Heart transplant (HTx), the gold standard therapy for advance heart failure, has been limited due to shortage of donor hearts which led to the development and growth of mechanical circulatory support devices such as left ventricular assist device (LVAD) and total artificial heart (TAH) [1–4]. This special issue describes several well-crafted manuscripts on LVADs and TAH use, their history of development, implant techniques, and management.
The LVADs, most used mechanical circulatory support (MCS) devices, were initially developed as a bridge to transplant therapy to hemodynamically support patients on waiting list for HTx. As the LVAD technology developed further, the pulsatile bulky devices were replaced by the smaller, durable, continuous flow devices [Bonde et al. this issue] and [5]. The use of LVADs improved the survival amongst patients on HTx waiting list [6]. In this “INFO-TORIAL” (informative editorial), we describe the impact of LVADs as bridge to transplant therapy and its evolution over time in the USA with use of United Network for Organ Sharing (UNOS) thoracic organ transplant database.
Data
The UNOS database is a publicly available database to academic researchers and healthcare quality organizations. We queried the UNOS thoracic transplant database between the years 2000 and 2020 for patients listed for HTx. Patients aged less than 18 years and listed for dual organ transplant were excluded. We then classified the patients in two major categories of LVAD and non-LVAD, which were patients with LVAD (both pulsatile and continuous flow) and no other MCS device, respectively. Patients supported by TAH, extracorporeal membrane oxygenation (ECMO), and other temporary devices were separately analyzed. A further sub-category analysis between pulsatile and continuous flow LVADs was also done.
We evaluated patient characteristics at time of listing and transplant. We computed Kaplan–Meier survival curves to evaluate differences between LVAD and non-LVAD groups while on waiting list and post HTx. All the analyses were done using SAS 9.4 software (SAS Inc., Cary, NC) at 95% confidence level. The data was presented as median (interquartile range) and N (%). Non-parametric statistical tests were used. The Wilcoxon rank-sum test was used for continuous variables and the chi-square test was used for categorical variables.
Waiting list characteristics and outcomes
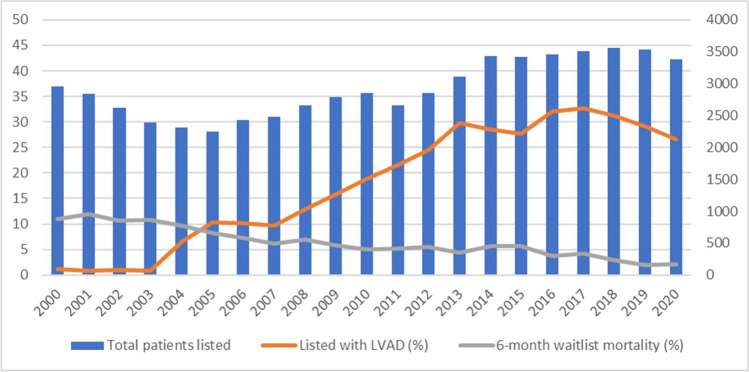
Between the years 2000 and 2020, 61,453 adults were listed for HTx in the USA of which 42,150 (68%) were transplanted. The use of LVAD at time of listing for HTx gradually increased from 1% in 2000 to 30% in 2018 while the 6-month waiting list mortality reduced from 11% in 2000 to 3% in 2020 (Fig. 1).
Fig. 1.
Overall patients listed (secondary axis), patients listed with LVAD (%, primary axis), and 6-month wait list mortality (%, primary axis). Abbreviations: LVAD, left ventricular assist device; BMI, body mass index; eGFR, estimated glomerular filtration rate; MCS, mechanical circulatory support
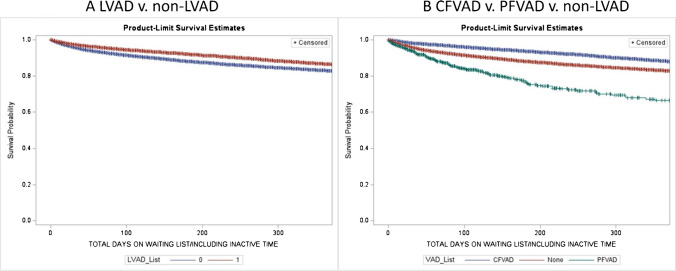
Of the 61,453 listed patients, 11,666 (19%) had an LVAD and 47,286 (77%) were in non-LVAD group and 2501 (4%) had other devices. Table 1A describes the differences between LVAD and non-LVAD groups at time of listing. In the LVAD group, there were more male and diabetes patients, while glomerular filtration rate (GFR) and mean pulmonary artery (PA) pressures were better (Table 1A). The waiting list mortality with use of LVAD was significantly lower compared to non-LVAD (6 months 7% vs 12%, p < 0.01) group (Fig. 2A). A sub-group analysis within the LVAD group showed that continuous flow devices had particularly lower mortality on waiting list (6% vs 23% vs 12% for continuous flow, pulsatile flow, and non-LVAD, respectively, p < 0.01) (Fig. 2B).
Table 1.
Recipient characteristics at time of initial listing (A) and transplant (B)
| Variables | A. Listing variables | B. Transplant variables | ||||
|---|---|---|---|---|---|---|
| Non-LVAD, n = 47,286 | LVAD, n = 11,666 | p-value | Non-LVAD, n = 26,811 | LVAD, n = 12,770 | p-value | |
| Age | 55 (45–62) | 55 (45–62) | 0.07 | 56 (46–63) | 56 (46–63) | 0.48 |
| Gender | 73% | 79% | < .01 | 71% | 81% | < .01 |
| BMI | 27 (23–31) | 29 (25–32) | < .01 | 26 (23–30) | 28 (25–32) | < .01 |
| eGFR | 62 (48–78) | 67 (52–85) | < .01 | 62 (47–78) | 65 (51–82) | < .01 |
| Mean PA pressure | 29 (22–36) | 25 (18–33) | < .01 | 28 (21–35) | 24 (18–32) | < .01 |
| Etiology | ||||||
| Dilated cardiomyopathy | 43% | 49% | < .01 | 42% | 51% | < .01 |
| Ischemic cardiomyopathy | 53% | 50% | 54% | 48% | ||
| Congenital heart defect | 3% | 0.50% | 4% | 1.00% | ||
| Other | 1% | 0.50% | 0% | 0.00% | ||
| Diabetes | 26% | 31% | < .01 | 24% | 30% | < .01 |
| On ventilation | 2% | 4% | < .01 | 2% | 1% | < .01 |
Fig. 2.
A Wait list survival between the LVAD and non-LVAD group (p < .01, data censored at 1 year). B Wait list survival between the continuous flow (CFVAD), pulsatile flow (PFVAD), and non-LVAD groups (p < .01, data censored at 1 year). Abbreviations: LVAD, left ventricular assist device; CFVAD, continuous flow ventricular assist device; PFVAD, pulsatile flow ventricular assist device; BMI, body mass index; eGFR, estimated glomerular filtration rate; MCS, mechanical circulatory support
Transplant characteristics and outcomes
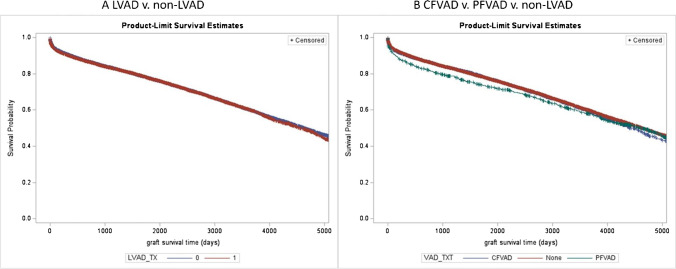
Of the 42,150 transplanted patients, 12,770 (30%) had LVAD and 26,811 (63%) were in the non-LVAD group and 2814 (7%) had other devices. Additional 1104 patients received LVAD after being listed for transplant. Table 1B describes the patients’ characteristics between the LVAD and non-LVAD groups at time of transplant. In the LVAD group, there were more male and diabetes patients while GFR and mean PA pressures were better. The 1- and 5-year post-transplant survival in the LVAD group was 90% and 77% respectively and in the non-LVAD group was 90% and 77% respectively which were not statistically different (p = 0.29, Fig. 3A). The survival stratified by the type of LVAD showed that patients with use of pulsatile flow had a survival disadvantage over continuous flow and non-LVAD patients at 1 year (85% vs 90% vs 90%, pulsatile flow ventricular assist device (PFVAD), continuous flow ventricular assist device (CFVAD), non-LVAD, respectively, p = 0.002, Fig. 3B).
Fig. 3.
A Post-transplant survival between the LVAD and non-LVAD groups (p = 0.29). B Post-transplant survival between the continuous flow LVAD (CFVAD), pulsatile flow LVAD (PFVAD), and non-LVAD groups (p = 0.002). Abbreviations: LVAD, left ventricular assist device; CFVAD, continuous flow ventricular assist device; PFVAD, pulsatile flow ventricular assist device; VAD_TXT, ventricular assist device at time of transplant; LVAD_TXT, left ventricular assist device at time of transplant; BMI, body mass index; eGFR, estimated glomerular filtration rate; MCS, mechanical circulatory support
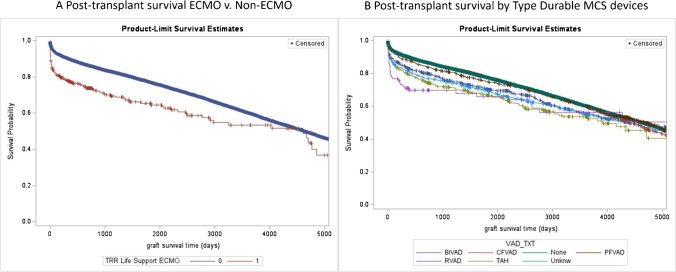
A sub-analysis of ECMO patients showed that use of ECMO as bridge to transplant was associated with higher post-transplant mortality (Fig. 4A). The use TAH, right ventricular assist device (RVAD), and biventricular assist device (BiVAD) was associated with poor post-transplant survival compared to LVAD and non-LVAD groups (Fig. 4B).
Fig. 4.
A Post-transplant survival ECMO vs non-ECMO. B Post-transplant survival by type durable MCS devices. Abbreviations: ECMO, extracorporeal membrane oxygenation; BiVAD, bi-ventricular assist device; CFVAD, continuous flow ventricular assist device; PFVAD, pulsatile flow ventricular assist device; TAH, total artificial heart; PA, pulmonary artery; BMI, body mass index; eGFR, estimated glomerular filtration rate; MCS, mechanical circulatory support
Discussion
Although the number of transplants performed have grown significantly, the donor organ shortage continues to remain the most significant challenge. The use of LVADs as a bridge to transplant therapy has significantly improved the wait list survival of patients and may have allowed more suitable donor organs to be transplanted in otherwise stable patients [6]. The better durability and improved waiting list outcomes with evolution of continuous flow LVADs have increased their use [7]. We have also observed that the LVAD patients had better kidney function and lower PA pressures compared to non-LVAD patients, which shows not only survival advantage on waiting list but also improved end-organ function and hemodynamic stability [8–10]. An important limitation to LVAD use has been the presence of congenital heart defects (both corrected and non-corrected) [11]. Use of TAH and BiVADs is still evolving and patients requiring biventricular support have not shown improved outcomes as described in this article, primarily due to their higher risk profile.
The high cost of the device and associated care may reduce their use as bridge to transplant strategy in India where healthcare insurance and payor market is still evolving [12, 13]. An organized, collective, and data-driven approach from the institutions involved in heart transplantation and LVAD implantation may help design and implement a policy for affordable LVAD implantation and effective ways to cover its cost either under a government-sponsored program or private sector [14]. A central data system such as Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) database may also help in clinical quality assessment, outcomes improvement, policy making, and collaborative research [14].
Conclusion
In summary, the use of continuous LVADs is associated with significant improvement in survival while waiting for HTx. LVAD therapy has several aspects from candidate selection to implantation technique to postoperative management which have been thoroughly described in this special issue of the journal in various articles. With an organized and collective approach, the high cost of LVADs could be mitigated to provide the best treatment options for advanced heart failure patients.
Funding
This work was supported in part by Health Resources and Services Administration contract HHSH250-2019-00001C. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Declarations
IRB approval
The university institutional review board approved this project.
Informed consent
The informed consent requirement was waived/exempted.
Conflict of interest
The authors of this manuscript have no competing interests or relevant financial disclosures. The work was self-funded and the data was obtained from the United Network for Organ Sharing (see “Funding”).
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Torregrossa G, Anyanwu A, Zucchetta F, Gerosa G. SynCardia: the total artificial heart. Ann Cardiothorac Surg. 2014;3:612–20. [DOI] [PMC free article] [PubMed]
- 2.Frazier OH, Rose EA, Oz MC, Dembitsky W, McCarthy P, Radovancevic B, et al. Multicenter clinical evaluation of the HeartMate vented electric left ventricular assist system in patients awaiting heart transplantation. J Thorac Cardiovasc Surg. 2001;122:1186–95. [DOI] [PubMed]
- 3.Miller LW, Pagani FD, Russell SD, John R, Boyle AJ, Aaronson KD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Eng J Med. 2007;357:885–96. [DOI] [PubMed]
- 4.Cowger JA. Addressing the growing US donor heart shortage: waiting for Godot or a transplant? J Am Coll Cardiol. 2017;69:1715–7. [DOI] [PubMed]
- 5.Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–51. [DOI] [PubMed]
- 6.Trivedi JR, Cheng A, Singh R, Williams ML, Slaughter MS. Survival on the heart transplant waiting list: impact of continuous flow left ventricular assist device as bridge to transplant. Ann Thorac Surg. 2014;98:830–4. [DOI] [PubMed]
- 7.Teuteberg JJ, Cleveland JC Jr., Cowger J, Higgins RS, Goldstein DJ, Keebler M, et al. The society of thoracic surgeons intermacs 2019 annual report: the changing landscape of devices and indications. Ann Thorac Surg. 2020;109:649–60. [DOI] [PubMed]
- 8.Pahwa S, Moore S, Slaughter MS, Trivedi JR. CARC17: the impact of persistent renal dysfunction in patients listed for heart transplant with temporary MCS device. ASAIO J. 2022;68:40.
- 9.Schumer EM, Gallo M, Rogers MP, Trivedi JR, Black MC, Massey HT, et al. The development of pulmonary hypertension results in decreased post-transplant survival. ASAIO J. 2018;64:508–14. [DOI] [PubMed]
- 10.Gallo M, Trivedi JR, Whited WM, Slaughter MS. Kidney function after left ventricular assist device placement. J Heart Lung Transplant. 2017;36:S98–9.
- 11.VanderPluym CJ, Cedars A, Eghtesady P, Maxwell BG, Gelow JM, Burchill LJ, et al. Outcomes following implantation of mechanical circulatory support in adults with congenital heart disease: an analysis of the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS). J Heart Lung Transplant. 2018;37:89–99. [DOI] [PubMed]
- 12.Slaughter MS, Bostic R, Tong K, Russo M, Rogers JG. Temporal changes in hospital costs for left ventricular assist device implantation. J Card Surg. 2011;26:535–41. [DOI] [PubMed]
- 13.Sivathasan C, Lim CP, Kerk KL, Sim DKL, Mehra MR. Mechanical circulatory support and heart transplantation in the Asia Pacific region. J Heart Lung Transplant. 2017;36:13–8. [DOI] [PubMed]
- 14.Kirklin JK, Naftel DC, Stevenson LW, Kormos RL, Pagani FD, Miller MA, et al. INTERMACS database for durable devices for circulatory support: first annual report. J Heart Lung Transplant. 2008;27:1065–72. [DOI] [PubMed]