Abstract
Objective:
To investigate the strength and reproducibility of the teratogenic impact of prenatal tobacco exposure (PTE) on child physical health and neurodevelopmental outcomes, in the context of intersecting sociodemographic and other prenatal correlates, and to see if early postnatal health mediates PTE associations with childhood outcomes.
Methods:
Among 9–10-year-olds (N = 8,803) in the Adolescent Brain Cognitive Development® Study, linear mixed-effect models tested PTE associations with birth and childhood outcomes of physical health, cognitive performance, and brain structure, controlling for confounding sociodemographic and prenatal health correlates. A mediation analysis tested the extent to which health at birth explained the associations between PTE and childhood outcomes.
Results:
PTE was reported by 12% of mothers (8% [n=738] pre-knowledge of pregnancy only, and 4% [n=361] pre- and post-knowledge of pregnancy). PTE was highest for children with risk for passive smoke exposure. Overall, children with any PTE had shorter breastfeeding durations than those without PTE, and PTE following knowledge of pregnancy was associated with being small for gestational age having lower birth weight, and obesity and lower cortical volume and surface area in childhood. Among children from high parent education households, any PTE was related to lower cognitive performance, which was partially mediated by duration of breastfeeding.
Conclusions:
PTE was linked to poorer health indicators at birth and neurodevelopmental outcomes at age 9–10 years in a large community cohort, independent of sociodemographic factors. Efficacious interventions for smoking-cessation during pregnancy are still needed and should incorporate support for breastfeeding to promote healthier development.
Keywords: Prenatal tobacco exposure, physical health, cognitive performance, brain structure, socioeconomic status
Introduction
In pregnancy, tobacco exposure continues to occur at higher rates than any other substance exposure, making prenatal tobacco exposure (PTE) among the top three preventable teratogens in our country (Forray, 2016). In 2016, 7% of pregnant women in the U.S. reported cigarette smoking post-recognition of pregnancy (Drake et al., 2018). PTE exposes the developing fetus to more than 7,000 chemicals, including nicotine and other known carcinogens (Holbrook, 2016). PTE even at moderate levels, is associated with adverse postnatal outcomes (Difranza et al., 2004; El Marroun et al., 2014; Olds et al., 1994; Sexton, 2011; U.S. Department of Health and Human Services, 2014, 2020). While, deleterious effects of PTE on physical and neurodevelopmental outcomes have been reported, the underlying mechanisms by which PTE may influence postnatal outcomes are less understood. For instance, PTE may alter neural mechanisms during fetal development, including altered fetal blood flow and protein metabolism (Zhou et al., 2014). PTE may also influence later postnatal development through its initial effect on health at birth, early postnatal factors (i.e., birthweight and breastfeeding), and postnatal exposure, although this has been less investigated. Further, PTE occurs in context of other intersecting socio-ecological and prenatal health factors which may also pose adversity to development (i.e., socioeconomic status and parental psychopathology), and thus these potential confounders must be considered in testing associations of PTE with childhood health (Gilman, Breslau, et al., 2008; Kodl & Wakschlag, 2004). While PTE is likely to occur concomitantly with other substances during pregnancy (Alberg et al., 2014; U.S. Department of Health and Human Services, 2014, 2020), use of cannabis during pregnancy has typically occurred via similar forms of ingestion as PTE, i.e., smoked (Schauer et al., 2016).
Children with PTE have increased risk for poorer physical health at birth, i.e., lower birthweight, and small for gestational age (birthweight ≤ 10th percentile for gestational age) (Chiolero et al., 2005; Lamm et al., 2020; Mitchell et al., 2007; Tong et al., 2017) and elevated rates of obesity (Chen et al., 2006) and asthma (Gilliland et al., 2001) later in childhood. PTE has also been linked to negative effects on breastfeeding, including high levels of nicotine in breastmilk, decreased breastmilk volume, and shorter duration of breastfeeding (for a review see Napierala et al., 2016). In turn, physical health indicators at birth have also been related to childhood health outcomes, including cognition during childhood (Zhou et al., 2014). Physical health in the early postnatal period (e.g., birthweight, small for gestational age, and breastfeeding) are health indicators most proximal to tobacco exposure during the prenatal period, and thus, it is important to consider how these early postnatal health indicators influence associations between PTE and later childhood outcomes.
PTE has been associated with poorer postnatal neurodevelopment, including decreased cognitive performance in school aged children (Cornelius et al., 2001; Cornelius & Day, 2009; Ernst et al., 2001; for a review see Huizink & Mulder, 2006) and lower total brain volumes among children with PTE relative to youth with no exposure (El Marroun et al., 2014, 2016). However, while some studies have demonstrated associations of PTE with cognitive performance in childhood after controlling for potential confounders, including socioeconomic status (SES; i.e., maternal education; Cornelius et al., 2001; Moore et al., 2020), some studies have reported undetectable effects of PTE on measures of cognitive functioning after accounting for SES and maternal intelligence (Baghurst et al., 1992; Batty et al., 2006; Breslau et al., 2005; Fergusson & Lloyd, 1991; Gilman, Gardener, et al., 2008; Huijbregts et al., 2006; R. & Stanton, 1994). Such findings suggest that the association between PTE and cognition was confounded by closely linked factors such as SES in the samples examined in these studies. These mixed and limited findings warrant further investigation to the detectable effects of PTE on cognitive performance and brain structure during childhood and possible mediating roles of indicators of health at birth.
In this study, we leverage the availability of a comprehensive array of developmental measures on a large and typically developing cohort of 8,803 children 9–10 years of age to test retrospective report of PTE in association with health at birth, postnatal physical health, cognitive performance and brain structure. We investigated the strengths of the associations of PTE with developmental measures when controlling for sociodemographic, perinatal health and psychosocial risk factors. We hypothesized that in the large and diverse ABCD study sample, there would be detectable associations of PTE with poorer postnatal outcomes, independent of potential confounders. In addition, we hypothesized that birth health outcomes would mediate PTE and neurodevelopmental associations. We report the relative effect sizes of the associations of PTE with postnatal outcomes in a large sample of typically developing 9-to-10-year-olds.
Method
This retrospective study used data collected for N = 8,803 children 9–10 years of age, born between 2005 and 2009, participating in the baseline visit for the longitudinal ABCD Study. The ABCD 2.0.1 data release was obtained from the NIMH Data Archive ABCD Collection (10.15154/1504041) with baseline data for a total of N = 11,875 children 9 – 10 years old. Brain and youth physical health and behavioral data were collected during the ABCD study baseline visit. At baseline, caregivers provided retrospective report of prenatal tobacco and other substance exposure, as well as perinatal health. The ABCD study recruitment approach and protocol have been described in detail previously (Barch et al., 2018; Garavan et al., 2018; Hagler et al., 2019; Luciana et al., 2018). Data were analyzed using R, version 4.0.0 (R Development Core Team, 2018).
Participants
ABCD used an epidemiologically informed recruitment strategy based on gender, race and ethnicity, and SES, to recruit a diverse sample similar to the American Community Survey (Garavan et al., 2018). Participants were recruited across 22 sites and followed at 21 sites (https://abcdstudy.org/study-sites/). Written informed consent from all parents and assent from all children was obtained to participate and the research study was approved by a centralized institutional review board at the University of California, San Diego. Participants with non-missing PTE data and non-missing demographic, behavioral, and brain imaging data were included in the analysis. Demographic characteristics are shown in Table 1.
Table 1.
ABCD study sample characteristics by levels of prenatal tobacco exposure during pregnancy.
| No Exposure (N = 7,704) |
PTE Pre-knowledge of pregnancy (N = 738) |
PTE Post-knowledge of pregnancy (N = 361) |
Overall (N = 8,803) |
|
|---|---|---|---|---|
|
| ||||
| Sociodemographic Variables | ||||
| Age, mean (SD), years | 118.88 (7.47) | 119.35 (7.51) | 119.45 (7.59) | 118.94 (7.48) |
| Females, n (%) | 3699 (48.0) | 356 (48.2) | 178 (49.3) | 4233 (48.1) |
| Household Income, n (%) | ||||
| <50K | 1921 (24.9) | 331 (44.9) | 227 (62.9) | 2479 (28.2) |
| 50K – 100K | 2147 (27.9) | 240 (32.5) | 98 (27.1) | 2485 (28.2) |
| >100K | 3636 (47.2) | 167 (22.6) | 36 (10.0) | 3839 (43.6) |
| Parental Education, n (%) | ||||
| Less than HS Diploma | 260 (3.4) | 42 (5.7) | 29 (8.0) | 331 (3.8) |
| HS Diploma/GED | 537 (7.0) | 83 (11.2) | 87 (24.1) | 707 (8.0) |
| Some College | 1724 (22.4) | 348 (47.2) | 174 (48.2) | 2246 (25.5) |
| Bachelor | 2158 (28.0) | 143 (19.4) | 55 (15.2) | 2356 (26.8) |
| Post Graduate Degree | 3025 (39.3) | 122 (16.5) | 16 (4.4) | 3163 (35.9) |
| Race-Ethnicity, n (%) | ||||
| White | 4416 (57.3) | 344 (46.6) | 188 (52.1) | 4948 (56.2) |
| Hispanic | 1513 (19.6) | 156 (21.1) | 44 (12.2) | 1713 (19.5) |
| Black | 928 (12.0) | 130 (17.6) | 79 (21.9) | 1137 (12.9) |
| Asian | 143 (1.9) | 5 (0.7) | 1 (0.3) | 149 (1.7) |
| Other/Mixed | 704 (9.1) | 103 (14.0) | 49 (13.6) | 856 (9.7) |
| How far along – pregnancy knowledge (weeks), mean (SD) | 6.68 (6.56) | 8.16 (7.37) | 9.42 (9.02) | 6.92 (6.78) |
| Maternal Age at Child’s Birth, mean (SD), years | 30.04 (5.99) | 26.85 (5.80) | 26.34 (5.54) | 29.62 (6.06) |
| Gestational Age, mean (SD), weeks | 39.07 (2.19) | 38.94 (2.28) | 38.90 (2.18) | 39.05 (2.20) |
| No. Prenatal Conditions, mean (SD) | 0.67 (1.05) | 0.85 (1.16) | 0.95 (1.13) | 0.70 (1.07) |
| Prenatal Cannabis, n (%) | ||||
| No-exposure | 7503 (97.4) | 567 (76.8) | 271 (75.1) | 8341 (94.8) |
| Pre-knowledge | 147 (1.9) | 135 (18.3) | 42 (11.6) | 324 (3.7) |
| Post-knowledge | 54 (0.7) | 36 (4.9) | 48 (13.3) | 138 (1.6) |
| Prenatal Other Substance, n (%) | ||||
| No-exposure | 5872 (76.2) | 319 (43.2) | 220 (60.9) | 6411 (72.8) |
| Pre-knowledge | 1682 (21.8) | 409 (55.4) | 112 (31.0) | 2203 (25.0) |
| Post-knowledge | 150 (1.9) | 10 (1.4) | 29 (8.0) | 189 (2.1) |
| Parent Psychopathology (ASR), mean (SD) | 42.17 (9.81) | 46.83 (10.77) | 49.38 (10.99) | 42.86 (10.12) |
| Passive Smoke Exposure, n (%) | 1021 (13.3) | 433 (58.7) | 306 (84.8) | 1760 (20.0) |
| Child Early Postnatal Health | ||||
| Small for Gestational Age a, n (%) | 1734 (18.1) | 180 (19.1) | 151 (27.4) | 2065 (18.6) |
| Birthweight, mean (SD), kg | 3.19 (0.66) | 3.18 (0.63) | 2.95 (0.67) | 3.18 (0.66) |
| Months Breastfed, mean (SD) | 8.52 (8.46) | 5.38 (7.26) | 3.27 (6.15) | 8.04 (8.38) |
| Childhood Physical Health | ||||
| Obesity (BMI > 95%) a, n (%) | 1558 (15.7) | 187 (19.3) | 174 (28.9) | 1919 (16.7) |
| Asthma a, n (%) | 1634 (16.4) | 200 (20.5) | 147 (24.3) | 1981 (17.2) |
| Childhood Neurodevelopment | ||||
| NIH Total Cognition, mean (SD) | 87.20 (8.86) | 85.11 (9.15) | 82.89 (8.37) | 86.85 (8.92) |
| Brain Structure, mean (SD) | ||||
| Mean Cortical Thickness (mm) | 2.78 (0.10) | 2.77 (0.10) | 2.77 (0.11) | 2.78 (0.10) |
| Total Cortical Surface Area (mm2) | 187047.62 (17879.80) |
184752.62 (17495.82) |
182091.96 (18531.87) |
186658.91 (17907.62) |
| Total Cortical Volume (mm3) | 599682.64 (56669.42) |
589179.97 (53639.62) |
580977.51 (58444.65) |
598061.74 (56668.89) |
Abbreviations: standard deviation (SD); thousands (K); high school (HS); general education diploma (GED); Adult Self-report (ASR); kilograms (kg); body-mass-index (BMI); millimeters (mm); millimeters squared (mm2); millimeters cubed (mm3).
N = 5,869 sample of singleton children for logistic models with binomial dependent variables.
Prenatal Tobacco Exposure
History of prenatal substance exposure was collected retrospectively via caregiver report at the baseline visit via the ABCD Developmental Questionnaire (Barch et al., 2018). Prenatal substance exposure was retrospectively reported by the child’s caregiver 9–10 years post birth, and were asked about maternal use of tobacco, cannabis, and other substances (i.e., alcohol, cocaine/crack, heroin/opioids, methamphetamine, and other drugs used recreationally) during pregnancy. Caregivers responded to two questions to assess prenatal substance exposure: (1) whether use occurred before maternal knowledge of pregnancy and (2) whether use occurred after maternal knowledge of pregnancy. The PTE categorical variable was coded into three levels to describe prenatal tobacco exposure: no exposure (n = 7,704), PTE pre-knowledge of pregnancy (n = 738), and PTE post-knowledge of pregnancy (n = 361).
Covariates
All covariates were chosen a priori based on previous studies investigating maternal prenatal tobacco use in association with developmental outcomes, for which important confounders were identified in the areas of sociodemographic factors (i.e., parental education, household income, race/ethnicity), perinatal health factors (i.e., prenatal health conditions, how far along in pregnancy recognition and gestational age), concomitant prenatal use of other substances, and psychosocial factors-- including parental mental health and risk for second-hand smoke exposure (Alberg et al., 2014; Gilman, Gardener, et al., 2008; Kodl & Wakschlag, 2004; U.S. Department of Health and Human Services, 2014, 2020)
Sociodemographic
Parent report for participant age in years, sex at birth, race and ethnicity (i.e., White, Hispanic/Latinx or Latino/a, Black, Asian, and Other), household income and parental educational attainment were assessed via the Parent Demographic Questionnaire (Barch et al., 2018). Household income was coded into three categorical brackets for SES levels, i.e., low household income (< $50K), mid household income ($50K – 100K), and high household income (> $100K). Highest parent education was coded into five categories: (1) less than a high school (HS) diploma, (2) HS diploma or a general education diploma (GED), (3) some college, (4) bachelor’s degree, or (5) post graduate degree (including master degrees, professional degrees, and doctorates).
Perinatal Health
In the ABCD study Developmental History Questionnaire (Barch et al., 2018), parents retrospectively reported how far along the pregnancy was upon knowing of the pregnancy (in weeks). Gestational age was reported as the number of weeks in pregnancy at the time of the child’s birth (up to 40+ weeks). We calculated the total number of endorsed prenatal conditions during pregnancy, including heavy bleeding, preeclampsia, gall bladder problems, persistent proteinuria, rubella, severe anemia, urinary infections, diabetes, high blood pressure, placenta complications, accidental injury, and a category of other medical conditions during pregnancy.
Prenatal Cannabis and Other Substance Exposure
Potential concomitant prenatal exposure to cannabis and other substances were considered by defining two categorical variables, one coding prenatal cannabis exposure and another for all other prenatal substance exposure. Both variables for prenatal cannabis and other prenatal substance exposure were each coded with three levels: (1) whether there was no exposure; (2) whether exposure occurred pre-knowledge; (3) whether exposure occurred post-knowledge of pregnancy. Other prenatal substance exposures queried whether alcohol, cocaine/crack, heroin/opioids, methamphetamine, or other drugs were used recreationally during pregnancy (Barch et al., 2018).
Psychosocial Factors
The Adult Self Report (ASR; Achenbach, 2009) completed by the study caregiver during the baseline visit was used to capture behavioral dimensions of parent psychopathology. The total problems ASR syndrome scale t-score was used, encompassing endorsement across dimensions of internalizing problems (e.g., anxiety and depression) and externalizing problems (e.g., aggressive behaviors and rule-breaking behavior), which is normed for each gender in ages 18–35 and 36–59 based on national probability samples. In addition, a binary measure (yes/no) for risk for postnatal passive smoke exposure was obtained from the ABCD screening questionnaire which inquired whether anyone living in the household with the child smoked cigarettes.
Child Early Postnatal Health
In the ABCD Developmental Questionnaire (Barch et al., 2018), information about perinatal health was assessed via parent report. Birth weight (pounds transformed to kilograms), and duration of months the child was breastfed were collected via parent report using the ABCD Developmental History Questionnaire. A categorical variable for small for gestational age (SGA) was calculated using the 2013 Fenton growth charts (Fenton & Kim, 2013) to classify children with SGA if their birth weight was lower than expected for the reported number of gestational weeks at birth based on standardized norms.
Childhood Physical Health
Body mass index (BMI) percentiles were calculated based on biological sex at birth and age using the Center for Disease Control (CDC) BMI percentile SAS program, a growth chart calculator based on a nationally representative reference population of children (Centers for Disease Control and Prevention (CDC), 2016). A categorical variable defined obesity as BMI percentiles greater than 95%. The number of asthma attacks in the recent year for the child was obtained via parent report using the ABCD Medical History Questionnaire (Barch et al., 2018).
Childhood Neurodevelopmental Outcomes
Cognition
The NIH Toolbox© Cognition Battery was administered as part of the baseline protocol (Luciana et al., 2018). Participants completed seven cognitive tasks in the areas of reading, vocabulary, working memory, processing speed, cognitive flexibility, episodic memory, and attention/inhibition. Cognitive performance was examined using the total composite t-scores, which provide an overall score for performance across all domains that is standardized for demographic factors, i.e., gender and race/ethnicity.
Brain Structure
The ABCD imaging protocol, acquisition and preprocessing procedures have been described previously (Hagler et al., 2019). Each ABCD site applied a standardized MRI protocol that included a T1 weighted scan. All imaging data was processed using FreeSurfer pipelines and procedures implemented by the ABCD Data Informatics and Resource Center. Quality control procedures are described in Hagler et al. 2019. Briefly, sMRI data was corrected for distortions and motion. Structural MRI data was manually reviewed by trained technicians pre and post undergoing processing pipelines to evaluate the integrity of the images in five artifact categories: intensity inhomogeneity, underestimation of white matter, pial overestimation, and magnetic susceptibility. An overall quality control (QC) score of 1 indicated the structural imaging data was usable, and a score of 0 indicated severe artifacts and were excluded. We investigated measures for whole-brain morphometry: total cortical volume (mm3), total cortical surface area (mm2), and mean cortical thickness (mm).
Statistical Analysis
Covariates
All covariates were entered simultaneously as independent variables into an ordinal logistic regression model to report the independent association of each covariate with PTE. We fitted ordinal logistic regression models using cumulative link models from the “ordinal” package, with PTE as the dependent variable, and all covariates for sociodemographic, perinatal health, prenatal cannabis and other substance exposure as independent variables. The adjusted odd ratios (AORs), i.e., independent association, between each covariate and the odds of PTE were reported.
PTE associations with postnatal developmental measures
Linear mixed-effect models were fitted using the “gamm4” package for each continuous dependent measure predicted by PTE (no exposure, PTE pre-knowledge or PTE post-knowledge of pregnancy). For binomial dependent variables, mixed-effect logistic regressions were fitted using the “gamm4” binomial (logit) function using only singletons, with a random effect for site, to allow for model convergence. Standard betas for continuous outcome variables and AORs for binomial outcome variables, and their corresponding 95% confidence intervals, were evaluated to assess the strength of the association between PTE and each dependent outcome. For each outcome variable, we tested three contrasts for PTE (post-knowledge vs. no exposure, pre-knowledge vs. no exposure, and post-knowledge vs. pre-knowledge of pregnancy), while including all demographic covariates of age, sex, race/ethnicity, SES (household income and parental education), gestational age, parent psychopathology, and risk for passive smoke exposure, and random intercepts of study site for behavioral measures or scanner identification (ID) for brain imaging measures, and family ID, as recommended for mixed-effect models to control for between-site/scanner and within-family correlations in the ABCD dataset (Dick et al., 2021).
SES Moderators
Given previously reported associations of SES (i.e., household income and parental education) on cognitive performance, including in the same cohort studied here, (Gonzalez et al., 2020), we tested the moderating role of SES on the associations between PTE and NIH total cognitive performance scores.
Early Postnatal Physical Health Mediators
PTE has been reported to have deleterious effects on early postnatal health (i.e., lower birth weight, small for gestational age, and shorter breastfeeding durations), while these early postnatal health indicators have also been shown to influence later outcomes in childhood (Chiolero et al., 2005; Horta et al., 2015; Lamm et al., 2020; Napierala et al., 2016; Shenkin et al., 2004; Yan et al., 2014). It is unknown to what extent PTE influences outcomes later in childhood through its initial effects on physical health at birth. Thus, birth health measures of birthweight, SGA, and breastfeeding were tested as potential mediators of possible associations of PTE with childhood outcomes (i.e., obesity, asthma, cognition and brain structure). While retrospective studies cannot prove causality, mediation analyses were utilized to identify potential important factors to be investigated in epidemiological prospective longitudinal studies. Mediation models were tested using the “mediation” R-package, controlling for all covariates, with site or scanner ID as a fixed effect, and random effect for family group ID, with PTE recoded to create binary exposure contrasts. Mediation effects were estimated using bootstrapping with quasi-Bayesian approximation (n = 1000 simulations).
RESULTS
In the sample analyzed (N = 8,803), 738 (8%) of children experienced PTE prior to maternal knowledge of pregnancy only, while 361 (4%) experienced PTE both prior to and after maternal knowledge of pregnancy. Descriptive statistics for all covariates and measures by PTE patterns (no exposure, PTE pre-knowledge, and PTE pre + post-knowledge of pregnancy) are shown in Table 1. A comparison of sample characteristics between the sample analyzed (n = 8,803 with complete data for PTE) and the sample excluded due to missing data (n = 3,072) showed modest differences such that missing data was higher among lower-income and parental education households, non-White and Hispanic participants, and participants with missing data showed one week earlier pregnancy knowledge on average, slightly greater percentage of prenatal exposure to cannabis and other substances throughout pregnancy, modestly higher parental psychopathology and greater percentage of participants with risk for passive smoke exposure compared to the sample analyzed (Supplementary Table 1).
Covariates
Sociodemographic factors, perinatal factors, and psychosocial factors were entered simultaneous in association with PTE to determine the adjusted odds of PTE. AOR values and 95% confidence intervals are shown in Figure 1. Children in households with mid- ($50K/year-100K/ year) and low-incomes (<$50K/year) were 1.78–2.32 times more likely to have PTE compared to high income households (>$100K/ year), with greater risk for the lowest income families. Children whose parents had 4 years of college or less were 1.38 to 4.79 times more likely to have PTE than children of parents with a post-graduate education, with risk highest among those with the lowest parental educational attainment. Children with a Hispanic ethnicity or Black race were 41–43% less likely to have PTE than non-Hispanic White children. Maternal endorsement of cannabis or other prenatal substance exposure after pregnancy recognition were 4 times more likely to have used tobacco during pregnancy. Children with risk for passive smoke exposure were 8 times more likely to have PTE. Odds of PTE were not associated with child age, sex, gestational age, number of prenatal conditions, and parent psychopathology (ASR).
Figure 1.
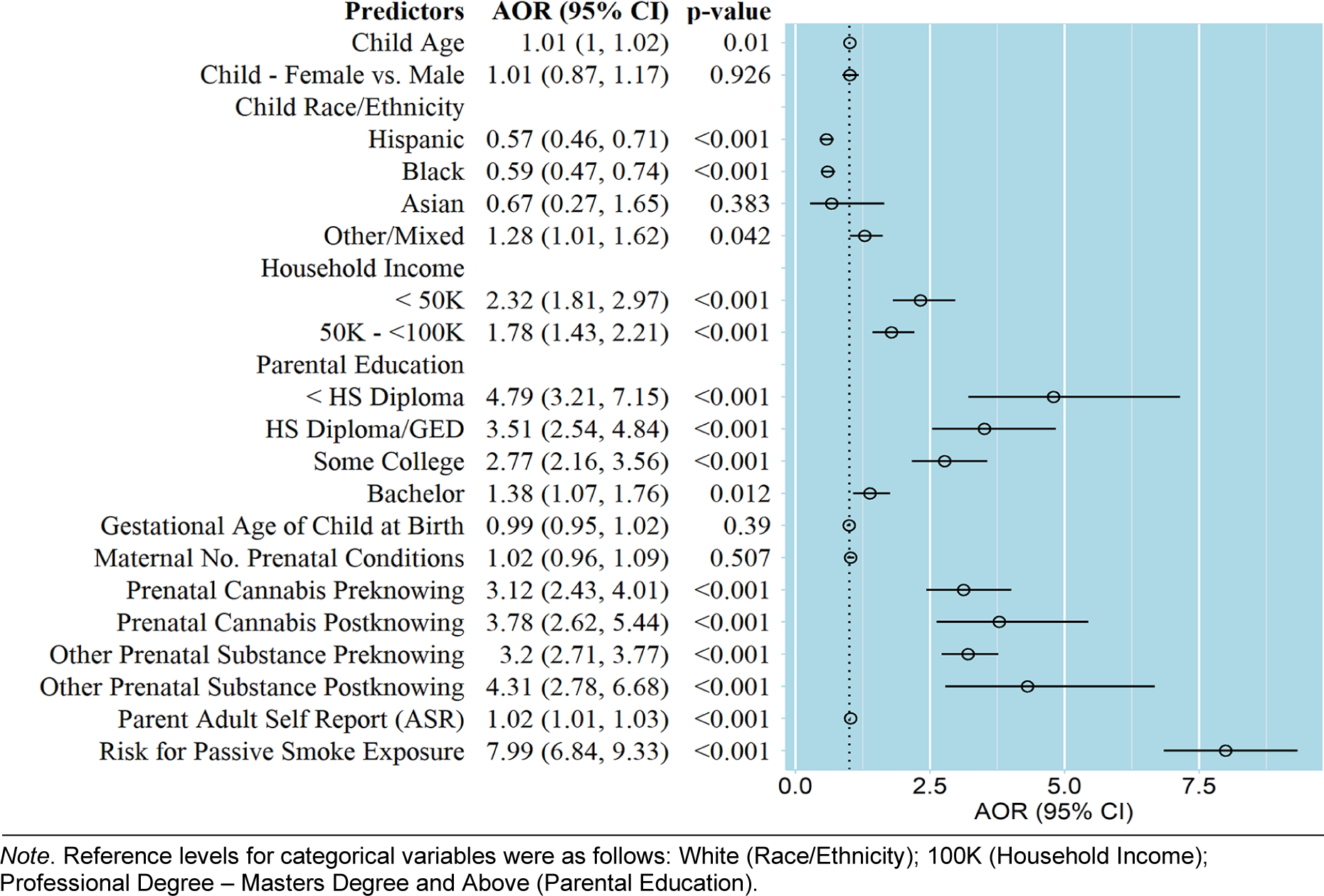
Adjusted odds ratio (AOR) and 95% confidence intervals (CI) for each covariate in relation to prenatal tobacco exposure.
PTE in association with Postnatal Birth and Physical Health
Children with PTE post-knowledge of pregnancy showed significantly lower birthweight compared to children with no-exposure, and also compared to children with PTE with only pre-knowledge of pregnancy (all β < −0.25 0.09, all p < 0.001; Figure 2A & 3), with no significant differences for PTE pre-knowledge compared to no exposure (p = 0.90; Figure 2A). Children with PTE post-knowledge of pregnancy were 1.8 times more likely to be considered small for gestational age (SGA; p = 0.003; Figure 2B), with no significant differences between other exposures (all p ≥ 0.08; Figure 2B). Breastfeeding duration was significantly shorter for each PTE contrast (pre-knowledge vs. no exposure, post-knowledge vs. no exposure, and pre-knowledge vs. post-knowledge), suggesting breastfeeding duration decreased incrementally with PTE (all β < −0.13, all p < 0.03; Figure 2A and 3).
Figure 2.

Associations of prenatal tobacco exposure (PTE) during pregnancy with physical health, psychological and behavioral problems, NIH total cognition, whole-brain structure measures. Shown are (A) standard betas and 95% confidence intervals (95% CI) for linear mixed-effect models and (B) adjusted odd ratios (AOR) and 95% CI for logistic regression models.
Figure 3.

Distribution of individual outcomes by exposure groups for PTE for continuous postnatal outcomes of (a) duration of breastfeeding, (b) birthweight, (c) total cortical volume, (d) total cortical surface area, (e) the interaction of PTE by parental educational attainment on NIH Toolbox total cognition scores; the means and 95% confidence intervals (black squares) and the dashed line corresponding to the mean of the non-exposed group.
PTE in association with Childhood Physical Health
Children with PTE through post-knowledge of pregnancy had 1.5 greater odds of obesity (BMI > 95%) compared to none or lesser exposure (Figure 2B; all p < 0.03), with no significant differences between PTE pre-knowledge compared to no exposure (Figure 2B). There were no significant associations of PTE with asthma, as reported as the number of attacks in the recent year (all p ≥ 0.05).
PTE in association with Childhood Neurodevelopment
Continued PTE post-knowledge of pregnancy, compared to no exposure, was associated with smaller total cortical volume and total cortical surface area, after adjusting for all potential confounders (all β < −0.12, all p < 0.03; Figure 2A and 3). We did not observe significant associations between any of the PTE contrasts with mean cortical thickness, or behavioral measures of attention problems, somatic complaints, anxiety/depression, and withdrawn/depression, (all |β| < 0.05, all p ≥ 0.48; Figure 2A) or asthma (all AOR < 1.5, all p ≥ 0.05; Figure 2B). The full model coefficients for all covariates are shown in Supplementary Table 2 and for all PTE contrasts on developmental outcomes in Supplementary Table 3.
While there was no significant association between PTE and cognition alone, there was an interaction of parental educational attainment by PTE on cognition scores for both exposure pre-knowledge (β= −1.65, [−2.54, −0.76], p = 0.003) and PTE post-knowledge of pregnancy (β= −2.94, [−3.27, −0.23], p = 0.02) compared to no PTE. Upon follow-up analyses for groups stratified by parental educational attainment, among n =5,076 children with higher parental education (4-year degree or higher), any PTE, i.e., pre-knowledge (β= −1.41, [−245, −0.36], p < 0.008) and post-knowledge (β= −2.94, [−4.88, −1.00], p = 0.003) of pregnancy was associated with lower cognition scores (Figure 3e). However, PTE was not significantly associated with cognition scores among children with lower parental educational attainment (n = 2,989; pre-knowledge exposure: β= 0.71, [−0.2, 1.62], p = 0.12; post-knowledge exposure: β= −0.13, [−1.32, 1.05], p = 0.82). There were no significant interactions between household income and PTE on cognition scores (all |β| < 1.83, all p > 0.08)
Mediating Role of Early Postnatal Health
Breastfeeding partially mediated the association between PTE and cognitive performance in all exposure comparisons (see Table 2). In post-hoc analyses, within both exposures pre-knowledge and post-knowledge of pregnancy, children who were breastfed showed higher cognitive scores than children who were never breastfed (p < 0.001; Supplementary Table 4). Exposure to longer durations of PTE (post-knowledge) was related to obesity, compared to none or lesser exposure (pre-knowledge), and this relationship was partially mediated by all three early postnatal health indicators (Table 2b), such that PTE, through a decrease in birthweight, shorter breastfeeding duration, and SGA at birth, was associated with greater odds of obesity. Duration of breastfeeding partially mediated all neurodevelopmental childhood outcomes (i.e., total cortical area, total volume, and cognition) specifically in children with longer duration of PTE (post-knowledge of pregnancy) compared to children with lesser PTE (none or exposure pre-knowledge; Supplementary Table 3). Similarly, birthweight partially mediated total volume and cognition differences in children with longer duration of PTE compared to less severe PTE, suggesting the effect of PTE on postnatal brain structure via birthweight and breastfeeding may be evident only among longer duration of PTE (post-knowledge) contrasted with less severe PTE.
Table 2.
Results of testing early postnatal physical health measures as mediators of childhood health outcomes among two contrasts, children with any PTE (pre + post-knowledge) compared to no exposure, and children with longer duration of PTE (post-knowledge only) compared to lesser PTE (none-to-Pre-knowledge).
| ANY PTE vs. No Exposure | |||||||||
| DV | n | Total Effect | Average Direct Effect | Average Indirect Effect | 95 % CI | Proportion Mediated (%) | |||
| LL | UL | ||||||||
| Total Cortical Area | Birthweight | 8,118 | −0.06 (p = 0.05) |
−0.06 (p = 0.05) |
−0.01 | −0.02 | 0.00 | 0.14 | NS (p = 0.17) |
| SGA b | 5,440 | −0.04 (p = 0.33) |
−0.03 (p = 0.43) |
−0.007 | −0.02 | 0.00 | 0.10 | NS (p = 0.38) |
|
| Breastfeeding (months) | 8,158 | −0.13 (p = 0.04) |
−0.05 (p = 0.18) |
−0.01 | −0.01 | 0.00 | <0.001 | NS (p = 0.13) |
|
| Total Cortical Area | Birthweight | 8,118 | −0.06 (p = 0.13) |
−0.06 (p = 0.08) |
−0.01 | −0.01 | 0.00 | <0.001 | NS (p = 0.06) |
| SGA b | 5,440 | −0.04 (p = 0.27) |
−0.12 (p = 0.06) |
−0.01 | −0.01 | 0.00 | 0.08 | NS (p = 0.34) |
|
| Breastfeeding (months) | 8,158 | −0.13 (p = 0.04) |
−0.06 (p = 0.08) |
−0.01 | −0.01 | 0.00 | <0.001 | NS (p = 0.05) |
|
| Cognition a | Birthweight | 5,357 | −0.06 (p = 0.05) |
−1.32 (p = 0.01) |
−0.02 | −0.06 | 0.02 | 0.41 | NS (p = 0.40) |
| SGA b | 3,553 | −1.30 (p = 0.03) |
−1.20 (p = 0.03) |
−0.03 | −0.11 | 0.01 | 0.26 | NS (p = 0.26) |
|
| Breastfeeding (months) | 5,392 | −1.80 (p = 0.14) |
−1.12 (p = 0.01) |
−0.15 | −0.25 | −0.07 | < 0.001 | 11% (p = 0.006) |
|
| Obesity b | Birthweight | 5,851 | 0.01 (p = 0.55) |
0.01 (p = 0.40) |
−0.003 | −0.01 | 0.00 | 0.02 | NS (p = 0.55) |
| SGA b | 5,851 | 0.01 (p = 0.47) |
0.01 (p = 0.40) |
−0.003 | −0.003 | 0.00 | 0.04 | NS (p = 0.50) |
|
| Breastfeeding (months) | 5,880 | 0.01 (p = 0.60) |
0.005 (p = 0.71) |
0.002 | 0.001 | 0.00 | 0.006 | NS (p = 0.60) |
|
| Longer Duration of PTE vs. Lesser Exposure (none-to-Pre-knowledge) | |||||||||
| DV | n | Total Effect | Average Direct Effect | Average Indirect Effect | 95 % CI | Proportion Mediated (%) | |||
| LL | UL | ||||||||
| Total Cortical Area | Birthweight | 8,118 | −0.11 (p = 0.03) |
−0.07 (p = 0.18) |
−0.05 | −0.07 | −0.03 | <0.001 | 39% (p = 0.03) |
| SGA b | 5,440 | −0.13 (p = 0.04) |
−0.11 (p = 0.07) |
−0.01 | −0.03 | 0.00 | 0.03 | NS (p = 0.07) |
|
| Breastfeeding (months) | 8,158 | −0.11 (p = 0.02) |
−0.10 (p = 0.04) |
−0.01 | −0.014 | 0.00 | <0.001 | 7% (p = 0.02) |
|
| Total Volume | Birthweight | 8,118 | −0.10 (p = 0.07) |
−0.05 (p = 0.30) |
−0.04 | −0.07 | −0.02 | <0.001 | NS (p = 0.07) |
| SGA b | 5,440 | −0.13 (p = 0.04) |
−0.12 (p = 0.06) |
−0.01 | −0.03 | 0.00 | 0.04 | NS (p = 0.07) |
|
| Breastfeeding (months) | 8,158 | 0.004 (p < 0.001) |
−0.11 (p < 0.001) |
−0.01 | −0.012 | −0.01 | <0.001 | 8% (p < 0.001) |
|
| Cognition a | Birthweight | 5,357 | −1.95 (p = 0.032) |
−1.86 (p = 0.01) |
−0.09 | −0.20 | −0.01 | 0.01 | 4% (p = 0.03) |
| SGA b | 3,553 | −1.80 (p = 0.14) |
−1.70 (p = 0.16) |
−0.07 | −0.26 | 0.02 | 0.19 | NS (p = 0.31) |
|
| Breastfeeding (months) | 5,392 | −2.15 (p = 0.02) |
−1.98 (p = 0.02) |
−0.17 | −0.33 | −0.03 | 0.02 | 8% (p = 0.04) |
|
| Obesity b | Birthweight | 5,851 | 0.06 (p = 0.001) |
0.08 (p =0.004) |
−0.01 | −0.02 | −0.01 | < 0.001 | −18% (p = 0.01) |
| SGA b | 5,851 | 0.06 (p = 0.01) |
0.07 (p = 0.01) |
−0.003 | −0.01 | 0.00 | 0.008 | −5% (p = 0.02) |
|
| Breastfeeding (months) | 5,880 | 0.004 (p = 0.01) |
0.05 (p = 0.036) |
0.004 | 0.001 | 0.00 | 0.008 | 7% (p = 0.036) |
|
Models tested in high SES sample due to SES X PTE interaction.
Models include only singletons (no siblings) to allow for logistic model convergence. SGA: small for gestational age; CI: confidence interval; LL: lower limit; UL: upper limit; NS: non-significant.
DISCUSSION
Within the large and diverse ABCD study cohort (N = 8,803), we investigated associations of PTE with postnatal outcomes in typically developing school-aged children 9 –10 years of age. We found that endorsement in the ABCD cohort of any PTE was 12%. PTE was associated with several postnatal measures, including lower birth weight, SGA, shorter duration of breastfeeding, higher odds of childhood obesity, and lower total cortical volume and surface area. Parental educational attainment moderated the association between PTE and cognitive performance, such that any PTE attenuated the benefit of higher parental education on cognitive performance. In this study, we present associations of PTE with developmental outcomes when controlling for other factors that could influence the associations with PTE, including sociodemographic factors, perinatal health, concomitant prenatal exposure to other substances, parent psychopathology, and risk for passive smoke exposure. Our findings suggest that the observed associations of PTE on developmental measures at birth and age 9–10 years are detectable, even after adjusting for potential confounders.
Importantly, given the diversity of the ABCD cohort, we took an intersectional approach to contextualize sociodemographic correlations with PTE, which considers how categorization of populations into social identities (i.e., SES or race or ethnicity) characterize lived experiences and are tied to social structures and systemic inequities (Else-Quest & Hyde, 2016). Thus, we examined all potential covariates of PTE simultaneously to determine adjusted odds ratios and interpret the unique odds of each correlate with PTE in context of intersecting identities (e.g., SES, race, ethnicity, and prenatal health profiles). Our analyses revealed youth who self-identified as Hispanic or Black were much less likely to have PTE compared to Whites, while lower SES households (lower household income and lower parental educational attainment) were more likely to have PTE, compared to children in higher SES households. This suggests that lower SES was an indicator of increased odds for PTE, while race or ethnicity were not indicators of increased odds. Maternal endorsement of prenatal exposure to other substances and endorsement of risk for postnatal second-hand smoke exposure showed the highest increased odds for PTE among all covariates. Other covariates, including parent psychopathology and concomitant other prenatal substance exposure have only recently been considered in associations of PTE and postnatal development, with even fewer studies accounting for risk for postnatal second-hand smoke exposure (Gilman, Gardener, et al., 2008; Lambe et al., 2006).
Previous studies demonstrate that PTE is associated with poorer physical health at birth, specifically lower birthweight and SGA with extended PTE (U.S. Department of Health and Human Services, 2020), and shorter durations of breastfeeding (Napierala et al., 2016), and here, we extended these findings to a non-clinical sample of children, representing national patterns of average PTE. Low birthweight has been linked to lower cognitive performance, most strongly among children in low SES households (Torche & Echevarría, 2011). In concordance with previous studies, PTE throughout both pre- and post-knowledge of pregnancy was associated with lower birth weight and greater odds of SGA, with no significant differences in birth weight or SGA for those with PTE pre-knowledge only compared to non-exposed children, suggesting cessation of smoking during pregnancy may eliminate risk for lower birth weight and SGA outcomes (U.S. Department of Health and Human Services, 2020). Studies show longer durations of breastfeeding can promote healthy physical and cognitive development (Anderson et al., 1999; Horwood & Fergusson, 1998; Kramer et al., 2008). Although there are likely to be many factors influencing breastfeeding patterns (Ladomenou et al., 2007), we found PTE was associated with shorter breastfeeding durations, even when controlling for other confounding factors, including prematurity and parental educational attainment. Further, breastfeeding mediated 11% of the association of PTE with cognitive performance in the higher SES sample, where an association of PTE was detectable. Differences in total cognition scores between patterns of PTE were less clear among children with reported lower parental education.
A few studies had suggested the effect of PTE on cognitive performance was no longer detectable when accounting for SES (i.e., maternal education), however, these studies did not test whether the association of PTE with cognitive outcomes differed between SES brackets (Breslau et al., 2005; Kafouri et al., 2009). Given that parental education is also found to be related to cognitive performance in the same cohort (Gonzalez et al., 2020), it is plausible that the associations of PTE are not distinguishable from the associations of lower SES on cognitive performance. In post-hoc analyses, children in high SES contexts who were breastfed showed higher cognitive scores compared to children who were never breastfed, even among children with PTE, suggesting that even for children with PTE, breastfeeding may still be a promotive factor for cognitive outcomes.
PTE throughout post-knowledge of pregnancy was associated with differences in children’s brain structure, i.e., lower total cortical volume and surface area in children exposed throughout pregnancy compared to children with no PTE, consistent with previous findings in substantially smaller cohorts (El Marroun et al., 2014, 2016; Gautam et al., 2015). Given the age group of the sample studied here (9–10 years of age), it is plausible that PTE associations with lower cortical volume and surface area map onto stable maturational differences in brain structure, or alternatively, to differences in the phase of maturation. During early childhood, cortical surface area shows curvilinear maturational trajectories that are regionally-specific, such that surface area generally increases during childhood and peaks in the pre-adolescent period, and thereafter decreasing for regional surface area (Jernigan et al., 2016; Wierenga et al., 2014). PTE was associated with lower cortical area and volume through shorter durations of breastfeeding when contrasting a longer duration of PTE, compared to no-exposure and lesser PTE. Similarly, PTE was related to cortical area via birthweight only among children with longer duration of PTE compared to no-exposure and lesser PTE. These findings suggest that for longer durations of PTE, birthweight could be a proximal measure of the severity of PTE while a shorter duration of breastfeeding, contributes to poorer childhood health outcomes. Similar to the 2020 Surgeon General’s report on Smoking Cessation, where causal links between PTE and outcomes are now accepted given large accumulation of observational evidence, our findings cannot conclude a causal link between PTE and these measure of early postnatal health, and should be investigated by future longitudinal studies using prospective designs. Future studies should examine how the pattern of PTE associations with brain structure change or remain stable during later developmental stages in adolescence.
We found that PTE after knowledge of pregnancy, compared to no PTE, was associated with obesity at age 9 – 10 years, even after controlling for important potential confounders, including SES. These findings are consistent with previous studies reporting increased odds of childhood obesity with PTE (Chen et al., 2006; Gilman, Gardener, et al., 2008). This finding does not apply to PTE prior to pregnancy recognition, suggesting that cessation of smoking in pregnancy may reduce or eliminate risk for childhood obesity. The prevalence of childhood obesity has increased in recent decades and is related to lower physical activity and improper nutrition, increasing risk for cardiovascular disease later in adulthood (Franks et al., 2010). We found the effect of PTE on obesity was partially mediated by a shorter duration of breastfeeding, decreased birthweight, and SGA at birth, suggesting PTE may influence childhood obesity through mechanisms related to health in the early postnatal period (Zhou et al., 2014). In contrast to previous findings, we did not find a significant association of PTE with asthma (episodes within the year), although it is plausible PTE may be associated with severity of asthma measured over longer periods of childhood (Gilliland et al., 2001; Zhou et al., 2014).
While the effect sizes for model comparisons were small, and of unknown relevance to daily functioning and development, our findings suggest that supporting mothers, and those close to them who also smoke tobacco, in reducing or stopping tobacco use during pregnancy, relative to no PTE, may be associated with overall healthier outcomes in children. While our findings suggest that a decrease in breastfeeding for children with PTE in high SES contexts may negatively influence cognitive outcomes, it is unknown to what extent other factors due to parenting styles associated with breastfeeding may contribute as well (e.g., attachment parenting style; Ladomenou et al., 2007). Importantly, given that the sample studied here was representative of a non-clinical community sample, with data not missing at random, the observed adverse associations of PTE on development may be conservative estimates of the potential harmful effects of PTE on developmental outcomes in children. Further investigations of how combined prenatal substance exposures that commonly occur with PTE, such as cannabis and alcohol, are needed to better capture the impact on development.
Conclusion
Understanding the complex relationship between PTE and developmental outcomes is difficult given that it may be associated with other socioeconomic, perinatal, and psychosocial risk factors. Here, we report PTE associations with physical health and neurodevelopmental measures, statistically independent of relevant potential confounders. Supporting women to stop smoking in pregnancy may have positive implications for their child’s health at birth and later in childhood, including increased likelihood of establishing breastfeeding, which in turn may influence better cognitive outcomes at age 9–10 years. Given that tobacco use patterns are highly correlated among couples, plus potential passive perinatal tobacco exposure resulting from the partner’s tobacco use, interventions should focus on smoking cessation during pregnancy and postnatal periods for the entire household, and not solely on the pregnant and/or nursing mother (Bottorff et al., 2005). Future studies are needed to investigate whether the associations between PTE and developmental outcomes measured here are consistent throughout adolescence or change with developmental periods.
Supplementary Material
Acknowledgments
The ABCD Study is supported by the National Institutes of Health and additional federal partners under award numbers U01DA041022, U01DA041028, U01DA041048, U01DA041089, U01DA041106, U01DA041117, U01DA041120, U01DA041134, U01DA041148, U01DA041156, U01DA041174, U24DA041123, U24DA041147, U01DA041093, and U01DA041025. A full list of supporters is available at https://abcdstudy.org/federal-partners.html. K.A.U. was supported by K01AA026889. M.R.G was supported by U01DA041048-05S1 and U01AA021692-09S1.A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/Consortium_Members.pdf. ABCD consortium investigators designed and implemented the study and/or provided data but did not all necessarily participate in analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators. The authors thank Natasha Akshoomoff, at the University of California, San Diego, and Martha Fuller at the University of San Diego who provided consultation on defining small for gestational age. We have no conflicts of interest to disclose. The ABCD 2.0.1 data release is available from the NIMH Data Archive ABCD Collection (10.15154/1504041).
Footnotes
CRediT Authors Statement: Marybel R. Gonzalez: Conceptualization, Methodology, Formal Analysis, Investigation, Data Curation, Writing – Original Draft, Writing – Review & Editing; Kristina Uban: Conceptualization, Writing – Review & Editing; Susan F. Tapert: Supervision, Writing – Review & Editing, Funding acquisition; Elizabeth R. Sowell: Conceptualization, Supervision, Writing –Review & Editing, Resources, Funding acquisition
References
- Achenbach TM (2009). The Achenbach System of Emprically Based Assessment (ASEBA): Development, Findings, Theory and Applications.
- Alberg AJ, Shopland DR, & Cummings KM (2014). The 2014 Surgeon General’s Report: Commemorating the 50th Anniversary of the 1964 Report of the Advisory Committee to the US Surgeon General and Updating the Evidence on the Health Consequences of Cigarette Smoking. In American Journal of Epidemiology (Vol. 179, Issue 4). Oxford Academic. 10.1093/AJE/KWT335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JW, Johnstone BM, & Remley DT (1999). Breast-feeding and cognitive development: A meta-analysis. American Journal of Clinical Nutrition, 70(4), 525–535. 10.1093/ajcn/70.4.525 [DOI] [PubMed] [Google Scholar]
- Baghurst PA, Tong S. -l, Woodward A, & McMichael AJ (1992). Effects of maternal smoking upon neuropsychological development in early childhood: importance of taking account of social and environmental factors. Paediatric and Perinatal Epidemiology, 6(4), 403–415. 10.1111/j.1365-3016.1992.tb00784.x [DOI] [PubMed] [Google Scholar]
- Barch DM, Albaugh MD, Avenevoli S, Chang L, Clark DB, Glantz MD, Hudziak JJ, Jernigan TL, Tapert SF, Yurgelun-Todd D, Alia-Klein N, Potter AS, Paulus MP, Prouty D, Zucker RA, & Sher KJ (2018). Demographic, physical and mental health assessments in the adolescent brain and cognitive development study: Rationale and description. In Developmental Cognitive Neuroscience (Vol. 32, pp. 55–66). 10.1016/j.dcn.2017.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batty GD, Der G, & Deary IJ (2006). Effect of Maternal Smoking During Pregnancy on Offspring’s Cognitive Ability: Empirical Evidence for Complete Confounding in the US National Longitudinal Survey of Youth. PEDIATRICS, 118(3), 943–950. 10.1542/peds.2006-0168 [DOI] [PubMed] [Google Scholar]
- Bottorff JL, Kalaw C, Johnson JL, Chambers N, Stewart M, Greaves L, & Kelly M (2005). Unraveling smoking ties: How tobacco use is embedded in couple interactions. Research in Nursing and Health, 28(4), 316–328. 10.1002/nur.20085 [DOI] [PubMed] [Google Scholar]
- Breslau N, Paneth N, Lucia VC, & Paneth-Pollak R (2005). Maternal smoking during pregnancy and offspring IQ. International Journal of Epidemiology, 34(5), 1047–1053. 10.1093/ije/dyi163 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). (2016). A SAS Program for the 2000 CDC Growth Charts (ages 0 to <20 years). https://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm
- Chen A, Pennell ML, Klebanoff MA, Rogan WJ, & Longnecker MP (2006). Maternal smoking during pregnancy in relation to child overweight: Follow-up to age 8 years. International Journal of Epidemiology, 35(1), 121–130. 10.1093/ije/dyi218 [DOI] [PubMed] [Google Scholar]
- Chiolero A, Bovet P, & Paccaud F (2005). Association between maternal smoking and low birth weight in Switzerland: The EDEN study. Swiss Medical Weekly, 135(35–36), 525–530. https://doi.org/2005/35/smw-11122 [DOI] [PubMed] [Google Scholar]
- Cornelius MD, & Day NL (2009). Developmental consequences of prenatal tobacco exposure. In Current Opinion in Neurology (Vol. 22, Issue 2, pp. 121–125). NIH Public Access. 10.1097/WCO.0b013e328326f6dc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius MD, Ryan CM, Day NL, Goldschmidt L, & Willford JA (2001). Prenatal tobacco effects on neuropsychological outcomes among preadolescents. Journal of Developmental and Behavioral Pediatrics, 22(4), 217–225. 10.1097/00004703-200108000-00002 [DOI] [PubMed] [Google Scholar]
- Dick AS, Lopez DA, Watts AL, Heeringa S, Reuter C, Bartsch H, Fan CC, Kennedy DN, Palmer C, Marshall A, Haist F, Hawes S, Nichols TE, Barch DM, Jernigan TL, Garavan H, Grant S, Pariyadath V, Hoffman E, … Thompson WK (2021). Meaningful associations in the adolescent brain cognitive development study. In NeuroImage (Vol. 239, p. 118262). Academic Press. 10.1016/j.neuroimage.2021.118262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Difranza JR, Aligne, ; Andrew C, & Weitzman M (2004). Prenatal and Postnatal Environmental Tobacco Smoke Exposure and Children’s Health. www.aappublications.org/news [PubMed]
- Drake P, Driscoll AK, & Mathews TJ (2018). Cigarette Smoking During Pregnancy: United States, 2016 Key findings Data from the National Vital Statistics System. NCHS Data Brief. https://www.cdc.gov/nchs/data/databriefs/db305.pdf [PubMed] [Google Scholar]
- El Marroun H, Schmidt MN, Franken IHA, Jaddoe VWV, Hofman A, Van Der Lugt A, Verhulst FC, Tiemeier H, & White T (2014). Prenatal tobacco exposure and brain morphology: A prospective study in young children. Neuropsychopharmacology, 39(4), 792–800. 10.1038/npp.2013.273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Marroun H, Tiemeier H, Franken IHA, Jaddoe VWV, van der Lugt A, Verhulst FC, Lahey BB, & White T (2016). Prenatal Cannabis and Tobacco Exposure in Relation to Brain Morphology: A Prospective Neuroimaging Study in Young Children. Biological Psychiatry, 79(12), 971–979. 10.1016/J.BIOPSYCH.2015.08.024 [DOI] [PubMed] [Google Scholar]
- Else-Quest NM, & Hyde JS (2016). Intersectionality in Quantitative Psychological Research: II. Methods and Techniques. 10.1177/0361684316647953, 40(3), 319–336. 10.1177/0361684316647953 [DOI] [Google Scholar]
- Ernst M, Moolchan ET, & Robinson ML (2001). Behavioral and neural consequences of prenatal exposure to nicotine. Journal of the American Academy of Child and Adolescent Psychiatry, 40(6), 630–641. 10.1097/00004583-200106000-00007 [DOI] [PubMed] [Google Scholar]
- Fenton TR, & Kim JH (2013). A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatrics, 13(1), 59. 10.1186/1471-2431-13-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson DM, & Lloyd M (1991). Smoking during pregnancy and its effects on child cognitive ability from the ages of 8 to 12 years. Paediatric and Perinatal Epidemiology, 5(2), 189–200. 10.1111/j.1365-3016.1991.tb00700.x [DOI] [PubMed] [Google Scholar]
- Forray A (2016). Substance use during pregnancy [version 1; referees: 2 approved]. In F1000Research (Vol. 5). Faculty of 1000 Ltd. 10.12688/F1000RESEARCH.7645.1 [DOI] [Google Scholar]
- Franks PW, Hanson RL, Knowler WC, Sievers ML, Bennett PH, & Looker HC (2010). Childhood Obesity, Other Cardiovascular Risk Factors, and Premature Death. New England Journal of Medicine, 362(6), 485–493. 10.1056/NEJMoa0904130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Bartsch H, Conway K, Decastro A, Goldstein RZ, Heeringa S, Jernigan T, Potter A, Thompson W, & Zahs D (2018). Recruiting the ABCD sample: Design considerations and procedures. Developmental Cognitive Neuroscience, 32, 16–22. 10.1016/j.dcn.2018.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam P, Warner TD, Kan EC, & Sowell ER (2015). Executive function and cortical thickness in youths prenatally exposed to cocaine, alcohol and tobacco. Developmental Cognitive Neuroscience, 16, 155–165. 10.1016/j.dcn.2015.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliland FD, Li Y-F, & Peters JM (2001). Effects of Maternal Smoking during Pregnancy and Environmental Tobacco Smoke on Asthma and Wheezing in Children. In Am J Respir Crit Care Med (Vol. 163). www.atsjournals.org [DOI] [PubMed] [Google Scholar]
- Gilman SE, Breslau J, Subramanian SV, Hitsman B, & Koenen KC (2008). Social factors, psychopathology, and maternal smoking during pregnancy. American Journal of Public Health, 98(3), 448–453. 10.2105/AJPH.2006.102772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman SE, Gardener H, & Buka SL (2008). Maternal smoking during pregnancy and children’s cognitive and physical development: A causal risk factor? American Journal of Epidemiology, 168(5), 522–531. 10.1093/aje/kwn175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez MR, Palmer CE, Uban KA, Jernigan TL, Thompson WK, & Sowell ER (2020). Positive Economic, Psychosocial, and Physiological Ecologies Predict Brain Structure and Cognitive Performance in 9–10-Year-Old Children. Frontiers in Human Neuroscience, 14, 436. 10.3389/fnhum.2020.578822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler DJ, Hatton SN, Cornejo MD, Makowski C, Fair DA, Dick AS, Sutherland MT, Casey BJ, Barch DM, Harms MP, Watts R, Bjork JM, Garavan HP, Hilmer L, Pung CJ, Sicat CS, Kuperman J, Bartsch H, Xue F, … Dale AM (2019). Image processing and analysis methods for the Adolescent Brain Cognitive Development Study. NeuroImage, 202, 116091. 10.1016/j.neuroimage.2019.116091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook BD (2016). The effects of nicotine on human fetal development. In Birth Defects Research Part C - Embryo Today: Reviews (Vol. 108, Issue 2, pp. 181–192). John Wiley and Sons Inc. 10.1002/bdrc.21128 [DOI] [PubMed] [Google Scholar]
- Horta BL, Loret De Mola C, & Victora CG (2015). Breastfeeding and intelligence: a systematic review and meta-analysis. Acta Paediatrica, 104, 14–19. 10.1111/APA.13139 [DOI] [PubMed] [Google Scholar]
- Horwood LJ, & Fergusson DM (1998). Breastfeeding and later cognitive and academic outcomes. Pediatrics, 101(1), e9–e9. 10.1542/peds.101.1.e9 [DOI] [PubMed] [Google Scholar]
- Huijbregts SCJ, Séguin JR, Zelazo PD, Parent S, Japel C, & Tremblay RE (2006). Interrelations between maternal smoking during pregnancy, birth weight and sociodemographic factors in the prediction of early cognitive abilities. Infant and Child Development, 15(6), 593–607. 10.1002/icd.480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizink AC, & Mulder EJH (2006). Maternal smoking, drinking or cannabis use during pregnancy and neurobehavioral and cognitive functioning in human offspring. In Neuroscience and Biobehavioral Reviews (Vol. 30, Issue 1, pp. 24–41). Neurosci Biobehav Rev. 10.1016/j.neubiorev.2005.04.005 [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Brown TT, Bartsch H, & Dale AM (2016). Toward an integrative science of the developing human mind and brain: Focus on the developing cortex. Developmental Cognitive Neuroscience, 18, 2–11. 10.1016/j.dcn.2015.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafouri S, Leonard G, Perron M, Richer L, Séguin JR, Veillette S, Pausova Z, & Paus T (2009). Maternal cigarette smoking during pregnancy and cognitive performance in adolescence. International Journal of Epidemiology, 38(1), 158–172. 10.1093/ije/dyn250 [DOI] [PubMed] [Google Scholar]
- Kodl MM, & Wakschlag LS (2004). Does a childhood history of externalizing problems predict smoking during pregnancy? Addictive Behaviors, 29(2), 273–279. 10.1016/j.addbeh.2003.08.003 [DOI] [PubMed] [Google Scholar]
- Kramer MS, Aboud F, Mironova E, Vanilovich I, Platt RW, Matush L, Igumnov S, Fombonne E, Bogdanovich N, Ducruet T, Collet JP, Chalmers B, Hodnett E, Davidovsky S, Skugarevsky O, Trofimovich O, Kozlova L, & Shapiro S (2008). Breastfeeding and child cognitive development: New evidence from a large randomized trial. Archives of General Psychiatry, 65(5), 578–584. 10.1001/archpsyc.65.5.578 [DOI] [PubMed] [Google Scholar]
- Ladomenou F, Kafatos A, & Galanakis E (2007). Risk factors related to intention to breastfeed, early weaning and suboptimal duration of breastfeeding. Acta Paediatrica, International Journal of Paediatrics, 96(10), 1441–1444. 10.1111/j.1651-2227.2007.00472.x [DOI] [PubMed] [Google Scholar]
- Lambe M, Hultman C, Torrång A, MacCabe J, & Cnattingius S (2006). Maternal smoking during pregnancy and school performance at age 15. Epidemiology, 17(5), 524–530. 10.1097/01.ede.0000231561.49208.be [DOI] [PubMed] [Google Scholar]
- Lamm SH, Ferdosi H, Boroje IJ, Afari-Dwamena NA, Qian L, Dash ED, Li J, Chen R, & Feinleib M (2020). Maternal tobacco use: A third-trimester risk factor for small-for-gestational-age pregnancy outcome. Preventive Medicine Reports, 18, 101080. 10.1016/j.pmedr.2020.101080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana M, Bjork JM, Nagel BJ, Barch DM, Gonzalez R, Nixon SJ, & Banich MT (2018). Adolescent neurocognitive development and impacts of substance use: Overview of the adolescent brain cognitive development (ABCD) baseline neurocognition battery. Developmental Cognitive Neuroscience, 32, 67–79. 10.1016/J.DCN.2018.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell E, Thompson J, Robinson E, Wild C, Becroft D, Clark P, Glavish N, Pattison N, & Pryor J (2007). Smoking, nicotine and tar and risk of small for gestational age babies. Acta Paediatrica, 91(3), 323–328. 10.1111/j.1651-2227.2002.tb01723.x [DOI] [PubMed] [Google Scholar]
- Moore BF, Shapiro AL, Wilkening G, Magzamen S, Starling AP, Allshouse WB, Adgate JL, & Dabelea D (2020). Prenatal Exposure to Tobacco and Offspring Neurocognitive Development in the Healthy Start Study. Journal of Pediatrics, 218, 28–34.e2. 10.1016/j.jpeds.2019.10.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napierala M, Mazela J, Merritt TA, & Florek E (2016). Tobacco smoking and breastfeeding: Effect on the lactation process, breast milk composition and infant development. A critical review. In Environmental Research (Vol. 151, pp. 321–338). 10.1016/j.envres.2016.08.002 [DOI] [PubMed] [Google Scholar]
- Olds DL, Henderson CR, & Tatelbaum R (1994). Intellectual Impairment in Children of Women Who Smoke Cigarettes During Pregnancy. Pediatrics, 93(2). https://pediatrics.aappublications.org/content/93/2/221.abstract?casa_token=zErsW9Btly8AAAAA:KxJ3ZP_Gb3WzRWz5bTATn4Ne1AoW_pPjJEAi4Lvw_pG9Mk-mkNLn8nVJwuHiwHIEY4mD7lB2sZPO [PubMed] [Google Scholar]
- R. M, & Stanton WR (1994). Smoking in pregnancy and child development to age 9 years. Journal of Paediatrics and Child Health, 30(3), 263–268. 10.1111/j.1440-1754.1994.tb00631.x [DOI] [PubMed] [Google Scholar]
- R Development Core Team. (2018). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, 2, https://www.R-project.org. http://www.r-project.org/ [Google Scholar]
- Schauer GL, King BA, Bunnell RE, Promoff G, & McAfee TA (2016). Toking, Vaping, and Eating for Health or Fun: Marijuana Use Patterns in Adults, U.S., 2014. American Journal of Preventive Medicine, 50(1), 1–8. 10.1016/J.AMEPRE.2015.05.027 [DOI] [PubMed] [Google Scholar]
- Sexton M (2011). A Clinical Trial of Change in Maternal Smoking and Its Effect on Birth Weight. JAMA: The Journal of the American Medical Association, 251(7), 911. 10.1001/jama.1984.03340310025013 [DOI] [PubMed] [Google Scholar]
- Shenkin SD, Starr JM, & Deary IJ (2004). Birth weight and cognitive ability in childhood: A systematic review. Psychological Bulletin, 130(6), 989–1013. 10.1037/0033-2909.130.6.989 [DOI] [PubMed] [Google Scholar]
- Tong VT, England LJ, Rockhill KM, & D’Angelo DV (2017). Risks of Preterm Delivery and Small for Gestational Age Infants: Effects of Nondaily and Low-Intensity Daily Smoking During Pregnancy. Paediatric and Perinatal Epidemiology, 31(2), 144–148. 10.1111/ppe.12343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torche F, & Echevarría G (2011). The effect of birthweight on childhood cognitive development in a middle-income country. International Journal of Epidemiology, 40(4), 1008–1018. 10.1093/ije/dyr030 [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. (2014). The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General.
- U.S. Department of Health and Human Services. (2020). Chapter 4 The Health Benefits of Smoking Cessation. In Smoking Cessation: A Report of the Surgeon General [Internet]. United States Public Health Service Office of the Surgeon General; National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health.United States Public Health Service Office of the Surgeon General; National Center for. [Google Scholar]
- Wierenga LM, Langen M, Oranje B, & Durston S (2014). Unique developmental trajectories of cortical thickness and surface area. NeuroImage, 87, 120–126. 10.1016/j.neuroimage.2013.11.010 [DOI] [PubMed] [Google Scholar]
- Yan J, Liu L, Zhu Y, Huang G, & Wang PP (2014). The association between breastfeeding and childhood obesity: A meta-analysis. BMC Public Health, 14(1), 1–11. 10.1186/1471-2458-14-1267/TABLES/3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Rosenthal DG, Sherman S, Zelikoff J, Gordon T, & Weitzman M (2014). Physical, behavioral, and cognitive effects of prenatal tobacco and postnatal secondhand smoke exposure. Current Problems in Pediatric and Adolescent Health Care, 44(8), 219–241. 10.1016/j.cppeds.2014.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


