Abstract
Background
Sporotrichosis is a mycosis frequently caused by Sporothrix schenckii, Sporothrix brasiliensis, and Sporothrix globosa. The cell wall is a species-specific fungal structure with a direct role in activating the host’s immune response. The current knowledge about anti-Sporothrix immunity comes from studies using S. schenckii or S. brasiliensis and murine cells. Macrophages and dendritic cells detect and eliminate pathogens, and although the function of these cells links innate with adaptive immunity, little is known about their interaction with Sporothrix spp.
Methods
S. schenckii, S. brasiliensis, and S. globosa conidia or yeast-like cells were co-incubated with human monocyte-derived macrophages or dendritic cells, and the phagocytosis and cytokine stimulation were assessed. These interactions were also performed in the presence of specific blocking agents of immune receptors or fungal cells with altered walls to analyze the contribution of these molecules to the immune cell-fungus interaction.
Results
Both types of immune cells phagocytosed S. globosa conidia and yeast-like cells to a greater extent, followed by S. brasiliensis and S. schenckii. Furthermore, when the wall internal components were exposed, the phagocytosis level increased for S. schenckii and S. brasiliensis, in contrast to S. globosa. Thus, the cell wall components have different functions during the interaction with macrophages and dendritic cells. S. globosa stimulated an increased proinflammatory response when compared to the other species. In macrophages, this was a dectin-1-, mannose receptor-, and TLR2-dependent response, but dectin-1- and TLR2-dependent stimulation in dendritic cells. For S. schenckii and S. brasiliensis, cytokine production was dependent on the activation of TLR4, CR3, and DC-SIGN.
Conclusion
The results of this study indicate that these species are recognized by immune cells differently and that this may depend on both the structure and cell wall organization of the different morphologies.
Keywords: cell wall, cytokine production, fungal morphologies, immune response, phagocytosis
Introduction
Sporotrichosis is a subcutaneous mycosis that affects humans and other mammals. Its etiological agents are members of the Sporothrix pathogenic clade, and currently, the infection is mainly caused by Sporothrix schenckii, Sporothrix brasiliensis, and Sporothrix globosa.1–3 This disease has global distribution; however, most of the animal and human cases are concentrated in countries located between the Tropics of Cancer and Capricorn, with exceptions reported in South Africa, China, Japan, and the anecdotic cases documented in European countries and the United States of America.3–10 The lymphocutaneous infection is the most frequent clinical form of sporotrichosis, which rarely becomes a life-threatening disseminated disease.11–13 However, when there is temporal or permanent immunosuppression in the host, the subcutaneous infection can develop into a systemic disease with high mortality rates in both humans and animals.5,10,14,15 Historically, S. schenckii was the first fungal species associated with sporotrichosis, and as a consequence, it is the Sporothrix species most thoroughly studied at both basic and clinical levels.3,16 S. globosa, like S. schenckii, is distributed worldwide;4 however, within the pathogenic clade is considered the least virulent species due to its limited growth at high temperatures.17–19 S. brasiliensis has been responsible for infectious outbreaks in Brazil and is considered the most virulent species.18–21 These three fungal species can grow as conidium-producer mycelia in the environment and undergo dimorphism to yeast-like cells when colonizing and damaging the host tissues. Thus, conidia and hyphae are the first fungal morphologies to establish contact with the host. However, the zoonotic and epizootic S. brasiliensis outbreaks have changed this paradigm, and currently, not only conidia but also yeast-like cells can infect host tissues.3,5,21,22
The study of antifungal immunity is nowadays an active research area because it is thought that the knowledge accumulated in this subject could be translated to immunotherapeutic approaches to help in the treatment of mycoses caused by etiological agents that commonly develop antifungal drug resistance; and also because it is clear that most of the severe presentations of mycosis and those associated with high mortality rates occur in individuals without an intact immunity, stressing the role of the immune response in controlling most of the aggressive manifestations of fungi when interacting with the host.23,24 Since the cell wall is the outermost Sporothrix structure and thus is the primary point of contact with components of the host immunity it has been thoroughly studied in recent years.19,22,25–32 Thus far, it is known that the S. schenckii and S. brasiliensis cell wall contains two layers. Chitin, β-1,6-, β-1,4-, and β-1,3-glucans are the main components of the inner layer;3,26,28 while N-linked and O-linked glycoproteins and peptidorhamnomannan are the main components of the outermost layer.5,28,33,34
Thus far, most of our knowledge about anti-Sporothrix immunity comes from the study of the interaction of S. schenckii with murine immune cells, and to a lesser extent the study of S. brasiliensis using the same experimental model.35 Cellular components of innate immunity, such as granulocytes, monocytes, macrophages, and dendritic cells are capable of detecting and kill fungal pathogens, recruit additional immune effectors, and to link innate and adaptive immunities.36 Currently, there are relatively few reports dealing with the Sporothrix-macrophage interaction.37–41 Using murine macrophages, it has been demonstrated that S. schenckii chitin-rich heteroglycan modulates cytokine production and promoted S. schenckii phagocytosis.42 Regarding immune receptors involved in this interaction, it was found that dectin-1 expression was increased in peritoneal murine macrophages after infection with S. schenckii, and there were nitric oxide and cytokines productions driven by this immune receptor;43 while TLR2 is required for fungal phagocytosis and the stimulation of IL-1β, IL-12, and TNF-α.44 TLR4 has also been involved in the sensing of S. schenckii by murine macrophages, and the absence of this receptor led to an increment in TGF-β production45 and a reduction of pro and anti-inflammatory cytokines.46 In the same line, TLR4 is required for phagocytosis of S. brasiliensis and stimulation of proinflammatory cytokines.47 Interestingly, TLR2 seems that does not contribute to cytokine stimulation when S. brasiliensis interacts with murine macrophages, but it is required for phagocytosis.48
The interaction of Sporothrix cells with human macrophages has also been studied. Thus far, it is known that S. schenckii N-linked and O-linked glycans promote uptake by human monocyte-derived macrophages in a dectin-1-, mannose receptor- (MR), complement receptor 3- (CR3), and TLR4-dependent mechanism;29,31 while peptidorhamnomannan seems to be the CR3 ligand for stimulation of IL-1β by both S. schenckii and S. brasiliensis cells.49 In addition, S. schenckii rhamnose-containing oligosaccharides are required for fungal uptake by human monocyte-derived macrophages in a TLR4-dependent pathway.22 The S. globosa-macrophage interaction has been lesser studied than that described for the other two species. Using murine macrophages, it has been reported that S. globosa melanin inhibits antigen presentation in macrophages, which could be an immunoevasion mechanism.50 When interacting with human macrophages, S. globosa melanin inhibited fungal phagocytosis, protected from radical oxygen and nitrogen species, negatively affected the production of proinflammatory cytokines, and modulated TLR2 and TLR4 expression.51
The information related to the Sporothrix-dendritic cell interactions is even more scarce. Immature human monocyte-derived dendritic cells were stimulated with S. schenckii yeast-like cells, and was found that fungal isolates from cutaneous origins were more potent to stimulate a strong Th1 response than strains from the visceral origin.52 Murine bone marrow-dendritic cells stimulated with S. schenckii cell wall proteins showed higher expression of CD80, CD86, and CD40, but not TLR-4, and Th1-prone cytokine profile with IL-6, IL-17A, and TNFα production.53 Similarly, S. schenckii yeast-like cells stimulated the production of a Th1-prone cytokine profile and phagocytosis when interacting with these immune cells.54,55
Despite all this information, the interaction between macrophages or dendritic cells with different Sporothrix species is poorly studied. Therefore, here we assessed the relevance of fungal morphology, cell wall components, and immune receptors on the interaction of different Sporothrix species when interacting with either human monocyte-derived macrophages or monocyte-derived dendritic cells.
Materials and Methods
Strains and Culturing Conditions
The following strains were used in this study: S. schenckii ATCC MYA-4821, S. brasiliensis ATCC MYA 4823, and S. globosa FMR 9624. All are clinical isolates characterized by molecular techniques elsewhere.32,56 These strains of S. schenckii and S. brasiliensis are considered model organisms of these species, and their genomes and transcriptome have been previously sequenced.57–59 Cells were grown in YPD medium, pH 4.5 (1% [w/v] yeast extract, 2% [w/v] gelatin peptone, and 3% [w/v] dextrose) at 28°C, and 2% [w/v] agar was added when the solid medium was required. For conidia propagation, cells were grown on YPD, pH 4.5 plates at 28°C and after seven days of incubation, 10 mL of deionized water was added to the surface of the plates; this was gently dispersed with a plastic spreader, and the conidia suspension was collected.60 Yeast-like cells were obtained by inoculating conidia in YPD, pH 7.8 broth, and incubating at 37°C and 120 rpm for four days.26 For both morphotypes, cells were thoroughly washed with deionized water and kept at −20°C until used. To inactivate cells by heating (heat-killed cells, HK), these were incubated at 60°C for 2 h, and the loss of viability was confirmed by incubation in YPD plates, pH 4.5, at 28°C for 5 days.26 The N-linked and O-linked glycans were removed from cell walls by incubating with endoglycosidase H and β-elimination, respectively, as reported.22,29
Ethics Statement
This study was conducted following the Declaration of Helsinki. In agreement, cells were obtained from healthy adult volunteers after information about the study was disclosed and written informed consent was signed. The use of human cells was approved by the Ethics Committee from Universidad de Guanajuato (reference CIBIUG-P36-2019).
Differentiation of Primary Human Cells to Macrophages and Dendritic Cells
Human PBMCs were isolated from venous blood using Histopaque-1077 (Sigma-Aldrich, Saint Louis, MO, USA), as described.61 For differentiation to macrophages, 1 mL aliquots containing 2×107 PBMCs in RPMI supplemented with 1% (v/v) penicillin-streptomycin solution (PS, Sigma-Aldrich) were seeded in flat bottom 12-well plates and incubated 1.5 h at 37°C, 5% (v/v) CO2. Non-adherent cells were discarded, and the remaining adherent cells were washed with PBS at 37°C, and 1 mL of X–VIVO serum-free medium (Lonza, Basel, Switzerland) supplemented with 1% (v/v) PS and 10 ng mL−1 recombinant human granulocyte-macrophage colony-stimulating factor (Sigma-Aldrich) were included per well.62 For differentiation to immature dendritic cells (DCs), adherent cells were added 1 mL X–VIVO serum-free medium supplemented with 1% (v/v) PS, 500 U mL−1 recombinant human IL-4 (Sigma-Aldrich), and 800 U mL−1 recombinant human granulocyte-macrophage colony-stimulating factor (Sigma-Aldrich) for 6–7 days.63,64 In both cases, plates were incubated for 6–7 days at 37 °C, 5% (v/v) CO2, replacing the medium every 2 days. Differentiation to DC cells was confirmed by flow cytometry, detecting DC-SIGN with FITC-conjugated mAb anti-human DC-SIGN (Thermo-Fisher Scientific, Waltham, MA, USA), as described elsewhere.63
Phagocytosis Assays
For fungal labeling, cells were incubated with 1 mg mL−1 Acridine Orange (Sigma-Aldrich) for 30 min at room temperature, the excess dye was washed with PBS, and cell concentration was adjusted at 3×107 cells mL−1.65 Six-well plates were used to perform the interactions at an immune cell: fungus ratio of 1:6 in 800 µL DMEM medium (Sigma-Aldrich). The plates were incubated for 2 h at 37°C and 5% (v/v) CO2,29,66 immune cells were detached from plates with chilled PBS and incubated with 1.25 mg mL−1 Trypan Blue.67 The phagocytic event was analyzed by cytometry using a FACSCanto II system (Becton Dickinson, Franklin Lakes, NJ, USA). Fifty thousand events were collected per sample through the FL1 and FL2 channels, which were previously calibrated with non-labeled immune cells.22,29,65,67 The following compounds were used in preincubation experiments: 200 μg mL−1 laminarin (Sigma-Aldrich),68 10 μg mL−1 anti-MR antibody (Thermo-Fisher Scientific, MA5-44033), 10 μg mL−1 anti-TLR4 antibody (Santa Cruz Biotechnology, Dallas, TX, sc-293072), 10 μg mL−1 anti-TLR2 antibody (Thermo-Fisher Scientific, 16–9922-82), 10 μg mL−1 anti-CD209 antibody (DC-SIGN; Thermo Fisher Scientific, 14–2099-82), 10 μg mL−1 anti-CD11b antibody (CR3, Thermo Fisher Scientific, MA5-16528), Isotype-matched, irrelevant IgG1 antibody (10 μg mL−1, Santa Cruz Biotechnology, Cat. No. sc-52003, used as a control in experiments where MR and TLR4 were blocked), 10 μg mL−1 IgG2aκ antibody (Thermo-Fisher Scientific, 14–4724-85, to control TLR2 and CD209 blocking experiments), and 10 μg mL−1 IgG2 antibody (R&D, Minneapolis, MN, USA, Cat. No. MAB9794, to control CD11b blocking assays).26,49,69 Immune cells were preincubated for 1 h at 37°C and 5% (v/v) CO2 with any of these compounds before interacting with fungal cells. Even though the above-listed reagents were negative for bacterial LPS, the preincubations were performed in the presence of 5 μg mL−1 polymyxin B (Sigma-Aldrich).70,71 Cells detected with the FL1 channel were considered in the early stage of phagocytosis, cells detected by both FL1 and FL2 channels were regarded as in the intermediate stage of phagocytosis, and those detected only with the FL2 channel were considered in the late stage of phagocytosis.22,29,65
Cytokine Stimulation
The cell-cell interactions were performed in 96-well microplates. Each well contained 100 µL of immune cells in RPMI 1640 Dutch modification (Sigma-Aldrich) at 1×106 cells mL−1 and 100 µL of fungal cells at 1×106 mL−1. Plates were incubated for 24 h at 37°C with 5% (v/v) CO2. Similar to the experiments described in the previous section, in some cases, immune cells were pre-incubated for 60 min at 37°C with 5% (v/v) CO2 with one of the antibodies already described or laminarin and the interactions were performed in the presence of 5 μg mL−1 polymyxin B (Sigma-Aldrich).70,71 After incubation, plates were centrifuged for 10 min at 1800 x g at 4°C, supernatants were collected and used for cytokine quantification by ELISA. The Standard ABTS ELISA Development kits (Peprotech, Cranbury, NJ, USA) were used for quantification of tumor necrosis factor-alpha (TNFα), interleukin 6 (IL-6), and interleukin 10 (IL-10); while DuoSet ELISA from R&D was used for interleukin 1β (IL-1β) and Interleukin 12 p70 (IL-12 p70) quantification. Immune cells suspended in RPMI 1640 Dutch modification were included in each plate as controls. In all cases, these control wells gave threshold levels that were subtracted from all the cytokine determinations.
Statistical Analysis
The Mann–Whitney U and Kruskal–Wallis tests from the GraphPad Prism 6 software were used for data analysis, establishing significance at P< 0.05. Since the Kruskal–Wallis analysis indicated that data had no normal distribution (P< 0.05), non-parametric tests were applied to the data. All experiments were carried out in duplicate with samples from eight healthy donors. Results are shown as the mean and standard deviation.
Results
Differential Phagocytosis of Sporothrix schenckii, Sporothrix brasiliensis, and Sporothrix globosa by Human Monocyte-Derived Macrophages
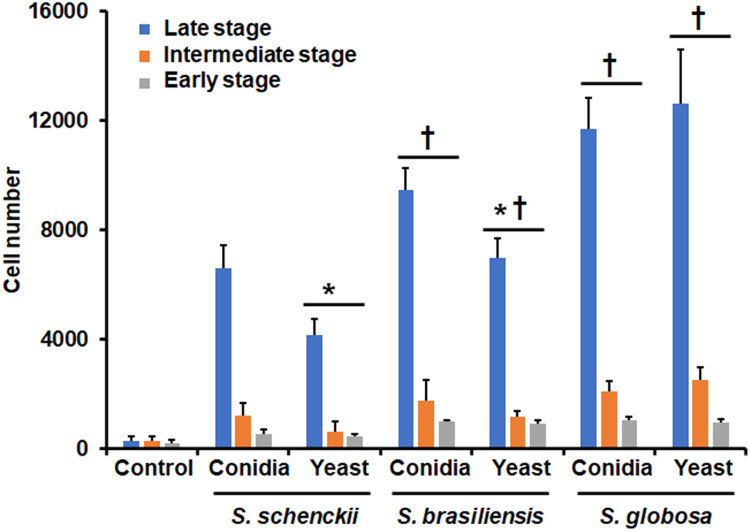
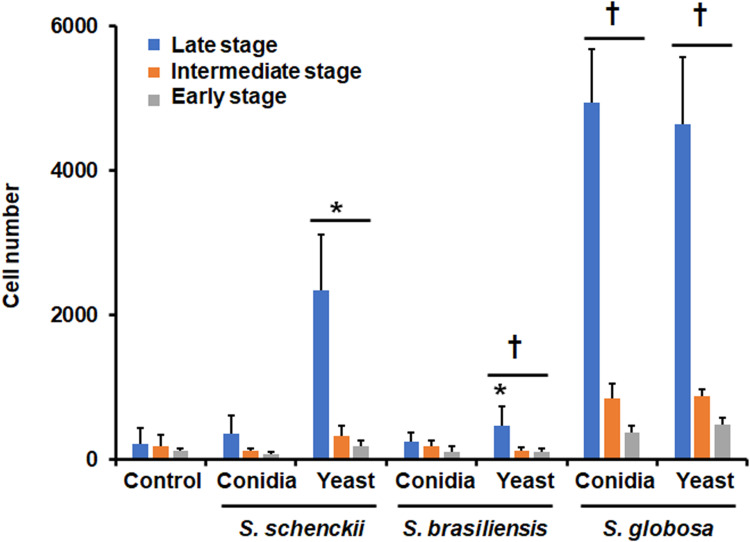
To compare the ability of primary human phagocytic cells to uptake fungi from the three most frequently isolated Sporothrix species from medical and veterinary samples, named S. schenckii, S. brasiliensis, and S. globosa, conidia or yeast-like cells were grown and co-incubated with the human cells. Since the phagocytic process was analyzed by flow cytometry, phagocytosis of germling could not be analyzed using this strategy, as hyphae can clothe the instrument pipes.72 When conidia were used in the interactions, this tended to be more phagocytosed than yeast-like cells in S. schenckii and S. brasiliensis, but for S. globosa, conidia, and yeast-like cells were similarly phagocytosed (Figure 1). When the phagocytosis of the three species was compared among themselves, S. schenckii cells were the less phagocytosed, followed by S. brasiliensis, and the most phagocytosed species was S. globosa. This observation applied to the two analyzed morphologies (Figure 1). In addition, these differences were observed only in the intermediate and late stages of phagocytosis (Figure 1). There were no significant differences in the early stage of the immune process when S. brasiliensis cells were compared with S. globosa cells (Figure 1). Control wells containing only the human cells showed threshold uptake levels (Figure 1).
Figure 1.
Phagocytosis of Sporothrix schenckii, Sporothrix brasiliensis, and Sporothrix globosa by human monocyte-derived macrophages. Interactions of human monocyte-derived macrophages and Sporothrix cells at a ratio 1:6 were incubated for 2 h at 37°C, and 5% (v/v) CO2. Macrophages were analyzed by flow cytometry, collecting 50,000 events, which were defined as a human cell interacting with at least one fluorescent fungal cell. Control, human cells were incubated only with PBS. *P < 0.05 when compared to conidia from the same species. †P < 0.05 when compared with S. schenckii cells of the same morphology. Cell numbers obtained with S. brasiliensis conidia and yeast-like cells at the intermediate and late stages were significantly different from those obtained with S. globosa cells (P < 0.05). Data are shown as means ± SD from eight donors analyzed by duplicate.
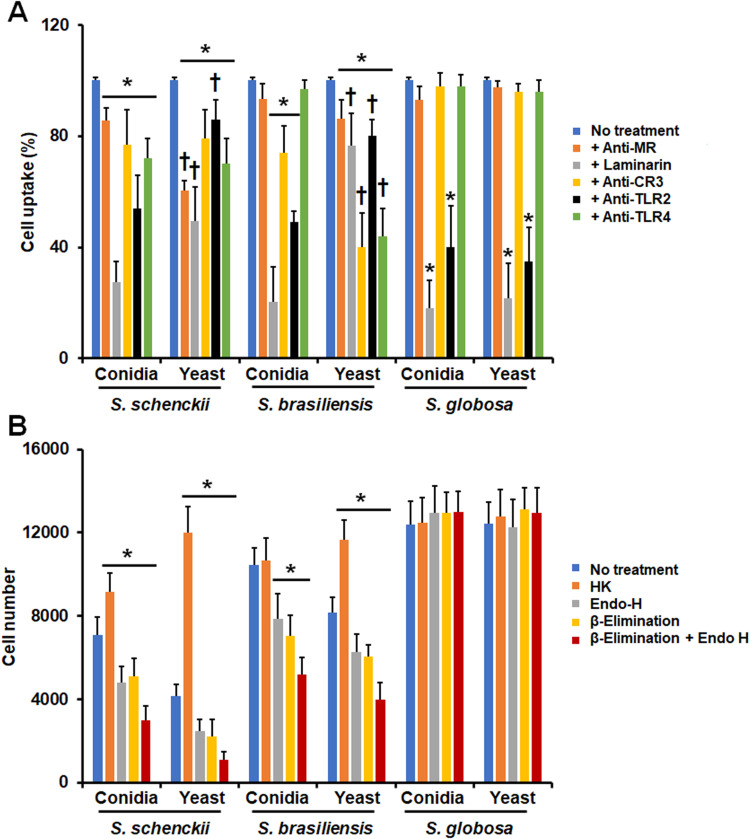
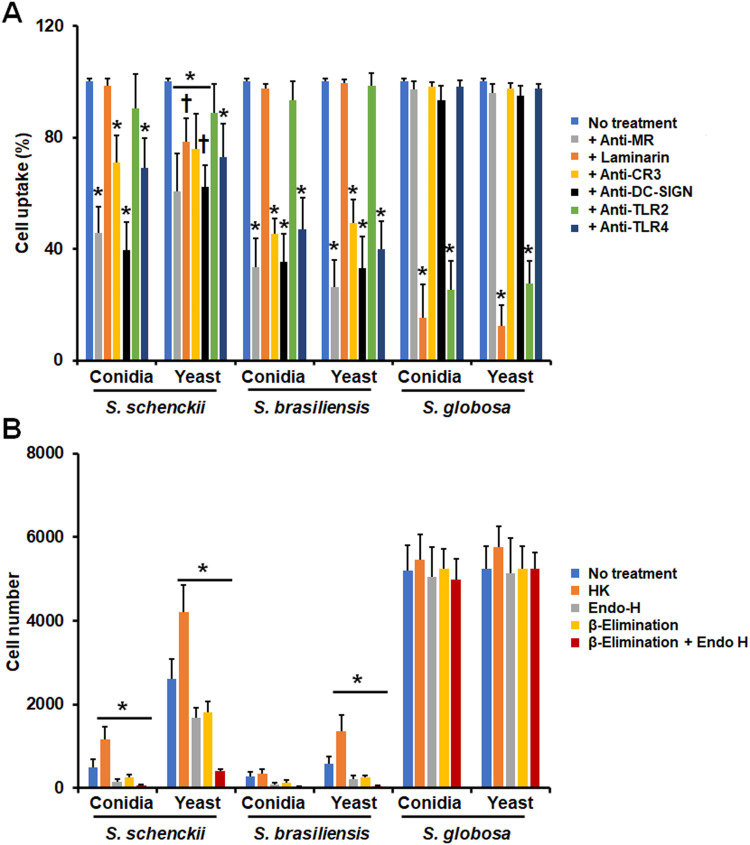
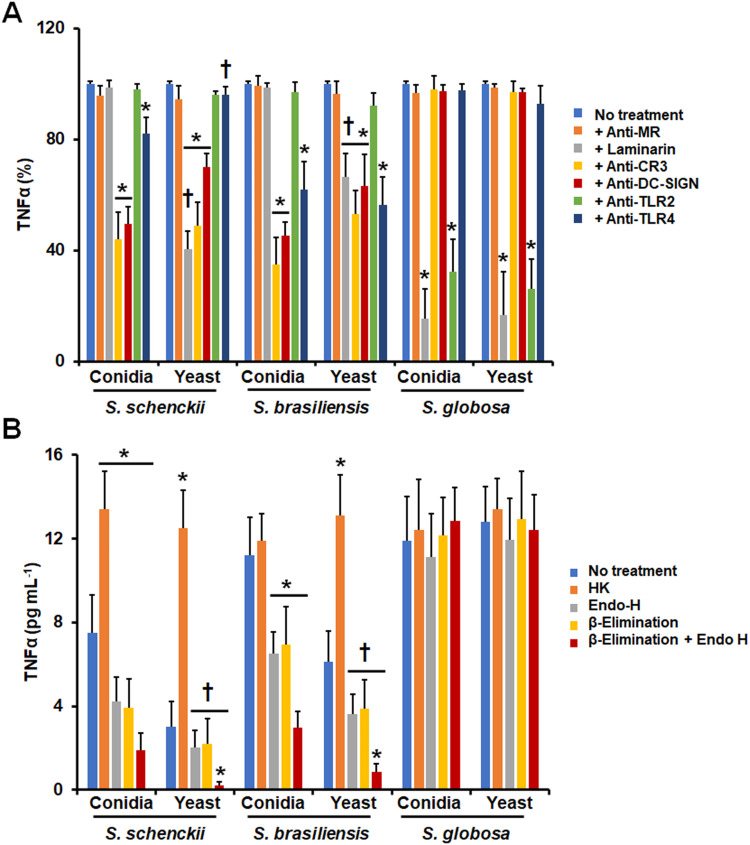
Next, we assessed the contribution of immune receptors on the uptake of either conidia or yeast-like cells from the three species under study. We focused on MR, dectin-1, CD11b (Complement receptor-3; CR3) TLR2, and TLR4, as these receptors have been previously involved in the recognition of S. schenckii and S. brasiliensis cells.26,43–46,49 For this, the immune cells were preincubated with monoclonal antibodies against particular receptors or with laminarin, an antagonist of dectin-1,62,69 before being challenged with the fungal cells. Since most of the fungal cells interacting with monocyte-derived macrophages are in the late stage of the phagocytic process, under our experimental conditions, we focused only on the analysis of this subset. For the case of S. schenckii conidia and yeast-like cells, the five receptors under analysis were required for fungal uptake, as the blocking of any of them significantly reduced the fungal uptake (Figure 2A). However, the contribution of receptors varied in conidia and yeast-like cells, being MR more relevant for yeast-like cell uptake than in conidia and dectin-1 and TLR2 for conidia uptake (Figure 2A). For the case of S. brasiliensis cells, the five receptors were required for proper yeast-like cells uptake, but MR was dispensable for conidia phagocytosis (Figure 2A). Like the observation in S. schenckii, the receptor relevance varied depending on the fungal morphology, being dectin-1, and TLR2 more relevant for conidia uptake and CR3 and TLR4 for yeast-like cells phagocytosis (Figure 2A). Interestingly, S. globosa conidia and yeast-like cells were phagocytosed via dectin-1 and TLR2, whilst MR, CR3, and TLR4 were dispensable for fungal uptake (Figure 2A). Isotype-matched irrelevant antibodies were used to preincubate human cells and did not affect the fungal phagocytosis of any of the morphologies or species under study (data not shown).
Figure 2.
Contribution of pattern recognition receptors and cell wall components on the phagocytosis of Sporothrix schenckii, Sporothrix brasiliensis, and Sporothrix globosa by human monocyte-derived macrophages. In (A), Human monocyte-derived macrophages were preincubated with 200 μg mL−1 laminarin or 10 μg mL−1 of any of the following antibodies: anti-mannose receptor (MR), anti-CR3, anti-TLR2, or anti-TLR4. Then, cells were coincubated with Acridine Orange-labeled conidia or yeast-like cells at a macrophage-fungus ratio of 1:6, for 2 h at 37°C and 5% (v/v) CO2. Human cells were analyzed by flow cytometry, collecting 50,000 events, which were defined as a human cell interacting with at least one fungal cell. All the interactions were performed in the presence of 5 μg mL−1 polymyxin B. No treatment, cells preincubated with PBS. Results correspond to cells in the late stage of phagocytosis. For all cases, 100% corresponds to human cells preincubated with PBS, and the absolute values were similar to those shown in Figure 1 or (B) of this figure. CR3, complement receptor 3. *P < 0.05 when compared to the no-treatment condition of the same strain. †P< 0.05 when compared to conidia from the same species. In (B), Similar experiments as described in (A), but human cells were not preincubated with any blocking agent. Before coincubation with human monocyte-derived macrophages, conidia, and yeast-like cells were inactivated by heat (HK), treated with endoglycosidase H (Endo-H), β-eliminated to remove O-linked glycans, or both treated with endoglycosidase H and β-elimination. No treatment refers to live cells without any treatment. *P < 0.05 when compared to the no-treatment condition of the same strain. For both panels, data were shown as means ± SD from eight donors analyzed by duplicate.
The contribution of cell wall components to fungal uptake by human monocyte-derived macrophages was also analyzed. For this, cells were inactivated by heat, as this treatment artifactually exposes internal cell wall components, such as β-1,3-glucan, and chitin at the cell wall surface.26,62,69 Moreover, cell wall N-linked or O-linked glycans were removed by treatment with endoglycosidase H or β-elimination, respectively.22,29,31 The S. schenckii HK conidia and yeast-like cells were more phagocytosed than live cells, but for the case of S. brasiliensis, this treatment has only a positive effect on the phagocytosis of yeast-like cells and had no effect on the S. globosa uptake (Figure 2B). Removal of N-linked glycans, O-linked glycans, or both kinds of glycans negatively affected the phagocytosis of the S. schenckii and S. brasiliensis conidia and yeast-like cells, but once again, phagocytosis of S. globosa was not affected by trimming of these cell wall components (Figure 2B). Collectively, these results suggest that conidia and yeast-like cells from the three species under analysis are differentially recognized by human monocyte-derived macrophages, and the pattern recognition receptors and cell wall components have species- and morphology-specific relevance.
Cytokine Production by Human Monocyte-Derived Macrophages Stimulated with Sporothrix schenckii, Sporothrix brasiliensis, and Sporothrix globosa
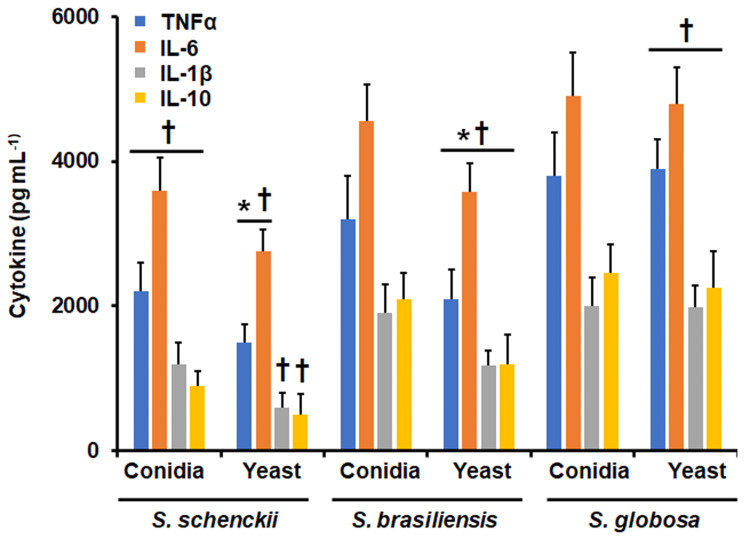
In addition to the study of the phagocytic process, we also assessed the ability of human monocyte-derived macrophages to produce cytokines after stimulation by the species under study. The TNFα and IL-6 levels stimulated by S. schenckii yeast-like cells were significantly lower when compared to the levels stimulated by S. schenckii conidia; whilst IL-1β and IL-10 levels were similar when cells were stimulated by any of these to S. schenckii morphologies (Figure 3). For S. brasiliensis, the four cytokines analyzed were stimulated at lower levels by yeast-like cells, when compared with conidia (Figure 3). However, when cells were stimulated with S. globosa conidia or yeast-like cells these produced similar levels of TNFα, IL-6, IL-1β, and IL-10 (Figure 3). When comparing the cytokine levels stimulated among the three different species, S. schenckii conidia stimulated the lowest TNFα, IL-6, IL-1β, and IL-10 levels, and conidia from S. brasiliensis and S. globosa stimulated similar levels of the four cytokines (Figure 3). Similarly, S. schenckii yeast-like cells stimulated the lowest cytokines levels, while S. brasiliensis intermediate and S. globosa yeast-like cells had the highest cytokine levels (Figure 3).
Figure 3.
Cytokine production by human monocyte-derived macrophages stimulated with Sporothrix schenckii, Sporothrix brasiliensis, and Sporothrix globosa. Human and fungal cells were coincubated for 24 h, and the supernatants were collected and used for cytokine quantification by ELISA. *P < 0.05, when compared with the cytokine level stimulated by conidia from the same species. †P < 0.05, when compared with the same morphology of the other two fungal species analyzed. Results are shown as mean ± standard deviation from data generated with samples from eight donors analyzed by duplicate.
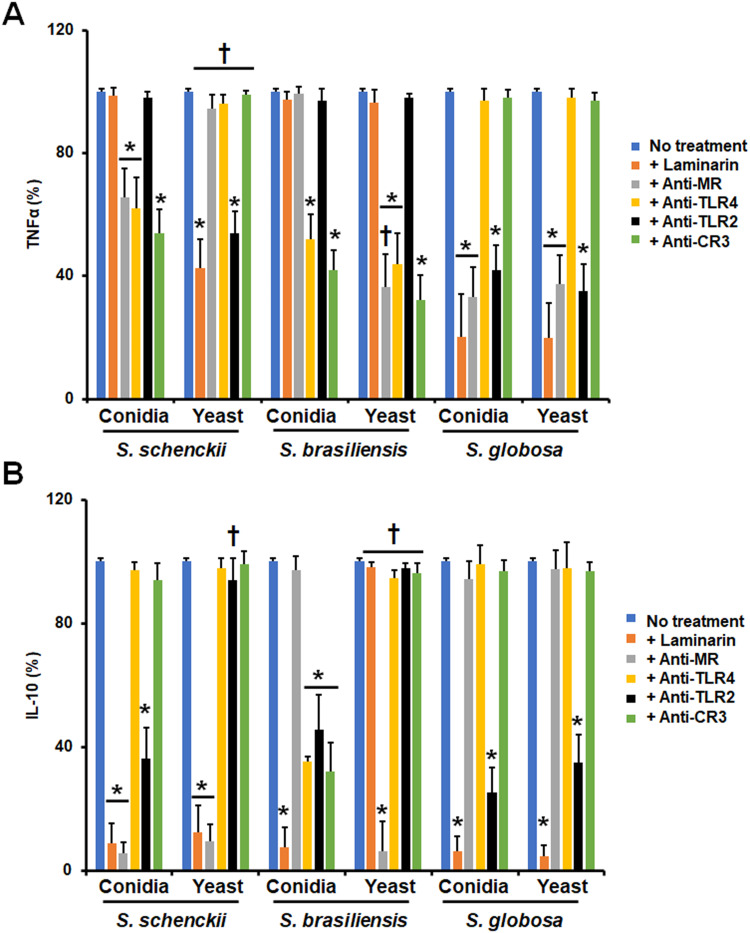
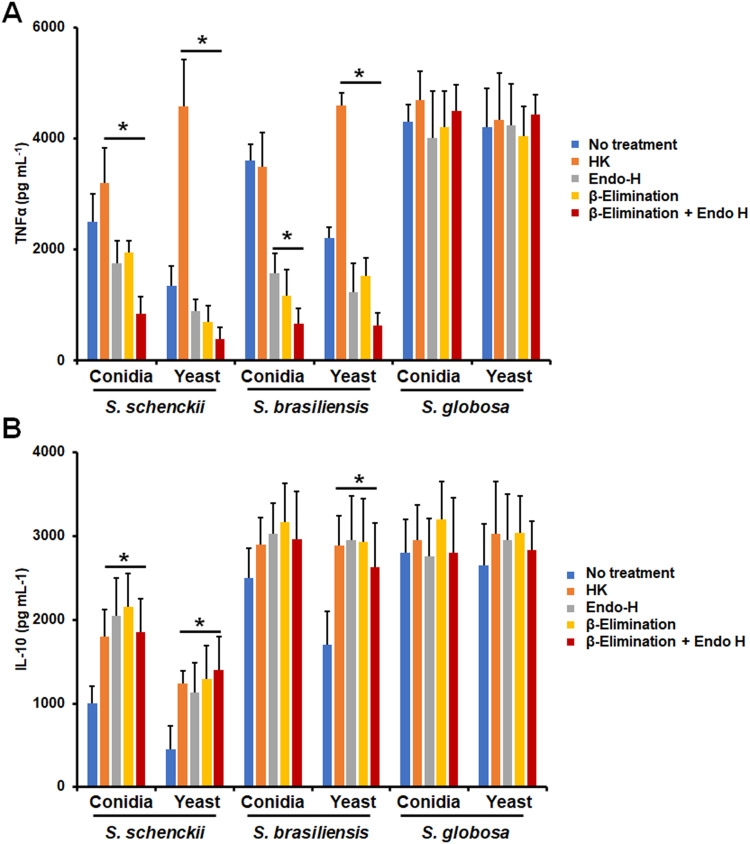
The TNFα and IL-10 production were taken as readouts of the host-fungus interaction and we also explored the contribution of different PRRs to the stimulation of both cytokines. For the case of TNFα, its stimulation by S. schenckii conidia was negatively influenced when MR, TLR4, or CR3 were blocked, suggesting a role of these receptors in the induction of this proinflammatory cytokine (Figure 4A). However, for S. schenckii yeast-like cells only dectin-1 and TLR2 were required for proper cytokine production, being dispensable the rest of the receptors analyzed (Figure 4A). S. brasiliensis conidia and yeast-like cells stimulated TNFα via TLR4 and CR3, but in the latter, there was also a contribution of MR (Figure 4 A); while in S. globosa, both conidia and yeast-like cells have a similar dependence in dectin-1, MR, and TLR2 for cytokine induction (Figure 4A). When a similar approach was used to analyze the relevance of receptors for IL-10 production, we found that S. schenckii conidia and yeast-like cells stimulated this cytokine via dectin-1 and MR, but in case of conidia, TLR2 was also involved (Figure 4B). Dectin-1, TRL2, TLR4, and CR3 were required for proper IL-10 stimulation by S. brasiliensis conidia, an observation that contrasted with the sole dependency on MR to IL-10 stimulation by S. brasiliensis yeast-like cells (Figure 4B). Finally, both morphologies of S. globosa did not show significant differences between them, both stimulating IL-10 via dectin-1 and TLR2 (Figure 4B). Isotype-matched irrelevant antibodies were used to preincubate human cells and did not affect the fungal phagocytosis of any of the morphologies or species under study (data not shown).
Figure 4.
Contribution of pattern recognition receptors to cytokine production by human monocyte-derived macrophages stimulated with Sporothrix schenckii, Sporothrix brasiliensis, and Sporothrix globosa. Human cells were preincubated with 200 μg mL−1 laminarin or 10 μg mL−1 of any of the following antibodies: anti-mannose receptor (MR), anti-complement receptor 3 (CR3), anti-TLR2, or anti-TLR4. Then, cells were coincubated with either conidia or yeast-like cells of the fungal species for 24 h at 37°C and 5% (v/v) CO2. Supernatants were collected and used to measure the levels of TNFα (A) or IL-10 (B) by ELISA. No treatment, cells preincubated with PBS. In all cases, 100% corresponds to the system with no treatment, and the absolute values were similar to those shown in Figure 3. *P < 0.05 when compared to the no-treatment condition of the same strain. †P< 0.05 when compared to conidia from the same species. Results are shown as mean ± standard deviation from data generated with samples from eight donors analyzed by duplicate.
We also assessed the contribution of cell wall components to TNFα and IL-10 stimulation by monocyte-derived macrophages. Both, S. schenckii HK conidia and yeast-like cells stimulated higher TNFα levels than live cells, but this cytokine production was significantly reduced when N-linked or O-linked glycans or the combination of both were removed from the wall (Figure 5A). Similarly, both, N-linked and O-linked glycans were required for proper TNFα stimulation by S. brasiliensis conidia or yeast-like cells, but only in the case of HK yeast-like cells was observed a stronger cytokine production when compared with live cells (Figure 5A). None of the cell wall treatments affected the ability of S. globosa conidia or yeast-like cells to stimulate TNFα (Figure 5A). Similar results were observed for IL-10 stimulation by S. globosa, but in the case of S. schenckii both conidia and yeast-like cells positively stimulated IL-10 production when HK cells were used for the interaction or when N-linked and O-linked glycans were removed from the wall (Figure 5B). For the case of S. brasiliensis cells, conidia did not affect the ability to stimulate IL-10 after modification of the cell wall, but yeast-like cells increased the ability to stimulate this cytokine when cells were heat-inactivated or N-linked and O-linked glycans were trimmed off from walls (Figure 5B). Collectively, these data indicate that monocyte-derived macrophages are capable to discriminate between cell morphologies and the different Sporothrix species, using different PRRs and having a differential response against these cells.
Figure 5.
Contribution of cell wall components on cytokine stimulation by Sporothrix schenckii, Sporothrix brasiliensis, and Sporothrix globosa interacting with human monocyte-derived macrophages. Macrophages were incubated with conidia or yeast-like cells at a macrophage-fungus ratio of 1:6, for 24 h at 37°C and 5% (v/v) CO2. Plates were centrifuged and supernatants were used to quantify TNFα (A) or IL-10 (B) by ELISA. Before coincubation with human monocyte-derived macrophages, conidia, and yeast-like cells were inactivated by heat (HK) treated with endoglycosidase H (Endo-H), β-eliminated to remove O-linked glycans, or both treated with endoglycosidase H and β-elimination. No treatment refers to live cells without any treatment. *P < 0.05 when compared to the no-treatment condition of the same strain. Results are shown as mean ± standard deviation from data generated with samples from eight donors analyzed by duplicate.
Human Monocyte-Derived Dendritic Cells Differentially Phagocyte Sporothrix schenckii, Sporothrix brasiliensis, and Sporothrix globosa
Next, we performed similar experiments but using monocyte-derived DCs. Using the same strategy to assess fungal uptake by macrophages, we found that S. schenckii and S. brasiliensis conidia were hardly phagocytosed by monocyte-derived DCs, but this was not the case for S. globosa, which were readily phagocytosed, in a similar rate than yeast-like cells (Figure 6). Both types of fungal cells generated the highest phagocytosis levels in the early, intermediate, and late stages (Figure 6). S. schenckii yeast-like cells were modestly phagocytosed by these immune cells but the levels were significantly different from those observed on S. brasiliensis yeast-like cells, which were the fungal cells with the lowest ability to be phagocytosed (Figure 6). Control reaction with immune cells incubated with buffer gave threshold detections (Figure 6).
Figure 6.
Phagocytosis of Sporothrix schenckii, Sporothrix brasiliensis, and Sporothrix globosa by human monocyte-derived dendritic cells. Fungal cells were labeled with Acridine Orange and incubated with human monocyte-derived dendritic cells at an immune cell-fungus ratio of 1:6, for 2 h at 37°C and 5% (v/v) CO2. Human cells were analyzed by flow cytometry, collecting 50,000 events, which were defined as a human cell interacting with at least one fluorescent fungal cell. Control, human cells were incubated only with PBS. *P < 0.05 when compared to conidia from the same species. †P < 0.05 when compared with the same morphology in the other two species. Results are shown as mean ± standard deviation from data generated with samples from eight donors analyzed by duplicate.
When the relevance of immune receptors for DCs uptake was analyzed, we found that S. schenckii conidia uptake was dependent on MR, CR3, DC-SIGN, and TLR4 (Figure 7). Similarly, yeast-like cells depended on the same receptor subsets, along with dectin-1 (Figure 7). The S. brasiliensis conidia and yeast-like cells depended on the engagement of MR, CR3, DC-SIGN, and TLR4 with its ligands, and both S. globosa morphologies were phagocytosed via dectin-1 and TLR2 (Figure 7). Isotype-matched irrelevant antibodies were used to preincubate human cells and did not affect the fungal phagocytosis of any of the morphologies or species under study (data not shown). These results indicate the three fungal species are sensed by different subsets of immune receptors found on the monocyte-derived DCs surface.
Figure 7.
Contribution of pattern recognition receptors and cell wall components on the phagocytosis of Sporothrix schenckii, Sporothrix brasiliensis, and Sporothrix globosa by human monocyte-derived Dendritic cells. In (A), Immune cells were preincubated with 200 μg mL−1 laminarin or 10 μg mL−1 of any of the following antibodies: anti-mannose receptor (MR), anti-CR3, anti-DCSIGN, anti-TLR2, or anti-TLR4. Then, cells were coincubated with Acridine Orange-labeled conidia or yeast-like cells at an immune cell-fungus ratio of 1:6, for 2 h at 37°C and 5% (v/v) CO2. Human cells were analyzed by flow cytometry, collecting 50,000 events, which were defined as a human cell interacting with at least one fluorescent fungal cell. All the interactions were performed in the presence of 5 μg mL−1 polymyxin B. No treatment refers to cells preincubated only with PBS. Results correspond to cells in the late stage of phagocytosis. For all cases, 100% corresponds to the system with no treatment, and the absolute values were similar to those shown in Figure 6 or (B) of this figure. CR3, complement receptor 3. *P < 0.05 when compared to the no-treatment condition of the same strain. †P< 0.05 when compared to conidia from the same species. In (B), Similar experiments as described in (A), but human cells were not preincubated with any blocking agent. Before coincubation with human monocyte-derived dendritic, conidia and yeast-like cells were inactivated by heat (HK) treated with endoglycosidase H (Endo-H), β-eliminated to remove O-linked glycans, or both treated with endoglycosidase H and β-elimination. No treatment refers to live cells without any treatment. *P < 0.05 when compared to the no-treatment condition of the same strain. Results are shown as mean ± standard deviation from data generated with samples from eight donors analyzed by duplicate.
Then, the relevance of cell wall components during the Sporothrix-monocyte-derived DCs interaction was analyzed. Both morphologies of S. schenckii were positively affected when HK cells were used in the interaction with immune cells, significantly increasing the number of phagocytosed cells (Figure 7B). However, the removal of N-linked, O-linked, or both types of glycans from the cell wall negatively affected cell uptake (Figure 7B). Similar results were observed for S. brasiliensis yeast-like cells, but no significant changes were observed for S. brasiliensis conidia subjected to these treatments (Figure 7B). S. globosa conidia and yeast-like cells did not show any effect on the phagocytosis when HK or deglycosylated cells were used to interact with monocyte-derived DCs. Collectively, these results suggest that the cell wall components play different roles during interaction with these immune cells.
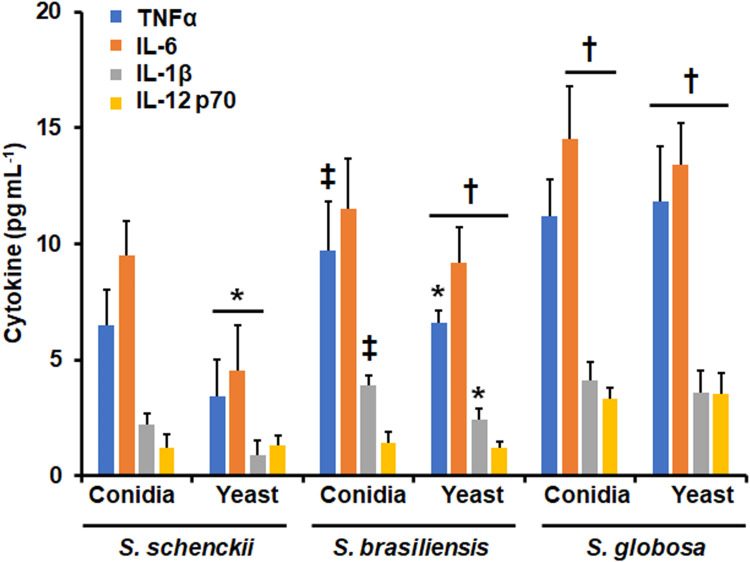
Next, to assess the ability of these fungal species to stimulate cytokine production by human monocyte-derived DCs, the proinflammatory cytokines TNFα, IL-6, IL-1β, and IL-12 p70 were quantified by ELISA, after the immune cell-fungus interaction. S. schenckii yeast-like cells stimulated lower TNFα, IL-6, and IL-1β levels than conidia, whilst both cell morphologies stimulated similar IL-12 p70 levels (Figure 8). For the case of S. brasiliensis yeast-like cells, only TNFα and IL-1β levels were lower when compared to those stimulated by conidia; while both S. globosa conidia and yeast-like cells stimulated similar levels of the four cytokines analyzed (Figure 8). When comparing the stimulation profiles among species, S. globosa stimulated the highest levels of the four cytokines followed by S. brasiliensis, and the lowest cytokine levels were associated with S. schenckii cells (Figure 8). For the case of conidia, only TNFα and IL-1β stimulated by S. brasiliensis conidia were higher than those stimulated by S. schenckii conidia (Figure 8).
Figure 8.
Cytokine production by human monocyte-derived dendritic cells stimulated with Sporothrix schenckii, Sporothrix brasiliensis, and Sporothrix globosa. Human and fungal cells were coincubated for 24 h., and the supernatants were collected and used for cytokines quantification by ELISA. *P < 0.05, when compared with the cytokine level stimulated by conidia from the same species. †P < 0.05, when compared with the same morphology of the other two fungal species analyzed. ‡P < 0.05, when compared with the same morphology of S. schenckii. Results are shown as mean ± standard deviation from data generated with samples from eight donors analyzed by duplicate.
Next, we assessed the contribution of PRRs to TNFα stimulation. CR3 and DC-SIGN were required to induce proper TNFα production by both S. schenckii conidia and yeast-like cells, although the latter also required dectin-1 engagement with its ligand and conidia of TLR4 signaling (Figure 9A). Similarly, S. brasiliensis conidia and yeast-like cells depended on interaction with CR3 and DC-SIGN for TNFα production but in these cases, TLR4 was also required (Figure 9A). Only S. brasiliensis yeast-like cells required dectin-1 signaling for proper TNFα production (Figure 9A). Finally, both S. globosa conidia and yeast-like cells stimulated TNFα via dectin-1 and TLR2 (Figure 9A). Isotype-matched irrelevant antibodies were used to preincubate human cells and did not affect the fungal phagocytosis of any of the morphologies or species under study (data not shown). Regarding the cell wall components involved in this stimulation, S. schenckii conidia and yeast-like HK cells stimulated higher cytokine levels than live cells (Figure 9B). When glycans were removed from the wall there was a negative effect on TNFα production by conidia, but in the case of yeast-like cells, this negative effect was more evident when both N-linked and O-linked glycans were removed from the wall (Figure 9B). For the case of S. brasiliensis, the removal of any of the two kinds of glycans or both together had a negative impact on the cytokine levels stimulated by conidia but not for yeast-like cells (Figure 9 B). Heat-inactivation had a positive effect only on S. brasiliensis yeast-like cells, being live and HK conidia similar in the ability to stimulate TNFα production (Figure 9B). For the case of S. globosa, none of the treatments affected the ability of conidia or yeast-like cells to stimulate TNFα (Figure 9B).
Figure 9.
Contribution of pattern recognition receptors and cell wall components to TNFα production by human monocyte-derived dendritic cells stimulated with Sporothrix schenckii, Sporothrix brasiliensis, and Sporothrix globosa. In A, Dendritic cells were preincubated with 200 μg mL−1 laminarin or 10 μg mL−1 of any of the following antibodies: anti-mannose receptor (MR), anti-complement receptor 3 (CR3), anti-DC-SIGN, anti-TLR2, or anti-TLR4. Then, cells were coincubated with either conidia or yeast-like cells of the fungal species for 24 h at 37°C and 5% (v/v) CO2, the supernatants were collected and used to measure the levels of TNFα. No treatment refers to cells preincubated with PBS. In all cases, 100% corresponds to the system with no treatment, and the absolute values were similar to those shown in Figure 8. *P < 0.05 when compared to the no-treatment condition of the same strain. †P< 0.05 when compared to conidia from the same species. In B, dendritic cells were incubated with conidia or yeast-like cells at an immune cell-fungus ratio of 1:6, for 24 h at 37°C and 5% (v/v) CO2 and supernatants were collected and used to quantify TNFα by ELISA. Before coincubation with human monocyte-derived dendritic cells, conidia, and yeast-like cells were inactivated by heat (HK) treated with endoglycosidase H (Endo-H), β-eliminated to remove O-linked glycans, or both treated with endoglycosidase H and β-elimination. No treatment refers to live cells without any treatment. *P < 0.05 when compared to the no-treatment condition of the same strain. †P< 0.05 when compared to conidia from the same species. Results are shown as mean ± standard deviation from data generated with samples from eight donors analyzed by duplicate.
Discussion
Members of the Sporothrix pathogenic clade have different cell wall components that are considered potential antigens.57,73,74 Previous studies in S. schenckii and S. brasiliensis determined that the cell wall composition and organization can change among species and morphologies;19,26,28 thus, it is feasible to conceive that immune cells interact differently with each species and morphotype. Thus far, few reports have assessed the interaction between Sporothrix and macrophages or DCs.42–45 Most of the knowledge about this interaction comes from the study of S. schenckii and S. brasiliensis,35 and little is known about the interactions of these immune cells with S. globosa. This work aimed to compare the importance of fungal morphology, cell wall components, and immune receptors during the interaction of S. schenckii, S. brasiliensis, and S. globosa with macrophages and DCs.
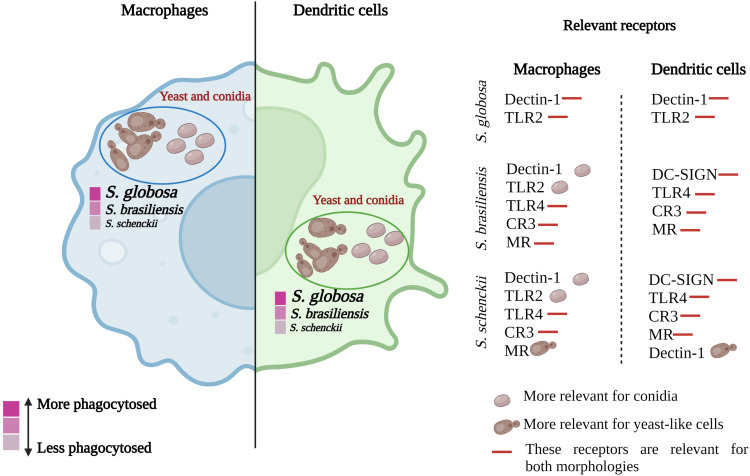
Phagocytosis assay with human monocyte-derived macrophages and DCs showed that S. globosa is the most phagocytosed species in both conidial and yeast-like cell morphologies, followed by S. brasiliensis and S. schenckii. The increased phagocytosis of S. globosa could be related to the organization of the cell wall components of this species. Phagocytosis is known to initiate with the recognition of β-1,3-glucan and chitin, through dectin-1, TLR2, and TLR4 receptors.75 So, it is likely that there could be an association between the proportion of these polysaccharides and phagocytosis. S. globosa has a higher amount of β-1,3-glucans exposed on the surface, which could favor its phagocytosis in both cell morphologies.19 In S. schenckii and S. brasiliensis, cells are less phagocytosed, and previous reports have shown that β-1,3-glucans are not exposed on the cell surface, and these are in a lower proportion than those of S. globosa, which hinders their recognition by these immune cells.19,26 When the contribution of cell wall components, such as β-glucans, N-glycans, and O-glycans of the three species was evaluated, it was found that HK S. schenckii and S. brasiliensis showed an increase in macrophage- and DCs-mediated phagocytosis of yeast-like cells, and a decrease in this when N-linked and O-linked glycans of these two species are removed. This suggests that N-glycans and O-glycans from S. schenckii and S. brasiliensis play an important role during the detection of immune cells to initiate phagocytosis. For the case of S. globosa, phagocytosis was not affected by any of the fungal treatments, and this could be related to the fact that both cell wall N-linked and O-linked glycans of this species are found in a lower proportion than in S. schenckii and S. brasiliensis.22,29 Furthermore, the absence of changes in phagocytosis of S. globosa could be because all components of the cell wall of this species contribute to fungal uptake by macrophages and DCs, and when altered by the treatments applied to cells the other wall components can compensate for the disturbance.
When the contribution of the different immune receptors in the uptake of conidia and yeasts was analyzed, it was found that all receptors are important in the detection of S. schenckii and S. brasiliensis by human monocyte-derived macrophages, mainly in yeast-like cells. However, some variations were observed in the case of S. brasiliensis conidia, where the essential receptors are dectin-1 and TLR2, a result that could be related to the cell wall organization of this morphology (Figure 10). 26,76
Figure 10.
Phagocytosis and receptors involved in the immune recognition of different species of the Sporothrix pathogenic clade. Macrophages and dendritic cells can phagocytose yeast-like cells and conidia of S. schenckii, S. brasiliensis, and S. globosa. S. globosa is the species most phagocytosed by both types of cells, followed by S. brasiliensis and, finally, S. schenckii. The receptors involved in such recognition vary between species and morphologies. S. globosa is recognized by dectin-1 and TLR2 receptors, while S. brasiliensis and S. schenckii are recognized by dectin-1, TLR2, TLR4, CR3, DC-SIGN, and MR.
Based on the results obtained, it can be observed that dectin-1 is a relevant receptor for the recognition of these three species by monocyte-derived macrophages. Most of the β-1,3-glucan required to trigger dectin-1-dependent signaling pathways may be already accessible on the cell surface, as reported with PBMCs in S. schenckii and S. brasiliensis species.26 Previous work has reported that the interaction of S. schenckii and S. brasiliensis with monocyte-derived macrophages depends on complement protein C3, which facilitates yeast phagocytosis.49 Here, we confirm this observation but interestingly, it does not apply to S. globosa, since the uptake of conidia and yeast-like cells was dependent on dectin-1 and TLR2 (Figure 10).
When the relevance of these receptors by human monocyte-derived DCs was assessed, it was found that the three species are detected by different sets of receptors. DCs play a key role in regulating the balance between pro- and anti-inflammatory responses, in addition, these cells can translate fungal-associated molecular signatures and coordinate an inflammatory response that protects against infection.54,77 For these immune cells, the receptors showed variation in the recognition of conidia and yeast-like cells of the three species, like that found in macrophages. However, it is striking that the dectin-1 receptor was dispensable for the recognition of conidia of S. schenckii and S. brasiliensis since it is a receptor that is involved in important signaling pathways and has a significant contribution in the recognition of fungal cells.78 According to the results obtained in this work, it is possible to hypothesize that the role of the different receptors analyzed may be influenced by the type of immune cell interacting with Sporothrix, highlighting the relevance of studying the immune cell-Sporothrix interaction with different subtypes of cellular immune effectors.
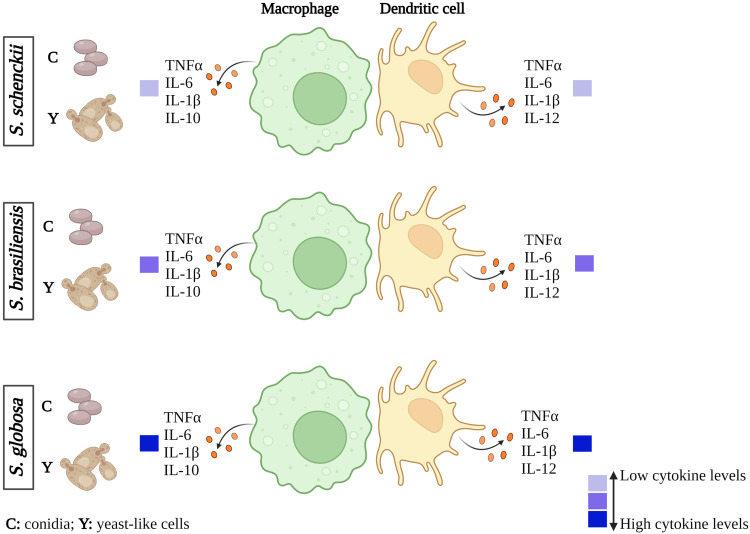
The interaction of either S. globosa conidia or yeast-like cells with human monocyte-derived macrophages and DCs induced a higher production of IL-6 and TNFα when compared to S. schenckii and S. brasiliensis. It is tempting to speculate that this proinflammatory trend could contribute to the lower ability to colonize tissues and establish the infection of this species. In agreement with this hypothesis, the contrary scenario was observed with the most virulent species of the clade, S. schenckii and S. brasiliensis, which stimulated lower pro-inflammatory levels (Figure 11).
Figure 11.
Cytokine profiles of macrophages and dendritic cells stimulated with Sporothrix schenckii, Sporothrix brasiliensis, or Sporothrix globosa. When human cells interact with S. globosa conidia or yeast-like cells, the production of the different cytokines analyzed increases. In the case of S. schenckii, there is a low stimulation of these cytokines compared to S. globosa and S. brasiliensis. These results were obtained from the inter-species comparison.
The analysis of different PRRs and cell wall components during the stimulation of TNFα and IL-10 by human monocyte-derived macrophages and DCs showed that the PRRs analyzed here have a morphology- and species-dependent role in this immunological event. When human-derived macrophages interacted with S. globosa conidia or yeast-like cells, dectin-1, MR, and TLR2 were required for TNFα stimulation, while dectin-1 and TLR-2 for IL-10 production. This contrast with the involvement of CR3 in the stimulation of TNFα by S. schenckii and S. brasiliensis cells, as reported previously and as shown here.49 Interestingly, IL-10 stimulation by S. brasiliensis yeast-like cells was solely dependent on MR, stressing again that glycan structures are different in these organisms, as reported previously for S. schenckii and S. brasiliensis.27,49 Finally, similar to other fungal species,63,64 DC-SIGN was required for stimulation of TNFα by S. schenckii and S. brasiliensis, but not for S. globosa, suggesting once again that β-1,3-glucan sensing by dectin-1 and TLR2 are the main PRRs-PAMPs interactions that these fungal cells establish with DCs.
Conclusion
In conclusion, this study reported that the cell wall components and the different morphologies of S. schenckii, S. brasiliensis, and S. globosa play an important role during recognition by human monocyte-derived macrophages and DCs. The interaction of these immune cells with the different Sporothrix species generates specific profiles of cytokines and uptake via different immune receptors. This study contributes to expanding the knowledge of the immune response against different species of the Sporothrix pathogenic clade.
Acknowledgments
This work was supported by Consejo Nacional de Ciencia y Tecnología (ref. FC 2015-02-834, Ciencia de Frontera 2019-6380), and Red Temática Glicociencia en Salud (CONACYT-México). The funding sources that supported this work did not have any involvement in the design, acquisition, and analysis of data and writing of the manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.de Beer ZW, Duong TA, Wingfield MJ. The divorce of Sporothrix and Ophiostoma: solution to a problematic relationship. Stud Mycol. 2016;83:165–191. doi: 10.1016/j.simyco.2016.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Beer ZW, Procter M, Wingfield MJ, Marincowitz S, Duong TA. Generic boundaries in the Ophiostomatales reconsidered and revised. Stud Mycol. 2022;101:57–120. doi: 10.3114/sim.2022.101.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopes-Bezerra LM, Mora-Montes HM, Zhang Y, et al. Sporotrichosis between 1898 and 2017: the evolution of knowledge on a changeable disease and on emerging etiological agents. Med Mycol. 2018;56(suppl_1):126–143. doi: 10.1093/mmy/myx103 [DOI] [PubMed] [Google Scholar]
- 4.Chakrabarti A, Bonifaz A, Gutierrez-Galhardo MC, Mochizuki T, Li S. Global epidemiology of sporotrichosis. Med Mycol. 2015;53(1):3–14. doi: 10.1093/mmy/myu062 [DOI] [PubMed] [Google Scholar]
- 5.Lopez-Romero E, Reyes-Montes MR, Perez-Torres A, et al. Sporothrix schenckii complex and sporotrichosis, an emerging health problem. Future Microbiol. 2011;6(1):85–102. doi: 10.2217/fmb.10.157 [DOI] [PubMed] [Google Scholar]
- 6.Lv S, Hu X, Liu Z, Lin Y, Wu H, Li F. Clinical epidemiology of sporotrichosis in Jilin province, China (1990–2019): a series of 4969 cases. Infect Drug Resist. 2022;15:1753–1765. doi: 10.2147/idr.S354380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takenaka M, Sato S, Nishimoto K. Survey of 155 sporotrichosis cases examined in Nagasaki Prefecture from 1951 to 2007. Nihon Ishinkin Gakkai Zasshi. 2009;50(2):101–108. doi: 10.3314/jjmm.50.101 [DOI] [PubMed] [Google Scholar]
- 8.Bongomin F, Adetona Fayemiwo S. Epidemiology of fungal diseases in Africa: a review of diagnostic drivers. Curr Med Mycol. 2021;7(1):63–70. doi: 10.18502/cmm.7.1.6246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barile F, Mastrolonardo M, Loconsole F, Rantuccio F. Cutaneous sporotrichosis in the period 1978–1992 in the province of Bari, Apulia, Southern Italy. Mycoses. 1993;36(5–6):181–185. doi: 10.1111/j.1439-0507.1993.tb00747.x [DOI] [PubMed] [Google Scholar]
- 10.Gold JA, Derado G, Mody RK, Benedict K. Sporotrichosis-associated hospitalizations, United States, 2000–2013. Emerg Infect Dis. 2016;22(10):1817–1820. doi: 10.3201/eid2210.160671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopes-Bezerra LM, Mora-Montes HM, Bonifaz A. Sporothrix and Sporotrichosis. In: Mora-Montes HM, Lopes-Bezerra LM, editors. Current Progress in Medical Mycology. Cham: Springer; 2017:309–331. [Google Scholar]
- 12.Mora-Montes HM, Dantas Ada S, Trujillo-Esquivel E, de Souza Baptista AR, Lopes-Bezerra LM. Current progress in the biology of members of the Sporothrix schenckii complex following the genomic era. FEMS Yeast Res. 2015;15:6. [DOI] [PubMed] [Google Scholar]
- 13.Orofino-Costa R, Macedo PM, Rodrigues AM, Bernardes-Engemann AR. Sporotrichosis: an update on epidemiology, etiopathogenesis, laboratory and clinical therapeutics. An Bras Dermatol. 2017;92(5):606–620. doi: 10.1590/abd1806-4841.2017279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Queiroz-Telles F, Buccheri R, Benard G. Sporotrichosis in immunocompromised hosts. J Fungi. 2019;5(1):8. doi: 10.3390/jof5010008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Miranda LHM, Meli M, Conceição-Silva F, et al. Co-infection with feline retrovirus is related to changes in immunological parameters of cats with sporotrichosis. PLoS One. 2018;13(11):e0207644. doi: 10.1371/journal.pone.0207644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mora-Montes HM. Special Issue “Sporothrix and Sporotrichosis 2.0”. J Fungi. 2022;8(8):821. doi: 10.3390/jof8080821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nava-Pérez N, Neri-García LG, Romero-González OE, Terrones-Cruz JA, García-Carnero LC, Mora-Montes HM. Biological and clinical attributes of Sporothrix globosa, a causative agent of sporotrichosis. Infect Drug Resist. 2022;15:2067–2090. doi: 10.2147/idr.s362099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arrillaga-Moncrieff I, Capilla J, Mayayo E, et al. Different virulence levels of the species of Sporothrix in a murine model. Clin Microbiol Infect. 2009;15(7):651–655. doi: 10.1111/j.1469-0691.2009.02824.x [DOI] [PubMed] [Google Scholar]
- 19.Lozoya-Pérez NE, Clavijo-Giraldo DM, Martínez-Duncker I, et al. Influences of the culturing media in the virulence and cell wall of Sporothrix schenckii, Sporothrix brasiliensis, and Sporothrix globosa. J Fungi. 2020;6(4):323. doi: 10.3390/jof6040323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodrigues AM, Gonçalves SS, de Carvalho JA, Borba-Santos LP, Rozental S, Camargo ZP. Current progress on epidemiology, diagnosis, and treatment of sporotrichosis and their future trends. J Fungi. 2022;8(8):776. doi: 10.3390/jof8080776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Etchecopaz A, Toscanini MA, Gisbert A, et al. Sporothrix brasiliensis: a review of an emerging South American fungal pathogen, its related disease, presentation and spread in Argentina. J Fungi. 2021;7(3):170. doi: 10.3390/jof7030170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tamez-Castrellón AK, van der Beek SL, López-Ramírez LA, et al. Disruption of protein rhamnosylation affects the Sporothrix schenckii-host interaction. Cell Surf. 2021;7:100058. doi: 10.1016/j.tcsw.2021.100058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lionakis MS, Drummond RA, Hohl TM. Immune responses to human fungal pathogens and therapeutic prospects. Nat Rev Immunol. 2023;1–20. doi: 10.1038/s41577-022-00826-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loh JT, Lam KP. Fungal infections: immune defense, immunotherapies and vaccines. Adv Drug Deliv Rev. 2023;196:114775. doi: 10.1016/j.addr.2023.114775 [DOI] [PubMed] [Google Scholar]
- 25.Gómez-Gaviria M, Vargas-Macías AP, García-Carnero LC, Martínez-Duncker I, Mora-Montes HM. Role of protein glycosylation in interactions of medically relevant fungi with the host. J Fungi. 2021;7(10):875. doi: 10.3390/jof7100875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martínez-Álvarez JA, Pérez-García LA, Mellado-Mojica E, et al. Sporothrix schenckii sensu stricto and Sporothrix brasiliensis are differentially recognized by human peripheral blood mononuclear cells. Front Microbiol. 2017;8:843. doi: 10.3389/fmicb.2017.00843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Villalobos-Duno HL, Barreto LA, Alvarez-Aular Á, et al. Comparison of cell wall polysaccharide composition and structure between strains of Sporothrix schenckii and Sporothrix brasiliensis. Front Microbiol. 2021;12:726958. doi: 10.3389/fmicb.2021.726958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopes-Bezerra LM, Walker LA, Niño-Vega G, et al. Cell walls of the dimorphic fungal pathogens Sporothrix schenckii and Sporothrix brasiliensis exhibit bilaminate structures and sloughing of extensive and intact layers. PLoS Negl Trop Dis. 2018;12(3):e0006169–e0006169. doi: 10.1371/journal.pntd.0006169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lozoya-Pérez NE, Casas-Flores S, de Almeida JRF, et al. Silencing of OCH1 unveils the role of Sporothrix schenckii N-linked glycans during the host-fungus interaction. Infect Drug Resist. 2019;12:67–85. doi: 10.2147/idr.S185037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bezerra L. Sporothrix schenckii cell wall peptidorhamnomannans. Mini Review. Front Microbiol. 2011;2:243. doi: 10.3389/fmicb.2011.00243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.López-Ramírez LA, Martínez-Duncker I, Márquez-Márquez A, Vargas-Macías AP, Mora-Montes HM. Silencing of ROT2, the encoding gene of the endoplasmic reticulum glucosidase II, affects the cell wall and the Sporothrix schenckii-host interaction. J Fungi. 2022;8(11):1220. doi: 10.3390/jof8111220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castro RA, Kubitschek-Barreira PH, Teixeira PAC, et al. Differences in cell morphometry, cell wall topography and Gp70 expression correlate with the virulence of Sporothrix brasiliensis clinical isolates. PLoS One. 2013;8(10):e75656. doi: 10.1371/journal.pone.0075656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lloyd KO, Bitoon MA. Isolation and purification of a peptido-rhamnomannan from the yeast form of Sporothrix schenckii. Structural and immunochemical studies. J Immunol. 1971;107(3):663–671. doi: 10.4049/jimmunol.107.3.663 [DOI] [PubMed] [Google Scholar]
- 34.García-Carnero LC, Salinas-Marín R, Lozoya-Pérez NE, et al. The Heat shock protein 60 and Pap1 participate in the Sporothrix schenckii-host interaction. J Fungi. 2021;7(11):960. doi: 10.3390/jof7110960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vargas-Macías AP, Gómez-Gaviria M, García-Carnero LC, Mora-Montes HM. Current models to study the Sporothrix-host interaction. Review. Front Fungal Biol. 2022;3:833111. doi: 10.3389/ffunb.2022.833111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ward RA, Vyas JM. The first line of defense: effector pathways of anti-fungal innate immunity. Curr Opin Microbiol. 2020;58:160–165. doi: 10.1016/j.mib.2020.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alegranci P, de Abreu Ribeiro LC, Ferreira LS, et al. The predominance of alternatively activated macrophages following challenge with cell wall peptide-polysaccharide after prior infection with Sporothrix schenckii. Mycopathologia. 2013;176(1–2):57–65. doi: 10.1007/s11046-013-9663-y [DOI] [PubMed] [Google Scholar]
- 38.Franco Dde L, Nascimento RC, Ferreira KS, Almeida SR. Antibodies against Sporothrix schenckii enhance TNF-α production and killing by macrophages. Scand J Immunol. 2012;75(2):142–146. doi: 10.1111/j.1365-3083.2011.02636.x [DOI] [PubMed] [Google Scholar]
- 39.Carlos IZ, Sgarbi DB, Santos GC, Placeres MC. Sporothrix schenckii lipid inhibits macrophage phagocytosis: involvement of nitric oxide and tumour necrosis factor-alpha. Scand J Immunol. 2003;57(3):214–220. doi: 10.1046/j.1365-3083.2003.01175.x [DOI] [PubMed] [Google Scholar]
- 40.Tachibana T, Matsuyama T, Mitsuyama M. Involvement of CD4+ T cells and macrophages in acquired protection against infection with Sporothrix schenckii in mice. Med Mycol. 1999;37(6):397–404. doi: 10.1046/j.1365-280x.1999.00239.x [DOI] [PubMed] [Google Scholar]
- 41.Oda LM, Kubelka CF, Alviano CS, Travassos LR. Ingestion of yeast forms of Sporothrix schenckii by mouse peritoneal macrophages. Infect Immun. 1983;39(2):497–504. doi: 10.1128/iai.39.2.497-504.1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang L, Zhang J, Du W, et al. Chitin-rich heteroglycan from Sporothrix schenckii sensu stricto potentiates fungal clearance in a mouse model of sporotrichosis and promotes macrophages phagocytosis. BMC Microbiol. 2021;21(1):190. doi: 10.1186/s12866-021-02243-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jellmayer JA, Ferreira LS, Manente FA, et al. Dectin-1 expression by macrophages and related antifungal mechanisms in a murine model of Sporothrix schenckii sensu stricto systemic infection. Microb Pathog. 2017;110:78–84. doi: 10.1016/j.micpath.2017.06.025 [DOI] [PubMed] [Google Scholar]
- 44.Negrini Tde C, Ferreira LS, Alegranci P, et al. Role of TLR-2 and fungal surface antigens on innate immune response against Sporothrix schenckii. Immunol Invest. 2013;42(1):36–48. doi: 10.3109/08820139.2012.719982 [DOI] [PubMed] [Google Scholar]
- 45.Sassá MF, Ferreira LS, Ribeiro LC, Carlos IZ. Immune response against Sporothrix schenckii in TLR-4-deficient mice. Mycopathologia. 2012;174(1):21–30. doi: 10.1007/s11046-012-9523-1 [DOI] [PubMed] [Google Scholar]
- 46.Sassá MF, Saturi AE, Souza LF, Ribeiro LC, Sgarbi DB, Carlos IZ. Response of macrophage Toll-like receptor 4 to a Sporothrix schenckii lipid extract during experimental sporotrichosis. Immunology. 2009;128(2):301–309. doi: 10.1111/j.1365-2567.2009.03118.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rossato L, Santos SSD, Ferreira LG, de Almeida SR. The importance of Toll-like receptor 4 during experimental Sporothrix brasiliensis infection. Med Mycol. 2019;57(4):489–495. doi: 10.1093/mmy/myy048 [DOI] [PubMed] [Google Scholar]
- 48.Rossato L, Silvana Dos Santos S, Ferreira LG, Rogério de Almeida S. The impact of the absence of Toll-like receptor-2 during Sporothrix brasiliensis infection. J Med Microbiol. 2019;68(1):87–94. doi: 10.1099/jmm.0.000876 [DOI] [PubMed] [Google Scholar]
- 49.Neves GWP, Wong SSW, Aimanianda V, et al. Complement-mediated differential immune response of human macrophages to Sporothrix species through interaction with their cell wall peptidorhamnomannans. Front Immunol. 2021;12:749074. doi: 10.3389/fimmu.2021.749074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Song Y, Yao L, Zhen Y, et al. Sporothrix globosa melanin inhibits antigen presentation by macrophages and enhances deep organ dissemination. Braz J Microbiol. 2021;52(1):19–31. doi: 10.1007/s42770-020-00345-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guan MQ, Yao L, Zhen Y, Song Y, Cui Y, Li SS. Melanin of Sporothrix globosa affects the function of THP-1 macrophages and modulates the expression of TLR2 and TLR4. Microb Pathog. 2021;159:105158. doi: 10.1016/j.micpath.2021.105158 [DOI] [PubMed] [Google Scholar]
- 52.Uenotsuchi T, Takeuchi S, Matsuda T, et al. Differential induction of Th1-prone immunity by human dendritic cells activated with Sporothrix schenckii of cutaneous and visceral origins to determine their different virulence. Int Immunol. 2006;18(12):1637–1646. doi: 10.1093/intimm/dxl097 [DOI] [PubMed] [Google Scholar]
- 53.Quinello C, Souza Ferreira L, Picolli I, et al. Sporothrix schenckii cell wall proteins-stimulated BMDCs are able to induce a Th1-prone cytokine profile in vitro. J Fungi. 2018;4(3):106. doi: 10.3390/jof4030106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Verdan FF, Faleiros JC, Ferreira LS, et al. Dendritic cell are able to differentially recognize Sporothrix schenckii antigens and promote Th1/Th17 response in vitro. Immunobiology. 2012;217(8):788–794. doi: 10.1016/j.imbio.2012.04.006 [DOI] [PubMed] [Google Scholar]
- 55.Kusuhara M, Qian H, Li X, et al. Mouse bone marrow-derived dendritic cells can phagocytize the Sporothrix schenckii, and mature and activate the immune response by secreting interleukin-12 and presenting antigens to T lymphocytes. J Dermatol. 2014;41(5):386–392. doi: 10.1111/1346-8138.12472 [DOI] [PubMed] [Google Scholar]
- 56.Madrid H, Cano J, Gené J, Bonifaz A, Toriello C, Guarro J. Sporothrix globosa, a pathogenic fungus with widespread geographical distribution. Rev Iberoam Micol. 2009;26(3):218–222. doi: 10.1016/j.riam.2009.02.005 [DOI] [PubMed] [Google Scholar]
- 57.Teixeira MM, de Almeida LG, Kubitschek-Barreira P, et al. Comparative genomics of the major fungal agents of human and animal sporotrichosis: Sporothrix schenckii and Sporothrix brasiliensis. BMC Genomics. 2014;15:943. doi: 10.1186/1471-2164-15-943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Giosa D, Felice MR, Giuffrè L, et al. Transcriptome-wide expression profiling of Sporothrix schenckii yeast and mycelial forms and the establishment of the Sporothrix Genome DataBase. Microb Genom. 2020;6(10):mgen000445. doi: 10.1099/mgen.0.000445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Giosa D, Giuffrè L, Felice MR, et al. P421 Whole-transcriptome analysis of Sporothrix brasiliensis grown in mold- and yeast-inducing conditions. Med Mycol. 2022;60(Supplement_1):myac072P421. doi: 10.1093/mmy/myac072.P421 [DOI] [Google Scholar]
- 60.Trujillo-Esquivel E, Martínez-Álvarez JA, Clavijo-Giraldo DM, et al. The Sporothrix schenckii gene encoding for the ribosomal protein L6 has constitutive and stable expression and works as an endogenous control in gene expression analysis. Front Microbiol. 2017;8:1676. doi: 10.3389/fmicb.2017.01676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Endres S, Ghorbani R, Lonnemann G, van der Meer JW, Dinarello CA. Measurement of immunoreactive interleukin-1 beta from human mononuclear cells: optimization of recovery, intrasubject consistency, and comparison with interleukin-1 alpha and tumor necrosis factor. Clin Immunol Immunopathol. 1988;49(3):424–438. doi: 10.1016/0090-1229(88)90130-4 [DOI] [PubMed] [Google Scholar]
- 62.Perez-Garcia LA, Csonka K, Flores-Carreon A, et al. Role of protein glycosylation in Candida parapsilosis cell wall integrity and host interaction. Front Microbiol. 2016;7:306. doi: 10.3389/fmicb.2016.00306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cambi A, Gijzen K, de Vries IJM, et al. The C-type lectin DC-SIGN (CD209) is an antigen-uptake receptor for Candida albicans on dendritic cells. Eur J Immunol. 2003;33(2):532–538. doi: 10.1002/immu.200310029 [DOI] [PubMed] [Google Scholar]
- 64.Cambi A, Netea MG, Mora-Montes HM, et al. Dendritic cell interaction with Candida albicans critically depends on N-linked mannan. J Biol Chem. 2008;283(29):20590–20599. doi: 10.1074/jbc.M709334200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hernandez-Chavez MJ, Franco B, Clavijo-Giraldo DM, Hernandez NV, Estrada-Mata E, Mora-Montes HM. Role of protein phosphomannosylation in the Candida tropicalis-macrophage interaction. FEMS Yeast Res. 2018. doi: 10.1093/femsyr/foy053 [DOI] [PubMed] [Google Scholar]
- 66.Lozoya-Pérez NE, Casas-Flores S, Martínez-Álvarez JA, et al. Generation of Sporothrix schenckii mutants expressing the green fluorescent protein suitable for the study of host-fungus interactions. Fungal Biol. 2018;122:1023–1030. doi: 10.1016/j.funbio.2018.07.004 [DOI] [PubMed] [Google Scholar]
- 67.Gonzalez-Hernandez RJ, Jin K, Hernandez-Chavez MJ, et al. Phosphomannosylation and the functional analysis of the extended Candida albicans MNN4-like gene family. Front Microbiol. 2017;8:2156. doi: 10.3389/fmicb.2017.02156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mora-Montes HM, Bates S, Netea MG, et al. A multifunctional mannosyltransferase family in Candida albicans determines cell wall mannan structure and host-fungus interactions. J Biol Chem. 2010;285(16):12087–12095. doi: 10.1074/jbc.M109.081513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Estrada-Mata E, Navarro-Arias MJ, Perez-Garcia LA, et al. Members of the Candida parapsilosis complex and Candida albicans are differentially recognized by human peripheral blood mononuclear cells. Front Microbiol. 2015;6:1527. doi: 10.3389/fmicb.2015.01527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Navarro-Arias MJ, Defosse TA, Dementhon K, et al. Disruption of protein mannosylation affects Candida guilliermondii cell wall, immune sensing, and virulence. Front Microbiol. 2016;7:1951. doi: 10.3389/fmicb.2016.01951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schwartz SN, Medoff G, Kobayashi GS, Kwan CN, Schlessinger D. Antifungal properties of polymyxin B and its potentiation of tetracycline as an antifungal agent. Antimicrob Agents Chemother. 1972;2(1):36–40. doi: 10.1128/AAC.2.1.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cossarizza A, Chang H-D, Radbruch A, et al. Guidelines for the use of flow cytometry and cell sorting in immunological studies. Eur J Immunol. 2021:51(12);2708–3145. doi: 10.1002/eji.202170126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tamez-Castrellón AK, Romeo O, García-Carnero LC, Lozoya-Pérez NE, Mora-Montes HM. Virulence factors in Sporothrix schenckii, one of the causative agents of sporotrichosis. Curr Protein Pept Sci. 2020;21(3):295–312. doi: 10.2174/1389203720666191007103004 [DOI] [PubMed] [Google Scholar]
- 74.Ruiz-Baca E, Mora-Montes HM, Lopez-Romero E, Toriello C, Mojica-Marin V, Urtiz-Estrada N. 2D-immunoblotting analysis of Sporothrix schenckii cell wall. Mem Inst Oswaldo Cruz. 2011;106(2):248–250. doi: 10.1590/S0074-02762011000200021 [DOI] [PubMed] [Google Scholar]
- 75.Thomas CJ, Schroder K. Pattern recognition receptor function in neutrophils. Trends Immunol. 2013;34(7):317–328. doi: 10.1016/j.it.2013.02.008 [DOI] [PubMed] [Google Scholar]
- 76.García-Carnero LC, Martínez-Duncker I, Gómez-Gaviria M, Mora-Montes HM. Differential recognition of clinically relevant Sporothrix species by human mononuclear cells. J Fungi. 2023;9(4):448. doi: 10.3390/jof9040448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bozza S, Perruccio K, Montagnoli C, et al. A dendritic cell vaccine against invasive aspergillosis in allogeneic hematopoietic transplantation. Blood. 2003;102(10):3807–3814. doi: 10.1182/blood-2003-03-0748 [DOI] [PubMed] [Google Scholar]
- 78.Reid DM, Gow NAR, Brown GD. Pattern recognition: recent insights from Dectin-1. Curr Opin Immunol. 2009;21(1):30–37. doi: 10.1016/j.coi.2009.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]