Summary
Background:
Intestinal epithelial stem cells (ISC) are responsible for epithelial regeneration and are critical to the intestine’s ability to regain barrier function following injury. Evaluating ISC biomarker expression in cases of small intestinal strangulation (SIS) may provide insight into clinical progression.
Objectives:
Intestinal resection margins from cases of SIS were evaluated to determine if (1) evidence of injury could be identified using histomorphometry, (2) ISC biomarker expression was decreased in the proximal resection margin compared to control and distal resection margin, and (3) the ISC biomarker expression was associated with the number of preoperative risk factors negatively related to outcome, postoperative complications, or case outcome.
Study design:
Retrospective cohort study.
Methods:
Intestinal samples were obtained intraoperatively from resection margins of adult horses with SIS and horses euthanised for reasons unrelated to colic. Preoperative risk factors negatively related to outcome, postoperative complications, and case outcome were obtained from medical records. Horses were grouped as euthanised intraoperatively, postoperatively, or survived to discharge. Histomorphometry and immunofluorescence were performed to evaluate tissue architecture and ISC and progenitor cell number. Groups were compared using one-way ANOVA. Associations between biomarker expression and the number of preoperative risk factors and postoperative complications negatively related to outcome were determined using linear regression modeling.
Results:
Thirty-six cases of SIS were evaluated. Ki67+ cell counts were decreased in the proximal (mean= 15.45 cells; 95% CI; 10.27–20.63, SD=4.17; p=0.02) and distal resection margins (mean=15.05; 95% CI= 8.46–21.64; SD=4.141; p=0.03) in horses euthanised postoperatively compared to control (mean=23.62 cells; 95% CI= 19.42–27.83; SD=5.883). In the distal resection margin, an increase in SOX9+Ki67+ cells were associated with a decrease in the total number of preoperative risk factors negatively related to outcome (95% CI; 0.236–1.123; p=0.008, SE=0.1393).
Main limitations:
Small population size.
Conclusions:
Proliferating cell and ISC numbers may be associated with case outcome.
Keywords: horse, small intestinal strangulation, immunofluorescence, Ki67, intestine, stem cell
1. Introduction
In recent years, the survival rate of horses with small intestinal strangulation (SIS) has improved.1 However, postoperative complications such as reflux, colic, and adhesions still present as important problems.2–4 In an attempt to determine the severity of intestinal compromise, a variety of preoperative, intraoperative, and postoperative assessments and case details have been studied. Yet, determining case prognosis and outcome continues to be challenging.5–8 Failure to predict outcome may be due to an underestimation of intestinal injury within the remaining in situ intestine post-resection and anastomosis.9–11 For example, intestine remaining after resection, while grossly normal in appearance, may be microscopically compromised due to prolonged, severe distension that may occur proximal to strangulation site. This distention may contribute to postoperative morbidity as increased luminal pressure has been shown to compress the intramural blood supply thereby causing mucosal injury.12,13 Furthermore, studies have suggested that in cases of SIS, bowel left in situ following the resection of grossly abnormal tissue had increased inflammation capable of compromising patient prognosis.14,15 To better predict case outcomes and to improve care of horses undergoing resection and anastomosis for SIS, a more accurate assessment of intestinal epithelial viability is needed.
One potential measurement of intestinal epithelial viability is assessment of intestinal epithelial stem cells (ISCs). These cells are responsible for homeostatic renewal of the luminal epithelium every 5–7 days.16 This rapid and ongoing renewal process maintains an intact epithelial lining which serves as a barrier to protect the systemic vasculature from noxious luminal contents.17,18 In strangulating lesions, barrier compromise occurs when small intestinal blood supply is reduced, leading to progressive epithelial cell loss.19 Cell loss is initiated at the villus tip and, as duration and severity of ischaemia progresses, extends into the crypt base where the ISCs reside,20 potentially impacting ISC capacity to renew epithelium. As previously demonstrated in a study of large colon volvulus, evaluation of ISC biomarker expression may be associated with severity of disease and could serve to indicate intestinal regenerative potential,21 therefore predicting outcome in cases of SIS. We hypothesised that a decrease in cellular expression of proliferative ISC biomarkers in intestinal segments adjacent to resected bowel would be associated with poor outcome in horses with SIS.
This retrospective clinical study aimed to identify if, in surgical cases of SIS, the intestine that remains after resection has an impaired regenerative potential denoted by a loss of ISCs, and if ISC loss is associated with poor outcome. Intestinal resection margins were evaluated to determine if (1) evidence of injury could be identified using H&E histomorphometry, (2) ISC biomarker expression was decreased in the proximal resection margin compared to control and the distal resection margin, and (3) the ISC biomarker expression was associated with the number of preoperative risk factors negatively related to outcome, postoperative complications, or case outcome.
2. Materials and Methods
2.1. Study population and inclusion criteria
Medical records from 2012 to 2020 were collected from the North Carolina State University Farm Animal and Equine Veterinary Medical Center for all SIS cases that underwent exploratory celiotomy and clinically warranted a surgical resection and anastomosis. Jejunal tissue was obtained from horses donated to the university for research that did not have any pre-existing gastrointestinal disease to serve as control (n=10). Control horses were not age matched and tissue collection occurred immediately after euthanasia. Horses with SIS were grouped into three categories based on surgical outcome: euthanised intra-operatively (n=15), euthanised postoperatively (n=5), and survived to discharge from hospital (n=16). No long-term follow-up was performed in these cases. Cases that were euthanised prior to surgery were excluded from this study. Horses were included in this study if SIS was identified intra-operatively and if at least one full thickness biopsy from the proximal or distal resection margin was obtained. Medical records were reviewed to compile a list of preoperative risk factors negatively related to outcome, type of anastomosis, postoperative complications, as well as case outcome.2,10,22,23 The preoperative risk factors that have been previously published to be negatively related to outcome included: tachycardia (>40 beats per minute), increased packed cell volume (PCV) >48%, increased systemic total protein (TP) >80 g/L, increased creatine kinase (CK) activity >470 IU/L, litres of net reflux >2L, increased systemic/peripheral lactate >2 mmol/L, increased peritoneal lactate >4mmol/L, and increased peritoneal TP >20 g/L. Postoperative complications included presence of laminitis, fever, diarrhoea, litres of reflux (>2L), and tachycardia (>40 beats per minute) within the initial three days after surgical intervention. Administration of total parenteral nutrition and requirement for a second surgery were also noted. A poor case outcome was defined as a horse that was euthanised prior to discharge.
2.2. Specimen preparation
Full thickness sections of jejunum from the proximal and distal site of the resection were collected at the time of surgery and immediately placed in either room-temperature 10% neutral-buffered formalin or refrigerated 4% paraformaldehyde (PFA). Formalin-fixed samples were then transferred to 70% ethanol solution and embedded in paraffin wax. PFA-fixed samples were transferred to 30% sucrose solution and embedded in optimal cutting temperature (OCT) compound (Tissue Tek). Blocks were sectioned at approximately 5 to 8 μm thickness and mounted on positively charged glass slides. Paraffin-embedded sectioned samples were stained with haematoxylin and eosin to evaluate crypt depth and villus height. Immunofluorescence was performed on OCT-embedded samples to analyse protein biomarker expression.
Three investigators (BV, GG, PB), blinded to specimen name, case outcome, and specific resection margin (proximal or distal), analysed immunofluorescence and histological slides. For each H&E slide evaluated for measurements of crypt depth and villus height, well-oriented crypts were identified as those with the crypt base closely opposed to the muscularis mucosa layer and that extended and opened fully into the intestinal lumen.
For analysis via immunofluorescence, heat-induced epitope retrieval was performed using a Pascal pressure chamber. Slides were cooled and then permeabilised with a detergent, 0.3%Triton X-100 PBS solution. Following permeabilisation, slides were incubated in a peptide-blocking agent, DakoProteinBlock (Agilent). Primary antibodies were used that have been previously shown to positively identify ISC and progenitor cells in normal equine tissue.21 The biomarker Ki67 identifies proliferating cells, SOX9 identifies progenitor cells, and PHH3 identifies cells actively undergoing mitosis.21 Co-localised cells were identified as a cell that simultaneously expressed both Ki67 and SOX9, consistent with an intestinal stem cell (ISC). Primary antibodies were diluted as follows: mouse α-Ki67 (Dako) (1:500), rabbit α-SOX9 (EMD Millipore) (1:250) and rabbit α-phospho-histone H3 (EMD Millipore) (PHH3; 1:200). Primary antibody incubation was performed for 24 hours at 4°C. Secondary antibodies, goat anti-mouse Alexa555 and goat anti-rabbit Alexa488, were diluted to 1:500. Nuclei were marked with bisbenzimide Hoechst 33258 nuclear stain (Abcam) (1:1000).
Images were captured on an inverted fluorescence microscope (Olympus IX83) fitted with a monochrome digital camera (ORCA-flash 4.0, Hamamatsu) and colour camera (DP26, Olympus). The objective lenses used were ×10, ×20, and ×40 with numerical apertures of 0.3, 0.45 and 0.6, respectively (LUC Plan FLN, Olympus). For each case of SIS, investigators analysed 6–10 well-oriented crypts per animal.19 For histomorphometric analyses, values of crypt depth or villus height were averaged for each animal. For immunofluorescence prepared slides, cells that positively expressed each protein biomarker (Ki67+, SOX9+, co-localised Ki67+SOX9+, and PHH3+) were counted and the numbers were averaged per total crypts counted.
2.3. Data analysis
Statistical analyses were performed in GraphPad Prism 7.0 (National Institutes of Health) and R statistical software version 4.0.2 (GraphPad Software). The three case outcome groups were used for comparison and data analysis. Within their respective outcome groups, the number of positive cells per crypt in the proximal and distal resection margins were compared using paired t tests in GraphPad Prism 7.0. The same software was then used to compare between case outcome group protein biomarker expression in the proximal resection margins and distal resection margins to control tissue using one-way ANOVAs (or Kruskal-Wallis test when residuals were not normally distributed as determined by the Anderson-Darling test).
Further analyses were conducted in R statistical software version 4.0.2. Analyses were conducted looking at the relationship between risk factors of poor outcome and protein biomarker expression or positive cell counts via Poisson regression. Analyses were conducted looking at relationships between complications and surgery descriptors using the Welch-corrected t test or Wilcoxon rank sum test as appropriate. Normality of data from risk factors and complications associated with poor outcome were assessed via visual inspection of histograms with emphasis on approximate symmetry and presence of extreme outliers. Where the Normality assumption held adequately, Welch’s t test was used to test differences between groups based upon the type of surgery that was performed, otherwise the Wilcoxon rank-sum test was used. The type of surgery a patient received (jejunojejunostomy, jejunoileostomy, or jejunocaecostomy) were intra-operative risk factors and therefore not considered in the comparisons of preoperative risk factors related to poor outcome. The total number of preoperative risk factors related to poor outcome for each patient was evaluated regarding its association with stem cell counts. Poisson models consisting of the number of complications as the dependent variable and each protein biomarker positive cell count as the independent variables were used to evaluate the relationships between complications and the number of cells co-expressing Ki67+SOX9+ (ISC). For inclusion of euthanasia, an interaction term was added between euthanasia and each cell count. A logistic model was fit with survival to discharge as the dependent variable and number of complications as the independent variable to examine the association between complications and outcome. Backward selection via the Akaike Information Criteria (AIC) was used to pare down logistic models to their most useful terms. Poisson model assumptions were evaluated based on visual inspection of residual and leverage plots before and after model selection. Bootstrapping was used to approximate power for comparisons between control and case patients.
3. Results
3.1. Study Population
A total of 36 horses that underwent exploratory celiotomy from 2012–2020 were included in this study. Full thickness small intestinal tissue biopsies were obtained from proximal and distal resection margins. One horse had poor tissue quality which prevented quantification of protein biomarkers SOX9 and Ki67. Two horses had incomplete medical records precluding analysis for preoperative risk factors and postoperative complications related to poor outcome, but tissue was obtained from surgery and evaluated for histomorphometric analysis and protein biomarker expression. Three horses underwent a jejunocaecostomy at the time of resection and one horse was euthanised intra-operatively with only proximal resection margin collected, therefore no representative distal resection margin was available for inclusion in the study (Figure 1). Of the horses included in this study, 15 were euthanised intra-operatively, 5 were euthanised postoperatively, and 16 survived to discharge. Ages ranged from 6 months to 27 years with a median of 15.5 years. There were 24 geldings, 10 mares, and two stallions. The breeds included: Morgan, Welsh Pony/Cob, Quarter Horse, Arabian, Appaloosa, Trakehner, Thoroughbred, Paint horse, Warmblood, Holsteiner, Oldenburg, Percheron, and Lipizzan. Control jejunal tissue was obtained from a total of 10 horses.
Figure 1:
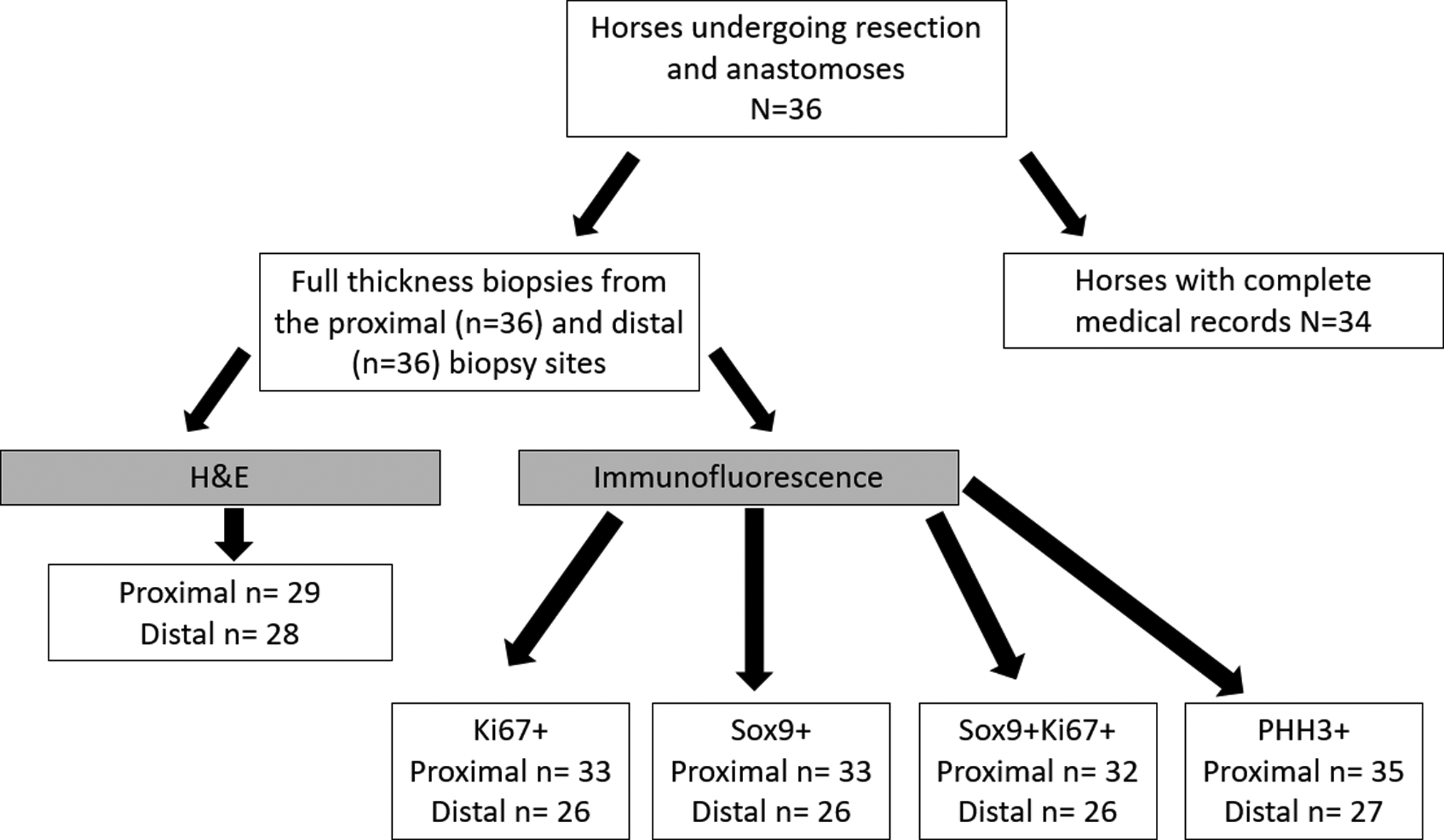
Flow chart describing process of sample selection for tissue processing and medical records.
3.2. Poor prognostic indicators, resection type, and resection length
Of the 36 horses included in this study, we identified the following most commonly occurring preoperative risk factors related to poor outcome: tachycardia (>40 beats per minute) in 30/34, abnormally increased CK (>470 IU/L) in 21/34, and increased systemic lactate (>2.0 mmol/l) in 21/34 (Table S1). Of the 21 horses that survived surgery, 19 had an end-to-end small intestinal anastomosis, and the remaining two horses had an end-to-side anastomosis (jejunocaecostomy). The most commonly occurring postoperative complications previously associated in the literature with poor outcome were reflux (>2L/h within a 24-hour period after surgery) in 13 of 21 horses and tachycardia (>60 beats per minute) in 15 of 21 horses (Table S2). Of the 16 horses that survived to discharge, nine had a jejunojejunostomy, five had a jejunoileostomy, and one had a jejunocaecostomy. The resection lengths ranged from 61 to 914 cm with a median of 182.9 cm.
3.3. Histomorphometric measures of crypt depth and villus height
Twenty-nine representative samples of proximal resection margin and 28 representative samples of distal resection margin were available for analysis. Overall, the crypt depth and villus height measurements did not show any significant differences between the resection margins and control (p>0.05) (Figure 2).
Figure 2:
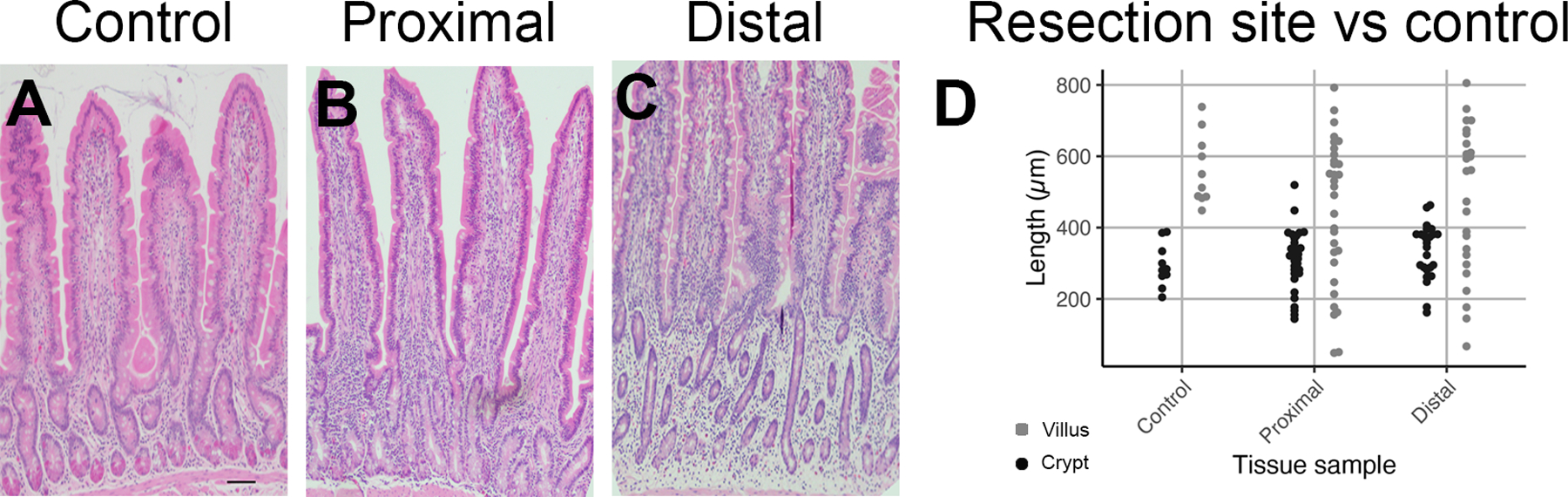
Representative H&E images from control horses and cases of SIS. A: Crypt-villus axis is shown in control, B: proximal, and C: distal resection margins. D: Boxplot graph demonstrating individual points of villus height and crypt depth in control, proximal, and distal resection margins. Scale bar is 100 micrometers.
3.4. Immunofluorescence analysis of resection margins
Thirty-five representative samples of proximal resection margins and 32 representative samples of distal resection margins were available for immunofluorescence analysis (Figure 3). Of the protein biomarkers evaluated between groups euthanised intra-operatively, euthanised postoperatively, and survived to discharge, only Ki67 cell counts were found to be significantly associated with case outcome (Table 1). Ki67 was significantly decreased in both the proximal resection margin (proximal resection mean=15.45 cells; 95% CI= 10.27–20.63; SD=4.17; Control proximal resection mean=23.62 cells; 95% CI= 19.42–27.83; SD=5.883; p=0.02) and distal resection margin of horses euthanised postoperatively compared to control tissue (distal resection mean=15.05; 95% CI= 8.46–21.64; SD= 4.141; Control distal resection mean=23.62 cells; 95% CI= 19.42–27.83; SD=5.883 p=0.03, respectively) (Figure 4).
Figure 3:
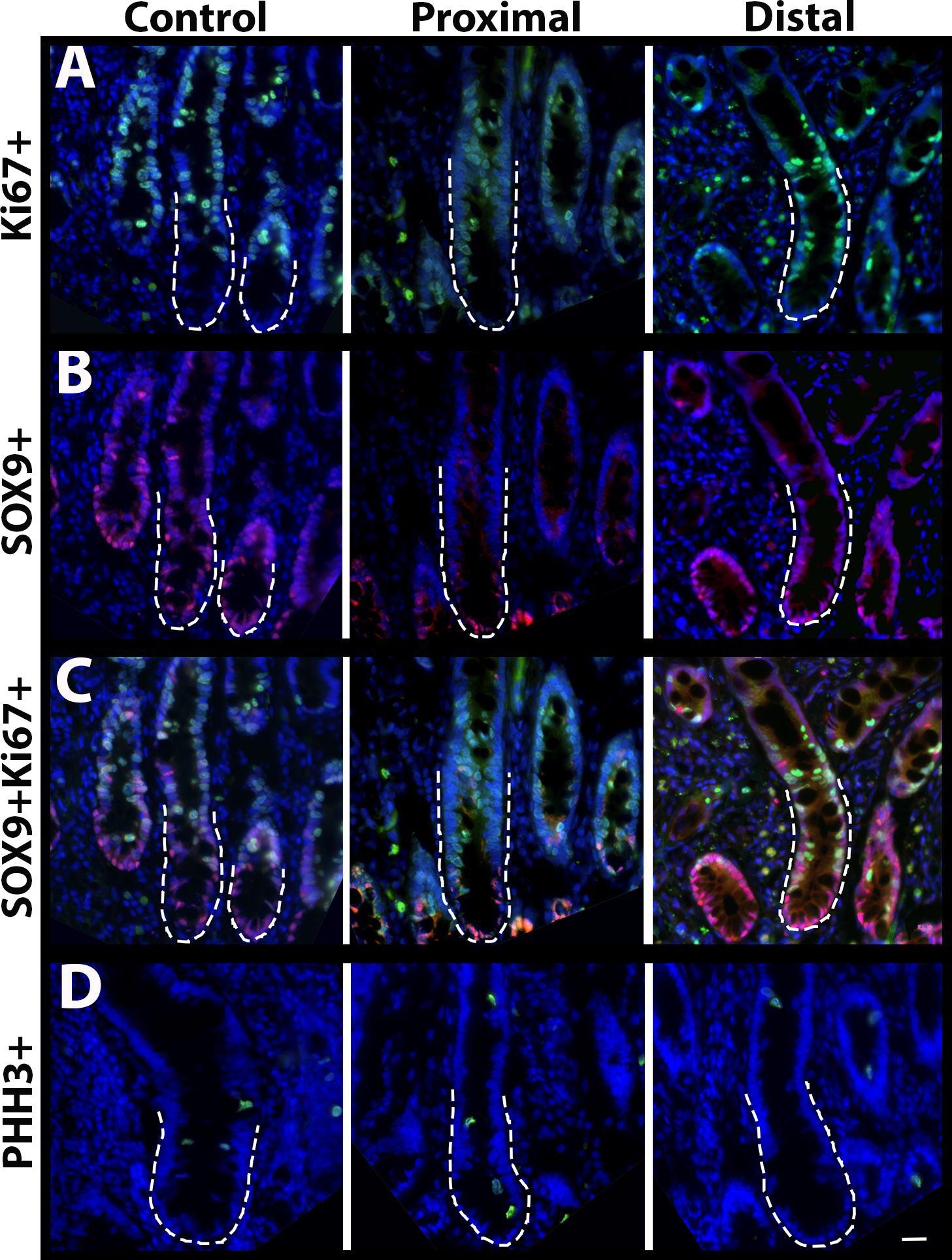
Representative immunofluorescence images from control horses and cases of SIS. Protein biomarker expression of A: Ki67, B: SOX9, C: SOX9 and Ki67 (Co-localised), and D: PHH3 from the control, proximal, and distal biopsies in all cases. Scale bar 50 micrometers.
Table 1:
Mean± s.d. values for average cell count of protein biomarker expression per crypt in control tissue and horses with SIS.
| Biomarker | Control Tissue (mean cells count ± SD) |
Resection margin | Euthanised Intra-operatively (mean cells count ± SD) | Euthanised Post-operatively (mean cells count ± SD) | Survived to discharge (mean cells count ± SD) |
|---|---|---|---|---|---|
| Ki67 | 23.62 ± 5.883 | Proximal | 21.16 ± 4.108 | 15.45 ± 4.174* | 20.52 ± 5.024 |
| Distal | 20.41 ± 3.098 | 15.05 ± 4.141* | 20.52 ± 5.191† | ||
| SOX9 | 23.97 ± 3.830 | Proximal | 25.63 ± 12.02† | 19.20 ± 5.411 | 21.89 ± 5.403 |
| Distal | 25.78 ± 13.34† | 18.45 ± 3.486 | 20.85 ± 5.648 | ||
| SOX9 and Ki67 (Co-localised) | 6.314 ± 2.590 | Proximal | 5.209 ± 2.903 | 3.155 ± 2.401 | 4.754 ± 2.542 |
| Distal | 5.500 ± 3.622 | 3.347 ± 2.186 | 4.169 ± 2.557 | ||
| PHH3 | 2.085 ± 2.051† | Proximal | 1.268 ± 1.688† | 1.848 ± 1.368† | 1.622 ± 1.098† |
| Distal | 1.397 ± 2.174† | 1.075 ± 0.6146 | 1.100 ± 0.7688 |
Denotes all values that were not normally distributed that KW test has been used instead of ANOVA
Denotes all values that were significantly different from control
Figure 4:
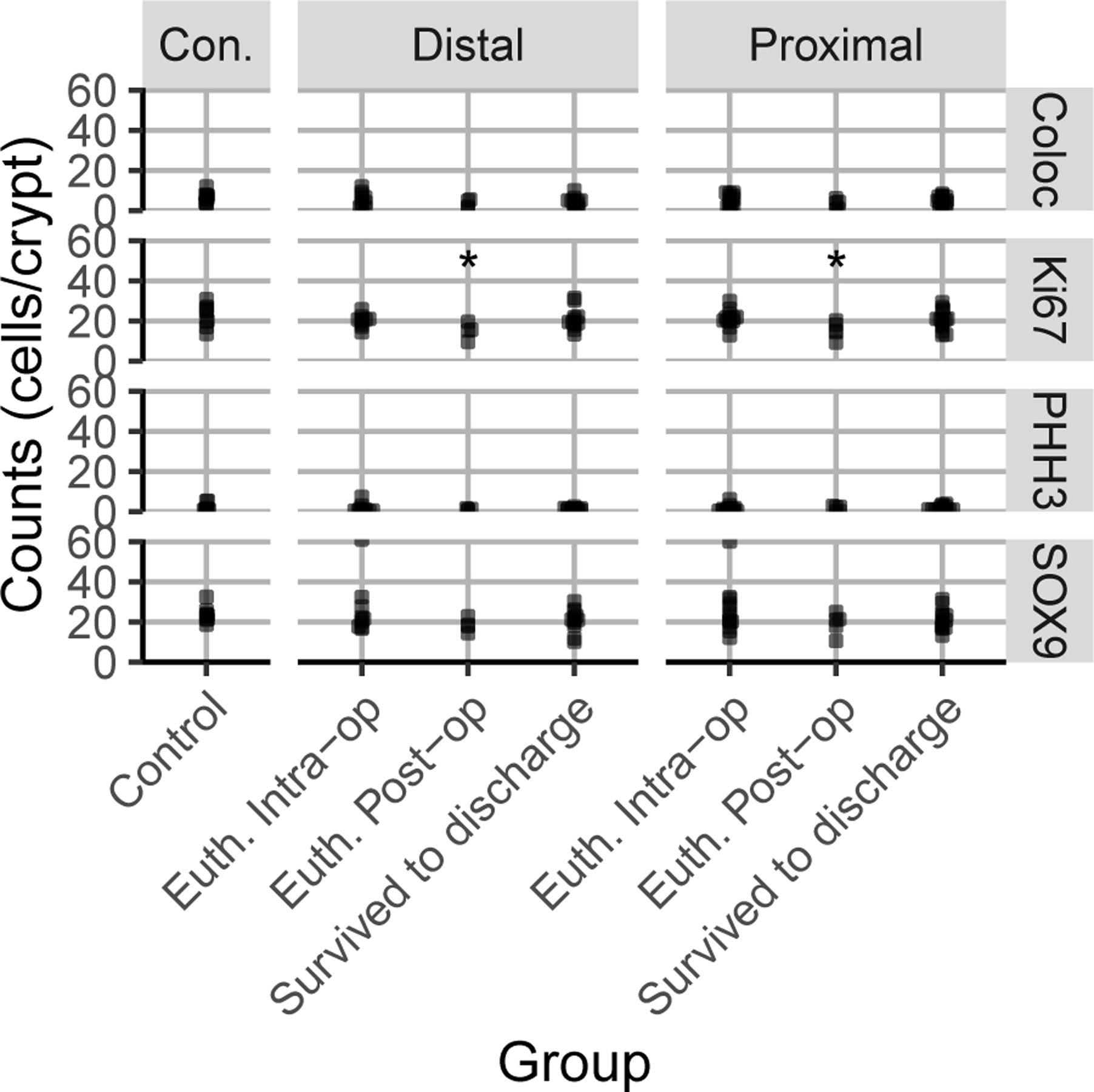
A boxplot graph demonstrating protein biomarker individual cell counts per crypt for control, euthanised intraoperatively (Euth Intra-op), euthanised postoperatively (Euth. Post-op) and survived to discharge in the proximal and distal resection margins. The asterisk denotes a p value of 0.02 for the proximal resection margin and 0.03 for the distal resection margin compared to control.
3.5. Determining an association of ISC biomarker expression with preoperative risk factors and postoperative complications
No relationship was found between the combined total number of preoperative risk factors and postoperative complications, postoperative complications alone, and any ISC biomarker in patients that were euthanised post-surgery compared to horses that survived until discharge. However, an increase in co-localised (SOX9+Ki67+) cell counts in the distal resection margin was associated with a significant decrease in the number of preoperative risk factors negatively related to outcome (mean=0.680; 95% CI; 0.236–1.123; SE= 0.1393; p=0.008). Post-hoc power calculations for each biomarker were performed and identified the following powers for proximal (PH3: 16.6%, Ki67: 34.4%, SOX9:30.6%, colocalised: 26.6%) and distal resection margins (PH3: 58%, Ki67: 40.2%, SOX9: 45.2%; colocalised: 34.7%). Consideration of these powers demonstrated that, to provide context for negative results, a minimum group size of 39 horses is required to detect significant differences between horses who did or did not receive surgery at a minimum power of 95%.
4. Discussion
The results of this study demonstrate that changes in proliferative cell and ISC protein biomarker expression occur at grossly normal resection margins in cases of SIS. This was characterised by a reduced number of Ki67+ proliferating cells in the resection margins of horses with SIS that were euthanised postoperatively. We surmise that this decrease in proliferating crypt-base epithelial cells may cause a reduction in the ability of the intestinal epithelium to renew and may increase an animal’s susceptibility to the negative sequelae of barrier dysfunction. In the absence of an intact barrier, translocation of luminal bacteria into the systemic vasculature may cause sepsis, multiple organ failure, and eventually death.
Our histomorphometric evaluation of the proximal and distal resection margins confirm that gross and histomorphometric appearance of tissue may not reflect the degree of ischaemic damage or the regenerative capacity of the intestine. This is consistent with a previous study indicating that there is more damage than epithelial morphology alone can demonstrate.14 In that study, the histological grade appeared normal despite increased neutrophilic injury at these sites. This finding is also supported by an earlier study which evaluated histologic evidence of injury in tissue proximal and distal to the obstruction site and determined that the lesion grade did not consistently indicate survival.24 Despite advances made in the evaluation of tissue biopsies as described in this and other studies,13 these injury determining techniques are disadvantaged by delays in tissue processing, decreasing their utility as an intraoperative tool. As a result, surgeons have relied on clinical assessment of bowel viability which include serosal appearance, motility, amount of intestine affected, and mesenteric artery pulsation.25
In this study, the only biomarker that demonstrated a significant difference in expression between control horses and those that were euthanised postoperatively was Ki67. The decreased Ki67+ cell count in the proximal and distal resection margins was associated with poor outcome. This finding differs from a study that evaluated a similar panel of ISC biomarker expression in cases of large colon volvulus which demonstrated that PHH3 expression could be used to predict case outcome.21 However, in that study, biopsies were obtained from the pelvic flexure, a site that is included within the strangulation, not from tissue that appeared grossly normal. In this study, the loss of Ki67+ cells in the tissue biopsies from the proximal resection margin may have been due to oedema, haemorrhage, and distension that can occur in the intestine proximal to the site of strangulation.26 Ischaemic injury can affect sites distant from grossly abnormal bowel and may be associated with inflammation. However, these effects are not well understood. It is plausible that abdominal inflammation could decrease cellular proliferation and therefore expression of Ki67. In our study population, we did not expect to see a change in biomarker expression within the tissue biopsied from the distal resection margin as this site is not normally distended or obviously affected from ischaemic injury. However, like our observations in the proximal resection margin, the decrease in Ki67+ cells measured in the distal resection site may further support the premise that tissue injury extends beyond the resection margins even if the gross appearance is normal. Furthermore, the progression of injury may occur following correction of a strangulating lesion due to the subsequent inflammation that occurs with reperfusion.14,27,28 In cases with devitalised distal ileum where it may not be feasible to resect all grossly affected intestine, we would expect a further decrease in Ki67 cell number. In these instances, surgeons may choose to perform a jejunocaecostomy despite being associated with a poorer outcome.14,27,29
A large limitation of this study was the small sample size of horses successfully recovered from surgery due to the large proportion of horses that were euthanised intraoperatively. This impacted our ability to independently evaluate the survivor groups to associate ISC biomarker expression with postoperative complications and surgical outcome. The large number of intraoperative euthanasia was due to owner’s request because of financial limitations. In those cases, it is difficult to draw conclusions related to ISC numbers and non-survival. An additional limitation was the lack of case details such as the duration of colic and time to surgical treatment. This pertinent clinical information could help convince owners and surgeons to not delay surgery as decreased ISC number may be associated with increased duration of colic.
The most common preoperative risk factors related to poor outcome in this study, tachycardia, increased systemic lactate, and increased CK activity, have been previously identified and used to indicate severe colic lesions such as SIS and ischaemic bowel.7,8,30 Novel to this study was the correlation of preoperative risk factors and ISC number. As expected, the number of preoperative risk factors associated with poor outcome were decreased in horses with a higher number of ISCs (SOX+Ki67+) in the distal resection margin. Horses with milder ischaemic injury retained their populations of ISCs, which likely facilitated regeneration and maintenance of the intestinal epithelial barrier, reducing the severity of systemic disease.
The results of this study demonstrate a change in expression of protein biomarkers at grossly normal resection margins in horses with SIS. Cases that were euthanised postoperatively had a reduced number of proliferating crypt-base epithelial cells (Ki67+). Therefore, the number of proliferating ISCs may be used along with other known prognostic indicators to help predict SIS case outcome postoperatively. Future directions may include a large, multicentre study for a more representative clinical picture or development of a point of care assay to quantify proliferating ISCs within the resection margins.
Supplementary Material
Table S1: Preoperative risk factors negatively related to outcome in horses that were euthanised intra-operatively, euthanised postoperatively, and survived to discharge
Table S2: Postoperative complications as well as requirement for total parenteral nutrition and second surgery in horses that were euthanised postoperatively and survived to discharge.
Acknowledgements
We thank the histopathology laboratory at NCSU College of Veterinary Medicine.
Sources of Funding
This work was funded by generous grants from George H. Hitchings New Investigator Award in Health Research (GAG) and the North Carolina Horse Council, T-35 Interdisciplinary Biomedical Research Training Program (GAG), Veterinary Scholars Program (GAG), and the NIH.
Footnotes
Authors’ declarations of Interest
No competing interests have been declared.
Ethical animal research
Procedures were approved by the North Carolina State University Institutional Care and Use Committee.
Informed consent
Owners gave consent for their animals’ inclusion in the study.
Data availability statement
The data that supports the findings of this study are available upon request to the corresponding author.
References
- 1.Freeman DE, Schaeffer DJ, Cleary OB. Long-term survival in horses with strangulating obstruction of the small intestine managed without resection. Equine Veterinary Journal. 2014;46(6):711–717. doi: 10.1111/evj.12216 [DOI] [PubMed] [Google Scholar]
- 2.Stewart S, Southwood LL, Aceto HW. Comparison of short- and long-term complications and survival following jejunojejunostomy, jejunoileostomy and jejunocaecostomy in 112 horses: 2005–2010. Equine Veterinary Journal. 2014;46(3):333–8. doi: 10.1111/evj.12143 [DOI] [PubMed] [Google Scholar]
- 3.Immonen IAM, Karikoski N, Mykkänen A, Niemelä T, Junnila J, Tulamo R-M. Long-term follow-up on recovery, return to use and sporting activity: a retrospective study of 236 operated colic horses in Finland (2006–2012). Acta Veterinaria Scandinavica. 2017/01/05 2017;59(1):5. doi: 10.1186/s13028-016-0273-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y, Wang Y, Du L, Liu P, Fei Z. Risk Factors, Recurrence and Short-Term Outcomes for Progressive Cerebral Infarction: A Retrospective Study. Report. Neurology India 2021;69:1675. [DOI] [PubMed] [Google Scholar]
- 5.van der Linden MA, Laffont CM, Sloet van Oldruitenborgh-Oosterbaan MM. Prognosis in equine medical and surgical colic. J Vet Intern Med. 2003;17(3):343–8. doi: 10.1111/j.1939-1676.2003.tb02459.x [DOI] [PubMed] [Google Scholar]
- 6.Blikslager AT, White NA II, Moore JN, Mair S. The Equine Acute Abdomen. John Wiley & Sons, Incorporated; 2017. [Google Scholar]
- 7.Kilcoyne I, Nieto JE, Dechant JE. Predictive value of plasma and peritoneal creatine kinase in horses with strangulating intestinal lesions. Veterinary Surgery. 2019;48(2):152–158. doi: 10.1111/vsu.13147 [DOI] [PubMed] [Google Scholar]
- 8.Morton AJ, Blikslager AT. Surgical and postoperative factors influencing short-term survival of horses following small intestinal resection: 92 cases (1994–2001). Equine Veterinary Journal. 2002;34(5):450–4. doi: 10.2746/042516402776117700 [DOI] [PubMed] [Google Scholar]
- 9.Freeman DE, Hammock P, Baker GJ, Goetz T, Foreman JH, Schaeffer DJ, Richter R-A, Inoue O, Magid JH. Short- and long-term survival and prevalence of postoperative ileus after small intestinal surgery in the horse. Equine Veterinary Journal. 2000;32(S32):42–51. doi: 10.1111/j.2042-3306.2000.tb05333.x [DOI] [PubMed] [Google Scholar]
- 10.Mair TS, Smith LJ. Survival and complication rates in 300 horses undergoing surgical treatment of colic. Part 2: Short-term complications. Equine Veterinary Journal. 2005;37(4):303–9. doi: 10.2746/0425164054529364 [DOI] [PubMed] [Google Scholar]
- 11.Abutarbush SM, Carmalt JL, Shoemaker RW. Causes of gastrointestinal colic in horses in western Canada: 604 cases (1992 to 2002). The Canadian veterinary journal = La revue veterinaire canadienne. 2005;46(9):800–805. [PMC free article] [PubMed] [Google Scholar]
- 12.Dabareiner RM, White NA, Donaldson LL. Effects of intraluminal distention and decompression on microvascular permeability and hemodynamics of the equine jejunum. Am J Vet Res. 2001;62(2):225–36. doi: 10.2460/ajvr.2001.62.225 [DOI] [PubMed] [Google Scholar]
- 13.Dabareiner RM, Sullins KE, White NA, Snyder JR. Serosal injury in the equine jejunum and ascending colon after ischemia-reperfusion or intraluminal distention and decompression. Vet Surg. 2001;30(2):114–25. doi: 10.1053/jvet.2001.21393 [DOI] [PubMed] [Google Scholar]
- 14.Gerard MP, Blikslager AT, Roberts MC, Tate LP Jr., Argenzio RA. The characteristics of intestinal injury peripheral to strangulating obstruction lesions in the equine small intestine. Equine Veterinary Journal. 1999;31(4):331–5. doi: 10.1111/j.2042-3306.1999.tb03826.x [DOI] [PubMed] [Google Scholar]
- 15.De Ceulaer K, Delesalle C, Van Elzen R, Van Brantegem L, Weyns A, Van Ginneken C. Morphological data indicate a stress response at the oral border of strangulated small intestine in horses. Res Vet Sci. 2011;91(2):294–300. doi: 10.1016/j.rvsc.2010.11.020 [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez LM. The mother of a gut cell: Intestinal epithelial stem cells. Equine Veterinary Education. 2015;27(11):559–560. doi: 10.1111/eve.12456 [DOI] [Google Scholar]
- 17.Gonzalez LM, Kinnin LA, Blikslager AT. Characterization of discrete equine intestinal epithelial cell lineages. Am J Vet Res. 2015;76(4):358–66. doi: 10.2460/ajvr.76.4.358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barker N, van de Wetering M, Clevers H. The intestinal stem cell. Genes Dev. 2008;22(14):1856–64. doi: 10.1101/gad.1674008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez LM, Moeser AJ, Blikslager AT. Animal models of ischemia-reperfusion-induced intestinal injury: progress and promise for translational research. Am J Physiol Gastrointest Liver Physiol. 2015;308(2):G63–75. doi: 10.1152/ajpgi.00112.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blikslager A, Gonzalez L. Equine Intestinal Mucosal Pathobiology. Annual Review of Animal Biosciences. 2018;6(1):157–175. doi: 10.1146/annurev-animal-030117-014748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kucera CR, Stranahan LW, Hughes F, Blikslager AT, Gonzalez LM. Protein biomarker of cell proliferation determines survival to discharge in cases of equine large colon volvulus. Equine Veterinary Journal. 2018;50(4):452–456. doi: 10.1111/evj.12767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mair TS, Smith LJ. Survival and complication rates in 300 horses undergoing surgical treatment of colic. Part 1: Short-term survival following a single laparotomy. Equine Veterinary Journal. 2005;37(4):296–302. doi: 10.2746/0425164054529409 [DOI] [PubMed] [Google Scholar]
- 23.Tennent-Brown BS. Interpreting lactate measurement in critically ill horses: diagnosis, treatment, and prognosis. Compend Contin Educ Vet. 2012;34(1):E2. [PubMed] [Google Scholar]
- 24.Meschter CL, Tyler DE, White NA, Moore J. Histologic findings in the gastrointestinal tract of horses with colic. American Journal of Veterinary Research. March 1986,2020-01-14 1986;47(3):598–606. [PubMed] [Google Scholar]
- 25.Fiege JK, Hackett ES, Rao S, Gillette SC, Southwood LL. Current Treatment of Ascending Colon Volvulus in Horses: A Survey of ACVS Diplomates. Veterinary Surgery. 2015;44(3):398–401. doi: 10.1111/j.1532-950X.2014.12195.x [DOI] [PubMed] [Google Scholar]
- 26.Allen D, White NA, Tyler DE. Morphologic effects of experimental distention of equine small intestine. Vet Surg. 1988;17(1):10–14. doi: 10.1111/j.1532-950x.1988.tb00269.x [DOI] [PubMed] [Google Scholar]
- 27.Moore RM, Muir WW, Granger DN. Mechanisms of gastrointestinal ischemia-reperfusion injury and potential therapeutic interventions: a review and its implications in the horse. J Vet Intern Med. 1995;9(3):115–32. doi: 10.1111/j.1939-1676.1995.tb03285.x [DOI] [PubMed] [Google Scholar]
- 28.Little D, Tomlinson JE, Blikslager AT. Post operative neutrophilic inflammation in equine small intestine after manipulation and ischaemia. Equine Veterinary Journal. 2005;37(4):329–35. doi: 10.2746/0425164054529472 [DOI] [PubMed] [Google Scholar]
- 29.Proudman CJ, Edwards GB, Barnes J. Differential survival in horses requiring end-to-end jejunojejunal anastomosis compared to those requiring side-to-side jejunocaecal anastomosis. Equine Veterinary Journal. 2007;39(2):181–5. doi: [DOI] [PubMed] [Google Scholar]
- 30.Krueger CR, Ruple-Czerniak A, Hackett ES. Evaluation of plasma muscle enzyme activity as an indicator of lesion characteristics and prognosis in horses undergoing celiotomy for acute gastrointestinal pain. BMC Veterinary Research. 2014;10(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Preoperative risk factors negatively related to outcome in horses that were euthanised intra-operatively, euthanised postoperatively, and survived to discharge
Table S2: Postoperative complications as well as requirement for total parenteral nutrition and second surgery in horses that were euthanised postoperatively and survived to discharge.
Data Availability Statement
The data that supports the findings of this study are available upon request to the corresponding author.


