ABSTRACT
In the EMPA-KIDNEY (The Study of Heart and Kidney Protection With Empagliflozin) trial, empagliflozin reduced cardiorenal outcomes by 28% (hazard ratio 0.72; 95% confidence interval 0.64–0.82; P < .0001) in a diverse population of over 6000 chronic kidney disease (CKD) patients, of whom >50% were not diabetic. It expanded the spectrum of CKD that may benefit from sodium-glucose cotransporter 2 (SGLT2) inhibition to participants with urinary albumin: creatinine ratio <30 mg/g and estimated glomerular filtration rate (eGFR) >20 mL/min/1.73 m2 or even lower (254 participants had an eGFR 15–20 mL/min/1.73 m2). EMPA-KIDNEY was stopped prematurely because of efficacy, thus limiting the ability to confirm benefit on the primary outcome in every pre-specified subgroup, especially in those with more slowly progressive CKD. However, data on chronic eGFR slopes were consistent with benefit at any eGFR or urinary albumin:creatinine ratio level potentially delaying kidney replacement therapy by 2–27 years, depending on baseline eGFR. The representation of diverse causes of CKD (>1600 participants with glomerular disease, >1400 with hypertensive kidney disease, >450 with tubulointerstitial disease and >600 with unknown cause) was higher than in prior SGLT2 inhibitor trials, although polycystic kidney disease was excluded. Around 15% (almost 1000) of participants were not on renin–angiotensin system blockade. The clinical characteristics of the cohort differed from DAPA-CKD (A Study to Evaluate the Effect of Dapagliflozin on Renal Outcomes and Cardiovascular Mortality in Patients With Chronic Kidney Disease), as did the frequency of individual components of the primary outcome in the placebo arm. Thus, rather than compare EMPA-KIDNEY with DAPA-CKD, the results of both trials should be seen as complementary to those of other SGLT2 inhibitor trials. Overall, EMPA-KIDNEY, a recent meta-analysis and post hoc analyses of participants with type 2 diabetes mellitus (T2DM) but no baseline CKD in other trials, indicates that SGLT2 inhibitor treatment will benefit an expanded CKD population with diverse baseline albuminuria or eGFR values, presence of T2DM or cause of CKD, as well as providing primary prevention of CKD in at least the T2DM setting.
Keywords: chronic kidney disease, normoalbuminuria, primary prevention, RAS blockers, SGLT2 inhibitor, treatment
BACKGROUND
Chronic kidney disease (CKD) is defined as abnormalities of kidney structure or function, present for >3 months, with implications for health [1]. The most common diagnostic criteria are a low estimated glomerular filtration rate (eGFR <60 mL/min/1.73 m2) or a high urinary albumin:creatinine ratio (UACR >30 mg/g) (Supplementary data, Fig. S1A). The implications for health include an increased risk of premature all-cause (Supplementary data, Fig. S1B) or cardiovascular death, CKD progression to kidney failure (Supplementary data, Fig. S1C) requiring kidney replacement therapy, and acute kidney injury (AKI) [2, 3]. Indeed, CKD is projected to become the fifth global cause of death by 2040 and diabetic kidney disease (DKD) is the largest contributor to this undesirable projection [4, 5]. Fortunately, following the demonstration of kidney and cardiovascular protection by sodium-glucose cotransporter 2 (SGLT2) inhibitors and the nonsteroidal mineralocorticoid receptor antagonist finerenone, both are currently recommended by clinical guidelines to treat persons with CKD and diabetes mellitus (DM) [6]. Additionally, the DAPA-CKD (A Study to Evaluate the Effect of Dapagliflozin on Renal Outcomes and Cardiovascular Mortality in Patients With Chronic Kidney Disease) trial enrolled which participants with and without type 2 DM (T2DM), demonstrated cardiorenal benefits in both groups and dapagliflozin is now licensed to treat adults with CKD [7, 8]. EMPA-KIDNEY (The Study of Heart and Kidney Protection With Empagliflozin) has largely reproduced the DAPA-CKD results for another SGLT2 inhibitor, empagliflozin, in a more diverse CKD population [9], begging the question, what exactly did we learn from EMPA-KIDNEY?
Despite evidence from DAPA-CKD [7], the adoption by DM guidelines of SGLT2 inhibitors to treat persons with DM and CKD, and the increasing uptake of dapagliflozin in routine clinical practice for non-diabetic CKD, nephrological guideline bodies have been slow to define the precise role of SGLT2 inhibition in the treatment of non-diabetic CKD [6, 10]. EMPA-KIDNEY now confirms the benefit of SGLT2 inhibition on risk of a primary endpoint consisting of progression of kidney disease or death from cardiovascular causes in non-diabetic CKD and expands the CKD population that may benefit from SGLT2 inhibitors to those with lower albuminuria and eGFR levels and to causes of CKD so far unexplored or underrepresented [9], decisively contributing to an updated meta-analysis of the kidney impact of SGLT2 inhibitors in the presence or absence of DM [11]. This should trigger a rapid update of guidelines for non-diabetic CKD, as has already been done for heart failure [12].
DAPA-CKD AND EMPA-KIDNEY ENROLLED DIFFERENT CKD POPULATIONS WHICH DIFFERED IN SURVIVAL AND CKD PROGRESSION OUTCOMES
DAPA-CKD and EMPA-KIDNEY are the only large-scale SGLT2 inhibitor trials which have tested a primary cardiorenal outcome in participants with non-diabetic and diabetic CKD [7, 9]. However, they had different inclusion and exclusion criteria (Supplementary data, Fig. S1D, Table 1) [7, 9, 13–17]. As a result, the 6609 participants in EMPA-KIDNEY had a lower prevalence of DM and cardiovascular disease (CVD) as well as lower albuminuria and eGFR values than the 4304 DAPA-CKD participants.
Table 1:
Key inclusion and exclusion criteria, baseline characteristics and outcomes in the placebo arm in DAPA-CKD and EMPA-KIDNEY (data are from references [7] and [9], unless otherwise specified). Colored cells indicate major differences between the two trials.
| DAPA-CKD | EMPA-KIDNEY | |
|---|---|---|
| Key trial characteristics | ||
| Trial metrics | ||
| N | 4304 | 6609 |
| Median follow-up | 2.4 | 2.0 |
| Inclusion criteria | ||
| eGFR (mL/min/1.73 m2) | 25 to 75 | 20 to <90 |
| UACR (mg/g) | 200 to 5000 | ≥200 if eGFR 45 to <90 |
| No limit for eGFR <45 | ||
| CVD | Atherosclerotic CVD within 12 weeks prior to enrolment | Prior atherosclerotic CVD in T2DM participants with an eGFR >60 mL/min/1.73 m2 |
| RAS blockade | Yes, if not contraindicated | Yes; also included participants not on RAS blockade when RAS blockade not indicated or not tolerated |
| Exclusion criteria | ||
| PKD | Excluded | Excluded |
| Lupus nephritis, vasculitis | Excluded | Included |
| Kidney transplantation | Excluded | Excluded |
| T1DM | Excluded | Initially included (69 randomized), later excludeda |
| Baseline data | ||
| Clinical characteristics | ||
| Age (years) | 62 | 64 |
| Women (%) | 33 | 33 |
| Non-DM (%) | 32.5 | 54 |
| CVD (%) | 37 | 27 (36 in DM, 19 in non-DM) |
| HF (%) | 11 | 10 (14 in DM, 6 in non-DM) |
| Causes of CKD | (16) | (17) |
| DKD, n (%) | 2510 (58.3) | 2057 (31) |
| Glomerular, n (%) | 695 (16.1) | 1669 (25) |
| Hypertensive/renovascular, n (%) | 687 (16.0) | 1445 (22) |
| Tubulointerstitial, n (%) | 53 (1.2) | 468 (7) |
| Unknown/other, n (%) | 214 (5.0) | 630 (10) |
| Baseline CKD status | ||
| KDIGO risk category very high, n (%) | 2336 (54) [15] | 4911(74) |
| eGFR (mL/min/1.73 m2) | 43 (±12) | 37 (±14) |
| eGFR category G4, n (%) | 624(14.5) | 2280 (34.5) |
| UACR (mg/g)b | 934–965 | 330 |
| UACR category A1, n (%) | 0 (0) | 1332 (20) |
| UACR category A2, n (%) | 444 (10) [15] | 1862 (28) |
| UACR > 1000 mg/g (%) | 48 | ND |
| RAS blockade, n (%) | 4174 (97) | 3399 (85) |
| Outcomes | ||
| Outcomes in the placebo arm (events/100 patient-years) | ||
| Primary outcome (events/100 patient-years)c | 7.5 | 9.0 |
| Death (any cause) (events/100 patient-years) | 6.8 | 2.6 |
| CVD death (events/100 patient-years) | 1.7 | 1.1 |
| HF hospitalization or CVD death (events/100 patient-years) | 3.0 | 2.4 |
| CKD progression (events/100 patient-years)d | 5.8 | 8.1 |
| CKD progression similar definition in both trials (events/100 patient-years)e | DM 6.0, non-DM 5.3 | DM 5.9, non-DM 4.7 |
| Primary outcomec in the placebo arm in subgroups of interest (%) [15] | ||
| All (%) | 14.5 | 16.9 |
| UACR category A1–A2 (%) | 4.4 | 7.5 |
| UACR category A3 (%) | 15.6 | 25.7 |
| eGFR category G2/G3a (%) | 10.5 | 9.5 |
| eGFR category G3b (%) | 14.1 | 12.0 |
| eGFR category G4 (%) | 26.3 | 27.5 |
| KDIGO risk category low, moderate, high (%) | 9.8 | 4.9 |
| KDIGO risk category very high (%) | 18.44 | 20.91 |
| DM (%) | 15.78 [34] | 20.20 |
| No DM (%) | 11.84 [34] | 14.08 |
| Chronic eGFR slopes in the placebo arm in subgroups of interest (mL/min/1.73 m2/year) | ||
| All | –3.83 [35] | −2.75 (no differences DM vs non-DM) |
| eGFR <30, 30–<45, ≥45 | ∼−3.5 to ∼−4 [15] | −2.85, −2.50, −3.60f |
| UACR <1000, 1000–3500, >3500 | ∼−2, ∼−4.5, ∼−7.5 [15] | ND |
| UACR <30, 30–300, >300 | ND | −0.89, −1.69, −4.11 |
Note that the higher incident of primary endpoint events in EMPA-KIDNEY as opposed to DAPA-CKD appears to be driven by a less stringent definition of sustained decrease in eGFR (≥40% vs ≥50% decrease in eGFR), as when the same definition is used, the incidence rate of kidney events is actually lower for EMPA-KIDNEY than for DAPA-CKD.
aSponsor request, not because of safety concern.
bMedian.
cDAPA-CKD: composite of sustained decline of ≥50% in eGFR from randomization, kidney replacement therapy, sustained decrease in eGFR to <15 mL/min/1.73 m2 and renal or cardiovascular death. EMPA-KIDNEY: composite of kidney disease progression (a sustained decline of ≥40% in eGFR from randomization, kidney replacement therapy, sustained decrease in eGFR to <10 mL/min/1.73 m2 or renal death) and cardiovascular death. Both outcomes differ in the threshold to define kidney failure (<15 mL/min/1.73 m2 in DAPA-CKD vs <10 mL/min/1.73 m2 in EMPA-KIDNEY), which they term end-stage kidney disease, and in threshold for the sustained decline in eGFR (≥50% in DAPA-CKD vs ≥40% in EMPA-KIDNEY).
dDAPA-CKD: Composite of decline in estimated GFR of ≥50%, kidney replacement therapy, sustained decrease in eGFR to <15 mL/min/1.73 m2 or death from renal causes. EMPA-KIDNEY: a sustained decline of ≥40% in eGFR from randomization, kidney replacement therapy, sustained decrease in eGFR to <10 mL/min/1.73 m2 or renal death.
eThe kidney disease progression was defined as a sustained decrease in eGFR (≥50%) from randomization in both trials. Data from [11]. In this analysis, kidney failure continued to be defined differently (<15 mL/min/1.73 m2 in DAPA-CKD vs <10 mL/min/1.73 m2 in EMPA-KIDNEY).
fParticipants with eGFR >45 mL/min/1.73 m2 had UACR >200 mg/g.
In EMPA-KIDNEY, eGFR and albuminuria inclusion criteria were wider than in DAPA-CKD or in Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) (which tested canagliflozin in DKD). EMPA-KIDNEY enrolled patients with eGFR ≥20 mL/min/1.73 m2, a lower threshold than in prior CKD trials of SGLT2 inhibitors. Additionally, it also enrolled patients with UACR <30 mg/g (i.e. normoalbuminuria) while CREDENCE and DAPA-CKD only enrolled those with UACR >200 mg/g [7, 9, 14]. A higher representation of non-diabetic participants was pre-specified in EMPA-KIDNEY, as was the exclusion of participants having T2DM, eGFR >60 mL/min/1.73 m2 and previous atherosclerotic CVD, thus excluding a subgroup of persons with DM at extremely high CVD and mortality risk. Though initially included, the trial later excluded type 1 DM (T1DM) [18]. In addition, more liberal inclusion of participants not on renin–angiotensin system (RAS) blockade was allowed in EMPA-KIDNEY.
As a consequence of the different inclusion and exclusion criteria, DAPA-CKD and EMPA-KIDNEY studied different populations that had different baseline clinical characteristics and profiles of CKD and CVD risk (Fig. 1A)and B) (Table 1) [16, 17]. EMPA-KIDNEY is the only CKD SGLT2 inhibitor trial to enroll patients from two full eGFR/albuminuria (GA) subcategories representing moderate CKD: G3bA1, which is the most common moderate KDIGO CKD risk category; and G2A3, overall representing over 50% of the global moderate CKD population (Supplementary data, Fig. S1A and D) [1]. Furthermore, EMPA-KIDNEY enrolled a larger representation of one mild CKD subcategory (G2A2) than DAPA-CKD, having expanded it to lower risk, higher eGFR patients. Additionally, the use of RAS blockade was lower and the representation of multiple non-DKD causes larger (>1600 participants with glomerular disease, >1400 with hypertensive kidney disease, >450 with tubulointerstitial disease and >600 with unknown cause) in EMPA-KIDNEY than in DAPA-CKD. These numbers in some cases dwarfed previous disease-specific trials, as for immunoglobulin A (IgA) nephropathy [19, 20]. Overall, the different profile of the participants supports the notion that DAPA-CKD and EMPA-KIDNEY data are complementary and should be integrated in meta-analyses, and against the notion that these trials can be compared.
Figure 1:
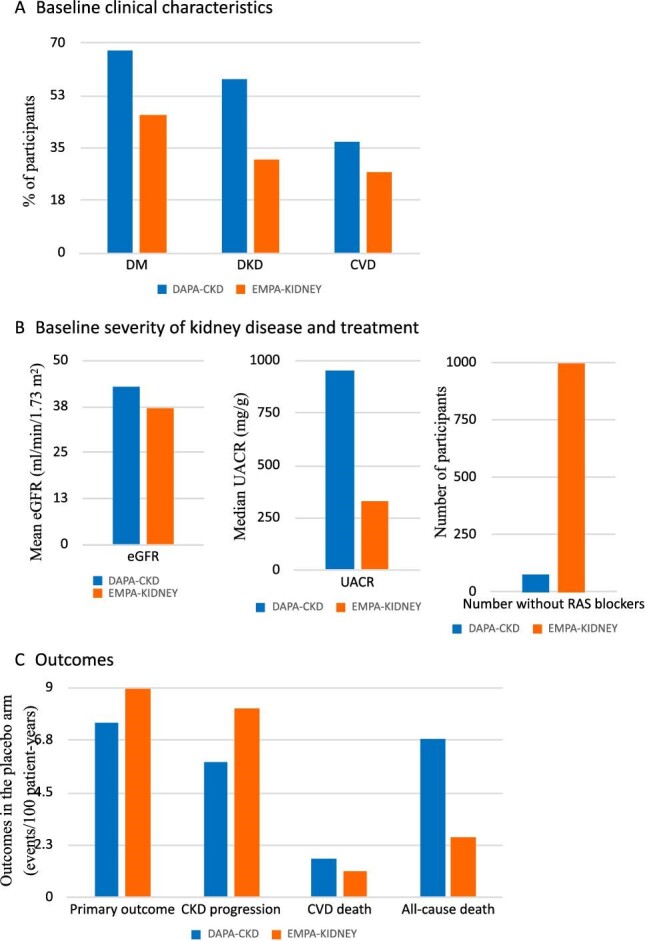
The DAPA-CKD and EMPA-KIDNEY trials enrolled different populations of participants with CKD and had different outcomes: their results should be integrated. (A) Baseline clinical characteristics. EMPA-KIDNEY participants had a lower prevalence of DM and DKD and a higher prevalence of non-diabetic cause of CKD than DAPA-CKD participants (see Table 1). Additionally, the prevalence of CVD was lower, likely because of the lower prevalence of DM and the exclusion of T2DM participants with baseline CVD when eGFR was >60 mL/min/1.73 m2. Thus, the population was less enriched for persons at higher risk of CVD and all-cause mortality than DAPA-CKD. (B) Baseline severity of kidney disease and treatment. In EMPA-KIDNEY participants, CKD was characterized by a lower baseline eGFR (i.e. potentially more severe CKD from the point of view of kidney function) but also by lower values of albuminuria, i.e. less severe CKD from the point of view of albuminuria. EMPA-KIDNEY enrolled a high number of participants not on RAS blockade. (C) While the incidence of the primary outcome was numerically higher in the placebo arm of EMPA-KIDNEY than in the placebo arm of DAPA-CKD, the incidence of mortality outcomes was higher in the placebo arm of DAPA-CKD than in the placebo arm of EMPA-KIDNEY. Note that in DAPA-CKD, kidney disease progression was defined as sustained decline of ≥50% in eGFR from randomization, kidney replacement therapy, sustained decrease in eGFR to <15 mL/min/1.73 m2 or renal death, while in EMPA-kidney, it was defined as a sustained decline of ≥40% in eGFR from randomization, kidney replacement therapy, sustained decrease in eGFR to <10 mL/min/1.73 m2 or renal death. In both cases, the composite primary outcome included both CKD progression and cardiovascular death. Potential differences highlighted in this figure are based on numerical data as no formal statistical comparisons were made [9, 17].
As expected from the different baseline clinical characteristics, the distribution of individual events that were part of the composite primary outcome and key secondary endpoints in the placebo arm differed for DAPA-CKD and EMPA-KIDNEY: mortality outcomes were more common in DAPA-CKD, despite a lower overall incidence of the composite primary outcome (Fig. 1C, Table 1). This further emphasizes that both trials are complementary rather than comparable.
EMPA-KIDNEY PROVIDES EVIDENCE FOR CARDIORENAL PROTECTION IN PARTICIPANTS WITH MULTIPLE CAUSES OF CKD AND WITH LOWER eGFR AND ALBUMINURIA VALUES THAN IN PRIOR CKD TRIALS OF SGLT2 INHIBITORS
The primary outcome of EMPA-KIDNEY was first occurrence of a composite of kidney disease progression that included a sustained decline of ≥40% in eGFR from randomization, kidney replacement therapy, sustained decrease in eGFR to <10 mL/min/1.73 m2 and cardiovascular death [9]. Of note, the sustained decrease in eGFR thresholds differ from the ≥50 and <15 mL/min/1.73 m2 used in DAPA-CKD [7]. Indeed, 15 mL/min/1.73 m2 was too close to the lower limit of inclusion eGFR criterion of 20 mL/min/1.73 m2 in EMPA-KIDNEY. In this regard, 254 participants had an eGFR 15–20 mL/min/1.73 m2 [11]. Additionally, 10 mL/min/1.73 m2 is a better representation of the eGFR threshold to consider kidney replacement therapy than 15 mL/min/1.73 m2.
At the end of the study, the primary outcome had occurred in 432 of 3304 (13.1%) participants in the empagliflozin arm vs 558 of 3305 (16.9%) participants in the placebo arm, representing a relative risk reduction (RRR) of 28% for empagliflozin [hazard ratio (HR) 0.72, 95% confidence interval (CI) 0.64–0.82, P < .001). Of note, the benefit was concentrated on the kidney outcomes of the primary endpoint. In this regard, CV mortality was low in both arms of the trial, being approximately 21% lower in the placebo arm of EMPA-KIDNEY than in the dapagliflozin arm of DAPA-CKD [7, 9]. Among secondary outcomes, there was an RRR of 14% for hospitalization for any cause with empagliflozin compared with placebo (HR 0.86, 95% CI 0.78–0.95, P = .003).
In prespecified subgroups, the benefit on the primary endpoint was consistent independently of DM status, baseline eGFR, age, sex, cause of CKD, baseline CVD or RAS blockade, although there was some impact of baseline UACR suggesting larger RRR for higher UACR values (P for trend = .02). Some have interpreted that no benefit was observed for participants with UACR <30 mg/g, as the point estimate HR for the primary endpoint was 1.01 (0.66–1.55). However, this was a lower risk group, as assessed by the low incidence of primary endpoint events (n = 42 as opposed to 438 in the A3 albuminuria subgroup) and the slow chronic eGFR slope in the placebo arm (Table 1), as discussed below. EMPA-KIDNEY was terminated early for benefit, as it met the prespecified efficacy rule for premature termination; this resulted in an overall median follow-up of 2.0 years, which was lower than that of CREDENCE and DAPA-CKD (2.6 years for both). As the empagliflozin-related benefit was driven by differences in the higher risk subgroups, the premature closure of the study may have limited the power to observe differences in events for lower risk subgroups. However, it would have been unethical to continue the trial once benefit had been demonstrated. In this regard, chronic eGFR slopes (i.e. eGFR slopes not considering the initial, hemodynamic and reversible dip in eGFR that is observed for SGLT2 inhibitors and for other kidney protective drugs) may better represent the impact of the intervention in low-risk groups. Indeed, chronic eGFR slopes data showed that empagliflozin slowed CKD progression across all eGFR and UACR categories and in patients with and without DM. Specifically, in participants with UACR <30 mg/g, the absolute difference in chronic eGFR slope was 0.78 (0.32–1.23) mL/min/1.73 m2/year (Fig. 2A); i.e. 8-fold slower in the empagliflozin than in the placebo arm (Fig. 2B), resulting in the most stable on-treatment eGFR slope of the different albuminuria categories (Fig. 2C). Thus, the sooner SGLT2 inhibition was initiated during the progression of albuminuria, the better the results observed in EMPA-KIDNEY in terms of preservation of kidney function. The message could be that the kidney protective effects can be observed regardless of the degree of albuminuria, and the presence of normoalbuminuria should not be a reason to defer treatment in conditions that are known to develop albuminuria or to not start treatment in conditions that are frequently non-albuminuric CKD such as tubulointerstitial kidney disease. These results are one of the important contributions of EMPA-KIDNEY as neither CREDENCE nor DAPA-CKD enrolled patients with UACR <200 mg/g and available post hoc analyses for DAPA-CKD only provide data for UACR thresholds of 1000 and 3500 mg/g (Table 1) [15]. To further contextualize the findings of EMPA-KIDNEY, the most widely used kidney protectants, RAS blockers, lack direct high-level evidence that by themselves they reduce hard renal outcomes in patients with normoalbuminuria.
Figure 2:
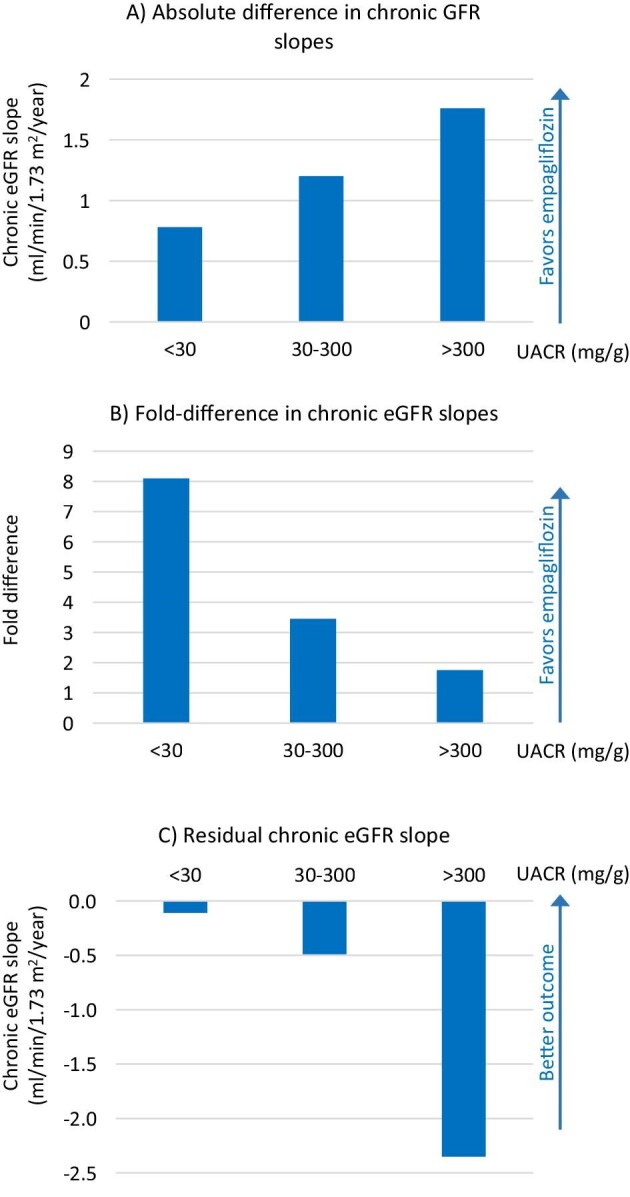
Chronic eGFR slopes according to baseline UACR category in EMPA-KIDNEY. (A) Absolute difference in chronic GFR slopes for albuminuria categories A1, A2 and A3 between the placebo and empagliflozin arms. Note that the largest difference is observed for participants with UACR >300 mg/g, i.e. in absolute terms, these participants obtained the largest preservation of chronic eGFR slope on empagliflozin vs placebo. (B) Fold-difference in chronic eGFR slopes for albuminuria categories A1, A2 and A3 between the placebo and empagliflozin arms. Note that in relative terms, the largest improvement in chronic eGFR slopes was observed for participants with UACR <30 mg/g. (C) On-treatment chronic eGFR slope. Note that the best outcome (i.e. the slowest eGFR slope) was observed in participants randomized to empagliflozin that had UACR <30 mg/g. These figures were generated using data from [9].
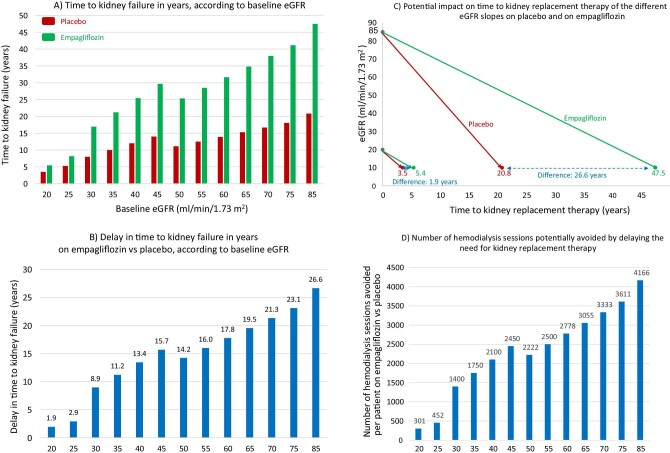
The same message of better results when SGLT2 inhibition is initiated early was delivered by the subgroup analysis of eGFR slopes by eGFR categories (Fig. 3). Chronic eGFR slopes allowed estimation of time to kidney failure, defined as eGFR 10 mL/min/1.73 m2, according to baseline eGFR (Fig. 3A). Empagliflozin would delay kidney failure, and thus, the need for kidney replacement therapy, from 1.9 years if it is initiated at eGFR 20 mL/min/1.73 m2 to 26.6 years if initiated when eGFR is 85 mL/min/1.73 m2 (Fig. 3B)and C). A delay of 1.9 years in initiating hemodialysis, apart from the expected mortality and morbidity benefits, would lead to the avoidance of 300 hemodialysis sessions per person starting SGLT2 inhibition when eGFR is 20 mL/min/1.73 m2, and the associated human suffering, healthcare costs, transportation costs, energy and water consumption, and plastic waste generation [21] (Fig. 3D). Starting empagliflozin when eGFR is 85 mL/min/1.73 m2 could (if the patient survives) avoid over 4000 hemodialysis sessions per patient and their associated impact. Even for patients never reaching the kidney failure stage, decreasing eGFR is associated with worsening outcomes and quality of life and with higher cardiovascular and mortality risks. Of note, once started, SGLT2 inhibitors have been continued in CKD randomized controlled trials (RCTs) until the initiation of kidney replacement therapy.
Figure 3:
Hypothetical transformation of chronic eGFR slopes into time to kidney failure, defined as eGFR 10 mL/min/1.73 m2, in the EMPA-KIDNEY trial. (A) Time to kidney failure in years, according to baseline eGFR, estimated from each baseline eGFR value by applying the chronic eGFR slopes corresponding to participants on placebo and on empagliflozin within the pre-specified eGFR subgroups (eGFR cut-off points to define subgroups set at 30 and 45 mL/min/1.73 m2) as per reference [9]. (B) Delay in time (years) to kidney failure on empagliflozin vs placebo, according to baseline eGFR, obtained by subtracting the time to kidney failure on empagliflozin from the time to kidney failure on placebo in (A). (C) Graphical presentation of representative chronic eGFR slopes from baseline to kidney failure, i.e. to the need for kidney replacement therapy. Hypothetical lines have been traced starting from extremes of the baseline eGFR inclusion criteria values (20 and 85 mL/min/1.73 m2) to eGFR 10 mL/min/1.73 m2, corresponding to chronic eGFR slopes of participants on placebo and on empagliflozin within each baseline eGFR subgroup, as per reference [9]. The difference in the time to kidney failure corresponds to the values in (B) for baseline 20 and 85 mL/min/1.73 m2. (D) Number of hemodialysis sessions potentially avoided by delaying the need for kidney replacement therapy by prescribing empagliflozin instead of placebo at each baseline eGFR value. The model assumes that patients will live up to the point where they need kidney replacement therapy and that they would continue hemodialysis throughout. While this is not expected to occur in every patient, it is a real possibility for some of them.
EMPA-KIDNEY PROVIDES EVIDENCE FOR CARDIORENAL PROTECTION BY SGLT2 INHIBITION MONOTHERAPY IN PARTICIPANTS WITH CKD
EMPA-KIDNEY provides some insight into kidney protection by SGLT2 inhibition monotherapy, i.e. in participants with CKD not on RAS blockade. Almost 1000 participants were not on RAS blockade, either due to absence of proved kidney benefit in patients with normoalbuminuria or to previous intolerance. As such, lack of RAS blockade was most common among non-diabetic participants with UACR <200 mg/g (22%) and least common among non-diabetic participants with UACR >200 mg/g (11%) [9, 17]. The data available so far are consistent with kidney protection afforded by SGLT2 inhibitors monotherapy being of similar magnitude to protection by RAS blockade monotherapy, although a detailed analysis of these subgroups is awaited (Supplementary data, Fig. S2).
NORMOALBUMINURIA, eGFR SLOPES AND CLINICAL RENAL OUTCOMES
As indicated above, EMPA-KIDNEY efficacy results in the subgroup of participants with normoalbuminuria were discordant for the primary endpoint or kidney events and for eGFR slope analyses [9], begging the question of which assessment may better represent the impact of SGLT2 inhibitors in this CKD population. A similar discordance had been previously reported by Packer et al. in another population at low risk of CKD and enriched for normoalbuminuria, i.e. persons with heart failure and preserved ejection fraction in the Empagliflozin in Heart Failure with a Preserved Ejection Fraction (EMPEROR-Preserved) trial, which had median UACR values of 30 mg/g among participants with DM and 16 mg/g among those without DM [11, 22]. Packer et al. concluded that the discrepancy suggests that eGFR slope analysis has limitations as a surrogate for predicting the effect of drugs on renal outcomes in patients with heart failure [22]. However, we respectfully disagree with this interpretation which appears to downplay the significance of eGFR slopes. We propose that the issue lies with trials or subanalyses lacking the appropriate design and power in terms of number of participants, number of events, definition of events and long-enough follow-up to be able to show an impact on specific events in low-risk subpopulations. In these scenarios, eGFR slopes may provide a better estimate of efficacy, as they incorporate data from the whole trial or subgroup population (i.e. from hundreds to thousands of participants), not just from a few scattered events [11, 22, 23, 36]. For kidney protective drugs that decrease hyperfiltration, the issue may be magnified by the initial decrease in eGFR that may contribute to any outcome defined as % decrease in eGFR (Supplementary data, Fig. S3) [24]. In this regard, the hypothesis by Packer et al. that events and eGFR data are discordant in certain populations with heart failure may not hold true when the definition of kidney event is slightly modified, for example, as done in a recent meta-analysis aiming at homogenizing the definition of “kidney event” across multiple SGLT2 inhibitor clinical trials [11]. Indeed, by changing the decrease in eGFR requirement from ≥40% to ≥50% (i.e. a more severe loss of eGFR, implying a harder endpoint), the eGFR slope results and kidney events results became aligned, showing a numerically larger benefit in Empagliflozin in Heart Failure with a Reduced Ejection Fraction (EMPEROR-Reduced) than in EMPEROR-Preserved, but overall numerical benefit in both trials for both eGFR and events assessments (Table 2). In another example, empagliflozin decreased kidney events in Empagliflozin, Cardiovascular Outcomes and Mortality in Type 2 Diabetes (EMPA-REG OUTCOME) participants with normoalbuminuria and eGFR >60 mL/min/1.73 m2, but as noted above, this was not the case for normoalbuminuric participants in EMPA-KIDNEY. Illustrating the consistency of chronic eGFR slope results even across trials, the placebo chronic eGFR slopes for EMPA-REG OUTCOME participants with normoalbuminuria and eGFR >60 mL/min/1.73 m2 were in the same range as for normoalbuminuric participants in EMPA-KIDNEY and also, the chronic eGFR slopes on empagliflozin and the difference between placebo and empagliflozin were in the same range, despite the different inclusion and exclusion criteria (Table 3).
Table 2:
eGFR slopes and kidney outcomes in EMPEROR-Reduced and EMPEROR-Preserved. Three outcomes are presented; eGFR slopes and two different definitions of events, that differ in the % of eGGR reduction (40% vs 50%).
| EMPEROR-Reduced [36] | EMPEROR-Preserved [23] | |
|---|---|---|
| Follow-up 16 months | Follow-up 26 months | |
| eGFR slope from baseline to 30 days post trial, off treatment (mL/min/1.73 m2/year and 95% CI) [22] | ||
| Placebo | –0.27 (–2.97 to –1.58) (n = 468) | –2.39 (–2.63 to –2.16) (n = 1608) |
| Empagliflozin | –0.50 (–1.19 to 0.19) (n = 467) | –1.46 (–1.70 to –1.22) (n = 1568) |
| Difference of empagliflozin versus placebo | 1.77 (0.80 to 2.74) | 0.94 (0.60 to 1.27) |
| Kidney events [22] | Sustained decrease in eGFR by ≥40% only | Sustained decrease in eGFR by ≥40% only |
| Sustained decrease in GFR to <10–15 mL/min/1.73 m2, dialysis | Sustained decrease in GFR to <10–15 mL/min/1.73 m2, dialysis | |
| Or renal transplantation | Or renal transplantation | |
| Placebo, n (%)/events/100 patient-years | 58/1867 (3.1)/3.1 | 112/2991 (3.7)/2.2 |
| Empagliflozin, n (%)/events/100 patient-years | 30/1863 (1.6)/1.6 | 108/2997 (3.6)/2.1 |
| Empagliflozin versus placebo, HR (95% CI) | 0.51 (0.33–0.79) | 0.95 (0.73–1.24) |
| Kidney events [11] | Sustained decrease in eGFR by ≥50% only | Sustained decrease in eGFR by ≥50% only |
| Sustained decrease in GFR to < 10–15 mL/min/1.73 m2, dialysis | Sustained decrease in GFR to <10–15 mL/min/1.73 m2, dialysis | |
| Or renal transplantation | Or renal transplantation | |
| Placebo, n (%)/events/100 patient-years | DM 23/929 (2.5%)/2.4 | DM 44/1472 (3.0%)/1.8 |
| Non-DM 10/938 (1.1%)/1.0 | Non-DM 18/1519 (1.2%)/0.7 | |
| Empagliflozin, n (%)/events/100 patient-years | DM 13/927 (1.4%)/1.3 | DM 38/1466 (2.6%)/1.5 |
| Non-DM 5/936 (0.5%)/0.5 | Non-DM 12/1531 (0.8%)/0.45 | |
| Empagliflozin versus placebo, HR (95% CI) | DM 0.52 (0.26, 1.03) | DM 0.82 (0.53, 1.27) |
| Non-DM 0.50 (0.17, 1.48) | Non-DM 0.68 (0.33, 1.40) |
Despite reports highlighting a discrepancy between eGFR slopes and kidney events results [22], the discrepancy depends on the specific definition of kidney events and disappears when a stricter definition is used. In any case, these trials were not designed or powered to assess kidney events during a short follow-up of a population at relatively low risk of CKD progression based on low median UACR values.
Table 3:
Kidney outcomes in participants with normoalbuminuria (UACR <30 mg/g) from EMPA-REG OUTCOME and EMPA-KIDNEY (data from [26] and [9]).
| EMPA-REG outcome, no CKD (eGFR ≥60 mL/min/1.73 m2, UACR <30 mg/g) | EMPA-KIDNEY (eGFR 20–45 mL/min/1.73 m2, UACR <30 mg/g) | |
|---|---|---|
| Follow-up 3.1 years | Follow-up 2.0 years | |
| Chronic eGFR slope (mL/min/1.73 m2/year and 95% CI) | ||
| Placebo | −0.95 (−1.34, −0.56) (n = 948) | −0.89 (−1.20, −0.58) (n = 663) |
| Empagliflozin | 0.22 (−0.02, 0.46) (n = 1946) | −0.11 (−0.44, 0.22) (n = 665) |
| Difference of empagliflozin versus placebo | 1.17 (0.71, 1.63) | 0.78 (0.32, 1.23) |
| Kidney events | Doubling of serum creatinine | Sustained decrease from baseline in the eGFR of ≥40% |
| Initiation of KRT | Initiation of KRT or sustained decrease in the eGFR to <10 mL/min/1.73 m2 | |
| Or death from kidney | Or death from renal causes | |
| Placebo | 20/1094 (1.8%) | 31/663 (4.7%) |
| Empagliflozin | 13/2205 (0.6%) | 30/665 (4.5%) |
| Empagliflozin versus placebo, HR (95% CI) | 0.31 (0.16, 0.63) | 0.97 (0.59–1.60) |
Note that despite differences between the datasets (prevalence of DM, baseline eGFR, follow-up time and other inclusion and exclusion criteria), placebo eGFR slope data are remarkably consistent in both trials and with the a priori expectation of persons with normoalbuminuria being populations at low risk for CKD progression. In both datasets there was also a remarkably similar response of chronic eGFR slopes to empagliflozin. However, there was a large difference in the impact of empagliflozin on the incidence of kidney events that, as expected, was low in both datasets. The differences noted in the HR for kidney events thus likely represent differences in the follow-up time (longer for EMPA-REG OUTCOME), number of participants (larger for EMPA-REG OUTCOME) and in the definition of the kidney events (stricter for EMPA-REG OUTCOME, as applied to the subgroup of participants in the present analysis), rather than different drug efficacy in participants with normoalbuminuria. As is the case for the results obtained when different definitions of kidney events are used in EMPEROR-Reduced and EMPEROR-Preserved (Table 2), the use of a stricter kidney endpoint may have contributed to observe differences between study arms in EMPA-REG OUTCOME. However, the definition of kidney event used in EMPA-KIDNEY may expect a larger contribution of the initial dip in eGFR towards reaching the threshold eGFR value that represents a kidney event (Supplementary data, Fig. S3), this being a source of confusion. A doubling of serum creatinine in a 60-year-old man with baseline eGFR of 60 mL/min/1.73 m2 represents a drop in eGFR of 34 mL/min/1.73 m2, while a 40% decrease in eGFR in a person with baseline eGFR of 20 mL/min/1.73 m2 represents a drop in eGFR of 8 mL/min/1.73 m2. In the same participants, reaching kidney failure would imply a loss of eGFR of 50 vs 10 mL/min/1.73 m2, and this drop would include the initial eGFR dip due to reduced hyperfiltration.
Overall, it is our opinion that when kidney events are few, likely resulting from a combination of low kidney risk and short follow-up for that low risk, and the definition of events is within the range of the dip in eGFR induced by SGLT2 inhibitors, chronic eGFR slopes provide a better assessment of kidney protection than kidney events. This knowledge should be incorporated into the design of future clinical trials.
EMPA-KIDNEY CONFIRMED THE SAFETY PROFILE OF SGLT2 INHIBITION IN PARTICIPANTS WITH CKD
The safety profile of empagliflozin in EMPA-KIDNEY was consistent with the safety profile of other SGLT2 inhibition trials. As in previous trials, the incidence of serious AKI was lower in the empagliflozin arm (HR 0.78, 95% CI 0.60–1.00) and none of the safety outcomes analyzed had a lower limit of the 95% CI above 1.0, including serious urinary tract infection and limb amputation. Lower limb amputation rates [empagliflozin 0.8% and placebo 0.6% (HR 1.43, 95% CI 0.80–2.57)] were similar to those seen in other SGLT2 inhibitor trials [11].
Ketoacidosis was defined by the coexistence of three criteria: symptoms or relevant presentation or relevant triggers, including missed insulin doses or intercurrent illness, and serum bicarbonate levels <15 mmol/L and blood beta-hydroxybutrate >1.5 mmol/L or high urine ketones. It was extremely uncommon with empagliflozin (6 events in 3304 participants, 0.2%) and even rarer in the placebo arm (1 in 3304 participants, who was diabetic, <0.1%). However, the manuscript does not clarify whether the five participants with DM, empagliflozin and ketoacidosis had T1DM or T2DM. In this regard, the European Medicines Agency indicates that empagliflozin should not be used in patients with T1DM. Ketoacidosis occurred in 1 of 1779 participants without DM in the empagliflozin group (<0.1%).
INTEGRATION OF KIDNEY OUTCOMES FROM LARGE PLACEBO-CONTROLLED TRIALS OF SGLT2 INHIBITORS
A collaborative meta-analysis that integrated the kidney outcomes from large placebo-controlled trials of SGLT2 inhibitors from the SGLT2 inhibitor Meta-Analysis Cardio-Renal Trialists’ Consortium (SMART-C) was made public at the time of publication of EMPA-KIDNEY [11]. For this analysis, kidney outcomes were slightly modified so as all trials shared the ≥50% sustained decrease in eGFR criterion. It concluded that in addition to the established cardiovascular benefits of SGLT2 inhibitors, RCTs support their use for modifying risk of CKD progression and AKI, in patients with T2DM at high cardiovascular risk, and in patients with CKD or heart failure irrespective of DM status, primary kidney disease or kidney function [11]. The relative risk of CKD progression on SGLT2 inhibitors was below 1.0 for IgA nephropathy, focal segmental glomerulosclerosis, other glomerular diseases and any glomerular disease.
Regarding baseline albuminuria values, the relative risk for CKD progression ranged from 0.51 to 0.61 for RCTs of canagliflozin, dapagliflozin or empagliflozin that had median UACR values <30 mg/g in DM participants and from 0.50 to 0.68 for RCTs of empagliflozin that had median UACR values <30 mg/g in non-DM participants, although 95% CIs were wide and crossed 1.0 in the latter, which had a lower sample size [11]. Similar findings were reported for risk of AKI: relative risk ranged from 0.41 to 0.69 in DM RCTs with median albuminuria <30 mg/g and from 0.56 to 0.80 in non-DM trials with low baseline UACR. Chronic eGFR slopes were not analyzed and no specific analysis of individual participants by baseline albuminuria was reported.
Among adverse effects, ketoacidosis was exceptional in non-DM participants (1/7788; i.e. 1 for 30 000 participant years of follow-up) and the risk was doubled in DM participants to reach an incidence of 0.29% (RR 2.12, 95% CI 1.49–3.04). The risk of lower limb amputation was mildly or not elevated [DM 1.15 (1.02–1.30), non-DM 1.06 (0.93–1.21), heterogeneity by DM status: P = .71] to an incidence lower than 1.70%.
Beyond this all-encompassing meta-analysis, EMPA-KIDNEY and EMPA-REG OUTCOME enrolled participants with a wide range of UACR values, from <30 mg/g to >300 mg/g, and a range of eGFR values (≥30 mL/min) that is complementary and partially overlapping (Supplementary data, Fig. S1D). As an example, 545 EMPA-REG OUTCOME participants had severe CKD and eGFR <60 mL/min/1.73 m2, fully overlapping with the population enrolled in EMPA-KIDNEY. In persons with DM, the combination of both trials covered the full spectrum from no-CKD to CKD up to an eGFR of 15 mL/min/1.73 m2, taking into account the enrolment of patients with eGFR <20 mL/min/1.73 m2 in EMPA-KIDNEY [11, 13]. The overall cardiorenal protection results were concordant for EMPA-KIDNEY and EMPA-REG OUTCOME and meta-analysis should combine the data to provide a holistic view of the impact of empagliflozin across different populations outside the heart failure scenario.
BEYOND NON-DIALYSIS CKD: SGLT2 INHIBITION FOR PRIMARY PREVENTION OF CKD AND FOR CKD PATIENTS ON DIALYSIS
While EMPA-REG and DAPA-CKD focused on persons who already had CKD, evidence has accumulated from post hoc analyses of cardiovascular outcomes trials on the potential of SGLT2 inhibitors for primary prevention of CKD and even to slow the age-associated loss of eGFR. Additionally, evidence for beneficial effects in persons with very low eGFR values and data on the mechanism of action for heart protection have opened the door for testing SGLT2 inhibitors in patients on dialysis, a once unthinkable possibility for drugs that have proximal tubule transporters as prime targets.
SGLT2 inhibition for primary prevention of CKD
Primary prevention of CKD implies prescribing SGLT2 inhibitors to patients not having CKD but at high risk of CKD to delay the onset or prevent the occurrence of CKD, in a similar manner that drugs may be prescribed to persons at high risk of CVD who do not have CVD in order to delay the onset or prevent the occurrence of CVD. Primary prevention of CVD has been highly successful and has contributed to the continued decline of the contribution of CVD to global causes of death [4, 5]. We hypothesize that a primary prevention approach may also decrease the burden of CKD and should be explored, and that SGLT2 inhibitors may be part of a holistic approach to this issue. Both Dapagliflozin Effect on Cardiovascular Events (DECLARE-TIMI 58) and EMPA-REG OUTCOME provided post hoc evidence that initiation of SGLT2 inhibitors in participants with T2DM and high cardiovascular risk who do not have CKD at baseline (i.e. eGFR >60 mL/min/1.73 m2 and UACR <30 mg/g) is clearly associated with primary prevention of CKD, as assessed by either CKD incidence, or kidney events or chronic eGFR slopes [25, 26] Specifically, in EMPA-REG a post hoc analysis disclosed that among persons with T2DM and high CVD risk but without baseline CKD (eGFR >60 mL/min/1.73 m2 and UACR <30 mg/g, n = 3322), the HR for incident nephropathy was 0.67 (0.47–0.94) and for doubling of serum creatinine, initiation of kidney replacement therapy or death from kidney it was 0.31 (0.16–0.63) [26] (Table 3). These data support the concept that empagliflozin offers kidney protection for persons with normoalbuminuria, at least for those with DM. A holistic assessment of the potential of SGLT2 inhibitors for primary prevention will require the integration through meta-analysis of data obtained in populations without baseline CKD, including the post hoc analyses of heart failure trials. Eventually new trials should directly address primary prevention of CKD by SGLT2 inhibitors in DM or outside DM. The design of these trial may be optimized by a careful analysis of information that is already available.
SGLT2 inhibition and age-associated loss of GFR
A topic related to primary prevention of CKD is the potential impact of SGLT2 inhibitors on the age-associated loss of eGFR, a hypothesis that should be validated in ad hoc–designed studies. The so-called age-associated decrease in GFR, usually estimated at around −1 mL/min/1.73 m2/year from age 40 years [27] and more recently estimated at −0.6 to −1.5 mL/min/1.73 m2 when measured in 1837 (53% women, aged 50–62 years) completely healthy persons [28]. In this regard, the combined assessment of the results of EMPA-REG OUTCOME and EMPA-KIDNEY disclosed overlapping eGFR slopes on empagliflozin of 0.22 (−0.02, 0.46) to −0.11 (−0.44, 0.22) mL/min/1.73 m2/year (Table 3) in 2611 low kidney risk participants (e.g. those with normoalbuminuria) mostly on RAS blockers, which is well below the expected age-associated loss of eGFR [9, 26]. Of note, in EMPA-REG OUTCOME, stable eGFR slopes (median 0.39 and 0.05 mL/min/1.73 m2/year, respectively) were also observed for 1729 additional participants with mild to moderate CKD as defined by KDIGO GA categories (Supplementary data, Fig. S1) [26].
SGLT2 inhibitors and dialysis
At dialysis initiation, patients still have residual renal function estimated at eGFR 5–10 mL/min/1.73 m2. In addition to contributing to clearing molecules that are also cleared by dialysis, residual kidney function may excrete molecules not easily dialyzed, such as protein-bound uremic toxins, as well as providing non-excretory kidney functions [29]. Indeed, residual renal function is inversely associated with cardiovascular deaths, despite what would be considered adequate dialysis. Thus, there is interest in preserving residual renal function. In this regard, RAS blockade preserved kidney function in a clinical trial in peritoneal dialysis [30]. Moreover, the mechanisms of cardiovascular protection by SGLT2 inhibitors have not been completely clarified. Thus, they may have direct action on the failing heart independent of changes in renal tubular function, and may, thus, potentially protect from cardiovascular events even in persons on dialysis and lacking residual renal function [31]. Thus, additional studies should explore potential benefits of SGLT2 inhibitors in persons on kidney replacement therapy, including those on peritoneal dialysis or hemodialysis, at least in those with residual renal function. The ongoing phase 2 DAPA-HD (NCT05179668) trial is evaluating hemodialysis patients with residual renal function with a primary endpoint of left ventricular mass.
CONCLUSION
In conclusion, EMPA-KIDNEY has provided evidence supporting a wider use of SGLT2 inhibitors for persons with CKD, encompassing persons with lower eGFR and albuminuria values and a wider range of causes of CKD than prior available evidence. It also supports the use of SGLT2 inhibitors in patients who are not on angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers for reasons of intolerance or others.
These results should be viewed in conjunction with prior evidence obtained in populations at high risk of CKD, but without baseline CKD, such as patients with DM and high cardiovascular risk in EMPA-REG OUTCOME and DECLARE-TIMI 58 [25, 26] (Fig. 4). Overall, SGLT2 inhibitors should be considered for both primary prevention of CKD in high-risk populations with DM and for treatment of CKD independent from the cause, baseline eGFR, albuminuria levels or presence of DM. It must be noted, however, that evidence in patients with normo- and micro-albuminuria without DM relates only to the analysis of eGFR slopes and not hard outcomes, due to reduced study power to capture difference in these lower risk subgroups.
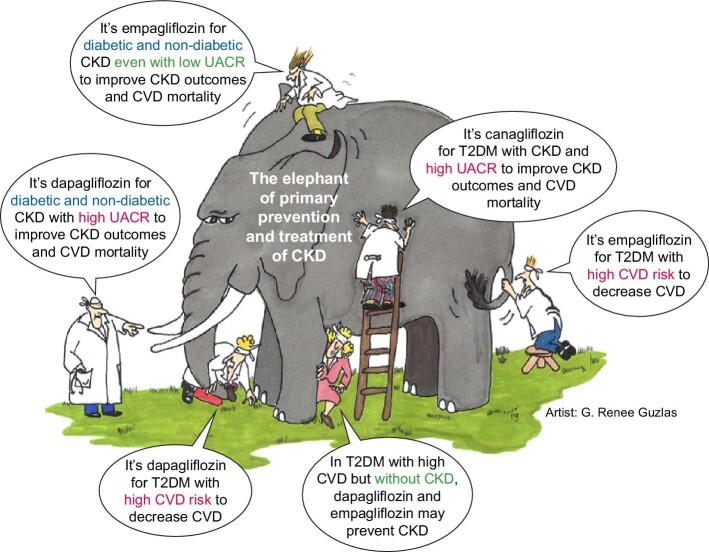
Figure 4:
Blind men and the elephant of CKD prevention and treatment. Over the years, different trials testing different SGLT2 inhibitors in different populations with different primary or secondary outcomes and post hoc analyses have provided insight into the potential of CKD inhibitors to prevent and treat CKD. The results of these trials should be integrated for a better appreciation of the role of SGLT2 inhibition in kidney protection, in a similar manner that the different touching experiences of the blind men in the Indian parable of the blind men and an elephant should be integrated to learn about the elephant. Adapted from reference [37] (artist: G. Renee Guzlas). All rights reserved ©. Reproduced by permission of J. Himmelfarb, P. Stenvinkel, T.A. Ikizler and R. M. Hakim.
Some areas require further research. So far, there is scarce evidence on SGLT2 inhibition for polycystic kidney disease and for immune-suppressed patients, including kidney transplant recipients, as these were excluded from large clinical trials [32, 33]. Additional subanalyses focused on specific causes are awaited, although these should be interpreted with caution, given that trials were not powered to detect differences in subgroups and they were stopped prematurely because of efficacy, potentially precluding observing differences in more slowly progressing causes of CKD. Specific clinical trials may be needed to settle unconvincing available evidence. Finally, primary CKD prevention by SGLT2 inhibitors should be characterized in high-risk individuals without DM (Supplementary data, Fig. S4) and their role, if any, in persons with CKD on dialysis should be explored.
Supplementary Material
ACKNOWLEDGEMENTS
FIS/Fondos FEDER (PI20/00744, PI19/00588, PI19/00815, PI21/01292, PI21/00251), ERA-PerMed-JTC2018 (KIDNEY ATTACK AC18/00064, ISCIII-RETIC REDinREN RD016/0009), ERA-PerMed-JTC 2022 (ONAKI-ICI AC22/00029) Sociedad Española de Nefrología, Sociedad Madrileña de Nefrología (SOMANE), FRIAT, Comunidad de Madrid en Biomedicina B2017/BMD-3686 CIFRA2-CM. Instituto de Salud Carlos III (ISCIII) RICORS program to RICORS2040 (RD21/0005/0001, RD21/0005/0016) funded by European Union—NextGenerationEU, Mecanismo para la Recuperación y la Resiliencia (MRR), SPACKDcPMP21/00109, FEDER funds, and Marató TV3 2020 421/C/2020, Marató TV3 2021 215/C/2021.
Contributor Information
Beatriz Fernández-Fernandez, Department of Nephrology and Hypertension, IIS-Fundacion Jimenez Diaz UAM, Madrid, Spain; RICORS2040, Madrid, Spain; Departamento de Medicina, Facultad de Medicina, Universidad Autónoma de Madrid, Madrid, Spain; GEENDIAB, Sociedad Española de Nefrología, Spain.
Pantelis Sarafidis, Department of Nephrology, Hippokration Hospital, Aristotle University of Thessaloniki, Greece.
Maria José Soler, RICORS2040, Madrid, Spain; GEENDIAB, Sociedad Española de Nefrología, Spain; Nephrology Department, Vall d'Hebron University Hospital, Vall d'Hebron Institue of Research, Barcelona, Spain.
Alberto Ortiz, Department of Nephrology and Hypertension, IIS-Fundacion Jimenez Diaz UAM, Madrid, Spain; RICORS2040, Madrid, Spain; Departamento de Medicina, Facultad de Medicina, Universidad Autónoma de Madrid, Madrid, Spain; GEENDIAB, Sociedad Española de Nefrología, Spain.
CONFLICT OF INTEREST STATEMENT
A.O. is one of the former Editors-in-Chief of Clinical Kidney Journal and has received grants from Sanofi and consultancy or speaker fees or travel support from Advicciene, Alexion, Astellas, Astrazeneca, Amicus, Amgen, Boehringer Ingelheim, Fresenius Medical Care, GSK, Bayer, Sanofi-Genzyme, Menarini, Mundipharma, Kyowa Kirin, Lilly, Freeline, Idorsia, Chiesi, Otsuka, Novo-Nordisk, Sysmex and Vifor Fresenius Medical Care Renal Pharma, and is Director of the CatedraMundipharma-UAM of diabetic kidney disease and the CatedraAstrazeneca-UAM of chronic kidney disease and electrolytes. He has stock in TelaraFarma. B.F.-F. has received grants from Esteve and consultancy or speaker fees or travel support from Astrazeneca, Bayer, Menarini, Novo-Nordisk Boehringer Ingelheim and Mundipharma. B.F.-F. is Editor for Nefroplus.
P.S. has received consultancy fees from AstraZeneca, Bayer, HealThink, Innovis Pharma, PrimeView, Menarini and ReCor Medical; speaker fees from Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Genesis Pharma, Menarini, PeerVoice, Springer and Win Medica; research grants from AstraZeneca, Boehringer Ingelheim, Elpen and Servier. M.J.S. reports honorarium for conferences, consulting fees and advisory boards from AstraZeneca, NovoNordisk, Esteve, Vifor, Bayer, Mundipharma, Ingelheim Lilly, Jansen, ICU Medical, Travere Therapeutics, GE Healthcare and Boehringer. M.J.S is also the former Editor-in-Chief of Clinical Kidney Journal.
REFERENCES
- 1. Perez-Gomez MV, Bartsch L-A, Castillo-Rodriguez Eet al. Clarifying the concept of chronic kidney disease for non-nephrologists. Clin Kidney J 2019;12:258–61. https://academic.oup.com/ckj/article/12/2/258/5320336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Disease Kidney: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013;3:1–150. [Google Scholar]
- 3. Ortiz A, Wanner C, Gansevoort Ret al. Chronic kidney disease as cardiovascular risk factor in routine clinical practice: a position statement by the Council of the European Renal Association. Nephrol Dial Transplant 2023;38:527–31. https://pubmed.ncbi.nlm.nih.gov/36216362/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ortiz A, Roger M, Jiménez VMet al. RICORS2040: the need for collaborative research in chronic kidney disease. Clin Kidney J 2021;15:372–87. https://pubmed.ncbi.nlm.nih.gov/35211298/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Foreman KJ, Marquez N, Dolgert Aet al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016–40 for 195 countries and territories. Lancet North Am Ed 2018;392:2052–90. 10.1016/S0140-6736(18)31694-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Boer IH, Khunti K, Sadusky Tet al. Diabetes management in chronic kidney disease: a consensus report by the American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2022;102:974–89. https://pubmed.ncbi.nlm.nih.gov/36202661/. [DOI] [PubMed] [Google Scholar]
- 7. Heerspink HJL, Stefánsson BV, Correa-Rotter Ret al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020;383:1436–46. https://pubmed.ncbi.nlm.nih.gov/32970396/. [DOI] [PubMed] [Google Scholar]
- 8. ANNEX I . Summary of product characteristics. Available from: https://www.ema.europa.eu/en/documents/product-information/forxiga-epar-product-information_en.pdf (22 April 2019, date last accessed).
- 9. Herrington WG, Staplin N, Wanner Cet al. Empagliflozin in patients with chronic kidney disease. N Engl J Med 2023;388:117–27. https://pubmed.ncbi.nlm.nih.gov/36331190/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rovin BH, Adler SG, Barratt Jet al. Executive summary of the KDIGO 2021 Guideline for the management of glomerular diseases. Kidney Int 2021;100:753–79. https://pubmed.ncbi.nlm.nih.gov/34556300/. [DOI] [PubMed] [Google Scholar]
- 11. Baigent C, Emberson J, Haynes Ret al. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet 2022;400:1788–801. https://pubmed.ncbi.nlm.nih.gov/36351458/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McDonagh TA, Metra M, Adamo Met al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–726. https://pubmed.ncbi.nlm.nih.gov/34447992/. [DOI] [PubMed] [Google Scholar]
- 13. Wanner C, Inzucchi SE, Lachin JMet al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016;375:323–34. http://www.ncbi.nlm.nih.gov/pubmed/27299675. [DOI] [PubMed] [Google Scholar]
- 14. Perkovic V, Jardine MJ, Neal Bet al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019:380:2295–306. http://www.nejm.org/doi/10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 15. Waijer SW, Vart P, Cherney DZIet al. Effect of dapagliflozin on kidney and cardiovascular outcomes by baseline KDIGO risk categories: a post hoc analysis of the DAPA-CKD trial. Diabetologia 2022;65:1085–97. https://pubmed.ncbi.nlm.nih.gov/35445820/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wheeler DC, Stefansson BV, Batiushin Met al. The dapagliflozin and prevention of adverse outcomes in chronic kidney disease (DAPA-CKD) trial: baseline characteristics. Nephrol Dial Transplant 2020;35:1700–11. https://pubmed.ncbi.nlm.nih.gov/32862232/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. EMPA-KIDNEY Collaborative Group . Design, recruitment, and baseline characteristics of the EMPA-KIDNEY trial. Nephrol Dial Transplant 2022;37:1317–29. https://pubmed.ncbi.nlm.nih.gov/35238940/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Herrington WG, Preiss D, Haynes Ret al. The potential for improving cardio-renal outcomes by sodium-glucose co-transporter-2 inhibition in people with chronic kidney disease: a rationale for the EMPA-KIDNEY study. Clin Kidney J 2018;11:749–61. https://academic.oup.com/ckj/article/11/6/749/5144684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Floege J. Mucosal corticosteroid therapy of IgA nephropathy. Kidney Int 2017;92:278–80. https://pubmed.ncbi.nlm.nih.gov/28709593/. [DOI] [PubMed] [Google Scholar]
- 20. Barratt J, Lafayette R, Kristensen Jet al. Results from part A of the multi-center, double-blind, randomized, placebo-controlled NefIgArd trial, which evaluated targeted-release formulation of budesonide for the treatment of primary immunoglobulin A nephropathy. Kidney Int 2023;103:391–402. https://pubmed.ncbi.nlm.nih.gov/36270561/. [DOI] [PubMed] [Google Scholar]
- 21. Vanholder R, Agar J, Braks Met al. The European Green Deal and nephrology: a call for action by the European Kidney Health Alliance (EKHA). Nephrol Dial Transplant 2023;38:1080–1088. https://pubmed.ncbi.nlm.nih.gov/35481547/. [DOI] [PubMed] [Google Scholar]
- 22. Packer M, Butler J, Zannad Fet al. Empagliflozin and major renal outcomes in heart failure. N Engl J Med 2021;385:1531–3. https://pubmed.ncbi.nlm.nih.gov/34449179/. [DOI] [PubMed] [Google Scholar]
- 23. Anker SD, Butler J, Filippatos Get al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021;385:1451–61. https://pubmed.ncbi.nlm.nih.gov/34449189/. [DOI] [PubMed] [Google Scholar]
- 24. Kraus BJ, Weir MR, Bakris GLet al. Characterization and implications of the initial estimated glomerular filtration rate “dip” upon sodium-glucose cotransporter-2 inhibition with empagliflozin in the EMPA-REG OUTCOME trial. Kidney Int 2021;99:750–62. https://pubmed.ncbi.nlm.nih.gov/33181154/. [DOI] [PubMed] [Google Scholar]
- 25. Mosenzon O, Raz I, Wiviott SDet al. Dapagliflozin and prevention of kidney disease among patients with type 2 diabetes: post hoc analyses from the DECLARE-TIMI 58 trial. Diabetes Care 2022;45:2350–9. https://pubmed.ncbi.nlm.nih.gov/35997319/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Levin A, Perkovic V, Wheeler DCet al. Empagliflozin and cardiovascular and kidney outcomes across KDIGO risk categories: post hoc analysis of a randomized, double-blind, placebo-controlled, multinational trial. Clin J Am Soc Nephrol 2020;15:1433–44. https://pubmed.ncbi.nlm.nih.gov/32994159/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rule AD, Glassock RJ.. The aging kidney. https://www.uptodate.com/contents/the-aging-kidney (25 January 2023, date last accessed).
- 28. Melsom T, Norvik JV, Enoksen ITet al. Sex differences in age-related loss of kidney function. J Am Soc Nephrol 2022;33:1891–902. https://pubmed.ncbi.nlm.nih.gov/35977806/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lowenstein J, Grantham JJ.. Residual renal function: a paradigm shift. Kidney Int 2017;91:561–5. https://pubmed.ncbi.nlm.nih.gov/28202171/. [DOI] [PubMed] [Google Scholar]
- 30. Li PKT, Chow KM, Wong TYHet al. Effects of an angiotensin-converting enzyme inhibitor on residual renal function in patients receiving peritoneal dialysis. A randomized, controlled study. Ann Intern Med 2003;139:105. https://pubmed.ncbi.nlm.nih.gov/12859160/. [DOI] [PubMed] [Google Scholar]
- 31. Packer M. SGLT2 inhibitors: role in protective reprogramming of cardiac nutrient transport and metabolism. Nat Rev Cardiol 2023. https://pubmed.ncbi.nlm.nih.gov/36609604/. [DOI] [PubMed] [Google Scholar]
- 32. Afsar B, Afsar RE, Demiray Aet al. Sodium-glucose cotransporter inhibition in polycystic kidney disease: fact or fiction. Clin Kidney J 2022;15:1275–83. https://pubmed.ncbi.nlm.nih.gov/35756735/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kanbay M, Demiray A, Afsar Bet al. Sodium-glucose cotransporter 2 inhibitors for diabetes mellitus control after kidney transplantation: review of the current evidence. Nephrology (Carlton) 2021;26:1007–17. https://pubmed.ncbi.nlm.nih.gov/34263502/. [DOI] [PubMed] [Google Scholar]
- 34. Wheeler DC, Stefánsson BV, Jongs Net al. Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and non-diabetic chronic kidney disease: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol 2021;9:22–31. https://pubmed.ncbi.nlm.nih.gov/33338413/. [DOI] [PubMed] [Google Scholar]
- 35. Heerspink HJL, Jongs N, Chertow GMet al. Effect of dapagliflozin on the rate of decline in kidney function in patients with chronic kidney disease with and without type 2 diabetes: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol 2021;9:743–54. https://pubmed.ncbi.nlm.nih.gov/34619108/. [DOI] [PubMed] [Google Scholar]
- 36. Packer M, Anker SD, Butler Jet al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413–24. https://pubmed.ncbi.nlm.nih.gov/32865377/. [DOI] [PubMed] [Google Scholar]
- 37. Himmelfarb J, Stenvinkel P, Ikizler TAet al. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int 2002;62:1524–38. https://pubmed.ncbi.nlm.nih.gov/12371953/. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.