Abstract
In mammals, molecules mainly secreted by white adipose tissue named adipokines are also synthetized locally in the reproductive tract and are able to influence reproductive functions. In avian species, previous studies indicated that the adipokine chemerin is highly abundant in the albumen, compared to the yolk and this was associated to high chemerin expression in the magnum. In addition, the authors observed that chemerin and its receptors are expressed by allantoic and amniotic membranes and chemerin is present in fluids during the embryo development. Here, we studied other adipokines, including adiponectin, visfatin, apelin, and adipolin in egg white and their known receptors in the active (egg-laying hen) and regressed (hen not laying) oviduct and embryonic annexes during embryo development. By using Western blot, RT-qPCR analysis and immunohistochemistry, we demonstrated the expression of different adipokines in the egg albumen (visfatin) and the reproductive tract (adiponectin, visfatin, apelin, adipolin, and their cognate receptors) according the position of egg in the oviduct. We showed that the expression of adipokines and adipokines receptors was strongly reduced in the regressed oviducts (arrested laying hen). Results indicated that visfatin and adiponectin appeared at ED11 to 14 and increased until ED18 in amniotic fluid whereas it was found from ED7 and was unchanged during embryo development in allantoic fluid. Taken together, adipokines and their receptors are expressed in the egg white, the reproductive tract and the embryonic annexes. Data obtained suggest important functions of theses metabolic hormones during the chicken embryo development. Thus, adipokines could be potential biomarkers to improve the embryo development.
Key words: Adipokines, egg, reproductive tract, embryonic annexes, chicken
INTRODUCTION
In mammals, several studies have already reported that molecules produced mainly by white adipose tissue named adipokines are involved not only in metabolic functions but also in reproductive functions (Barbe et al., 2019, Estienne et al., 2019). In addition, some of these adipokines and their receptors are expressed throughout the reproductive tract suggesting a local and/or systemic action. In avian species, less adipokines have been described in the literature (Bernardi et al., 2021). The identification of leptin in avian genome has long been controversial. Characterization for the first leptin in chicken and duck genomes was reported by Seroussi et al. (2016). Unfortunately, its role is still unclear because leptin is not detectable in blood circulation. They observed that leptin has an autocrine/paracrine mode of action for avian species. More data are available about the following adipokines in birds: adiponectin, chemerin, visfatin and to a lesser extent adipolin and apelin (Mellouk et al., 2018a; Bernardi et al., 2021).
It is well known that adiponectin is mainly expressed by theca cells compared with granulosa cells from follicles in chicken (Chabrolle et al., 2007). In addition, chicken theca cells are able to synthetize but also to secrete the heavy molecular weight (HMW) isoform of adiponectin (Hadley et al., 2020). Concerning adiponectin receptors, AdipoR1 is highly expressed in granulosa cells and AdipoR2 has equivalent levels in both ovarian cells that could be associated with potential effects on ovarian functions (Chabrolle et al., 2007; Ramachandran et al., 2013). Adiponectin and its receptors are also expressed in hypothalamic-pituitary axis in laying hens (Li et al., 2021). Moreover, Mellouk et al. (2018b) showed that adiponectin system is expressed in chicken embryo during the early embryo development. More data are available about chicken chemerin. A previous study has already shown the expression of chemerin in ovarian cells in avian species (Mellouk et al., 2018c; Estienne et al., 2020a). Moreover, Estienne et al., 2022 showed that chemerin was more expressed in magnum, where the albumen is formed, compared to the other compartments of the hen oviduct. They indicated that, chemerin receptors CMKLR1, GPR1, and CCRL2, are found in hen tract with a maximum expression in the vagina, magnum and uterus, respectively. In addition, several studies identified chemerin within the albumen and perivitelline membranes of chicken eggs (Mann, 2007; Bílková et al., 2018; Estienne et al., 2022). Also, it was observed that chemerin system is expressed by the allantoic and amniotic membranes and fluids during the early embryo development in chicken (Estienne et al., 2022). In birds, even if visfatin is considered mainly as a myokine (Krzysik-Walker et al., 2008), it also presents in ovarian cortex in particular in theca cells compared to granulosa cells from hierarchical follicles in hens (Ons et al., 2010; Diot et al., 2015a). Visfatin could alter in vivo folliculogenesis and steroidogenesis in hens since it reduces in vitro IGF1-induced production of progesterone in granulosa cells (Diot et al., 2015a). Only one study suggested an implication of adipolin in the regulation of reproduction in broiler breeds (Bornelöv et al., 2018). Concerning apelin, Bello et al. (2021) noted that apelin signaling pathways is present in hen ovary and involved in egg production (Bello et al., 2021). Unfortunately, no more information is available in the literature concerning the expression of apelin and its receptor in the reproductive tract of avian species.
In conclusion, adiponectin and apelin system, visfatin and adipolin are expressed in the ovary of various mammalian species but their distribution in reproductive tract, unfertilized eggs and fertilized eggs during early embryo development is unknown in chicken. Therefore, the aims of the present study were to 1) investigate the presence of adiponectin, visfatin, apelin, adipolin, and their cognate receptors in the egg albumen and different compartments of the hen oviduct and 2) determine the expression of theses adipokines and their receptors in embryonic annexes (allantoic and amniotic membranes and fluids) to better understand their distribution and potential role during the embryo development in chicken.
MATERIALS AND METHODS
Ethical Issues
According to the ethical issues for the protection of animals, this project does not require the consent of the competent ethics committee for animal experiments. The UEPEAT experimental unit is registered by the Ministry of Agriculture with the license number D-37-175-1 for animal experimentation. All experiments were performed in accordance with the European Communities Council Directive 2010/63/UE.
Animals and Samples Collection
About 25 hens of Rhode Island breed (35-wk-old) were reared at the UEPEAT experimental unit under conventional breeding conditions. For each egg, an albumen sample was collected and stored at −20°C until analyses. At the end of the experiment, hens were euthanized by electrical stunning as recommended by the ethical committee. Their oviducts were collected, and a sample of each part (infundibulum, magnum, isthmus, uterus, and vagina) was collected and stored at −80°C until analyses. Also, abdominal adipose tissue and leg muscle samples were collected and stored at −80°C until analyses.
About 50 fertile chicken eggs were conventionally incubated at UEPEAT according to the following protocol. Eggs were stored in a room at 15°C to 16°C and 80 to 85% humidity for 1 wk. Then, all eggs were placed in alternative rows on each shelf of the incubator. They were maintained at 37.8°C and 56% relative humidity and automatically turned every hour. At d 7 and 14 of incubation, all eggs were candled, and infertile eggs, along with dead embryo eggs, were eliminated at ED18 of incubation. Six fertile eggs were retrieved at ED7, ED9, ED11, ED14, ED16, and ED18 of incubation. Embryos from each of these stages were sacrificed by decapitation, and the embryonic annexes (amniotic and allantoic membranes and amniotic and allantoic fluids) were immediately, snap-frozen and stored at −80°C until analyses.
Reverse Transcription and Quantitative Polymerase Chain Reaction (RT-qPCR)
Total RNA was extracted from the infundibulum, magnum, isthmus, uterus and vagina of 35-wk-old hens (6 animals) and from amniotic membranes and amniotic membranes of 35-wk-old hens (10 and 8 animals, respectively) by the homogenization of 100 mg of tissue in lysis buffer reagent, using a total RNA extraction kit according to the manufacturer's recommendations (NucleoSpin RNA, Macherey-Nagel, Hoerdt, France). The complementary DNA (cDNA) was generated by reverse transcription (RT) of total RNA (1 µg) in a mixture comprised of 0.5 mM of each deoxyribonucleotide triphosphate (dATP, dGTP, dCTP, and dTTP), 2 M of RT buffer, 15 µg/µL of oligodT, 0.125 U of ribonuclease inhibitor, and 0.05 U of Moloney murine leukemia virus (MMLV) reverse transcriptase for 1 h at 37°C. Real-time PCR was performed using the CFX384 Touch Real-Time PCR Detection System (Bio-Rad, Marnes-la-Coquette, France) in a mixture containing SYBR Green Supermix 1× reagent (Bio-Rad, Marnes la Coquette, France), 250 nM specific chicken primers (Invitrogen by Life Technologies, Villebon sur Yvette, France, see Table 1) and 5 µL of cDNA (diluted 5-fold) for a total volume of 20 µL. The samples were duplicated on the same plate, and the following PCR procedure was used. After an incubation of 2 min at 50°C and a denaturation step of 10 min at 95°C, samples were subjected to 40 cycles (30 s at 95°C, 30 s at 60°C, and 30 s at 72°C). The expression levels of messenger RNA were standardized to 3 reference genes (Eukaryotic translation elongation factor 1 alpha 1 (EEF1), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and β-actin). Adipokines expression was calculated according to primer efficiency (E) and quantification cycle (Cq), where expression = E − Cq. Then, the relative expression of the target genes to the geometric mean of the 3 reference genes was analyzed.
Table 1.
List of primers (Gallus gallus) used for RT-qPCR.
| Gene | Primer forward | Primer reverse |
|---|---|---|
| Adiponectin | ACAGGTGCAGAAGGACCGAG | AAGACAGAGCCGCTTGCTTG |
| AdipoR1 | GAATACACACCGAGACGGGC | GCCCAAGACGCAGACAATGG |
| AdipoR2 | GAGACTGGCAACATCTGGAC | TGCGATGCCCAGGACACAAA |
| Visfatin | CAGATCCGGAGTGTTACTGG | CCTATGCCAGCAGTCTCTTG |
| Apelin | CTGGAGCTGCCCTGTGG | TGACAGACACGGTGACCAG |
| APJ | CTCGGGCAGCTTCTCCTC | GCGGAAGAGCGTCTCCTG |
| Adipolin | CCTATCCAACAGCGAGGAGC | CATCTGGGCGGTGGACAAAG |
| Chemerin | CGCGTGGTGAAGGATGTG | CGACTGCTCCCTAAAGAGGAACT |
| CMKLR1 | CGGTCAACGCCATTTGGT | GGGTAGGAAGATGTTGAAGGAA |
| GPR1 | ACCTGCCTGAGGAAGAAGAA | AAAGGCCAGTGGAAGCCCAT |
| CCRL2 | CACGCAGTGTTTGCTTTAAAAGC | CAACAGCCCACGTGACAATG |
| β-Actin | ACGGAACCACAGTTTATCATC | GTCCCAGTCTTCAACTATACC |
| GAPDH | TGCTGCCCAGAACATCATCC | ATCAGCAGCAGCCTTCACTACC |
| EEF1a | AGCAGACTTTGTGACCTTGCC | TGACATGAGACAGACGGTTGC |
Detection of Adipokines by Western Blot Analysis
White adipose tissue (WAT), muscle (M), and egg white (EG) samples were lysed using and Ultraturax (Invitrogen by Life Technologies, Villebon sur Yvette, France) in lysis buffer 50% (vol/vol, Tris 1 M (pH 7.4), NaCl 0.15 M, EDTA 1.3 mM, EGTA 1 mM, sodium orthovanadate 2 mM mM, NaF 0.1 M, NH2PO4 1%, Triton 0.5%). The protein concentration of lysates was measured using the bicinchoninic acid (BCA) protein assay (Interchim, Montluçon, France). Samples (80 µg) were mixed with Laemmli buffer 5× and proteins were denatured for 5 min by heating at 95°C. Proteins were loaded on an electrophoresis sodium dodecyl sulfate-polyacrylamide gel and then proteins were transferred to a nitrocellulose membrane. Membranes were blocked with Tris-Buffered Saline Tween buffer containing 0.05% of Tween 20 and 5% of milk for 30 min at room temperature. Membranes were incubated overnight at 4°C with the appropriate primary antibodies (antiadiponectin, antivisfatin, antiadipolin, and antichemerin antibodies as described in Table 2). Then, membranes were incubated for 90 min at room temperature with a Horse Radish Peroxidase-conjugated antimouse IgG. Protein of interest was detected by enhanced chemiluminescence (ECL, Western Lightning Plus-ECL, Perkin Elmer, Villebon-sur-Yvette, France) with a G-box SynGene (Ozyme, St Quentin en Yvelines, France) and the GeneSnap software (Ozyme, St Quentin en Yvelines, France). Then, protein amounts were quantified with GeneTools software (Ozyme, St Quentin en Yvelines, France). The results were expressed as the intensity signal in arbitrary units after normalization of protein signals with the total protein amount as evaluated by Ponceau-red staining (Sigma-Aldrich, Saint Quentin Fallavier, France).
Table 2.
List of primary and secondary antibodies used for protein detection by Western blot.
| Antibodies | Dilution | Supplier | Host | Molecular weight (kDa) | Specific target species | Validation for avian species |
|---|---|---|---|---|---|---|
| Chemerin | 1/1000 | Agrobio | Mouse | 18 | Chicken | (Estienne et al., 2020b) |
| Adiponectin | 1/1000 | Agrobio | Rabbit | 25–28 | Bovine | (Diot et al., 2015b) |
| Visfatin | 1/1000 | Agrobio | Rabbit | 52 | Chicken | (Diot et al., 2015b, 2015a) |
| Adipolin | 1/1000 | Novus Biological | Rabbit | 25–32 | Human | |
| Rabbit | 1/50000 | Biorad | Goat | |||
| Mouse | 1/50000 | Interchim | Goat |
Immunofluorescence
For different adipokines immunodetection experiments within the oviduct, samples of the infundibulum, magnum, isthmus, uterus and vagina from adult hens (35-wk-old) were fixed in formalin, paraffin-embedded and finally sectioned (10 µm). Sections were dewaxed and rehydrated in xylene and in decreasing concentrations of alcohol (100, 95, 90, 75, and 50%). Antigen retrieval was performed by steaming the sections in a microwave in citrate buffer (0.01 M) with a pH of 6.0 for 5 min, then cooling for 20 min after three 5-min washes in PBS. Sections were incubated at 4°C overnight with the rabbit monoclonal antichicken interest antibody as described in Table 2 diluted 1:200 in PBS-bovine serum albumin (BSA). After washes in PBS-1% BSA, slides were incubated in goat antimouse Alexa 488(diluted at 1:500 in PBS for 1 h). After three 5-min washes in PBS, sections were mounted with DAPI. Negative controls were performed by replacing antibody with rabbit IgG.
Adiponectin and Visfatin Enzyme-Linked Immunosorbent Assay
Adiponectin and visfatin concentrations were measured from amniotic and allantoic fluids of eggs incubated at ED7 to ED18 by using Enzyme-Linked Immunosorbent Assay (ELISA) kit as previously described by Mellouk et al. (2018d). Briefly, MBS269004 (sensitivity 5 pg/mL) and MBS016609 (sensitivity 0.1 μg/mL) (My BioSource, San Diego, CA) kits were used to determine adiponectin and visfatin concentrations. The experiment was performed following the manufacturer's protocol with an intra-assay coefficient of variation ≤8% (adiponectin assay), and <10% (visfatin assay).
Statistical Analysis
The GraphPad Prism software (version 6) was used for all analyses. All data are represented as means ± standard error of mean (SEM) with a level of significance less or equal than 0.05 (P ≤ 0.05). Data were compared by 1-way ANOVA with Tukey-Kramer multiple comparisons tests. Different letters indicate significant differences (P ≤ 0.05).
RESULTS
Expression of Different Adipokines and Cognate Receptors Within the Reproductive Tract
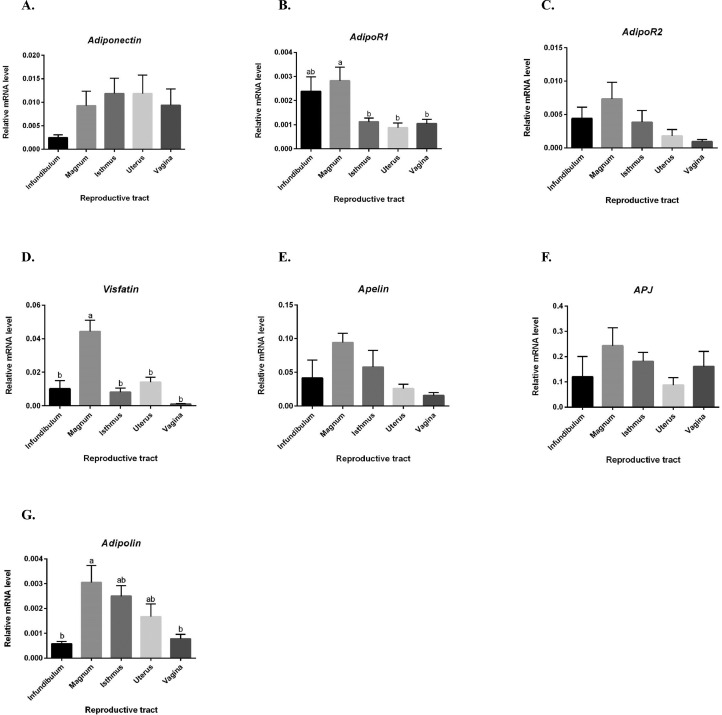
We first determined by RT-qPCR the expression of several adipokines and their cognate receptors within 5 parts of the reproductive tract: infundibulum, magnum, isthmus, uterus and vagina (Figure 1). As shown in Figure 1A and C, adiponectin and adipoR2 gene expression was no significantly different in the hen oviduct (P = 0.283) while adipoR1 was significantly higher in magnum compared to the isthmus, uterus and vagina (P = 0.004) (Figure 1B). Visfatin and adipolin gene expression were significantly higher in the magnum of oviduct hen as compared to infundibulum and vagina (adipolin) or to the other part of the reproductive tract (visfatin) (Figure 1D and G) whereas the expression of apelin and its receptor APJ genes was similar in the different parts of the oviduct (Figure 1E and F).
Figure 1.
Expression of different adipokines and cognate receptors within the reproductive tract. Relative expression of different adipokines and their cognate receptors (A–G) in the different parts of the hen reproductive tract (infundibulum, magnum, isthmus, uterus, and vagina) quantified by RT-qPCR (n = 6 animals for each part of oviduct). Values are expressed as mean ± SEM. Different letters indicate significant differences at P < 0.05.
We also investigated by immunofluorescence, the presence of adipokines and their cognate receptors in different parts of the hen oviduct with different levels of immunostaining between the 5 compartments (Table 3 and Figure 1 in supplementary data). Adiponectin immunostaining was highly abundant in infundibulum compared to the gene expression. AdipoR1 and visfatin expression were significantly higher in magnum whereas their protein levels were found with a stronger immunostaining in uterus and vagina for adipoR1 and infundibulum, isthmus and uterus for visfatin, respectively (Table 3 and Figure 1A–D in supplementary data). Positive immunostaining for apelin was observed in isthmus, uterus and vagina (Table 3 and Figure 1E in supplementary data) whereas APJ receptor and adipolin were mostly found in the isthmus and vagina (Table 3 and Figure 1F and G in supplementary data).
Table 3.
Level of immunostaining of different adipokines and their cognate receptors in different compartments of reproductive tract.
| Protein | Infundibulum | Magnum | Isthmus | Uterus | Vagina |
|---|---|---|---|---|---|
| Adiponectin | ++ | - | + | + | + |
| AdipoR1 | + | +/- | + | ++ | ++ |
| AdipoR2 | +/- | - | + | + | + |
| Visfatin | ++ | + | ++ | ++ | - |
| Apelin | +/- | +/- | + | + | + |
| APJ | + | + | ++ | + | ++ |
| Adipolin | +/- | - | ++ | + | ++ |
Expression of Different Adipokines and Cognate Receptors in Magnum According to the Egg Position in the Reproductive Tract
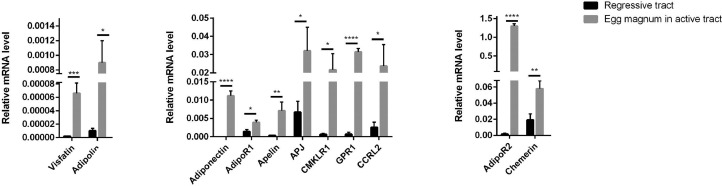
We next studied the expression of adipokines and cognate receptors genes in the magnum tissue during the laying period (peak of laying) in according to the egg position in the hen oviduct (egg in magnum, isthmus, soft egg in uterus, and hard egg in uterus (Table 4). In addition, we compared this mRNA expression in regressive tract as arrested laying hen and in active tract in laying hens (Figure 2). We observed that adipoR1 and chemerin expression in magnum were different according to the egg position within the oviduct whereas those of other adipokines was similar (Table 4). AdipoR1 gene expression in the magnum was highly abundant when hard egg was in uterus as compared to other egg positions in the tract (P < 0.0001) (Table 4). Moreover, chemerin expression in magnum was significantly higher when the egg was located in magnum and with a hard-shelled uterine egg (P = 0.05) (Table 4). We also analyzed adipokines and adipokine receptors expression in magnum of regressed tract. The expression of all adipokines and adipokine receptors gene expression in magnum were significantly lower in regressed oviduct compared to the active of reproductive tract in a laying hen and this whatever the position of egg (Figure 2 where adipokines expression in magnum is represented only in regressed tract and active oviduct (when egg is located in magnum)).
Table 4.
Expression of different adipokines and their receptors in magnum depending on the egg position in oviduct.
| Gene | Egg magnum | Egg isthmus | Soft egg uterus | Hard egg uterus | P value |
|---|---|---|---|---|---|
| Adiponectin | 0.0112 ± 0.0013 | 0.0085 ± 0.0017 | 0.0043 ± 0.0018 | 0.0060 ± 0.0015 | 0.09 |
| AdipoR1 | 0.0038 ± 0.0006b | 0.0024 ± 0.0003b | 0.0029 ± 0.0002b | 0.0087 ± 0.0007a | <0.0001 |
| AdipoR2 | 1.299 ± 0.065 | 0.635 ± 0.192 | 0.472 ± 0.203 | 0.549 ± 0.170 | 0.11 |
| Visfatin | 6.59e-5 ± 1.53e-5 | 4.32e-5 ± 1.01e-5 | 5.11e-5 ± 8.33e-6 | 5.68e-5 ± 8.94e-6 | 0.57 |
| Apelin | 0.0071 ± 0.0024 | 0.0080 ± 0.0019 | 0.0035 ± 0.0009 | 0.0084 ± 0.0016 | 0.13 |
| APJ | 0.0321 ± 0.0128 | 0.0393 ± 0.0082 | 0.0221 ± 0.0062 | 0.0430 ± 0.0071 | 0.21 |
| Adipolin | 0.0009 ± 0.0003 | 0.0011 ± 0.0002 | 0.0005 ± 0.0002 | 0.0007 ± 0.0001 | 0.21 |
| Chemerin | 0.0578 ± 0.0098a | 0.0415 ± 0.0056ab | 0.0360 ± 0.0050b | 0.0530 ± 0.0046a | 0.05 |
| CMKLR1 | 0.0217 ± 0.0088 | 0.0214 ± 0.0069 | 0.0153 ± 0.0049 | 0.0259 ± 0.0062 | 0.65 |
| GPR1 | 0.0315 ± 0.0018 | 0.0190 ± 0.0039 | 0.0233 ± 0.0060 | 0.0194 ± 0.0025 | 0.24 |
| CCRL2 | 0.0237 ± 0.0118 | 0.0211 ± 0.0064 | 0.0168 ± 0.0053 | 0.0399 ± 0.0090 | 0.16 |
Data are shown as the mean ± SEM; n = 6 to 12 samples per egg position. Groups showing different superscript letters are significantly different (P ≤ 0.05).
In bold are indicated the significant P value
Figure 2.
Expression of different adipokines and cognate receptors in magnum in regressive and active oviduct (egg located in magnum). Relative expression of different adipokines and their cognate receptors in the magnum in regressed tract as arrested laying hen and in active tract in laying hen with an egg located in the magnum quantified by RT-qPCR (n = 10 animals). Values are expressed as mean ± SEM. Different letters indicate significant differences at P < 0.05.
Adipokines in Egg White
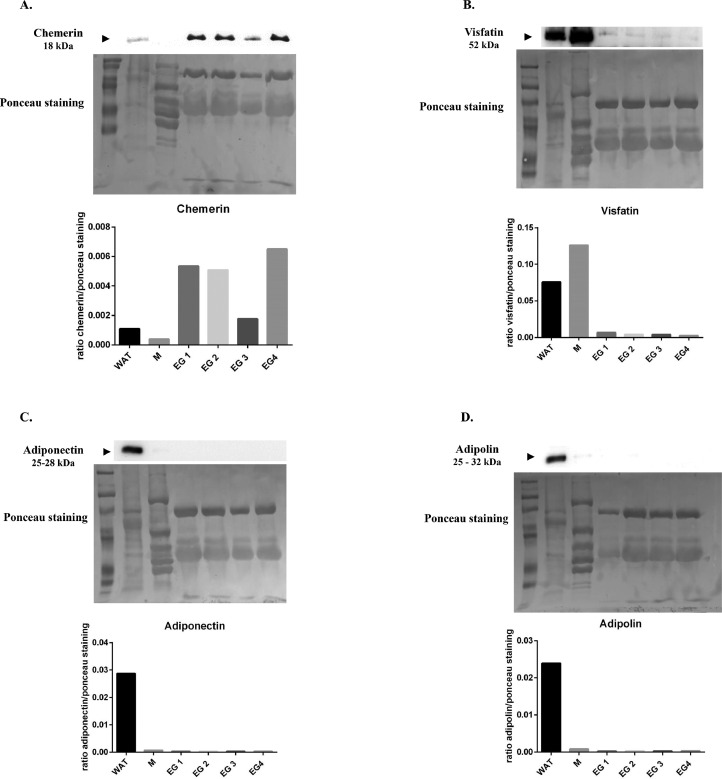
Since the egg white proteins are mainly secreted by the epithelial cells of the magnum (Chousalkar and Roberts, 2008; Jung et al., 2011) and we showed that adipokines are expressed in magnum, we investigated if adipokines including adiponectin, visfatin, and adipolin could be released from magnum. As shown in Figure 3A, we confirmed as previously described by Estienne et al., 2022 that chemerin protein was highly abundant in egg white samples as compared to the WAT and muscle (M) samples. By immunoblot, we observed that visfatin is also present in egg white but at lower levels than those in WAT and muscle (Figure 3B). However, we failed to detect adiponectin and adipolin in egg white whereas we found them in WAT that was used as positive control (Figure 3C and D). Unfortunately, we could not reveal apelin because of the lack of the cross-reaction of the antibodies.
Figure 3.
Abundance of different adipokines in egg white. Protein abundance of different adipokines (A–D) in white adipose tissue (WAT), muscle (M), and egg white (EG) samples detected by Western blotting. The ratio protein/ponceau is represented (n = 4 egg whites (EG) from four different hens).
Expression of Different Adipokines and Cognate Receptors in Embryonic Annexes and Fluids
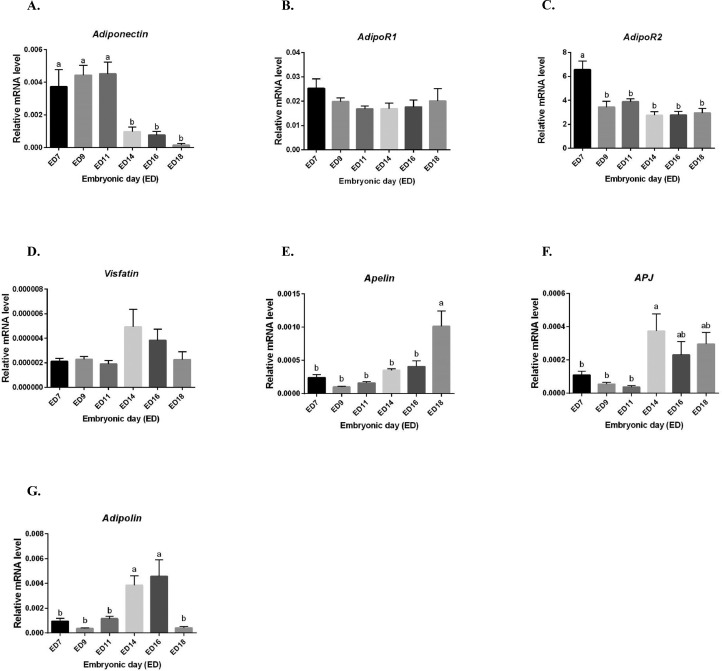
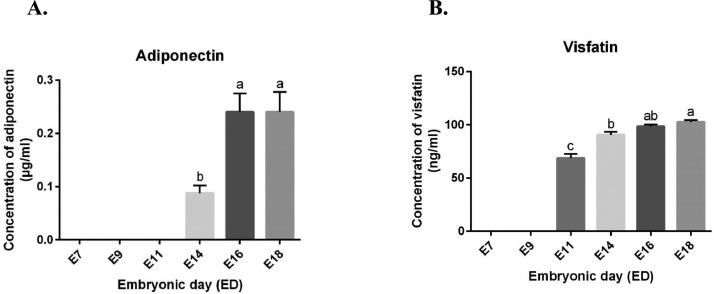
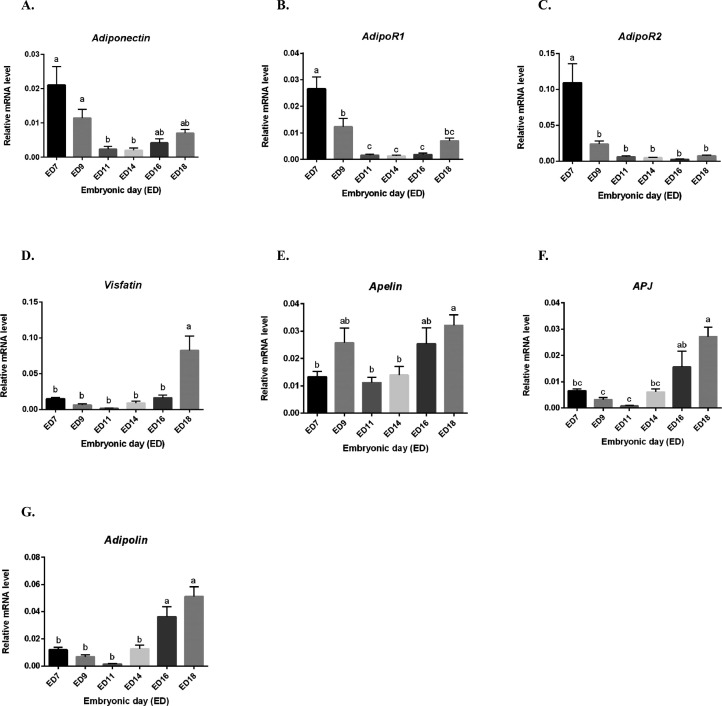
We next determined by RT-qPCR whether the adipokines and their cognate receptors could be expressed in the embryonic annexes. Thus, we analyzed amniotic and chorioallantoic membranes at different embryonic days (EDs): ED7, 9, 11, 14, 16 and 18. For amniotic membranes, we demonstrated that adiponectin gene is higher expressed from ED7 to ED11 than from ED14 to ED18 (P < 0.0001) (Figure 4A). Then, adipoR1 gene expression was unchanged for the entire incubation period (P = 0.3649), whereas those of adipoR2 was higher at ED7 and significantly decreased at ED9 from ED18 (P < 0.0001) (Figure 4B and C). Visfatin was significantly different during all the embryonic development without difference between EDs (P = 0.0472), whereas we observed maximum gene expression at ED14 following by a reduced expression (Figure 4D). Apelin gene expression was lower from ED7 to ED16 and then significantly increased at ED18 (P < 0.0001) (Figure 4E). The expression of its APJ receptor gene was higher at ED14 and remained stable until ED18 (P = 0.0004) (Figure 4F). In contrast, adipolin gene expression was lower from ED7 to ED11 and significantly increased at ED14 and ED16 following by an important reduced expression at ED18 (P < 0.0001) (Figure 4G). We also measured adiponectin and visfatin concentration in amniotic fluids at different EDs: ED7, 9, 11, 14, 16 and 18 using ELISA assay. As shown in Figure 5A, adiponectin was not detectable from ED7 to E11 and increased with maximum concentration 0.24 ng/mL at ED16 and ED18 (P = 0.0079). Visfatin protein was also detected in amniotic fluids at ED11 (69 ng/mL) and increased until ED18 with maximum concentration at 103 ng/mL) (P < 0.0001) (Figure 5B).
Figure 4.
Adipokines and cognate receptors expression in amniotic membranes. Relative expression of different adipokines and their cognate receptors (A–G) in the amniotic membranes of incubated eggs at different embryonic days (EDs): 7, 9, 11, 14, 16, and 18 quantified by RT-qPCR (n = 10 animals at each ED stage). Values are expressed as mean ± SEM. Different letters indicate significant differences at P < 0.05.
Figure 5.
Adipokines concentration in amniotic fluids. Adiponectin (A) and visfatin (B) concentrations in the amniotic fluids of incubated eggs at different embryonic days (EDs): 7, 9, 11, 14, 16 and 18 determined by ELISA assay (n = 6 animals at each ED). Values are expressed as mean ± SEM. Different letters indicate significant differences at P < 0.05.
For allantoic membranes, adiponectin, adipoR1, and adipoR2 gene expression was higher at ED7 following by a reduced expression until ED18 (P < 0.0001) (Figure 6A–C). In contrast, visfatin gene expression was lower from ED7 to ED16 and increased at ED18 (P < 0.0001) (Figure 6D). For apelin system and adipolin, we observed a significant increase at ED16 and maximum expression at the end of embryonic incubation ED18 (Figure 6E–G). We also measured adiponectin and visfatin concentration in allantoic fluids at different EDs by ELISA assay. Both adipokines were stable during the entire period of incubation. In allantoic fluids, the concentrations of adiponectin were lower than those of visfatin fluids (0.14 ng/mL and 58 ng/mL, respectively) (Figure 2 in supplementary data).
Figure 6.
Adipokines and cognate receptors expression in allantoic membranes. Relative expression of different adipokines and their cognate receptors (A–G) in the allantoic membranes of incubated eggs at different embryonic days (EDs): 7, 9, 11, 14, 16, and 18 quantified by RT-qPCR (n = 8 animals at each ED). Values are expressed as mean ± SEM. Different letters indicate significant differences at P < 0.05.
DISCUSSION
In the present study, the authors investigated for the first time the presence of several adipokines and their cognate receptors in different parts of the reproductive tract in hen. They also observed that these adipokines were present in embryonic annexes especially in allantoic and amniotic membranes and fluids.
Adipokines and Adipokines Receptors Expression in Oviduct and Their Potential Role(s)
In broiler chickens, it is well known that excessive amounts of visceral adipose tissue lead to often excessive follicular recruitment, double ovulation, or increased incidence of ovarian regression (Yu et al., 1992). Since adipokines are mainly produced by adipose tissue, they could participate to this dysregulation of the reproductive tract. In hen, we also know that adipokines can modulate steroids production by ovarian cells at least in vitro for adiponectin (Chabrolle et al., 2007; Li et al., 2021) and visfatin (Diot et al., 2015). The functions and development of avian oviduct are largely regulated by the steroids. In addition, the adipokines and their receptor are also expressed in the oviduct in mammals. In hen, the authors showed that chemerin concentration in egg white produced by the magnum is negatively correlated with egg performances (egg weight, albumen weight, and yolk weight, Bernardi et al., 2022). However, they did not observe significant correlation with the laying rate. However, other adipokines produced locally by the oviduct could be associated with the egg production. Thus, it appears important to identify the adipokines and their receptors in the different part of the avian oviduct. In the present study, the authors focused on 5 adipokines (adiponectin, chemerin, visfatin, apelin, and adipolin) that have been described in the literature (Bernardi et al., 2021). They did not investigate the expression of leptin because in avian species it is still a debate if leptin is an adipokine since it shows no expression in adipose tissue of the few avian species (Prokop et al., 2014) and it is undetectable in blood circulation (Seroussi et al., 2016). In birds, only the left oviduct is functional. It is a long tubular organ with histologically and functionally 5 distinct segments: infundibulum, magnum, isthmus, uterus (shell gland), and vagina that are involved in egg maturation and transportation, fertilization and egg formation (Roberts, 2004; Sah and Mishra, 2018). The infundibulum captures the ovulated oocyte. It is also the place where ovum fertilization as well as deposition of the outer layer of the yolk membrane and the first layer of egg albumen occurs (Olsen et al., 1948; Rahman et al., 2013). The magnum produces most of the egg albumen. In the isthmus, inner and outer eggshell membranes are formed, whereas the shell gland is responsible for the addition of fluid with electrolytes to the egg albumen and for the formation of calcified eggshell. The vagina participates in pushing the egg out. In the present study, results indicated that adiponectin, adipoR1, adipoR2, visfatin, apelin, apelin receptor, and adipolin are expressed in the hen oviduct. However, only adipoR1, visfatin and adipolin were differently expressed according the part of the reproductive tract. Indeed, adipoR1 was higher expressed in magnum than in isthmus, uterus and vagina whereas visfatin mRNA was more abundant in magnum as compared to the other oviduct part. In addition, adipolin was more expressed in magnum as compared to infundibulum and vagina. Thus, these data suggest that the magnum is the main part of the oviduct where adipoR1, visfatin and adipolin could play a role. So, one hypothesis is that visfatin and adipolin would be secreted by the epithelial cells of the magnum and consequently would be present in albumen. However, after Western blot the authors detected only visfatin in the egg white. Visfatin, also called nicotinamide phosphoribosyltransferase (NAMPT) or pre-B-cell colony-enhancing factor, is an adipocytokine and cytosolic enzyme with NAMPT activity (Dahl et al., 2012). Thus, visfatin, can act extracellularly as a cytokine-like molecule or intracellularly as a NAMPT, regulating NAD biosynthesis in the NAD salvage pathway (Dahl et al., 2012). In mammalian cells, visfatin regulates physiological processes such as cell proliferation and glucose metabolism and specifically increases glucose uptake (Carbone et al., 2017). Thus, one hypothesis is that visfatin/NAMPT in epithelial cells of the magnum could have the same functions as those described for mammalian cells. In albumen, visfatin could contribute to facilitate the transport of nutrients into the developing embryo. In the present study the authors showed that adiponectin and adipolin, the 2 most prominent anti-inflammatory adipokines in mammals, are expressed in magnum but they are not detectable in the albumen whereas they are present in adipose tissue. However, it is not possible to rule out the presence of these proteins in extracellular vesicles, which could be released by magnum epithelial cells in small quantities and therefore not detectable by the antibodies used. Indeed, in mammals, there are extracellular vesicles produced from the oviduct epithelial cells in the oviductal fluid. These vesicles have been shown to improve the fertilization process, prevent polyspermy, and improve embryo development (Harris et al., 2020). In addition, adipokines such as adiponectin are found in mammalian extracellular vesicles (Camino et al., 2023). The authors recently showed that another adipokine named chemerin was also highly expressed in magnum (Estienne et al., 2022). Within the oviduct, the egg spends about 3 h in the magnum, and 20 to 21 h in the uterus (Eyal-Giladi and Kochav, 1976). The entire process requires luminal and glandular epithelial proliferation and differentiation in the magnum and shell gland. In chicken, visfatin is able to increase the expression of adipocyte differentiation markers (Li et al., 2017) suggesting that it could be involved in the cell differentiation of the magnum. The authors also found that adipokines and their cognate receptors are less expressed in the magnum of a regressed tract (arrested laying hens) as compared to an active oviduct. This can be explained by the steroid production. Indeed, the glandular epithelial proliferation and differentiation in the magnum and shell gland are largely under the control of ovarian steroid hormones mainly estrogen (E2) and progesterone (P4). Thus, one hypothesis is that the expression of adipokines and adipokines receptors are steroid dependent. Estienne et al. have already shown that a treatment of P4 and E2 on magnum explants induced an in vitro chemerin expression and secretion (Estienne et al., 2022). A treatment with E2 upregulated visfatin expression in ovariectomized mice (Annie et al., 2019) and E2 induced in vitro visfatin expression in 3T3-L1 cells (Zhou and Seidel, 2010). Such studies are currently lacking in avian (nonmammalian) species. However, chicken visfatin gene has a high homology of sequence with mammalian visfatin gene (Li et al., 2012). So, the current results suggest that adipokines and adipokines receptors expression in the avian reproductive tract could be dependent on steroid hormones.
Presence of Adipokines in Egg White?
The presence of adiponectin and visfatin in the magnum suggests that these proteins could be secreted in the egg white as we previously described for chemerin (Estienne et al., 2022). Current data show that adiponectin and adipolin are undetectable whereas visfatin is present but at low concentration in the egg white. The albumen is the primary source of nutrients and a barrier to the pathogenic infections of the developing embryo (Stevens, 1996). Thus, visfatin in egg white could participate to protect microbial infections. Indeed, visfatin is also a nicotinamide phosphoribosyl transferase (NAMPT) and Mohanty et al. reported that some phosphoribosyltransferase acts as a virulence and immunomodulatory factor, and consequently could constitute a novel target for antimycobacterial drugs (Mohanty et al., 2015).
Adipokines and Adipokines Receptors Expression in Embryonic Annexes: Role in Embryo Development?
In the present study, the authors observed that visfatin and adiponectin were detectable around ED ED11 to 14. For visfatin, this can be explained by the transfer of egg white in the amniotic sac, since around ED12 (Sugimoto et al., 1989), egg white proteins are transferred in mass into the amniotic sac where they are absorbed orally by the embryo as a protein source (Yoshizaki et al., 2002). The adiponectin concentration observed in amniotic liquid was higher than those observed for visfatin. However, these concentrations were lower than those found in blood plasma at least in chicken adult (Mellouk et al., 2018c; Barbe et al., 2020). The authors also detected these 2 adipokines in the allantoic liquid at similar concentrations as those observed in amniotic liquid. However, they were at similar concentration from ED7 to ED18. In mammals, adiponectin and visfatin are also physiologic constituent of amniotic fluid (Chervenak et al., 2018; Musilova et al., 2021). Interestingly, in human, the concentration of amniotic fluid visfatin increases with advancing gestational age and it is elevated in patients with microbial invasion of the amniotic cavity, regardless of the membrane status, suggesting that visfatin could participate in the host response against infection (Mazaki-Tovi et al., 2008). These data support the idea that visfatin in the amniotic fluid of birds may be involved in the defense of the embryo. In both amniotic and chorioallantoic membrane (CAM), the authors showed that adipokines and adipokines receptors are differentially expressed during the chicken embryo development. Indeed, in amniotic membrane, adiponectin and adipoR2 expression decreases whereas apelin, APJ and adipolin increase. In CAM, the gene expression of adiponectin and its 2 receptors decreases whereas those of visfatin, apelin and APJ increases. In amniotic vertebrates including birds and reptile, CAM functions as respiratory organ for embryonic development. The CAM is derived from fusion between 2 pre-existing membranes, the allantois, a hindgut diverticulum and a reservoir for metabolic waste, and the chorion which marks the embryo's external boundary. Chorioallantoic fusin generates the embryonic respiratory organ in birds and reptiles and the placenta in mammals (Nagai et al., 2022). In mammals, several reports described the presence and the secretion of adipokines by the placenta (Pérez-Pérez et al., 2018). Adipokines receptors are also found in the placental cells where they can regulate placental functions (proliferation, protein synthesis, invasion, and apoptosis in placental cells) in an autocrine or paracrine manner. In CAM, adipokines could exert similar functions.
CONCLUSIONS
Taken together, the authors show for the first time that adipokines mainly produced by the adipose tissue in mammals and their cognate receptors present in metabolic and reproductive tissues are expressed by the hen oviduct. Moreover, visfatin can accumulate in egg white and visfatin and adiponectin in amniotic and allantoic fluids during the embryo development. Adipokines and adipokine receptors are also expressed and differentially regulated in amniotic and allantoic membranes during embryogenesis. However, it still necessary to perform experiments to better understand their roles during chicken embryo development to potentially use them as biomaker of the quality of embryo for genetic selection.
ACKNOWLEDGMENTS
The authors are grateful to the persons from the experimental unit (INRAE, PEAT, Centre Val de Loire DOI: 10.15454/1.5572326250887292E12) especially Joël Delaveau and Christophe Rat for egg incubation and Yannick Beaumard who take care of animals. The authors thank the “ANRT (Association nationale de la recherche et de la technologie)” and SYSAAF (Syndicat des Sélectionneurs Avicoles et Aquacoles Français) grant number 2020/0697 for the Ophelie Bernardi's financial support.
DISCLOSURES
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in the present study.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2023.102908.
Appendix. Supplementary materials
REFERENCES
- Annie L., Gurusubramanian G., Roy V.K. Estrogen and progesterone dependent expression of visfatin/NAMPT regulates proliferation and apoptosis in mice uterus during estrous cycle. J. Steroid Biochem. Mol. Biol. 2019;185:225–236. doi: 10.1016/j.jsbmb.2018.09.010. [DOI] [PubMed] [Google Scholar]
- Barbe A., Bongrani A., Mellouk N., Estienne A., Kurowska P., Grandhaye J., et al. Mechanisms of Adiponectin Action in Fertility: An overview from gametogenesis to gestation in humans and animal models in normal and pathological conditions. Int. J. Mol. Sci. 2019;20:1526. doi: 10.3390/ijms20071526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbe A., Mellouk N., Ramé C., Grandhaye J., Staub C., Venturi E., Cirot M., Petit A., Anger K., Chahnamian M., Ganier P., Callut O., Cailleau-Audouin E., Metayer-Coustard S., Riva A., Froment P., Dupont J. A grape seed extract maternal dietary supplementation in reproductive hens reduces oxidative stress associated to modulation of plasma and tissue adipokines expression and improves viability of offsprings. PLoS One. 2020;15 doi: 10.1371/journal.pone.0231131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello S.F., Xu H., Guo L., Li K., Zheng M., Xu Y., Zhang S., Bekele E.J., Bahareldin A.A., Zhu W., Zhang D., Zhang X., Ji C., Nie Q. Hypothalamic and ovarian transcriptome profiling reveals potential candidate genes in low and high egg production of white Muscovy ducks (Cairina moschata) Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi O., Estienne A., Reverchon M., Bigot Y., Froment P., Dupont J. Adipokines in metabolic and reproductive functions in birds: an overview of current knowns and unknowns. Mol. Cell. Endocrinol. 2021;534 doi: 10.1016/j.mce.2021.111370. [DOI] [PubMed] [Google Scholar]
- Bernardi O., Reverchon M., Estienne A., Baumard Y., Ramé C., Brossaud A. Chicken white egg chemerin as a tool for genetic selection for egg weight and hen fertility. Front. Physiol. 2022;13 doi: 10.3389/fphys.2022.1012212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bílková B., Świderská Z., Zita L., Laloë D., Charles M., Beneš V., Stopka P., Vinkler M. Domestic fowl breed variation in egg white protein expression: application of proteomics and transcriptomics. J. Agric. Food Chem. 2018;66:11854–11863. doi: 10.1021/acs.jafc.8b03099. [DOI] [PubMed] [Google Scholar]
- Bornelöv S., Seroussi E., Yosefi S., Benjamini S., Miyara S., Ruzal M., Grabherr M., Rafati N., Molin A.M., Pendavis K., Burgess S.C., Andersson L., Friedman-Einat M. Comparative omics and feeding manipulations in chicken indicate a shift of the endocrine role of visceral fat towards reproduction. BMC Genom. 2018;19:295. doi: 10.1186/s12864-018-4675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camino T., Lago-Baameiro N., Pardo M. Extracellular vesicles as carriers of adipokines and their role in obesity. Biomedicines. 2023;11:422. doi: 10.3390/biomedicines11020422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone F., Liberale L., Bonaventura A., Vecchiè A., Casula M., Cea M. Regulation and function of extracellular nicotinamide phosphoribosyltransferase/visfatin. Compr. Physiol. 2017;7:603–621. doi: 10.1002/cphy.c160029. [DOI] [PubMed] [Google Scholar]
- Chabrolle C., Tosca L., Crochet S., Tesseraud S., Dupont J. Expression of adiponectin and its receptors (AdipoR1 and AdipoR2) in chicken ovary: potential role in ovarian steroidogenesis. Domest. Anim. Endocrinol. 2007;33:480–487. doi: 10.1016/j.domaniend.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Chervenak J., Sawai M., Kabab L.E., Lohana R., Skupski D., Witkin S.S. Adiponectin concentration in mid-trimester amniotic fluid varies with the α-amylase level and maternal and neonatal outcomes. J. Perinat. Med. 2018;46:317–321. doi: 10.1515/jpm-2017-0094. [DOI] [PubMed] [Google Scholar]
- Chousalkar K.K., Roberts J.R. Ultrastructural changes in the oviduct of the laying hen during the laying cycle. Cell Tissue Res. 2008;332:349–358. doi: 10.1007/s00441-007-0567-3. [DOI] [PubMed] [Google Scholar]
- Dahl T.B., Holm S., Aukrust P., Halvorsen B. Visfatin/NAMPT: a multifaceted molecule with diverse roles in physiology and pathophysiology. Annu. Rev. Nutr. 2012;32:229–243. doi: 10.1146/annurev-nutr-071811-150746. [DOI] [PubMed] [Google Scholar]
- Diot M., Reverchon M., Ramé C., Baumard Y., Dupont J. Expression and effect of NAMPT (visfatin) on progesterone secretion in hen granulosa cells. Reproduction. 2015;150:53–63. doi: 10.1530/REP-15-0021. [DOI] [PubMed] [Google Scholar]
- Diot M., Reverchon M., Rame C., Froment P., Brillard J.P., Brière S., Levêque G., Guillaume D., Dupont J. Expression of adiponectin, chemerin and visfatin in plasma and different tissues during a laying season in turkeys. Reprod. Biol. Endocrinol. 2015;13:81. doi: 10.1186/s12958-015-0081-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estienne A., Bongrani A., Reverchon M., Ramé C., Ducluzeau P.H., Froment P., et al. Involvement of novel adipokines, Chemerin, visfatin, resistin and apelin in reproductive functions in normal and pathological conditions in humans and animal models. Int. J. Mol. Sci. 2019;20:4431. doi: 10.3390/ijms20184431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estienne A., Brossaud A., Ramé C., Bernardi O., Reverchon M., Rat C., Delaveau J., Chambellon E., Helloin E., Froment P., Dupont J. Chemerin is secreted by the chicken oviduct, accumulates in egg albumen and could promote embryo development. Sci. Rep. 2022;12:8989. doi: 10.1038/s41598-022-12961-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estienne A., Brossaud A., Reverchon M., Ramé C., Froment P., Dupont J. Adipokines expression and effects in oocyte maturation, fertilization and early embryo development: lessons from mammals and birds. Int. J. Mol. Sci. 2020;21:3581. doi: 10.3390/ijms21103581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estienne A., Reverchon M., Partyka A., Bourdon G., Grandhaye J., Barbe A., Caldas-Silveira E., Rame C., Niżański W., Froment P., Dupont J. Chemerin impairs in vitro testosterone production, sperm motility, and fertility in chicken: possible involvement of its receptor CMKLR1. Cells. 2020;9:1599. doi: 10.3390/cells9071599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyal-Giladi H., Kochav S. From cleavage to primitive streak formation: a complementary normal table and a new look at the first stages of the development of the chick. Dev. Biol. 1976;49:321–337. doi: 10.1016/0012-1606(76)90178-0. [DOI] [PubMed] [Google Scholar]
- Hadley J.A., Ocón-Grove O., Ramachandran R. Adiponectin is secreted by theca layer cells isolated from chicken ovarian follicles. Reproduction. 2020;159:275–288. doi: 10.1530/REP-19-0505. [DOI] [PubMed] [Google Scholar]
- Harris E.A., Stephens K.K., Winuthayanon W. Extracellular vesicles and the oviduct function. Int. J. Mol. Sci. 2020;21:8280. doi: 10.3390/ijms21218280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J.G., Lim W., Park T.S., Kim J.N., Han B.K., Song G., Han J.Y. Structural and histological characterization of oviductal magnum and lectin-binding patterns in Gallus domesticus. Reprod. Biol. Endocrinol. 2011;9:62. doi: 10.1186/1477-7827-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzysik-Walker S.M., Ocón-Grove O.M., Maddineni S.R., Hendricks G.L., Ramachandran R. Is visfatin an adipokine or myokine? Evidence for greater visfatin expression in skeletal muscle than visceral fat in chickens. Endocrinology. 2008;149:1543–1550. doi: 10.1210/en.2007-1301. [DOI] [PubMed] [Google Scholar]
- Li C., Li Q., Li J., Zhang N., Li Y., Li Y., Li H., Yan F., Kang X., Liu X., Tian Y. Expression and localization of adiponectin and its receptors (AdipoR1 and AdipoR2) in the hypothalamic-pituitary-ovarian axis of laying hens. Theriogenology. 2021;159:35–44. doi: 10.1016/j.theriogenology.2020.10.020. [DOI] [PubMed] [Google Scholar]
- Li J., Meng F., Song C., Wang Y., Leung F.C. Characterization of chicken visfatin gene: cDNA cloning, tissue distribution, and promoter analysis. Poult. Sci. 2012;91:2885–2894. doi: 10.3382/ps.2012-02315. [DOI] [PubMed] [Google Scholar]
- Li Z., Wang Y., Tian X., Shang P., Chen H., Kang X., Tian Y., Han R. Characterization of the visfatin gene and its expression pattern and effect on 3T3-L1 adipocyte differentiation in chickens. Gene. 2017;632:16–24. doi: 10.1016/j.gene.2017.08.025. [DOI] [PubMed] [Google Scholar]
- Mann K. The chicken egg white proteome. Proteomics. 2007;7:3558–3568. doi: 10.1002/pmic.200700397. [DOI] [PubMed] [Google Scholar]
- Mazaki-Tovi S., Romero R., Kusanovic J.P., Erez O., Gotsch F., Mittal P., Than N.G., Nhan-Chang C.L., Hamill N., Vaisbuch E., Chaiworapongsa T., Edwin S.S., Nien J.K., Gomez R., Espinoza J., Kendal-Wright C., Hassan S.S., Bryant-Greenwood G. Visfatin/Pre-B cell colony-enhancing factor in amniotic fluid in normal pregnancy, spontaneous labor at term, preterm labor and prelabor rupture of membranes: an association with subclinical intrauterine infection in preterm parturition. J. Perinat. Med. 2008;36:485–496. doi: 10.1515/JPM.2008.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellouk N., Ramé C., Barbe A., Grandhaye J., Froment P., Dupont J. Chicken is a useful model to investigate the role of adipokines in metabolic and reproductive diseases. Int. J. Endocrinol. 2018;2018:1–19. doi: 10.1155/2018/4579734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellouk N., Ramé C., Delaveau J., Rat C., Marchand M., Mercerand F., Travel A., Brionne A., Chartrin P., Ma L., Froment P., Dupont J. Food restriction but not fish oil increases fertility in hens: role of RARRES2? Reproduction. 2018;155:321–331. doi: 10.1530/REP-17-0678. [DOI] [PubMed] [Google Scholar]
- Mellouk N., Ramé C., Delaveau J., Rat C., Maurer E., Froment P., Dupont J. Adipokines expression profile in liver, adipose tissue and muscle during chicken embryo development. Gen. Comp. Endocrinol. 2018;267:146–156. doi: 10.1016/j.ygcen.2018.06.016. [DOI] [PubMed] [Google Scholar]
- Mellouk N., Ramé C., Marchand M., Staub C., Touzé J.L., Venturi É., Mercerand F., Travel A., Chartrin P., Lecompte F., Ma L., Froment P., Dupont J. Effect of different levels of feed restriction and fish oil fatty acid supplementation on fat deposition by using different techniques, plasma levels and mRNA expression of several adipokines in broiler breeder hens. PLoS One. 2018;13 doi: 10.1371/journal.pone.0191121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty S., Jagannathan L., Ganguli G., Padhi A., Roy D., Alaridah N., Saha P., Nongthomba U., Godaly G., Gopal R.K., Banerjee S., Sonawane A. A mycobacterial phosphoribosyltransferase promotes bacillary survival by inhibiting oxidative stress and autophagy pathways in macrophages and zebrafish. J. Biol. Chem. 2015;290:13321–13343. doi: 10.1074/jbc.M114.598482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musilova I., Kolackova M., Andrys C., Drahosova M., Baranová I., Chmelarova M., Stranik J., Jacobsson B., Kacerovsky M. Nicotinamide phosphoribosyltransferase and intra-amniotic inflammation in preterm prelabor rupture of membranes. J. Matern. Fetal Neonatal Med. 2021;34:736–746. doi: 10.1080/14767058.2019.1615049. [DOI] [PubMed] [Google Scholar]
- Nagai H., Tanoue Y., Nakamura T., Chan C.J.J., Yamada S., Saitou M., Fukuda T., Sheng G. Mesothelial fusion mediates chorioallantoic membrane formation. Philos. Trans. R. Soc. B Biol. Sci. 2022;377 doi: 10.1098/rstb.2021.0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen M.W., Neher B.H. The site of fertilization in the domestic fowl. J. Exp. Zool. 1948;109:355–366. doi: 10.1002/jez.1401090303. [DOI] [PubMed] [Google Scholar]
- Ons E., Gertler A., Buyse J., Lebihan-Duval E., Bordas A., Goddeeris B., Dridi S. Visfatin gene expression in chickens is sex and tissue dependent. Domest. Anim. Endocrinol. 2010;38:63–74. doi: 10.1016/j.domaniend.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Pérez-Pérez A., Toro A., Vilariño-García T., Maymó J., Guadix P., Dueñas J.L., et al. Leptin action in normal and pathological pregnancies. J. Cell. Mol. Med. 2018;22:716–727. doi: 10.1111/jcmm.13369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokop J.W., Schmidt C., Gasper D., Duff R.J., Milsted A., Ohkubo T., Ball H.C., Shawkey M.D., Mays H.L., Jr, Cogburn L.A., Londraville R.L. Discovery of the elusive leptin in birds: identification of several 'missing links' in the evolution of leptin and its receptor. PLoS One. 2014;9:e92751. doi: 10.1371/journal.pone.0092751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M.A. An introduction to morphology of the reproductive system and anatomy of hen's egg. J. Life Earth Sci. 2013;8:1–10. [Google Scholar]
- Ramachandran R., Maddineni S., Ocón-Grove O., Hendricks G., Vasilatos-Younken R., Hadley J.A. Expression of adiponectin and its receptors in avian species. Gen. Comp. Endocrinol. 2013;190:88–95. doi: 10.1016/j.ygcen.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Roberts J.R. Factors affecting egg internal quality and egg shell quality in laying hens. J. Poult. Sci. 2004;41:161–177. [Google Scholar]
- Sah N., Mishra B. Regulation of egg formation in the oviduct of laying hen. Worlds Poult. Sci. J. 2018;74:509–522. [Google Scholar]
- Seroussi E., Cinnamon Y., Yosefi S., Genin O., Smith J.G., Rafati N., Bornelöv S., Andersson L., Friedman-Einat M. Identification of the long-sought leptin in chicken and duck: expression pattern of the highly GC-rich avian leptin fits an autocrine/paracrine rather than endocrine function. Endocrinology. 2016;157:737–751. doi: 10.1210/en.2015-1634. [DOI] [PubMed] [Google Scholar]
- Stevens L. Egg proteins: what are their functions? Sci. Prog. 1996;79(Pt. 1):65–87. [PubMed] [Google Scholar]
- Sugimoto Y., Saito A., Kusakabe T., Hori K., Koga K. Flow of egg white ovalbumin into the yolk sac during embryogenesis. Biochim. Biophys. Acta BBA - Gen. Subj. 1989;992:400–403. doi: 10.1016/0304-4165(89)90104-9. [DOI] [PubMed] [Google Scholar]
- Yoshizaki N., Ito Y., Hori H., Saito H., Iwasawa A. Absorption, transportation and digestion of egg white in quail embryos. Dev. Growth Differ. 2002;44:11–22. doi: 10.1046/j.1440-169x.2002.00620.x. [DOI] [PubMed] [Google Scholar]
- Yu M.W., Robinson F.E., Charles R.G., Weingardt R. Effect of feed allowance during rearing and breeding on female broiler breeders: 2. Ovarian morphology and production. Poult. Sci. 1992;71:1750–1761. doi: 10.3382/ps.0711750. [DOI] [PubMed] [Google Scholar]
- Zhou J., Seidel E.R. Estrogens induce visfatin expression in 3T3-L1 cells. Peptides. 2010;31:271–274. doi: 10.1016/j.peptides.2009.12.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.