Abstract
Purpose:
The popularity of dental amalgam arises from its excellent long-term performance, ease of use, and low cost. However, there is a concern about the potential adverse health effects arising from exposure to mercury in amalgam. This review article critically discusses the safety of dental amalgam as a restorative material and our preparedness for a mercury-free road ahead.
Materials and methods:
A database search was performed on PubMed and Google scholar using the keywords: “mercury-free dentistry”, “mercury toxicity”, “amalgam substitutes”, “amalgam mercury toxicity”. Inclusion and exclusion criteria were specified clearly. Relevant literature was also searched in the dental textbooks.
Results:
Around 40 articles, highlighting mercury exposure among dental professionals and patients were included. Despite the overwhelming body of scientific evidence demonstrating amalgam to be a safe restorative material, concerns about the toxic effects of mercury persist.
Conclusion:
The real challenge is to find a suitable amalgam substitute and to follow the mercury hygiene measures closely.
Keywords: amalgam, mercury toxicity, Minamata convention, amalgam substitutes, amalgam alternatives
Introduction
Technically, an amalgam is a mixture of mercury (Hg) and another metal. Dental amalgam is a combination of mercury and a silver-tin (Ag-Sn) alloy (1). Amalgam was first used in Chinese literature in 659, and it has remained the most widespread and successful restorative material in dentistry for the past 160 years (2). Amalgam’s popularity stems out of its superior long-term results, convenience of use, and affordability (1, 3). Prior to the 1970s, more than three-fourths of total restorations were made with amalgam. However, over the last 30 years, the use of amalgam has decreased globally. This is due to a decrease in caries incidence, an increase in the use of cast restorations such as crowns, ceramic inlays, and onlays, and the availability of direct filling tooth-coloured alternate restoratives for certain applications (2).
Despite its widespread use and prominence as a restorative material, there have been apprehensions about the potential negative health effects of mercury exposure in amalgam (2). Mercury, like all other materials, can be dangerous if not handled correctly. To ascertain that mercury does not dissipate into the oral cavity, the alloying reaction of mercury with the Ag-Sn alloy must be completed. Once the reaction is finished, incredibly low levels of mercury, well below the existing health standard, can be released (4).
The impact of mercury obtained from amalgam to the total body load has been much debated, but it seems to be minimal. The key point to remember is that mercury gets into the body on a daily basis, irrespective of the restorative materials used in the oral cavity. Under normal physiological conditions, mercury undergoes biochemical processing and is excreted from the body. As long as the levels are low, there is no danger of mercury toxicity (4). Shortly after amalgam came into use in the United States, there were reports of mercury problems.
Materials and methods
A search of the PubMed indexed database (www.ncbi.nlm. nih.gov/pubmed) over the last 37 years (custom range: 1983 – 2020) was conducted using keywords/phrases such as “mercury-free dentistry” (yielded 23 results); “mercury-free” (195 results); “mercury toxicity” (10,862 results); “amalgam substitutes” (132 results); “amalgam mercury toxicity” (514 results); “amalgam alternatives” (455 results) and Google scholar database was conducted using phrases “mercury- free dentistry” (yielded 60,300 results); “amalgam substitutes” (31,100 results); “mercury-free dentistry era” (17,900 results); “amalgam alternatives” (71,900 results). Relevant literature on dental amalgam and its substitutes and alternatives and mercury toxicity was also searched in dental textbooks. Around 40 of these articles were deemed appropriate for inclusion. All review articles, original articles, in vivo/in vitro studies, and controlled clinical trials were considered for inclusion in this review. More emphasis was laid on studies focusing on mercury exposures among dental professionals and dental assistants and exposure to patients from mercury present in amalgam restorations. Appropriate data was collected, pooled, and finally synthesized.
Brief history of amalgam
In 1603, Tobias Dorn Kreilius created Amalgam fillings by dissolving copper sulfide with strong acids and mercury. In 1818, Louis Regnart (who is called Father of Amalgam) lowered the temperature of D’ Arcet’s Mineral Cement by adding more mercury to it. D’ Arcet cement was similar to the mixture produced by Kreilius and was earlier introduced in France. It had to be boiled and then poured into the tooth. In 1826, M. Auguste Taevaeu (5) developed the silver paste-mercury mixture. In 1833, the Crawcour duo (4), British businessmen, recognized the potential of Taevaeu’s silver-mercury mixture, brought the concept to New York, and endorsed the material as a cost-effective and easy-touse restoration material.
However, no consideration was given to the appropriate mercury-to-alloy proportions or the alloy form used. The alloy-mercury mix was created majority of the time by rubbing fragments of varying composition silver coins with a file. Slow-setting amalgams were created due to inconsistencies in materials and techniques, transferring mercury from the unset mass into exposed dentine. Although no casualties have been published, multiple incidents of pulp death occurred (4).
First amalgam war
The American Society of Dental Surgeons issued a warning about the dangers of amalgam in 1845 (6). This society declared all filling materials other than gold to be toxic, igniting a complex battle between dental professionals using gold foil restorations and those employing amalgam. Due to known toxicity of mercury, the members of this group swore to refrain from its use. The numerous discussions and assertions that erupted over dental mercury marked the beginning of an era known as the “Amalgam War.”
The American Society of Dental Surgeons had dissolved by 1856 as a result of the Amalgam War, and the American Dental Association (ADA), an organization that endorsed the usage of amalgam, became the nation’s new face. Nevertheless, concerns about dental amalgam remained. An article published in Chicago Medical Journal article in 1873 warned of “thousands of individuals all over the globe being poisoned by pernicious sublimate produced in the oral cavity from dental amalgam inserts in the teeth” (7).
According to Dr. E. Talbot’s (8) research published in the Ohio State Journal of Dental Science (1882), amalgam will generate mercury fumes. F. Flagg’s work, on the contrary, promoted the use of amalgam. In 1896, Dr. G.V Black (9) also published a comprehensive research article recommending the use of amalgam. Nonetheless, it took years for the dental profession to acknowledge Dr. Black’s insights.
Second amalgam war
Due to potentially harmful mercury release, there were periodic calls to ban the use of amalgam. Dr. J. Tuthill’s (7) study on “Mercurial necrosis caused by amalgam fillings,” which was released in The Brooklyn Medical Journal in 1898, was a watershed moment. The dental amalgam debate raged on into the early 20th century, when technological progress allowed for numerous studies to confirm that mercury poses a hazardous risk when triturated with silver alloy and filled in teeth. In 1926, Alfred Stock, a German doctorate and chemist by profession, launched the so-called Second Amalgam War. Dr. Stock was exposed to significant amounts of mercury while operating in his facility because the amalgam tablet used at that time had to be warmed up in a ladle until the crystals of mercury popped up before being moved to a mortar and pestle for trituration. The above process resulted in the emission of a large amount of mercury vapour (9). Dr. Stock’s concerns prompted the formation of an inquiry commission to look into his accusations. In its report in 1930, the commission validated the safety of newer amalgam formulations that did not require heating and were rapidly replacing older compositions (9).
Third amalgam war
Another major dispute arose in 1980, when Dr. Hal Huggins, a Colorado dentist, began spreading the notion that amalgam restorations were responsible for a variety of ailments. He authored a work in 1985 stating unequivocally that amalgam restorations emit sufficient mercury to induce neural, cardiac, autoimmune, connective tissue, psychological, and inflammatory disorders (9). According to a 1995 study, 8.7% of dental professionals sought to abolish amalgam from their practices while 14.3% were unsure about its safety (9). For nearly 10 years, a dedicated group of consumers and allied health professionals worked towards getting amalgam banned.
The media played a significant role in fuelling opposition to amalgam, particularly in its “1 Hour” section that aired on television in 1990 (9). Clinicians having a large social following, such as Dr. Robert Atkins, and Dr. Andrew Weil, wrote best-selling health books warning the masses concerning possible dangers of amalgam restorations. In response to public uproar, dental professionals, the National Institute of Health-National Institute for Dental Research (NIH-NIDR), the Food and Drug Administration (FDA), and many other prominent organizations convened a meeting with world-renowned scientists and clinicians. It was unanimously agreed that, while more research into amalgam was needed, it could not be assumed that amalgam posed a serious health risk (4). Those people strongly advised against removing amalgam restorations due to this fear.
Allegations about the dangers of amalgam keep appearing in daily papers, non-scientific magazines, and, on occasion, journals. However, all documented research indicates that no causal connection exists between dental amalgam and other medical issues.
Mercury generation potential
Mercury is ubiquitous in nature. Each year, between 2,700 x103 Kg and 6,000 x103 Kg of mercury are discharged into the atmosphere from the ocean waters and the Earth’s crust. Human activities such as the combustion of household and industrial wastes, as well as the combustion of fossils fuels, emit nearly 2,000 - 3,000 tonnes. In 2000, Asian countries contributed approximately 54% of global mercury emissions from anthropogenic sources, accounting for more than half of total production. Mercury waste is also produced during various dental procedures. With so many dental colleges and institutions in India, estimating the amount of mercury waste generated and its proper disposal is difficult. According to one study, dental offices produced approximately 4000 kg of mercury per year, with 1000 kg of mercury flowing into the region’s waste water (10).
Mercury toxicity and exposure recommendations
Mercury can be found in three different chemical forms: elemental (valence 0), inorganic (valence +1 and +2), and organic (alkyl and aryl). The physiochemical properties, absorption and excretion levels, tissue distribution patterns, as well as toxicological profile of these forms differ (2). The most volatile of the three is elemental/metallic mercury, with mercury vapour in air being its most common form. A careful examination of mercury hygiene procedures confirms that the most dangerous times are when elemental mercury is present as a liquid or vapour. As a vapor, metallic mercury is easily absorbed through lungs at 80% efficiency (Table 1). Inorganic mercury is typically quarried as an inorganic sulphide (cinnabar ore), which is burned in air to oxidize and help push off sulphur while collecting liquid mercury. There are many other water-soluble inorganic compounds of mercury which release mercury ions into solution. They are used in pharmaceuticals and are sparsely absorbed through the respiratory tract but rapidly absorbed through the digestive system (4). The organic compounds of mercury are found in food and drinking water and are particularly associated with sea food. This type of mercury is the primary source of mercury exposure for the vast majority of people. Although methyl mercury is easily absorbed from food, it is excreted less efficiently than all the other forms of mercury. This type of mercury tends to accumulate in the brain, liver, and kidney (4). The Environmental Protection Agency (EPA) estimates total daily exposure to methyl mercury (organic mercury compound) at 5.8 micrograms (ug), while Clarkson and colleagues (2) estimate 2.3 ug. The amount of elemental mercury inhaled from the air is estimated to be between 40 and 120 nanograms each day (2).
It has been proposed that microbes in the oral or digestive tract can convert metallic mercury to methyl mercury. Specific microorganisms found in seawater are often also believed to be capable of this conversion. This organic mercury then builds up in the organs of fish and other marine creatures, eventually reaching humans who consume seafood. A classic prototype of this phenomenon is Minamata disease. For many years, waste material with significant levels of metallic mercury was dumped into the ocean surrounding Minamata Bay in Japan. Fish from all these bodies of water became adulterated with methyl mercury, resulting in severe toxicosis that caused death and prolonged overdoses that ultimately led to neurological disturbances, which is currently recognized as Minamata disease. Congenital Minamata disease was another teratogenic effect. The minimum dose of methyl mercury required to develop these Minamata disease symptoms is estimated to be 5 milligrams per day (9).
The toxic effects of different mercury forms have been well recorded and studied, primarily in communities exposed to high levels of occupational or environmental mercury (Figure 1). As a result, various regulations for limiting work - related mercury exposure have been established. The National Institute for Occupational Safety and Health (NIOSH), and the Occupational Safety and Health Administration (OSHA) have adopted a threshold limit value (TLV) of 50μg mercury vapour per cubic metre of the breathing zone air for eight hours per day, 40 hours per week. The World Health Organization (WHO) has established a TLV of 25ug/m3 for work - related mercury exposure (2).
Figure 1.
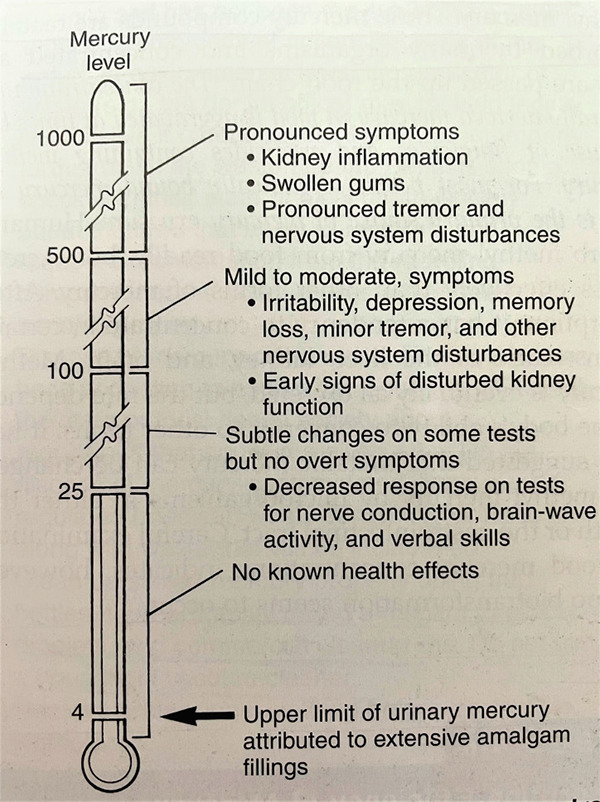
Mercury thermometer portraying different levels of mercury.
Fawer et al. (11) reported in 1983 that industrial workers (n=26) exposed to work - related mercury at a time-weighted estimate of 26ug/m3 in the place of work for a mean of 15.3 years had a substantial rise in hand tremors versus a control group. Mackert and Berglund (12) delved deeper and discovered that the hand-tremor trial was not blinded and the participants’ medical and prior records were unknown. There was also hardly any reference of any additional sources of mercury ingestion or egestion. The specimen size was limited, and no dosimetric relationship had been identified. Using Fawer and colleagues’ study as a guide, the Agency for Toxic Substances and Disease Registry established the minimum risk level (MRL) for prolonged human mercury inhalation in ambient air at 0.3ug/m3. The MRL is defined as the level of mercury vapour to which a person can be consistently subjected without suffering any health problems. The environmental protection agency also recognizes 0.3ug/m3 as the inhalation benchmark concentration for metallic mercury in air.
Dental professionals and mercury exposure from amalgam
The health hazards associated with amalgam use are definitely higher for dentists instead of patients. Dental personnel are exposed to mercury in several ways, including: • Through direct physical contact with liquid mercury / newly triturated dental amalgam • Inhaling mercury vapours (at the rate of 2-28 μg/ facet surface/ day) through - Inadvertent mercury splash (Figure 2) - Defective amalgamators - Faulty amalgam capsules (Figure 3) - Broken bulk mercury dispensers - Trituration and amalgam condensation - Polishing or removal of amalgam restorations (Figure 4) - Mercury vaporization from tainted instruments and - Open collection of amalgam scrap or used capsules with inadequate alloy to entirely consume the available mercury (Figure 5)
Figure 2.

Inadvertent mercury splash.
Figure 3.

Amalgam capsules.
Figure 4.
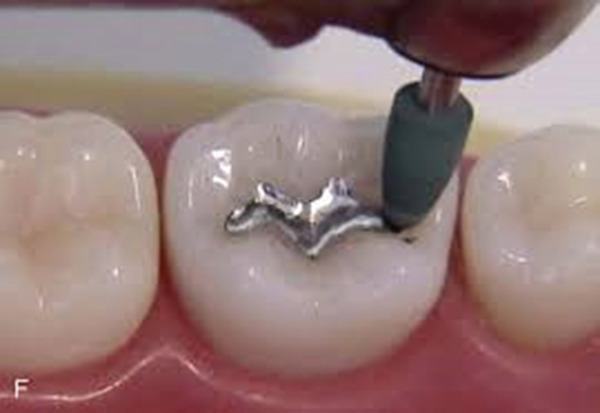
Mercury vapour released during polishing of amalgam restorations.
Figure 5.

Chair side methods of trapping amalgam.
A case of Idiopathic Thrombocytopenic Purpura (ITP) caused by vacuuming spilled mercury has been reported in the literature. Research shows that mercury exposure among dentists has been slowly declining, most likely due to the American Dental Association’s recommended mercury handling practices. Urinary mercury concentrations in dental professionals averaged 19.5ug/L in 1980, 6.7ug/L in 1986, and 4.9ug/L in 1991 (13). Decharat et al. (14) measured airborne and urinary mercury exposure among Thai dental healthcare staff and discovered that urinary mercury levels averaged 8.24±1.89ug/g creatinine (range 2.0-22.84ug/g creatinine). The majority among them had urinary levels of mercury below 20ug/g creatinine, which is the American Conference of Governmental Industrial Hygienists (ACGIH) recommendation for mercury in urine. Urinary mercury concentrations in dental caregivers exposed to unsafe working conditions reached 22.84ug/g creatinine. According to the authors, mercury exposure was found to be directly related to hygienic practices. Ferracane et al. in 1994 (2) determined that exposure to metallic mercury vapour from mercury discharges in the dental clinic survived about ten to twenty minutes in a well-ventilated operatory; and in poor ventilation, mercury vapour densities reverted to levels below NIOSH’s TLV in less than 30 minutes. The researchers indicated that mercury stayed in vapour state just for a short time due to its density and predilection for substrates, implying that a single unforeseen spill would presumably not constitute a key cause of mercury in a properly ventilated dental operatory. When proper infection-control practices were followed, inhalation of toxic vapours during amalgam restoration placement was found to be negligible. Even though substantial amounts of mercury may be produced during restorative treatment, high-volume evacuation can eliminate approximately 90% of them. Langworth et al. (1997) (15) observed that the levels of mercury generated during restorative procedures in dental clinics averaged around 2ug mercury/m3, with no adverse health effects on the personnel. When the high-volume evacuator was used, mercury vapour thresholds with in dentist’s ambient environment were minimal (1 to 2ug/m3); without it, mercury vapour thresholds were 2 to 15 times greater than the WHO TLV. According to these researchers, the mercury level fluctuated significantly during the removal process, with peaks lasting only a few seconds (2).
Nagpal et al. (16) conducted a study and found that while some professional practices enhanced the level of mercury exposure, it still was well below prescribed standards. Dentists mentioned greater medical issues than the control subjects, many of which were neurological symptoms. Initial symptoms revealed by dental practitioners may be related to low-level, prolonged occupational mercury exposure, but they could even be the result of ageing, work - related abuse, and anxiety. The study encouraged dental professionals, researchers, and educators to use good work practices.
Rowland et al. in 1994 (2) studied female dental assistants and noted that females exposed to high levels of occupational mercury vapour were less procreant than those who were not exposed. Low mercury exposed subjects, on the other hand, were more fertile than unexposed comparison group. Mocevic et al. in 2017 (17) conducted a cross-sectional study in 529 males from Greenland, Poland, and Ukraine to examine semen properties and serum concentrations of androgens in connection with ambient mercury exposure. They found no evidence that environmental mercury exposure in men with median whole blood concentrations up to 10 ng ml-1 has an adverse effect on male reproductive health biological markers.
Mercury exposure from amalgam restorations in dental patients
It has been well understood that amalgam restoration placement and removal can lead to large intraoral mercury vapour peaks. Engle and colleagues, in an in vitro study demonstrated that dry polishing of amalgam restorations released 44ug of mercury fumes for every restoration (2). In-vivo amalgam removal on the other hand, resulted in releasing 15 to 20 ug of mercury vapour from one restoration. The brief period of these inhalation exposures, however, is deemed insufficient to induce any adverse health effects and the placement and removal of dental amalgam does not seem to pose a serious health risk to patients. Sällsten et al. (18) investigated the effect of protracted repeated nicotine gum chewing on plasma and urine mercury content. Mercury levels in gum chewers were significantly higher (nearly four times) than the average reading in Swedish dental personnel, but lower than the levels that could cause harmful effects. This research furthermore highlights the importance of carefully selecting a comparison group in clinical studies when establishing standard mercury exposure levels.
Chronic mercury exposure of the patients due to mercury generated from dental amalgam post insertion has also been studied. It was long believed that hardened amalgam did not generate mercury. However, numerous researches in the 1970s and 1980s found it otherwise (19). Early estimates of the average daily dose in people who have not been exposed to mercury at work ranged from 1.24 to 1.27ug/day, but latest evidence indicates a comparatively lower mercury dose from amalgam (2). According to Halbach in 1995 (20), mercury release was proportional to restoration time and surface area.
The possible implications of mercury discharge from amalgam on pregnant females’ foetuses and new-borns have been studied. The findings revealed that the mercury content in foetal and infant liver, kidney, and brain samples correlated positively with said dental amalgam restorations in mothers. However, the study design and data analysis method are highly dubious. A variety of methods can be used to estimate the average diurnal amount of mercury released from set amalgam restorations. Several variables, such as the number and age of restorations, the form of dental amalgam used, the surface area of the restoration, the quality of the restoration, methods of measuring mercury, and data analysis, all may be responsible for reported differences in results from different studies. Certain foods have been demonstrated to lower intraoral mercury vapour levels. If all masticatory period is assumed to increase vapour levels, then everyday mercury exposure from amalgam is overstated (2).
Mercury hypersensitivity
Mercury hypersensitivity is a response of the immune system to extremely low levels of mercury (4).Though poorly understood; it has been at times claimed as a potential hazard. However, the percentage of people who have been identified as plausibly hypersensitive is small, and the sensitivity reaction is mild and not serious. Mackert et al. (4) and Mandel et al. (4) studied this condition and scientifically disproved the hypothesised problems.
Amalgam illness
Amalgam illness refers to a condition that is typically self-reported by patients and is attributed to mercury vapour inhalation from established amalgam restorations (19). Symptoms differ from those seen with traditional mercury poisoning and include lethargy, poor concentration, muscle aches, and immunologic disorders. There is a lot of uncertainty about the establishment of amalgam illness because of absence of diagnostic symptoms, as well as any approved clinical test for its detection. This is complicated further by the fact that several mental illnesses demonstrate symptoms identical to ones caused by amalgam restorations. According to two surveys, 70% of sufferers asserting amalgam illness had a psychiatric condition, in contrast to 14% in a comparison group.
National and international events
The discussion over dental amalgam extends well beyond a single country’s borders; in fact, countries all over the world are dealing with the same issues (21).
Denmark’s regulation on mercury-containing products
According to a document released by an ad hoc working group of the European Commission (EC), the Environment Ministry released an order (No. 520) on June 9, 1994, prohibiting the trade of all mercury-containing commodities, including dental amalgam, to improve national climatic conditions. It was emphasized that the prohibition action will not go into effect unless there is a sufficient amount of clinically acceptable alternative restorative materials (non-amalgam substitutes) available.
Mercury regulations in Sweden
Barring isolated incidents of allergic reactions, there is no research evidence that mercury in dental amalgam has a negative impact on the body. According to the advisory committee, there is no medical basis to recommend amalgam removal to alleviate symptoms of general ailment.
Amalgam alerts in Germany
The German Ministry of Health issued a consensus statement on July 1, 1997, prohibiting any placement or removal of amalgam fillings from pregnant women’s mouths.
Amalgam Advisory issued in Norway
The Norwegian Health Board has advised pregnant women to avoid “extensive amalgam therapy.”
European Commission submit on amalgam
There is currently no evidence that mercury from dental amalgam poses an undesirable health threat to the wider public. Moreover, there is an insufficient data to suggest that clinically acceptable dental amalgam restorations should be removed unless a confirmed allergy to this material is present.
Regulation by the US Food and Drug Administration (FDA)
Encapsulated dental amalgam was classified as a Class II medical device by the US FDA in 2009. Encapsulated amalgam was previously not classified, and dental mercury (Class I) and alloy (Class II) were each given their own classifications. Amalgam is classified in the same category as the majority of other restorative materials, including composite and direct gold restorations, under this regulation. Furthermore, the FDA reaffirms that it is a reliable and practical restorative option for patients.
The Minamata convention, a historical change
The Minamata Convention on Mercury is a world treaty aimed at protecting both people and the environment from mercury’s harmful consequences (22, 23). The Intergovernmental Negotiating Committee on Mercury held its fifth session in Geneva, Switzerland, on January 19, 2013. The Minamata Agreement took effect on August 16, 2017 (24).
The following are the key points of the Minamata Convention to curtail the usage of dental amalgam: • Establishing national objectives for dental caries prevention and oral health advancement, thus reducing the requirement for dental restoration. • Fostering research and development of high-quality non-mercury dental restoration products (25).
The World Health Organization issued a report titled Future Use of Materials for Dental Restorations in 2009, advising that: • Moving away from dental amalgams was contingent on adequate quality of alternative cost- effective dental restorative materials. • Since current restorative options are not long-lasting, it would be appropriate to reduce rather than eliminate the use of dental amalgam during this time • Encourage professional groups and dental colleges to sensitise practitioners and students about mercury- free restoration options, as well as to promote Best Management Practices (BMP). BMPs are a set of protocols for controlling as well as disposing of amalgam waste that includes, but are not limited to: - Use of ISO 1114 compliant amalgam separators (Figure 5) - Launching large-scale mercury recovery initiatives - Making use of chair side traps (Figure 5) - Vacuum retrieval - Examining and cleaning traps - Recyclability - Discarding of the collected amalgam by a commercial waste disposal provider • Opposing health coverage policies and measures that favour dental amalgam over mercury-free dental restoration. • Promoting medical coverage initiatives that encourage the adoption of high-quality dental amalgam substitutes for dental restoration. • Using only encapsulated dental amalgam. • Encouraging dental services to employ best environmental measures to minimize mercury and mercury compound releases into water and soil (Figure 6) (21).
Figure 6.

IISD-ELA: Promoting best environmental practices.
Best Environmental Practices (IISD-ELA)
The IISD Experimental Lakes Area (Figure 6) is a natural laboratory in Ontario, Canada, consisting of 58 small lakes and their watersheds reserved for scientific research. The International Institute for Sustainable Development (IISD) manages and operates the facility, which has a mandate to investigate the aquatic effects of a wide range of stresses on lakes and their catchments. According to IISD-ELA research, ecosystems can recover quickly from mercury poisoning. We simply need to reduce the amount of mercury entering that ecosystem.
Amalgam alternatives and substitutes
Amalgam alternatives are any materials that can be used to restore a tooth instead of amalgam (e.g., composite, glass-ionomer, gold, cast gold alloys, and ceramics) (4). Amalgam substitutes (e.g., cast gold alloys) are materials that are believed to have properties similar to or superior than the amalgam restoration they are replacing (4, 26). Each of these materials has relative advantages, disadvantages, and costs associated with its use. Gold is an inert metal, but it is not generally regarded as aesthetically pleasing. It is also costly. Composites and glass ionomers though aesthetic; lack the strength, durability and longevity of amalgam. Hence, they are unsuitable for large restorations. Furthermore, studies have suggested that Bisphenol A, which is present in composites, may pose a health threat. It has been identified as an oestrogen mimicker and has been linked to male infertility, as well as prostate and breast cancer (27, 28). European Commission’s Scientific Committee concluded in May 2008 that dental amalgams are an efficacious and useful option for both the patients and dental professionals, and that the alternative materials have clinical limitations and toxicological risks (29). Cast/ Indirect restorations, though superior in strength and in establishing contours and contacts, are much more technique sensitive, time consuming and costlier than amalgam restorations.
A few compositions contain few amalgam constituents (e.g., Ag-Sn alloy particles) but no mercury (30). Gallium alloys made with Ag-Sn particles in Gallium-Indium (Ga- In). Gallium melts at 28°C and when combined with Indium, can create molten alloys at ambient temperature. In amalgam, Ga-In has been used in place of mercury. Other systems are being researched in which gold is combined with certain other noble metals to create the restoration structure (4). These systems seem to be extremely expensive, and little is known about their performance.The American Dental Association patented a mercury-free direct-filling alloy based on mercury-coated Ag-Sn particles that can be self-welded by compaction to create a restoration in collaboration with the National Institute of Standards and Technology. This method has been suggested as a replacement for amalgam, but is to be commercialised (4). If alloy particle sizes are carefully selected to load together well, the amount of mercury necessary for mixing can be limited to the 15% to 25% range. The clinical properties of these low-mercury amalgams are not clear (4).
Table 1.
Absorption efficiency of mercury.
| Skin | Lungs | Gastrointestinal tract | |
|---|---|---|---|
| Elemental | _ | 80% | 0.01% |
| Inorganic | _ | 80% | 7% |
| Organic | _ | _ | 95-98% |
Conclusion
The road towards mercury free dentistry is long and arduous and we need to tread carefully on it. Despite the fact that the enormous amount of evidence indicates amalgam to be a reliable and effective restorative material, recent publications and the Minamata Convention have put a halt to all discussions. The debate is not about whether mercury released from various sources is toxic or not or whether dental amalgam should remain the restorative material of choice or not. The consensus is now clear, but the real challenge is to find a suitable alternative restorative material that either matches amalgam in its physical properties or is superior to it. Till then, amalgam remains the undisputed king of all restorative materials. However, the point that needs consideration is that until the time amalgam is being used or even being phased-down, proper mercury hygiene measures should be employed by the entire dental office team.
Footnotes
Ethics committee approval: Not required.
Informed consent:Not required.
Peer review: Externally peer-reviewed.
Author contributions: PD, KKD, SG, HS participated in designing the study. PD, KKD, HS participated in generating the data for the study. PD, ZJ participated in gathering the data for the study. PD wrote the majority of the original draft of the paper. KKD, HS participated in writing the paper. PD has had access to all of the raw data of the study. PD has reviewed the pertinent raw data on which the results and conclusions of this study are based. PD, KKD, SG, HS, ZJ have approved the final version of this paper. PD guarantees that all individuals who meet the Journal’s authorship criteria are included as authors of this paper.
Conflict of interest:The authors declared that they have no conflict of interest.
Financial disclosure:The authors declared that this study received no financial support.
References
- 1.Phillips RW, Skinner EW. Skinner’s science of dental materials. 11th ed. Philadelphia: Saunders; 1991. p. 496. [Google Scholar]
- 2.Dental amalgam: update on safety concerns. ADA council on Scientific Affairs. J Am Dent Assoc. 1998. Apr;129(4):494–503. 10.14219/jada.archive.1998.0252 [DOI] [PubMed] [Google Scholar]
- 3.U.S. Public Health Service, Committee to Coordinate Environmental Health and Related Programs . Dental amalgam: A scientific review and recommended public health service strategy for research, education and regulation (Final report of the Subcommittee on Risk Management. Washington, D.C.:U.S. Government Printing Office, 1993; PHS publication no. 342-322/60025). [Google Scholar]
- 4.Sturdevant. Biomaterials. In: Roberson TM, Heymann HO, Swift EJ, editors. Art and Science of Operative Dentistry. 5th Ed., India: Mosby; Reed Elsevier India Pvt. Ltd., 2006, p. 135-242.
- 5.Mahalaxmi S. Dental Amalgam. In: Mahalaxmi S, editor. Materials Used in Dentistry. 1st ed. India: Wolters Kluwer Health (India); 2013. pp. 195–221. [Google Scholar]
- 6.Hyson JM Jr. Amalgam: its history and perils. J Calif Dent Assoc. 2006. Mar;34(3):215–29. 10.1080/19424396.2006.12222190 [DOI] [PubMed] [Google Scholar]
- 7.Tuthill JY. Mercurial necrosis resulting from amalgam fillings. The Brooklyn Medical Journal. 1898;XII:725–42. [PMC free article] [PubMed] [Google Scholar]
- 8.Talbot ES. The chemistry and physiological action of mercury as used in amalgam fillings. The Ohio State J Dent Sci. 1882;2:1–12. [PMC free article] [PubMed] [Google Scholar]
- 9.Dodes JE. The amalgam controversy. An evidence-based analysis. J Am Dent Assoc. 2001. Mar;132(3):348–56. 10.14219/jada.archive.2001.0178 [DOI] [PubMed] [Google Scholar]
- 10.Soni R, Bhatnagar A, Vivek R, Singh R, Chaturvedi TP, Singh A. A systematic review on mercury toxicity from dental amalgam fillings and its management strategies. J Sci Res. 2012;56:81–92. [Google Scholar]
- 11.Fawer RF, de Ribaupierre Y, Guillemin MP, Berode M, Lob M. Measurement of hand tremor induced by industrial exposure to metallic mercury. Br J Ind Med. 1983. May;40(2):204–8. 10.1136/oem.40.2.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mackert JR Jr, Berglund A. Mercury exposure from dental amalgam fillings: absorbed dose and the potential for adverse health effects. Crit Rev Oral Biol Med. 1997;8(4):410–36. 10.1177/10454411970080040401 [DOI] [PubMed] [Google Scholar]
- 13.Bernhoft RA. Mercury toxicity and treatment: a review of the literature. J Environ Public Health. 2012;2012:460508. 10.1155/2012/460508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Decharat S, Phethuayluk P, Maneelok S, Thepaksorn P. Determination of Mercury Exposure among Dental Health Workers in Nakhon Si Thammarat Province, Thailand. J Toxicol. 2014;2014:401012. 10.1155/2014/401012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langworth S, Sällsten G, Barregård L, Cynkier I, Lind ML, Söderman E. Exposure to mercury vapor and impact on health in the dental profession in Sweden. J Dent Res. 1997. Jul;76(7):1397–404. 10.1177/00220345970760071001 [DOI] [PubMed] [Google Scholar]
- 16.Nagpal N, Bettiol SS, Isham A, Hoang H, Crocombe LA. A Review of Mercury Exposure and Health of Dental Personnel. Saf Health Work. 2017. Mar;8(1):1–10. 10.1016/j.shaw.2016.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mocevic E, Specht IO, Marott JL, Giwercman A, Jönsson BA, Toft G, et al. Environmental mercury exposure, semen quality and reproductive hormones in Greenlandic Inuit and European men: a cross-sectional study. Asian J Androl. 2013. Jan;15(1):97–104. 10.1038/aja.2012.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sällsten G, Thorén J, Barregård L, Schütz A, Skarping G. Long-term use of nicotine chewing gum and mercury exposure from dental amalgam fillings. J Dent Res. 1996. Jan;75(1):594–8. 10.1177/00220345960750011301 [DOI] [PubMed] [Google Scholar]
- 19.Roberts HW, Charlton DG. The release of mercury from amalgam restorations and its health effects: a review. Oper Dent. 2009;34(5):605–14. 10.2341/08-072-LIT [DOI] [PubMed] [Google Scholar]
- 20.Halbach S. Combined estimation of mercury species released from amalgam. J Dent Res. 1995. Apr;74(4):1103–9. 10.1177/00220345950740041101 [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization . Petersen, Poul Erik, Baez, Ramon, Kwan, Stella & Ogawa, Hiroshi. (2010). Future use of materials for dental restoration: report of the meeting convened at WHO HQ, Geneva, Switzerland 16th to 17th November 2009. World Health Organization. https://apps.who.int/iris/handle/10665/202500
- 22.Wikipedia contributors. Minamata Convention on Mercury. Wikipedia, The Free Encyclopedia. December 29, 2020, 22:42 UTC. Available at: https://en.wikipedia.org/w/index.php?title=Minamata_Convention_on_Mercury&oldid=997082190. Accessed January 8, 2021.
- 23.Yang L, Zhang Y, Wang F, Luo Z, Guo S, Strähle U. Toxicity of mercury: molecular evidence. Chemosphere. 2020. Apr;245:125586. 10.1016/j.chemosphere.2019.125586 [DOI] [PubMed] [Google Scholar]
- 24.Balaji SM. Mercury, dentistry, minamata convention and research opportunities. Indian J Dent Res. 2019;30(6):819. 10.4103/ijdr.IJDR_924_19 [DOI] [PubMed] [Google Scholar]
- 25.Lutz F, Krejci I. Amalgam substitutes: a critical analysis. J Esthet Dent. 2000;12(3):146–59. 10.1111/j.1708-8240.2000.tb00214.x [DOI] [PubMed] [Google Scholar]
- 26."British Dental Health Foundation Policy Statement Dental Amalgam" (PDF). Dentalhealth.org. Archived from the original (PDF) on 2015-January-13. Retrieved 2015-06-13.
- 27.Wahl MJ. Amalgam—resurrection and redemption. Part 2: the medical mythology of anti-amalgam. Quintessence Int. 2001. Oct;32(9):696–710. [PubMed] [Google Scholar]
- 28.SCENIHR (Scientific Committee on Emerging and Newly-Identified Health Risks) , Scientific opinion on the Safety of Dental Amalgam and Alternative Dental Restoration Materials for Patients and Users (update), 29 April 2015.
- 29.Yousefi H. Replacing dental amalgam by mercury-free restorative materials; it’s time to take action. Daru. 2018. Sep;26(1):1–3. 10.1007/s40199-018-0212-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fisher J, Varenne B, Narvaez D, Vickers C. The Minamata Convention and the phase down of dental amalgam. Bull World Health Organ. 2018. Jun;96(6):436–8. 10.2471/BLT.17.203141 [DOI] [PMC free article] [PubMed] [Google Scholar]


