Abstract
Objectives:
Managing multimorbidity as aging stroke patients is complex; standard self-management programs necessitate adaptations. We used visual analytics to examine complex relationships among aging stroke survivors’ comorbidities. These findings informed pre-adaptation of a component of the Chronic Disease Self-Management Program.
Methods:
Secondary analysis of 2013–2014 Medicare claims with stroke as an index condition, hospital readmission within 90 days (n=42,938), and 72 comorbidities. Visual analytics identified patient subgroups and co-occurring comorbidities. Guided by the framework for reporting adaptations and modifications to evidence-based interventions (FRAME), an interdisciplinary team developed vignettes that highlighted multimorbidity to customize the self-management program.
Results:
There were five significant subgroups (z=6.19, p<.001) of comorbidities such as obesity and cancer. We constructed 6 vignettes based on the 5 subgroups.
Discussion:
Aging stroke patients often face substantial disease-management hurdles. We used visual analytics to inform pre-adaptation of a self-management program to fit the needs of older adult stroke survivors.
Keywords: stroke, self-management, visual analytics, adaptation
Introduction
Stroke is a leading cause of disability, institutionalization, readmission, and death (Mozzafarian et al., 2015). While improvements in the early detection of stroke and its rapid treatment have helped reduce mortality rates in stroke patients, many often face substantial disease-management hurdles once they return to the community (Mozaffarian et al., 2015). In other words, aging with multiple chronic conditions coupled with increased burden of disease is a challenge that needs management. There is evidence that these challenges frequently result in negative and costly outcomes, such as exacerbation of existing comorbidities, development of new chronic conditions, and unplanned hospital readmissions (Lui & Nguyen, 2018; Pearson et al., 2011). Participating in rehabilitation is one way aging stroke survivors can decrease the negative impacts of stroke (Cramer et al., 2017). Such rehabilitation should be goal-directed, multidimensional, interdisciplinary, and patient centered (Kristensen et al., 2016). Rehabilitation treatment also should contain an education component, given its importance in post-stroke care (Eames et al., 2010). Education should be individualized, clearly presented, and also offered to caregivers (Eames et al., 2010).
A constantly growing focus area in stroke rehabilitation is chronic disease management (Wagner et al., 2001). Patient education has been the core of chronic disease management for many years (Wagner et al., 2001). Patient education programs focus on compliance with disease-specific concerns and address broad problems experienced by individuals with the disease (McGowan, 2012). However, over the past twenty years there has been a shift from not only providing the needed information to patients about their chronic diseases and their consequences, but also instructing them on how they can manage their own condition over time (Eames et al., 2010). This improves not only the patient’s education of the chronic condition, but also facilitates the patient’s self-efficacy and self-confidence in the management of their disease (Eames et al., 2010). Self-management programs focus on self-efficacy and self-confidence by having patients identify problems that are specific to their situations. During self-management, patients are taught problem solving with dialogue from peers and healthcare workers to support personal behavior change (Lorig & Holman, 2003). Current analyses of self-management studies recommend a combination of both patient education and self-efficacy-oriented problem solving (McGowan, 2012).
Of the numerous chronic disease management programs in the literature, the Stanford Chronic Disease Self-Management Program (CDSMP; Lorig et al., 2001) has been widely researched and has been identified as an exceptional model for geriatric care (Ory et al., 2015). The CDSMP has been shown to be effective in improving outcomes for patients with chronic conditions such as arthritis, diabetes, cardiovascular diseases, and respiratory diseases (Adepoju et al., 2014; T. J. Brady et al., 2013; T. M. Brady et al., 2011; Cameron-Tucker et al., 2014; Lorig et al., 1999; Smeulders et al., 2009). The core component shared by all models is patient self-management support, which includes goal setting, problem solving, and lifestyle behavior education (Lorig & Holman, 2003). The CDSMP content is delivered in a group format, following a published manual and includes six self-management skills: problem solving, decision-making, appropriate resource utilization, forming a patient-provider partnership, action planning, and implementing action necessary to manage health issues autonomously (Lorig & Holman, 2003). The intent of the program is to help patients better manage chronic conditions with a program that can be applicable to several chronic conditions. Overall, the CDSMP establishes an important connection between clinical practice and the community experiences of older adults managing comorbidities.
Although the intent of the CDSMP was developed for several chronic conditions and not intended to be disease specific (Franek, 2013), it has been used with certain populations (Horrell & Kneipp, 2017), including stroke survivors (Lorig et al., 2001). To our knowledge, there are only two studies that identified the need to make modifications to the CDSMP specifically intended for people with stroke (Fugazzaro et al., 2021; Wolf et al., 2017). Wolf et al., used the standard CDSMP intervention in persons with mild stroke and modified some aspects but did not customize the program specifically to those with stroke. Unfortunately, the results did not demonstrate significantly better outcomes compared to the control group who received no active intervention (Wolf et al., 2017). The authors conclude that a tailored version of the CDSMP may be necessary and could then be effective for use in stroke survivors with multiple chronic conditions (Wolf et al., 2017). Second, Fugazzaro et al. incorporated five adaptations into an Italian version of the CDSMP (Fugazzaro et al., 2021). An example of one adaptation is the delivery of simplified content because of cognitive impairments that stroke survivors frequently experience. The study concluded that the adaptations helped patients to adhere to the program, utilize the resources that the CDSMP manual provides, and supported the implementation of structured self-management interventions (Fugazzaro et al., 2021). These findings support the need for a tailored, stroke-specific CDSMPs to optimize patient outcomes.
To successfully modify a program to be stroke-specific, it is important to follow an established adaptation framework based in implementation science. The FRAME, or framework for reporting adaptations and modifications to evidence-based interventions, was developed to help guide and report modifications to evidence-based programs. The FRAME helps create purposeful alterations of an intervention, and it is a necessity in implementation science (Stirman et al., 2013). In FRAME, the goal of the modification, when the modification occurs, what is modified, and what level are among some of the questions considered in the framework. Additionally, adaptations can be either pre-adapted or in response to challenges during the implementation phase. FRAME allows interventions to be continually tailored to meet individuals’ needs (i.e., aging adults living with multiple comorbidities), in attempts to improve the effectiveness of the intervention (Kirk et al., 2020).
It is well documented that aging individuals with stroke have a substantial number of comorbidities (Gallacher et al., 2014; Pearson et al., 2011) and that these comorbidities have a negative effect on patient outcomes (Karatepe et al., 2008; McCann & Lawrence, 2020; Pearson et al., 2011). For example, more than half of stroke survivors have greater than four comorbidities resulting in more cost and use of healthcare services (Gruneir et al., 2016). Therefore, it is important to consider these comorbidities and address them by evaluating existing models of care for older patients, developing new models or making adaptations to existing ones such as self-management.
Currently, there is a research gap on how to successfully facilitate a self-management program for older adults living with multiple comorbidities in addition to the acute stroke event. Visual analytics is a data-driven technique to identify and visualize patient subgroups and their most frequently co-occurring comorbidities. While this is the first study to use bipartite networks to examine subgroups of older stroke survivors based on frequently co-occurring comorbidities, previous studies have used this method to analyze heterogeneity in other diseases with a wide range of molecular and clinical variables (S. Bhavnani et al., 2014; S. K. Bhavnani, Dang, et al., 2014; S. K. Bhavnani, Drake, et al., 2014; S. K. Bhavnani et al., 2011, 2012, 2013, 2015, 2018). Hence, bipartite networks have been widely found to be beneficial for identifying co-occurring comorbidities. For patients with stroke, co-existing comorbidities may individually appear less clinically severe, but together can impose health and psychosocial limitations that have medical and functional consequences.
Many older adults are living with complex multimorbidity after stroke. Initial attempts to use standardized self-management program suggested a need to customize the program for stroke. Our goal was to fill this gap using a data-driven visual analytics method that captures the complexity of comorbidities in older adults with stroke. Armed with this information, we pre-adapted the CDSMP with the inclusion of vignettes and activities to facilitate problem solving, decision-making, and action planning among older adult stroke survivors. The overall goal of this project was to develop a customized self-management program for older individuals with stroke. Therefore, the specific study objectives were to (1) use visual analytics to characterize the combinations of chronic conditions from a secondary dataset of stroke survivors, and (2) use these combinations to inform the pre-adaptation of a component of the CDSMP. The anticipated outcome of the study is an adaptation to the CDSMP that better aligns with the complexity of aging stroke patients.
Materials and Methods
The focus of this study is the pre-adaptation of an evidence-based program used for older adults to benefit the health of stroke survivors (Ory et al., 2015). We used findings from visual analytics to inform the customized pre-adaptations. Therefore, our methods are broken into two sections that correspond to each of the objectives of the study: visual analytics and pre-adaptations. These allow us to provide a practical application to translate complex data into actionable interventions.
Visual Analytics
Design and Dataset
This study was a retrospective analysis of United States Medicare claims records from the Medicare Provider Analysis and Review and Master Beneficiary Summary files 2013–2014, of older adult patients >65 years old following an index stroke as defined by MS-DRG 61–66. Medicare is a comprehensive dataset with detailed information on beneficiary comorbidities (Hong et al., 2018). We identified beneficiaries readmitted to the hospital within 90 days of discharge for inclusion in the analysis. The CDSMP is most often implemented in community settings, and the 90-day window allowed us to incorporate stroke patients who were living in the community, whereas a commonly-used 30-day window may have included rehabilitation service delivery (Middleton et al., 2018). This resulted in 42,938 beneficiaries. This study was approved by the Institutional Review Board as an expedited review at University of Texas Medical Branch; therefore, no consent was required.
Variables
The outcome variable, 90-day readmission, was dichotomized. We captured all comorbidities of readmitted patients. To categorize these, we first applied the Centers for Medicare and Medicaid Services (CMS) Hierarchical Conditions Categories (HCCs) based on the quality measure specifications for readmission (Evans, 2011). Next, the remaining comorbidities were grouped by clinical experts in neurology, social work, psychology, and stroke rehabilitation. For example, CMS quality measure specifications group six cancer diagnostic codes, and our clinical experts added the diagnostic code of “other neoplasm” to this grouping. Therefore, there were seven total diagnostic codes for the group labeled “Cancer”. The final number used in the analyses was 72 comorbidities (Appendix 1), which were dichotomized.
Analysis
To identify patient subgroups and their most frequently co-occurring comorbidities, we used bipartite networks, a visual analytical approach (Newman, 2010). Bipartite network analysis can automatically identify and visualize biclusters (referred to as “subgroups” here) —groups made up of two categories of things—consisting of both patients and their characteristics such as comorbidities (Newman, 2010). The quantitative output provides the number, size, and statistical significance of the subgroups consisting of patients and their most frequently co-occurring comorbidities. The visual output generates a network which looks like a spider web where patients and comorbidities are represented as circles and triangles called “nodes”, and the association between a patient and a comorbidity is represented with a line or “edge” between the respective pair of patient and comorbidity. Furthermore, patients and their most frequently co-occurring comorbidities are separated into the quantitatively-identified subgroups. The resulting network visualization enables domain experts and clinicians to more quickly interpret the frequently co-occurring comorbidities of patients in a subgroup, to infer their clinical phenotypes and underlying mechanisms, and to design potential interventions.
To analyze the data, we used R (version 3.6.2; The R Foundation, 2019) to perform the following steps. First, we represented the data as a bipartite network, where nodes represent either patients (circles) or comorbidities (triangles), and the edges (lines connecting them) represent the presence of a comorbidity for that patient. Second, we identified patient subgroups and their most frequently co-occurring comorbidities using bicluster modularity (Chauhan et al., 2016; Treviño et al., 2015). Modularity is a number that ranges from −0.5 to +1, which indicates how strongly clustered a network is, with higher numbers indicating stronger clusteredness (Chauhan et al., 2016; Treviño et al., 2015). Bipartite modularity code is available online from https://cran.r-project.org/web/packages/BipartiteModularityMaximization/index.html. Next, we tested the significance of the clusteredness by randomly generating 1,000 networks while preserving the number of patients and comorbidities and the distribution of comorbidity prevalence in the data. Then we compared the modularity generated from the real network to the distribution of modularities generated from the above random networks to test whether the clusteredness in the real network was statistically significant. Although patients may have comorbidities in other clusters within the network, this test shows that the separation is statistically significant, such that patients and comorbidities belong in their respective subgroups more than expected by chance alone. Finally, we used the ExplodeLayout algorithm (S. K. Bhavnani et al., 2017; Dang et al., 2016) to separate the biclusters (i.e., subgroups) for reducing the overlap between the subgroups, while preserving the distances within the subgroups, with the goal of improving their interpretability. This algorithm modifies only the visualization of the network, but not the underlying data or the boundaries and members of the subgroups. ExplodeLayout is an algorithm written in R and available online from (https://github.com/UTMB-DIVA-Lab/epl; S. K. Bhavnani et al., 2017; Dang et al., 2016).
Pre-adaptations
Network Interpretation
The network of subgroups was then presented to 11 individuals including nine clinicians and two researchers on the multidisciplinary stroke team. The individuals included stroke neurologists, psychologists, social workers, rehabilitation clinicians (i.e., occupational and physical therapists), and research scientists who study older adults. The team provided written feedback where they named the cluster (i.e., subgroup) and evaluated the meaningfulness of the subgroup to stroke rehabilitation.
Vignette Development
The FRAME guided the team’s modification to the existing, evidence-based CDSMP with the goal of adapting the program for older adult stroke survivors. With FRAME, we address five components: 1) who makes the adaptation, 2) what content is modified, 3) what context is modified, 4) for whom the modification is made, and 5) what the nature of the content modification is. The interdisciplinary team of experts were those making the pre-planned adaptations. The modification of content was the guided discussion of multimorbidity. The context modification was the vignette format added for a specified CDSMP session. The adaptations targeted individual participants at the group level. The nature of the adaptation was the added element of the vignette. The vignettes described a fictional example of a stroke survivor living with at least two comorbidities and detailed a scenario on a topic which would occur in the CDSMP curriculum, thus preserving the fidelity of the CDSMP. Overall, the reason for the modification was to better fit older adult stroke survivors’ comorbidity self-management. In the standard CDSMP, the discussions are designed as open-ended group discussions in which all group members may engage. Now, these structured conversations could provide more context around self-management of multiple comorbidities in addition to the stroke, which is based on real data from visual analytics and supported by topics written about in the literature (Benjamin, 2020; Prior & Suskin, 2018).
Results
Visual Analytics
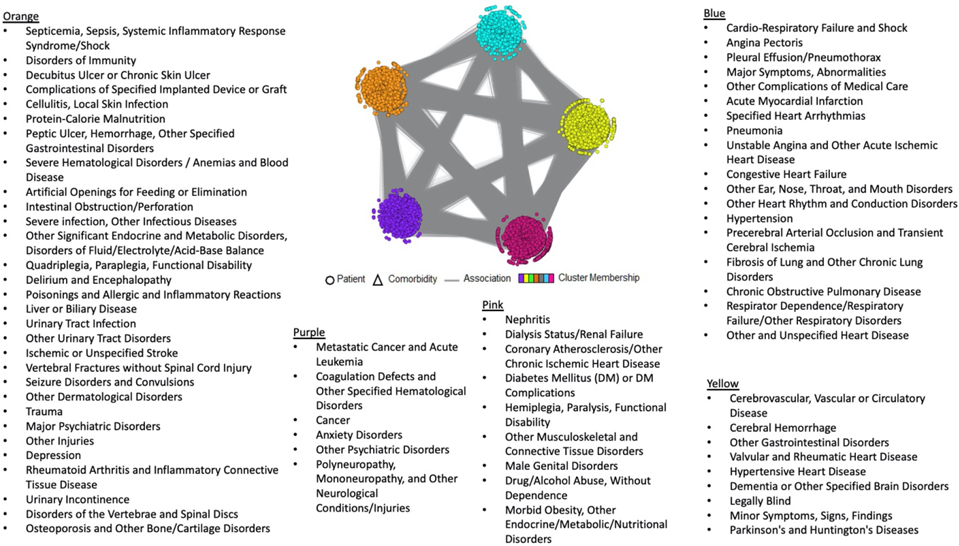
Table 1 presents the demographics of the patients in the cohort and the 72 comorbidities. The bipartite network analysis revealed five biclusters (i.e., “subgroups”, Figure 1). The analysis showed significant clusteredness (Modularity=0.17, z=6.19, p<.001). As noted in the methods, the visualization of the subgroups preserves the network’s edges and nodes. Therefore, the gray edges seen between subgroups in Figure 1 represent relationships between patients and comorbidities in other subgroups. However, as the network revealed significant clusteredness, these edges are visual in nature and do not affect the significance of the clustering.
Table 1:
Demographics of the stroke cohort (N=42,938).
| N (%) | ||
|---|---|---|
| Sex | ||
| Male | 17,966 (41.8) | |
| Female | 24,972 (58.2) | |
| Age | ||
| 66–69 | 4,803 (11.2) | |
| 70–74 | 7,430 (17.3) | |
| 75–79 | 8,158 (19.0) | |
| 80–84 | 8,974 (20.9) | |
| 85+ | 13,573 (31.6) | |
| Race | ||
| Non-Hispanic White | 33,856 (78.8) | |
| Black | 5,447 (12.7) | |
| Hispanic | 2,358 (5.5) | |
| Asian/Pacific Islander | 780 (1.8) | |
| American Indian/Alaska Native | 190 (0.4) | |
| Other | 228 (0.5) | |
| Unknown | 79 (0.2) | |
| Stroke Type | ||
| Arterial Ischemic Stroke | 26,884 (62.6) | |
| Intracerebral Hemorrhage | 2,498 (5.8) | |
| Subarachnoid Hemorrhage | 552 (1.3) | |
| Transient Ischemic Attack | 8,526 (19.9) | |
| Other* | 4,478 (10.4) |
‘Other’ stroke type included: 433.x0 (occlusion and stenosis of precerebral arteries without mention of cerebral infarction); 434.x0 (occlusion of cerebral arteries without mention of cerebral infarction).
Figure 1.
The five subgroups from the visual analytics output.
Pre-adaptations
Network Interpretation
Our multidisciplinary team conceptualized and characterized the five patient subgroups based on comorbidities in each subgroup as follows: 1) Obesity/Diabetes (Pink), 2) Immunity/Infection, Neurological Diseases, Major Psychiatric/Depression (Orange), 3) Brain/Vascular, Neurodegenerative Diseases (Yellow), 4) Heart, Lungs/Pulmonary Diseases (Blue), and 5) Cancer, Other Psychiatric Disorders (Purple) (Figure 1).
Vignette Development
The team selected multiple pairs of comorbidities from each subgroup consistent with the findings from visual analytics. They were selected for the purpose of developing clinical case vignettes to facilitate discussions and activities during CDSMP sessions. Table 2 gives examples of these case vignettes. It also describes how the case vignettes correspond to each weekly session of the CDSMP, when they are used, and during which specific activities they appear in the CDSMP curriculum. For example, the sixth vignette incorporates the activity of “talking to your healthcare provider” using a fictional stroke survivor also living with Parkinson’s and legal blindness. In this scenario, we said that this person uses technology to write down anything that they need to remember from the appointment. The technology is speech-to-text on an iPad. This way, the person does not have to struggle to hold the pen (Parkinson’s), they can see what they wrote (legal blindness), and they can remember all that was discussed (stroke).
Table 2.
Chronic Disease Self-Management Program sections and corresponding adaptations based on visual analytic results
| Self-Management sections and specific activity and topic | Examples of adapting the activity using a stroke case vignette to guide the discussion | Visual Analytic cluster color and co-morbidities that our case vignette stroke survivor also was experiencing |
|---|---|---|
| Session 1, Activity 4: Getting a good night sleep | In this case vignette, the stroke survivor has hemiparesis. The suggestion includes taking a water pill/diuretic (which was prescribed by her doctor because of the hypertension) during the day, so that the person will not be interrupted at night because of the need for frequent urination. | Blue cluster, hypertension and lung fibrosis |
| Session 2, Activity 3: Physical exercise brainstorm | In this case vignette, the stroke survivor has difficulty thinking clearly and remembering things. The suggestion includes exercising with friends, keep the exercises scheduled in her calendar, use reminders in her cell phone, and keep the exercises routine the same time of day. | Blue cluster, pneumonia and chronic obstructive pulmonary disorder |
| Session 3, Activity 3: Pain and fatigue | In this case vignette, the stroke survivor cannot independently walk or complete self-care tasks because of weakness. The suggestion is to mix activity with rest, and use relaxation and meditation techniques when in bed, first thing in the morning. | Purple cluster, cancer and anxiety |
| Session 4, Activity 3: Healthy eating | In this case vignette, the stroke survivor lives alone and also has double vision. The suggestion is to use an online meal service or online food delivery, this way she can check the food labels by enlarging the font, using techniques to reduce the double vision, in order to make healthy choices. | Pink cluster, morbid obesity and diabetes mellitus |
| Session 5, Activity 5: Dealing with depression | In this case vignette, the stroke survivor has vertigo from the stroke. The suggestion to how she can feel less depressed would be to call a friend, ask her doctor if home counseling sessions would be appropriate, complete deep breathing/meditation exercises, or even have a friend visit her in her home and do manicures. | Orange cluster, depression and osteoporosis |
| Session 6, Activity 2: Working with your healthcare provider and having good communication | In this case vignette, the stroke survivor has a spastic hand because of the stroke. The suggestion included the person using technology such as an iPad to write questions, because he could use the speak command option, instead of writing and make the font larger. | Yellow cluster, legally blind and Parkinson’s |
Discussion
Stroke is complex, and national guidelines recommend the use of self-management programs to support stroke survivors in coping with the long-term consequences and barriers to activity participation after the event (Holman & Lorig, 2004; van Veenendaal et al., 1996; Winstein et al., 2016). However, standardized self-management programs may not acknowledge multimorbidity among older stroke survivors. Therefore, this study fills this gap by pre-adapting the CDSMP, a self-management program shown to be very effective in older adults. To do this, we used a data-driven visual analytics approach that captured the complexity of relationships between older adult stroke survivors and their comorbidities. Based on these relationships, we included vignettes and activities to facilitate problem solving, decision-making, and action planning tailored to this population.
Our large data visual analytics approach revealed unique combinations of chronic comorbidities for stroke survivors readmitted to the hospital within 90 days of discharge. Specifically, we found five significant and specific profiles comprised of multiple co-occurring comorbidities, characterized as: 1) Obesity/Diabetes, 2) Immunity/Infection, Neurological Diseases, Major Psychiatric/Depression, 3) Brain/Vascular, Neurodegenerative Diseases, 4) Heart, Lungs/Pulmonary Diseases, and 5) Cancer, Other Psychiatric Disorders. These represent a complex combination of multiple comorbidities associated with readmission in a stroke group. Our findings confirm that stroke survivors have multiple comorbidities that co-occur in statistically and clinically significant patterns.
Exploring co-occurring comorbidities among subgroups of stroke survivors is necessary to improve our understanding of the challenges that impact self-management and to enhance medical management and rehabilitation following stroke. Clinicians and specialists must consider the application of the clusters to self-management strategies. However, clustered comorbidities could be interpreted as a pre-established group of conditions known to be related, a new relationship that has yet to be considered, or a relationship with limited clinical implications. Therefore, the quantitative analysis should be accompanied by qualitative analysis by domain experts. Implementing precision rehabilitation driven by large data visual analytics represents a promising technique with the potential to improve functional and medical outcomes while improving self-efficacy.
Comorbidities from each subgroup were used to pre-adapt an aspect of the CDSMP. Instead of the current approach of targeting single comorbidities (Fugazzaro et al., 2021; Wolf et al., 2017), our pre-adapted program facilitates the management of multiple commonly occurring comorbidities across stroke patients. The program includes traditional patient education with self-management tools in a peer group to encourage discussion in a supported environment led by CDSMP-trained facilitators. The purpose of including these adaptations was to ensure that the self-management intervention meets the needs of older adults who likely have multiple comorbidities. This is significant because we know comorbidities commonly co-occur in aging stroke survivors (Pearson et al., 2011). Adaptations were made to the discussion section that occurred prior to a CDSMP activity. Our approach to adaptation was to create fictional case vignettes using the visual analytics output and supported by literature to create a focused and tailored discussion instead of using an open discussion format. Consistent with the CDSMP, a case vignette was developed for each of the six sessions. The anticipated impact is that the pre-adapted self-management program will be more appropriate than standard self-management programs. This is in line with the findings from Ferretti’s work with adults aging with developmental disabilities where a modified CDSMP showed higher completion rates (Ferretti & McCallion, 2019).
There are some limitations to this study. The analysis was applied only to comorbidities within one dataset. Each patient subgroup has other characteristics (e.g., demographics and functional outcomes) which are not highlighted in the network. Such information could help in better understanding the patient subgroups, which could inform the design of the tailored interventions. Additionally, our data did not show whether patients received PAC services after their acute discharge. However, as the goal of this study was to pre-adapt a self-management program based on comorbidities, the comorbidities characterized would be applicable to those who received or did not receive PAC after discharge.
The main implication of this study is that the pre-adaptation provides a tailored program that bridges big data with actionable interventions for older adult stroke survivors. These pre-adaptations can be performed by community workers who are facilitating the management of comorbidities in this population. Pre-adaptation of the CDSMP acknowledges to the patients, families, caregivers, and providers that these comorbidities relate to one another.
There are many avenues for future research. Although our results were found to be statistically and clinically significant, the analysis should be repeated in an independent dataset to test whether the pattern of comorbidity co-occurrence replicates, using methods such as the Rand Index. Furthermore, as stroke disproportionately affects older adults, women, and ethnic minorities, future analyses should include not only comorbidities but also demographics and other relevant variables. Future clinical trials are needed to test the hypothesis that treating multiple symptoms and comorbidities with an adapted CDSMP will prevent future complications and health problems when managing multiple comorbidities. Finally, future research should include implementation studies to determine the feasibility of the now-adapted CDSMP for stroke survivors. In other words, methods of visual analytics and implementation science together may help inform the development of specific strategies that can address disparities in stroke care and improve healthcare outcomes.
In conclusion, our study provides an innovative application of visual analytics to target individualized self-management programs based on older adults’ commonly co-occurring comorbidities. Our work suggests an approach to improve upon patient education programs (Jones & Riazi, 2011) and capitalizes on self-management with a pre-adapted, tailored program for aging individuals with stroke. This approach in the future should allow more effective pre-adaptations of self-management programs and may help to increase self-efficacy and overall rehabilitation outcomes for older adults following stroke.
Supplementary Material
Funding:
National Institutes of Health (UL 1TR001439, P30 AG024832, K12 HD055929, P30 AG059301, K01 HD101589, K01 AG065492) and Agency for Healthcare Research and Quality (T32 HS026133).
Footnotes
Declaration of Conflicting Interests: The authors declare that there is no conflict of interest.
Data Availability:
The data used in this study were obtained from the Research Data Assistance Center (ResDAC) through a Data Use Agreement with the Centers for Medicare and Medicaid Services (CMS) and are not publicly available.
References
- Adepoju OE, Bolin JN, Phillips CD, Zhao H, Ohsfeldt RL, McMaughan DK, Helduser JW, & Forjuoh SN (2014). Effects of diabetes self-management programs on time-to-hospitalization among patients with type 2 diabetes: A survival analysis model. Patient Education and Counseling, 95(1), 111–117. 10.1016/j.pec.2014.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin SE (2020). Sleep in Patients With Neurologic Disease. Continuum (Minneapolis, Minn.), 26(4), 1016–1033. 10.1212/CON.0000000000000887 [DOI] [PubMed] [Google Scholar]
- Bhavnani S, Dang B, Caro M, Saade G, & Visweswaran S (2014). 698: Genetic differences reveal heterogeneity in spontaneous preterm birth pathophysiology: a visual analytical approach. American Journal of Obstetrics and Gynecology, 210, S343–S344. 10.1016/j.ajog.2013.10.731 [DOI] [Google Scholar]
- Bhavnani SK, Bellala G, Victor S, Bassler KE, & Visweswaran S (2012). The role of complementary bipartite visual analytical representations in the analysis of SNPs: A case study in ancestral informative markers. Journal of the American Medical Informatics Association: JAMIA, 19(e1), e5–e12. 10.1136/amiajnl-2011-000745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhavnani SK, Chen T, Ayyaswamy A, Visweswaran S, Bellala G, Rohit D, & Kevin E, B. (2017). Enabling Comprehension of Patient Subgroups and Characteristics in Large Bipartite Networks: Implications for Precision Medicine. AMIA Summits on Translational Science Proceedings, 2017, 21–29. [PMC free article] [PubMed] [Google Scholar]
- Bhavnani SK, Dang B, Bellala G, Divekar R, Visweswaran S, Brasier AR, & Kurosky A (2015). Unlocking proteomic heterogeneity in complex diseases through visual analytics. PROTEOMICS, 15(8), 1405–1418. 10.1002/pmic.201400451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhavnani SK, Dang B, Caro M, Bellala G, Visweswaran S, Mejias A, & Divekar R (2014). Heterogeneity within and across Pediatric Pulmonary Infections: From Bipartite Networks to At-Risk Subphenotypes. AMIA Joint Summits on Translational Science Proceedings. AMIA Joint Summits on Translational Science, 2014, 29–34. [PMC free article] [PubMed] [Google Scholar]
- Bhavnani SK, Dang B, Kilaru V, Caro M, Visweswaran S, Saade G, Smith AK, & Menon R (2018). Methylation Differences Reveal Heterogeneity in Preterm Pathophysiology: Results from Bipartite Network Analyses. Journal of Perinatal Medicine, 46(5), 509–521. 10.1515/jpm-2017-0126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhavnani SK, Drake J, Bellala G, Dang B, Peng B-H, Oteo JA, Santibañez-Saenz P, Visweswaran S, & Olano JP (2013). How Cytokines Co-occur across Rickettsioses Patients: From Bipartite Visual Analytics to Mechanistic Inferences of a Cytokine Storm. AMIA Joint Summits on Translational Science Proceedings. AMIA Joint Summits on Translational Science, 2013, 15–19. [PMC free article] [PubMed] [Google Scholar]
- Bhavnani SK, Drake J, & Divekar R (2014). The role of visual analytics in asthma phenotyping and biomarker discovery. Advances in Experimental Medicine and Biology, 795, 289–305. 10.1007/978-1-4614-8603-9_18 [DOI] [PubMed] [Google Scholar]
- Bhavnani SK, Victor S, Calhoun WJ, Busse WW, Bleecker E, Castro M, Ju H, Pillai R, Oezguen N, Bellala G, & Brasier AR (2011). How cytokines co-occur across asthma patients: From bipartite network analysis to a molecular-based classification. Journal of Biomedical Informatics, 44, S24–S30. 10.1016/j.jbi.2011.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady TJ, Murphy L, O’Colmain BJ, Beauchesne D, Daniels B, Greenberg M, House M, & Chervin D (2013). A meta-analysis of health status, health behaviors, and health care utilization outcomes of the Chronic Disease Self-Management Program. Preventing Chronic Disease, 10, 120112. 10.5888/pcd10.120112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady TM, Beauchesne D, bhalakia A, Chervin D, Daniels B, Greenberg M, House M, & O’Colmain B (2011). Sorting through the evidence for the arthritis self management program and the chronic disease self management program: Executive summary of ASMP/CDSMP meta-analyses. Centers for Disease Control and Prevention. https://stacks.cdc.gov/view/cdc/23175 [Google Scholar]
- Cameron-Tucker HL, Wood-Baker R, Owen C, Joseph L, & Walters EH (2014). Chronic disease self-management and exercise in COPD as pulmonary rehabilitation: A randomized controlled trial. International Journal of Chronic Obstructive Pulmonary Disease, 9, 513–523. 10.2147/COPD.S58478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan R, Ravi J, Datta P, Chen T, Schnappinger D, Bassler KE, Balázsi G, & Gennaro ML (2016). Reconstruction and topological characterization of the sigma factor regulatory network of Mycobacterium tuberculosis. Nature Communications, 7, 11062. 10.1038/ncomms11062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer SC, Wolf SL, Adams HP, Chen D, Dromerick AW, Dunning K, Ellerbe C, Grande A, Janis S, Lansberg MG, Lazar RM, Palesch YY, Richards L, Roth E, Savitz SI, Wechsler LR, Wintermark M, & Broderick JP (2017). Stroke Recovery & Rehabilitation Research: Issues, Opportunities, and the NIH StrokeNet. Stroke, 48(3), 813–819. 10.1161/STROKEAHA.116.015501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang B, Mathew J, Chen T, & Bhavnani S (2016). ExplodeLayout: Comprehending Patient Subgroups in Large Networks. AMIA. [Google Scholar]
- Eames S, Hoffmann T, Worrall L, & Read S (2010). Stroke patients’ and carers’ perception of barriers to accessing stroke information. Topics in Stroke Rehabilitation, 17(2), 69–78. 10.1310/tsr1702-69 [DOI] [PubMed] [Google Scholar]
- Evans M (2011). National Bureau of Economic Research Overview of software for the version 22 CMS-HCC risk-adjustment model. https://www.cms.gov/Medicare/Health-Plans/MedicareAdvtgSpecRateStats/downloads/evaluation_risk_adj_model_2011.pdf
- Ferretti LA, & McCallion P (2019). Translating the Chronic Disease Self-Management Program for Community-Dwelling Adults With Developmental Disabilities. Journal of Aging and Health, 31(10_suppl), 22S–38S. 10.1177/0898264318822363 [DOI] [PubMed] [Google Scholar]
- Franek J (2013). Self-management support interventions for persons with chronic disease: An evidence-based analysis. Ontario Health Technology Assessment Series, 13(9), 1–60. [PMC free article] [PubMed] [Google Scholar]
- Fugazzaro S, Denti M, Accogli MA, Costi S, Pagliacci D, Calugi S, Cavalli E, Taricco M, & Bardelli R (2021). Self-Management in Stroke Survivors: Development and Implementation of the Look after Yourself (LAY) Intervention. International Journal of Environmental Research and Public Health, 18(11), 5925. 10.3390/ijerph18115925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallacher KI, Batty GD, McLean G, Mercer SW, Guthrie B, May CR, Langhorne P, & Mair FS (2014). Stroke, multimorbidity and polypharmacy in a nationally representative sample of 1,424,378 patients in Scotland: Implications for treatment burden. BMC Medicine, 12, 151. 10.1186/s12916-014-0151-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruneir A, Griffith LE, Fisher K, Panjwani D, Gandhi S, Sheng L, Patterson C, Gafni A, Ploeg J, & Markle-Reid M (2016). Increasing comorbidity and health services utilization in older adults with prior stroke. Neurology, 87(20), 2091–2098. 10.1212/WNL.0000000000003329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman H, & Lorig K (2004). Patient Self-Management: A Key to Effectiveness and Efficiency in Care of Chronic Disease. Public Health Reports, 119(3), 239–243. 10.1016/j.phr.2004.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong I, Karmarkar A, Chan W, Kuo Y-F, Mallinson T, Ottenbacher KJ, Goodwin JS, Andersen CR, & Reistetter TA (2018). Discharge Patterns for Ischemic and Hemorrhagic Stroke Patients Going From Acute Care Hospitals to Inpatient and Skilled Nursing Rehabilitation. American Journal of Physical Medicine & Rehabilitation, 97(9), 636–645. 10.1097/PHM.0000000000000932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrell LN, & Kneipp SM (2017). Strategies for recruiting populations to participate in the chronic disease self-management program (CDSMP): A systematic review. Health Marketing Quarterly, 34(4), 268–283. 10.1080/07359683.2017.1375240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones F, & Riazi A (2011). Self-efficacy and self-management after stroke: A systematic review. Disability and Rehabilitation, 33(10), 797–810. 10.3109/09638288.2010.511415 [DOI] [PubMed] [Google Scholar]
- Karatepe AG, Gunaydin R, Kaya T, & Turkmen G (2008). Comorbidity in patients after stroke: Impact on functional outcome. Journal of Rehabilitation Medicine, 40(10), 831–835. 10.2340/16501977-0269 [DOI] [PubMed] [Google Scholar]
- Kirk MA, Moore JE, Wiltsey Stirman S, & Birken SA (2020). Towards a comprehensive model for understanding adaptations’ impact: The model for adaptation design and impact (MADI). Implementation Science, 15(1), 56. 10.1186/s13012-020-01021-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen HK, Tistad M, Koch L. von, & Ytterberg C (2016). The Importance of Patient Involvement in Stroke Rehabilitation. PLOS ONE, 11(6), e0157149. 10.1371/journal.pone.0157149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorig KR, & Holman H (2003). Self-management education: History, definition, outcomes, and mechanisms. Annals of Behavioral Medicine: A Publication of the Society of Behavioral Medicine, 26(1), 1–7. 10.1207/S15324796ABM2601_01 [DOI] [PubMed] [Google Scholar]
- Lorig KR, Ritter P, Stewart AL, Sobel DS, Brown BW, Bandura A, Gonzalez VM, Laurent DD, & Holman HR (2001). Chronic disease self-management program: 2-year health status and health care utilization outcomes. Medical Care, 39(11), 1217–1223. [DOI] [PubMed] [Google Scholar]
- Lorig KR, Sobel DS, Stewart AL, Brown BW, Bandura A, Ritter P, Gonzalez VM, Laurent DD, & Holman HR (1999). Evidence suggesting that a chronic disease self-management program can improve health status while reducing hospitalization: A randomized trial. Medical Care, 37(1), 5–14. [DOI] [PubMed] [Google Scholar]
- Lui SK, & Nguyen MH (2018). Elderly Stroke Rehabilitation: Overcoming the Complications and Its Associated Challenges. Current Gerontology and Geriatrics Research, 2018. 10.1155/2018/9853837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann SK, & Lawrence CB (2020). Comorbidity and age in the modelling of stroke: Are we still failing to consider the characteristics of stroke patients? BMJ Open Science, 4(1), e100013. 10.1136/bmjos-2019-100013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan PT (2012). Self-management education and support in chronic disease management. Primary Care, 39(2), 307–325. 10.1016/j.pop.2012.03.005 [DOI] [PubMed] [Google Scholar]
- Middleton A, Kuo Y-F, Graham JE, Karmarkar A, Lin Y-L, Goodwin JS, Haas A, & Ottenbacher KJ (2018). Readmission Patterns Over 90-Day Episodes of Care Among Medicare Fee-for-Service Beneficiaries Discharged to Post-acute Care. Journal of the American Medical Directors Association, 19(10), 896–901. 10.1016/j.jamda.2018.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després J-P, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, … American Heart Association Statistics Committee and Stroke Statistics Subcommittee. (2015). Heart disease and stroke statistics--2015 update: A report from the American Heart Association. Circulation, 131(4), e29–322. 10.1161/CIR.0000000000000152 [DOI] [PubMed] [Google Scholar]
- Mozzafarian D, Benjamin EJ, Go AS, & et al. on behalf of the American Heart Association Statistics Committee ande Stroke Statistics Subcommittee. (2015). Heart disease and stroke statistics—2015 update: A report from the American Heart Association. Circulation, 131, e29–e322. [DOI] [PubMed] [Google Scholar]
- Newman M (2010). Networks: An Introduction. In Networks (1st ed.). Oxford University Press. https://oxford.universitypressscholarship.com/view/10.1093/acprof:oso/9780199206650.001.0001/acprof-9780199206650 [Google Scholar]
- Ory MG, Ahn S, Towne SD, & Smith ML (2015). Chronic Disease Self-Management Education: Program Success and Future Directions. In Malone ML, Capezuti EA, & Palmer RM (Eds.), Geriatrics Models of Care: Bringing “Best Practice” to an Aging America (pp. 147–153). Springer International Publishing. 10.1007/978-3-319-16068-9_12 [DOI] [Google Scholar]
- Pearson WS, Bhat-Schelbert K, & Probst JC (2011). Multiple Chronic Conditions and the Aging of America: Challenge for Primary Care Physicians. Journal of Primary Care & Community Health. 10.1177/2150131911414577 [DOI] [PubMed] [Google Scholar]
- Prior PL, & Suskin N (2018). Exercise for stroke prevention. Stroke and Vascular Neurology, 3(2), 59–68. 10.1136/svn-2018-000155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeulders ESTF, van Haastregt JCM, Ambergen T, Janssen-Boyne JJJ, van Eijk JTM, & Kempen GIJM (2009). The impact of a self-management group programme on health behaviour and healthcare utilization among congestive heart failure patients. European Journal of Heart Failure, 11(6), 609–616. 10.1093/eurjhf/hfp047 [DOI] [PubMed] [Google Scholar]
- Stirman SW, Miller CJ, Toder K, & Calloway A (2013). Development of a framework and coding system for modifications and adaptations of evidence-based interventions. Implementation Science: IS, 8, 65. 10.1186/1748-5908-8-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The R Foundation. (2019). R: The R Project for Statistical Computing. R Core Team. https://www.r-project.org/ [Google Scholar]
- Treviño S, Nyberg A, Genio CID, & Bassler KE (2015). Fast and accurate determination of modularity and its effect size. Journal of Statistical Mechanics: Theory and Experiment, 2015(2), P02003. 10.1088/1742-5468/2015/02/P02003 [DOI] [Google Scholar]
- van Veenendaal H, Grinspun DR, & Adriaanse HP (1996). Educational needs of stroke survivors and their family members, as perceived by themselves and by health professionals. Patient Education and Counseling, 28(3), 265–276. 10.1016/0738-3991(95)00853-5 [DOI] [PubMed] [Google Scholar]
- Wagner EH, Austin BT, Davis C, Hindmarsh M, Schaefer J, & Bonomi A (2001). Improving chronic illness care: Translating evidence into action. Health Affairs (Project Hope), 20(6), 64–78. 10.1377/hlthaff.20.6.64 [DOI] [PubMed] [Google Scholar]
- Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, Deruyter F, Eng JJ, Fisher B, Harvey RL, Lang CE, MacKay-Lyons M, Ottenbacher KJ, Pugh S, Reeves MJ, Richards LG, Stiers W, Zorowitz RD, & American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Quality of Care and Outcomes Research. (2016). Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke, 47(6), e98–e169. 10.1161/STR.0000000000000098 [DOI] [PubMed] [Google Scholar]
- Wolf TJ, Spiers MJ, Doherty M, & Leary EV (2017). The effect of self-management education following mild stroke: An exploratory randomized controlled trial. Topics in Stroke Rehabilitation, 24(5), 345–352. 10.1080/10749357.2017.1289687 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this study were obtained from the Research Data Assistance Center (ResDAC) through a Data Use Agreement with the Centers for Medicare and Medicaid Services (CMS) and are not publicly available.