ABSTRACT
Background
Long-term high-dose lithium therapy in bipolar disorder is known to adversely affect kidney function. However, recent animal studies have revealed that low amounts of lithium are beneficial for the kidney when it is damaged by exposure to nephrotoxic compounds, inflammation or oxidative stress. This study aimed to investigate whether urinary lithium excretion, reflecting dietary lithium intake, is associated with adverse long-term kidney graft outcomes and patient survival.
Methods
Urinary lithium concentration was measured using inductively coupled plasma mass spectrometry in 642 stable kidney transplant recipients (KTRs). Graft failure was defined as the start of dialysis or retransplantation and kidney function decline was defined as a doubling of serum creatinine.
Results
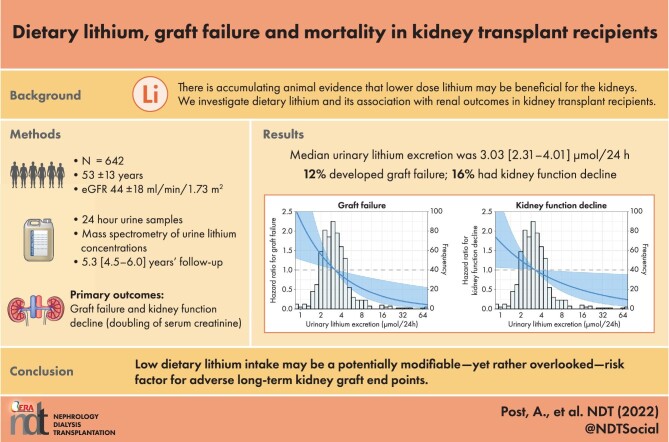
The median urinary lithium excretion was 3.03 μmol/24 h [interquartile range (IQR) 2.31–4.01]. Urinary lithium excretion was associated with energy, plant protein and water intake. During a median follow-up of 5.3 years (IQR 4.5–6.0), 79 (12%) KTRs developed graft failure and 127 (20%) KTRs developed kidney function decline. Higher urinary lithium excretion was associated with a lower risk of graft failure {hazard ratio [HR] per doubling 0.54 [95% confidence interval (CI) 0.38–0.79]} and kidney function decline [HR per doubling 0.73 (95% CI 0.54–0.99)]. These associations remained independent of adjustment for potential confounders and in sensitivity analyses. There was a significant effect modification with the use of proliferation inhibitors (P = .05) and baseline estimated glomerular filtration rate (eGFR; P < .001), with higher urinary lithium excretion being more protective in KTRs not using proliferation inhibitors and in KTRs with lower baseline eGFR. Furthermore, higher urinary lithium excretion was associated with a reduced risk of all-cause mortality [HR 0.64 (95% CI 0.49–0.83); P = .001].
Conclusion
Dietary lithium intake may be a potentially modifiable, yet rather overlooked, risk factor for adverse long-term kidney graft outcomes and patient survival.
Trial registration
Keywords: graft failure, kidney transplant recipients, lithium, mortality
Graphical Abstract
Graphical Abstract.
 Watch the video of this contribution at
https://academic.oup.com/ndt/pages/author_videos.
Watch the video of this contribution at
https://academic.oup.com/ndt/pages/author_videos.
KEY LEARNING POINTS.
What is already known about this subject?
There is accumulating evidence from animal studies that lower-dose lithium may be beneficial for the kidneys.
What this study adds?
The current study demonstrates that a lower dietary lithium intake is robustly and independently associated with an increased risk of graft failure and mortality in kidney transplant recipients.
What impact this may have on practice or policy?
The current study paves the way for further studies investigating whether stimulating dietary lithium intake or low-dose lithium supplementation may represent a novel risk management strategy to decrease the burden of long-term kidney graft failure and premature mortality.
INTRODUCTION
Kidney transplantation is the gold standard for end-stage kidney disease (ESKD) and the number of transplantations increases yearly. Nonetheless, many patients still spend years on waiting lists or depend on a living donation by a family member or friend. Hence it is a major clinical as well as ethical necessity to improve long-term graft and patient survival. In the past decades, advances in transplantation medicine have greatly improved short-term graft and patient survival after transplantation [1]. However, long-term graft and patient survival remains largely unchanged and kidney transplant recipients (KTRs) continue to be at increased risk of graft failure, kidney function decline and premature mortality, thereby warranting a search for novel potentially modifiable risk factors [2, 3]. One potential modifiable risk factor is dietary lithium intake.
To many nephrologists, lithium is known for the severe renal side effects of long-term high-dose lithium therapy for bipolar disorder, which include nephrogenic diabetes insipidus and ESKD [4]. However, low-grade lithium exposure may have opposite effects, as several animal studies have revealed that administration of small amounts of lithium prevents kidney damage resulting from nephrotoxic compounds [5–8], inflammation [9, 10] and oxidative stress [5, 11, 12]. A potential mechanism is lithium-induced inhibition of glycogen synthase kinase type 3β (GSK3β), a serine/threonine protein that otherwise prevents tubular repair and induces pro-inflammatory gene transcription and apoptosis/necrosis [4]. A recent study in mice demonstrated that targeted inhibition of GSK3β using low-dose lithium was able to intercept the senescence signalling, mitigate podocyte senescence and slow kidney aging [13]. To date, it is unknown whether alleviation of kidney damage by low-grade lithium exposure as observed in animals can be extended to humans.
Prior to investigating whether low-dose lithium therapy possesses renoprotective effects in humans, it is imperative to know whether variations in dietary lithium intake are associated with long-term outcomes in humans with susceptible kidneys, like those living with a transplanted kidney. While beneficial effects of higher dietary lithium intake have been reported for all-cause mortality and suicide rates [14, 15] in the general population, no epidemiological studies have investigated whether variations in dietary lithium intake are associated with long-term outcomes in KTRs. Classic dietary assessments have many limitations, including under- and overreporting, illiteracy and motivation requirements, changes in diet due to self-reflections, errors in portion size estimates and socially desirable answers [16, 17]. After being virtually entirely absorbed in the small intestine, lithium is distributed relatively uniformly in body water, with only small differences between intra- and extracellular concentrations [18]. Given the small size of lithium, it is freely filtered in the glomeruli, after which it is largely reabsorbed in the proximal tubules via transcellular and paracellular transport [4, 19]. In steady-state circumstances, >95% of ingested lithium is excreted by the kidney in the urine, while a minority of lithium leaves the body through faeces and sweat [20–22], thereby making 24-h urinary lithium excretion a suitable marker for assessing the daily intake of lithium, with the benefit of not being affected by the aforementioned limitations of classic dietary assessments.
The primary aim of the current study was to investigate the prospective association of dietary lithium intake with graft failure and kidney function decline in a cohort of 642 KTRs. We additionally performed effect modification analyses to determine whether the associations are stronger in specific subgroups of KTRs. In secondary analyses we investigated the association of dietary lithium intake with all-cause mortality.
MATERIALS AND METHODS
Study population
This observational prospective study was conducted in the TransplantLines Food and Nutrition Biobank and Cohort Study, a cohort of kidney transplant recipients that has been described in detail previously [23–25]. In short, all adult (≥18 years of age) prevalent KTRs without known or apparent systemic illnesses (i.e. malignancies, opportunistic infections) who visited the outpatient clinic of the University Medical Center Groningen between November 2008 and June 2011 were invited to participate. Geographically, the cohort is based on KTRs living in the northern part of the Netherlands. Included KTRs were all transplanted at the University Medical Center Groningen and had no history of drug or alcohol addiction. Of 817 initially invited KTRs, 706 (87%) signed written informed consent to participate in the study. After excluding KTRs with missing data on urinary lithium excretion, a total of 642 KTRs were eligible for statistical analyses. A flowchart of KTRs through the study is shown in Supplementary Fig. S1. The study protocol was approved by the University Medical Center Groningen institutional ethical review board (medical ethical committee 2008/186) and was conducted in accordance with the Declaration of Helsinki and Declaration of Istanbul. The reporting of the current study conforms to the Strengthening the Reporting of Observational Studies in Epidemiology guidelines [26].
Clinical parameters
Information on health status, medical history and medication use was obtained from patient records. Information on smoking behaviour and alcohol intake was obtained from a questionnaire. Participants were classified as current, former or never smokers. Alcohol intake was split into 0–10 g/24 h, 10–30 g/24 h and >30 g/24 h. Body weight and height were measured with participants wearing indoor clothing without shoes. Body mass index (BMI) was calculated as weight (in kg) divided by height (in m2) and body surface area (BSA) was calculated using the formula of Du Bois and Du Bois [27]. All measurements were performed during a morning visit to the outpatient clinic after an 8- to 12-h overnight fasting period. Blood pressure (BP) was measured (in mmHg) with a semiautomatic device (Dinamap 1846, Critikon, Tampa, FL, USA) according to a strict protocol as previously described [28]. Diabetes mellitus was diagnosed according to American Diabetes Association criteria (2017) as having a fasting plasma glucose concentration ≥7.0 mmol/l or the use of antidiabetic medication [29]. Hypertension was defined as systolic BP >140 mmHg and/or diastolic BP >90 mmHg.
Laboratory measurements
All participants were instructed to collect a 24-h urine sample according to a strict protocol the day before their visit to the outpatient clinic. Urine was collected under oil and chlorhexidine was added as an antiseptic agent. Upon completion of the 24-h urine collection, fasting venous blood samples anticoagulated with lithium heparin, sodium fluoride and potassium ethylenediaminetetraacetic acid (EDTA) were obtained in the morning. Samples were stored in small aliquots at −80°C for later use.
Lipids, electrolytes, creatinine, high-sensitivity C-reactive protein (hs-CRP), glucose, haemoglobin A1c and urinary protein were measured using routine clinical laboratory methods (Roche Diagnostics, Basel, Switzerland). Serum cystatin C was measured with the Gentian Cystatin C Immunoassay (Gentian, Moss, Norway) on a Roche modular analyser and was calibrated directly with the standard supplied by the manufacturer. Plasma neutrophil gelatinase–associated lipocalin (NGAL) concentrations were measured in EDTA plasma using a validated particle-enhanced turbidimetric immunoassay (Gentian). Urinary liver-type fatty acid-binding protein (LFABP) was measured with an enzyme-linked immunosorbent assay (ELISA; human uL-FABP assay kit 96 test; CMIC Holdings, Tokyo, Japan). Urinary epidermal growth factor (EGF) was measured using an ELISA (R&D Systems, Minneapolis, MN, USA). Plasma malondialdehyde concentration was measured using a high-performance liquid chromatography assay [30]. Free sulfhydryl groups in serum were quantified using Ellman's reagent. Human leukocyte antigen I (HLA-I) and HLA-II antibodies were quantified using an ELISA (LATM205, One Lambda, Canoga Park, CA, USA). Kidney function was assessed by the estimated glomerular filtration rate (eGFR) based on the Chronic Kidney Disease Epidemiology Collaboration creatinine cystatin C equation from 2012 [31, 32].
Analysis of urinary lithium excretion
Urinary lithium concentrations were determined using an inductively coupled plasma mass spectrometry (ICP-MS) ICAP Q instrument (Thermo Fisher Scientific, Waltham, MA, USA). Measurements were conducted in accordance with DIN EN ISO 17294-2: 2017-01 [33]. In short, samples were decomposed with nitric acid and lithium concentrations were detected in 1:50 diluted urinary samples. Rhodium (2 μg/l, corresponding to 19.4 nmol/l) was added as the internal standard. Blank samples have been applied to trace sources of potential lithium contaminations within sample preparation and following ICP-MS analysis. Under the selected conditions, the intra-assay coefficient of variation was 0.7% (n = 6). The recovery of lithium in spiked samples was 98.8% (n = 6). The lower limit of quantitation (LOQ) was 11.5 nmol/l and the limit of detection (LOD) was 2.9 nmol/l.
Food frequency questionnaires (FFQs)
Information on dietary intake was obtained from a validated semiquantitative FFQ, which was linked to the Dutch food composition table (NEVO) [34]. The FFQ inquired about the intake of 177 food items during the past month, with seasonal variations taken into account. For each item, the frequency was recorded in times per day, week or month. The number of servings was expressed in natural units (e.g. slice of bread or apple) or household measures (e.g. cup or spoon). The questionnaire was self-administered and filled out at home. Every FFQ was checked for completeness by a trained researcher and inconsistent answers were verified with the patients. Validation of the FFQ in KTRs was assessed as previously reported [35]. Dietary data were converted into daily nutrient intakes with the use of the Dutch Food Composition Table of 2006 [36]. Dietary intakes were adjusted for total energy intake (kcal/24 h) according to the residual method [37].
Outcomes
The primary outcomes of the study were graft failure, defined as the start of dialysis or retransplantation, and kidney function decline, defined as a doubling of serum creatinine. The primary outcomes were censored for death. These outcomes were chosen to adhere to current recommendations and state of the art in the field of nephrology [38]. The surveillance system of the outpatient program at our university hospital ensures updated information on graft endpoints as assessed by a nephrologist. Within the aforementioned system, patients visit the outpatient clinic with declining frequency after transplantation, in accordance with the guidelines of the American Society of Transplantation [39]. The secondary outcomes were all-cause mortality and cause-specific mortality. Cardiovascular mortality was defined as death due to cerebrovascular disease, ischaemic heart disease, heart failure or sudden cardiac death according to International Classification of Diseases, Ninth Revision (ICD-9) codes 410–447 [40]. Cancer mortality was defined according to a previously specified list of ICD-9 codes [41], whereas infectious disease mortality was identified according to ICD-9 codes 1–139 [42]. Endpoints were recorded until September 2015. General practitioners or referring nephrologists were contacted in case the status of a patient was unknown. No patients were lost to follow-up. An overview of the outcomes throughout follow-up is shown in Supplementary Table S1. A cross-tabulation of the renal outcomes is shown in Supplementary Table S2.
Statistical analysis
All data analyses and computations were performed with R version 4.1.1 (R Foundation for Statistical Computing, Vienna, Austria). Baseline data are presented as mean ± standard deviation (SD) for normally distributed data, median [interquartile range (IQR)] for non-normally distributed data and number (percentage) for nominal data. Two-sided P-values <.05 were considered statistically significant. Univariable linear regression analyses were used to demonstrate associations between urinary lithium excretion and variables at baseline. Regression coefficients were given as standardized β values, the latter referring to the number of SDs a dependent variable changes per SD increase of the independent variable, thereby allowing for comparison of the strength of the associations of different variables. A log2 transformation was performed for urinary lithium excretion prior to the analyses. Since there are no clinically relevant cut-offs available for urinary lithium excretion, to prevent loss of power and in alignment with expert consensus [43, 44], all analyses are performed with continuous variables rather than tertiles, quartiles or quantiles.
To account for potential bias that could result from the exclusion of participants with missing values [45], multiple imputation using fully conditional specification was performed using the ‘mice’ package to obtain five imputed data sets. The algorithm was run 30 iterations and convergence of the Markov chains was evaluated with trace plots of the mean and SD. To confirm that imputed values were biologically plausible, the distributions of the imputed values were visually investigated and compared with the distribution of the observed values. Analyses were performed in each of the data sets and results were pooled using Rubin's rules [45, 46]. An overview of missing values that are imputed is shown in Supplementary Table S2. Time-to-event analyses were performed using imputed data sets.
To study whether log2 transformed urinary lithium excretion was prospectively associated with the outcomes, multivariable-adjusted Cox proportional hazards regression models were fitted to the data. Time 0 was defined as the baseline visit of the study, which was a median 5.3 years (IQR 1.9–12.1) after transplantation. To adjust for the prevalent KTR design, in multivariable analyses, adjustments were made for the time between transplantation and baseline, described below. The Schoenfeld residuals were inspected to determine that the proportionality of hazards assumption was not violated. Non-linearity was assessed by comparing linear and non-linear models (natural spline with 3 degrees of freedom) using an analysis of deviance table, based on the log partial likelihood. Adjustments were made for a priori selected variables and for potentially relevant variables identified from the baseline table by P < .05. Since dietary intakes tend to be higher in larger patients, adjustment was performed for BSA, as this incorporates both weight and height and was associated more strongly with lithium excretion than BMI. Initial adjustments consist of the basic potential confounders age, sex, BSA, eGFR and urinary protein excretion (model 3). To avoid overfitting and inclusion of too many variables for the number of events, additional models were created using additive adjustments to model 3 [47]. These adjustments include alcohol intake and smoking status (model 4) and time between transplantation and baseline, the number of transplantations up to baseline, the presence of anti-HLA antibodies, pre-emptive transplantation, deceased donor versus living donor, age of the donor, history of rejection, warm ischaemia time, calcineurin inhibitors and proliferation inhibitors (model 5).
To assess whether the associations of urinary lithium excretion with graft failure and kidney function decline were driven by dietary intake, inflammation/oxidation, tubular damage or muscle mass, additional Cox regression analyses were performed by adjusting a base model for the dietary intakes (total energy intake, plant protein intake, water intake and urinary sodium excretion), inflammation and oxidation markers (hs-CRP, plasma malondialdehyde and free sulfhydryl groups), tubular damage markers (EGF, LFABP and NGAL) and muscle mass (as reflected by urinary creatinine excretion).
Potential effect modification for covariates was assessed by adding product terms of the covariate and log2 transformed urinary lithium excretion to models with the covariate and log2 transformed urinary lithium excretion. P for interaction <.05 was considered to indicate a significant effect modification. Stratified Cox proportional hazards regression analyses were performed to assess the association of urinary lithium excretion with outcomes if significant effect modification was present. The effect of urinary lithium excretion on the hazard of the outcomes of interest was displayed visually to facilitate its interpretation.
In order to determine the robustness of the findings, several sensitivity analyses were performed. The sensitivity analyses were conducted by performing Cox regression analyses excluding KTRs with outlying values of urinary lithium excretion (defined values deviating >3 SD from the mean), with an alcohol intake >30 g/24 h, who are current smokers, >70 years of age, with a BMI <17.5 or >30 kg/m2, with a dialysis vintage >6 years, with multiple transplantations and with a history of rejection. Lastly, we also investigated whether urinary lithium concentration, rather than excretion, was associated with primary and secondary outcomes.
Primary analyses explored the association of urinary lithium excretion with graft failure and kidney function decline. Secondary analyses explored the association of urinary lithium excretion with all-cause mortality and cause-specific mortality.
RESULTS
Baseline characteristics
A total of 642 patients (53 ± 13 years, 56% male, eGFR 44 ± 18 ml/min/1.73 m2) were included at a median 5.3 years (IQR 1.9–12.1) after kidney transplantation. The median urinary lithium excretion was 3.03 μmol/24 h (IQR 2.31–4.01). An overview of baseline characteristics and univariable linear regression analyses of urinary lithium excretion with baseline characteristics is shown in Table 1. Urinary lithium excretion was positively associated with male sex, weight, height, BSA, current smoker, higher alcohol intake, living kidney donation, urinary sodium excretion, urinary creatinine excretion, free sulfhydryl groups, total energy intake, plant protein intake and water intake (all P < .05). Urinary lithium excretion was negatively associated with having more than one transplantation before baseline, cold ischaemia time, the presence of HLA-II antibodies and plasma malondialdehyde (all P < .05). Urinary lithium excretion was not associated with the fractional sodium excretion.
Table 1:
Characteristics and associations of characteristics with urinary lithium excretion in 642 KTRs at baseline.
| Characteristics | KTR cohort (n = 642) | Std. β | 95% CI of Std. β | P for trend |
|---|---|---|---|---|
| Urinary lithium excretion (μmol/24 h), median (IQR) | 3.03 (2.31–4.01) | – | – | – |
| Demographics | ||||
| Age (years), mean ± SD | 53 ± 13 | −0.01 | −0.09–0.07 | .8 |
| Sex (male), n (%) | 362 (56) | 0.25 | 0.10–0.41 | .001 |
| Smokers, n (%) | ||||
| Never | 257 (42) | −0.02 | −0.10–0.06 | .59 |
| Past | 279 (46) | −0.03 | −0.11–0.05 | .40 |
| Current | 75 (12) | 0.08 | 0.01–0.16 | .04 |
| Alcohol (g/24 h), n (%) | ||||
| 0–10 | 437 (74) | −0.10 | −0.19 to −0.03 | .005 |
| 10–30 | 124 (21) | 0.05 | −0.03–0.13 | .24 |
| >30 | 28 (4) | 0.14 | 0.06–0.22 | <.001 |
| Body composition, mean ± SD | ||||
| Weight (kg) | 80.3 ± 16.4 | 0.10 | 0.02–0.18 | .01 |
| Height (cm) | 173.5 ± 9.6 | 0.13 | 0.05–0.20 | .001 |
| BMI (kg/m2) | 26.6 ± 4.8 | 0.04 | −0.04–0.11 | .4 |
| BSA (m2) | 1.94 ± 0.21 | 0.13 | 0.05–0.20 | .001 |
| Primary kidney disease, n (%) | ||||
| Primary glomerulosclerosis | 185 (29) | 0.07 | −0.01–0.15 | .08 |
| Glomerulonephritis | 51 (8) | 0.03 | −0.05–0.11 | .4 |
| Tubulointerstitial nephritis | 71 (11) | −0.02 | −0.10–0.06 | .7 |
| Polycystic kidney disease | 130 (20) | −0.05 | −0.12–0.03 | .2 |
| Hypo- or dysplasia | 25 (4) | −0.07 | −0.15–0.01 | .07 |
| Renovascular disease | 37 (6) | −0.01 | −0.08–0.07 | .09 |
| Diabetes | 32 (5) | 0.03 | −0.04–0.11 | .4 |
| Other | 111 (17) | −0.02 | −0.10–0.06 | .6 |
| Cardiovascular parameters, mean ± SD | ||||
| Systolic BP (mmHg) | 135.9 ± 17.5 | 0.03 | −0.05–0.10 | .5 |
| Diastolic BP (mmHg) | 82.3 ± 10.9 | 0.05 | −0.03–0.12 | .2 |
| Mean arterial pressure (mmHg) | 106.9 ± 14.9 | 0.03 | −0.05–0.10 | .5 |
| Heart rate (bpm) | 68.6 ± 12.0 | −0.02 | −0.10–0.06 | .6 |
| Hypertensiona, n (%) | 261 (41) | 0.01 | −0.07–0.09 | .8 |
| Antihypertensive drugs, n (%) | 564 (88) | 0.08 | −0.15–0.32 | .5 |
| Lipids, mean ± SD | ||||
| Total cholesterol (mmol/l) | 5.14 ± 1.14 | −0.04 | −0.12–0.04 | .3 |
| HDL cholesterol (mmol/l) | 1.39 ± 0.48 | 0.03 | −0.05–0.11 | .5 |
| LDL cholesterol (mmol/l) | 2.99 ± 0.95 | −0.03 | −0.10–0.05 | .5 |
| Triglycerides (mmol/l), median (IQR) | 1.68 (1.25–2.29) | −0.03 | −0.11–0.05 | .5 |
| Statins, n (%) | 340 (53) | 0.04 | −0.11–0.20 | .6 |
| Glucose homeostasis, median (IQR) | ||||
| Glucose (mmol/l) | 5.3 (4.8–6.0) | 0.01 | −0.08–0.08 | .9 |
| HbA1c (%) | 5.8 (5.5–6.2) | 0.01 | −0.08–0.09 | .9 |
| Diabetes, n (%) | 149 (23) | −0.12 | −0.31–0.06 | .2 |
| Antidiabetic drugs, n (%) | 96 (15) | −0.05 | −0.27–0.16 | .6 |
| Transplantation-related | ||||
| Time since transplantation (years), median (IQR) | 5.3 (1.9–12.1) | −0.02 | −0.10–0.06 | .7 |
| >1 transplantation up to baseline, n (%) | 65 (10) | −0.32 | −0.57 to −0.06 | .02 |
| Pre-emptive transplantation (yes), n (%) | 99 (15) | 0.01 | −0.21–0.22 | .9 |
| Living donor, n (%) | 219 (34) | 0.23 | 0.07–0.39 | .006 |
| Donor age (years), mean ± SD | 43 ± 15 | 0.01 | −0.08–0.08 | .9 |
| Dialysis vintage (months), median (IQR) | 27 (9–53) | −0.02 | −0.10–0.06 | .6 |
| Cold ischaemia time (hours), mean ± SD | 14.2 ± 10.0 | −0.11 | −0.18 to −0.03 | .008 |
| Warm ischaemia time (minutes), mean ± SD | 43.2 ± 15.5 | 0.03 | −0.05–0.11 | .5 |
| HLA antibodies, n (%) | ||||
| HLA-I | 100 (16) | −0.16 | −0.37–0.05 | .1 |
| HLA-II | 113 (18) | −0.28 | −0.48 to −0.08 | .007 |
| History of rejection, n (%) | 169 (26) | −0.06 | −0.24–0.12 | .5 |
| Calcineurin inhibitors, n (%) | 371 (58) | −0.05 | −0.21–0.11 | .5 |
| Proliferation inhibitors, n (%) | 531 (83) | −0.05 | −0.26–0.16 | .6 |
| Kidney function and proteinuria | ||||
| eGFR (ml/min/1.73 m2)b, mean ± SD | 44 ± 18 | 0.07 | −0.01–0.14 | .1 |
| Urinary protein excretion (g/24 h), median (IQR) | 0.2 (0.0–0.4) | 0.01 | −0.07–0.08 | .9 |
| Proteinuria (>0.5 g/24 h), n (%) | 148 (23) | 0.05 | −0.13–0.24 | .6 |
| Tubular damage markers, median (IQR) | ||||
| Urinary LFABP (μg/24 h) | 2.2 (1.0–7.3) | 0.02 | −0.07–0.10 | .7 |
| Urinary EGF (ng/mg creatinine) | 4.0 (1.8–7.8) | 0.01 | −0.07–0.08 | .9 |
| Plasma NGAL (μg/l) | 172 (134–232) | −0.03 | −0.11–0.05 | .4 |
| Miscellaneous parameters | ||||
| Plasma sodium (mmol/l), mean ± SD | 141 ± 3 | −0.04 | −0.12–0.03 | .3 |
| Plasma potassium (mmol/l), mean ± SD | 4.0 ± 0.5 | −0.07 | −0.15–0.01 | .06 |
| Plasma urea (mmol/l), median (IQR) | 9.6 (7.3–13.4) | −0.06 | −0.13–0.02 | .2 |
| Plasma albumin (g/l), median (IQR) | 43 (41–45) | 0.04 | −0.03–0.12 | .3 |
| Urinary sodium excretion (mmol/24 h), mean ± SD | 157 ± 62 | 0.25 | 0.18–0.33 | <.001 |
| Urinary creatinine excretion (mmol/24 h), mean ± SD | 11.6 ± 3.4 | 0.26 | 0.19–0.34 | <.001 |
| Fractional sodium excretion (%), median (IQR) | 1.18 (0.85–1.61) | 0.04 | −0.07–0.15 | .5 |
| Inflammation and oxidative stress | ||||
| hs-CRP (mg/l), median (IQR) | 1.6 (0.8–4.6) | −0.03 | −0.12–0.05 | .4 |
| Malondialdehyde (μmol/l), median (IQR) | 2.5 (1.9–3.8) | −0.10 | −0.18 to −0.03 | .008 |
| Free sulfhydryl groups (μmol/l), mean ± SD | 130 ± 48 | 0.10 | 0.03–0.18 | .008 |
| FFQs, mean ± SD | ||||
| Energy intake (kcal/24 h) | 2161 ± 620 | 0.12 | 0.04–0.20 | .003 |
| Total protein intake (g/24 h) | 82 ± 13 | 0.08 | −0.01–0.16 | .06 |
| Plant protein intake (g/24 h) | 31 ± 6 | 0.09 | 0.01–0.17 | .02 |
| Animal protein intake (g/24 h) | 51 ± 13 | 0.03 | −0.05–0.11 | .4 |
| Total carbohydrate intake (g/24 h) | 248 ± 44 | 0.03 | −0.05–0.11 | .5 |
| Total fat intake (g/24 h) | 88 ± 17 | 0.04 | −0.04–0.12 | .3 |
| Vegetable intake (g/24 h) | 93 ± 57 | 0.05 | −0.03–0.13 | .2 |
| Fruit intake (g/24 h) | 151 ± 113 | 0.03 | −0.05–0.11 | .5 |
| Dairy intake (g/24 h) | 118 ± 84 | −0.04 | −0.12–0.04 | .4 |
| Fish intake (g/24 h), median (IQR) | 12 (4–21) | 0.06 | −0.01–0.12 | .07 |
| Meat intake (g/24 h) | 96 ± 38 | 0.02 | −0.07–0.09 | .7 |
| Water intake (g/24 h) | 2028 ± 474 | 0.23 | 0.15–0.30 | <.001 |
Hypertension defined as systolic BP >140 mmHg and/or diastolic BP >90 mmHg.
Assessed using the Chronic Kidney Disease Epidemiology Collaboration formula based on creatinine and cystatin C.
HDL, high-density lipoprotein; LDL, low-density lipoprotein; HbA1c, haemoglobin A1c; TIA, transient ischaemic attack; CVA, cerebrovascular accident.
Primary analyses
During a median follow-up of 5.3 years (IQR 4.5–6.0), 79 (12%) KTRs developed graft failure and 127 (20%) KTRs developed kidney function decline. An overview of Cox regression analyses of urinary lithium excretion with the risk of graft failure and kidney function decline is shown in Table 2. In univariable analyses, higher urinary lithium excretion was associated with a reduced risk of graft failure [HR per doubling of urinary lithium excretion 0.54 (95% CI 0.38–0.79); P = .002] as well as a reduced risk of kidney function decline [HR 0.73 (95% CI 0.54–0.99); P = .041]. The assumption of proportional hazards was not violated in any of the models. Comparisons of linear and non-linear models using an analysis of deviance table demonstrated that a linear fit was the optimal fit (comparison linear and non-linear P > .05). After adjusting for age, sex, BSA, eGFR and urinary protein excretion, urinary lithium excretion remained associated with a reduced risk of graft failure [HR 0.59 (95% CI 0.41–0.86); P = .006] and kidney function decline [HR 0.73 (95% CI 0.54–0.98); P = .031]. Further adjustment for potential confounders, including smoking status, alcohol intake, time between transplantation and baseline, the number of transplantations up to baseline, HLA antibodies, pre-emptive transplantation, deceased donor, donor age, history of rejection, warm ischaemia time, calcineurin inhibitor usage and proliferation inhibitor usage did not materially change the associations.
Table 2:
Cox regression analyses of urinary lithium excretion with graft failure and kidney function decline in 642 KTRs.
| Graft failure | Kidney function decline | |||
|---|---|---|---|---|
| Model | HR (95% CI) per log2 μmol/24 h | P-value | HR (95% CI) per log2 μmol/24 h | P-value |
| Model 1 | 0.54 (0.38–0.79) | .002 | 0.73 (0.54–0.99) | .041 |
| Model 2 | 0.55 (0.38–0.79) | .002 | 0.72 (0.53–0.97) | .028 |
| Model 3 | 0.59 (0.41–0.86) | .006 | 0.73 (0.54–0.98) | .031 |
| Model 4 | 0.59 (0.41–0.85) | .005 | 0.71 (0.53–0.95) | .021 |
| Model 5 | 0.62 (0.42–0.91) | .016 | 0.73 (0.54–1.00) | .054 |
| Events, n (%) | 79 (12) | 102 (16) | ||
Graft failure was defined as start of dialysis or retransplantation and kidney function decline was defined as a doubling of serum creatinine.
Urinary lithium excretion was log2 transformed prior to analyses.
Model 1: crude. Model 2: adjusted for age and sex. Model 3: model 2, additionally adjusted for BSA, eGFR (Chronic Kidney Disease Epidemiology Collaboration equation based on both serum creatine and cystatin C) and urinary protein excretion. Model 4: model 3, additionally adjusted for smoking status and alcohol intake. Model 5: model 3, additionally adjusted for time between transplantation and baseline, the number of transplantations up to baseline, HLA antibodies, pre-emptive transplantation, deceased donor, donor age, history of rejection, warm ischaemia time, calcineurin inhibitors and proliferation inhibitors.
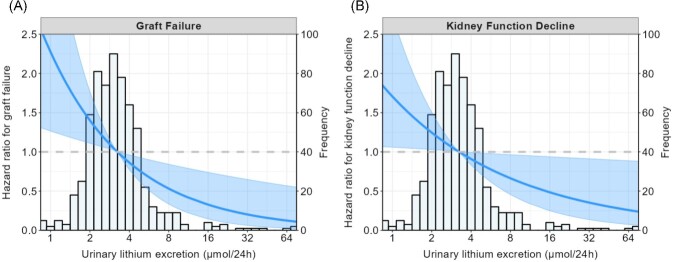
To assess whether the associations of urinary lithium excretion with graft failure and kidney function decline were driven by dietary intake, inflammation/oxidation, tubular damage or muscle mass, additional Cox regression analyses were performed by adjusting a base model (model 3) for dietary intakes (total energy intake, plant protein intake and water intake), inflammation and oxidation markers (hs-CRP, plasma malondialdehyde and free sulfhydryl groups) and tubular damage markers (EGF, LFABP and NGAL). These analyses are shown in Supplementary Table S3 and demonstrated that the association of urinary lithium excretion with graft failure and kidney function decline remained mostly significant despite these adjustments. A graphical representation of the association of urinary lithium excretion with the risk of graft failure and kidney function decline is shown in Fig. 1.
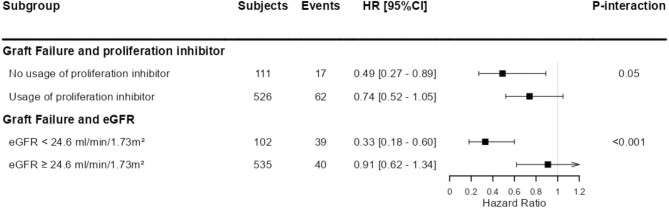
Figure 1:
Stratified analysis of the association of urinary lithium excretion with risk of graft failure, according to effect modifiers. P for interaction <.05 was considered to indicate a significant effect modification. Stratified Cox proportional hazards regression analyses were performed to assess the association of log2 transformed urinary lithium excretion with the risk of graft failure and kidney function decline according to significant effect modifiers. The used cut-offs for eGFR for stratified analyses were chosen to obtain a comparable number of events per group. Coefficient estimates are shown with adjustment for age, sex, BSA, eGFR and urinary protein excretion. The arrow indicates the upper limit of the CI is larger than the figure limit of 1.2.
The association between urinary lithium excretion and graft failure was modified by the use of proliferation inhibitors (P = .05) and eGFR (P < .001). Urinary lithium excretion was more strongly associated with graft failure in KTRs not using proliferation inhibitors and in KTRs with a lower baseline eGFR. For other covariates, there was no significant effect modification (all P > .05). An overview of stratified analyses according to effect modifiers is shown in Fig. 2.
Figure 2:
Graphical representation of the association of urinary lithium excretion with the risk of (A) graft failure and (B) kidney function decline. Graft failure was defined as the start of dialysis or retransplantation and kidney function decline was defined as a doubling of serum creatinine. The lines show the adjusted HR and the shaded area corresponds to the pointwise 95% CI. The analyses were adjusted for age, sex, BSA, eGFR and urinary protein excretion. P-values for effects are .006 and .03 for graft failure and kidney function decline, respectively.
Sensitivity analyses were performed to determine the robustness of the findings. The associations of urinary lithium excretion with graft failure remained materially unchanged after excluding KTRs with outlying values of urinary lithium excretion, an alcohol intake >30 g/24 h, current smokers, >70 years of age, a BMI <17.5 or >30 kg/m2, a dialysis vintage >6 years, multiple transplantations and a history of rejection (all P < .05). A summary of the sensitivity analyses is shown in Table 3. Lastly, analyses using urinary lithium concentration rather than urinary lithium excretion are shown in Supplementary Table S4. These analyses demonstrated that a higher urinary lithium concentration was associated with a reduced risk of graft failure.
Table 3:
Sensitivity analyses of the Cox regression analyses of urinary lithium excretion with graft failure and kidney function decline in 642 KTRs.
| Graft failure | Kidney function decline | ||||
|---|---|---|---|---|---|
| Excluded | n | HR (95% CI) per log2 μmol/24 h | P-value | HR (95% CI) per log2 μmol/24 h | P-value |
| None (base model) | 642 | 0.59 (0.41–0.86) | .006 | 0.73 (0.54–0.98) | .031 |
| Outliersa | 629 | 0.59 (0.39–0.91) | .019 | 0.81 (0.60–1.15) | .235 |
| Alcohol intake >30 g/24 h | 614 | 0.57 (0.39–0.83) | .004 | 0.76 (0.57–1.02) | .069 |
| Current smoker | 567 | 0.47 (0.30–0.72) | <.001 | 0.75 (0.54–1.03) | .077 |
| >70 years of age | 601 | 0.61 (0.42–0.88) | .009 | 0.71 (0.53–0.97) | .031 |
| BMI <17.5 or >30 kg/m2 | 505 | 0.65 (0.43–0.98) | .039 | 0.69 (0.49–0.97) | .033 |
| Dialysis vintage >6 years | 570 | 0.64 (0.44–0.92) | .018 | 0.71 (0.52–0.97) | .033 |
| Multiple transplantations | 577 | 0.62 (0.42–0.91) | .016 | 0.71 (0.51–0.99) | .042 |
| History of rejection | 473 | 0.48 (0.30–0.76) | .002 | 0.73 (0.52–1.03) | .071 |
Graft failure was defined as the start of dialysis or retransplantation and kidney function decline was defined as a doubling of serum creatinine.
Urinary lithium excretion was log2 transformed prior to analyses.
All analyses are adjusted for age, sex, BSA, eGFR and urinary protein excretion.
Outliers in lithium excretion were defined as deviating ≥3 SD from the mean after log2 transformation.
Secondary analyses
During a median follow-up of 5.4 years (IQR 4.8–6.1), 138 (21%) KTRs died. An overview of Cox regression analyses of urinary lithium excretion with the risk of all-cause mortality is shown in Table 4. Higher urinary lithium excretion was associated with a reduced risk of all-cause mortality [HR per doubling of urinary lithium excretion 0.64 (95% CI 0.49–0.83); P = .001]. The assumption of proportional hazards was not violated in any of the models. Comparisons of linear and non-linear models using an analysis of deviance table demonstrated that a linear fit was optimal (comparison of linear and non-linear P > .05). The effect of lithium excretion on the hazard of all-cause mortality was of comparable magnitude after adjustment for age, sex, BSA, eGFR and urinary protein excretion [HR 0.66 (95% CI 0.51–0.86); P = .003]. Further adjustment for potential confounders did not materially change the association for all-cause mortality.
Table 4:
Cox regression analyses of urinary lithium excretion with all-cause mortality.
| All-cause mortality | ||
|---|---|---|
| Model | HR (95% CI) per log2 μmol/24 h | P-value |
| Model 1 | 0.64 (0.49–0.83) | .001 |
| Model 2 | 0.64 (0.48–0.84) | .002 |
| Model 3 | 0.67 (0.51–0.87) | .003 |
| Model 4 | 0.66 (0.51–0.87) | .002 |
| Model 5 | 0.67 (0.51–0.90) | .007 |
| Events, n (%) | 138 (21) | |
Urinary lithium excretion was log2 transformed prior to analyses.
Model 1: crude. Model 2: adjusted for age and sex. Model 3: model 2, additionally adjusted for BSA, eGFR (Chronic Kidney Disease Epidemiology Collaboration equation based on both serum creatine and cystatin C) and urinary protein excretion. Model 4: model 3, additionally adjusted for smoking status and alcohol intake. Model 5: model 3, additionally adjusted for time between transplantation and baseline, the number of transplantations up to baseline, HLA antibodies, pre-emptive transplantation, deceased donor, donor age, history of rejection, warm ischaemia time, calcineurin inhibitors and proliferation inhibitors.
Cause-specific mortality analyses are shown in Supplementary Table S5. These analyses demonstrated that the association of urinary lithium excretion with mortality seems mostly based on infectious mortality. Analyses using urinary lithium concentration rather than urinary lithium excretion are shown in Supplementary Table S6. These analyses demonstrated that a higher urinary lithium concentration is borderline associated with lower all-cause mortality.
DISCUSSION
The primary finding of the current study is that higher urinary lithium excretion, reflecting a higher dietary lithium intake, is associated with a reduced risk of graft failure and kidney function decline in a large cohort of stable KTRs. The association of urinary lithium excretion and graft failure remained independent of potential confounders and was robust in sensitivity analyses. A secondary finding is that higher urinary lithium excretion is also associated with a reduced risk of all-cause mortality. The findings of the current study suggest that dietary lithium intake may be a potentially modifiable—yet rather overlooked—risk factor for adverse long-term kidney graft outcomes and patient survival.
Lithium is the lightest naturally occurring alkali metal in the earth's crust and uptake of trace amounts of lithium via foodstuffs [22] and water [48] is essential for physical and mental health, with experimentally induced lithium deficiency leading to chronic inflammation, abnormalities in reproduction and behaviour and higher mortality in animals [18]. In humans, lithium was detected in organs and foetal tissues in the late 19th century, leading to early suggestions of possible specific functions in humans [18]. However, medical applications overshadowed research on lithium as a potential micronutrient. Initially, lithium was used, with no success, as a treatment for gout and to dissolve bladder stones. Later it was successfully employed to treat bipolar disorder [18]. Despite many efforts of pharmaceutical companies to develop alternatives, it remains the most effective treatment for bipolar disorder to date. Unfortunately, however, it also comes with serious renal side effects. In up to 20% of lithium-treated patients, over the course of months to years, lithium can cause nephrogenic diabetes insipidus, a urinary concentrating defect [4]. Furthermore, depending on the dosage and treatment duration, over the course of many years, lithium therapy increases the chance of developing ESKD by 6- to 8-fold [49, 50]. While the exact mechanisms leading to ESKD are not fully elucidated, it is assumed that tubular atrophy and chronic interstitial fibrosis are essential steps. Furthermore, glomerulosclerosis, occurring after the onset of tubular atrophy and chronic interstitial fibrosis, as well as the development of renal microcysts have also been implicated [4, 51–53].
It should be noted, however, that daily intake of lithium in patients with bipolar disorder lies in an order of magnitude of 1000 mg, which is >40 000 times higher than the median dietary lithium intake of the KTRs in the current study (21 μg). Whereas chronic intake of such high lithium amounts may cause kidney damage, there is accumulating evidence from animal studies indicating that low lithium amounts are beneficial in preventing kidney damage caused by exposure to nephrotoxic compounds, inflammation and oxidative stress. In mice, a single dose of lithium (40 mg/kg) 3 days after cisplatin-induced acute kidney injury was able to promote renal tubular epithelia repopulation, improve kidney repair and accelerate renal function recovery [5]. In another murine study, lithium therapy (40 mg/kg) improved podocyte injury and proteinuria in mice treated with lipopolysaccharide or adriamycin, demonstrated by reduced albuminuria and concomitant suppressed expression of podocytopathic mediators B7-1 and MCP-1 [6]. Yet another study in mice demonstrated that mice treated with lithium (40 mg/kg) showed decreased mortality, decreased renal tubular dilation and decreased renal cell apoptosis in response to endotoxemia [9]. Furthermore, a study in rats demonstrated that lithium injection (50 mg/kg) 30 minutes before ischaemia–reperfusion was able to reduce renal oxidative stress by reducing the mitochondrial membrane depolarization and production of reactive oxygen species (ROS) [11]. In each of the aforementioned animal studies, the authors speculated that the renoprotective effects of lithium are attributable to inhibition of GSK3β, a key transducer involved in a large number of cellular signalling pathways. Inhibition of GSK3β may increase cell repair by activating pro-proliferative pathways, leading to, among others, increased expression of cyclinD1, c-Myc and HIF-1α [54]. Inhibition of GSK3β may also reduce the inflammation by fine-tuning nuclear factor κB expression, a mediator of pro-inflammatory gene transcription [55]. Furthermore, GSK3β inhibitors have been shown to prevent ROS-induced apoptosis of mesangial cells and proximal epithelial cells [56, 57]. Lastly, a very recent study in mice demonstrated that targeted inhibition of GSK3β in podocytes via pharmacologic blockade with lithium was able to intercept the senescence signalling, mitigate podocyte senescence and slow kidney aging. Higher nutritional levels of lithium may possibly improve the long-term outcome of the graft kidney via anti-aging activity. Although the studied lithium dosages in the aforementioned studies are lower than the regular lithium dosage for bipolar disorder, they are still much higher than the environmental intake of lithium.
To date, no intervention studies have been published investigating the effect of lithium therapy on preventing kidney injury in humans. It is worth noting, however, that one clinical trial is currently being performed, investigating whether lithium treatment can prevent cardiac surgery–induced acute kidney injury [58]. It is of great interest to know whether variations in dietary lithium intake are also associated with renoprotection, especially for KTRs, for whom long-term risk of graft failure remains high, thus necessitating the search for novel potentially modifiable risk factors. To our knowledge, this is the first study demonstrating a relationship between dietary lithium intake and long-term kidney graft survival. Higher urinary lithium excretion was associated with a reduced risk of graft failure and kidney function decline, independent of potential confounders. Furthermore, these associations were shown to be robust in multiple sensitivity analyses. Upon assessment of the HRs, it becomes clear that urinary lithium excretion is more strongly associated with graft failure than with doubling of serum creatinine. The decision to start with dialysis or transplantation is multifactorial and cannot be explained by serum creatinine alone [38]. Start of dialysis or transplantation, which is equivalent to graft failure as an outcome in KTRs is less susceptible to bias by non-GFR factors than the change in serum creatinine as an outcome. The reason is that advancing of kidney failure is often accompanied by protein energy wasting and loss of muscle mass, leading to underestimation of the true decline in kidney function if it is evaluated through the change in serum creatinine. For that reason, authoritative institutions have advised using independent markers of muscle mass for estimation of GFR, like cystatin C, as an alternative for serum creatinine if it has not been excluded that an intervention or exposure under evaluation for a potential effect on the change in kidney function can also have an effect on muscle mass [59, 60]. Importantly, for lithium as an exposure, this cannot be excluded because lithium is known as an inhibitor of GSK3β [61], which in turn is a known inhibitor of development of muscle hypertrophy, together suggesting that lithium could play role in the preservation of muscle mass [62, 63], potentially explaining the difference in HRs between the two primary outcomes. Unfortunately, we do not have data on doubling of serum cystatin C, so it is not possible to perform the recommended analysis alternative to doubling of serum creatinine.
Due to the observational nature, we were unable to directly investigate the underlying mechanisms of the associations of urinary lithium excretion with graft failure and kidney function decline. Yet, using linear regression, an inverse association was found between urinary lithium excretion and plasma malondialdehyde, a biomarker in which higher concentrations reflect more systemic oxidative stress [64]. Furthermore, a positive association was found between urinary lithium excretion and free sulfhydryl (thiol) groups of organosulfur compounds, which have been established as robust and accurate biomarkers for systemic redox status [65–67]. Higher levels of free thiols are generally representative of a more favourable redox status. Since circulating ROS lead to the oxidation of sulfhydryl groups, lower free sulfhydryl groups are indicative of systemic oxidative stress. The aforementioned associations imply that higher urinary lithium excretion may be associated with less oxidative stress and points toward oxidative stress as a potential mechanism underlying the association of urinary lithium excretion with graft failure and kidney function decline. It is unknown, however, whether dietary lithium intake can also exert effects through GSK3β inhibition or whether the underlying mechanisms of the found associations are different altogether.
Dietary lithium comes mostly from drinking water, grains, vegetables and animal-derived foods [18]. In general, diets rich in grains and vegetables may be expected to provide more lithium than diets rich in animal proteins. However, due to the uneven distribution of lithium in the earth's crust, a predominantly vegetarian diet is not necessarily lithium rich [18]. Accordingly, the estimated dietary lithium intake varies greatly by country. The estimated dietary lithium intake in the current study (21 μg/24 h) was lower than the dietary lithium intake in China (1560 μg/24 h), Mexico (1485 μg/24 h) and Sweden (1090 μg/24 h), yet higher than the dietary of lithium intake in Poland (10.7 μg/24 h) and Belgium (8.6 μg/24 h) [18, 22, 68]. While these data should be interpreted with a large degree of prudence, it clearly demonstrates that dietary lithium intake greatly differs by country. Although there is no well-established recommended dietary allowance (RDA), it has been stated that the minimal human adult lithium requirement is ≈100 μg/24 h, but higher intakes may be necessary for beneficial effects [18]. Schrauzer et al. [18] proposed an RDA of 1 mg/24 h for a 70-kg adult, corresponding to 14.3 mg/24 h/kg bodyweight. With 95% of dietary lithium intake being excreted renally, in the current cohort only 16 (2%) KTRs would have an intake >100 μg/24 h and none would reach the RDA proposed by Schrauzer et al. While the dietary lithium intake in the current cohort can be considered relatively low, it is unclear what constitutes a deficiency, given the lack of sufficient research into the dietary lithium requirements of humans, let alone a specific population such as KTRs
Using FFQ data, in the current study a positive association was found between urinary lithium excretion and total energy, plant protein and water intake. Yet, despite adjusting for these dietary intakes in the Cox regression analyses, the association of urinary lithium excretion with graft failure and kidney function decline remained significant. The association between urinary lithium excretion and water intake led us to perform additional sensitivity analyses using urinary lithium concentration rather than urinary lithium excretion to uncover whether the effects might have been mediated by urinary volume. These analyses demonstrated that a higher urinary lithium concentration was also associated with a lower risk of graft failure and kidney function decline.
Analyses on potential effect mediation demonstrated multiple effect mediators, including the use of proliferation inhibitors. Higher lithium excretion was more strongly associated with a reduced risk of graft failure in patients not using proliferation inhibitors. One speculative mechanism could be that patients on proliferation inhibitors already have less active GSK3β, as mycophenolate is known to increase the amount of phosphorylated GSK3β, which is the inactive form of GSK3β [69]. Furthermore, our interaction analyses also demonstrated that the association of urinary lithium excretion was strongest in KTRs with a lower baseline eGFR. A similar effect modification was found in the association between urinary lithium excretion and kidney function decline. These findings appear to show that higher dietary lithium intake is especially beneficial in patients with lower kidney function, who are already at a higher risk of graft failure.
The analyses of the current study demonstrate that higher urinary lithium excretion is associated with a reduced risk of all-cause mortality, independent of the potential confounders included in this study. This finding is in alignment with previous cohort studies in the general population that have demonstrated an inverse association between lithium concentrations in drinking water and the risk of all-cause mortality [15].
Strengths of the current study include the large sample size of this well-defined and specific patient group of KTRs and the long follow-up on graft and patient survival. In addition, extensive data collection of many demographic and laboratory parameters enabled adjustment for many potential confounders. However, several limitations of this study need to be addressed. Due to the observational design of this study, we were unable to investigate whether the relationship between urinary lithium excretion and graft and patient outcomes in KTRs is causal or associative. Similarly, the observational design of this study does not allow for further studies to elucidate the biological mechanisms underlying the association of urinary lithium excretion and graft and patient outcomes. Also, unfortunately we did not have data on doubling of serum cystatin C as an outcome. Furthermore, we were unable to adjust for the expanded criteria donor status due to the unavailability of data necessary to define this status other than donor age. It is also important to note that even though we adjusted for many potential confounders, the possibility of residual confounding remains. Lastly, while it is known that the lithium concentration of inland surface water in the Netherlands is relatively low (3.5 μg/l [70], as compared with 18 μg/l in the USA [71]), we did not have access to data on geographical differences in water concentrations of lithium, which could be a potential confounder.
CONCLUSION
In conclusion, the present study shows that in a Dutch cohort of outpatient KTRs, higher urinary lithium excretion, reflecting higher dietary lithium intake, was independently and robustly associated with a reduced risk of graft failure and kidney function decline. The association with graft failure was strongest in KTRs not using proliferation inhibitors and KTRs with a lower baseline eGFR. Furthermore, higher urinary lithium excretion was also associated with a reduced risk of all-cause mortality. Lithium intake may be a potentially modifiable—yet rather overlooked—risk factor for adverse long-term kidney graft endpoints and patient survival. Whether stimulating dietary lithium intake or low-dose lithium supplementation may represent a novel risk management strategy to decrease the burden of long-term kidney graft failure and premature mortality remains to be investigated in interventional studies.
FUNDING
The generation of this cohort and its underlying biobank, known as the TransplantLines Food and Nutrition Biobank and Cohort Study (trial registration NCT02811835), was funded by the Top Institute Food and Nutrition (grant A-1003).
Supplementary Material
Contributor Information
Adrian Post, Department of Internal Medicine, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands.
Daan Kremer, Department of Internal Medicine, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands.
Dion Groothof, Department of Internal Medicine, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands.
Ulrike Seidel, Institute of Human Nutrition and Food Science, University of Kiel, Kiel, Germany.
Patricia Huebbe, Institute of Human Nutrition and Food Science, University of Kiel, Kiel, Germany.
Casper F M Franssen, Department of Internal Medicine, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands.
Ido P Kema, Department of Laboratory Medicine, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands.
Kai Lüersen, Institute of Human Nutrition and Food Science, University of Kiel, Kiel, Germany.
Gerald Rimbach, Institute of Human Nutrition and Food Science, University of Kiel, Kiel, Germany.
Stephan J L Bakker, Department of Internal Medicine, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands; Prinicipal Investigator of the TransplantLines cohort studies.
AUTHORS’ CONTRIBUTIONS
A.P. and S.J.L.B. designed the study, wrote the manuscript and had primary responsibility for the final content. K.L., U.S., P.H. and G.R. performed the laboratory analysis. A.P. analysed the data. A.P., D.K., D.G., U.S., P.H., C.F.M.F., I.P.K., K.L., G.R. and S.J.L.B. revised and edited the manuscript. All authors substantially contributed to the study and manuscript design, data analyses, data interpretation and/or revision and approved the final version of the work. The authors have agreed to take accountability for all aspects of this study.
DATA AVAILABILITY STATEMENT
Data described in the manuscript, code book and analytic code will be made available upon request.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Abramowicz D, Oberbauer R, Heemann Uet al. Recent advances in kidney transplantation: a viewpoint from the Descartes advisory board. Nephrol Dial Transplant 2018;33:1699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jardine AG, Gaston RS, Fellstrom BCet al. Prevention of cardiovascular disease in adult recipients of kidney transplants. Lancet 2011;378:1419–27. [DOI] [PubMed] [Google Scholar]
- 3. Lamb KE, Lodhi S, HU Meier-Kriesche. Long-term renal allograft survival in the United States: a critical reappraisal. Am J Transplant 2011;11:450–62. [DOI] [PubMed] [Google Scholar]
- 4. Alsady M, Baumgarten R, Deen PMTet al. Lithium in the kidney: friend and foe? J Am Soc Nephrol 2016;27:1587–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bao H, Ge Y, Wang Zet al. Delayed administration of a single dose of lithium promotes recovery from AKI. J Am Soc Nephrol 2014;25:488–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bao H, Ge Y, Peng Aet al. Fine-tuning of NFκB by glycogen synthase kinase 3β directs the fate of glomerular podocytes upon injury. Kidney Int 2015;87:1176–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xu W, Ge Y, Liu Zet al. Glycogen synthase kinase 3β dictates podocyte motility and focal adhesion turnover by modulating paxillin activity implications for the protective effect of low-dose lithium in podocytopathy. Am J Pathol 2014;184:2742–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Plotnikov EY, Grebenchikov OA, Babenko VAet al. Nephroprotective effect of GSK-3β inhibition by lithium ions and δ-opioid receptor agonist dalargin on gentamicin-induced nephrotoxicity. Toxicol Lett 2013;220:303–8. [DOI] [PubMed] [Google Scholar]
- 9. Wang Y, Huang WC, Wang CYet al. Inhibiting glycogen synthase kinase-3 reduces endotoxaemic acute renal failure by down-regulating inflammation and renal cell apoptosis. Br J Pharmacol 2009;157:1004–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lenz SP, Izui S, Benediktsson Het al. Lithium chloride enhances survival of NZB/W lupus mice: influence of melatonin and timing of treatment. Int J Immunopharmacol 1995;17:581–92. [DOI] [PubMed] [Google Scholar]
- 11. Plotnikov EY, Kazachenko AV, Vyssokikh MYet al. The role of mitochondria in oxidative and nitrosative stress during ischemia/reperfusion in the rat kidney. Kidney Int 2007;72:1493–502. [DOI] [PubMed] [Google Scholar]
- 12. Talab SS, Emami H, Elmi Aet al. Chronic lithium treatment protects the rat kidney against ischemia/reperfusion injury: the role of nitric oxide and cyclooxygenase pathways. Eur J Pharmacol 2010;647:171–7. [DOI] [PubMed] [Google Scholar]
- 13. Fang Y, Chen B, Liu Zet al. Age-related GSK3β overexpression drives podocyte senescence and glomerular aging. J Clin Invest 2022;132:e141848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Memon A, Rogers I, Fitzsimmons SMDDet al. Association between naturally occurring lithium in drinking water and suicide rates: systematic review and meta-analysis of ecological studies. Br J Psychiatry, 2020;217:667–78. [DOI] [PubMed] [Google Scholar]
- 15. Zarse K, Terao T, Tian Jet al. Low-dose lithium uptake promotes longevity in humans and metazoans. Eur J Nutr 2011;50:387–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shim JS, Oh K, Kim HC.. Dietary assessment methods in epidemiologic studies. Epidemiol Health 2014;36:e2014009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Naska A, Lagiou A, Lagiou P.. Dietary assessment methods in epidemiological research: current state of the art and future prospects. F1000Res 2017;6:926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schrauzer GN. Lithium: occurrence, dietary intakes, nutritional essentiality. J Am Coll Nutr 2002;21:14–21. [DOI] [PubMed] [Google Scholar]
- 19. Boer WH, Fransen R, Shirley DGet al. Evaluation of the lithium clearance method: direct analysis of tubular lithium handling by micropuncture. Kidney Int 1995;47:1023–30. [DOI] [PubMed] [Google Scholar]
- 20. Amdisen A. Serum level monitoring and clinical pharmacokinetics of lithium. Clin Pharmacokinet 1977;2:73–92. [DOI] [PubMed] [Google Scholar]
- 21. Trautner EM, Morris R Noack, CHet al. The excretion and retention of ingested lithium and its effect on the ionic balance of man. Med J Aust 1955;2:280–91. [PubMed] [Google Scholar]
- 22. Szklarska D, Rzymski P.. Is lithium a micronutrient? From biological activity and epidemiological observation to food fortification. Biol Trace Elem Res 2019;189:18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van den Berg E, Pasch A, Westendorp WHet al. Urinary sulfur metabolites associate with a favorable cardiovascular risk profile and survival benefit in renal transplant recipients. J Am Soc Nephrol 2014;25:1303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van den Berg E, Engberink MF, Brink EJet al. Dietary acid load and metabolic acidosis in renal transplant recipients. Clin J Am Soc Nephrol 2012;7:1811–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Post A, Said MY, Gomes-Neto AWet al. Urinary 3-hydroxyisovaleryl carnitine excretion, protein energy malnutrition and risk of all-cause mortality in kidney transplant recipients: results from the TransplantLines cohort studies. Clin Nutr 2021;40:2109–20. [DOI] [PubMed] [Google Scholar]
- 26. von Elm E, Altman DG, Egger Met al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007;147:573–7. [DOI] [PubMed] [Google Scholar]
- 27. D Du Bois, EF Du Bois. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 1989;5:303–11; discussion 312–3. [PubMed] [Google Scholar]
- 28. van den Berg E, Geleijnse JM, Brink EJet al. Sodium intake and blood pressure in renal transplant recipients. Nephrol Dial Transplant 2012;27:3352–9. [DOI] [PubMed] [Google Scholar]
- 29. American Diabetes Association . 2. Classification and diagnosis of diabetes. Diabetes Care 2017;40(Suppl 1):S11–24. [DOI] [PubMed] [Google Scholar]
- 30. Yepes-Calderón M, Sotomayor CG, Neto AWGet al. Plasma malondialdehyde and risk of new-onset diabetes after transplantation in renal transplant recipients: a prospective cohort study. J Clin Med 2019;8:453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Inker LA, Schmid CH, Tighiouart Het al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012;367:20–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Terpos E, Christoulas D, Kastritis Eet al. The Chronic Kidney Disease Epidemiology Collaboration cystatin C (CKD-EPI-CysC) equation has an independent prognostic value for overall survival in newly diagnosed patients with symptomatic multiple myeloma; is it time to change from MDRD to CKD-EPI-CysC equations? Eur J Haematol 2013;91:347–55. [DOI] [PubMed] [Google Scholar]
- 33. Seidel U, Jans K, Hommen Net al. Lithium content of 160 beverages and its impact on lithium status in drosophila melanogaster. Foods 2020;9:795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Feunekes GI, van Staveren WA, de Vries JHet al. Relative and biomarker-based validity of a food-frequency questionnaire estimating intake of fats and cholesterol. Am J Clin Nutr 1993;58:489–96. [DOI] [PubMed] [Google Scholar]
- 35. van den Berg E, Engberink MF, Brink EJet al. Dietary protein, blood pressure and renal function in renal transplant recipients. Br J Nutr 2013;109:1463–70. [DOI] [PubMed] [Google Scholar]
- 36. National Institute for Public Health and the Environment . Dutch Food Composition Table of 2006. Bilthoven: National Institute for Public Health and the Environment, 2013. [Google Scholar]
- 37. Willett WC, Howe GR, Kushi LH.. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997;65(4 Suppl):1220S–8S; discussion 1229S–31S. [DOI] [PubMed] [Google Scholar]
- 38. Weldegiorgis M, de Zeeuw D, Heerspink HJL.. Renal end points in clinical trials of kidney disease. Curr Opin Nephrol Hypertens 2015;24:284–9. [DOI] [PubMed] [Google Scholar]
- 39. Kasiske BL, Vazquez MA, Harmon WEet al. Recommendations for the outpatient surveillance of renal transplant recipients. J Am Soc Nephrol 2000;11(Suppl 15):S1–86. [PubMed] [Google Scholar]
- 40. Minovic I, van der Veen A, van Faassen Met al. Functional vitamin B-6 status and long-term mortality in renal transplant recipients. Am J Clin Nutr 2017;106:1366–74. [DOI] [PubMed] [Google Scholar]
- 41. Weiner MG, Livshits A, Carozzoni Cet al. Derivation of malignancy status from ICD-9 codes. AMIA Annu Symp Proc 2003;2003:1050. [PMC free article] [PubMed] [Google Scholar]
- 42. Deen CPJ, van der Veen A, Gomes-Neto AWet al. Urinary excretion of N(1)-methylnicotinamide and N(1)-methyl-2-pyridone-5-carboxamide and mortality in kidney transplant recipients. Nutrients 2020;12:2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bennette C, Vickers A.. Against quantiles: categorization of continuous variables in epidemiologic research, and its discontents. BMC Med Res Method 2012;12:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Greenland S. Avoiding power loss associated with categorization and ordinal scores in dose-response and trend analysis. Epidemiology 1995;6:450–4. [DOI] [PubMed] [Google Scholar]
- 45. Sterne JA, White IR, Carlin JBet al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Harel O, Zhou XH.. Multiple imputation: review of theory, implementation and software. Stat Med 2007;26:3057–77. [DOI] [PubMed] [Google Scholar]
- 47. Harrell Jr FE, Lee KL, Mark DB.. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15:361–87. [DOI] [PubMed] [Google Scholar]
- 48. Seidel U, Baumhof E, Hägele FAet al. Lithium-rich mineral water is a highly bioavailable lithium source for human consumption. Mol Nutr Food Res 2019;63:e1900039. [DOI] [PubMed] [Google Scholar]
- 49. Bendz H, Schön S, Attman POet al. Renal failure occurs in chronic lithium treatment but is uncommon. Kidney Int 2010;77:219–24. [DOI] [PubMed] [Google Scholar]
- 50. Aiff H, Attman PO, Aurell Met al. Effects of 10 to 30 years of lithium treatment on kidney function. J Psychopharmacol 2015;29:608–14. [DOI] [PubMed] [Google Scholar]
- 51. Markowitz GS, Radhakrishnan J, Kambham Net al. Lithium nephrotoxicity: a progressive combined glomerular and tubulointerstitial nephropathy. J Am Soc Nephrol 2000;11:1439–48. [DOI] [PubMed] [Google Scholar]
- 52. Ottosen PD, Sigh B, Kristensen Jet al. Lithium induced interstitial nephropathy associated with chronic renal failure. Reversibility and correlation between functional and structural changes. Acta Pathol Microbiol Immunol Scand A 1984;92:447–54. [DOI] [PubMed] [Google Scholar]
- 53. Walker RJ, Leader JP, Bedford JJet al. Chronic interstitial fibrosis in the rat kidney induced by long-term (6-mo) exposure to lithium. Am J Physiol Renal Physiol 2013;304:F300–7. [DOI] [PubMed] [Google Scholar]
- 54. Howard C, Tao S, Yang HCet al. Specific deletion of glycogen synthase kinase-3β in the renal proximal tubule protects against acute nephrotoxic injury in mice. Kidney Int 2012;82:1000–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sanz AB, Sanchez-Niño MD, Ramos AMet al. NF-κB in renal inflammation. J Am Soc Nephrol 2010;21:1254–62. [DOI] [PubMed] [Google Scholar]
- 56. Wang Z, Havasi A, Gall Jet al. GSK3β promotes apoptosis after renal ischemic injury. J Am Soc Nephrol 2010;21:284–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lin CL, Wang JY, Huang YTet al. Wnt/β-catenin signaling modulates survival of high glucose-stressed mesangial cells. J Am Soc Nephrol 2006;17:2812–20. [DOI] [PubMed] [Google Scholar]
- 58. Sharif S, Chen B, Brewster Pet al. Rationale and design of assessing the effectiveness of short-term low-dose lithium therapy in averting cardiac surgery-associated acute kidney injury: a randomized, double blinded, placebo controlled pilot trial. Front Med (Lausanne) 2021;8:639402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Levey AS, Inker LA, Matsushita Ket al. GFR decline as an end point for clinical trials in CKD: a scientific workshop sponsored by the national kidney foundation and the US food and drug administration. Am J Kidney Dis, 2014;64:821–35. [DOI] [PubMed] [Google Scholar]
- 60. Levey AS, Gansevoort RT, Coresh Jet al. Change in albuminuria and GFR as end points for clinical trials in early stages of CKD: a scientific workshop sponsored by the National Kidney Foundation in collaboration with the US Food and Drug Administration and European Medicines Agency. Am J Kidney Dis 2020;75:84–104. [DOI] [PubMed] [Google Scholar]
- 61. Freland L, Beaulieu JM.. Inhibition of GSK3 by lithium, from single molecules to signaling networks. Front Mol Neurosci 2012;5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rommel C, Bodine SC, Clarke BAet al. Mediation of IGF-1-induced skeletal myotube hypertrophy by Pl(3)K/Alt/mTOR and Pl(3)K/Akt/GSK3 pathways. Nat Cell Biol 2001;3:1009–13. [DOI] [PubMed] [Google Scholar]
- 63. Vyas DR, Spangenburg EE, Abraha TWet al. GSK-3β negatively regulates skeletal myotube hypertrophy. Am J Physiol Cell Physiol 2002;283:C545–51. [DOI] [PubMed] [Google Scholar]
- 64. Nielsen F, Mikkelsen BB, Nielsen JBet al. Plasma malondialdehyde as biomarker for oxidative stress: reference interval and effects of life-style factors. Clin Chem 1997;43:1209–14. [PubMed] [Google Scholar]
- 65. Turell L, Radi R, Alvarez B.. The thiol pool in human plasma: the central contribution of albumin to redox processes. Free Radic Biol Med 2013;65:244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cortese-Krott MM, Koning A, Kuhnle GGCet al. The reactive species interactome: evolutionary emergence, biological significance, and opportunities for redox metabolomics and personalized medicine. Antioxid Redox Signal 2017;27:684–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Banne AF, Amiri A, Pero RW.. Reduced level of serum thiols in patients with a diagnosis of active disease. J Anti Aging Med 2003;6:327–34. [DOI] [PubMed] [Google Scholar]
- 68. van Cauwenbergh R, Hendrix P, Robberecht Het al. Daily dietary lithium intake in Belgium using duplicate portion sampling. Eur Food Res Technol 1999;208:153–5. [Google Scholar]
- 69. Ryu S, Lee Y, Hyun MYet al. Mycophenolate antagonizes IFN-γ-induced catagen-like changes via β-catenin activation in human dermal papilla cells and hair follicles. Int J Mol Sci 2014;15:16800–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Osté, L. Derivation of dissolved background concentrations in Dutch surface water based on a 10th percentile of monitoring data. https://rvs.rivm.nl/sites/default/files/2018-05/1206111-005-BGS-0005-DEF-Derivation%20of%20dissolved%20background%20concentrations%20in%20Dutch%20surface%20water%20based%20on%20a%2010th%20percentile%20-%20achtergrondconcentraties%20metalen_0.pdf (29 January 2023, date last accessed). [Google Scholar]
- 71. Sharma N, Westerhoff P, Zeng C.. Lithium occurrence in drinking water sources of the United States. Chemosphere 2022;305:135458. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book and analytic code will be made available upon request.