Abstract
We have studied the effects of CC-chemokines on human immunodeficiency virus type 1 (HIV-1) infection, focusing on the infectivity enhancement caused by RANTES. High RANTES concentrations increase the infectivity of HIV-1 isolates that use CXC-chemokine receptor 4 for entry. However, RANTES can have a similar enhancing effect on macrophagetropic viruses that enter via CC-chemokine receptor 5 (CCR5), despite binding to the same receptor as the virus. Furthermore, RANTES enhances the infectivity of HIV-1 pseudotyped with the envelope glycoprotein of murine leukemia virus or vesicular stomatitis virus, showing that the mechanism of enhancement is independent of the route of virus-cell fusion. The enhancing effects of RANTES are not mediated via CCR5 or other known chemokine receptors and are not mimicked by MIP-1α or MIP-1β. The N-terminally modified derivative aminooxypentane RANTES (AOP-RANTES) efficiently inhibits HIV-1 infection via CCR5 but otherwise mimics RANTES by enhancing viral infectivity. There are two mechanisms of enhancement: one apparent when target cells are pretreated with RANTES (or AOP-RANTES) for several hours, and the other apparent when RANTES (or AOP-RANTES) is added during virus-cell absorption. We believe that the first mechanism is related to cellular activation by RANTES, whereas the second is an increase in virion attachment to target cells.
The infection of CD4+ target cells by human immunodeficiency virus type 1 (HIV-1) is initially mediated by interactions between the viral envelope glycoproteins, the CD4 receptor, and coreceptors. The most commonly used coreceptors are CXC-chemokine receptor 4 (CXCR4) and CC-chemokine receptor 5 (CCR5) (2, 9, 10, 15, 20, 24–26, 29, 51, 81, 89, 92). Virus entry can be inhibited by either CXC- or CC-chemokines, depending on whether fusion is mediated by CXCR4 or by CCR5 (11, 17, 20, 26, 60, 61). The most active of these chemokines are the CCR5 ligands RANTES, MIP-1α, and MIP-1β (71, 72) and the CXCR4 ligand SDF-1α (11, 60). Natural or synthetic modifications of the N termini of these chemokines to alter the nature of their interactions with receptors can affect their anti-HIV-1 activity (5, 37, 62, 70, 76). A few other CC-chemokines, macrophage chemotactic proteins 2 and -3 (MCP-2 and MCP-3), macrophage-derived chemokine (MDC), and I-309 have also been reported to inhibit HIV-1 infection (30, 40, 64, 75). The inhibitory effects of MDC and MCP-3 are probably not exerted via a known HIV-1 coreceptor (64, 75), and the status of MDC as an antiviral agent is now in question (6, 21, 46).
How chemokines inhibit HIV-1 infection is complex. The cognate chemokines can directly inhibit the interaction between the viral gp120 glycoprotein and the coreceptor (79, 89). Down-regulation of coreceptors as a result of chemokine binding reduces the efficiency of viral entry (3, 4, 35, 47, 81). In addition, signals transduced into the cell by the receptor interactions of chemokines might interfere with transcription of the HIV-1 genome, although this has not been demonstrated (31). We have also found significant donor-to-donor variation in the effect of CC-chemokines on HIV-1 replication in primary CD4+ T cells (80, 81). Contributing to this variation could be the considerable range in CCR5 expression (50, 65, 79, 90), and/or in the extent of endogenous CC-chemokine production (65, 66), that occurs between different individuals.
Adding to the complexity are cell-type-dependent variations in the sensitivity of HIV-1 replication to CC-chemokines. This is manifested by studies of macrophages/monocytes. RANTES, MIP-1α, and/or MIP-1β have been variously described as strong inhibitors (2, 12, 91), weak inhibitors (22, 26, 33, 36, 42, 58, 76), or activators (74) of HIV-1 Env-mediated membrane fusion and/or replication in these cells. HIV-1 infection of human osteosarcoma cells is also insensitive to CC-chemokines (20). In addition, high concentrations of RANTES, MIP-1α, or MIP-1β can increase the replication of T-cell line-tropic (X4 or R5X4) HIV-1 strains that enter CD4+ T cells via CXCR4, which is not a receptor for CC-chemokines (22, 43, 56, 75). Lower concentrations of MIP-1α also have an enhancing effect on X4 or R5X4 virus replication in tissue blocks (48). These effects of CC-chemokines are presumably exerted independently of any direct interaction with a functioning HIV-1 coreceptor, although it is uncertain whether a single mechanism can account for all of the observations (22, 43, 48, 56, 75).
The interactions between HIV-1, chemokines, and target cells are clearly multifaceted. To understand some of the underlying phenomena, we have studied the effects of CC-chemokines on HIV-1 replication in nonlymphoid cell lines expressing different coreceptors. We conclude that RANTES and AOP-RANTES, but not MIP-1α or MIP-1β, increases HIV-1 infection independently of the nature of the coreceptor used for viral entry. Indeed, RANTES and the N-terminally modified derivative aminooxypentane RANTES (AOP-RANTES) enhance the infectivity of pseudotyped viruses that do not enter cells via known chemokine receptors. The activation of HIV-1 infection by RANTES could help explain why CC-chemokines are often found to be relatively poor inhibitors of HIV-1 replication in macrophages or other nonlymphoid cells; any inhibitory action of RANTES on virus-cell fusion mediated via CCR5 may be counteracted, wholly or in part, by an increase in the efficiency of other processes involved in viral infection.
MATERIALS AND METHODS
Cells.
HeLa-CD4 and HeLa-CD4-CCR5 cell lines were obtained from D. Kabat (44). GHOST cell lines and U87MG-CD4 cell lines stably expressing CCR5 and CXCR4 were provided by V. KewalRamani and D. Littman (38, 92). COS-CD4 and 3T3-CD4 cells were from T. Dragic (26), and 293T cells were from the American Type Culture Collection (Rockville, Md.) (20). All cell lines were maintained in Dulbecco’s minimal essential medium containing 10% fetal calf serum, glutamine, and antibiotics and were split twice a week. The medium for GHOST cell lines was supplemented with G418 (500 μg/ml), puromycin (1 μg/ml), and hygromycin (100 μg/ml). The medium for U87MG-CD4 cell lines was supplemented with puromycin (1 μg/ml) and neomycin (300 μg/ml). All of these chemicals were obtained from Sigma Chemicals, Inc. (St. Louis, Mo.).
Chemokines.
The recombinant human chemokines MIP-1α, MIP-1β, MCP-1, MCP-3, and RANTES were purchased from R&D Systems Inc. (Minneapolis, Minn.). RANTES was also provided by Serono Pharmaceutical Research Institute (Geneva, Switzerland). Since the two preparations gave identical results, we do not indicate which was used in what experiments. AOP-RANTES was also provided by Serono (76). SDF-1α was from Gryphon Sciences (San Francisco, Calif.).
Viruses.
Pseudotyped luciferase reporter viruses were produced by the calcium phosphate technique (14, 18, 20, 26). Thus, 293T cells were cotransfected with the envelope-deficient NL4-3 construct pNL-Luc and with a pSV vector expressing viral envelope glycoproteins (14, 18, 20). The pNL-Luc virus carries the luciferase reporter gene; the various pSV vectors express envelope glycoproteins derived from HIV-1 isolate ADA or HxB2, the amphotropic murine leukemia virus (MuLV), or the vesicular stomatitis virus (VSV) G protein (VSV-G) (18, 20, 26, 27).
Pseudotyped viruses carrying the guanine-hypoxanthine phosphoribosyltransferase (Gpt) resistance gene (R7-3/gpt viruses) were prepared by cotransfecting 293T cells with the envelope-deficient R7-3/HIV-gpt vector and the pSV vector expressing the amphotropic MuLV envelope glycoproteins (45, 87). Pseudotyped Moloney MuLV (MoMuLV) carrying the puromycin resistance gene (MoMuLV/puromycin) was prepared by cotransfecting 293T cells with the puromycin vector pBABEpuro, the MoMuLV gag-pol core vector, and the pSV vector expressing the MoMuLV envelope (45, 52).
In each case, fresh medium was added to the cells 20 h after transfection. The supernatant was collected 48 h posttransfection, centrifuged for 10 min at 6,000 rpm, and filtered through a 0.22-μm-pore-size filter. Virus stocks were analyzed for p24 antigen concentration by an in-house p24 antigen enzyme-linked immunosorbent assay as described previously (82). Pseudotyped MoMuLV was used as a 1:25 dilution of the stock. This virus preparation was not adjusted for core protein concentration.
Infection assay with luciferase readout.
The extent of HIV-1 entry was determined by an assay based on single-cycle infection (14, 18, 20, 26). One day before infection, cells were seeded into 96-well tissue culture plates at a density of 104 per well unless otherwise stated. After 24 h, the cells were infected with virus (5 ng of p24 antigen unless otherwise stated) for 2 h at 37°C in the presence or absence of chemokines, in a total infection volume of 100 μl. Unbound virus was then removed by washing, and fresh medium containing or lacking chemokines (as appropriate) in a total volume of 100 μl was added back to the cells, unless otherwise stated. Seventy-two hours postinfection, the cells were washed once with phosphate-buffered saline and lysed in 50 μl of 1× reporter lysis buffer (Promega Inc.). The luciferase activity in a mixture of 100 μl of luciferase substrate (Promega) and 30 μl of cell lysate was measured in relative light units, using a DYNEX MLX microplate luminometer.
Infection assay with virus carrying the Gpt resistance gene.
One day before infection, HeLa-CD4 cells were seeded into 24-well tissue culture plates at a density of 2 × 104 per well. Infection was with 10 ng of virus (as determined by p24 antigen content) in a total volume of 300 μl, in the absence of Polybrene. RANTES was added to the cells simultaneously at a concentration of 2.5 or 5 μg/ml, and the infection was allowed to proceed for 2 h at 37°C. The cells were then washed once with medium and fed with fresh medium containing the appropriate concentration of RANTES. After a further 48 h, the cells were split 1/16, 1/32, 1/320, 1/3,200, 1/32,000, and 1/320,000 into Gpt selection medium (45) and then fed every 3 days with selection medium. On day 17 postinfection, Gpt-resistant colonies were stained with an aqueous solution of 0.2% crystal violet–25% isopropanol–5% acetic acid and counted.
Infection assay with virus carrying puromycin resistance gene.
The procedure was as used with the Gpt-resistant virus except that after the 48-h incubation, the cells were split into selection medium containing 2 μg of puromycin per ml (52). The cells were then fed every 3 days with selection medium. On day 13 postinfection, puromycin-resistant colonies were stained as described above and counted.
RESULTS
Differential effect of RANTES and AOP-RANTES on HIV-1 infection of human cell lines.
Previous studies had suggested that HIV-1 replication in several nonlymphoid cells, including macrophages, was relatively insensitive to inhibition by RANTES (22, 29, 33, 36, 42, 58, 74, 76). In contrast, AOP-RANTES, a modified RANTES molecule with altered biological properties, is a potent inhibitor of HIV-1 replication in CD4+ T cells, macrophages, and CD4− and CCR5-transfected GHOST cells (47, 76). We therefore compared the effects of these two CCR5 ligands on the early stages of HIV-1 replication in different human, nonlymphoid cell lines, engineered to express CCR5 (Fig. 1). For these experiments, we used a single-cycle assay with Env-pseudotyped, luciferase-expressing HIV-1 recombinants (14, 18, 20, 26). In such an assay, a positive luciferase readout requires that the pseudotyped virus enter the target cell, integrate its genome into the host DNA, and have its genome (including the luciferase enzyme) transcribed and translated.
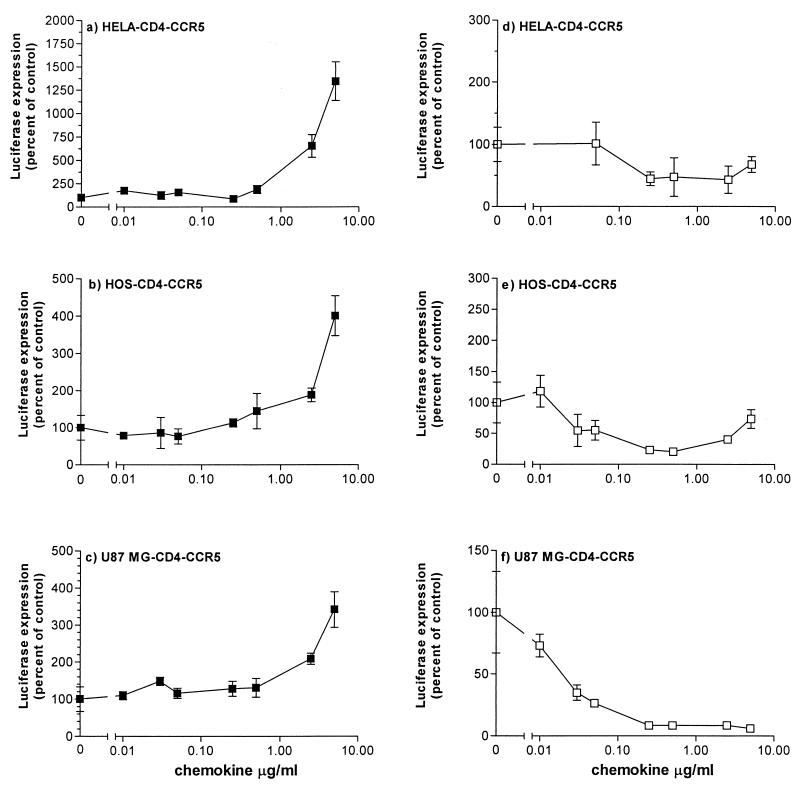
FIG. 1.
Effects of RANTES and AOP-RANTES on infection of different target cells by HIV-1 ADA pseudotypes. Cells were infected with HIV-1 ADA pseudotypes in the presence or absence of the indicated concentrations of RANTES (a to c, ■) or AOP-RANTES (d to f, □). Unbound virus was removed after a 2-h incubation, and cultures were replenished with medium containing the same concentration of CC-chemokine. Luciferase expression was measured on day 3 postinfection and presented as percentage of control (no RANTES, considered 100%). a and d, HeLa-CD4-CCR5 cells; b and e, HOS-CD4-CCR5 cells; c and f, U87MG-CD4-CCR5 cells.
Using pseudotypes containing the envelope glycoproteins of HIV-1 ADA, a classic macrophagetropic, R5 virus (23), we observed that the simultaneous addition of RANTES strongly increased luciferase expression in HeLa-CD4-CCR5 (cervical carcinoma) cells (Fig. 1a), in HOS-CD4-CCR5 (human osteosarcoma) cells (Fig. 1b), and in U87MG-CD4-CCR5 (human glioblastoma-astrocytoma) cells (Fig. 1c). The extent of enhancement was significant; in some experiments involving HIV-1 ADA infection of HeLa-CD4-CCR5 cells, the increase in luciferase activity was >100-fold (see below), but a 5- to 20-fold enhancement was more common in HeLa and HOS cells at the highest RANTES concentrations routinely tested (5 μg/ml) (Fig. 1). A lesser but still obvious effect was seen with U87MG-CD4-CCR5 cells (Fig. 1c). For all cell types, the enhancement of viral infectivity by RANTES was dose dependent in the range 0.5 to 5.0 μg/ml (Fig. 1).
In contrast to RANTES, AOP-RANTES was almost invariably an inhibitor of HIV-1 ADA infection under the same assay conditions. Dose-dependent inhibitory effects were observed at AOP-RANTES concentrations above approximately 50 ng/ml, the extent of suppression being usually around 10-fold (Fig. 1d to f). However, in HOS-CD4-CCR5 cells there was a modest but consistent reversal of the inhibitory effect of AOP-RANTES at the highest concentrations tested (Fig. 1e).
RANTES, but neither MIP-1α nor MIP-1β, enhances HIV-1 infection independently of the coreceptor used for entry.
The HIV-1 ADA pseudotypes did not detectably infect HeLa-CD4, HOS-CD4, or U87-CD4 cells that did not express CCR5, whether RANTES was present or not, indicating that entry of this virus was strictly CCR5 dependent (data not shown). The enhancement of luciferase expression by RANTES in the CCR5-expressing cells therefore presents an apparent paradox: RANTES should occlude and/or down-regulate CCR5 and prevent HIV-1 ADA pseudotypes from using it for entry, but instead it significantly increases the extent of CCR5-dependent HIV-1 infection. The pattern of data observed therefore suggests that RANTES must have multiple effects on the early stages of the HIV-1 life cycle.
To reduce the complexity of the experimental system and facilitate an analysis of the underlying phenomena, we studied the actions of chemokines on infection mediated by the envelope glycoproteins of the T-lymphotropic X4 virus HxB2. The HxB2-pseudotyped virus enters HeLa-CD4 cells via CXCR4 that is endogenously expressed by these cells, and so infection is CCR5 independent. Infection of HeLa-CD4 cells by HxB2 pseudotypes was enhanced by the simultaneous addition of RANTES, but MIP-1α, MIP-1β, MCP-1, and MCP-3 had no effect, and the CXCR4 ligand SDF-1α inhibited infection (Fig. 2a and b). The same pattern of data was found when HeLa-CD4 cells which stably expressed transfected CCR5 were used instead (Fig. 2c and data not shown). These results therefore establish that the enhancing effect of RANTES is independent of the coreceptor used by HIV-1 to enter the target cells (in this case, CXCR4) and that the presence of CCR5 on the target cells is not necessary for RANTES to exert its effect (since it occurs both in HeLa-CD4 cells and HeLa-CD4-CCR5 cells). The latter conclusion is consistent with the inability of MIP-1α and MIP-1β to mimic the enhancing effect of RANTES (Fig. 2a to c).
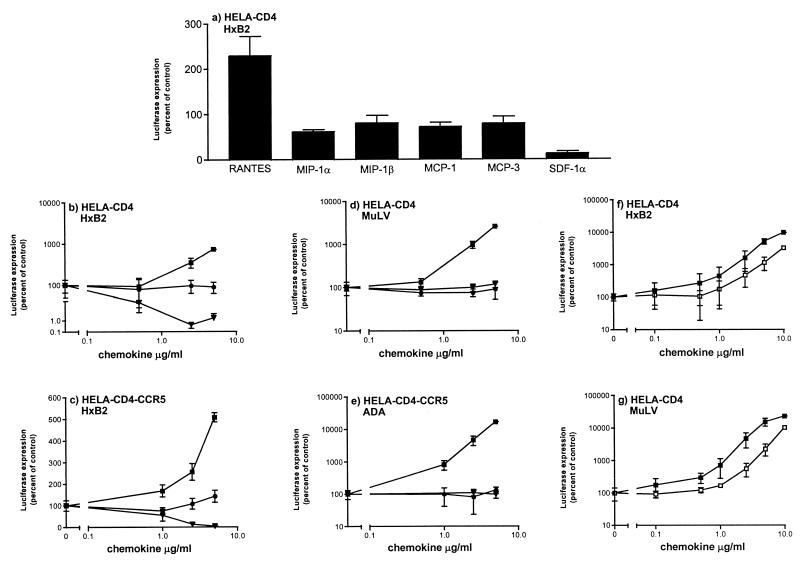
FIG. 2.
Effects of CC- and CXC-chemokines on infection of different target cells by HIV-1 pseudotypes. Cells were infected with pseudotyped viruses in the presence or absence of indicated concentrations of chemokines. Unbound virus was removed after a 2-h incubation, and cultures were replenished with medium containing the same concentration of chemokine. Luciferase expression was measured on day 3 postinfection and presented as percentage of control (no chemokine, considered 100%). (a) HeLa-CD4 cells were infected with HIV-1 HxB2-pseudotyped in the presence of 2.5 μg of RANTES, MIP-1α, MIP-1β, MCP-1, MCP-3, or SDF-1α per ml or with no added chemokine. (b to g) The cells were incubated with the indicated concentrations of RANTES (■), AOP-RANTES (□), MIP-1β (•), or SDF-1α (▾). The target cells were HeLa-CD4 (a, b, d, f, and g) and HeLa-CD4-CCR5 (c and e). The Env pseudotypes were HIV-1 HxB2 (a, b, c, and f) MuLV (d and g), and HIV-1 ADA (e). Viral inocula were the equivalents of 5 ng (a, b, and d), 1.5 ng (c), 3.5 ng (e), 30 ng (f), and 4 ng (g) of p24 antigen.
RANTES enhances infection by MuLV and VSV pseudotypes of HIV-1.
To investigate whether RANTES could enhance viral infectivity independently of the use of any chemokine receptor, we studied its effect on the entry of HIV-1 pseudotyped by the envelope glycoproteins of amphotropic MuLV. This virus does not possess HIV-1 envelope glycoproteins and is not known to use a chemokine receptor for entry; its receptor is an ubiquitously expressed phosphate transporter (49).
Infection of HeLa-CD4 cells mediated by the MuLV envelope glycoproteins was enhanced by RANTES to approximately the same extent as infection supported by envelope glycoproteins from HIV-1 HxB2 or HIV-1 ADA (Fig. 2). Indeed, MuLV Env-mediated infection of HeLa-CD4 cells was indistinguishable from HIV-1 ADA infection of HeLa-CD4-CCR5 cells in respect of its responsiveness to RANTES, MIP-1β, and SDF-1α (compare Fig. 2d and e). Unlike HxB2 entry, however, infection of HeLa-CD4 cells by the MuLV envelope pseudotypes was not inhibited by SDF-1α, since it is CXCR4 independent (compare Fig. 2b and d). The presence of CCR5 was not necessary for RANTES enhancement of MuLV pseudotypes, since similar results were obtained in HeLa-CD4 and HeLa-CD4-CCR5 cells (data not shown).
In contrast to RANTES, AOP-RANTES is an efficient inhibitor of CCR5-mediated entry of HIV-1 ADA pseudotypes (Fig. 1). However, when viral infection was independent of CCR5, AOP-RANTES mimicked RANTES by causing infectivity enhancement. This was observed for both HIV-1 HxB2 pseudotypes (Fig. 2f) and MuLV pseudotypes (Fig. 2g), again showing that the enhancement was not dependent on the receptor used for virus entry. Slightly higher concentrations of AOP-RANTES than of RANTES were required for infectivity enhancement; typically, the dose-response curves for AOP-RANTES were shifted to the right about threefold (Fig. 2f and g; see also Fig. 1b and e).
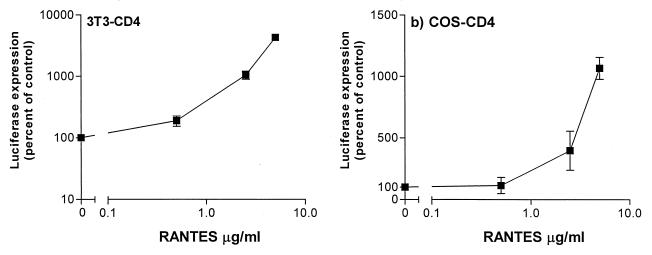
HIV-1 pseudotyped by the MuLV envelope glycoproteins will also efficiently enter nonhuman cells. The infection of 3T3-CD4 (murine) and COS-CD4 (simian) cells by MuLV pseudotypes was also enhanced by RANTES, showing that the effects of this chemokine are not restricted to human cells (Fig. 3).
FIG. 3.
RANTES enhances infection of nonhuman cells by MuLV pseudotypes. Murine 3T3-CD4 cells (5 × 104 per well of a 24-well plate) (a) and simian COS-CD4 cells (104 per well of a 96-well plate) (b) were infected with MuLV Env-pseudotyped HIV-1 in the presence or absence of the indicated concentrations of RANTES. Viral inocula were the equivalents of 200 ng of HIV-1 p24 antigen for the 3T3-CD4 cells 11 ng for the COS-CD4 cells. Unbound virus was removed after a 2-h incubation, and cultures were replenished with medium containing the same concentration of RANTES. Luciferase expression was measured on day 3 postinfection and presented as percentage of control (no RANTES, considered 100%).
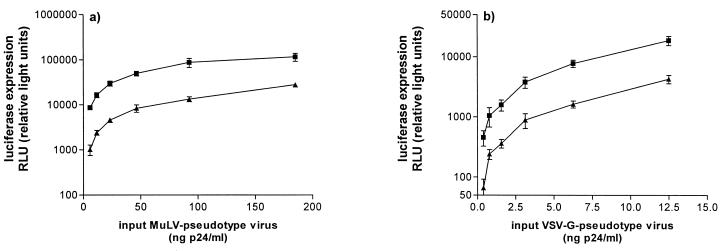
Both the HIV-1 and MuLV envelope glycoproteins enable the pseudotyped virus to enter the host cell via fusion at the cell membrane. To see whether RANTES was able to enhance viral replication irrespective of the route of entry, we prepared pseudotypes that contained VSV-G. VSV enters cells by receptor-mediated endocytosis triggered by an interaction of VSV-G with its cell surface receptor. The viral core is then released into the cytoplasm by a pH-dependent fusion reaction with the endosomal membrane (1). When RANTES was added with the viral inoculum, there was significantly enhanced infection of HeLa-CD4 cells by the VSV Env-pseudotyped virus (Fig. 4b). This was indistinguishable from the enhancement seen with MuLV Env pseudotypes (Fig. 4a). The enhancing effect of RANTES is therefore independent of the mechanism of virus-cell fusion, not just of the receptor system used to mediate fusion at the plasma membrane.
FIG. 4.
RANTES enhances the infectivity of both VSV and MuLV pseudotypes. HeLa-CD4 cells were infected with various concentrations of MuLV-pseudotyped (a) and VSV-G-pseudotyped (b) viruses in the presence (■) or absence (▴) of 5 μg of RANTES per ml. Unbound virus was removed after a 2-h incubation, and cultures were replenished with medium containing the same concentration of RANTES.
RANTES does not increase transcription of the HIV-1 genome postentry.
RANTES can be a comitogen for T-cell proliferation stimulated via CD3 (41, 78, 83, 88). A unifying explanation for the enhancement of viral replication by RANTES would therefore be that the chemokine promotes cell proliferation, leading to an increase in the efficiency of HIV-1 genome transcription. Such an effect would enhance luciferase expression from the Env-pseudotyped reporter viruses we have used, in addition to increasing the replication of wild-type virus in more conventional assays used by others (22, 44, 48, 56, 75). However, when RANTES was added at 2.5 μg/ml to HeLa-CD4 cells under the culture conditions used in the infection experiments, there was no increase in [3H]thymidine incorporation (data not shown). It therefore seems unlikely that a nonspecific effect of RANTES on cellular metabolism could account for the enhancement of viral replication.
To test more directly whether RANTES increased HIV-1 genome transcription, we used two separate envelope-defective proviral constructs: the HxB2 derivative R7-3/gpt, which encodes the Gpt resistance gene (44), and MoMuLV/puromycin (53). Both viruses were pseudotyped with the amphotropic MuLV envelope glycoproteins and used in single-round infection assays in the presence and absence of RANTES. The addition of RANTES increased by 25- to 30-fold the number of HeLa-CD4 cells transduced by the HIV-1 and MoMuLV drug-resistant pseudotypes (Table 1).
TABLE 1.
Integration of pseudotyped HIV and MoMuLV in the presence of RANTESa
| RANTES concn (μg/ml) | MuLV Env + R7-3/gpt
|
MuLV Env + MoMuLV/puromycin
|
||
|---|---|---|---|---|
| No. of resistant colonies | % Control | No. of resistant colonies | % Control | |
| 0 (untreated control) | 464 | 100 | 2,695 | 100 |
| 2.5 | 11,840 | 2,552 | ND | ND |
| 5.0 | 13,760 | 2,966 | 33,050 | 1,226 |
HeLa-CD4 cells were infected with MuLV-pseudotyped R7-3/gpt and MoMuLV/puromycin constructs in the presence or absence of the indicated concentrations of RANTES (see Materials and Methods). Drug-resistant colonies were counted after 13 to 17 days of drug treatment, to provide a measurement of viral integration. ND, not determined.
The readout of these assays should be unaffected by any factors that influence transcription efficiency, since the number of drug-resistant cell colonies, not just the amount of a specific gene product (e.g., p24 antigen or luciferase), is recorded. However, to confirm that there was no effect of RANTES at the transcription level, we performed control experiments in which RANTES-treated and untreated cells were transfected with the R7-3/gpt and MoMuLV/puromycin proviral constructs used in the infection experiments. In these experiments, RANTES had no effect on the number of colonies scored in either system (data not shown). Hence, these assays provide a direct measure of virus entry and integration and not of transcription. The enhancing effect of RANTES is therefore independent of the transcriptional level. Furthermore, the nature of the viral core is also irrelevant, since similar degrees of enhancement were observed with the R7-3gpt virus (HIV-1 core) and the MoMuLV/puromycin (MoMuLV core) (Table 1).
RANTES and AOP-RANTES have multiple effects on early stages of the HIV-1 life cycle.
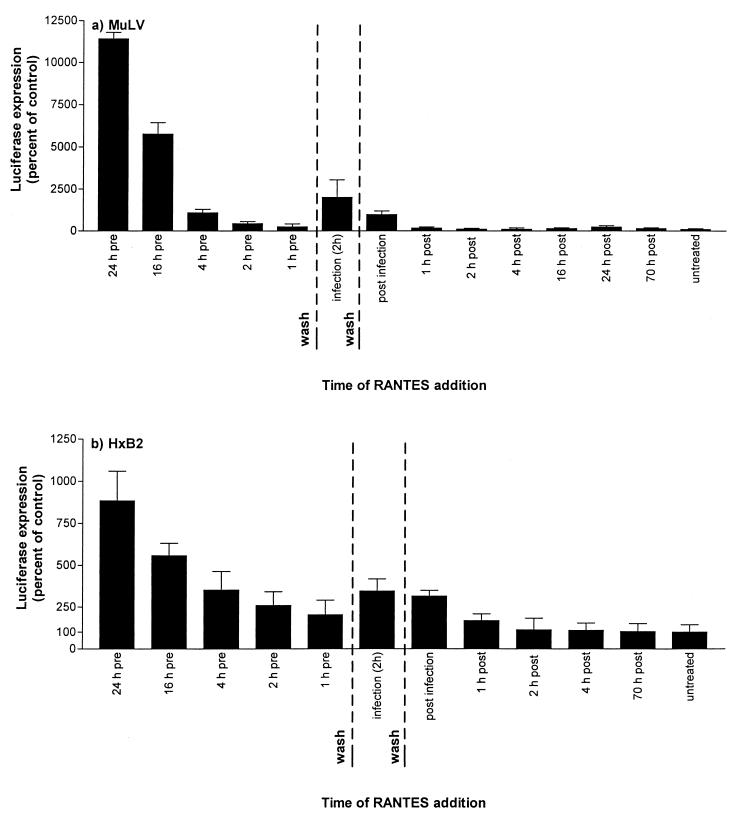
Taken together, the above experiments show that RANTES and AOP-RANTES activate a stage in the virus life cycle that is independent of the specific receptors and fusion mechanism and that does not occur at the transcriptional stage. To gain further insights into the nature of the enhancement effect, we performed experiments in which the time of RANTES addition to the target cells was varied. In one such experiment (Fig. 5a), the infecting virus was MuLV Env-pseudotyped HIV-1 and the target cells were HeLa-CD4. The enhancement of viral infection was greatest when the target cells were pretreated with RANTES for 24 h and then washed prior to virus addition. In the experiment shown, MuLV Env-pseudotyped HIV-1 infection was increased 114-fold under these conditions (Fig. 5a). Decreasing the RANTES preincubation period to 16 h reduced the extent of enhancement to 58-fold; after a 1-h preincubation, only a 2.5-fold increase in viral infection occurred in the presence of RANTES. However, when the virus inoculum and RANTES were added simultaneously and left together on the cells for the standard 2-h infection period, the extent of the RANTES-induced enhancement increased to 20-fold (Fig. 5a). When RANTES was added immediately after the cells were washed to remove free virus, some enhancement of infection occurred (9.7-fold). However, little or no enhancement occurred when RANTES was added as soon as 1 h postinfection, in the absence of any cell-free virus (1.6-fold).
FIG. 5.
Time of addition affects the extent of RANTES-mediated infectivity enhancement. RANTES (2.5 μg/ml) was added to HeLa-CD4 cells at the indicated times before, during, or after infection with MuLV (a) or HIV-1 HxB2 (b) Env pseudotypes. Viral inocula were the equivalents of 3 ng (a) and 5.8 ng (b) of HIV-1 p24 antigen. The infection period, defined as the time when the virus inoculum was in contact with the cells, lasted 2 h. For the pretreatment time points, RANTES was added to the cells at the indicated time before infection was initiated, and then the RANTES-containing medium was removed immediately before the addition of virus. RANTES was absent for the 2-h infection period and subsequently. For the “infection” time point, RANTES was added during the 2-h infection period but was not present before or after this time. For the time point marked “post infection,” RANTES was added to the cells immediately after the virus inoculum had been removed at the end of the 2-h infection period. For all other postinfection time points, RANTES was added at the indicated times after removal of the viral inoculum. For all postinfection time points, RANTES remained in the cultures until the end of the experiment. Luciferase expression was measured on day 3 postinfection and presented as percentage of control (no RANTES, considered 100%).
Qualitatively very similar results were obtained with HIV-1 HxB2 pseudotypes and with both HeLa-CD4 and HeLa-CD4-CCR5 cells (Fig. 5b and data not shown), indicating that the effects recorded above were independent of the virus-cell fusion mechanism. Quantitatively, less enhancement was generally observed with HxB2 pseudotypes than with MuLV pseudotypes, for reasons that are not yet clear.
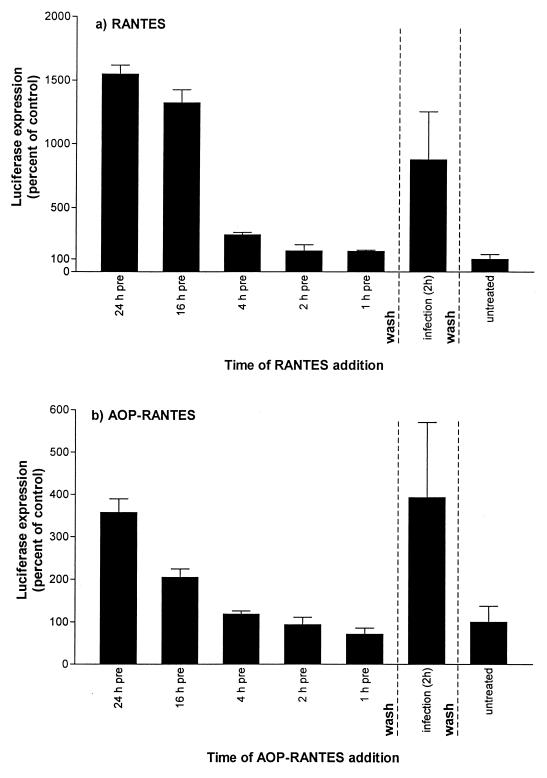
Preincubation of HeLa-CD4 cells with MIP-1α or MIP-1β (2.5 μg/ml) caused no enhancement of MuLV infectivity under the conditions of the experiment shown in Fig. 5a (data not shown). Thus, the observed effect is not a general property of CC-chemokines. However, AOP-RANTES mimicked RANTES in enhancing viral infectivity, both when it was used to pretreat the target cells and when it was added simultaneously with the inoculum (Fig. 6). The magnitude of the enhancement observed with AOP-RANTES was less than occurred with RANTES when each chemokine was added at 2.5 μg/ml, as predicted by Fig. 2f and g, but the temporal patterns were qualitatively indistinguishable (Fig. 6).
FIG. 6.
AOP-RANTES mimics RANTES by enhancing viral infectivity. For design of the experiment, see the legend to Fig. 5. RANTES (a) or AOP-RANTES (b), each at 2.5 μg/ml, was added to HeLa-CD4 cells at various times prior to or during infectivity with MuLV Env pseudotypes (3.5 ng of p24 antigen per ml). Note that the scales on the abscissa differ in the two panels, reflecting the lesser enhancement of infectivity caused by AOP-RANTES.
The magnitude of RANTES-induced enhancement varied between experiments, but the pattern of responses recorded in Fig. 5 and 6 was consistent; in multiple experiments RANTES always had a greater effect when added simultaneously with the infecting virus than when added to the cells for 1 to 4 h prior to the virus and then removed before virus addition. However, prolonged (≥16-h) exposure of the cells to RANTES always caused a greater increase in virus infection.
DISCUSSION
We have confirmed previous reports that the CC-chemokine RANTES is a weaker inhibitor of HIV-1 infection via CCR5 than the derivative AOP-RANTES (47, 76). We have also confirmed that RANTES can enhance the infectivity of some HIV-1 strains under certain circumstances (22, 43, 56, 74, 75). RANTES clearly has multiple, opposing effects on the HIV-1 life cycle. It can inhibit viral entry mediated by CCR5, as a direct (i.e., competition) or indirect (i.e., down-modulation) consequence of its interaction with the same receptor (3, 4, 35, 47, 79, 81, 89). Countering this inhibition are at least two mechanisms by which RANTES can increase the efficiency of HIV-1 infection; one involves a sustained effect of the chemokine on the cells, and the other is a process which occurs when the virus initially interacts with the cells in the presence of RANTES. The consistent lack of enhancement seen when RANTES is added as early as 1 h postinfection may indicate that stages in the virus life cycle which involve nuclear import, integration, or transcription are not affected, since all of these steps are not completed until at least 12 h postinfection. This conclusion is supported by the experiments showing that the enhancing effect of RANTES is independent of the transcriptional level (Table 1). Alternatively, some facets of the early postentry events in the HIV-1 life cycle may be inherently labile; their timing may be such that RANTES does not affect them unless it is in contact with the cells for many hours prior to virus addition.
Which of the various opposing effects dominates the overall responsiveness of HIV-1 infection to RANTES will depend upon the virus strain, its coreceptor of choice, and other facets of the target cell that remain to be determined. The particular in vitro assay system used will probably be another variable, given the complexity of the underlying phenomena. For instance, quantitatively different results are likely to be derived from assays of viral entry, viral replication, and Env-mediated membrane fusion. Under some circumstances, enhancing effects will reduce the inhibitory actions of RANTES on CCR5-mediated entry from what they might have been; under others, such as when X4 or R5X4 viruses enter via CXCR4, an overall increase in viral infectivity may occur. There can even be net enhancement of macrophagetropic HIV-1 infection via CCR5 (for example, HIV-1 ADA in HeLa-CD4-CCR5 cells [Fig. 1]); despite the competition that must occur between RANTES and the virus for CCR5, the infection-promoting effects of RANTES dominate overall.
In contrast to RANTES, with AOP-RANTES the infection-inhibiting component is paramount for the infection of macrophagetropic viruses via CCR5 (Fig. 1). This is probably due to the altered interactions of AOP-RANTES with CCR5 created by the N-terminal modification, which cause an increased rate of CCR5 down-modulation and diminished recycling (47, 76). AOP-RANTES should not, however, be described as a CCR5 antagonist since true antagonists are not able to drive chemokine receptor internalization (77). Like RANTES, AOP-RANTES enhances the infection of HIV-1 HxB2 via CXCR4 and that of MuLV via a different receptor system. The altered interactions of AOP-RANTES with CCR5 are irrelevant to the enhancement phenomenon, since CCR5 is not involved in the entry of HxB2 or MuLV and is not expressed on the target cells (Fig. 2f and g; Fig. 6). Other properties of the RANTES and AOP-RANTES molecule must therefore be involved in the enhancement effects, which are notably not induced by other CC-chemokines such as MIP-1α and MIP-1β.
RANTES is often reported to cause only very limited inhibition, or an overall enhancement, of HIV-1 infection in macrophages (22, 26, 33, 36, 42, 58, 74, 76). However, strong inhibition by RANTES in macrophages is sometimes observed (2, 12, 91), the conflicting results perhaps reflecting variation in CCR5 expression due to the differentiation state of the cells (28, 42, 59). Another variable might be the cell surface content of heparan sulfate binding sites for chemokines (39, 63, 84). We have found that in CD4+ T cells, RANTES can either inhibit or enhance infection by X4 and R5X4 viruses and that what happens is donor dependent (80). The reasons for this variation are not known.
We observed enhancing effects of RANTES on viral infection in multiple human and nonhuman cells of different lineages; human cervical carcinoma, osteosarcoma, and gliobastoma-astrocytoma cells, simian kidney cells, and murine fibroblasts. In all cells, the dose-response curves for RANTES-mediated infectivity enhancement were very similar. This commonality suggests the presence of a ubiquitously expressed, low-affinity RANTES receptor(s). All of the known RANTES receptors would be saturated by G-protein-coupled RANTES at concentrations at least 10-fold, and perhaps 100-fold, lower than the concentration at which enhancement is observed (69, 86). We have tried to identify the relevant receptor(s) but have not yet succeeded. None of the presently characterized G-protein RANTES receptors is expressed in all of the cells in which viral infectivity enhancement can be observed (34).
In vitro, the enhancing effects of RANTES occur at relatively high input concentrations, typically above 500 ng/ml (60 nM). This raises a legitimate question as to the physiological relevance of the enhancement phenomenon which is difficult to answer; information on localized concentrations of RANTES in relevant tissues is lacking, and the biologically active form of RANTES may be, in any case, a glycosoaminoglycan complex of unknown tissue concentration (39, 63, 84). Although CC- and CXC-chemokines usually signal through their receptors at concentrations in the nanomolar range (69, 86), Bacon et al. have reported that RANTES transduces two separate signals in human CD4+ T cells (7, 8, 19). Via an undefined receptor(s), RANTES induces a transient, pertussis toxin-sensitive Ca2+ signal when added in the range 1 to 100 nM (8 to 800 ng/ml). However, at higher concentrations (1 μM, equivalent to 8 μg/ml), RANTES stimulates a much more sustained increase in intracellular Ca2+ that is sensitive to the protein tyrosine kinase inhibitor herbimycin and is driven by the influx of extracellular Ca2+ (7). Again, the receptor(s) through which RANTES triggers this second signal is not known. The concentration range in which we observe RANTES-mediated enhancement of viral infectivity in a variety of cell types corresponds closely (0.5 to 5.0 μg/ml) to that eliciting the sustained, tyrosine kinase-mediated Ca2+ signal (Fig. 1 and 2). This finding suggests, but does not prove, that at least part of the enhancement process is related to events that trigger a second signal such as that described by Bacon et al. (7, 8, 19). Of note is that neither MIP-1α nor MIP-1β activates the second signal (7, 8, 19), nor does either enhance viral infectivity in our experimental systems. Dairaghi et al. have suggested that there may be an association between RANTES signaling and CD3 expression (19). However, the involvement of CD3 can be ruled out in our experimental systems, since all of the various cell lines that we used are CD3 negative, as determined by fluorescence-activated cell sorting analysis (data not shown).
When one is considering how RANTES and AOP-RANTES enhance HIV-1 infection, two sets of data are relevant. First, the route of viral entry is not a relevant variable, since we observed enhancement of infectivity mediated by HIV-1 envelope glycoproteins via CCR5 or CXCR4, by the MuLV envelope independently of these coreceptors, and by VSV-G, which fuses within endosomes. Second, we could find no evidence that RANTES could enhance a postfusion stage of the viral life cycle: there was no effect of RANTES on gene transcription, and RANTES did not increase HIV-1 replication when added after virus entry was complete. How then might RANTES increase the efficiency of virus-cell fusion in a way that is independent of the mechanism of fusion and of the viral glycoproteins used to achieve it? One possibility is that RANTES (and AOP-RANTES) increases the absorption of virions to cells in a way that is independent of the viral envelope glycoproteins. Since the efficiency of the absorption step is limiting for the infectability of retroviruses (16, 52, 68, 85), any process that increased virion attachment to cells would undoubtedly enhance infectivity. We are presently investigating this hypothesis as an explanation for the enhancement that occurs when RANTES is added to cells simultaneously with virus. Of note is that any such process would have to be specific to RANTES (and AOP-RANTES), since we did not observe enhancement with MIP-1α, MIP-1β, MCP-1, or MCP-3 under the same conditions.
A rather greater enhancement of infectivity was observed when the target cells were pretreated for several hours with RANTES or AOP-RANTES prior to virus addition. The time course of the effect suggests that signal-induced alterations of cellular gene expression might be responsible, perhaps triggered by the sustained, tyrosine kinase-mediated Ca2+ signal noted above. We have not yet been successful with experiments that use inhibitors of G-protein-mediated Ca2+ signaling (e.g., pertussis toxin and herbimycin) because we find that these inhibitors can have toxic effects on the target cells over a multihour period. Toxicity issues need to be taken into account when one is considering whether an observed effect of an inhibitor on a Ca2+ signal in the short term is necessarily the explanation of an inhibition of HIV-1 replication, measured many hours later.
As well as further dissection of the biological mechanisms by which RANTES enhances viral infectivity, it would be prudent to consider whether the phenomena that we are studying could contribute to the restrictions on HIV-1 and simian immunodeficiency virus replication in certain cell types. These include macrophages (13, 54, 73) and some subclones of U937 cells (55, 57). The enhancement of cell-cell fusion mediated by different viral envelope glycoproteins upon expression of the cytomegalovirus-encoded chemokine receptor US28 might also be a related phenomenon (67).
Extrapolations from in vitro studies to in vivo situations must always be made with care. However, the observations that RANTES and derivatives such as AOP-RANTES have multiple effects on target cells, and can enhance viral infectivity in general, should be taken into account when one considers whether to use chemokines as systemic antiviral agents for HIV-1 infection and whether chemokines are a correlate of protection against HIV-1 disease progression (17, 32).
ACKNOWLEDGMENTS
We are very grateful to David Kabat, Dan Littman, and Tanya Dragic for providing cell lines, to Michael Siani for SDF-1α, and to Robin Offord and Brigitte Dufour for the synthesis of AOP-RANTES. We appreciate the technical assistance of Fréderic Borlat, Francis Kajumo, Tom Ketas, Jamie Matthews, Elizabeth Maxwell, and Alexandra Meyer.
This study was supported by NIH grant AI41420 and by the Pediatric AIDS Foundation. A.T. is a Fellow of the Austrian Program for Advanced Research and Technology; J.P.M. is an Elizabeth Glaser Scientist of the Pediatric AIDS Foundation.
REFERENCES
- 1.Aiken C. Pseudotyping human immunodeficiency virus type 1 (HIV-1) by the glycoprotein of vesicular stomatitis virus targets HIV-1 entry to an endocytic pathway and suppresses both the requirement for Nef and the sensitivity to cyclosporin A. J Virol. 1998;71:5871–5877. doi: 10.1128/jvi.71.8.5871-5877.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 3.Amara A, Le Gall S, Schwartz O, Salamero J, Montes M, Loetscher P, Baggiolini M, Virelizier J-L, Arenzana-Seisdedos F. HIV coreceptor downregulation as antiviral principle: SDF-1α-dependent internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication. J Exp Med. 1997;186:139–146. doi: 10.1084/jem.186.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aramori I, Ferguson S S G, Bieniasz P D, Cullen B R, Caron M G. Molecular mechanism of desensitization of the chemokine receptor CCR-5: receptor signalling and internalization are dissociable from its role as an HIV-1 co-receptor. EMBO J. 1997;16:4606–4616. doi: 10.1093/emboj/16.15.4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arenzana-Seisedos F, Virelizier J-L, Rousset D, Clark-Lewis I, Loetscher P, Moser B, Baggiolini M. HIV blocked by chemokine antagonist. Nature. 1996;383:400. doi: 10.1038/383400a0. [DOI] [PubMed] [Google Scholar]
- 6.Arenzana-Seisedos F, Amara A, Thomas D, Virelizier J-L, Baleux F, Clark-Lewis I, Legler D F, Moser B, Baggiolini M. β-Chemokine MDC and HIV-1 infection. Science. 1998;281:486. [Google Scholar]
- 7.Bacon K B, Premack B A, Gardner P, Schall T J. Activation of dual T cell signaling pathways by the chemokine RANTES. Science. 1995;269:1727–1730. doi: 10.1126/science.7569902. [DOI] [PubMed] [Google Scholar]
- 8.Bacon K B, Schall T J, Dairaghi D J. RANTES activation of phospholipase D in Jurkat T cells: requirement of GTP-binding proteins ARF and RhoA. J Immunol. 1998;160:1894–1900. [PubMed] [Google Scholar]
- 9.Berger, E. A. 1997. HIV entry and tropism: the chemokine receptor connection. AIDS 11(Suppl. A):S3–S16. [PubMed]
- 10.Bieniasz P D, Cullen B R. Chemokine receptors and human immunodeficiency virus infection. Front Biosci. 1998;3:44–58. doi: 10.2741/a265. [DOI] [PubMed] [Google Scholar]
- 11.Bleul C C, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer T A. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 12.Capobianchi M R, Abbate I, Antonelli G, Turriziani O, Dolei A, Dianzani F. Inhibition of HIV type 1 BaL replication by MIP-1α, MIP-1β, and RANTES in macrophages. AIDS Res Hum Retroviruses. 1998;14:233–240. doi: 10.1089/aid.1998.14.233. [DOI] [PubMed] [Google Scholar]
- 13.Chackerian B, Long E M, Luciw P A, Overbaugh J. Human immunodeficiency virus type 1 coreceptors participate in postentry stages in the virus replication cycle and function in simian immunodeficiency virus infection. J Virol. 1997;71:3932–3939. doi: 10.1128/jvi.71.5.3932-3939.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen B K, Saksela K, Andino R, Baltimore D. Distinct modes of human immunodeficiency virus type 1 proviral latency revealed by superinfection of nonproductively infected cell lines with recombinant luciferase-encoding viruses. J Virol. 1994;68:654–660. doi: 10.1128/jvi.68.2.654-660.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard G, Sodroski J. The β-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 16.Chuck A S, Palsson B O. Consistent and high rates of gene transfer can be obtained using flow-through transduction over a wide range of retroviral titers. Hum Gene Ther. 1996;7:743–750. doi: 10.1089/hum.1996.7.6-743. [DOI] [PubMed] [Google Scholar]
- 17.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Identification of RANTES, MIP-1 alpha and MIP-1 beta as the major HIV suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 18.Connor R I, Sheridan K E, Ceradini D, Choe S, Landau N R. Change in coreceptor use correlates with disease progression in HIV-1 infected individuals. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dairaghi D J, Soo K S, Oldham E R, Premack B A, Kitamura T, Bacon K B, Schall T J. RANTES-induced T cell activation correlates with CD3 expression. J Immunol. 1998;160:426–433. [PubMed] [Google Scholar]
- 20.Deng H K, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major coreceptor 5 for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 21.DeVico A L, Pal R, Markham P D, Garzino-Demo A, Gallo R C. β chemokine MDC and HIV-1 infection. Science. 1998;281:486. doi: 10.1126/science.278.5338.695. [DOI] [PubMed] [Google Scholar]
- 22.Dolei A, Biolchini A, Serra C, Currali S, Gomes E, Dianzani F. Increased replication of a T-cell-tropic HIV strain and CXC-chemokine receptor-4 induction in T cells treated with macrophage inflammatory protein (MIP)-1α, MIP-1β and RANTES β-chemokines. AIDS. 1998;12:183–190. doi: 10.1097/00002030-199802000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Doms R W, Moore J P. HIV-1 coreceptor use: a molecular window into viral tropism. In: Korber B, Brander C, Moore J, Walker B, Koup R, Haynes B, editors. HIV molecular immunology database 1997, part III. Los Alamos, N.Mex: Theoretical Biology and Biophysics, Los Alamos National Laboratory; 1997. pp. 1–12. [Google Scholar]
- 24.Doms R W, Peiper S C. Unwelcome guests with master keys: how HIV uses chemokine receptors for cellular entry. Virology. 1997;235:179–190. doi: 10.1006/viro.1997.8703. [DOI] [PubMed] [Google Scholar]
- 25.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S, Parmentier M, Collman R G, Doms R W. A dual-tropic, primary HIV-1 isolate that uses fusin and the β-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1159. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 26.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 27.Dragic T, Trkola A, Lin S W, Nagashima K A, Kajumo F, Zhao L, Olson W C, Wu L, Mackay C R, Allaway G P, Sakmar T P, Moore J P, Maddon P J. Amino-terminal substitutions in the CCR5 coreceptor impair gp120 binding and human immunodeficiency virus type 1 entry. J Virol. 1998;72:279–285. doi: 10.1128/jvi.72.1.279-285.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fear W R, Kesson A M, Naif H, Lynch G W, Cunningham A L. Differential tropism and chemokine receptor expression of human immunodeficiency virus type 1 in neonatal monocytes, monocyte-derived macrophages, and placental macrophages. J Virol. 1998;72:1334–1344. doi: 10.1128/jvi.72.2.1334-1344.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 30.Frade J M R, Llorente M, Mellado M, Alcami J, Gutierrez-Ramos J C, Zaballos A, del Real G, Martinez A C. The amino-terminal domain of the CCR2 chemokine receptor acts as a coreceptor for HIV-1 infection. J Clin Investig. 1997;100:497–502. doi: 10.1172/JCI119558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garzino-Demo A, Arya S K, De Vico A L, Cocchi F, Lusso P, Gallo R C. C-C chemokine RANTES and HIV long terminal repeat-driven gene expression. AIDS Res Hum Retroviruses. 1997;13:1367–1371. doi: 10.1089/aid.1997.13.1367. [DOI] [PubMed] [Google Scholar]
- 32.Garzino-Demo, A., A. L. De Vico, F. Cocchi, and R. C. Gallo. 1998. β-chemokines and protection from HIV type 1 disease. AIDS Res. Hum. Retroviruses 14(Suppl. 2):S-177–S-184. [PubMed]
- 33.Ghorpade A, Xia M Q, Hyman B T, Persidsky Y, Nukuma A, Bock P, Che M, Limoges J, Gendelman H E, Mackay C R. Role of the β-chemokine receptors CCR3 and CCR5 in human immunodeficiency virus type 1 infection of monocytes and microglia. J Virol. 1998;72:3351–3361. doi: 10.1128/jvi.72.4.3351-3361.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gordon, C., A. Trkola, J. P. Moore, C. A. Power, and A. E. I. Proudfoot. Unpublished results.
- 35.Haribabu B, Richardson R M, Fisher I, Sozzani S, Peiper S C, Horuk R, Ali H, Synderman R. Regulation of human chemokine receptor CXCR4: Role of phosphorylation in desensitization and internalization. J Biol Chem. 1997;272:28726–28731. doi: 10.1074/jbc.272.45.28726. [DOI] [PubMed] [Google Scholar]
- 36.He J, Chen Y, Farzan M, Choe H, Ohagen A, Gartner S, Busciglio J, Yang X, Hofmann W, Newman W, Mackay C R, Sodroski J, Gabuzda D. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature. 1997;385:645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- 37.Heveker N, Montes M, Germeroth L, Amara A, Trautmann A, Alizon M, Schneider-Mergener J. Dissociation of the signalling and antiviral properties of SDF-1-derived small peptides. Curr Biol. 1998;8:369–376. doi: 10.1016/s0960-9822(98)70155-1. [DOI] [PubMed] [Google Scholar]
- 38.Hill C M, Deng H-K, Unutmaz D, KewalRamani V, Bastiani L, Gorny M K, Zolla-Pazner S, Littman D R. Envelope glycoproteins from human immunodeficiency virus types 1 and 2 and simian immunodeficiency virus can use human CCR5 as a coreceptor for viral entry and make direct CD4-dependent interactions with this chemokine receptor. J Virol. 1997;71:6296–6304. doi: 10.1128/jvi.71.9.6296-6304.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoogewerf A J, Kuschert G S, Proudfoot A E, Borlat F, Clark-Lewis I, Power C A, Wells T N. Glycosaminoglycans mediate cell surface oligomerization of chemokines. Biochemistry. 1997;36:13570–13578. doi: 10.1021/bi971125s. [DOI] [PubMed] [Google Scholar]
- 40.Horuk R, Hesselgesser J, Zhou Y, Faulds D, Halks-Miller M, Harvey S, Taub D, Samson M, Parmentier M, Rucker J, Doranz B J, Doms R W. The CC chemokine I-309 inhibits CCR8 dependent infection by diverse HIV-1 strains. J Biol Chem. 1998;273:386–391. doi: 10.1074/jbc.273.1.386. [DOI] [PubMed] [Google Scholar]
- 41.Karpus W J, Lukacs N W, Kennedy K J, Smith W S, Hurst S D, Barrett T A. Differential CC chemokine-induced enhancement of T helper cell cytokine production. J Immunol. 1997;158:4129–4136. [PubMed] [Google Scholar]
- 42.Kelly M D, Naif H M, Adams S L, Cunningham A L, Lloyd A R. Dichotomous effects of β-chemokines on HIV replication in monocytes and monocyte-derived macrophages. J Immunol. 1998;160:3091–3095. [PubMed] [Google Scholar]
- 43.Kinter A, Catanzaro A, Monaco J, Ruiz M, Justement J, Moir S, Arthos J, Oliva A, Ehler L, Mizell S, Jackson R, Ostrowski M, Hoxie J, Offord R, Fauci A S. CC-chemokines enhance the replication of T-tropic strains of HIV-1 in CD4+ T cells: role of signal transduction. Proc Natl Acad Sci USA. 1998;95:11880–11885. doi: 10.1073/pnas.95.20.11880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kozak S L, Platt E J, Madani N, Ferro F E, Jr, Peden K, Kabat D. CD4, CXCR-4, and CCR-5 dependencies for infection by primary patient and laboratory-adapted isolates of human immunodeficiency virus type 1. J Virol. 1997;71:873–882. doi: 10.1128/jvi.71.2.873-882.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Landau N R, Page K A, Littman D R. Pseudotyping with human T-cell leukemia virus type 1 broadens the human immunodeficiency virus host range. J Virol. 1991;65:162–169. doi: 10.1128/jvi.65.1.162-169.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee B, Rucker J, Doms R W, Tsang M, Hu X, Dietz M, Bailer R, Montaner L J, Gerard C, Sullivan N, Sodroski J, Stantchev T S, Broder C C. β-Chemokine MDC and HIV-1 infection. Science. 1998;281:486. [Google Scholar]
- 47.Mack M, Luckow B, Nelson P J, Lihak J, Simmons G, Clapham P R, Signoret N, Marsh M, Stangassinger M, Borlat F, Wells T N C, Schlöndorff D, Proudfoot A E I. Aminooxypentane-RANTES induces CCR5 internalization but inhibits recycling: a novel inhibitory mechanism of HIV infectivity. J Exp Med. 1998;187:1215–1224. doi: 10.1084/jem.187.8.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Margolis L B, Glushakova S, Grivel J-C, Murphy P M. Blockade of CC chemokine receptor 5 (CCR5)-tropic human immunodeficiency virus-1 replication in human lymphoid tissue by CC chemokines. J Clin Investig. 1998;101:1876–1880. doi: 10.1172/JCI2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miller D G, Miller A D. A family of retroviruses that utilize related phosphate transporters for cell entry. J Virol. 1994;68:8270–8276. doi: 10.1128/jvi.68.12.8270-8276.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moore J P. Co-receptors: implications for HIV pathogenesis and therapy. Science. 1997;276:51–52. doi: 10.1126/science.276.5309.51. [DOI] [PubMed] [Google Scholar]
- 51.Moore J P, Trkola A, Dragic T. Co-receptors for HIV-1 entry. Curr Opin Immunol. 1997;9:551–562. doi: 10.1016/s0952-7915(97)80110-0. [DOI] [PubMed] [Google Scholar]
- 52.Morgan J R, LeDoux J M, Snow R G, Tompkins R G, Yarmush M L. Retrovirus infection: effect of time and target cell number. J Virol. 1995;69:6994–7000. doi: 10.1128/jvi.69.11.6994-7000.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morgenstern J P, Land H. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990;18:3587–3592. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mori K, Ringler D J, Desrosiers R C. Restricted replication of simian immunodeficiency virus strain 239 in macrophages is determined by env but is not due to restricted entry. J Virol. 1993;67:2807–2814. doi: 10.1128/jvi.67.5.2807-2814.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moriuchi H, Moriuchi M, Arthos J, Hoxie J, Fauci A S. Promonocytic U937 subclones expressing CD4 and CXCR4 are resistant to infection with and cell-to-cell fusion by T-cell-tropic human immunodeficiency virus type 1. J Virol. 1997;71:9664–9671. doi: 10.1128/jvi.71.12.9664-9671.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moriuchi H, Moriuchi M, Fauci A S. Factors secreted by human T lymphotropic virus type I (HTLV-I)-infected cells can enhance or inhibit replication of HIV-1 in HTLV-I-uninfected cells: implications for in vivo coinfection with HTLV-I and HIV-1. J Exp Med. 1998;187:1689–1697. doi: 10.1084/jem.187.10.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moriuchi H, Moriuchi M, Fauci A S. Differentiation of promonocytic U937 subclones into macrophagelike phenotypes regulates a cellular factor(s) which modulates fusion/entry of macrophagetropic human immunodeficiency virus type 1. J Virol. 1998;72:3394–3400. doi: 10.1128/jvi.72.4.3394-3400.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moriuchi M, Moriuchi H, Combadiere C, Murphy P M, Fauci A S. CD8+ T cell-derived factor(s), but not β-chemokines RANTES, MIP-1α, and MIP-1β, suppress HIV-1 replication in monocyte/macrophages. Proc Natl Acad Sci USA. 1996;93:15341–15345. doi: 10.1073/pnas.93.26.15341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Naif H M, Li S, Alali M, Sloane A, Wu L, Kelly M, Lynch G, Lloyd A, Cunningham A L. CCR5 expression correlates with susceptibility of maturing monocytes to human immunodeficiency virus type 1 infection. J Virol. 1998;72:830–836. doi: 10.1128/jvi.72.1.830-836.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier J-L, Arenzana-Seisdedos F, Schwartz O, Heard J-M, Clark-Lewis I, Legler D F, Loetscher M, Baggiolini M, Moser B. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 61.Oravecz T, Pall M, Norcross M A. β-Chemokine inhibition of monocytotropic HIV-1 infection. Interference with a postbinding fusion step. J Immunol. 1996;157:1329–1332. [PubMed] [Google Scholar]
- 62.Oravecz T, Pall M, Roderiquez G, Gorrell M D, Ditto M, Nguyen N Y, Boykins R, Unsworth E, Norcross M A. Regulation of the receptor specificity and function of the chemokine RANTES (regulated on activation, normal T cell expressed and secreted) by dipeptidyl peptidase IV (CD26)-mediated cleavage. J Exp Med. 1997;186:1865–1872. doi: 10.1084/jem.186.11.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oravecz T, Pall M, Wang J, Roderiquez G, Ditto M, Norcross M A. Regulation of anti-HIV-1 activity of RANTES by heparan sulfate proteoglycans. J Immunol. 1997;159:4587–4592. [PubMed] [Google Scholar]
- 64.Pal R, Garzino-Demo A, Markham P D, Burns J, Brown M, Gallo R C, DeVico A L. Inhibition of HIV-1 infection by the β-chemokine MDC. Science. 1997;278:695–698. doi: 10.1126/science.278.5338.695. [DOI] [PubMed] [Google Scholar]
- 65.Paxton W A, Liu R, Kang S, Wu L, Gingeras T R, Landau N R, Mackay C R, Koup R A. Reduced HIV-1 infectability of CD4+ lymphocytes from exposed-uninfected individuals: association with low expression of CCR5 and high production of β-chemokines. Virology. 1998;244:66–73. doi: 10.1006/viro.1998.9082. [DOI] [PubMed] [Google Scholar]
- 66.Paxton W A, Martin S R, Tse D, O’Brien T R, Skurnick J, VanDevanter N L, Padian N, Braun J F, Kotler D P, Wolinsky S M, Koup R A. Relative resistance to HIV-1 infection of CD4 lymphocytes from persons who remain uninfected despite multiple high-risk sexual exposures. Nat Med. 1996;2:412–417. doi: 10.1038/nm0496-412. [DOI] [PubMed] [Google Scholar]
- 67.Pleskoff O, Treboute C, Alizon M. The cytomegalovirus-encoded chemokine receptor US28 can enhance cell-cell fusion mediated by different viral proteins. J Virol. 1998;72:6389–6397. doi: 10.1128/jvi.72.8.6389-6397.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Porter C D, Lukacs K V, Box G, Takeuchi Y, Collins M K L. Cationic liposomes enhance the rate of transduction by a recombinant retroviral vector in vitro and in vivo. J Virol. 1998;72:4832–4840. doi: 10.1128/jvi.72.6.4832-4840.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Premack B A, Schall T J. Chemokine receptors: gateways to inflammation and infection. Nat Med. 1996;2:1174–1178. doi: 10.1038/nm1196-1174. [DOI] [PubMed] [Google Scholar]
- 70.Proost P, De Meester I, Schols D, Struyf S, Lambeir A-M, Wuyts A, Oppendakker G, De Clercq E, Scharpe S, van Damme J. Amino-terminal truncation of chemokines by CD26/dipeptidyl-peptidase IV. Conversion of RANTES into a potent inhibitor of monocyte chemotaxis and HIV-1-infection. J Biol Chem. 1998;273:7222–7227. doi: 10.1074/jbc.273.13.7222. [DOI] [PubMed] [Google Scholar]
- 71.Raport C J, Gosling J, Schweickart V L, Gray P W, Charo I F. Molecular cloning and functional characterization of a novel human CC chemokine receptor (CCR5) for Rantes, MIP-1beta, and MIP 1alpha. J Biol Chem. 1996;271:17161–17166. doi: 10.1074/jbc.271.29.17161. [DOI] [PubMed] [Google Scholar]
- 72.Samson M, Labbe O, Mollereau C, Vassart G, Parmentier M. Molecular cloning and functional expression of a new CC-chemokine receptor gene, CC-CKR5. Biochemistry. 1996;11:3362–3367. doi: 10.1021/bi952950g. [DOI] [PubMed] [Google Scholar]
- 73.Schmidtmayerova H, Alfano M, Nuovo G, Bukrinsky M. Human immunodeficiency virus type 1 T-lymphotropic strains enter macrophages via a CD4- and CXCR4-mediated pathway: replication is restricted at a postentry level. J Virol. 1998;72:4633–4642. doi: 10.1128/jvi.72.6.4633-4642.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schmidtmayerova H, Sherry B, Bukrinsky M. Chemokines and HIV replication. Nature. 1996;382:767. doi: 10.1038/382767a0. [DOI] [PubMed] [Google Scholar]
- 75.Schols D, Proost P, van Damme J, De Clercq E. RANTES and MCP-3 inhibit the replication of T-cell-tropic human immunodeficiency virus type 1 strains (SF-2, MN, and HE) J Virol. 1997;71:7300–7304. doi: 10.1128/jvi.71.10.7300-7304.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Simmons G, Clapham P R, Picard C, Offord R E, Rosenkilde M M, Schwartz T W, Buser R, Wells T N C, Proudfoot A E I. Potent inhibition of HIV-1 infectivity in macrophages and lymphocytes by a novel CCR5 antagonist. Science. 1997;276:276–279. doi: 10.1126/science.276.5310.276. [DOI] [PubMed] [Google Scholar]
- 77.Solari R, Offord R E, Remy S, Aubry J-P, Wells T N C, Whitehorn E, Oung T, Proudfoot A E I. Receptor-mediated endocytosis of CC-chemokines. J Biol Chem. 1997;272:9617–9620. doi: 10.1074/jbc.272.15.9617. [DOI] [PubMed] [Google Scholar]
- 78.Taub D D, Turcovski-Corrales S M, Key M L, Longo D L, Murphy W J. Chemokines and T lymphocyte activation. J Immunol. 1996;156:2095–2103. [PubMed] [Google Scholar]
- 79.Trkola A, Dragic T, Arthos J, Binley J M, Olson W C, Allaway G P, Cheng-Mayer C, Robinson J, Maddon P J, Moore J P. CD4 dependent, antibody sensitive interactions between HIV-1 and its co-receptor CCR5. Nature. 1996;384:184–186. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 80.Trkola, A., and J. P. Moore. Unpublished observations.
- 81.Trkola A, Paxton W A, Monard S P, Hoxie J A, Siani M A, Thompson D A, Wu L, Mackay C R, Horuk R, Moore J P. Genetic subtype independent inhibition of human immunodeficiency virus type 1 replication by CC and CXC chemokines. J Virol. 1998;72:396–404. doi: 10.1128/jvi.72.1.396-404.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Trkola A, Pomales A B, Yuan H, Korber B, Maddon P J, Allaway G P, Katinger H, Barbas III C F, Burton D R, Ho D D, Moore J P. Cross-clade neutralization of primary isolates of human immunodeficiency virus type 1 by human monoclonal antibodies and tetrameric CD4-IgG. J Virol. 1995;69:6609–6617. doi: 10.1128/jvi.69.11.6609-6617.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Turner L, Ward S G, Westwick J. RANTES-activated human T lymphocytes. J Immunol. 1995;155:2437–2444. [PubMed] [Google Scholar]
- 84.Wagner L, Yang O O, Garcia-Zepeda E A, Ge Y, Kalams S A, Walker B D, Pasternak M S, Luster A D. β-Chemokines are released from HIV-1 specific cytotoxic T-cell granules complexed to proteoglycans. Nature. 1998;391:908–911. doi: 10.1038/36129. [DOI] [PubMed] [Google Scholar]
- 85.Wang H, Paul R, Burgeson R E, Keene D R, Kabat D. Plasma membrane receptors for ecotropic murine retroviruses require a limiting accessory factor. J Virol. 1991;65:6468–6477. doi: 10.1128/jvi.65.12.6468-6477.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wells T N C, Power C A, Lusti-Narasimhan M, Hoogewerf A J, Cooke R M, Chung C-w, Peitsch M C, Proudfoot A E I. Selectivity and antagonism of chemokine receptors. J Leukoc Biol. 1996;59:53–60. doi: 10.1002/jlb.59.1.53. [DOI] [PubMed] [Google Scholar]
- 87.Wiskerchen M, Muesing M A. Human immunodeficiency virus type 1 integrase: effects of mutations on viral ability to integrate, direct viral gene expression from unintegrated viral DNA templates, and sustain viral propagation in primary cells. J Virol. 1995;69:376–386. doi: 10.1128/jvi.69.1.376-386.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wong M, Fish E N. RANTES and MIP-1α activate stats in T cells. J Biol Chem. 1998;273:309–314. doi: 10.1074/jbc.273.1.309. [DOI] [PubMed] [Google Scholar]
- 89.Wu L, Gerard N P, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardoso A A, Desjardin E, Newman W, Gerard C, Sodroski J. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature. 1996;384:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 90.Wu L, Paxton W A, Kassam N, Ruffing N, Rottman J B, Sullivan N, Choe H, Sodroski J, Newman W, Koup R A, Mackay C. CCR5 levels and expression pattern correlate with infectability by macrophage tropic HIV-1, in vitro. J Exp Med. 1997;185:1681–1691. doi: 10.1084/jem.185.9.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ylisastigui L, Vizzavona J, Drakopoulou E, Paindavoine P, Calvo C-F, Parmentier M, Gluckman J C, Vita C, Benjouad A. Synthetic full-length and truncated RANTES inhibit HIV-1 infection of primary macrophages. AIDS. 1998;12:977–984. [PubMed] [Google Scholar]
- 92.Zhang Y-J, Dragic T, Cao Y, Kostrikis L, Kwon D S, Littman D R, KewalRamani V N, Moore J P. Use of co-receptors other than CCR5 by non-syncytium-inducing adult and pediatric isolates of human immunodeficiency virus type 1 is rare in vitro. J Virol. 1998;72:9337–9344. doi: 10.1128/jvi.72.11.9337-9344.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]