Abstract
An abdominal inflammatory myofibroblastic tumor (AIMT), is a rare benign tumor composed of inflammatory and other mesenchymal cells. It can affect the entire body, predominantly in children and young adults. The diagnosis is challenging considering the wide clinical presentation and can often be mistaken for malignant tumors.
We report a rare case of a 46-year-old female patient, who presented with intermittent abdominal pain weight loss, and an abdominal palpable mass. Abdominal ultrasound found a well-defined 18 cm, rounded mass, with solid and cystic components. Abdominal CT demonstrated a well-defined, hypodense, retro gastric mass of 20 cm, with thickened wall and heterogenous enhancement. The mass had contact with the pancreatic tail, transverse colon, spleen, left kidney pedicles, abdominal aorta, superior mesenteric vein, and mesaraic trunk with no invasion signs. The mass was initially thought to be pancreatic cancer, but given the large size, other diagnoses like sarcoma, lymphoma, or abdominal hydatid cyst were suggested. Endoscopic ultrasound found a rounded retro gastric mass of 18/12 cm, with a thickened wall and well-limited calcifications. The content was both cystic and solid with mobile vegetations, with no visible Doppler flow. The mass had contact with the body and tail of the pancreas, spleen hilum, the upper pole of the spleen, and the hepatic pedicle behind, with no invasion sign. After a multidisciplinary team meeting, a decision was taken to perform surgical resection with mass resection, distal splenopancreatectomy, and transverse and sigmoid colectomy. Pathological and immunostaining results were consistent with inflammatory pseudotumor. The postoperative recovery was uncomplicated. The patient remains asymptomatic with no obvious signs of metastasis or recurrence.
AIMT represents a reel diagnostic challenge. Clinical symptoms are unspecific. Radiological and endoscopic features can often be mistaken for malignant tumors. Surgical management remains to be the best therapeutic option. We report a rare case of AIMT treated by surgery with complete resection. We suggested a long-term follow-up given the local recurrence risk.
Keywords: case report, surgery, myofibroblastic, pseudotumor, inflammatory
Introduction
An inflammatory myofibroblastic tumor (IMT), also known as an inflammatory pseudotumor or myofibroblastic pseudotumor, is a rare benign tumor composed of various inflammatory and mesenchymal cells [1]. It can affect the entire body and occurs predominantly in children and young adults [2]. The diagnosis is challenging given the wide clinical presentation and can often be mistaken for malignant tumors. We report a clinical case of abdominal IMT (AIMT) presenting as a pancreatic mass with a review of the relevant literature.
Case presentation
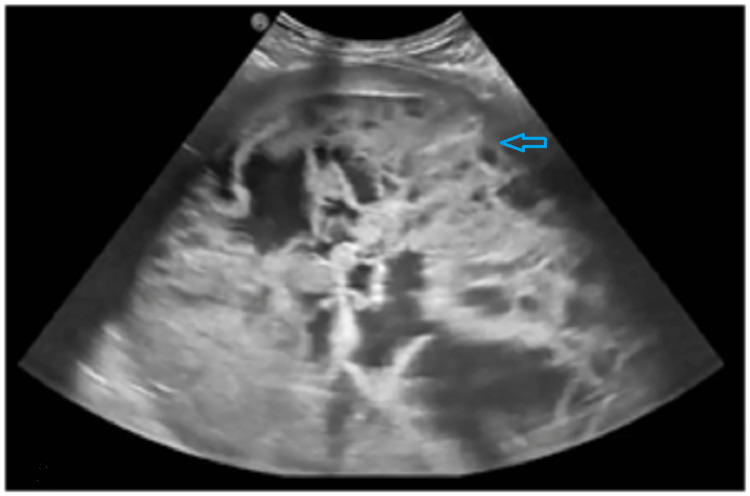
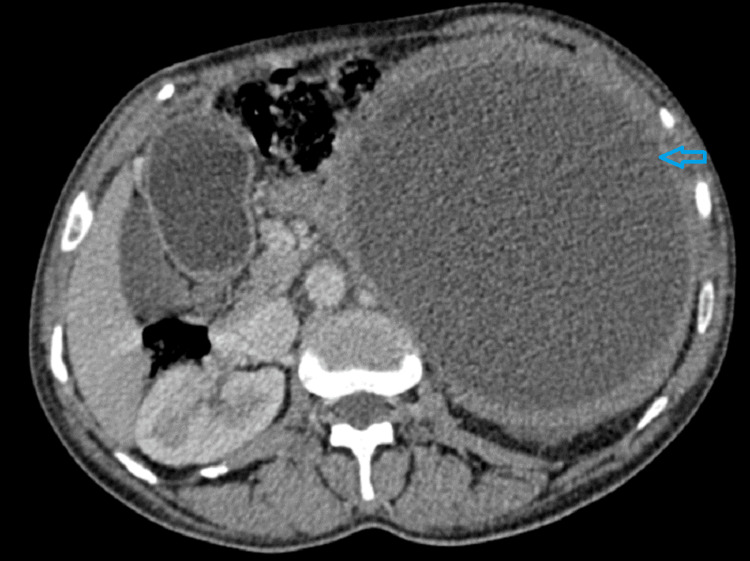
A 46-year-old woman without any past medical history was referred to our gastroenterology unit in February 2022 to evaluate an intra-abdominal mass detected by ultrasound in a nearby hospital. The patient had intermittent abdominal pain for three months. She reported a weight loss of 2 Kg over the past two months. Physical examination revealed a palpable mass of 18 cm, extending from the left hypochondrium to the left flank. Laboratory results, including complete blood count, C-reactive protein, and tumor markers, were all within reference ranges. Abdominal ultrasound found a well-defined, heterogeneous, rounded abdominal mass with a solid and cystic component measuring 18 cm (Figure 1). Abdominal computed tomography (CT) demonstrated a well-defined, hypodense retrogastric mass of 20 cm with thickened wall and heterogeneous enhancement (Figure 2). The mass was in contact with the pancreatic tail, transverse colon, spleen, left kidney pedicles, abdominal aorta, superior mesenteric vein, and mesaraic trunk without invasion signs. The mass origin was difficult to specify, given its size. Several differential diagnoses were suggested, including pancreatic adenocarcinoma, sarcoma, lymphoma, spleen epithelial cyst, and abdominal hydatid cyst.
Figure 1. Abdominal ultrasound image.
Ultrasound showed a well-defined, heterogeneous, rounded abdominal mass, with solid and cystic components.
Figure 2. Abdominal computed tomography image.
The image is showing a well-defined, hypodense, retrogastric 20-cm mass with thickened wall and heterogenous enhancement.
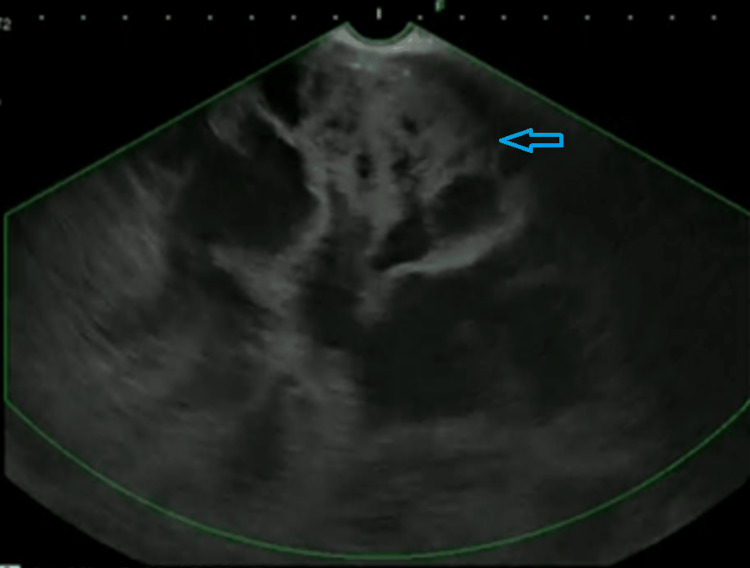
Endoscopic ultrasound (EUS) revealed a round retrogastric mass of 18 cm by 12 cm, with flaky and well-limited calcifications; the content was both cystic and solid with mobile vegetation. It had no visible Doppler flow, and the mass had contact with the body and tail of the pancreas, the spleen hilum, the upper pole of the spleen, and the hepatic pedicle behind without invasion sign. The mass was in contact with the gastric wall with no invasion sign (Figure 3).
Figure 3. Endoscopic ultrasound image.
Endoscopic ultrasound showed a rounded retrogastric mass with thickened wall. The content was both cystic and solid with mobile vegetation.
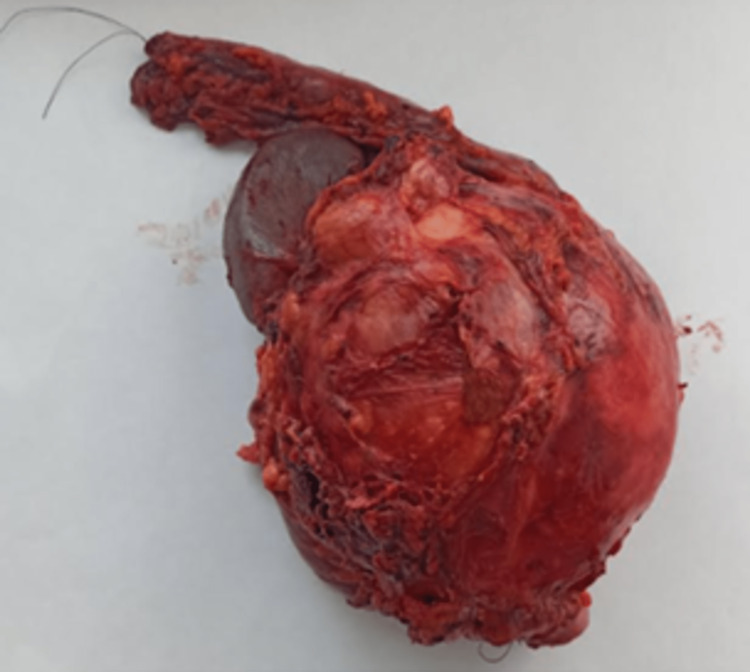
After a multidisciplinary team meeting, the decision was made to perform a laparotomy for diagnosis and treatment. Exploration revealed a large cystic mass of 20 cm in close contact with the tail pancreas, spleen, left kidney, transverse, and sigmoid colon, with multiple peritoneal adhesions. No liver lesions or peritoneal carcinomatosis were detected. It was decided to perform the mass resection, with distal splenopancreatectomy and transverse and sigmoid colectomy (Figure 4).
Figure 4. A large 20-cm mass completely resected by laparotomy.
Macroscopically, the mass had a cystic aspect with dark fluid content, thickened fibrous wall, and vegetation. Histological study revealed a well-limited myofibroblastic tumor proliferation, with bundles of regular fibroblasts and clusters of dense collagen. Congestive vessels and inflammatory infiltrate of lymphocytes, plasma cells, and eosinophils were detected. Tumor proliferation adheres intimately to the pancreas, spleen, and transverse and sigmoid colon with no invasion signs. The immunohistochemical study stained negatively for PS100, STAT6, ALK, desmin, CD117, and CD34. A diagnosis of an intraabdominal pseudo-inflammatory tumor was determined, given the histopathological results. The patient’s postoperative recovery was uncomplicated. She was discharged from the hospital on day seven. The patient is asymptomatic with no obvious signs of metastasis or recurrence over the 15-month follow-up.
Discussion
IMT is rare and benign and often occurs in young patients [1,2]. AIMT is extremely rare and includes the retroperitoneum, pancreas, liver, mesentery, kidney, esophagus, stomach, small intestine, and colon [3-5]. While the etiology of AIMT is unknown, several causes are suspected, including infection with the Epstein-Barr virus, cytomegalovirus, mycobacteria, trauma, irradiation, or surgery [6,7]. Our patient had no past medical history and none of these etiologies. Although AIMTs are benign neoplasms, a rare incidence of malignant transformation has been reported [7]. World Health Organization Classification of Soft Tissue and Bone currently classifies IMT as intermediate neoplasms [8,9]. The clinical symptoms vary according to the involved organ. Although digestive symptoms in AIMT are nonspecific, patients can experience abdominal discomfort, dyspepsia, weight loss, and palpable mass (as was seen in our case). Laboratory findings are nonspecific, and iron deficiency anemia can be detected [10]. The similarities with malignant tumors represent a diagnostic challenge. Additionally, the radiological characteristics of AIMT are not specific. After CT contrast enhancement, AIMT can appear as a large heterogeneous mass with necrotic hypodense areas, well-defined margins, and well-vascularized solid tumors [11,12]. Calcifications and fatty components have been described in nonenhancement CTs [12]. In our case, an ultrasound found a well-defined heterogeneous, large abdominal mass, and abdominal CT demonstrated a well-defined, hypodense retrogastric mass of 20 cm, with heterogeneous enhancement.
EUS is performed for better diagnostic accuracy if radiological results were inconclusive for subsequent treatment. The role of EUS in diagnosing pancreatic masses, non-pancreatic, and retroperitoneal lesions is well established [13]. Erikson et al. found that EUS can provide superior results to CT about the precise origin of retroperitoneal masses and/or its involvement of adjacent organs in 38% of cases [14].
However, the diagnosis of AIMT in EUS remains challenging, and fine needle aspiration biopsy is ineffective [15]. A surgical approach is usually indicated for a definitive histological diagnosis [15,16]. AIMT is characterized histologically by myofibroblastic spindle cells and inflammatory cells, such as lymphocytes, histiocytes, and eosinophils in the collagenated stroma [17]. Molecular aberrations of anaplastic lymphoma kinase (ALK) receptors on chromosome 2p23 were found in 50% to 70% of AIMT cases [18]. Therefore, AIMT is a neoplasm with specific histological characteristics of inflammatory sclerosing and fibrosing processes [18]. However, no relationship has been established between ALK expression and prognosis [9]. In an immunohistochemical study, AIMT is positive for actin, desmin, vimentin, CD68, and CD34 [3].
Surgery with complete resection is recommended as the first therapeutic option of AIMT. Chemotherapy and radiotherapy have limited roles as adjunctive treatments. High-dose steroids or imatinib may be indicated in inoperable tumors [9]. AIMT is regarded as an intermediate neoplasm with a favorable prognosis [3]. However, local recurrence after surgery occurs in 15% to 37% of cases, which requires long-term follow-up [19,20]. In our series, the patient had surgery with complete tumor resection after a multidisciplinary team discussion. No postoperative treatment was administered, and the prognosis was favorable.
Conclusions
This case highlights that AIMTs are a diagnostic challenge because they usually mimic malignant tumors. Surgical management remains the best therapeutic option. The prognosis is often favorable but requires long-term monitoring, given the risk of recurrence. The case presented was treated by surgery with complete resection and a long-term follow-up was suggested considering the local recurrence risk.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study
References
- 1.Inflammatory myofibroblastic tumor (plasma cell granuloma). Clinicopathologic study of 20 cases with immunohistochemical and ultrastructural observations. Pettinato G, Manivel JC, De Rosa N, Dehner LP. Am J Clin Pathol. 1990;94:538–546. doi: 10.1093/ajcp/94.5.538. [DOI] [PubMed] [Google Scholar]
- 2.Inflammatory myofibroblastic tumour of the lung: a reactive lesion or a true neoplasm? Panagiotopoulos N, Patrini D, Gvinianidze L, Woo WL, Borg E, Lawrence D. J Thorac Dis. 2015;7:908–911. doi: 10.3978/j.issn.2072-1439.2015.04.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inflammatory myofibroblastic tumor of the pancreatic neck: a case report and review of literature. Chen ZT, Lin YX, Li MX, Zhang T, Wan DL, Lin SZ. World J Clin Cases. 2021;9:6418–6427. doi: 10.12998/wjcc.v9.i22.6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mesenteric inflammatory myofibroblastic tumor as a rare cause of small intestinal intussusception. Alabbas Z, Issa M, Omran A, Issa R. J Surg Case Rep. 2020;2020:0. doi: 10.1093/jscr/rjaa322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Renal inflammatory myofibroblastic tumor: a case report. Ivanov AS, Antonov PA, Chitalov ZR. Urol Case Rep. 2021;37:101620. doi: 10.1016/j.eucr.2021.101620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The enigmatic inflammatory pseudotumours: the current state of our understanding, or misunderstanding. Dehner LP. J Pathol. 2000;192:277–279. doi: 10.1002/1096-9896(200011)192:3<277::AID-PATH749>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 7.Inflammatory pseudotumor of the alimentary tract: clinical and surgical experience. Sanders BM, West KW, Gingalewski C, Engum S, Davis M, Grosfeld JL. J Pediatr Surg. 2001;36:169–173. doi: 10.1053/jpsu.2001.20045. [DOI] [PubMed] [Google Scholar]
- 8.Inflammatory myofibroblastic tumours: a pictorial review. Cantera JE, Alfaro MP, Rafart DC, Zalazar R, Muruzabal MM, Barquín PG, Pérez IV. Insights Imaging. 2015;6:85–96. doi: 10.1007/s13244-014-0370-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdominal inflammatory myofibroblastic tumor a clinicopathologic study with reappraisal of biologic behavior. Fragoso AC, Eloy C, Estevão-Costa J, Campos M, Farinha N, Lopes JM. J Pediatr Surg. 2011;46:2076–2082. doi: 10.1016/j.jpedsurg.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Abdominal inflammatory myofibroblastic tumor: report on four cases and review of literature. Mirshemirani A, Tabari AK, Sadeghian N, Shariat-Torbaghan S, Pourafkari M, Mohajerzadeh L. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3446124/ Iran J Pediatr. 2011;21:543–548. [PMC free article] [PubMed] [Google Scholar]
- 11.Inflammatory pseudotumour of the pancreas in a child. Slavotinek JP, Bourne AJ, Sage MR, Freeman JK. Pediatr Radiol. 2000;30:801–803. doi: 10.1007/s002470000331. [DOI] [PubMed] [Google Scholar]
- 12.Spontaneous regression of an inflammatory pseudotumor of the liver presenting as an obstructing malignant biliary tumor. Lévy S, Sauvanet A, Diebold MD, Marcus C, Da Costa N, Thiéfin G. Gastrointest Endosc. 2001;53:371–374. doi: 10.1016/s0016-5107(01)70422-1. [DOI] [PubMed] [Google Scholar]
- 13.Endoscopic ultrasound guided fine needle aspiration of non-pancreatic lesions: an institutional experience. Anand D, Barroeta JE, Gupta PK, Kochman M, Baloch ZW. J Clin Pathol. 2007;60:1254–1262. doi: 10.1136/jcp.2006.045955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clinical utility of endoscopic ultrasound and endscopic ultrasound-guided fine needle aspiration in retroperitoneal neoplasms. Erickson RA, Tretjak Z. Am J Gastroenterol. 2000;95:1188–1194. doi: 10.1111/j.1572-0241.2000.02008.x. [DOI] [PubMed] [Google Scholar]
- 15.Retroperitoneal inflammatory pseudotumor presenting as a pancreatic mass. Poves I, Alonso S, Jimeno M, Bessa X, Burdío F, Grande L. http://www.serena.unina.it/index.php/jop/article/view/788/925. JOP. 2012;13:308–311. [PubMed] [Google Scholar]
- 16.Laparoscopic splenectomy for inflammatory pseudotumor of the spleen. Suzumura K, Okada T, Satake M, Fujimito J. Hepatogastroenterology. 2011;58:1909–1911. doi: 10.5754/hge10250. [DOI] [PubMed] [Google Scholar]
- 17.Abdominal inflammatory myofibroblastic tumor. Groenveld RL, Raber MH, Oosterhof-Berktas R, Eijken E, Klaase JM. Case Rep Gastroenterol. 2014;8:67–71. doi: 10.1159/000360843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Coffin CM, Hornick JL, Fletcher CD. Am J Surg Pathol. 2007;31:509–520. doi: 10.1097/01.pas.0000213393.57322.c7. [DOI] [PubMed] [Google Scholar]
- 19.Recurrence of inflammatory pseudotumor in the distal bile duct: lessons learned from a single case and reported cases. Lopez-Tomassetti Fernandez EM, Luis HD, Malagon AM, Gonzalez IA, Pallares AC. World J Gastroenterol. 2006;12:3938–3943. doi: 10.3748/wjg.v12.i24.3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pancreatic inflammatory myofibroblastic tumor presenting with extracolonic obstruction. İflazoğlu N, Kaplan Kozan S, Biri T, Ünlü S, Gökçe H, Doğan S, Gökçe ON. Turk J Surg. 2020;36:233–237. doi: 10.5578/turkjsurg.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]