Abstract
Background
The approval of long-acting injectable cabotegravir/rilpivirine (LAI CAB/RPV) heightened the urgency of ensuring effective implementation. Our study assesses readiness and barriers to implement LAI CAB/RPV across Ryan White–funded clinics in the United States.
Methods
We conducted a cross-sectional survey between December 2020 and January 2021 using validated 4-item measures: acceptability of intervention measure (AIM), intervention appropriateness measure (IAM), and feasibility of intervention measure (FIM). Associations between measures and clinic characteristics were evaluated via Spearman rank correlations. A 5-point Likert scale ranked potential barriers of implementation responses. Open-ended questions were analyzed through a thematic approach.
Results
Of 270 clinics, 44 (16%) completed the survey: 38% federally qualified health centers, 36% academic, 20% community-based organizations, 14% hospital outpatient, and 9% nonprofit. Means (SD; range) were as follows: AIM, 17.6 (2.4; 12–20); IAM, 17.6 (2.4; 13–20); and FIM, 16.8 (2.9; 7–20). Twenty percent were not at all ready to implement LAI CAB/RPV, and 52% were slightly or somewhat ready. There was a significant association between AIM and the proportion of Medicaid patients (AIM, rho = 0.312, P = .050). Community-based organizations scored the highest readiness measures (mean [SD]: AIM, 19.50 [1.41]; IAM, 19.25 [1.49]; FIM, 19.13 [1.36]) as compared with other clinics. Implementation barriers were cost and patients’ nonadherence to visits.
Conclusions
There is variability of readiness yet high levels of perceived acceptability and appropriateness of implementing LAI CAB/RPV among Ryan White clinics, necessitating tailored interventions for successful implementation. A special focus on addressing the barriers of adherence and the cost of implementation is needed.
Keywords: HIV, implementation, long-acting antiretroviral, viral suppression
A cross-sectional survey of Ryan White clinics in January 2021 showed that only 52% were slightly or somewhat ready to implement long-acting antiretroviral therapy.
In 2019, the US Department of Health and Human Services proposed the Ending the HIV Epidemic initiative, which involved scaling up HIV diagnosis, treatment, and prevention services [1–3]. The initiative requires collaborative efforts across health agencies such as the Health Resources and Services Administration to rapidly treat HIV infection among people with HIV by ensuring antiretroviral therapy (ART) receipt and achieving sustained viral suppression [3]. Despite this call to action, many people with HIV continue to encounter barriers to successful treatment, including failure or delay in ART initiation [4], poor adherence [5], and development of HIV drug resistance [6].
Adherence to ART is critical to ending the HIV epidemic, yet incomplete adherence remains a persistent barrier to successful therapy [7]. In the treatment of chronic illnesses requiring daily pharmacotherapy, novel pharmacologic therapies such as long-acting injectables (LAIs) have been shown to improve adherence [8, 9]. Cabotegravir (CAB) and rilpivirine (RPV) long-acting formulation is considered a paradigm shift in the treatment of HIV and is efficacious in the treatment of naive and experienced patients as an intramuscular injection administered monthly or every 2 months [10–12]. This regimen was approved by the US Food and Drug Administration (FDA) in January 2021 and is recommended per the guidelines of the Department of Health and Human Services as a switch option for patients who are virologically suppressed while undergoing a stable oral antiretroviral regimen [13].
Previous studies highlight potential advantages and barriers to LAI CAB/RPV adoption. Advantages for patients may include convenience, increased confidentiality, and removal of the need for daily oral dosing [14, 15]. Barriers for patients and providers include concerns around safety and efficacy, increased clinic visits, and cost [16]. Furthermore, at the organizational level, clinical and nonclinical stakeholders expressed concerns that LAI CAB/RPV would disrupt workflow by placing an increased demand on staff capacity [17]. Although studies have documented a variety of facilitators and barriers to consider for LAI CAB/RPV implementation, most prior research has focused primarily on patient-related barriers, such as discomfort or pain associated with injections [18], and patients’ perceptions and preferences of LAI CAB/RPV advantages [19, 20].
To successfully implement LAI CAB/RPV, it is essential to gain clinical and organizational leadership buy-in. The present study explores organizational readiness to implement LAI CAB/RPV. Organizations such as Ryan White (RW)–funded clinics (Health Resources and Services Administration), which provide direct health care and support services for more than half a million people with HIV [15], present a unique opportunity to gain insight into organizational readiness to implement LAI CAB/RPV. There is potential for LAI antiretroviral formulations to improve engagement in care and positively contribute to treatment goals of ending the HIV epidemic. However, it is critical to explore LAI CAB/RPV implementation strategies and recognize any potential barriers to their uptake. This study was designed to identify the levels of readiness by exploring the perceived appropriateness, acceptability, and feasibility to implement LAI CAB/RPV in RW clinics shortly after drug approval. To our knowledge, there is no similar study following the initiatives to end the HIV epidemic and the approval of LAI CAB/RPV.
METHODS
Study Setting and Population
An anonymous online cross-sectional survey of RW Part C grantees was developed, piloted, and conducted with input from affiliated experts (eg, medical providers and grant directors). The REDCap platform (Research Electronic Data Capture) [21] was used to collect responses on a 48-item self-administered questionnaire collecting clinic information, HIV practice characteristics, and measures related to readiness for and barriers to LAI CAB/RPV implementation. The survey was emailed to RW Part C programs in December 2020 with 3 reminder emails sent at 1-week intervals thereafter. Participant responses were securely stored on REDCap’s web application, which has a built-in safeguard that prevents participants from submitting multiple responses. Participants did not receive incentives to complete the surveys. The target population included 353 RW Part C program medical directors identified by the organization Target HIV (www.targethiv.org). Eighty-three programs were excluded because of invalid emails, leading to a total sample of 270 programs. Inclusion criteria for the study were RW Part C clinics that provided primary HIV services to adult patients who are ≥18 years old and located within the United States or its territories. Exclusion criteria were RW Part C clinics that provided services to pediatric patients (<18 years of age).
Participant Consent Statement
This survey was approved by the Institutional Review Board of the Nebraska Medical Center's Office of Regulatory Affairs, wherein completion of the survey implied participant consent.
Participants were told that their participation is voluntary and that they can stop or withdraw at any time and for any reason.
Measures
The primary measures of interest were readiness for implementation based on the acceptability of intervention measure (AIM), the intervention appropriateness measure (IAM), and the feasibility of intervention measure (FIM) [22]. These implementation outcome measures are commonly used as early indicators of implementation success in formative research or pilot studies [23]. Acceptability is the perception among stakeholders that the intervention is agreeable, palatable, and satisfactory. Appropriateness is the perceived fit, relevance, or compatibility of the innovation for a given setting, provider, or consumer. Feasibility is the extent to which the intervention can be successfully carried out within the given setting. The implementation measures were scored with a Likert scale ranging from 1 to 5: 1 = strongly disagree, 2 = disagree, 3 = neither agree nor disagree, 4 = agree, and 5 = strongly agree. Higher average scores indicate greater readiness for implementation. In a study validating the measures, Cronbach alpha coefficients were relatively large (AIM = 0.85, IAM = 0.91, FIM = 0.89), indicating high reliability within each scale [22].
Questions regarding barriers to implementation were based on 5-point Likert scales where respondents were asked to rate each potential barrier with responses ranging from 1 (not a barrier at all) to 5 (extreme barrier). Respondents were also asked to report percentage distributions for their clinic's patient population characteristics, such as race, gender, poverty level, insurance coverage, viral suppression rates, and retention in care rates. Using Roger's diffusion of innovation theory, we asked respondents to rate their clinic’s readiness to adopt new therapies by choosing from 5 responses that range from describing the clinic as an active seeker of new clinical ideas to a change averter [24].
Data Analysis
Most survey items were summarized with means and standard deviations for continuous measures and frequencies and percentages for categorical measures. For primary outcomes, AIM, IAM, and FIM were calculated by summing the responses for the individual questions. Each scale consists of 4 items with each item scored 1 to 5; the scale range is restricted to 5 to 20. Means, standard deviations, and ranges were calculated for each measure. Associations of AIM, IAM, and FIM with other measures, such as clinic characteristics, were assessed via Spearman rank correlations for continuous measures and Wilcoxon rank sum tests or Kruskal-Wallis tests for categorical measures. Associations between some characteristics (eg, clinic type and type of RW funding received) and AIM, IAM, and FIM were not formally evaluated, given that some clinics fell into >1 category. For these, we reported means and standard deviations for all clinics in each category. For barrier measures, mean values were calculated for each potential barrier, with higher scores indicating a larger barrier. We used Stata statistical software (version 17; StataCorp) for data analysis. Respondents’ answers to a single open-ended question soliciting any additional comments that they had on the subject were analyzed via a deductive thematic analysis approach. We reviewed the raw responses and began the process of preliminary data coding using deductive codes drawn from the implementation measures. As the coding process progressed, we discussed initial codes and grouped the open-ended responses into categories of acceptability, appropriateness, and feasibility of LAI CAB/RPV.
The study was deemed exempt by the University of Nebraska Medical Center's institutional review board.
RESULTS
Respondent and Clinic Characteristics
Out of the total sample size (N = 270), 44 RW Part C programs completed the survey (16% completion rate). Of the 44 surveys received, 4 were submitted after LAI CAB/RPV was approved, with the last one received 2 weeks after approval. However, the majority (n = 40) were completed prior to the approval of LAI CAB/RPV. Workplace roles of the respondents at their clinics included program manager (n = 13), medical director (n = 10), physician (n = 10), fiscal manager (n = 7), nurse practitioner (n = 7), nurse (n = 3), physician assistant (n = 3), pharmacist (n = 1), social worker/case manager (n = 1), and other (n = 7). These clinics had a mean (SD) collective active patient population of 899 (697) and ranged from 47 to 3700 patients. Approximately 38% of clinics identified as federally qualified health centers, 36% academic medical centers, 20% AIDS service/community-based organizations, 14% hospital outpatient clinics, and/or 9% nonprofit private clinics.
Clinics shared their experiences providing LAI therapies: 50% provide LAI antipsychotics and 82% provide LAI contraceptives. Approximately 90% of the clinics surveyed have participated in clinical trials (n = 28). However, only 23% indicated their specific participation in an LAI CAB/RPV trial.
Clinics’ Implementation Readiness Based on AIM, IAM, and FIM
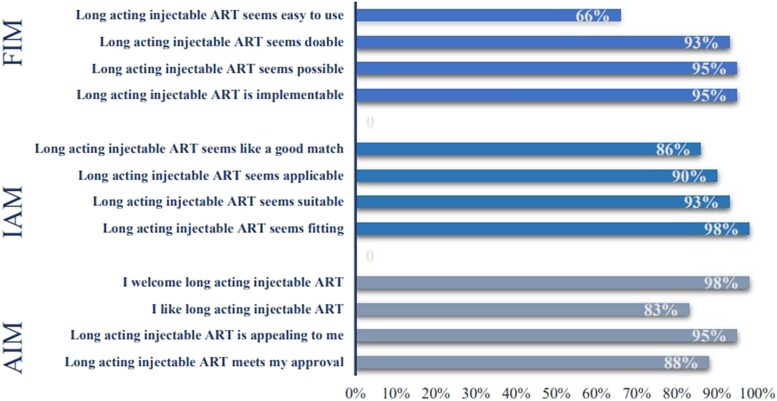
Using the implementation measures (AIM, IAM, and FIM), respondents shared their clinics’ acceptability, appropriateness, and feasibility of implementing LAI ART in their clinics. The means (SD; range) for the measures were as follows: AIM, 17.6 (2.4; 12–20); IAM, 17.6 (2.4; 13–20); and FIM, 16.8 (2.9; 7–20). Figure 1 reports the proportions of respondents who agreed or completely agreed with the implementation measures. Ninety-eight percent of respondents agreed/completely agreed to welcoming the use of LAI CAB/RPV in their clinic. However, only 66% agreed/completely agreed that LAI ART is easy to use.
Figure 1.
Proportion of respondents who agreed/completely agreed with survey questions about the acceptability, appropriateness, and feasibility of long-acting injectable cabotegravir/rilpivirine. AIM, acceptability of intervention measure; ART, antiretroviral therapy; FIM, feasibility of intervention measure; IAM, intervention appropriateness measure.
Clinic Experiences, Readiness, and Patient Selection for LAI CAB/RPV
When asked to rate their clinic's overall readiness to implement LAI CAB/RPV, 20% of respondents identified their clinic as not ready at all, 32% slightly ready, 20% somewhat ready, 20% fairly ready, and 7% extremely ready. Table 1 shows the respondents’ experience and readiness for implementing LAI CAB/RPV.
Table 1.
Clinics’ Readiness for LAI CAB/RPV and Strategies for Implementation
| No. (%) | |
|---|---|
| Has the clinic conducted a patient needs assessment to prepare for the implementation of LAI CAB/RPV? | 44 |
| Yes | 2(5) |
| No | 38 (86) |
| Unsure | 4 (9) |
| Has the clinic conducted a staff needs assessment to prepare for the implementation of LAI CAB/RPV? | 44 |
| Yes | 4 (9) |
| No | 37 (84) |
| Unsure | 3 (7) |
| Has the clinic developed policy and procedures for the implementation of LAI CAB/RPV? | 43 |
| Yes | 2 (5) |
| No | 38 (88) |
| Unsure | 3 (7) |
| What is the clinic's general willingness to provide new therapies?a | 43 |
| This clinic actively seeks out new clinical ideas and initiatives to integrate into the practice | 24 (56) |
| This clinic plays an active role in implementing new clinical initiatives and is one of the first among HIV clinics to try these new services | 12 (28) |
| This clinic waits for other HIV clinics to try out new clinical services prior to adopting the service | 6 (14) |
| This clinic does not provide new clinical services unless it is required (eg, by Ryan White policies or HIV treatment guidelines) | 1 (2) |
| This clinic prefers not to change its practice | 0 (0) |
| For which of the following patient groups is your clinic likely to prescribe LAI ART?b | 44 |
| Patients who are adherent to daily oral ART | 28 (64) |
| Patients who are not adherent to daily oral ART | 37 (84) |
| Patients who experience pill fatigue | 38 (86) |
| Patients who experience pill aversion | 37 (84) |
| Patients who express interest in LAI ART | 42 (95) |
| Patients who are concerned about HIV disclosure | 22 (50) |
| Other | 2 (5) |
| At which of the following locations does your clinic plan to implement LAI ART?b | 44 |
| The clinic | 43 (98) |
| Patient's home | 5 (11) |
| Retail pharmacy | 2 (5) |
| Infusion center | 1 (2) |
| Other | 3 (7) |
| Rate your clinic's overall readiness to implement LAI ART | 44 |
| Not at all ready | 9 (20) |
| Slightly ready | 14 (32) |
| Somewhat ready | 9 (20) |
| Fairly ready | 9 (20) |
| Extremely ready | 3 (7) |
Abbreviations: ART, antiretroviral therapy; CAB/RPV, cabotegravir/rilpivirine; LAI, long-acting injectable.
Choose 1 statement.
Select all that apply.
The assessment of readiness included the clinic's preparedness based on needs assessments, policies, and the patient settings in which the clinic planned to implement LAI CAB/RPV. Of the 44 respondents, 5% (n = 2) reported that their clinic had conducted a needs assessment on patients to prepare for the implementation of LAI CAB/RPV, while 9% (n = 4) indicated that they were unsure. Similarly, 9% (n = 4) reported completing such a needs assessment for staff. Of the 43 respondents who answered the question, 2 (5%) said that their clinic had developed a policy for such implementation. Clinics identified the patient population that they were more likely to prescribe LAI CAB/RPV (Table 1).
When asked about the patient population to which clinics will likely prescribe LAI CAB/RPV, approximately 95% of the respondents identified patients who express an interest in the LAI. However, 50% were likely to prescribe to patients who were concerned about HIV disclosure, and 64% were likely to prescribe to patients who were adherent to their daily oral ART. Most respondents identified their clinic as the location in which they plan to implement LAI CAB/RPV (98%), and very few identified patients’ homes, retail pharmacies, and infusion centers (5%, 2%, and 7%, respectively).
Associations Between Implementation Measures and Clinic Characteristics
The associations between implementation measures and clinic characteristics are shown in Table 2. There was a significant association between the AIM measure and the proportion of patients insured by Medicaid (AIM, rho = 0.312, P = .050). There was a significant negative association between feasibility implementation measures and the proportion of cisgender females served (rho = −0.403, P = .010). There were no significant associations between any of the 3 measures of implementation and the proportion of cisgender male patients (AIM, rho = 0.192, P = .235; IAM, rho = 0.153, P = .346; FIM, rho = 0.289, P = .071), the proportion of patients in the ≤100% poverty level income group (AIM, rho = 0.154, P = .402; IAM, rho = 0.254, P = .160; FIM, rho = 0.187, P = .306), or the proportion of patients receiving AIDS Drug Assistance Program–sponsored insurance in clinics (AIM, rho = 0.237, P = .225; IAM, rho = 0.156, P = .430).
Table 2.
Association Between Clinic Characteristics and Acceptability, Appropriateness, and Feasibility of Implementing
| AIM | IAM | FIM | ||||
|---|---|---|---|---|---|---|
| Spearman | P Value | Spearman | P Value | Spearman | P Value | |
| Characteristics of clinic population | ||||||
| Race | ||||||
| White | 0.063 | .699 | 0.137 | .398 | 0.146 | .368 |
| Black or African American | 0.022 | .895 | −0.082 | .615 | −0.093 | .567 |
| American Indian or Alaska Native | 0.029 | .880 | 0.077 | .686 | 0.083 | .663 |
| Asian | 0.336 | .070 | 0.377 | .040 | 0.172 | .363 |
| Native Hawaiian or other Pacific Islander | 0.300 | .129 | 0.365 | .061 | 0.303 | .124 |
| Other | 0.013 | .949 | 0.016 | .936 | −0.082 | .684 |
| Gender | ||||||
| Cisgender male | 0.192 | .235 | 0.153 | .346 | 0.289 | .071 |
| Cisgender female | −0.255 | .113 | −0.198 | .221 | −0.403 | .010 |
| Transgender person | 0.084 | .612 | 0.140 | .394 | 0.074 | .653 |
| Other | 0.218 | … | … | … | … | … |
| Unknown | … | .520 | 0.218 | .520 | −0.135 | .693 |
| Poverty level, % | ||||||
| ≤100 | 0.154 | .402 | 0.254 | .160 | 0.187 | .306 |
| 100–200 | 0.086 | .645 | 0.068 | .715 | −0.163 | .382 |
| 201–300 | −0.303 | .117 | −0.261 | .180 | −0.180 | .360 |
| 301–400 | −0.096 | .657 | −0.123 | .566 | 0.001 | .995 |
| >400 | −0.192 | .393 | −0.274 | .217 | −0.141 | .530 |
| Insurance coverage | ||||||
| Uninsured | −0.091 | .593 | −0.012 | .946 | 0.014 | .932 |
| Private/employer | −0.130 | .437 | −0.148 | .374 | −0.072 | .666 |
| ADAP sponsored | 0.237 | .225 | 0.156 | .430 | −0.080 | .685 |
| Medicare/Tricare/other federal | −0.151 | .373 | −0.142 | .403 | −0.219 | .193 |
| Medicaid | 0.312 | .050 | 0.282 | .078 | 0.214 | .185 |
| Other | −0.068 | .825 | 0.061 | .844 | 0.052 | .865 |
| Wilcoxon Rank-Sum and Kruskal-Wallis Tests | ||||||
|---|---|---|---|---|---|---|
| Mean (SD) | P Value | Mean (SD) | P Value | Mean (SD) | P Value | |
| Facility type | … | … | … | |||
| Academic medical center | 16.93 (2.64) | 17.07 (2.59) | 15.93 (3.63) | |||
| AIDS service organization/community-based organization | 19.50 (1.41) | 19.25 (1.49) | 19.13 (1.36) | |||
| Federally qualified health center | 17.69 (2.52) | 17.88 (2.39) | 16.75 (2.65) | |||
| Hospital outpatient clinic | 18.00 (2.35) | 18.40 (2.19) | 17.00 (2.00) | |||
| Nonprofit private clinic | 16.50 (2.52) | 16.25 (2.63) | 16.50 (2.38) | |||
| Other | 17.00 (2.65) | 16.33 (3.51) | 17.00 (2.65) | |||
| Clinic setting | .796 | .657 | .816 | |||
| Urban | 17.45 (2.58) | 17.73 (2.27) | 16.55 (3.28) | |||
| Suburban | 18.09 (2.30) | 18.09 (2.39) | 17.55 (2.34) | |||
| Rural | 17.33 (2.40) | 16.89 (2.80) | 16.44 (2.51) | |||
| Clinic provision of LA therapies | .623 | .719 | .679 | |||
| Yes | 17.71 (2.33) | 17.67 (2.31) | 17.14 (2.52) | |||
| No | 17.19 (2.69) | 17.38 (2.66) | 16.44 (3.56) | |||
| Unsure | 18.40 (2.19) | 18.40 (2.19) | 16.40 (2.07) | |||
| Clinic provision of LA contraceptives | .298 | .586 | .452 | |||
| Yes | 17.76 (2.23) | 17.76 (2.20) | 16.97 (2.53) | |||
| No | 16.88 (3.23) | 17.13 (3.23) | 16.00 (4.17) | |||
| Clinic provision of other LAI | .836 | .753 | .767 | |||
| Yes | 17.50 (2.72) | 17.90 (2.23) | 17.30 (2.16) | |||
| No | 17.89 (2.03) | 18.00 (2.00) | 16.56 (2.96) | |||
| Unsure | 17.45 (2.07) | 17.18 (2.23) | 17.09 (2.17) | |||
| Clinic participates in CAB/RPV clinical trials | .576 | .547 | .441 | |||
| Yes | 17.20 (1.93) | 17.20 (1.93) | 16.50 (2.68) | |||
| No | 17.65 (2.59) | 17.71 (2.55) | 16.77 (2.96) | |||
| Unsure | 20.00 (—) | 20.00 (—) | 20.00 (—) | |||
| Clinic conducted a patient need assessment to prepare for the implementation of LAI-ART | .316 | .309 | .519 | |||
| Yes | 18.00 (2.83) | 18.00 (2.83) | 18.00 (2.83) | |||
| No | 17.39 (2.48) | 17.42 (2.44) | 16.56 (2.94) | |||
| Unsure | 19.25 (1.50) | 19.50 (1.00) | 18.25 (2.22) | |||
| Clinic conducted a staff needs assessment to prepare for the implementation of LAI-ART | .482 | .477 | .875 | |||
| Yes | 18.00 (2.31) | 18.00 (2.31) | 16.75 (2.22) | |||
| No | 17.43 (2.50) | 17.46 (2.47) | 16.69 (3.00) | |||
| Unsure | 19.00 (1.73) | 19.33 (1.15) | 18.00 (2.65) | |||
| Clinic has developed policies and procedures for the implementation of LAI-ART | .529 | .510 | .589 | |||
| Yes | 16.00 (0.00) | 16.00 (0.00) | 16.00 (0.00) | |||
| No | 17.64 (2.51) | 17.69 (2.47) | 16.72 (3.03) | |||
| Unsure | 18.67 (2.31) | 18.67 (2.31) | 18.33 (2.08) | |||
| Degree of adoption | ||||||
| This clinic actively seeks out new clinical ideas and initiatives to integrate into the practice | 18.63 (1.97) | … | 18.71 (1.83) | … | 17.63 (2.45) | … |
| This clinic plays an active role in implementing new clinical initiatives and is one of the first among HIV clinics to try these new services | 16.82 (2.14) | .013 | 16.82 (2.14) | .019 | 16.55 (1.92) | .147 |
| This clinic waits for other HIV clinics to try out new clinical services prior to adopting the service | 15.50 (2.66) | … | 15.17 (2.79) | … | 14.00 (4.47) | … |
| This clinic does not provide new clinical services unless it is required (eg, by Ryan White policies or HIV treatment guidelines) | 14.00 (—) | … | 16.00 (—) | … | 16.00 (—) | … |
| This clinic prefers not to change its practice | … | … | … | … | … | … |
Bold indicates P ≤ .05.
Abbreviations: ADAP, AIDS Drug Assistance Program; AIM, acceptability of intervention measure; ART, antiretroviral therapy; CAB, cabotegravir; FIM, feasibility of intervention measure; IAM, intervention appropriateness measure; LA, long-acting; LAI, long-acting injectable; RPV, rilpivirine.
Clinics that are AIDS service/community-based organizations consistently scored the highest on each of the 3 measures of implementation (mean [SD]: AIM, 19.50 [1.41]; IAM, 19.25 [1.49]; FIM, 19.13 [1.36]). An increase in the degree of adoption was associated with higher scores in AIM, IAM, and FIM. Clinics actively seeking new clinical ideas and initiatives to integrate into their practice had a higher mean (SD) per measure: 18.63 (1.97) for AIM, 18.71 (1.83) for IAM, and 17.63 (2.45) for FIM. In contrast, clinics that provide new clinical services only when required had lower means: 14 for AIM, 16 for IAM, and 16 for FIM. The associations between the clinic's degree of adoption and the implementation measures were significant for AIM and IAM (P = .013 and .019, respectively).
Barriers to Implementation of LAI CAB/RPV
The 5 top barriers were as follows: concerns about drug resistance in patients who do not adhere to monthly injection visits (mean, 2.72), patient adherence to monthly injection visits (2.63), cost of implementing LAI ART (2.58), patient transportation for monthly injection visits (2.33), and tracking patients who do not show for injection visits (2.28).
Qualitative analysis of 18 participants’ responses to the open-ended survey regarding the implementation of LAI CAB/RPV at their clinics is presented in Table 3. Participants shared their general acceptability toward LAI CAB/RPV and enthusiasm for its approval. To ensure the feasible implementation of LAI CAB/RPV, participants shared the types of resources that will be beneficial for their clinics, such as implementation strategy updates to RW-funded clinics.
Table 3.
Open-ended Survey Responses Regarding Ryan White–Funded Clinics’ Readiness to Implement Long-acting Injectable Cabotegravir/Rilpivirine
| Response Category | Respondent Quotes |
|---|---|
| Acceptability of implementing LAI CAB/RPV | Patients who have participated in the clinical trials have been very accepting of the long-acting injectable therapy. I am anxious to see if injectables improve adherence with the folks who are less optimally adherent to daily oral therapy.—Respondent 6 We are looking forward to offering this option to our patients. Once a rollout date is on the horizon, we will work quickly to develop and implement appropriate clinical workflows. Our strong HIV case management program will be an important support in educating patients and ensuring they adhere to regular visits.—Respondent 8 We are very interested in implementing an ART injectable clinic for our patients especially those that are adherent and virally suppressed.—Respondent 10 We have been waiting on this opportunity and are looking forward to FDA approval, training, and implementation.—Respondent 11 We have patients who have been asking about when this therapy will be available. The staff has pinpointed patients who we think would be a good fit for the therapy, especially those with pill fatigue that is affecting their VL [viral load] suppression. Overall, I think we are cautiously excited to begin offering it.—Respondent 15 |
| Appropriateness of implementing LAI CAB/RPV | The option to provide long-acting injectable ART therapy is a major step forward in the treatment arsenal we can provide to our patients and an additional tool for ending the HIV epidemic.—Respondent 7 While located in an urban setting, we have a large catchment area that serves a vast amount of rural population. Many of these patients experience hardships that result from lack of infrastructure within our state—specifically lack of public transportation especially from rural to urban areas, medical providers within these rural areas, and stigmatization within the medical facilities that service those areas.—Respondent 17 |
| Feasibility of implementing LAI CAB/RPV | We have many patients who receive ARVs from ADAP pharmacy, and I am uncertain what the logistics will be to acquire [LAI CAB/RPV] from ADAP in a timely manner so that it is not a bottleneck if patients present for an injection.—Respondent 12 Will be helpful to share and update RW [Ryan White] Clinics re the issue as there's a very limited knowledge re injectable ART's other than it's becoming available. –Respondent 18 We have no information about how the medication will be shipped, billed or associated co-pays and prior authorization requirements. Shipping directly to patients WILL NOT work. PAs [prior authorizations] take time and consume a lot of resources.—Respondent 13 As long as the state ADAP Program, Medicaid Program and private insurers incorporate the therapy in their formularies, making the cost of the therapy manageable, we should have no major barriers associated with implementing this therapeutic approach.—Respondent 1 |
Abbreviations: ADAP, AIDS Drug Assistance Program; ART, antiretroviral therapy; ARV, antiretroviral; FDA, Food and Drug Administration; LAI CAB/RPV, long-acting injectable cabotegravir/rilpivirine.
DISCUSSION
Our study assessed RW clinics’ readiness to implement LAI CAB/RPV. Specifically, it examined the acceptability, appropriateness, and feasibility of administration; the barriers to implementation; and the experiences providing LAIs among RW Part C clinics right before FDA approval of LAI CAB/RPV. Despite the clinics’ experiences in providing LAI therapies such as contraceptives and antipsychotics, our findings showed that RW-funded clinics were not ready to implement LAI CAB/RPV. Our respondents shared a high level of acceptability and perceived appropriateness of implementing LAI CAB/RPV. Specifically, community-based organizations and academic medical centers demonstrated the highest levels of acceptability, appropriateness, and feasibility of implementation as compared with hospital outpatient clinics and nonprofit private clinics. Common barriers to the implementation of LAI CAB/RPV were identified as patient-related and health systems factors, such as adherence to clinic appointments and the cost of implementation, respectively.
Our study findings are supported by a preimplementation study examining the willingness of consumer, clinical, and nonclinical stakeholders to adopt LAI CAB/RPV as a treatment option; this study showed that clinical providers were willing to adopt LAI CAB/RPV as a treatment option [17]. More than 90% of our respondents agreed or completely agreed that they welcome the implementation of LAI CAB/RPV in their clinic setting and that the therapy is appealing to them. Similarly, 90% of our respondents agreed/completely agreed that LAI CAB/RPV is applicable, fitting, and suitable to their clinic settings. However, as compared with the acceptability and appropriateness of LAI CAB/RPV, the feasibility of implementation was reported with less agreeability. The low perceived feasibility of implementing LAI CAB/RPV vs the high implementation measures of acceptability and appropriateness of implementation is congruent with the clinic's readiness to implement LAI CAB/RPV. Our findings indicate that only 27% of clinics were fairly or extremely ready to implement LAI CAB/RPV. Further assessment of clinics’ readiness found that >80% of our respondents’ clinics had neither conducted a patient or staff needs assessment nor developed policies and procedures for the implementation.
Our findings revealed that the implementation measures for applicability and acceptability had a significant association with clinics’ degree of adoption of new therapies. LAI CAB/RPV is a novel therapy; as such, clinics that are not early adopters of new HIV therapies will be lagging in its implementation. The significant association between clinics with a high degree of adoption of new therapies and measures of acceptability and applicability, rather than feasibility of implementation, can be attributed to several factors. Clinics with a high degree of adoption indicate that the new therapies are well received and align with the needs and preferences of health care providers and patients, which contributes to their acceptance and perceived applicability within the clinic. Feasibility of implementation, though, focuses more on the logistical aspects and resources required for implementation, which may have already been addressed by clinics with a high degree of adoption. The adoption of new therapies is influenced by contextual factors, such as organizational culture and available resources, which may play a more influential role in driving adoption than feasibility alone. Future research could explore the interplay among acceptability, applicability, feasibility, and contextual factors to gain a comprehensive understanding of the factors influencing the adoption of new therapies in clinics.
Our study showed significant correlations between clinics with Asian race and IAM, clinics with cisgender females and FIM, and clinics with Medicaid patients and AIM. While our study did not examine the factors behind these correlations, it is possible that clinics serving a larger Asian population, cisgender females, and patients receiving Medicaid may have culturally sensitive practices and approaches that account for the role of social determinants of health. These practices and approaches could have influenced the perceived acceptability, feasibility, and appropriateness.
Early implementation reports of LAI CAB/RPV at a southern RW-funded clinic described some of the barriers that our participants anticipated [25]. These included logistical challenges to attaining medications, administering injections, and supporting the enrollment of patients. Additional barriers were pursuit of prior authorization and unavailability of medication on formularies, leading to delays in initiation of therapy. Some of these are system-level barriers that are beyond clinics’ ability to resolve and will likely affect wider adoption of LAI CAB/RPV. Insurance prior authorization, cost, staffing, and acquisition of drug are likely to continue to be barriers to new injectable therapies. Therefore, it is prudent that HIV clinics advocate strongly for removal of these barriers to enable patients to access this important modality for delivering ART. A recent review article of challenges to LAI ART implementation identified similar barriers, which include injection training for staff and managing appointment reminders [26].
In the CUSTOMIZE study, health care staff found LAI CAB/RPV to be acceptable, appropriate, and feasible, with most staff describing “optimal” implementation within 1 to 3 months [27]. Successful implementation strategies leading to patient adherence included good communication about target dosing window, appointment reminder systems, and designated staff accountable for appointment tracking. At the clinic level, strategies for successful clinic implementation were identified as good staff communication, teamwork, and web-based treatment plans. In addition, infrastructure changes were included as part of the implementation, such as extended clinic hours, tracking and reminder systems, calling patients 2 days before first injection, and creating capacity for walk-in appointments [27].
Other concerns expressed by clinics include potential patient nonadherence to monthly visits and development of resistance in those who do not adhere to monthly visits. Several studies found that LAI ART modality is acceptable to people with HIV, including those not enrolled into clinical trials [9, 10, 12, 28]. In addition, treatment satisfaction in patients enrolled in LAI CAB/RPV clinical trials was high [16, 20]. Participants in the LATTE-2 trial described the convenience of LAI CAB/RPV vs daily pills and the emotional benefits, such as minimized potential for HIV disclosure and eliminating the “daily reminder of living with HIV,” as some of the reasons behind acceptability [29]. However, it is unclear whether patients’ acceptability of LAI CAB/RPV translates into better adherence and clinical outcomes. Therefore, implementation science studies focused on approaches to maintain adherence to injection visits are crucial to ensure equitable implementation while accounting for the potential impacts of implicit bias.
Limitations of our study include the small sample size, which could be explained by the timing of the study—specifically, during the second wave of COVID-19 in the United States, when HIV clinics were overwhelmed by pandemic operation and planning. Our study was conducted shortly before the FDA approved LAI CAB/RPV; thus, it is possible that the timing is insufficient for some clinics to adequately assess their readiness for implementation. Additionally, the surveys were sent to RW clinic directors based on information from public databases, which may be outdated. Rigorous recruitment techniques, such as up-front monetary incentives, postal surveys, and precontact with a phone call [30], are recommended to improve response rates. Our survey did not include questions regarding LAI CAB/RPV administration every 2 months, given that this dosing frequency was not under consideration by the FDA at the time of survey distribution. Despite our study being conducted before the approval of LAI CAB/RPV, the findings remain timely and relevant due to the current low uptake of this medication. A retrospective study conducted in a single clinic revealed important insights into the reasons behind the limited initiation of LAI CAB/RPV. The study highlighted the most commonly reported reasons as inconsistent clinic attendance, difficulty in reaching patients, and patient choice not to start [31]. These findings shed light on the barriers and considerations that influence the decision-making process surrounding the use of LAI CAB/RPV and can inform future strategies to improve its uptake and adherence.
Our study assessed readiness for implementing LAI CAB/RPV prior to FDA approval. We plan to conduct follow-up assessments of the same measures within the initial 5 years postapproval to facilitate a comprehensive evaluation by collaborating with organizations engaged in widespread implementation of LAI CAB/RPV.
Additionally, there are growing concerns regarding implicit bias among health care providers and the possibility of favoring “ideal candidates,” which could result in an unequal distribution of LAI CAB/RPV among patients in need. Our studies have identified various barriers that contribute to potential nonadherence, exacerbating the issue of inequitable access. Therefore, future implementation studies should use health equity frameworks to comprehensively comprehend these barriers, particularly those that disproportionately affect patients who are nonadherent. It is critical to develop strategies aimed at addressing these barriers, ensuring that patients who desire LAI CAB/RPV have equitable access to this medication.
CONCLUSION
The success of LAI CAB/RPV implementation is highly dependent on the readiness of clinics to provide the novel therapy. Our findings are helpful in assessing readiness, to inform implementation considerations for HIV clinics as they anticipate scale-up of LAI across the United States. Clinics can be adequately prepared for implementation by addressing patient-related barriers to implementation, such as adherence to clinic visits and transportation to clinics. Clinic staff may benefit from adequate education concerning medication access and coverage determination/billing processes.
Supplementary Material
Contributor Information
Adati Tarfa, School of Pharmacy, University of Wisconsin–Madison, Madison, Wisconsin, USA.
Harlan Sayles, College of Public Health, University of Nebraska Medical Center, University of Nebraska, Omaha, Nebraska, USA.
Sara H Bares, College of Medicine, University of Nebraska Medical Center, University of Nebraska, Omaha, Nebraska, USA.
Joshua P Havens, College of Medicine, University of Nebraska Medical Center, University of Nebraska, Omaha, Nebraska, USA; College of Pharmacy, University of Nebraska Medical Center, University of Nebraska, Omaha, Nebraska, USA.
Nada Fadul, College of Medicine, University of Nebraska Medical Center, University of Nebraska, Omaha, Nebraska, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments . We thank Matt Anderson at the University of Nebraska Medical Center for the REDCap survey development.
Author contributions. N. F., J. P. H., and S. H. B. designed the study. N. F. and H. S. contributed to data collection. H. S. analyzed the data. A. T., H. S., and N. F. interpreted the data. A. T. and N. F. drafted the manuscript. All authors reviewed, critically revised, and approved the final manuscript.
Financial support. This work was supported by institutional funds at the University of Nebraska Medical Center, College of Medicine.
References
- 1. Kazi DS, Katz IT, Jha AK. PrEParing to end the HIV epidemic—California's route as a road map for the United States. N Engl J Med 2019; 381:2489–91. [DOI] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention. Gaps in HIV testing and treatment hinder efforts to stop new infections. Press release. 2019. https://www.cdc.gov/media/releases/2019/p0315-gaps-hinder-hiv-testing.html.
- 3. Fauci AS, Redfield RR, Sigounas G, Weahkee MD, Giroir BP. Ending the HIV epidemic: a plan for the United States. JAMA 2019; 321:844–5. [DOI] [PubMed] [Google Scholar]
- 4. Bunda BA, Bassett IV. Reaching the second 90: the strategies for linkage to care and antiretroviral therapy initiation. Curr Opin HIV AIDS 2019; 14:494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tarfa A, Pecanac K, Shiyanbola O. Patients, social worker, and pharmacists’ perceptions of barriers to providing HIV care in community pharmacies in the United States. Pharmacy 2021; 9:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Whiteley LB, Olsen EM, Haubrick KK, Odoom E, Tarantino N, Brown LK. A review of interventions to enhance HIV medication adherence. Curr HIV/AIDS Rep 2021; 18:443–57. [DOI] [PubMed] [Google Scholar]
- 7. Rudolph AE, Dembo RS, Tobin K, Latkin C. Perceived HIV treatment norms modify the association between HIV-related stigma and adherence to antiretroviral therapy among persons living with HIV in Baltimore, Maryland. AIDS Behav 2022; 26:537–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chaudhary K, Patel MM, Mehta PJ. Long-acting injectables: current perspectives and future promise. Crit Rev Ther Drug Carrier Syst 2019; 36:137–81. [DOI] [PubMed] [Google Scholar]
- 9. Brissos S, Veguilla MR, Taylor D, Balanzá-Martinez V. The role of long-acting injectable antipsychotics in schizophrenia: a critical appraisal. Ther Adv Psychopharmacol 2014; 4:198–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Swindells S, Andrade-Villanueva JF, Richmond GJ, et al. Long-acting cabotegravir and rilpivirine for maintenance of HIV-1 suppression. N Engl J Med 2020; 382:1112–23. [DOI] [PubMed] [Google Scholar]
- 11. Jaeger H, Overton ET, Richmond G, et al. Long-acting cabotegravir and rilpivirine dosed every 2 months in adults with HIV-1 infection (ATLAS-2 M), 96-week results: a randomised, multicentre, open-label, phase 3b, non-inferiority study. Lancet HIV 2021; 8:e679–89. [DOI] [PubMed] [Google Scholar]
- 12. Orkin C, Arasteh K, Górgolas Hernández-Mora M, et al. Long-acting cabotegravir and rilpivirine after oral induction for HIV-1 infection. N Engl J Med 2020; 382:1124–35. [DOI] [PubMed] [Google Scholar]
- 13. Durham SH, Chahine EB. Cabotegravir-rilpivirine: the first complete long-acting injectable regimen for the treatment of HIV-1 infection. Ann Pharmacother 2021; 55:1397–409. [DOI] [PubMed] [Google Scholar]
- 14. Kerrigan D, Mantsios A, Gorgolas M, et al. Experiences with long acting injectable ART: a qualitative study among PLHIV participating in a phase II study of cabotegravir + rilpivirine (LATTE-2) in the United States and Spain. PLoS One 2018; 13:e0190487-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Philbin MM, Bergen S, Parish C, et al. Long-acting injectable ART and PrEP among women in six cities across the United States: a qualitative analysis of who would benefit the most. AIDS Behav 2022; 26:1260–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mantsios A, Murray M, Karver TS, et al. Multi-level considerations for optimal implementation of long-acting injectable antiretroviral therapy to treat people living with HIV: perspectives of health care providers participating in phase 3 trials. BMC Health Serv Res 2021; 21:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jolayemi O, Bogart LM, Storholm ED, et al. Perspectives on preparing for long-acting injectable treatment for HIV among consumer, clinical and nonclinical stakeholders: a qualitative study exploring the anticipated challenges and opportunities for implementation in Los Angeles County. PLoS One 2022; 17:e0262926-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Murray M, Antela A, Mills A, et al. Patient-Reported outcomes in ATLAS and FLAIR participants on long-acting regimens of cabotegravir and rilpivirine over 48 weeks. AIDS Behav 2020; 24:3533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mantsios A, Murray M, Karver TS, et al. Efficacy and freedom: patient experiences with the transition from daily oral to long-acting injectable antiretroviral therapy to treat HIV in the context of phase 3 trials. AIDS Behav 2020; 24:3473–81. [DOI] [PubMed] [Google Scholar]
- 20. Mantsios A, Murray M, Karver TS, et al. “I feel empowered”: women's perspectives on and experiences with long-acting injectable antiretroviral therapy in the USA and Spain. Cult Health Sex 2021; 23:1066–78. [DOI] [PubMed] [Google Scholar]
- 21. Harris PA, Taylor R, Minor BL, et al. The REDCap Consortium: building an international community of software platform partners. J Biomed Inform 2019; 95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weiner BJ, Lewis CCN, Stanick C, et al. Acceptability of intervention measure (AIM), intervention appropriateness measure (IAM), and feasibility of intervention measure (FIM). In: Psychometric Assessment of Three Newly Developed Implementation Outcome Measures. Creative Common License; 2017.
- 23. Weiner BJ, Lewis CC, Stanick C, et al. Psychometric assessment of three newly developed implementation outcome measures. Implement Sci 2017; 12:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bertrand JT. Diffusion of innovations and HIV/AIDS. J Health Commun 2004; 9:113–21. [DOI] [PubMed] [Google Scholar]
- 25. Collins LF, Corbin-Johnson D, Asrat M, et al. Early experience implementing long-acting injectable cabotegravir/rilpivirine for HIV-1 treatment at a Ryan White–funded clinic in the US South. Open Forum Infect Dis 2022; 9:ofac455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Waters L, Sparrowhawk A. Clinical implementation of long-acting antiretroviral treatment in high-income countries: challenges and advantages. Curr Opin HIV AIDS 2022; 17:121–6. [DOI] [PubMed] [Google Scholar]
- 27. Czarnogorski M, Garris CP, Dalessandro M, et al. Perspectives of healthcare providers on implementation of long-acting cabotegravir plus rilpivirine in US healthcare settings from a hybrid III implementation-effectiveness study (CUSTOMIZE). J Int AIDS Soc 2022; 25:e26003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Spreen WR, Margolis DA, Pottage JC. Long-acting injectable antiretrovirals for HIV treatment and prevention. Curr opin HIV AIDS 2013; 8:565–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Margolis DA, Gonzalez-Garcia J, Stellbrink HJ, et al. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet 2017; 390:1499–510. [DOI] [PubMed] [Google Scholar]
- 30. Pit SW, Vo T, Pyakurel S. The effectiveness of recruitment strategies on general practitioner's survey response rates—a systematic review. BMC Med Res Methodol 2014; 14:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hill LA, Abulhosn KK, Yin JF, Bamford LP. Single-center experience evaluating and initiating people with HIV on long-acting cabotegravir/rilpivirine. AIDS 2023; 37:605–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.