Abstract
Treatment of heart failure needs a firm understanding of anatomy and physiology of the circulatory system and the heart. Ancient India takes credit for the “modern concepts” of human circulation. This short review encompasses futuristic perspectives on mechanical circulatory devices (MCS). The heart is a complex structure which has evolved over millennia both in its structure and mechanical functionality. Evolving from a simple tube with peristaltic action such as in annelids, it evolved rapidly to form a more complexity as animals evolved from oceanic to terrestrial adaptation. The major advance is the innovation of placing the actuation mechanism within the blood flow path, such as in continuous flow technology (axial or centrifugal) when contrasted to the positive displacement pumps. We present novel concepts but also touch upon what we would consider as fundamental problems or paradigms that need to be addressed to move this field ahead. Finally, we propose what would be termed a “futuristic” MCS device.
Keywords: Heart failure, Mechanical circulatory support, Ventricular assist device
Introduction
Background: historical understanding of anatomy and physiology of the circulatory system
Treatment of heart failure needs a firm understanding of anatomy and physiology of the circulatory system and the heart. Anatomical knowledge based on human cadaveric dissection with technical standardization (Sushruta, twelfth to sixth century BC) gave ancient Indian medicine distinct edge over western counterparts where anatomical knowledge was mostly based on animal dissection since cadaveric dissection remained prohibited until 1500 AD (Andreas Vesalius) in the western hemisphere. Ancient Indian medicine predating western understanding by centuries understood cardiovascular anatomy and physiology by considering the heart transmitting energy around the body via “nadis” as channels. Further explanations of “siras” as veins, “dhamini” as arteries, and “srotas” as channels with flow were quite revealing when looked through the prism of what we understand is a “modern” concept of cardiovascular physiology. However, the absence of knowledge about pulmonary circulation (presumably due to reliance on fetal and still born cadavers for dissection) and its role in cardiovascular physiology remained limited for centuries (in ancient Indian treaties), stymying further advances.
When one considers designs of cardiovascular systems, one may need to look at designs not just limited to human species. The remarkable cardiovascular endurance of bar-headed geese (Anser indicus, word origin “Anser”: from proto-Latin: hāns; from proto-Indo-European ǵʰh2éns’ cognates in Sanskrit “hansa”, old English: gos, English: goose) was aptly noted by Kalidasa in Raghuvamsa. Belonging to Anatidae (family of water birds), the bar-headed geese make the remarkable feat of flying over Mount Everest (first noted by George Lowe who accompanied Tenzing Norgay and Edmund Hillary to the summit in 1953, Mount Everest is approximately 29,000 ft high) non-stop and can fly 1000 miles in a single day. A remarkable example of adaptability for a rarified hypoxic atmosphere and endurance, both qualities that would be highly relevant for considering an artificial mechanical circulatory support (MCS) design.
Evolution of heart and MCS designs
When considering a futuristic MCS design, one needs to understand the evolution of the heart structure. Evolving from a simple tube with peristaltic action such as in annelids, it evolved rapidly to form a more complexity as animals evolved from oceanic to terrestrial adaptation. The mammalian and avian heart is unique in pumping the blood at a right angle to its return by complex folding over and rotation of the primitive circulatory tube structure to generate differential pressure and flow to systemic and pulmonary beds respectively. This understanding is important as to why a ventricular assist device (VAD) that is optimized by pump principles to support the left ventricle may not be an appropriate choice to support the right ventricle. In this article, VAD is used to define a concept of ventricular assistance (right vs left, single vs dual chamber support or artificial heart support), instead of using myriad of acronyms for each since most of these systems in development principally use positive displacement or rotary (bulky axial, centrifugal, or mixed rotor) principles for operation except those mentioned below which use differential actuation principles. Historically, external compression of ventricles find mention from time to time, which, frankly, don’t take into account myriad of biological factors (myocardial irritability, thickness, and space within the pericardial space) which would prohibit clinical use and are not discussed specifically. Placement of devices from extracorporeal (actuation chamber outside the body) to intracorporeal (pumping chamber within body) has evolved with miniaturization from abdominal, pre-peritoneal, left pleural, pericardial to intracardiac placements and merely represents incremental technological progress at miniaturization and hence is not specifically commented on. However, a future device should be intracardiac and not be cumbersomely put in extracardiac spaces as biological factors can complicate adverse events.
The major advance that opened the feasibility of VAD therapy to be offered to end-stage heart failure patients on wider scale irrespective of body size is the innovation of placing the actuation mechanism within the blood flow path when contrasted to the positive displacement pumps (which worked on displacing stroke volume by rate of ejection mimicking heart rate × stroke volume to achieve output) where the actuation mechanism essentially sat outside the blood flow path. Naturally, this came at the price of reduced stroke volume but compensated by revolutions per minute by the rotor within the blood flow path contributing fraction of stroke volume per revolution. This led to careful assessment of shear stress on circulating blood cells in terms of hemolysis and trauma to platelets while designing these pumps.
As we sat down to write this article, we felt that enumerating various past, present, and under development devices would not do justice to the title chosen for this manuscript. As several designs or concepts that we came across may be classed as natural evolution of a central idea, so, we sought out what would be defined as “novel” design and concepts. Here, we present not only such novel concepts but also touch upon what we would consider as fundamental problems or paradigms that need to be addressed. In the context of the last comment, we feel one paradigm shift that is needed desperately is to move away from “bridge to bridge” strategies; an ideal device should be agnostic of acuity (acute vs chronic), type of failure (heart failure with reduced ejection fraction (HFrEF) or heart failure with preserved ejection fraction (HFpEF)), early vs late heart failure stages, and indication (bridge to transplant or destination therapy); for this to achieve; device invasiveness should be minimal and ability to switch off operation in situ when not needed (keeping in mind the cyclic nature of heart failure exacerbation) and by inference such device should be durable to meet this challenge.
Usually, any novel idea is supposed to critique or draw attention to the drawbacks inherent in the current deigns. The purpose of this article is not meant to criticize the various limitations and adverse events associated with current and past VAD designs but provide technological and innovative insights in addressing such drawbacks. The author at outset wants to acknowledge and applaud the immense contribution of innovators, engineers, surgeons, physicians, VAD professionals, regulatory bodies, and entrepreneurs/business leaders in saving lives of the critically ill population with heart failure who have very few options. If it were not for the VADs in clinical use now and in the past, many would not have had the chance to live or be candidates for a heart transplantation. In many ways, VAD therapy is unique, in that no single person can influence the decision-making process, which remains very robust with several checks and balances and is least affected by any commercial bias unlike any other pharmaceutical or device use. The often-cited comparison between heart transplantation and VAD therapy is inevitable and in fact both therapies are complementary to each other and innovation in one influence usage of other therapy, as we will outline in the later part of the article.
Evolution from pulsatile to rotary pumps and vestiges of the past
Rotary pumps have emerged as the mainstay therapy for chronic heart failure with vast experience, good performance and safety, albeit at a high cost. This is an indication of an incremental progress in rotary design improving upon prior positive displacement pumps in terms of durability. However, many features of pulsatile pumps which were used for the last 50 years have been retained; like, the driveline, peripherals, inflow, and outflow connections have largely remained unchanged. At the same time, the market is better defined (both acute and chronic heart failure) and remains a niche area with little competition so far. A lot has been learned along the way in terms of patient management and selection. Culmination of these is seen with the successful introduction of HeartMate 3 (Abbott Cardiovascular, Plymouth, MN), in clinical practice, with surgical ease of implantation, MagLev Technology with artificial induced rotor washout [1]. Based on the clinical outcomes, a long due agnostic preimplant strategy (independent of transplant candidacy) has been advocated as a better approach for durable VADs [2]. The final frontier of achieving 1% device thrombosis in HeartMate 3 (Abbott Cardiovascular, Plymouth, MN) is indeed a remarkable engineering achievement (1 and 2). A less invasive approach is feasible using HeartMate 3(Abbott Cardiovascular, Plymouth, MN), for all cases needing a VAD support [3].
The attachment of inflow cannula to the left ventricle apex originated due to the need to fill the historic, large pulsatile devices is retained to feed the powerful rotary pump which is set at a constant speed and is unable to identify and prevent suction should it be used in any other position. This means approach to either side of the heart is necessary, one for left ventricular apex and another to the ascending aorta for unobstructed return and prevent stasis within aortic root if it were to be connected to the descending aorta leading to the increased extent of invasiveness of the procedure. These facts together with unchanged peripherals remain a limitation even if one were to improve upon the pump design itself by miniaturization [4]]. The extent of invasiveness for a frail heart failure patient with organ dysfunction has obvious implications and contrasts with the achievement in the field of transcatheter valves (compared to surgical aortic valves).
The above fact is compounded and exemplified by incidence (% rounded for ease) of stroke (10–15%), driveline infections (25%), bleeding (40–50%), right ventricular dysfunction/failure (30%), respiratory complications (25%), and combined burden of infections that affects > 50% of patients receiving durable VADs [1, 2, 5, 6]]. Thus, invasiveness and not being self-contained remain major technological limitations. Contrast this with some of the patients who undergo cardiac resynchronization therapy/automatic implantable cardiac defibrillator (CRT/AICD) devices (self-contained and transcatheter delivery with no protruding power lines communicating with exterior) which many patients undergo just months prior to VADs have only < 1–2% infectious complications. Feasibility of such self-contained VAD systems was reported for historic VAD systems such as Arrow LionHeart [7] and AbioCor TAH [8] in the past with minimal adverse events attributable to the wireless powering of such devices and significant reduction in infectious adverse events.
A pump is a pump: temporary versus durable
Temporary MCS has seen significant progress in the management strategies and more tilt towards transcatheter approach; however, the designs have mostly remained unchanged for the last decade or so except attempts at deployment via percutaneous catheter–based application [9, 10]. The duration for support is usually limited due to thrombosis, hemolysis, and durability. The progress in durable VADs hasn’t necessarily translated to the temporary MCS field. However, an explosive growth in utilization of endovascular devices may be attributed to innovative business strategy of addressing unmet needs in the interventional catheterization laboratories where the pre-emptive or anticipatory usage has been particularly novel [11, 12].
Total artificial heart (TAH) systems
Attempts in earlier era were to remove both ventricles and replace them with the pumping chambers by pulsatile pumps. Most of the designs of left ventricular assist device (LVAD) actually originated from this earlier work on pulsatile pumps that supported both ventricles. However, it quickly became apparent that not all heart failure patients need both ventricles to be replaced and they can function well even with just left ventricle be replaced by a pump. The only Federal Drug Administration (FDA)–approved device for TAH is the Syncardia TAH system. One of the major limitations of TAH systems is limited patients who may benefit from such therapy (giant cell myocarditis, unresectable cardiac tumors, and profound biventricular failure with no possibility for recovery) and up to 15% of patients needed renal support due to lack of natriuretic peptide as a result of removal of the both ventricles. Continuous flow technology has been applied with differential impeller profile but the same actuation mechanism has been utilized in designs of BiVACOR, Cleveland TAH, Rein Heart, Helical Flow TAH, and Oregon Heart. In an attempt to reduce thromboembolic events, pericardium clad pulsatile devices (CarMat TAH) have been developed. All the TAH systems usually need approach via median sternotomy and excision of both ventricles. Attempts have been made to use LVAD to support/bypass both ventricles with mixed results (HeartWare, HeartMate II, and HeartMate3), the main problem is using a pump optimized for use for high pump head and flow which has the potential to overflood the pulmonary system, and any attempts to reduce outflow diameter can result in increased thrombosis of pumps used for the right side. These attempts are best described as biventricular support devices than TAH systems.
Dynamics of heart failure therapies in the current era
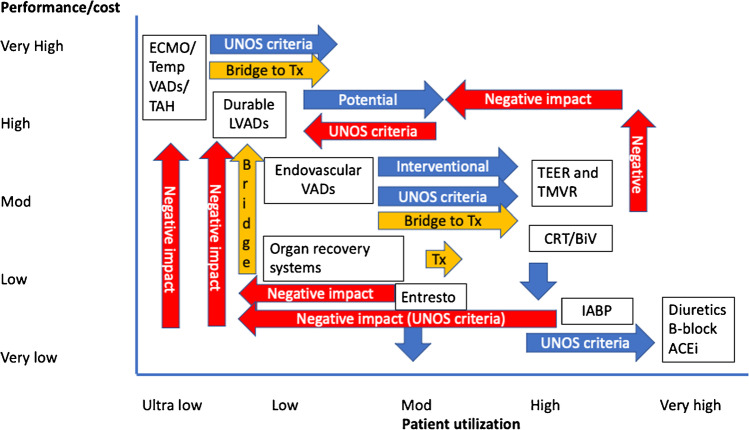
Therapy for heart failure ranges from oral medications to VAD/transplant. Currently, there are several changes that affect the potential of durable devices directly or indirectly (Fig. 1). Besides recent change in United Network for Organ Sharing (UNOS) listing criteria which has obvious implications (in United States (US) but comparable to European and Asian context), indirect effect of increased usage of temporary devices as a bridge to transplant and disease course alterations with emerging therapies such as transcatheter edge to edge repair (TEER) and mitral valve replacement (TMVR), and novel drug combination such as Entresto (Novartis, East Hanover, NJ) is increasingly impacting durable device use (Fig. 1). Several other therapies such as ambulatory counter pulsation, increasing renal flow with impellers placed within the descending aorta, self-expanding axial pumps, vena cava occlusion to reduce preload to right ventricle, and inter atrial shunting devices to manipulate left atrial pressures have been under investigation and some are in clinical trials; their disease-modifying potential is yet to have a major impact on natural history of end-stage heart failure and VAD use. Transcatheter therapies discussed above are trying to address a therapeutic gap before temporary and durable VADs to ameliorate the symptoms of heart failure. Bridging patients from temporary support to durable support remains an area of interest to optimize patients for durable device implant in this population [13]; however, the new UNOS criteria for listing will have continued impact on such strategies in US population; however, the lessons are generalizable where durable VADs are still sparingly used as bridge to transplant.
Fig. 1.
Various heart failure therapies and its effect on the utilization and potential. The x-axis shows patient utilization and y-axis shows performance and cost of each therapy. Therapies and interventions are plotted to signify their relative impact for easier understanding of the landscape of heart failure therapies and cost and utilization has been generalized to convey the central message. Abbreviations: UNOS, United Network for Organ Sharing; IABP, intraaortic balloon pumping; LVAD, left ventricular assist device; VAD, ventricular assist device; TMVR, transcatheter mitral valve replacement; TEER, transcatheter edge to edge repair; Tx, transplant; TAH, total artificial heart; B-block, beta blockers; ACEi, angiotensin-converting enzyme inhibitors; CRT, continuous renal replacement therapy; BiV, biventricular pacing; Tempo, temporary
An ideal VAD system
An ideal VAD device should incorporate four F’s. It should make the patient “free” from surgical invasiveness, stroke, bleeding events by reducing shear, eliminate drivelines, and external controllers/batteries for a 24/7 operation by implantable components that gives total freedom for several hours. It should be “flexible”, and indication agnostic, such as HFrEF, HFpEF, cardiogenic shock, or end-stage congestive heart failure (CHF). The device should be “forgetful”; instead of constantly reminding the patient of myriad of restrictions, it should allow the patient good quality of life with least amount of time commitment for daily upkeep and reminders and have automated remote monitoring capabilities. Such a device should be “forceful”, and it needs to be able to provide the cardiac output with least amount of power consumed.
Innovations to help achieve the ideal VAD
In series vs parallel operation
When one contemplates VAD support for failing heart, one should remember the caveat that the heart (left or right ventricle) isn’t an inert chamber with no output or ability to generate pump head (pressure). The clinically accepted indication to support an anerobic physiology where there is consistent evidence of cardiac index less than 1.8 Lt/min/M2 means the native failed heart is not simply a passive reservoir and this has implications when considering novel ways to augment its function. So, when one considers the failed heart as a pump and adds a VAD in series, the resulting combined pump performance curve results in additive effect on the pump head (pressure in mm of Hg) with same pump flow (Lt/min). Alternatively, when the heart is attached to a VAD in parallel fashion, then the resulting pump performance curve (combined) results in additive effect on the cumulative flow (Lt/min) at the same pressure head (mm of Hg). The fact that pumps used for left-sided support (current parallel use) needs optimization to have an effective pump head is reflective of this and contrast with aim of keeping pump head low and flow larger when supporting right ventricle. These are some fundamentals which are important when trying to use a pump optimized for left-sided support for augmenting right-sided output. Clinical evidence shows troublesome outcomes when one uses a simplistic approach of using left-sided pumps for right-sided use, thinking that all we need is increased overall flow to meet physiological demand. Historically and experimentally, aortic and mitral positions have been tried for ventricular flow augmentation with mixed outcomes. However, for simplicity of understanding (in series vs parallel pumps), one can compare axial pumps placed in transaortic position (Impella family for left-sided support, Abiomed, Danvers, MA) to left ventricular apex to aorta of durable devices (HeartMate 3, Abbott Cardiovascular, Plymouth, MN), for understanding. A summary of innovative features of currently used systems is presented in Table 1.
Table 1.
Current clinically utilized devices and special innovation specific to the device
| Device | Special innovation |
|---|---|
| HeartMate II | Minimal moving parts and actuation mechanism within blood path, reducing mechanical failure |
| HeartMate 3 | Automated periodic washout of rotor by modulating speed: reduction in clot formation |
| Impella family devices | Miniaturization and percutaneous deployment, preemptive use prior to development of cardiogenic shock as in PCI procedures |
| MicroMed Debakey | Ultrasonic flow probe for real-time flow measurement |
| Jarvik 2000 flowmaker | Skull-based pedestal for reducing driveline infections. And wireless powering in two patients reducing driveline infections |
Actuation mechanism
CorWave (CorWave, Clichy, France) employs the concept of simulating the marine animal’s undulating movement for propulsion and is powered by driveline connected to external controller and batteries. It utilizes a standard surgical procedure as any rotary pump with left ventricle to aorta pumping. And possibly can be placed within the pericardium without need for a pump pocket.
TorVAD (Windmill Cardiovascular Systems Inc, Austin, TX) exploits unique abilities of rotary principle but produces a pulsative flow by synchronously shuttling two pistons to create a pulsed output with gated electrocardiogram (ECG) operation. It has a potential for reducing shear and create an adaptive flow. It does need to be connected to left ventricular apex for adequate filling with return to aorta. However, the positive displacement will need extracardiac placement. It is intended to be fully implantable with integrated battery and controller module with likely wireless charging of the batteries.
Bio-H: open helicoid impeller geometry
To reduce shear stress and allow a more physiologic blood path (akin to medium-sized vessels which have a diameter of 6 mm or more), a biologically inspired impeller geometry is proposed that allows majority of the blood path via a large open path [14]. The small footprint allows for miniaturization without compromising flow (contrast with bulky axial/centrifugal rotors that pose limitations in miniaturization) with least amount of hemolysis to achieve a transcatheter delivery. A power consumption of 1.25 Watts at 6 L/min of flow (a maximum flow 8 Lt/min with 8-mm pump) and maximum shear stress that barely exceeds 300 pascals (400 pascals being a hemolytic threshold). A unique non-axial actuation mechanism is the key innovation that allows the critical reduction in power consumption, and this has huge implication with 12 h of implanted battery operation on single charge to provide truly “free” existence.
Eliminate driveline by wireless power transfer
One distinct difference between any implantable medical device such as pacemakers, artificial knee or hips, and VAD is the presence of a permanent electrical cable that penetrates the abdominal wall to connect to a controller and batteries. Besides acting as a constant nidus for infection, this leads to overall less acceptance by patients and poor quality and leads to a cumbersome tethered existence. Personal hygiene is another issue which can drive bacterial colonization in VAD patients.
When an electrical current is passed through a copper (or similar conducting material), it creates a magnetic field, and if another coil is brought close enough to this magnetic field, it can induce magnetic field within the second coil and allow power transfer (think of transformer drums on power lines); such “inductive” power transfer termed transcutaneous electrical transfer (TETS) has been used in couple of historical devices. However, traditional TETS or induction-based power transfer generates heat if misaligned or due to angulation; this drawback is actually exploited in designs of cooking stoves based on inductive power transfer!
Most materials that are electrically conductive exhibit a natural phenomenon called resonance. Electrically conductive materials exhibit natural frequency at which they resonate. One can exploit this by tuning two coils to a specific frequency which when electrically stimulated to form a very tight bond even if separated by meter distances to allow efficient electrical transfer. This fact is exploited in Free Range Resonant Electrical Delivery system (FREE-D system). This is an ideal wireless electrical power transfer regimen for VAD patients as it is minimally influenced by alignment or angulation between implanted and external supply coil. Feasibility of wireless power delivery allows for a totally implantable system [15–17], but for maximum benefit, this needs to be coupled with ultra-fast charging of the implanted batteries (unpublished data) without excessive heat generation for a truly “free” operation. Historical devices such as AbioCor TAH and Arrow LionHeart showed as much as 30% reduction in overall infections when using an older TETs-based wireless power transfer module with 20 min battery time.
Battery technology beyond lithium-ion chemistry
Lithium-ion (and polymer) batteries have been a major advance in both medical and consumer world since its commercial use. However, heat generation, risk of fire, and size limitation for implanted components for VAD would limit the duration of support (although feasible up to 4 h without unduly increasing implant weight if theoretically supporting a commercially available durable VAD device) with concerns for heat generation. Newer technologies based on sodium-ion appear promising, with abundance of sodium in nature and in the human body as well. Current indications show that it would be economical and with longer durations and certain advantages over fouling of the cathodes used in lithium batteries. Futuristic power sources may be able to exploit the ionic composition of body to generate power. Currently, such attempts are limited to generating microwatts for limited applications.
Co-rhythmic or pulsed operation
Phasic ejection from cardiac chambers is ubiquitous in nature across phyla. The clinical indicator of such a pulsatile flow is pulse pressure. Extreme reductions in pulse pressure due to severe aortic stenosis lead to increased shear stress, acquired von Willebrand factor deficiency, and subsequent gastrointestinal bleeding related to arteriovenous malformations. On the other hand, high pulse pressure has been implicated in end organ damage as seen in renal failure, hypertension, and aortic insufficiency.
The reduction in surplus energy with continuous flow relative to pulsatile pumping is one potential drawback of the current devices and reduced pulse pressure is hypothesized in the causation of gastrointestinal bleeding due to arterio-venous malformation, stroke, thromboembolic phenomena, hemolysis, and aortic insufficiency. Theoretically, pulsed operation of current rotary VADs could mitigate these adverse events; however, the optimal quantity of pulse pressure needed is not known. Some degree of pulsatility may be potentially restored with speed-modulated changes in pump speed but this can be accomplished in many ways, whether it be co-pulsation or counter-pulsation and no consensus has been reached regarding the appropriate pulse width or amplitude of speed modulation to produce the greatest hemodynamic benefit. One should note that the artificial pulse in HeartMate 3 device is primarily meant for washout of the rotor and not a true pulsed operation.
With the goal of improving clinical care of people with VADs, wave intensity analysis (WIA) has the potential to inform clinicians of the strength of left ventricular (LV) contraction and relaxation and precisely differentiate between systolic and diastolic dysfunction. Furthermore, WIA can inform the design of future VADs by quantifying the interactions that occur with the LV throughout the cardiac cycle. WIA is far superior to other methods used to define the amount of pulsatility in the LV or aorta (e.g., pulse pressure, pulse index, energy equivalent pressure, or surplus hemodynamic energy) because it quantifies the fundamental energy of waves that create pressure and flow waveforms. One of the major advantages of WIA is that the accurate timing of waves can be measured and related to events that occur throughout the cardiac cycle.
Smart controller with integrated chip technology allows for an adaptive gated electrocardiogram (ECG) pulsed operation [18] to customize beat to beat pump output which is desirable both from engineering perspective for rotor washout and creating more physiologic operation [19, 20]. Health Insurance Portability and Accountability Act (HIPAA) compliant wireless communication allows for remote VAD professional and physician monitoring and call back ability in real time for 24/7 (akin to home security systems) [21].
Maintenance in situ
Innovative feature of future implanted VAD systems would be capability of physician-directed outpatient maintenance of the pump by inspection, self-isolation, and cleaning to eliminate periodic nidus formation of thrombotic material and the risk of stroke (unpublished data).
Transcatheter delivery
Above innovations allow for a transcatheter delivery with a least path to bypass the left ventricle (drawing blood from the left atrium via the interatrial septum and return to the aorta) with intentional trans-caval-aortic puncture which has shown good safety profile when utilized for delivery of transcatheter valves (unpublished data). This also eliminates the issue of blood stasis around cannulas placed in the left ventricle.
Autonomous operation of an implanted VAD
Although VAD addresses the immediate issue of low LV output, patients continue to suffer from CHF and have periodic fluid shifts that result in changes in cardiac chamber sizes. At present, patients are discharged home at a fixed rpm for a given VAD. Ventricular chamber size is measured by echo to adjust speed, typically every 3–5 months. Continuous flow VAD has lower pre-load sensitivity compared to a natural heart; this poses particular challenge while preventing LV suction and maintaining adequate perfusion. Current algorithms can detect but are unable to prevent such suction events which lead to arrhythmia. Repeated suction events can adversely affect the right ventricular function due to septal shift. Implantation of flow and pressure probes are impractical due to risk of thrombus formation, infection, and sensor drift or failure. Sensorless control strategies based on motor current and fuzzy logic are unable to consistently provide adequate perfusion over a range of clinical conditions/activities. Such algorithm assumes a linear relationship between heart rate and flow. Centrifugal VADs are afterload sensitive; the higher the pressure against which they work, the lower the flow at given rpm and this has significant implications in clinical setting of hypertension.
When one contemplates a totally implantable VAD system, the ideal innovation is to transmit power across the skin/tissues and develop sensors that can detect and measure physiological criteria which can then help autonomously run a VAD device. Such an innovation has eluded the heart failure community since different physiological parameters need differing technologies (piezoelectric, ultrasound, infra-red, etc.) to detect, which would make the implant volume prohibitive and impractical. Resonantly coupled sensors that are placed on outflow graft and LV apical cannulation region can accurately predict changes in the heart size with microsecond accuracy without increasing implant volume and complexity and robust lack of senor drift due to tuned single frequency operation [22].
Anticoagulation: systemic vs modifying surfaces
Generally, systemic anticoagulation is used to avoid thrombosis from foreign metal surfaces. This poses the body in a unique physiological milieu of diastolic hypertension, increasing direct connections at capillary level of av malformations and subsequently risk of bleeding as well as thrombosis related to fluctuating anticoagulation levels. Coumadin and antiplatelet agents such as aspirin remain the recommended anticoagulation strategy in VAD patients. Although the contact bearing area exposed to blood remains limited, the entire body is subjected to a state of anticoagulation leading to adverse events. At the same time, adequate anticoagulation is important to avoid device-related strokes and device thrombosis. An interesting paradigm would be to only modify the surface that is exposed to blood to a non-thrombotic chemistry. Several experimental studies show promising results (unpublished data); however, the ability to maintain coatings, presence of fluorinated compounds which can be toxic to the human body, and permanence of the surfaces and interactions with platelets have remained unanswered. However, the idea seems appealing and has gained wide acceptance in consumer products for creating a non-fouling surface [23].
Conclusion
It is clear that there are several advantages to having a transcatheter durable device that meets the physiological demand and is autonomously controlled and wirelessly powered that meets the criteria for an ideal VAD device. The next phase of VAD therapy needs to focus on treating earlier stages of heart failure to arrest the progression as current therapies mostly are symptom-targeted therapies with inability to change course, earlier intervention promises to change the natural history akin to conditions such as aortic stenosis or left main disease.
Funding
None.
Declarations
Ethics
This is a review article and no ethics approval or human ethics approval of consent was needed.
Conflict of interest
The senior author serves on the board of American Society for Artificial Internal Organs. The author is a founder and inventor of Corisma MCS Systems Inc, Hamden, CT, and owns financial interest in the entity. The author is founder of R2TPS LLC, KaNa-Dh LLC, and partner in JSimple LLC with financial interests. The article discusses technologies not yet approved by FDA. The author participated in the MOMENTUM 3 clinical trial of HeartMate 3 device. The author owns several patents related to transcatheter technologies in the domain of mechanical circulatory devices, aortic, mitral, and other intraluminal technologies. No conflict of interest: Riya Bonde.
Footnotes
Novel (def: Merriam Webster): New and not resembling something formerly known or used. Not previously identified. Original or striking especially in conception or style.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mehra MR, Uriel N, Naka Y, Cleveland JC Jr, Yuzefpolskaya M, Salerno CT, et al. MOMENTUM 3Investigators. A fully magnetically levitated left ventricular assist device -final report. N Engl J Med. 2019;380:1618–1627. 10.1056/NEJMoa1900486. [DOI] [PubMed]
- 2.Goldstein DJ, Naka Y, Horstmanshof D, Ravichandran AK, Schroder J, Ransom J, et al. Association of clinical outcomes with left ventricular assist device use by bridge to transplant or destination therapy intent: the multicenter study of MagLev Technology in patients undergoing mechanical circulatory support therapy with HeartMate 3 (MOMENTUM 3) randomized clinical trial. JAMA Cardiol. 2020;5:411-419. 10.1001/jamacardio.2019.5323. [DOI] [PMC free article] [PubMed]
- 3.Bonde P, Stawiarski S, Agboola O, Geirsson A. Less invasive extra-pericardial placement of left ventricular device on reduction of right ventricular failure in the early postoperative period. Presented at the 99th Annual Meeting of AATS, Toronto, CA, 2019.
- 4.Bellumkonda L, Bonde P. Ventricular assist device therapy for heart failure--past, present, and future. Int Anesthesiol Clin. 2012 Summer;50:123–45. [DOI] [PubMed]
- 5.Milano CA, Rogers JG, Tatooles AJ, Bhat G, Slaughter MS, Birks EJ, et al. ENDURANCE Investigators. HVAD: the ENDURANCE Supplemental Trial. JACC Heart Fail. 2018;6:792–802. 10.1016/j.jchf.2018.05.012. [DOI] [PubMed]
- 6.Aaronson KD, Slaughter MS, Miller LW, McGee EC, Cotts WG, Acker MA, et al. HeartWare Ventricular Assist Device (HVAD) Bridge to Transplant ADVANCE Trial Investigators. Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation. 2012;125:3191–200. 10.1161/CIRCULATIONAHA.111.058412. [DOI] [PubMed]
- 7.Pae WE, Connell JM, Adelowo A, Boehmer JP, Korfer R, El-Banayosy A, et al. Clinical Utility Baseline Study (CUBS) Group. Does total implantability reduce infection with the use of a left ventricular assist device? The LionHeart experience in Europe. J Heart Lung Transplant. 2007;26:219–29. 10.1016/j.healun.2006.12.007. [DOI] [PubMed]
- 8.Dowling RD, Gray LA Jr, Etoch SW, Laks H, Marelli D, Samuels L, et al. Initial experience with the AbioCor implantable replacement heart system. J Thorac Cardiovasc Surg. 2004;127:131-41. 10.1016/j.jtcvs.2003.07.023. [DOI] [PubMed]
- 9.Saffarzadeh A, Bonde P. Options for temporary mechanical circulatory support. J Thorac Dis. 2015;7:2102–2111. doi: 10.3978/j.issn.2072-1439.2015.09.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Letzen B, Park J, Tuzun Z, Bonde P. Design and development of a miniaturized percutaneously deployable wireless left ventricular assist device: early prototypes and feasibility testing. ASAIO J. 2018;64:147–153. doi: 10.1097/MAT.0000000000000669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stretch R, Sauer CM, Yuh DD, Bonde P. National trends in the utilization of short-term mechanical circulatory support: incidence, outcomes, and cost analysis. J Am Coll Cardiol. 2014;64:1407–1415. doi: 10.1016/j.jacc.2014.07.958. [DOI] [PubMed] [Google Scholar]
- 12.Sauer CM, Yuh DD, Bonde P. Extracorporeal membrane oxygenation use has increased by 433% in adults in the United States from 2006 to 2011. ASAIO J. 2015;61:31–36. doi: 10.1097/MAT.0000000000000160. [DOI] [PubMed] [Google Scholar]
- 13.Mori M, McCloskey G, Geirsson A, Mangi AA, Yun JJ, Jacoby D, et al. Improving outcomes in INTERMACS category 1 patients with pre-LVAD, awake venous-arterial extracorporeal membrane oxygenation support. ASAIO J. 2019;65:819-826. [DOI] [PubMed]
- 14.Park J, Oki K, Hesselmann F, Geirsson A, Kaufmann T, Bonde P. Biologically inspired, open, helicoid impeller design for mechanical circulatory assist. ASAIO J. 2020;66:899–908. doi: 10.1097/MAT.0000000000001090. [DOI] [PubMed] [Google Scholar]
- 15.Waters BH, Smith JR, Bonde P. Innovative Free-range Resonant Electrical Energy Delivery system (FREE-D System) for a ventricular assist device using wireless power. ASAIO J. 2014;60:31–37. doi: 10.1097/MAT.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 16.Wang JX, Smith JR, Bonde P. Energy transmission and power sources for mechanical circulatory support devices to achieve total implantability. Ann Thorac Surg. 2014;97:1467–1474. doi: 10.1016/j.athoracsur.2013.10.107. [DOI] [PubMed] [Google Scholar]
- 17.Waters BH, Park J, Bouwmeester JC, Valdovinos J, Geirsson A, Sample AP, et al. Electrical power to run ventricular assist devices using the Free-range Resonant Electrical Energy Delivery system. J Heart Lung Transplant. 2018;37:1467-1474. 10.1016/j.healun.2018.08.007. [DOI] [PMC free article] [PubMed]
- 18.Asgari SS, Bonde P. Implantable physiologic controller for left ventricular assist devices with telemetry capability. J Thorac Cardiovasc Surg. 2014;147:192–202. doi: 10.1016/j.jtcvs.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Bouwmeester JC, Park J, Geirsson A, Valdovinos J, Bonde P. Quantification of pulsed operation of rotary left ventricular assist devices with wave intensity analysis. ASAIO J. 2019;65:324–330. doi: 10.1097/MAT.0000000000000821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bouwmeester JC, Park J, Valdovinos J, Bonde P. Wave intensity analysis of right ventricular function during pulsed operation of rotary left ventricular assist devices. ASAIO J. 2019;65:465–472. doi: 10.1097/MAT.0000000000000835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giebisch G, Gallant N, Bonde P. iOS based app for control and communication of a wireless LVAD. American Society of Artificial Internal Organs 61st Annual Meeting, Chicago, IL; 2015.
- 22.Palagani Y, Sorkin E, Bahel P, Bonde R, Bonde P. Resonantly coupled high efficiency sensors for assessment of chamber size in LVADs. ASAIO J. 2022;68:2, 129. [DOI] [PubMed]
- 23.Tchouta LN, Bonde PN. The quest for non thrombotic surface modifications to achieve hemocompatibility of implantable devices. ASAIO J. 2015;61:623-34. [DOI] [PubMed]