Alzheimer's disease (AD) is a neurodegenerative disease characterized by progressive cognitive dysfunction and behavioral impairment that occurs in old age and pre-old age [1]. Apolipoprotein4 (ApoE4) is the most important genetic risk factor for late-onset AD [2]. Peripheral apoE4 is separated from that in the CNS by the blood-brain barrier (BBB) and is abundantly produced by the liver and macrophages and released into the blood to modulate lipid-related events. However, it is still unclear how peripheral apoE4 affects the pathology and cognitive function of AD patients due to its abundant expression in the brain and periphery. Fortunately, Liu et al. [3] have provided a precise answer to this question in a paper published in the journal Nature Neuroscience. They initially demonstrated that liver-expressed apoE4 disrupts synaptic plasticity and cognitive function by impairing cerebrovascular function and exacerbating brain amyloid pathology. However, apoE3, as a polymorphism of the apoE genotype, can reduce brain amyloid pathology, providing a strong theoretical basis for targeted apoE treatment of AD.
There is no doubt that the findings of Liu et al. are important for understanding the pathology and target strategy of AD. Previous studies have confirmed that apoE4 significantly increases the risk of AD and reduces the age of AD onset in a gene dose-dependent manner. In addition, apoE4 accelerates aging-related cognitive decline in non-demented individuals [4]. Compared to other isoforms, apoE4 drives earlier and more extensive amyloid-β (Aβ) pathology [5]. However, it is difficult to assess the exact role of peripheral apoE4 in brain function and AD pathology because of its presence in both central and peripheral areas. To date, Liu et al. developed animal models that allow for cell-type-specific and inducible expression of apoE3 or apoE4. Animals were bred to albumin-Cre mice to remove the loxP-flanked Neo gene, leading to the expression of human apoE3 or apoE4 in the liver in an apoE-knockout background. Their findings demonstrated that peripheral apoE4 may impair cognitive behavior by disrupting cerebrovascular function, especially the BBB integrity, while peripheral apoE3 has beneficial effects on brain function. In addition, human-induced pluripotent stem cell-derived apoE4-expressing astrocytes display intracellular lipid dysregulation, which leads to vascular dysfunction. And this demonstrates that disruption of key molecules in lipid metabolism may lead to the increased disease risk associated with the apoE4 genotype.
Does peripheral apoE affect cerebrovascular function to different degrees? What is its pathogenesis? Prior studies have shown that cerebrovascular dysfunction, including leakage of the BBB and impairment of microcirculation, is strongly associated with neuronal dysfunction [6]. To examine how peripheral expression of apoE3 or apoE4 affects brain function, Liu et al. constructed liver-specific expression of human apoE3 (iE3) or apoE4 (iE4) in mice in the context of apoE knockout, ensuring that apoE3 or apoE4 was expressed only in the liver and secreted into the blood. They found that the memory of iE3 mice was improved while iE4 mice showed more severe memory deficits and significantly inhibited synaptic plasticity via electrophysiological studies. These results suggested that peripheral apoE3 has beneficial effects, whereas peripheral apoE4 has deleterious effects on cognition. To further explore the underlying pathological mechanism by which liver-expressed apoE4 disrupts brain function, Liu et al. studied cerebral vascular permeability in mice. Increased leakage of the BBB and levels of albumin in perivascular areas were found in iE4 mice. Moreover, decreased levels of tight junction protein claudin-5 and zonula occludens 1 in endothelial cells also occurred in iE4 mice. Meanwhile, increased deposition of perivascular IgG was also found in iE4 mice. In addition, iE4 mice were also found to have a lower frequency vasodilation of cerebral arterioles and significantly reduced numbers of vascular branches, which may lead to decreased arterial cerebral blood flow and an impaired auto-regulation response. In conclusion, these results suggested that peripheral apoE4 has negative effects on the brain function by disrupting the integrity of the BBB. Later, Liu et al. isolated vascular-related cell populations from the brain of iE3 and iE4 mice and conducted scRNA-seq analysis, which showed up-regulated genes of innate immunity, antigen processing, vascular endothelial growth factor signal, matrix remodeling and migration, oxidative stress, and transforming growth fact-β signal in iE4 astrocytes and down-regulated genes for energy homeostasis. These results showed that peripheral apoE4 promotes the immune response of endothelial cells and increases vascular associated gliosis.
This study by Liu et al. confirmed that liver-expressed apoE4 may impair synaptic plasticity and cognitive function by disrupting cerebrovascular function. So, do peripheral apoE isoforms have different effects on brain amyloid deposition? Previous studies have shown that brain amyloid deposition is dependent on apoE isoforms (apoE4 > apoE3 > apoE2). However, whether peripheral apoE isoforms affect amyloid pathology and AD-related pathways remained unclear. Therefore, Liu et al. hybridized iE3 or iE4 mice with APP/PS1 mice in the context of apoE knockout. The results revealed increased deposition of Aβ40 and Aβ42 and decreased synapse-associated postsynaptic density protein 95 in APP/iE4 mice. However, the changes in APP/iE3 mice were opposite to those in APP/iE4 mice. Peripheral apoE4 may disrupt the efficiency of perivascular drainage of Aβ from the brain by disrupting basement membrane proteins and endothelial functions in cerebral blood vessels. The peripheral expression of apoE4 may promote vascular inflammation. In addition, peripheral apoE4 expression can lead to decreased blood flow in the brain, and increased deposition of Aβ in the brain. More importantly, emerging evidence suggests that peripheral systemic factors such as blood components, microbial metabolites, and immune cells can modulate brain function [7,8]. A recent clinical trial evaluating the effects of plasma protein replacement therapy also showed positive effects on reducing cognitive impairment in mild and moderate AD [9]. Liu et al. injected plasma from young iE3 mice or iE4 mice into older WT mice and found that the plasma of iE3 mice improved the cognition and BBB dysfunction of aged mice, while the injection of plasma from iE4 mice into older mice increased leakage of the BBB. It is possible that apoE4 abrogated the beneficial effects of young plasma on old mice. This also provides new possibilities for apoE targeted treatment of AD.
ApoE4 increases AD risk in several ways [10,11]. Liu et al. confirmed that peripheral apoE could affect cognitive function and pathological changes in AD; specifically, apoE3 was protective while apoE4 was destructive (Fig. 1). This study also opens a promising horizon for future studies. A better understanding of how peripheral apoE isoforms affect brain function and AD-related pathways could allow us to elucidate why apoE4 increases AD risk. Not only that, but they also provide new insights into how to target apoE isoforms for individualized treatment of AD.
Fig. 1.
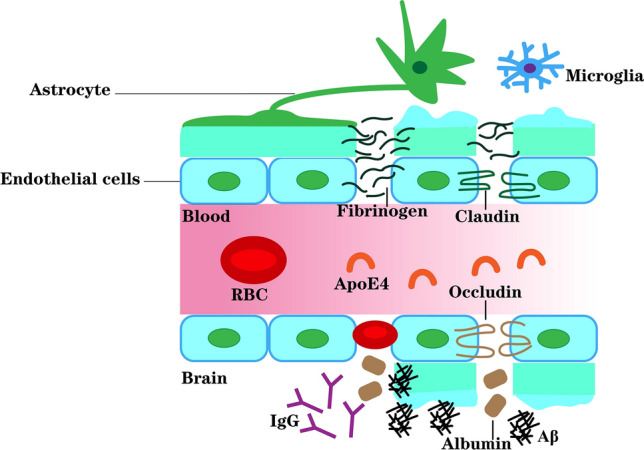
Peripheral apoE4 drives Alzheimer’s pathology by impairing cerebrovascular function. ApoE4 is the most important genetic risk factor for late-onset AD. Liu et al. constructed liver-specific expression of human apoE3 or apoE4 in mice in the context of apoE knockout, ensuring that apoE3 or apoE4 was expressed only in the liver and secreted into the blood. To further explore the underlying pathological mechanism by which liver-expressed apoE4 disrupts brain function, Liu et al. studied cerebral vascular permeability. Increased leakage of the BBB and levels of albumin in perivascular areas were found in iE4 mice. Then, decreased levels of the tight junction proteins claudin-5 (CLDN5) and zonula occludens1 (ZO1) in endothelial cells were also found in iE4 mice. Meanwhile, increased deposition of perivascular IgG also occurred in iE4 mice. Moreover, iE4 mice were also found to have a lower frequency of vasodilation of cerebral arterioles and significantly reduced numbers of vascular branches, which may lead to decreased arterial cerebral blood flow. In addition, the peripheral apoE4 may promote vascular inflammation, thereby transducing inflammatory signals to the brain and the activation of astrocytes and microglia. These together lead to increased Aβ deposition. In conclusion, liver-expressed apoE4 disrupts synaptic plasticity and cognitive function by impairing cerebrovascular function and exacerbating brain amyloid pathology.
However, some basic questions still need further clarification.
First, apoE4 can cause AD-related cognitive impairment by disrupting BBB integrity. However, the leakage of fibrinogen after BBB disruption has been shown to trigger perivascular microglial aggregation and cognitive impairment [12]. Therefore, further studies are needed to determine whether microglia and their responses play a role in peripheral apoE-mediated brain function and AD pathogenesis. In addition, previous studies have demonstrated that the cyclophilin A and matrix metalloprotein-9 mechanism is primarily involved in the process of apoE4 driving BBB damage to impair AD cognition. The current study demonstrated that peripheral apoE4 mediates the pathogenesis of AD by affecting cerebrovascular function. However, the specific mechanism has not been clarified and further research is needed.
Second, peripheral apoE4 increases the deposition of Aβ in the brain. However, the precise mechanism that mediates the exosmosis or influx of Aβ in the brain and the interaction between the apoE isoforms and Aβ receptors remain unclear.
Third, peripheral apoE4 affects cerebrovascular function and AD pathology in multiple ways, but what is the interaction between them?
To conclude, although there are still many unanswered questions, the work of Liu et al. reveals the potential mechanism by which peripheral apoE isoforms affect brain function, explores the possibility of targeting apoE in the treatment of AD, and plays a key role in the in-depth study of the pathophysiological mechanism of cognitive dysfunction in AD patients. As such, the current work may be a milestone in the construction of individualized therapy for AD in the future.
Acknowledgements
This research highlight was supported by the Science and Technology Innovation 2030 Major Projects (2022ZD0211600), the National Natural Science Foundation of China (82271574, 82071204, and 81871069), the Key Projects of Commission of Health, Jiangsu Province (ZDB2020008).
Conflict of interest
All authors declare no competing interest.
References
- 1.JackJr CRB, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brandon JA, Farmer BC, Williams HC, Johnson LA. APOE and alzheimer’s disease: Neuroimaging of metabolic and cerebrovascular dysfunction. Front Aging Neurosci. 2018;10:180. doi: 10.3389/fnagi.2018.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu CC, Zhao J, Fu Y, Inoue Y, Ren Y, Chen Y, et al. Peripheral apoE4 enhances Alzheimer’s pathology and impairs cognition by compromising cerebrovascular function. Nat Neurosci. 2022;25:1020–1033. doi: 10.1038/s41593-022-01127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamazaki Y, Zhao N, Caulfield TR, Liu CC, Bu G. Apolipoprotein E and Alzheimer disease: Pathobiology and targeting strategies. Nat Rev Neurol. 2019;15:501–518. doi: 10.1038/s41582-019-0228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carbonell F, Zijdenbos AP, McLaren DG, Iturria-Medina Y, Bedell BJ, Alzheimer’s Disease Neuroimaging Initiative. Modulation of glucose metabolism and metabolic connectivity by β-amyloid. J Cereb Blood Flow Metab 2016, 36: 2058–2071. [DOI] [PMC free article] [PubMed]
- 6.Miners JS, Schulz I, Love S. Differing associations between Aβ accumulation, hypoperfusion, blood-brain barrier dysfunction and loss of PDGFRB pericyte marker in the precuneus and parietal white matter in Alzheimer’s disease. J Cereb Blood Flow Metab. 2018;38:103–115. doi: 10.1177/0271678X17690761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bulgart HR, Neczypor EW, Wold LE, Mackos AR. Microbial involvement in Alzheimer disease development and progression. Mol Neurodegener. 2020;15:42. doi: 10.1186/s13024-020-00378-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pluvinage JV, Wyss-Coray T. Systemic factors as mediators of brain homeostasis, ageing and neurodegeneration. Nat Rev Neurosci. 2020;21:93–102. doi: 10.1038/s41583-019-0255-9. [DOI] [PubMed] [Google Scholar]
- 9.Boada M, López OL, Olazarán J, Núñez L, Pfeffer M, Paricio M, et al. A randomized, controlled clinical trial of plasma exchange with albumin replacement for Alzheimer’s disease: Primary results of the AMBAR Study. Alzheimers Dement. 2020;16:1412–1425. doi: 10.1002/alz.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montagne A, Nation DA, Sagare AP, Barisano G, Sweeney MD, Chakhoyan A, et al. APOE4 leads to blood-brain barrier dysfunction predicting cognitive decline. Nature. 2020;581:71–76. doi: 10.1038/s41586-020-2247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi Y, Holtzman DM. Interplay between innate immunity and Alzheimer disease: APOE and TREM2 in the spotlight. Nat Rev Immunol. 2018;18:759–772. doi: 10.1038/s41577-018-0051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hussain B, Fang C, Chang J. Blood-brain barrier breakdown: An emerging biomarker of cognitive impairment in normal aging and dementia. Front Neurosci. 2021;15:688090. doi: 10.3389/fnins.2021.688090. [DOI] [PMC free article] [PubMed] [Google Scholar]


