ABSTRACT
Background
The European Renal Association (ERA) Registry collects data on kidney replacement therapy (KRT) in patients with ESKD. This paper is a summary of the ERA Registry Annual Report 2020, also including comparisons among primary renal disease (PRD) groups.
Methods
Data were collected from 52 national and regional registries from 34 European countries and countries bordering the Mediterranean Sea: 35 registries from 18 countries providing individual level data and 17 registries from 17 countries providing aggregated data. Using this data, KRT incidence and prevalence, kidney transplantation rates, expected remaining lifetimes and survival probabilities were calculated.
Results
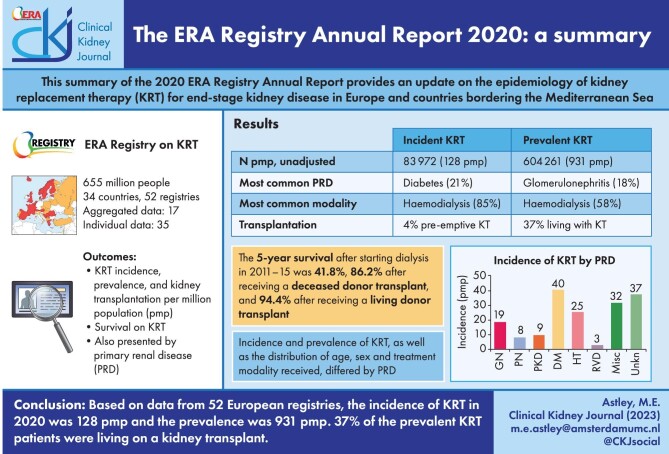
A general population of 654.9 million people was covered by the ERA Registry in 2020. The overall incidence of KRT was 128 per million population (p.m.p.). In incident KRT patients, 54% were older than 65 years, 63% were men and the most common PRD was diabetes mellitus (21%). Regarding initial treatment modality in incident patients, 85% received haemodialysis (HD), 11% received peritoneal dialysis (PD) and 4% received a pre-emptive kidney transplant. On 31 December 2020, the prevalence of KRT was 931 p.m.p. In prevalent patients, 45% were older than 65 years, 60% were men and glomerulonephritis was the most common PRD (18%). Of these patients, 58% were on HD, 5% on PD and 37% were living with a kidney transplant. The overall kidney transplantation rate in 2020 was 28 p.m.p., with a majority of kidney grafts from deceased donors (71%). The unadjusted 5-year survival, based on incident dialysis patient from 2011–15, was 41.8%. For patients having received a deceased donor transplant, the unadjusted 5-year survival probability was 86.2% and for patients having received a living donor transplant it was 94.4%. When comparing data by PRD group, differences were found regarding the distribution of age groups, sex and treatment modality received.
Keywords: dialysis, epidemiology, ESKD, kidney transplantation, patient survival
Graphical Abstract
Graphical Abstract.
INTRODUCTION
The European Renal Association (ERA) Registry Annual Report 2020 [1] (Supplementary data) provides updates on the epidemiology of kidney replacement therapy (KRT) for end-stage kidney disease (ESKD) in Europe and countries bordering the Mediterranean Sea. This paper will provide a summary of these data. Individual or aggregated data were provided by 52 national or regional registries from 34 countries. Thirty-five registries from 18 countries provided individual level data, the remaining registries provided aggregated data. The general population covered by the participating registries was 654.9 million people, 76.8% of the total European population. This article will present data on the incidence and prevalence of KRT, and kidney transplantation rates in 2020. Additionally, patient and graft survival using data from 2011–18 will be presented. For the first time we will also present comparisons across primary renal disease (PRD) groups. Information on methods used for analysis are described in the ERA Registry Annual Report 2020.
RESULTS
KRT incidence
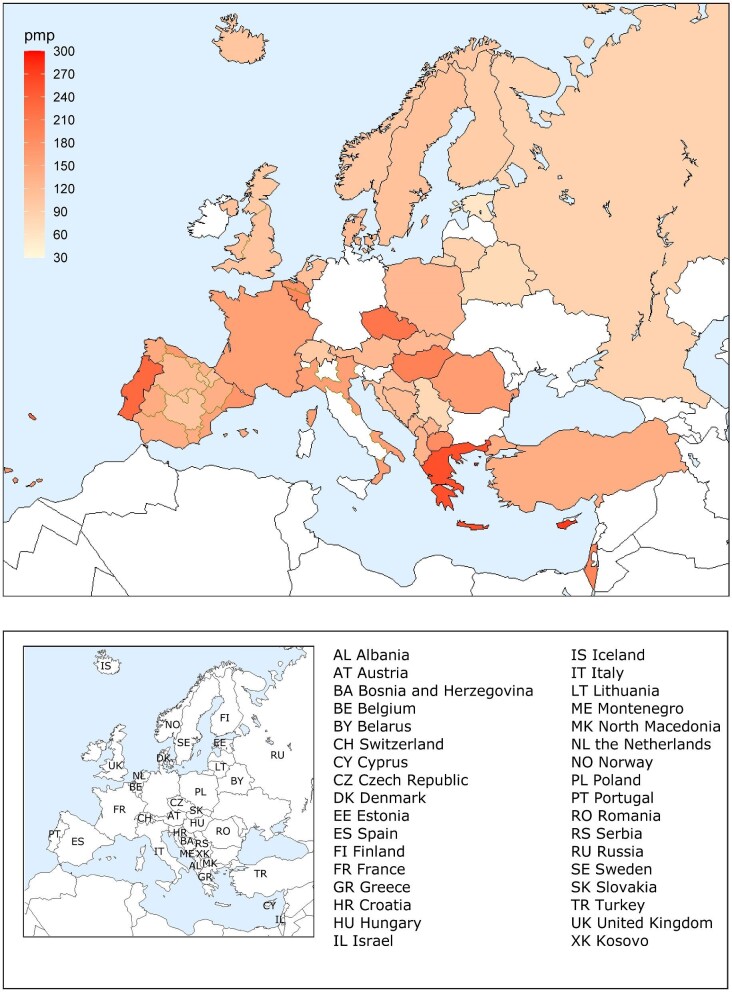
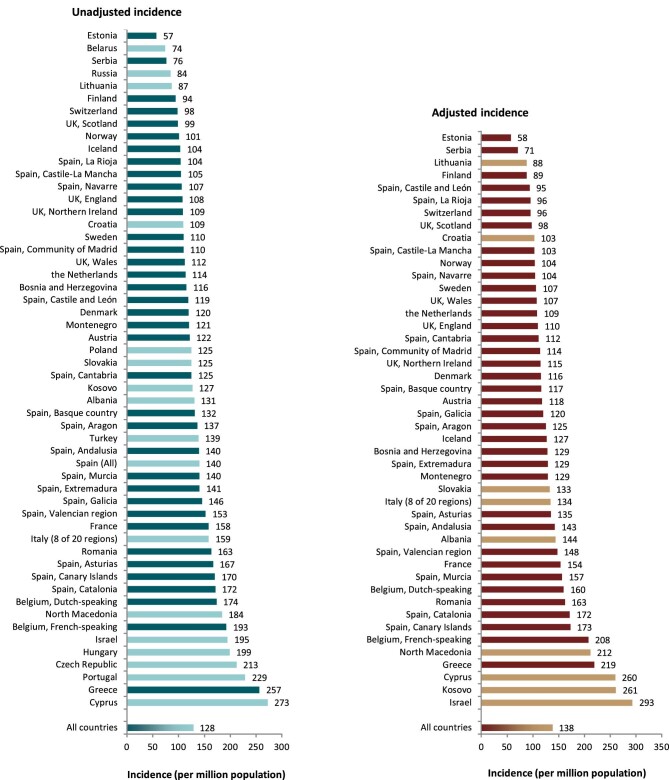
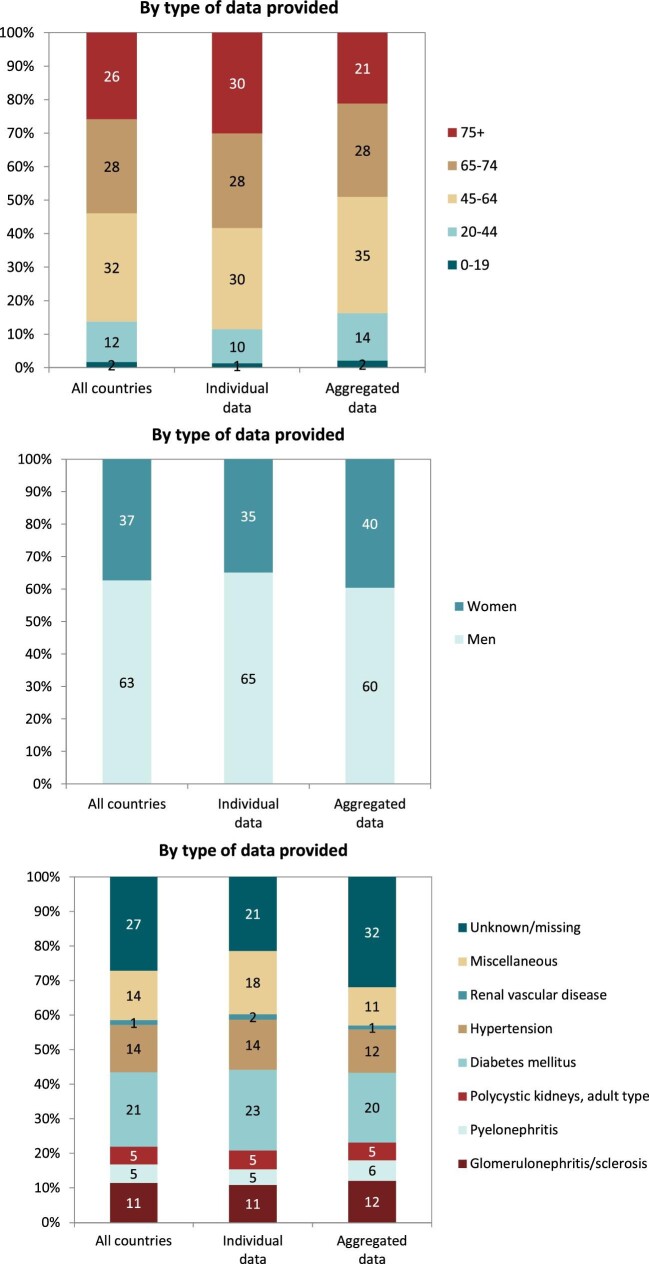
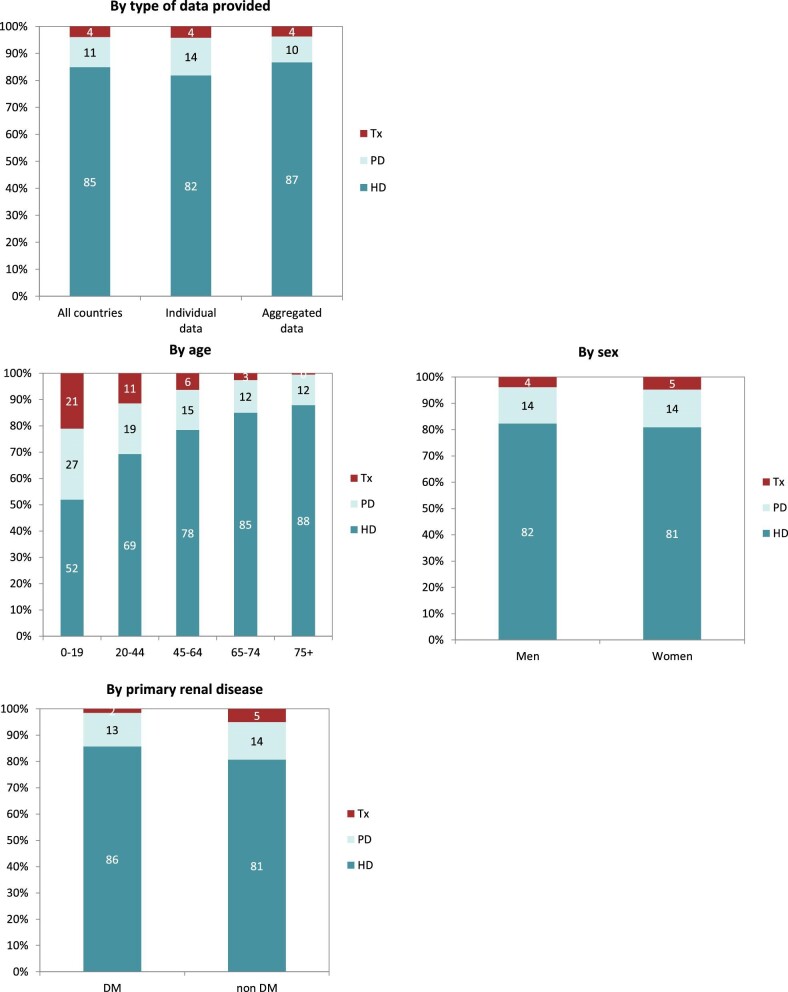
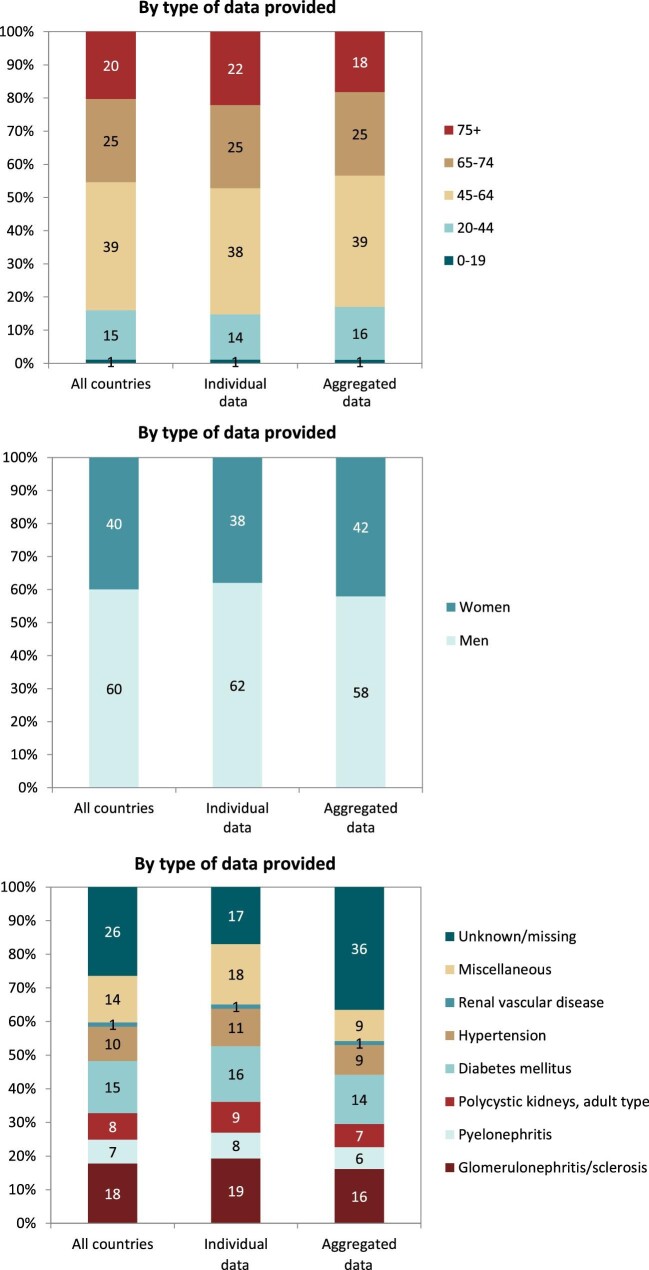
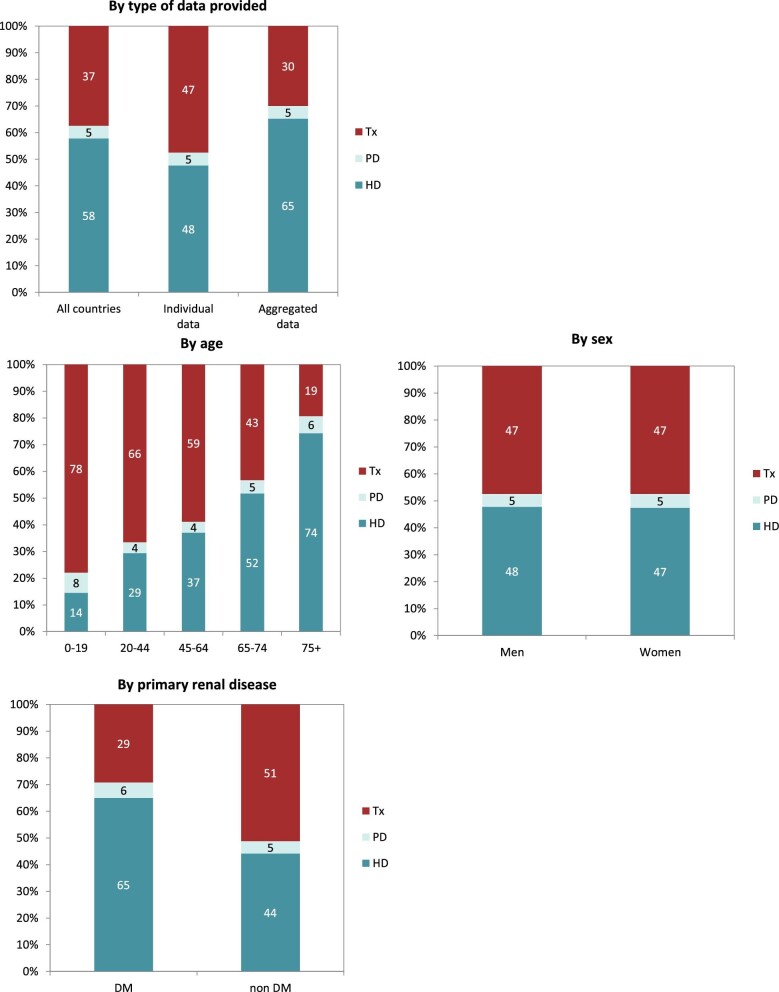
Out of a population of 654.9 million patients, 83 972 patients with ESKD initiated KRT. This corresponds to a KRT incidence rate of 128 per million population (p.m.p.) or about 1 per 7800 Europeans (Table 1). This was slightly lower compared with the KRT incidence rate in 2019 (132 p.m.p.). The lowest unadjusted incidence was 1 per 17 500 inhabitants in Estonia (57 p.m.p.) and the highest unadjusted incidence was 1 per 3670 inhabitants in Cyprus (273 p.m.p., Table 1 and Figs 1 and 2). When adjusted to age and sex using the distribution of the EU28 population [2], the overall adjusted incidence of KRT for the available 46 countries/regions was 138 p.m.p. (Fig. 2). Adjusted incidence ranged from 1 per 17 300 inhabitants in Estonia (58 p.m.p.) to 1 per 3410 inhabitants in Israel (293 p.m.p., Fig. 2). The overall median age at KRT initiation was 65.6 years and spanned a range of 62.6 years old in Lithuania to 74.2 years in Greece (Table 1). In countries providing individual data, 58% of patients starting KRT were aged over 65 years (Fig. 3). This was slightly lower in countries providing aggregated data, where 49% of patients starting KRT were aged over 65 years old (Fig. 3). The overall KRT incidence for men was 1 per 6275 (159.3 p.m.p.), and for females 1 per 11 200 (89.0 p.m.p.). This differed between countries providing individual data (men: 180.4 p.m.p., women: 93.0 p.m.p.) and countries providing aggregated data (men: 141.3 p.m.p., women: 84.6 p.m.p.). On average, 63% of patients initiating KRT were men (Fig. 3), and this was 65% for countries providing individual data and 60% for countries providing aggregated data (Fig. 3). The overall unadjusted incidence of KRT for ESKD due to diabetes mellitus (DM) was 31 p.m.p. and ranged from 14 p.m.p. in Estonia to 114 p.m.p. in Cyprus (Table 1). The most common known PRDs for patients initiating KRT were DM (21%) and hypertension (HT, 14%, Fig. 3). PRD cause was unknown/missing for 27% and categorized as miscellaneous for 14% of incident patients (Fig. 3). Most patients initiating KRT received haemodialysis (HD, 85%), followed by peritoneal dialysis (PD, 11%) and pre-emptive kidney transplant (4%, Fig. 4). In registries providing individual level data, the proportion of pre-emptive kidney transplants were highest in patients under 20 years old (21%, Fig. 4). Patients aged over 75 most commonly started with HD (88%) while in patients under 19 years old this was much less common (52%). KRT modality did not differ by sex (Fig. 4). Patients with DM as PRD had slightly fewer pre-emptive kidney transplants (2%) compared with non-DM patients (5%). HD as first treatment modality was higher in patients with DM than non-DM (86% compared with 81%), but PD was similar in DM and non-DM patients (13% compared with 14%, Fig. 4). At Day 91, 82% of incident KRT patients were on HD, 14% on PD and 4% living with a kidney transplant (Fig. 5).
Table 1:
Summary data on the unadjusted incidence of KRT in 2020 on Day 1, by country or region, the mean and median age at the start of KRT, and the incidence of KRT of patients with DM as PRD.
| Incidence of KRT in 2020, at Day 1 | |||||||
|---|---|---|---|---|---|---|---|
| Country/region | General population covered by the registry in thousands | All (n) | All (p.m.p.) | Mean age (years) | Median age (years) | DM (n) | DM (p.m.p.) |
| Albania | 2818 | 368 | 131 | 62.5 | 65.0 | 84 | 30 |
| Austriaa | 8901 | 1085 | 122 | 65.0 | 68.6 | 272 | 31 |
| Belarusb | 9380 | 696 | 74 | 145 | 15 | ||
| Belgium, Dutch-speakingc | 6654 | 1156 | 174 | 71.0 | 73.5 | 236 | 35 |
| Belgium, French-speakingc | 4890 | 944 | 193 | 67.6 | 69.6 | 208 | 43 |
| Bosnia and Herzegovina | 3531 | 408 | 116 | 63.0 | 65.0 | 107 | 30 |
| Croatiad | 3441 | 376 | 109 | 69.1 | 71.0 | 105 | 31 |
| Cyprus | 888 | 242 | 273 | 70.0 | 72.0 | 101 | 114 |
| Czech Republicd | 10 488 | 2230 | 213 | ||||
| Denmark | 5888 | 707 | 120 | 64.3 | 67.1 | 193 | 33 |
| Estonia | 1330 | 76 | 57 | 60.2 | 63.4 | 18 | 14 |
| Finland | 5530 | 522 | 94 | 61.0 | 64.6 | 175 | 32 |
| France | 67 488 | 10 696 | 158 | 67.5 | 70.5 | 2486 | 37 |
| Greece | 10 699 | 2749 | 257 | 71.5 | 74.2 | 659 | 62 |
| Hungary | 9672 | 1923 | 199 | 63.7 | 527 | 54 | |
| Iceland | 366 | 38 | 104 | 65.3 | 72.2 | 5 | 14 |
| Israel | 9215 | 1797 | 195 | 67.0 | 69.2 | 804 | 87 |
| Italy (8 of 20 regions) | 21 471 | 3405 | 159 | 68.6 | 71.5 | 520 | 24 |
| Kosovo | 1688 | 214 | 127 | 61.1 | 64.0 | 68 | 40 |
| Lithuania | 2794 | 242 | 87 | 60.8 | 62.6 | 39 | 14 |
| Montenegroc | 621 | 75 | 121 | 61.3 | 63.0 | 14 | 23 |
| North Macedonia | 2069 | 381 | 184 | 63.9 | 66.0 | 96 | 46 |
| Norway | 5379 | 543 | 101 | 62.3 | 65.9 | 90 | 17 |
| Poland | 38 354 | 4781 | 125 | 1484 | 39 | ||
| Portugale | 10 298 | 2361 | 229 | 773 | 75 | ||
| Romania | 18 302 | 2990 | 163 | 62.2 | 64.6 | 378 | 21 |
| Russiad | 143 248 | 12 103 | 84 | 2900 | 20 | ||
| Serbia | 6209 | 474 | 76 | 60.3 | 63.1 | 138 | 22 |
| Slovakiad | 4324 | 540 | 125 | 64.4 | 66.0 | 163 | 38 |
| Spain (All) | 47 451 | 6643 | 140 | 63.8 | 68.3 | 1668 | 35 |
| Spain, Andalusia | 8490 | 1185 | 140 | 64.4 | 67.7 | 316 | 37 |
| Spain, Aragon | 1331 | 182 | 137 | 64.5 | 68.2 | 46 | 35 |
| Spain, Asturias | 1016 | 170 | 167 | 67.1 | 69.6 | 50 | 49 |
| Spain, Basque country | 2188 | 288 | 132 | 65.4 | 69.3 | 73 | 33 |
| Spain, Canary Islands | 2241 | 382 | 170 | 64.0 | 66.5 | 126 | 56 |
| Spain, Cantabriac | 583 | 73 | 125 | 65.4 | 67.0 | 21 | 36 |
| Spain, Castile and Leónc | 2395 | 285 | 119 | 69.0 | 70.9 | 70 | 29 |
| Spain, Castile-La Manchac | 2047 | 215 | 105 | 65.1 | 66.2 | 62 | 30 |
| Spain, Catalonia | 7780 | 1336 | 172 | 66.8 | 70.4 | 255 | 33 |
| Spain, Community of Madrid | 6780 | 745 | 110 | 64.5 | 68.3 | 195 | 29 |
| Spain, Extremadura | 1064 | 150 | 141 | 65.9 | 66.7 | 48 | 45 |
| Spain, Galicia | 2700 | 393 | 146 | 67.5 | 69.6 | 109 | 40 |
| Spain, La Rioja | 316 | 33 | 104 | 68.2 | 69.2 | 6 | 19 |
| Spain, Murcia | 1511 | 212 | 140 | 65.0 | 66.9 | 49 | 32 |
| Spain, Navarrec | 657 | 70 | 107 | 63.4 | 68.7 | 26 | 40 |
| Spain, Valencian region | 5057 | 773 | 153 | 66.5 | 70.3 | 177 | 35 |
| Sweden | 10 353 | 1137 | 110 | 64.0 | 68.5 | 281 | 27 |
| Switzerland | 8638 | 846 | 98 | 65.2 | 68.9 | 159 | 18 |
| the Netherlands | 16 395 | 1861 | 114 | 63.9 | 67.7 | 389 | 24 |
| Turkeyf | 83 614 | 11 596 | 139 | 707 | 45 | ||
| UK, England | 56 550 | 6097 | 108 | 61.0 | 63.5 | 1618 | 29 |
| UK, Northern Ireland | 1896 | 206 | 109 | 60.1 | 64.0 | 39 | 21 |
| UK, Scotland | 5466 | 542 | 99 | 60.0 | 63.0 | 161 | 29 |
| UK, Wales | 3170 | 356 | 112 | 61.5 | 65.4 | 92 | 29 |
| All countries | 654 932 | 83 972 | 128 | 65.1 | 65.6 | 18 068 | 31 |
DM = diabetes mellitus as PRD.
When cells are left empty, the data are unavailable and could not be used for the calculation of the summary data.
aThe incidence is underestimated by approximately 1% due to one HD centre not submitting data.
bPatients younger than 18 years of age are not reported.
cPatients younger than 20 years of age are not reported: the true incidence counts are therefore slightly higher than the counts reported here.
dData include dialysis patients only.
eData on PRD are available for dialysis patients only (99.1% of total N = 2361).
fData on the incidence of PRD (DM) is based on 2180 dialysis patients (18.8% of total).
Figure 1:
Incidence of KRT in 2020 on Day 1 by country or region, unadjusted.
Figure 2:
Incidence of KRT in 2020 on Day 1 by country or region, unadjusted (left panel) and adjusted (right panel). Registries providing individual patient data are shown as dark bars, and registries providing aggregated data as light bars. See Appendix 1 for a list of countries and regions providing individual patient data or aggregated data. Adjustment of the incidence was performed by standardizing the incidence to the age and sex distribution of the EU28 population.
Figure 3:
Distribution of (A) age, (B) sex and (C) PRD by type of data provided for incident patients accepted for KRT in 2020, on Day 1, unadjusted.
Figure 4:
Distribution of treatment modality by (A) type of data provided, (B) age, (C) sex and (D) PRD (DM and non-DM) for incident patients accepted for KRT in 2020, on Day 1, unadjusted. Panels (B), (C) and (D) are only based on the data from registries providing individual patient data. See Appendix 1 for a list of countries and regions providing individual patient data or aggregated data. Abbreviations: Tx: transplant.
Figure 5:
Distribution of treatment modality by (A) type of data provided, (B) age, (C) sex and (D) PRD (DM and non-DM) for incident patients accepted for KRT in 2020, on Day 91, unadjusted. Panels (B), (C) and (D) are only based on the data from registries providing individual patient data. See Appendix 1 for a list of countries and regions providing individual patient data or aggregated data. Abbreviations: Tx: transplant.
KRT prevalence
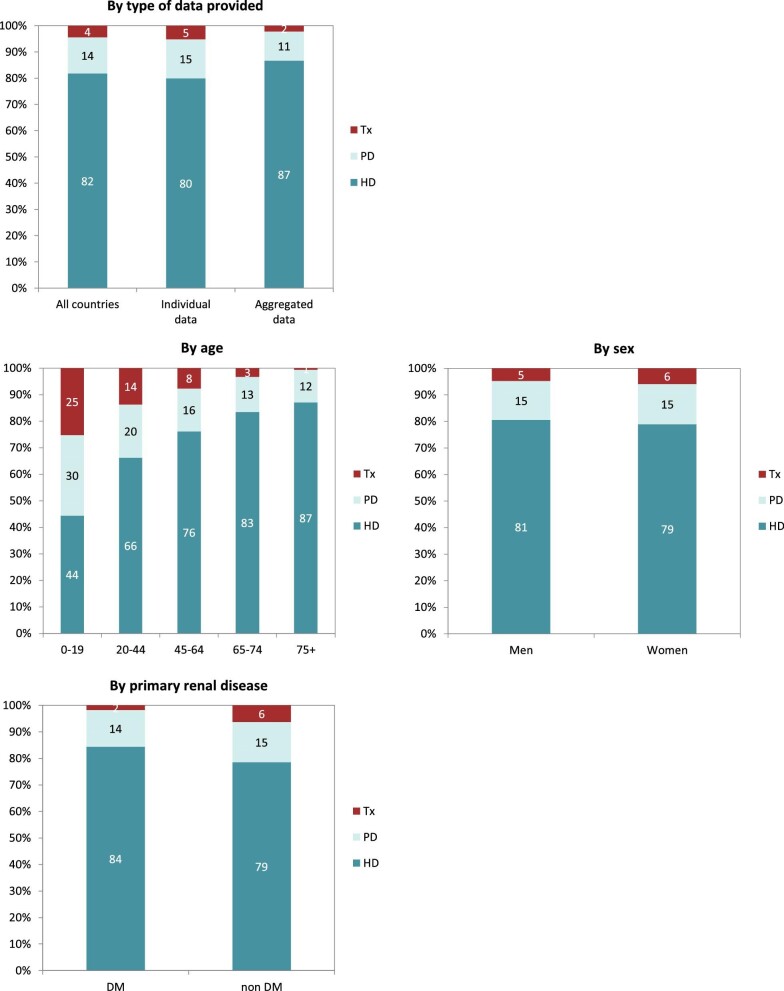
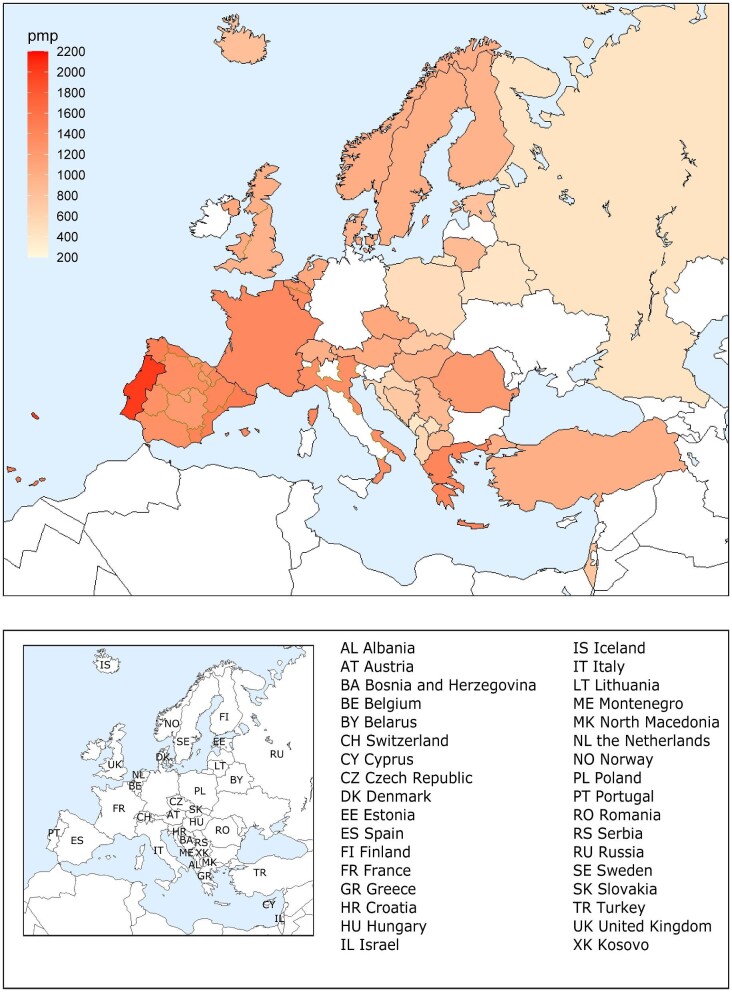
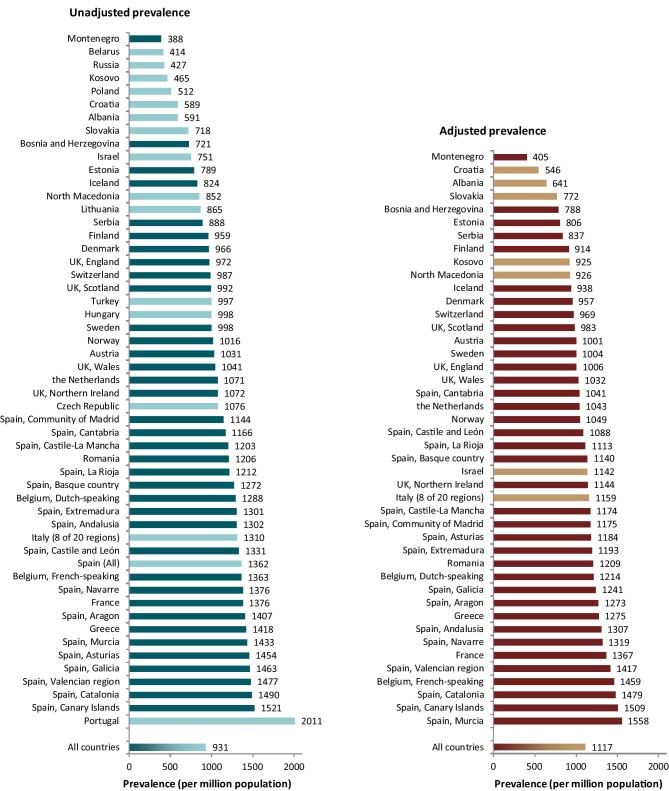
A total of 604 261 patients with ESKD were receiving KRT on 31 December 2020. This corresponds to an unadjusted KRT prevalence of around 1 per 1070 Europeans (931 p.m.p., Table 2), which is slightly higher than in 2019 (893 p.m.p.). The unadjusted prevalence of KRT ranged from 1 per 2580 inhabitants (388 p.m.p.) in Montenegro to 1 per 497 inhabitants (2011 p.m.p.) in Portugal (Table 2 and Figs 6 and 7). When adjusted to age and sex using the distribution of the EU28 population, the overall prevalence of KRT for the available 44 countries/regions was 1 per 895 Europeans (1117 p.m.p., Fig. 7). Adjusted prevalence ranged from 1 per 2470 inhabitants (405 p.m.p.) in Montenegro to 1 per 642 (1558 p.m.p.) in the Murcia region (Spain) (Fig. 7). The median age for patients on KRT was 59.3 years old, ranging from 51.0 years in Albania to 69.3 in Israel, although this latter country only included dialysis patients (Table 2). A majority of prevalent patients were aged over 45 years (84%, Fig. 8). The overall prevalence of KRT among men was 1 per 882 (1133.5 p.m.p.) and among women 1 per 1405 (711.9 p.m.p.). For countries providing individual data this was 1482.5 p.m.p. for men and 873.9 p.m.p. for women, and for countries providing aggregated data it was 880.9 p.m.p. for men and 589.2 p.m.p. for women. Overall, 60% of all prevalent patients were men (Fig. 8). The prevalence of patients with DM as PRD was 159 p.m.p., ranging from 55 p.m.p. in Belarus to 411 p.m.p. in the Canary Islands region (Spain) (Table 2). The most common PRD was glomerulonephritis/sclerosis (18%), followed by DM (15%) and HT (10%, Fig. 8). Twenty-six percent of PRD causes were unknown/missing and 14% were miscellaneous. A majority of prevalent patients were receiving HD (58%), followed by kidney transplantation (37%) and PD (5%, Fig. 9). Seventy-eight percent of patients under 20 years old were living with a kidney transplant, and this proportion progressively decreased with increasing age groups (Fig. 9). Only 19% of patients over 75 years were living with a kidney transplant and the majority of those over 75 years were receiving HD (74%). Treatment modality for prevalent patients was similar between men and women (Fig. 9). Patients with DM as PRD were less likely to be living with a kidney transplant (29%) compared with those not having DM as PRD (51%) and more likely to be receiving HD (65%) compared with those not having DM as PRD (44%, Fig. 9).
Table 2:
Summary data on the unadjusted prevalence of KRT in 2020 on 31 December 2020, by country or region with the mean and median age at 31 December 2020, and the prevalence of KRT of patients with DM as PRD.
| Prevalent patients on KRT in 2020 | |||||||
|---|---|---|---|---|---|---|---|
| Country/region | General population covered by the registry in thousands | All (n) | All (p.m.p.) | Mean age (years) | Median age (years) | DM (n) | DM (p.m.p.) |
| Albania | 2818 | 1664 | 591 | 52.2 | 51.0 | 356 | 126 |
| Austriaa | 8901 | 9181 | 1031 | 62.4 | 63.8 | 1728 | 194 |
| Belarusb | 9380 | 3881 | 414 | 514 | 55 | ||
| Belgium, Dutch-speakingc | 6654 | 8571 | 1288 | 66.5 | 68.4 | 1389 | 209 |
| Belgium, French-speakingc | 4890 | 6663 | 1363 | 65.5 | 67.0 | 1186 | 243 |
| Bosnia and Herzegovina | 3531 | 2547 | 721 | 59.9 | 61.4 | 478 | 135 |
| Croatiad | 3441 | 2028 | 589 | 66.4 | 69.0 | 510 | 148 |
| Cyprus | 888 | ||||||
| Czech Republic | 10 488 | 11 287 | 1076 | ||||
| Denmark | 5888 | 5685 | 966 | 59.3 | 60.8 | 962 | 163 |
| Estonia | 1330 | 1049 | 789 | 59.0 | 60.0 | 200 | 150 |
| Finland | 5530 | 5303 | 959 | 60.1 | 62.5 | 1323 | 239 |
| France | 67 488 | 92 864 | 1376 | 63.4 | 65.6 | 15 361 | 228 |
| Greece | 10 699 | 15 169 | 1418 | 65.9 | 68.0 | 2815 | 263 |
| Hungary | 9672 | 9651 | 998 | ||||
| Iceland | 366 | 302 | 824 | 58.5 | 59.7 | 33 | 90 |
| Israeld | 9215 | 6916 | 751 | 67.4 | 69.3 | 3254 | 353 |
| Italy (8 of 20 regions) | 21 471 | 28 127 | 1310 | 63.1 | 65.0 | 2930 | 136 |
| Kosovo | 1688 | 784 | 465 | 60.2 | 62.0 | 231 | 137 |
| Lithuania | 2794 | 2417 | 865 | ||||
| Montenegroc | 621 | 241 | 388 | 58.2 | 61.5 | 35 | 56 |
| North Macedonia | 2069 | 1762 | 852 | 59.8 | 61.0 | 312 | 151 |
| Norway | 5379 | 5464 | 1016 | 60.2 | 62.3 | 757 | 141 |
| Polandd | 38 354 | 19 647 | 512 | 5449 | 142 | ||
| Portugal | 10 298 | 20 713 | 2011 | 67.7 | 3684 | 358 | |
| Romania | 18 302 | 22 074 | 1206 | 63.4 | 65.3 | 2104 | 115 |
| Russia | 143 248 | 61 164 | 427 | 11 102 | 78 | ||
| Serbia | 6209 | 5516 | 888 | 60.7 | 63.0 | 976 | 157 |
| Slovakiad | 4324 | 3105 | 718 | 64.0 | 66.0 | 928 | 215 |
| Spain (All) | 47 451 | 64 621 | 1362 | 60.4 | 63.7 | 10 560 | 223 |
| Spain, Andalusia | 8490 | 11 053 | 1302 | 61.4 | 62.7 | 1848 | 218 |
| Spain, Aragon | 1331 | 1872 | 1407 | 65.0 | 66.7 | 328 | 246 |
| Spain, Asturias | 1016 | 1477 | 1454 | 64.6 | 66.2 | 272 | 268 |
| Spain, Basque country | 2188 | 2783 | 1272 | 62.4 | 64.5 | 356 | 163 |
| Spain, Canary Islands | 2241 | 3409 | 1521 | 62.5 | 63.6 | 921 | 411 |
| Spain, Cantabriac | 583 | 680 | 1166 | 63.7 | 65.3 | 111 | 190 |
| Spain, Castile and Leónc | 2395 | 3187 | 1331 | 66.0 | 67.0 | 547 | 228 |
| Spain, Castile-La Manchac | 2047 | 2462 | 1203 | 63.7 | 64.3 | 424 | 207 |
| Spain, Catalonia | 7780 | 11 593 | 1490 | 63.5 | 65.3 | 1628 | 209 |
| Spain, Community of Madrid | 6780 | 7753 | 1144 | 62.2 | 63.7 | 1338 | 197 |
| Spain, Extremadura | 1064 | 1384 | 1301 | 63.1 | 63.7 | 226 | 212 |
| Spain, Galicia | 2700 | 3951 | 1463 | 63.7 | 64.8 | 669 | 248 |
| Spain, La Rioja | 316 | 383 | 1212 | 62.7 | 63.7 | 48 | 152 |
| Spain, Murcia | 1511 | 2165 | 1433 | 63.0 | 63.8 | 334 | 221 |
| Spain, Navarrec | 657 | 904 | 1376 | 63.7 | 65.7 | 149 | 227 |
| Spain, Valencian region | 5057 | 7470 | 1477 | 64.1 | 66.0 | 1127 | 223 |
| Sweden | 10 353 | 10 332 | 998 | 60.3 | 62.5 | 1784 | 172 |
| Switzerland | 8638 | 8528 | 987 | 62.8 | 64.7 | 1269 | 147 |
| the Netherlands | 16 744 | 17 931 | 1071 | 61.2 | 63.2 | 2393 | 143 |
| Turkeye | 83 614 | 83 350 | 997 | 2638 | 347 | ||
| UK, England | 56 550 | 54 971 | 972 | 58.3 | 59.5 | 9908 | 175 |
| UK, Northern Ireland | 1896 | 2032 | 1072 | 58.3 | 59.2 | 298 | 157 |
| UK, Scotland | 5466 | 5421 | 992 | 57.3 | 59.0 | 940 | 172 |
| UK, Wales | 3170 | 3300 | 1041 | 58.6 | 59.9 | 599 | 189 |
| All countries | 649 818 | 604 261 | 931 | 60.7 | 59.3 | 89 006 | 159 |
When cells are left empty, the data are unavailable and could not be used for the calculation of the summary data.
aThe prevalence is underestimated by approximately 1% due to one HD centre not submitting data.
bPatients younger than 18 years of age are not reported.
cPatients younger than 20 years of age are not reported. The true prevalent counts are therefore slightly higher than the counts reported here.
dData on prevalence include dialysis patients only.
eData on the prevalence of PRD (DM) is based on 7585 dialysis patients (9.1% of total).
DM = diabetes mellitus as PRD.
Figure 6:
Prevalence of KRT on 31 December 2020 by country or region, unadjusted.
Figure 7:
Prevalence of KRT on 31 December 2020 by country/region, unadjusted (left panel) and adjusted (right panel).
Figure 8:
Distribution of (A) age, (B) sex and (C) PRD by type of data provided for prevalent patients on KRT on 31 December 2020, unadjusted.
Figure 9:
Distribution of treatment modality by (A) type of data provided, (B) age, (C) sex and (D) PRD (DM and non-DM) for prevalent patients on KRT on 31 December 2020, unadjusted. Panels (B), (C) and (D) are only based on the data from registries providing individual patient data. See Appendix 1 for a list of countries and regions providing individual patient data or aggregated data. Abbreviations: Tx: transplant.
Kidney transplantation
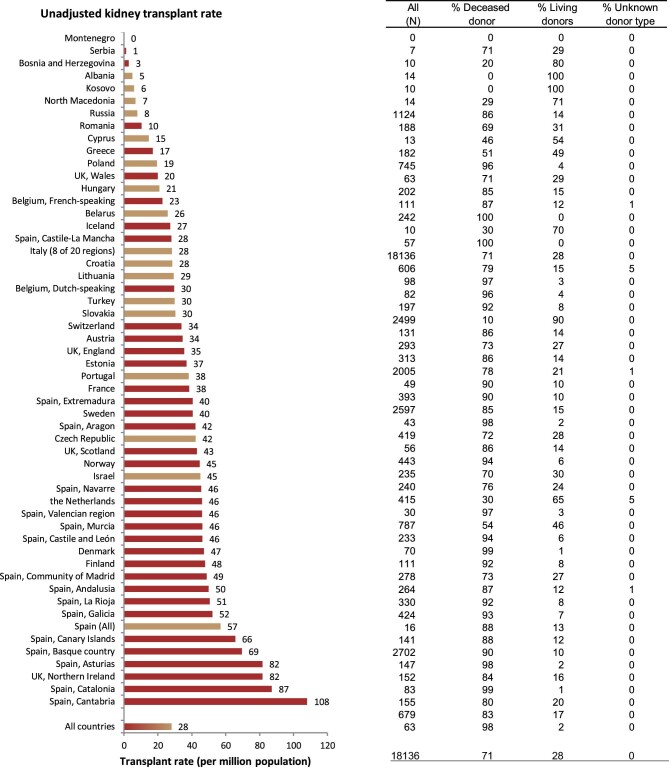
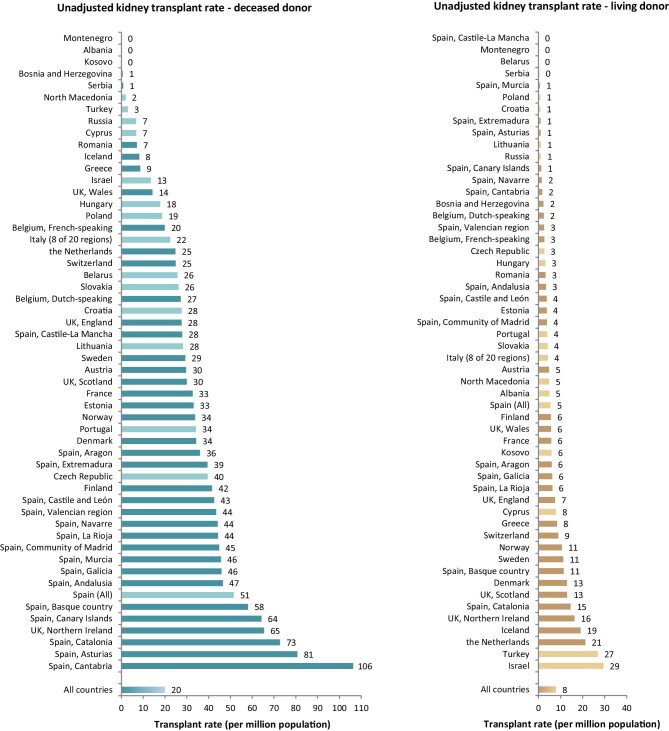
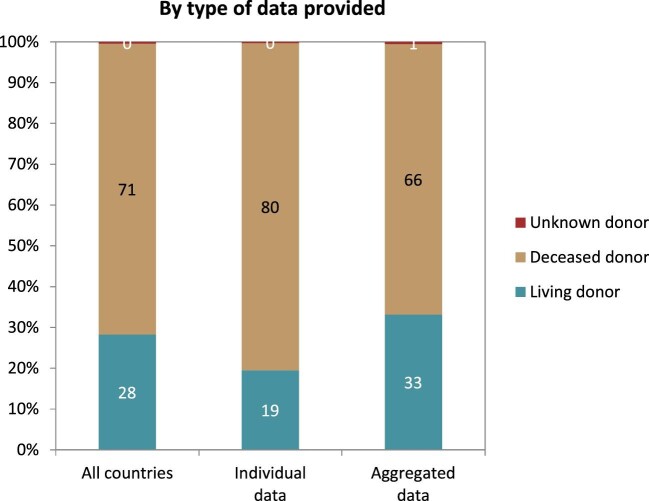
In 2020, a total of 18 136 kidney transplantations were performed, which corresponds to an unadjusted kidney transplantation rate of 1 per 35 800 Europeans (28 p.m.p., Fig. 10) and is lower than in 2019 (35 p.m.p.). Kidney transplantation rate ranged from 0 p.m.p. in Montenegro to 108 p.m.p. in the Cantabria region (Spain) (Fig. 10). Overall, most kidney transplantations were from deceased donors (71%). Albania and Kosovo had no kidney transplantations from deceased donors while Belarus and the Spanish region of Castile-La Mancha had all kidney grafts from deceased donors. The overall transplantation rate from deceased donors (20 p.m.p.) was more than twice that from living donors (8 p.m.p., Fig. 11). The deceased donor transplantation rate was highest in the Cantabria region (Spain) (106 p.m.p.). Eighty percent of kidney transplants from countries providing individual level data were from deceased donors, which is a larger proportion compared with that within the countries providing aggregated data (66%, Fig. 12). The highest rate of living donor transplantations was in Israel (29 p.m.p., Fig. 11).
Figure 10:
Kidney transplantations performed in 2020 by country or region, unadjusted. Registries providing individual patient data are shown as red bars, and registries providing aggregated data as orange bars.
Figure 11:
Kidney transplantations performed in 2020 for deceased donor (left panel) and living donor (right panel) transplantations, unadjusted. Registries providing individual patient data are shown as dark bars, and registries providing aggregated data as light bars.
Figure 12:
Distribution of donor type by type of data provided for kidney transplantations performed in 2020, unadjusted.
Survival probability of patients receiving KRT
Using data on patients starting KRT during the period 2011–15, the unadjusted 5-year survival probability was 51.8% [95% confidence interval (95% CI) 51.6–52.0, Table 3]. The 5-year unadjusted survival probability for patients receiving a first kidney transplant during the period 2011–15 from either a deceased (86.2%; 95% CI 85.8–86.5) or living donor (94.4%; 95% CI 94.0–94.8) was higher than the survival probability for patients starting dialysis (41.8%; 95% CI 41.5–42.1). Patients initiating dialysis with HD had a slightly higher 5-year adjusted survival probability (47%) than those initiating dialysis with PD (44%) (Fig. 13). In patients that received a kidney transplant, the 5-year survival probability of those having received a deceased donor transplant (92.1%; 95% CI 91.9–92.4) was lower than for those having received a living donor transplant (95.0%; 95% CI 94.6–95.4, Table 3 and Fig. 14). Five-year adjusted graft survival was also lower after deceased donor kidney transplantations (81.3%; 95% CI 80.9–81.8) than after living donor kidney transplantations (87.3%; 95% CI 86.6–87.9, Table 3).
Table 3:
One-, 2- and 5-year survival probabilities by treatment modality and cohort, from Day 1 of the start of KRT, dialysis, or from the day of first kidney transplantation.
| Survival probabilities as percentage (95% CI) | |||||
|---|---|---|---|---|---|
| Cohort: 2011–15 | Cohort: 2014–18 | ||||
| Survival type | 1 year | 2 years | 5 years | 1 year | 2 years |
| Patient survival on KRT | |||||
| Unadjusted | 84.8 (84.6–85.0) | 74.8 (74.6–75.1) | 51.8 (51.6–52.0) | 85.5 (85.4–85.7) | 75.6 (75.4–75.8) |
| Adjusteda | 87.6 (87.4–87.7) | 78.5 (78.3–78.7) | 53.9 (53.6–54.2) | 88.1 (88.0–88.3) | 79.0 (78.8–79.2) |
| Patient survival on dialysis | |||||
| Unadjusted | 83.7 (83.5–83.9) | 72.2 (72.0–72.5) | 41.8 (41.5–42.1) | 84.5 (84.3–84.6) | 72.9 (72.7–73.1) |
| Adjusteda | 85.9 (85.8–86.1) | 75.6 (75.4–75.8) | 46.8 (46.5–47.1) | 86.8 (86.7–87.0) | 76.6 (76.4–76.8) |
| Patient survival after a first kidney transplantation (deceased donor) | |||||
| Unadjusted | 96.2 (96.0–96.4) | 94.1 (93.8–94.3) | 86.2 (85.8–86.5) | 96.3 (96.2–96.5) | 94.1 (93.9–94.3) |
| Adjustedb | 98.0 (97.9–98.1) | 96.8 (96.7–97.0) | 92.1 (91.9–92.4) | 98.1 (97.9–98.2) | 96.8 (96.7–97.0) |
| Graft survival after a first kidney transplantation (deceased donor) | |||||
| Unadjusted | 91.0 (90.7–91.3) | 87.9 (87.5–88.2) | 77.3 (76.9–77.7) | 91.3 (91.0–91.6) | 88.0 (87.7–88.4) |
| Adjustedb | 92.9 (92.6–93.1) | 90.3 (90.0–90.6) | 81.3 (80.9–81.8) | 93.1 (92.8–93.3) | 90.4 (90.1–90.7) |
| Patient survival after a first kidney transplantation (living donor) | |||||
| Unadjusted | 99.0 (98.8–99.1) | 98.1 (97.8–98.3) | 94.4 (94.0–94.8) | 98.9 (98.7–99.0) | 98.0 (97.8–98.3) |
| Adjustedb | 99.1 (98.9–99.3) | 98.3 (98.1–98.5) | 95.0 (94.6–95.4) | 99.1 (98.9–99.2) | 98.4 (98.1–98.6) |
| Graft survival after a first kidney transplantation (living donor) | |||||
| Unadjusted | 96.6 (96.3–97.0) | 94.9 (94.5–95.3) | 88.1 (87.5–88.7) | 96.7 (96.3–97.0) | 95.2 (94.8–95.5) |
| Adjustedb | 96.4 (96.0–96.7) | 94.5 (94.1–95.0) | 87.3 (86.6–87.9) | 96.5 (96.1–96.8) | 94.9 (94.5–95.3) |
aAnalyses were adjusted using fixed values: age (67 years), sex (63% men) and PRD (24% DM, 19% HT/RVD, 11% GN and 46% other causes)
bAnalyses were adjusted using fixed values: age (50 years), sex (63% men) and PRD (14% DM, 10% HT/RVD, 23% GN and 53% other causes)
This table is based on data from the following registries providing individual patient data: Austria, Belgium (Dutch-speaking), Belgium (French-speaking), Bosnia and Herzegovina, Denmark, Estonia, Finland, France, Greece, Iceland, Norway, Spain (Andalusia), Spain (Aragon), Spain (Asturias), Spain (Basque country), Spain (Cantabria), Spain (Castile and León), Spain (Castile-La Mancha), Spain (Catalonia), Spain (Community of Madrid), Spain (Extremadura), Spain (Galicia), Spain (Murcia), Spain (Valencian Region), Sweden, the Netherlands, the UK (England/Northern Ireland/Wales) and the UK (Scotland).
Figure 13:
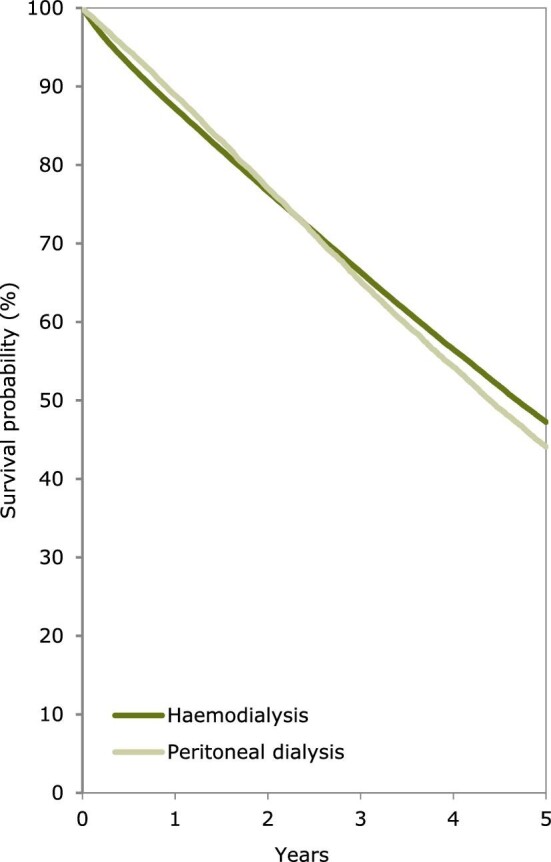
Patient survival by modality, either HD or PD, in incident dialysis patients from Day 91. Survival was adjusted using fixed values for age (67 years), sex (63% men) and PRD (24% diabetes mellitus, 19% hypertension/renal vascular disease, 11% glomerulonephritis and 46% other causes). This figure is based on patients starting dialysis between 2011 and 2015 from the following registries providing individual patient data: Austria, Belgium (Dutch-speaking), Belgium (French-speaking), Bosnia and Herzegovina, Denmark, Estonia, Finland, France, Greece, Iceland, Norway, Spain (Andalusia), Spain (Aragon), Spain (Asturias), Spain (Basque country), Spain (Cantabria), Spain (Castile and León), Spain (Castile-La Mancha), Spain (Catalonia), Spain (Community of Madrid), Spain (Extremadura), Spain (Galicia), Spain (Murcia), Spain (Valencian Region), Sweden, the Netherlands, the UK (England/Northern Ireland/Wales) and the UK (Scotland).
Figure 14:
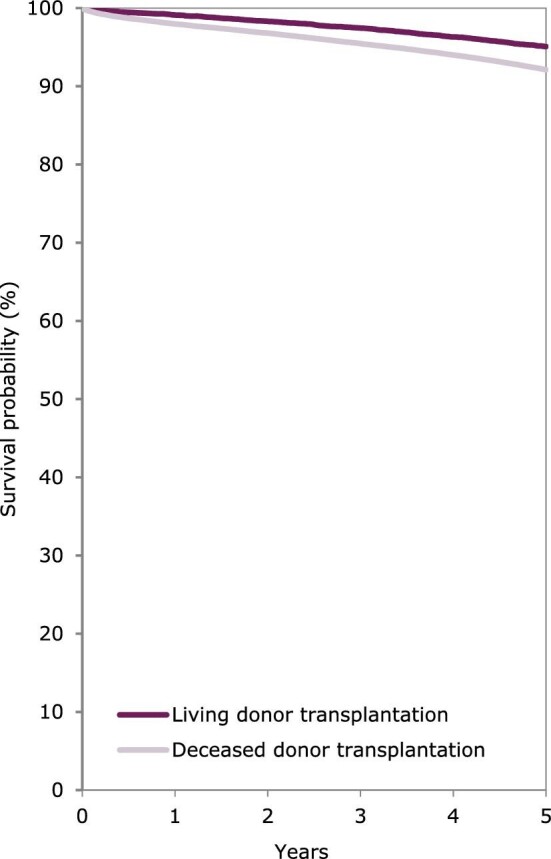
Patient survival in kidney transplant recipients by donor type, either deceased or living donors, from day of transplantation. Survival was adjusted using fixed values for age (50 years), sex (63% men) and PRD (14% diabetes mellitus, 10% hypertension/renal vascular disease, 23% glomerulonephritis and 53% other causes). This figure is based on patients receiving a kidney transplant between 2011 and 2015 from the following registries providing individual patient data: Austria, Belgium (Dutch-speaking), Belgium (French-speaking), Bosnia and Herzegovina, Denmark, Estonia, Finland, France, Greece, Iceland, Norway, Spain (Andalusia), Spain (Aragon), Spain (Asturias), Spain (Basque country), Spain (Cantabria), Spain (Castile and León), Spain (Castile-La Mancha), Spain (Catalonia), Spain (Community of Madrid), Spain (Extremadura), Spain (Galicia), Spain (Murcia), Spain (Valencian Region), Sweden, the Netherlands, the UK (England/Northern Ireland/Wales) and the UK (Scotland).
Expected remaining lifetime
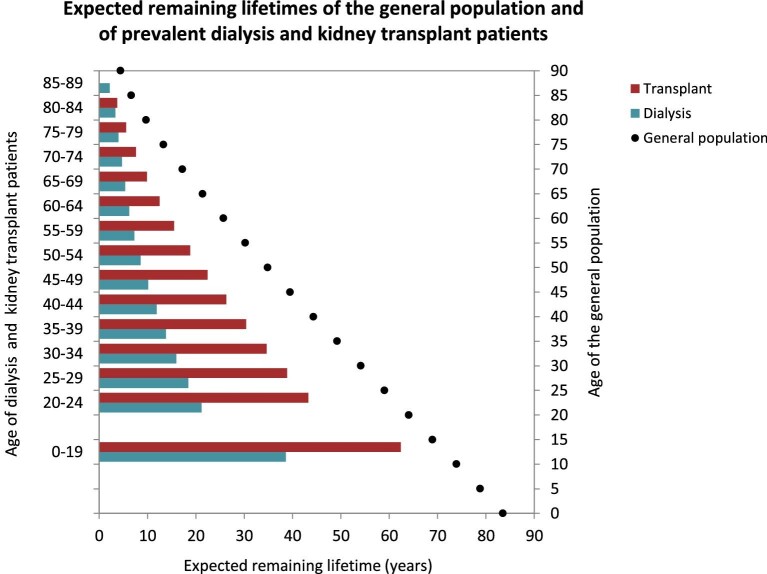
Using data from 2016–20, the expected remaining lifetime of KRT patients was consistently lower than the general population across all age groups (Fig. 15). Patients on dialysis had about a 50% lower expected remaining lifetime compared with patients with a kidney transplant. However, in patients above the age of 70 years the gap in expected remaining lifetime between those on dialysis and those with a transplant became smaller.
Figure 15:
Expected remaining lifetime, in years, of the general population (cohort 2016–20) and of prevalent dialysis and kidney transplant patients (cohort 2016–20) by age. This figure is based on data from the following registries providing individual patient data: Austria, Belgium (Dutch-speaking), Belgium (French-speaking), Bosnia and Herzegovina, Denmark, Estonia, Finland, France, Greece, Iceland, Norway, Spain (Andalusia), Spain (Aragon), Spain (Asturias), Spain (Basque country), Spain (Cantabria), Spain (Castile and León), Spain (Castile-La Mancha), Spain (Catalonia), Spain (Community of Madrid), Spain (Extremadura), Spain (Galicia), Spain (Murcia), Spain (Valencian Region), Sweden, the Netherlands, the UK (England/Northern Ireland/Wales) and the UK (Scotland).
Comparisons by primary renal disease
For the first time, comparisons across PRD groups are presented. Using individual patient-level data from 35 registries from 18 countries in Europe, Figs 16–23 show comparisons across PRD groups. Causes of PRD were grouped into eight categories: glomerulonephritis/sclerosis (GN), pyelonephritis (PN), adult type polycystic kidneys (PKD), DM, HT, renal vascular disease (RVD), miscellaneous causes (Appendix 2) and unknown/missing PRD.
Figure 16:
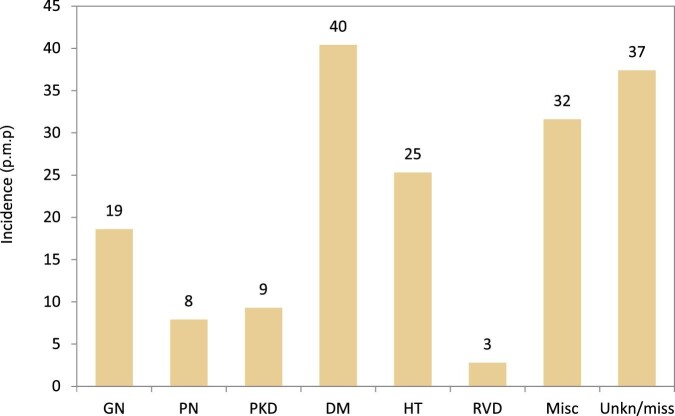
Incidence of KRT in 2020 on Day 1 by PRD, unadjusted. Abbreviations: Misc: miscellaneous; Unkn/miss: unknown/missing.
Figure 23:
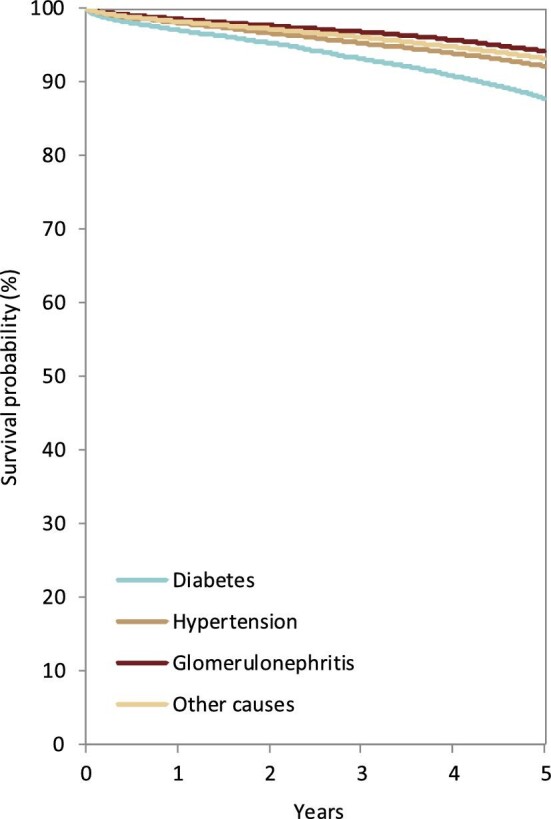
Patient survival by PRD in kidney transplant recipients from day of transplantation. Survival was adjusted using fixed values for age (50 years) and sex (63% men). This figure is based on patients receiving a kidney transplant between 2011 and 2015 from the following registries providing individual patient data: Austria, Belgium (Dutch-speaking), Belgium (French-speaking), Bosnia and Herzegovina, Denmark, Estonia, Finland, France, Greece, Iceland, Norway, Spain (Andalusia), Spain (Aragon), Spain (Asturias), Spain (Basque country), Spain (Cantabria), Spain (Castile and León), Spain (Castile-La Mancha), Spain (Catalonia), Spain (Community of Madrid), Spain (Extremadura), Spain (Galicia), Spain (Murcia), Spain (Valencian Region), Sweden, the Netherlands, the UK (England/Northern Ireland/Wales) and the UK (Scotland).
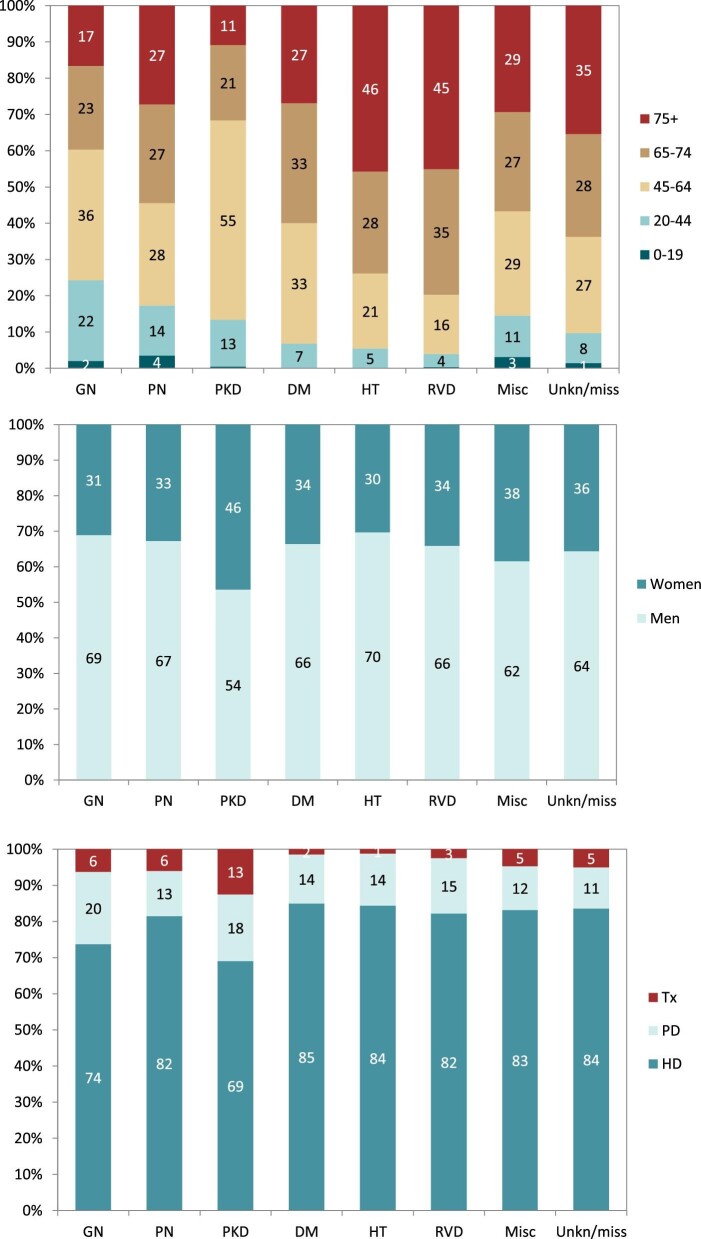
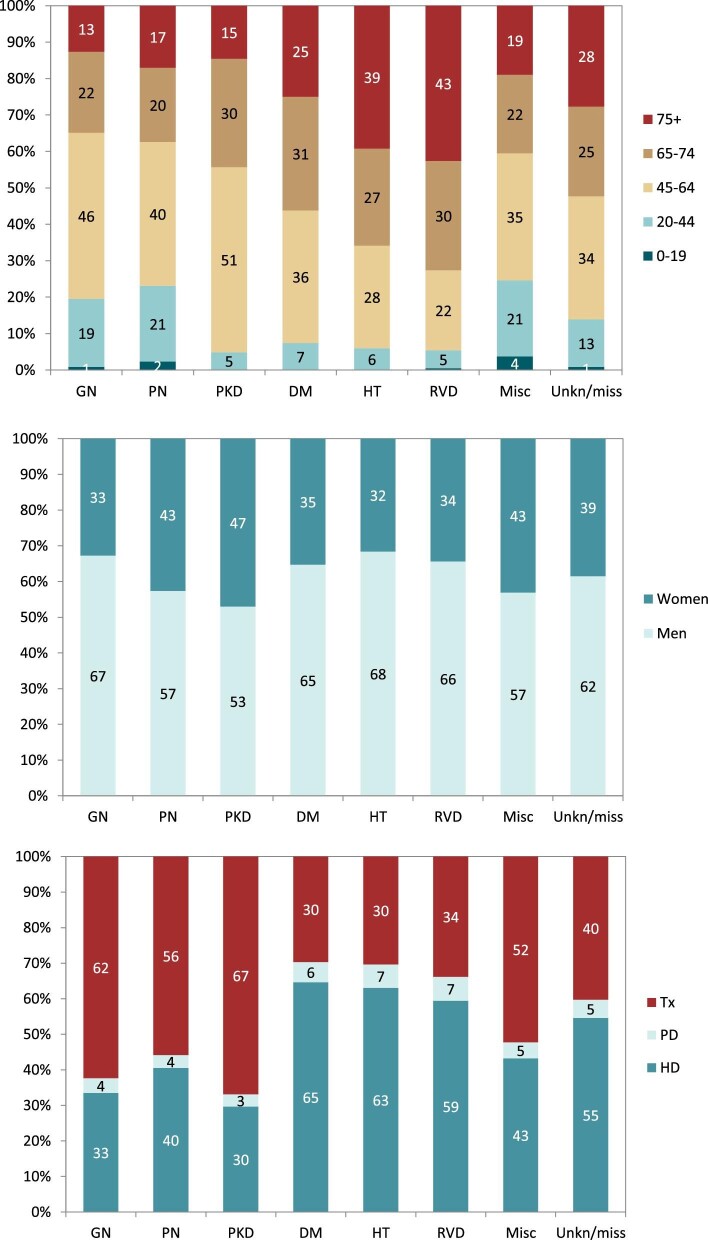
In 2020, DM was the most common PRD in incident KRT patients (40 p.m.p.), followed by HT (25 p.m.p.) and GN (19 p.m.p., Fig. 16). Patients aged over 75 comprised the highest proportion of patients with HT (46%) and RVD (45%, Fig. 17). Patients younger than 45 years old comprised the lowest proportion of incident patients in most PRD groups, except for GN and PKD. The proportion of women was highest in patients with PKD (46%), and in other PRD groups the prevalence of women ranged from 30% to 38% (Fig. 17). Regardless of PRD, HD was the most common form of KRT in incident patients, with the proportion of patients using HD ranging from 69%–85% among the PRD groups (Fig. 17). The proportion of patients receiving a pre-emptive kidney transplant was 2-fold higher for those with PKD (13%) compared with other PRD groups (1% to 6%, Fig. 17). Survival probabilities of incident dialysis patients (Day 91) by PRD group adjusted to age and sex are shown in Fig. 18. After 5 years, the highest 5-year survival probability was found for patients with GN (57%), and the lowest for DM (41%, Fig. 18).
Figure 17:
Distribution of (A) age, (B) sex and (C) treatment modality by PRD for incident patients accepted for KRT in 2020, on Day 1, unadjusted. Abbreviations: Misc: miscellaneous; Unkn/miss: unknown/missing; Tx: transplant.
Figure 18:
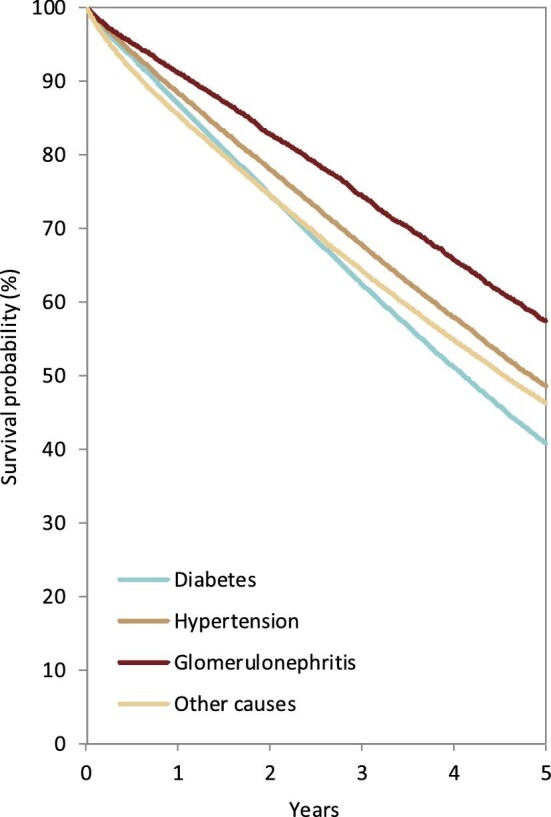
Patient survival by PRD in incident dialysis patients from Day 91. Survival was adjusted using fixed values for age (67 years) and sex (63% men). This figure is based on patients starting dialysis between 2011 and 2015 from the following registries providing individual patient data: Austria, Belgium (Dutch-speaking), Belgium (French-speaking), Bosnia and Herzegovina, Denmark, Estonia, Finland, France, Greece, Iceland, Norway, Spain (Andalusia), Spain (Aragon), Spain (Asturias), Spain (Basque country), Spain (Cantabria), Spain (Castile and León), Spain (Castile-La Mancha), Spain (Catalonia), Spain (Community of Madrid), Spain (Extremadura), Spain (Galicia), Spain (Murcia), Spain (Valencian Region), Sweden, the Netherlands, the UK (England/Northern Ireland/Wales) and the UK (Scotland).
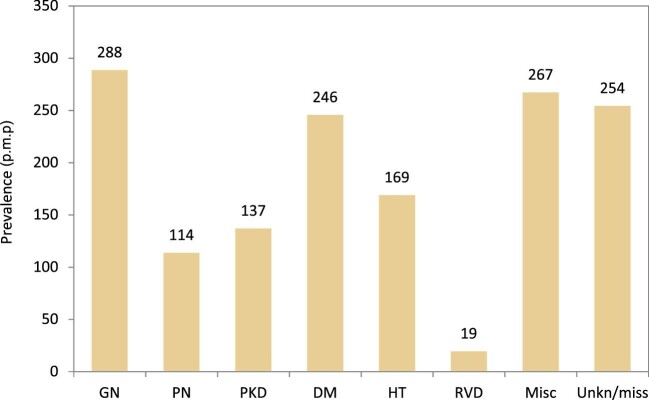
The most common PRDs in prevalent KRT patients on 31 December 2020 were GN (288 p.m.p.), DM (246 p.m.p.) and HT (169 p.m.p., Fig. 19). Prevalent KRT patients under 45 years old comprised 20% of patients with GN and 23% of patients with PN, but a significantly smaller proportion of patients with PKD (5%), DM (7%), HT (6%) and RVD (5%, Fig. 20). Prevalent KRT patients over 75 years old comprised the largest proportion of patients with RVD (43%) or HT (39%). The proportion of women in PRD groups ranged from 32% in patients with HT to 47% in patients with PKD (Fig. 20). Kidney transplantation was the most common KRT for prevalent patients with PKD (67%), GN (62%) and PN (56%, Fig. 20). For the remaining PRD groups, HD was the most common form of KRT for prevalent patients, with the use of HD ranging from 59% to 65% (Fig. 20).
Figure 19:
Prevalence of KRT on 31 December 2020 by PRD, unadjusted. Abbreviations: Misc: miscellaneous; Unkn/miss: unknown/missing.
Figure 20:
Distribution of (A) age, (B) sex and (C) treatment modality distribution by PRD in prevalent patients on KRT on 31 December 2020, unadjusted. Abbreviations: Misc: miscellaneous; Unkn/miss: unknown/missing; Tx: transplant.
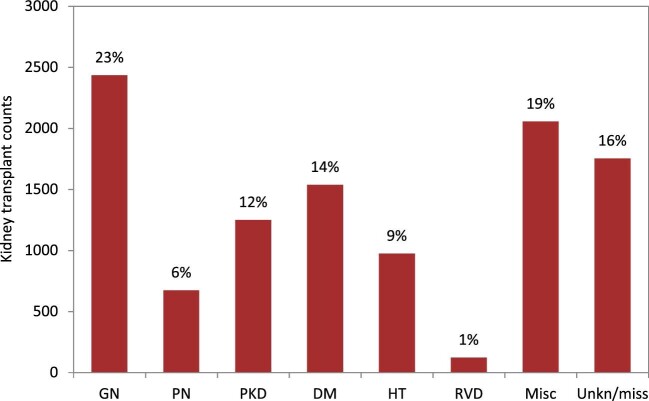
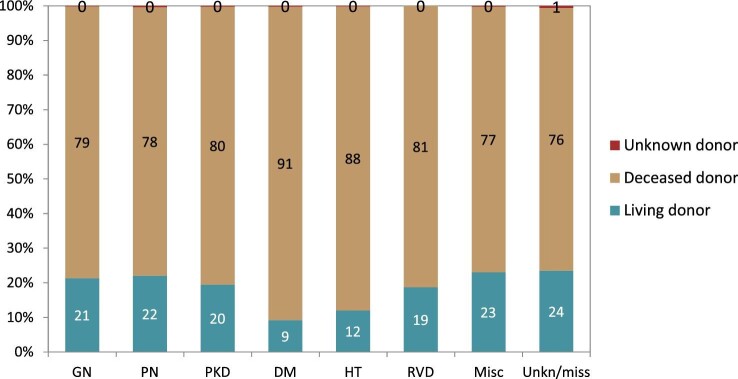
Patients with GN received most of the available kidney transplants (23%, Fig. 21). The percentage of kidney transplants available was lower for patients with DM (14%), PKD (12%), HT (9%) and PN (6%). Only 1% of all kidney transplants was available for patients with RVD. A majority of transplants were from deceased donors regardless of PRD group (76%–91%, Fig. 22). Patients with PN, GN or PKD had the highest proportion of living donor transplantations (22%, 21% and 20%, respectively). Comparatively, patients with DM or HT had about 50% lower proportions of living donor transplants (9% and 12%). Age- and sex-adjusted survival probabilities after first transplantation slightly differed by PRD group (Fig. 23), with DM patients having the lowest survival probability after 5 years (88%).
Figure 21:
Kidney transplantations in both prevalent and incident KRT patients performed in 2020 by PRD, unadjusted. The percentages in this figure sum up to 100% for all PRD groups together. Abbreviations: Misc: miscellaneous; Unkn/miss: unknown/missing.
Figure 22:
Distribution of donor type by PRD for kidney transplantations performed in 2020, unadjusted. Abbreviations: Misc: miscellaneous; Unkn/miss: unknown/missing.
AFFILIATED REGISTRIES
We would like to thank the patients and the staff of the dialysis and transplant units for contributing the data via their national and regional renal registries. Furthermore, we gratefully acknowledge the following registries and persons for their contribution of the data: Albanian Renal Registry (M. Rroji and E. Likaj); Austrian Dialysis and Transplant Registry (OEDTR) (F. Engler, R. Kramar, G. Mayer, and the Austrian Society of Nephrology); Belarus Renal Registry (K.S. Kamisarau and A. Kalachyk); Dutch-speaking Belgian Society of Nephrology (NBVN) (J. De Meester); French-speaking Belgian Society of Nephrology (GNFB) (J.M. des Grottes and F. Collart); Renal Registry Bosnia and Herzegovina (H. Resić and E. Mešić); Croatian Renal Registry (D. Katicic and K. Altabas); Cyprus Renal Registry (S. Glyki, E. Magiroudi and A. Saccidou); Czech Republic: Registry of Dialysis Patients (RDP) (F. Lopot, I. Rychlík and L. Francová); Danish Nephrology Registry (DNS) (K. Hommel); Estonian Society of Nephrology (Ü. Pechter, M. Ots-Rosenberg and K. Lilienthal); France: The Epidemiology and Information Network in Nephrology (REIN) (C. Couchoud); Hellenic Renal Registry (G. Moustakas); Hungarian Renal Registry (I. Kulcsar, L. Wagner and L. Rosivall); Icelandic End-Stage Renal Disease Registry (R. Palsson); Montenegro Renal Registry (M. Ratkovic, D. Radunović and F. Tomović); Israel National Registry of Renal Replacement Therapy (L. Keinan-Boker and R. Dichtiar); Italian Registry of Dialysis and Transplantation (RIDT) (M. Postorino and A. Limido); Kosovo Renal Registry (S. Selmani and M. Tolaj-Avdiu); Lithuanian Renal Registry (I. Nedzelskiene and V. Vainauskas); North Macedonian Renal Registry (I. Rambabova Bushljetikj); Norwegian Renal Registry (A.V. Reisæter); Renal Registry of Poland (A. Debska-Slizien, P. Jagodzinski and R. Gellert); Portuguese Renal Registry (A. Ferreira); Romanian Renal Registry (RRR) (G. Mircescu, L. Garneata and E. Podgoreanu); Russian Renal Registry (A. Andrusev, H. Zakharova and N. Tomilina); Renal Registry in Serbia (M. Lausevic, R. Naumovic, all of the Serbian renal units, and the Serbian Society of Nephrology); Slovakian Renal Registry (I. Lajdová, V. Spustová and A. Oksa); Spain Renal Registry (B. Mahillo Durán); Swedish Renal Registry (SRR) (K.G. Prütz, M. Stendahl, M. Evans, S. Schön, T. Lundgren, H. Rydell and M. Segelmark); Swiss Dialysis Registry (P. Ambühl); Dutch Renal Registry (RENINE) (L. Heuveling); Registry of the Nephrology, Dialysis and Transplantation in Turkey (TSNNR) (K. Ateş and G. Süleymanlar); UK Renal Registry (all the staff of the UK Renal Registry and of the renal units submitting data); Scottish Renal Registry (SRR) (all of the Scottish renal units); and the regional registries of Andalusia (SICATA) (on behalf of all users of SICATA), Asturias (P. Beltrán, M. Rodríguez, J.R. Quirós, and RERCA Working Group), Basque country (UNIPAR) (Á. Magaz, J. Aranzabal, M. Rodrigo and I. Moina), Canary Islands (I. Santana Gil and C. Torres Medina), Cantabria (J.C. Ruiz San Millán), Castile and León (M.A. Palencia García and P. Ucio Mingo), Castile-La Mancha (G. Gutiérrez Ávila and I. Moreno Alía), Catalonia (RMRC) (J. Tort), Community of Madrid (M.I. Aparicio de Madre and F. Tornero Molina), Extremadura (all the renal units (Nephrology and Dialysis)), La Rioja (E. Huarte Loza, M. Artamendi Larrañaga and H. Hernández Vargas), Murcia (I. Marín Sánchez), Navarre (Manrique Escola) and Valencian region (O. Zurriaga).
ERA REGISTRY COMMITTEE MEMBERS
C. Wanner, Germany (ERA President); A. Ortiz, Spain (Chairman); P. Ambühl, Switzerland; M. Arici, Turkey; M. Arnol, Slovenia; S. Bakkaloglu, Turkey; P.M. Ferraro, Italy; J. Helve, Finland; J.E. Sánchez-Alvarez, Spain; M. Segelmark, Sweden; S.S. Sørensen, Denmark; E. Vidal, Italy.
ERA REGISTRY OFFICE STAFF
K.J. Jager (Managing Director), M.E. Astley, B.A. Boerstra, M. Bonthuis, R. Boenink, N.C. Chesnaye, R. Cornet, A. Kramer, V.S. Stel and A.J. Weerstra.
Supplementary Material
ACKNOWLEDGEMENTS
The ERA Registry would like to thank the patients and staff of all the dialysis and transplant units who have contributed data via their national and regional renal registries. In addition, we would like to thank the persons and organizations listed in the paragraph ‘Affiliated registries’ for their contribution to the work of the ERA Registry.
Appendix 1
Countries or regions providing individual patient data to the ERA Registry
Austria, Belgium (Dutch-speaking), Belgium (French-speaking), Bosnia and Herzegovina, Denmark, Estonia, Finland, France, Greece, Iceland, Montenegro, Norway, Romania, Serbia, Spain (Andalusia), Spain (Aragon), Spain (Asturias), Spain (Basque country), Spain (Canary Islands), Spain (Cantabria), Spain (Castile and León), Spain (Castile-La Mancha), Spain (Catalonia), Spain (Community of Madrid), Spain (Extremadura), Spain (Galicia), Spain (La Rioja), Spain (Murcia), Spain (Navarre), Spain (Valencian region), Sweden, Switzerland, the Netherlands, the UK (England/Northern Ireland/Wales) and the UK (Scotland).
Countries or regions providing aggregated data to the ERA Registry
Albania, Belarus, Croatia, Cyprus, Czech Republic, Hungary, Israel, Italy (8 of 20 regions), Kosovo, Lithuania, North Macedonia, Poland, Portugal, Russia, Slovakia, Spain, Turkey.
Countries part of the European Union (EU-28) population in 2015 (used as reference population)
Austria, Belgium, Bulgaria, Croatia, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, the Netherlands, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden, the UK.
Appendix 2
Miscellaneous primary renal disease
Nephropathy (interstitial) due to analgesic drugs, nephropathy (interstitial) due to cis-platinum, nephropathy (interstitial) due to cyclosporin A, lead-induced nephropathy (interstitial), drug-induced nephropathy (interstitial) not mentioned above, cystic kidney disease—type unspecified, polycystic kidneys; infantile (recessive), medullary cystic disease; including nephronophtisis, cystic kidney disease—other specified type, hereditary/familial nephropathy—type unspecified, hereditary nephritis with nerve deafness (Alport's Syndrome), cystinosis, primary oxalosis, Fabry's disease, hereditary nephropathy—other specified type, renal hypoplasia (congenital)—type unspecified, oligomeganephronic hypoplasia, congenital renal dysplasia with or without urinary tract malformation, syndrome of agenesis of abdominal muscles (prune belly syndrome), renal vascular disease due to polyarteritis, Wegener's granulomatosis, ischaemic renal disease/cholesterol embolism, glomerulonephritis related to liver cirrhosis, cryoglobulinaemic glomerulonephritis, myelomatosis/light chain deposit disease, amyloidosis, lupus erythematosus, Henoch–Schoenlein purpura, Goodpasture's Syndrome, systemic sclerosis (scleroderma), haemolytic uremic syndrome (including Moschcowitz syndrome), multi-system disease—other (not mentioned above), tubular necrosis (irreversible) or cortical necrosis (different from haemolytic uraemic syndrome), tuberculosis gout, nephrocalcinosis and hypercalcaemic nephropathy, Balkan nephropathy, kidney tumour, traumatic or surgical loss of kidney, other identified renal disorders.
Contributor Information
Megan E Astley, ERA Registry, Department of Medical Informatics, Amsterdam UMC location University of Amsterdam, Amsterdam, The Netherlands; Amsterdam Public Health, Health Behaviours & Chronic Diseases and Methodology, Amsterdam, The Netherlands.
Rianne Boenink, ERA Registry, Department of Medical Informatics, Amsterdam UMC location University of Amsterdam, Amsterdam, The Netherlands; Amsterdam Public Health, Quality of Care and Ageing & Later Life, Amsterdam, The Netherlands.
Samar Abd ElHafeez, ERA Registry, Department of Medical Informatics, Amsterdam UMC location University of Amsterdam, Amsterdam, The Netherlands; Epidemiology Department, High Institute of Public Health, Alexandria University, Alexandria, Egypt.
Sara Trujillo-Alemán, Health Quality Assessment and Information System Service, Dirección General de Programas Asistenciales, Servicio Canario de la Salud, Canary Islands, Spain.
Federico Arribas, Department of Aragon Health, General Direction of Health Care, Zaragoza, Spain.
Anders Åsberg, The Norwegian Renal Registry, Department of Transplantation Medicine, Oslo University Hospital – Rikshospitalet, Oslo, Norway.
Pazit Beckerman, Sheba Medical Center, Ramat Gan, and School of Medicine, Tel Aviv University, Tel Aviv, Israel.
Samira Bell, Scottish Renal Registry, Public Health Scotland, Glasgow, UK; Division of Population Health & Genomics, University of Dundee, Ninewells Hospital, Dundee, United Kingdom.
María Encarnación Bouzas-Caamaño, Regional Transplant Coordination of Galicia, Galician Health Service, Santiago de Compostela, Spain.
Jordi Comas Farnés, Catalan Renal Registry, Catalan Transplant Organization, Health Department, Generalitat of Catalonia, Barcelona, Spain.
Ana Amélia Galvão, Portuguese Society of Nephrology, Coimbra, Portugal.
Nikola Gjorgjievski, Hospital of Nephrology, Skopje, N. Macedonia; Faculty of Medicine, University “SS Cyril and Methodius” Skopje, Skopje, N. Macedonia.
Vjollca Godanci Kelmendi, Nephrology Clinic, University Clinical Centre of Kosova, Prishtina, Kosova.
Rebecca Guidotti, Institute of Nephrology, City Hospital Zurich, Zurich, Switzerland.
Jaakko Helve, Finnish Registry for Kidney Diseases and Abdominal Center Nephrology, University of Helsinki and Helsinki University Central Hospital, Helsinki, Finland.
Alma Idrizi, Service of Nephrology, UHC Mother Teresa, Tirana, Albania.
Ólafur S Indriðason, Division of Nephrology, Landspitali–The National University Hospital of Iceland, Reykjavik, Iceland.
Kyriakos Ioannou, Cyprus Renal Registry, Nicosia, Cyprus.
Julia Kerschbaum, Austrian Dialysis and Transplant Registry, Department of Internal Medicine IV–Nephrology and Hypertension, Medical University Innsbruck, Innsbruck, Austria.
Kirill Komissarov, Minsk Scientific and Practical Center of Surgery, Transplantation and Hematology, Minsk, Belarus.
Pablo Castro de la Nuez, SICATA: Information System of the Autonomous Coordination of Transplants of Andalusia, Seville, Andalucia, Spain.
Mathilde Lassalle, REIN Registry, Agence de la Biomédecine, Saint-Denis La Plaine, France.
Maurizio Nordio, Division of Nephrology, AULSS 2, Treviso General Hospital, Treviso, Italy.
Olga Lucía Rodríguez Arévalo, Registry of Renal Patients of the Valencian Community, General Directorate of Public Health and Addictions, Ministry of Universal Health and Public Health, Valencia, Spain; Health and Well-being Technologies Program, Polytechnic University of Valencia, Valencia, Spain.
Carmen Santiuste, Murcia Renal Registry, Department of Epidemiology, Murcia Regional Health Council, IMIB-Arrixaca, Murcia, Spain; CIBER Epidemiología y Salud Pública (CIBERESP), Madrid, Spain.
Nurhan Seyahi, Department of Nephrology, Cerrahpaşa Medical Faculty, Istanbul University-Cerrahpaşa, Istanbul, Turkey.
María Fernanda Slon Roblero, Hospital Universitario de Navarra, Pamplona, Navarra, Spain.
Retha Steenkamp, UK Renal Registry, Bristol, UK.
Marc A G J ten Dam, Dutch Registry RENINE, Nefrovisie, Utrecht, The Netherlands.
Elena V Zakharova, Nephrology, Botkin Hospital, Moscow, Russia; Nephrology and Hemodialysis, Russian Medical Academy of Continuing Professional Education, Moscow, Russia.
Edita Ziginskiene, Lithuanian Nephrology, Dialysis and Transplantation Association, Kaunas, Lithuania; Nephrology Department, Medical Academy, Lithuanian University of Health Sciences, Kaunas, Lithuania.
Marjolein Bonthuis, ERA Registry, Department of Medical Informatics, Amsterdam UMC location University of Amsterdam, Amsterdam, The Netherlands; Amsterdam Public Health, Quality of Care, Amsterdam, The Netherlands; ESPN/ERA Registry, Amsterdam UMC location University of Amsterdam, Department of Medical Informatics, Amsterdam, The Netherlands.
Vianda S Stel, ERA Registry, Department of Medical Informatics, Amsterdam UMC location University of Amsterdam, Amsterdam, The Netherlands; Amsterdam Public Health, Quality of Care and Ageing & Later Life, Amsterdam, The Netherlands.
Alberto Ortiz, Department of Nephrology and Hypertension, IIS-Fundacion Jimenez Diaz UAM, Madrid, Spain; Department of Medicine, Universidad Autonoma de Madrid, Madrid, Spain.
Kitty J Jager, ERA Registry, Department of Medical Informatics, Amsterdam UMC location University of Amsterdam, Amsterdam, The Netherlands; Amsterdam Public Health, Quality of Care and Ageing & Later Life, Amsterdam, The Netherlands.
Anneke Kramer, ERA Registry, Department of Medical Informatics, Amsterdam UMC location University of Amsterdam, Amsterdam, The Netherlands.
FUNDING
The ERA Registry is funded by the European Renal Association (ERA). This article was written by M.E.A. et al. on behalf of the ERA Registry, which is an official body of the ERA. In addition, A.O. reports grants from Sanofi and personal fees from Astellas, AstraZeneca, Amicus, Bayes, Fresenius Medical care and Idorsia, outside the submitted work. K.J.J. reports grants from ERA, during the conduct of the study, and lecture and presentation fees from Fresenius. V.S.S. reports receiving grants from the ERA. A.Å. reports receiving study grants from AstraZeneca and CEPI, and speaker fees from Orifarm and Sandoz. O.S.I. reports grants from the Landspitali University Hospital Science Fund. M.F.S.R. reports receiving consulting fees and payment from Baxter and Fresenius. J.C.F. reports receiving payment for presentation from the TPM-DTI Foundation. P.A. reports receiving consulting fees from Astellas and CSL-Vifor. P.C.N. reports receiving support from Sistema Sanitario Público de Andalucía.
CONFLICT OF INTEREST STATEMENT
A.O. is the previous Editor-in-Chief of CKJ.
DATA AVAILABILITY STATEMENT
The data underlying this article cannot be shared publicly due to privacy laws, such as the General Data Protection Regulation (GDPR).
REFERENCES
- 1. ERA Registry: ERA Registry Annual Report 2020. Amsterdam, The Netherlands:Amsterdam UMC, location AMC, Department of Medical Informatics, 2022. [Google Scholar]
- 2. Eurostat . Available from: www.ec.europa.eu/eurostat/data/database (14 March 2022, date last accessed).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly due to privacy laws, such as the General Data Protection Regulation (GDPR).