Abstract
Persistent infection with mouse hepatitis virus (MHV) strain A59 in murine DBT (delayed brain tumor) cells resulted in the emergence of host range variants, designated V51A and V51B, at 210 days postinfection. These host range mutants replicated efficiently in normally nonpermissive Chinese hamster ovary (CHO), in human hepatocarcinoma (HepG2), and to a lesser extent in human breast carcinoma (MCF7) cell lines. Little if any replication was noted in baby hamster kidney (BHK), green African monkey kidney (COS-7), feline kidney (CRFK), and swine testicular (ST) cell lines. By fluorescent antibody (FA) staining, persistent viruses V10B and V30B, isolated at days 38 and 119 days postinfection, also demonstrated very low levels of replication in human HepG2 cells. These data suggest that persistence may rapidly select for host range expansion of animal viruses. Pretreatment of HepG2 cells with a polyclonal antibody directed against human carcinoembryonic antigens (CEA) or with some monoclonal antibodies (Col-1, Col-4, Col-12, and Col-14) that bind human CEA significantly inhibited V51B infection. Under identical conditions, little or no blockade was evident with other monoclonal antibodies (kat4c or Col-6) which also bind the human CEA glycoproteins. In addition, an antibody (EDDA) directed against irrelevant antigens did not block V51B replication. Pretreatment with the Col-4 and Col-14 antibodies did not block Sindbis virus replication in HepG2 cells or MHV infection in DBT cells, suggesting that one or more CEA glycoproteins likely functioned as receptors for V51B entry into human cell lines. To test this hypothesis, the human biliary glycoprotein (Bgp) and CEA genes were cloned and expressed in normally nonpermissive BHK cell lines by using noncytopathic Sindbis virus replicons (pSinRep19). By growth curves and FA staining, human CEA and to a much lesser extent human Bgp functioned as receptors for V51B entry. Furthermore, V51B replication was blocked with polyclonal antiserum directed against human CEA and Bgp. Under identical conditions, the parental MHV strain A59 failed to replicate in BHK cells expressing human Bgp or CEA. These data suggest that MHV persistence may promote virus cross-species transmissibility by selecting for virus variants that recognize phylogenetic homologues of the normal receptor.
Animal virus host range specificity and the evolution of new viral diseases are complex phenomena involving interactions between the virus, the host, and the environment (2). Although some new diseases may have resulted from mutations that altered virus tissue tropism, virulence and pathogenesis in the normal host, many new human viruses probably arose by cross-species transmissibility from animal reservoirs (2, 45). Recent examples of emerging viruses include equine morbillivirus, human immunodeficiency viruses (HIVs), hantavirus, hemorrhagic fever viruses, arboviruses, and influenza viruses (27, 45, 47). While these emerging viruses are highly heterogeneous in their structures and replication strategies, they probably have bridged the species barrier by evolving the capacity to interact with specific cellular factors which regulate virus entry, replication, or transmissibility in the new host species. Unfortunately, few studies have attempted to link specific conditions of environmental change with the molecular targets and evolutionary mechanisms that promote the emergence and cross-species transmissibility of animal viruses.
Coronaviridae include a diverse group of highly species specific avian and mammalian viruses and are excellent models to study the fundamental principles governing virus cross-species transmissibility and xenotropism (4, 21, 22, 41). For mouse hepatitis virus (MHV), host range specificity is almost exclusively mediated at entry since the genomic RNA is infectious in nonpermissive cell lines and expression of the MHV receptor (MHVR), a biliary glycoprotein (Bgp1), converts nonpermissive hamster, human, and primate cell lines into susceptible hosts for virus replication (21, 22, 38). In addition to MHVR, a codominant Bgp1 allele (Bgp1b), a second biliary glycoprotein (Bgp2), and a brain pregnancy-specific carcinoembryonic antigen (CEA) glycoprotein may also function as weak receptors for MHV entry in the mouse (11, 51, 70). Although MHV is highly species specific, intracranial inoculation of MHV-JHM into the primate central nervous system (CNS) has resulted in the rapid emergence of primate-adapted strains of MHV that induced encephalitis and demyelination in the new host (50). Using an in vitro model that may reflect conditions present in heavily immunosuppressed xenograph recipients, we have shown that MHV rapidly evolves the capacity to replicate efficiently in many different species (4).
MHV normally causes an acute, self-limited cytolytic infection, but persistent infections can be established in neonatal or immunosuppressed mice and in cultured cells in vitro (12, 26, 53, 55). Following infection, MHV may also persist for a year or more in the CNS of susceptible mice (26). Although the molecular mechanisms of MHV persistence in vivo are unknown, persistent infection in DBT (delayed brain tumor) cells is likely mediated by virus selection for host cells that resist infection by downregulating expression of MHVR (12, 55). In response to emerging host cell resistance, persistent viruses evolve increased virulence and display increased affinity for MHVR and perhaps other Bgp receptors for entry (12). In this report, we demonstrate that persistent MHV infection rapidly promotes the emergence of host range mutants of MHV which replicate efficiently in normally nonpermissive Chinese hamster ovary (CHO) and human cell lines. These variants replicated poorly in baby hamster kidney (BHK) and swine testicular (ST) cells. Using antibody blockade and transfection studies, we showed that persistent viruses use human CEAs (hCEAs) as receptors for entry into nonpermissive hosts. Persistent infection may promote MHV cross-species transmissibility by selecting for viruses that recognize phylogenetic homologues of the normal receptor.
MATERIALS AND METHODS
Virus and cell lines.
DBT cells were originally established from a delayed brain tumor in a CDF1 mouse inoculated intracerebrally with the Schmidt-Ruppin strain of Rous sarcoma virus (12). DBT cells were maintained in Eagle’s minimum essential medium (MEM) containing 8% fetal clone II supplemented with 5% tryptose phosphate broth (TBP), gentamicin (0.05 μg/ml), and kanamycin (0.25 μg/ml). BHK cells were kindly provided by Robert E. Johnston (University of North Carolina at Chapel Hill) and maintained within 12 passages of the original stock in alpha MEM supplemented with 7% fetal calf serum (FCS), 10% TBP, and 1% penicillin-streptomycin. CHO cells were maintained in MEM containing 10% FCS, 5% TPB, gentamicin (0.05 μg/ml), and kanamycin (0.25 μg/ml) at 37°C. Human breast carcinoma (MCF7) cell lines were maintained in dMEM-H (MEM containing 7% FBS, 10% TBP, insulin [10 μg/ml], and 1% penicillin-streptomycin) at 37°C. Human hepatocarcinoma (HepG2) cells were maintained in MEM containing 10% FCS, 5% TPB, gentamicin (0.05 μg/ml), and kanamycin (0.25 μg/ml) at 37°C. ST cell lines were kindly provided by Brenda Hogue (Baylor College of Medicine) and maintained in Eagle’s MEM containing 10% FBS, 1× nonessential amino acids, and 1% penicillin-streptomycin at 37°C.
MHV-A59 and all persistent viruses were propagated in DBT cells as previously described (3, 4). The MHV-H2 BHK-adapted virus variant was isolated from mixed cultures of DBT and BHK cells as previously described (4). All plaque assays were performed in DBT cells cultured in 60-mm2 dishes at 37°C. Cultures were overlaid with 0.8% agarose (LE agarose; SeaKem) in 1× minimal essential medium containing 5% fetal clone II, 5% TPB, gentamicin (0.05 μg/ml), and kanamycin (0.25 μg/ml). Viable cells were stained with neutral red, and the viral plaques were enumerated between 36 and 48 h postinfection.
Isolation of persistent viruses and growth curves.
The characterization of DBT cultures persistently infected with MHV-A59 have been previously reported by our laboratory (12). Viruses were isolated from persistently infected cultures on days 28 (passage 8), 38 (passage 10), 75 (passage 19), 119 (passage 30), and 210 (passage 51) postinfection. Viruses were plaque purified in DBT cells and subsequently repurified by additional rounds of plaque purification. Individual plaques were then inoculated into 60-mm2 dishes, and supernatants were harvested at ∼18 to 24 h postinfection when syncytium formation approached 100%. Virus stocks were propagated in DBT-9 cells grown in 75-cm2 flasks and then stored for subsequent use. Persistent variants V8A and V8B were isolated on day 28, while variants V10A and V10B were isolated at day 38 postinfection. Persistent variant pairs V19A-V19B, V30A-V30B, and V51A-V51B were isolated on days 75, 119, and 210 postinfection, respectively. V30A and V30B were previously designated V1 and V16, respectively (12).
Cultures of DBT-9, BHK, CHO, HepG2, MCF7, and COS-7 cell lines were seeded at densities of 4.0 × 104 to 5.0 × 104 cells/well in eight-well LabTek chamber slides. The cultures were infected at a multiplicity of infection (MOI) of 5 for 1 h, rinsed two to three times with phosphate-buffered saline, (PBS), and overlaid with complete medium. The cultures were incubated at 37°C, and virus samples were harvested at the indicated times for plaque assay as previously described (3, 4).
Detection of viral antigens by immunofluorescence.
Different cell clones grown on LabTek chamber slides were infected with MHV-A59 or persistent virus isolates at an MOI of 5 for 1 h at room temperature. The inocula were removed, and the cells were washed twice with PBS to remove residual virus. At different times postinfection, infected cells were fixed with acetone-methanol (1:1) and stored at 4°C. Fixed cells were rehydrated by several washes in PBS and then incubated with a 1:200 dilution of a polyclonal antibody against MHV-A59 for 30 min at room temperature. After three washes with PBS, the cells were incubated with a 1:100 dilution of goat anti-mouse immunoglobulin G (IgG)-fluorescein isothiocyanate (FITC) conjugate (Sigma) for 30 min at room temperature. Following three washes with PBS, the cells were incubated for 10 min with Evans blue counterstain (Sigma) at room temperature, washed three times with PBS, and then examined under a Nikon FXA fluorescence microscope. To determine the percentage of infected cells, three to five fields were photographed and the numbers of infected cells were counted.
Blockade of persistent virus entry into human cell lines.
HepG2 cells were seeded at densities of 5 × 104 cells into eight-chamber LabTek slides overnight and then incubated with 100 μl of a 1:4 dilution of a monoclonal (Kat4c) or polyclonal (pCEA) antibody for 1 h at 37°C. These antibodies will bind with many different hCEA glycoproteins (DAKO Corp., Carpinteria, Calif.). Alternatively, cells were incubated with monoclonal antibodies varying in CEA reactivity (kindly provided by J. Schlom National Cancer Institute) (33, 46). Seeded cells (5 × 104) were incubated with 100 μl of a 1:4 dilution of the hybridoma supernatants for 1 h prior to infection. The antibodies were then removed, and the cells were inoculated with virus at an MOI of 5 for 1 h at room temperature. Virus was removed, the cultures washed twice with PBS to remove residual virus, and 400 μl of complete medium containing a 1:20 dilution of the appropriate antibody was added to the cultures. Virus samples were harvested at different times postinfection and stored at −70°C for plaque assay in DBT-9 cells. As a control, cells were pretreated with an equivalent amount of a monoclonal antibody (EDDA or R501) directed against an irrelevant antigen.
Cloning and transfection studies.
Human Bgp (hBgp) was cloned from HepG2 cells by using reverse transcription-PCR and was subcloned into TA cloning vectors as instructed by the manufacturer (Promega). Briefly, total intracellular RNA was isolated from HepG2 cells by using RNA STAT-60 reagents as instructed by the manufacturer (Tel-Test “B”, Inc.). To clone the hBgp1 gene, reverse transcription was performed with Superscript 2 reverse transcriptase and an oligodeoxynucleotide primer located just downstream from the C terminus of the Bgp1 open reading frame (ORF) (5′-ACAGAGTAATCCTAGAGG-3′) (5). Following cDNA synthesis at 42°C for 1 h, the cDNA was denatured for 5 min at 94°C and amplified by PCR with Taq polymerase (28 cycles of denaturation at 94°C for 30 s, 58°C for 40 s, and 72°C for 105 s). The forward (5′-CAGGGCCAGCAGGAGACAC-3′) and reverse primer pairs derived from the reported sequence of the hBgp1 gene (GenBank accession no. J03858) (5). The 1.6-kb products were separated in 1.0% agarose gels and isolated by using Qiagen reagents (Qiagen Inc., Chatsworth, Calif.) prior to subcloning into the TA cloning vector. Positive clones were identified by restriction digestion profiles and sequence analysis. The hBgp1 ORF was reamplified by using primers that contained a 5′ XbaI site (5′-CAGTCATCTAGAAGACACCATGGGGCACCTCTC-3′) and a 3′ SphI site (5′-CAGTCAGCATGCCAGGACAGGTTTCATTACTGC-3′), isolated from gels, and inserted in the pSinRep19 expression vector at the XbaI/SphI site. Positive clones were identified by restriction analysis and PCR.
hCEA was subcloned from plasmid DNA kindly provided by J. Schlom. Primers containing a 5′ XbaI site (5′-CAGTCATCTAGAACCATGGAGTCTCCCTCGGCC-3′) and a 3′ SphI site (5′-CAGTCAGCATGCCTGCTATATCAGAGCAACCCC-3′) that spanned the CEA ORF were used for PCR amplification of the approximate 1.5-kb insert. Following restriction analysis, these products were inserted into the pSinRep19 expression vector downstream of a 26S promoter element. The pSinRep19 replicons encoding the T7 RNA polymerase (pSinRep19/T7pol) and green fluorescent protein (GFP) (pSinRep19/GFP) were kindly provided by C. Rice, Washington University.
Selection for stable cell lines expressing human Bgp or CEA.
T7 transcripts were synthesized as instructed by the manufacturer (Stratagene) from XhoI- or NotI-linearized plasmid pSinRep-Bgp1, pSinRep-CEA, pSinRep19/T7pol, or pSinRep19/GFP and electroporated into BHK cells. Briefly, 5 × 106 BHK cells were mixed with GFP, Bgp, or CEA transcripts and electroporated with three pulses at a setting of 850 kV and a capacitor setting of 25 μF, with an approximate time constant after the pulse of 0.6 ms in a 0.2-cm cuvette. Following a 10-min incubation at room temperature, the cells were transferred into a 60-mm2 dish in complete medium. Transfection efficiencies averaged 60 to 80% as determined with the pSinRep19/GFP control. After 12 h, medium containing 5 μg of puromycin per ml was added to the cultures, and cell lines expressing each gene were isolated over the following week.
Cell lines stably expressing similar amounts of human Bgp or CEA were isolated by cell sorting. Briefly, cultures of cells were trypsinized from 75-cm2 flasks, washed twice in PBS, and incubated with monoclonal antibody Kat4c for 30 min at room temperature. The cells were precipitated by centrifugation at 2,500 rpm in an Eppendorf centrifuge for 2 min and washed three times with 1 ml of PBS. The cells were then incubated with FITC-conjugated rabbit anti-mouse IgG antiserum for 30 min at room temperature, washed three times with PBS, and sorted in a Cytomation MoFlo cell sorter (INK, Fort Collins, Colo.). Approximately, 2 × 105 to 5 × 105 cells that displayed low or high levels of CEA or Bgp were collected and cultivated as previously described. Fluorescence-activated cell sorting (FACS) analysis on sorted cultures that stably expressed low Bgp levels (BHK-hBgp1), high Bgp levels (BHK-hBgp1+), or hCEA (BHK-hCEA) were performed as previously described (12), using monoclonal antibody Kat4c.
RESULTS
Persistence selects MHV host range mutants.
MHV infection in DBT cells rapidly established a carrier-state culture in which only a portion of the cells expressed viral antigen (∼5 to 20%) and virus titers persisted at around 106 PFU/ml throughout the first 210 days of culture (12). As the CEA gene family is highly homologous in mammals (5, 6, 10, 22, 51, 54) and we have previously demonstrated that adaptation of MHV to BHK cells resulted in the emergence of mutants with broad host range specificity (4), we determined if persistent infections selected for host range mutants of MHV. Persistent viruses V8A and -B, V10A and -B, V19A and -B, V30A and -B, and V51A and -B were isolated on days 28, 38, 78, 119, and 210 postinfection, respectively. All persistent viruses replicated efficiently in DBT cell lines, approaching titers of 107 to 108 PFU/ml within about 24 to 30 h postinfection. None of the persistent viruses replicated efficiently in BHK cell lines, although a rare V51A- or V51B-infected BHK cell could be detected by fluorescent antibody (FA) staining at frequencies of less than 0.01%. Presumably too few cells were productively infected to increase virus titers above background levels (∼104) associated with the inoculating dose (see Fig. 7). All persistent viruses infected more than 95% of the DBT cells in culture, as evidenced by FA staining (data not shown).
FIG. 7.
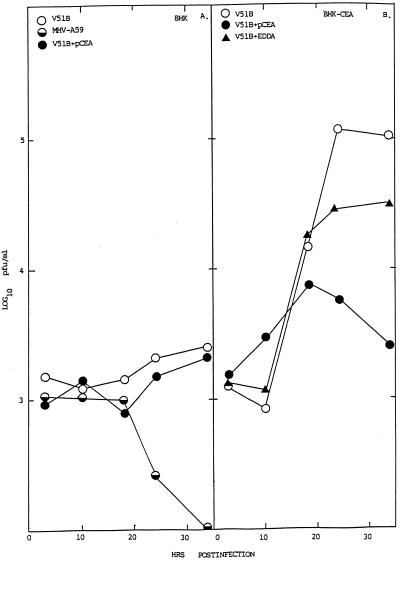
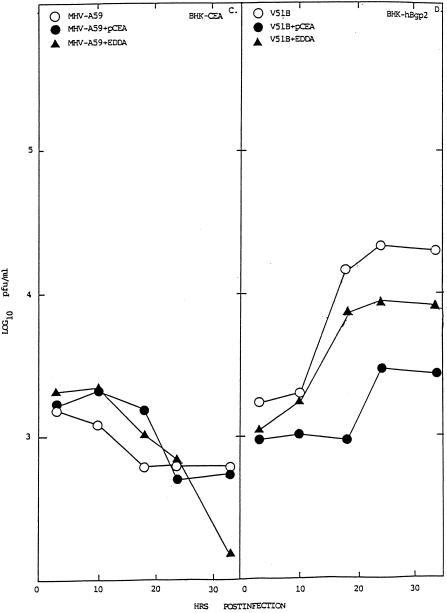
MHV replication in BHK cells expressing hCEA glycoproteins. Cultures of BHK, BHK-hCEA, BHK-hBgp1, and BHK-hBgp1+ cells were infected with V51B or MHV-A59, as indicated, at an MOI of 5 for 1 h at room temperature. In some experiments, the cells had been pretreated with a 1:4 dilution of pCEA or nonspecific antibody EDDA for 30 min prior to infection. Following infection, the cultures were washed extensively and virus were samples taken at the indicated times.
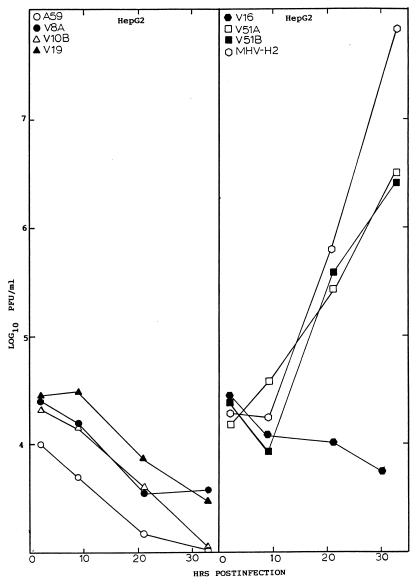
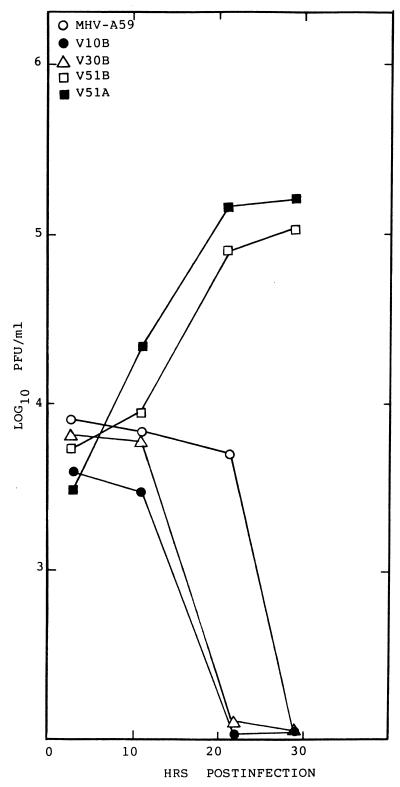
To determine if persistent infection selected for the emergence of viruses that replicated more efficiently in other mammalian hosts, virus replication was examined in hamster (CHO), primate (COS-7), human (HepG2 and MCF7), feline (CRFK), and swine (ST) cell lines. These cell lines were chosen for their susceptibility (CHO, HepG2, COS-7, and MCF7) or resistance (ST and CRFK) to infection with the hamster-adapted isolate, MHV-H2 (4). The V51A and V51B persistent isolates replicated to titers approaching ∼106 PFU/ml in CHO cells (data not shown). Both viruses also plaqued efficiently in CHO cells (data not shown). In addition, the V51A and V51B isolates replicated to titers that approached ∼5 × 106 PFU/ml in HepG2 cells within 36 h postinfection (Fig. 1). Under identical conditions, neither the MHV-A59 parent nor any of the persistent viruses isolated at or before 119 days postinfection replicated efficiently in these cells (Fig. 1). While low levels of V51B replication were noted in human breast carcinoma MCF7 cells with virus titers approaching 104 PFU/ml, no replication was evident in ST or CRFK cells (data not shown).
FIG. 1.
Persistent virus replication in HepG2 cells. Cultures of HepG2 cells (105) were infected with V8A, V10B, V30B, V51A, V51B, MHV-A59, or the MHV-H2 host range variant at an MOI of 5 for 1 h at room temperature. Virus samples were harvested at the designated times and assayed by plaque assay.
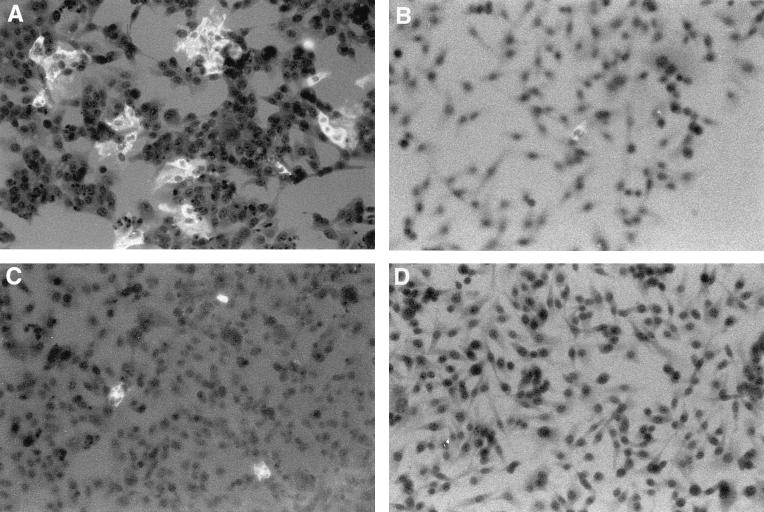
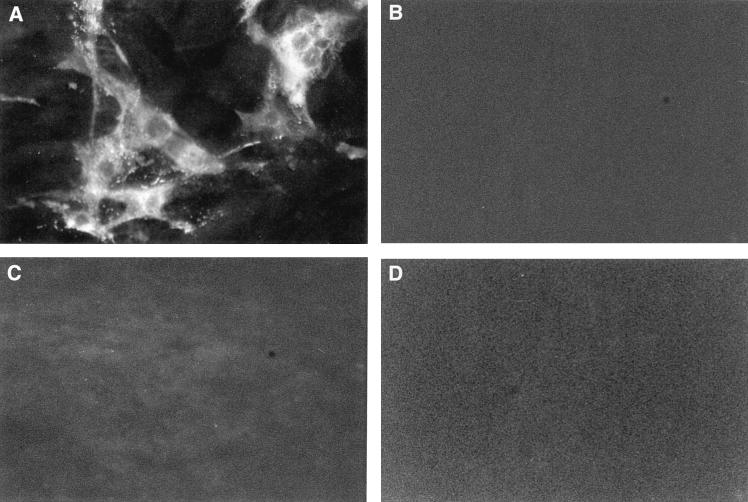
Viral titers directly correlated with the percentage of infected cells noted in culture. By FA staining techniques, V51A and V51B replication was clearly evident in HepG2 cell lines, with about 10 to 20% of the cells, respectively, productively expressing viral antigens, at 18 h postinfection (Fig. 2). At later times, more than 70% of the cells expressed viral antigens (data not shown). Importantly, an occasional (at frequencies of less than 0.1%) antigen-positive HepG2 cell was noted in V10B- or V30B-infected cultures, suggesting that limited MHV host range expansion into human cells may rapidly evolve within the first 38 days of persistence. However, the rare productively infected cell likely failed to produce sufficient progeny virions to increase titers above background levels of the residual inoculum (Fig. 1). These data demonstrated that persistent viruses between 119 and 210 days postinfection had evolved mutations that conferred efficient cross-species transmissibility to some human and hamster cell lines.
FIG. 2.
Persistent virus antigen expression in HepG2 cells. Cultures of HepG2 cell lines in eight-chamber LabTek chambers were infected with V51B (A), V10B (B), V30B (C), or MHV-A59 (D) at an MOI of 5 for 1 h. The cultures were stained with polyclonal MHV-A59 antiserum at 18 h postinfection.
hCEA antiserum blocks persistent virus entry into human cell lines.
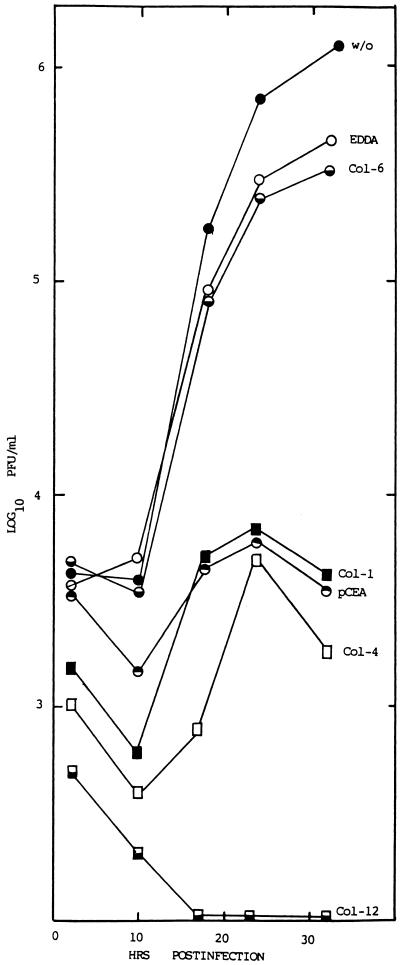
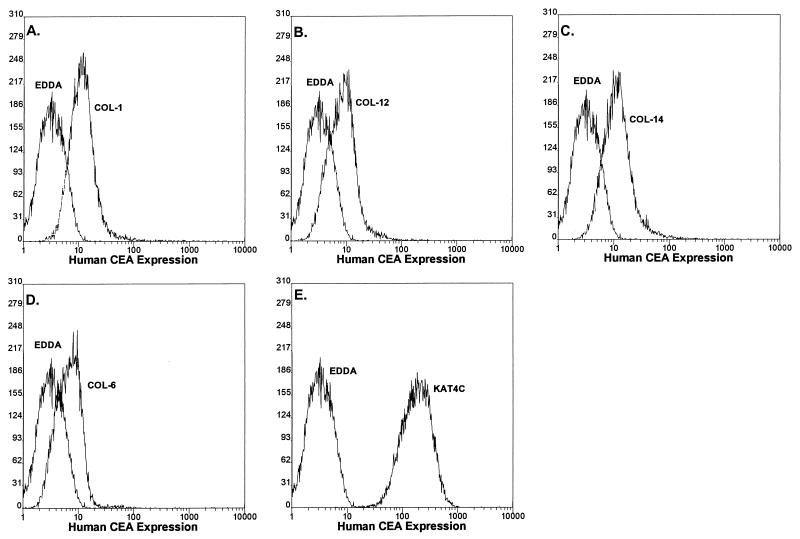
In humans, at least 22 CEA-related genes have been demonstrated to cluster on chromosome 19 (5, 6, 10). These genes have been subdivided into the CEA, Bgp, nonspecific cross-reacting antigen, and pregnancy-specific glycoprotein subgroups based on expression patterns, gene structure, and sequence homologies (6). Sequence comparisons revealed that the human CEA and Bgp glycoproteins are well conserved with MHVR and to a slightly lesser extent with Bgp2 (10) (data not shown). Since previous studies have demonstrated that abundantly expressed hCEA and Bgp may serve as receptors for MHV entry into nonpermissive cells (10), persistent viruses may recognize phylogenetic homologues of the normal receptor to gain entry into human cells. To address this question, we analyzed whether different monoclonal and polyclonal antisera that bind hCEA glycoproteins could block V51B infection in HepG2 cells (16). Incubation of HepG2 cells with pCEA, with monoclonal antibody Kat4c, or with the Col-1, Col-4, Col-6, Col-12, and Col-14 hybridoma supernatants, which displayed more discrete cross-reactivity among the different hCEA genes, produced mixed results (Table 1). Control antibodies against irrelevant antigens (EDDA) failed to block virus replication in HepG2 cells, as virus titers approached those of untreated controls (Fig. 3). Monoclonal antibodies Col-6 and Kat4c also failed to efficiently block V51B replication. Under identical conditions, however, measurable levels of blockade were evident with Col-1, Col-4, Col-12, Col-14, and pCEA (Fig. 3; Table 1). Importantly, these antibodies failed to block Sindbis virus replication in HepG2 cells or block MHV replication in DBT cells (data not shown). FA staining revealed significant reductions in the percentages of infected cells also in cultures pretreated with Col-4 and pCEA (>95% reduction) but not with the EDDA or Kat4c antibody, suggesting that inhibition of virus replication occurred early in infection (data not shown). By FACS analysis, the monoclonal antibodies used in these studies were shown to recognize epitopes in one or more hCEA glycoproteins expressed on HepG2 cells (Fig. 4). Comparisons between the antibody cross-reactivities among the different hCEA glycoproteins and their capacity to block V51B infection in HepG2 cells suggested that one or more hCEA glycoproteins, possibly CEA itself, were likely candidate receptors for V51B entry (Table 1).
TABLE 1.
hCEA antibodies
| Antibody | V51B blockade in HepG2 cells | hCEA antigen cross-reactivitya
|
|||||
|---|---|---|---|---|---|---|---|
| Bgp1 | CGM6 | NCA | CGM1 | CEA | CGM7 | ||
| Monoclonal | |||||||
| Col-1 | + | − | − | − | + | + | − |
| Col-6 | − | − | ? | − | ? | + | ? |
| Col-12 | + | ?a | ? | ? | ? | + | ? |
| Col-4 | + | + | − | − | + | + | − |
| Col-14 | + | ? | ? | ? | ? | + | ? |
| Kat4c | − | + | + | + | − | + | − |
| pCEA (polyclonal) | + | + | + | + | + | + | + |
NCA, non-cross-reacting antigen; ?, not known.
FIG. 3.
Blockade experiments in HepG2 cells. Cultures of HepG2 cells in eight-chamber LabTek slides were untreated (w/o) or treated with various polyclonal or monoclonal antibodies against hCEA genes at a 1:4 dilution for 1 h at room temperature. The antibodies were removed, and the cultures were infected with V51B at an MOI of 5 for 1 h. The virus inoculum was removed, and complete medium containing a 1:20 dilution of the same antibody was added to the chamber. Virus samples were taken at the indicated times, and virus titers were determined by plaque assay in DBT-9 cells.
FIG. 4.
hCEA glycoprotein expression in HepG2 cells. HepG2 cells were pretreated with various antisera for 30 min at room temperature. After extensive washing, FITC-conjugated rabbit anti-mouse IgG antiserum was added for 30 min at room temperature. Following additional washing, the cells were analyzed by FACS techniques. (A) Col-1; (B) Col-12; (C) Col-14; (D) Col-6; (E) Kat4c.
Persistent viruses recognize hCEA glycoproteins as receptors.
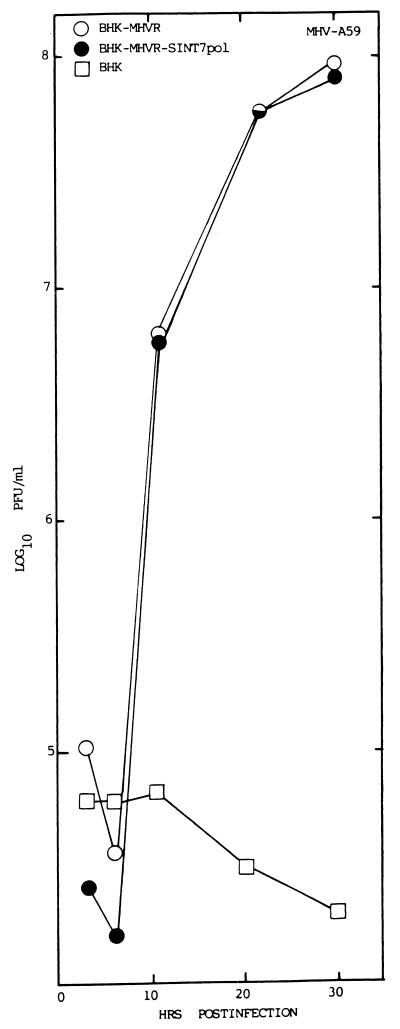
To determine if hCEA glycoproteins could function as receptors for persistent virus entry into human cells, the hBgp gene was cloned from HepG2 cells by using primer pairs obtained from the published sequence (5). The cDNA to the hBgp mRNA was then subcloned downstream of a 26S promoter element in the pSinRep19 noncytopathic Sindbis virus replicons kindly provided by C. Rice (29, 30, 64). In contrast to other Sindbis virus replicons lacking the structural genes (30), these replicons are noncytopathic in BHK cells due to adaptive mutations in the nonstructural protein nsP2 of Sindbis virus (67). Consequently, they establish persistent infections in BHK and other hamster cell lines without apparent deleterious effects. These replicons also encode puromycin resistance from a second downstream 26S promoter, allowing rapid selection of puromycin-resistant cells (67). To determine if these vectors display any toxicity to MHV replication, BHK-MHVR cells were transfected with the pSinRep19/T7pol replicons, and puromycin-resistant cells were isolated and designated BHK-MHVR/SinT7pol cells. MHV-A59 replication was equally efficient in both BHK-MHVR and BHK-MHVR/SinT7pol cells, with peak virus titers approaching 108 PFU/ml by 30 h postinfection, respectively (Fig. 5). These data demonstrate that the Sindbis virus replicons were not inhibitory or antagonistic to efficient MHV infection. Similar findings were noted with several other MHV strains (data not shown).
FIG. 5.
pSinRep19 replicons do not inhibit MHV infection. To determine if the noncytopathic pSinRep19 replicons block MHV replication, cultures of BHK-MHVR cells were transfected with the pSinRep19/T7pol transcripts and selected with puromycin (5 μg/ml) for several days. Cultures of BHK-MHVR/SinT7pol, BHK-MHVR, and BHK cells were infected with MHV-A59 at an MOI of 5 for 1 h. Virus samples were harvested at the indicated times and assayed by plaque assay in DBT cells.
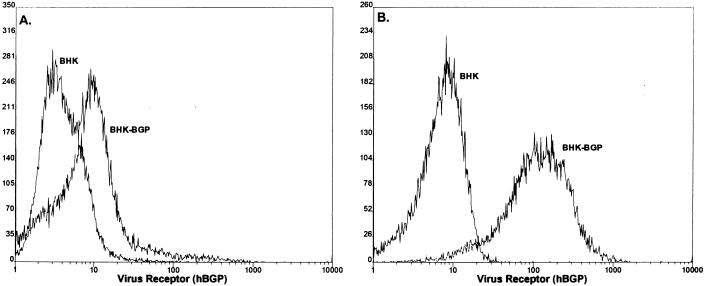
Transcripts were synthesized from pSinRep-CEA and pSinRep-Bgp and electroporated into nonpermissive BHK cells. Following selection with puromycin, cell lines expressing levels of human CEA and Bgp comparable to that noted in HepG2 cells were isolated with monoclonal antibody Kat4c by FACS. We reasoned that by isolating cell lines that expressed levels of CEA and Bgp comparable to those seen in susceptible cells, we would prevent possible receptor-mediated overexpression blockade of MHV replication and allow direct comparisons of receptor usage under normal levels of receptor bioavailability (13). A single cell line expressing hCEA (BHK-hCEA) and two different cell lines expressing either low (BHK-hBgp1) or high (BHK-hBgp1+) levels of hBgp were isolated (Fig. 6). FACs analysis with monoclonal antibody Kat4c demonstrated that hCEA and hBgp were expressed in BHK cell lines at levels comparable with that detected in HepG2 cells (Fig. 4 and 6). Stable hCEA and hBgp expression has been noted for about 2 months in culture.
FIG. 6.
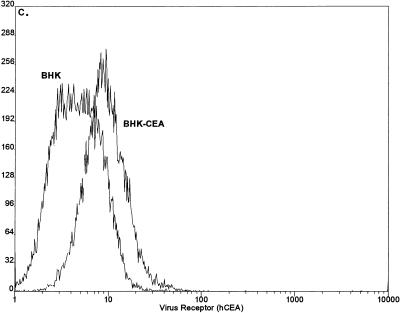
Expression of hCEA and hBgp in BHK cells. BHK cells were transfected with transcripts from the pSinRep19-hBgp and pSinRep19-hCEA replicons and selected with puromycin (5 μg/ml) for several days. By using monoclonal antibody Kat4c, the cultures were sorted into cell lines BHK-hBgp1 (A), BHK-hBgp1+ (B), and BHK-hCEA (C). These cell lines were grown into large stocks of cell lines expressing relatively uniform levels of hCEA or hBgp. FACS analysis was performed with monoclonal antibody Kat4c as previously described (12).
Cultures of BHK, BHK-hBgp1, BHK-hBgp1+, and BHK-hCEA cell lines were infected with MHV-A59 and V51B at an MOI of 5 for 1 h at room temperature. The cultures were washed extensively to remove unabsorbed viruses, and growth curves were analyzed over the next ∼30 h. Efficient V51B virus replication was noted in the BHK-hCEA cell line, with virus titers exceeding 105 PFU/ml—an approximate 2-log increase above background levels (Fig. 7). By FA staining, significant numbers of BHK-hCEA cells expressed viral antigens compared to the controls (Fig. 8). A lower level of V51B replication (∼1 log) was noted in the BHK-hBgp-1+ cell line, suggesting that hBgp may also function as a receptor for entry although with much less efficiency than hCEA (Fig. 7). Similar results were seen following V51B infection in BHK-Bgp1 cells (data not shown). Little if any replication of V51B was noted in BHK cells (Fig. 7). In contrast to previous reports suggesting that hBgp and CEA may function as receptors for MHV-A59 entry (10), no evidence for MHV-A59 replication was detected in the BHK, BHK-hCEA, BHK-hBgp1, and BHK-hBgp1+ cell lines (Fig. 7 and 8). To determine if other V51 isolates or persistent viruses could replicate in the BHK-hCEA cell lines, cultures were infected with V51A, V51B, V30B, V10B, and MHV-A59. Under conditions in which the V51A and V51B isolates replicated to titers approaching 105 PFU/ml, little if any replication was noted with V30B, V10B or MHV-A59 (Fig. 9).
FIG. 8.
Virus antigen in BHK cells expressing hCEA glycoproteins. Cultures treated as described for Fig. 7 were fixed and FA stained for the presence of viral antigen. (A) BHK-hCEA cells infected with V51B; (B) BHK cells infected with V51B; (C) BHK-hCEA cells infected with MHV-A59; (D) BHK cells infected with MHV-A59.
FIG. 9.
Persistent virus replication in BHK-hCEA cells. Cultures of BHK-hCEA cells (106) were infected with MHV-A59, V10B, V30B, V51A, and V51B at an MOI of 5 for 1 h at room temperature. Virus titers were harvested at the designated times for analysis by plaque assay.
To provide additional evidence that hCEA and hBgp were functioning as receptors for V51B entry, blockade experiments were performed with rabbit polyclonal antiserum directed against the hCEA glycoprotein family. Pretreatment of cells with pCEA significantly blocked V51B replication in BHK-hCEA and BHK-hBgp1+ cell lines by ∼1 to 2 logs of titer (Fig. 7). A decrease in the number of V51B-infected cells as detected by FA staining was also noted, consistent with the hypothesis that both hCEA and hBgp could function as receptors for entry into BHK cells (data not shown). These data suggest that persistence may promote MHV cross-species transmissibility by selecting for variants that recognize human homologues of the normal murine Bgp receptor for entry into human cell lines.
DISCUSSION
Virus persistence and cross-species transmissibility.
MHV is highly species specific, yet host range mutants have been isolated from mixed cell cultures in vitro under conditions that may reflect mechanisms of exogenous virus cross-species transmissibility in human xenograph recipients, following intracranial inoculation and persistence in the primate CNS, and following persistent infection in cell lines derived from outbred and inbred mouse strains in vitro (4, 50, 56). As MHV species specificity is likely mediated at entry, these model systems are uniquely positioned to identify the virus-receptor interactions that mediate virus cross-species transmissibility and host range expansion. These models may also allow comparison of the molecular mechanisms that allow viral cross-species transmissibility under a variety of different environmental conditions and in the setting of persistent viral infections. In contrast to the picornavirus, myxovirus, and paramyxovirus host range mutants (14, 32, 48, 57, 61, 69), the MHV models are uniquely positioned for the examination of the molecular mechanisms regulating cross-species transmissibility of zoonotic viruses to human, primate, and other mammalian hosts.
The emergence of new viral diseases in humans is usually attributable to environmental, cultural, or behavior changes that provide new opportunities for virus replication and transmissibility between hosts (45). A prediction of high mutation frequency suggest that RNA viruses evolve rapidly in the face of changing environmental conditions (3, 4, 37, 42). It is less clear, however, whether such changes select for the emergence of viruses from preexisting pools of host range mutants or select for advantageous mutations in a virus which permit cross-species transmissibility into the new host. In this report, we demonstrate that persistent infections rapidly result in a ready source of host range mutants of animal viruses in the absence of any alternate host species. In persistently infected murine 17Cl1 cells, similar findings have been reported by Schickli et al. (56) with the isolation of MHV host range variants at passage 600 (∼3 to 4 years postinfection) that replicate efficiently (∼105 PFU/ml) in some hamster cell lines. In an extension of these studies, we demonstrate that MHV host range expansion evolves within the first 38 days postinfection (passage 10), although efficient infection and replication of human cell lines was evident only with persistent viruses that emerged sometime between 119 and 210 days postinfection (passages 30 to 51). Thus, persistence in DBT cells, which were derived from outbred CD1 mice, rapidly selects for host range mutants of animal viruses which can replicate efficiently in some human, primate, and hamster cell lines. Since many other RNA and DNA viruses persist by selecting for increased host cell resistance and viruses that replicate more efficiently in these resistant cells (12, 17, 19, 28, 36), it will be of interest to determine if cross-species transmissibility represents a common consequence of persistence. Such a hypothesis may not be unprecedented, as substitutions in the capsid of persistent polioviruses also confer a neurovirulent phenotype on the Mahoney type 1 strain in mice (15). As many emerging viruses, such as retroviruses, morbilliviruses, arenaviruses, and many arthropod-borne viruses, rapidly establish persistent infections in their natural hosts, persistent infection in vivo may produce preexisting pools of host range mutants that are rapidly selected following a change in the natural ecology of the host-parasite interaction.
MHV rapidly results in a carrier persistent culture in vitro in which only a small percentage of cells are infected and actively producing progeny virions. Virus infection selects for host cell populations that express little if any MHVR and are resistant to infection (12, 55). Over time, more virulent viruses with altered receptor specificities rapidly emerge, subsequently infecting and replicating in these highly resistant host cell populations (12). Consequently, MHV persistence represents a model for identifying sites of virus-receptor interaction at the cellular level that regulate the coevolution of virus entry, virulence, and host cell resistance. Two general mechanisms may account for host range expansion during persistent MHV infection in vitro and in vivo. During infection, cells expressing little MHVR may select for variants with altered receptor specificities driving cross-species transmissibility (12). While such a mechanism may account for the emergence of host range mutants during persistent infection in vitro, MHV infection is normally acute and self-limiting in vivo, suggesting that host range mutants would have little opportunity to evolve before immunologic clearance. In some cases, however, MHV may reach immunologically privileged sites like the CNS and persist in tissues where MHVR or other alternative Bgp or CEA receptors are expressed at low levels (26, 31, 38, 53). In the murine CNS, MHV sequences may persist for over 1 year, although little if any infectious virus has been demonstrated under these conditions (26). Persistent coronavirus infections in the CNS of humans and primates have also been described (49, 50, 60). Long-term persistence in the CNS may also downregulate receptor expression and effectively select for virus variants with altered receptor specificities and tissue tropisms that fortuitously extend host range specificity. Organ-specific selection of viral variants following chronic infection in mice has been described, and virus infection may downregulate expression of the receptor (1, 36).
As an alternative to this hypothesis, MHV persistence in DBT cells may also be analogous to natural conditions where outbred mice encode multiple Bgp receptor alleles that confer increased host cell susceptibility or resistance to MHV infection (12, 51, 70). For example, the Bgp1b allele is not as efficient a receptor as MHVR, and it confers resistance to MHV-A59 infection in SJL mice (12, 52). In DBT cells which encode the Bgp1a and Bgp1b alleles, persistent viruses rapidly evolve the capacity to efficiently utilize the Bgp1b glycoprotein as a receptor for entry into cells (12, 70). MHV infection in outbred mice that encode several different polymorphic Bgp receptor alleles might also coselect for virus receptor mutants during either an acute or a persistent infection. Over time, such variants may fortuitously extend host range and be amplified following exposure in a new host ecology. Although speculative, both hypotheses provide ample opportunity for the selection of virus variants with altered receptor specificities resulting in host range expansion of MHV. As receptor/entry mutants of many DNA and RNA viruses have been isolated from persistently infected cells (12, 19, 20, 28), persistence may represent an important pathway for host range expansion of many animal and plant viruses. The detection of persistent MHV-like sequences in the CNS of multiple sclerosis patients also supports the disturbing hypothesis that MHV host range expansion may also occur in vivo (8, 49).
Molecular mechanisms of MHV cross-species transmissibility.
We and others have shown that MHV persistence in vitro is maintained by the epigenetic expression of MHVR and by the emergence of persistent viruses which display increased affinity for MHVR and other polymorphic Bgp alleles as receptors for entry (12, 55). All of the persistent viruses use MHVR as a receptor (4a). Consequently, persistent viruses may have an increased affinity for highly conserved domains within all murine Bgp genes, may tolerate increased residue heterogeneity within a common virus binding domain in different Bgps, or may bind to completely different receptor residues in different Bgps. Since persistent viruses also evolve efficient host range expansion between 119 and 210 days postinfection, virus scanning for murine Bgp receptor homologues may have inadvertently expanded the MHV host range by increasing virus affinity for highly homologous domains in the CEA glycoproteins from different species. Since the N-terminal domain of S likely contains the MHVR binding residues, it seems likely that S glycoprotein gene mutations may be responsible for expanded host range (62).
The murine and human CEA genes are well conserved in nature. MHV-A59 but not MHV-JHM can utilize the human Bgp and CEA glycoproteins as receptors for entry when expressed at extremely high levels in nonpermissive cells (10). Using blockade experiments with hCEA antiserum and expression of human CEA and Bgp in nonpermissive BHK cells, V51B entry into human cell lines likely occurs by high-affinity interactions with one or more phylogenetic homologues of the normal viral receptor. Although blockade experiments suggest that the V51 variants may use one or more CEA or Bgp glycoproteins as receptors, we have not identified the actual receptor for persistent virus entry into human cell lines. The hCEA glycoprotein is a more likely choice than Bgp, since it functions much more efficiently as a receptor for V51B entry into BHK cells. It should be noted, however, that not all CEA- or Bgp-expressing cells became productively infected, suggesting that other CEA glycoproteins or that combinations of hCEA glycoproteins may function in persistent virus entry into human cells. Such findings are not unprecedented, as different human chemokine coreceptors function as receptors for HIV entry into different host cell populations (9, 23, 25, 43, 58). The presence of a conserved consensus motif which may function as a common binding domain in murine Bgp, hBgp, and hCEA glycoproteins further supports the possibility that several CEA family members function as receptors for V51B entry (10). Since human and porcine coronaviruses recognize entirely unique portions of their aminopeptidase receptors for entry, considerable latitude might exist in defining the CEA glycoprotein receptor residues that bind V51B and mediate host range expansion into alternative species (40). Detailed analysis of receptor binding residues in the N-terminal domain of the MHV-A59 S glycoprotein gene coupled with identification of virus binding residues in murine and human CEA glycoproteins should reveal fundamental mechanisms regulating the emergence of new viral diseases and adaptation of zoonotic viruses to the human host.
In contrast to previous reports following transient expression of human Bgp and CEA in COS cells (10), we have shown that these glycoproteins do not serve as receptors for MHV-A59 entry when expressed at normal levels of bioavailability. Although speculative, it seems likely that the different results in the two systems may be related to actual levels of human Bgp or CEA receptor bioavailability in the different nonpermissive host backgrounds. Our results, however, are consistent with the inability of MHV to infect any known human cell lines in culture. Additional studies are clearly needed to identify the exact CEA receptor that functions as a receptor for V51 entry into human cell lines and to elucidate the exact conditions of receptor bioavailability required for efficient MHV-A59 infection.
Molecular mechanisms of virus cross-species transmissibility.
The cellular receptor is an essential component for virus entry and a major determinant of host range specificity, tissue tropism, and pathogenesis (35, 65, 66). Since little information is available regarding receptor utilization among phylogenetically related viruses that replicate in distinct species (65), the molecular and evolutionary mechanisms that regulate virus cross-species transmissibility are obscure. Focusing at the level of entry, we propose that new viral diseases may emerge by either a homologue scanning or a receptor switching mechanism. The homologue scanning model predicts that phylogenetic homologues of the normal receptor function as natural conduits for cross-species transmissibility and entry into alternative host species. For example, HIV likely evolved from simian immunodeficiency virus (27). Both viruses utilize CD4 and various related chemokine glycoproteins as receptors and coreceptors for entry into cells (9, 58). Among the group I coronaviruses, transmissible gastroenteritis virus, feline infectious peritonitis virus, and human coronavirus 229E use the aminopeptidase N glycoprotein as a receptor, and they cause similar enteric or upper respiratory tract infections in their hosts (18, 40, 63, 68). Moreover, the feline aminopeptidase N glycoprotein serves as a receptor for entry of all three coronaviruses into cells (63). In these virus families, it seems likely that host range expansion evolved along phylogenetically related receptor molecules.
Receptor switching suggests that host range expansion may occur by virus recognition of entirely new receptor molecules. Murine-adapted strains of poliovirus do not recognize the murine homologue of the poliovirus receptor; rather, these host range mutants recognize some alternative receptor for entry into murine cells (7, 44). Concomitant with virus recognition of unique receptor moieties for entry into murine cells, these viruses cause highly variable disease syndromes (65, 66). Although the speculation is controversial, HIV may also expand its cell tropism in vivo by recognizing galactosyl ceramide as a receptor in neuronal cells and intestinal epithelium (34). Tissue culture adaptation of foot-and-mouth disease virus also results in the emergence of virus variants that recognize heparan sulfate as a receptor for virus entry (39). Clearly, mechanisms regulating virus cross-species transmissibility may be extremely plastic and heavily influenced by the prevailing environmental conditions, selective pressures, and sites of virus-host interaction which regulate species specificity. The same theoretical mechanisms which allow viruses to circumvent the natural barriers of cross-species transmissibility at the level of virus-receptor interaction may also affect the ability of a virus to cross the species barrier by overcoming blocks at the level of transcription, replication, assembly, or release (59, 69). For example, Vif (virus infectivity factor) function is somehow host cell restricted and may represent a critical determinant in the ability of HIV to switch host species (24). Understanding the fundamental molecular mechanisms for coronavirus host range expansion may shed considerable insight into the molecular and evolutionary mechanisms of virus cross-species transmission and the emergence of new diseases in humans and animals.
ACKNOWLEDGMENTS
We thank Sheila Peel, Nancy Davis, and Robert E. Johnston for helpful comments during the course of this research. Special thanks go to Larry Arnold for assisting in the sorting of cell lines expressing hCEA.
This study was supported by a research grant from National Institutes of Health (AI 23964) and a fellowship to W.C. from the Public Health Service (5 T32 A107151-16).
REFERENCES
- 1.Ahmed R, Hahn C S, Somasundaram T, Villarette L, Matloubian M, Strauss J H. Molecular basis of organ-specific selection of viral variants during chronic infection. J Virol. 1991;65:4242–4247. doi: 10.1128/jvi.65.8.4242-4247.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ampel N M. Plagues—what’s past is present: thoughts on the origin and history of new infectious diseases. Rev Infect Dis. 1991;13:658–665. doi: 10.1093/clinids/13.4.658. [DOI] [PubMed] [Google Scholar]
- 3.Baric R S, Fu K S, Schaad M C, Stohlman S A. Establishing a genetic recombination map for MHV-A59 complementation groups. Virology. 1990;177:646–656. doi: 10.1016/0042-6822(90)90530-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baric R S, Yount B, Hensley L, Peel S, Chen W. Episodic evolution mediates interspecies transfer of a murine coronavirus. J Virol. 1997;71:1946–1955. doi: 10.1128/jvi.71.3.1946-1955.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4a.Baric, R. S., et al. Unpublished data.
- 5.Barnett T, Drake L, Pickle W., II Human biliary glycoprotein gene: characterization of a family of novel alternatively spliced RNAs and their expressed proteins. Mol Cell Biol. 1993;13:1273–1282. doi: 10.1128/mcb.13.2.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnett T, Zimmermann W. Workshop report: proposed nomenclature for the carcinoembryonic antigen (CEA) gene family. Tumor Biol. 1990;11:59–63. doi: 10.1159/000217643. [DOI] [PubMed] [Google Scholar]
- 7.Bernhardt, G., J. Harber, A. Zibert, M. deCrombrugghe, and E. Wimmer. The poliovirus receptor: identification of domains and amino acid residues critical for virus binding. Virology 203:344–356. [DOI] [PubMed]
- 8.Burks J S, Pelletier B L, Calvez V, Colbere-Garapin F. Two coronaviruses isolated from central nervous system tissue of two multiple sclerosis patients. Science. 1980;209:933–934. doi: 10.1126/science.7403860. [DOI] [PubMed] [Google Scholar]
- 9.Chackerian B, Long E M, Luciw P A, Overbaugh J. Human immunodeficiency virus type 1 coreceptors participate in postentry stages in the virus replication cycle and function in simian immunodeficiency virus infection. J Virol. 1997;71:3932–3939. doi: 10.1128/jvi.71.5.3932-3939.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen C S, Asanaka M, Chen F S, Shively J E, Lai M M C. Human carcinoembryonic antigen and biliary glycoprotein can serve as mouse hepatitis virus receptors. J Virol. 1997;71:1688–1691. doi: 10.1128/jvi.71.2.1688-1691.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen C S, Asanaka M, Yokomori K, Wang F, Hwang S B, Li H P, Lai M M C. A pregnancy specific glycoprotein is expressed in the brain and serves as a receptor for mouse hepatitis virus. Proc Natl Acad Sci USA. 1995;92:12095–12099. doi: 10.1073/pnas.92.26.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen W, Baric R S. Molecular anatomy of mouse hepatitis virus persistence: coevolution of increased host resistance and virus virulence. J Virol. 1996;70:3947–3960. doi: 10.1128/jvi.70.6.3947-3960.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen W, Madden V J, Bagnell C R, Baric R S. Host derived intracellular immunization against mouse hepatitis virus infection. Virology. 1997;228:318–322. doi: 10.1006/viro.1996.8402. [DOI] [PubMed] [Google Scholar]
- 14.Couderc T, Delpeyrous F, LeBlay H, Blondel B. Mouse adaptation determinants of poliovirus type 1 enhance viral uncoating. J Virol. 1996;70:305–312. doi: 10.1128/jvi.70.1.305-312.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Couderc T, Guedo N, Calvez V, Pelletier I, Hogle J, Colbere-Garapin F, Blondel B. Substitutions in the capsids of poliovirus mutants selected in human neuroblastoma cells confer on the Mahoney type 1 strain a phenotype neurovirulent in mice. J Virol. 1994;68:8386–8391. doi: 10.1128/jvi.68.12.8386-8391.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daams G M, Von Dem Borne A, Van der Shoot C E. Presented at the Seventh International Workshop on Leukocyte Typing, 1996. 1996. Characterization of the CD66/CD67 panel mAB. [Google Scholar]
- 17.De La Torre J C, Marinez-Sales E, Diez J, Villaverde F, Gebauer E, Rocha M, Davilia M, Domingo E. Coevolution of cells and viruses in a persistent infection of foot-and-mouth disease virus in cell culture. J Virol. 1988;62:2050–2058. doi: 10.1128/jvi.62.6.2050-2058.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delmas B, Gelfi J, L’Haridon R, Vogel L K, Sjostrom H, Noren O, Laude H. Aminopeptidase N is a major receptor for the enteropathogenic coronavirus TGEV. Nature. 1992;357:417–420. doi: 10.1038/357417a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dermody T S, Nibert M L, Wetzel D, Tong X, Fields B N. Cells and viruses with mutations affecting viral entry are selected during persistent infections of L cells with mammalian reoviruses. J Virol. 1993;67:2055–2063. doi: 10.1128/jvi.67.4.2055-2063.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duncan G, Pelletier I, Colbere-Garapin F. Two amino acid substitutions in the type 3 poliovirus capsid contribute to the establishment of persistent infection in HEp-2c cells by modifying virus-receptor interactions. Virology. 1998;241:14–29. doi: 10.1006/viro.1997.8955. [DOI] [PubMed] [Google Scholar]
- 21.Dveksler G S, Pensiero C W, Cardellichio C B, McCuaig K, Pensiero M N, Jiang G-S, Beauchemin N, Holmes K V. Several members of the mouse carcinoembryonic antigen-related glycoprotein family are functional receptors for the coronavirus mouse hepatitis virus-A59. J Virol. 1993;67:1–8. doi: 10.1128/jvi.67.1.1-8.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dveksler G S, Pensiero M N, Cardellichio C B, Williams R K, Jiang G-S, Holmes K V, Dieffenbach C W. Cloning of the mouse hepatitis virus (MHV) receptor: expression in human and hamster cell lines confers susceptibility to MHV. J Virol. 1991;65:6881–6891. doi: 10.1128/jvi.65.12.6881-6891.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edinger A L, Amedee A, Miller K, Doranz B J, Endres M, Sharron M, Samson M, Lu Z H, Clements J E, Murphey-Corb M, Peiper S C, Parmentier M, Broder C C, Doms R W. Differential utilization of CCR5 by macrophage and T cell tropic immunodeficiency virus strains. Proc Natl Acad Sci USA. 1997;94:4005–4010. doi: 10.1073/pnas.94.8.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan L, Peden K. Cell-free transmission of vif mutants of HIV-1. Virology. 1992;190:19–29. doi: 10.1016/0042-6822(92)91188-z. [DOI] [PubMed] [Google Scholar]
- 25.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 26.Fleming J O, Houtman J J, Alaca H, Hinze H C, McKenzie D, Aiken J, Bleasdale T, Baker S. Persistence of viral RNA in the CNS of mice inoculated with MHV-4. In: Laude H, Vautherot F J, editors. Coronaviruses. New York, N.Y: Plenum Press; 1994. pp. 327–332. [DOI] [PubMed] [Google Scholar]
- 27.Franchini G, Gurgo C, Guo H G, Collalti E, Fargnoli K A, Hall L F, Wong-Staal F, Reitz M S. Sequence of simian immunodeficiency virus and its relationship to the human immunodeficiency viruses. Nature. 1987;328:539–543. doi: 10.1038/328539a0. [DOI] [PubMed] [Google Scholar]
- 28.Freimuth P. A human cell line selected for resistance to adenovirus infection has reduced levels of the virus receptor. J Virol. 1996;70:4081–4085. doi: 10.1128/jvi.70.6.4081-4085.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frolov I, Hoffman T A, Pragai B M, Dryga S A, Huang H V, Schlesinger S, Rice C M. Alphavirus-based expression systems: strategies and applications. Proc Natl Acad Sci USA. 1996;93:11371–11377. doi: 10.1073/pnas.93.21.11371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frolov I, Schlesinger S. Comparison of the effects of Sindbis virus and Sindbis virus replicons on host cell protein synthesis and cytopathogenicity in BHK cells. J Virol. 1994;68:1721–1727. doi: 10.1128/jvi.68.3.1721-1727.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Godfraind C, Langreth S G, Cardellichio C B, Knobler R, Coutelier J P, Dubois-Dacq M, Holmes K V. Tissue and cellular distribution of an adhesion molecule in the carcinoembryonic antigen family that serves as a receptor for mouse hepatitis virus. Lab Investig. 1995;73:615–627. [PubMed] [Google Scholar]
- 32.Griffin D E, Mullinex J, Nasayan O, et al. Age dependence of viral expression: comparative pathogenesis of two rodent-adapted strains of measles virus in mice. Infect Immun. 1974;9:690–695. doi: 10.1128/iai.9.4.690-695.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grunert F, Soubt M, Jantscheff P, Nagel G. Presented at the Seventh International Workshop on Leukocyte Typing, 1996. 1996. M14.4 specificity and epitope localization of CD66/CD 67 mAB. [Google Scholar]
- 34.Harouse J M, Collman R G, Gonzalez-Scarano F. Human immunodeficiency virus type 1 infection of SK-N-MC cells: domains of gp120 involved in entry into a CD4-negative, galactosyl ceramide/3′ sulfo-galactosyl ceramide-positive cell line. J Virol. 1995;69:7383–7390. doi: 10.1128/jvi.69.12.7383-7390.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haywood A M. Virus receptors: binding, adhesion strengthening, and changes in viral structure. J Virol. 1994;68:1–5. doi: 10.1128/jvi.68.1.1-5.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirano A, Yant S, Iwata K, Korte-Sarfaty J, Seya T, Nagasawa S, Wong T C. Human cell receptor CD46 is down regulated through recognition of a membrane-proximal region of the cytoplasmic domain in persistent measles virus infection. J Virol. 1996;70:6929–6936. doi: 10.1128/jvi.70.10.6929-6936.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holland J J, De La Torre J C, Steinhauer D A. RNA virus populations as quasispecies. Curr Top Microbiol Immunol. 1992;176:1–43. doi: 10.1007/978-3-642-77011-1_1. [DOI] [PubMed] [Google Scholar]
- 38.Holmes K V, Dveksler D G. Specificity of coronavirus receptor interactions. In: Wimmer E, editor. Cell receptors for animal viruses. Plainview, N.Y: Cold Spring Harbor Press; 1994. pp. 403–441. [Google Scholar]
- 39.Jackson T, Ellard F M, Ghazaleh R A, Brookes S M, Blakemore W E, Corteyn A H, Stuart D I, Newman J W, King A M. Efficient infection of cells in culture by type O foot-and-mouth disease virus requires binding to cell surface heparan sulfate. J Virol. 1996;70:5282–5287. doi: 10.1128/jvi.70.8.5282-5287.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klobe A F, Hegyl A, Siddell S G. Identification of residues critical for the human coronavirus 229E receptor function of human aminopeptidase N. J Gen Virol. 1997;78:2795–2802. doi: 10.1099/0022-1317-78-11-2795. [DOI] [PubMed] [Google Scholar]
- 41.Lai M M C. Coronaviruses: organization, replication and expression of genome. Annu Rev Microbiol. 1990;44:303–333. doi: 10.1146/annurev.mi.44.100190.001511. [DOI] [PubMed] [Google Scholar]
- 42.Lai M M C. Genetic recombination in RNA viruses. Curr Top Microbiol Immunol. 1992;176:21–32. doi: 10.1007/978-3-642-77011-1_2. [DOI] [PubMed] [Google Scholar]
- 43.Malcon L, Choe H, Martin K A, Farzan M, Ponath P D, Wu L, Newmann W, Gerard N, Gerard C, Sodroski J. Utilization of C-C chemokine receptor 5 by the envelope glycoproteins of a pathogenic simian immunodeficiency virus, SIVmac239. J Virol. 1997;71:2522–2527. doi: 10.1128/jvi.71.3.2522-2527.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morrison M E, Racaniello V R. Molecular cloning and expression of a murine homolog of the human poliovirus receptor gene. J Virol. 1992;66:2807–2813. doi: 10.1128/jvi.66.5.2807-2813.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morse S S. Factors in the emergence of infectious diseases. Emerg Infect Dis. 1995;1:7–15. doi: 10.3201/eid0101.950102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murano R, Wunderlich A, Thor A, Lundy J, Noguchi R, Cunningham R, Schlom J. Definition by monoclonal antibodies of a repetoire of epitopes on carcinoembryonic antigen differentially expressed in human colon carcinomas versus normal adult tissues. Cancer Res. 1985;45:5769–5780. [PubMed] [Google Scholar]
- 47.Murray K, Selleck P, Hooper P, Hyatt A, Gould A, Gleeson L, Westbury H, Hiley L, Selvey L, Rodwell B, Ketterer P. A morbillivirus that caused fatal disease in horses and humans. Science. 1995;268:94–97. doi: 10.1126/science.7701348. [DOI] [PubMed] [Google Scholar]
- 48.Murray M G, Bradley J, Yang X-F, Wimmer E, Moss E G, Racaniello V R. Poliovirus host range is determined by a short amino acid sequence in neutralization antigenic site I. Science. 1988;241:213–215. doi: 10.1126/science.2838906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murray R S, Brown B, Brian D, Gabirac G F. Detection of coronavirus RNA and antigen in multiple sclerosis brain. Ann Neurol. 1992;31:525–533. doi: 10.1002/ana.410310511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murray R S, Cal G-Y, Hoel K, Zhang J-Y, Soike K F, Cabirac G F. Coronavirus infects and causes demyelination in the primate central nervous system. Virology. 1992;188:274–284. doi: 10.1016/0042-6822(92)90757-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nedellec P, Dveksler G S, Daniels E, Turbide C, Chow B, Basile A A, Holmes K V, Beauchemin N. Bgp2, a new member of the carcinoembryonic antigen-related gene family, encodes an alternative receptor for mouse hepatitis viruses. J Virol. 1994;68:4525–4537. doi: 10.1128/jvi.68.7.4525-4537.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohtsuka N, Yamada Y K, Taguchi F. Difference of virus-binding activity of two receptor proteins for mouse hepatitis virus. J Gen Virol. 1996;77:1683–1692. doi: 10.1099/0022-1317-77-8-1683. [DOI] [PubMed] [Google Scholar]
- 53.Perlman S, Jacobson G, Olson L A, Afifi A. Identification of the spinal cord as a major site of persistence during chronic infection with a murine coronavirus. Virology. 1990;175:418–426. doi: 10.1016/0042-6822(90)90426-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rubert F, Saunders A M, Rebstock S, Thompson J A, Zimmerman W. Characterization of murine carcinoembryonic antigen gene family members. Mamm Genome. 1992;3:262–273. doi: 10.1007/BF00292154. [DOI] [PubMed] [Google Scholar]
- 55.Sawicki S G, Lu J-H, Holmes K V. Persistent infection of cultured cells with mouse hepatitis virus (MHV) results from the epigenetic expression of the MHV receptor. J Virol. 1995;69:5535–5543. doi: 10.1128/jvi.69.9.5535-5543.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schickli J H, Zelus B D, Wentworth D E, Sawicki S G, Holmes K V. The murine coronavirus mouse hepatitis virus strain A59 from persistently infected murine cells exhibits an extended host range. J Virol. 1997;71:9499–9507. doi: 10.1128/jvi.71.12.9499-9507.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scholtissek C, Koennecke I, Rott R. Host range recombinants of fowl plaque (influenza A) virus. Virology. 1978;91:79–85. doi: 10.1016/0042-6822(78)90356-2. [DOI] [PubMed] [Google Scholar]
- 58.Signoret N, Poignard P, Blanc D, Sattentau Q J. Human and simian immunodeficiency viruses: virus-receptor interactions. Trends Microbiol. 1993;1:328–333. doi: 10.1016/0966-842x(93)90072-y. [DOI] [PubMed] [Google Scholar]
- 59.Spandidos D A, Graham A F. Nonpermissive infection of L cells by an avian reovirus: restricted transcription of the viral genome. J Virol. 1976;19:977–984. doi: 10.1128/jvi.19.3.977-984.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stewart J N, Mounir S, Talbot P J. Human coronavirus gene expression in the brains of multiple sclerosis patients. Virology. 1992;191:502–505. doi: 10.1016/0042-6822(92)90220-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Subbarao E K, London W, Murphy B R. A single amino acid in the PB2 gene of influenza A virus is a determinant of host range. J Virol. 1993;67:1761–1764. doi: 10.1128/jvi.67.4.1761-1764.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Suzuki H, Taguchi F. Analysis of the receptor binding site of murine coronavirus spike glycoprotein. J Virol. 1996;70:2632–2636. doi: 10.1128/jvi.70.4.2632-2636.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Treshnan D B, Levis R, Holmes K V. Feline aminopeptidase N serves as a receptor for feline, canine, porcine, and human coronaviruses in serogroup I. J Virol. 1996;70:8669–8674. doi: 10.1128/jvi.70.12.8669-8674.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Washington University Alphavirus Group. Unpublished data.
- 65.Wimmer E. Cellular receptors for viruses. In: Wimmer E, editor. Cellular receptors for viruses. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1994. pp. 1–14. [Google Scholar]
- 66.Wimmer E, Harber J J, Bibb J A, Gromeier M, Lu H-H, Bernhardt G. Poliovirus receptors. In: Wimmer E, editor. Cellular receptors for animal viruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1994. pp. 101–127. [Google Scholar]
- 67.Vara J A, Portela A, Ortin J, Jimenez A. Expression in mammalian cells of a gene from Streptomyces alboniger conferring puromycin resistance. Nucleic Acids Res. 1986;14:4617–4624. doi: 10.1093/nar/14.11.4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yeager C L, Ashmun R A, Williams R K, Cardellichio C B, Shapiro L H, Look A T, Holmes K V. Human aminopeptidase N is a receptor for human coronavirus 229E. Nature. 1992;357:420–422. doi: 10.1038/357420a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yin F H, Lomax N B. Host range mutants of human rhinoviruses in which nonstructural genes are altered. J Virol. 1983;48:410–418. doi: 10.1128/jvi.48.2.410-418.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yokomori K, Lai M M C. Mouse hepatitis virus utilizes two carcinoembryonic antigens as alternative receptors. J Virol. 1992;66:6194–6199. doi: 10.1128/jvi.66.10.6194-6199.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]