Abstract
Background and Objective
Historically, dosing of tacrolimus is guided by therapeutic drug monitoring (TDM) of the whole blood concentration, which is strongly influenced by haematocrit. The therapeutic and adverse effects are however expected to be driven by the unbound exposure, which could be better represented by measuring plasma concentrations.
Objective
We aimed to establish plasma concentration ranges reflecting whole blood concentrations within currently used target ranges.
Methods
Plasma and whole blood tacrolimus concentrations were determined in samples of transplant recipients included in the TransplantLines Biobank and Cohort Study. Targeted whole blood trough concentrations are 4–6 ng/mL and 7–10 ng/mL for kidney and lung transplant recipients, respectively. A population pharmacokinetic model was developed using non-linear mixed-effects modelling. Simulations were performed to infer plasma concentration ranges corresponding to whole blood target ranges.
Results
Plasma (n = 1973) and whole blood (n = 1961) tacrolimus concentrations were determined in 1060 transplant recipients. A one-compartment model with fixed first-order absorption and estimated first-order elimination characterised observed plasma concentrations. Plasma was linked to whole blood using a saturable binding equation (maximum binding 35.7 ng/mL, 95% confidence interval (CI) 31.0–40.4 ng/mL; dissociation constant 0.24 ng/mL, 95% CI 0.19–0.29 ng/mL). Model simulations indicate that patients within the whole blood target range are expected to have plasma concentrations (95% prediction interval) of 0.06–0.26 ng/mL and 0.10–0.93 ng/mL for kidney and lung transplant recipients, respectively.
Conclusion
Whole blood tacrolimus target ranges, currently used to guide TDM, were translated to plasma concentration ranges of 0.06–0.26 ng/mL and 0.10–0.93 ng/mL for kidney and lung transplant recipients, respectively.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40262-023-01259-x.
Key Points
| The relationship between plasma and whole blood tacrolimus concentrations indicates that binding of tacrolimus to erythrocytes is saturable. |
| A haematocrit correction of the whole blood target range was insufficient to account for changes in the total erythrocyte volume. Therefore, a more complex non-linear correction of the whole blood target range would be required for therapeutic drug monitoring in clinical practice. |
| The currently used target ranges based on whole blood concentrations translate in a broad target range of comparable plasma concentrations. |
Introduction
Tacrolimus reduces the risk of allograft rejection in solid organ transplant recipients by suppression of the T-cell-mediated immune response [1]. As such, it is generally considered the first choice immunosuppressive drug after solid organ transplantation. However, there is a delicate balance between insufficient immunosuppression (with an increased risk of rejection) and excessive immunosuppression (with an increased risk of infection and tacrolimus-induced adverse effects) [2]. In clinical practice, we aim to keep this delicate balance by monitoring whole blood trough tacrolimus concentrations [3].
Whole blood concentration measurements should however be carefully interpreted because this concentration is expected to change with the erythrocyte fraction in blood (i.e. haematocrit) [4–6]. Moreover, the unbound plasma tacrolimus exposure is independent of fluctuations in haematocrit and is presumed to drive the therapeutic effect [4, 6, 7]. Therefore, haematocrit corrections of the whole blood target concentration range have been proposed to avoid incorrect dose adjustments due to fluctuations in haematocrit [4, 8].
The proposed haematocrit correction of the whole blood target concentration range by Størset et al. assumes that the plasma concentration is proportional to the whole blood concentration [4]. In contrast, a study of heart and lung transplant recipients suggested that binding of tacrolimus to erythrocytes could be saturated [9], which questions whether a proportional correction of the whole blood target concentration range is appropriate. A non-linear, saturable, haematocrit correction of the whole blood target range has been proposed by Schijvens et al., but this correction has not been externally validated [8]. Therefore, we aimed to re-evaluate the relationship between plasma and whole blood tacrolimus concentrations in a large population of kidney and lung transplant recipients using a joint population pharmacokinetic model. Subsequently, with this model, we aimed to establish a plasma concentration range that reflects whole blood concentrations within currently used uncorrected target concentration ranges.
Methods
Patient Population and Study Design
For the current study, we used data and samples from patients enrolled in the ongoing TransplantLines Biobank and Cohort Study (NCT03272841) from the University Medical Center Groningen, Groningen, The Netherlands. The rationale and design of the TransplantLines Biobank and Cohort Study have previously been described [10]. In short, all candidates for solid organ transplantation (≥ 18 years of age) and living donors were eligible for participation. The study protocol of the Biobank and Cohort Study has been approved by the local Medical Ethics Review Committee (METc 2014/077). Furthermore, the study adheres to the local Biobank Regulation and was conducted in accordance with the Declarations of Helsinki and Istanbul. All participants provided written informed consent upon enrolment.
Demographic and clinical data were extracted from medical records. All measurements were performed during a visit to the outpatient clinic. Blood samples were drawn shortly before the study visit after an overnight fasting period of 8–12 h. Moreover, patients were instructed to take immunosuppressive medication, including tacrolimus, after blood was drawn. Laboratory measurements were performed using routine laboratory methods.
Samples from kidney and lung transplant recipients with documented tacrolimus use were included in this analysis. Most transplant recipients received tapering triple immunosuppressive therapy with prednisolone, tacrolimus and mycophenolate mofetil. Dosing of tacrolimus was guided by therapeutic drug monitoring (TDM), which aimed at targeting whole blood trough concentrations in the range of 6–10 ng/mL at 3 months after transplantation and 4–6 ng/mL at 6 months or longer after transplantation for kidney transplant recipients. For lung transplant recipients, TDM aimed at a whole blood trough concentration range of 7–10 ng/mL at 3 months or longer after transplantation. If necessary, dose adjustments were carried out assuming linear pharmacokinetics. No correction of the whole blood target ranges for fluctuations in haematocrit were performed.
Tacrolimus Analyses
Tacrolimus concentrations were determined from 10 mL EDTA blood samples collected at several time points after transplantation. Blood sample collection was scheduled at 8.00 a.m. and patients were instructed to administer tacrolimus at 10.00 a.m. for once-daily dosing regimens, and at 10.00 a.m. and 10.00 p.m. for twice-daily dosing regimens. Blood sampling was assumed at 10 or 22 h after dosing. Blood samples for whole blood tacrolimus monitoring were drawn at the same time as the samples for tacrolimus plasma analysis, and were processed and analysed within 4 h. EDTA blood samples drawn for plasma tacrolimus measurements were kept at room temperature and within 4 h were processed to plasma by temperature-controlled centrifugation (21°C) at 1300g for 10 min, and subsequently stored at − 80°C until analysis. Haemolysed samples, as determined by visual inspection, were not analysed.
All tacrolimus analyses were performed by the Laboratory of the department of Clinical Pharmacy and Pharmacology at the University Medical Center Groningen. The laboratory participates in the LGC proficiency testing programme (LGC Group, Bury, UK) to verify accuracy, precision and specificity. Concentrations were measured using a TSQ Quantiva mass spectrometer with a Vanquish UHPLC system, both from Thermo Fisher Scientific (Waltham, MA, USA).
For whole blood tacrolimus analysis, a validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) method was used as reported previously, with minor alterations [11, 12]. The linear range was 1.00–50.0 ng/mL, with between-day and within-day imprecision, as measured on 3 separate days, of < 10% for all four quality control samples (1.0 µg/L, 5.0 µg/L, 15.0 mg/L, 40.0 µg/L; n = 5), with an overall bias of 1.3–11.3%. All samples were analysed within 4 h, which covers the validated benchtop stability period of 74 h.
For plasma tacrolimus analysis, a validated LC-MS/MS method was used [13]. The linear range was 0.05–5.00 ng/mL, with between-day and within-day imprecision, as measured on 3 separate days, of <10% for all four QCs (0.05, 0.1, 2.0, 4.0 µg/L; n = 5), with an overall bias of −5.1 to 3.0%. Samples were prepared and frozen within the allowed 5-h benchtop stability, and analysed within three freeze-thaw cycles.
Development of the Population Pharmacokinetic Model
Data pre- and postprocessing was performed using R (R version 4.0.3, The R Foundation for Statistical Computing, Vienna, Austria). The joint population pharmacokinetic model was developed using NONMEM (version 7.5; ICON Development Solutions, Ellicott City, MD, USA). Model parameters were simultaneously estimated using the first-order conditional estimation with interaction method.
Plasma concentrations (Cplasma) were modelled using a one-compartment model with first-order absorption and first-order elimination, which was implemented with the built-in model library in NONMEM (ADVAN2/TRANS2). This model structure was selected based on a review of tacrolimus population pharmacokinetic models in adult transplant recipients [14] (see Eq. 1).
| 1 |
In this equation, D is the tacrolimus dose, V is the volume of distribution, and CL is the clearance. The absorption rate constant (ka) was fixed to 3.5 h-1 and bioavailability (F) was fixed to 25%, as we anticipated that insufficient data were available to estimate these parameters from the data [14].
Haematocrit represents the fraction of erythrocytes in a blood sample and was previously proposed as a factor that explains variability in whole blood concentrations [4]. To simultaneously describe the plasma and whole blood tacrolimus concentrations, we assumed that the whole blood concentration (Cwhole) was the weighted sum of the plasma concentration and the bound concentrations (Cbound), using haematocrit as a weighting factor (Eq. 2).
| 2 |
Missing haematocrit values were imputed using the last observation carried forward method. Furthermore, it was assumed that the plasma and whole blood tacrolimus concentrations were in equilibrium at the time of sample collection. To estimate the bound concentration, models for linear binding and saturable binding were evaluated (Eqs. 3 and 4):
| 3 |
| 4 |
In these equations, in the case of linear binding kinetics, fractionbound represents the fraction of the plasma concentration bound to erythrocytes, and, in the case of saturable binding kinetics, Bmax represents the maximum plasma concentration bound to erythrocytes and Kd represents the dissociation constant (i.e. the plasma concentration where 50% binding occurs).
Interindividual variability was explored on all structural model parameters and was assumed to be log-normally distributed. Covariance between the interindividual variability estimates was explored by estimating a full covariance block. Intraindividual variability was assumed to be normally distributed and was described by additive, proportional, or a combined additive and proportional error model. Correlations between the intraindividual variability for plasma and whole blood concentrations that result from both compounds being measured at the same sampling time was accounted for by the L2 data item in NONMEM [15].
Covariate selection was performed in three steps. First, the influence of time after transplantation, dose, formulation, measures for body size (body weight and fat-free mass) and haematocrit on model parameters was evaluated using a forward selection approach, as these covariates were included in the majority of the whole blood population pharmacokinetic models in the literature and were considered to have a theoretic mechanistic basis [14]. Second, other available covariates, identified in previous population pharmacokinetic models as summarised by Kirubakaran et al. or based on potential correlation with random effects, were evaluated using a similar forward selection approach [14]. These included: age, albumin, alkaline phosphatase (ALP), alanine transaminase (ALT), aspartate transaminase (AST), bilirubin, body surface area, body mass index, estimated glomerular filtration rate (eGFR; calculated using the Chronic Kidney Disease Epidemiology Collaboration [CKD-EPI] formula), ethnicity, intake conditions (fasting or non-fasting state), haptoglobin, low-density lipoprotein cholesterol, sex, total cholesterol, total plasma protein, transplantation type, vitamin B12 concentration and concomitant use of calcium channel blockers, predniso(lo)n, sulfonylurea derivatives, proton pump inhibitors, vitamin D3 and mycophenolic acid. Third, a backward elimination was conducted for all covariates included in the first and second steps. Details regarding which covariate effects were evaluated per structural model parameter are provided in electronic supplementary material (ESM) Table 1. Categorical covariates were modelled proportionally and continuous covariates were median normalised and modelled using a power model. Time after transplantation was additionally explored using an exponential model structure. Partially missing covariates were imputed using the last observation carried forward approach. For completely missing continuous covariates, median imputation was performed, and for categorical covariates, mode imputation was performed if no value was available within patients.
Modifications to the structural, stochastic and covariate models were assessed using the objective function value (OFV) and were accepted in the case of a drop of 6.63 points (p < 0.01), or in the backward elimination step, 10.83 (p < 0.001) [16]. Numeric model evaluation was further performed in terms of the relative standard error of model parameters and plausibility of the model estimates [16]. Standard goodness-of-fit plots and prediction-corrected visual predictive checks were used to graphically evaluate the model [16, 17].
Simulations
Simulations were performed to evaluate the relationship between plasma and whole blood trough concentrations. A simulation dataset was created by extracting covariate values, for all significant covariates in the population pharmacokinetic model, of all patients included in the model development dataset. Subsequently, for plasma trough concentrations in the sequence of 0.0–0.5 ng/mL and in steps of 0.01 ng/mL, whole blood trough concentrations were simulated using the developed population pharmacokinetic model. For each patient, 1000 simulations were performed and summarised on a population level. The number of whole blood concentrations in the target range was calculated from the simulated data.
Simulations were also performed to evaluate the marginal effects of significant covariates on the plasma and whole blood trough concentration. For these simulations, a typical individual was defined as reference using median values for continuous variables and most frequent value for categorical covariates. This reference typical individual was compared with a typical individual where covariate values approached the minimum or maximum observed covariate value. One thousand simulations were performed for each typical individual. The RxODE package (version 1.0.9) in R was used for all simulations.
Results
Demographics
A total of 1060 transplant recipients (845 kidney and 215 lung transplant recipients) were included in this analysis (Table 1). Transplant recipients had a mean (standard deviation [SD]) age of 54.7 (± 13.6) years, bodyweight of 81.0 (± 16.3) kg, eGFR of 50.2 (± 19.2) mL/min/1.73m2 and haematocrit of 39.0 (± 5.5) %. Most frequently used concomitant medications were mycophenolic acid (83.7%), predniso(lo)n (97.8%) and proton pump inhibitors (86.4%).
Table 1.
Demographic characteristics at time of first sample collection
| Transplant type | |||
|---|---|---|---|
| Kidney [n = 845] | Lung [n = 215] | Total [n = 1060] | |
| Time after transplantation (days) | 42 [2–409] | 68 [3–318] | 47 [2–409] |
| Sex | |||
| Male | 527 (62.4) | 114 (53.0) | 641 (60.5) |
| Ethnicity | |||
| Asian | 16 (1.9) | 5 (2.3) | 21 (2.0) |
| Black | 14 (1.7) | 0 (0.0) | 14 (1.3) |
| Caucasian | 784 (92.8) | 181 (84.2) | 965 (91.0) |
| Other or missing | 31 (3.6) | 29 (13.5) | 60 (5.7) |
| Age (years) | 54.6 ± 13.8 | 54.7 ± 12.8 | 54.7 ± 13.6 |
| Bodyweight (kg) | 82.7 ± 16.1 | 74.4 ± 15.6 | 81.0 ± 16.3 |
| Body surface area (m2) | 1.98 ± 0.22 | 1.87 ± 0.22 | 1.95 ± 0.22 |
| BMI (kg/m2) | 27.1 ± 4.7 | 25.0 ± 4.5 | 26.7 ± 4.8 |
| eGFR (mL/min/1.73 m2) | 49.0 ± 17.5 | 54.9 ± 24.1 | 50.2 ± 19.2 |
| Haematocrit (%) | 39.5 ± 5.6 | 37.2 ± 4.6 | 39.0 ± 5.5 |
| Albumin (g/L) | 43.7 ± 3.0 | 43.4 ± 3.3 | 43.6 ± 3.0 |
| ALT (U/L) | 22.5 ± 14.4 | 20.8 ± 10.5 | 22.2 ± 13.7 |
| AST (U/L) | 21.9 ± 8.2 | 23.1 ± 7.7 | 22.2 ± 8.1 |
| ALP (U/L) | 87.5 ± 45.8 | 72.2 ± 38.1 | 84.3 ± 44.8 |
| Bilirubin (μmol/L) | 7.4 ± 4.4 | 6.7 ± 3.7 | 7.3 ± 4.3 |
| Total plasma protein (g/L) | 69.2 ± 4.1 | 68.5 ± 4.2 | 69.0 ± 4.1 |
| Haptoglobin (g/L) | 1.5 ± 0.7 | 1.4 ± 0.7 | 1.5 ± 0.7 |
| Total cholesterol (mmol/L) | 4.7 ± 1.1 | 4.9 ± 1.3 | 4.8 ± 1.1 |
| LDL cholesterol (mmol/L) | 2.9 ± 0.9 | 2.9 ± 1.1 | 2.9 ± 1.0 |
| Vitamin B12 (pmol/L) | 380.7 ± 232.3 | 476.0 ± 322.9 | 401.2 ± 257.4 |
| Concomitant medication | |||
| Calcium channel blockers | 382 (45.2) | 17 (7.9) | 399 (37.6) |
| Predniso(lo)n | 824 (97.5) | 213 (99.1) | 1037 (97.8) |
| SU derivative | 42 (5.0) | 8 (3.7) | 50 (4.7) |
| Proton pump inhibitor | 717 (84.9) | 199 (92.6) | 916 (86.4) |
| Colecalciferol | 337 (39.9) | 80 (37.2) | 417 (39.3) |
| Mycophenolic acid | 709 (83.9) | 178 (82.8) | 887.0 (83.7) |
| Plasma samples | Kidney [n = 1670] | Lung [n = 303] | Total [n = 1973] |
|---|---|---|---|
| Samples per patient | 2.7 [1–6] | 1.8 [1–4] | 2.5 [1–6] |
| Dose (mg) | 2.9 ± 1.9 | 3.2 ± 1.8 | 2.9 ± 1.9 |
| Formulation | |||
| Prograft | 1214 (72.7) | 260 (82.2) | 1474 (74.7) |
| Advagraf | 326 (19.5) | 39 (12.9) | 365 (18.5) |
| Envarsus | 107 (6.4) | 0 (0.0) | 107 (5.4) |
| Missing | 23 (1.4) | 4 (1.3) | 27 (1.4) |
| Intake condition | |||
| Fasted | 1019 (61.0) | 85 (28.1) | 1104 (56.0) |
| Whole blood samples | Kidney [n = 1666] | Lung [n = 295] | Total [n = 1961] |
|---|---|---|---|
| Samples per patient | 2.6 [1–6] | 1.8 [1–4] | 2.5 [1–6] |
| Dose (mg) | 2.9 ± 1.9 | 3.1 ± 1.8 | 2.9 ± 1.9 |
| Formulation | |||
| Prograft | 1201 (72.1) | 255 (86.4) | 1456 (74.3) |
| Advagraf | 331 (19.9) | 36 (12.2) | 367 (18.7) |
| Envarsus | 110 (6.6) | 0 (0.0) | 110 (5.6) |
| Missing | 24 (1.4) | 4 (1.4) | 28 (1.4) |
| Intake condition | |||
| Fasted | 1017 (61.0) | 85 (28.8) | 1102 (56.2) |
Results are presented as number of patients (percentage) for categorical variables, or mean ± SD or mean [minimum–maximum] for continuous variables
BMI body mass index, eGFR estimated glomerular filtration rate, ALT alanine transaminase, AST aspartate transaminase, ALP alkaline phosphatase, LDL low-density lipoprotein, SU sulfonylurea
Observed Tacrolimus Concentrations
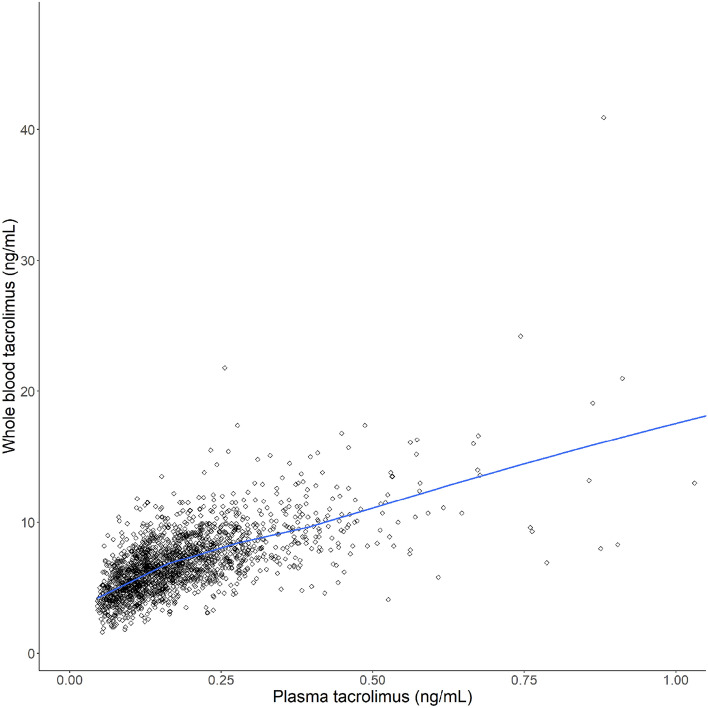
A total of 1973 plasma concentrations were available (1670 and 303 from kidney and lung transplant recipients, respectively) [Table 1]. One plasma sample was excluded due to laboratory errors. A total of 1961 whole blood concentrations were determined, of which 1666 and 295 were determined in samples obtained from kidney and lung transplant recipients, respectively (Table 1; Fig. 1). No whole blood samples were excluded.
Fig. 1.
Observed plasma versus whole blood tacrolimus concentration. Blue line represents a LOESS smoother. LOESS locally estimated scatterplot smoothing
In kidney transplant recipients, the geometric mean (minimum–maximum, coefficient of variation [CV]) whole blood tacrolimus concentration was 7.6 (1.0–17.4, 27.5%) ng/mL and 5.8 (1.0–47.4, 38.4%) ng/mL at 3 months and 6 months or longer after transplantation, respectively. A total of 74.2 and 39.8% of the whole blood concentrations were within the target range at 3 months and 6 months or longer after transplantation, respectively. In lung transplant recipients, the geometric mean (minimum–maximum) whole blood tacrolimus concentration was 9.6 (3.9–21.0, 30.1%) ng/mL and 8.1 (2.1–40.9, 38.3%) ng/mL at 3 months and 6 months or longer after transplantation, respectively. A total of 35.9 and 55.9% of the whole blood concentrations were within the target range at 3 and 6 months or longer after transplantation, respectively.
In kidney transplant recipients, the geometric mean (minimum–maximum, CV) plasma concentrations were 0.20 (0.05–1.47, 58.1%) ng/mL and 0.14 (0.05–1.12, 60.9%) ng/mL at 3 months and 6 months or longer after transplantation, respectively. In lung transplant recipients, the geometric mean (minimum–maximum) plasma concentrations were 0.34 (0.17–0.91, 44.3%) ng/mL and 0.22 (0.06–0.90, 54.4%) ng/mL at 3 months and 6 months or longer after transplantation, respectively.
Development of the Population Pharmacokinetic Model
For the structural model, the model with saturable binding (Eq. 4) fitted the whole blood observations better than the model with linear binding (ΔOFV = − 572.8 points) [Eq. 3]. Parameter estimates of both models are presented in the ESM. For the stochastic model, interindividual variability could be identified on the relative bioavailability (F) and the parameter representing the Bmax. Intraindividual variability was described using a proportional error for both plasma and whole blood observations. Covariance between both error terms was estimated, which significantly improved the overall model fit (ΔOFV = − 536.9 points).
During the first covariate modelling step, effects of dose (per formulation) on F (ΔOFV = − 483.3 points), haematocrit on Bmax (ΔOFV = − 127.4 points) and CL (ΔOFV = − 34.6 points), and time after transplantation on F (ΔOFV = − 210.7 points) were included in the model. In the second covariate step, type of transplantation on CL (ΔOFV = − 111.2 points) and on Bmax (ΔOFV = − 37.5), mycophenolic acid on Bmax (ΔOFV = − 62.7 points), total cholesterol on Bmax (ΔOFV = −46.0 points), vitamin D3 on Bmax (ΔOFV = − 18.4 points), haptoglobin on Bmax (ΔOFV = − 16.2 points) and V (ΔOFV = − 18.1 points), vitamin B12 on Bmax (ΔOFV = − 14.6) and eGFR on CL (ΔOFV = − 11.0 points). No covariates were removed during the backward elimination step.
Model parameters were estimated with adequate precision (Table 2). The final model equations for the model parameters are displayed in Eqs. (5–10) and the structural model is displayed in Eqs. 1, 2 and 4:
| 5 |
| 6 |
| 7 |
| 8 |
| 9 |
| 10 |
Table 2.
Parameter estimates of the population pharmacokinetic model
| Structural model parameters | Parameter estimate | RSE (%) |
|---|---|---|
| Apparent plasma CL (L/h) | 257.6 | 3.6 |
| Apparent plasma Vd (L) | 4213.2 | 8.3 |
| Bmax (ng/mL) | 35.7 | 6.7 |
| C50 (ng/mL) | 0.24 | 10.4 |
| Parameter affected | Covariate parameters | Structure | Parameter estimate | RSE (%) |
|---|---|---|---|---|
| F | Dose | |||
| Prograft | Power | − 0.64 | 4.7 | |
| Envarsus | Power | − 0.74 | 9.7 | |
| Advagraf | Power | − 0.62 | 9.4 | |
| Time after transplantation (days) | Exponential | − 0.32 | 8.9 | |
| Food effect | ||||
| Prograft, fasted | Proportional | − 0.11 | 22.4 | |
| CL | Haematocrit | Power | 0.49 | 16.4 |
| eGFR | Power | 0.07 | 40.2 | |
| Transplantation type (lung recipient) | Proportional | − 0.22 | 10.9 | |
| V | Haptoglobin | Power | 0.13 | 31.2 |
| Bmax | Haematocrit | Power | − 0.43 | 14.6 |
| Total cholesterol | Power | − 0.18 | 16.4 | |
| Haptoglobin | Power | − 0.05 | 22.6 | |
| Vitamin B12 | Power | 0.04 | 32.2 | |
| Transplantation type (lung recipient) | Proportional | 0.10 | 21.9 | |
| Mycophenolic acid | Proportional | 0.14 | 16.3 | |
| Vitamin D use | Proportional | 0.05 | 28.3 |
| Stochastic model parameters | Structure | Estimate (CV%) | RSE (%) | Shrinkage (%) |
|---|---|---|---|---|
| Between-subject variability on F | Exponential | 0.13 (37.3) | 8.5 | 18.2 |
| Between-subject variability on Bmax | Exponential | 0.02 (14.4) | 13.8 | 34.6 |
| Covariance between F and Bmax | − 0.02 (r = −0.39) | |||
| Residual variability for plasma tacrolimus observations | Proportional | 0.14 (37.1) | 4.9 | 13.2 |
| Residual variability for whole blood tacrolimus observations | Proportional | 0.06 (25.3) | 5.4 | 12.6 |
| Covariance between residual variability for plasma and whole blood tacrolimus observations | 0.06 (r = 0.60) |
RSE relative standard error, CL clearance, CV coefficient of variation [estimated as sqrt(exp(estimate) − 1) for between-subject variability and sqrt(estimate) for within-subject variability parameters], r correlation coefficient, Vd volume of distribution, Bmax maximum plasma concentration bound to erythrocytes, C50 plasma concentration resulting in half of maximum binding, F bioavailability, eGFR estimated glomerular filtration rate
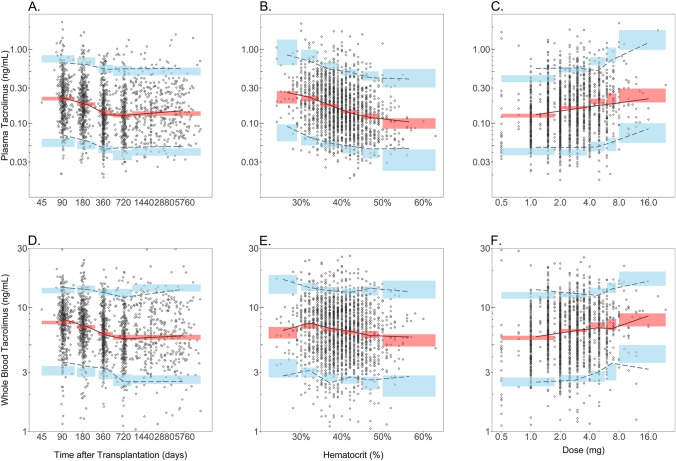
Intraindividual variability (CV) was estimated to be 37.3% for plasma and 25.3% for whole blood. Good accordance between prediction-corrected observed and simulated percentiles was observed, which indicates adequate predictive performance of the joint population pharmacokinetic model in describing the studied population (Fig. 2).
Fig. 2.
Prediction-corrected visual predictive check stratified by plasma and whole blood tacrolimus concentrations. The prediction-corrected plasma tacrolimus concentration is displayed versus time after transplantation (a), haematocrit (b) and dose (c). The prediction-corrected whole blood tacrolimus concentration is also displayed versus time after transplantation (d), haematocrit (e) and daily dose (f). The prediction-corrected observed tacrolimus concentration is displayed as grey open circles. The dashed lines represent the 5th, 50th and 95th percentile of the prediction-corrected observed tacrolimus concentration. The red and blue shaded areas represent the 95% confidence interval for the simulated 5th, 50th and 95th percentiles of the prediction-corrected predicted tacrolimus concentration
Simulations
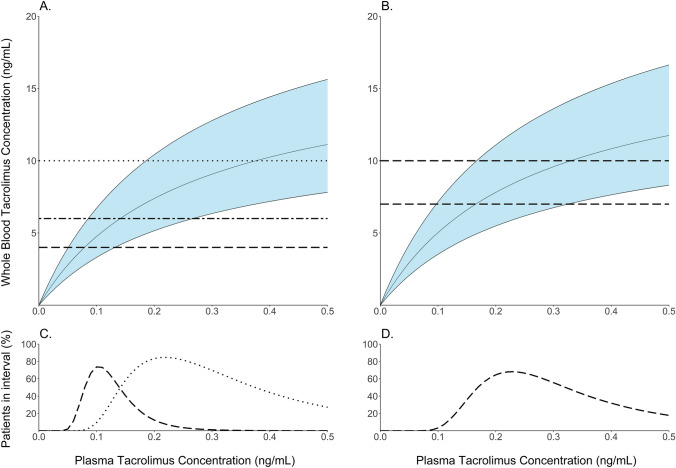
The relationship between plasma and whole blood concentrations was quantified using model simulations (Fig. 3). For kidney transplant recipients, when targeting whole blood concentrations of 6–10 ng/mL at month 3 and 4–6 ng/mL at month 6 and longer, model simulations indicate that 95% of predicted plasma concentrations are expected to fall within 0.10-1.18 ng/mL and 0.06–0.26 ng/mL, respectively. For lung transplant recipients, when targeting whole blood concentrations of 7–10 ng/mL at month 3 and longer, model simulations indicate that 95% of predicted plasma concentrations are expected to fall within 0.10–0.93 ng/mL.
Fig. 3.
Relationship between plasma and whole blood tacrolimus concentrations. The relationship between the plasma and predicted whole blood tacrolimus concentration is displayed, stratified by kidney (a) or lung (b) transplant recipient. Predicted percentage of patients within the whole blood reference range versus the plasma tacrolimus concentration is displayed, stratified by kidney (c) and lung (d) transplant recipient. The solid lines in the top plots represent the 2.5th, 50th and 97.5th percentiles of the predicted whole blood concentration. Shaded area displays the 95% prediction interval. Dotted lines represent the whole blood reference range at month 3 (kidney), and dashed lines represent the whole blood reference range at month 6 (kidney) at month 3 (lung) or later
The marginal effects of significant covariates on the whole blood and plasma concentration were visualised using model simulations (ESM Figs. 1 and 2). The most influential covariate for both plasma and whole blood trough concentrations is dose. For the reference individual, a 3.0 mg dose translates to a plasma concentration of 0.09 (95% PI 0.05–0.19) ng/mL and whole blood concentration of 4.4 (95% PI: 2.7–7.0) ng/mL. A low dose of 0.5 mg and high dose of 25 mg translate to plasma concentrations of 0.05 (95% prediction interval [PI] 0.02–0.10) ng/mL and 0.20 (95% PI 0.10–0.41) ng/mL, respectively, and to whole blood concentrations of 2.7 (95% PI 1.6–4.6) ng/mL and 7.2 (95% PI 4.9–10.6) ng/mL, respectively. Other influential covariates are type of transplantation and haematocrit. Lung transplant recipients had a 22.2% (95% confidence interval [CI] 17.4–27.0%) lower CL compared with kidney transplant recipients, and correspondingly, a higher plasma and whole blood concentration. The effects of haematocrit are more pronounced on the plasma concentrations. A low haematocrit of 20% and high haematocrit of 60% translate to plasma concentrations of 0.14 (95% PI 0.07–0.29) and 0.07 (95% PI 0.04–0.14) ng/mL, respectively, and whole blood concentrations of 4.1 (95% PI 2.7–6.3) ng/mL and 4.5 (95% PI 2.7–7.4) ng/mL, respectively.
Discussion
In this study, we successfully developed a joint population pharmacokinetic model that describes the variability in both plasma and whole blood tacrolimus concentrations in a large heterogeneous population of kidney and lung transplant recipients. The relationship between plasma and whole blood concentrations was best described using a non-linear equation, which indicates that binding to erythrocytes is saturable. Plasma target ranges comparable with currently used uncorrected whole blood target ranges were derived. Additionally, between-subject variability in the plasma concentration could partially be explained by dose, formulation, time after transplantation, food intake, haematocrit, eGFR, transplantation type and haptoglobin. Variability in the whole blood concentration was additionally influenced by haematocrit, total cholesterol, haptoglobin, vitamin B12 level, transplantation type, mycophenolic acid use and vitamin D use.
After organ transplantation, the delicate balance between risks of graft rejection versus adverse effects is monitored through TDM of whole blood tacrolimus concentrations. Tacrolimus has high affinity for erythrocytes and therefore changes in haematocrit are expected to influence the whole blood concentration, but not overall plasma tacrolimus exposure. This formed the basis of the proposed haematocrit correction of the whole blood target range by Størset et al. [4]. In our analysis, we scaled both the bound and plasma concentration by haematocrit and its inverse, to account for changes in the total erythrocyte and plasma volume, respectively. After this correction, we found that the relationship between the plasma and whole blood concentration was best described using a non-linear binding equation. This is in line with a previously published joint population pharmacokinetic model, where binding to erythrocytes was also described using a non-linear binding equation after correcting for haematocrit [9]. This model was however based on a relatively small and clinically unstable population of heart and lung transplant recipients in their first 6 days after transplantation. With the present study, we can confirm that saturable binding is also relevant in our large and more heterogeneous population of stable kidney and lung transplant recipients. A non-linear haematocrit correction of the whole blood target range, as proposed by Schijvens et al., would thus be more appropriate to account for fluctuations in haematocrit when TDM is based on whole blood tacrolimus concentrations [8].
A haematocrit correction of the whole blood target concentration range does however not account for between-subject variability in plasma exposure. As an alternative, the individual plasma exposure can directly be estimated by measuring plasma concentrations in clinical practice. In previous studies, the plasma concentration was however found to poorly correlate with clinical outcomes [18] .This, as well as technical challenges to routinely measure tacrolimus plasma concentrations, hampered implementation of TDM based on plasma concentrations in clinical practice. The poor correlation between plasma concentration and clinical outcomes was attributed to substantial variability in the plasma concentration, which was believed to be a consequence of the used bioanalytical method [18]. A more sensitive bioanalytical assay was used in this study to determine the plasma tacrolimus concentration. Nonetheless, we found that the plasma concentration was still more variable than the whole blood tacrolimus concentration. The larger variability in the plasma concentration is however expected to be an artifact of the data, as TDM based on the uncorrected whole blood target range will only reduce variability in the whole blood concentration. This will come at the cost of increased variability in the haematocrit-corrected whole blood concentration, and even more pronounced variability in the plasma concentration. It is currently unknown whether dose titration using TDM based on plasma concentrations would translate into more predictable clinical outcomes.
Haematocrit and dose showed the most clinically relevant effects on the plasma concentration in the population pharmacokinetic analysis. After correction for changes in the erythrocyte volume, we found that the apparent plasma CL, which is inversely proportional to the plasma exposure, increases with an increase in haematocrit. For whole blood concentrations, the effect of haematocrit on the apparent CL is counteracted by the effect of haematocrit on the maximum binding capacity, which explains the discrepancy in effects. It is not completely understood how haematocrit influences the pharmacokinetic parameters. Haemolytic samples were excluded before analysis, but it cannot be excluded that less pronounced haemolysis had an influence on the analysis. This finding could indicate that a more complex correction of the whole blood target range would be required to account for fluctuations in haematocrit and, in addition, haemolysis. With respect to dose, tacrolimus is deemed to have dose proportional pharmacokinetics, but we observed a non-linear relationship between dose and plasma concentration [19]. Dose proportionality assessments are limited to the whole blood concentration, and therefore it is currently unclear how these findings can be translated to plasma tacrolimus pharmacokinetics.
For abovementioned reasons, it seems easier to measure plasma concentrations in clinical practice. Besides extensive binding to erythrocytes, tacrolimus also binds to several plasma proteins, such as albumin, lipoproteins and α1-acid-glycoprotein [20]. Measuring the total plasma concentration, as done in this study, could thus also be affected by plasma proteins. However, in vitro studies demonstrated that binding of tacrolimus to plasma proteins is not concentration-dependent [21]. Furthermore, in the population pharmacokinetic analysis by Sikma et al., no concentration dependency was observed in the relationship between the total plasma concentration and the unbound plasma concentration [9]. The effect of plasma proteins in that study could however be underestimated as the plasma protein ranges were within the normal reference ranges. In our study, more heterogeneous levels of plasma proteins were observed. Albumin was no significant covariate in our population pharmacokinetic model, but we did identify haptoglobin and total cholesterol as factors that influenced model parameters. However, model simulations indicated that the overall effect of both factors on the plasma and whole blood concentration was limited. Measurement of total plasma concentrations may thus be an accurate enough reflection of the therapeutically active unbound exposure as compared with the currently used whole blood concentrations. However, at this moment it is unknown whether the derived target plasma concentrations translate into more predictable clinical outcomes.
This study has several limitations, of which most pronounced was the observational nature of the study. In the data collection, only nominal times between dosing and sampling were recorded. This hampers interpretation of the dynamic changes in plasma and whole blood concentration during a dosing interval. This is not expected to influence the characterised relationship between plasma and whole blood tacrolimus concentration because binding to erythrocytes is expected to be very rapid. Additionally, only trough concentrations were collected, but due to a switch of dosing interval in some patients, i.e. both once-daily and twice-daily dosing observations were present within subjects, there is some information present in the elimination phase. The developed model should however be limited to predictions of the plasma and whole blood concentration following current practice of trough sample collection as formulation effects also appear to be present. External validation of the model would be required to ensure adequate predictive performance of our model for use in clinical practice. Furthermore, the cytochrome P450 (CYP) 3A genotype is known to influence the tacrolimus pharmacokinetics, but no genotyping of patients has currently been performed [14]. The variability appears to be adequately characterised by the model, but the detected covariate effects (e.g. the difference between kidney and lung transplant recipients) could be confounded by the influence of CYP3A genotype. With respect to the bioanalytical method, it has previously been described that the whole blood to plasma ratio depends on the temperature of incubation [22, 23]. In this study, the influence of temperature was standardised on being incubated and processed at room temperature (21°C). The plasma concentration target ranges proposed in this manuscript depend on the centrifugation temperature used in our laboratory (21°C). In our understanding, standard operating procedures in other laboratories are similar and centrifugation at 21°C is not uncommon. Nevertheless, care should be taken when generalising our results to situations where a different temperature standardisation is implemented.
Conclusion
This study is one of the first steps towards implementation of TDM based on plasma tacrolimus concentrations, rather than whole blood concentrations. We demonstrated that the plasma concentration changes disproportionally with the whole blood concentration due to saturation in binding of tacrolimus to erythrocytes. A haematocrit correction, to account for changes in the total erythrocyte volume, was insufficient to completely remove the influence of haematocrit on several model parameters, which indicates that a more complex non-linear correction of the whole blood target range would be required for TDM in clinical practice. Alternatively, we propose that the plasma concentration can be directly measured. We hypothesise that the plasma concentration is a better estimation of the pharmacologically active moiety. However, the currently used target ranges based on whole blood concentrations translate in a broad target range of comparable plasma concentrations. Future studies are needed to evaluate whether dose titrations based on the plasma concentration would translate into more predictable clinical outcomes.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Funding
The TransplantLines Biobank and Cohort Study was supported by a grant from Astellas BV (TransplantLines Biobank and Cohort Study) and Chiesi Pharmaceuticals BV (PA-SP/PRJ-2020-9136), and was co-financed by the Dutch Ministry of Economic Affairs and Climate Policy by means of the public-private partnership (PPP) allowance made available by the Top Sector Life Sciences & Health to stimulate PPPs. The funders had no role in the design of the study or writing of the manuscript.
Conflict of interest
Jeroen V. Koomen, Tim J. Knobbe, Tanja R. Zijp, Daan Kremer, C. Tji Gan, Erik A.M. Verschuuren, Stephan J.L. Bakker, Daan J. Touw, and Pieter J. Colin declare that they have no financial or non-financial interests that are directly or indirectly related to the work submitted for publication.
Ethics approval
The study protocol of the TransplantLines Biobank and Cohort Study has been approved by the local medical Ethics Review Committee (METc 2014/077).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Availability of data and material
Public sharing of individual participant data was not included in the informed consent of the TransplantLines Biobank and cohort study, but data can be made available to interested researchers upon reasonable request by mailing to the data manager of the TransplantLines Biobank and Cohort study (datarequest.transplantlines@umcg.nl).
Code availability
Model code can be made available by contacting the corresponding author.
Author contributions
J.V. Koomen and P.J. Colin: conception and design of the study, data analysis, modelling, interpretation of data and manuscript preparation. T.J. Knobbe, D. Kremer, C.T. Gan, E.A.M. Verschuren, S.J.L. Bakker: acquisition, interpretation of data, and revised the manuscript. T.R. Zijp, D.J. Touw: interpretation of data, bioanalysis, and revised the manuscript.
References
- 1.Vincenti F, Jensik SC, Filo RS, Miller J, Pirsch J. A long-term comparison of tacrolimus (FK506) and cyclosporine in kidney transplantation: evidence for improved allograft survival at five years. Transplantation. 2002;73:775–782. doi: 10.1097/00007890-200203150-00021. [DOI] [PubMed] [Google Scholar]
- 2.Kasiske BL, Zeier MG, Chapman JR, et al. KDIGO clinical practice guideline for the care of kidney transplant recipients: a summary. Kidney Int. 2010;77:299–311. doi: 10.1038/ki.2009.377. [DOI] [PubMed] [Google Scholar]
- 3.Brunet M, van Gelder T, Åsberg A, et al. Therapeutic drug monitoring of tacrolimus-personalized therapy: second consensus report. Ther Drug Monit. 2019;41:261–307. doi: 10.1097/FTD.0000000000000640. [DOI] [PubMed] [Google Scholar]
- 4.Størset E, Holford N, Midtvedt K, Bremer S, Bergan S, Åsberg A. Importance of hematocrit for a tacrolimus target concentration strategy. Eur J Clin Pharmacol. 2014;70:65–77. doi: 10.1007/s00228-013-1584-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Möller A, Iwasaki K, Kawamura A, et al. The disposition of 14C-labeled tacrolimus after intravenous and oral administration in healthy human subjects. Drug Metab Dispos. 1999;27:633–636. [PubMed] [Google Scholar]
- 6.Hebert MF, Zheng S, Hays K, et al. Interpreting tacrolimus concentrations during pregnancy and postpartum. Transplantation. 2013;95:908–915. doi: 10.1097/TP.0b013e318278d367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benet LZ, Hoener B. Changes in plasma protein binding have little clinical relevance. Clin Pharmacol Ther. 2002;71:115–121. doi: 10.1067/mcp.2002.121829. [DOI] [PubMed] [Google Scholar]
- 8.Schijvens AM, van Hesteren FHS, Cornelissen EAM, et al. The potential impact of hematocrit correction on evaluation of tacrolimus target exposure in pediatric kidney transplant patients. Pediatr Nephrol. 2019;34:507–515. doi: 10.1007/s00467-018-4117-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sikma MA, Van Maarseveen EM, Hunault CC, et al. Unbound plasma, total plasma, and whole-blood tacrolimus pharmacokinetics early after thoracic organ transplantation. Clin Pharmacokinet. 2020;59:771–780. doi: 10.1007/s40262-019-00854-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eisenga MF, Gomes-Neto AW, van Londen M, et al. Rationale and design of TransplantLines: a prospective cohort study and biobank of solid organ transplant recipients. BMJ Open. 2018;8:e024502-2018-024502. [DOI] [PMC free article] [PubMed]
- 11.Koster RA, Dijkers ECF, Uges DRA. Robust, high-throughput LC-MS/MS method for therapeutic drug monitoring of cyclosporine, tacrolimus, everolimus, and sirolimus in whole blood. Ther Drug Monit. 2009;31:116–125. doi: 10.1097/FTD.0b013e318192304c. [DOI] [PubMed] [Google Scholar]
- 12.Zijp TR, van Hateren K, Kuiper H, Jongedijk EM, Touw DJ. Ultra-high throughput dual channel LC–MS/MS for quantification of four immunosuppressants in whole blood for therapeutic drug monitoring. J Chromatogr A. 2023 May 20;1702:464086. [DOI] [PubMed]
- 13.Zijp TR, Knobbe T, van Hateren K, Roggeveld J, Blokzijl H, Tji Gan C, Jl Bakker S, Jongedijk EM, Touw DJ. Expeditious quantification of plasma tacrolimus with liquid chromatography tandem mass spectrometry in solid organ transplantation. J Chromatogr B Analyt Technol Biomed Life Sci. 2023 May 1;1222:123709. [DOI] [PubMed]
- 14.Kirubakaran R, Stocker SL, Hennig S, Day RO, Carland JE. Population pharmacokinetic models of tacrolimus in adult transplant recipients: a systematic review. Clin Pharmacokinet. 2020;59:1357–1392. doi: 10.1007/s40262-020-00922-x. [DOI] [PubMed] [Google Scholar]
- 15.Bauer RJ. NONMEM tutorial part I: description of commands and options, with simple examples of population analysis. CPT Pharmacometrics Syst Pharmacol. 2019;8:525–537. doi: 10.1002/psp4.12404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen THT, Mouksassi M, Holford N, et al. Model evaluation of continuous data pharmacometric models: metrics and graphics. CPT Pharmacometrics Syst Pharmacol. 2017;6:87–109. doi: 10.1002/psp4.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13:143–151. doi: 10.1208/s12248-011-9255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winkler M, Ringe B, Baumann J, Loss M, Wonigeit K, Pichlmayr R. Plasma vs whole blood for therapeutic drug monitoring of patients receiving FK 506 for immunosuppression. Clin Chem. 1994;40:2247–2253. doi: 10.1093/clinchem/40.12.2247. [DOI] [PubMed] [Google Scholar]
- 19.Bekersky I, Dressler D, Mekki QA. Dose linearity after oral administration of tacrolimus 1-mg capsules at doses of 3, 7, and 10 mg. Clin Ther. 1999;21:2058–2064. doi: 10.1016/S0149-2918(00)87237-9. [DOI] [PubMed] [Google Scholar]
- 20.Zahir H, Nand RA, Brown KF, Tattam BN, McLachlan AJ. Validation of methods to study the distribution and protein binding of tacrolimus in human blood. J Pharmacol Toxicol Methods. 2001;46:27–35. doi: 10.1016/S1056-8719(02)00158-2. [DOI] [PubMed] [Google Scholar]
- 21.Piekoszewski W, Jusko WJ. Plasma protein binding of tacrolimus in humans. J Pharm Sci. 1993;82:340–341. doi: 10.1002/jps.2600820325. [DOI] [PubMed] [Google Scholar]
- 22.Nagase K, Iwasaki K, Nozaki K, Noda K. Distribution and protein binding of FK506, a potent immunosuppressive macrolide lactone, in human blood and its uptake by erythrocytes. J Pharm Pharmacol. 1994;46(2):113–117. doi: 10.1111/j.2042-7158.1994.tb03752.x. [DOI] [PubMed] [Google Scholar]
- 23.Wallemacq PE, Firdaous I, Hassoun A. Improvement and assessment of enzyme-linked immunosorbent assay to detect low FK506 concentrations in plasma or whole blood within 6 hours. Clin Chem. 1993;39(6):1045–1049. doi: 10.1093/clinchem/39.6.1045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.