Abstract
Bone healing is a complex process orchestrated by various factors, such as mechanical, chemical and electrical cues. Creating synthetic biomaterials that combine several of these factors leading to tailored and controlled tissue regeneration, is the goal of scientists worldwide. Among those factors is piezoelectricity which creates a physiological electrical microenvironment that plays an important role in stimulating bone cells and fostering bone regeneration. However, only a limited number of studies have addressed the potential of combining piezoelectric biomaterials with state-of-the-art fabrication methods to fabricate tailored scaffolds for bone tissue engineering. Here, we present an approach that takes advantage of modern additive manufacturing techniques to create macroporous biomaterial scaffolds based on a piezoelectric and bioactive ceramic-crystallised glass composite. Using binder jetting, scaffolds made of barium titanate and 45S5 bioactive glass are fabricated and extensively characterised with respect to their physical and functional properties. The 3D-printed ceramic-crystallised glass composite scaffolds show both suitable mechanical strength and bioactive behaviour, as represented by the accumulation of bone-like calcium phosphate on the surface. Piezoelectric scaffolds that mimic or even surpass bone with piezoelectric constants ranging from 1 to 21 pC/N are achieved, depending on the composition of the composite. Using MC3T3-E1 osteoblast precursor cells, the scaffolds show high cytocompatibility coupled with cell attachment and proliferation, rendering the barium titanate/45S5 ceramic-crystallised glass composites promising candidates for bone tissue engineering.
Keywords: Bone regeneration, 3D printing, Piezoelectricity, Bioactivity, Barium titanate
Graphical abstract
Highlights
-
•
3D printing of bioactive and piezoelectric barium titanate (BT) and bioactive glass (BG) composites.
-
•
BT/BG-composites show piezoelectric properties comparable to bone or higher.
-
•
3D-printed BT/BG scaffolds show the formation of a hydroxycarbonated apatite layer after incubation in SBF.
-
•
BT/BG scaffolds with enhanced cell-cell and cell-material interaction.
1. Introduction
The last few years have seen an increased interest in bioactive and smart biomaterials for hard tissue repair and regeneration. Several complex material systems have been developed by tailoring physical, chemical and biological properties to maintain control over cellular behaviour and functional tissue regeneration. Biomaterials are considered smart if they can respond to external or internal stimuli or if they possess the ability to actively stimulate or trigger effects on tissue or cells. The stimuli can vary, ranging from reactions to pH changes, temperature changes, the response to mechanical stimuli or ion exchange [1,2]. Bioactive glasses are one of the best-known and most extensively researched examples of bioactive materials. The research field of bioactive glasses was established in 1969 b y Prof. Larry Hench, who developed a glass composition of 46.1 mol.% SiO2, 24.4 mol.% Na2O, 26.9 mol.% CaO and 2.6 mol.% P2O5 (later called 45S5 Bioglass™) which created a solid bond to the bone after a short implantation time [3,4]. The rapid and strong bonding in the implant-bone interface is caused by the formation of a hydroxycarbonated apatite (HCA) layer on the surface of the glass when in contact with body fluids, induced by the dissolution of the glass and the release of ions such as calcium and phosphate ions into the interface. However, it is not only the formation of an HCA layer that characterises BGs in their effect. BG, especially when provided with various dopants (e.g. Ag+, Cu2+, Zn2+, Sr2+, Mg2+), can trigger several beneficial biological reactions through ion release. Osteogenic [[5], [6], [7], [8], [9]], angiogenic [[8], [9], [10], [11], [12]], antibacterial [9,[13], [14], [15]] and anti-inflammatory [9,[16], [17], [18]] properties have been demonstrated by several authors. Through targeted chemical adaptation, therapeutic effects can be induced, such as the inhibition of osteoporosis or the promotion of wound healing [9,19]. Although BG has proven its beneficial properties in various applications and has been processed in a wide variety of forms, e.g. through molding techniques, as 3D-printed ceramic scaffolds [20] or in the form of polymer-BG composites or BG fillers in hydrogels [21], they have not yet been able to replace autologous bone replacement as the gold standard.
Another interesting, smart biomaterial class that plays an increasing role in bone tissue engineering (BTE), are piezoelectric biomaterials [[22], [23], [24], [25]]. Piezoelectric materials generate charges due to small deformations induced by load. The deformation of the material causes an asymmetric shift of dipoles or ions in the crystal lattice and a change in the overall electrical polarisation which generates piezoelectricity [26]. This piezoelectricity is also generated by biological tissues and seems essential in tissue development and regeneration. We already know the importance of bioelectricity in the organism in the context of neuronal signal transmission or in cardiology. But it also plays a vital role in bone and bone remodelling [[27], [28], [29], [30]]. Recently, Kreller et al. showed the possibility of using biomaterials and bioelectrically stimulation to induce a cellular response to improve bone regeneration and affect bone remodelling processes [31]. Piezoelectricity in bone is achieved by collagen's highly ordered triple helix structure which exhibits an inherent polarisation. Since bone is a composite of densely packed collagen fibrils and hydroxyapatite, deformation or shearing of the collagen fibrils leads to piezoelectricity [22,32]. The collagen represents the main piezoelectric component in the bone, even though Tofail et al. demonstrated piezoelectric properties in sintered hydroxyapatite using piezo force microscopy [33]. However, the biological effects of piezoelectricity and bioelectricity orchestrating bone remodelling and regeneration continue to be discussed in the scientific community. The basic idea is that load induces deformation in the bone and generates an electric field due to the piezoelectric properties, which leads to an accumulation of ions, macromolecules and cells that then promote bone remodelling [23,24,32]. However, this theory seems to apply to dry bone mainly. In moist bone or bone in physiological environments, the piezoelectric effect cannot explain stress-generated potentials exclusively. Instead, streaming potentials, meaning potentials generated by the flow of ion-rich intestinal fluids, seem to play an important role and interact with the piezoelectric effect. In particular, a complex interplay of the streaming potential and piezoelectricity coupled via the zeta potential is being discussed. An increase in the surface charge density due to strain increases the zeta potential, thus intensifying the streaming potential and fostering bone regeneration and remodelling [22,34]. It is, therefore, reasonable to transfer the piezoelectric properties of bone to engineered biomaterials and thus, facilitate bone regeneration in a biomimicry approach. Recent studies have explored the use of lead-free piezoelectric ceramics such as barium titanate (BaTiO3) [[35], [36], [37], [38], [39], [40], [41], [42]], lithium niobate (LN) [[43], [44], [45]], lithium potassium sodium niobate (LNKN) [23,46,47] and potassium sodium niobate (KNN) [48,49] showing enhanced protein adsorption on charged surfaces and increased cell proliferation and metabolic activity. Therefore, combining a piezoelectric ceramic which causes autonomous stimulation of bone cells through bioelectric cues with a classical bioactive material that leads to rapid biomineralisation in the interface is quite promising. Recently, a research group led by Saeidi et al. demonstrated the beneficial properties of a freeze-casted BaTiO3/BG composite scaffold with high cytocompatibility and high cell viability of up to 98% compared to the control group [50]. In another approach by Zhao et al. the combination of a poly (vinylidene fluoride) (PVDF) membrane with electro-spun collagen fibres with incorporated microscale BG particles showed promising bone regenerative results both in vitro and in vivo [51]. The increased bone healing is attributed to the increased accumulation of calcium ions on the polarised surface of the PVDF membrane. The authors found that the high concentration of Ca2+ simultaneously leads to increased activity of the calcium-sensing receptor of the osteoblasts and thus, further promotes osteogenesis [51,52]. However, combining piezoelectric and bioactive materials with modern manufacturing methods such as 3D printing has not yet been studied.
In this study, we combine the advantages of piezoelectric (BaTiO3) and bioactive (45S5® Bioglass) biomaterials in an additive manufacturing process to achieve functional, 3D biomaterials for bone regeneration. We show dense specimens and scaffolds with defined pore sizes can be produced using a binder jetting process. Through subsequent thermal post-treatment, we achieve dense composite materials with mechanical properties in the range of physiological spongy bone [[53], [54], [55]]. In this case, bioactive glass serves not only as a bioactive mediator but also as a matrix stabilising component: by adding bioactive glass, we significantly improved the mechanical properties of 3D-binder-jetted BaTiO3 scaffolds compared to previously published BaTiO3-hydroxyapatite (BaTiO3/HA) composites [40]. Afterwards, we assessed the piezoelectric and bioactive properties of the fabricated scaffolds. Finally, we investigated cell adhesion and cytocompatibility of the materials toward MC3T3-E1 murine pre-osteoblast cells. As a result, the bioactive and piezoelectric multi-functional composite materials shown in this study advance the field of composite biomaterials for bone regeneration by offering scaffolds for piezoelectric and bioactive stimulation of bone cells.
2. Materials and methods
2.1. Material and fabrication
To produce a printable powder mixture, BaTiO3 powder (d50: 3,0 μm, Sigma-Aldrich/Merck KGaA, Darmstadt, Germany), 45S5 bioactive glass powder (d50: 4.0 ± 1.0 μm, composition: 45 wt% SiO2−24.5 wt% CaO−24.5 wt% Na2O−6 wt% P2O5, Schott Vitryxx™, Schott AG, Germany) and an additional SiO2-based flowing agent (Aerosil R8200™) were mixed with Polyethylenmethacrylate (PEMA, DEGACRYL™, Evonik Industries, Essen, Germany) in a jar rolling mill WTR 295 W/R (Welte Mahltechnik GmbH, Ransbach-Baumbach, Germany). The powders were dried and sieved in advance to avoid any agglomerations. The exact composition of the powder mixtures is shown in Table 1. Cylindrical samples, dense and with interconnected macropores, as published previously, were designed using CAD software (SolidWorks, Dassault systems, Waltham, USA) [40]. The powder mixture was 3D-printed on a Voxeljet VX500 binder-jet printer (Voxeljet AG, Friedberg, Germany) with a layer thickness of 100 μm and a printing resolution of 250 dpi. In brief, the printer deposits a binder fluid (SOLUPOR, Voxeljet AG, Friedberg, Germany) in a layer-by-layer process according to the *.STL file, and partially dissolves the polymeric phase. The binder consists of a solvent mixture using hexane-1-ol, 2-ethylhexyl acetate, and hexyl acetate, as published in previous protocols [40,56]. After a waiting period of 24 h to ensure that the solvent evaporated, they were removed from the powder bed. Samples were stored for at least 24 h in a drying cabinet (Heraeus T6060, Hanau, Germany) at 40 °C for complete curing.
Table 1.
Powder mixture compositions used for 3D printing.
| BaTiO3 | BG (45S5) | SiO2 | PEMA | |
|---|---|---|---|---|
| BaTiO3/5%BG | 81.8 wt% | 5 wt% | 1.5 wt% | 11.7 wt% |
| BaTiO3/15%BG | 73.2 wt% | 15 wt% | 1.3 wt% | 10.5 wt% |
2.2. Post-processing
Finding a suitable process window for sintering BaTiO3/BG composites is critical in producing final scaffolds. BaTiO3 is conventionally sintered at high temperatures around 1300 °C [37,[57], [58], [59], [60]]. At these temperatures, BG is already present as a melt and generally loses its bioactive properties (degree of crystallinity increases) due to excessive heat treatment [61,62]. We have relied closely on existing literature on the sintering of BaTiO3 and BaTiO3 composites to determine the sintering curve used here and on our own research and experiments [40,63]. After drying, the samples were thermally post-processed for 1 h at 300 °C and 2 h at 500 °C in a furnace to remove the polymer matrix. The post-processed specimens were then sintered in a furnace under an ambient atmosphere to obtain dense ceramic-crystallised glass composites. The sintering curves are visualised in Fig. 1.
Fig. 1.
Sintering curves for the respective composites BaTiO3/5% BG and BaTiO3/15%BG. Both were sintered with a conventional furnace in air.
2.3. Morphological and microstructural characterisation
To determine the shrinkage after sintering, the samples were measured with a digital calliper (conforms to DIN 682) before and after thermal post-processing (n = 5 per group). The density and porosity of the sintered samples (n = 5 per group) were determined with the Archimedes' principle using a specially equipped precision scale (LA230s, Sartorius AG, Göttingen, Germany). Scanning electron microscopy (SEM, Merlin VP compact, Carl Zeiss AG, Jena, Germany) and electron dispersive X-ray spectroscopy (XFlash 6/30 Co., Bruker, Berlin, Germany) were used to assess the microstructure and chemical composition. X-ray diffraction (XRD) powder patterns were recorded on a Panalytical X'Pert θ/2θ -diffractometer equipped with an Xcelerator detector using automatic divergence slits and Cu kα1/α2 radiation (40 kV, 40 mA; λ = 0.15406 nm, 0.154443 nm). Cu beta-radiation was excluded using a nickel filter foil. Measurements were performed with 0.086°s−1, respectively. Finely pestled samples were mounted on silicon zero background holders. After data collection, obtained intensities were converted from automatic to fixed divergence slits (0.25°) for further analysis. Peak positions and profiles were fitted with the Pseudo-Voigt function using the HighScore Plus software package (Panalytical). Phase identification was done by using the PDF-2 database of the International Center of Diffraction Data (ICDD).
2.4. Mechanical characterisation
Compression tests on cylindrical samples (n = 5 per group) were performed using a uniaxial testing machine (Zwick Roell Z5.0, ZwickRoell GmbH, Ulm, Germany) with a 5 kN load cell and a crosshead speed of 0.5 mm min−1. Prior to the compression test, the top and bottom surfaces of the scaffolds were ground to achieve optimal testing conditions in a uniaxial testing state. The Young's modulus was calculated between 1.5% and 2% strain.
2.5. Poling and piezoelectric characterisation
The fabricated cylindrical samples were polarised in a strong electrical field. The polarisation setup consists of a sample holder with silver (Ag) electrodes on both sides that were connected to a high voltage power supply (HNCs 10,000–180 pos., Heinzinger, Rosenheim, Germany). The sample holder was placed in a heated silicon oil bath (ELBESIL, L. Böwing GmbH, Hochheim) to prevent any sparks and to polarise the scaffolds shortly below the Curie temperature (120 °C). The samples were polarised applying a field strength of 1.25 kV/mm for 30 min. The piezoelectric constant d33 was measured with the Berlincourt method [64] using a d33 piezometer (PM300, PIEZOTEST, Singapore). For the piezoelectric assessment n = 5 samplers per group were used.
2.6. Bioactivity testing
To investigate the bioactivity of BaTiO3/BG composite scaffolds, simulated body fluid (SBF) was produced according to (Kokubo) and ISO 23317 [65]. 3D-printed discs with a diameter of d = 5 mm and h = 2 mm height were fabricated with n = 3 samples per group. The samples were placed in SBF according to previously published protocols [55], and placed on a shaking incubator (Heidolph Unimax 1010, Heidolph Instruments GmbH & CO. KG, Germany) at 37 °C and 90 rpm. SBF was changed every two days. Samples were removed after 7, 14, 21, and 28 days of immersion in SBF. The samples were rinsed with ultrapure water and dried under a fume hood at room temperature (22 °C, RT). Subsequently, the scaffolds were analysed by Fourier-transform infrared spectroscopy (FTIR) and SEM/EDS analyses as described elsewhere [55]. In brief, FTIR (IRAffinity-1S, Shimadzu Europa GmbH) absorbance spectra were recorded for the BaTiO3/BG composite scaffolds before incubation in SBF and after each incubation time point. SEM (Auriga Crossbeam, Carl Zeiss Microscopy GmbH, Germany) and EDS (X-MaxN, Oxford Instruments) were used to assess the surface morphology as well as to determine the elemental surface composition of the BaTiO3/BG scaffolds, respectively.
2.7. Cell culture studies
2.7.1. Cell culture and maintenance
Mouse calvaria pre-osteoblast cells (MC3T3-E1, Sigma Aldrich, Germany) were cultured in alpha-modified minimum essential medium (α-MEM) (Gibco®, Life Technologies™, Germany), supplemented with 10% (v/v) fetal bovine serum (FBS), 1% (v/v) penicillin/streptomycin (all Sigma-Aldrich, Germany) and 1% (v/v) l-Glutamine (Thermo Fisher Scientific Inc., USA). Cells were passaged in T75 cell culture flasks (Sarstedt, Germany) and maintained at 37 °C, 95% air, 5% CO2 humidified atmosphere in an incubator (Galaxy® 170 R, Eppendorf AG, Germany). Trypsin/EDTA (Sigma Aldrich, Germany) was used for cell detachment, while cell counting was performed via the trypan blue exclusion method using Neubauer chambers (Paul Marienfeld GmbH & Co. KG).
To assess the cytocompatibility of 3D-printed BaTiO3/BG and BaTiO3/HA (as a benchmark, previously published [40]) scaffolds, 100.000 cells/ml MC3T3-E1 were seeded on 3D-printed cell culture discs (1 ml/scaffold) in 24-well cell culture plates. The samples were cultured for 24 h to assess initial cell attachment and in vitro cytocompatibility. Tissue culture polystyrene (TCPS) substrates served as an additional control to the 3D-printed BaTiO3/BG discs. To assess pH change due to potential BG dissolution from the scaffolds, the cell culture medium (5 ml, n = 4 scaffolds) was removed 24 h after cell seeding, and the pH was recorded.
2.8. LIVE/DEAD staining
Cell viability was determined by performing a LIVE/DEAD staining assay as described previously [55]. After 7 days, cells were stained using calcein acetoxymethyl ester (Calcein AM, LIVE) (InvitrogenTM, Molecular probes by Life technologiesTM, USA) and propidium iodide (PI, DEAD) (InvitrogenTM, Molecular probes by Life technologiesTM, USA), to indicate viable and dead/apoptotic cells, respectively. BaTiO3/BG samples were washed with phosphate-buffered saline (DPBS, Thermo Fisher, USA) and incubated with 1 ml of DPBS stock solution containing 4 μl/ml Calcein AM and 5 μl/ml PI for 45min. Samples were washed (DPBS) and fixed using 500 μl of fixation solution (0.1 M PIPES (Piperazine-N,N′- bis(2-ethanesulfonic acid), Merck, Germany), 1 mM EGTA (Ethylene glycol tetraacetic acid, Merck, Germany), 4% (w/v) polyethyleneglycol, 3.7% (w/v) paraformaldehyde (all Sigma Aldrich, Germany) in Hank's balanced salt solution (HBSS)).
2.9. Multiphoton fluorescence microscopy
After 7 days of incubation, samples of BaTiO3/5%BG and TCPS control (n = 3) were washed using HBSS and fixed using 4% formaldehyde solution (in HBSS) for 5 min. The cells were permeabilised (0.1% TritonX-100 for 5 min) and washed twice using HBSS. Subsequently, the cells were stained using 5 and 1 μl/ml of Rhodamine-phalloidin F-Actin/4′,6-Diamidino-2-phenylindol (DAPI) (both Life Technologies, Thermo Fisher, USA), in HBSS, for 1 h and 5 min, respectively. The samples were imaged using a multiphoton microscope (TriMScope II, LaVision BioTec, Bielefeld, Germany) equipped with a HC FLUOTAR L 25x/0.95 W VISIR objective (Leica Microsystems GmbH, Germany). DAPI was excited using a mode-locked ps-pulsed Ti:Sa laser (Chameleon Vision II, Coherent, Santa Clara, USA) at 810 nm. The sample fluorescence was recorded using the following single bandpass filters: 450/30 nm (DAPI), 525/50 nm (Calcein-AM), and 620/60 nm (Rhodamine-Phalloidine), all Chroma Technology group (Acal BFi Germany GmbH, Germany). Different photomultiplier tubes (PMT) were used for each channel. Every image had a field-of-view of 409.6 x 409.6 μm2. The imaging depth was adapted according to sample roughness (TCPS, flat; 3D-printed BaTiO3/5% BG, rough), in order to capture all cells in the field-of-view. Maximum intensity z-projections were made for a qualitative assessment. Cell number and density were quantified using ImageJ (ImageJ software package, Fiji, ImageJ 1.52i). For the analysis, n = 6 samples of BaTiO3/BG composite and n = 3 samples of positive TCPS controls were used.
2.10. Cell material interaction
The interaction of MC3T3-E1 cells with BaTiO3/BG scaffold surfaces was assessed using SEM. Briefly, cells were fixed after 7 days of incubation on the samples and transferred to two SEM-fixation solutions [66] for 1 h, respectively. The samples were then dehydrated using an ethanol series by subsequent incubation in 30%, 50%, 70%, 75%, 80%, 85%, 90%, 95% and 99% EtOH/H2O solutions for 10 min each. Prior to imaging, the samples were dried using a critical point drier (EM CPD300, Leica, Wetzlar, Germany). SEM images were recorded using an Auriga CrossBeam SEM (Carl Zeiss, Oberkochen, Germany).
2.11. Extracellular lactate dehydrogenase release assay (LDH)
To assess the potential cytotoxicity of BaTiO3/BG scaffolds, extracellular LDH release was determined from cell culture supernatants using the TOX7 in vitro toxicity kit (Sigma-Aldrich). Aliquots of 500 μl were removed from MC3T3-E1 cells cultured for 24 h on TCPS, BaTiO3/HA, BaTiO3/5%BG, and BaTiO3/15%BG samples (n = 6), and frozen at −21 °C until further use. All samples were treated according to the manufacturer's instructions and as described in previous studies [67]. Briefly, samples were thawed on the day of analysis and 140 μl of the supernatants were transferred into UV–vis cuvettes (path length: 10 mm, Sarstedt, Germany). LDH master-mix solution (60 μl) containing equal amounts of substrate solution, LDH cofactor solution, and dye solution were added to the cuvettes and incubated at RT for 30 min in the dark. The samples were diluted using 700 μl UPW, and the absorption was immediately measured (λ = 490 nm, 690 nm) using a UV–vis spectrophotometer (NanoDrop One, ThermoFisher, USA) in cuvette mode.
2.12. PicoGreen proliferation assay
Cells were grown for seven days on TCPS, BaTiO3/HA, BaTiO3/5%BG, and BaTiO3/15%BG samples. Cells grown BaTiO3 composites (n = 6) and TCPS controls were washed using HBSS and frozen at −80 °C to facilitate cell lysis and DNA release. On the day of the experiment, samples were thawed and washed using 1 ml of 1 × TE PicoGreen assay buffer solution (Quant-iT PicoGreen ds- DNA Assay-Kit, Invitrogen, Life Technologies, ThermoFisher, USA). Equal amounts of Quant-iT PicoGreen working solution and sample (50/50) were mixed and incubated for 3 min at RT without light. The relative fluorescence (RFU) was recorded using a CFX connect spectrofluorometer (Bio-Rad, Germany). LambdaDNA assay standard served as the calibration curve (R2 = 0.99). DsDNA data for all samples were normalised to cells grown on TCPS for 24 h as a control.
2.13. Statistical analysis
All statistical analyses were performed using GraphPad Prism v9.1.0 software. Data were checked for normal distribution using Shapiro-Wilk tests. Normally distributed data were subjected to unpaired Student t-tests (Fig. 7b) for pair-wise comparisons or one-way ANOVA tests (Fig. 7c, e). Non-normally distributed data (Fig. 7d) was analysed using the Mann-Whitney test. p < 0.05 was considered as statistically significant differences between groups.
Fig. 7.
FTIR of 3D-printed BaTiO3/BG-composite scaffolds after incubation in SBF. A) FTIR spectrum for BaTiO3/5%BG over 28d in SBF with minor PO43− bending vibrations and C032− bending vibrations. B) FTIR spectrum for BaTiO3/15%BG with more pronounced bending vibrations for P043− and C032− compared to the 5%BG composite and an additional asymmetric stretch vibration at 1000 cm−1 which can also be assigned to PO43−.
3. Results and discussion
3.1. Scaffold fabrication and morphology
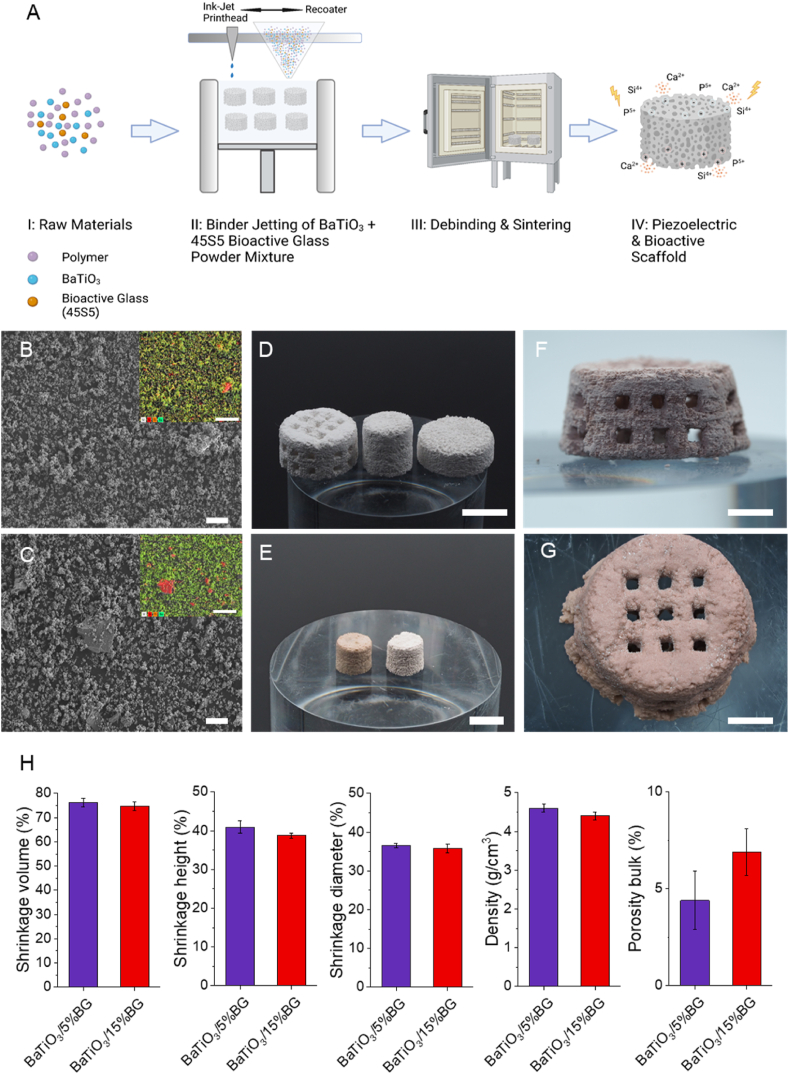
Fig. 2 shows the fabrication route we used to produce bioactive and piezoelectric scaffolds. The first important step was the selection of the starting materials and preparing a homogeneous and flowable powder blend. For the piezoelectric components, we chose BaTiO3 which is lead-free and has already shown its suitability as a biomaterial in several publications [32,35,39]. As a bioactive component, we used 45S5 Bioglass which, despite the possibility of improved bioactive glasses, is still the typical benchmark material in the context of classical bioactivity [68]. As previously described, both were mixed with polyethylene methacrylate (PEMA) to form a flowable blend [40,69,70]. Fig. 2B and C show SEM images for both powder blends BaTiO3/BG5% and BaTiO3/BG15%. The supplementary material section shows representative SEM images of the individual base materials (Fig. S1). The apparent difference in particle size shows microscopic BaTiO3 particles (d50 3 μm) and much larger BG flakes (d50 20 μm). In addition, the EDS element mappings show the distribution of the elements in colour, with silicon shown in red, as it is the primary component in the BG, and barium shown in green (corresponding spectra are shown in Figs. S2 and S3). The powder blend prepared in this way was deposited in homogeneous layers by the 3D printer, and scaffolds were successfully produced using a binder jetting process. In the binder jetting process, the polymer component of the powder blend is dissolved by a solvent applied based on the sliced CAD file, and the ceramic particles are firmly integrated into the polymer matrix during evaporation. Fig. 2,D shows exemplary green parts of the BaTiO3/5%BG composited fabricated via binder jetting. We produced dense solid cylinders and interconnected porous scaffolds with a pore size of about 1 mm. After printing, it was necessary to thermally post-process and sinter the fabricated scaffolds (Fig. 1, A III) to obtain a ceramic-crystallised glass composite. However, thermal post-treatment is critical, as the starting materials show very different sintering behaviour. BaTiO3 conventionally sinters in a temperature range around 1300° [71,72], while BG (45S5) already passes two glass transition temperatures (Tg1, Tg2) at 550 °C and 850 °C and two melting temperatures at 1190 °C and 1230 °C [61,73]. Fig. 1, E shows two printed and thermally post-treated cylindrical samples of the BaTiO3/5%BG composite on the left and the BaTiO3/15%BG composite on the right. Both samples show a very similar appearance, with only slight differences in terms of shrinkage and a slight colour difference (Fig. 2, H). Notably, the samples of the BaTiO3/15% BG group could not be sintered at 1320 °C due to the high content of BG and were therefore, sintered just below the first melting temperature of 45S5 BG at 1150 °C. The macroporous scaffolds were also successfully sintered and are shown in Fig. 2, F (BaTiO3/5%BG) and G (BaTiO3/15%BG). Noticeably, the scaffolds are not completely accurate in shape after sintering and are expanding at the base. Since the glass transition temperatures were exceeded during sintering, which is associated with a strong reduction in the viscosity of the BG, as well as the melting point of the BG for the BaTiO3/5%BG, the microstructure is strongly influenced by viscous flow [61,74]. The scaffolds are largely able to compensate for this, but presumably do not remain accurate in shape as a result. In this context, BG also acts as a sintering aid, allowing the sintering process to occur earlier at lower temperatures and providing greater compaction. However, the pores of the scaffolds remain free and interconnected, which is essential in terms of osteo-conduction and vascularisation [75].
Fig. 2.
Processing route for piezoelectric and bioactive BaTiO3/BG scaffolds and 3D-printed results. A) Schematic illustration (B–C) SEM powder analysis of both compositions containing 5% (B) and 15% (C) BG (scale bar = 20 μm). (D) Green parts printed via Binder Jetting based on the BaTiO3/5%BG composition (scale bar = 10 mm). (E) Post-processed cylindrical samples of the BaTiO3/5%BG (left) and BaTiO3/15%BG (right) composite (scale bar = 10 mm). (F) Front view of a BaTiO3/5%BG scaffold and (G) top view of a BaTiO3/15%BG scaffold (scale bar = 5 mm). (H) Morphological and physical properties of 3D-printed BaTiO3/BG composites. Data are shown as mean ± SD (n = 5). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Microstructure
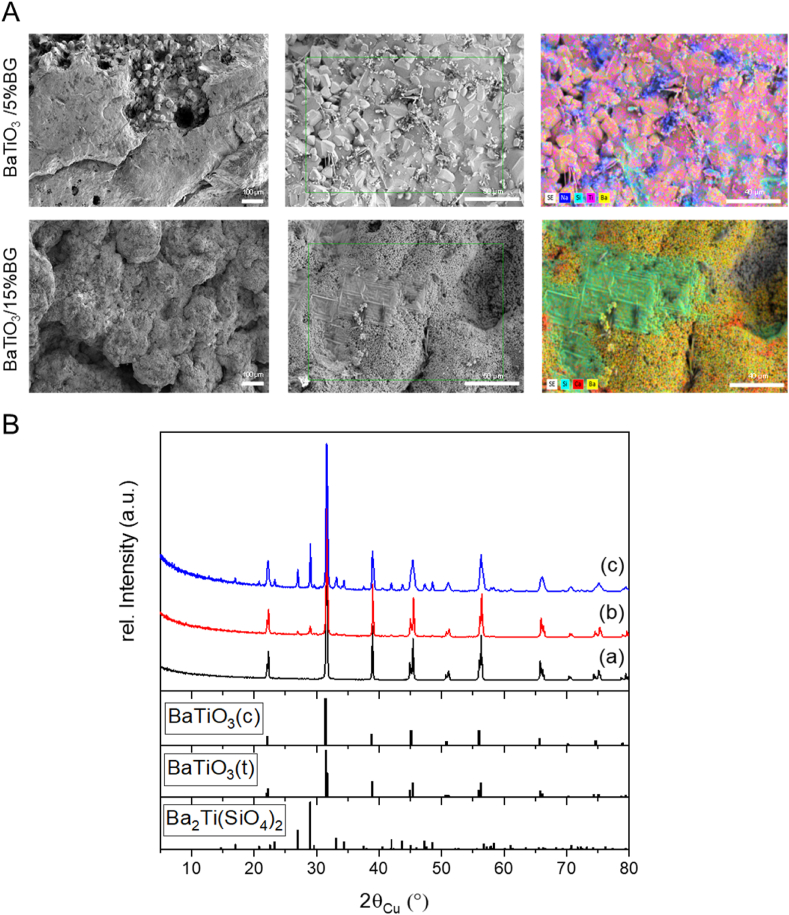
The microstructural composition of ceramic-crystallised glass composites substantially influences the mechanical, dielectric and bioactive properties and is significantly affected by the necessary thermal post-treatment. Fig. 3, A shows SEM images of both composites after the respective thermal post-treatment. Both composites offer a highly compacted surface, corresponding to the porosity shown in Fig. 1, H. Only the BaTiO3/15% BG composite sintered at 1150 °C still shows intergranular porosity and indicates that the sintering process of the BaTiO3 is not finished. Nevertheless, with 6.9%, the bulk porosity is low, and the microstructure is largely densified. From the literature, it is known that 45S5 BG already undergoes rapid densification from the first Tg1 (550 °C) due to the reduction of the viscosity of the glass and the onset of viscous flow. This process continues until about 600 °C when glass-in-glass separation and crystallisation processes are initiated and prevent further densification. From Tg2 onwards, the remaining amorphous phases reach a viscosity low enough again to allow viscous flow or even liquid phase sintering, thus causing further densification of the BG and the composite [61,74]. We assume that the densification process of the BG plays a decisive role in the sintering process of the composite, especially for the higher BG loading of 15%. The intergranular pores that the BaTiO3/15% BG composite still showed, are no longer present in the BaTiO3/5% BG composite sintered at 1320 °C. Here, the sintering process of the BaTiO3 particles seems to be completed. The EDS maps show portions of BG, especially Si, Na and Ca, on the surface of both composites (corresponding spectra are shown in Figs. S4 and S5). In both composites, the BG particles present in the starting material (Fig. 2 B, C) have lost their shape, which supports the assumption of viscous flow of the BG within the composite. However, the premises described in the literature refer to the sintering of pure 45S5 BG and already define dependencies on factors such as particle size, heating rates and holding times. These also apply to the composites presented here, including the presence of BaTiO3 as a ceramic phase which interacts with the BG, e.g. through the exchange of second-order ions, significantly influencing the sintering process and the degree of crystallisation of the resulting ceramic-crystallised glass composite.
Fig. 3.
Microstructural properties of thermal post-processed BaTiO3/BG composites. (A) Representative SEM images with coupled EDS element mappings. (B) Powder X-ray diffraction pattern of utilised untreated BaTiO3 (a), BaTiO3/5%BG (sintered at 1320 °C; b) and BaTiO3/15%BG (sintered at 1150 °C; c), respectively, and reference Bragg peak positions of identified main phases (cubic BaTiO3 ICDD pdf 01-089-2475, tetragonal BaTiO3 ICDD pdf 01-074-7965, Ba2Ti(SiO4)2 05-00-0075).
Powder X-ray diffraction has been applied to gain information about crystal phase and their potential change within the sample. For BaTiO3, two crystalline polymorphs of cubic and tetragonal symmetry can be identified. The tetragonal phase, typically characterised by a sharp double peak at 45° 2theta, is the phase of interest for achieving desired ferroelectric and piezoelectric properties [76,77]. Obtained diffraction data of processed and sintered BaTiO3/BG composite samples and pure BaTiO3 are summarised in Fig. 3, B. Supporting information regarding the raw materials and the Miller indices and interplanar spacing of the major identified phases in the final composites can be found in Fig. S7 and Tables 1–3 of the supplementary information file. BaTiO3 can still be identified from both datasets independent of the amount of BG added and treatment temperature. Notable additional phases were identified, where Ba2TiSi2O8 (Fresnoite) is the most prominent one. This glass-ceramic phase which exhibits piezoelectric properties, is formed to a greater extent in BaTiO3/BG15% compared to BaTiO3/BG5% but shows no ferroelectric properties and cannot be polarised by an external electric field [78]. Besides, some low-intensity peaks remain unexplained in the BaTiO3/BG15% diffraction pattern if BaTiO3 and Ba2TiSi2O8 are considered. These Bragg peaks (Fig. S6) can finally be assigned to the presence of CaTiO3 and Na6Ca3Si6O18 (Combeite). As well as for Ba2TiSi2O8, the presence of CaTiO3 can be explained by the reaction of BaTiO3 with BG, whereas Na6Ca3Si6O18 might result from the crystallisation of the latter. This clearly illustrated that BG undergoes structural changes from the amorphous to the crystalline state if it was treated at high temperatures. In particular, temperatures above Tg2 (850 °C) induce the formation of crystalline sodium-calcium-silicate phases and lead to high densification of the matrix [73,79]. Recently, pseudo-piezoelectric properties for CaTiO3 were described as well as the application as possible bone substitute material or implant coating [[80], [81], [82]].
4.1. Mechanical properties
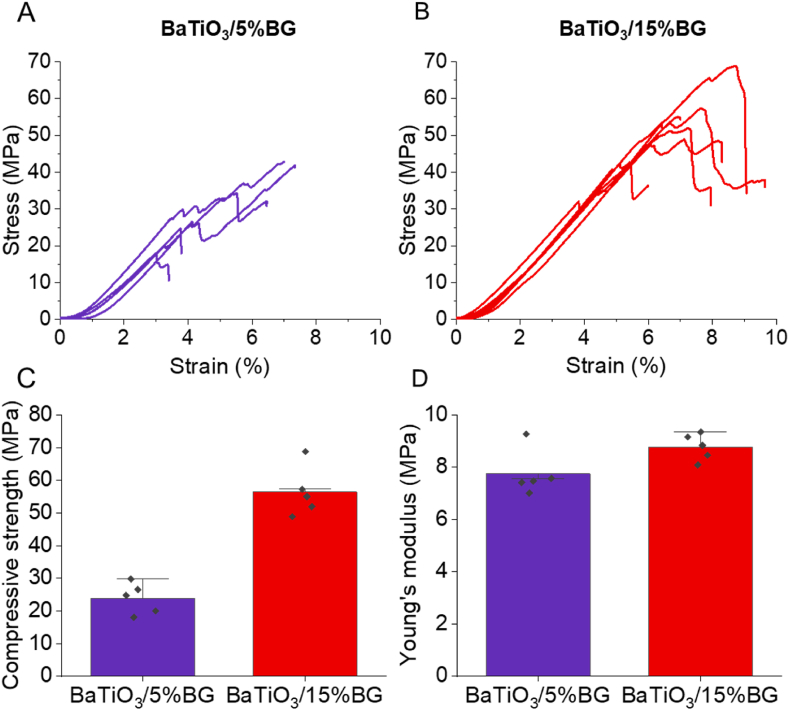
We performed compression tests to assess the mechanical properties of the fabricated composites. The stress-strain curves for both ceramic-crystallised glass composites are presented in Fig. 4A and B. The curves for both composites show a similar shape but differ with respect to the time of fracture. The BaTiO3/5%BG fails significantly earlier at lower compressive strength than the BaTiO3/15%BG composite (Fig. 4, C). In fact, the BaTiO3/5%BG composite exhibited an average compressive strength of 23.8 ± 4.8 MPa, and the composite with a higher BG content of 15% reached a compressive strength of 56.4 ± 7.6 MPa. The Young's moduli of both composites are very comparable, with 7.7 ± 0.9 MPa and 8.8 ± 0.5 MPa for the BaTiO3/5%BG and the BaTiO3/15%BG composites, respectively (Fig. 4, D). It is known that bioactive glasses can be used to enhance the mechanical properties of bioceramics such as HA and beta-TCP. Goller et al. achieved an average compressive strength of 83 MPa with a content of 5–10 wt% 45S5 in a BG/HA composite sintered between 1200 and 1300 °C [83]. Adding 5 wt% more BG to the composite increased the compressive strength of about 20 MPa, but this was dependent on the sintering temperature and could only be demonstrated for 1200 °C. For 1300 °C sintered samples, an opposite effect occurred as decomposition of the HA occurred, and unwanted reactions between the HA and the BG arose [83,84]. Similar results can be seen in the composites shown here. Increased BG content and sintering at lower temperatures increase compressive strength to 30 MPa. The higher proportion of BG in the BaTiO3/BG15% composite and the use of lower sintering temperatures which lead to significantly more crystalline phases, appears to be the reason for the increase in compressive strength. Saeidi et al. showed comparable results for freeze-casted BaTiO3/BG composites with 10 and 25 wt% BG loading, where the compressive strength increased similarly with the BG content [50]. Due to the very high porosity of 77%, Saeidi et al. achieved a compressive strength of 16 MPa with the BaTiO3/BG composite with 25 wt% BG content. Shokrollahi et al. fabricated freeze-casted BaTiO3/Akermanite (Ca2MgSi2O7) scaffolds with comparable compressive strength values around 20 MPa without observing a considerable reinforcement of the composites due to an increase in the akermanite content [85]. The mechanical properties presented by us are in a similar range to those presented by other groups working with BaTiO3-composites and in a range that is very similar to cancellous bone, making the scaffolds appealing for use in BTE, at least from a mechanical point of view. Compared to our first scaffold generation based on BaTiO3/HA, we significantly increased the mechanical values by using BG instead of HA as a partner for BaTiO3 [40].
Fig. 4.
Mechanical properties of 3D-printed BaTiO3/BG composites. (A-B) Qualitative stress-strain diagrams from compression tests of BaTiO3/5%BG (A) and BaTiO3/15%BG (B). (C-D) Compressive strength and elastic modulus of 3D-printed BaTiO3/5%BG (C) and BaTiO3/15%BG (D). Data are shown as mean ± SD (n = 5).
4.2. Piezoelectricity
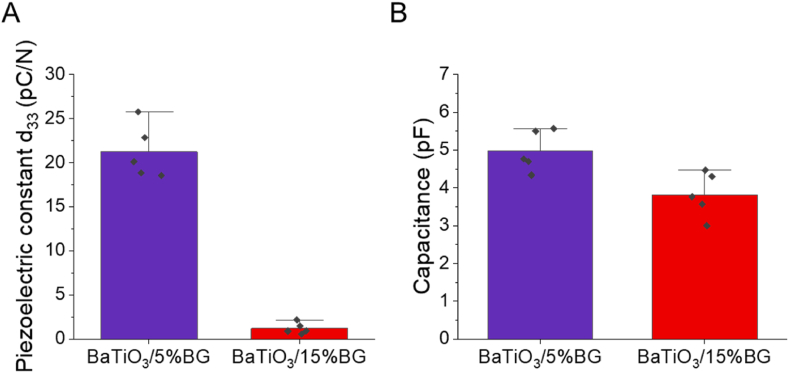
We assessed the piezoelectric properties by using the Berlincourt method exposing the polarised scaffolds to a cyclic load and measured the piezoelectric constant d33 and the capacitance of the scaffolds (Fig. 5A and B). The BaTiO3/5%BG composites exhibited an average piezoelectric constant d33 of 21.2 ± 3.1 pC/N and a capacitance of 5.0 ± 0.5 pF, whereas the BaTiO3/15%BG composites exhibited a d33 of 1.2 ± 0.6 pC/N and a capacitance of 3.8 ± 0.6 pF. As expected, the increase in the BG content leads to a significant reduction of the ferroelectric and piezoelectric properties of the composite [37,50,85]. Saeidi et al. have also shown piezoelectric properties of similar magnitude for BaTiO3/BG composites and found a decrease in the charge constant d33 with an increase in BG content. Nevertheless, they were able to measure a d33 of 24 pC/N up to a BG load of 25 wt% [50]. Shokrollahi et al. reported d33 values in the range of 0.5–4 pC/N for BaTiO3-akermanite composites, likewise decreasing with the increase of the non-piezoelectric phase fraction. Even small amounts of non-piezoelectric phase significantly change the microstructure of the BaTiO3 composites, causing a pronounced degradation of the piezoelectric properties compared to pure BaTiO3 ceramics [85]. One reason for this is the change in the composition of the material and the formation of a ceramic-crystallised glass composite. For instance, the XRD in Fig. 2, B shows the formation of different silicate phases due to the high temperature during the sintering process. The barium-titanium silicates formed, among other things, are potentially piezoelectric but not ferroelectric. This means that their dipoles do not align in the polarisation process, so they only contribute to directional piezoelectricity to a limited extent [78]. In addition, especially for the higher loaded BaTiO3/BG15% composite, the tetragonal crystal structure which is necessary for the piezoelectric properties of BaTiO3, seems to degrade, which is evident from the lack of a characteristic double peak at 2theta 45° [76]. Furthermore, due to the material inhomogeneity (pores) and the different resulting permittivities, a uniform polarisation field is not established in the scaffolds, which can lead to an inhomogeneous remanent polarisation and thus, low piezoelectricity [86]. Overall, the results are in a range in the order of magnitude of natural bone tissue or even higher, making the scaffolds suitable candidates in a bone mimicking tissue engineering approach [87,88]. Tang et al. presented very promising results with a composite of BaTiO3/HA with a d33 of around 2.7 pC/N, which showed significantly increased alkaline phospatase (ALP) activity compared to pure BaTiO3 and HA after 7 days of dynamic loading [37].
Fig. 5.
Piezoelectric properties of 3D-printed BaTiO3/BG composites. (A) Piezoelectric constant d33, (B) Capacitance. Data are shown as mean ± SD (n = 5).
4.3. Bioactivity
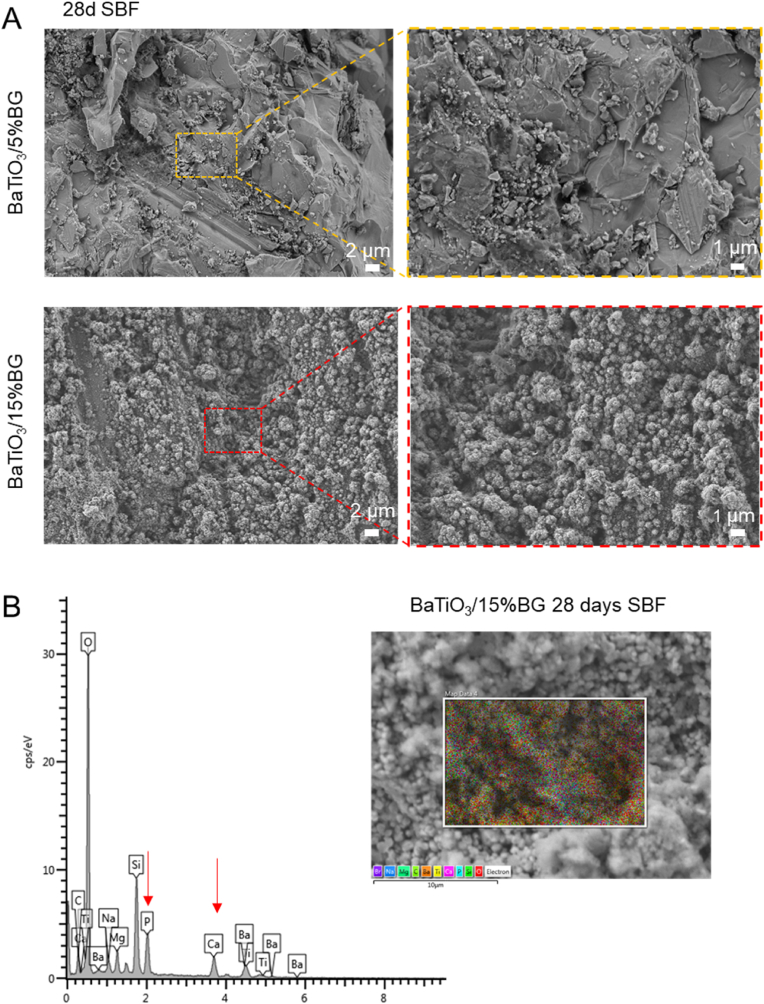
Having established the fabrication route for BaTiO3/BG composites, and after characterisation of the major physical properties of the scaffolds, we became interested in the bioactive potential of the scaffolds. To investigate bioactivity, we incubated scaffolds of both composites for 28 days in SBF and characterised their ability to form an HCA layer [65]. An apparent change in the surface morphology became visible in the BaTiO3/15%BG composite, where a cauliflower-like morphology developed after 28 d, indicating calcium phosphate species on the surface (Fig. 6, A) [55,89]. The BaTiO3/5% BG scaffolds however, did not display this behaviour. On the surface of the BaTiO3/5%BG scaffolds, particles resembling calcium phosphates are sporadically visible. The prominent formation of the characteristic cauliflower-like morphology is presumably attributed to the increased BG load and the lower sintering temperature. The EDS analysis confirms the presence of Ca and P on the surface, in addition to the prominent peaks for O, Si and Ba (Fig. 6, B).
Fig. 6.
Bioactivity assessment of BaTiO3/BG composites. (A) SEM images of BaTiO3/BG composites after 28 days of immersion in SBF showing cauliflower-like apatite formation on the surface of the BaTiO3/15%BG composite. (B) EDS spectra on the surface of the BaTiO3/15%BG composite.
We performed FTIR spectroscopy to assess further the formation of calcium phosphates on the surface of the scaffolds (Fig. 7). The spectra were recorded after 0 d, 7 d, 14 d, 21 d and 28 d of immersion in SBF. The BaTiO3/15%BG composite shows more explicit indications of the formation of HCA in the spectra than the BaTiO3/5%BG composite. From about 14 days, mid-infrared vibrations associated with P043− appear, represented by a bending vibration around 500 cm−1 wavelength and an asymmetric stretch vibration at about 1000 cm−1 [90,91]. However, the characteristic PO43− vibrations at 560 cm−1 and 604 cm−1 were not detected in either composite even after 28 days in SBF [91]. A further indication of the formation of an HCA layer is the distinct detection of asymmetric CO32− bend vibrations which increase considerably within the 28 days in SBF for the BaTiO3/15%BG composite [91,92]. The lower BG-loaded BaTiO3/5%BG composite shows a reduced bioactive potential compared to the BaTiO3/15%BG composite. The PO43− bending vibrations at 500 cm−1 are only visible to a lesser extent, and the asymmetric stretch vibrations at 1000 cm−1 are absent. The CO32− bend vibration is likewise reduced compared to the BaTiO3/15%BG composite. The results support the insights gained from the SEM. Both composites show bioactive potential, albeit limited according to the BG content.
4.4. Cytocompatibility
An essential requirement for biomaterials is to be non-toxic and to promote cell growth. We have, therefore, intensively investigated cytocompatibility and cell-material interaction for the 3D-printed BaTiO3/BG composites and benchmarked them against our previous piezoelectric composite of BaTiO3/HA [40]. For this purpose, we cultured scaffolds with MC3T3-E1 pre-osteoblasts for a period of 7 d. The results are shown in Fig. 8. After 7 d in cell culture, high growth of cells up to confluence is visible for BaTiO3/5%BG composition, similar to the TCPS positive control (Fig. 8, A). On the BaTiO3/HA composite, the cell layer is not as dense, and the BaTiO3/15%BG composite shows signs of cytotoxicity due to a barely pronounced cell layer and a round cytoskeleton of the cells. The image-based quantification (Fig. 8, B) of the cell number confirms this. It reveals a significantly increased cell number of the BaTiO3/5%BG composite compared to the BaTiO3/HA composite of the first generation. The number of cells growing on the BaTiO3/5%BG composite was similar to that on the TCPS pos. Control, with no significant differences. As an indicator for cell death, we used an LDH assay and quantified the extracellular LDH level (Fig. 8, C). The LDH level was significantly increased on BaTiO3/15%BG samples, again indicating the cytotoxicity of the composition. We hypothesise that the cytotoxic effect of the BaTiO3/15%BG composite is due to a strong burst release of hydroxide ions (OH−) from the ceramic-crystallised glass matrix, raising the pH value to a cytotoxic range. A measurement of the pH in the cell cultures showed a significant increase for the BaTiO3/15BG composites compared to the scaffolds (Fig. 8, E). Such behaviour is known for BG scaffolds and can be countered with the preconditioning of the scaffolds. Therefore, it does not necessarily exclude the applicability of the higher-loaded composite as a potential biomaterial [93]. To assess the proliferation of the cells, we determined the relative DNA content using a PicoGreen QuantiT dsDNA assay. This corresponds with the image analysis and confirms a significantly higher dsDNA content on the BaTiO3/5%BG scaffolds compared to the BaTiO3/HA scaffolds, indicating a substantially enhanced proliferation of MC3T3 cells on the ceramic-crystallised glass composite. The SEM Image analysis (Fig. 8, F) of cells grown on BaTiO3/5% BG, after 7 days of incubation, indicates a good cell-material and cell-cell interaction (F2, F3) with filopodia visible on the material granules from printing. The cells grow on the material, and its roughness originates from 3D printing (F3). Moreover, the cells grow into the micropores (F1) and partially bridge them, forming a cell layer on top (F4). A 3D render (Fig. 8, G) of the cell layer from confocal microscopy confirms the density of the layer and shows how much the cells can bridge the microporosity. To study the interaction of the cells with the material and the cell layer in more detail, we subsequently used two-photon microscopy (Fig. 8, H). The cells on the BaTiO3/5% BG grow directly on the surface and form a levitating cell layer where the cells bridge microporosities (H′). Such a double-layered cell film explains why the relative DNA content increased compared to the TCPS pos. Control which only shows a monolayer (H″). This shows the potential of the BaTiO3/BG composite and the 3D printing approach to specifically influence cell growth on and into the scaffolds by hierarchically built structures with designed macro and microporosity. Saeidi et al. also demonstrated a high cytocompatibility of freeze-cast BaTiO3/BG composites of up to 98% using human mesenchymal stem cells (hMSCs) [50]. In contrast to our data, the cytocompatibility could be significantly increased with the increase in the BG fraction. The authors do not describe any signs of adverse shifts in pH due to ion release. However, they washed the scaffolds three times with PBS and presumably preconditioned them. Overall, the in vitro results show that especially the BaTiO3/5%BG composite, although with limited performance in the SBF test, is an excellent candidate as a material for BTE. It is also the material combination with a higher piezoelectric charge constant d33, which makes it an ideal choice for use in the combined approach as a responsive piezoelectric and bioactive material. However, other material combinations, especially in the range between 5 wt% and 15 wt% BG loading, should be considered in future studies. Finding a sweet spot that allows a better balance between piezoelectric properties, biomechanics, and bioactivity might be possible in this range. A load of 15 wt% BG should not be exceeded, as it would sacrifice the piezoelectric properties of the composite and, therefore, the possibility of further stimulating cells electrically.
Fig. 8.
In-vitro cytocompatibility assessment of MC3T3-E1 cells on BaTiO3/BG and first-generation BaTiO3/HA composites. (A) Maximum intensity z-projections of DAPI (nuclei), Calcein-AM (live cells), and Rhodamine-Phalloidin (F-Actin) stained cells after seven days of incubation. (B) Number of cells/mm grown on the different materials, quantified via image analysis (n = 6 for BaTiO3/HA or BaTiO3/BG samples, n = 3 for TCPS). Data are shown as mean ± SD. Statistical analysis between groups was performed using an unpaired two-tailed Students t-test. All data were subjected to tests for normality prior to applying students t-test. (C) Quantification of extracellular LDH level as an indicator for cell death (n = 6). Data are shown as mean ± SD. Statistical analysis between groups was performed using an one-way ANOVA test. (D) The relative DNA content of samples with cells grown for seven days was quantified using PicoGreen QuantiT dsDNA assay as an indicator for cell proliferation (n = 6). Data are shown as mean ± SD statistical analysis between groups was performed using a Mann-Whitney test. (E) pH of the medium after 24h of cell culture (n = 6). Data are shown as mean ± SD. Statistical analysis was performed using one-way ANOVA analysis. (F) SEM Image analysis of cells grown on BatiO3/5% BG, after 7 days of incubation. 3D-render image of such cell-layer grown on BatiO3/5%BG for 7 days of incubation. Bottom: z-y cross-section. Scale bar = 100 μm. (H) Detailed image of cells grown on BatiO3/BG analysed via two-photon microscopy imaging, including an evaluation of the fluorescence intensity of TCPS and BaTiO3/5%BG. Scale bars: 100 μm.
5. Conclusion
In conclusion, we demonstrate the fabrication of piezoelectric and bioactive BaTiO3/BG composite scaffolds by binder jetting for use in BTE. Starting with the description of the entire process line for the fabrication of the scaffolds, we provide a comprehensive characterisation of essential physical and functional parameters which are critical in its use as a biomaterial. The study can be summarised with the following main points:
-
I)
We demonstrate, for the first time, the 3D printing of BaTiO3/BG ceramic-crystallised glass composites for biomaterial application.
-
II)
The manufactured specimens and scaffolds are easy to handle and show the necessary compressive strength to be a suitable bone substitute material in non-load-bearing applications.
-
III)
The prepared BaTiO3/BG composites show ferroelectric and piezoelectric behaviour. The generated piezoelectric charge constant d33 is of the same order of magnitude or higher than that of natural bone and strongly depends on the load of BG.
-
IV)
An increased content of BG in the composite (BaTiO3/15%BG) combined with a lower sintering temperature results in a pronounced HCA layer formation during SBF testing. Due to a lower BG content in the BaTiO3/5%BG ceramic-crystallised glass composite, the HCA layer formation is limited compared to an increased load of BG.
-
V)
The in vitro experiments also indicate a dependence on BG load. In particular, the scaffolds based on the BaTiO3/5%BG composite show great potential in the in vitro experiment with no signs of cytotoxicity and strong proliferation of MC3T3 cells. BaTiO3/15%BG scaffolds showed cytotoxic behaviour due to burst ion release and an increase in pH value. However, this could be counteracted with preconditioning, but this was not investigated in this study.
-
VI)
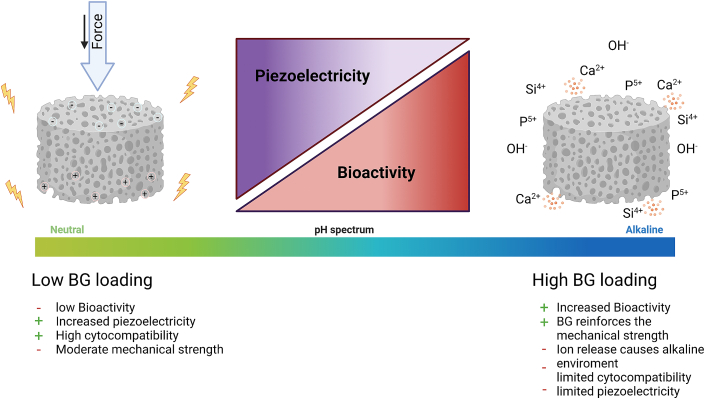
We were able to identify limits for the use of BG in a piezoelectric and bioactive composite based on BaTiO3 and outline a possible field of conflict between individual properties, in particular bioactivity and piezoelectricity (Fig. 9).
Fig. 9.
Influence of BG loading on essential properties of 3D printed BaTiO3/BG composites.
6. Limitations and outlook
The study presented here was conceptualised as the first study exploiting the potential of piezoelectric and bioactive 3D-printed scaffolds for bone applications. The main objective was to establish a 3D printing process for piezoelectric and bioactive materials and to investigate the limitations of our material and fabrication process, especially in terms of BG loading, trying to match bioactivity with piezoelectric properties, as it is known that non-piezoelectric phases actively limit the piezoelectric properties. Therefore, we focused on low and high BG loading to find the limits of our material and process. For the material and process presented here, we were able to render a field of conflict when designing piezoelectric and bioactive 3D printed scaffolds identifying a lower and upper limit of BG load for the composites developed (Fig. 9). However, we have not yet investigated BG loadings between the loadings presented here, but these offer the potential to further improve the targeted properties of the material. Nevertheless, the BG content should be kept moderate. Otherwise, the piezoelectric properties and, thus, the possibility to electrically stimulate bone cells are lost. We will perform follow-up studies to investigate compositions between 5% and 15% BG load to tailor the material further and fine-tune the material- and process properties. Furthermore, we aim to examine the potential of scaffolds for BTE in more depth and are planning dynamic cell culture studies under targeted mechanical loading and active triggering of the piezoelectric effect. In addition, the chorioallantoic membrane (CAM) assay and in vivo studies in small animals are planned to investigate the biocompatibility and bioactivity of the presented composites in detail. An important focus will be to explore the potential release of Ba2+−ions from the BaTiO3/BG composite matrix over a prolonged period of time in cell culture or in vivo to investigate possible long-term toxic effects and possible inflammatory response of the tissue [94,95]. Furthermore, we anticipate significant potential in the further development of the process. We aspire to transfer the material into much more complex structures, such as triple periodic minimal surface (TPMS) designs and integrate the structural properties described by numerous authors [[96], [97], [98]]. Taken together, we envision combining piezoelectric and bioactive materials with modern additive manufacturing techniques to create customised biomaterials with a tailored and controllable biological response.
Credit author contributions
Christian Polley: Conceptualisation, Methodology, Investigations, Visualisation, Formal Analysis, Writing - Original Draft, Review and Editing Thomas Distler: Investigations, Visualisation, Formal Analysis, Writing - Original Draft Caroline Scheufler: Investigations Rainer Detsch: Conceptualisation, Writing - Reviewing and Editing Henrik Lund: Investigations, Writing - Reviewing and Editing Armin Springer: Investigations, Writing - Reviewing and Editing Dominik Schneidereit: Investigations, Writing - Reviewing and Editing Oliver Friedrich: Investigations, Writing - Reviewing and Editing Aldo R. Boccaccini: Conceptualisation, Project Administration, Funding Acquisition, Supervision, Writing - Reviewing and Editing Hermann Seitz: Conceptualisation, Project Administration, Funding Acquisition, Supervision, Writing - Reviewing and Editing.
Ethics approval and consent to participate
It is a research manuscript and human subjects or animals were not involved. Therefore no “ethics approval and consent” is needed.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was funded by Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – SFB 1270/1,2–299150580 to Hermann Seitz and Aldo R. Boccaccini and TRR225 subproject B08/Z02 to Oliver Friedrich. We also thank the technical staff of all institutes who successfully helped with the investigations. The Graphical Abstract and Fig. 1A & Fig. 9 were created with Biorender.com.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mtbio.2023.100719.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Zhang K., Wang S., Zhou C., Cheng L., Gao X., Xie X., Sun J., Wang H., Weir M.D., Reynolds M.A., Zhang N., Bai Y., Xu H.H.K. Advanced smart biomaterials and constructs for hard tissue engineering and regeneration. Bone Res. 2018;6 doi: 10.1038/s41413-018-0032-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holzapfel B.M., Reichert J.C., Schantz J.T., Gbureck U., Rackwitz L., Nöth U., Jakob F., Rudert M., Groll J., Hutmacher D.W. How smart do biomaterials need to be? A translational science and clinical point of view. Adv. Drug Deliv. Rev. 2013;65:581–603. doi: 10.1016/j.addr.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Hench L.L., Splinter R.J., Allen W.C., Greenlee T.K. Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. 1971;5:117–141. doi: 10.1002/JBM.820050611. [DOI] [Google Scholar]
- 4.Hench L.L. The story of Bioglass. J. Mater. Sci. Mater. Med. 2006:967–978. doi: 10.1007/s10856-006-0432-z. [DOI] [PubMed] [Google Scholar]
- 5.Radin S., Reilly G., Bhargave G., Leboy P.S., Ducheyne P. Osteogenic effects of bioactive glass on bone marrow stromal cells. J. Biomed. Mater. Res., Part A. 2005;73:21–29. doi: 10.1002/jbm.a.30241. [DOI] [PubMed] [Google Scholar]
- 6.Westhauser F., Wilkesmann S., Nawaz Q., Hohenbild F., Rehder F., Saur M., Fellenberg J., Moghaddam A., Ali M.S., Peukert W., Boccaccini A.R. Effect of manganese, zinc, and copper on the biological and osteogenic properties of mesoporous bioactive glass nanoparticles. J. Biomed. Mater. Res., Part A. 2021;109:1457–1467. doi: 10.1002/jbm.a.37136. [DOI] [PubMed] [Google Scholar]
- 7.Moura J., Teixeira L.N., Ravagnani C., Peitl O., Zanotto E.D., Beloti M.M., Panzeri H., Rosa A.L., De Oliveira P.T. In vitro osteogenesis on a highly bioactive glass-ceramic (Biosilicate®) J. Biomed. Mater. Res., Part A. 2007;82:545–557. doi: 10.1002/jbm.a.31165. [DOI] [PubMed] [Google Scholar]
- 8.Weng L., Boda S.K., Teusink M.J., Shuler F.D., Li X., Xie J. Binary doping of strontium and copper enhancing osteogenesis and angiogenesis of bioactive glass nanofibers while suppressing osteoclast activity. ACS Appl. Mater. Interfaces. 2017;9:24484–24496. doi: 10.1021/acsami.7b06521. [DOI] [PubMed] [Google Scholar]
- 9.Hoppe A., Güldal N.S., Boccaccini A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials. 2011;32:2757–2774. doi: 10.1016/j.biomaterials.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Miguez-Pacheco V., Hench L.L., Boccaccini A.R. Bioactive glasses beyond bone and teeth: emerging applications in contact with soft tissues. Acta Biomater. 2015;13:1–15. doi: 10.1016/j.actbio.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Gerhardt L.C., Widdows K.L., Erol M.M., Burch C.W., Sanz-Herrera J.A., Ochoa I., Stämpfli R., Roqan I.S., Gabe S., Ansari T., Boccaccini A.R. The pro-angiogenic properties of multi-functional bioactive glass composite scaffolds. Biomaterials. 2011;32:4096–4108. doi: 10.1016/j.biomaterials.2011.02.032. [DOI] [PubMed] [Google Scholar]
- 12.Bührer G., Rottensteiner U., Hoppe A., Detsch R., Dafinova D., Fey T., Greil P., Weis C., Beier J.P., Boccacini A.R., Horch R.E., Arkudas A. Biomed. Glas. Walter de Gruyter GmbH; 2016. Evaluation of in vivo angiogenetic effects of copper doped bioactive glass scaffolds in the AV loop model; pp. 111–117. [DOI] [Google Scholar]
- 13.Fernandes J.S., Gentile P., Pires R.A., Reis R.L., Hatton P.V. Multifunctional bioactive glass and glass-ceramic biomaterials with antibacterial properties for repair and regeneration of bone tissue. Acta Biomater. 2017;59:2–11. doi: 10.1016/J.ACTBIO.2017.06.046. [DOI] [PubMed] [Google Scholar]
- 14.Jones J.R., Ehrenfried L.M., Saravanapavan P., Hench L.L. Controlling ion release from bioactive glass foam scaffolds with antibacterial properties. J. Mater. Sci. Mater. Med. 2006:989–996. doi: 10.1007/s10856-006-0434-x. [DOI] [PubMed] [Google Scholar]
- 15.Kargozar S., Montazerian M., Hamzehlou S., Kim H.W., Baino F. Mesoporous bioactive glasses: promising platforms for antibacterial strategies. Acta Biomater. 2018;81:1–19. doi: 10.1016/J.ACTBIO.2018.09.052. [DOI] [PubMed] [Google Scholar]
- 16.Zheng K., Torre E., Bari A., Taccardi N., Cassinelli C., Morra M., Fiorilli S., Vitale-Brovarone C., Iviglia G., Boccaccini A.R. Antioxidant mesoporous Ce-doped bioactive glass nanoparticles with anti-inflammatory and pro-osteogenic activities. Mater. Today Bio. 2020;5 doi: 10.1016/j.mtbio.2020.100041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Majumdar S., Hira S.K., Tripathi H., Kumar A.S., Manna P.P., Singh S.P., Krishnamurthy S. Synthesis and characterization of barium-doped bioactive glass with potential anti-inflammatory activity. Ceram. Int. 2021;47:7143–7158. doi: 10.1016/j.ceramint.2020.11.068. [DOI] [Google Scholar]
- 18.Barrak F.N., Li S., Mohammed A.A., Myant C., Jones J.R. Anti-inflammatory properties of S53P4 bioactive glass implant material. J. Dent. 2022;127 doi: 10.1016/J.JDENT.2022.104296. [DOI] [PubMed] [Google Scholar]
- 19.Brauer D.S. Bioactive glasses - structure and properties. Angew. Chem. Int. Ed. 2015;54:4160–4181. doi: 10.1002/anie.201405310. [DOI] [PubMed] [Google Scholar]
- 20.Baino F., Fiume E., Barberi J., Kargozar S., Marchi J., Massera J., Verné E. Processing methods for making porous bioactive glass-based scaffolds—a state-of-the-art review. Int. J. Appl. Ceram. Technol. 2019;16:1762–1796. doi: 10.1111/IJAC.13195. [DOI] [Google Scholar]
- 21.Zeimaran E., Pourshahrestani S., Fathi A., Razak N.A. bin A., Kadri N.A., Sheikhi A., Baino F. Advances in bioactive glass-containing injectable hydrogel biomaterials for tissue regeneration. Acta Biomater. 2021;136:1–36. doi: 10.1016/J.ACTBIO.2021.09.034. [DOI] [PubMed] [Google Scholar]
- 22.Rajabi A.H., Jaffe M., Arinzeh T.L. Piezoelectric materials for tissue regeneration: a review. Acta Biomater. 2015;24:12–23. doi: 10.1016/j.actbio.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 23.Tandon B., Blaker J.J., Cartmell S.H. Piezoelectric materials as stimulatory biomedical materials and scaffolds for bone repair. Acta Biomater. 2018;73:1–20. doi: 10.1016/J.ACTBIO.2018.04.026. [DOI] [PubMed] [Google Scholar]
- 24.Kapat K., Shubhra Q.T.H., Zhou M., Leeuwenburgh S. Piezoelectric nano-biomaterials for biomedicine and tissue regeneration. Adv. Funct. Mater. 2020;30 doi: 10.1002/adfm.201909045. [DOI] [Google Scholar]
- 25.J. Jacob, N. More, K. Kalia, G. Kapusetti, Piezoelectric Smart Biomaterials for Bone and Cartilage Tissue Engineering, Inflamm. Regen. (n.d.). https://doi.org/10.1186/s41232-018-0059-8. [DOI] [PMC free article] [PubMed]
- 26.Curie J., Curie P. Développement par compression de l’électricité polaire dans les cristaux hémièdres à faces inclinées. Bull. Mineral. 1880;3:90–93. doi: 10.3406/BULMI.1880.1564. [DOI] [Google Scholar]
- 27.Fukada E., Yasuda I. On the piezoelectric effect of bone. J. Phys. Soc. Japan. 1957;12:1158–1162. doi: 10.1143/JPSJ.12.1158. [DOI] [Google Scholar]
- 28.Fukada E., Yasuda I. Piezoelectric effects in collagen. Jpn. J. Appl. Phys. 1964;3:117–121. doi: 10.1143/JJAP.3.117. [DOI] [Google Scholar]
- 29.Andrew C., Bassett L. 1968. Biologic Significance of Piezoelectricity*. [DOI] [PubMed] [Google Scholar]
- 30.Sahm F., Jakovljevic A., Bader R., Detsch R., Jonitz-Heincke A. Is there an influence of electrically stimulated osteoblasts on the induction of osteoclastogenesis? Appl. Sci. 2022;12 doi: 10.3390/app122211840. [DOI] [Google Scholar]
- 31.Kreller T., Zimmermann J., van Rienen U., Boccaccini A.R., Jonitz-Heincke A., Detsch R. Alternating electric field stimulation: phenotype analysis and osteoclast activity of differentiated RAW 264.7 macrophages on hydroxyapatite-coated Ti6Al4V surfaces and their crosstalk with MC3T3-E1 pre-osteoblasts. Biomater. Adv. 2023;146 doi: 10.1016/j.bioadv.2023.213285. [DOI] [PubMed] [Google Scholar]
- 32.Khare D., Basu B., Dubey A.K. Electrical stimulation and piezoelectric biomaterials for bone tissue engineering applications. Biomaterials. 2020:258. doi: 10.1016/j.biomaterials.2020.120280. [DOI] [PubMed] [Google Scholar]
- 33.Tofail S.A.M., Haverty D., Cox F., Erhart J., Hána P., Ryzhenko V. Dielectric relaxation in monoclinic hydroxyapatite: observation of hydroxide ion dipoles. Cit. J. Appl. Phys. 2009;105 doi: 10.1063/1.3093863. [DOI] [Google Scholar]
- 34.A.C. Ahn, A.J. Grodzinsky, Relevance of collagen piezoelectricity to "Wolff's Law": a critical review., Med. Eng. Phys.. 31 (2009) 733–41. 10.1016/j.medengphy.2009.02.006. [DOI] [PMC free article] [PubMed]
- 35.Park J.B., Kelly B.J., Kenner G.H., von Recum A.F., Grether M.F., Coffeen W.W. Piezoelectric ceramic implants:in vivo results. J. Biomed. Mater. Res. 1981;15:103–110. doi: 10.1002/jbm.820150114. [DOI] [PubMed] [Google Scholar]
- 36.Liu W., Yang D., Wei X., Guo S., Wang N., Tang Z., Lu Y., Shen S., Shi L., Li X., Guo Z. Fabrication of piezoelectric porous BaTiO3 scaffold to repair large segmental bone defect in sheep. J. Biomater. Appl. 2020 doi: 10.1177/0885328220942906. [DOI] [PubMed] [Google Scholar]
- 37.Tang Y., Wu C., Wu Z., Hu L., Zhang W., Zhao K. Fabrication and in vitro biological properties of piezoelectric bioceramics for bone regeneration. Sci. Rep. 2017;7 doi: 10.1038/srep43360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baxter F.R., Turner I.G., Bowen C.R., Gittings J.P., Chaudhuri J.B. An in vitro study of electrically active hydroxyapatite-barium titanate ceramics using Saos-2 cells. J. Mater. Sci. Mater. Med. 2009;20:1697–1708. doi: 10.1007/s10856-009-3734-0. [DOI] [PubMed] [Google Scholar]
- 39.Poon K.K., Wurm M.C., Evans D.M., Einarsrud M., Lutz R., Glaum J. Biocompatibility of (Ba,Ca)(Zr,Ti)O 3 piezoelectric ceramics for bone replacement materials. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020;108:1295–1303. doi: 10.1002/jbm.b.34477. [DOI] [PubMed] [Google Scholar]
- 40.Polley C., Distler T., Detsch R., Lund H., Springer A., Boccaccini A.R., Seitz H. 3D printing of piezoelectric barium titanate-hydroxyapatite scaffolds with interconnected porosity for bone tissue engineering. Materials. 2020;13:1773. doi: 10.3390/ma13071773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poon K.K., Schafföner S., Einarsrud M.A., Glaum J. Barium titanate-based bilayer functional coatings on Ti alloy biomedical implants. J. Eur. Ceram. Soc. 2021;41:2918–2922. doi: 10.1016/j.jeurceramsoc.2020.12.023. [DOI] [Google Scholar]
- 42.Riaz A., Polley C., Lund H., Springer A., Seitz H. A novel approach to fabricate load-bearing Ti6Al4V-Barium titanate piezoelectric bone scaffolds by coupling electron beam melting and field-assisted sintering. Mater. Des. 2023;225 doi: 10.1016/J.MATDES.2022.111428. [DOI] [Google Scholar]
- 43.Vaněk P., Kolská Z., Luxbacher T., García J.A.L., Lehocký M., Vandrovcová M., Bačáková L., Petzelt J. Electrical activity of ferroelectric biomaterials and its effects on the adhesion, growth and enzymatic activity of human osteoblast-like cells. J. Phys. D Appl. Phys. 2016;49 doi: 10.1088/0022-3727/49/17/175403. [DOI] [Google Scholar]
- 44.Marchesano V., Gennari O., Mecozzi L., Grilli S., Ferraro P. Effects of lithium niobate polarization on cell adhesion and morphology. ACS Appl. Mater. Interfaces. 2015;7:18113–18119. doi: 10.1021/ACSAMI.5B05340/ASSET/IMAGES/LARGE/AM-2015-05340A_0012. [DOI] [PubMed] [Google Scholar]
- 45.Vilarinho P.M., Barroca N., Zlotnik S., Félix P., Fernandes M.H. Are lithium niobate (LiNbO3) and lithium tantalate (LiTaO3) ferroelectrics bioactive? Mater. Sci. Eng., C. 2014;39:395–402. doi: 10.1016/J.MSEC.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 46.Wang Q., Chen Q., Zhu J., Huang C., Darvell B.W., Chen Z. Effects of pore shape and porosity on the properties of porous LNKN ceramics as bone substitute. Mater. Chem. Phys. 2008;109:488–491. doi: 10.1016/J.MATCHEMPHYS.2007.12.022. [DOI] [Google Scholar]
- 47.Dubey A.K., Kinoshita R., Kakimoto K.I. Piezoelectric sodium potassium niobate mediated improved polarization and in vitro bioactivity of hydroxyapatite. RSC Adv. 2015;5:19638–19646. doi: 10.1039/C5RA00771B. [DOI] [Google Scholar]
- 48.Gaukås N.H., Huynh Q.S., Pratap A.A., Einarsrud M.A., Grande T., Holsinger R.M.D., Glaum J. In vitro biocompatibility of piezoelectric K0.5Na0.5NbO3Thin films on platinized silicon substrates. ACS Appl. Bio Mater. 2020;3:8714–8721. doi: 10.1021/acsabm.0c01111. [DOI] [PubMed] [Google Scholar]
- 49.Chen W., Yu Z., Pang J., Yu P., Tan G., Ning C. Fabrication of biocompatible potassium sodium niobate piezoelectric ceramic as an electroactive implant. Materials. 2017;10:345. doi: 10.3390/MA10040345. 10 (2017) 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saeidi B., Derakhshandeh M.R., Delshad Chermahini M., Doostmohammadi A. Novel porous barium titanate/nano-bioactive glass composite with high piezoelectric coefficient for bone regeneration applications. J. Mater. Eng. Perform. 2020;29:5420–5427. doi: 10.1007/s11665-020-05016-0. [DOI] [Google Scholar]
- 51.Zhao F., Zhang C., Liu J., Liu L., Cao X., Chen X., Lei B., Shao L. Periosteum structure/function-mimicking bioactive scaffolds with piezoelectric/chem/nano signals for critical-sized bone regeneration. Chem. Eng. J. 2020;402 doi: 10.1016/j.cej.2020.126203. [DOI] [Google Scholar]
- 52.Goltzman D., Hendy G.N. The calcium-sensing receptor in bone-mechanistic and therapeutic insights. Nat. Rev. Endocrinol. 2015;11:298–307. doi: 10.1038/nrendo.2015.30. [DOI] [PubMed] [Google Scholar]
- 53.Martens M., Van Audekercke R., Delport P., De Meester P., Mulier J.C. The mechanical characteristics of cancellous bone at the upper femoral region. J. Biomech. 1983;16:971–983. doi: 10.1016/0021-9290(83)90098-2. [DOI] [PubMed] [Google Scholar]
- 54.Vahey J.W., Lewis J.L., Vanderby R. Elastic moduli, yield stress, and ultimate stress of cancellous bone in the canine proximal femur. J. Biomech. 1987;20:29–33. doi: 10.1016/0021-9290(87)90264-8. [DOI] [PubMed] [Google Scholar]
- 55.Distler T., Fournier N., Grünewald A., Polley C., Seitz H., Detsch R., Boccaccini A.R. Polymer-bioactive glass composite filaments for 3D scaffold manufacturing by fused deposition modeling: fabrication and characterization. Front. Bioeng. Biotechnol. 2020;8:552. doi: 10.3389/fbioe.2020.00552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Polzin C., Spath S., Seitz H. Characterization and evaluation of a PMMA-based 3D printing process. Rapid Prototyp. J. 2013;19:37–43. doi: 10.1108/13552541311292718. [DOI] [Google Scholar]
- 57.Takahashi H., Numamoto Y., Tani J., Matsuta K., Qiu J., Tsurekawa S. Lead-free barium titanate ceramics with large piezoelectric constant fabricated by microwave sintering. Jpn. J. Appl. Phys., Part 2. 2006;45 doi: 10.1143/JJAP.45.L30. [DOI] [Google Scholar]
- 58.Gaytan S.M., Cadena M.A., Karim H., Delfin D., Lin Y., Espalin D., MacDonald E., Wicker R.B. Fabrication of barium titanate by binder jetting additive manufacturing technology. Ceram. Int. 2015 doi: 10.1016/j.ceramint.2015.01.108. [DOI] [Google Scholar]
- 59.Wang W., Sun J., Guo B., Chen X., Ananth K.P., Bai J. Fabrication of piezoelectric nano-ceramics via stereolithography of low viscous and non-aqueous suspensions. J. Eur. Ceram. Soc. 2020;40:682–688. doi: 10.1016/j.jeurceramsoc.2019.10.033. [DOI] [Google Scholar]
- 60.Dai B., Hu X., Yin R., Bai W., Wen F., Deng J., Zheng L., Du J., Zheng P., Qin H. Piezoelectric grain-size effects of BaTiO3 ceramics under different sintering atmospheres. J. Mater. Sci. Mater. Electron. 2017;28:7928–7934. doi: 10.1007/s10854-017-6494-5. [DOI] [Google Scholar]
- 61.Boccaccini A.R., Chen Q., Lefebvre L., Gremillard L., Chevalier J. Sintering, crystallisation and biodegradation behaviour of Bioglass®-derived glass-ceramics. Faraday Discuss. 2007;136:27–44. doi: 10.1039/b616539g. [DOI] [PubMed] [Google Scholar]
- 62.Bretcanu O., Chatzistavrou X., Paraskevopoulos K., Conradt R., Thompson I., Boccaccini A.R. Sintering and crystallisation of 45S5 Bioglass® powder. J. Eur. Ceram. Soc. 2009;29:3299–3306. doi: 10.1016/j.jeurceramsoc.2009.06.035. [DOI] [Google Scholar]
- 63.Polley C., Schulze S., Distler T., Detsch R., Boccaccini A.R., Seitz H. 2020. Sintering Behavior of 3D Printed Barium Titanate Composite Scaffolds for Bone Repair. [DOI] [Google Scholar]
- 64.Stewart M., Battrick W., Cain M. 2001. Measurement Good Practice Guide No. 44 Measuring Piezoelectric D 33 Coefficients Using the Direct Method Measuring Piezoelectric D 33 Coefficients Using the Direct Method Contents. [Google Scholar]
- 65.T. Kokubo, Bioactive Glass Ceramics: Properties and Applications, n.d. [DOI] [PubMed]
- 66.Distler T., Polley C., Shi F., Schneidereit D., Ashton M.D., Friedrich O., Kolb J.F., Hardy J.G., Detsch R., Seitz H., Boccaccini A.R. Electrically conductive and 3D-printable oxidized alginate-gelatin polypyrrole:PSS hydrogels for tissue engineering. Adv. Healthc. Mater. 2021;10 doi: 10.1002/adhm.202001876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Distler T., Polley C., Shi F., Schneidereit D., Ashton M.D., Friedrich O., Kolb J.F., Hardy J.G., Detsch R., Seitz H., Boccaccini A.R. Electrically conductive and 3D-printable oxidized alginate-gelatin polypyrrole:PSS hydrogels for tissue engineering. Adv. Healthc. Mater. 2021;10 doi: 10.1002/adhm.202001876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rizwan M., Hamdi M., Basirun W.J. Bioglass® 45S5-based composites for bone tissue engineering and functional applications. J. Biomed. Mater. Res., Part A. 2017;105:3197–3223. doi: 10.1002/jbm.a.36156. [DOI] [PubMed] [Google Scholar]
- 69.Polley C., Distler T., Rüffer D., Detsch R., Boccaccini A.R., Seitz H. 3D printing of smart materials for bone regeneration. Trans. Addit. Manuf. Meets Med. 2019;1 doi: 10.18416/AMMM.2019.1909S07T03. [DOI] [Google Scholar]
- 70.Schult M., Buckow E., Seitz H. Experimental studies on 3D printing of barium titanate ceramics for medical applications. Curr. Dir. Biomed. Eng. 2016;2:95–99. doi: 10.1515/cdbme-2016-0024. [DOI] [Google Scholar]
- 71.Maison W., Kleeberg R., Heimann R.B., Phanichphant S. Phase content, tetragonality, and crystallite size of nanoscaled barium titanate synthesized by the catecholate process: effect of calcination temperature. J. Eur. Ceram. Soc. 2003;23:127–132. doi: 10.1016/S0955-2219(02)00071-7. [DOI] [Google Scholar]
- 72.Choi S.Y., Kang S.J.L. Sintering kinetics by structural transition at grain boundaries in barium titanate. Acta Mater. 2004;52:2937–2943. doi: 10.1016/J.ACTAMAT.2004.02.039. [DOI] [Google Scholar]
- 73.Bellucci D., Cannillo V., Sola A. An overview of the effects of thermal processing on bioactive glasses. Sci. Sinter. 2010;42:307–320. doi: 10.2298/SOS1003307B. [DOI] [Google Scholar]
- 74.Lefebvre L., Gremillard L., Chevalier J., Zenati R., Bernache-Assolant D. Sintering behaviour of 45S5 bioactive glass. Acta Biomater. 2008;4:1894–1903. doi: 10.1016/j.actbio.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 75.Karageorgiou V., Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26:5474–5491. doi: 10.1016/J.BIOMATERIALS.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 76.Lee H.W., Moon S., Choi C.H., Kim D.K. Synthesis and size control of tetragonal barium titanate nanopowders by facile solvothermal method. J. Am. Ceram. Soc. 2012;95:2429–2434. doi: 10.1111/J.1551-2916.2012.05085.X. [DOI] [Google Scholar]
- 77.Chen C., Hao H., Wang T., Cheng J., Luo Z., Zhang L., Cao M., Yao Z., Liu H. Nano-BaTiO3 phase transition behavior in coated BaTiO3-based dielectric ceramics. Ceram. Int. 2019;45:7166–7172. doi: 10.1016/J.CERAMINT.2018.12.223. [DOI] [Google Scholar]
- 78.Wisniewski W., Thieme K., Rüssel C. Fresnoite glass-ceramics – a review. Prog. Mater. Sci. 2018;98:68–107. doi: 10.1016/j.pmatsci.2018.05.002. [DOI] [Google Scholar]
- 79.Lefebvre L., Chevalier J., Gremillard L., Zenati R., Thollet G., Bernache-Assolant D., Govin A. Structural transformations of bioactive glass 45S5 with thermal treatments. Acta Mater. 2007;55:3305–3313. doi: 10.1016/j.actamat.2007.01.029. [DOI] [Google Scholar]
- 80.Riaz A., Witte K., Bodnar W., Hantusch M., Schell N., Springer A., Burkel E. Structural changes and pseudo-piezoelectric behaviour of field assisted sintered calcium titanate. Materialia. 2021;15 doi: 10.1016/j.mtla.2021.100998. [DOI] [Google Scholar]
- 81.Cheng H., Hu H., Li G., Zhang M., Xiang K., Zhu Z., Wan Y. Calcium titanate micro-sheets scaffold for improved cell viability and osteogenesis. Chem. Eng. J. 2020;389 doi: 10.1016/J.CEJ.2020.124400. [DOI] [Google Scholar]
- 82.Yamaguchi S., Akeda K., Shintani S.A., Sudo A., Matsushita T. Drug-releasing gelatin coating reinforced with calcium titanate formed on Ti–6Al–4V alloy designed for osteoporosis bone repair. Coatings. 2022;12 doi: 10.3390/coatings12020139. [DOI] [Google Scholar]
- 83.Goller G., Demirkiran H., Oktar F.N., Demirkesen E. Processing and characterization of bioglass reinforced hydroxyapatite composites. Ceram. Int. 2003;29:721–724. doi: 10.1016/S0272-8842(02)00223-7. [DOI] [Google Scholar]
- 84.Luginina M., Angioni D., Montinaro S., Orrù R., Cao G., Sergi R., Bellucci D., Cannillo V. Hydroxyapatite/bioactive glass functionally graded materials (FGM) for bone tissue engineering. J. Eur. Ceram. Soc. 2020;40:4623–4634. doi: 10.1016/j.jeurceramsoc.2020.05.061. [DOI] [Google Scholar]
- 85.Shokrollahi H., Salimi F., Doostmohammadi A. The fabrication and characterization of barium titanate/akermanite nano-bio-ceramic with a suitable piezoelectric coefficient for bone defect recovery. J. Mech. Behav. Biomed. Mater. 2017;74:365–370. doi: 10.1016/j.jmbbm.2017.06.024. [DOI] [PubMed] [Google Scholar]
- 86.Yap E.W., Glaum J., Oddershede J., Daniels J.E. Effect of porosity on the ferroelectric and piezoelectric properties of (Ba0.85Ca0.15)(Zr0.1Ti0.9)O3 piezoelectric ceramics. Scripta Mater. 2018;145:122–125. doi: 10.1016/j.scriptamat.2017.10.022. [DOI] [Google Scholar]
- 87.Fukada E. Piezoelectricity in polymers and biological materials. Ultrasonics. 1968;6:229–234. doi: 10.1016/0041-624X(68)90132-7. [DOI] [PubMed] [Google Scholar]
- 88.Marino A.A., Gross B.D. Piezoelectricity in cementum, dentine and bone. Arch. Oral Biol. 1989;34:507–509. doi: 10.1016/0003-9969(89)90087-3. [DOI] [PubMed] [Google Scholar]
- 89.Boccardi E., Philippart A., Melli V., Altomare L., De Nardo L., Novajra G., Vitale-Brovarone C., Fey T., Boccaccini A.R. Bioactivity and mechanical stability of 45S5 bioactive glass scaffolds based on natural marine sponges. Ann. Biomed. Eng. 2016;44:1881–1893. doi: 10.1007/S10439-016-1595-5/FIGURES/8. [DOI] [PubMed] [Google Scholar]
- 90.Aguiar H., Serra J., González P., León B. Structural study of sol-gel silicate glasses by IR and Raman spectroscopies. J. Non-Cryst. Solids. 2009;355:475–480. doi: 10.1016/j.jnoncrysol.2009.01.010. [DOI] [Google Scholar]
- 91.Zheng K., Solodovnyk A., Li W., Goudouri O.M., Stähli C., Nazhat S.N., Boccaccini A.R. Aging time and temperature effects on the structure and bioactivity of gel-derived 45S5 glass-ceramics. J. Am. Ceram. Soc. 2015;98:30–38. doi: 10.1111/jace.13258. [DOI] [Google Scholar]
- 92.Lin K., Chang J., Liu Z., Zeng Y., Shen R. Fabrication and characterization of 45S5 bioglass reinforced macroporous calcium silicate bioceramics. J. Eur. Ceram. Soc. 2009;29:2937–2943. doi: 10.1016/j.jeurceramsoc.2009.04.025. [DOI] [Google Scholar]
- 93.Ciraldo F.E., Boccardi E., Melli V., Westhauser F., Boccaccini A.R. Tackling bioactive glass excessive in vitro bioreactivity: preconditioning approaches for cell culture tests. Acta Biomater. 2018;75:3–10. doi: 10.1016/j.actbio.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 94.Ball J.P., Mound B.A., Nino J.C., Allen J.B. Biocompatible evaluation of barium titanate foamed ceramic structures for orthopedic applications. J. Biomed. Mater. Res., Part A. 2014;102:2089–2095. doi: 10.1002/jbm.a.34879. [DOI] [PubMed] [Google Scholar]
- 95.Dubey A.K., Thrivikraman G., Basu B. Absence of systemic toxicity in mouse model towards BaTiO3 nanoparticulate based eluate treatment. J. Mater. Sci. Mater. Med. 2015;26 doi: 10.1007/s10856-015-5414-6. [DOI] [PubMed] [Google Scholar]
- 96.Dong Z., Zhao X. Application of TPMS structure in bone regeneration. Eng. Regen. 2021;2:154–162. doi: 10.1016/j.engreg.2021.09.004. [DOI] [Google Scholar]
- 97.Polley C., Radlof W., Hauschulz F., Benz C., Sander M., Seitz H. Morphological and mechanical characterisation of three-dimensional gyroid structures fabricated by electron beam melting for the use as a porous biomaterial. J. Mech. Behav. Biomed. Mater. 2021 doi: 10.1016/j.jmbbm.2021.104882. [DOI] [PubMed] [Google Scholar]
- 98.Cai Z., Liu Z., Hu X., Kuang H., Zhai J. The effect of porosity on the mechanical properties of 3D-printed triply periodic minimal surface (TPMS) bioscaffold. Bio-Design Manuf. 2019;2:242–255. doi: 10.1007/s42242-019-00054-7. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.