Abstract
Nearly half of new HIV cases in the United States are among youth. Little is known about the willingness of young adults living with HIV (YLWH) to participate in HIV cure-related research. In 2021, we recruited 271 YLWH aged 18–29 for an online survey. We asked questions about willingness to participate in HIV cure research, perceived risks and benefits, acceptable trade-offs, and perceptions on analytical treatment interruptions. We conducted descriptive analyses to summarize data and bivariate analyses to explore correlations by demographics. Most respondents (mean age = 26) identified as men (86%) and Black Americans (69%). YLWH expressed high willingness to consider participating in cell- and gene-based approaches (75%) and immune-based approaches (71%). Approximately 45% would be willing to let their viral load become detectable for a period of time during an HIV cure study, 27% would not be willing, and 28% did not know. The social risk most likely to deter participation was the possibility of transmitting HIV to sex partners while off HIV medications (65% of respondents would be deterred a great deal or a lot). Compared to the 25–29 age group (n = 192), the 18–24 age group (n = 79) was more likely to indicate that having to disclose HIV status would matter a great deal in considering participation in HIV cure research (38% vs. 21%, p = .003). Inclusion and engagement of YLWH are critical for advancing novel HIV curative agents. Our article concludes with possible considerations for engaging YLWH in HIV cure research. Physical, clinical, and social risks will need to be kept to a minimum, and research teams will need to proactively mitigate the possibility of transmitting HIV to sex partners while off HIV medications.
Keywords: young adults, HIV, HIV cure research, sociobehavioral sciences, racial and ethnic minorities, United States
Introduction
Current HIV therapies limit active viral replication but do not purge and eliminate the latent reservoir, a pool of cells that store transcriptionally inactive (latent) yet replication-competent HIV proviruses.1 These latent proviruses can spontaneously emerge and support viral replication if antiretroviral therapy (ART) is interrupted. In HIV cure-related research, the goal is to identify strategies that would either completely eliminate HIV from the body (cure) or permanently suppress the virus in the absence of ART (remission) (hereafter referred to as HIV cure research).2 However, most HIV cure studies are in the early stages of development3 and carry risks to research participants that must be considered.
One notable feature of HIV cure research is the need for participants to interrupt ART—called analytical treatment interruptions (ATIs)4—to test the efficacy of cure strategies that would replace daily oral ART and induce long-term HIV suppression or remission.4 ATIs may pose risks to participants, such as viral resistance and disease progression.5,6 ATIs may also pose risks to sex partners of participants, who may be at risk of acquiring HIV due to exposure to potentially prolonged periods of viremia.7,8 Yet, these viremic periods currently are the only way to evaluate the efficacy of a potential HIV curative strategy,9 and the research community must constantly monitor risks of ATIs to ensure safety of this approach.10,11
In the United States, Europe, and Australia, sociobehavioral sciences on HIV cure research has predominantly been conducted among older White men.12–19 Studies in Thailand,20–22 China,23–25 and Brazil26 have enrolled more diverse demographics and younger populations. Few studies, however, have investigated the motivations, deterrents, perceptions, and experiences of diverse young persons living with HIV (PLWH) related to HIV cure research.
In 2018, in the United States, young adults between the ages of 25 and 29 and 20 and 24 carried the first and second highest rates, respectively, of new HIV infections.27 Most of these cases were among gay, bisexual, and other men in same-gender loving relationships and Black American and Latinx men.28 However, current HIV cure research participants do not reflect demographically young adults living with HIV (YLWH), particularly from groups historically underrepresented in research.29,30 Given that YLWH represent nearly half of all newly reported HIV cases in the United States,28 are less likely to be retained in care and achieve virologic suppression compared to older PLWH,31 and likely will be the group most directly affected by scientific advancements in HIV therapies in the coming years,32 it is critical to understand their perceptions toward HIV cure research.
YLWH may perceive the personal, clinical, and social risks and benefits of HIV cure research differently from older populations of PLWH.32 In this study, we explored potential deterrents and motivators of HIV cure research participation among YLWH in the United States. The main objectives were to comprehensively understand the willingness of demographically YLWH to accept potential risks when engaging in HIV cure studies and how YLWH perceive risks and benefits surrounding these studies.
Materials and Methods
Study design
We conducted a nationwide online survey among YLWH (18–29 years old) to quantify their willingness, motivators, and deterrents to participating in HIV cure research and their perceptions about undergoing ATIs. All participants provided consent, and the study was approved by the University of California, San Francisco Institutional Review Board (study #20-29992 approved on February 1, 2021).
Recruitment
Between April and August 2021, we recruited participants using social media posts (on Twitter and Facebook), paid ads on mobile dating apps (e.g., Jack'd), and through organizations and clinics that serve YLWH. Potential participants completed the online screening survey, called, or sent a text message to the study mobile phone and were individually screened by the study coordinator. Eligible participants were 18–29 years old, living in the United States, living with HIV, able to complete the study survey in English, and willing to give consent. Participant age was verified with a survey-uploaded or text-messaged photo of their identification card showing their name and date of birth. HIV status was verified with a photo of the participant's antiretroviral medication bottle or laboratory report or healthcare provider letter showing their name and HIV status or HIV viral load.33,34
Eligible participants were sent consent information and a unique individual link to complete the online survey by text or e-mail. The survey was programmed using Qualtrics software (Provo, UT). Skip logic was programmed to allow participants to skip certain questions that were not applicable after responding to a preceding question in a certain way. Upon completion of the survey, participants received $40 through cash transfer mobile app or e-gift card, based on their preference.
Procedures and measures
Demographics and socioeconomic status
Demographic variables included participant age, sex assigned at birth and current gender identity, race and ethnicity, sexual orientation, and state of residence. Socioeconomic status items included educational attainment, employment status, perceived financial situation (I have enough money to live comfortably; I can barely get by on the money I have; I cannot get by on the money I have), current living situation, history of homelessness, and current health insurance status.
Willingness to consider participation in HIV cure research
Participants were asked whether they would consider participating in different types of current HIV cure studies (e.g., using cell and gene therapy, immune-based approaches, latency reversing agents), procedures (e.g., basic blood draw studies, hair donations), as well as HIV cure social science studies (e.g., interviews or focus groups).
To convey HIV cure research strategies and HIV cure mechanisms, we used comic-style illustrations prepared by professional graphic artists and illustrators (35). The illustrations included: latency reversing agents, which we described as “a type of study that would reactivate HIV inside your cells so that your immune system can identify and fight the virus”; immune-based approaches, which we described as “a type of study that would help strengthen your immune system's ability to fight HIV”; and cell and gene therapies, which we described as “a type of study that would make your cells better able to fight HIV.” Response options for all questions around willingness to consider participation were: “Yes, I am participating now or have done so in the past”; “Yes, in the future”; “No, I would not”; or “I don't know.”
Perceived benefits and risks of HIV cure research participation
Participants were asked to choose the extent to which potential benefits, risks, and burdens would affect their consideration of participating in HIV cure research.12,13,36 Survey questions included perceived social benefits (e.g., helping find a cure for HIV, helping other people living with HIV), personal benefits (e.g., feeling good about contributing to HIV cure research, gaining knowledge about HIV), and personal clinical benefits (e.g., preserving immune system's ability to fight HIV, controlling viral load in the absence of treatment).
Potential barriers included physical risks with a high chance of happening (e.g., virus going up unexpectedly, stomach discomfort), physical risks with a small chance of happening (e.g., developing dementia, developing drug resistance to HIV treatment, illness caused by overactive immune system), other personal risks and burdens (e.g., time commitments, study visit commitments, need for invasive procedures, potential side effects), social risks (e.g., risk of transmitting HIV to sex partners, being treated poorly by study staff, discrimination, and stigma), perceived burdens (e.g., transportation issues, time away from work or school), and psychosocial and/or emotional risks (e.g., anxiety). Response options included: a great deal, a lot, a moderate amount, a little, not at all, and I don't know.
HIV cure research scenarios and trade-offs
Participants were asked to consider making trade-offs13 in being willing to accept a new HIV cure strategy if it meant that they would no longer have to take daily pills (“Yes, I would accept that tradeoff”; “No, I would not accept that tradeoff”). Potential trade-offs included: (1) having to go to the doctor more often, (2) an increased chance of transmitting HIV to a partner, (3) initial but temporary moderate side effects (such as itchy rash, blurred vision), (4) a chance of developing other health problems years later, (5) no increase in years they live, (6) no improvement in quality of life, and (7) causing mental side effects (such as anxiety or depression).
Additional considerations and logistical factors
Participants were also asked to indicate the importance of the following variables on their decision to participate in HIV cure research: (1) the race and ethnicity of research staff and other study participants, (2) staff trained in working with young populations, (3) language proficiency of research staff, (4) assistance with transportation, (5) flexible research hours, (6) incentives (e.g., cash, gift cards), (7) study being conducted at a university known to lead HIV cure research, and (8) the amount of background research done on HIV cure.
Perceptions of ATIs and partner protection measures
Participants were asked to indicate whether they ever heard of the expression U = U – meaning that PLWH who are undetectable for HIV cannot sexually transmit the virus and whether they would be willing to allow their HIV viral load to become detectable for any period of time during an HIV cure study. Those who indicated that they would be willing were asked to choose the longest period they would be prepared to have a detectable viral load. Finally, we asked participants to choose the reason(s) they would want to end the ATI and restart ART. These included decline in CD4 cell count, being asked by HIV care provider to start ART, researchers letting them know they are detectable for HIV, and being able to transmit HIV.
Because ATIs have the potential to increase the risk of HIV transmission to sex partners,9,37 participants indicated how important various partner protections would be, including: providing pre-exposure prophylaxis (PrEP) and postexposure prophylaxis (PEP) medications and/or service referral, condoms, and HIV testing and counseling for partner(s); and assistance with HIV disclosure to partner(s).
Statistical analyses
We conducted descriptive analyses to summarize the results. We report the number of analyzable responses (denominator) for each question. In addition, we conducted bivariate analyses to explore whether gender identity, age groups, racial identity, region of residency, and financial status were individually statistically significantly correlated with the above dependent variables (e.g., willingness to consider participation in HIV cure research, perceived benefits and risks of HIV cure research participation, HIV cure research scenarios and trade-offs, additional considerations and logistical factors, and perceptions of ATIs and partner protection measures). We reported p values, which were calculated using Chi-squared tests for categorical comparisons, or Fisher's exact tests if the sample within a cell was five or fewer.
To determine if multivariate analyses were possible, we tested the correlations between the five demographic and socioeconomic variables. We found that gender identity, age groups, and regions of residency were statistically significantly correlated with one another. Racial identity was also correlated with region of residency. Financial status was not correlated with any of the demographic variables. To control for the effects of confounding variables, multivariate analyses were used to test each of the correlations that were determined to be statistically significant in the bivariate analyses by controlling for each of the other demographic variables that are correlated with the key demographic variable in the analysis. When a statistically significant bivariate correlation becomes insignificant after controlling for another correlated demographic variable, the change in significance was noted in the Results. Due to the small number of participants in particular regions of residency, racial identity, gender identity, and age groups, a comprehensive multivariate analysis controlling for multiple variables was not possible due to lack of variability.
Cisgender men were defined as participants who selected male to identify their sex and gender. For bivariate analyses, gender identity options included participants who only selected man or male to identify their gender versus all others. We categorized participants into two age groups: 18–24 and 25–29 years (age 25 represents the cut-off at which YLWH are transferred to adult services).38
Due to the racial composition of the sample, participants were categorized into three mutually exclusive racial identity categories: Hispanic or Latinx; Black American and non-Latinx; and one or more other non-Latinx ethnicity (including multiracial non-Latinx). Regions of residency were South, West, Midwest, and Northeast. Financial status was indicated by one of three response options: cannot get by on the money they have; can barely get by; and have enough money to live comfortably. Participants who indicated that they could not get by on the money they had were categorized as financially constrained. All bivariate analyses were conducted using Stata (version 11). All “Don't Know” and “Prefer not to answer” responses were not included in the bivariate analyses.
Results
Survey respondents
There were 271 respondents, including 14 who partially completed the questionnaire. Most identified as men (86%) and Black Americans/non-Latinx (69%). The mean age of respondents was 26 years old, and 59% had some college education. Respondents were recruited from across the United States, with 49% from the South. Of total participants, over half (52%) were employed, 22% were financially constrained, 55% were barely getting by on the money that they had, and 54% reported having ever been homeless (Table 1).
Table 1.
Demographic Characteristics of Survey Respondents (United States, 2021)
| All ages n (%) | 18–24 years Age group n (%) | 25–29 years Age group n (%) | |
|---|---|---|---|
| Gender (select all that apply) | n = 271 | n = 79 | n = 192 |
| Man | 233 (86) | 63 (80) | 170 (89) |
| Woman | 15 (6) | 7 (9) | 8 (4) |
| Gender non-binary | 16 (6) | 6 (8) | 10 (5) |
| Transgender woman | 11 (4) | 5 (6) | 6 (3) |
| Other, transgender man, genderqueer, questioning, prefer not to answer | 10 (4) | 0 (0) | 10 (5) |
| Sex assigned at birth | n = 271 | n = 79 | n = 192 |
| Male | 254 (94) | 72 (91) | 182 (95) |
| Female | 14 (5) | 7 (9) | 7 (4) |
| Other/prefer not to answer | 3 (1) | 0 (0) | 3 (2) |
| Sexual orientation (select all that apply) | n = 271 | n = 79 | n = 192 |
| Gay | 208 (77) | 60 (76) | 148 (77) |
| Bisexual | 51 (19) | 13 (16) | 38 (20) |
| Straight | 16 (6) | 7 (9) | 9 (5) |
| Other, lesbian, prefer not to answer | 17 (6) | 6 (8) | 11 (6) |
| Region of residency | n = 271 | n = 79 | n = 192 |
| Northeast | 26 (10) | 8 (10) | 18 (9) |
| Midwest | 46 (17) | 21 (27) | 25 (13) |
| South | 134 (49) | 40 (51) | 94 (49) |
| West | 65 (24) | 10 (13) | 55 (29) |
| Racial identity (select all that apply) | n = 271 | n = 79 | n = 192 |
| Black American non-Latinx | 187 (69) | 60 (76) | 127 (66) |
| Latinx | 48 (18) | 10 (13) | 38 (20) |
| White non-Latinx | 27 (10) | 6 (8) | 21 (11) |
| Asian non-Latinx | 9 (3) | 1 (1) | 8 (4) |
| Other non-Latinx, American Indian non-Latinx, Native Hawaiian non-Latinx, Prefer not to answer | 20 (7) | 8 (10) | 12 (6) |
| Age | n = 271 | n = 79 | n = 192 |
| Mean (years) | 26 | 22 | 27 |
| Standard Deviation (years) | 2.8 | 1.7 | 1.4 |
| Age groups (years) | n = 271 | n = 79 | n = 192 |
| 18–20 | 13 (5) | 13 (16) | 0 (0) |
| 21–23 | 46 (17) | 46 (58) | 0 (0) |
| 24–26 | 77 (28) | 20 (25) | 57 (30) |
| 27–29 | 135 (50) | 0 (0) | 135 (70) |
| Highest level of education completed | n = 271 | n = 79 | n = 192 |
| Did not complete high school or G.E.D. | 16 (6) | 8 (10) | 8 (4) |
| Completed high school or G.E.D. | 95 (35) | 33 (42) | 62 (32) |
| Some college, less than a bachelor's degree | 102 (38) | 28 (35) | 74 (39) |
| Bachelor's degree or higher | 58 (21) | 10 (13) | 48 (25) |
| Current work situation | n = 271 | n = 79 | n = 192 |
| Employed | 142 (52) | 38 (48) | 104 (54) |
| Unemployed or laid off | 91 (34) | 31 (39) | 60 (31) |
| Disabled or sick leave | 17 (6) | 3 (4) | 14 (7) |
| Student | 13 (5) | 5 (6) | 8 (4) |
| Other/prefer not to answer | 8 (3) | 2 (3) | 6 (3) |
| Financial situation | n = 271 | n = 79 | n = 192 |
| I have enough money to live comfortably | 56 (21) | 13 (16) | 43 (22) |
| I can barely get by on the money I have | 149 (55) | 42 (53) | 107 (56) |
| I cannot get by on the money I have (financially constrained) | 60 (22) | 22 (28) | 38 (20) |
| Prefer not to answer | 6 (2) | 2 (3) | 4 (2) |
| No. of times had ever been in jail or prison | n = 271 | n = 79 | n = 192 |
| None | 186 (69) | 58 (73) | 128 (67) |
| Once | 34 (13) | 9 (11) | 25 (13) |
| More than once | 47 (17) | 10 (13) | 37 (19) |
| Prefer not to answer | 4 (1) | 2 (3) | 2 (1) |
| Current health insurance situation | n = 271 | n = 79 | n = 192 |
| Public plan | 139 (51) | 37 (47) | 102 (53) |
| Own, student, or spouse insurance plan | 77 (28) | 16 (20) | 61 (32) |
| Parent's insurance plan | 16 (6) | 13 (16) | 3 (2) |
| None/no insurance | 35 (13) | 11 (14) | 24 (13) |
| Other/Prefer not to answer | 4 (1) | 2 (3) | 2 (1) |
| Current living situation (select all that apply) | n = 271 | n = 79 | n = 192 |
| Own or rent housing | 162 (60) | 44 (56) | 118 (61) |
| Parent's housing | 51 (19) | 17 (22) | 34 (18) |
| Someone else's housing | 43 (16) | 11 (14) | 32 (17) |
| Boarding or welfare housing | 16 (6) | 6 (8) | 10 (5) |
| Living outside | 13 (5) | 6 (8) | 7 (4) |
| Other/prefer not to answer | 2 (1) | 0 (0) | 2 (1) |
| Was ever homeless | n = 271 | n = 79 | n = 192 |
| Yes | 145 (54) | 38 (48) | 107 (56) |
| No | 124 (46) | 40 (51) | 84 (44) |
| Prefer not to answer | 2 (1) | 1 (1) | 1 (1) |
Willingness to consider participation in HIV cure research
Figure 1 shows the preference hierarchy of seven different types of HIV cure-related studies or procedures for which 268 respondents indicated whether they might consider joining or in which they had previously participated. Respondents reported high willingness to consider participating in basic blood draw studies (74%) and hair donation studies (68%). In addition, YLWH expressed high willingness to consider participating in cell- and gene-based approaches (75%) and immune-based approaches (71%), based on the illustrations provided.38
FIG. 1.
Willingness to consider participating in different types of HIV cure studies (n = 268 YLWH in the United States).
Perceived benefits and risks of HIV cure research participation
Figure 2 shows the degree to which perceived clinical, social, or personal benefits would motivate participation in HIV cure research (n = 266–267). The most prevalent perceived motivators and benefits (combining “a lot” and “a great deal”) included the ability to help one's immune system fight HIV (88%), feeling they are helping future generations (84%), controlling HIV without the need for HIV medications (82%), hoping HIV disease will improve (82%), and feeling they are contributing to research (82%).
FIG. 2.
Degree to which factors would affect respondents' willingness to participate in HIV cure research (n = 266 – 267 YLWH in the United States).
Figure 3 shows the degree to which perceived physical health risks, social risks, and burdens would deter participation in HIV cure research (n = 262–266). Physical health risks that would most deter potential participation (combining “a lot” and “a great deal” responses) were those that were quite rare. These included the following: dementia (68%), developing drug resistance to HIV treatment (64%), illnesses caused by overactive immune system (63%), problems with bones or muscles (58%), or severe side effects such as allergic reactions or trouble breathing (58%). Furthermore, some physical health risks with a high chance of occurring would deter participation a lot or a great deal, including virus levels going up unexpectedly (59%), stomach discomfort (45%), and physical pain/discomfort (33%). Social risks most likely to deter participants were the possibility of transmitting HIV to sex partners while off HIV medications (65%), being treated poorly by study staff (65%), and financial risks (62%). In general, respondents appeared less deterred by potential participation burdens, such as having to take time off work or school.
FIG. 3.
Degree by which factors might prevent respondents from participating in HIV cure research (n = 262 – 266 YLWH in the United States).
HIV cure research scenarios and trade-offs
We asked respondents to indicate acceptability for various HIV cure scenarios and trade-offs compared with taking oral daily ART pills. As shown in Figure 4, the most acceptable trade-offs were having to go to the clinic more often (e.g., every month) and moderate initial side effects that would eventually go away, with 63% and 58% of respondents, respectively, willing to accept these trade-offs compared with oral daily ART. The least acceptable trade-offs (the “unacceptable” response choice) were the chance of passing HIV to a sex partner (68%), mental side effects (65%), no increase in quality of life (61%), and developing health risks later in life (59%).
FIG. 4.
HIV cure research scenarios and trade-offs (n = 259–260 YLWH in the United States).
Additional considerations and logistical factors
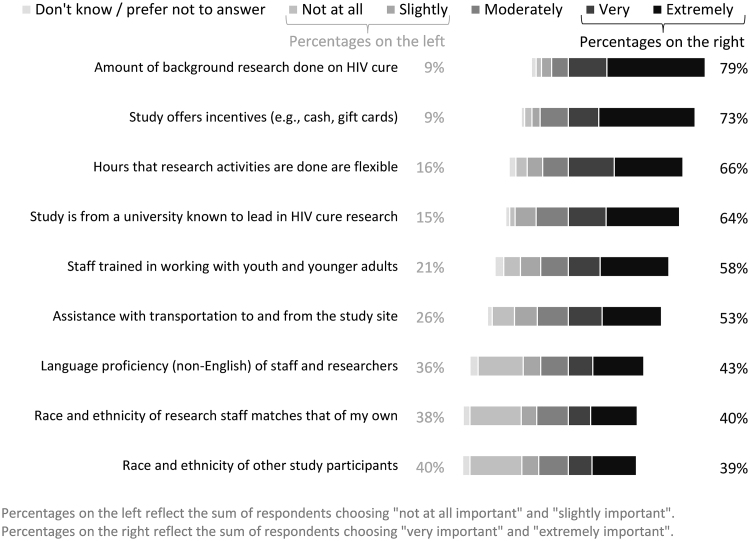
As shown in Figure 5, the most important factor that would affect willingness to consider participating in HIV cure research (the “very likely” and “extremely likely” responses) was the amount of background research conducted on a particular cure strategy (79%), followed by study incentives offered (73%), and flexible hours of research activities (66%). While 58% of participants found that it was very or extremely important for staff to be trained in working with young populations, the race and ethnicity of other participants and the race and ethnicity of research staff matching their own were the least important factors, with 40% and 38% of respondents indicating that this would matter slightly or not at all, respectively.
FIG. 5.
Importance of factors influencing wanting to participate in HIV cure research (n = 258 YLWH in the United States).
Perceptions of ATIs and partner protection measures
Of the 261 respondents completing this section, 84% had ever heard of the expression U = U. Out of 255 respondents, 45% would be willing to let their viral load become detectable for a period of time during an HIV cure study, 27% would not be willing, and 28% did not know. Of those who would be willing to have detectable HIV viral load (n = 115), 23% would prefer the period of having a detectable viral load to be shorter than 3 months, 25% between 3 and 5 months, 10% between 6 and 8 months, 7% between 9 and 12 months, 13% more than 12 months, and 23% did not know. Preferred reasons for restarting ART following an ATI included a decline in CD4 cell count (43%), being asked by HIV care provider to start ART (41%), researchers letting them know that their viral load is detectable (26%), and being able to transmit HIV (24%).
As shown in Figure 6, the following partner protection measures were perceived as very or extremely important: providing HIV testing and counseling (81%) and/or PrEP or PEP for partners (80%); referring partners for PrEP and PEP services (78%), providing condoms for partners (75%), and assistance with HIV disclosure to partners (71%).
FIG. 6.
Importance of partner protection strategies to participate in studies involving HIV treatment interruptions (n = 258 YLWH in the United States).
Exploratory bivariate analyses
Table 2 provides a summary of statistically significant exploratory bivariate results. Statistically significant (p < .1) correlations are identified and then retested in a multivariate analysis by controlling for each of the other demographic variables that are correlated with the key demographic variable. Where a bivariate correlation was no longer statistically significant after the inclusion of a control variable, the change in p value is noted in Table 2.
Table 2.
Summary of Exploratory Bivariate Results (Youth4Cure Survey, United States, 2021)
| Age groups |
Region |
Financial status |
Gender |
Race/Ethnicity |
|
|---|---|---|---|---|---|
| Group of participants 18–24 years old (29% of n = 271) compared to group of participants 25–29 years old (71%) | Comparing participants who are in the Midwest (“M”: 17% of n = 271), Northeast (“NE”: 10%), South (“S”: 49%), and West (“W”: 24%) | Comparing participants who cannot get by on the money they have (“financially constrained”: 23% of n = 265) to participants that can barely get by on the money they have or that have enough money to live comfortably (77%). Excludes six participants who did not specify financial status | Cisgender men (84% of n = 269) compared to all other genders (16%). Excludes two participants that did not specify gender | Comparing participants who are Hispanic or Latinx (“H”: 18% of n = 266), Black or African American and non-Hispanic (“B”: 68%), and other or multiracial and non-Hispanic (“O”: 14%). Excludes five participants that did not specify race/ethnicity | |
| Willingness to consider participating in different types of HIV cure studies | No differences | No differences | Financially constrained participants less likely to consider participating in an HIV cure study involving cell and gene-based approaches (81% vs. 91%, p = .047) | No differences | Hispanic/Latinx and Black non-Hispanic participants less likely to consider participating in an HIV cure study that involves hair donation (H: 81% vs. B: 76% vs. O: 94%, fe = 0.051) |
| Motivating factors influencing willingness to participate in HIV cure studies | No differences | Participants in the West less likely to consider getting more medical attention in a study than the average person living with HIV as mattering “a great deal” (M: 49% vs. NE: 70% vs. S: 57% vs. W: 40%, p = .049) Participants in the West less likely to consider controlling HIV without the need for HIV meds as mattering “a lot or a great deal” (M: 82% vs. NE: 95% vs. S: 89% vs. W: 76%, fe = 0.059) |
Financially constrained participants more likely to consider the following as mattering “a great deal”: Having regular access to special medical doctors/nurses/researchers because of being in a study (69% vs. 54%, p = .057) Engaging with research teams to advance science (68% vs. 51%, p = .024) Feeling they are helping people like themselves such as other young people, women, other people of color, other LGBTQ people, etc. (80% vs. 65%, p = .045) Hope that their HIV disease will improve (83% vs. 64%, p = .007) Helping own immune system's ability to fight HIV (85% vs. 69%, p = .014) Controlling HIV without the need for HIV meds (80% vs. 67%, p = .070) Receiving payment for participation (85% vs. 63%, p = .002) Financially constrained participants more likely to consider the following as mattering “a lot or a great deal”: Gaining special knowledge about HIV (87% vs. 74%, p = .037) Gaining special knowledge about own personal health from being in the study (88% vs. 74%, p = .027) |
Cisgender men less likely to consider feeling they are helping future generations of people with HIV as mattering “a great deal” (68% vs. 83%, p = .066) | No differences |
| Risk factors influencing willingness to participate in HIV cure studies | Younger youth age group more likely than older youth age group to consider the following as mattering “a great deal”: Virus level going up unexpectedly (60% vs. 42%, p = .007) Developing dementia or other problems with ability to think or remember (64% vs. 50%, p = .050). Not statistically significant when controlling for region. Illnesses that can occur if immune system becomes overly active (59% vs. 47%, p = .073). Not statistically significant when controlling for region. Possibility of current HIV meds stop working because the virus will become resistant (65% vs. 50%, p = .031). Not statistically significant when controlling for region. Needing to delay having children temporarily (36% vs. 20%, p = .011) Possibility of permanently being unable to have children in the future (57% vs. 39%, p = .007) Transmitting HIV to others if off HIV meds during the study (64% vs. 48%, p = .023) Having to disclose HIV status (38% vs. 21%, p = .003) Being treated poorly by the study staff (61% vs. 45%, p = .020) Facing stigma or discrimination (47% vs. 32%, p = .026) Transportation challenges in getting to and from study visits (36% vs. 17%, p = .001) |
Participants in the Northeast and Midwest more likely than others to consider the following as mattering “a great deal”: Developing dementia or other problems with ability to think or remember (M: 69% vs. NE: 63% vs. S: 50% vs. W: 48%, p = .091) Psychological side effects such as depression and anxiety (M: 45% vs. NE: 58% vs. S: 34% vs. W: 30%, p = .053) Possibility of current HIV meds stop working because the virus will become resistant (M: 68% vs. NE: 61% vs. S: 54% vs. W: 44%, p = .081). Not statistically significant when controlling for gender or age groups. Financial risks such as losing health insurance (M: 63% vs. NE: 63% vs. S: 43% vs. W: 48%, p = .084) Participants in the South and Midwest more likely than others to consider having to disclose HIV status matters “a great deal” (M: 27% vs. NE: 17% vs. S: 32% vs. W: 15%, fe = 0.051). Not statistically significant when controlling for race/ethnicity. Participants in the Northeast more likely than others to consider being treated poorly by the study staff matters “a lot or a great deal” (M: 60% vs. NE: 92% vs. S: 68% vs. W: 59%, fe = 0.018) |
Financially constrained participants more likely to consider the following as mattering “a great deal”: Having to disclose HIV status (40% vs. 22%, p = .007) Financial risks such as losing health insurance (60% vs. 46%, p = .066) |
Cisgender men less likely to consider stomach discomforts as mattering “a great deal” (25% vs. 38%, p = .095). Not statistically significant when controlling for age groups. | Black non-Hispanic participants more likely than others to consider the following as mattering “a great deal”: Having to disclose HIV status (H: 17% vs. B: 32% vs. O: 11%, fe = 0.012) Finding childcare while going to a study visit (H: 2% vs. B: 13% vs. O: 6%, fe = 0.072). Not statistically significant when controlling for region. Hispanic/Latinx participants less likely than others to consider the following as mattering “a great deal”: Stomach discomforts (H: 13% vs. B: 31% vs. O: 30%, p = .041) Hispanic/Latinx and Black non-Hispanic participants more likely than others to consider the following as mattering “a lot or a great deal”: Possibility of current HIV meds stop working because the virus will become resistant (H: 66% vs. B: 72% vs. O: 51%, p = .050) Need to temporarily delay having children (H: 26% vs. B: 35% vs. O: 19%, p = .098) Time commitment required for study visits (H: 30% vs. B: 34% vs. O: 11%, fe = 0.020) Transportation challenges in getting to and from study visits (H: 30% vs. B: 39% vs. O: 14%, fe = 0.010) Having study visits take time away from work or school (H: 32% vs. B: 45% vs. O: 20%, p = .014) |
| Willingness to let HIV viral load go to a detectable level for a period of time during an ATI study | Younger youth age group less willing to let HIV viral load go to a detectable level for a period of time during an HIV cure study (52% vs. 66%, p = .072) | No differences | No differences | No differences | No differences |
| Accepting trade-offs of alternative HIV control strategies instead of taking daily ARTs | Younger youth age group less likely than older youth age group to choose alternative control strategy over continuing daily ARTs if control strategy includes: Increased chance of passing on HIV to a sex partner (16% vs. 28%, p = .055) Moderate side effects initially (e.g., itchy rash, blurred vision) but then went away after a few weeks (52% vs. 66%, p = .038) No increase in the number of years lived (31% vs. 44%, p = .070) |
No differences | No differences | Cisgender men less likely to choose alternative control strategy over continuing daily ARTs if control strategy involves going to the clinic much more often, such as monthly (66% vs. 89%, fe = 0.005) | Hispanic/Latinx and Black non-Hispanic participants less likely than others to choose alternative control strategy over continuing daily ARTs if control strategy includes: Moderate side effects initially (e.g., itchy rash, blurred vision) but then went away after a few weeks (H: 70% vs. B: 57% vs. O: 81%, p = .019) No increase in the number of years lived (H: 42% vs. B: 33% vs. O: 71%, p < .001) No increase in the quality of life (H: 33% vs. B: 29% vs. O: 50%, p = .058) |
| Willingness to participate in HIV cure studies if it means being unable to participate in future HIV cure studies that may be more effective in achieving a cure | No differences | No differences | No differences | No differences | No differences |
| Importance of partner protection strategies in participating in ATI studies | Younger youth age group more likely than older youth age group to consider the following as “extremely important”: Providing condoms for partners (76% vs. 59%, p = .010) Assistance with HIV disclosures to partners (65% vs. 51%, p = .047) |
No differences | Financially constrained participants more likely to consider the following as “extremely important”: Providing condoms for partners (78% vs. 61%, p = .029) HIV testing and counseling for partners (80% vs. 60%, p = .008) Assistance with HIV disclosure to partners (70% vs. 50%, p = .012) |
Cisgender men less likely to consider referrals for pre-exposure prophylaxis and postexposure prophylaxis for sex partners as “extremely important” (62% vs. 82%, p = .018) | Hispanic/Latinx and Black non-Hispanic participants more likely than others to consider assistance with HIV disclosures to partners as “very or extremely important” (H: 76% vs. B: 78% vs. O: 60%, p = .084) |
| Other logistical factors influencing decision to participate in HIV cure research | Younger youth age group more likely than older youth age group to consider having staff trained in working with youth and younger adults as “extremely important” (51% vs. 38%, p = .071). Not statistically significant when controlling for region. | Participants in the Northeast more likely to consider that a study is being offered from a university known to lead research in HIV cure research is “very or extremely important” (M: 62% vs. NE: 88% vs. S: 62% vs. W: 66%, fe = 0.093) | Financially constrained participants more likely to consider assistance with transportation to and from the study site as “extremely important” (50% vs. 31%, p = .011) Financially constrained participants more likely to consider race and ethnicity of research staff matching that of participant as “very or extremely important” (54% vs. 38%, p = .045) Financially constrained participants less likely to consider the following factors as “very or extremely important”: Study is being offered from a university known to lead research in HIV cure research (55% vs. 68%, p = .075) Amount of background research done on HIV cure (71% vs. 84%, p = .037) |
No differences | Black non-Hispanic participants more likely than others to consider the following factors as “extremely important”: Race and ethnicity of research staff matching that of participant (H: 17% vs. B: 33% vs. O:22%, p = .081). Not statistically significant when controlling for region. Assistance with transportation to and from the study site (H: 26% vs. B: 41% vs. O: 25%, p = .050). Not statistically significant when controlling for region. Hours that research activities are done are flexible (H: 32% vs. B: 47% vs. O: 31%, p = .075) Hispanic/Latinx participants less likely than others to consider that staff training in working with youth and younger adults as “extremely important” (H: 22% vs. B: 47% vs. O: 39%, p = .011). Not statistically significant when controlling for region. Hispanic/Latinx and Black non-Hispanic participants more likely than others to consider studies offering incentives (e.g., cash, gift cards) as “extremely important” (H: 51% vs. B: 62% vs. O: 39%, p = .030) |
All correlations remain statistically significant (p < .1) when controlling for confounding variables in multivariate analyses unless specified otherwise. Confounding variables were gender, age groups and region for each other, and race/ethnicity with region.
ART, antiretroviral therapy; ATI, analytical treatment interruption; fe, Fisher's exact value.
Bivariate analyses by age groups (18–24 years vs. 25–29 years)
Compared to the 25–29 age group (n = 192), the 18–24 age group (n = 79) was more likely to indicate that the following risk factors would matter a great deal in considering participating in HIV cure studies: high HIV viral load (60% vs. 42%, p = .007); the possibility of developing HIV drug resistance (65% vs. 47%, p = .031; not significant when controlling for regions of residence); transmitting HIV to others while off ART (64% vs. 48%, p = .023); and having to disclose HIV status (38% vs. 21%, p = .003). The 18–24 age group was more likely to indicate that assistance with HIV disclosures (65% vs. 51%, p = .047) and providing condoms for sex partners (76% vs. 59%, p = .010) were extremely important strategies to protect partners while participating in a cure study, including an ATI.
Bivariate analyses by racial identity
Black American participants (n = 180) were more likely to find that disclosing HIV status mattered a great deal (Black non-Hispanic 32%, Hispanic 17%, Other non-Hispanic 11%, Fisher's exact test = 0.012) and taking time away from work or school (Black non-Hispanic 45%, Hispanic 32%, Other non-Hispanic 20%, p = .014) mattered a lot or a great deal compared to other racial/ethnic identity groups.
Bivariate analyses by financial status
Respondents who were financially constrained (n = 60) were more likely to indicate that: receiving payment mattered a great deal in motivating them to participate in HIV cure research (85% vs. 63%, p = .002); having to disclose HIV status (40% vs. 22%, p = .007) and financial risks such as losing health insurance (60% vs. 46%, p = .066) mattered a great deal; and assistance with transportation was extremely important to participate (50% vs. 31%, p = .011) compared to those who were not as financially constrained (n = 205).
Additional bivariate analyses
Gender and sex
We found very few differences in perspectives between cisgender men (n = 227) and people of other genders. Cisgender men were less likely to choose a nonoral-ART HIV cure strategy if it meant having to go to the clinic more often, such as monthly clinic visits (66% cisgender men vs. 89% other genders, Fisher's exact test = 0.005), and less likely to indicate that referrals for PrEP and PEP for sex partners were extremely important to participating in a study involving ATIs (62% cisgender men vs. 82% other genders, p = .018).
Regions of residency
We found very little differences by region; other than that participants in the Midwest and South were more likely than those from other regions to indicate that having to disclose HIV status mattered a great deal as a risk factor (Midwest 27%, Northeast 17%, South 32%, West 15%, Fisher's exact test = 0.051; not statistically significant when controlling for racial identity).
Discussion
We found high levels of consideration for participating in HIV cure research among YLWH. The most important deterrents were related to treatment interruption, particularly the possibility of transmitting HIV to sex partners. Disclosure of HIV status was also a significant barrier, particularly for younger, Black American participants, who may face greater risks of race-based stigma and discrimination.
The high willingness of YLWH to consider participating in different types of HIV cure research corroborates findings from previous research focused on older PLWH.12,13,39 YLWH were willing to consider participating in immune-, cell-, and gene-based approaches when these methods were explained clearly. This finding is encouraging, given that cell and gene therapy represents a target product profile for a globally scalable HIV cure,40 and research investments in these approaches will be augmented in the coming years.41 Further research is needed to determine whether willingness translates into actual participant enrollment and retention in this research.42 A study by Prins et al conducted in the Netherlands found that hypothetical questionnaires overestimate willingness to participate in HIV cure research [i.e., 67% (n = 111) hypothetical willingness to participate compared with 43% actual willingness (n = 135) to participate].43
We found that some physical health and psychosocial risks present in HIV cure studies might be deterrents for YLWH. The finding that YLWH would be demotivated by the potential to develop ART drug resistance is salient because YLWH face a lifelong prospect of contending with HIV. Moreover, nearly two-thirds of respondents reported mental health side effects as unacceptable, possibly reflecting high rates of mental health challenges among YLWH44,45 and underscoring the need to avoid undue psychosocial harm.
In our sample, we found almost no difference in perspectives between cisgender men versus people of other gender identities. This may have been a reflection of the overwhelmingly male-identified sample and is surprising because previous research found several statistically significant gender differences in perceptions of risks, such as cisgender and transgender women being less willing to tolerate physical risks than cisgender men.13 Our survey did reveal statistically significant differences by age and racial identity groups. Black YLWH seemed more risk averse than other racial identity groups on some factors and burdens, such as physical side effects, disclosing HIV status, and finding transportation, consistent with previous surveys with older PLWH.12,46 One possible explanation is that marginalized communities disproportionately manage multiple intersecting vulnerabilities (e.g., racism, homelessness, homophobia, HIV stigma, substance use),32 which may lead to increased risk aversion.
Furthermore, the majority of YLWH were deterred by the possibility of transmitting HIV during ATIs and noted the importance of partner protection measures, particularly in the younger (18–24 years) age group. The finding of YLWH exhibiting HIV prevention altruism47 is noteworthy, because the field of HIV cure research is moving toward less restrictive ATIs and prolonged periods of viremia to test promising cure strategies.4 This result corroborates results from our previous survey work showing that participants younger than 50 years were more demotivated by potential social risks (e.g., stigma, discrimination, HIV disclosure, and fear of transmitting HIV during a treatment interruption) than were older PLWH.31 This result is also consistent with a survey conducted in the Netherlands which showed reluctance to interrupt ART for extended periods among people diagnosed during acute HIV.48 Likewise, trial designs requiring extended viremia to determine intervention efficacy may not be as appealing to the newer generation of PLWH who will be at the forefront of research toward an HIV cure.
Another important finding from our study is that the 18–24 age group required support in disclosing HIV status to partners during ATIs. This result is not unanticipated because older PLWH may have had more time and opportunities to develop disclosure strategies. In addition to helping participants disclose HIV status and ATI, research teams will need to design partner protection strategies37,49 with young people in mind—including PrEP/PEP for partners, skill building for behavioral risk reduction, counseling, and mental health services that are attentive to the needs of young adults.
Social and behavioral science experts are crucial research partners in efforts to enhance protective measures for YLWH to disclose HIV status to their sex partners. In the era of U = U,50 ATIs assume that HIV (and ATI) disclosure will inherently occur, but YLWH have likely not had as much opportunity to prepare and execute that process relative to older PLWH. Furthermore, ATIs may send mixed messages to YLWH who often struggle with ART adherence.51 Protocols requiring participants to restart ART may require additional medication adherence safeguards for young participants. Helping YLWH navigate ART interruptions and restarts in a manner that enhances research trustworthiness52 will be critical to prevent compounding ART nonadherence and potential drug resistance.
Additional factors to address include the following: providing incentives to participation that recognize YLWH's time without creating undue influence or distorting judgments around risks and benefits;53 ensuring staff well-versed in working with young populations; and assisting younger participants with transportation needs.
Summary of key considerations for engaging racially diverse YLWH in HIV cure research
To successfully implement HIV cure research programs with racially diverse YLWH, the following considerations are important, based on our findings:
Because YLWH face a lifelong prospect of HIV, physical, clinical, and social risks will need to be kept to a minimum and irreversible side effects avoided at all cost.
Research teams will need to carefully and proactively mitigate social risks as well, such as the possibility of transmitting HIV to sex partners.
YLWH involved in ATI trials will require robust psychosocial support, and more research is necessary to understand the psychosocial and mental health risks of interrupting ART.
Adequate support around HIV disclosure will be necessary.
When designing risk mitigation strategies for partners, research teams will need to help young adults overcome barriers to HIV prevention (e.g., PrEP access), particularly in communities that remain underserved.
As YLWH navigate lower ART adherence and transition into adult HIV care, they may require more adherence support around interrupting and restarting ART.
Research teams will need to pay close attention to financial risks and logistical factors for YLWH (e.g., providing access to transportation) and adopt equity frameworks54 when implementing trials with historically marginalized groups.
Research staff should be attuned to the health needs of young people and trained in youth cultures.
Limitations
Our survey is limited by its hypothetical nature (i.e., questions relied on stated as opposed to revealed preferences). Our sample was skewed toward Black American cisgender gay male YLWH and also may have been biased toward those connected to the recruitment platforms and with internet access. The low percentage of cisgender and transgender women was likely a result of our recruitment strategy. More research will be necessary to identify differences in willingness to consider participating in HIV cure research by sex and gender. Due to lack of variability in the demographics of participants, we were not able to conduct more comprehensive multivariate analyses. We focused on descriptive and mainly bivariate analyses; however, future research should further examine these data using conjoint analyses which may yield further insights. The survey was only offered in English and this likely resulted in under-representation of Hispanic or Latinx YLWH.
Furthermore, our HIV cure research illustrations35 did not include information on specific risks, procedures, and time commitments involved for each strategy, and more research will be needed to ascertain how these may affect stated preferences. We may have underexplored perceptions of ATIs as a potential motivator or benefit to participation among YLWH (e.g., not having to take HIV medication). Measures were self-reported, which may have resulted in social desirability and recall biases. Although these data cannot be used to predict YLWH's enrollment rates in cure studies, findings can inform engagement, education, and communication efforts. The main strength of the study is that respondents were young and were predominantly Black and Latinx sexual minority individuals. To our knowledge, this is the first time a sociobehavioral survey deliberately engaged racially diverse YLWH in HIV cure research in the United States. Compared with previous surveys,12,13 this study is more representative of the demographics of YLWH in the United States.
Conclusion
YLWH remain under-represented in HIV cure research yet will be key decision-makers in determining which novel HIV therapies are acceptable for the future. New paradigms are urgently needed to engage younger and more diverse participants in HIV cure research. The field of HIV cure research may need to rethink barriers to entry and construct studies that embrace and prioritize the involvement of diverse YLWH, paying attention to intersecting issues of race, gender, stigma, discrimination, partner protections, logistical issues, and economic vulnerability.
Acknowledgments
The authors thank all of our participants, the UCSF Youth Advisory Panel members who guided the research, and the Youth4Cure Scientific Advisory Board who provided consultations. Special thanks to Harrison Glazer for his guidance on advertising.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Consent to Participate
Informed consent was obtained from all individual participants included in this study.
Availability of Data and Material
All relevant data related to this study have been included in this article.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
The authors' work was supported by the National Institute of Mental Health (award number R21MH122280).
References
- 1. Archin NM, Sung JM, Garrido C, et al. Eradicating HIV-1 infection: Seeking to clear a persistent pathogen. Nat Rev Microbiol 2014;12(11):750–764; doi: 10.1038/nrmicro3352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Deeks SG, Lewin SR, Ross AL, et al. International AIDS society global scientific strategy: Towards an HIV cure 2016. Nat Med 2016;22(8):839–850; doi: 10.1038/nm.4108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dubé K, Henderson GE, Margolis DM. Framing expectations in early HIV cure research. Trends Microbiol 2014;22(10):547–549; doi: 10.1016/j.tim.2014.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Julg B, Dee L, Ananworanich J, et al. Recommendations for analytical antiretroviral treatment interruptions in HIV research trials-report of a consensus meeting. Lancet HIV 2019;6(4):e259–e268; doi: 10.1016/S2352-3018(19)30052-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stecher M, Claßen A, Klein F, et al. Systematic review and meta-analysis of treatment interruptions in human immunodeficiency virus (HIV) type 1-infected patients receiving antiretroviral therapy: implications for future HIV cure trials. Clin Infect Dis 2020;70(7):1406–1417; doi: 10.1093/cid/ciz417 [DOI] [PubMed] [Google Scholar]
- 6. Lau JSY, Smith MZ, Lewin SR, et al. Clinical trials of antiretroviral treatment interruption in HIV-infected individuals. AIDS 2019;33(5):773–791; doi: 10.1097/QAD.0000000000002113 [DOI] [PubMed] [Google Scholar]
- 7. Lelièvre J-D, Hocqueloux L. Unintended HIV-1 transmission to a sex partner in a study of a therapeutic vaccine candidate. J Infect Dis 2019;220(Suppl 1):S5–S6; doi: 10.1093/infdis/jiz012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ugarte A, Romero Y, Tricas A, et al. Unintended HIV-1 infection during analytical therapy interruption. J Infect Dis 2020;221(10):1740–1742; doi: 10.1093/infdis/jiz611 [DOI] [PubMed] [Google Scholar]
- 9. Peluso MJ, Dee L, Campbell D, et al. A collaborative, multidisciplinary approach to HIV transmission risk mitigation during analytic treatment interruption. J Virus Erad 2020;6(1):34–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Richart V, Fernández I, de Lazzari E, et al. High rate of long-term clinical events after antiretroviral therapy resumption in HIV-positive patients exposed to antiretroviral therapy interruption. AIDS 2021;35(15):2463–2468; doi: 10.1097/QAD.0000000000003058 [DOI] [PubMed] [Google Scholar]
- 11. Wen Y, Bar KJ, Li JZ. Lessons learned from HIV Antiretroviral treatment interruption trials. Curr Opin HIV AIDS 2018;13(5):416–421; doi: 10.1097/COH.0000000000000484 [DOI] [PubMed] [Google Scholar]
- 12. Dubé K, Evans D, Sylla L, et al. Willingness to participate and take risks in HIV cure research: Survey results from 400 people living with HIV in the US. J Virus Erad 2017;3(1):40–50.e2150e21; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dubé K, Eskaf S, Evans D, et al. The dose response: Perceptions of people living with HIV in the United States on alternatives to oral daily antiretroviral therapy. AIDS Res Hum Retroviruses 2020;36(4):324–348; doi: 10.1089/AID.2019.0175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dubé K, Taylor J, Sylla L, et al. ‘Well, it's the risk of the unknown… right?’: A qualitative study of perceived risks and benefits of HIV cure research in the United States. PLoS One 2017;12(1):e0170112; doi: 10.1371/journal.pone.0170112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dubé K, Evans D, Dee L, et al. ‘We need to deploy them very thoughtfully and carefully’: Perceptions of analytical treatment interruptions in HIV cure research in the United States-A qualitative inquiry. AIDS Res Hum Retroviruses 2018;34(1):67–79; doi: 10.1089/AID.2017.0067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Protière C, Spire B, Mora M, et al. Patterns of patient and healthcare provider viewpoints regarding participation in HIV cure-related clinical trials. Findings from a Multicentre French Survey Using Q Methodology (ANRS-APSEC). PLoS One 2017;12(11):e0187489; doi: 10.1371/journal.pone.0187489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Protiere C, Arnold M, Fiorentino M, et al. Differences in HIV cure clinical trial preferences of French people living with HIV and physicians in the ANRS-APSEC study: A discrete choice experiment. J Int AIDS Soc 2020;23(2):e25443; doi: 10.1002/jia2.25443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Power J, Dowsett GW, Westle A, et al. The significance and expectations of HIV cure research among people living with HIV in Australia. PLoS One 2020;15(3):e0229733; doi: 10.1371/journal.pone.0229733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Power J, Westle A, Dowsett GW, et al. Perceptions of HIV cure research among people living with HIV in Australia. PLoS One 2018;13(8):e0202647; doi: 10.1371/journal.pone.0202647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Henderson GE, Waltz M, Meagher K, et al. Going off antiretroviral treatment in a closely monitored HIV ‘cure’ trial: Longitudinal assessments of acutely diagnosed trial participants and decliners. J Int AIDS Soc 2019;22(3):e25260; doi: 10.1002/jia2.25260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Henderson GE, Peay HL, Kroon E. et al. Ethics of treatment interruption trials in HIV cure research: Addressing the conundrum of risk/benefit assessment. J Med Ethics 2018;44(4):270–276; doi: 10.1136/medethics-2017-104433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peay HL, Ormsby NQ, Henderson GE, et al. Recommendations from Thai stakeholders about protecting HIV remission (‘cure’) trial participants: Report from a participatory workshop. Int Health 2020;12(6):567–574; doi: 10.1093/inthealth/ihaa067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu F, Zhang A, Babbitt A, et al. Overcoming HIV stigma? A qualitative analysis of HIV cure research and stigma among men who have sex with men living with HIV. Arch Sex Behav 2018;47(7):2061–2069; doi: 10.13039/100000060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ma Q, Wu F, Henderson G, et al. ‘I can coexist with HIV’: A qualitative study of perceptions of HIV cure among people living with HIV in Guangzhou, China. J Virus Erad 2016;2(3):170–174; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chu CE, Wu F, He X, et al. Exploring the social meaning of curing HIV: A qualitative study of people who inject drugs in Guangzhou, China. AIDS Res Hum Retroviruses 2015;31(1):78–84; doi: 10.1089/AID.2014.0200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wozniak RJ, Cerqueira NB, Dantas MCS, et al. Factors associated with attitudes towards HIV cure research among transgender women and travestis: A cross-sectional survey in São Paulo, Brazil. BMJ Open 2020;10(11):e040092; doi: 10.1136/bmjopen-2020-040092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. CDC. HIV Surveillance Report. Diagnoses of HIV Infection in the United States and Dependent Areas, 2018. (Updated). Available from https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2018-updated-vol-31.pdf [Last accessed: November 2, 2022].
- 28. CDC. HIV and Youth. 2021. Available from: https://www.cdc.gov/hiv/group/age/youth/index.html [Last accessed: November 2, 2022].
- 29. Curno MJ, Rossi S, Hodges-Mameletzis I, et al. A systematic review of the inclusion (or exclusion) of women in HIV research: From clinical studies of antiretrovirals and vaccines to cure strategies. J Acquir Immune Defic Syndr 2016;71(2):181–188; doi: 10.1097/QAI.0000000000000842 [DOI] [PubMed] [Google Scholar]
- 30. Dubé K, Kanazawa J, Campbell C, et al. Considerations for increasing racial, ethnic, gender, and sexual diversity in HIV cure-related research with analytical treatment interruptions: A qualitative inquiry. AIDS Res Hum Retrovir 2022;38(1):50–63; doi: 10.1089/aid.2021.0023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Saberi P, Eskaf S, Sauceda J, et al. Perceptions of HIV virologic control strategies among younger and older age groups of people living with HIV in the United States: A cross-sectional survey. AIDS Res Hum Retroviruses 2020;36(7):606–615; doi: 10.1089/AID.2020.0023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Saberi P, Campbell CK, Venegas M, et al. Time to engage young people in HIV cure research. AIDS Res Hum Retroviruses 2022;38(1):2–4; doi: 10.1089/AID.2020.0268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saberi P, Lisha NE, Erguera XA, et al. A Mobile Health App (WYZ) for engagement in care and antiretroviral therapy adherence among youth and young adults living with HIV: Single-arm pilot intervention study. JMIR Form Res 2021;5(8):e26861; doi: 10.2196/26861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Saberi P, McCuistian C, Agnew E, et al. Video-counseling intervention to address HIV care engagement, mental health, and substance use challenges: A pilot randomized clinical trial for youth and young adults living with HIV. Telemed Rep 2021;2(1):14–25; doi: 10.1089/tmr.2020.0014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. UCSF. Youth4Cure. HIV Cure Research Illustrations. 2021. Available from: https://youth4cure.ucsf.edu/hiv-cure-research-illustrations [Last accessed: November 2, 2022].
- 36. Dubé K, Ramirez C, Handibode J, et al. Participation in HIV cure-related research: A scoping review of the proxy literature and implications for future research. J Virus Erad 2015;1:250–256; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dubé K, Kanazawa J, Dee L, et al. Ethical and practical considerations for mitigating risks to sexual partners during analytical treatment interruptions in HIV cure-related research. HIV Res Clin Pract 2021;22(1):14–30; doi: 10.1080/25787489.2021.1902116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hussen SA, Chahroudi A, Boylan A, et al. Transition of youth living with HIV from pediatric to adult-oriented healthcare: A review of the literature. Future Virol 2015;9(10):921–929; doi: 10.2217/fvl.14.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kwan TH, Chan CP, Wong NS, et al. Awareness of HIV functional cure and willingness in participating in related clinical trials: Comparison between antiretroviral naïve and experienced men who have sex with men living with HIV. BMC Infect Dis 2022;22(1):383; doi: 10.1186/s12879-022-07346-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lewin SR, Attoye T, Bansbach C, et al. Multi-stakeholder consensus on a target product profile for an HIV cure. Lancet HIV 2021;8(1):e42–e50; doi: 10.1016/S2352-3018(20)30234-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dybul M, Attoye T, Baptiste S, et al. The case for an HIV cure and how to get there. Lancet HIV 2021;8(1):e51–e58; doi: 10.1016/S2352-3018(20)30232-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Buchbinder SP, Metch B, Holte SE, et al. Determinants of enrollment in a preventive HIV vaccine trial: Hypothetical versus actual willingness and barriers to participation. J Acquir Immune Defic Syndr 2004;36(1):604–612; doi: 10.1097/00126334-200405010-00009 [DOI] [PubMed] [Google Scholar]
- 43. Prins H, Paulus MR, Rokx C, et al. Hypothetical questionnaires may overestimate willingness to participate in HIV cure research: Comparison of a cross-sectional survey to actual willingness to participate in an HIV cure study in the Netherlands. J Virus Erad 2020;6(4):100014; doi: 10.1016/j.jve.2020.100014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vreeman RC, McCoy BM, Lee S. Mental health challenges among adolescents living with HIV: Vreeman RC et al. J Int AIDS Soc 2017;20(9590):21497; doi: 10.13039/100000025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Benton TD, Kee Ng WY, Leung D, et al. Depression among youth living with HIV/AIDS. Child Adolesc Psychiatr Clin N Am 2019;28(3):447–459; doi: 10.1016/j.chc.2019.02.014 [DOI] [PubMed] [Google Scholar]
- 46. Arnold MP, Evans D, Vergel N. Recruitment and ethical considerations in HIV cure trials requiring treatment interruption. J Virus Erad 2015;1(1):43–48; doi: 10.1016/S2055-6640(20)31148-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. O'Dell B, Rosser S, Miner M, et al. HIV prevention altruism and sexual risk behavior in HIV-positive men who have sex with men. AIDS Behav 2012;12(5);713–720; doi: 10.1007/s10461-007-9321-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Grijsen ML, Steingrover R, Wit FW, et al. No treatment versus 24 or 60 weeks of antiretroviral treatment during primary HIV infection: The randomized primo-SHM trial. PLoS Med 2012;9(3):e1001196; doi: 10.1371/journal.pmed.1001196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Peluso MJ, Dee L, Shao S, et al. Operationalizing HIV cure-related trials with analytic treatment interruptions during the SARS-Cov-2 pandemic: A collaborative approach. Clin Infect Dis 2021;72(10):1843–1849; doi: 10.1093/cid/ciaa1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Prevention Access Campaign. U = U. Available from: https://preventionaccess.org/ [Last accessed: November 2, 2022].
- 51. Foster C, Ayers S, Fidler S. Antiretroviral adherence for adolescents growing up with HIV: Understanding real life, drug delivery and forgiveness. Ther Adv Infect Dis 2020;7:2049936120920177; doi: 10.1177/2049936120920177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wilkins CH. Effective engagement requires trust and being trustworthy. Med Care 2018;56 (10 suppl 1):S6–S8; doi: 10.1097/MLR.0000000000000953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Brown B, Galea JT, Davidson P, et al. Transparency of participant incentives in HIV research. Lancet HIV 2016;3(10):e456–e457; doi: 10.1016/S2352-3018(16)30150-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bowleg L. Towards a critical health equity research stance: Why epistemology and methodology matter more than qualitative methods. Health Educ Behav 2017;44(5):677–684; doi: 10.1177/1090198117728760 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data related to this study have been included in this article.