Abstract
Declining blood CD4+ T-cell counts mark the progress of simian immunodeficiency virus (SIV) disease in macaques and model the consequences of untreated human immunodeficiency virus infection in humans. However, blood lymphocytes are only a fraction of the recirculating lymphocyte pool, and their numbers are affected by cell synthesis, cell depletion, and distribution among blood and lymphoid tissue compartments. Asymptomatic, SIV-infected macaques maintained constant and nearly normal numbers of recirculating lymphocytes despite the decline in CD4+ T-cell counts. Substantial depletion was detected only when blood CD4+ T-cell counts fell below 300/μl. In asymptomatic animals, changes in CD4+ T-cell distribution were more important than lymphocyte depletion for controlling the blood cell levels.
The hallmark of human immunodeficiency virus type 1 (HIV-1) infection in humans or simian immunodeficiency virus (SIV) infection in macaques is a progressive loss of blood CD4+ T lymphocytes that is associated with evolving immune deficiency. The empirical value of blood CD4+ T cells as a surrogate marker of disease progression has been established in numerous clinical studies (5, 6, 18). Current models hold that decreasing blood CD4+ T-cell counts result from virus-mediated cell destruction or decreased cell replenishment rates (9, 11, 22, 38). A “tap and drain” model was proposed to explain declining blood CD4+ T-cell counts during HIV disease progression (9, 38). In this conceptualization, blood CD4+ T-cell counts reflect differences between the supply of newly synthesized cells entering through the “tap” and cells lost through the “drain.” Cell loss includes normal cell turnover and cell destruction by HIV or SIV infection. Loss was hypothesized to slightly exceed the supply of new cells, and this difference was thought to explain the slowly declining blood CD4+ T-cell counts.
Models of this type do not adequately account for the complexity of lymphocyte populations or for the mechanisms controlling distribution of CD4+ lymphocytes among various pools (4, 15, 25, 32). Blood lymphocyte populations are regulated in a complex manner that includes control of cell distribution among blood and other compartments along with cell supply and cell loss mechanisms. Extensive accounting of cells throughout the body has demonstrated that 1 to 2% of the total population of lymphocytes are in the blood at any given time (35). Thoracic duct cannulation experiments in sheep, calves, and dogs showed that about 10 times more cells drain through the thoracic duct than are in the blood (30), suggesting that the total recirculating population greatly exceeds the fraction present at any one time in blood. These concepts have formed a framework for the theoretical distribution of lymphocyte populations. It is usually stated that lymphocytes in blood represent approximately 1 to 2% of the body’s lymphocytes, although it is more accurate to view the total lymphocyte population as being divided among the recirculating pool, which is estimated to be 10% of the total body lymphocytes, and the “tissue resident” pool, containing 90% of lymphocytes in organs and tissues (30, 35, 39). Recirculating lymphocytes include cells present in blood (one-fifth of the population, equivalent to 2% of total body lymphocytes) and cells present transiently in lymphoid tissues, spleen, or lymph (four-fifths of the recirculating population). The fraction of recirculating cells present in blood is affected by the number of newly synthesized cells entering the pool, cell turnover, and trafficking between blood and other compartments that is regulated by cell adhesion molecules and chemokine signals (3, 33, 39).
We studied whether SIV infection in the macaque can alter lymphocyte sequestration among blood and tissue compartments, thereby altering blood CD4+ T-cell counts by a mechanism other than cell supply or cell destruction. Few methods are available for assessing the recirculating pool of lymphocytes in vivo. Cannulation of the thoracic duct has been used to study lymphocyte traffic from nodes to blood in mice and sheep (24, 30). However, this method is unsuitable for routine studies of humans or nonhuman primates. Another approach uses pertussis toxin to interrupt the trafficking of recirculating lymphocytes and thus allow direct measurements of the size and composition of this pool. Intravenous pertussis toxin induces a substantial lymphocytosis in all mammals tested thus far (8, 12) by blocking chemokine signals normally transduced through 7-transmembrane receptors and their associated heterotrimeric G proteins (2). Pertussis toxin catalyzes ADP ribosylation on the alpha subunit of heterotrimeric G proteins from the Gαi, Gαo, and Gαt subclasses (7). Modified Gαi proteins fail to transduce the chemokine signal, and pertussis toxin-treated cells do not cross the endothelial barrier (2, 3). Pertussis toxin does not stimulate cellular proliferation in vivo, and the flux of lymphocytes from tissues to blood through the thoracic duct continues at the normal rate until the tissues become depleted and the flux declines (13, 14, 23). In the mouse, 3 × 108 cells were collected during 48 h of thoracic duct cannulation with only small numbers of cells in lymph after this point (31). In mice treated with pertussis toxin, lymphocyte counts increased from 1.5 × 107 to 1 × 108 cells/ml of blood (1). Assuming that each 20-g mouse contains a total blood volume of 2 ml, the recirculating lymphocyte population measured after pertussis toxin injection was approximately 2 × 108 lymphocytes. Estimates for the total recirculating lymphocyte population after thoracic duct cannulation and by pertussis toxin studies were very similar. Pertussis toxin injection is a useful method for studying recirculating lymphocyte populations in vivo.
Effects of pertussis toxin on lymphocyte counts are transient and last approximately 28 days in rhesus macaques. Peak lymphocytosis occurs by 5 days after pertussis toxin injection, and organized lymphoid tissues are mostly depleted of parafollicular lymphocytes by this time (21). The kinetics of lymphocytosis after pertussis toxin treatment were similar for SIV-infected and uninfected animals (8, 21). Pertussis toxin treatment of macaques provides a convenient tool for transiently accumulating the recirculating lymphocyte pool in blood. Using this approach, we can assess the possibility that the SIV disease affects the mechanisms for controlling distribution of lymphocytes among tissue and blood compartments.
MATERIALS AND METHODS
Animals and virus infection.
Rhesus macaques were housed at the University of Wisconsin Regional Primate Research Center. Protocols for these macaque studies were reviewed and approved by the University of Wisconsin Animal Care and Use Committee. Animals were 4 to 6 years old at the time of the study. A total of 19 animals were each infected intravenously with 20 50% tissue culture infectious doses of SIVmac (equivalent to 1 animal infective dose) (20). After infection, blood cell counts, flow cytometry analyses, virus titer assays, and physical examinations (among other tests) were performed routinely. Infection status was confirmed by at least three positive virus isolation assays, PCR assays, plasma antigenemia tests, and correlated clinical signs, including declining blood CD4+ T-cell counts.
Pertussis toxin sources and administration.
Pertussis toxin (List Biological Laboratories Inc., Campbell, Calif., or Pasteur Mereieux Connaught, Ontario, Canada) was administered intravenously. Pertussis toxin from List Biological Laboratories was provided as a lyophilized protein that was resuspended in sterile saline solution (100 μg/ml). Pertussis toxin from Pasteur Mereieux Connaught was in 50% glycerol-sterile saline solution and was diluted in sterile saline solution to the working concentration (100 μg/ml). Both stocks were tested in uninfected animals at various concentrations to determine the minimum amount needed to induce maximal lymphocytosis; this amount was determined to be 25 μg/kg of body mass. Fifteen animals, including all uninfected animals, received pertussis toxin from List Biological Laboratories, and 12 animals received pertussis toxin from Pasteur Mereieux Connaught. There were no differences in the magnitude or duration of lymphocytosis for animals treated with either product. Pertussis toxin was administered 4 to 6 months after SIV infection. Injection was into the saphenous vein at the rate of 1 ml/min, and there were no signs of injury at the site of injection.
Lymphocyte sample collection and analysis.
Blood was drawn under anesthesia into heparinized collection tubes. Blood draws were all performed in the morning between 0900 and 1100 h. Complete blood cell counts (automated) were performed at General Medical Laboratories (Madison, Wis.), a certified clinical laboratory with experience analyzing rhesus blood samples. For flow cytometry studies, peripheral blood mononuclear cells were purified by gradient centrifugation on Ficoll-Hypaque and fixed in 1% paraformaldehyde. Inguinal lymph node biopsies were collected under anesthesia, and single-cell suspensions were prepared by teasing apart the lymph node tissue and purifying mononuclear cells on Ficoll-Hypaque. Purified cells were fixed in 1% paraformaldehyde. Autopsies were performed immediately after euthanasia by intravenous injection of 50 mg of sodium pentobarbital/kg. Lymph nodes and spleen sections were fixed in buffered formaldehyde and embedded in paraffin for histology or were used for mononuclear cell preparation. CD2+, CD4+, CD8+, and CD20+ subsets of the lymph node mononuclear cells were analyzed by flow cytometry with a Becton Dickinson FACScan. Antibodies to CD2, CD4, CD8 (Antigenix-America, Franklin Square, N.Y.), and CD20 (Becton Dickinson, Boston, Mass.) cell surface markers, and the appropriate isotype controls, were conjugated to R-phycoerytherin, except anti-CD2 and the control immunoglobulin G1 isotype (fluoroscein isothiocyanate). Analyses were performed using Cellquest (Becton Dickinson) software.
Statistical analysis.
JMP software (SAS, Research Triangle Park, N.C.) was used for statistical analyses. Student’s t test was used where applicable. Correlations and linear fit were analyzed by using the Pearson product-moment correlation coefficient. Differences were considered statistically significant when P was <0.05.
RESULTS
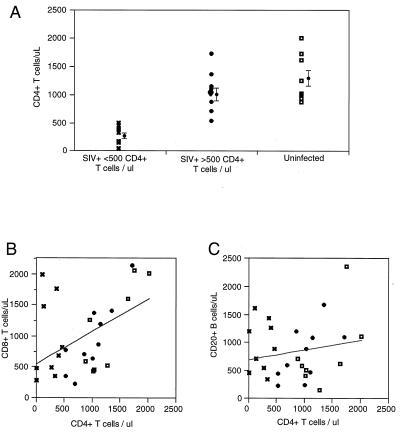
We administered pertussis toxin to 8 uninfected and 19 SIV-infected rhesus macaques that were matched for age. Total lymphocyte counts were determined by automated clinical blood counting of heparinized blood samples. For these samples, the absolute numbers of CD4+, CD8+, and CD20+ lymphocytes did not always equal the total lymphocyte count. These differences were due to cells staining dimly for the CD8 cell surface marker (presumed to be natural killer and γδ T cells), γδ T cells that are negative for subset markers, and other undefined cells. The proportion of cells in these undesignated categories was variable and was not consistently related to the CD4+ cell count, response to pertussis toxin, infection status, or stage of disease progression. Within these animal cohorts (including all infected and uninfected macaques), there was a positive relationship between absolute CD8+ T-cell count and absolute CD4+ T-cell count (Fig. 1B). It was not possible to discern a clear relationship between CD4+ T-cell count and the levels of CD20+ B cells in blood (Fig. 1C).
FIG. 1.
Lymphocyte counts for 25 rhesus macaques before administration of intravenous pertussis toxin. (A) CD4+ lymphocyte counts for uninfected animals (◘), SIV-infected animals with >500 CD4+ T cells/μl ( ), and SIV-infected animals with <500 CD4+ T cells/μl (✖). The relationships between CD8+ T lymphocytes (B) and CD20+ B lymphocytes (C) versus reference (before pertussis toxin) CD4+ lymphocyte counts were also determined (symbols as for panel A). Correlations and linear fits were analyzed using the Pearson product-moment correlation coefficient for CD8+ T-cell and CD20+ B-cell subsets in relation to the CD4+ count before pertussis toxin treatment.
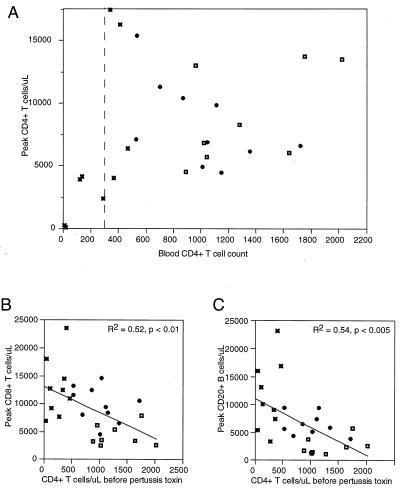
Lymphocyte counts in blood increased approximately 4.3-fold by 5 days after pertussis toxin injection in uninfected macaques (Table 1). The average CD4+ T-cell count in blood for uninfected macaques increased from 1,330 cells per μl to the peak level of 8,900 cells per μl (6.7-fold increase) after pertussis toxin injection (Table 1). Assuming that peak lymphocytosis represents the majority of recirculating lymphocytes, a 6.7-fold increase means that 15% (15% × 6.7 = 100%) of the recirculating CD4+ T-cell pool was in the blood compartment under normal circumstances and the remaining 85% was in lymphoid tissues, spleen, and lymph.
TABLE 1.
Lymphocyte levels before and after pertussis toxin treatmenta
| Group (n)b | Total no. of
lymphocytes
|
No. (%) of CD4+ T
cells
|
No. (%) of CD8+ T
cells
|
No. (%) of CD20+ B cells
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Fold increasec | Before | After | Fold increase | Before | After | Fold increase | Before | After | Fold increase | |
| Uninfected (8) | 5,025 | 21,624 | 4.3 | 1,330 (29) | 8,900 (42) | 6.7 | 1,110 (19) | 4,313 (21) | 3.9 | 800 (15) | 2,500 (11) | 3.1 |
| SIV+ >500 CD4/μl (11) | 3,180 | 27,209 | 8.5 | 1,000 (32) | 8,300 (31) | 8.3 | 960 (29) | 10,000 (36) | 10.4 | 780 (24) | 5,880 (22) | 7.5 |
| SIV+ <500 CD4/μl (8) | 2,896 | 33,369 | 11.5 | 250 (10) | 6,080 (27) | 24.3 | 920 (31) | 12,800 (42) | 14 | 930 (37) | 11,570 (36) | 12.4 |
Lymphocyte counts were taken for blood samples before pertussis toxin treatment and at peak lymphocytosis (3 to 5 days after pertussis toxin administration).
SIV+ >500 CD4/μl, SIV-infected animals with >500 CD4+ T cells/μl of blood; SIV+ <500 CD4/μl, SIV-infected animals with <500 CD4+ T cells/μl of blood.
Fold increase is the ratio of each value at peak lymphocytosis to the respective value before treatment.
SIV-infected macaques with high blood CD4+ T-cell counts (greater than 500 CD4+ T cells per μl of blood) had an average blood CD4+ T-cell count of 1,000 per μl (Fig. 1A). At peak lymphocytosis, the CD4+ T-cell count increased approximately 8.3-fold (Table 1), indicating that 12% of recirculating CD4+ lymphocytes were present in blood and the remaining 88% were present in other compartments. Although there were only small differences between uninfected animals and those with SIV and high CD4+ T-cell counts, the trend of our data shows a decreasing proportion of recirculating CD4+ T cells present in blood after infection compared to that for healthy, uninfected macaques.
SIV-infected macaques with low CD4+ T-cell counts (less than 500 CD4+ T cells per μl of blood) had an average count of 250 CD4 cells per μl (Fig. 1A). Pertussis toxin raised the blood CD4+ T-cell count an average of 24.3-fold (Table 1), showing that SIV-infected macaques with low CD4+ T-cell counts had only 4% of recirculating CD4+ T cells in blood; this value was significantly different from the 12% figure for infected animals with CD4+ T-cell counts of >500 and from the value of 15% recirculating cells in blood for uninfected macaques (P < 0.001). During the time when the number of blood CD4+ T cells declined from an average of 1,330 per μl in uninfected animals to an average of 250 per μl in the low CD4+ T-cell group (a 5.3-fold decrease), the total recirculating pool of CD4+ T cells declined by a factor of only 1.5-fold (Table 1; Fig. 2A). These results show that blood CD4+ T-cell counts represented an increased proportion of cells in tissues and a concomitantly decreased proportion of cells in the blood.
FIG. 2.
Peak cell counts after intravenous pertussis toxin administration compared to reference (before pertussis toxin) CD4+ lymphocyte counts. Symbols: ◘, uninfected animals; , SIV-infected animals with >500 CD4+ T cells/μl; ✖, SIV-infected animals with <500 CD4+ T cells/μl. (A) CD4+ T-lymphocyte counts. Note the critical boundary at 300 CD4+ T cells/μl. (B) CD8+ T-lymphocyte counts at peak lymphocytosis. (C) CD20+ B-lymphocyte counts at peak lymphocytosis. Correlations and linear fits were analyzed by using the Pearson product-moment correlation coefficient.
The observation of a changing CD4+ T-cell distribution prompted us to ask whether this pathologic event was specific or whether it affected all lymphocyte subsets. Recirculating CD8+ T- and CD20+ B-cell pools increased substantially as disease progressed. We compared the CD8+ T-cell and CD20+ B-cell peak lymphocytosis with the CD4+ T-cell count before pertussis toxin injection. The recirculating pools for both CD8+ T cells (Fig. 2B) and CD20+ B cells (Fig. 2C) increased as the reference CD4+ T-cell count decreased. The increased sizes of the recirculating CD8+ T- and CD20+ B-cell pools without corresponding changes in the blood absolute counts were similar to what was observed for CD4+ T cells, and we concluded that lymphocytes from all subsets were sequestered preferentially in lymphoid tissues.
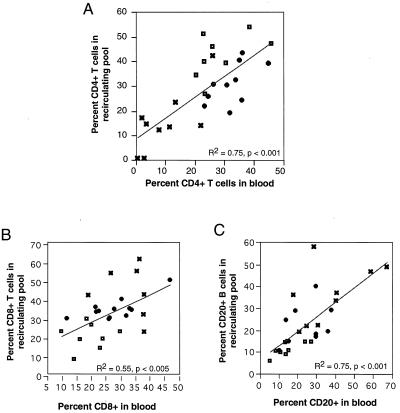
Our data on the distribution of recirculating lymphocytes showed that analysis of distribution in blood did not provide an accurate quantitative representation, but it may still present an accurate qualitative view of lymphocyte subpopulations. Accordingly, we compared the proportions of lymphocyte populations before and during lymphocytosis. Percentages for each lymphocyte subset were very similar in blood and in the total recirculating pool (Fig. 3). Analysis of CD4+ T-cell counts before and at peak lymphocytosis (Fig. 3A) produced a Pearson product-moment correlation coefficient of 0.75 (P < 0.001); CD8+ T cells (Fig. 3B) had a correlation coefficient of 0.55 (P < 0.005), and CD20+ B cells (Fig. 3C) had a correlation coefficient of 0.75 (P < 0.001). The percentages of CD4+ T cells in both blood and the total recirculating pool decreased because of increases in the proportion and number of CD8+ T and CD20+ B lymphocytes. Despite changes in the quantitative distribution of recirculating lymphocytes, the blood provided a reasonably accurate sample of the proportions for three major lymphocyte subpopulations.
FIG. 3.
Proportional distributions of lymphocyte subpopulations are similar in blood and the total recirculating pool. Symbols: ◘, uninfected animals; , SIV-infected animals with >500 CD4+ T cells/μl; ✖, SIV-infected animals with <500 CD4+ T cells/μl. Correlations and linear fits were analyzed by using the Pearson product-moment correlation coefficient.
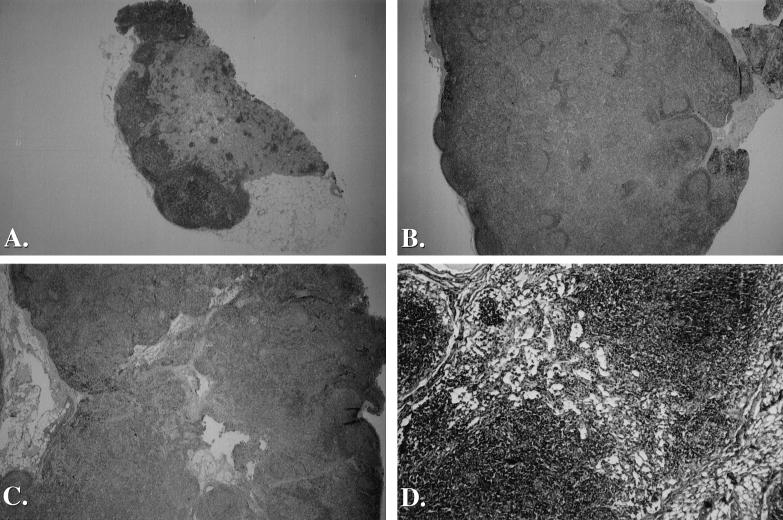
We decided to perform lymph node biopsies on uninfected and SIV-infected macaques to compare tissue and blood populations with and without pertussis toxin treatment. A major concern in this type of study was whether individual lymph nodes are representative of the majority of lymphoid tissues. A lengthy necropsy study involving 19 SIV-infected animals showed that peripheral lymphoid tissues were altered by SIV infection and that multiple peripheral lymph node samples from an individual animal were mostly uniform in appearance. The pattern of lymph node pathology among infected macaques (Fig. 4) was divided into three distinct stages (types I to III) which are comparable with the pathology seen after HIV infection (10, 19). Type I pathology is marked by acute lymphadenitis with follicular hyperplasia and splenomegaly (Fig. 4B). Type II pathology is represented by subacute lymphadenitis and follicular involution (Fig. 4C). Type III pathology shows severe lymphatic cell depletion associated with vascular and histiocytic proliferation (Fig. 4D). We examined postmortem tissues from 19 necropsies of SIV-infected animals and characterized multiple tissues as type I, II, or III. Lymph nodes from uninfected animals were morphologically normal and contained few active germinal centers (Fig. 4A).
FIG. 4.
Microscopic anatomy of lymph node specimens from uninfected and SIV-infected macaques. Inguinal lymph node biopsy specimens were fixed in buffered formalin before being embedded in paraffin. Sections were stained with hemotoxylin and eosin. (A) Lymph node tissue from an uninfected macaque. This small node is morphologically normal and contains only a few active germinal centers. Magnification, ×6.25. (B) Lymph node tissue from an SIV-infected macaque exhibiting follicular hyperplasia. Numerous germinal centers are present with extensive lymphocyte proliferation. Magnification, ×6.25. (C) Lymph node tissue from an SIV-infected macque exhibiting follicular involution. Extensive lymphocyte proliferation and poor germinal center morphogy are present. Magnification, ×6.25. (D) A higher-magnification view of lymph node tissue from an SIV-infected macque exhibiting severe lymphocyte depletion. T-cell zones are severely depleted and only remnant germinal centers are present in this end-stage specimen. Magnification, ×25.
Four of 19 SIV infection cases fit the type I pathology. Uniform swelling of all deep and superficial lymphoid sites, including ileal-cecal, mesenteric, retroperitoneal, inguinal, axillary, and popliteal lymph nodes, was noted along with pronounced splenomegaly. These tissues were hyperplastic and exhibited enlargement of the subcortical regions and expansion of the germinal centers. Six cases fit the type II pathology, with swelling of ileal, mesenteric, inguinal, and axillary lymph nodes. Microscopic observation showed that inguinal and axillary lymph node follicles were involuted and had some atrophy of germinal centers and initial depletion of cortical cells. All six type II cases exhibited splenomegaly and lymphadenopathy. Nine cases fit the type III pathology. Lymphoid tissues were slightly swollen and atrophic, the ileal lymph nodes and deep nodes were larger than the inguinal and axillary sites, and only small islands of intact lymphatic tissue remained throughout the body. Many lymph nodes had extensive lymphocytic depletion and stromal hyalinization (Fig. 4D). Five spleens were atrophic with the remaining four cases exhibiting marginal splenomegaly. Type III pathology occurred only when blood CD4+ T-cell counts were very low, often less than 100/μl. Importantly, lymph node pathology was manifested uniformly in all lymph nodes from a single animal. This study justified the use of individual lymph node biopsies as representative of most peripheral lymphoid tissues.
We also collected complete inguinal lymph node biopsies from four uninfected and five SIV-infected animals for analysis of lymphocyte subsets (Table 2). On average, inguinal lymph nodes from SIV-infected macaques contained more than 10 times the number of lymphocytes found in lymph nodes from uninfected animals, and the number of CD4+ T cells was also 7.5 times higher in the tissues from infected animals. The numbers of CD8+ T cells and CD20+ B cells were also increased. The absolute numbers of lymphocytes in SIV-infected animals were significantly larger than in uninfected animals (Student’s t test, P < 0.05). These results are consistent with measurements of the recirculating pools based on pertussis toxin treatment in that lymph nodes from infected animals were sequestering large numbers of lymphocytes despite decreased CD4+ T cells in blood.
TABLE 2.
Cell counts from inguinal lymph node biopsies compared to blood and recirculating cell countsa
| Group | No. of lymphocytes/lymph node or μl of blood | No. (%) of CD4+ T cells | No. (%) of CD8+ T cells | No. (%) of CD20+ B cells |
|---|---|---|---|---|
| SIV negative | ||||
| 90004 | ||||
| LN | 2.8 × 107 | 9.5 × 106 (34) | 3.2 × 106 (12) | 1.2 × 107 (42) |
| Blood count | 2,916 | 893 (31) | 586 (20) | 707 (24) |
| Recirculating | 11,525 | 5,436 (39) | 3,164 (27) | 1,711 (15) |
| 90116 | ||||
| LN | 7.0 × 105 | 2.9 × 105 (42) | 1.1 × 105 (17) | 2.8 × 105 (40) |
| Blood count | 2,785 | 1,281 (46) | 516 (17) | 151 (5) |
| Recirculating | 17,544 | 8,281 (42) | 5,368 (31) | 1,056 (6) |
| 89033 | ||||
| LN | 8.5 × 106 | 3.7 × 106 (43) | 1.6 × 106 (19) | 1.3 × 106 (15) |
| Blood count | 2,645 | 1,026 (39) | 431 (16) | 403 (15) |
| Recirculating | 12,654 | 6,809 (54) | 2,476 (20) | 1,367 (11) |
| AS04 | ||||
| LN | 3.0 × 107 | 1.2 × 107 (39) | 5.7 × 106 (19) | 7.8 × 106 (26) |
| Blood count | 3,100 | 1,209 (39) | 992 (32) | 682 (22) |
| Avg LN | 1.7 × 107 | 6.4 × 106 (40) | 2.6 × 106 (17) | 5.4 × 106 (31) |
| Avg blood count | 2,862 | 1,102 (39) | 631 (21) | 486 (17) |
| Avg recirculating | 13,907 | 6,842 (45) | 3,669 (26) | 1,378 (11) |
| SIV positive | ||||
| 91021 | ||||
| LN | 1.6 × 108 | 2.3 × 107 (14) | 5.2 × 107 (33) | 6.8 × 107 (42) |
| Blood count | 2,508 | 290 (12) | 479 (19) | 531 (21) |
| Recirculating | 17,632 | 2,380 (14) | 7,615 (43) | 3,395 (19) |
| 93028 | ||||
| LN | 3.2 × 108 | 7 × 107 (22) | 9.4 × 107 (20) | 1.1 × 108 (35) |
| Blood count | 3,285 | 870 (25) | 702 (21) | 1,193 (36) |
| Recirculating | 34,018 | 10,386 (31) | 12,389 (36) | 6,521 (20) |
| 92043 | ||||
| LN | 1.5 × 108 | 3.7 × 107 (25) | 2.6 × 107 (17) | 7.5 × 107 (50) |
| Blood count | 7,015 | 1,726 (26) | 2130 (30) | 1,087 (15) |
| Recirculating | 25,680 | 6,610 (26) | 10,490 (41) | 3,793 (15) |
| 91084 | ||||
| R. Ing.b | 3.0 × 108 | 1 × 108 (32) | 4.7 × 107 (16) | 1.3 × 108 (45) |
| L. Ing.b | 7.2 × 107 | 3.0 × 107 (41) | 1.3 × 107 (19) | 2.6 × 107 (36) |
| Blood count | 2,666 | 765 (29) | 331 (12) | 267 (10) |
| 91023 | ||||
| LN | 3.8 × 107 | 1.5 × 107 (40) | 8.4 × 106 (22) | 1.0 × 107 (25) |
| Blood count | 2,854 | 742 (26) | 743 (26) | 571 (20) |
| Avg LN | 1.7 × 108 | 4.8 × 107 (29) | 4.0 × 107 (22) | 4.8 × 107 (39) |
| Avg blood | 3,666 | 879 (24) | 877 (22) | 730 (20) |
| Avg recirculating | 25,687 | 6,457 (24) | 10,165 (40) | 4,570 (18) |
Values in scientific notation are numbers of lymphocytes per lymph node (LN). AS04, 91084, and 91023 did not receive pertussis toxin, so no measurement of recirculating cells (recirculating) is provided.
Right (R.) and left (L.) inguinal (Ing.) lymph nodes were analyzed separately.
DISCUSSION
Quantitative analysis of the recirculating lymphocyte population was accomplished for SIV-infected and uninfected rhesus macaques. Intravenous pertussis toxin provided a means of trapping recirculating cells in blood, where they could be sampled accurately and characterized by flow cytometry and other methods. By using this approach it was possible to demonstrate three important characteristics for the recirculating lymphocyte population in this animal model for AIDS. First, disease progression during the asymptomatic period is attended by an increasing proportion of CD4+ lymphocytes residing in tissue or lymph and a decreasing proportion of recirculating CD4+ cells present in blood. Thus, the drop in blood CD4+ T cells during asymptomatic infection is due mainly to redistribution of CD4+ T cells and not to a bulk depletion of this population. Second, the changes in CD4+ lymphocyte distribution are similar to the changes in CD8+ and CD20+ lymphocyte distributions within the recirculating pool. These findings suggest that the pathologic effect of SIV infection on lymphocyte distribution is not specific for the population of virus-susceptible cells but rather is a general feature of the immune system during infection. Third, we noted that bulk losses of recirculating CD4+ lymphocytes occurred only after the blood CD4+ T-cell count dropped below 300 cells/μl. These data indicate a fundamental change in function for the reticuloendothelial system at the late stage of disease, wherein recirculating cells are no longer sequestered and are depleted rapidly. The initial evidence for bulk depletion of recirculating CD4+ lymphocytes is roughly equivalent to the stage of disease progression at which clinical signs become evident, suggesting that loss of reticuloendothelial system integrity, bulk depletion of CD4+ lymphocytes, and frank disease are directly related. These findings also argue strongly against models claiming that declining blood CD4+ T-cell counts during asymptomatic infection are a direct result of virus infection and cell destruction.
The recognized splenomegaly and lymphadenopathy that occur during HIV or SIV infection, and studies of SIV-infected rhesus and HIV-infected patients showing that the loss of CD4+ T cells in the tissue population is less rapid than cell losses in the blood compartment, attest to altered properties of the recirculating lymphocyte populations (27–29). Early studies found that the percentage of CD4+ T cells in SIV-infected animals was similar to that in uninfected animals until the CD4/CD8 ratio dropped below 0.5. This ratio was affected by increases in the percentages of CD69−, interleukin 2R−, and CD45RA-dim CD8+ lymphocytes (27, 28). In HIV infection, the CD4/B cell ratio in asymptomatic patient tonsillar tissue was similar to that in uninfected controls, but the CD4/B cell ratio decreased markedly in symptomatic patients. Changes in the CD4/CD8 ratio occurred in asymptomatic patients due to increases in the number of CD8+ T cells, whereas the CD4/CD8 ratio in symptomatic patients was affected by losses of CD4+ T cells (29). Another example of lymphocyte compartmentalization was provided recently by studies of acute SIV infection in macaques showing that the decreased percentage of jejunal CD4+ T cells was not reflected by substantial changes in peripheral blood CD4+ T-cell counts during acute infection (36). Our work is different but complementary to these studies because we assessed the recirculating pool that specifically contributes to the blood cell counts rather than the total tissue pool which has many cells that migrate only rarely. We also assessed quantitative changes in CD8+ T- and CD20+ B-cell populations within the recirculating pool rather than using simple percentages and CD4/CD8 ratios that can be altered by either losses in one subset or increases in another subset. Our work also answers questions raised about the nature of the increased uptake of the DNA analog 5′-bromodeoxyuridine (BrdU) in SIV-infected macaques compared to that in uninfected animals (11). In that study, all major lymphocyte populations had increased uptake of BrdU. The turnover of BrdU was also similar in all lymphocyte subsets. These studies asked how a virus that principally infects CD4+ T cells could affect these other subsets with or without virus-mediated destruction and questioned where these populations could be reserved during the infection process. Overall, there is substantial support for a view of AIDS pathogenesis that emphasizes lymphocyte redistribution over bulk depletion as an important feature of asymptomatic infection.
Quantitative studies of rat and human tissue lymphocyte populations have shown that rats (200 g) have 2.5 × 1010 total (recirculating and tissue resident) lymphocytes per kg and humans (60 kg) have 7 × 109 total lymphocytes per kg (35). Therefore, the total number of lymphocytes is actually inversely proportional to body mass. We found that at peak lymphocytosis there are 2 × 107 lymphocytes/μl of blood in uninfected macaques. Assuming there is 100 ml of blood per kg, our estimates of 10% recirculating cells show that 2 × 109 cells/100 ml is the peak of lymphocytosis. Assuming that the entire recirculating pool is 10% of the total lymphocyte pool, we obtain an estimate of 2 × 1010 cells/kg as the total body lymphocyte count. This estimate fits well with earlier measurements of total lymphocyte populations in rats and humans.
The recirculating and tissue resident pools are clearly distinct. CD20+ B cells are underrepresented in the recirculating pool (∼15 to 20% in uninfected juvenile rhesus monkeys) compared with the tissue resident pool (∼35% in lymph nodes from the same animals in our studies). These pools are also quite different in regard to diseases such as Hodgkin’s lymphoma, where the spleen accumulates large numbers of lymphocytes but the blood cell counts are normal or only slightly increased (37). Recirculating and resident lymphocyte populations remain distinct as a result of regulated lymphocyte trafficking and sequestration. Individual properties of these populations may be altered by disease. The potential impact on peripheral blood cell counts of the proportional distribution of recirculating lymphocytes among blood and other compartments has not been well studied. Considering the importance of blood cell monitoring for many clinical problems, it is valuable to identify conditions that alter the proportional distribution of these cell populations and to show whether these effects are specific to individual lymphocyte subpopulations. Our data further emphasize the problems inherent in extrapolating lymphocyte population dynamics from blood samples. However, we note that the distribution of recirculating CD4+ T cells changes gradually over the period from infection to symptomatic disease and that this change will have only a small impact on calculations related to virus half-life and virus replication rates, as these studies assume a short-term, steady-state condition (9, 22).
The apparent paradox between decreasing blood cell counts and increasing tissue pools of lymphocytes is likely to be another consequence of persistent immune activation after HIV or SIV infection. Chronic stimulation of lymphocytes leads to increased levels of adhesion molecules and shifts the balance toward a greater proportion of cells being sequestered in lymphoid tissues (16, 17, 26, 34). Altered distribution of recirculating lymphocytes is an important contributing factor in the pathogenesis of AIDS and highlights critical cell adhesion and chemokine signaling mechanisms (3) as novel drug targets for therapy.
ACKNOWLEDGMENTS
This study was supported by PHS grants AI38491 and AI36643 (C.D.P.) and by grant RR00167 to the Wisconsin Regional Primate Research Center.
We thank James Thompson and the staff at the Wisconsin Regional Primate Research Center for assistance with the animal studies, and we are grateful to J. M. McCune for a critical reading of the manuscript.
Footnotes
Publication 38010 from the Wisconsin Regional Primate Research Center.
REFERENCES
- 1.Athanassiades T J, Morse S I. Lymphocytosis induced in mice by supernatant fluids of Bordetella pertussis cultures: a histopathological study. Blood. 1973;42:611–621. [PubMed] [Google Scholar]
- 2.Bargatze R F, Butcher E C. Rapid G protein-regulated activation event involved in lymphocyte binding to high endothelial venules. J Exp Med. 1993;178:367–372. doi: 10.1084/jem.178.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butcher E C, Picker L J. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 4.Dimitrov D S, Martin M A. HIV results in the frame. CD4+ cell turnover. Nature. 1995;375:194–195. doi: 10.1038/375194b0. [DOI] [PubMed] [Google Scholar]
- 5.Eyster M E, Rabkin C S, Hilgartner M W, Aledort L M, Ragni M V, Sprandio J, White G C, Eichinger S, de Moerloose P, Andes W A, Cohen A R, Manco-Johnson M, Bray G L, Schramm W, Hatzakis A, Lederman M M, Kessler C M, Goedert J J. Human immunodeficiency virus-related conditions in children and adults with hemophilia: rates, relationship to CD4 counts and predictive value. Blood. 1993;81:828–834. [PubMed] [Google Scholar]
- 6.Fahey J L, Taylor J M, Detels R, Hofmann B, Melmed R, Nishanian P, Giorgi J V. The prognostic value of cellular and serologic markers in infection with human immunodeficiency virus type 1. New Engl J Med. 1990;322:166–172. doi: 10.1056/NEJM199001183220305. [DOI] [PubMed] [Google Scholar]
- 7.Gierschik P. ADP-ribosylation of signal transducing guanine nucleotide-binding proteins by pertussis toxin. Curr Top Microbiol Immunol. 1992;175:69–98. doi: 10.1007/978-3-642-76966-5_4. [DOI] [PubMed] [Google Scholar]
- 8.Hinds P W I, Yin C, Salvato M S, Pauza C D. Pertussis toxin induces lymphocytosis in rhesus macaques. J Med Primatol. 1996;25:375–381. doi: 10.1111/j.1600-0684.1996.tb00032.x. [DOI] [PubMed] [Google Scholar]
- 9.Ho D D, Neumann A U, Perelson A S, Chen W, Leonard J M, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 10.Ioachim H L. Pathology of AIDS. J. B. Philadelphia, Pa: Lippincott Co.; 1989. [Google Scholar]
- 11.Mohri H, Bonhoeffer S, Monard S, Perelson A S, Ho D D. Rapid turnover of T lymphocytes in SIV-infected rhesus macaques. Science. 1998;279:1223–1227. doi: 10.1126/science.279.5354.1223. [DOI] [PubMed] [Google Scholar]
- 12.Morse S, Riester S. Studies on the leukocytosis and lymphocytosis induced by Bordetella pertussis. II. The effect of pertussis vaccine on the thoracic duct lymph and lymphocytes of mice. J Exp Med. 1967;125:619–628. doi: 10.1084/jem.125.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morse S I, Barron B A. Studies on the leukocytosis and lymphocytosis induced by Bordetella pertussis. III. The distribution of transfused lymphocytes in pertussis treated and normal mice. J Exp Med. 1970;129:663–672. doi: 10.1084/jem.132.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morse S I, Riester S K. Studies on the leukocytosis and lymphocytosis induced by Bordetella pertussis. I. Radioautographic analysis of the circulating cells in mice undergoing pertussis induced lymphocytosis. J Exp Med. 1967;125:401–411. doi: 10.1084/jem.125.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mosier D E. HIV results in the frame. CD4+ cell turnover. Nature. 1995;375:193–194. doi: 10.1038/375193b0. [DOI] [PubMed] [Google Scholar]
- 16.Muro-Cacho C A, Pantaleo G, Fauci A S. Analysis of apoptosis in lymph nodes of HIV-infected persons. Intensity of apoptosis correlates with the general state of activation of the lymphoid tissue and not with stage of disease or viral burden. J Immunol. 1995;154:5555–5566. [PubMed] [Google Scholar]
- 17.Ng T T C, Guntermann C, Nye K E, Parkin J M, Anderson J, Norman J E, Morrow W J W. Adhesion co-receptor expression and intracellular signalling in HIV disease: implications for immunotherapy. J Acquir Immune Defic Syndr. 1995;9:337–343. [PubMed] [Google Scholar]
- 18.O’Brien W A, Hartigan P M, Martin D, Esinhart J, Hill A, Benoit S, Rubin M, Simberkoff M S, Hamilton J D. Changes in plasma HIV-1 RNA and CD4+ lymphocyte counts and the risk of progression to AIDS. New Engl J Med. 1996;334:426–431. doi: 10.1056/NEJM199602153340703. [DOI] [PubMed] [Google Scholar]
- 19.O’Hara C J. Lymphoid system. In: Harawai S J, O’Hara C J, editors. Pathology and pathophysiology of AIDS and HIV-related diseases. C. V. St. Louis, Mo: Mosby Co.; 1989. pp. 136–199. [Google Scholar]
- 20.Pauza C D, Emau P, Salvato S, Trivedi P, MacKenzie D, Schultz K T, Malkovsky M. Transmission of SIV by intrarectal inoculation in rhesus monkeys. In: Girard M, Vallette L, editors. Proceedings: retroviruses of human AIDS and related animal diseases. Lyons, France: Foundation Marcel Merieux; 1993. pp. 151–156. [Google Scholar]
- 21.Pauza C D, Hinds P W, Yin C, Mckechnie T S, Hinds S B, Salvato M S. The lymphocytosis-promoting agent pertussis toxin affects virus burden and lymphocyte distribution in the SIV-infected rhesus macaque. AIDS Res Hum Retroviruses. 1997;13:87–95. doi: 10.1089/aid.1997.13.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perelson A S, Neumann A U, Markowitz M, Leonard J M, Ho D D. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science. 1996;271:1582–1586. doi: 10.1126/science.271.5255.1582. [DOI] [PubMed] [Google Scholar]
- 23.Rai K R, Chanana A D, Cronkite E P, Joel D D, Stevens J B. Studies on lymphocytes. XVIII. Mechanism of lymphocytosis induced by supernatant fluids of Bordetella pertussis cultures. Blood. 1971;38:49–59. [PubMed] [Google Scholar]
- 24.Reynolds J, Heron I, Dudler L, Trnka Z. T-cell recirculation in the sheep: migratory properties of cells from lymph nodes. Immunology. 1982;47:415–421. [PMC free article] [PubMed] [Google Scholar]
- 25.Roederer M. Getting to the HAART of T cell dynamics. Nat Med. 1998;4:145–146. doi: 10.1038/nm0298-145. [DOI] [PubMed] [Google Scholar]
- 26.Rosenberg Y J, Cafaro A, Brennan T, Greenhouse J G, Villinger F, Ansari A A, Brown C, McKinnon K, Bellah S, Yalley-Ogunro J, Elkins W R, Gartner S, Lewis M G. Virus-induced cytokines regulate circulating lymphocyte levels during primary SIV infections. Int Immunol. 1997;9:703–712. doi: 10.1093/intimm/9.5.703. [DOI] [PubMed] [Google Scholar]
- 27.Rosenberg Y J, Zack P M, Leon E C, White B D, Papermaster S F, Hall E, Greenhouse J J, Eddy G A, Lewis M G. Immunological and virological changes associated with decline in CD4/CD8 ratios in lymphoid organs of SIV-infected macaques. AIDS Res Hum Retroviruses. 1994;10:863–872. doi: 10.1089/aid.1994.10.863. [DOI] [PubMed] [Google Scholar]
- 28.Rosenberg Y J, Zack P M, White B D, Papermaster S F, Elkins W R, Eddy G A, Lewis M G. Decline in the CD4+ lymphocyte population in the blood of SIV-infected macaques is not reflected in lymph nodes. AIDS Res Hum Retroviruses. 1993;9:639–646. doi: 10.1089/aid.1993.9.639. [DOI] [PubMed] [Google Scholar]
- 29.Rosok B I, Bostad L, Voltersvik P, Bjerknes R, Olofsson J, Asjo B, Brinchmann J E. Reduced CD4 cell counts in blood do not reflect CD4 cell depletion in tonsillar tissue in asymptomatic HIV-1 infection. AIDS. 1996;10:F35–F38. [PubMed] [Google Scholar]
- 30.Schnappauf H, Schnappauf U. Drainage of the thoracic duct and amount of the “easily mobilized” lymphocytes in calves, sheep and dogs. Blut. 1968;16:209–220. doi: 10.1007/BF01631670. [DOI] [PubMed] [Google Scholar]
- 31.Sprent J. Circulating T and B lymphocytes of the mouse. I. Migratory properties. Cell Immunol. 1973;7:10–39. doi: 10.1016/0008-8749(73)90180-9. [DOI] [PubMed] [Google Scholar]
- 32.Sprent J, Tough D. HIV results in the frame. CD4+ cell turnover. Nature. 1995;375:194. doi: 10.1038/375194a0. [DOI] [PubMed] [Google Scholar]
- 33.Springer T A. Traffic signals for lymphocye recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 34.Stent G, Cameron P U, Crowe S M. Expression of CD11/CD18 and ICAM-1 on monocytes and lymphocytes of HIV-1 infected individuals. J Leukocyte Biol. 1994;56:304–309. doi: 10.1002/jlb.56.3.304. [DOI] [PubMed] [Google Scholar]
- 35.Trepel F. Number and distribution of lymphocytes in man. A critical analysis. Klin Wochenschn. 1974;52:511–515. doi: 10.1007/BF01468720. [DOI] [PubMed] [Google Scholar]
- 36.Veazey R S, DeMaria M, Chalifoux L V, Shvetz D E, Pauley D R, Knight H L, Rosenzweig M, Johnson R P, Desrosiers R C, Lackner A A. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 37.Westermann J, Pabst R. Lymphocyte subsets in the blood: a diagnostic window on the lymphoid system? Immunol Today. 1990;11:406–410. doi: 10.1016/0167-5699(90)90160-b. [DOI] [PubMed] [Google Scholar]
- 38.Wolthers K C, Bea G, Wisman A, Otto S A, de Roda Husman A M, Schaft N, de Wolf F, Goudsmit J, Coutinho R A, van der Zee A G, Meyaard L, Miedema F. T cell telomere length in HIV-1 infection: no evidence for increased CD4+ T cell turnover. Science. 1996;274:1543–1547. doi: 10.1126/science.274.5292.1543. [DOI] [PubMed] [Google Scholar]
- 39.Young A J, Hay J B, MacKay C R. Lymphocyte recirculation and life span in vivo. Curr Top Microbiol Immunol. 1993;184:160–173. doi: 10.1007/978-3-642-78253-4_13. [DOI] [PubMed] [Google Scholar]