Matching for the major histocompatibility complex (MHC)—and more specifically, the human leukocyte antigen (HLA) system—has been the gold standard in transplantation for decades. Selecting an HLA-matched donor is the preferred and straightforward choice for most immunologists, hematologists, and organ transplant specialists. Conversely, HLA-mismatched donors have been less accepted in hematopoietic stem cell transplantation (HSCT) due to unacceptable severe graft-versus-host disease (GvHD) and/or host-versus-graft (HvG) rejection. However, in the past three decades, a new approach has emerged: haploidentical HSCT (haplo-HSCT), which uses a related donor who is only partially matched to the recipient, such as parents and offspring. This new treatment has been shown to have similar or even better outcomes than classical HSCT of HLA-identical sibling donors (MSDs). Haploidentical donors have become a significant donor source for stem cell transplantation around the world in the past decade, and they now account for more than 60% of all donors in China and ~20% of donors in Europe and North America [1, 2]. This has led many to question whether mismatch could lead to better transplantation outcomes, and if so, for whom, how, and why?
In contrast to organ transplants, HSCT has a desired shadow power parallel to GvHD, known as the graft-versus-tumor (GvT) effect, which reduces the risk of relapse in various hematological malignancies after HSCT. It seems logical to maximize this effect by selecting donors with a significant HLA mismatch to the donor. Nevertheless, it has been considered too risky to use this power without controlling GvHD and graft rejection, which nearly resulted in the abandonment of haplo-HSCT three decades ago. The breakthrough of T-cell depletion (TCD) allografts, as well as two T-cell-replete (TCR) haplo-HSCT platforms—including the Beijing protocol (G-CSF-mobilized allografts and anti-thymocyte globulin based) and the Baltimore Protocol (posttransplant cyclophosphamide-based, PT-CY)—have resulted in promising outcomes in terms of acceptable GvHD and engraftment rates. The outcomes of patients following haplo-HSCT, especially by TCR protocols, have gradually become comparable to the outcomes of patients using either HLA-identical sibling donors or matched unrelated donors in the past two decades [2]. Although preliminary results from 2011 showed that the new haplo-HSCT protocol might exert more potent GvT effects in high-risk acute leukemia patients [3], this idea was not widely accepted, and registry studies reported downbeat results [4]. Since 2017, there has been increasing evidence to suggest that haplo-HSCT has overtaken HLA-matched HSCT in the treatment of high-risk patients. Chang et al. reported that AML patients with pre-HSCT measurable residual disease (MRD) receiving haploidentical HSCT following the Beijing protocol had a lower relapse rate (19% vs. 55%, P < 0.001) and longer disease-free survival (DFS, 74% vs. 33%, P < 0.001) than those receiving HLA-matched sibling HSCT [5]. In patients with high-risk ALL, haploidentical HSCT was associated with a lower 3-year relapse rate (23% vs. 47%, p = 0.006) and longer DFS (65% vs. 43%, p = 0.023) [6]. These results were further verified by haplo-HSCT following Baltimore protocols in high-risk AML and lymphoma [7, 8]. This has caused the question to evolve from “Is there a stronger GvT effect using HLA-haploidentical donors?” to “Why is haplo-HSCT taking the lead?” The report by Guo et al. opened up a new era of research to answer this exciting question [9].
Several vital points help to answer this question. First, the report used primary mouse AML cells carrying green fluorescent protein (GFP)-expressing human fusion genes and MHC molecules on T cells that were matched or haploidentical to leukemia cells, which enabled research of the immune cell dynamic response during leukemia development in vivo and best mimics human leukemia pathogenesis. Second, without the interference of GvHD, the report identified increased numbers and cytotoxic cytokine secretion of T cells and natural killer (NK) cells following haplo-HSCT in the mouse model. For example, T cells from the haplo group secreted higher TNF-α and IFN-γ levels than those from the MHC-matched group, and NK cells from the haplo group secreted higher IFN-γ, perforin and CD107a levels than those from the MHC-matched group. Finally, the report confirmed disparities in T cells from patients 1 year after HSCT, with T cells from haplo-HSCT patients showing more significant cytotoxic activity against leukemia cells than T cells from HLA-matched HSCT patients. Together, these results revealed the role of immune cells in the superior antileukemia effects of haplo-HSCT over HLA/MHC-matched HSCT [9].
Considering the positive role of chronic GvHD (cGvHD) in mediating the GvT effect, one may ask whether haplo-HSCT reduces relapse by increasing cGvHD at the expense of patients’ quality-of-life (QoL). Guo demonstrated that the fold reduction in the leukemia burden of patients after haplo-HSCT was significantly lower than that after MSD-HSCT at day 180, even excluding patients with cGvHD. In a recent study by Wang, patients with pre-HSCT MRD + AML who underwent MSD-HSCT demonstrated similar cGvHD rates (66.1% vs. 68.0%, p = 0.726), high relapse rates (35.6% vs. 13.0%, p = 0.001) and lower relapse-free survival rates (51.3%vs. 73.8%, p = 0.008) than patients who underwent haplo-HSCT [10]. Long-term follow-up of patients following haplo-HSCT demonstrated comparable QoL. In particular, similar physical and mental component summaries in adults and physical, mental, social, and role well-being in children at 5 years post-HSCT [11]. These findings are promising, as they suggest that haplo-HSCT may not have a negative impact on long-term QoL outcomes compared to HLA-matched HSCT. Based on research by Guo and other colleagues, the latest Chinese consensus of HSCT conditioning and donor selection suggests that “Haploidentical donors are the preferred donor choice over matched sibling donors for patients with high-risk leukemia in experienced centers” [12]. This is the first global consensus that recommends the use of haplo-HSCT to replace the “gold standard—HLA-matched sibling donor” by pursuing more substantial GvT effects, better disease-free survival, and excellent QoL following HSCT. These findings provide clarity regarding for whom and how mismatch could lead to better transplantation outcomes [13].
In addition to Guo’s work, researchers worldwide continually ask why mismatch makes perfect GvT. As a stronger immune cell reaction was observed when the HLA molecules on T cells were haploidentical with leukemia cells compared with those that were matched with leukemia cells, this finding highlights the importance of HLA genes on leukemia cells in mediating GvT effects. On the other hand, one could anticipate that genomic loss of the unshared HLA haplotype in leukemia might be related to relapse after haplo-HSCT. Indeed, among patients with HLA loss at relapse, treatment including donor lymphocyte infusion or interferon-alpha would result in harmful GvHD, as the patient’s nonhematopoietic tissues did not experience HLA loss as the tumor cells did [14]. As HLA loss may be one of the most significant “Achilles’ Heels” for haplo-HSCT, further studies are needed to address this tempting question. First, it is crucial to determine whether HLA loss can be detected at lower levels, such as in the state of measurable residual disease. Second, potential strategies to reverse or prevent this process by targeting the immune environment should be explored. Third, in the event of HLA loss, it is essential to investigate whether salvage infusion of gene-modified immune cells is a viable alternative to a second HSCT from other donors. These investigations can potentially improve the efficacy of haplo-HSCT.
On the other hand, we might trigger more substantial GvT effects by leukemia-associated antigens (LAAs); hopefully, LAAs are highly expressed in leukemia cells but not in most healthy tissues. By infusion of donor-derived multiple leukemia antigen-specific T cells targeting leukemia-associated antigens frequently expressed in ALL (PRAME, WT1, and survivin) after HSCT, patients could strengthen their tumor-reactive T cells against known antigens by in vivo amplification of T cells and prevent relapse [15]. Future research in this area may lead to the development of novel immunotherapies that harness the power of LAAs to further improve the power of GvT by haplo-HSCT.
In summary, the superiority of haploidentical HSCT over traditional HLA-matched HSCT in the fight against hematological malignancies has initiated a new era of research and treatment options (Fig. 1). Continued research is needed to optimize haplo-HSCT protocols by research into the mechanisms underlying the GvT effect and the development of more effective and targeted immunotherapies.
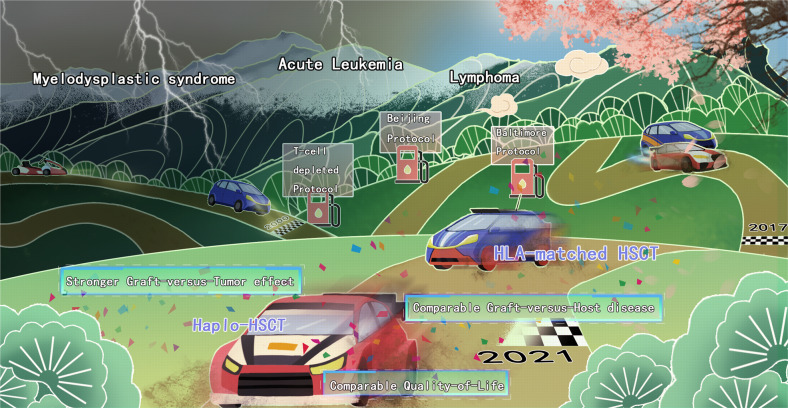
Fig. 1.
Haplo-HSCT has surpassed HLA-matched HSCT in the last three decades. Haplo-HSCT lagged significantly behind HLA-matched HSCT before 2000. With the breakthrough of the T-cell depletion protocol, the Beijing protocol and the Baltimore protocol, haplo-HSCT has caught up with the outcomes of HLA-matched HSCT. As growing evidence suggests a stronger graft-versus-tumor effect, comparable graft-versus-host disease and comparable quality-of-life when comparing haplo-HSCT with HLA-matched HSCT since 2017, there is now a consensus that haplo-HSCT be given priority in certain indications in 2021, and the story of “a perfect mismatch” continues after then
Acknowledgements
National Key Research and Development Program of China (No.2022YFA1103300, 2021YFA1100902); Major Program, Key Program and General Program of the National Natural Science Foundation of China (82293630, 81930004, 82070182); Beijing Nova Program of Science and Technology (No. Z191100001119120); Fund of China Scholarship Council (202106015007).
Author contributions
XJH designed the comment. ML and HDG drafted the manuscript and prepared the figures. All authors read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Meng Lv, Hui-Dong Guo.
References
- 1.Chang YJ, Pei XY, Huang XJ. Haematopoietic stem-cell transplantation in China in the era of targeted therapies: current advances, challenges, and future directions. Lancet Haematol. 2022;9:e919–e929. doi: 10.1016/S2352-3026(22)00293-9. [DOI] [PubMed] [Google Scholar]
- 2.Lv M, Gorin NC, Huang XJ. A vision for the future of allogeneic hematopoietic stem cell transplantation in the next decade. Sci Bull (Beijing) 2022;67:1921–4. doi: 10.1016/j.scib.2022.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Liu DH, Xu LP, Liu KY, Chen H, Chen YH, et al. Superior graft-versus-leukemia effect associated with transplantation of haploidentical compared with HLA-identical sibling donor grafts for high-risk acute leukemia: an historic comparison. Biol Blood Marrow Transpl. 2011;17:821–30. doi: 10.1016/j.bbmt.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 4.Ringden O, Labopin M, Ciceri F, Velardi A, Bacigalupo A, Arcese W, et al. Is there a stronger graft-versus-leukemia effect using HLA-haploidentical donors compared with HLA-identical siblings? Leukemia. 2016;30:447–55. doi: 10.1038/leu.2015.232. [DOI] [PubMed] [Google Scholar]
- 5.Chang YJ, Wang Y, Liu YR, Xu LP, Zhang XH, Chen H, et al. Haploidentical allograft is superior to matched sibling donor allograft in eradicating pre-transplantation minimal residual disease of AML patients as determined by multiparameter flow cytometry: a retrospective and prospective analysis. J Hematol Oncol. 2017;10:134. doi: 10.1186/s13045-017-0502-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang YJ, Wang Y, Xu LP, Zhang XH, Chen H, Chen YH, et al. Haploidentical donor is preferred over matched sibling donor for pre-transplantation MRD positive ALL: a phase 3 genetically randomized study. J Hematol Oncol. 2020;13:27. doi: 10.1186/s13045-020-00860-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorin NC, Labopin M, Blaise D, de Groot M, Socié G, Bourhis JH, et al. Stem cell transplantation from a haploidentical donor versus a genoidentical sister for adult male patients with acute myelogenous leukemia in first remission: A retrospective study from the acute leukemia working party of the European Society for Blood and Marrow Transplantation. Cancer. 2020;126:1004–15. doi: 10.1002/cncr.32629. [DOI] [PubMed] [Google Scholar]
- 8.Nagler A, Labopin M, Houhou M, Aljurf M, Mousavi A, Hamladji RM, et al. Outcome of haploidentical versus matched sibling donors in hematopoietic stem cell transplantation for adult patients with acute lymphoblastic leukemia: a study from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. J Hematol Oncol. 2021;14:53. doi: 10.1186/s13045-021-01065-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo H, Chang YJ, Hong Y, Xu LP, Wang Y, Zhang XH, et al. Dynamic immune profiling identifies the stronger graft-versus-leukemia (GVL) effects with haploidentical allografts compared to HLA-matched stem cell transplantation. Cell Mol Immunol. 2021;18:1172–85. doi: 10.1038/s41423-020-00597-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang M, Wang B, Xu LP, Wang Y, Zhang XH, Cheng Y, et al. The lower relapse rate and better survival advantages of haploidentical allograft compared with HLA-matched sibling donor allografts for intermediate- and adverse-risk AML patients with pretransplantation minimal residual disease. Bone Marrow Transpl. 2023;58:215–8. doi: 10.1038/s41409-022-01872-7. [DOI] [PubMed] [Google Scholar]
- 11.Xu ZL, Xu LP, Wu DP, Wang SQ, Zhang X, Xi R, et al. Comparable long-term outcomes between upfront haploidentical and identical sibling donor transplant in aplastic anemia: a national registry-based study. Haematologica. 2022;107:2918–27. doi: 10.3324/haematol.2022.280758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang XH, Chen J, Han MZ, Huang H, Jiang EL, Jiang M, et al. The consensus from The Chinese Society of Hematology on indications, conditioning regimens and donor selection for allogeneic hematopoietic stem cell transplantation: 2021 update. J Hematol Oncol. 2021;14:145. doi: 10.1186/s13045-021-01159-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lv M, Shen M, Mo X. Development of allogeneic hematopoietic stem cell transplantation in 2022: Regenerating “Groot” to heal the world. Innov (Camb) 2023;4:100373. doi: 10.1016/j.xinn.2023.100373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu H, Shi J, Luo Y, Yu J, Lai X, Liu L, et al. Assessment of Patient-Specific Human Leukocyte Antigen Genomic Loss at Relapse After Antithymocyte Globulin–Based T-Cell–Replete Haploidentical Hematopoietic Stem Cell Transplant. JAMA Netw Open. 2022;5:e226114. doi: 10.1001/jamanetworkopen.2022.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naik S, Vasileiou S, Tzannou I, Kuvalekar M, Watanabe A, Robertson C, et al. Donor-derived multiple leukemia antigen-specific T-cell therapy to prevent relapse after transplant in patients with ALL. Blood. 2022;139:2706–11. doi: 10.1182/blood.2021014648. [DOI] [PMC free article] [PubMed] [Google Scholar]