Recognizing and characterizing bradykinesia is a critical issue in movement disorders. Bradykinesia is the core symptom for the definition of parkinsonism and for the diagnosis of Parkinson’s disease (PD) and atypical parkinsonism (AP).1–7 To some extent, however, the debate on the terminology and definition of bradykinesia has never been settled.8,9 Bradykinesia literally means slowness of movement. However, the term is still used interchangeably to indicate low amplitude movement (hypokinesia) or no movement (akinesia),10 and it is often used to apply to both voluntary and spontaneous/automatic movements.11–13 The current bradykinesia definition in the context of diagnostic criteria for PD includes both slowness and progressive reduction in movement amplitude and velocity when performing repetitive movements, that is, decrement or sequence effect.3,4,10 The current concept of bradykinesia, including potentially distinct motor abnormalities, is reflected in the evaluation scales routinely used in clinical practice.14,15
The current bradykinesia definition has some clinical and pathophysiological inconsistencies.10,16,17 In this paper, we first review the historical background of the definition of bradykinesia and related terms. We then summarize the various inconsistencies that undermine the current bradykinesia definition and its applicability in different conditions and propose redefining bradykinesia. We finally emphasize the usefulness of a new bradykinesia definition and discuss the potential implications of this proposal.
Historical Background on Terminology
The terminology of bradykinesia, hypokinesia, and akinesia has long been debated.8 Interestingly, none of these terms were first introduced with specific reference to parkinsonism. The term bradykinesia was introduced in 1907 to describe slowed voluntary movements in dystonia.18 About 20 years later, the so-called “bradykinetic syndrome” has been described in PD and other conditions to specifically refer to slowness at the beginning and during the execution of voluntary movements.19 The term hypokinesia was introduced in 187820 as opposite to hyperkinesia.8 In the context of PD, hypokinesia was used for the first time in the early 1900s, with reference to the lack of “associated, successive, reactive and expressive movements.”21 Later on, it was suggested that hypokinesia could be adopted only when an amplitude decrement was present.22 The term akinesia was introduced in the second half of the 19th century to describe decreased motion, including paralysis. Subsequently, the term akinesia has been mainly adopted in psychiatric patients,23 and it was used for the first time in PD only in 1870, to indicate the “paralysis” occurring in the advanced disease stages.24
In the early 1990s, Gibb and Lees defined bradykinesia as “slowness of voluntary movement with a progressive reduction in speed and amplitude of repetitive actions.”3,25 Accordingly, the current diagnostic criterion for PD4 defines bradykinesia as “slowness of movement AND decrement in amplitude or speed (or progressive hesitations/halts) as movements are continued,” due to two main clinical assumptions: (1) these signs are generally present during the examination of a PD patient and (2) in parkinsonism caused by PD, a decline in either speed or amplitude or both is seen as repetitive movements are continued, a feature usually not observed in other parkinsonisms.4 The concept of bradykinesia is even more complicated in AP.10 The diagnostic criterion for progressive supranuclear palsy (PSP)5 indicates akinesia, not bradykinesia, as a core feature and used this term synonymously with parkinsonism. The criteria for multiple system atrophy (MSA)1 diagnosis require bradykinesia to be associated with rigidity and tremor, without specifying what features would qualify as bradykinesia. Finally, the criteria for corticobasal syndrome (CBS)2 use bradykinesia and akinesia interchangeably. Only the clinical criteria for dementia with Lewy bodies (DLB)6 refer to bradykinesia as slowness of movement and decrement in amplitude or speed as in PD.
Critical Issues with the Current Definition of Bradykinesia
Clinical Inconsistencies
First, the conceptualization of bradykinesia according to the current criteria associates this specific movement disorder with parkinsonism.4 This peculiarity does not apply to other disorders of movement, that is, dystonia, chorea, myoclonus, and tremor, which may instead underlie a wide spectrum of etiologies with no a priori restriction.26,27 In this regard, it should also be acknowledged that the current definition of bradykinesia does not necessarily apply to all cases of parkinsonism. Clinical experience indicates that bradykinesia features can be variable in patients, even in those who have a clear diagnosis of PD.
Another important issue of bradykinesia concerns AP. Although a decline in velocity or amplitude is usually seen with ongoing voluntary movements in PD, this feature is uncommon in other parkinsonisms.4,10,28 PSP is usually characterized by a marked reduction in velocity and amplitude without a sequence effect.28,29 Note that in PSP, or PD patients when there is marked hypokinesia, the amplitude of movement may be reduced, and the number of taps appears to increase during the task, a phenomenon also referred to as tachykinesia.13 In these cases, however, the speed at which the individual movements are performed is nonetheless slow. Thus, the current definition of bradykinesia, which includes a sequence effect, does not necessarily fit all cases of parkinsonism. Again, studies in patients with MSA, CBS, or DLB are too limited to allow even preliminary conclusions for these conditions.28 However, it should be emphasized that these other degenerative parkinsonisms have motor signs that hamper the motor assessment, for example, the presence of bradyphrenia in DLB, myoclonic jerks in MSA, and apraxia in CBS.30,31
If we broaden the field of observation and include non-parkinsonian neurological disorders, several clinical studies specifically refer to the presence of bradykinesia in these disorders.17 Examples of these conditions include motor neuron diseases, psychiatric conditions, other neurodegenerative diseases, and even some hyperkinetic disorders like dystonia (for which the term was originally coined), chorea, and essential tremor (ET),17 where slowness of movement has even been included among the so-called “soft signs” needed for the diagnosis of “ET-plus.”26,32 In these conditions, experimental studies indicate that bradykinesia can be explained by pathophysiological mechanisms that differ from those observed in parkinsonism.10,16,33–36 We can therefore question whether its use is appropriate.17 In particular, the sequence effect is often absent in most non-parkinsonian conditions, although this has not been adequately investigated.17
In addition, there is a discontinuity between the current bradykinesia definition and the bradykinesia assessment in patients using the MDS-UPDRS.14 The assessment of spontaneous and voluntary movements in the MDS-UPDRS scale is properly kept separate. Again, concerning voluntary movements the scale requires separate examination for speed, amplitude, hesitations, halts, or decrement (eg, sequence effect). However, it is assumed that the various movement abnormalities have the same weight on the global score. This implies that a subject can be classified as bradykinetic irrespective of the specific abnormality that is identified to score MDS-UPDRS ≥ 1 in the specific items, that is, even when there is no evidence of the sequence effect on examination. Finally, concerning the patient evaluation, the clinical criteria emphasize the presence of limb bradykinesia for parkinsonism definition.4 However, clinical and experimental evidence documented that bradykinesia may affect other body regions. Involvement of the face, voice, or walking is missing from the current bradykinesia definition.4 For example, although progressive shortening of step length or festination has been reported in PD, and often, though not always, precedes a “motor block” (another term for hesitations/halts), also known as freezing of gait,37,38 the sequence effect of voluntary facial movement or the voice has not been documented.14,15,39
Pathophysiological Inconsistencies
A point of criticism regarding the current definition of bradykinesia is to use a single term to denote motor alterations that depend on the impaired control of both spontaneous and voluntary movements, which are instead physiologically distinct. Furthermore, the alterations that fall under the definition of bradykinesia of voluntary movements are often tangled together despite distinct underlying mechanisms. The observation that bradykinesia and hypokinesia often coexist in the same patient is in part explained by biomechanical principles. In healthy individuals there is an almost-linear relationship between movement velocity and amplitude40,41; that is, movement velocity is proportional to movement amplitude, and the relationship is approximately constant over a wide range of values,41 though the relationship can be lost in PD.42 Also, bradykinesia and hypokinesia improve significantly in PD when patients are treated with dopaminergic drugs or deep brain stimulation (DBS) indicating that these two motor abnormalities share a common pathophysiological background.43–46 However, bradykinesia and hypokinesia may vary in terms of sensitivity to change in response to dopaminergic medication or surgery, possibly due to the multi-level network effects of these treatments.10,45,47–49 Thus, it seems more appropriate to interpret bradykinesia and hypokinesia as separate movement abnormalities.
Other aspects to consider relate to impaired performance of repetitive and continuous movements, that is, the sequence effect. This abnormality has never been documented during spontaneous movements,10,39 for example, spontaneous facial expressions or arm pendulum movements during walking, and therefore the current definition of bradykinesia does not fit with spontaneous movement abnormalities in parkinsonism. Again, concerning voluntary movements, the current bradykinesia definition cannot be applied when single movements are tested because the sequence effect can be documented only during repetitive and continuous movements.10,16 Nevertheless, experimental findings indicate that in some cases, PD patients may not have a measurable sequence effect during finger tapping or writing.10,28,29,46,50,51 Some experimental evidence supports a common pathophysiological mechanism between bradykinesia, hypokinesia, and sequence effect in PD that involves β oscillations at the basal ganglia level.52,53 Thus, the increased power of β-band oscillations (and the overall duration of β bursts) possibly represents a fundamental pathophysiological substrate of PD, to which further mechanisms superimpose, culminating in specific motor abnormalities.52,53 However, there is also evidence indicating that the pathophysiological mechanisms underlying bradykinesia, hypokinesia, and sequence effect are distinct. The most convincing observation again derives from differences in response to dopaminergic drugs or DBS. Although dopaminergic replacement therapy improves movement velocity and amplitude, there is no evidence showing that dopaminergic replacement improves the sequence effect.10,45,46,50,51,54 Furthermore, experimental studies using neurophysiological techniques or neuroimaging have demonstrated pathophysiological differences between PD patients with and without the sequence effect. These differences are primarily observed at the cortical and cerebellar levels, which are two of the most important nodes in the pathophysiology of PD.10,40,45,51,55
Finally, progressive hesitations/halts specifically refer to increased variability in the regularity and timing of repetitive movements, particularly those characterized by the alternating contraction and relaxation of agonist/antagonist muscle groups.12 Similar alterations are also frequently observed in cerebellar disorders. Therefore, the interpretation of possible underlying pathophysiological mechanisms remains speculative. For example, it is possible that progressive hesitations/halts in parkinsonism reflect the central timing impairment during the performance of voluntary movements or the pathophysiological involvement of the cerebellum.55,56
Why Redefine Bradykinesia
A new bradykinesia definition would have important practical implications. First, it would allow us to harmonize the concept of bradykinesia with other disorders of movement, where a certain disorder is not necessarily linked to a specific etiology.26,27 Again, it would help avoid terminological contradictions that result in confusion in the characterization of patients’ motor alterations both in clinical practice and in scientific work. In this regard, considering the various alterations separately, rather than incorporating them into a single term, would make it possible to better characterize a clinical phenotype, ideally with the aid of neurophysiology or novel technologies. Redefining bradykinesia would allow a better evaluation of patients and a more accurate description of the effect of therapies, which is variable in relation to the specific motor abnormality observed.10
Toward a New Definition of Bradykinesia
We suggest redefining bradykinesia by following three major principles.
Distinguishing voluntary and automatic/spontaneous movements.
Although bradykinesia and its associated features would refer to voluntary movement abnormalities, an alternative term is necessary to specifically refer to automatic/spontaneous movement abnormalities. We propose the term “oligokinesia” to describe the latter phenomena (Table 1).
TABLE 1.
The “bradykinesia complex”
| Feature | Definition | Types of movements being affected |
|---|---|---|
| Bradykinesia | Reduced velocity of movements | Single or repetitive, alternating and continuous movements (limbs and body axis) |
| Hypokinesia | Reduced amplitude of movements | Single or repetitive, alternating and continuous movements (limbs and body axis) |
| Sequence effect | Progressive reduction in amplitude and/or velocity | Repetitive, alternating, and continuous movements (limbs) |
| Hesitations/halts | Irregularities in movement timing | Repetitive, alternating, and continuous movements (limbs) |
| Akinesia | Inability to perform a movement | Single, repetitive, alternating, and continuous movements (limbs and body axis) |
| Oligokinesia | Reduction/lack of spontaneous/ automatic movements | Spontaneous blinking, spontaneous facial expressions, pendular movements of the upper limbs during walking |
Note: The definitions of bradykinesia and related features (hypokinesia, sequence effect, and hesitations/halts) are summarized.
Dissecting the “bradykinesia complex.”
Bradykinesia-related terms are best used in their original etymological meaning10 (Table 1). In line with this reasoning, normal movement is indicated by the term eukinesia (Fig. 1). The term bradykinesia would specifically refer to the slowness of voluntary movements, which, however, may be accompanied by associated features, depending on the motor task performed. These include (1) hypokinesia, (2) sequence effect, and (3) hesitations/halts, in variable combinations. Note that in this work, we have used the term sequence effect instead of decrement. Decrement specifically refers to the progressive decrease of a movement parameter; however, the deterioration of motor performance in PD during repetitive movements may also manifest itself in the form of an increase in the time required to execute the motor performance.10 We, therefore, believe that the correct term to use, which is all-encompassing, is sequence effect and not decrement. Whenever present, the various movement abnormalities should be separately specifically reported in the description of the clinical phenotype. Therefore, if slowness of movement is present, then there is “bradykinesia.” If a sequence effect is also present, this would be referred to as “bradykinesia with sequence effect.” Additional elements, for example, hypokinesia, hesitations/halts, or oligokinesia, can also be added to complete a full movement description (see Axis 1 later). The term akinesia should be used for conditions in which the assessment is marred by the patient’s inability to perform voluntary movement, and therefore a more detailed phenomenological description is not possible.
FIG. 1.
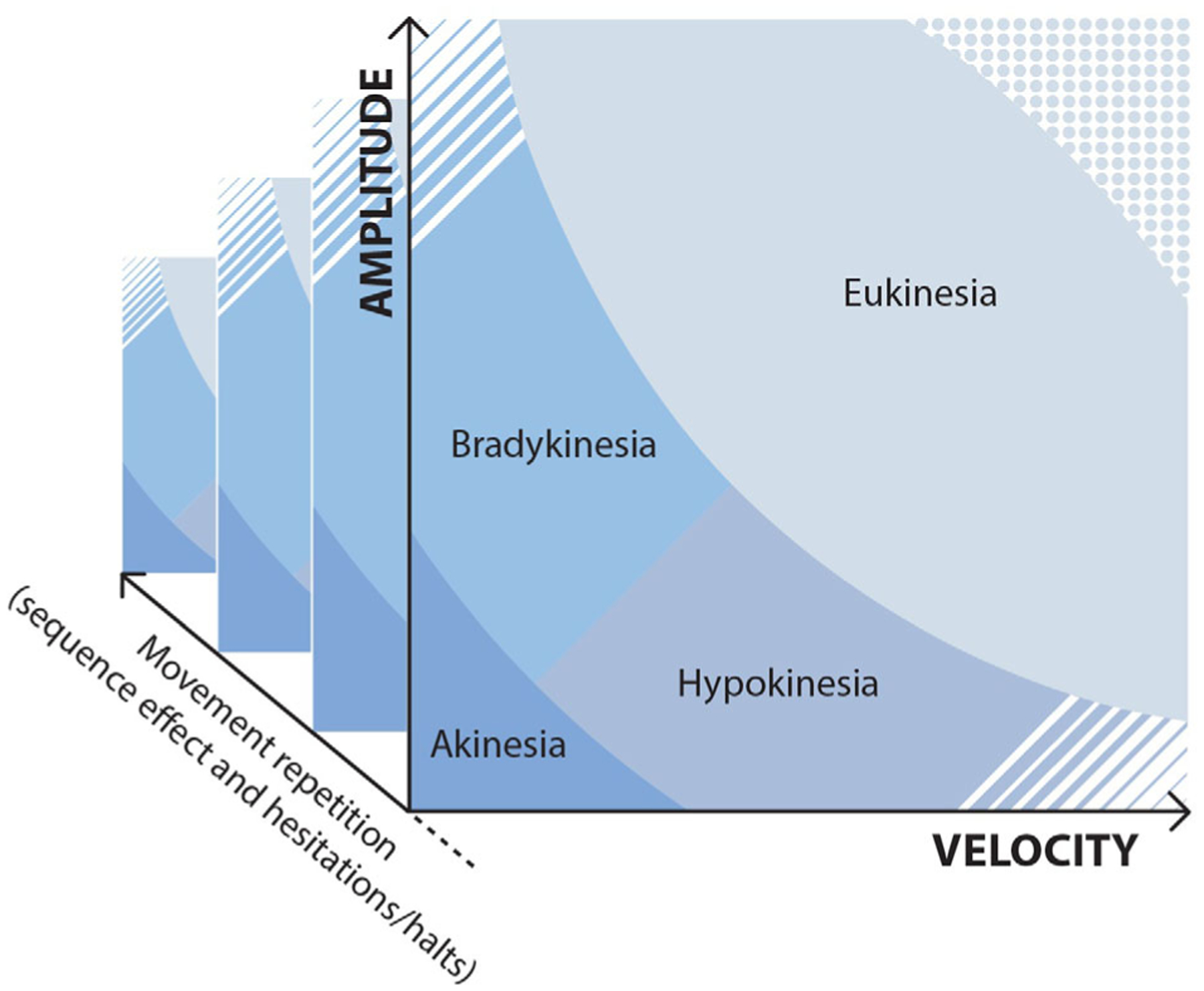
The figure schematically represents the two main dimensions of voluntary movement, namely velocity (x-axis) and amplitude (y-axis). Based on these two values, the movement can be considered predominantly bradykinetic if of reduced velocity or hypokinetic if of reduced amplitude. If both parameters are markedly reduced, the movement is defined as akinetic. Conversely, amplitude and velocity may be within a normal range (eukinesia). In some cases, velocity and amplitude may have values that exceed normal limits, as observed in some hyperkinesias, for example, ballismus. The dashed area at the lower right indicates markedly reduced amplitude but high velocity movements. The dashed area at the upper left indicates markedly reduced velocity but high amplitude movements. Dotted and dashed areas overall indicate unlikely movement values. With the repetition of the movement (z-axis), changes in motor performance can be observed in terms of amplitude and/or velocity reduction (sequence effect) or motor hesitations/halts. Note that although in this figure distinct limits are depicted to differentiate the various areas, these limits may be blurred in the reality. Again, the limits for the definition of movement abnormalities are arbitrary and may be modified in future experimental studies, though the conceptual framework of the new definition of bradykinesia elaborated here should remain unchanged.
Establishing a dual-axis approach.
Redefining bradykinesia according to the aforementioned principles implies that the phenomenological description of the patient is valid, regardless of the underlying etiology. As is already the case with other movement disorders,26,27 this description can fit in the first of two distinct axes for the approach to bradykinesia: Axis I, which describes the major phenomenology of bradykinesia and related terms in a given patient, and Axis II, which addresses the etiology (Table 2). Axis I may include (1) type of movements (voluntary vs. spontaneous movement and single vs. repetitive movements), (2) major movement features, (3) body distribution (limbs and/or axial districts), and (4) possible associated features (isolated and/or combined with other neurological disorders). Axis II may instead describe the possible etiology underlying the motor abnormality, that is, (1) parkinsonism, (2) non-parkinsonian conditions (due to a known specific cause), or (3) unknown etiology.
TABLE 2.
Proposed classification of bradykinesia and related motor abnormalities
| Axis I. Phenomenology | |
|---|---|
| • Type of movements | Voluntary (single and repetitive or continuous) vs. spontaneous and semi-automatic movement (eg, face expressions, walking) |
| • Major movement features | Bradykinesia (movement slowness), hypokinesia (reduced movement amplitude), sequence effect, hesitations/halts (only repetitive movements), akinesia, oligokinesia (only spontaneous movements) |
| • Body distribution | Limbs and/or body axis (face, voice, trunk) |
| • Associated clinical features | Isolated or combined with other neurological symptoms |
| Axis II. Etiology | |
| • Parkinsonism | PD, PSP, MSA, CBS, DLB, and others |
| • Non-parkinsonian conditions | Hyperkinetic movement disorders and other neurological conditions |
| • Unknown etiology | |
Abbreviations: PD: Parkinson’s disease; PSP: progressive supranuclear palsy; MSA: multiple system atrophy; CBS: corticobasal syndrome; DLB: dementia Lewy body.
Tentative Implications and Conclusions
In this paper we addressed the general structure of a new approach to defining bradykinesia, and we analyzed the phenomenological characteristics of bradykinesia (Axis I). We suggest that when there is a combination of motor alterations, that is, bradykinesia with sequence effect and any additional features, the clinical picture is highly suggestive of parkinsonism. In contrast, isolated bradykinesia is a non-specific finding that may be present in various non-parkinsonian conditions of known or unknown etiology. Further studies will be needed to further clarify the relationships between Axis I and Axis II for the purpose of bradykinesia definition. The combination of these two sets of descriptors may provide meaningful information on any bradykinetic patient, avoid the inclusion of distinct motor alterations with different pathophysiological backgrounds in one single term and the use of inconsistent terminology, and serve as a basis for the development of better research and treatment strategies.
It should be noted that the approach to the definition of bradykinesia proposed here may be applicable to both clinical and experimental settings. Although bradykinesia, hypokinesia, sequence effect, hesitations/halts, akinesia, and oligokinesia can be identified in many patients on clinical grounds only, there is a proportion of patients in whom these abnormalities can be hard to distinguish. Laboratory methods of objective movement quantification, such as kinematic analysis, could be applied in these cases.57,58
In conclusion, the proper recognition and classification of bradykinesia and related features, that is, the “bradykinesia complex,” may improve further research efforts and increase the accuracy of its use in distinguishing different parkinsonisms from one another and between parkinsonian from non-parkinsonian conditions.
Acknowledgment:
The authors wish to thank Marina Milano (marina. milano3@gmail.com) for the graphic design of Figure 1. Open Access Funding provided by Universita degli Studi di Roma La Sapienza within the CRUI-CARE Agreement.
Footnotes
Conflict of Interest
None of the authors have any potential conflicts of interest to disclose.
Relevant conflicts of interest/financial disclosures: Nothing to report.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- 1.Wenning GK, Stankovic I, Vignatelli L, et al. The movement disorder society criteria for the diagnosis of multiple system atrophy. Mov Disord 2022;37(6):1131–1148. 10.1002/mds.29005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong MJ, Litvan I, Lang AE, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology 2013;80(5):496–503. 10.1212/WNL.0b013e31827f0fd1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berardelli A, Wenning GK, Antonini A, et al. EFNS/MDS-ES/ENS [corrected] recommendations for the diagnosis of Parkinson’s disease. Eur J Neurol 2013;20(1):16–34. 10.1111/ene.12022 [DOI] [PubMed] [Google Scholar]
- 4.Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 2015;30(12):1591–1601. 10.1002/mds.26424 [DOI] [PubMed] [Google Scholar]
- 5.Höglinger GU, Respondek G, Stamelou M, et al. Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov Disord 2017;32(6):853–864. 10.1002/mds.26987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB consortium. Neurology 2017;89(1):88–100. 10.1212/WNL.0000000000004058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bloem BR, Okun MS, Klein C. Parkinson’s disease. Lancet 2021; 397(10291):2284–2303. 10.1016/S0140-6736(21)00218-X [DOI] [PubMed] [Google Scholar]
- 8.Schilder JCM, Overmars SS, Marinus J, van Hilten JJ, Koehler PJ. The terminology of akinesia, bradykinesia and hypokinesia: past, present and future. Parkinsonism Relat Disord 2017;37:27–35. 10.1016/j.parkreldis.2017.01.010 [DOI] [PubMed] [Google Scholar]
- 9.Goedert M, Compston A. Parkinson’s disease—the story of an eponym. Nat Rev Neurol 2018;14(1):57–62. 10.1038/nrneurol.2017.165 [DOI] [PubMed] [Google Scholar]
- 10.Bologna M, Paparella G, Fasano A, Hallett M, Berardelli A. Evolving concepts on bradykinesia. Brain 2020;143(3):727–750. 10.1093/brain/awz344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu T, Hallett M, Chan P. Motor automaticity in Parkinson’s disease. Neurobiol Dis 2015;82:226–234. 10.1016/j.nbd.2015.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hess CW, Hallett M. The phenomenology of Parkinson’s disease. Semin Neurol 2017;37(2):109–117. 10.1055/s-0037-1601869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hobson DE. Clinical manifestations of Parkinson’s disease and parkinsonism. Can J Neurol Sci 2003;30(Suppl 1):S2–S9. 10.1017/s0317167100003188 [DOI] [PubMed] [Google Scholar]
- 14.Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-sponsored revision of the unified Parkinson’s disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 2008;23(15):2129–2170. 10.1002/mds.22340 [DOI] [PubMed] [Google Scholar]
- 15.Goetz CG, Fahn S, Martinez-Martin P, et al. Movement disorder society-sponsored revision of the unified Parkinson’s disease rating scale (MDS-UPDRS): process, format, and clinimetric testing plan. Mov Disord 2007;22(1):41–47. 10.1002/mds.21198 [DOI] [PubMed] [Google Scholar]
- 16.Berardelli A, Rothwell JC, Thompson PD, Hallett M. Pathophysiology of bradykinesia in Parkinson’s disease. Brain 2001;124(Pt 11): 2131–2146. [DOI] [PubMed] [Google Scholar]
- 17.Paparella G, Fasano A, Hallett M, Berardelli A, Bologna M. Emerging concepts on bradykinesia in non-parkinsonian conditions. Eur J Neurol 2021;1:2403–2422. 10.1111/ene.14851 [DOI] [PubMed] [Google Scholar]
- 18.Verger H, Cruchet R. Trait e de Torticolis Spasmodiques. Paris: Masson & Cie; 1907. [Google Scholar]
- 19.Verger H, Cruchet R. Les etats parkinsoniens et le syndrome bradykinetique; Paris: Librairie J.-B. Baillière et Fils; 1925:15–19.
- 20.Ross J A Treatise on the Diseases of the Nervous System. London: William Wood; 1883:1. [Google Scholar]
- 21.Foerster O Zur Analyse und Pathophysiologie der striaren Bewegungsstorungen. Z Gesamte Neurol Psychiatr 1921;73:1–169. [Google Scholar]
- 22.Lotmar F Das hypokinetisch-hypertonische syndrom 5. In: Bumke OF, ed. Handbuch der Neurologie. Vol. 5; Berlin: Springer; 1936:429–430. [Google Scholar]
- 23.Mobius P Ueber akinesia algera. Dtsch Z für Nervenheilkd 1891;1: 121–135. [Google Scholar]
- 24.Jaccoud S Trait e de pathologie interne. Paris: Adrien Delahaye; 1870:410–426. [Google Scholar]
- 25.Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry 1988;51(6):745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhatia KP, Bain P, Bajaj N, et al. Consensus statement on the classification of tremors. From the task force on tremor of the International Parkinson and Movement Disorder Society: IPMDS task force on tremor consensus statement. Mov Disord 2018;33(1):75–87. 10.1002/mds.27121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albanese A, Bhatia K, Bressman SB, et al. Phenomenology and classification of dystonia: a consensus update: dystonia: phenomenology and classification. Mov Disord 2013;28(7):863–873. 10.1002/mds.25475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bologna M, Suppa A, Di Stasio F, Conte A, Fabbrini G, Berardelli A. Neurophysiological studies on atypical parkinsonian syndromes. Parkinsonism Relat Disord 2017;42:12–21. 10.1016/j.parkreldis.2017.06.017 [DOI] [PubMed] [Google Scholar]
- 29.Ling H, Massey LA, Lees AJ, Brown P, Day BL. Hypokinesia without decrement distinguishes progressive supranuclear palsy from Parkinson’s disease. Brain 2012;135(Pt 4):1141–1153. 10.1093/brain/aws038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zadikoff C, Lang AE. Apraxia in movement disorders. Brain 2005; 128(Pt 7):1480–1497. 10.1093/brain/awh560 [DOI] [PubMed] [Google Scholar]
- 31.Okuma Y, Fujishima K, Miwa H, Mori H, Mizuno Y. Myoclonic tremulous movements in multiple system atrophy are a form of cortical myoclonus. Mov Disord 2005;20(4):451–456. 10.1002/mds.20346 [DOI] [PubMed] [Google Scholar]
- 32.Lenka A, Pandey S. Essential tremor: five new things. Neurol Clin Pract 2022;12(2):183–186. 10.1212/CPJ.0000000000001145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bologna M, Guerra A, Colella D, et al. Bradykinesia in Alzheimer’s disease and its neurophysiological substrates. Clin Neurophysiol 2020;131(4):850–858. 10.1016/j.clinph.2019.12.413 [DOI] [PubMed] [Google Scholar]
- 34.Colella D, Guerra A, Paparella G, et al. Motor dysfunction in mild cognitive impairment as tested by kinematic analysis and trans-cranial magnetic stimulation. Clin Neurophysiol 2020;132(2): 315–322. [DOI] [PubMed] [Google Scholar]
- 35.Paparella G, Ceccanti M, Colella D, et al. Bradykinesia in motoneuron diseases. Clin Neurophysiol 2021;132(10):2558–2566. 10.1016/j.clinph.2021.08.006 [DOI] [PubMed] [Google Scholar]
- 36.Bologna M, Paparella G, Colella D, et al. Is there evidence of bradykinesia in essential tremor? Eur J Neurol 2020;12:1501–1509. 10.1111/ene.14312 [DOI] [PubMed] [Google Scholar]
- 37.Fasano A, Bloem BR. Gait disorders. Continuum 2013;19(5): 1344–1382. 10.1212/01.CON.0000436159.33447.69 [DOI] [PubMed] [Google Scholar]
- 38.Iansek R, Huxham F, McGinley J. The sequence effect and gait festination in Parkinson disease: contributors to freezing of gait? Mov Disord 2006;21(9):1419–1424. 10.1002/mds.20998 [DOI] [PubMed] [Google Scholar]
- 39.Bologna M, Fabbrini G, Marsili L, Defazio G, Thompson PD, Berardelli A. Facial bradykinesia. J Neurol Neurosurg Psychiatry 2013;84(6):681–685. 10.1136/jnnp-2012-303993 [DOI] [PubMed] [Google Scholar]
- 40.Guerra A, Colella D, Giangrosso M, et al. Driving motor cortex oscillations modulates bradykinesia in Parkinson’s disease. Brain 2022;145(1):224–236. 10.1093/brain/awab257 [DOI] [PubMed] [Google Scholar]
- 41.Fitts PM. The information capacity of the human motor system in controlling the amplitude of movement. 1954. J Exp Psychol Gen 1992; 121(3):262–269. 10.1037//0096-3445.121.3.262 [DOI] [PubMed] [Google Scholar]
- 42.Hallett M, Khoshbin S. A physiological mechanism of bradykinesia. Brain 1980;103(2):301–314. [DOI] [PubMed] [Google Scholar]
- 43.Suppa A, Bologna M, Conte A, Berardelli A, Fabbrini G. The effect of L-dopa in Parkinson’s disease as revealed by neurophysiological studies of motor and sensory functions. Expert Rev Neurother 2017;17(2):181–192. 10.1080/14737175.2016.1219251 [DOI] [PubMed] [Google Scholar]
- 44.Timmermann L, Braun M, Groiss S, et al. Differential effects of levodopa and subthalamic nucleus deep brain stimulation on bradykinesia in Parkinson’s disease. Mov Disord 2008;23(2):218–227. 10.1002/mds.21808 [DOI] [PubMed] [Google Scholar]
- 45.Bologna M, Guerra A, Paparella G, et al. Neurophysiological correlates of bradykinesia in Parkinson’s disease. Brain 2018;141(8): 2432–2444. 10.1093/brain/awy155 [DOI] [PubMed] [Google Scholar]
- 46.Bologna M, Latorre A, Di Biasio F, et al. The effect of L-Dopa/carbidopa intestinal gel in Parkinson disease assessed using neurophysiologic techniques. Clin Neuropharmacol 2016;39(6):302–305. 10.1097/WNF.0000000000000184 [DOI] [PubMed] [Google Scholar]
- 47.Michely J, Volz LJ, Barbe MT, et al. Dopaminergic modulation of motor network dynamics in Parkinson’s disease. Brain 2015;138(Pt 3):664–678. 10.1093/brain/awu381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kimber TE, Tsai CS, Semmler J, Brophy BP, Thompson PD. Voluntary movement after pallidotomy in severe Parkinson’s disease. Brain 1999;122(Pt 5):895–906. 10.1093/brain/122.5.895 [DOI] [PubMed] [Google Scholar]
- 49.Espay AJ, Giuffrida JP, Chen R, et al. Differential response of speed, amplitude, and rhythm to dopaminergic medications in Parkinson’s disease. Mov Disord 2011;26(14):2504–2508. 10.1002/mds.23893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bologna M, Leodori G, Stirpe P, et al. Bradykinesia in early and advanced Parkinson’s disease. J Neurol Sci 2016;369:286–291. 10.1016/j.jns.2016.08.028 [DOI] [PubMed] [Google Scholar]
- 51.Wu T, Zhang J, Hallett M, Feng T, Hou Y, Chan P. Neural correlates underlying micrographia in Parkinson’s disease. Brain 2016; 139(Pt 1):144–160. 10.1093/brain/awv319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lofredi R, Tan H, Neumann WJ, et al. Beta bursts during continuous movements accompany the velocity decrement in Parkinson’s disease patients. Neurobiol Dis 2019;127:462–471. 10.1016/j.nbd.2019.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steiner LA, Neumann WJ, Staub-Bartelt F, et al. Subthalamic beta dynamics mirror Parkinsonian bradykinesia months after neurostimulator implantation. Mov Disord 2017;32(8):1183–1190. 10.1002/mds.27068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kang SY, Wasaka T, Shamim EA, et al. Characteristics of the sequence effect in Parkinson’s disease. Mov Disord 2010;25(13): 2148–2155. 10.1002/mds.23251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu T, Hallett M. The cerebellum in Parkinson’s disease. Brain 2013;136(Pt 3):696–709. 10.1093/brain/aws360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fasano A, Laganiere SE, Lam S, Fox MD. Lesions causing freezing of gait localize to a cerebellar functional network. Ann Neurol 2017;81(1):129–141. 10.1002/ana.24845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heldman DA, Espay AJ, LeWitt PA, Giuffrida JP. Clinician versus machine: reliability and responsiveness of motor endpoints in Parkinson’s disease. Parkinsonism Relat Disord 2014;20(6): 590–595. 10.1016/j.parkreldis.2014.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hasan H, Athauda DS, Foltynie T, Noyce AJ. Technologies assessing limb bradykinesia in Parkinson’s disease. J Parkinsons Dis 2017;7(1):65–77. 10.3233/JPD-160878 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


