Abstract
Background
Multiple myeloma is a malignancy of plasma cells accounting for approximately 1% of cancers and 12% of haematological malignancies. The first‐in‐class proteasome inhibitor, bortezomib, is commonly used to treat newly diagnosed as well as relapsed/refractory myeloma, either as single agent or combined with other therapies.
Objectives
We conducted a systematic review and meta‐analysis to assess the effects of bortezomib on overall survival (OS), progression‐free survival (PFS), response rate (RR), health‐related quality of life (HRQoL), adverse events (AEs) and treatment‐related death (TRD).
Search methods
We searched MEDLINE, the Cochrane Central Register of Controlled Trials and EMBASE (till 27 January 2016) as well as conference proceedings and clinical trial registries for randomised controlled trials (RCTs).
Selection criteria
We included randomised controlled trials (RCTs) that compared i) bortezomib versus no bortezomib with the same background therapy in each arm; ii) bortezomib versus no bortezomib with different background therapy in each arm or compared to other agent(s) and iii) bortezomib dose comparisons and comparisons of different treatment administrations and schedules.
Data collection and analysis
Two review authors independently extracted outcomes data and assessed risk of bias. We extracted hazard ratios (HR) and their confidence intervals for OS and PFS and odds ratios (OR) for response rates, AEs and TRD. We contacted trial authors to provide summary statistics if missing. We estimated Logrank statistics which were not available. We extracted HRQoL data, where available.
Main results
We screened a total of 3667 records, identifying 16 relevant RCTs involving 5626 patients and included 12 trials in the meta‐analyses. All trials were randomised and open‐label studies. Two trials were published in abstract form and therefore we were unable to assess potential risk of bias in full.
There is moderate‐quality evidence that bortezomib prolongs OS (four studies, 1586 patients; Peto OR 0.77, 95% CI 0.65 to 0.92) and PFS (five studies, 1855 patients; Peto OR 0.65, 95% CI 0.57 to 0.74) from analysing trials of bortezomib versus no bortezomib with the same background therapy in each arm.
There is high‐quality evidence that bortezomib prolongs OS (five studies, 2532 patients; Peto OR 0.76, 95% CI 0.67 to 0.88) but low‐quality evidence for PFS (four studies, 2489 patients; Peto OR 0.67, 95% CI 0.61 to 0.75) from analysing trials of bortezomib versus no bortezomib with different background therapy in each arm or compared to other agent(s).
Four trials (N = 716) examined different doses, methods of administrations and treatment schedules and were reviewed qualitatively only.
We identified four trials in the meta‐analysis that measured time to progression (TTP) and were able to extract and analyse PFS data for three of the studies, while in the case of one study, we included TTP data as PFS data were not available. We therefore did not analyse TTP separately in this review.
Patients treated with bortezomib have increased risk of thrombocytopenia, neutropenia, gastro‐intestinal toxicities, peripheral neuropathy, infection and fatigue with the quality of evidence highly variable. There is high‐quality evidence for increased risk of cardiac disorders from analysing trials of bortezomib versus no bortezomib with different background therapy in each arm or versus other agents. The risk of TRD in either comparison group analysed is uncertain due to the low quality of the evidence.
Only four trials analysed HRQoL and the data could not be meta‐analysed.
Subgroup analyses by disease setting revealed improvements in all outcomes, whereas for therapy setting, an improved benefit for bortezomib was observed in all outcomes and subgroups except for OS following consolidation therapy.
Authors' conclusions
This meta‐analysis found that myeloma patients receiving bortezomib benefited in terms of OS, PFS and response rate compared to those who did not receive bortezomib. This benefit was observed in trials of bortezomib versus no bortezomib with the same background therapy and in trials of bortezomib versus no bortezomib with different background therapy in each arm or compared to other agent(s). Further evaluation of newer proteasome inhibitors is required to ascertain whether these agents offer an improved risk‐benefit profile, while more studies of HRQoL are also required.
Plain language summary
The role of bortezomib treatment for patients with multiple myeloma
Background
Multiple myeloma is a type of blood cancer, affecting plasma cells inside the bone marrow. Bortezomib is a type of treatment for myeloma called a proteasome inhibitor. Proteasomes are enzymes found in all cells and play an important role in cell function and growth. Cancer cells are more sensitive to the effects of bortezomib, causing cancer cells to die or not grow any further.
Study characteristics
We wanted to know the benefits and harms from bortezomib treatment for myeloma. We searched medical databases and trial registries until January 2016. We included studies of bortezomib compared to no bortezomib, with either the same or different background therapy or compared to other drugs. Studies of newly diagnosed and relapsed myeloma were included as well as those that compared different doses, ways of administering bortezomib and treatment schedules.
Key results
We found 16 studies involving 5626 myeloma patients. The results of this review suggest that bortezomib can lead to better survival, a longer time without progression and better response rates compared to those not receiving bortezomib. Treatment with bortezomib causes a number of side effects including: low levels of some blood cells; gastro‐intestinal effects such as constipation, diarrhoea, nausea and vomiting; nerve pain and tingling in hands and feet, as well as infection. A greater risk of heart problems was seen in one of the comparison groups studied. Risk of death from bortezomib treatment was uncertain in either group analysed. Only four studies assessed quality of life and could not be analysed together.
Quality of the evidence
We judged quality of the evidence as high to moderate for mortality or number of deaths, whereas it was considered low‐quality evidence for progression‐free survival. the quality of evidence for adverse events was highly variable (low to high). For assessment of treatment‐related death, there was no evidence of a difference, with low‐quality evidence in one comparison (bortezomib compared to no bortezomib with the same background therapy) and very low‐quality evidence in comparison two (bortezomib compared to no bortezomib with different background therapy or compared to other drugs).
Conclusion
Patients receiving bortezomib had better response rates, longer time without progression and appeared to live longer compared to those not receiving bortezomib, however patients receiving bortezomib experienced more side effects. Other proteasome inhibitor drugs have also been developed, therefore further research should focus on whether these newer drugs provide additional benefits and fewer side effects than bortezomib. More studies on health‐related quality of life are also needed.
Summary of findings
Summary of findings for the main comparison. Bortezomib versus no bortezomib for the treatment of multiple myeloma.
| Bortezomib versus no bortezomib | ||||||
| Patient or population: All patients with a diagnosis of multiple myeloma Setting: International multicentre studies Intervention: Bortezomib Comparison: Bortezomib versus no bortezomib (same or different background therapy or other agents) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk without bortezomib | Risk with bortezomib | |||||
| Overall Survival | Study population | Peto Odds Ratio 0.77 (0.69 to 0.86) | 4118 (9 RCTs) | ⨁⨁⨁⨁ HIGH | ||
| 215 per 1000 | 166 per 1000 (148 to 185) | |||||
| Overall Survival ‐ Bortezomib versus no bortezomib (same background therapy) Follow‐up 36 to 60 months |
Study population | Peto Odds Ratio 0.77 (0.65 to 0.92) | 1586 (4 RCTs) | ⨁⨁⨁◯ MODERATE 1 | ||
| 354 per 1000 | 273 per 1000 (230 to 326) | |||||
| Overall Survival ‐ Bortezomib versus no bortezomib (different background therapy or other agents) Follow‐up 7.5 to 67 months |
Study population | Peto Odds Ratio 0.76 (0.67 to 0.88) | 2532 (5 RCTs) | ⨁⨁⨁⨁ HIGH | ||
| 129 per 1000 | 98 per 1000 (87 to 114) | |||||
| Progression‐Free Survival | Study population | Peto Odds Ratio 0.67 (0.61 to 0.72) | 4344 (9 RCTs) | ⨁⨁◯◯ LOW 2 3 | ||
| 523 per 1000 | 350 per 1000 (319 to 377) | |||||
| Progression‐Free Survival ‐ Bortezomib versus no bortezomib (same background therapy) Follow‐up 30 to 60 months |
Study population | Peto Odds Ratio 0.65 (0.57 to 0.74) | 1855 (5 RCTs) | ⨁⨁⨁◯ MODERATE 2 | ||
| 324 per 1000 | 211 per 1000 (185 to 240) | |||||
| Progression‐Free Survival ‐ Bortezomib versus no bortezomib (different background therapy or other agents) Follow‐up 22 to 67 months |
Study population | Peto Odds Ratio 0.67 (0.61 to 0.75) | 2489 (4 RCTs) | ⨁⨁◯◯ LOW 2 4 | ||
| 669 per 1000 | 448 per 1000 (408 to 501) | |||||
| Treatment‐related death | Study population | OR 0.76 (0.43 to 1.34) | 2389 (5 RCTs) | ⨁⨁◯◯ LOW 6 | ||
| 22 per 1000 | 17 per 1000 (10 to 29) | |||||
| Moderate | ||||||
| 27 per 1000 | 21 per 1000 (12 to 36) | |||||
| Treatment‐related death ‐ Bortezomib versus no bortezomib (same background therapy) | Study population | OR 0.81 (0.30 to 2.16) | 737 (2 RCTs) | ⨁⨁◯◯ LOW 6 | ||
| 22 per 1000 | 18 per 1000 (7 to 47) | |||||
| Moderate | ||||||
| 35 per 1000 | 29 per 1000 (11 to 73) | |||||
| Treatment‐related death ‐ Bortezomib versus no bortezomib (different background therapy or other agents) | Study population | OR 0.73 (0.36 to 1.48) | 1652 (3 RCTs) | ⨁◯◯◯ VERY LOW 6 7 | ||
| 22 per 1000 | 16 per 1000 (8 to 32) | |||||
| Moderate | ||||||
| 27 per 1000 | 20 per 1000 (10 to 40) | |||||
| Health‐related quality of life | see comment | see comment | see comment | 717 (4 RCTs) |
see comment | Each trial used the same validated quality of life instrument (European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire (QLQ‐C30), whereas the time points of administration of the questionnaire varied between the four trials. |
| Adverse Events: Thrombocytopenia | Study population | OR 2.05 (1.70 to 2.48) | 3791 (8 RCTs) | ⨁⨁◯◯ LOW 8 | ||
| 114 per 1000 | 209 per 1000 (180 to 242) | |||||
| Moderate | ||||||
| 48 per 1000 | 94 per 1000 (79 to 111) | |||||
| Adverse Events: Thrombocytopenia ‐ Bortezomib versus no bortezomib (same background therapy) | Study population | OR 1.51 (1.13 to 2.00) | 1196 (3 RCTs) | ⨁⨁⨁⨁ HIGH | ||
| 197 per 1000 | 271 per 1000 (217 to 330) | |||||
| Moderate | ||||||
| 47 per 1000 | 70 per 1000 (53 to 90) | |||||
| Adverse Events: Thrombocytopenia ‐ Bortezomib versus no bortezomib (different background therapy or other agents) | Study population | OR 2.60 (2.01 to 3.35) | 2595 (5 RCTs) | ⨁⨁◯◯ LOW 9 | ||
| 76 per 1000 | 176 per 1000 (142 to 216) | |||||
| Moderate | ||||||
| 49 per 1000 | 118 per 1000 (93 to 147) | |||||
| Adverse Events: Diarrhoea | Study population | OR 2.44 (1.74 to 3.43) | 3788 (8 RCTs) | ⨁⨁◯◯ LOW 5 10 | ||
| 26 per 1000 | 62 per 1000 (45 to 85) | |||||
| Moderate | ||||||
| 17 per 1000 | 39 per 1000 (28 to 54) | |||||
| Adverse Events: Diarrhoea ‐ Bortezomib versus no bortezomib (same background therapy) | Study population | OR 6.24 (2.79 to 13.98) | 1670 (4 RCTs) | ⨁⨁⨁◯ MODERATE 5 | ||
| 8 per 1000 | 50 per 1000 (23 to 106) | |||||
| Moderate | ||||||
| 7 per 1000 | 41 per 1000 (19 to 87) | |||||
| Adverse Events: Diarrhoea ‐ Bortezomib versus no bortezomib (different background therapy or other agents) | Study population | OR 1.80 (1.22 to 2.65) | 2118 (4 RCTs) | ⨁⨁⨁◯ MODERATE 10 | ||
| 40 per 1000 | 71 per 1000 (49 to 100) | |||||
| Moderate | ||||||
| 23 per 1000 | 40 per 1000 (28 to 58) | |||||
| Adverse Events: Peripheral Neuropathy | Study population | OR 3.71 (2.92 to 4.70) | 4636 (10 RCTs) | ⨁⨁⨁◯ MODERATE 5 | ||
| 44 per 1000 | 145 per 1000 (118 to 176) | |||||
| Moderate | ||||||
| 80 per 1000 | 244 per 1000 (203 to 291) | |||||
| Adverse Events: Peripheral Neuropathy ‐ Bortezomib versus no bortezomib (same background therapy) | Study population | OR 5.10 (3.37 to 7.72) | 2040 (5 RCTs) | ⨁⨁◯◯ LOW 5 10 | ||
| 31 per 1000 | 139 per 1000 (96 to 196) | |||||
| Moderate | ||||||
| 139 per 1000 | 453 per 1000 (353 to 556) | |||||
| Adverse Events: Peripheral Neuropathy ‐ Bortezomib versus no bortezomib (different background therapy or other agents) | Study population | OR 3.09 (2.30 to 4.14) | 2596 (5 RCTs) | ⨁⨁⨁⨁ HIGH | ||
| 54 per 1000 | 149 per 1000 (116 to 190) | |||||
| Moderate | ||||||
| 21 per 1000 | 62 per 1000 (47 to 81) | |||||
| Adverse Events: Infections (All) | Study population | OR 1.51 (1.27 to 1.79) | 4266 (9 RCTs) | ⨁⨁⨁◯ MODERATE 11 | ||
| 128 per 1000 | 181 per 1000 (157 to 207) | |||||
| Moderate | ||||||
| 254 per 1000 | 339 per 1000 (302 to 378) | |||||
| Adverse Events: Infections (All) ‐ Bortezomib versus no bortezomib (same background therapy) | Study population | OR 1.37 (0.97 to 1.93) | 1670 (4 RCTs) | ⨁⨁⨁⨁ HIGH | ||
| 77 per 1000 | 103 per 1000 (75 to 139) | |||||
| Moderate | ||||||
| 57 per 1000 | 77 per 1000 (56 to 105) | |||||
| Adverse Events: Infections (All) ‐ Bortezomib versus no bortezomib (different background therapy or other agents) | Study population | OR 1.55 (1.27 to 1.90) | 2596 (5 RCTs) | ⨁⨁◯◯ LOW 9 | ||
| 160 per 1000 | 228 per 1000 (194 to 265) | |||||
| Moderate | ||||||
| 209 per 1000 | 291 per 1000 (251 to 335) | |||||
| Adverse Events: Cardiac Disorders | Study population | OR 1.74 (1.17 to 2.58) | 2191 (5 RCTs) | ⨁⨁⨁⨁ HIGH | ||
| 38 per 1000 | 65 per 1000 (44 to 93) | |||||
| Moderate | ||||||
| 30 per 1000 | 51 per 1000 (35 to 74) | |||||
| Adverse Events: Cardiac Disorders ‐ Bortezomib versus no bortezomib (same background therapy) | Study population | OR 1.17 (0.39 to 3.52) | 736 (2 RCTs) | ⨁⨁⨁◯ MODERATE 5 | ||
| 16 per 1000 | 19 per 1000 (6 to 55) | |||||
| Moderate | ||||||
| 14 per 1000 | 17 per 1000 (6 to 49) | |||||
| Adverse Events: Cardiac Disorders ‐ Bortezomib versus no bortezomib (different background therapy or other agents) | Study population | OR 1.84 (1.21 to 2.81) | 1455 (3 RCTs) | ⨁⨁⨁⨁ HIGH | ||
| 49 per 1000 | 87 per 1000 (59 to 127) | |||||
| Moderate | ||||||
| 49 per 1000 | 86 per 1000 (58 to 126) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded one level due to heterogeneity 55%
2 Downgraded one level because TTP was analysed instead of PFS in one trial.
3 Downgraded one level due to heterogeneity 56%.
4 Downgraded one level due to heterogeneity 70% .
5 Downgraded one level due to low number of events, wide CI.
6 Downgraded two levels due to very low number of events, very wide CI.
7 Downgraded one level due to heterogeneity 53%.
8 Downgraded one level due to heterogeneity 81%.
9 Downgraded one level due to heterogeneity 86%.
10 Downgraded one level due to heterogeneity 57%.
11 Downgraded one level due to heterogeneity 76%.
Background
Description of the condition
Multiple myeloma is a bone marrow‐based malignancy characterised by the clonal proliferation of neoplastic plasma cells, the presence of a monoclonal paraprotein in the blood or urine and organ dysfunction (Palumbo 2011). An estimated 102,000 people were diagnosed with myeloma globally in 2008, accounting for approximately 1% of all cancers diagnosed and 12% of all haematological malignancies (Ferlay 2010). The median age at diagnosis is approximately 70 years (Palumbo 2011). Recent advances in treatment have led to significant improvements in relative survival rates at five and 10 years, improving from 32.8% and 15% in the period from 1998 to 2002, to 40.3% and 20.8%, respectively, in the years between 2003 and 2007 (Pulte 2011). Myeloma remains an incurable condition, however, and therefore the primary goal of treatment is therefore to control the disease, attain sustainable remissions and optimise quality of life.
Description of the intervention
Until relatively recently, treatment for myeloma consisted of either single agent or combination regimens of chemotherapy drugs such as melphalan, doxorubicin and vincristine, and the glucocorticosteroids, prednisone and dexamethasone (Raab 2009). The introduction of stem cell transplantation for certain subgroups in the 1990s led to improvements in disease‐free and overall survival (OS) (Raab 2009). More recently, the development of targeted therapies such as the immunomodulatory drugs, thalidomide and lenalidomide, and proteasome inhibitors of which bortezomib was the first available, has considerably expanded therapeutic options for myeloma patients (Raab 2009).
The proteasome inhibitor, bortezomib, has been a major advance in the treatment of myeloma. Bortezomib was first approved for clinical use based on an overall response rate (ORR) of 35% and a median time to progression (TTP) of seven months observed in a phase II trial of patients with relapsed and refractory disease who were treated with single‐agent bortezomib (Richardson 2003). An international randomised phase III trial evaluating bortezomib versus high‐dose dexamethasone subsequently demonstrated superior response rates, an improved TTP and a superior median OS of 29.8 months versus 23.7 months in those receiving bortezomib (Richardson 2005; Richardson 2007).
A number of trials evaluating bortezomib in combination with other therapeutic agents have also been reported (Moreau 2012). Preclinical and clinical data on various combination regimens have provided support for the hypothesis that bortezomib sensitises myeloma cells to other therapies, resulting in additive or even synergistic activity (Shah 2009).
While clinically effective, some myeloma patients are unable to tolerate treatment with bortezomib due to side effects such as nausea, fatigue, diarrhoea, peripheral neuropathy and thrombocytopenia (a decreased number of platelets in the blood) (Kyle 2009). Most of these side effects are predictable and manageable, but in some cases they may be life‐threatening (Bertolotti 2008). Ongoing trials investigating bortezomib in combination with other agents aim to identify regimens that will provide a more favourable risk‐benefit profile (Palumbo 2011).
A number of new ‘second generation’ proteasome inhibitors (carfilzomib, marizomib and ixazomib), each with distinct chemical properties, have also been developed and are undergoing evaluation in clinical trials (Moreau 2012). The most clinically advanced of these agents is carfilzomib, which was approved for use in patients with multiple myeloma who are progressing on or after treatment with bortezomib and an immunomodulatory agent. This licence was based on a phase II trial of patients with relapsed/refractory multiple myeloma treated with single agent carfilzomib. An ORR of 23.7% (95% confidence interval (CI) 18.7% to 29.4%), a median response duration of 7.8 months and a median OS of 15.6 months was observed (Siegel 2012). It is anticipated, that, in addition to superior efficacy, these agents will also offer a more acceptable adverse‐event profile compared to bortezomib and will be clinically useful in patients with myeloma resistant to bortezomib (Chen 2011a).
As these newer proteasome inhibitors are still under evaluation, this review was restricted to the use of bortezomib in the treatment of myeloma.
How the intervention might work
Bortezomib belongs to a new generation of anti‐cancer drugs that work by targeting specific cell receptors, proteins and signalling pathways. Proteasomes are 26S ATP‐dependent protein complexes within the ubiquitin‐proteasome pathway. They are present in all cells and are responsible for processing the majority of intracellular proteins (Moreau 2012). Cancer cells generally have higher levels of proteasome activity when compared with normal cells and are therefore more sensitive to proteasome inhibition (Moreau 2012), leading to disruption of cellular growth and survival. This is due to both the de‐regulation of signalling pathways within the myeloma cell as well as inhibition of the interaction between the myeloma cells and the bone marrow microenvironment (Chen 2011a).
Bortezomib is a dipeptidyl boronic acid, reversible proteasome inhibitor that primarily targets the chymotrypsin‐like and caspase‐like active sites of the proteasome with minimal effect on trypsin‐like activity (Lawasut 2012). Through proteasome inhibition, bortezomib acts via multiple mechanisms to suppress tumour survival pathways and to arrest tumour growth, tumour spread, and angiogenesis (Moreau 2012).
Why it is important to do this review
Bortezomib is commonly used for the treatment of myeloma at all stages of the disease and in all major myeloma treatment settings. A systematic review is important to evaluate the accumulated clinical evidence for the clinical efficacy and tolerability of treatment with bortezomib.
Randomised controlled trials (RCTs) investigating bortezomib have demonstrated that its use is associated with statistically significant improvements in response rates and event‐free survival. These, however, are primarily surrogate outcome measures for OS. A systematic review and meta‐analysis of relevant similar trials will therefore analyse its effect on OS while analysis of combined data from similar RCTs will also enable greater precision in making an unbiased estimate of the effects of treatment.
Objectives
We assessed the effects of bortezomib treatment in comparison to other therapies, different doses, treatment administration and schedules of bortezomib, on overall survival (OS), progression‐free survival (PFS), response rate (RR), health‐related quality of life (HRQoL), adverse events (AE) and treatment‐related death (TRD).
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) in which the intervention consisted of bortezomib for the treatment of myeloma.
Types of participants
Patients of any age, gender or ethnic origin and with any diagnosis of multiple myeloma (according to either the Durie‐Salmon staging system or International Staging System (ISS) (Kyle 2009) were included in this review. We included patients who were either newly diagnosed (had received no prior therapy) or patients with relapsed disease. We also included patients who were considered to be either transplant eligible or ineligible. Patient eligibility for stem cell transplant is determined primarily by age, as well as performance status, frailty, and presence of comorbidities. We did not define transplant eligibility for this review and therefore selected studies that included all types of patients.
Types of interventions
We included RCTs that investigated the following comparisons.
Bortezomib versus no bortezomib with the same background therapy in each arm
Bortezomib versus no bortezomib with different background therapy in each arm or compared to other agent(s)
Bortezomib dose comparisons and comparisons of different treatment administrations and schedules
We combined two of the comparison groups (studies of bortezomib versus no bortezomib with different background therapy in each arm and studies of bortezomib versus other agents(s)) as these studies included complex combination regimens/therapies, with some studies considered as belonging to either comparison.
Types of outcome measures
Primary outcomes
Overall survival (OS): time from date of randomisation to date of death (from any cause)
Progression‐free survival (PFS): time from date of randomisation to date of progression or death (from any cause)
Secondary outcomes
Overall response rate (ORR), complete response rate (CRR) and partial response rate (PRR): the proportion of patients with overall, complete or partial response
Time to progression (TTP): time from randomisation to date of progression. As TTP may also be referred to as PFS, we planned to only analyse TTP separately, if it were defined differently
Treatment‐free interval (TFI): time from randomisation to date of initiation of next treatment regimen or similar
Treatment‐related death: death due to treatment‐related toxicity and not disease progression
Adverse events (AE): as defined by the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE)
Health‐related quality of life (HRQoL): as defined by the validated quality of life measures or instruments used in each trial
Search methods for identification of studies
Electronic searches
We performed a systematic search of the following electronic databases, using comprehensive search strategies incorporating key search terms.
The Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library) till 27 January 2016 (Appendix 1)
MEDLINE (Ovid) till 27 January 2016 (Appendix 2)
EMBASE (Elsevier) till 31 July 2015 (Appendix 3)
Databases were searched using the Cochrane Highly Sensitive Search Strategy (Lefebvre 2011) to identify randomised trials in MEDLINE combined with selected MeSH terms and free text terms. Language restrictions were not imposed. Search strategies were tailored to the other databases. The search strategies for databases are shown in Appendix 1, 2 and 3.
Electronic searches of MEDLINE and CENTRAL were conducted by the CHMG Trial Search Co‐ordinator and of EMBASE by the first author of the review with support from a librarian. Results of the electronic databases were collated into a single reference library using the reference manager software Endnote X6 (EndNote 2012) and independently screened by two review authors (KS and AH).
Searching other resources
In addition, we searched for ongoing and unpublished clinical trials in the following clinical trial registries using key words ‘bortezomib’, ‘multiple ‘myeloma’ and ‘randomised’:
National Institute of Health (NIH) Register http://clinicaltrials.gov (search date: 04 May 2015)
International Standard Randomised Controlled Trial Number (ISRCTN) register http://www.controlledtrials.com (search date: 04 May 2015)
We searched also online archives of conference proceedings for relevant meeting abstracts:
American Society of Hematology (ASH) 2012 to 2015
American Society of Clinical Oncology (ASCO) 2012 to 2015
European Hematology Association (EHA) 2012 to 2015
European Society of Medical Oncology (ESMO) 2011 to 2015
We also searched reference lists of relevant studies and review articles and contacted principal investigators and trial sponsors by e‐mail regarding status of unpublished or incomplete trials.
Data collection and analysis
Selection of studies
Two review authors (KS and AH) independently screened the abstracts of retrieved articles for eligibility according to pre‐determined criteria. Any inconsistencies between the review authors during the screening process were discussed. If a decision could not be made on the basis of the abstract, a full‐text article of the study in question was retrieved and assessed independently by the two authors to make the final decision regarding study eligibility. No articles or studies required discussion with a third review author. The number of studies identified, the number of included and excluded studies and reasons for inclusion/exclusion were documented according to PRISMA guidelines (Moher 2009).
Data extraction and management
For each eligible trial, two review authors (KS and AH) independently extracted data using a data extraction form, which included the following.
Trial identification: title, authors, journal name, publication date, countries, sponsor, funding
Trial design: type of trial design, treatment setting, number of arms, number of centres, sample size and rationale, randomisation method, allocation concealment, blinding, stratification factors, analysis methods, pre‐specified alpha error, beta error, effect size, analysis types (e.g. intention‐to‐treat (ITT), per protocol)
Trial comparisons: Experimental and control arms, number of courses of treatment, doses, timing and route of administration, other treatments received
Trial participants: age (median/mean and age range), sex, stage (Durie‐Salmon, International or both), inclusion criteria, exclusion criteria
Trial progress and follow‐up: duration of accrual and follow‐up periods, number of participants per arm, number of participants lost to follow‐up, and excluded from analysis
Outcomes:
Overall survival (OS)
Progression‐free survival (PFS)
Overall response rate (ORR); complete response rate (CRR); partial response rate (PRR)
Time to progression (TTP)
Treatment‐free interval
Treatment‐related death (TRD)
Adverse events (AEs)
Health‐related quality of life (HRQoL)
Review author KS entered data into the Characteristics of Studies tables in RevMan and AH checked these tables for accuracy. AH entered the outcomes data into RevMan and KS checked these data for accuracy. Any inconsistencies or disagreements were resolved through discussion between the two authors.
For studies with more than one publication, we extracted data from all publications as per recommendations provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a), however we considered the final or updated version of each trial as the primary source for the extraction of outcomes data.
For studies with a 3‐arm randomisation, data were extracted for just the unconfounded comparison (bortezomib versus no bortezomib with the same background therapy) in that randomisation, or by grouping arms containing bortezomib.
Assessment of risk of bias in included studies
We used the Rrisk of bias' assessment tool as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b), and classified trials at low, high or unclear risk of bias for the following.
Random sequence generation (selection bias)
Allocation concealment (selection bias)
Blinding of participants (performance bias)
Blinding of outcome assessment (detection bias)
Incomplete outcome data (attrition bias)
Selective reporting (reporting bias)
Other sources of bias.
For each type of bias, a judgement was made using one of three categories:
low risk: if the criterion was adequately fulfilled;
high risk: if the criterion was not fulfilled;
unclear risk: if the report did not provide sufficient information to allow for a judgement of high or low risk or if the risk of bias is unknown.
We assessed individual outcomes e.g. OS, RR according to the above criteria (see Characteristics of included studies and 'Risk of bias' tables).
Measures of treatment effect
Time‐to‐event outcomes data: We extracted the hazard ratios (HR) and 95% confidence intervals (CI) for OS and PFS from included studies and calculated the overall odds ratio (OR) and 95% CI for combined studies using methods recommended in theCochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Dichotomous outcomes data
Response and progression of disease were reported in each included trial according to either the International Myeloma Working Group (IMWG) uniform response criteria or European Group for Blood and Bone Marrow Transplantation (EBMT) criteria (Kyle 2009). The IMWG uniform response criteria were developed similarly to the EBMT criteria with some notable modifications (Kyle 2009). For the purposes of this review and meta‐analysis, we assumed that complete response (CR) and overall response were similar regardless of the response criteria used. We extracted the CRR and ORR as reported in each trial and analysed these data as odds ratios (OR) with 95% CIs.
AEs were reported in each included trial according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE). AEs may occur more than once in the same individual, particularly during different treatment cycles. The number of grade 3/4 AEs were reported as a percentage (%) of the total number of patients on each arm in each included trial. Therefore, we assumed that each AE was counted once and analysed these data as dichotomous data.
Treatment‐related deaths were extracted from the text of the trial publication where reported and analysed as dichotomous data.
Continuous outcomes data: We planned to extract HRQoL data where this was reported as an outcome measure. A variety of quality of life measurement instruments may be used and may also be measured at differing time points. We could not conduct meta‐analysis due to variation in reporting and incomplete data and therefore we summarised these data only.
Unit of analysis issues
We did not anticipate any unit of analysis issues.
Dealing with missing data
We approached dealing with missing data according to recommendations provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a) and classified data as either 'missing at random' or 'not missing at random'. In the case of data considered to be missing at random, we analysed the available data. For data considered to be not missing at random, we contacted the trial authors for further information.
If data were still not available, we stated the assumptions made for the analysis. Logrank statistics that were not available from the published articles were estimated. Where possible we used previously reported methods (Parmar 1998; Tierney 2007). The methods followed and estimates made were verified by an independent statistician. In one case (MD Anderson Study), original data were recreated based on the Kaplan‐Meier plot.
Assessment of heterogeneity
The presence of statistical heterogeneity of included studies was assessed using the Chi2 test at a significance level of P < 0.10 (Deeks 2011). The I2 statistic was used to quantify heterogeneity according to the following thresholds described in theCochrane Handbook for Systematic Reviews of Interventions (Deeks 2011):
0% to 40% (heterogeneity possibly not important);
30% to 60% (may represent moderate heterogeneity);
50% to 90% (may represent substantial heterogeneity);
75% to 100% (considerable heterogeneity).
Where we identified heterogeneity, we conducted subgroup analyses as outlined in the section Subgroup analysis and investigation of heterogeneity.
Assessment of reporting biases
To assess the likelihood of reporting bias, funnel plots were produced according to methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Sterne 2011). This was done for the primary outcomes only.
Data synthesis
We used the latest version of the software package RevMan 5.3 (RevMan 5.3) to enter data and combined results from included studies.
Standard statistical methods for the meta‐analysis of dichotomous, time‐to‐event and continuous variables were used. If time‐to‐event outcomes were not available, we calculated summary estimates. Fixed‐effect methods for meta‐analysis were utilised. The O‐E and V method was used to calculate the effect size, a commonly used method in meta‐analysis which produces a Peto odds ratio (OR) rather than a Hazard Ratio (HR). In some cases, the published HR was used to calculate O‐E and V, and therefore the Peto OR obtained was the same as the HR; if the HR was not available, the statistics were estimated from other data provided (e.g. P value and number of events). This method was therefore used to estimate the effect size when a publication did not adequately report the HR and CIs.
We produced a 'Summary of findings' table using GRADE software (Schünemann 2011) and summarised the results for OS, PFS, TRD, HRQoL and major AEs. We pooled results where the data were sufficiently similar to be combined and performed a meta‐analysis for each comparison. We analysed comparisons 1 and 2 together and comparison 3 was analysed qualitatively only.
Subgroup analysis and investigation of heterogeneity
We analysed the following subgroups.
Disease setting
Newly diagnosed (transplant eligible)
Newly diagnosed (transplant ineligible)
Relapsed and/or relapsed/refractory
Therapy setting
Induction therapy (pre‐transplant)
Consolidation therapy (post‐transplant)
Maintenance therapy (post‐transplant)
Tests for heterogeneity were used to investigate whether the treatment effect was greater in some subgroups than in others. Tests for interactions were used to verify subgroup differences.
Where a trial used bortezomib in more than one therapy setting, it was included in all relevant subgroups. Consequently only subgroup totals and no overall totals were calculated for these analyses.
Sensitivity analysis
We did not conduct a sensitivity analysis excluding trials considered to be at an overall high risk of bias (see Differences between protocol and review).
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Ongoing studies.
Results of the search
The primary electronic searches of each database (CENTRAL, MEDLINE, and EMBASE) performed in January 2016 yielded a total of 33761 records after the removal of internal duplicates. Results from each database were collated into a single EndNote library, with additional duplicates removed, leaving 3667 records in total to be screened according to the eligibility criteria. A total of 3382 records were excluded as irrelevant. Reasons for exclusion included: non‐clinical studies; clinical studies unrelated to bortezomib and/or multiple myeloma; non‐randomised studies, retrospective studies or case studies. Sixteen additional duplicate records were also removed and 55 records were considered to be not the definitive articles. The remaining 38 records were then sourced in full text for more detailed evaluation. A further 12 records were excluded at this stage.
Sixty‐four additional records were identified through other sources from the screening of conference proceedings, clinical trials registries, checking of reference lists of relevant studies and review articles and handsearching. One full‐text article that was identified through handsearching was added after the date of the electronic searches as it provided relevant updated outcomes data for one of the included trials (GIMEMA‐MM‐03‐05 Study).
At least three trials were identified as completed in 2013 through searches of clinical trials registries (Consolidation (61‐75 years) Study; Consolidation (less than 60 years) Study; King Fasail Hospital Study), however these trials are not yet published in full and no further data have been made available.
Finally, a total of 16 studies were considered eligible for inclusion and a total of six studies excluded. The process and results of study identification are outlined in a flow diagram according to the PRISMA statement (Moher 2009) (Figure 1).
1.
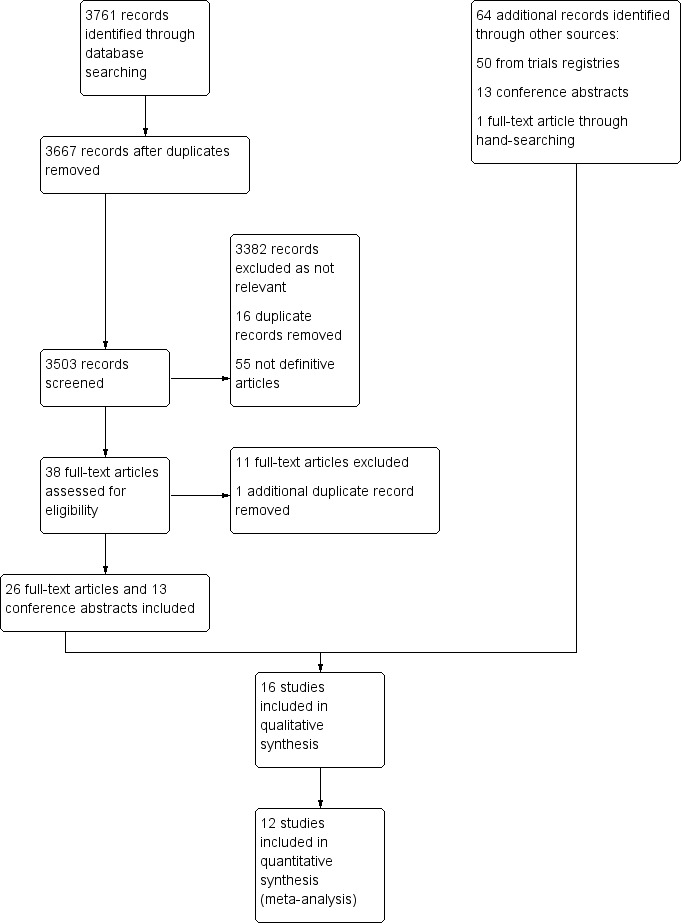
Study flow diagram.
Included studies
The number of included trials according to our eligibility criteria is 16 trials involving 5626 patients. The characteristics of the included studies are summarised in the table Characteristics of included studies.
Among these trials, six trials involving 2247 patients evaluated bortezomib versus no bortezomib with the same background therapy in each arm (GEM05MENOS65 Study; GIMEMA‐MMY‐3006 Study; MD Anderson Study; MMVAR/IFM 2005‐04 Study; NMSG 15/05 Study; VISTA Study). Another six trials involving 2663 patients evaluated bortezomib versus no bortezomib with either different background therapy in each arm or compared to other agents (All India Institute Study; APEX Study; GIMEMA‐MM‐03‐05 Study; HOVON‐65/GMMG‐HD4 Study; IFM 2005‐01 Study; NMSG 17/07 Study). Meta‐analyses were performed on these two groups of trials (12 trials in total).
Finally, four trials involving 716 patients assessed bortezomib dose comparisons, methods of administrations and treatment schedules (CREST Study; GEM2010MAS65 Study; IFM 2007‐02 Study; MMY‐3021 Study). The trials in this group were too dissimilar for meta‐analysis. These trials were therefore assessed qualitatively only.
Design
All trials included in this review were randomised and open‐label. The majority of trials were two‐armed randomised controlled trials (RCTs), while two trials were three‐armed RCTs (GEM05MENOS65 Study; MD Anderson Study) and one trial was a four‐arm RCT (IFM 2005‐01 Study). The GEM05MENOS65 Study randomised patients to one of three induction therapy arms (Arm A (VTD: bortezomib, thalidomide, dexamethasone), Arm B (TD: thalidomide, dexamethasone), and Arm C (VBMCP/VBAD/B: vincristine, BCNU, melphalan, cyclophosphamide, prednisone/vincristine, BCNU, doxorubicin, dexamethasone/bortezomib). Following completion of stem cell transplantation, eligible patients were randomised a second time to one of three maintenance arms: Arm A (TV: thalidomide, bortezomib, Arm B (T: thalidomide only), and Arm C (alfa2‐IFN: Interferon alpha‐2b). We extracted data for two arms only i.e. VTD versus TD alone in the induction phase and TV versus T in the maintenance phase, as the third arm was confounded by the administration of other therapies. In the MD Anderson Study, patients were randomised in a 1:1:1 ratio to one of three arms (20 patients per group): the conditioning regimen with no bortezomib (Group 1), the conditioning regimen and 1 mg/m2 of bortezomib (Group 2) or the conditioning regimen and 1.5mg/m2 of bortezomib (Group 3). We combined the extracted data from Groups 2 and 3 for the meta‐analysis and we re‐created the original data from the published Kaplan‐Meier plots. In the case of the IFM 2005‐01 Study, patients were randomised to receive vincristine Adriamycin dexamethasone (VAD) induction, with or without dexamethasone, cyclophosphamide, etoposide and cisplatin (DCEP) consolidation or bortezomib and dexamethasone (BD) induction, with or without DCEP consolidation and we were therefore able to combine data as a two‐arm comparison.
Sample sizes
The smallest trial had a sample size of 43 patients (All India Institute Study), while the largest trial was the HOVON‐65/GMMG‐HD4 Study with a sample size of 827 patients.
Setting
The majority of trials were multi‐centre trials conducted either within a single country or in several countries. Two trials were conducted in single centres only (MD Anderson Study; All India Institute Study). Seven trials were conducted in newly diagnosed transplant eligible patients (GEM05MENOS65 Study; GIMEMA‐MMY‐3006 Study; HOVON‐65/GMMG‐HD4 Study; IFM 2005‐01 Study; IFM 2007‐02 Study; MD Anderson Study; NMSG 15/05 Study), three trials in transplant ineligible patients (GIMEMA‐MM‐03‐05 Study; GEM2010MAS65 Study; VISTA Study), and five trials in patients with relapsed/refractory myeloma (APEX Study; CREST Study; MMVAR/IFM 2005‐04 Study; MMY‐3021 Study; NMSG 17/07 Study). One trial studied myeloma patients with light chain induced acute renal failure (All India Institute Study).
Participants
All trials included male and female patients with a diagnosis of multiple myeloma according to either the Durie‐Salmon staging system or International Staging System (ISS) and who were at least 18 years of age. Upper age limits were reported in some trials; patients less than 65 years of age were included in transplant eligible trials, whereas patients greater than 65 years were included in transplant ineligible trials. We did not extract or analyse age because of the variation across trials and instead conducted subgroup analysis of trials of transplant eligible and ineligible patients.
Interventions
Interventions included bortezomib in combination with other agents, such as chemotherapy drugs e.g. cyclophosphamide, melphalan; corticosteroids e.g. dexamethasone and prednisone and immuno‐modulatory agents e.g. thalidomide and included two‐, three‐ and four‐drug combinations, given orally and/or by intravenous administration, or both. Bortezomib as a single agent was evaluated in four trials (APEX Study; CREST Study; NMSG 15/05 Study; MMY‐3021 Study). The APEX Study compared bortezomib and high‐dose dexamethasone with high‐dose dexamethasone only in patients with relapsed disease who had received one to three prior therapies. In this study 62% of patients on the high‐dose dexamethasone arm crossed over to the bortezomib arm following disease progression. The CREST Study was the first published randomised study of bortezomib to evaluate the safety and efficacy of two doses of bortezomib in 54 patients who had relapsed after or were refractory to frontline therapy and received intravenous bortezomib at doses of 1.0 mg/m2 or 1.3 mg/m2 twice weekly for two weeks every three weeks for up to eight cycles. The MMY‐3021 Study was a non‐inferiority trial that compared subcutaneous versus intravenous administration of bortezomib, while the NMSG 15/05 Study evaluated bortezomib as consolidation therapy versus no treatment. Interventions including bortezomib were administered as induction therapy (prior to stem cell transplantation) in five trials, of which two trials also included maintenance therapy (GEM05MENOS65 Study; HOVON‐65/GMMG‐HD4 Study) and one trial included consolidation therapy (GIMEMA‐MM‐03‐05 Study) post‐transplant. One trial evaluated maintenance therapy in transplant ineligible patients (GIMEMA‐MM‐03‐05 Study). Comparator(s) included either: no therapy, chemotherapy drugs, corticosteroids and/or combination treatment regimens.
Outcomes
Overall survival (OS) and progression‐free survival (PFS) data were available from nine trials (APEX Study; GIMEMA‐MM‐03‐05 Study; GIMEMA‐MMY‐3006 Study; HOVON‐65/GMMG‐HD4 Study; IFM 2005‐01 Study; MD Anderson Study; MMVAR/IFM 2005‐04 Study; NMSG 15/05 Study; VISTA Study). Time to progression (TTP) was reported in four trials (APEX Study; GIMEMA‐MMY‐3006 Study; MMVAR/IFM 2005‐04 Study; VISTA Study). Treatment‐free interval (TFI) or time to next treatment or therapy was reported in two trials (NMSG 17/07 Study; VISTA Study). Response rates were reported in all trials, although not all response categories (overall, complete and partial response) were reported, therefore we prioritised the extraction and analysis of overall response rate (ORR) and complete response rate (CRR) data. Adverse events (AEs) were also reported in all trials, although the level of AE reporting varied. We therefore prioritised the extraction and analysis of common grade 3 and grade 4 AEs. Treatment‐related deaths (TRD) were reported in five trials (APEX Study; GIMEMA‐MM‐03‐05 Study; IFM 2005‐01 Study; MD Anderson Study; VISTA Study), while four trials included health‐related quality of life (HRQoL) outcomes (APEX Study, NMSG 15/05 Study, NMSG 17/07 Study and VISTA Study).
See Characteristics of included studies for further details.
Excluded studies
A total of six studies were excluded (Characteristics of excluded studies). The study by Chen 2011b compared bortezomib and dexamethasone (BD) versus vincristine, doxorubicin and dexamethasone (VAD) as induction therapy followed by thalidomide as maintenance therapy in newly diagnosed myeloma. The study design was described as 'retrospective randomised' involving 46 patients that were randomised according to date of hospitalisation. We decided to exclude this study as it was a small study incorporating a quasi‐randomisation method. The remaining five studies were excluded because each trial involved bortezomib treatment at the same dose or schedule on both arms.
Risk of bias in included studies
Results of the overall 'Risk of bias' assessment is presented in Figure 2 and a summary of the risk of bias for each included trial is presented in Figure 3. Some criteria are assessed for individual outcomes e.g. blinding, intention‐to‐treat (ITT) analysis.
2.
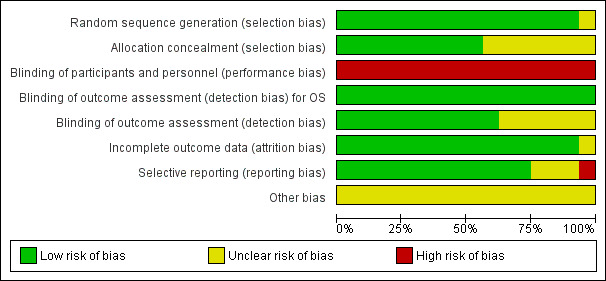
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
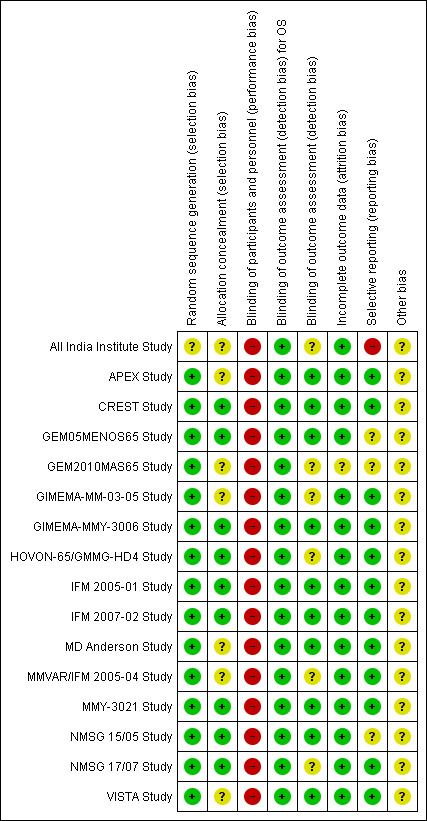
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
The funnel plots for the primary outcomes of OS and PFS did not suggest publication bias.
Allocation
We judged the potential risk of selection bias for random sequence generation as low for most included trials with the exception of one trial (All India Institute Study). This study was published as a conference abstract and was described as 'randomised' in the title, however provided no additional information regarding randomisation. All other trial publications provided a clearer description of randomisation and therefore we considered these trials to be at low risk of bias for random sequence generation.
We judged the potential risk of bias as low for allocation concealment for nine of the included trials, as the type of randomisation system used was adequately reported e.g. centrally randomised or web‐based system, whereas the potential risk of bias for seven trials was considered to be as unclear as no details were provided. One trial used randomisation envelopes, and was judged to be at somewhat higher risk of bias (CREST Study). However, this was still allocated low risk as per Cochrane guidelines.
Blinding
All trial allocations were open‐label, with both participants and trial personnel aware of the treatments administered. Blinding in cancer trials poses ethical considerations and is generally difficult to implement because of the different adverse‐event profiles, routes of administration and schedules between treatment arms. Open‐label studies are more susceptible to performance bias, therefore we judged the potential risk of bias for blinding of participants and personnel to be high.
Blinding of outcomes assessment was employed in 10 of the 16 trials, wherein outcomes were assessed independently e.g. disease response assessment performed by central laboratory analysis and/or results adjudicated by an independent committee of experts or data analysts. We therefore considered these trials to be at low risk of detection bias for outcome assessment. For the remaining six trials, we considered the risk of detection bias as unclear as these trials did not report the use of central or independent review of outcomes data.
Incomplete outcome data
We judged the potential risk of attrition bias as low for the included trials with the exception of the GEM2010MAS65 Study. This study has only been published as a conference abstract to date, therefore did not provide sufficient information to fully assess this criterion. We considered the potential risk of attrition bias as unclear. It was noted that this trial is ongoing at the time of preparing this review. The majority of trials provided a detailed participant flow chart with the rates of withdrawal, drop‐out and loss to follow‐up being generally acceptable and the completeness of follow‐up data considered adequate.
Selective reporting
We judged the risk of reporting bias as low in 12 of the 16 trials as the benefits and side effects of treatment were adequately reported for each arm. We considered the risk of reporting bias as unclear for three trials. Two trials reported selected AEs only (GEM05MENOS65 Study and NMSG 15/05 Study), while one trial has only been published as a conference abstract and did not have sufficient information available (GEM2010MAS65 Study). It was noted that this trial is registered on a clinical trials registry and is ongoing at the time of preparing this review. We considered one trial to be at high risk of reporting bias (All India Institute Study). This trial was also published as a conference abstract and did not report the key eligibility criteria or details of baseline characteristics in each arm. Selected AEs were reported. We could not find this trial registered on a clinical trials registry and we were unable to contact the study authors.
It was noted that 14 of the 16 trials were registered on a publicly accessible clinical trials registry. There were two exceptions (All India Institute Study and CREST Study). The latter study was conducted in 2001 when registration was not yet routine and may have not been registered for this reason. The number of relevant trials identified through searches of clinical trials registries were in line with that conducted at the protocol stage of this review (preliminary searches were conducted in 2012 and in 2013).
Other potential sources of bias
We did not identify any other potential sources of bias in the 16 trials. We extracted sponsorship and funding details for each included trial where it was reported and did not identify any particular bias regarding either of these sources. It was noted that four of the 16 trials were sponsored by pharmaceutical companies (APEX Study; CREST Study; MMY‐3021 Study; VISTA Study), while 11 trials were sponsored either by academic groups, research institutes, hospitals or investigators and one trial's sponsorship was unknown (All India Institute Study). Four trials sponsored by academic groups reported sources of funding from pharmaceutical companies (GEM05MENOS65 Study; GIMEMA‐MMY‐3006 Study; HOVON‐65/GMMG‐HD4 Study; MD Anderson Study).
Effects of interventions
See: Table 1
See Table 1 for the main comparisons.
Comparison 1 and 2: Bortezomib versus no bortezomib with the same background therapy in each arm/bortezomib versus no bortezomib with different background therapy in each arm or compared with other agent(s)
Primary Outcomes
Overall survival (OS)
We estimated OS from nine of 12 trials (All India Institute Study; APEX Study; GIMEMA‐MM‐03‐05 Study; GIMEMA‐MMY‐3006 Study; HOVON‐65/GMMG‐HD4 Study; IFM 2005‐01 Study; MD Anderson Study; NMSG 15/05 Study; VISTA Study). We included a total of 4118 patients with 821 reported deaths. Two studies (All India Institute Study; IFM 2005‐01 Study) reported the number of events (number of deaths) per arm, however the publications did not include a Hazard Ratio (HR) comparing the treatments with either a corresponding P value or a 95% confidence interval (CI) for the outcome of OS and therefore were considered non‐estimable for the pooled estimate. The Peto odds ratio (OR) is 0.77 (95% CI 0.69 to 0.86, P < 0.00001) in favour of bortezomib (Analysis 1.1) (Figure 4). In the comparison of bortezomib versus no bortezomib with the same background therapy in each arm, an analysis of 1586 patients produced a statistically significant OS benefit (Peto OR = 0.77 (95% CI 0.65 to 0.92, P < 0.00001) for patients receiving bortezomib. The comparison of bortezomib versus no bortezomib with different background therapy in each arm or compared to other agent(s) included 2532 patients and produced a statistically significant OS benefit (Peto OR = 0.76 (95% CI 0.67 to 0.88, P < 0.00001) for patients receiving bortezomib. Moderate heterogeneity was observed in the studies of bortezomib versus no bortezomib with the same background therapy (I2 = 55%), while no heterogeneity was observed in the studies of different background therapy or when compared to other agents (I2 = 0%), resulting in minor heterogeneity across both groups (I2 = 18%). The test for subgroup differences was not significant (P = 0.92). This meta‐analysis indicates that there is evidence of a significant beneficial effect upon OS in favour of bortezomib.
1.1. Analysis.
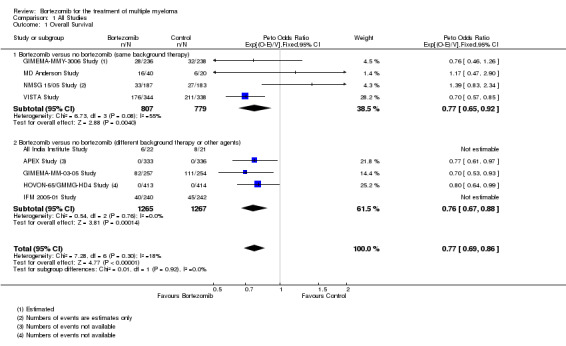
Comparison 1 All Studies, Outcome 1 Overall Survival.
4.
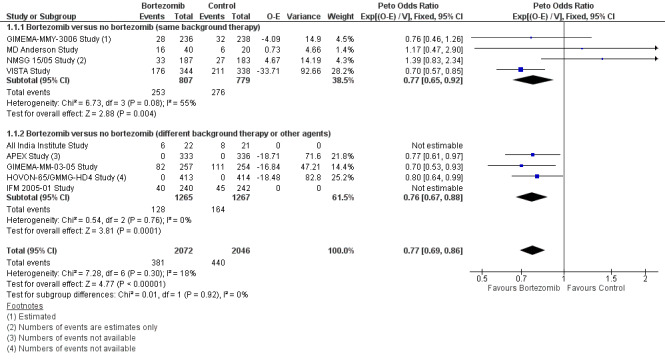
Forest plot of comparison: 3 All studies, outcome: 3.1 Overall Survival.
Progression‐free (PFS) survival
We estimated PFS from nine of 12 trials (APEX Study; GIMEMA‐MM‐03‐05 Study; GIMEMA‐MMY‐3006 Study; HOVON‐65/GMMG‐HD4 Study; IFM 2005‐01 Study; MD Anderson Study; MMVAR/IFM 2005‐04 Study; NMSG 15/05 Study; VISTA Study). We included a total of 4344 patients and an estimated 2063 progression events. The Peto OR is 0.67 (95% CI 0.61 to 0.72, P < 0.00001) in favour of bortezomib (Analysis 1.2) (Figure 5). In the comparison of bortezomib versus no bortezomib with the same background therapy in each arm, an analysis of 1855 patients produced a statistically significant PFS benefit (Peto OR = 0.65 (95% CI 0.57 to 0.74, P < 0.00001) for patients receiving bortezomib. The comparison of bortezomib versus no bortezomib with different background therapy in each arm or compared to other agent(s) included 2489 patients and produced a statistically significant PFS benefit (Peto OR = 0.67 (95% CI 0.61 to 0.75, P < 0.00001) for patients receiving bortezomib. Moderate heterogeneity was observed in the studies of bortezomib versus no bortezomib with the same background therapy (I2 = 49%), while substantial heterogeneity was observed in the other comparison group (I2 = 70%), resulting in moderate heterogeneity across both groups (I2 = 56%). The test for subgroup differences was not significant (P = 0.68). This meta‐analysis indicates that there is evidence of a significant beneficial effect upon PFS for bortezomib.
1.2. Analysis.
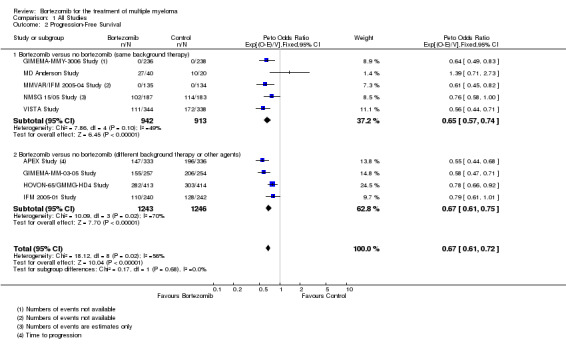
Comparison 1 All Studies, Outcome 2 Progression‐Free Survival.
5.
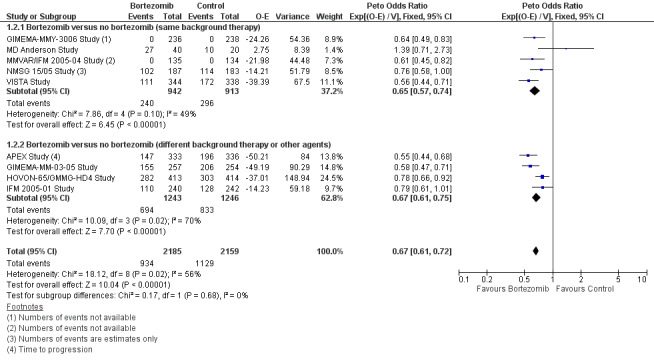
Forest plot of comparison: 3 All studies, outcome: 3.2 Progression Free Survival.
Secondary Outcomes
Complete response rate (CRR)
We estimated CRR from 12 trials. We included a total of 4630 patients with 1093 complete responses.The odds ratio (OR) is 2.35 (95% CI 2.02 to 2.73, P < 0.00001) in favour of bortezomib (Analysis 1.3) (Figure 6). In the comparison of bortezomib versus no bortezomib with the same background therapy in each arm, an analysis of 2064 patients produced a statistically significant benefit for CRR (OR = 2.63 (95% CI 2.13 to 3.24, P < 0.00001) for patients receiving bortezomib. The comparison of bortezomib versus no bortezomib with different background therapy in each arm or compared to other agent(s) included 2566 patients and produced a statistically significant benefit for CRR (OR = 2.08 (95% CI 1.67 to 2.58, P < 0.00001) for patients receiving bortezomib. Substantial heterogeneity was observed in the studies of bortezomib versus no bortezomib with the same background therapy (I2 = 86%), and moderate heterogeneity observed in the studies of different background therapy or when compared to other agents (I2 = 53%), resulting in substantial heterogeneity across both groups (I2 = 77%). The test for subgroup differences was not significant (P = 0.13). This meta‐analysis indicates that there is evidence of a significant beneficial effect upon CRR in favour of bortezomib.
1.3. Analysis.
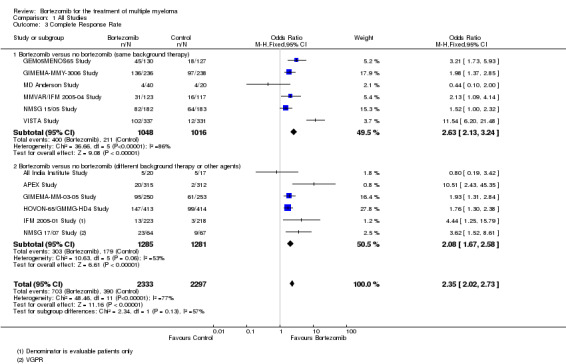
Comparison 1 All Studies, Outcome 3 Complete Response Rate.
6.
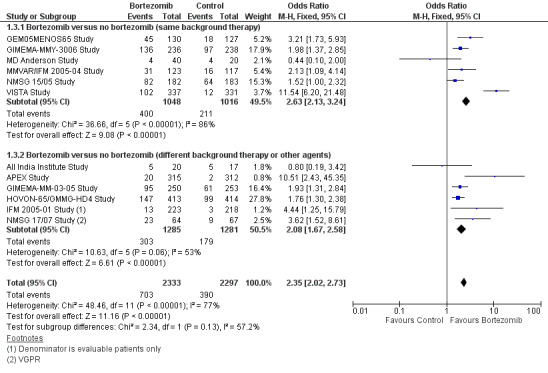
Forest plot of comparison: 3 All studies, outcome: 3.3 Complete Response Rate.
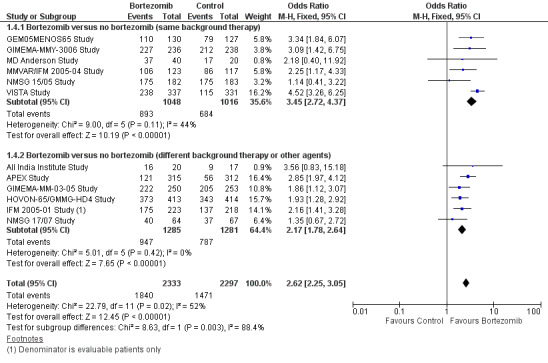
Overall response rate (ORR)
We estimated ORR from 12 trials. We included a total of 4630 patients with 3311 overall responses. The OR is 2.62 (95% CI 2.25 to 3.05, P < 0.00001) in favour of bortezomib (Analysis 1.4) (Figure 7). In the comparison of bortezomib versus no bortezomib with the same background therapy in each arm, an analysis of 2064 patients produced a statistically significant benefit for ORR (OR = 3.45 (95% CI 2.72 to 4.37, P < 0.00001) for patients receiving bortezomib. The comparison of bortezomib versus no bortezomib with different background therapy in each arm or compared to other agent(s) included 2566 patients and produced a statistically significant benefit for ORR (OR = 2.17 (95% CI 1.78 to 2.64, P < 0.00001) for patients receiving bortezomib. Moderate heterogeneity was observed in the studies of bortezomib versus no bortezomib with the same background therapy (I2 = 44%), and no heterogeneity observed in the other comparison group (I2 = 0%), resulting in moderate heterogeneity across both groups (I2 = 52%). The test for subgroup differences was significantly different (P = 0.00). The direction of treatment effect, however, favoured bortezomib in both groups. This meta‐analysis indicates that there is evidence of a significant beneficial effect upon ORR in favour of bortezomib.
1.4. Analysis.
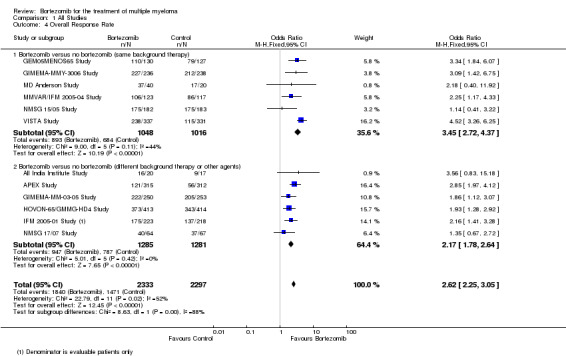
Comparison 1 All Studies, Outcome 4 Overall Response Rate.
7.
Forest plot of comparison: 3 All studies, outcome: 3.4 Overall Response Rate.
Time to progression (TTP)
We identified four trials in the meta‐analysis that measured TTP as an outcome (APEX Study; GIMEMA‐MMY‐3006 Study, MMVAR/IFM 2005‐04 Study; VISTA Study). We were able to extract and analyse PFS data for the VISTA Study, GIMEMA‐MMY‐3006 Study and MMVAR/IFM 2005‐04 Study, while in the case of the APEX Study, we included TTP data as PFS data were not available. We therefore did not analyse TTP separately.
Treatment‐free interval (TFI)
Treatment‐free interval (TFI) or time to next treatment or therapy was reported in two of 16 trials (NMSG 17/07 Study; VISTA Study). Each trial belonged to different comparison groups, therefore it was not possible to conduct meta‐analysis on this outcome.
In the NMSG 17/07 Study, patients were randomised to bortezomib and dexamethasone (BD) or thalidomide and dexamethasone (TD) and assessed the time to start of next line of treatment which was similar for both groups (median of 8.5 months (95% CI 4.5 to 11.8) for BD and 9.7 months (95% CI 5.3 to 11.4) for TD). In the VISTA Study, patients were randomised to receive bortezomib, melphalan and prednisone (VMP) or melphalan and prednisone (MP) alone. The TFI was significantly longer in the bortezomib group than in the control group (median 19.4 versus 9.1 months, HR = 0.573, P = 0.001).
Treatment‐related death (TRD)
We estimated TRD from five of 12 trials. We included a total of 2389 patients with 46 TRDs. The OR is 0.76 (95% CI 0.43 to 1.34, P = 0.34) (Analysis 1.5). In the comparison of bortezomib versus no bortezomib with the same background therapy in each arm, only two trials were included, with no statistically significant difference between the groups (OR = 0.81 (95% CI 0.30 to 2.16, P = 0.67). The comparison of bortezomib versus no bortezomib with different background therapy in each arm or compared to other agent(s) included three trials and also produced a non‐statistically significant difference between the groups (OR = 0.73 (95% CI 0.36 to 1.48, P = 0.12). Minor heterogeneity was observed in the studies of bortezomib versus no bortezomib with the same background therapy (I2 = 9%), and moderate heterogeneity observed in the other comparison group (I2 = 53%), resulting in low heterogeneity across both groups (I2 = 24%). The test for subgroup differences was not significant (P = 0.87). This meta‐analysis indicates that there is no significant difference in TRD between bortezomib and bortezomib‐containing therapies versus non‐bortezomib containing control groups.
1.5. Analysis.
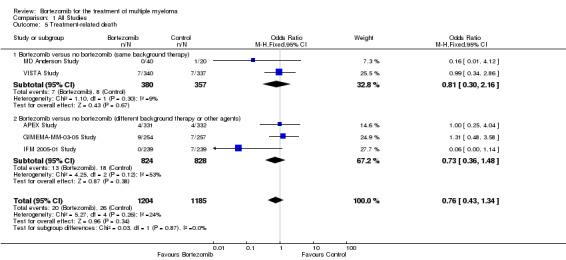
Comparison 1 All Studies, Outcome 5 Treatment‐related death.
Adverse events (AEs)
Thrombocytopenia
Eight of the 12 trials reported on the frequency of grade three or four thrombocytopenia. There were 380 cases (20.0%) in 1897 patients in the bortezomib group and 216 cases (11.4%) in 1894 patients in the control group. The increased risk of thrombocytopenia in patients treated with bortezomib was statistically significant (OR = 2.05, 95% CI 1.70 to 2.48, P < 0.00001) (Analysis 1.6). In the comparison of bortezomib versus no bortezomib with the same background therapy in each arm, OR = 1.51 (95% CI 1.13 to 2.00, P = 0.0048), and for the comparison of bortezomib versus no bortezomib with different background therapy in each arm or compared to other agent(s), OR = 2.60 (95% CI 2.01 to 3.35, P < 0.00001). Minor heterogeneity was observed in the studies of bortezomib versus no bortezomib with the same background therapy (I2 = 12%), and substantial heterogeneity was observed in the studies of bortezomib versus no bortezomib with different background therapy or versus other agent(s) (I2 = 86%), resulting in substantial heterogeneity across both groups (I2 = 81%). The test for subgroup differences was significantly different (P = 0.01). The risk of thrombocytopenia, however, was greater with bortezomib in both groups.
1.6. Analysis.
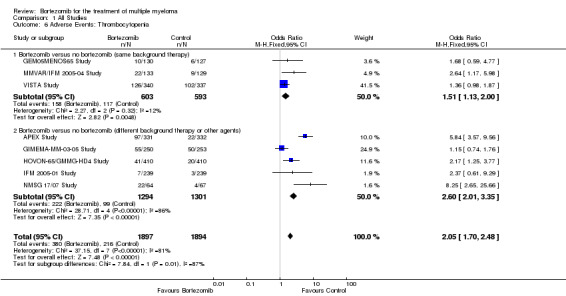
Comparison 1 All Studies, Outcome 6 Adverse Events: Thrombocytopenia.
Neutropenia
Eight of the 12 trials reported on the frequency of grade three or four neutropenia. There were 343 cases (18.1%) in 1897 patients in the bortezomib group and 279 cases (14.8%) in 1894 patients in the control group. The increased risk of neutropenia in patients treated with bortezomib was statistically significant (OR = 1.33, 95% CI 1.10 to 1.60, P = 0.003) (Analysis 1.7). In the comparison of bortezomib versus no bortezomib with the same background therapy in each arm, OR = 0.95 (95% CI 0.73 to 1.24, P = 0.73) and for the comparison of bortezomib versus no bortezomib with different background therapy in each arm or compared to other agent(s), OR = 1.85 (95% CI 1.41 to 2.41, P < 0.00001). Minor heterogeneity was observed in the studies of bortezomib versus no bortezomib with the same background therapy (I2 = 23%), and substantial heterogeneity observed in the studies of bortezomib versus no bortezomib with different background therapy or versus other agent(s) (I2 = 87%), resulting in substantial heterogeneity across both groups (I2 = 82%). The test for subgroup differences was significantly different (P = 0.00). The risk of neutropenia was not significantly different in the studies of bortezomib versus no bortezomib with the same background therapy, whereas it was significantly different in the other comparison group of bortezomib versus no bortezomib with different background therapy or versus other agent(s).
1.7. Analysis.
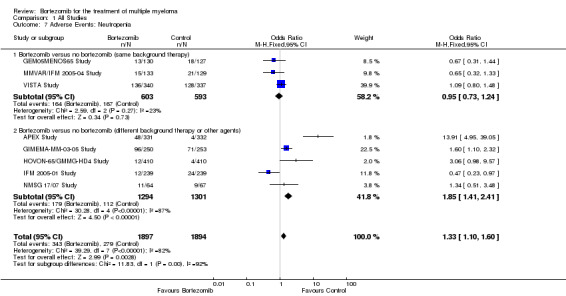
Comparison 1 All Studies, Outcome 7 Adverse Events: Neutropenia.
Anaemia
Six of the 12 trials reported on the frequency of grade three or four anaemia. There were 173 cases (10.2%) in 1703 patients in the bortezomib group and 208 cases (12.2%) in 1701 patients in the control group. In the comparison of bortezomib versus no bortezomib with the same background therapy in each arm, OR = 0.67 (95% CI 0.48 to 0.94, P = 0.02) and for the comparison of bortezomib versus no bortezomib with different background therapy in each arm or compared to other agent(s), OR = 0.92 (95% CI 0.69 to 1.21, P = 0.54). The differential risk of anaemia between the two groups was close to reaching statistical significance between the two groups (OR = 0.80, 95% CI 0.65 to 1.00, P = 0.05) (Analysis 1.8). Substantial heterogeneity was observed in the studies of bortezomib versus no bortezomib with the same background therapy (I2 = 70%), and minor heterogeneity observed in the studies of bortezomib versus no bortezomib with different background therapy or versus other agent(s) (I2 = 26%), resulting in moderate heterogeneity across both groups (I2 = 47%). The test for subgroup differences was not significantly different (P = 0.16).
1.8. Analysis.
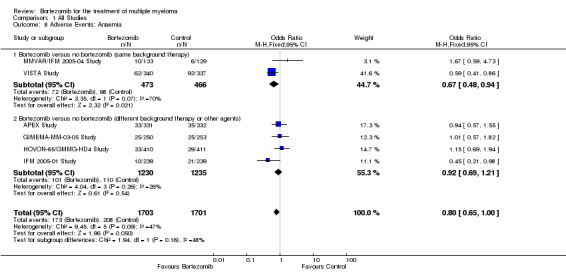
Comparison 1 All Studies, Outcome 8 Adverse Events: Anaemia.
Gastro‐intestinal adverse events (GI AEs)
Eight of the 12 trials included in the meta‐analysis reported GI AEs. We were unable, however, to extract data on individual GI AEs from each trial, and the data therefore include counts of all GI AEs, except where indicated.
Nausea/Vomiting
There were 99 cases (5.2%) in 1894 patients in the bortezomib group and 44 cases (2.3%) in 1894 patients in the control group. The increased risk of nausea/vomiting in patients treated with bortezomib was statistically significant (OR = 2.37, 95% CI 1.64 to 3.42, P < 0.00001) (Analysis 1.9). In the comparison of bortezomib versus no bortezomib with the same background therapy in each arm, OR = 4.55 (95% CI 1.99 to 10.42, P = 0.00033) and for the comparison of bortezomib versus no bortezomib with different background therapy in each arm or compared to other agent(s), OR = 1.93 (95% CI 1.28 to 2.93, P = 0.0018). No heterogeneity was observed in the studies of bortezomib versus no bortezomib with the same background therapy (I2 = 0%), and moderate heterogeneity observed in the other comparison group (I2 = 37%), resulting in minor heterogeneity across both groups (I2 = 27%). The test for subgroup differences was not significantly different (P = 0.07).
1.9. Analysis.
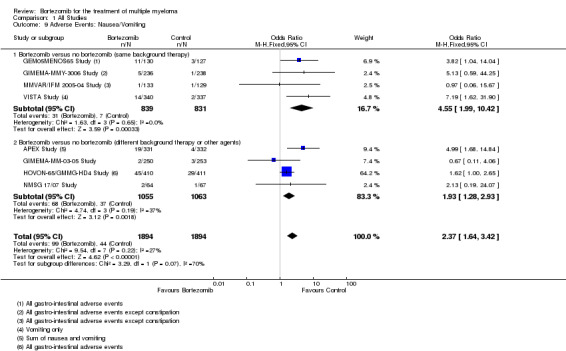
Comparison 1 All Studies, Outcome 9 Adverse Events: Nausea/Vomiting.
Diarrhoea
There were 116 cases (6.1%) in 1894 patients in the bortezomib group and 50 cases (2.6%) in 1894 patients in the control group. The increased risk of diarrhoea in patients treated with bortezomib was statistically significant (OR = 2.44, 95% CI 1.74 to 3.43, P < 0.00001) (Analysis 1.10). In the comparison of bortezomib versus no bortezomib with the same background therapy in each arm, OR = 6.24 (95% CI 2.79 to 13.98, P < 0.00001) and for the comparison of bortezomib versus no bortezomib with different background therapy in each arm or compared to other agent(s), OR = 1.80 (95% CI 1.22 to 2.65, P = 0.0031). Minor heterogeneity was observed in the studies of bortezomib versus no bortezomib with the same background therapy (I2 = 10%), and moderate heterogeneity observed in the other comparison group (I2 = 57%), resulting in moderate heterogeneity across both groups (I2 = 57%). The test for subgroup differences was significantly different (P = 0.01), however risk of diarrhoea was greater with bortezomib for both groups.
1.10. Analysis.
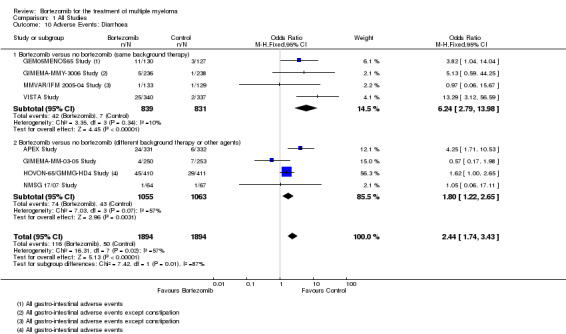
Comparison 1 All Studies, Outcome 10 Adverse Events: Diarrhoea.
Constipation
There were 93 cases (4.9%) in 1894 patients in the bortezomib group and 60 cases (3.2%) in 1894 patients in the control group. The increased risk of constipation in patients treated with bortezomib was statistically significant (OR = 1.59, 95% CI 1.14 to 2.22, P = 0.0064) (Analysis 1.11). In the comparison of bortezomib versus no bortezomib with the same background therapy in each arm, OR = 1.88 (95% CI 1.04 to 3.41, P = 0.037) and for the comparison of bortezomib versus no bortezomib with different background therapy in each arm, or compared to other agent(s), OR = 1.47 (95% CI 0.98 to 2.20, P = 0.063). No heterogeneity was observed in either the studies of bortezomib versus no bortezomib with the same background therapy or in the other comparison group (I2 = 0%). The test for subgroup differences was not statistically significant (P = 0.50).
1.11. Analysis.
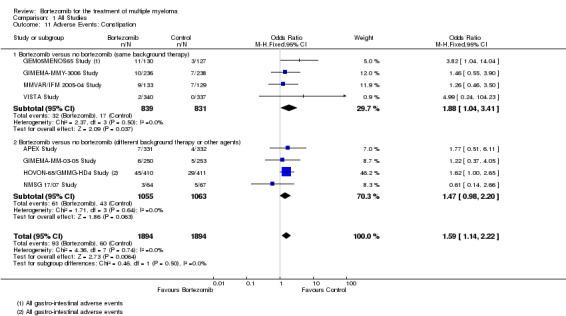
Comparison 1 All Studies, Outcome 11 Adverse Events: Constipation.
Peripheral neuropathy
Ten of the 12 trials reported on the frequency of grade three or four peripheral neuropathy. There were 319 cases (13.75%) in 2320 patients in the bortezomib group and 101 cases (4.4%) in 2316 patients in the control group. The increased risk of peripheral neuropathy in patients treated with bortezomib was statistically significant (OR = 3.71, 95% CI 2.92 to 4.70, P = P < 0.00001) (Analysis 1.12). In the comparison of bortezomib versus no bortezomib with the same background therapy in each arm, OR = 5.10 (95% CI 3.37 to 7.72, P < 0.00001), and for the comparison of bortezomib versus no bortezomib with different background therapy in each arm or compared to other agent(s), OR = 3.09 (95% CI 2.30 to 4.14, P < 0.00001). Moderate heterogeneity was observed in both the studies of bortezomib versus no bortezomib with the same background therapy (I2 = 57%), and in the other comparison group (I2 = 30%), resulting in moderate heterogeneity across both groups (I2 = 40%). The test for subgroup differences was approaching statistical significance (P = 0.05), however, risk of peripheral neuropathy was significantly greater with bortezomib in both groups.
1.12. Analysis.
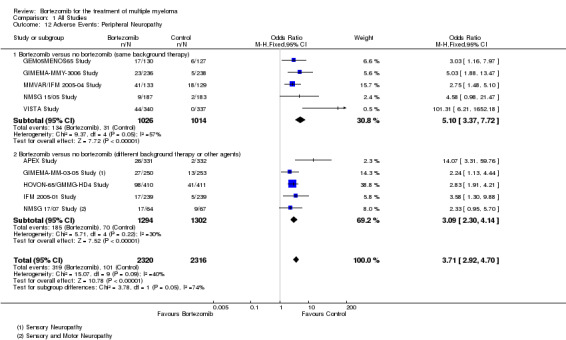
Comparison 1 All Studies, Outcome 12 Adverse Events: Peripheral Neuropathy.
Infections (all)
Nine of the 12 trials reported on the frequency of grade three or four infections. There were 377 cases (17.7%) in 2133 patients in the bortezomib group and 272 cases (12.75%) in 2133 patients in the control group. The increased risk of infection in patients treated with bortezomib was statistically significant (OR = 1.51, 95% CI 1.27 to 1.79, P < 0.00001) (Analysis 1.13). In the comparison of bortezomib versus no bortezomib with the same background therapy in each arm, OR = 1.37 (95% CI 0.97 to 1.93, P = 0.071) and for the comparison of bortezomib versus no bortezomib with different background therapy in each arm or compared to other agent(s), OR = 1.55 (95% CI 1.27 to 1.90, P < 0.00001). Minor heterogeneity was observed in the studies of bortezomib versus no bortezomib with the same background therapy (I2 = 22%) and substantial heterogeneity in the studies of bortezomib versus no bortezomib with different background therapy or versus other agent(s) (I2 = 86%), resulting in substantial heterogeneity across both groups (I2 = 76%). The test for subgroup differences was not statistically significant (P = 0.54).
1.13. Analysis.
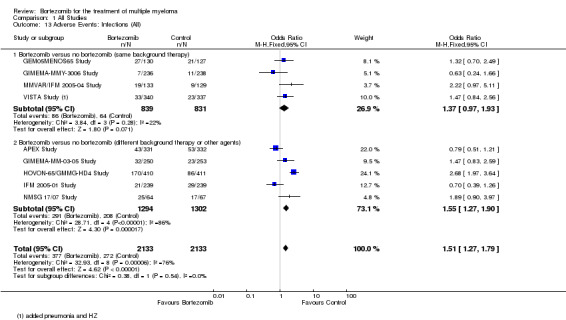
Comparison 1 All Studies, Outcome 13 Adverse Events: Infections (All).
Herpes zoster infection
Grade three or four herpes zoster infections were reported in only four of 12 trials included in the meta‐analysis (two trials in each group). There were 22 cases (2.5%) in 868 patients in the bortezomib group and 12 cases (1.4%) in 865 patients in the control group. The increased risk of herpes zoster infection in patients treated with bortezomib was not statistically significant (OR = 1.83, 95% CI 0.91 to 3.67, P = 0.091) (Analysis 1.14). In the comparison of bortezomib versus no bortezomib with the same background therapy in each arm, OR = 1.93 (95% CI 0.74 to 5.03, P = 0.79) and for the comparison of bortezomib versus no bortezomib with different background therapy in each arm or compared to other agent(s), OR = 1.71 (95% CI 0.62 to 4.74, P = 0.30). No heterogeneity was observed in the studies of bortezomib versus no bortezomib with the same background therapy (I2 = 0%), while very minor heterogeneity was present in the other comparison group (I2 = 2%), resulting in no heterogeneity across both groups (I2 = 0%). The test for subgroup differences was not statistically significant (P = 0.87). It should be noted that an increased risk of herpes zoster infection associated with bortezomib treatment has been reported. In the APEX Study, a subset analysis found that bortezomib was associated with a significantly higher incidence of herpes zoster compared with dexamethasone treatment (13% versus 5%, P = 0.0002), with most herpes zoster infections classified as either grade one or two infections, whereas incidences of grade three or four events and infections that were considered SAEs, were similar between treatment arms.
1.14. Analysis.
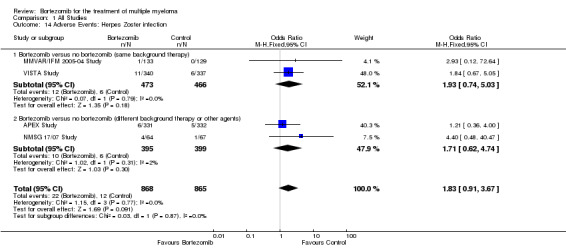
Comparison 1 All Studies, Outcome 14 Adverse Events: Herpes Zoster infection.
Cardiac disorders
Grade three and four cardiac disorders were reported in only five of 12 trials included in the meta‐analysis. There were 70 cases (6.4%) in 1093 patients in the bortezomib group and 42 cases (3.8%) in 1098 patients in the control group. There was no statistically significant increased risk of cardiac disorders in patients treated with bortezomib compared to the control group in the studies of bortezomib versus no bortezomib with the same background therapy comprising two trials (OR = 1.17, 95% CI 0.39 to 3.52, P = 0.78). A significantly elevated risk, however, was detected in the studies of bortezomib versus no bortezomib with different background therapy or versus other agent(s) that comprised three trials (OR = 1.84, 95% CI 1.21 to 2.81, P = 0.006) (Analysis 1.15). No heterogeneity was observed in either or across both groups (I2 = 0%). The test for subgroup differences was not statistically significant (P = 0.45).
1.15. Analysis.
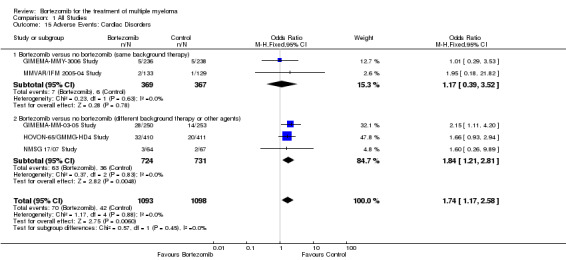
Comparison 1 All Studies, Outcome 15 Adverse Events: Cardiac Disorders.
Fatigue
Fatigue was reported in only five of 12 trials included in the meta‐analysis. There were 84 cases (5.7%) in 1464 patients in the bortezomib group and 44 cases (3.0%) in 1462 patients in the control group. The increased risk of fatigue in patients treated with bortezomib compared to the control group was statistically significant (OR = 1.96, 95% CI 1.35 to 2.84, P = 0.0004) (Analysis 1.16). In the comparison of bortezomib versus no bortezomib with the same background therapy in each arm, OR = 3.30 (95% CI 1.66 to 6.58, P = 0.00069) and for the comparison of bortezomib versus no bortezomib with different background therapy in each arm or compared to other agent(s), OR = 1.52 (95% CI 0.97 to 2.38, P = 0.070). No heterogeneity was observed in the studies of bortezomib versus no bortezomib with the same background therapy (I2 = 0%), with moderate heterogeneity in the other comparison group (I2 = 39%), resulting in moderate heterogeneity across both groups (44%). The test for subgroup differences was borderline statistically significant (P = 0.06).
1.16. Analysis.
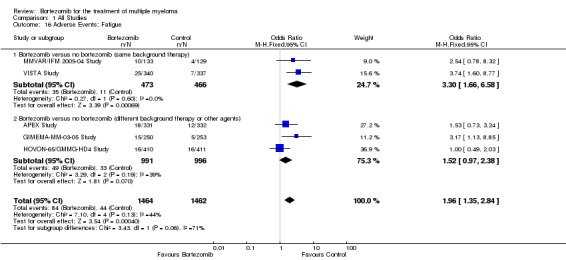
Comparison 1 All Studies, Outcome 16 Adverse Events: Fatigue.
Health‐related quality of life (HRQoL)
Only four trials included HRQoL outcomes (APEX Study; NMSG 15/05 Study; NMSG 17/07 Study; VISTA Study,). Each trial used the same validated quality of life instrument (European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire (QLQ‐C30), whereas the time points of administration of the questionnaire varied between the four trials. Detailed quality of life analyses were performed in the VISTA Study and in the APEX Study. The APEX study also measured neurotoxicity using the Functional Assessment of Cancer Therapy/Gynecologic Oncology Group–Neurotoxicity (NTX) side‐effects questionnaire. As the number of trials with quality of life outcomes data was relatively small (four of 12 trials) and the data reported for some trials insufficient, we could not perform meta‐analysis on this outcome.
In summary, data from the APEX Study (bortezomib versus no bortezomib with different background therapy or versus other agent(s)) indicated that patients treated with bortezomib had significantly better mean Global Health Status when compared to patients receiving dexamethasone. Patients treated with bortezomib also had significantly better physical health, role, cognitive, and emotional functioning scores, lower dyspnoea and sleep symptom scores. Better NTX questionnaire scores were observed on the bortezomib arm when compared to the dexamethasone arm, despite a significantly greater incidence of greater than or equal to grade three peripheral neuropathy in those who received bortezomib. This observation could be due to the range of measures assessed by the NTX scale that are not related to peripheral neuropathy.
Limited quality of life data was reported in the NMSG 15/05 Study comparing bortezomib consolidation with no consolidation therapy post transplant setting (bortezomib versus no bortezomib with the same background therapy). After eight weeks of study treatment, a statistically significant increase in fatigue and nausea or vomiting was observed in the bortezomib group (P < 0.01), with fatigue reported as reaching the cut‐off for clinical relevance. However, there were no significant differences in HRQoL scores between the bortezomib arm and the control arm. In the NMSG 17/07 Study of bortezomib and dexamethasone versus thalidomide and dexamethasone in melphalan‐refractory patients (bortezomib versus no bortezomib with different background therapy or versus other agent(s)), no difference was seen for any of the quality of life domains measured, with the exception of fatigue which was observed to be worse in the bortezomib arm (P = 0.04). A significantly higher score for sleep disturbance was observed in the bortezomib arm at 12 weeks of treatment (P < 0.01).
Finally, in the VISTA Study of bortezomib, melphalan and prednisone (VMP) versus melphalan and prednisone (MP) in transplant ineligible myeloma (bortezomib versus no bortezomib with the same background therapy), patients experienced clinically meaningful lower quality of life domain scores after four cycles of treatment with VMP. Improvements on the VMP arm compared to the baseline and to the MP arm were observed from cycle five, while mean scores generally improved by the end‐of‐treatment assessment versus baseline in both arms. Improved HRQoL was observed in the analyses of patients receiving a lower dose intensity of bortezomib, while multivariate analysis suggested clinically and statistically significant improvements in domains of global health status. In addition, it was found that lower scores for pain, appetite loss and diarrhoea may occur in patients who respond to treatment and in particular those patients who achieve CR.
Comparison 3: Dose and Schedule Studies
Four trials assessed bortezomib dose comparisons, methods of administrations and treatment schedules (CREST Study; GEM2010MAS65 Study; IFM 2007‐02 Study; MMY‐3021 Study), and were assessed qualitatively only.
The CREST Study was the first published randomised study of bortezomib to evaluate the safety and efficacy of two doses of bortezomib in 54 patients who had relapsed after, or were refractory to frontline therapy. Patients received intravenous bortezomib at doses of 1.0 mg/m2 or 1.3 mg/m2 twice weekly for two weeks every three weeks for up to eight cycles. Dexamethasone was permitted in patients with either progressive or stable disease after two or four cycles, respectively. The primary outcomes were response rates, and secondary outcomes were response rate to bortezomib in combination with dexamethasone, TTP on bortezomib alone and in combination with dexamethasone.
The GEM2010MAS65 Study was a randomised open‐label phase III trial that compared VMP to Lenalidomide plus dexamethasone (Rd) in a sequential versus an alternating scheme in 242 elderly patients > 65 years with newly diagnosed myeloma. Patients received either a sequential scheme of nine cycles of VMP followed by nine cycles of Rd or the same regimens in an alternating approach (one cycle of VMP alternating with one Rd (half of the patients started by VMP and half by Rd) for up to 18 cycles. The primary outcomes were TTP and toxicity and secondary outcomes were response, genomics analysis, duration of response, PFS, time to next therapy and OS. This study has only been published by conference abstract to date.
The IFM 2007‐02 Study was a randomised open‐label phase III trial and compared bortezomib‐dexamethasone (VD) as induction therapy versus a combination of reduced doses of bortezomib and thalidomide plus dexamethasone (vtD) in 199 patients with newly diagnosed myeloma. The primary outcome was post‐induction complete response rate (CRR) and secondary outcomes were CR plus very good partial response (VGPR) rates after cycle two, after induction and after autologous stem cell transplant, overall response rates (ORR) (≥ partial response (PR)) after cycle two, after induction and after autologous stem cell transplant., safety and toxicity.
The MMY‐3021 Study was a randomised open‐label phase III non‐inferiority trial of subcutaneous versus intravenous administration of bortezomib in 222 patients with measurable progressive disease who had received one to three prior therapies. The primary outcome was ORR after four cycles and secondary outcomes were CR, nCR and VGPR after four cycles, ORR after eight cycles, time to response, duration of response, TTP, PFS, one‐year OS, safety and tolerability, pharmacokinetics and pharmacodynamics. The initial trial report demonstrated non‐inferior efficacy with subcutaneous versus intravenous bortezomib for ORR after four cycles of single‐agent bortezomib.
Overall Survival (OS)
In the CREST Study, one‐ and five‐year survival rates were 82% and 32%, respectively, in the 1.0 mg/m2 group and 81% and 45%, respectively, in the 1.3 mg/m2 group. In the GEM2010MAS65 Study, after a median follow‐up of 27 months, OS was not reached. Patients who achieved a complete response had significantly longer OS (and PFS) when compared with patients who did not achieve CR in both arms and patients younger than 75 years demonstrated significantly improved survival when compared to patients ≥ 75 years, however there was no significant difference between the arms. In the IFM 2007‐02 Study, there was no difference regarding OS between the two arms (VD versus vtD). In the MMY‐3021 Study, which tested for non‐inferiority, one‐year OS was not significantly different between the treatment arms of subcutaneous versus intravenous bortezomib (72.6%, 95% CI 63.1 to 80.0 versus 76.7%, 95% CI 64.1 to 85.4, P = 0.504).
Progression‐Free Survival (PFS)
In the CREST Study, median TTP was 7.0 months and 11.0 months in the 1.0 mg/m2 and 1.3 mg/m2 groups respectively. In the GEM2010MAS65 Study, after a median follow‐up of 27 months, PFS was not significantly different between the arms (30 months in both arms). In the IFM 2007‐02 Study, there was no difference in median PFS between the two arms (30 months in the VD arm versus 26 months in the vtD arm, P = 0.22). In the MMY‐3021 Study, there were no significant differences in TTP between the treatment arms of subcutaneous versus intravenous bortezomib (median 10.4 months, 95% CI 8.5 to 11.7 versus 9.4 months, 95% CI 7.6 to 10.6; P = 0·387).
Adverse Events (AEs)
The most commonly reported adverse events (AEs) in the CREST Study were fatigue (70%), nausea (54%), diarrhoea (44%), pyrexia (41%), constipation (37%), peripheral neuropathy (41%), arthralgia (35%), insomnia (35%), headache (31%), limb pain (31%), thrombocytopenia (30%) and upper respiratory tract infection (30%). In the GEM2010MAS65 Study, no significant differences were observed between the sequential and alternating arms in the frequency of grade three or four neutropenia (19% and 22%), thrombocytopenia (21% and 20%), and 3% and 6% of patients in the sequential and alternating arms had grade three or four infections. No differences were observed in the incidence of peripheral neuropathy in the sequential and alternating arms (4% and 3%, respectively), nor in the rate of grade three or four thrombotic events (1% and 2%). In the IFM 2007‐02 Study, the proportion of patients with at least one AE of grade three or higher was not different between the two groups. Grade three or four haematological or non‐haematological toxicities, with the exception of peripheral neuropathy were rare, with no significant differences between the arms. The incidence of peripheral neuropathy was 70% in the VD arm versus 53% in the vtD arm (P = 0.01). Grade two peripheral neuropathy was much higher in the VD arm than vtD arm and grade three peripheral neuropathy was seen in 11% of patients with VD compared to 3% with vtD (P = 0.03), with four patients discontinuing treatment because of peripheral neuropathy in the VD arm versus none in the vtD arm.
In the MMY‐3021 Study, grade three or worse AEs were reported in 84 (57%) patients in the subcutaneous group versus 52 (70%) in the intravenous group. The most common AEs were thrombocytopenia (19 (13%) versus 14 [(19%)), neutropenia (26 (18%) versus 13 (18%)), and anaemia (18 (12%) versus six (8%)). Peripheral neuropathy of any grade (56 (38%) versus 39 (53%); P = 0·044), grade two or worse (35 (24%) versus 30 (41%); P = 0·012), and grade three or worse (nine (6%) versus 12 (16%); P = 0·026) was significantly less common with subcutaneous than with intravenous administration.
Subgroup analysis ‐ disease setting
We considered three subgroups for myeloma disease setting: transplant eligible, transplant ineligible and relapsed/refractory disease and included 11 trials in this subgroup analysis (we did not include the All India Institute Study as the disease setting was unclear). We performed subgroup analyses for OS, PFS, CRR and ORR (Analysis 2.1; Analysis 2.2; Analysis 2.3; Analysis 2.4).
2.1. Analysis.
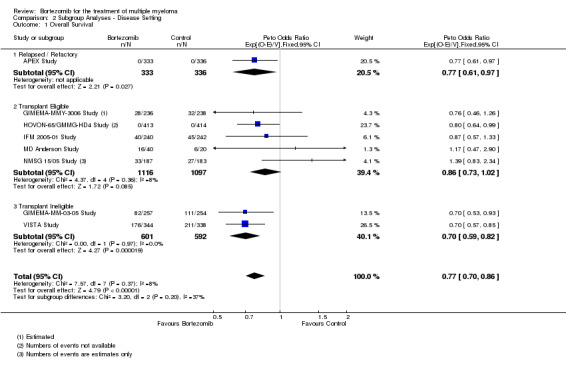
Comparison 2 Subgroup Analyses ‐ Disease Setting, Outcome 1 Overall Survival.
2.2. Analysis.
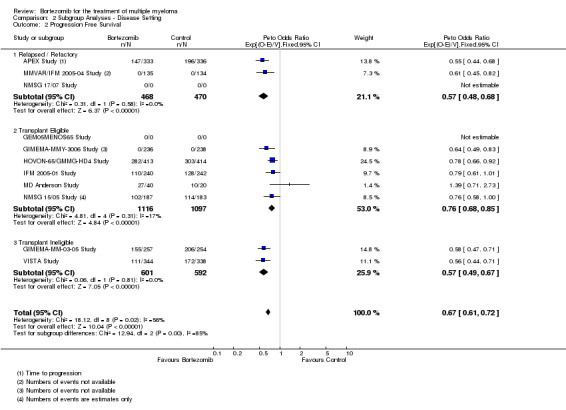
Comparison 2 Subgroup Analyses ‐ Disease Setting, Outcome 2 Progression Free Survival.
2.3. Analysis.
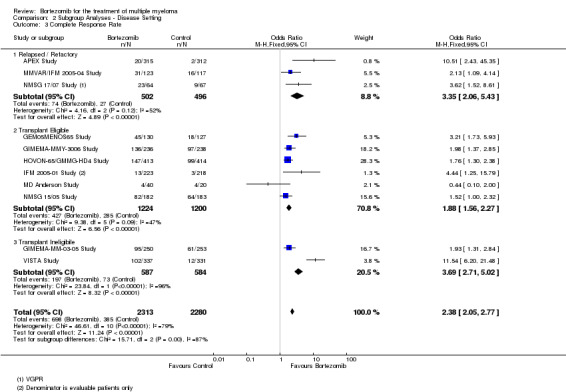
Comparison 2 Subgroup Analyses ‐ Disease Setting, Outcome 3 Complete Response Rate.
2.4. Analysis.
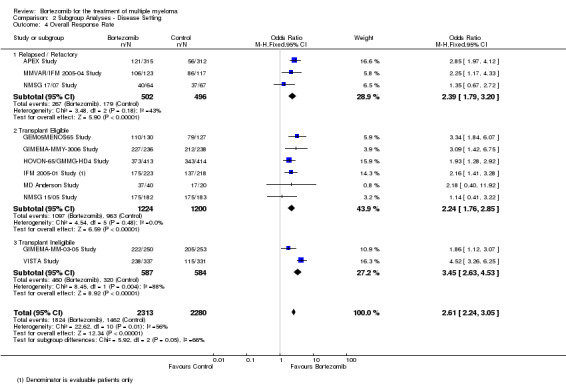
Comparison 2 Subgroup Analyses ‐ Disease Setting, Outcome 4 Overall Response Rate.
For OS, a statistically significant benefit with bortezomib treatment was observed in all groups, with the smallest benefit observed in the transplant eligible group. Considering this group alone, the benefit was not statistically significant with a Peto odds ratio (OR) of 0.86 (95% CI 0.73 to 1.02) (Analysis 2.1). For PFS, the observed benefit for bortezomib was lower in the transplant eligible group than the other two groups but still statistically significant (Analysis 2.2). There was evidence of heterogeneity between subgroups for PFS (P = 0.002, I² = 84.5%).
Subgroup analysis ‐ therapy setting
We considered three subgroups for myeloma therapy setting: induction, consolidation and maintenance and included six trials in the subgroup analysis for therapy setting. We also performed subgroup analyses for OS, PFS, CRR and ORR (Analysis 3.1; Analysis 3.2; Analysis 3.3; Analysis 3.4).
3.1. Analysis.
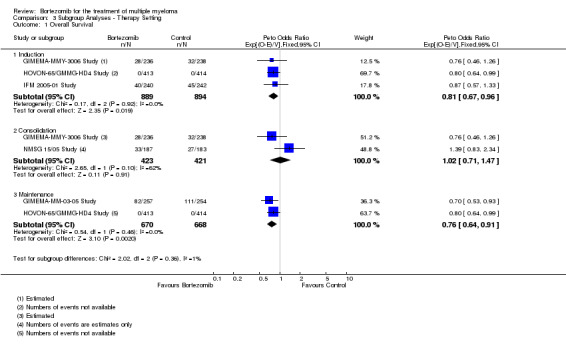
Comparison 3 Subgroup Analyses ‐ Therapy Setting, Outcome 1 Overall Survival.
3.2. Analysis.
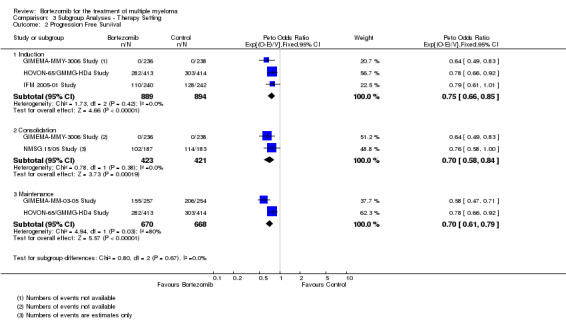
Comparison 3 Subgroup Analyses ‐ Therapy Setting, Outcome 2 Progression Free Survival.
3.3. Analysis.
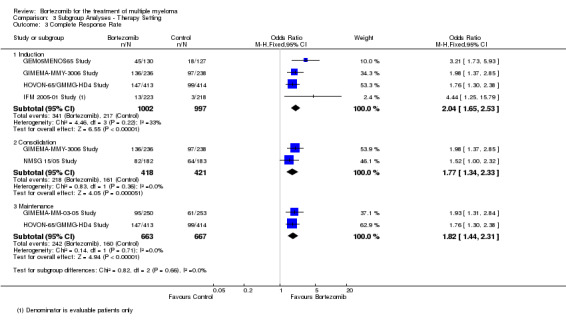
Comparison 3 Subgroup Analyses ‐ Therapy Setting, Outcome 3 Complete Response Rate.
3.4. Analysis.
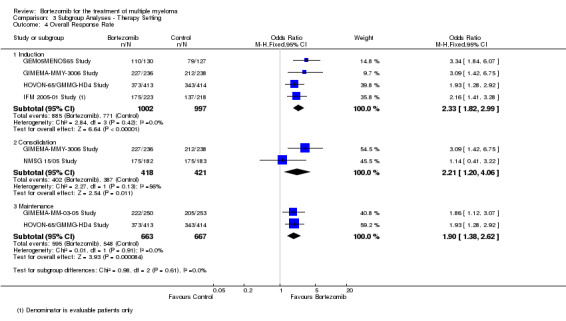
Comparison 3 Subgroup Analyses ‐ Therapy Setting, Outcome 4 Overall Response Rate.
A statistically significant benefit for bortezomib was observed in all outcomes and subgroups except for OS following consolidation therapy. Heterogeneity tests between subgroups were non‐significant for all outcomes.
Discussion
Summary of main results
The goal of this systematic review and meta‐analysis was to synthesise all available data on the effects of bortezomib treatment for multiple myeloma. We identified 16 relevant randomised controlled trials (RCTs) involving 5626 patients and 12 trials were included in the meta‐analyses. Among these 12 trials, six trials involving 2247 patients evaluated bortezomib versus no bortezomib with the same background therapy in each arm, while another six trials involving 2663 patients evaluated bortezomib versus no bortezomib with either different background therapy in each arm or compared to other agents. An additional four trials involving 716 patients assessed bortezomib dose comparisons, methods of administrations and treatment schedules. The trials in this group were too dissimilar for meta‐analysis and were therefore assessed qualitatively. We identified four trials in the meta‐analysis that measured time to progression (TTP) as an outcome and were able to extract and analyse progression‐free survival (PFS) data for three of the studies, while in the case of the APEX Study, we included TTP data as PFS data were not available. We therefore did not analyse TTP separately.
From this systematic review and meta‐analysis we can summarise the following.
A clear benefit in overall and progression‐free survival (PFS) in favour of bortezomib is observed in the pooled analysis of trials and for each pf the comparison groups analysed.
Patients treated with bortezomib also had overall (ORR) and complete response rates (CRR) that were significantly higher than in controls in the pooled analysis and also for each of the comparison groups analysed.
Patients treated with bortezomib had significantly greater risk of thrombocytopenia, neutropenia, gastro‐intestinal toxicities, peripheral neuropathy, infection and fatigue. A greater risk of cardiac disorders was observed only in the studies of bortezomib versus no bortezomib with different background therapy in each arm or versus other agents and there was no evidence of increased risk of treatment‐related death (TRD) in either of the groups analysed.
From the qualitative analysis of four trials that evaluated dose and schedule studies, an improved benefit was observed with a dose of 1.3 mg/m2 compared to 1.0 mg/m2 in the CREST Study; and this dose has been the approved starting dose level for bortezomib. The MMY‐3021 Study also showed that subcutaneous administration of bortezomib is non‐inferior to intravenous administration and also a significantly lower incidence of peripheral neuropathy was observed with subcutaneous administration.
Health‐related quality of life (HRQoL) was only examined in four trials. Improved Global Health Status was observed with bortezomib in the APEX Study, while no difference in quality of life measures were observed between arms in the NMSG 15/05 Study and NMSG 17/07 Study, with some symptoms e.g. fatigue, sleep disturbance significantly worse in the bortezomib group. In the VISTA Study, quality of life was worse in the bortezomib group after four cycles, but improved after five cycles of treatment and also at the end‐of‐treatment assessment.
Subgroup analyses by disease setting revealed improvements in all outcomes. Moderate to substantial heterogeneity was observed between the subgroups, with the smallest benefit seen in transplant eligible patients. In the case of overall survival (OS), the benefit for bortezomib was not statistically significant.
In the subgroup analyses by therapy setting, a statistically significant benefit for bortezomib was observed in all outcomes and subgroups, except for that of OS following consolidation therapy. However, the heterogeneity test comparing the treatment effect in this subgroup with the other groups was not significant. As such, there is little justification for treating this subgroup as different from the others, and therefore we are unable to conclude that there was no OS benefit for bortezomib in this specific setting.
Overall completeness and applicability of evidence
Sixteen published RCTs are included in this review of bortezomib treatment for multiple myeloma. Of the included studies, six RCTs assessed the efficacy and safety of bortezomib versus no bortezomib, in the setting of identical background therapy, whereas another six RCTs had different background therapy in each arm. Both of these groups of trials (a total of 12 studies) were included in the meta‐analysis. Another four RCTs assessed different bortezomib dose, administration and treatment schedules, but could not be included in the meta‐analysis. A majority of included studies (14 of 16) were published as full‐text articles and only two studies in abstract form. Of the 12 studies included in the meta‐analysis, eight studies provided data on OS, nine studies provided data on PFS and 12 studies provided data on response rates. All studies reported adverse effect (AE) data, although not for all of the individual AEs reported in this review. Only five of 12 trials reported the incidence of TRD and only four trials included analyses of health‐related quality of Life (HRQoL).
We therefore conclude that the completeness and applicability of the evidence in this review to be generally moderate to high for the outcomes relevant to this review. We are aware of 15 ongoing or unpublished studies (see Characteristics of studies awaiting classification and Characteristics of ongoing studies) from a review of clinical trials registries that may be included in a future update of this review. At least three trials were identified as completed in 2013 through searches of clinical trials registries (Consolidation (61‐75 years) Study; Consolidation (less than 60 years) Study; King Fasail Hospital Study), however these trials are not yet published and no further data have been provided by the authors. An assessment of the published articles, however, will be required in order to determine their eligibility for inclusion.
Quality of the evidence
The risk of bias in all 16 studies included in this review has been analysed in detail. Two included trials were published in abstract form (All India Institute Study; GEM2010MAS65 Study). We were therefore unable to fully assess the potential risk of bias in these studies. All included studies had an open‐label design, while seven studies had unclear allocation concealment that could suggest selection and performance biases. Six studies had unclear blinding of outcome assessment, which could lead to detection bias. Attrition and reporting bias were considered to be at low risk for the majority of studies. One aspect was considered to be at high risk of bias in one study (All India Institute Study, where reporting bias was felt to be a potential issue. We judged the overall risk of bias of included trials as generally low and therefore could be considered to be of adequate methodological quality. Collectively, the quality of evidence for the main comparisons was high for OS (mortality) but low for PFS, primarily due to trial heterogeneity. Significant heterogeneity was observed in the analysis of PFS, particularly in the comparison group of bortezomib versus no bortezomib with different background therapy or other agents. This heterogeneity may have arisen as a result of this comparison being confounded by the presence of other treatments. Also, differences in the methods used for response assessment across trials may have also contributed to variability in the data; trials either employed the IMWG (International Myeloma Working Group guidelines) or EBMT (European Group for Blood and Marrow Transplant) response criteria. In addition, only some trials conducted central or independent review of response assessments, which may also have been a contributory factor. Quality of evidence for TRD was moderate due to low number of events and wide confidence intervals. Quality of evidence for HRQoL could not be evaluated due to few trials evaluating this outcome.
Potential biases in the review process
To prevent potential biases in the review process, we considered only RCTs. We attempted to avoid biases by conducting all review processes (trial searching, data extraction and analysis) in duplicate, by two review authors working independently. Any disagreements were discussed in order to reach consensus. Overall, we are confident that all relevant studies were identified and included and all review processes were followed according to Cochrane recommendations.
We did not identify any evidence of publication bias, however a number of trials were identified as ongoing and three trials were reported as complete in 2013 but not yet published, therefore we could not include data from these trials in this review.
Agreements and disagreements with other studies or reviews
To our knowledge this is the first comprehensive systematic review and meta‐analysis of bortezomib treatment for multiple myeloma across all disease and therapy settings. We searched for published reviews and/or health technology reports with systematic searches of databases and identified the following publications.
We identified three systematic reviews and meta‐analyses that have been conducted to evaluate the efficacy of bortezomib for the treatment of myeloma in specific disease or therapy settings.
Nooka 2013 performed a meta‐analysis of RCTs of bortezomib‐containing induction regimens (BICR) in transplant eligible myeloma patients, and identified four eligible trials, all of which are included in our review. In their review, the impact of BCIR versus non‐bortezomib‐containing induction regimens (NBCIR) on responses rates, PFS and OS and on regimen‐related grade three toxicities was analysed. The pooled hazard ratios (HR) for three‐year PFS and OS were 0.71 (95% confidence interval (CI), 0.60 to 0.83, P < 0.00001) and 0.79 (95% CI, 0.66 to 0.96, P = 0.014), respectively in favour of BCIR. Response rates were statistically significantly in favour of BICR. These findings would also be in agreement with our subgroup analyses of induction therapy trials (Analysis 3.1 to Analysis 3.4).
Sonneveld 2013 also performed a meta‐analysis of clinical trials involving induction regimens containing bortezomib versus no bortezomib, and included the same four trials in the review by Nooka 2013, also included in our review. They analysed patient‐level data from three of the trials and study‐level data from a fourth trial due to legal restrictions on data access. Complete response rates were significantly higher post‐transplant following bortezomib‐based versus non bortezomib‐based induction therapy (38% versus 24%; OR = 2.05 (95% CI, 1.64 to 2.56, P = 0.001) and this benefit remained when the fourth trial data was included (pooled odds ratio (OR) = 1.96). Median PFS was 35.9 months versus 28.6 months with bortezomib‐based versus non bortezomib‐based induction, respectively (HR = 0.75, P = 0.001) and three‐year OS (HR = 0.81, P = 0.0402).
Finally, Zeng 2013 performed a systematic review and meta‐analysis of clinical trials of bortezomib for patients with previously untreated myeloma and included five trials, all of which are included in our review. They included three trials that compared bortezomib with no bortezomib, and two that compared bortezomib with other treatments (vincristine/Adriamycin‐based chemotherapy) in their analysis. Compared with no bortezomib or vincristine‐based chemotherapy, the bortezomib‐based regimen significantly improved OS: HR = 0.71 (95 % CI 0.55 to 0.93) and HR = 0.77 (95 % CI 0.60 to 0.99), respectively. However, they found when compared with the vincristine plus Adriamycin‐based regimen, the OS was similar (HR = 0.87, 95% CI 0.57 to 1.33). Other efficacy outcomes such as TTP, PFS, and response rates were also improved in patients receiving the bortezomib‐based regimen.
All three reviews reported significantly higher rates of adverse events, especially peripheral neuropathy with bortezomib‐based regimens. The results of these three systematic reviews and meta‐analysis are therefore in agreement with the findings in our review.
A Health Technology Assessment (HTA) conducted in the UK summarised RCT evidence for clinical effectiveness as a narrative summary and cost‐effectiveness analysis of bortezomib or thalidomide in combination with an alkylating agent and a corticosteroid for first‐line treatment (Picot 2011). This review included only one trial of bortezomib treatment (VISTA Study), and found that VMP (bortezomib, melphalan, and prednisone) could be considered more clinically effective than MP (melphalan and prednisone alone) for the first‐line treatment of myeloma in patients ineligible for high‐dose therapy and stem cell transplant.
Authors' conclusions
Implications for practice.
This systematic review and meta‐analysis focused on clinically relevant outcomes, such as response rates, survival and adverse events. Our review found that treatment of myeloma with bortezomib leads to statistically significant improvements in response rates and in the duration of progression‐free and overall survival across myeloma disease and therapy settings. Bortezomib, however, also induces significant toxicity that may be dose‐limiting. As a result, recommended dose modification schedules as well as appropriate evidence‐based prophylaxis and supportive care regimens should be used for the duration of therapy. Premature discontinuation of therapy due to toxicity such as peripheral neuropathy will prevent such patients benefiting from this effective agent.
We conclude that bortezomib should be considered to be a standard therapy for multiple myeloma.
There is insufficient evidence, however, to draw any conclusions regarding the optimal combination therapy involving bortezomib.
Implications for research.
While substantial clinical evidence has accumulated to support the use of bortezomib as a treatment for multiple myeloma, clinical trials of newer proteasome inhibitors are also needed. A number of novel proteasome inhibitor drugs are in clinical development, the most advanced of which is carfilzomib. A global assessment of novel agents should encompass not only survival and response outcomes but also adverse effects and patient quality of life. In addition, given the increasing cost of anti‐cancer therapies on health budgets, a formal evaluation of the cost‐effectiveness of these newer proteasome inhibitor drugs should be routinely included in cost‐benefit analyses. In summary, further research should encompass the following.
The optimal proteasome inhibitor to be included in combination regimens for the treatment of myeloma in each disease and therapy setting.
Further evaluation of clinical and biologic prognostic markers, for example fluorescence in situ hybridisation (FISH) cytogenetic profiles and their influence on response to treatment with proteasome inhibitors.
Further evaluation on the dose and scheduling of proteasome inhibitors in order to improve the toxicity profile of this class of agent and for optimal health‐related quality of life.
Mechanisms of resistance to proteasome inhibitors should be identified and strategies to overcome resistance developed.
Predictors of response to proteasome inhibitor treatment should be identified, such that treatment can be tailored to individual myeloma patients.
Acknowledgements
The authors thank: Ina Monsef, Trial Search Co‐ordinator of the Cochrane Haematological Malignancies Review Group for developing the search strategies and conducting database searches; Andrew Howman for advice and expertise on statistical methods and Cochrane review methodology, searching for trials, selection of studies, data extraction, statistical analysis and data presentation, input to drafting the protocol and review; Greg Sheaf, librarian at Trinity College Dublin for advice on searching and reference management; Veronica Moroz, biostatistician at CRCTU, University of Birmingham for double checking some data extracted on survival outcomes; Andrea Will and the rest of the staff at the editorial base of the Cochrane Haematological Malignancies Group for their comments on this review.
Appendices
Appendix 1. Cochrane Central Register of Controlled Trials search strategy
| ID | Search |
| 1 | MeSH descriptor: [Multiple Myeloma] explode all trees |
| 2 | myelom* |
| 3 | MeSH descriptor: [Plasmacytoma] explode all trees |
| 4 | plasm*cytom* |
| 5 | plasmozytom* |
| 6 | plasm* cell myelom* |
| 7 | myelomatosis |
| 8 | MeSH descriptor: [Leukemia, Plasma Cell] explode all trees |
| 9 | (plasma* near/3 neoplas*) |
| 10 | kahler* |
| 11 | #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 |
| 12 | (proteasom* near/2 inhibitor*) |
| 13 | bortezomib* |
| 14 | proscript* |
| 15 | (PS‐341* or PS341*) |
| 16 | (LDP‐341* or LDP341* or MLN‐341* or MLN341* or MG‐341* or MG341*) |
| 17 | velcad* |
| 18 | #12 or #13 or #14 or #15 or #16 or #17 |
| 19 | #11 and #18 |
Appendix 2. MEDLINE search strategy
| ID | Search |
| 1 | exp MULTIPLE MYELOMA/ |
| 2 | myelom$.tw,kf,ot. |
| 3 | exp PLASMACYTOMA/ |
| 4 | plasm?cytom$.tw,kf,ot. |
| 5 | plasmozytom$.tw,kf,ot. |
| 6 | plasm$ cell myelom$.tw,kf,ot. |
| 7 | myelomatosis.tw,kf,ot. |
| 8 | LEUKEMIA, PLASMA CELL/ |
| 9 | (plasma$ adj3 neoplas$).tw,kf,ot. |
| 10 | kahler.tw,kf,ot. |
| 11 | or/1‐10 |
| 12 | (proteasom$ adj2 inhibitor$).tw,kf,ot. |
| 13 | bortezomib$.tw,kf,ot,nm. |
| 14 | proscript$.tw,kf,nm,ot. |
| 15 | (PS‐341 or PS341).tw,kf,nm,ot. |
| 16 | (LDP‐341 or LDP341 or MLN‐341 or MLN341 or MG‐341 or MG341).tw,kf,nm,ot. |
| 17 | velcad$.tw,kf,ot. |
| 18 | or/12‐17 |
| 19 | 11 and 18 |
| 20 | randomized controlled trial.pt. |
| 21 | controlled clinical trial.pt. |
| 22 | randomi?ed.ab. |
| 23 | placebo.ab. |
| 24 | clinical trials as topic.sh. |
| 25 | randomly.ab. |
| 26 | trial.ti. |
| 27 | or/20‐26 |
| 28 | humans.sh. |
| 29 | 27 and 28 |
| 30 | 19 and 29 |
Appendix 3. EMBASE search strategy
| ID | Search |
| 1 | randomization/exp |
| 2 | (factorial AND design) |
| 3 | (crossover AND procedure/exp) |
| 4 | placebo/exp |
| 5 | (double AND blind/exp AND procedure/exp) |
| 6 | (single AND blind/exp AND procedure/exp) |
| 7 | assign* |
| 8 | allocat* |
| 9 | volunteer* |
| 10 | (randomized AND controlled AND trial) |
| 11 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 |
| 12 | (multiple AND myeloma/exp) |
| 13 | myelom* |
| 14 | plasmacytoma/exp |
| 15 | (plasm* AND cell/exp AND myelom*) |
| 16 | (plasma/exp AND cell/exp AND leukemia/exp) |
| 17 | (plasma* NEAR/3 neoplas*) |
| 18 | 12 or 13 or 14 or 15 or 16 or 17 |
| 19 | (proteasome/exp AND inhibitor) |
| 20 | bortezomib/exp |
| 21 | velcade/exp |
| 22 | velcad* |
| 23 | PS 341/exp OR PS341/exp |
| 24 | LDP 341/exp OR LDP341/exp or MLN 341/exp OR MLN341/exp OR MG 341/exp OR MG341/exp |
| 25 | 19 or 20 or 21 or 22 or 23 or 24 |
| 26 | 11 and 18 and 25 |
Data and analyses
Comparison 1. All Studies.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall Survival | 9 | 4118 | Peto Odds Ratio (95% CI) | 0.77 [0.69, 0.86] |
| 1.1 Bortezomib versus no bortezomib (same background therapy) | 4 | 1586 | Peto Odds Ratio (95% CI) | 0.77 [0.65, 0.92] |
| 1.2 Bortezomib versus no bortezomib (different background therapy or other agents) | 5 | 2532 | Peto Odds Ratio (95% CI) | 0.76 [0.67, 0.88] |
| 2 Progression‐Free Survival | 9 | 4344 | Peto Odds Ratio (95% CI) | 0.67 [0.61, 0.72] |
| 2.1 Bortezomib versus no bortezomib (same background therapy) | 5 | 1855 | Peto Odds Ratio (95% CI) | 0.65 [0.57, 0.74] |
| 2.2 Bortezomib versus no bortezomib (different background therapy or other agents) | 4 | 2489 | Peto Odds Ratio (95% CI) | 0.67 [0.61, 0.75] |
| 3 Complete Response Rate | 12 | 4630 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.35 [2.02, 2.73] |
| 3.1 Bortezomib versus no bortezomib (same background therapy) | 6 | 2064 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.63 [2.13, 3.24] |
| 3.2 Bortezomib versus no bortezomib (different background therapy or other agents) | 6 | 2566 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.08 [1.67, 2.58] |
| 4 Overall Response Rate | 12 | 4630 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.62 [2.25, 3.05] |
| 4.1 Bortezomib versus no bortezomib (same background therapy) | 6 | 2064 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.45 [2.72, 4.37] |
| 4.2 Bortezomib versus no bortezomib (different background therapy or other agents) | 6 | 2566 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.17 [1.78, 2.64] |
| 5 Treatment‐related death | 5 | 2389 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.43, 1.34] |
| 5.1 Bortezomib versus no bortezomib (same background therapy) | 2 | 737 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.30, 2.16] |
| 5.2 Bortezomib versus no bortezomib (different background therapy or other agents) | 3 | 1652 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.36, 1.48] |
| 6 Adverse Events: Thrombocytopenia | 8 | 3791 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.05 [1.70, 2.48] |
| 6.1 Bortezomib versus no bortezomib (same background therapy) | 3 | 1196 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.13, 2.00] |
| 6.2 Bortezomib versus no bortezomib (different background therapy or other agents) | 5 | 2595 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.60 [2.01, 3.35] |
| 7 Adverse Events: Neutropenia | 8 | 3791 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.10, 1.60] |
| 7.1 Bortezomib versus no bortezomib (same background therapy) | 3 | 1196 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.73, 1.24] |
| 7.2 Bortezomib versus no bortezomib (different background therapy or other agents) | 5 | 2595 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.85 [1.41, 2.41] |
| 8 Adverse Events: Anaemia | 6 | 3404 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.65, 1.00] |
| 8.1 Bortezomib versus no bortezomib (same background therapy) | 2 | 939 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.48, 0.94] |
| 8.2 Bortezomib versus no bortezomib (different background therapy or other agents) | 4 | 2465 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.69, 1.21] |
| 9 Adverse Events: Nausea/Vomiting | 8 | 3788 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.37 [1.64, 3.42] |
| 9.1 Bortezomib versus no bortezomib (same background therapy) | 4 | 1670 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.55 [1.99, 10.42] |
| 9.2 Bortezomib versus no bortezomib (different background therapy or other agents) | 4 | 2118 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.93 [1.28, 2.93] |
| 10 Adverse Events: Diarrhoea | 8 | 3788 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.44 [1.74, 3.43] |
| 10.1 Bortezomib versus no bortezomib (same background therapy) | 4 | 1670 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.24 [2.79, 13.98] |
| 10.2 Bortezomib versus no bortezomib (different background therapy or other agents) | 4 | 2118 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.80 [1.22, 2.65] |
| 11 Adverse Events: Constipation | 8 | 3788 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.59 [1.14, 2.22] |
| 11.1 Bortezomib versus no bortezomib (same background therapy) | 4 | 1670 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.88 [1.04, 3.41] |
| 11.2 Bortezomib versus no bortezomib (different background therapy or other agents) | 4 | 2118 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.98, 2.20] |
| 12 Adverse Events: Peripheral Neuropathy | 10 | 4636 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.71 [2.92, 4.70] |
| 12.1 Bortezomib versus no bortezomib (same background therapy) | 5 | 2040 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.10 [3.37, 7.72] |
| 12.2 Bortezomib versus no bortezomib (different background therapy or other agents) | 5 | 2596 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.09 [2.30, 4.14] |
| 13 Adverse Events: Infections (All) | 9 | 4266 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.27, 1.79] |
| 13.1 Bortezomib versus no bortezomib (same background therapy) | 4 | 1670 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.97, 1.93] |
| 13.2 Bortezomib versus no bortezomib (different background therapy or other agents) | 5 | 2596 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.55 [1.27, 1.90] |
| 14 Adverse Events: Herpes Zoster infection | 4 | 1733 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.91, 3.67] |
| 14.1 Bortezomib versus no bortezomib (same background therapy) | 2 | 939 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.93 [0.74, 5.03] |
| 14.2 Bortezomib versus no bortezomib (different background therapy or other agents) | 2 | 794 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.62, 4.74] |
| 15 Adverse Events: Cardiac Disorders | 5 | 2191 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.74 [1.17, 2.58] |
| 15.1 Bortezomib versus no bortezomib (same background therapy) | 2 | 736 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.39, 3.52] |
| 15.2 Bortezomib versus no bortezomib (different background therapy or other agents) | 3 | 1455 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.84 [1.21, 2.81] |
| 16 Adverse Events: Fatigue | 5 | 2926 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.96 [1.35, 2.84] |
| 16.1 Bortezomib versus no bortezomib (same background therapy) | 2 | 939 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.30 [1.66, 6.58] |
| 16.2 Bortezomib versus no bortezomib (different background therapy or other agents) | 3 | 1987 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.97, 2.38] |
Comparison 2. Subgroup Analyses ‐ Disease Setting.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall Survival | 8 | 4075 | Peto Odds Ratio (95% CI) | 0.77 [0.70, 0.86] |
| 1.1 Relapsed / Refactory | 1 | 669 | Peto Odds Ratio (95% CI) | 0.77 [0.61, 0.97] |
| 1.2 Transplant Eligible | 5 | 2213 | Peto Odds Ratio (95% CI) | 0.86 [0.73, 1.02] |
| 1.3 Transplant Ineligible | 2 | 1193 | Peto Odds Ratio (95% CI) | 0.70 [0.59, 0.82] |
| 2 Progression Free Survival | 11 | 4344 | Peto Odds Ratio (95% CI) | 0.67 [0.61, 0.72] |
| 2.1 Relapsed / Refactory | 3 | 938 | Peto Odds Ratio (95% CI) | 0.57 [0.48, 0.68] |
| 2.2 Transplant Eligible | 6 | 2213 | Peto Odds Ratio (95% CI) | 0.76 [0.68, 0.85] |
| 2.3 Transplant Ineligible | 2 | 1193 | Peto Odds Ratio (95% CI) | 0.57 [0.49, 0.67] |
| 3 Complete Response Rate | 11 | 4593 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.38 [2.05, 2.77] |
| 3.1 Relapsed / Refactory | 3 | 998 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.35 [2.06, 5.43] |
| 3.2 Transplant Eligible | 6 | 2424 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.88 [1.56, 2.27] |
| 3.3 Transplant Ineligibile | 2 | 1171 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.69 [2.71, 5.02] |
| 4 Overall Response Rate | 11 | 4593 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.61 [2.24, 3.05] |
| 4.1 Relapsed / Refactory | 3 | 998 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.39 [1.79, 3.20] |
| 4.2 Transplant Eligible | 6 | 2424 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.24 [1.76, 2.85] |
| 4.3 Transplant Ineligibile | 2 | 1171 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.45 [2.63, 4.53] |
Comparison 3. Subgroup Analyses ‐ Therapy Setting.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall Survival | 5 | Peto Odds Ratio (95% CI) | Subtotals only | |
| 1.1 Induction | 3 | 1783 | Peto Odds Ratio (95% CI) | 0.81 [0.67, 0.96] |
| 1.2 Consolidation | 2 | 844 | Peto Odds Ratio (95% CI) | 1.02 [0.71, 1.47] |
| 1.3 Maintenance | 2 | 1338 | Peto Odds Ratio (95% CI) | 0.76 [0.64, 0.91] |
| 2 Progression Free Survival | 5 | Peto Odds Ratio (95% CI) | Subtotals only | |
| 2.1 Induction | 3 | 1783 | Peto Odds Ratio (95% CI) | 0.75 [0.66, 0.85] |
| 2.2 Consolidation | 2 | 844 | Peto Odds Ratio (95% CI) | 0.70 [0.58, 0.84] |
| 2.3 Maintenance | 2 | 1338 | Peto Odds Ratio (95% CI) | 0.70 [0.61, 0.79] |
| 3 Complete Response Rate | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Induction | 4 | 1999 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.04 [1.65, 2.53] |
| 3.2 Consolidation | 2 | 839 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.77 [1.34, 2.33] |
| 3.3 Maintenance | 2 | 1330 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.82 [1.44, 2.31] |
| 4 Overall Response Rate | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Induction | 4 | 1999 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.33 [1.82, 2.99] |
| 4.2 Consolidation | 2 | 839 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.21 [1.20, 4.06] |
| 4.3 Maintenance | 2 | 1330 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.90 [1.38, 2.62] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
All India Institute Study.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study design described as 'randomised' in title however randomisation methods not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study. |
| Blinding of outcome assessment (detection bias) for OS | Low risk | Low risk of bias for OS. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Objective outcomes measured (e.g. response rate and progression according to IMWG guidelines, number of patients alive). Independent blinded outcomes assessment not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Patient status reported at median follow‐up of 7.5 months. |
| Selective reporting (reporting bias) | High risk | Key eligibility criteria not reported. Baseline characteristics per arm not reported. Selected adverse events reported only. |
| Other bias | Unclear risk | Not reported. |
APEX Study.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study design described as 'randomised'. |
| Allocation concealment (selection bias) | Unclear risk | Not specified. Central randomisation probably performed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study. |
| Blinding of outcome assessment (detection bias) for OS | Low risk | Low risk of bias for OS. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objective endpoints measured (e.g. response rate and progression by the European Group for Blood and Bone Marrow Transplant (EBMT) criteria, overall survival). Response data based on central laboratory analysis. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data for the final analyses of the time to disease progression and the response were censored. Full details of censoring reported. |
| Selective reporting (reporting bias) | Low risk | Benefits and harms reported. |
| Other bias | Unclear risk | Not reported. |
CREST Study.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study design described as 'randomised'. |
| Allocation concealment (selection bias) | Low risk | Randomisation envelopes at each centre selected based on stage of disease and front‐line chemo‐therapeutic regimen. Type of envelope used e.g. opaque and who had access to the envelopes not adequately reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study. |
| Blinding of outcome assessment (detection bias) for OS | Low risk | Low risk of bias for OS. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objective endpoints measured (e.g. response rate and progression by the European Group for Blood and Bone Marrow Transplant (EBMT) criteria, overall survival). Response data assessed by independent review committee of 3 myeloma experts independent of trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Patient status at > 5‐year median follow‐up reported. |
| Selective reporting (reporting bias) | Low risk | Benefits and harms reported. |
| Other bias | Unclear risk | Not reported. |
GEM05MENOS65 Study.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study design described as 'randomised'. |
| Allocation concealment (selection bias) | Low risk | Patients were 'centrally randomised'. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study. |
| Blinding of outcome assessment (detection bias) for OS | Low risk | Low risk of bias for OS. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objective endpoints measured (e.g. response rate according to the European Group for Blood and Bone Marrow Transplant (EBMT) criteria, overall survival). Response data and toxicity monitored by an external contract research organisation and centrally reviewed by the principal investigators. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Acceptable rates of withdrawal during induction therapy reported. |
| Selective reporting (reporting bias) | Unclear risk | Benefits and harms reported, however selected adverse events reported only. |
| Other bias | Unclear risk | Not reported. |
GEM2010MAS65 Study.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study design described as 'randomised'. |
| Allocation concealment (selection bias) | Unclear risk | Not known. Central randomisation probably performed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study. |
| Blinding of outcome assessment (detection bias) for OS | Low risk | Low risk of bias for OS. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Objective endpoints measured (e.g. response rate according to the European Group for Blood and Bone Marrow Transplant (EBMT) criteria, overall survival). Not known if blinded/independent outcomes assessment conducted. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not clear if any patients lost to follow‐up, withdrawn etc (abstract only). |
| Selective reporting (reporting bias) | Unclear risk | Not clear (abstract only). Benefits and harms reported. |
| Other bias | Unclear risk | Not reported. |
GIMEMA‐MM‐03‐05 Study.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study design described as 'randomised'. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. Multi‐centre trial, therefore probably centrally randomised. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study. |
| Blinding of outcome assessment (detection bias) for OS | Low risk | Low risk of bias for OS. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Objective endpoints measured (e.g. response rate and progression according to International Uniform Response Criteria, overall survival) however independent blinded outcomes assessment not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Detailed flow chart accounting for all patients. Acceptable rates of withdrawal/lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Benefits and harms reported. |
| Other bias | Unclear risk | Not reported. |
GIMEMA‐MMY‐3006 Study.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study design described as 'randomised'. |
| Allocation concealment (selection bias) | Low risk | Patients were randomised via 'web‐based system' at central coordinating centre. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study. |
| Blinding of outcome assessment (detection bias) for OS | Low risk | Low risk of bias for OS. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objective endpoints measured (e.g. response rate and progression according to the European Group for Blood and Bone Marrow Transplant (EBMT) criteria, overall survival). Responses monitored by external contract research organisation and centrally reassessed by central coordinating team. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Detailed flow chart accounting for all patients. Acceptable rates of withdrawal/lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Benefits and harms reported. |
| Other bias | Unclear risk | Not reported. |
HOVON‐65/GMMG‐HD4 Study.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study design described as 'randomised'. |
| Allocation concealment (selection bias) | Low risk | Patients were randomised via 'web‐based' system. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study. |
| Blinding of outcome assessment (detection bias) for OS | Low risk | Low risk of bias for OS. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Objective endpoints measured (e.g. response rate and progression according to modified European Group for Blood and Bone Marrow Transplant (EBMT) criteria, overall survival), however, independent/blinded outcomes assessment not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Detailed flow chart accounting for all patients included. |
| Selective reporting (reporting bias) | Low risk | Benefits and harms reported. |
| Other bias | Unclear risk | None reported. |
IFM 2005‐01 Study.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study design described as 'randomised'. |
| Allocation concealment (selection bias) | Low risk | Patients were 'centrally randomly assigned'. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study. |
| Blinding of outcome assessment (detection bias) for OS | Low risk | Low risk of bias for OS. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objective endpoints measured (e.g. response rate and progression according to modified European Group for Blood and Bone Marrow Transplant (EBMT) criteria, overall survival). Responses confirmed by independent review committee. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Detailed flow chart accounting for all patients included. Acceptable rates of withdrawal. |
| Selective reporting (reporting bias) | Low risk | Benefits and harms reported. |
| Other bias | Unclear risk | None reported. |
IFM 2007‐02 Study.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study design described as 'randomised'. |
| Allocation concealment (selection bias) | Low risk | Patients were 'centrally randomised'. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study. |
| Blinding of outcome assessment (detection bias) for OS | Low risk | Low risk of bias for OS. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objective endpoints measured (e.g. response rate and progression according to International Myeloma Working Group Uniform Criteria, overall survival). Laboratory samples to evaluate response data were centrally evaluated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Detailed flow chart accounting for all patients included. Acceptable rates of withdrawal. |
| Selective reporting (reporting bias) | Low risk | Benefits and harms reported. |
| Other bias | Unclear risk | None reported. |
MD Anderson Study.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study design described as 'randomised'. |
| Allocation concealment (selection bias) | Unclear risk | Not specified. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study. |
| Blinding of outcome assessment (detection bias) for OS | Low risk | Low risk of bias for OS. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objective endpoints measured (e.g. response rate, overall survival). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 patients withdrew early (from group 1 and group 3). No lost to follow‐up patients reported. |
| Selective reporting (reporting bias) | Low risk | Benefits and harms reported. |
| Other bias | Unclear risk | None reported. |
MMVAR/IFM 2005‐04 Study.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study design described as 'randomised'. |
| Allocation concealment (selection bias) | Unclear risk | Not specified. Central randomisation most probably performed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study. |
| Blinding of outcome assessment (detection bias) for OS | Low risk | Low risk of bias for OS. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Objective endpoints measured (e.g. response rate and progression according to European Group for Blood and Bone Marrow Transplant (EBMT) criteria, overall survival). Independent outcomes assessment not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow chart with all randomised patients accounted for. Low rate of lost to follow‐up. All patients included in intention‐to‐treat Analysis. |
| Selective reporting (reporting bias) | Low risk | Benefits and harms reported. |
| Other bias | Unclear risk | None reported. |
MMY‐3021 Study.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study design described as 'randomised', employing a 'computer‐generated randomisation schedule' based on 'permuted blocks'. |
| Allocation concealment (selection bias) | Low risk | Central randomisation using an 'interactive voice response system' (IVRS). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study. |
| Blinding of outcome assessment (detection bias) for OS | Low risk | Low risk of bias for OS. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objective endpoints measured (e.g. response rate and progression by European Group for Blood and Bone Marrow Transplant (EBMT) criteria, overall survival). Response data based on central laboratory analysis and blinded response evaluation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Detailed flow chart with all randomised patients accounted for. No lost to follow‐up patients reported. |
| Selective reporting (reporting bias) | Low risk | Benefits and harms reported. |
| Other bias | Unclear risk | None reported. |
NMSG 15/05 Study.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study design described as 'randomised'. |
| Allocation concealment (selection bias) | Low risk | Central computer randomisation performed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study. |
| Blinding of outcome assessment (detection bias) for OS | Low risk | Low risk of bias for OS. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objective endpoints measured (e.g. response rate and progression according to European Group for Blood and Bone Marrow Transplant (EBMT) criteria, overall survival). Independent outcomes assessment not reported but data monitored by independent contract research organization. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Clear flow chart with all randomised patients accounted for. No lost to follow‐up reported. All patients included in intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Unclear risk | Benefits and harms reported however selected adverse events reported (peripheral neuropathy and neuropathic pain) only. 2 secondary malignancies reported (1 on treatment arm; 1 on control arm). |
| Other bias | Unclear risk | None reported. |
NMSG 17/07 Study.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study design described as 'randomised'. |
| Allocation concealment (selection bias) | Low risk | Central web‐based randomisation performed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study. |
| Blinding of outcome assessment (detection bias) for OS | Low risk | Low risk of bias for OS. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Objective endpoints measured (e.g. response rate and progression according to International Myeloma Working Group (IMWG) guidelines, overall survival). Independent outcomes assessment not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Detailed flow chart for randomised patients included. Acceptable rates of withdrawal, no lost to follow‐up patients reported. All patients included in intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | Benefits and harms reported. |
| Other bias | Unclear risk | None reported. |
VISTA Study.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study design described as 'randomised'. |
| Allocation concealment (selection bias) | Unclear risk | Not specified. Central randomisation most probably performed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study. |
| Blinding of outcome assessment (detection bias) for OS | Low risk | Low risk of bias for OS. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objective endpoints measured (e.g. e.g. response rate and progression according to European Group for Blood and Bone Marrow Transplant (EBMT) criteria, overall survival). Response data based on central laboratory analysis of blood and urine samples. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Detailed flow chart. Low numbers lost to follow‐up/excluded from analysis. |
| Selective reporting (reporting bias) | Low risk | Benefits and harms reported. |
| Other bias | Unclear risk | None reported. |
IV: intravenous SC: subcutaneous
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chen 2011b | Described as a 'retrospective randomised' study. Small study of 46 patients randomised according to date of hospitalisation. |
| Goldschmidt 2012 | Same dose of bortezomib on same days on both arms (PAD regimen = 28 day cycle and VCD regimen = 21‐day cycle). |
| Kumar 2012 | Same dose/schedule of bortezomib on each arm. |
| Mateos 2010 | Same dose/schedule of bortezomib on each arm. |
| Niesvisky 2010 | Same dose/schedule of bortezomib on each arm. |
| Orlowski 2007 | Same dose/schedule of bortezomib on each arm. |
PAD: VCD:
Characteristics of studies awaiting assessment [ordered by study ID]
Mayo Clinic Study.
| Methods |
Design: Randomised, open‐label, phase II trial. Overall sample size: N = 150 patients. |
| Participants | Patients with newly diagnosed multiple myeloma who have completed stem cell transplant. |
| Interventions | Arm A (bortezomib). Patients receive bortezomib subcutaneously (SC) on days 1 and 15 of courses 1 to 12 and day 1 of courses 13 to 24. Arm B (bortezomib, cyclophosphamide, dexamethasone). Patients receive bortezomib SC as in Arm A, cyclophosphamide orally on days 1 and 15 of courses 1 to12 and day 1 of courses 13 to 24, and dexamethasone orally on days 1 and 15 of courses 1 to 12 and day 1 of courses 13 to 24. Arm C (bortezomib, lenalidomide). Patients receive bortezomib SC as in Arm A and lenalidomide orally on days 1 to 28. |
| Outcomes |
Primary: Proportion of patients with stringent complete response. Secondary: Survival time, progression‐free survival, adverse events. |
| Notes | Sponsor: Mayo Clinic, US. This study is not eligible for inclusion (same dose/schedule of bortezomib on each arm). To be verified post‐publication for addition to next update of this review. |
Velcade Consolidation Bone Study.
| Methods |
Design: Randomised, open‐label, phase II trial. Overall sample size: N = 106 patients. |
| Participants | Patients with multiple myeloma who have received high‐dose chemotherapy and autologous stem cell transplantation. |
| Interventions |
Experimental arm: Bortezomib 1.6 mg/m² bolus injection on Days 1, 8, 15 and 22 every 5 weeks for 4 cycles. Control arm: Observation only. |
| Outcomes |
Primary: Change From baseline in Bone Mineral Density (BMD). Secondary: Progression‐free survival, bone markers, skeletal events, appearance of new bone lesions, Karnosfsky performance status, overall survival. |
| Notes | Sponsor: Janssen‐Cilag International NV. BMD data published by abstract only (European Haematology Association (EHA) Congress, June 2014). To consider eligibility of study for inclusion in future update of review if progression‐free survival and/or overall survival data are published. |
Characteristics of ongoing studies [ordered by study ID]
CLARION Study.
| Trial name or title | CLARION Study |
| Methods |
|
| Participants | Transplant‐ineligible patients with multiple myeloma. |
| Interventions | Carfilzomib, Melphalan, and Prednisone (CMP) versus Bortezomib, Melphalan, and Prednisone (VMP). |
| Outcomes |
|
| Starting date | 2013 |
| Contact information | Onyx Pharmaceuticals |
| Notes | Study in recruitment phase. November 2017 (final data collection date for primary outcome measure). Last update on Clinicaltrials.gov April 2015. |
Consolidation (61‐75 years) Study.
| Trial name or title | CR006127 Study |
| Methods |
|
| Participants | Patients with multiple myeloma aged 61 to 75. |
| Interventions | Bortezomib 1.6 mg/m2 IV on days 1, 8, 15 and 22 of 35‐day cycle for 4 cycles as consolidation therapy versus observation. |
| Outcomes |
|
| Starting date | 2006 |
| Contact information | Janssen‐Cilag G.m.b.H |
| Notes | May 2013 (final data collection date for primary outcome measure). Last update on Clinicaltrials.gov March 2015. Contact with company confirmed study not yet published. |
Consolidation (less than 60 years) Study.
| Trial name or title | CR006124 Study |
| Methods |
|
| Participants | Patients with multiple myeloma aged less than 60 years. |
| Interventions | Bortezomib 1.6 mg/m2 IV on days 1, 8, 15 and 22 of 35‐day cycle for 4 cycles as consolidation therapy versus observation. |
| Outcomes |
|
| Starting date | 2006 |
| Contact information | Janssen‐Cilag G.m.b.H |
| Notes | May 2013 (final data collection date for primary outcome measure). Last update on Clinicaltrials.gov March 2015. Contact with company confirmed study not yet published. |
E1A11 Study.
| Trial name or title | ECOG E1A11 Study |
| Methods |
|
| Participants | Patients with newly diagnosed symptomatic multiple myeloma. |
| Interventions | Bortezomib, Lenalidomide and Dexamethasone (VRd) versus Carfilzomib, Lenalidomide, Dexamethasone (CRd) followed by limited or indefinite Lenalidomide maintenance. |
| Outcomes |
|
| Starting date | 2013 |
| Contact information | Dr SK Kumar, Eastern Cooperative Oncology Group (ECOG) |
| Notes | May 2016 (final data collection date for primary outcome measure). Last update on Clinicaltrials.gov September 2014. |
ENDEAVOR Study.
| Trial name or title | ENDEAVOR Study |
| Methods |
|
| Participants | Patients with relapsed multiple myeloma |
| Interventions | Carfilzomib and Dexamethasone versus Bortezomib and Dexamethasone |
| Outcomes | Primary: Progression‐free survival |
| Starting date | 2012 |
| Contact information | Onyx Pharmaceuticals |
| Notes | January 2016 (final data collection date for primary outcome measure). Last update on Clinicaltrials.gov March 2015. |
Hackensack University Study.
| Trial name or title | PRO# 1307 Study |
| Methods |
|
| Participants | Patients with multiple myeloma 65 years or older. |
| Interventions | Autologous Stem Cell Transplantation with high‐dose Melphalan versus high‐dose Melphalan and Bortezomib. |
| Outcomes |
|
| Starting date | 2010 |
| Contact information | Dr M Donato, John Theurer Cancer Center, Hackensack University Medical Center, New Jersey, US. |
| Notes | Study open to recruitment. November 2014 (final data collection date for primary outcome measure). Last update on Clinicaltrials.gov Aug 2014. |
HOVON 95 Study.
| Trial name or title | HOVON 95 Study |
| Methods |
|
| Participants | Patients with newly diagnosed multiple myeloma. |
| Interventions | Bortezomib, Melphalan, Prednisone (VMP) With high‐dose Melphalan followed by Bortezomib, Lenalidomide, Dexamethasone (VRD) Consolidation and Lenalidomide Maintenance. |
| Outcomes |
|
| Starting date | 2011 |
| Contact information | Prof. P Sonneveld, Stichting Hemato‐Oncologie voor Volwassenen Nederland |
| Notes | April 2021 (final data collection date for primary outcome measure). Last update on Clinicaltrials.gov March 2015. |
King Fasail Hospital Study.
| Trial name or title | 2081‐113 Study |
| Methods |
|
| Participants | Patients with previously untreated multiple myeloma without an intent for immediate autologous stem cell transplant. |
| Interventions | Lenalidomide and low dose dexamethasone (LLD) versus bortezomib, lenalidomide and low dose dexamethasone (BLLD) as induction therapy. |
| Outcomes | Progression‐free survival. |
| Starting date | 2009 |
| Contact information | Dr N Chaudri, King Faisal Specialist Hospital & Reseach Center, Riyadh, Saudi Arabia |
| Notes | January 2013 (Final data collection date for primary outcome measure). Recruitment status unknown; last update on Clinicaltrials.gov Feb 2012. |
Optimized Retreatment Study.
| Trial name or title | CR018796 Study |
| Methods |
Overall sample size: N = 80 patients. |
| Participants | Patients with multiple myeloma in first or second relapse. |
| Interventions |
|
| Outcomes |
|
| Starting date | 2013 |
| Contact information | Janssen‐Cilag International NV |
| Notes | January 2016 (final data collection date for primary outcome measure). Last update on Clinicaltrials.gov April 2015. |
Subcutaneous Bortezomib Maintenance Study.
| Trial name or title | Subcutaneous bortezomib maintenance Study |
| Methods |
|
| Participants | Patients with relapsed and refractory multiple myeloma after salvage with bortezomib‐based therapy. |
| Interventions |
|
| Outcomes |
|
| Starting date | 2013 |
| Contact information | Stichting Hemato‐Oncologie voor Volwassenen Nederland. |
| Notes | Study open to recruitment. November 2016 (final data collection date for primary outcome measure). Last update on Clinicaltrials.gov March 2015. |
SWOG‐S0777 Study.
| Trial name or title | SWOG‐S0777 Study |
| Methods |
|
| Participants | Previously untreated multiple myeloma without intent for immediate Autologous Stem Cell Transplant. |
| Interventions | Lenalidomide and Low Dose Dexamethasone (LLD) versus Bortezomib, Lenalidomide and Low Dose Dexamethasone (BLLD) as induction therapy. |
| Outcomes |
|
| Starting date | 2008 |
| Contact information | Dr BG Durie, Southwest Oncology Group (SWOG). |
| Notes | Follow‐up continuing. Last update on Clinicaltrials.gov September 2014. |
VCAT Study.
| Trial name or title | CR018751 Study |
| Methods |
|
| Participants | Patients with multiple myeloma after receiving Bortezomib, Cyclophosphamide, Dexamethasone (VCD) Induction and Autologous Stem Cell Transplant. |
| Interventions | Bortezomib Consolidation (With Thalidomide and Prednisolone) versus Thalidomide and Prednisolone Alone. |
| Outcomes |
|
| Starting date | 2012 |
| Contact information | Janssen Scientific Affairs, LLC |
| Notes | October 2015 (Final data collection date for primary outcome measure). Last update on Clinicaltrials.gov March 2015. |
Wuerzburg University Hospital Study.
| Trial name or title | DSMM XIV Study |
| Methods |
|
| Participants | Patients with newly diagnosed multiple myeloma. |
| Interventions | Lenalidomide, Adriamycin, Dexamethasone (RAD) versus Lenalidomide, Bortezomib, Dexamethasone (VRD) as induction therapy followed by response‐adapted consolidation and lenalidomide maintenance. |
| Outcomes |
|
| Starting date | 2012 |
| Contact information | Dr Stefan Knop, Wuerzburg University Hospital. |
| Notes | Study still recruiting. Last update on Clinicaltrials.gov September 2012. |
Differences between protocol and review
We edited the background section.
We combined two of the comparison groups in the meta‐analysis (studies of bortezomib versus no bortezomib with different background therapy in each arm and studies of bortezomib versus other agents(s)) as these studies included complex combination regimens/therapies, with some studies considered as belonging to either comparison.
We planned to only analyse time to progression (TTP) separately, if it were defined differently from progression‐free survival (PFS). We identified four trials in the meta‐analysis that measured TTP as an outcome (VISTA Study, GIMEMA‐MMY‐3006 Study, MMVAR/IFM 2005‐04 Study and APEX Study). We were able to extract and analyse PFS data for the VISTA Study, GIMEMA‐MMY‐3006 Study and MMVAR/IFM 2005‐04 Study, while in the case of the APEX Study, we included TTP data as PFS data were not available. We therefore did not analyse TTP separately.
We extracted grade three or four adverse events only and not all grades.
We did not perform sensitivity analysis to exclude trials that were overall at high risk of bias, because all trials were considered to be overall at low to moderate risk of bias.
The number of subgroups were reduced and simplified for the review compared to that outlined in the protocol into two main categories: clinical setting and therapy setting as these settings/subgroups were considered to be more clinically relevant and reflected the trial settings much more accurately of the studies included in this review. We performed subgroup analyses on clinical outcomes only: overall survival (OS), PFS, complete response rate (CRR) and overall response rate (ORR). We did not analyse age as a subgroup as each trial enrolled a population with an age range and we deemed it more clinically relevant to analyse trials of transplant eligible versus ineligible populations.
Contributions of authors
Kathleen Scott: drafting the protocol, searching for trials, trial selection, data extraction, statistical analysis, data presentation and drafting the review.
Andrea Will: creating the 'Summary of findings' table.
Keith Wheatley: advice and expertise on statistical methods and Cochrane review methodology, statistical analysis and data presentation, input to drafting the protocol and review.
Patrick J Hayden: provision of clinical expertise and interpretation of trial data, input to drafting the protocol and review.
Imelda Coyne: Cochrane fellowship supervisor to Kathleen Scott, advice and guidance on Cochrane review methodology, input to drafting the protocol and review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Health Research Board, Ireland.
Kathleen Scott has received a Cochrane Training Fellowship from the Health Research Board, Ireland
Declarations of interest
Kathleen Scott: None known.
Andrea Will: None known.
Keith Wheatley: None known.
Patrick J Hayden: Received payment for talks given at educational meetings from Janssen Cilag, Celgene, Sanofi; received payment for participation in advisory board meetings from Janssen Cilag, Celgene.
Imelda Coyne: None known.
New
References
References to studies included in this review
All India Institute Study {published data only}
- Sahai S, Kumar L, Raina V, Sharma A, Gupta R, Mahajan S, et al. Multiple myeloma with light chain‐induced acute renal failure ‐ role of bortezomib‐dexamethasone & cyclophosphamide‐thalidomide & dexamethasone: a prospective randomized study (Abstract 3648). Proceedings of the European Cancer Congress. Amsterdam, 27 Sep ‐ 01 Oct 2013.
APEX Study {published data only}
- Chanan‐Khan A, Sonneveld P, Schuster MW, Stadtmauer EA, Facon T, Harousseau JL, et al. Analysis of herpes zoster events among bortezomib‐treated patients in the phase III APEX study. Journal of Clinical Oncology 2008;26(29):4784‐90. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Richardson PG, Sonneveld P, Schuster MW, Irwin D, San Miguel JF, et al. Bortezomib is associated with better health‐related quality of life than high‐dose dexamethasone in patients with relapsed multiple myeloma: results from the APEX study. British Journal of Haematology 2008;143(4):511‐9. [DOI] [PubMed] [Google Scholar]
- Richardson PG, Sonneveld P, Schuster M, Irwin D, Stadtmauer EA, Facon T, et al. Bortezomib or high‐dose dexamethasone for relapsed multiple myeloma. New England Journal of Medicine 2005;352(24):2487‐98. [DOI] [PubMed] [Google Scholar]
- Richardson PG, Sonneveld P, Schuster M, Irwin D, Stadtmauer EA, Facon T, et al. Extended follow‐up of a phase 3 trial in relapsed multiple myeloma: final time‐to‐event results of the APEX trial. Blood 2007;110(10):3557‐60. [DOI] [PubMed] [Google Scholar]
CREST Study {published data only}
- Jagannath S, Barlogie B, Berenson J, Siegel D, Irwin D, Richardson PG, et al. A phase 2 study of two doses of bortezomib in relapsed or refractory myeloma. British Journal of Haematology 2004;127(2):165‐72. [DOI] [PubMed] [Google Scholar]
- Jagannath S, Barlogie B, Berenson JR, Siegel DS, Irwin D, Richardson PG, et al. Updated survival analyses after prolonged follow‐up of the phase 2, multicenter CREST study of bortezomib in relapsed or refractory multiple myeloma. British Journal of Haematology 2008;143(4):537‐40. [DOI] [PubMed] [Google Scholar]
GEM05MENOS65 Study {published data only}
- Rosinol L, Oriol A, Teruel AI, Hernandez D, Lopez‐Jiminez J, Rubia J, et al. Superiority of bortezomib, thalidomide, and dexamethasone (VTD) as induction pretransplantation therapy in multiple myeloma: a randomized phase 3 PETHEMA/GEM study. Blood 2012;120(8):1589‐96. [DOI] [PubMed] [Google Scholar]
- Rosinol L, Oriol A, Teruel AL, Hernandez D, Lopez‐Jimenez J, Rubia J, et al. Maintenance therapy after stem‐cell transplantation for multiple myeloma with bortezomib/thalidomide vs.thalidomide vs. alfa2b‐interferon: final results of a phase iii pethema/gem randomized trial. Blood (ASH Annual Meeting Abstracts) Abstract 334. 2012; Vol. 120, issue 21.
GEM2010MAS65 Study {published data only}
- Mateos MV, Martinez‐Lopez J, Hernandez MT, Martinez R, Rosinnol L, Ocio EM, et al. Comparison of sequential vs alternating administration of bortezomib, melphalan and prednisone (VMP) and lenalidomide plus dexamethasone (Rd) in elderly patients with newly diagnosed multiple myeloma (MM) patients: GEM2010MAS65 trial. Blood (ASH Annual Meeting Abstracts) Abstract 178. 2014; Vol. 124.
- Mateos MV, Martinez‐Lopez J, Hernandez MT, Martinez R, Rosinnol L, Ocio EM, et al. Comparison of sequential vs alternating administration of bortezomib, melphalan and prednisone (VMP) and lenalidomide plus dexamethasone (Rd) in elderly patients with newly diagnosed multiple myeloma (MM) patients: GEM2010MAS65 trial. Blood (ASH Annual Meeting Abstracts) Abstract 403. 2013; Vol. 122, issue 21.
GIMEMA‐MM‐03‐05 Study {published data only}
- Palumbo A, Bringhen S, Paoli L, Cotzia E, Ria R, Gentili S, et al. Bortezomib‐melphalan‐prednisone‐thalidomide followed by continuous bortezomib thalidomide (VMPT‐VT) improved survival. Clinical Lymphoma, Myeloma and Leukemia 2013;13:S106‐107. [Google Scholar]
- Palumbo A, Bringhen S, Larocca A, Rossi D, Raimondo F, Magarotto V, et al. Bortezomib‐melphalan‐prednisone‐thalidomide followed by maintenance with bortezomib‐thalidomide compared with bortezomib‐melphalan‐prednisone for initial treatment of multiple myeloma: updated follow‐up and improved survival. Journal of Clinical Oncology 2014;32(7):634‐40. [DOI] [PubMed] [Google Scholar]
- Palumbo A, Bringhen S, Rossi D, Cavalli M, Larocca A, Ria R, et al. Bortezomib‐melphalan‐prednisone‐thalidomide followed by maintenance with bortezomib‐thalidomide compared with bortezomib‐melphalan‐prednisone for initial treatment of multiple myeloma: a randomized controlled trial. Journal of Clinical Oncology 2010;28(34):5101‐9. [DOI] [PubMed] [Google Scholar]
GIMEMA‐MMY‐3006 Study {published data only}
- Cavo M, Galli M, Pantani L, Raimondo F, Crippa C, Offidani M, et al. Bortezomib‐thalidomide‐dexamethasone incorporated into autotransplantation is associated with more favorable outcomes after relapse in comparison with thalidomide‐dexamethasone plus autotransplantation in multiple myeloma. Blood (ASH Annual Meeting Abstracts) Abstract 4210. 2012; Vol. 120.
- Cavo M, Galli M, Pezzi A, Raimondo F, Crippa C, Offidani M, et al. Persistent improvement in clinical outcomes with bortezomib‐thalidomide‐dexamethasone vs thalidomide‐dexamethasone incorporated into double autologous transplantation for multiple myeloma: an updated analysis of phase 3 Gimema‐MMY‐3006 study. Blood (ASH Annual Meeting Abstracts) Abstract 2090. 2013; Vol. 122, issue 21.
- Cavo M, Pantani L, Petrucci MT, Patriarca F, Zamagni E, Donnarumma D, et al. Bortezomib‐thalidomide‐dexamethasone is superior to thalidomide‐dexamethasone as consolidation therapy after autologous hematopoietic stem cell transplantation in patients with newly diagnosed multiple myeloma. Blood 2012;120(1):9‐19. [DOI] [PubMed] [Google Scholar]
- Cavo M, Tacchetti P, Patriarca F, Petrucci MT, Pantani L, Galli M, et al. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem‐cell transplantation in newly diagnosed multiple myeloma: a randomised phase 3 study. Lancet 2010;376(9758):2075‐85. [DOI] [PubMed] [Google Scholar]
HOVON‐65/GMMG‐HD4 Study {published data only}
- Sonneveld P, Scheid C, Holt B, Jarari L, Bertsch U, Salwender H, et al. Bortezomib induction and maintenance treatment improves survival in patients with newly diagnosed multiple myeloma: extended follow‐up of the HOVON‐65/GMMG‐HD4 trial. Blood (ASH Annual Meeting Abstracts) Abstract 404. 2013; Vol. 122, issue 21.
- Sonneveld P, Schmidt‐Wolf IGH, Holt B, Jarari L, Bertsch U, Salwender H, et al. Bortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: results of the randomized phase III HOVON‐65/ GMMG‐HD4 trial. Journal of Clinical Oncology 2012;30(24):2946‐55. [DOI] [PubMed] [Google Scholar]
IFM 2005‐01 Study {published data only}
- Harousseau JL, Attal M, Avet‐Loiseau H, Marit G, Caillot D, Mohty M, et al. Bortezomib plus dexamethasone is superior to vincristine plus doxorubicin plus dexamethasone as induction treatment prior to autologous stem‐cell transplantation in newly diagnosed multiple myeloma: results of the IFM 2005‐01 phase III trial. Journal of Clinical Oncology 2010;28(30):4621‐9. [DOI] [PubMed] [Google Scholar]
IFM 2007‐02 Study {published data only}
- Moreau P, Avet‐Loiseau H, Facon T, Attal M, Tiab M, Hulin C, et al. Bortezomib plus dexamethasone versus reduced‐dose bortezomib, thalidomide plus dexamethasone as induction treatment before autologous stem cell transplantation in newly diagnosed multiple myeloma. Blood 2011;118(22):5752‐8. [DOI] [PubMed] [Google Scholar]
MD Anderson Study {published data only}
- Sharma M, Khan H, Thall PF, Orlowski RZ, Bassett Jr RL, Shah N, et al. A randomized phase 2 trial of a preparative regimen of bortezomib, high‐dose melphalan, arsenic trioxide, and ascorbic acid. Cancer 2012;118(9):2507‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
MMVAR/IFM 2005‐04 Study {published data only}
- Garderet L, Iacobelli S, Moreau P, Mamoun D, Lafon I, Niederwieser D, et al. Superiority of the triple combination of bortezomib‐thalidomide‐dexamethasone over the dual combination of thalidomide‐dexamethasone in patients with multiple myeloma progressing or relapsing after autologous transplantation: the MMVAR/IFM 2005‐04 Randomized Phase III Trial from the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Journal of Clinical Oncology 2012;30(20):2475‐82. [DOI] [PubMed] [Google Scholar]
MMY‐3021 Study {published data only}
- Arnulf B, Pylypenko H, Grosicki S, Karamanesht I, Leleu X, Velde H, et al. Updated survival analysis of a randomized phase III study of subcutaneous versus intravenous bortezomib in patients with relapsed multiple myeloma. Haematologica 2012;97(12):1925‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau P, Pylypenko H, Grosicki S, Karamanesht I, Leleu X, Grishunina M, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non‐inferiority study. Lancet Oncology 2011;12(5):431‐40. [DOI] [PubMed] [Google Scholar]
NMSG 15/05 Study {published data only}
- Mellqvist UH, Gimsing P, Hjertner O, Lenhoff S, Laane E, Remes K, et al. Bortezomib consolidation after autologous stem cell transplantation in multiple myeloma: a Nordic Myeloma Study Group randomized phase 3 trial. Blood 2013;121(23):4647‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
NMSG 17/07 Study {published data only}
- Hjorth M, Hjertner O, Knudsen LM, Gulbrandsen N, Homberg E, Pedersen PT, et al. Thalidomide and dexamethasone vs. bortezomib and dexamethasone for melphalan refractory myeloma: a randomized study. European Journal of Haematology 2012;88(6):485‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
VISTA Study {published data only}
- Delforge M, Dhawan R, Robinson D Jr, Meunier J, Regnault A, Esseltine DL, et al. Health‐related quality of life in elderly, newly diagnosed multiple myeloma patients treated with VMP vs. MP: results from the VISTA trial. European Journal of Haematology 2012;89(1):16‐27. [DOI] [PubMed] [Google Scholar]
- Mateos MV, Richardson PG, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, et al. Bortezomib plus melphalan and prednisone compared with melphalan and prednisone in previously untreated multiple myeloma: updated follow‐up and impact of subsequent therapy in the phase III VISTA trial. Journal of Clinical Oncology 2010;28(13):2259‐66. [DOI] [PubMed] [Google Scholar]
- San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. New England Journal of Medicine 2008;359(9):906‐17. [DOI] [PubMed] [Google Scholar]
- San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, et al. Persistent overall survival benefit and no increased risk of second malignancies with bortezomib‐melphalan‐prednisone versus melphalan‐prednisone in patients with previously untreated multiple myeloma. Journal of Clinical Oncology 2013;31(4):448‐55. [DOI] [PubMed] [Google Scholar]
- Spicka I, Mateos MV, Redman K, Dimopoulos MA, Richardson PG. An overview of the VISTA trial: newly diagnosed, untreated patients with multiple myeloma ineligible for stem cell transplantation. Immunotherapy 2011;3(9):1033‐40. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Chen 2011b {published data only}
- Chen RA, Tu Y, Cao Y, Liu L, Liang Y. Bortezomib‐dexamethasone or vincristine‐doxorubicin‐dexamethasone as induction therapy followed by thalidomide as maintenance therapy in untreated multiple myeloma patients. Journal of International Medical Research 2011;39(5):1975‐84. [DOI] [PubMed] [Google Scholar]
Goldschmidt 2012 {published data only}
- Goldschmidt H, Salwender H, Bertsch U, et al. GMMG MM5 trial in newly diagnosed multiple myeloma to evaluate PAD vs VCD induction prior to HDT followed by lenalidomide consolidation and maintenance ‐ first interim analysis on induction. Haematologica 2012;97(115):Abstract 285. [Google Scholar]
Kumar 2012 {published data only}
- Kumar S, Flinn I, Richardson PG, Hari P, Callander N, Noga SJ, et al. Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in previously untreated multiple myeloma. Blood 2012;119(19):4375‐82. [DOI] [PubMed] [Google Scholar]
Mateos 2010 {published data only}
- Mateos MV, Oriol A, Martínez‐López J, Gutiérrez N, Teruel AI, Paz R, et al. Bortezomib, melphalan, and prednisone versus bortezomib, thalidomide, and prednisone as induction therapy followed by maintenance treatment with bortezomib and thalidomide versus bortezomib and prednisone in elderly patients with untreated multiple myeloma: a randomised trial. Lancet Oncology 2010;11(10):934‐41. [DOI] [PubMed] [Google Scholar]
Niesvisky 2010 {published data only}
- Niesvizky R, Flinn IW, Rifkin RM, Gabrail NY, Charu V, et al. Phase 3b UPFRONT study: safety and efficacy of weekly bortezomib maintenance therapy after bortezomib‐based induction regimens in elderly, newly diagnosed multiple myeloma patients. American Society of Hematology. 2010.
Orlowski 2007 {published data only}
- Orlowski R, Nagler A, Sonneveld P, Bladé J, Hajek R, Spencer A, et al. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. Journal of Clinical Oncology 2007;25(25):3892‐901. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Mayo Clinic Study {unpublished data only}
Velcade Consolidation Bone Study {unpublished data only}
References to ongoing studies
CLARION Study {unpublished data only}
- CLARION Study. Ongoing study 2013.
Consolidation (61‐75 years) Study {unpublished data only}
- CR006127 Study. Ongoing study 2006.
Consolidation (less than 60 years) Study {unpublished data only}
- CR006124 Study. Ongoing study 2006.
E1A11 Study {unpublished data only}
- ECOG E1A11 Study. Ongoing study 2013.
ENDEAVOR Study {unpublished data only}
- ENDEAVOR Study. Ongoing study 2012.
Hackensack University Study {unpublished data only}
- PRO# 1307 Study. Ongoing study 2010.
HOVON 95 Study {unpublished data only}
- HOVON 95 Study. Ongoing study 2011.
King Fasail Hospital Study {unpublished data only}
- 2081‐113 Study. Ongoing study 2009.
Optimized Retreatment Study {unpublished data only}
- CR018796 Study. Ongoing study 2013.
Subcutaneous Bortezomib Maintenance Study {unpublished data only}
- Subcutaneous bortezomib maintenance Study. Ongoing study 2013.
SWOG‐S0777 Study {unpublished data only}
- SWOG‐S0777 Study. Ongoing study 2008.
VCAT Study {unpublished data only}
- CR018751 Study. Ongoing study 2012.
Wuerzburg University Hospital Study {unpublished data only}
- DSMM XIV Study. Ongoing study 2012.
Additional references
Bertolotti 2008
- Bertolotti P, Bilotti E, Colson K, Curran K, Doss D, Faiman B, et al. Management of side effects of novel therapies for multiple myeloma: consensus statements developed by the International Myeloma Foundation's Nurse Leadership Board. Clinical Journal of Oncology Nursing 2008;12(3):9‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2011a
- Chen D, Frezza M, Schmitt S, Kanwar J, Dou QP. Bortezomib as the first proteasome inhibitor anticancer drug: current status and future perspectives. Current Cancer Drug Targets 2011;11(3):239‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. Cochrane Handbook for Systematic Reviews of Interventions. Vol. 5.1.0, http://www.cochrane‐handbook.org: The Cochrane Collaboration, 2011. [Google Scholar]
EndNote 2012 [Computer program]
- Thomson Reuters. EndNote. Version X6. Thomson Reuters, 2012.
Ferlay 2010
- Ferlay J, Shin H, Bray F, Forman D, Mathers CD, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International Journal of Cancer 2010;127:2893‐917. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC. Chapter 16: Special topics in statistics. Cochrane Handbook for Systematic Reviews of Interventions. Vol. 5.1.0, Available from http://www.cochrane‐handbook.org: The Cochrane Collaboration, 2011. [Google Scholar]
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions. Vol. 5.1.0, Available from http://www.cochrane‐handbook.org: The Cochrane Collaboration, 2011. [Google Scholar]
Kyle 2009
- Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia 2009;23(1):3‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lawasut 2012
- Lawasut P, Chauhan D, Laubach J, Hayes C, Fabre C, Maglio M, et al. New proteasome inhibitors in myeloma. Current Haematologic Malignancy Reports 2012;7:258‐66. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for Studies. Cochrane Handbook for Systematic Review of Interventions. Vol. 5.1.0, Available from http://www.cochrane‐handbook.org: The Cochrane Collaboration, 2011. [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta‐Analyses: The PRISMA Statement. PLOS Medicine 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moreau 2012
- Moreau P, Richardson PG, Cavo M. Proteasome inhibitors in multiple myeloma: 10 years later. Blood 2012;120(5):947‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nooka 2013
- Nooka AK, Kaufman JL, Behera M, Langston A, Waller EK, Flowers CR, et al. Bortezomib‐containing induction regimens in transplant‐eligible myeloma patients: a meta‐analysis of phase 3 randomized clinical trials. Cancer 2013;119(23):4119‐28. [DOI] [PubMed] [Google Scholar]
Palumbo 2011
- Palumbo A, Anderson KC. Multiple myeloma. New England Journal of Medicine 2011;364:1046‐60. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar M, Torri V, Stewart L. Extracting summary statistics to perform meta‐analysis of the published literature for survival endpoints. Statistics in Medicine 1998;17:2815‐34. [DOI] [PubMed] [Google Scholar]
Picot 2011
- Picot J, Cooper K, Bryant J, Clegg AJ. The clinical effectiveness and cost‐effectiveness of bortezomib and thalidomide in combination regimens with an alkylating agent and a corticosteroid for the first‐line treatment of multiple myeloma: a systematic review and economic evaluation. Health Technology Assessment 2011;15(41):1‐204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pulte 2011
- Pulte D, Gondos A, Brenner H. Improvement in survival of older adults with multiple myeloma: results of an updated period of analysis of SEER data. Oncologist 2011;16:1600‐03. [DOI] [PMC free article] [PubMed] [Google Scholar]
Raab 2009
- Raab MS, Podar K, Breitkreutz I, Richardson PG, Anderson KC. Multiple myeloma. Lancet 2009;374:324‐39. [DOI] [PubMed] [Google Scholar]
RevMan 5.3 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Richardson 2003
- Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D. A phase 2 study of bortezomib in relapsed, refractory myeloma. New England Journal of Medicine 2003;348:2609‐17. [DOI] [PubMed] [Google Scholar]
Richardson 2005
- Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadmauer EA, Facon T, et al. Bortezomib or high‐dose dexamethasone for relapsed multiple myeloma. New England Journal of Medicine 2005;352(24):2487‐98. [DOI] [PubMed] [Google Scholar]
Richardson 2007
- Richardson PG, Sonneveld P, Schuster P, Irwin D, Stadmauer EA, Facon T, et al. Extended follow‐up of a phase 3 trial in relapsed multiple myeloma: final time‐to‐event results of the APEX trial. Blood 2007;110(10):3557‐60. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. Cochrane Handbook for Systematic Reviews of Interventions. 5.1.0. http://www.cochrane‐handbook.org: The Cochrane Collaboration, 2011. [Google Scholar]
Shah 2009
- Shah JJ, Orlowski RZ. Proteasome inhibitors in the treatment of multiple myeloma. Leukemia 2009;23:1964‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Siegel 2012
- Siegel SG, Martin T, Wang M, Vij R, Jakubowiak AJ, Lonial S, et al. A phase 2 study of single‐agent carfilzomib (PX‐171‐003‐A1) in patients with relapsed and refractory multiple myeloma. Blood 2012;120(14):2817‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sonneveld 2013
- Sonneveld P, Goldschmidt H, Rosinol L, Blade J, Lahuerta JJ, Cavo M, et al. Bortezomib‐based versus nonbortezomib‐based induction treatment before autologous stem‐cell transplantation in patients with previously untreated multiple myeloma: a meta‐analysis of phase III randomized, controlled trials. Journal of Clinical Oncology 2013;31(26):3279‐87. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D. Chapter 10: Addressing reporting biases. Cochrane Handbook for Systematic Reviews of Interventions. Vol. 5.1.0, http://www.cochrane‐handbook.org: The Cochrane Collaboration, 2011. [Google Scholar]
Tierney 2007
- Tierney J, Stewart L, Ghersi D, Burdett S, Sydes M. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8:16. [DOI: 10.1186/1745-6215-8-16] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zeng 2013
- Zeng Z, Lin J, Chen J. Bortezomib for patients with previously untreated multiple myeloma: a systematic review and meta‐analysis of randomized controlled trials. Annals of Hematology 2013;92:935‐43. [DOI] [PubMed] [Google Scholar]


