Abstract
The incidence and prevalence of end-stage heart failure continue to rise; however, the number of donor hearts available for transplantation continues to be limited. Therefore, alternatives to transplantation, such as the use of total artificial hearts (TAH), are necessary. The long and winding road to the development and implantation of the ideal TAH remains under construction. Although efforts have been ongoing for almost a century, researchers and clinicians continue to improve currently available TAHs and design and construct new models. With mortality and morbidity rates decreasing, particularly at high-volume centers with a dedicated team and carefully selected patients, the use of TAHs as a bridge to transplantation, and even destination therapy in clinical trials, the future of TAHs is bright.
Keywords: Heart failure, Transplantation , Implantation
The total artificial heart through the ages
A fascination with, and a quest to find a replacement for, the human heart spans decades, centuries, and even millennia. As Galen wrote in the second century, “No other instrument performs such continuous, hard work as the heart.” [1]. Not surprisingly, therefore, this hard-working organ sometimes fails even with the best medical care, leaving the only available option for rectifying end-stage biventricular heart failure to be heart replacement. Although heart transplantation is most often the ultimate solution, a shortage of donor organs has limited this option over the past 50 + years, since Christian Barnard performed the world’s first human heart transplant in Cape Town, South Africa, in 1967. Therefore, scientists, engineers, and clinicians have spent the greater part of a century developing and improving various versions of the total artificial heart (TAH).
The development of a functional TAH has taken a long and circuitous journey, at times progressing in fits and starts. In the 1930s, Charles Lindbergh and Alexis Carrel attempted to create a TAH, but in reality, they like others at the time created instead a perfusion pump, initiating the work that would eventually become the heart-lung bypass.
In 1937, a 21-year-old Russian biology student, Vladimir Petrovich Demikhov, toiled in relative obscurity in a laboratory at a remote university, developing and ultimately implanting the first TAH in a warm-blooded animal. In this seminal work, he first ligated the coronary arteries of the dog’s native heart; 12 min later, he replaced the heart with a device of his design (Fig. 1); and, 16 min after Demikhov started the device with an electromotor, the “dog’s artificial circulation continued for 90 min (without the native heart), [during which] time all signs of life were observed.” Demikhov repeated this experiment twice in 1938 and 5 more times in 1958 after he had joined Bryukhonenko in Moscow. Having refined his equipment, he subsequently kept the animals alive for up to 5 h and 30 min [2] before abandoning his search for a TAH to take up research into heart and lung transplantation.
Fig. 1.
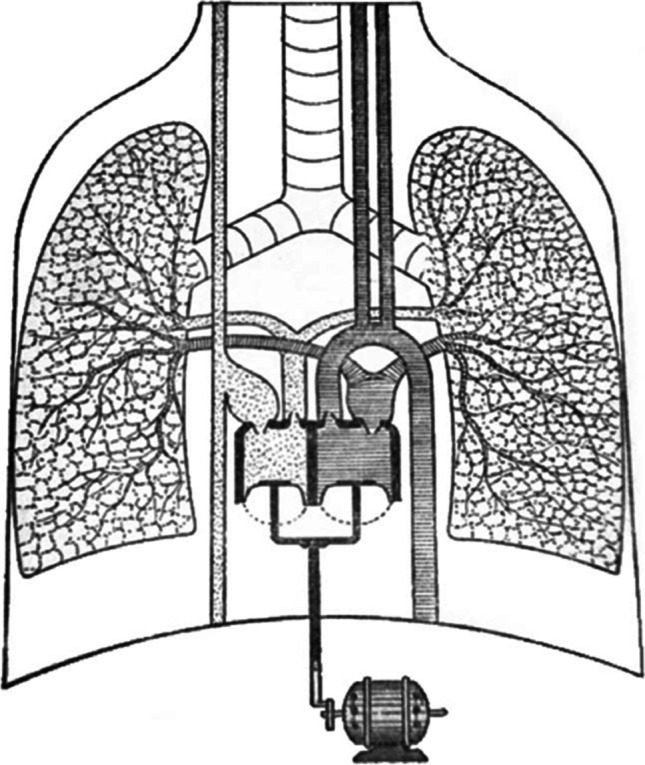
Demikhov total artificial heart. Reprinted with permission
Two decades later, a collaboration between Willem Kolff and Tetsuzo Akutso at the Cleveland Clinic led to the implantation of a mechanical heart into a living animal. Building upon Kolff’s previous work, in which he had developed an artificial kidney, and Akutso’s research into artificial heart-lung bypass, the pair implanted their plastic TAH into a dog, keeping the animal “alive” for 90 min (Fig. 2) [1]. Kolff eventually decamped to Utah, accompanied by Kwan-Gett.
Fig. 2.
Kolff and Atsuku total artificial heart. Reprinted with permission. https://blog.lib.utah.edu/father-artificial-organs-willem-j-kolff/
In the meantime, DeBakey and colleagues had developed a temporary assist device rather than a true TAH. They successfully deployed their device, which was paracorporeal, as opposed to implanted, several times, allowing their human patients’ native hearts to heal while being temporarily supported.
Kantrowitz, who is probably best known for performing the first heart transplant in a human in the United States (USA) on December 6, 1967, also developed what he termed a booster heart, which he tested and refined in dogs in the laboratory before implanting it into two humans in 1966. This two-layered device was sutured into place in the aortic arch, utilized compressed air in the outer layer of the tube to forcefully expel the blood in the inner tube, and was intended to be a permanent solution. Both patients survived the initial operation but succumbed at 20 h and 12 days, respectively. Although the booster heart ultimately was found to not afford a permanent solution, it did provide the framework for Kantrowitz’s left ventricular assist device (LVAD), which he termed a “dynamic aortic patch,” and the first, and ultimately widely deployed, intra-aortic balloon pump.
After being trounced by Christian Barnard in the race to be the first to perform a human heart transplant, Denton Cooley was not about to let another surgeon beat him to implant the first TAH into a human. He did so in 1969, implanting the Liotta TAH (Fig. 3) in an operation that generated not only a great deal of controversy but also a lifelong schism between Cooley and DeBakey. The patient survived with the TAH for 64 h, conscious and responding to commands, before receiving an orthotopic transplant. Thirty-two hours after the transplant, “cardiac action ceased.”
Fig. 3.
Liotta heart. Reprinted with permission. https://www.texasheart.org/50th-anniversary-of-the-worlds-first-total-artificial-heart/
Researchers made remarkable strides during the 1970s, developing various TAHs that kept animals alive from 150 days in Cleveland, more than 6 months in Berlin, to 221 days at the University of Utah. Much of this early work was funded through the National Institutes of Health (NIH)’s artificial heart program, which had kicked off in 1964 under the premise that, with sufficient funding and collaboration with private industry, medical researchers could build a viable implantable heart within 5 years. The goal during the first 3 years was to have various competing companies develop final designs for materials and components, lay out hardware specifications, and create plans for facilities to install and test the devices. Over the next 2 years, the winner of this competition was to have designed and developed several prototypes of the TAH. Finally, by the end of the 5 years, everything would be in place to manufacture, install, and maintain these TAHs—5 years total from the start of the competition to scores of TAHs rolling off the assembly lines—a lofty goal. Several reasons have been put forth to answer why these ambitious projections were not met, but suffice it to say that creating biocompatible materials, designing a small implantable device, and identifying an energy source for the devices were challenging, to say the least. Although as initially created, the program was to be exclusively devoted to creating a TAH, the reality was that funds were almost immediately diverted to other cardiovascular research, and, before the 5 years was up, the advisory panel recommended broadening the scope of the program to also support the development of left ventricular assist devices and intra-aortic balloon pumps. In less than 10 years, NIH disbanded the program, but research and progress have continued unabated.
One of the early TAHs was the AbioCor TAH (Abiomed, Inc., MA), an implantable TAH that utilized transcutaneous delivery of energy. Because the 1-year survival among the 14 patients in whom it was implanted was 1% (thromboembolic events), further development of this device was abandoned.
US Food and Drug Administration (FDA)-approved TAH
The SynCardia Total Artificial Heart – temporary (TAH-t) (SynCardia Systems, Tucson, AZ), nee CardioWest TAH, nee Symbion TAH, nee Jarvik-7 TAH
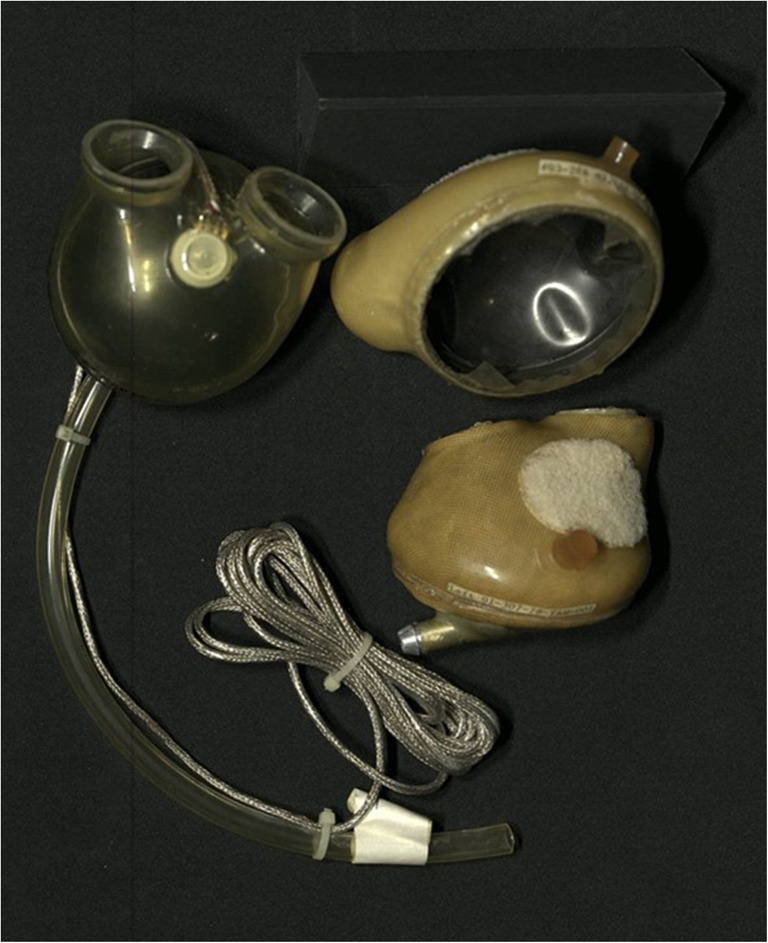
Perhaps the most widely known TAH was Jarvik 7, primarily because the media captured the attention of the general public through their daily updates on Barney Clark, the 61-year-old recipient of the first TAH as destination therapy (DT) in 1984. All eyes were on Dr. Clark as he took his daily laps around the University of Utah tethered to a 350-pound console during the 112 days he lived after receiving the Jarvik-7 TAH. This TAH (Fig. 4) was originally implanted only as DT in several patients throughout the world, with the cause of death most often attributed to sepsis or stroke. In 1985, the group in Arizona headed by Jack Copeland was the first to implant the Jarvik-7 TAH as bridge to transplantation (BTT). Over the ensuing 6 years, they implanted 198 Symbion TAHs, as it became to be known as BTT. Outcomes improved dramatically both in Arizona and elsewhere, with 72% of the Arizona patients surviving transplantation and 59% of those transplanted surviving to discharge to home. In 1991, the FDA canceled the Symbion TAH investigation device exemption (IDE), with subsequent transfer of both the manufacturing and intellectual property to another Utah-based organization and the University of Arizona, where the device was renamed CardioWest TAH. After a new IDE was obtained for a 70-cc device, more than 100 CardioWest TAHs were implanted as BTT throughout the world over the next 5 years, with 63% of patients surviving transplantation and 92% of those who survived to transplant being discharged home.
Fig. 4.

Jarvik heart. Reprinted with permission. https://invention.si.edu/podcast-robert-jarvik-mends-broken-hearts
Subsequently, the 70-cc model SynCardia TAH-t was approved by the FDA in 2004 and Health Canada in 2005 (Fig. 5). The 50-cc Syncardia TAH-t (SynCardia, Tucson, AZ, USA) for use in patients with a body surface area ≤ 1.85m2 was approved in Europe in 2014, Canada in 2016, and the USA in 2020 (Fig. 6). Both TAHs are pneumatically driven diaphragm pumps that completely replace the native ventricles. The drivelines from the TAH are attached to 1 of 2 drive systems—one for use in the hospital (Fig. 7), and a Freedom driver (Fig. 8), which is portable, allowing the patient greater mobility and the opportunity to return home while awaiting transplantation.
Fig. 5.
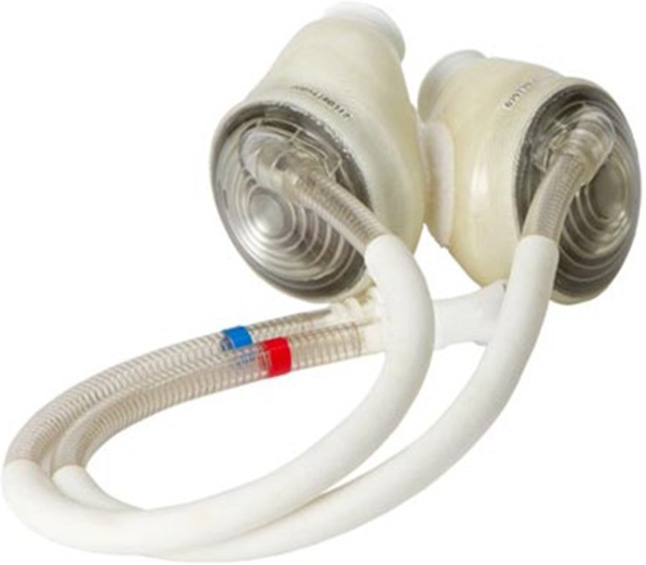
Syncardia TAH 70 cc. Reprinted with permission. https://syncardia.com/clinicians/our-products/see-all-our-products/
Fig. 6.

Syncardia TAH 50 cc. Reprinted with permission. https://syncardia.com/clinicians/our-products/see-all-our-products/
Fig. 7.

Syncardia Companion 2 (C2) hospital driver. Reprinted with permission. https://syncardia.com/clinicians/our-products/see-all-our-products/
Fig. 8.
Syncardia Freedom driver. Reprinted with permission. https://syncardia.com/clinicians/our-products/see-all-our-products/
According to SynCardia, more than 1700 of their TAHs have been implanted in patients worldwide. The 2020 Interagency Registry for Mechanically Assisted Circulatory Support (Intermacs) report indicates that 449 TAH systems were implanted in the USA between 2010 and 2020 as BTT [2].
TAHs are currently in development
An ongoing clinical trial employing the 70-cc Syncardia TAH as DT under an IDE seeks to enroll a total of 19 patients with biventricular failure who are not eligible for transplantation.
Aeson
The autoregulating, electrodynamically driven, pulsatile Carmat total artificial heart (C-TAH; subsequently named the Aeson®, CARMAT SA, Vélizy-Villacoublay, France) with bioprosthetic blood-contacting material was developed through a collaboration between Alain Carpentier, MD, Ph.D., and Airbus (Fig. 9) [3]. Carpentier et al. [4] published the first data on the use of this device in 2 patients with irreversible heart failure who were not eligible to receive a transplant and had been implanted with the C-TAH in 2013 and 2014, respectively. Patient 1 survived for 70 days in the hospital, dying from device failure; patient 2 was discharged from the hospital on day 150 and subsequently lived for 4 additional months at home before succumbing to multiorgan failure. Data from these patients were also included in a single-arm, prospective, nonblinded, nonrandomized feasibility study [5] in 4 patients, 3 of whom received the device as DT and 1 as BTT. One of these patients was discharged home for 3 months. The cause of death was device related in 2 patients and respiratory failure and multiorgan failure in the remaining 2.
Fig. 9.

Aeson© TAH. Reprinted with permission. https://www.carmatsa.com/en/
A clinical trial of the C-TAH in the first cohort of 10 patients with advanced heart failure, the PIVOTAL trial, began in 2016; 10 more patients were added as a second cohort. The primary outcome measure was 180-day survival or successful heart transplantation. According to the authors, “Explant analysis of all devices and autopsy examinations revealed no evidence of thrombotic deposits anywhere,” leading to “Conformite Europeenne Mark” (CE) approval as a BTT indication for the Aeson® TAH in December 2020. A subsequent analysis of the hemodynamic impact of the C-TAH on blood found a lack of thrombosis, only 1 case of bleeding, and no change in the von Willibrand factor profile among the 10 patients implanted with this device, indicating a superiority in this regard, compared with continuous flow and even centrifugal LVADs [6].
In early 2020, the FDA approved a 10-patient study of the C-TAH for implantation, followed by a May 2020 Centers for Medicare & Medicaid Services (CMS) approval for reimbursement of the device and associated services. In February 2021, the FDA approved the use of C-TAH in an early feasibility study of 10 transplant-eligible patients with advanced heart failure. The 20th C-TAH ever and the first in the USA was implanted in a patient with heart failure in July 2021; the patient subsequently received a heart transplant.
The Early Feasibility Clinical Study (EFICAS), which is anticipated to enroll 52 participants with advanced heart failure, is a multicenter prospective study comparing a cohort eligible to receive the C-TAH with standard therapy in a cohort of patients whose anatomy is anatomically incompatible with the C-TAH. Seven of these TAHs were subsequently implanted in Europe before the company voluntarily suspended implantation due to quality issues.
ReinHart
The development of the ReinHeart TAH (ReinHart, Aachen, Germany) has been ongoing since 2009 [7]. The current rendition employs an implanted battery that is charged via a transcutaneous energy system, thereby obviating the risk of infection associated with the use of devices that require skin incisions (Fig. 10). In addition, the system is lightweight, leading to enhanced quality of life associated with less mobility restriction. The ReinHeart TAH has undergone extensive testing in vivo.
Fig. 10.
ReinHeart TAH. Reprinted with permission. https://www.reinheart.de/
BiVACOR TAH
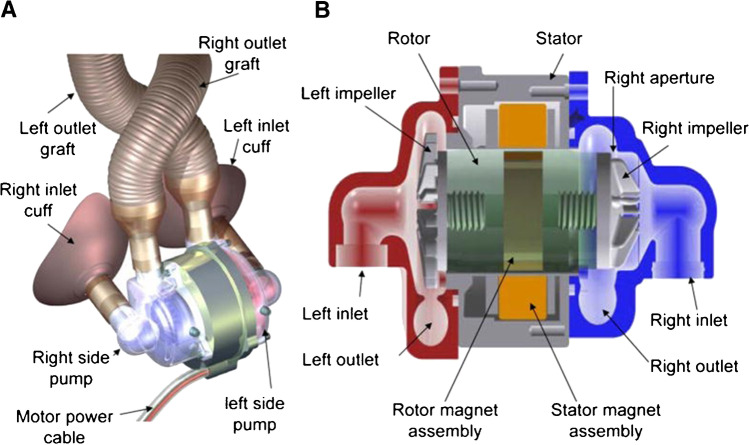
BiVACOR (BiVACOR Inc., Houston, TX), in collaboration with researchers in the USA and Australia, is developing a TAH that incorporates a newly designed technology. Instead of relying on a mechanical pump, as do other TAHs, the BiVACOR heart employs a rotary (magnetic levitation) technology to propel blood into the aorta (Fig. 11). The biocompatible device, which is encased in a titanium shell, has 2 additional qualities that make it particularly appealing: it is small enough to fit into the chest cavity of a woman or child (weighs ~ 650 g), and it incorporates flow adaptation, allowing it to respond to patients’ changing requirements without outside input [8]. As of this publication, many of these devices have been implanted in calves, which all seem to be developing and gaining weight at normal rates.
Fig. 11.

BiVACOR TAH. Reprinted with permission. https://bivacor.com/
Cleveland Clinic Continuous Flow TAH
The adult version of the Cleveland Clinic Continuous Flow TAH (CFTAH) has been studied in an animal model (calf) over the long term, and a pediatric version, known as the p-CFTAH, is also in development (Fig. 12). Both are valveless sensorless devices that are composed of a single, centrally located, rotating assembly with bilateral centrifugal pumps. The differential forces between the filling pressures of the 2 pumps not only determine the position of the rotating assembly but also balance the left and right circulations, thereby negating the need for electronic intervention. An algorithm in the controller automatically adjusts the speed, which can be modulated to produce a pulsatile flow. Ultimately, therefore, this TAH has a single moving part and a single electromechanical component.
Fig. 12.
Cleveland Clinic Continuous Flow TAH (CFTAH). Reprinted with permission
Indications for and outcomes of TAH implantation
As noted previously, the FDA-approved indication, and the criteria for clinical trials, for TAH implantation is as BTT for use in patients with severe biventricular failure who are at risk for imminently dying and for whom a suitable donor heart is not available. The most common etiology of biventricular failure in patients receiving TAHs is dilated or ischemic cardiomyopathy, although many other diagnoses may result in biventricular failure necessitating a TAH (Table 1).
Table 1.
Indications for total artificial heart implantation
| Cardiac malignancy |
| Cardiomyopathy |
| Restrictive |
| Infiltrative |
| Chagas disease |
| Congenital heart disease |
| Malignant arrhythmia |
| Failing heart transplant |
| Postinfarction septal wall defect |
| Ventricular thrombosis |
Careful selection of patients, the timing of implantation, and meticulous management by an experienced team are the key factors in optimizing outcomes for patients requiring TAHs. A 2018 study examined data from the INTERMACS database regarding patients who had a TAH as BTT or bridge to decision [9]. Among the 450 who had a TAH implanted between 2006 and 2017, 266 survived transplantation (53%), 162 died on support (34%), and the remaining 13% of patients remained on support with the TAH at 12 months after implant. The 3 most common causes of death were multisystem organ failure, neurologic injury, and elective withdrawal of support.
Risk factors for death included older patient age (i.e., > 40 years), need for dialysis preimplant, higher creatinine, and lower albumin levels, and implantation at a less-experienced center (i.e., performing ≤ 10 TAH). Six-month survival was only 50% among those patients requiring dialysis before TAH implantation; the 12-month survival rates at higher-volume vs lower-volume centers were 64.8% and 36.7%, respectively. The center volume also affected the likelihood of transplantation, with 58% of patients at the higher-volume centers vs 43% at the lower-volume centers receiving a cardiac transplant by 12 months. There are between 5 and 20% of patients that can benefit from biventricular support. The use of 2 ventricular assist devices (VADs) for total support is also a major surgical procedure as complex as placing a TAH. The use of two VADs with a single controller is possible but the industry has been reluctant in designing. The absence of ventricles does not reduce the incidence of strokes because the artificial surfaces can also be a source of thrombus. The use of two VADs has been used in a TAH configuration by removing the ventricles but there have been no clinical studies to prove clinical superiority.
The TAH has primarily been used as BTT in patients who exhibit irreversible biventricular failure in need of heart transplantation. There are a few reports in the medical literature about using the TAH in patients with severe Right ventricle (RV) failure post LVAD placement. However, it is known that some of these patients can be re-bridged successfully. The advantage of the TAH over LVAD + temporary support is that the TAH patient can be discharged home and managed as an outpatient. This is not the case for a patient with a temporary device. There are several publications regarding the preparation of the mediastinum post-device implantation to facilitate explanation at the time of transplantation. These techniques can be utilized for all device explanations. Several manuscripts are being prepared to describe the medical management of a patient with a TAH.
Conclusion
As TAHs have been refined, and surgical and medical experience have grown, outcomes for patients who have received TAHs as BTT have improved, with survival to transplantation in high-volume centers reaching ~ 65% [10]. Nonetheless, the quest for ever-better devices continues, with the hope of reaching the goal that Robert Jarvik so eloquently stated more than 40 years ago, “If the artificial heart is ever to achieve its objective, it must be more than a pump. It must also be more than functional, reliable, and dependable. It must be forgettable.”
Funding
None.
Declarations
Informed Consent
All patients that received a SynCardia TAH gave informed consent.
Statement of human rights
All clinical trials were approved by their respective Internal Review Boards and Ethical Committees at each institution.
Statement of animal rights
All experimental procedures involving animals were conducted by the Institutional Animal Care guidelines.
Conflict of interest
Dr. Francisco Arabia serves as a consultant to Syncardia Systems, LLC, Carmat TAH, and BiVACOR TAH. Catherine Friederich Murray, MNLM has nothing to disclose.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McKellar S. The value of multiple approaches: the early years of artificial heart research. Artif Organs. 2018;42:473–475. doi: 10.1111/aor.13167. [DOI] [PubMed] [Google Scholar]
- 2.Molina EJ, Shah P, Kiernan MS, Cornwell 3rd WK, Copeland H, Takeda K, et al. The Society of Thoracic Surgeons Intermacs 2020 annual report. Ann Thorac Surg. 2021;111:778–792. [DOI] [PubMed]
- 3.Han JJ. Aeson-The Carmat total artificial heart is approved for enrollment in the United States. Artif Organs. 2021;45:445–446. doi: 10.1111/aor.13959. [DOI] [PubMed] [Google Scholar]
- 4.Carpentier A, Latremouille C, Cholley B, Smadja DM, Roussel JC, Boissier E, et al. First clinical use of a bioprosthetic total artificial heart: report of two cases. Lancet. 2015;386:1556–1563. [DOI] [PubMed]
- 5.Latremouille C, Carpentier A, Leprince P, Roussel J-C, Cholley B, Boissier E, et al. A bioprosthetic total artificial heart for end-stage heart failure: results from a pilot study. J Heart Lung Transplant. 2018;37:33–37. [DOI] [PubMed]
- 6.Poitier B, Chocron R, Peronino C, Philippe A, Pya Y, Rivet N, et al. Bioprosthetic total artificial heart in autoregulated mode is biologically hemocompatible: insights for multimers of von Willebrand factor. Arterioscler Thromb Vasc Biol. 2022;42:470–480. [DOI] [PubMed]
- 7.Arabia FA. The total artificial heart: where are we? Cardiol Rev. 2020;28:275–282. doi: 10.1097/CRD.0000000000000322. [DOI] [PubMed] [Google Scholar]
- 8.Greatrex N, Kleinheyer M, Nestler F, Timms D. The Maglev Heart could keep cardiac patients alive. Reference. Institute of Electrical and Electronics Engineers Spectrum. 2019;56:22–29.
- 9.Beasley GS, Allen K, Pahl E, Jackson L, Eltayeb O, Monge M, et al. Successful bridge to transplant in a pediatric patient using the SynCardia 50 cc total artificial heart. ASAIO J. 2020;66:e33–e35. [DOI] [PubMed]
- 10.Carrier M, Moriguchi J, Shah KB, Anyanwu AC, Mahr C, Skipper E, et al. Outcomes after heart transplantation and total artificial heart implantation: a multicenter study. J Heart Lung Transplant. 2021;40:220–228. [DOI] [PubMed]