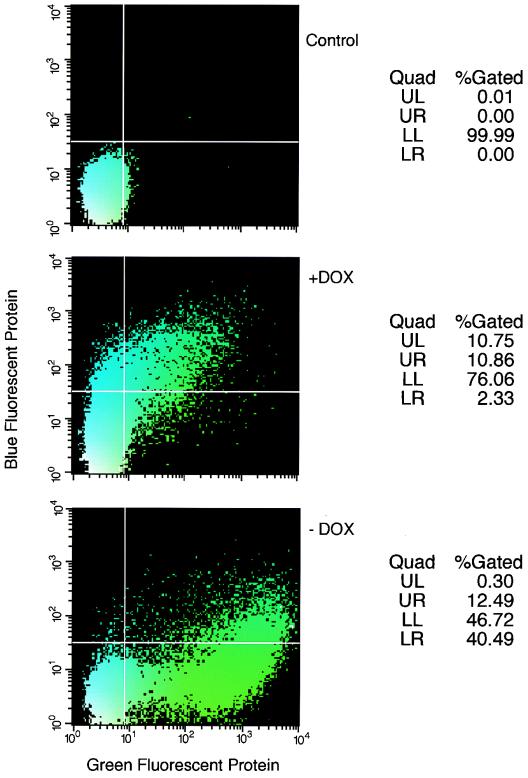
The extensive characterization of the tetracycline-inducible system encouraged us to use this regulatory control in the development of the lentivirus vector packaging cell line. As a first step, we generated a 293 (human embryonic kidney) cell line that constitutively expresses the tTA. The tTA is a fusion product of the amino terminal-DNA binding domain of the tet repressor and the carboxy-terminal activation domain of VP-16 from herpes simplex virus (8). In the absence of tetracycline, tTA binds to the tet-responsive elements (TRE) in the tet operator (tet0) and efficiently activates transcription from downstream minimal promoters. The association between tTA and the TRE is prevented by tetracycline; therefore, in the presence of low concentrations of tetracycline or its derivative deoxycycline, transcription from TRE is turned off. We cotransfected 293 cells with a plasmid, ptTAΔn, that expresses tTA from the early promoter of CMV and with plasmid pSRαBSR expressing the blastocidine resistance gene from the SRα promoter (21). Stably transfected clones were selected by culturing in the presence of blastocidine, and 20 cell clones whose growth rates did not differ significantly from that of the parental 293 cells were screened for tTA expression. Each clone was cotransfected with plasmid pBIGF that expresses GFP from a tetracycline-regulated promoter and plasmid pcDNABFP expressing BFP from a CMV promoter. The transfected cells were cultured either in the presence or the absence of doxycycline, a potent derivative of tetracycline (9). By fluorescence microscopy and FACS analysis we could evaluate the relative expression of GFP (as a reporter for tTA activity) and the expression of BFP (as a standard for transfection efficiency and transcription activity). The FACS analysis of SODk0 cells transfected with pBIGF and pcDNABFP in the presence of doxycycline shows little GFP, whereas in the absence of deoxycycline, intense GFP staining can be identified. Based on the relative induction of GFP and the efficiency of transfection (Fig. 1), we chose SODk0 as a parental cell line for development of inducible lentiviral vector packaging cell line.
FIG. 1.
Screening for tTA-expressing 293 cells. Human embryonic kidney 293 cell clones that stably expressed the tTA gene were transiently cotransfected with the pBIGF plasmid that produced the GFP under the control of the tetracycline-regulated promoter and the pcDNABFP plasmid that expressed the BFP under the control of the CMV promoter. Activation of the tetracycline-regulated promoter by the tTA in the presence and the absence of doxycycline (DOX) was evaluated by GFP expression and was determined by FACS analysis (x axis). BFP expression from the CMV promoter served as a standard for transfection efficiency and transcription activity and was determined by FACS analysis (y axis). Nontransfected human embryonic kidney 293 cells that expressed the tTA gene served as a negative control for either GFP or BFP expression.
SODk1 packaging cell line.
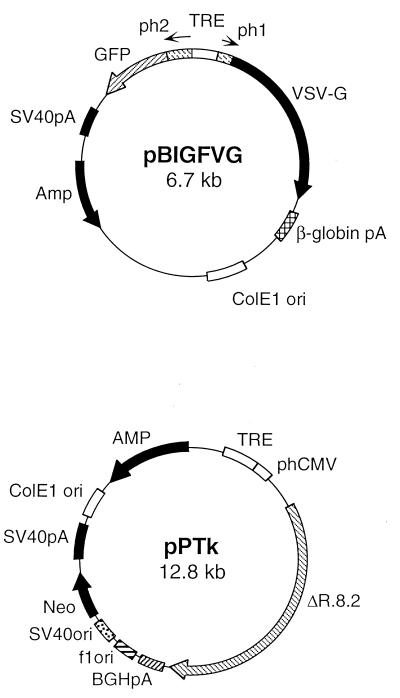
To generate an inducible VSV-G pseudotyped lentivirus packaging cell line, we molecularly cloned the VSV-G gene and the entire HIV packaging cassette into tetracycline-inducible vectors. The VSV-G gene was cloned into pBIGF to generate pBIGFVG from which the GFP and the VSV-G are transcribed under the regulation of a bidirectional tetracycline-inducible promoter (Fig. 2). The HIV-1 packaging cassette was cloned into phCMVn to generate pPTK, from which all HIV-1 genes (except the HIV-1 envelope gp120) are expressed under the control of a tetracycline-regulated promoter (Fig. 2). Before establishing a producer cell line, we wanted to evaluate the potential of inducible packaging systems to produce virus particles. Therefore, we transiently cotransfected SODk0 cells with the inducible packaging plasmid pPTK, the inducible envelope plasmid pBIGFVS, or the previously described pMDG plasmid that expresses the VSV-G envelope under the regulation of the CMV promoter and the vector plasmid pCLCG (12). To standardize vector production, we also generated vector particles by transiently transfecting 293T cells with the vector plasmid pCLCG, pMDG (VSV-G envelope), and the ΔR8.2 (packaging) plasmids that were previously described (14). Based on p24 (viral gag) levels in the conditioned media of transfected cells and the actual vector titers (Table 1), we conclude that vector production by the tetracycline-regulated packaging and envelope constructs is similar if not more efficient than the transient three-plasmid transfection method previously employed (10, 12–15).
FIG. 2.
Inducible envelope and packaging constructs. pBIGFVG is an inducible plasmid that expresses the VSV-G envelope and the GFP genes under the control of a bidirectional tetracycline-inducible promoter. This promoter contains two human CMV minimal promoters (ph1 and ph2) separated by the TRE. Arrows indicate transcription directions. The simian virus 40 (SV40) and β-globin polyadenylation signals are indicated. The pPTK plasmid contains an HIV-1 genomic fragment that encodes all HIV-1 genes excluding the HIV-1 envelope (ΔR.8.2) under the regulation of an inducible promoter. This promoter contains the TRE and the human CMV minimal promoter (pHCMV). Also indicated are the bovine growth hormone polyadenylation signal (BGHpA), the SV40 promoter and origin (SV40ori), the neomycin resistance gene (Neo), the SV40 polyadenylation signal (SV40pA), the ColE1 origin (ColE1 ori), and the f1 origin (f1ori).
TABLE 1.
Vector production by transient three-plasmid transfection of SODk0 cellsa
| Cells | Packaging | Envelope | p24gag level (ng/ml) | Vector titer (IU/ml) |
|---|---|---|---|---|
| SODk0 | pPTK | pMDG | 206 | 3.5 × 106 |
| SODk0 | pPTK | pBIGFVG | 114 | 2.0 × 106 |
| 293T | ΔR8.2 | pMDG | 53 | 1.5 × 106 |
To investigate vector production by the inducible envelope and packaging constructs, the vector-producing plasmid pCLCG and the inducible packaging plasmid pPTK were cotransfected to SODk0 cells with either the inducible VSV-G envelope plasmid pBIGFVG or the CMV promoter-regulated VSV-G envelope-producing plasmid pMDG. As a positive control, 293T cells were transiently cotransfected with the vector-producing plasmid pCLCG, the Δr8.2 and the pMDG plasmids that expressed the VSV-G envelope and the HIV-1 packaging genes, respectively, under the control of the CMV promoter. Forty-eight hours posttransfection, vector titers and p24gag levels in conditioned media were determined by serial dilutions on 293T cells and by ELISA, respectively.
Encouraged by these results, we embarked on the establishment of a stable packaging cell line. The pPTK packaging construct that also expresses the neomycin resistance gene (Fig. 2) and the pBIGFVG envelope construct were cotransfected into the SODk0 cell line. Thirty-five stably transfected cell clones were isolated and screened for inducible regulation of the VSV-G–GFP and the HIV packaging cassettes. Twenty cell clones in which at least 90% of the cells turned green 48 h after the withdrawal of doxycycline were selected. The second stage of the screening was aimed at the isolation of the clone in which the highest expression levels of the HIV-1 packaging proteins were obtained following induction. The cells were cultured in the presence or absence of doxycycline, and HIV gag protein production was determined by p24gag ELISA. The SODk1 clone was found to be most promising since the p24gag concentration in the media was below detection level in the presence of doxycycline and higher than 800 ng/ml without it.
Induction by sodium butyrate.
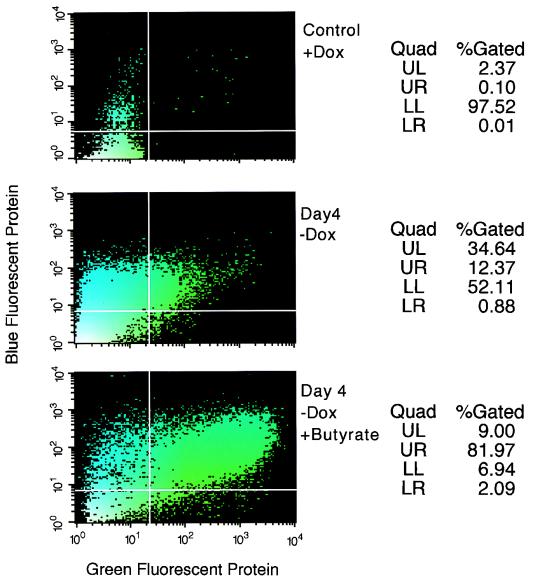
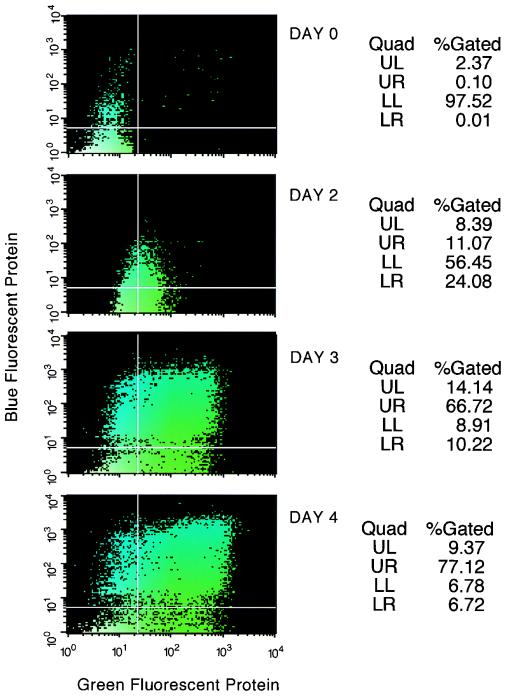
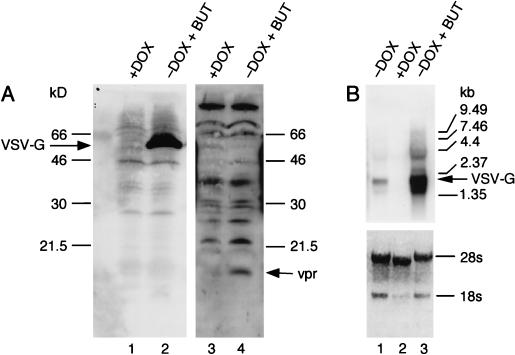
Surprisingly, the percentage of SODk1 cells that turned green upon doxycycline withdrawal gradually declined and within 1 month of the isolation of the SODk1 clone, less than 10% of the cells could be induced to express GFP. In parallel, we observed a prolongation in the time required to reach maximal levels of p24gag. Interestingly, this phenomenon was common to all 20 cell clones. The fact that the SODk1 cells were resistant to blastocidine and neomycin indicated that the tTA and the packaging plasmids were still integrated in their genome. We attributed the changes in the kinetics and magnitude of SODk1 cells induction to chromatin remodeling. To test this assumption, SODk1 cells were transduced with the pLBFPL vector in which the expression of the BFP is controlled by the HIV-1 LTR. Since the activation of the HIV LTR in 293 cells is tat dependent, we could monitor the induction of the pPTK packaging plasmid by monitoring BFP expression. Expression of pPTK will provide the tat protein, which in turn will activate HIV LTR to transcribe and express BFP. We hypothesized that a potent inhibitor of deacetylation, such as sodium butyrate (22, 24), could revert some of the repressive effects of chromatin on tetracycline-regulated promoters. To test our hypothesis, SODk1Blue cells were cultured in the absence of tetracycline and 48 h later, 5 mM of sodium butyrate was added to half of the samples. By FACS analysis, we evaluated the induction of the pBIGFVG envelope and the pPTK packaging constructs by the expression of the GFP and the BFP, respectively. Figure 3 demonstrates that sodium butyrate has a dramatic effect on the magnitude of induction in SODk1 cells. The time course analysis of SODk1 cells in the presence of sodium butyrate shows that the activation of the pPTK packaging plasmid and the pBIGFVG envelope is relatively synchronized and reaches maximal levels 3 to 4 days after doxycycline withdrawal (Fig. 4). To further evaluate the efficiency of doxycycline regulation of gene expression, we measured the amount of both the Vpr (one of the products of pPTK) and the VSV-G (encoded by pBIGFVG) proteins by Western blot analysis. Constitutive expression of these proteins is not compatible with long-term cell culturing because both proteins are cytostatic or cytotoxic (7, 17, 20). Results shown in Fig. 5A indicate that, although the expression of both proteins is nearly undetectable in the presence of doxycycline (lanes 1 and 3), it is dramatically increased upon doxycycline withdrawal and butyrate treatment (lanes 2 and 4). We therefore conclude that the SODk1 cell line allows regulated production of both HIV viral proteins required for packaging and VSV-G-encoded envelope protein. We next tested if the increased amount of VSV-G protein was in fact due to enhanced transcription of VSV-G gene in the presence of sodium butyrate. Figure 5B (lane 3) shows that, in the absence of deoxycycline, the addition of sodium butyrate leads to a greater-than-10-fold increase in VSV-G mRNA. Thus, the addition of sodium butyrate directly affects transcription, which is reflected in the increased virus titers (see below).
FIG. 3.
Induction by sodium butyrate. SODk1Blue cells were induced by doxycycline (DOX) withdrawal in the presence or absence of sodium butyrate. Expression of the BFP and GFP was determined by FACS analysis (y and x axis, respectively) and reflected the activation of the inducible HIV-1 packaging and the VSV-G envelope cassettes, respectively. Noninduced SODk1Blue cells served as a negative control.
FIG. 4.
Induction of SODk1Blue cells. SODk1Blue cells were induced by doxycycline withdrawal in the presence of sodium butyrate. Activation of the VSV-G envelope and the HIV-1 packaging cassettes was reflected by the expression levels of the GFP and the BFP genes, respectively, and was determined at days 0, 2, 3, and 4 postinduction by FACS analysis.
FIG. 5.
(A) Western blot analysis of Vpr and VSV-G protein induction. SODk1 cells were induced by doxycycline (DOX) withdrawal and sodium butyrate (BUT) treatment. Three days postinduction, production of the HIV-1 Vpr (lanes 3 and 4) and the VSV-G envelope protein (lanes 1 and 2) in noninduced (lanes 1 and 3) and induced (lanes 2 and 4) cells was determined by Western blot analysis. (B) Northern analysis. Total RNA was extracted from either noninduced SODk1 cells that were cultured in the presence of doxycycline (+DOX) or from SODk1 cells that were induced by doxycycline withdrawal in the presence (−DOX + BUT) or the absence (−DOX) of 5 mM sodium butyrate (upper part of figure). RNA samples were ethidium bromide stained and electrophoresed in a 1.2% agarose gel (lower part of figure). The efficiency of RNA transfer to positively charged membrane was determined by UV fluorescence. The position of the 1.6-kb VSV-G mRNA is indicated with an arrow.
Lentivirus vector producer cell line.
To generate a producer cell line, SODk1 cells were transduced with CLCG lentivirus vector at an MOI of 2. The CLCG vector in the three-plasmid transfections (12) expresses GFP under the control of the CMV promoter; therefore, we could use fluorescence microscopy to determine that between 80 and 90% of SODK1 cells were transduced. Since uninduced SODK1 cells were not producing VSV-G protein, there was presumably no interference to transduction by the CLCG vector containing VSV-G protein. To induce vector production, the cells were cultured in the absence of doxycycline for 24 h followed by the addition of 5 mM of sodium butyrate to half of the samples. Culture medium was replenished every 24 h, and vector production was determined by p24gag ELISA and by serial dilutions on 293T cells. Table 2 shows that 2 × 105 IU/ml of vector can be generated in 24 h following the addition of sodium butyrate by the stable lentivirus vector producer cell lines. By day 4, nearly 3 × 106 IU/ml of recombinant viruses can be obtained; however, vector production at later time points was significantly reduced due to lower cell viability. The producer cells fuse to form syncytia (7). Furthermore, there was a correlation between the actual vector titers that were determined by serial dilutions on 293T cells and the expected titer based on p24gag concentration. In the absence of sodium butyrate, the increase in p24gag concentration was significantly slower. Although the maximal p24gag concentration was not affected by the absence of sodium butyrate, the actual vector titer as determined by serial dilutions on 293T cells was more than 100-fold lower than the titer obtained in the presence of butyrate. Since less than 90% of the SODk1CG1 cells were transduced with the GFP-expressing lentivirus vector, we could increase the titers by reinfecting the cells with the same lentivirus vector (data not shown).
TABLE 2.
Effect of sodium butyrate on the induction of SODk1CG1 producer cell linea
| Time of assay (days postinduction) | Result under conditions of −Dox and +sodium butyrate
|
Result under conditions of −Dox and −sodium butyrate
|
||
|---|---|---|---|---|
| Vector titer (IU/ml) | p24gag level (ng/ml) | Vector titer (IU/ml) | p24gag level (ng/ml) | |
| 2 | 2 × 105 | 120 | 0 | 12 |
| 3 | 1 × 106 | 971 | 1 × 102 | 332 |
| 4 | 3 × 106 | 1,150 | 3 × 104 | 805 |
| 5 | 6 × 104 | 1,370 | ||
SODk1CG1 cells were induced by the withdrawal of doxycycline (−Dox) in the presence (+) or absence (−) of 5 mM sodium butyrate. Media were replenished every 24 h. Conditioned media were assayed daily for vector titers and p24gag levels were assayed by serial dilutions on 293T cells and by ELISA, respectively. The titers and p24gag levels are averages of results from two independent experiments; there was no more than 25% variation between experiments.
We next examined the lentivirus vector preparations from the SODk1CG1 cell line for the presence of helper virus. As in the past, we have relied on three independent methods: (i) the marker rescue assay; (ii) the tat transfer assay on vector-transduced P4.2 cells; and (iii) the p24gag ELISA on vector-transduced P4.2 cells. No replication-competent helper virus could be detected by these methods (15).
Properties of viral vectors obtained from the packaging cell line SODk1CG1.
To test the transduction of nondividing cells with lentivirus vectors produced by the SODk1CG1 packaging cell line, human embryonic fibroblasts (HEF) and HeLa cells were growth arrested at the G1/S boundary of the cell cycle by culturing in low-serum media (0.1% fetal calf serum) or by aphidicolin treatment, respectively. Table 3 shows that lentivirus vector that was generated by the SODk1CG1 packaging cell line was as efficient at transducing G1-arrested cells as proliferating cells.
TABLE 3.
In vitro transduction of nondividing cellsa
| Cell type | Vector titer (IU/ml) under conditions of:
|
|
|---|---|---|
| Cycling | G1/S arrest | |
| HEF | 5 × 105 | 4 × 105 |
| HeLa | 1 × 106 | 8 × 105 |
Lentivirus vectors generated by the SODk1CG1 producer cell line were titrated by serial dilutions on HEF and HeLa cells arrested in G1/S by serum starvation for 48 h or by aphidicolin treatment, respectively. Vector titration on nontreated HEF and HeLa cells served as a control. The titers are averages of results from two independent experiments; there was no more than 25% variation between the experiments.
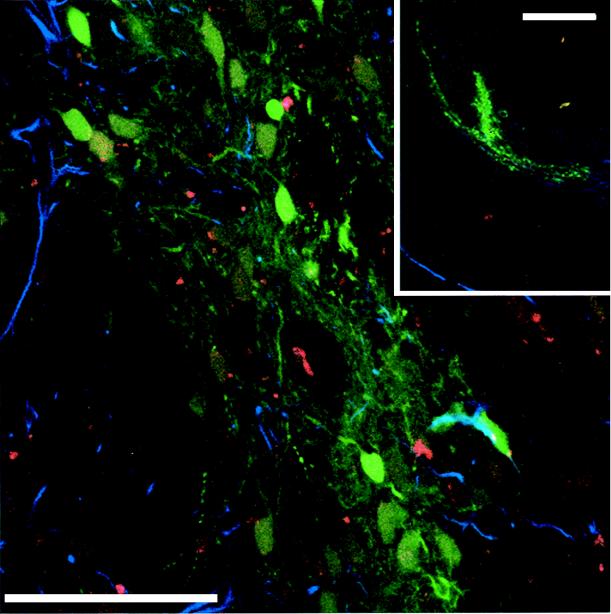
To investigate if the vector produced by SODk1CG1 packaging cell line can transduce nondividing cells in vivo, we concentrated the vector by ultracentrifugation to titers of 109 IU/ml. Two microliters of concentrated recombinant vector was injected into the corpus striatum of adult rat brain. Four weeks postinjection, the brains were sectioned and analyzed for GFP expression by confocal microscopy. Using immunofluorescence staining with antibodies for NeuN and GFAP, we could determine whether the transduced cells were neurons or astrocytes. The results shown in Fig. 6 demonstrate that the vector produced by the SODk1CG1 cell line can efficiently transduce terminally differentiated nondividing neurons. About 60% of the transduced cells were neurons, 32% of the transduced cells were astrocytes, and the remaining 8% showed neither marker. Preferential transduction of neurons over glial cells by lentivirus vectors was previously reported (15), and the mechanism responsible for that has not yet been characterized.
FIG. 6.
Transduction of brain of immunocompetent rat. Representative coronal section (40 μm thick) of a rat brain with unilateral striatal injection of 2 μl of vector expressing GFP 4 weeks after injection. Low magnification (bar = 1 mm) and high magnification (inset) (bar = 100 μm) are shown. Double labelling for NeuN (red) and GFAP (blue) was used.
DISCUSSION
Lentivirus vectors have the ability to deliver and to maintain long-term expression of transgenes in a broad range of tissues in vivo. To date, these vectors have been generated by transient three-plasmid transfections (10, 12–15). Although this method allows the generation of high-titer vectors, it limits the amount of vector particles that can be produced. It was important, therefore, to develop a stable cell line that can reproducibly generate high-titer lentivirus vectors in amounts that allow lentivirus-based gene therapy on large animal models.
The fact that HIV protease, VSV-G, and HIV vpr genes have been reported to be cytotoxic or cytostatic when constitutively expressed mandated the use of an inducible system. Alternatively, we could substitute the VSV-G envelope with a nontoxic envelope gene (e.g., amphotropic env) and delete the accessory proteins from the HIV packaging cassette. This could allow us to screen for cell clones resistant to the HIV protease and yet constitutively produce vector particles. Our decision to establish an inducible cell line was based on two major considerations. First, we did not want to lose the major advantages offered by the VSV-G envelope, namely, the broad range of target tissues and the ability for vector concentration by ultracentrifugation (2). Second, we did not want to limit the use of the lentivirus vector produced by the packaging cell line to tissues that may need complementation for the lack of HIV accessory proteins. Additionally we assumed that the cytotoxic effect of the HIV protease could eventually induce negative selection for cells that express this protein even if the parental cell clone was resistant to the protease. We chose to develop a regulated packaging cell line based on the well-defined and characterized tetracycline-inducible system (8, 9).
In the process of establishing the inducible lentivirus vector packaging cell line, we observed a gradual decline in the reactivation of tetracycline-regulated promoters. The fact that addition of 5 mM sodium butyrate to the culture media overrode the silencing process indicated that this phenomenon was in part the result of changes in chromatin remodeling in the tetracycline-regulated promoters. Interestingly, in the absence of sodium butyrate, p24gag production reached its maximal level within 4 to 6 days of doxycycline withdrawal. This result was in accordance with data presented in an earlier report showing that expression of HIV packaging proteins from a tetracycline-regulated promoter reached its maximal levels 6 to 7 days after tetracycline withdrawal (25). Although maximal production of the HIV packaging proteins in the absence of sodium butyrate was not lower than the maximal level obtained in the presence of butyrate, vector titers were almost 100-fold lower in the absence of butyrate. This observation can be explained by inefficient induction of the bidirectional tetracycline-regulated promoter from which the GFP and the VSV-G envelope protein are expressed. Clearly, sodium butyrate enhances transcription (Fig. 5B). However, we cannot rule out the possibility that part of the increase in vector titers stems from the effect of butyrate on HIV LTR or other endogenous genes.
Interestingly, secondary transduction of SODk1CG1 cells with the GFP lentivirus vector results in a significant increase in vector titers (9a), which correlated with proportional increase of p24gag levels in conditioned media. It is interesting, therefore, to investigate additional rounds of vector transduction and see if p24gag levels and vector titers increase. The uncloned populations of SODk1CG1 packaging cell line that have been generated in this study produce high-titer VSV-G pseudotyped lentivirus vectors (up to 3 × 106 IU/ml). It is therefore reasonable to assume that even higher titers can be obtained once individual clones can be screened for the highest producer.
In spite of the high-titer vector production, we did not detect helper virus in any of our vector preparations. There are several elements in our cell line that reduce the probability of generating replication competent virus. (i) The HIV packaging unit, the envelope, and the vector RNA are transcribed from separate expression units. (ii) The VSV-G envelope does not share any sequence homology with the vector or the packaging transcripts. (iii) The transcription of the vector, the envelope, and the packaging unit is limited to the time of induction. (iv) The VSV-G envelope is cytotoxic and therefore reduces the probability of being constitutively expressed. We believe these are important safety features. Further removal of additional viral genes (e.g., vpr, vpu, nef, vif, etc.) will add safety features to the lentivirus vector.
In summary, we report here the establishment of a tetracycline-regulated lentivirus vector packaging cell line that reproducibly generates helper-free, high-titer VSV-G pseudotyped vectors. These vectors have a broad spectrum of target tissues and can be concentrated by ultracentrifugation to very high titers (higher than 109 IU/ml). Vectors produced by the new packaging cell lines efficiently transduced G1/S arrested cells in vitro and terminally differentiated neurons in brain of immunocompetent rat. The new packaging cell line allows large-scale production of lentivirus vectors and therefore will facilitate human gene therapy efforts.
ACKNOWLEDGMENTS
We are grateful to D. Chambers for help with FACS analysis and to J. Simon, L. Grabowski, M. Gage, and B. Coyne for help in preparation of the manuscript; we also thank members of the Verma and Gage laboratories for their interest in this work.
This work was supported by grants from the National Institutes of Health, National Institute for Aging, the American Paralysis Association, and the Hollfelder Foundation. T.K. is supported by a fellowship from the Cystic Fibrosis Foundation. We gratefully acknowledge the support of the Frances Berger Foundation and the March of Dimes. I.M.V. is an American Cancer Society Professor of Molecular Biology.
REFERENCES
- 1.Buchschacher G L, Jr, Panganiban A T. Human immunodeficiency virus vectors for inducible expression of foreign genes. J Virol. 1992;66:2731–2739. doi: 10.1128/jvi.66.5.2731-2739.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burns J C, Friedmann T, Driever W, Burrascano M, Yee J K. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci USA. 1993;90:8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carroll R, Lin J-T, Dacquel E J, Mosca J D, Burke D S, St. Louis D C. A human immunodeficiency virus type 1 (HIV-1)-based retroviral vector system utilizing stable HIV-1 packaging cell lines. J Virol. 1994;68:6047–6051. doi: 10.1128/jvi.68.9.6047-6051.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charneau P, Mirambeau G, Roux P, Paulous S, Buc H, Clavel F. HIV-1 reverse transcription. A termination step at the center of the genome. J Mol Biol. 1994;241:651–662. doi: 10.1006/jmbi.1994.1542. [DOI] [PubMed] [Google Scholar]
- 5.Chen C A, Okayama H. Calcium phosphate-mediated gene transfer: a highly efficient transfection system for stably transforming cells with plasmid DNA. BioTechniques. 1988;6:632–638. [PubMed] [Google Scholar]
- 6.Corbeau P, Kraus G, Wong-Staal F. Efficient gene transfer by a human immunodeficiency virus type 1 (HIV-1)-derived vector utilizing a stable HIV packaging cell line. Proc Natl Acad Sci USA. 1996;93:14070–14075. doi: 10.1073/pnas.93.24.14070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eidelman O, Schlegel R, Tralka T S, Blumenthal R. pH-dependent fusion induced by vesicular stomatitis virus glycoprotein reconstituted into phospholipid vesicles. J Biol Chem. 1984;259:4622–4628. [PubMed] [Google Scholar]
- 8.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gossen M, Freundlieb S, Bender G, Muller G, Hillen W, Bujard H. Transcriptional activation by tetracyclines in mammalian cells. Science. 1995;268:1766–1769. doi: 10.1126/science.7792603. [DOI] [PubMed] [Google Scholar]
- 9a.Kafri, T. Unpublished results.
- 10.Kafri T, Blomer U, Peterson D A, Gage F H, Verma I M. Sustained expression of genes delivered directly into liver and muscle by lentiviral vectors. Nat Genet. 1997;17:314–317. doi: 10.1038/ng1197-314. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan A H, Swanstrom R. The HIV-1 gag precursor is processed via two pathways: implications for cytotoxicity. Biomed Biochim Acta. 1991;50:647–653. [PubMed] [Google Scholar]
- 12.Miyoshi H, Blömer U, Takahashi M, Gage F H, Verma I M. Development of a self-inactivating lentivirus vector. J Virol. 1998;72:8150–8157. doi: 10.1128/jvi.72.10.8150-8157.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyoshi H, Takahashi M, Gage F H, Verma I M. Stable and efficient gene transfer into the retina using an HIV-based lentiviral vector. Proc Natl Acad Sci USA. 1997;94:10319–10323. doi: 10.1073/pnas.94.19.10319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naldini L, Blomer U, Gage F H, Trono D, Verma I M. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci USA. 1996;93:11382–11388. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage F H, Verma I M, Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 16.Ory D S, Neugeboren B A, Mulligan R C. A stable human-derived packaging cell line for production of high titer retrovirus/vesicular stomatitis virus G pseudotypes. Proc Natl Acad Sci USA. 1996;93:11400–11406. doi: 10.1073/pnas.93.21.11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Planelles V, Bachelerie F, Jowett J B M, Haislip A, Xie Y, Banooni P, Masuda T, Chen I S Y. Fate of the human immunodeficiency virus type 1 provirus in infected cells: a role for vpr. J Virol. 1995;69:5883–5889. doi: 10.1128/jvi.69.9.5883-5889.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richardson J H, Child L A, Lever A M L. Packaging of human immunodeficiency virus type 1 RNA requires cis-acting sequences outside the 5′ leader region. J Virol. 1993;67:3997–4005. doi: 10.1128/jvi.67.7.3997-4005.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rizvi T A, Panganiban A T. Simian immunodeficiency virus RNA is efficiently encapsidated by human immunodeficiency virus type 1 particles. J Virol. 1993;67:2681–2688. doi: 10.1128/jvi.67.5.2681-2688.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rogel M E, Wu L I, Emerman M. The human immunodeficiency virus type 1 vpr gene prevents cell proliferation during chronic infection. J Virol. 1995;69:882–888. doi: 10.1128/jvi.69.2.882-888.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takebe Y, Seiki M, Fujisawa J, Hoy P, Yokota K, Arai K, Yoshida M, Arai N. SR alpha promoter: an efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and the R-U5 segment of human T-cell leukemia virus type 1 long terminal repeat. Mol Cell Biol. 1988;8:466–472. doi: 10.1128/mcb.8.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turner B M. Histone acetylation and control of gene expression. J Cell Sci. 1991;99:13–20. doi: 10.1242/jcs.99.1.13. [DOI] [PubMed] [Google Scholar]
- 23.Verma I M, Somia N. Gene therapy—promises, problems and prospects. Nature. 1997;389:239–242. doi: 10.1038/38410. [DOI] [PubMed] [Google Scholar]
- 24.Wade P A, Pruss D, Wolffe A P. Histone acetylation: chromatin in action. Trends Biochem Sci. 1997;22:128–132. doi: 10.1016/s0968-0004(97)01016-5. [DOI] [PubMed] [Google Scholar]
- 25.Yu H, Rabson A B, Kaul M, Ron Y, Dougherty J P. Inducible human immunodeficiency virus type 1 packaging cell lines. J Virol. 1996;70:4530–4537. doi: 10.1128/jvi.70.7.4530-4537.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]