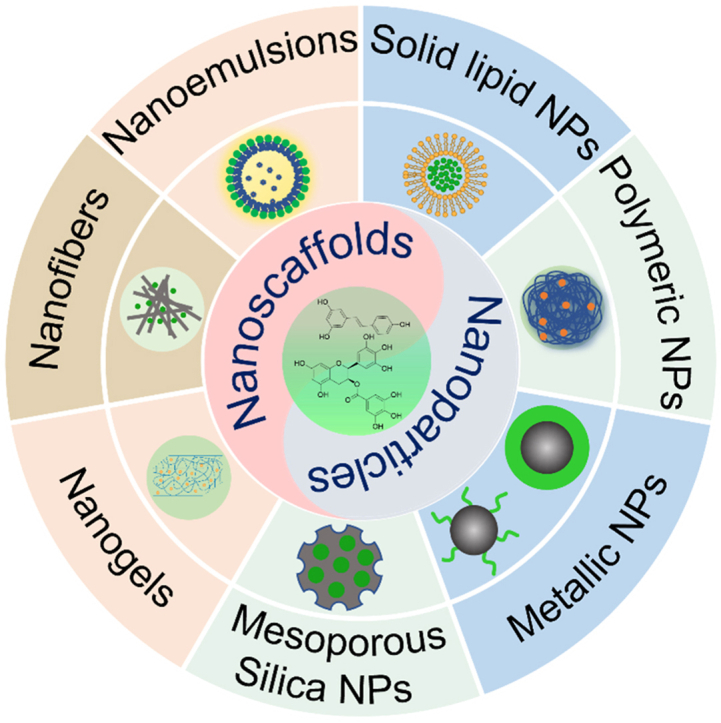
Abstract
Infectious disease is one of the top 10 causes of death worldwide, especially in low-income countries. The extensive use of antibiotics has led to an increase in antibiotic resistance, which poses a critical threat to human health globally. Natural products such as polyphenolic compounds and their derivatives have been shown the positive therapeutic effects in antibacterial therapy. However, the inherent physicochemical properties of polyphenolic compounds and their derivatives limit their pharmaceutical effects, such as short half-lives, chemical instability, low bioavailability, and poor water solubility. Nanoformulations have shown promising advantages in improving antibacterial activity by controlling the release of drugs and enhancing the bioavailability of polyphenols. In this review, we listed the classification and antibacterial mechanisms of the polyphenolic compounds. More importantly, the nanoformulations for the delivery of polyphenols as the antibacterial agent were summarized, including different types of nanoparticles (NPs) such as polymer-based NPs, metal-based NPs, lipid-based NPs, and nanoscaffolds such as nanogels, nanofibers, and nanoemulsions. At the same time, we also presented the potential biological applications of the nano-system to enhance the antibacterial ability of polyphenols, aiming to provide a new therapeutic perspective for the antibiotic-free treatment of infectious diseases.
Keywords: Polyphenols, Nanoformulations, Antibacterial activity, Drug delivery systems
Graphical abstract
In this review, the delivery nanoformulations were comprehensively summarized, including nanoparticles (solid lipid nanoparticles, metallic nanoparticles, and mesoporous silica nanoparticles) and nanoscaffolds (nanogels, nanofibers, and nanoemulsions) which performed the antibacterial functions of polyphenols.
In recent research, polyphenolic compounds have been proved to have potential antibacterial activity. Nanoformulations can overcome the shortcomings of polyphenols such as the low-water solution, ease to degraded and oxidized.
1. Introduction
According to the statistics and forecast of the World Health Organization (WHO), the annual mortality of infectious diseases caused by drug resistance exceeds 700,000 all over the world, and the number will be up to 10 million by 2050 without better treatment [1]. However, the excessive and long-term use of conventional antibiotics has increased the occurrence of drug resistance, which has gradually led to increased mortality and healthcare costs. WHO listed multi-drug resistant (MDR) bacteria as one of the top ten hazards for the health of humans in 2019 [2,3]. Due to the emergence of new resistance mechanisms, the development of new antibiotics is much slower than the emergence of resistant bacteria. Therefore, it is a severe problem to develop new non-antibiotic antibacterial agents to provide a new option for the treatment of infectious diseases.
Over the past decades, natural products have raised hopes for the treatment of infectious diseases due to their promising antibacterial activity. Among these natural compounds, more than 49% of them have been approved by the U.S. Food and Drug Administration (FDA) and regulatory agencies in other regions to treat bacterial infections [3]. Polyphenolic compounds are widely present in vegetables, flowers, stems, and seeds [2]. They play an important role in the growth of plants of their radiation and microbial resistance function [4]. In recent years, a growing number of researchers have focused on the positive effects of polyphenolic compounds in vitro and in vivo studies. The biological properties include antibacterial, anticancer, anti-inflammation, antioxidant, cardioprotection, and anti-major depression activity [[5], [6], [7], [8], [9]]. Due to the potential versatile therapeutic effects and wide range of natural sources, polyphenols are expected to be a potential biomedical material for multi-purpose therapeutic applications. Particularly for bacterial infections, as a class of natural products, polyphenols hold great promise as a therapeutic alternative to antibiotics. A series of polyphenolic compounds with antibacterial effects have been reported, such as tannic acid (TA), curcumin, epigallocatechin gallate (EGCG), and resveratrol. The antibacterial properties of these compounds include direct antibacterial activity, suppression of bacterial virulence, and synergism with antibiotics [10]. Recently, dietary polyphenols have been made into healthcare products for healthcare functions and have been identified as therapeutic drugs for prophylaxis and regulation of antibiotic activity [11]. Several formulations containing polyphenols (either alone or in combination with other compounds) are undergoing preclinical and clinical evaluation [12].
However, the inherent properties of polyphenols limit their therapeutic activity. Low water solubility and poor stability to environmental conditions are important reasons for the low absorption rate of polyphenolic compounds [13]. The water solubility of polyphenols depends on their affinity with water molecules and molecular weight (MW). Higher MW had been reported to result in poorer aqueous solubility [14]. The instability in the gastrointestinal tract is affected by several factors such as enzymes, pH, microbiota, and the occurrence of other nutrients [15]. For example, resveratrol is mostly soluble in alcohol while poorly soluble in water (21 μg/mL at pH 7.4), which affects its bioavailability. At the same time, after oral administration, resveratrol is quickly absorbed in the gastrointestinal tract, but its bioavailability is less than 1% due to a general first-pass effect [16]. On the other hand, polyphenolic compounds are also susceptible to environmental factors such as heat, moisture, oxygen, and light irradiation [17,18]. Consequently, the low bioavailability of polyphenols limits their uptake of potential health benefits. Therefore, polyphenols are often loaded into carriers to increase biocompatibility and prevent environmental degradation.
As an emerging nanotechnology, nanoformulations can overcome the existing shortcomings of polyphenols and have the following advantages: (1) Enhance the aqueous solution of low-water soluble polyphenols. Due to the mutual repulsion and hydration of polar groups on the surface, NPs can maintain good stability in dispersed systems [13]. Curcumin, for example, is a polyphenolic compound present mainly in the spice turmeric (Curcuma longa). Many types of polymer-based NPs are designed to encapsulate and deliver curcumin due to their insolubility in the water. Compared with free curcumin, curcumin-loaded chitosan NPs showed better concentration and dispersity in aqueous solutions [19]. (2) Reduce the risk of oxidation and degradation of polyphenols. For example, EGCG has been proven to be unstable under alkaline and neural pH condition while relatively stable in acidic solutions, resulting in low bioavailability in the gut and blood [20]. However, in simulated gastric and intestinal fluids studies, the stability of hordein NPs encapsulated-EGCG was significantly improved compared to free EGCG [21]. (3) Provide controlled and stable release with therapeutic drug loading. By way of example, curcumin-loaded nanofibers prepared by electrospinning amphiphilic-block segmented polyurethanes showed a steady curcumin release during 432 h after a burst release in 24 h and played continued antibacterial ability to Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) [22]. Based on the positive therapeutic effects of polyphenols in various fields and the advantages of nanotechnology, a series of related reviews have been conducted. The previous reviews mainly focus on 1) the encapsulation technology of polyphenols to improve the bioavailability; 2) other biomedical applications such as anti-cancer, anti-diabetes, anti-inflammation, and cardiovascular protection of polyphenol-based nanomaterials; 3) nanomaterials to improve the antibacterial function of a certain type of polyphenols (such as tea polyphenols, curcumin, and resveratrol, etc.). However, a comprehensive review of the nanoformulations for enhanced antibacterial effects is still lacking. This review highlighted the design and antibacterial applications of polyphenol-based nanoformulations in the biomedical field. The delivery nanoformulations were systematically reviewed, including NPs (SLNs, metallic NPs, and mesoporous silica NPs) and nanoscaffolds (nanogels, nanofibers, and nanoemulsions) which performed the enhanced antibacterial functions of polyphenols (Fig. 1). We hoped to provide a new strategy for treating infectious diseases and promoting the clinical application of polyphenols.
Fig. 1.
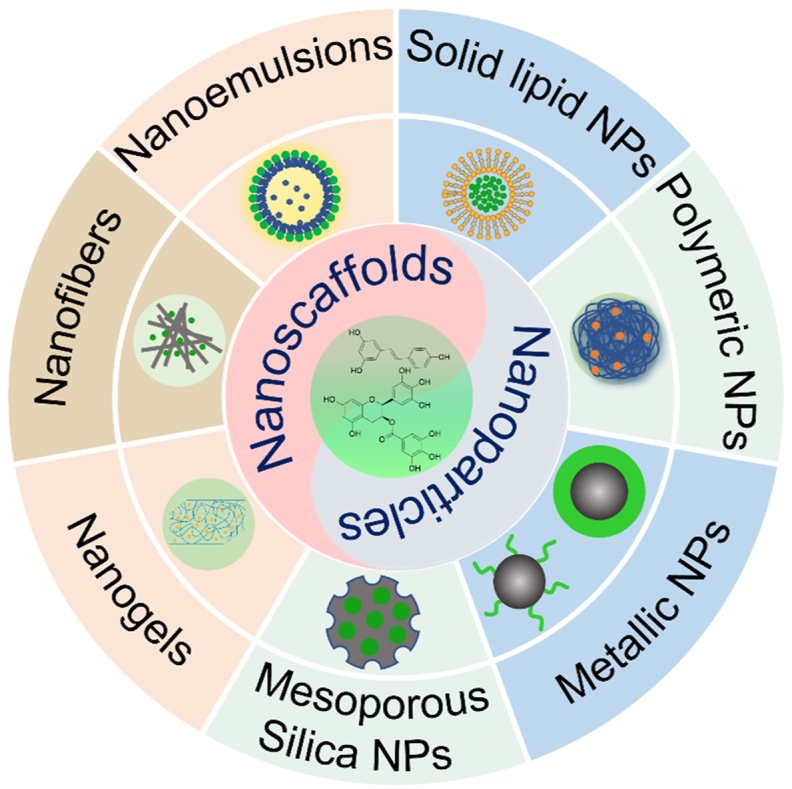
Different types of nanoformulations for delivery of polyphenols with antibacterial activity.
2. Classification and antibacterial activity of polyphenols
Many secondary metabolites of plant origin, such as polyphenols, alkaloids, and terpenes, have antimicrobial effects. Various antibacterial mechanisms have been proved in plant-derived compounds, including disruption of bacterial membranes, inhibition of biofilm formation and efflux pump, and suppression of DNA and protein synthesis [23]. Among them, alkaloids are organic nitrogenous bases generally synthesized from amino acids. These compounds are water-soluble at acidic pH, which is beneficial to play a role in bacterial infection environment [24,25]. Terpenes are a diverse group of organic compounds produced by a variety of animals and plants, which are hydrocarbons with the 5-carbon isoprene unit as the main structural component of their biosynthesis [26]. By contrast, polyphenols have a prominent antibacterial activity. For example, a comparative study of terpenoids, alkaloids, and polyphenols against MDR showed that the antibacterial activity of terpenoids was not as active as that of polyphenols. In addition, terpenoids also have some difficulties in crossing through the bacterial membrane barrier [24,27]. Furthermore, the toxicity of carvacrol (a monoterpenoid compound) should be further considered due to the toxicity of its antibacterial therapeutic concentration on animal cells [25]. The high antimicrobial effect of polyphenols can be mainly attributed to the presence of phenolic hydroxyl groups and through multiple antibacterial mechanisms. In this section, we classified polyphenols according to their chemical structures and presented their properties and main biological activities.
2.1. Classification of polyphenols
Polyphenolic compounds consist of one or more aromatic rings connected to at least two phenolic hydroxyl groups and can be classified as flavonoids and non-flavonoids [28]. Polyphenolic compounds are a variety of molecules, including catechin, EGCG, TA, resveratrol, curcumin, etc. Fig. 2 showed the formula for the structure of common polyphenols.
Fig. 2.
The structural formula for common polyphenolic compounds.
2.1.1. Flavonoids
Flavonoids are comprising 15 carbons with two benzene rings connected through a three-carbon chain (C6–C3–C6). The two benzene rings are linked by a heterocyclic pyran ring. Depending on the degree of oxidation, hydroxylation, saturation, and the hydroxylation pattern of the heterocyclic pyran rings, as well as their junction position with the benzene rings, flavonoids can be subclassified as flavones, flavanols, flavanones, isoflavones, and anthocyanins. The bioavailability and chemical properties are related to the substitution of chemical groups of the flavonoid compound structures [29]. Tea polyphenols, including flavonoids, flavanols, phenolic acids, and anthocyanins, are the main polyphenolic substances that exist in green tea with many positive biological effects [30]. Flavanol compounds, the most studied are catechins including epicatechin (EC), epigallocatechin (EGC), epicatechin gallate (ECG), and epigallocatechin gallate (EGCG) [31].
2.1.2. Non-flavonoids
Non-flavonoids include phenolic acids (hydroxycinnamic and hydroxybenzoic acids based on C6–C3 and C6–C1), stilbenes (C6–C2–C6), and lignans (C6–C3–C3–C6). In addition, tannins (C6–C1) and xanthones (C6–C6–C6) are also involved in this category. These compounds have two or more benzene rings in their structure, whereas tannins consist of multiple benzene rings.
Phenolic acids have a carboxyl group attached to a benzene ring and include cinnamic acid and benzoic acid. Examples of hydroxycinnamic derivatives are ferulic, caffeic, sinapic, and p-coumaric acids, whereas gallic, vanillic, syringic, and p-hydroxybenzoic acids belong to hydroxybenzoic acid [32]. Hydroxycinnamic is mainly present in fruits (especially those red ones), vegetables, coffee, tea, and whole grains [33]. Hydroxycinnamates exist as monomers, dimers, and as the bound form ester or amides. For example, chlorogenic acid is an ester formed by condensation of one or more trans-cinnamic acids (caffeic acid, ferulic acid, erucic acid) and quinine or their derivatives. Chlorogenic acid mainly exists in green coffee beans, which is widely concerned due to their potential health benefits, including anti-inflammatory, anti-bacterial, anti-diabetic, and anti-carcinogenic effects [34]. Besides chlorogenic acid, rosmarinic acid is another ester of 3,4-dihydroxy phenyl lactic acid and caffeic acid, which is widely found in the plant kingdom [35]. It has potent antibacterial activity against S. aureus strains, methicillin-resistant Staphylococcus. aureus (MRSA), Enterobacteriaceae spp., and Pseudomonas spp [36]. Gallic acid (3,4,5 trihydroxy benzoic acid) is a representative of the hydroxybenzoic acid group, which can be extracted from natural plants or synthesized chemically. Gallic acid widely exists in, as an organic material, widely used in medicine, food, and dyes [37]. It also shows broad antibacterial effects. For example, gallic acid inhibits the growth and formation of biofilm of Escherichia coli and Staphylococcus mutans. Furthermore, it possesses an inhibitory effect on the growth and the capsular polysaccharide biosynthesis of Klebsiella pneumoniae [38].
Stilbenes exist widely in grapes, grapevines, wines, blueberries, cranberries, mulberries, almonds, and beans. The absorption and metabolism rates of stilbenes are determined by their various phenolic structures (polymerization and chemical substituents) [39]. Stilbenes have been reported to have medicinal properties, such as anti-inflammation and antioxidant activity [39,40]. The main component of stilbene is resveratrol (3,5,4′-trihydroxystilbene), which has been widely studied for its potent antibacterial activity against a broad range of bacteria. Resveratrol can reduce toxin expression and inhibit the biofilm formation of bacteria. Furthermore, in combination with antibiotics, it can enhance the effect of aminoglycosides against Staphylococcus aureus [41].
Tannins are water-soluble polyphenols rich in benzene rings and hydroxyl groups. Tannins are generally classified into hydrolyzable (ellagitannins and gallotannins) and non-hydrolyzable (condensed) according to their chemical structures and constitutive monomers [42]. Condensed tannins are derivatives of flavanols where the 2 position is bound to catechol or phloroglucinol. Gallotannis, also called tannic acid, contains multiple hydroxyl groups forming hydrogen bonds with other molecules, and is popular in biomedical research and used in the development of new drugs for many infectious diseases [43].
2.2. Mechanisms of antibacterial activity of polyphenols
The broad range of antibacterial activity of polyphenols is attributed to the following mechanism: inhibiting bacterial DNA replication and bacterial energy metabolism, altering the function of the bacterial membrane, reducing the cell attachment and formation of biofilm, suppressing the porin protein on the membrane of cells and changing the permeability, and synergizing with other agents [[44], [45], [46], [47], [48]].
At the genetic level, polyphenols act as antibacterial agents by inhibiting the DNA replication of bacteria. The inhibition of DNA gyrase is the dominant mechanism in this category. Green tea polyphenols and chebulinic acid showed an inhibitory ability against gyrase and can modify the DNA topology [49]. EGCG from green tea exerts antibacterial effects by inhibiting the B-subunit of gyrase at the ATP binding site. In addition, EGCG has attracted wide interest and is considered a promising antibacterial agent through multiple antimicrobial mechanisms (inhibition of efflux pump and chromosomal penicillinase) [50]. Curcumin has been reported to inhibit bacterial DNA expression, in combination with interactions with DNA molecules. The oxidation products of curcumin form many free radicals, some of which are necessary for bacterial synthesis, thus interfering with the replication of bacterial DNA [51]. When bacterial DNA is damaged, it activates the SOS response, which is an antibacterial self-protection response and also a factor in bacterial drug resistance [52]. An early study reported that curcumin exerted antibacterial effects against Salmonella typhimurium and Escherichia coli by inhibiting the SOS induction and genetic mutation by UV light. At the same time, curcumin also effectively inhibited phage regeneration [53].
The structural integrity of cell walls and membranes and the stability of membrane potentials play key roles in the spatial structure of mitotic and cytoskeletal proteins in bacteria. The hydroxyl group of polyphenols affects the instability of cell membrane potentials and pH gradients, both of which contribute to antibacterial activity. α-Mangosteen (a polyphenolic compound) has an antibacterial effect on resistant strains of MRSA. Under scanning electron microscopy, it can destroy the integrity of the cell membrane, and destroy bacterial cell components [54]. The present study showed the antibacterial activity of curcumin against Gram-positive bacteria (Enterococcus faecalis and Staphylococcus aureus) and Gram-negative bacteria (Pseudomonas aeruginosa and Escherichia coli) [55]. Scanning electron microscopy, fluorescence microscopy, and membrane permeabilization assays confirmed the membrane damage of bacteria on exposure to curcumin [56]. Besides, polyphenols can play antibacterial activity by destroying the biosynthesis of the cell wall and inhibiting the key enzymes including dihydrofolate reductase, urease, and sortase [57]. d-alanine: d-alanine ligase is a key enzyme in the assembly of the peptidoglycan precursors to bacterial cell walls. Quercetin is a flavonoid compound that could destroy the activity of E. coli and H. pylori by inhibiting the d-alanine: d-alanine ligase [58]. Some other polyphenols can interact directly with peptidoglycan to inhibit the biosynthesis of the cell wall [59].
In addition, biofilms are collections of microbial tissues that surround bacterial cells. It has been shown that the bacteria in biofilms grow more slowly than planktonic bacteria, resulting in resistance to the biotic antiproliferative activity. Curcumin has been proven to inhibit the formation, reduce the thickness, and loosen the structure of biofilm by various pathogens in host organisms [7,57,60]. A study was conducted to directly observe the urinary tract pathogenic biofilm and the amount of microorganisms in the biofilm by laser confocal scanning confocal microscopy. The curcumin-treated urinary tract pathogens were identified to have reduced biofilm thickness. Biofilms of E. coli and Proteus mirabilis were reduced to varying degrees. In terms of other antibacterial mechanisms, polyphenols (extract of glycyrrhiza inflata) could inhibit oxygen consumption and electron transfer in the respiratory chain of sensitive bacteria and interfere with the energy metabolism of bacteria [61].
3. Different types of nanoformulations for the delivery of polyphenols
3.1. Nanoparticles
The advantages of NPs include the size and surface can be easily modified, the release under certain environments can be maintained and controlled, and distribution and clearance from the body of the drug can be altered [62,63]. Thanks to their versatile properties, NPs are increasingly being used in drug delivery systems for both active and passive drug targeting. According to the literature, a variety of materials have been used to produce NPs for the delivery of polyphenols, ranging from lipid and polymeric molecules to inorganic compounds [64]. In this section, the properties and antibacterial applications of the most commonly studied NPs for polyphenol delivery were summarized in the field of biomedicine.
3.1.1. Solid lipid NPs (SLNs)
Considerations for encapsulation include the size and solubility of the loaded compound, the characteristics of the carrier, and the route of delivery. Lipid carriers are a suitable choice for the delivery of polyphenolic compounds due to their affinity for hydrophobic compounds and their similar constitution to biological membranes. In 1991, Muller developed an encapsulation method, SLNs, which remain solid at the temperature of the human body and room [65]. The particle size of SLNs typically ranges in size from 40 to 1000 nm and meets a variety need of nanoparticle possibilities [66]. Compared with other colloidal carriers, the advantages of SLNs include higher stability with both lipophilic and hydrophilic compounds, non-toxicity, and ease of large-scale production [67]. Particularly, the properties of the reduced particle size, controlled and targeted drug release, and cheaply manufactured, make SLNs one of the most popular systems in drug delivery [66]. SLNs are made of lipids, which are usually physiological lipids, including glycerides, fatty acids, sterols, partial glycides, and waxes. These lipids are dispersed in water and stabilized by surfactants, including neutral, ionic, and non-ionic can help prevent the agglomeration of particles [67]. Herein, we reviewed the primary preparation methods, enhanced antibacterial ability, and the major biomedical applications of polyphenol-loaded SLNs.
High-pressure homogenization (HPH) is the most used method in SLNs preparation for the encapsulation of polyphenolic compounds. Shtay et al. prepared EGCG-loaded SLNs by a hot HPH method. In vitro release studies were conducted using simulated gastric juice (pH 1.2) and intestinal juice (pH 6.8), and longer release times were observed under both conditions, which might improve the bioavailability of EGCG [68]. At the same time, solvent emulsification/evaporation is also a satisfactory method due to its good solubility in the organic solvent for lipids without any thermal stress [69]. On account of the lipid matrix of SLNs being hydrophobic, the double-emulsion technique seems to be a promising approach to encapsulate the hydrophilic compounds [66]. Parisa et al. prepared stable curcumin-loaded SLNs by the HPH method with mannitol as cryoprotectant. The size of the particle was 112 nm and the loading efficiency was 70.4 ± 1.2%. The curcumin particles showed increased antibacterial activity against E. coli and S. aureus compared to free curcumin at the same concentration. More than 85% and 92% of the curcumin was released after 36 and 48 h. They demonstrated that 5% mannitol was the preferred cryoprotectant compared to 15% mannitol due to their particle size being 163 nm and 306 nm [70]. To improve the loaded capacity and stability, He et al. used propylene glycol monoesters (PGMEs) instead of glyceryl tripalmitate to fabricate carvacrol-loaded SLNs (Car-SLNs) against S. aureus and E. coli O157: H7. The size of the Car-SLNs remained stable and remained smaller than 200 nm after 30 days of storage. Since purified PGMEs and glyceryl monostearate could form stable α-form crystals, they could provide stably controlled release scaffoldings for drug delivery [71].
In addition, Lin et al. prepared curcumin-loaded SLNs with Chinese white wax (Cur-cwSLNs) using high-shear homogenization and ultrasound technique. In vitro antibacterial study, the (minimum inhibitory concentration, MIC) of Cur-cwSLNs and free curcumin was 62.5 μg/mL and 500 μg/mL respectively against S. aureus. At the same time, colorimetric assays of biofilms showed that Cur-cwSLNs significantly inhibited biofilm formation in S. aureus compared to free curcumin. Furthermore, the Cur-cwSLNs had a slow release of curcumin without burst release over 96 h under both pH 4.5 and pH 7.4 conditions [72]. The higher release rate in acid conditions suggested that these SLNs might be more suitable for acid-infected environments, such as skin, dental caries, and the vagina. For topical infection, SLNs are best suited for transdermal delivery due to their property of combining lipid and polymeric NPs. Fakhara et al. designed a curcumin-loaded SLNs patch for transdermal delivery to enhance the anti-microbial effect [73]. For oral mucosal infection, Heba et al. designed a curcumin-loaded SLNs with five lipids as surfactants to evaluate the antibacterial properties against S. aureus, Streptococcus mutans, E. coli, Viridansstrept, and Lactobacillus acidophilus. Compared with the free curcumin, CurSLNs showed an increased antibacterial effect by eightfold, fourfold, twofold, fourfold, and twofold, respectively [74].
3.1.2. Polymeric NPs
Among organic macromolecular-based drug carriers, polymeric NPs have been widely chosen as drug delivery vectors due to their offered advantages: firstly, chemical and physical stability under physiological environments and storage conditions; secondly, excellent biocompatibility and biodegradability, low toxicity, and immunogenicity; thirdly, surface modification with ligands; and finally ease of production [75]. Depending on the nature of the polymer, various substances such as macromolecules and hydrophobic drugs can be delivered to cells and specialized organs. Polymer-based NPs include two main categories, natural and synthetic origin. Natural polymers mainly include chitosan (CS), dextran, hyaluronic acid, starch, cellulose, and gelatin. Synthetic polymers mainly include polylactic acid (PLA), polyethylene glycol (PEG), polylactic-glycolic acid (PLGA), and polycaprolactone (PCL). In this section, we reviewed the most widely used natural and synthetic polymeric NPs as polyphenol delivery carriers for antibacterial applications.
3.1.2.1. Chitosan-based NPs
CS, a natural origin polymer, is derived from chitin (extraction with the exoskeleton of crustaceans) and has advantages including non-toxic and low-cost. CS is generally recognized as safe and approved by FDA for dietary and medical use [76]. In addition, due to the inherent character of positive charge, mucoadhesion, in situ gelling, permeation enhancing, controlled release, and efflux pump inhibition, CS has become a preferred candidate for antimicrobial delivery [77].
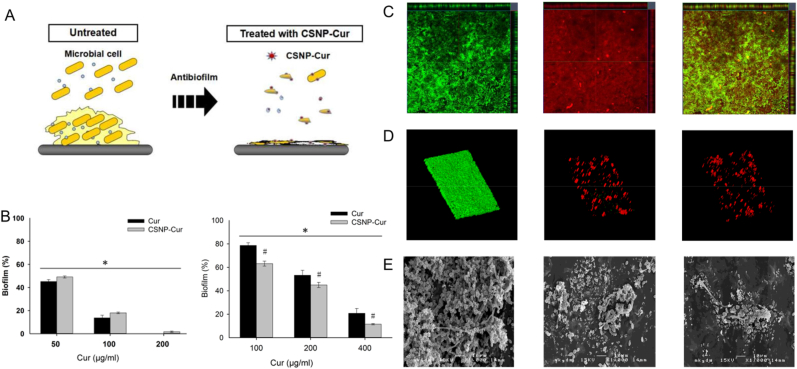
Nanoencapsulation and chemical modification are two main techniques to produce drug-loaded chitosan NPs (CSNPs) [78]. The capsulation process may occur based on the interaction of hydrophobic, electrostatic, and/or hydrogen bonding between CS and drug molecules [79]. Ionic gelation is the most studied preparation method for CSNPs synthesis [80]. This method is based on the electrostatic interaction between CS positively charged amino sugar monomer unit and negatively charged polyanion such as tripolyphosphate (TPP) [81]. Tan et al. used the ionic gelation method to encapsulate curcumin in CSNPs for polymicrobial biofilm-related infection (Fig. 3). The NPs played a role in enhancing antibacterial activity against S. aureus and a sustained release of curcumin under different pH conditions (7.4 and 5.4) and inhibited biofilm formation due to the binding of the positively charged CS and anionic biofilm components [82].
Fig. 3.
Antibacterial activity of curcumin-loaded CSNP (CSNP-Cur). (A) Antibiofilm graphical abstract of CSNP-Cur. (B) Inhibition and disruption activity of CSNP-Cur on biofilm of S. aureus. (C) Penetration of biofilm by CSNPs labeled by Syto 9 (green) and Rhodamine B isothiocyanate (red). (D) Confocal laser scanning microscopy (CLSM) images of polymicrobial biofilm with and without treatment of CSNP-Cur. (E) SEM images of polymicrobial biofilm with and without the treatment of CSNP-Cur (Reprinted from Ref. [81] with permission from ELSEVIER).
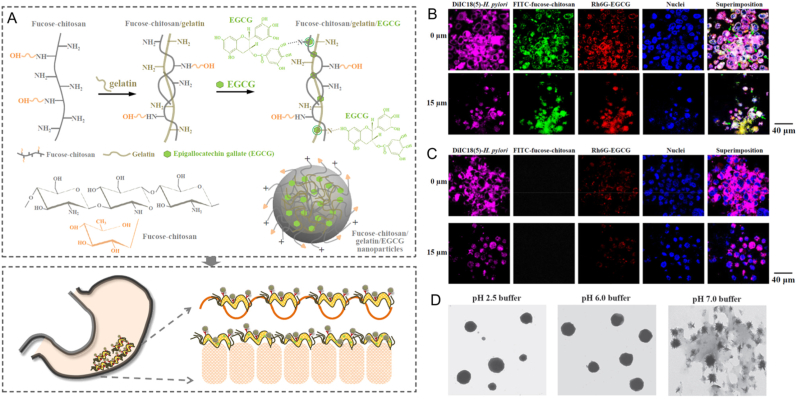
As an example of chemical modification, curcumin, a natural pigment, has also been used in photodynamic therapy for antibacterial applications. Zhao et al. prepared a novel nanomaterial by the esterification reaction of curcumin and carboxylated chitosan [83], which had an effective ROS scavenging ability as well as photodynamic antibacterial activity against S. aureus and E. coli. Numerous studies have shown that CS had desirable properties of mucoadhesion, through the electrostatic interaction between the positively charged amino groups of CS and the N-acetyl neuraminic acid in the gastric mucus [84,85]. In addition, CSNPs with sizes less than 500 nm may contribute to crossing the intestinal mucous barrier and interact with the underlying epithelial cells. In particular, when the zeta potential of NPs was positive, the adhesion ability of intestinal tissue would be facilitated [86]. Therefore, for oral administration, drug delivery systems with CS shells have often been used to increase the residence time in the gastrointestinal tract and for the purpose of controlled release of drugs. For example, Lin et al. developed NPs composed of fucose-conjugated CS with gelatin of EGCG and proved that the EGCG-loaded NPs had a significantly higher infectious targeting and greater clearance effect of Helicobacter pylori (H. pylori) in vitro and in vivo studies than that of EGCG solution (Fig. 4A–C). At the same time, the NPs showed a relatively stable morphology under the acidic environment (pH 2.5 and 6.0) but collapsed and led to the release of EGCG at pH 7.0 (Fig. 4D) [87].
Fig. 4.
Preparation of fucose-CS/gelatin/EGCG NPs and their antibacterial ability. (A) Scheme of preparation and gastric epithelium targeting of fucose-CS/gelatin/EGCG NPs. (B) Fluorescence images of H. pylori-infected AGS cell (human gastric adenocarcinoma cell line) monolayers treated with fucose-CS/gelatin/EGCG NPs at different depths. (C) Fluorescence images of H. pylori-infected AGS cell monolayers treated with EGCG solution at different depths. (D) TEM micrograph of fucose-CS/gelatin/EGCG NPs under different pH environments (Reprinted from Ref. [86] with permission from National Institute for Materials Science).
Smaller size and suitable surface discharges of NPs are critical for antibacterial effects, as they can provide desirable stability and penetration into bacterial cells. According to the literature, CS with low MW has a stronger antibacterial ability for resistant strains [88,89]. To obtain a smaller size and a higher encapsulation efficiency (EE) of CSNPs, Zhang et al. used β-CS with a MW of ∼160 kDa and a ratio to TPP of 5:1. Compared with catechin solution, catechin-loaded β-CSNPs played a better antibacterial effect against L. innocua and E. coli [90]. In addition to the MW of CS, the number of polyphenol hydroxyl groups loaded onto it also affects the properties of NPs and their antibacterial effects. Madureira et al. capsulated protocatechuic acid (PA), rosmarinic acid (RA), and 2,5-dihydroxybenzoic acid (DHBA) into CSNPs with low and high MW of CS to investigate their antibacterial effect against B. cereus, E. coli, L. innocua, Y. enterocolitica, S. aureus, and S. typhimurium. Their outcomes showed that low MW chitosan with RA obtained the higher EE and the best inhibitory effects [91]. Some other recent examples of polyphenol-containing CSNPs are summarized in Table 1.
Table 1.
Antibacterial effect of chitosan NPs loaded with active polyphenols.
| Polyphenols-loaded Nanoformulations |
Antibacterial against | Ref. |
|---|---|---|
| Tannin-lysozyme@ CSNPs |
S.aureus; Salmonella enteritidis; Listeria monocytogenes |
[92] |
| Green tea oil-loaded CSNPs | E. coli | [93] |
| Ferulic acid encapsulated CSNPs | Pseudomonas aeruginosa | [94] |
| Curcumin-loaded CSNPs |
S. aureus; C. albicans |
[76] |
| Catechin and quercetin-loaded CSNPs |
E. coli; S. aureus; Bacillus subtilis |
[95] |
| Carvacrol-loaded CSNPs |
E. coli; S. aureus; Bacillus cereus |
[78] |
| P. dactylifera extract-loaded CSNPs |
Bacillus subtilis; Bacillus cereus |
[96] |
3.1.2.2. Poly (lactic-co-glycolic acid) (PLGA) based NPs
PLGA-based NPs are another widely studied and applied polymeric nanoformulations in nanomedicine due to their biodegradability and low toxicity, which was approved by FDA for human therapy [97]. In line with the development of encapsulation techniques, PLGA polymers have been successfully used to load natural products including polyphenols. Several in vitro and in vivo studies have proved that nanoencapsulation of polyphenols with PLGA polymer was a potential method to protect and enhance their bioactivity.
PLGA polymers can be hydrolyzed in the body into lactic acid and glycolic acid monomers, both of which are endogenous and easily metabolized via the Kreb cycle. Emulsification-solvent evaporation is applicable to liposoluble drugs and is the most employed method for the preparation of PLGA-based NPs. This technique includes oil in water (O/W) and water in the oil-water (W/O/W) method [97]. For example, Duranoglu et al. used a single emulsion-solvent evaporation technique to encapsulate hesperetin (a flavonoid compound) into PLGA-based NPs for the antibacterial therapeutic application. Compared with free hesperidin, the optimized hesperetin-loaded NPs showed significant inhibition against S. aureus and E. coli [98]. In addition, Arasoglu et al. used the same method to produce quercetin-loaded PLGA-based NPs with different initial quercetin amounts. The results showed that cell walls got wrinkled and damaged under TEM observation after 24 h exposure to quercetin-loaded PLGA-based NPs, which indicated the antibacterial mechanism might be attributed to the abruption of cell walls and surface charges of microorganisms [99].
PLGA polymer is a polyester of PLA and polyglycolic acid (PGA) and comprises different formations according to the ratio of the two components. The higher the content of glycolide, the more hydrophilic and easier to degrade, while the higher the content of lactic acid, the more hydrophobic and more gradual to degrade [100]. This feature can be used to increase the EE of a hydrophilic or hydrophobic substance in a drug delivery system [101,102]. Pereira et al. prepared PLGA-based NPs from natural plant extracts rich in hydrophobic polyphenols, including epicatechin, gallic acid and ellagic acid, and assessed their inhibitory effects against Listeria innocua. Their result showed the lactide: glycolide ratio of 65: 35 exhibited a significantly higher EE and more gradual initial burst compared with ratio of 50: 50 [103].
Despite the favorable biodegradability and biocompatibility, PLGA-based NPs are susceptible to being cleared from circulation by macrophages in the mononuclear phagocyte system after being administrated through an intravenous route [104]. To overcome this dilemma, PLGA-based NPs are imparted with surface modifications, that may prevent them from being cleared by the natural defense systems. PEG, a hydrophilic polymer, is the most commonly used technique due to its advantages of high molecular weight, long chain length, and high surface density. With the help of PEG polymer, PLGA-based NPs could increase the solubility and retention time in vivo [105]. For example, Deepika et al. loaded rutin (a flavonoid) in the PEG-modified PLGA-based NPs, which exhibited the synergetic antibacterial ability against MDR last for several days [106]. Antibacterial analysis showed that the MIC of PLGA-based NPs was 2 times lower than that of the free drugs, and their disruption of biofilms was observed.
3.1.2.3. Other synthesized polymer-based NPs
A recent study used three synthetic polymers (polyethyleneimine (PEI), polyvinyl alcohol (PVA), and polyacrylic acid (PAA)) loaded with curcumin to enhance their antibacterial activity against MRSA. The antibacterial ability of the three curcumin-loaded NPs was significantly improved when used alone or in combination with methicillin, and PEI-based NPs exhibited the strongest inhibitory effect [107]. Gutierrez et al. synthesized curcumin-loaded anionic and cationic NPs using PLA and dextran sulfate, combined with photodynamic therapy (PDT), to improve the solubility of curcumin and its antibacterial ability against Streptococcus mutans and MRSA [108]. Synthesized nanoformulations showed high antibacterial photodynamic activity against planktonic bacteria, and even in the absence of light, cationic NPs revealed an enhanced antibacterial effect.
3.1.3. Inorganic NPs
Inorganic NPs are widely preferred due to their advantages of adjustable size and shape. In addition, the high specific surface allows the NPs to attach high concentrations of functional ligands, and act as carriers to deliver other active compounds, improving their interaction with target bacteria [109]. Inorganic NPs consist of both metallic and non-metallic NPs. Silver and gold NPs are increasingly being used among metallic NPs, and some typical examples of polyphenol-containing metallic NPs were summarized in Table 2. Metal oxides and mesoporous silica NPs (MSNs) have also been attracted to use as delivery of natural products attributed to their various surface modifications [110].
Table 2.
Antibacterial effects of polyphenols in the synthesis of metallic NPs.
| Polyphenols | Composition | Bacteria | Ref. |
|---|---|---|---|
| Curcumin | Cur-AgNPs | P. aeruginosa; E. coli; S. aureus | [111] |
| Curcumin | Cur-AgNPs | E. coli; B. subtilis | [112] |
| Tannic acid | TA-AgNPs | Gram-negative bacteria | [113] |
| Tea polyphenol | Ag@TP NPs | E. coli | [114] |
| Gallic acid | AuNPs | E. coli; S. aureus | [115] |
| Resveratrol | Res-AuNPs; Res-AgNPs | Streptococcus pneumoniae | [116] |
| EGCG | AuNPs; AgNPs | S. aureus; K. pneumoniae | [117] |
| Curcumin | Cur@TA-CuII Cur@TA-FeIII |
E. coli; S. aureus | [118] |
| Tea polyphenol | Cu@TPNPs | S. aureus | [119] |
| Tannic acid | TA-CuNPs | E. coli; S. aureus | [120] |
3.1.3.1. Metallic NPs
Potential antibacterial properties have been demonstrated in metallic NPs such as Ag, Au, Cu, and Zn particles. The antibacterial mechanisms of metallic NPs include (1) Disruption of bacterial metabolism by binding with sulfhydryl or disulfide groups of essential enzymes. (2) Interruption of bacterial membranes by releasing ions from metallic NPs. (3) Inhibition of DNA replication, induction of protein denaturation, and destruction of the cell wall by generating reactive oxygen species (ROS) [121].
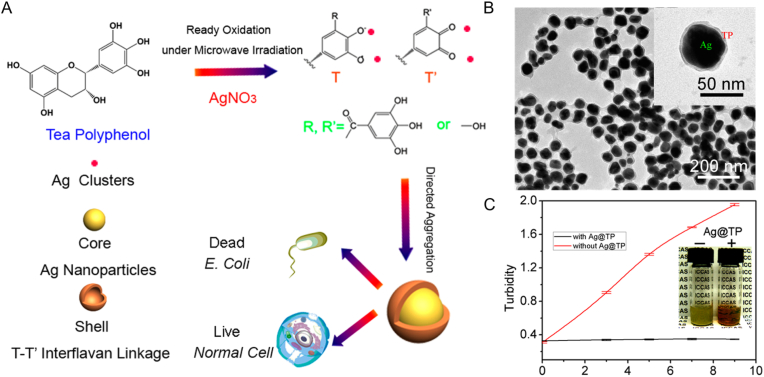
Silver NPs (AgNPs) and gold NPs (AuNPs) are the most widely used NPs for antibacterial purposes due to their large specific surface area and good contact with microorganisms. For example, in the research of Song et al. the curcumin-AgNPs (cAgNPs) had an increased inhibition activity against E. coli and B. subtilis than that of free curcumin. More ROS was generated by cAgNPs, resulting in membrane damage and leakage of intracellular contents. The bacteria treated with cAgNPs showed dramatically changed shape and shrunken cell walls observed under SEM [108]. Fei et al. produced a core-shell structure in which the core was AgNPs and the shell was the polymerized tea polyphenol (TP) [114]. The Ag@TP NPs were produced by the one-pot microwave self-assembly method within 1 min and the core-shell structure was proved by SEM (Fig. 5A–B). The NPs strongly inhibited E. coli without significant toxicity to normal cells (Fig. 5C).
Fig. 5.
Antibacterial ability of TP-loaded AgNPs. (A) Self-assembly scheme of Ag@TP NPs. (B) SEM image of the core-shell nanostructure. (C) Turbidity at 600 nm in E. coli cocultured with and without Ag@TP NPs (Reprinted from Ref. [113] with permission from American Chemical Society).
Reducing agents and stabilizers are associated with the size and dispersion of metallic NPs [122]. Polyphenols, known as powerful antioxidants, have opened the way for “green synthesis” by providing efficient reduction and capping potential instead of traditional chemical agents during the procedure of metallic NPs [123]. In Onitsuka's study, catechin-rich tea leaves were used as reducing and capping agents to prepare AgNPs and AuNPs. The outcome showed that the synthetic nanoformulations enhanced antibacterial activity against S. aureus and K. pneumoniae compared with commercially available silver nanopowder [117].
The size and dispersion of NPs play an important role in their antibacterial effectiveness. Generally, AgNPs and AuNPs follow the pattern that the smaller the size, the stronger the antibacterial ability [109]. However, the antimicrobial performance of smaller particles can be limited, because the smaller particles tend to aggregate and reduce the contact area with bacteria [124]. Polyphenols can act as stabilizers during the process of metallic NPs by enhancing their stability. For instance, Podstawczyk et al. produced stable catechin-capped CuNPs with a size of less than 5 nm at a pH value of 11 (Fig. 6A) [125]. In addition, Park and his colleagues synthesized the antibacterial resveratrol-delivered AuNPs and AgNPs with a size range from ∼8.3 nm to ∼21.8 nm, and the as-prepared NPs maintained a good dispersion state after ultracentrifugation and re-dispersion (Fig. 6B). Besides, Fourier-transform infrared spectra (FTIR) indicated the C C and hydroxyl groups in the phenyl ring of resveratrol were participated in the reduction reaction [116].
Fig. 6.
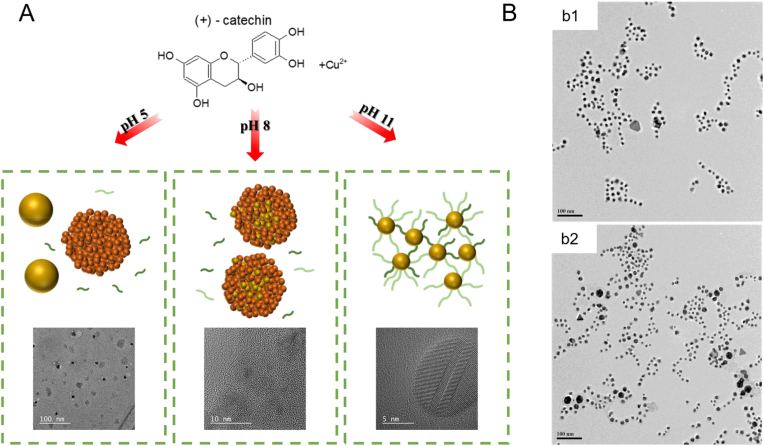
(A) Preparation of stable and small CuNPs (Reprinted with permission from Yang Qin et al. [122]). (B) High-resolution transmission electron microscopy (HR-TEM) images of resveratrol-AuNPs before (b1) and after (b2) the ultracentrifugation and re-dispersion process (Reprinted from Ref. [124] with permission from American Chemical Society).
Metal-Phenolic Networks (MPNs), inorganic-organic networks based on coordination between electron-dense phenolic hydroxyl groups and positively charged metal ions, have been widely considered as new powerful antibacterial agents [126]. The formation of MPNs chelated with metals enhances the antibacterial efficacy of polyphenols compared to polyphenols alone. The efficacy of polyphenols in MPNs is attributed to the following advantages of polyphenols (1) Playing a synergetic antibacterial role with metal ions. (2) Providing a slow release of metal ions and achieving the sustained antibacterial effect. (3) Easy to dissociate with metal ions and plays an antibacterial effect under the environment of acidic conditions (bacterial infection) [127]. For instance, Zhang et al. developed a biocompatible composite incorporating TA and magnesium chloride (MgCl2) into bacterial cellulose for antibacterial and antibiofilm purposes (Fig. 7A) [128]. With the help of coordination with Mg2+, TA revealed a sustained release from the nanoformulation. Qin and his colleagues developed MPNs coated NPs based on the coordination between TA and iron ions, which showed significant antibacterial effects. TA revealed a highly efficient release under acidic conditions (Fig. 7B). [129]. Additionally, MPN can be fabricated into ROS-response nanoformulations, which can be used to act as antibacterial agents in infected environments. In the study of Li et al., copper-poly (TA) scaffold could degrade under high ROS levels, followed by sustained release of Cu2+ and the peroxidase/peroxidase-mimic effect, with a powerful antibacterial material for the infectious wound [130]. Moreover, the addition of carboxylic acid to a phenol-Fe nano-network could effectively control the peroxidase-like activity through the acidic microenvironment and the proximity effect of the carboxyl group. For example, Wang et al. tested the peroxidase-like activity of a nano-network constructed by five kinds of polyphenols and Fe. They found that gallic acid with a carboxylic acid structure was more likely to catalyze hydrogen peroxide to generate hydroxyl radicals under acidic conditions and play an antibacterial role [131].
Fig. 7.
Fabrication and the antibacterial effect of MPNs. (A) The coordination interaction between TA and Mg2+ in bacterial cellulose and its inhibitory effect on biofilm formation (Reprinted from Ref. [127] with permission from ELSEVIER). (B) MPNs based on coordination between TA and ferric ion (Fe3+) and the release of TA under different pH values (Reprinted from Ref. [128] with permission from American Chemical Society). (C) Preparation of GNPs@MPNs and their antibacterial effect against S. aureus and E. coli under near-infrared light (NIR) (Reprinted from Ref. [132] with permission from ELSEVIER). (D) Hybrid nanoformulation based on the coordination between TA and Fe3+ and its antibacterial effect under NIR (Reprinted from Ref. [134] with permission from WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim).
In addition, the photothermal effect of metallic NPs also plays an important role in enhancing their antibacterial capabilities. For example, gold nanorods (AuNRs) as photothermal agents (PTAs) have been widely researched due to their remarkable photon harvesting properties, which can transform NIR into heat and kill the bacteria by increasing the temporal temperature of the focus infection [132]. Sun et al. encapsulated AuNRs in the MPNs which coordinated iron with polyphenols (EGCG, TA, and procyanidins) [133,134]. The results showed a remarkably potent role in destroying cell membranes and biomolecules of E. coli and S. aureus and accelerated the healing of the wound infected by MRSA (Fig. 7C). Yu et al. demonstrated a hybrid nanoformulation by the composite of TA, Fe3+, CS, and silk fibroin for wound dressing applications. The nanoformulation exhibited an excellent antibacterial ability for both Gram-positive and Gram-negative bacteria, benefiting from the synergistic and high photothermal transition effect of the TA/Fe3+ (Fig. 7D) [135].
3.1.3.2. Non-metallic NPs
Metal oxide NPs. Metal oxide NPs, such as ZnO, TiO2, Fe2O3, Fe3O4, and CuO, exert antibacterial effects against Gram-positive and Gram-negative bacteria [136,137]. Among metal oxide NPs, zinc oxide NPs (ZnO NPs) have presented excellent antibacterial properties and thus have been of great interest because of their good biocompatibility at the same time. ZnO NPs have been approved by FDA for drug delivery applications due to their easy synthesis, tailoring of shape, and non-toxic nature [110]. Lee et al. fabricated ZnO@GA NPs by coordinated covalent bonding of GA to ZnO NPs surfaces. The functionalized ZnO NPs showed good antibacterial activity against MRSA and E. coli [138]. To target the site of infection, Ghaffari et al. prepared the pH-dependent ZnO NPs to enhance the antibacterial activity of curcumin which was released faster under mildly acidic conditions compared with the neutral pH condition [139,140]. In recent years, titanium dioxide NPs (TiO2 NPs) have also attracted great attention due to their significant antibacterial ability. For instance, Dessai et al. used rutin (derivatives of flavonoids) to fabricate TiO2 NPs, which was an eco-friend and cost-effective material with antibacterial properties [141]. Additionally, iron oxide NPs (IONPs) were also an attractive candidate for antimicrobial preparation due to the balance of antibacterial effect and biocompatibility [142]. Synthetic methods with the application of natural extracts used as reducing agents have attracted considerable interest. By way of example, Aliya et al. used polyphenols of Nigella sativa seeds as reducing agents to produce IONPs with sizes 5–6 nm, which had a prominent effect on E. coli and Salmonella typhi [143]. In a recent study by Ke et al. TA was used as forming coordination bonds with Fe3O4 NPs [144]. The nanoformulation contributed synergistically to local wound infection, benefiting from the adhesion ability and photothermal properties.
Silica NPs. Studies in the past decade have shown that silica materials, especially ordered MSNs, are attractive due to their unique tunable particle size, polar volume, and high loading capacity [140]. The structure of silica nanomaterials (such as particle size and shape, pore characteristics, and morphology) and surface traits (surface functionalities and surface roughness) affect drug loading and release rates [145]. In an earlier study, the researchers formed silica NPs by condensation of the carboxyl or hydroxyl groups of GA to improve the stability and antibacterial effect of the polyphenolic compounds. Compared with free GA, the antibacterial activity of silica NPs against Paenibacillus larvae was significantly improved [146]. Moreover, Petrisor et al. loaded GA in MSNs with different ratios and observed their inhibitory ability of gram-negative bacteria [147]. The release of GA from the MSNs was rapid in both simulated gastric fluid and simulated intestinal fluid. To overcome the shortcomings of rapid release, the optimization approach was the functionalized surface of the mesoporous materials [148]. For example, Shabana et al. synthesized PEG-coated MSNs to deliver quercetin [149]. The PEG-coated MSNs showed strong antibacterial effects against S. agalactiae and K. pneumoniae by oral route and a sustained release for more than 48 h.
3.2. Scaffolds (or matrix)-based formulations
3.2.1. Nanogels
Hydrogels are a kind of material synthesized by water-soluble natural or synthetic polymers, which form gels under the change of temperature, ion strength, pH value, ultraviolet radiation, and other different signals [150]. The properties such as good mucoadhesion, biocompatibility, controlled release, and enhance bioavailability of drugs by encapsulating or surface-adsorbing make nanogels promising biological materials for polyphenol delivery in local infection [151,152].
Nanogels can also entrap other nanostructures, known as “nanohybrid hydrogel” or “functionalized nanogels”. Metallic and metal oxide NPs are frequently embedded into the hydrogels to improve their mechanical, physicochemical, and biological properties by increasing the specific surface area and stability. For example, to increase the loading capacity, Dhanya et al. inserted ZnO NPs into the quercetin-loaded nanogels and significantly increased the quercetin loading by 39% [153]. These hybrid nanogels showed significant inhibitory effects against the strain of S. aureus and Trychophyton rubrum. Additionally, the introduction of metallic ions with a photothermal effect can play a cooperative role in antibacterial activity. As an example, Cao et al. prepared thermo-response functionalized nanogels against drug-resistant bacteria infection in local wounds [154]. The high temperature induced by AgNPs accelerated the release of TA and Ag+ under NIR, exerting a strong antibacterial function (∼97% of MRSA and∼96% of E. coli were killed) (Fig. 8A). The preparation of stimulus-responsive nanogels involved hydrogen bonds, ionic bonds, van der Waals interactions, etc. For instance, in another study on localized infections, Tao et al. prepared thermal-response and on-demand release of polydopamine (PDA)@curcumin nanogels for antibacterial activity of wound healing [155]. Under NIR laser irradiation, curcumin released sharply increased attributed to π-π stacking, and hydrogen bonding interactions changed with increasing temperature of NIR (Fig. 8B).
Fig. 8.
(A) The preparation of TA-loaded hybrid nanogel and the enhanced antibacterial activity under NIR (Reprinted from Ref. [153] with permission from ELSEVIER). (B) The synthesis route of curcumin-loaded nanogel and the bacterial inactivation effect under NIR (Reprinted from Ref. [154] with permission from ELSEVIER).
In recent years, stimuli-responsive (such as pH, glucose, and ROS) nanogels have gained much attention due to their potential applications in infectious wounds. Controlled and triggered drug release is a desired property of responsive nanogels. In general, nanogels with ionizable groups tend to exhibit pH-sensitive swelling and release properties. Ninan et al. produced the TA/carboxylated agarose nanogels cross-linked with zinc ions for pH-responsive and controlled release of TA. At low pH conditions, phenolic hydroxyl groups of TA were protonated, and relatively few polyphenols bonded to metal ions to facilitate the release of TA and exert antibacterial activity against E coli, S. aureus, and P. aeruginosa [156]. In addition to the weak acidic pH of the locally infected wound microenvironment, the pH-responsive nature of the nanogels is required for drug delivery from the gastrointestinal tract. For example, Hu et al. bonded EGCG with amyloid fibrils to form reversible nanogels [157]. Under human small intestine pH (pH = 6), EGCG could be released well and exerted antibacterial effects on gram-positive bacteria (Listeria monocytogenes, MRSA, and Streptococcus oralis) and gram-negative bacteria (Pseudomonas aeruginosa, E. coli, and Klebsiella pneumoniae) coating the mucosal surface of the small intestine, without obvious cytotoxic effect on the colon epithelium. The ROS-responsive nanogels are based on the forming of ROS-responsive chemical bonds such as borate easter [158]. For example, Ni et al. prepared a multistage ROS-responsive nanogel based on the phenylborate ester between TA, PVA, and phenylboronic acid-modified polyphosphazene [159]. The nanogel could inhibit the growth of E. coli within 4 h and effectively scavenge ROS in vitro study. For another example, Wu et al. fabricated a pH/ROS dual-responsive injectable nanogel based on a boronic ester bond formed between the catechol of caffeic acid and phenylboronic acids [160]. The nanogel had a spatiotemporal release of the natural polyphenol mangiferin and showed the effective against infection during the early stages of wound repair.
3.2.2. Nanofibers
Nanofibers are polymeric materials with diameters on the nanometer scale and lengths in meters. In recent years, taking advantage of the high mesh porosity and specific surface area of nanofibers, some scholars have reported using nanofibers as drug delivery carriers to improve the therapeutic activity of polyphenols [161]. Nanofibers with therapeutic activity are usually manufactured by combining selected polymers with polyphenolic compounds through different methods, such as (1) Blending polyphenolic compounds with the polymers before spinning. (2) Coaxial spinning fabrication of core/shell structures. (3) Pre-treatment of encapsulated polyphenols mixed with polymers. (4) Post-treatment of the nanofibers. (5) Attachment of polyphenolic compounds to the surface of nanofibers [162]. To improve production efficiency and reduce production time and cost, several techniques for preparing nanofibers have been developed, such as electrospinning, solution blow spinning, centrifugal spinning, and carbon dioxide laser supersonic drawing [[163], [164], [165], [166], [167]]. Wang et al. used an electrospinning technique to load curcumin in zein fibers successfully with a high EE of nearly 100%, and the nanofibers manifested a remained antioxidant capability and an enhanced antibacterial effect against S. aureus and E. coli [168].
The polymers fabricating nanofibers include natural polymers (such as CS, collagen, zein, gelatin, silk fibroin, and cellulose) and synthetic polymers. Among the synthetic polymers, PLGA, PLA, PGA, PCL, and PVA are commonly used to improve the mechanical properties and degradation kinetics due to their biocompatible and biodegradable characteristics [[169], [170], [171]]. For example, Zhang et al. prepared nanofibers with antibacterial and ultraviolet protection application potential based PVA and tea polyphenol [172]. This composite was blended through H-binding interaction and had good biodegradability and mechanical property.
The parameters affecting polyphenols release from nanofibers are related to the physical properties of fibers, polymers, and polyphenolic compounds. Firstly, the diameter of nanofibers affects the drug release, following the pattern that the thinner the nanofibers, the faster the release. Fallha et al. used the electrospinning technique to prepare PCL/gelatin/curcumin nanofibers to exert antibacterial activity against Gram-positive and Gram-negative bacteria [173]. As the voltage increased, the diameter of nanofibers drastically increased, thereby providing a longer-term antimicrobial environment. Secondly, the composition of polymers and the porosity also affect the kinetics of drug release. According to the study of Wang et al. curcumin cumulative release from nanofibers increased with the decrease in the weight ratio of PEG: PLA [174]. At the same time, due to the porous structure, curcumin was released from the nanofibers in a controlled manner, which resulted in sustained antibacterial effects. Trug-loaded nanofibers prepared by single electrospinning usually exhibited an initial burst release due to the high density of drug distribution on the nanofiber surface. To prevent the burst release of the drug, Wang et al. produced core-shell structure nanofibers with tea polyphenol as the core and PLA as the shell material [175]. This nanofiber showed a better release of tea polyphenol for 141 h. Finally, the loading parameters of polyphenols such as loading amount, solubility, and polymer-compound interaction, can also affect the release behavior of nanofibers [162,176]. For local wound dressings, besides long-term antibacterial activity, satisfactory biocompatibility, and skin adaptability should also be considered. Wu et al. established a kind of nanofibrous mats with CS and polydopamine on the surface [177], which resulted in a durable tensile stress of 6.7 MPa and a powerful inhibition against S. aureus and E. coli for 14 days. A recent study used different contents of GA to fabricate cellulose acetate nanofibers and investigated their potential antibacterial effects and release characteristics as wound dressing applications [178]. The researchers found that the cumulative release of 20% GA was greater than that of 40% GA because the average diameter of 20% GA was smaller and easier to release from nanofibers.
3.2.3. Nanoemulsions
Emulsions are widely used as one of the most desirable encapsulation and delivery systems for hydrophilic, lipophilic, and amphiphilic bioactive compounds due to their high encapsulation efficiency, viscoelastic, and visual properties, as well as the maintenance of the bioactive stability of encapsulated molecules. The droplet sizes of nanoemulsions range from ten to several hundred nanometers. Emulsions for encapsulation and delivery of polyphenols can be classified according to the literature into nanoemulsions (droplets size 200–500 nm) and microemulsions (droplets size<100 nm) [179,180]. The oil phase usually contains 5–20% oil/lipid droplets, sometimes significantly up to 70%. Re-esterified sections derived from soybean oil, cottonseed oil, safflower oil, and rice-bran oil (labeled as long-chain triglycerides, medium-chain triglycerides, and short-chain triglycerides according to the chain length) are used alone or in combination to fabricate nanoemulsions [[181], [182], [183]]. McClements et al. explored the influence of formative oil/lipid and droplet size on the bioavailability of curcumin [184]. Their biorelevant tests showed that nanoemulsions made with long-chain triglycerides and medium-chain triglycerides achieved maximum systemic availability.
Different ratios of the aqueous phase and organic phase, as well as the concentration of surfactant, can affect the stability of nanoemulsions [179,185]. In the study of Kaur et al., curcumin-loaded nanoemulsions were prepared to use against drug-resistant strains of uropathogenic bacteria [186]. The nanoemulsions with the composition of soybean oil (13.09%) and water (71.22%) showed higher inhibitory ability on bacteria and biofilm formation than the natural compounds. Lou et al. used low-energy methods to produce trans-cinnamic acid nanoemulsion to enhance the antibacterial and antibiofilm effect of Pseudomonas aeruginosa [187]. They found that as the concentration of tween 80 (surfactant) increased from 1% to 5%, the particle size decreased from more than 100 nm–∼47 nm, while the polydispersity index (PDI) decreased from 0.58 to 0.29 as the concentration of tween 80 increased from 3% to 5%.
Several factors, such as pH, temperature, enzyme activity, and ionic concentration of the surrounding media, may trigger drug release from the nanoemulsion system. Very lipophilic drugs are released when the oily or lipid components have been digested. The small droplet size of nanoemulsions suggests that the diffusion rate of the drug across the oil phase may be quite rapid. Therefore, nanoemulsions are always fixed in structured carriers such as organogel or coated by thick layers of polymer to provide controlled release methods [188]. In a recent study, curcumin-loaded nanoemulsions were encapsulated in a hybrid hydrogel of CS, alginate, gelatin, and polyethylene oxide to enhance the antibacterial activity of curcumin [189]. At the same time, curcumin was released faster in simulated gastric fluid (pH 2.1) than in simulated intestinal fluid (pH 7.4) because alginate was protonated and curcumin was beneficial to the release under the low pH. That was, the hydrogel covering of nanoemulsion had a significant effect on drug release kinetics under an infectious environment.
4. Conclusion and outlook
With the increasing incidence of drug-resistant bacteria around the world, considerations regarding the application of antibiotic-free strategies will be a focus of future research. Polyphenolic compounds have been shown to have a positive effect on antibacterial therapy, however, their poor stability, low water solubility, and low bioavailability characteristics have created several limitations for their therapeutic application. By overcoming these limitations and controlling their release under specific conditions, nanoformulations have made important contributions. In this review, we have summarized the nanoformulations which enhanced the antibacterial effect of polyphenolic compounds and the advantages of different nano-systems and their potential application fields. For instance, polyphenol-loaded antibacterial nanogels can be applied to wound dressings, catheter-associated infections, urinary tract coating, etc. due to their local administration, controlled and prolonged release, and stimulated switch-on and off release. SLNs and CS-based nanoformulations are useful for systemic administration due to their low toxicity and good biocompatibility. Nanoemulsions provide greater opportunities to increase the bioavailability of lipophilic polyphenols.
However, some crucial challenges are still faced in the clinical translation of polyphenolic antibacterial nanoformulations: (1) Based on the excellent performance of polyphenolic compounds, considerations regarding antibiotic-free strategies should be a focus and be expanded from biomedicine to other fields such as environmental protection, animal welfare, and food safety. Simple, general, and cost-effective methods are needed to prepare polyphenolic nanoformulations. (2) Numerous studies have shown that polyphenolic nanoformulations have good antibacterial effects, but there is a lack of cross-sectional comparisons of the antibacterial capabilities of different formulations, and the mechanism of their antibacterial effect needs to be further explored and confirmed. At the same time, similar to the shortcomings of traditional drug delivery systems, polyphenolic compounds suffer from a relatively low effective utility. Smart nanoformulations designed to target infectious sites and to release favorably under infectious microenvironments would further enhance their effectiveness [190]. (3) The biological safety of nanoformulations is an important consideration for further application, especially for the use of inorganic nanoformulations. Although CS and other food-grade biopolymers are considered safe, the process of preparation often involves organic chemicals, which may create a potential risk upon ingestion [39]. Strategies that use hybrid nanoformulations or combined therapy such as photothermal therapy should be considered to reduce the amount of metal ions use. Moreover, the processes of degradation, digestion, and elimination of nanoformulations are still not well understood, and their accumulation in different organs is also an important concern for human health. Nevertheless, a systematic and careful investigation of the biosafety of the nanoformulations is required.
On all accounts, nanotechnology has paved the promising path for drug delivery, and polyphenolic nanoformulations have potential prospects in antibacterial biomedical applications. The diversity of procedures from manufacture to the analysis of polyphenolic nanoformulations affects their physical properties, chemical behavior, and biological interactions, and therefore their applications. With a further understanding of the intrinsic properties of polyphenols, smart nanoformulations with low cost, ease of assembly, and facilitated site-specific targeting will play a critical role in the antibacterial applications. We expect this review to provide researchers with a framework for the advanced development of polyphenol-based nanomaterials and to guide more exploration in the field of antibiotic-free strategies to fight against bacterial infections.
4.1. Study highlight
-
(1)
Polyphenols are rich in nature and possess excellent antibacterial potential. Most polyphenols have poor water solubility and are liable disrupted by environmental acidity, temperature, enzymes, etc, which limit their therapeutic activity.
-
(2)
Nanoformulations (nanoparticles and nanoscaffolds) have played an important role in improving the bioavailability and antibacterial effect of polyphenols. Polyphenol-loaded nanoformulations have a promising prospect in non-biotic antibacterial applications in the biomedical field.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are thankful to the National Natural Science Foundation of China (52073278), “Medical Science + X″ Cross-innovation Team of the Norman Bethune Health Science of Jilin University (2022JBGS10), Jilin Province Science and Technology Development Program (20230101045JC), The Education Department of Jilin Province (JJKH20231205KJ), Health Commission of Jilin Province (2021JC036), and “Special Funding for Fundamental Scientific Research Business Expenses of Central Universities”.
Data availability
No data was used for the research described in the article.
References
- 1.Sierra J.M., Fuste E., Rabanal F., Vinuesa T., Vinas M. An overview of antimicrobial peptides and the latest advances in their development. Expet Opin. Biol. Ther. 2017;17(6):663–676. doi: 10.1080/14712598.2017.1315402. [DOI] [PubMed] [Google Scholar]
- 2.Ayaz M., Ullah F., Sadiq A., Ullah F., Ovais M., Ahmed J., Devkota H.P. Synergistic interactions of phytochemicals with antimicrobial agents: potential strategy to counteract drug resistance. Chem. Biol. Interact. 2019;308:294–303. doi: 10.1016/j.cbi.2019.05.050. [DOI] [PubMed] [Google Scholar]
- 3.Watkins R., Wu L., Zhang C., Davis R.M., Xu B. Natural product-based nanomedicine: recent advances and issues. Int. J. Nanomed. 2015;10:6055–6074. doi: 10.2147/IJN.S92162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mierziak J., Kostyn K., Kulma A. Flavonoids as important molecules of plant interactions with the environment. Molecules. 2014;19(10):16240–16265. doi: 10.3390/molecules191016240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stanisavljevic N.S., Ilic M.D., Matic I.Z., Jovanovic Z.S., Cupic T., Dabic D., Natic M.M., Tesic Z. Identification of phenolic compounds from seed coats of differently colored european varieties of pea (Pisum sativum L.) and characterization of their antioxidant and in vitro anticancer activities. Nutr. Cancer. 2016;68(6):998–1000. doi: 10.1080/01635581.2016.1190019. [DOI] [PubMed] [Google Scholar]
- 6.Poti F., Santi D., Spaggiari G., Zimetti F., Zanotti I. Polyphenol health effects on cardiovascular and neurodegenerative disorders: a review and meta-analysis. Int. J. Mol. Sci. 2019;20(2):351. doi: 10.3390/ijms20020351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng D., Huang C., Huang H., Zhao Y., Khan M.R.U., Zhao H., Huang L. Antibacterial mechanism of curcumin: a review. Chem. Biodivers. 2020;17(8) doi: 10.1002/cbdv.202000171. [DOI] [PubMed] [Google Scholar]
- 8.Ibrahim N., Wong S.K., Mohamed I.N., Mohamed N., Chin K.Y., Ima-Nirwana S., Shuid A.N. Wound healing properties of selected natural products. Int. J. Environ. Res. Publ. Health. 2018;15(11):2360. doi: 10.3390/ijerph15112360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rothenberg D.O., Zhang L. Mechanisms underlying the anti-depressive effects of regular tea consumption. Nutrients. 2019;11(6):1361. doi: 10.3390/nu11061361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jubair N., Rajagopal M., Chinnappan S., Abdullah N.B., Fatima A. Review on the antibacterial mechanism of plant-derived compounds against multidrug-resistant bacteria (MDR) Evid-Based Compl. Alt. 2021;2021 doi: 10.1155/2021/3663315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barbieri R., Coppo E., Marchese A., Daglia M., Sobarzo-Sanchez E., Nabavi S.F., Nabavi S.M. Phytochemicals for human disease: an update on plant-derived compounds antibacterial activity. Microbiol. Res. 2017;196:44–68. doi: 10.1016/j.micres.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Murthy N.T.V., Agrahari V., Chauhan H. Polyphenols against infectious diseases: controlled release nano-formulations. Eur. J. Pharm. Biopharm. 2021;161:66–79. doi: 10.1016/j.ejpb.2021.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Li Z., Jiang H., Xu C., Gu L. A review: using nanoparticles to enhance absorption and bioavailability of phenolic phytochemicals. Food Hydrocolloids. 2015;43:153–164. doi: 10.1016/j.foodhyd.2014.05.010. [DOI] [Google Scholar]
- 14.Ou K., Percival S.S., Zou T., Khoo C., Gu L., Agric J. Transport of cranberry A-type procyanidin dimers, trimers, and tetramers across monolayers of human intestinal epithelial Caco-2 cells. Food Chem. 2012;60(6):1390–1396. doi: 10.1021/jf2040912. [DOI] [PubMed] [Google Scholar]
- 15.Grgic J., Selo G., Planinic M., Tisma M., Bucic-Kojic A. Role of the encapsulation in bioavailability of phenolic compounds. Antioxidants. 2020;9(10):923. doi: 10.3390/antiox9100923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Modica M.N., Intagliata S., Santagati L.M., Montenegro L. Strategies to improve resveratrol systemic and topical bioavailability: an update. Antioxidants. 2019;8(8):244–277. doi: 10.3390/antiox8080244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang Z., Bhandari B. Encapsulation of polyphenols-a review. Trends Food Sci. Technol. 2010;21(10):510–523. doi: 10.1016/j.tifs.2010.08.003. [DOI] [Google Scholar]
- 18.Bartosz T., Irene T. Polyphenols encapsulation-application of innovation technologies to improve stability of natural products. Phys. Sci. Rev. 2016;1(2) doi: 10.1515/psr-2015-0005. [DOI] [Google Scholar]
- 19.Guzman-Villanueva D., El-Sherbiny I.M., Herrera-Ruiz D., Smyth H.D. Design and in vitro evaluation of a new nano-microparticulate system for enhanced aqueous-phase solubility of curcumin. BioMed Res. Int. 2013;2013 doi: 10.1155/2013/724763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dai W., Ruan C., Zhang Y., Wang J., Han J., Shao Z., Sun Y., Liang J. Bioavailability enhancement of EGCG by structural modification and nano-delivery: a review. J. Funct.Foods. 2020;65 doi: 10.1016/j.jff.2019.103732. [DOI] [Google Scholar]
- 21.Song H., Wang Q., He A., Li S., Guan X., Hu Y., Feng S. Antioxidant activity, storage stability and in vitro release of epigallocatechin-3-gallate (EGCG) encapsulated in hordein nanoparticles. Food Chem. 2022;388 doi: 10.1016/j.foodchem.2022.132903. [DOI] [PubMed] [Google Scholar]
- 22.Shababdoust A., Zandi M., Ehsani M., Shokrollahi P., Foudazi R. Controlled curcumin release from nanofibers based on amphiphilic-block segmented polyurethanes. Int. J. Pharm. 2020;575 doi: 10.1016/j.ijpharm.2019.118947. [DOI] [PubMed] [Google Scholar]
- 23.Aljelehawy Q.H.A., Mohammadi S., Mohamadian E., Allah O.Raji Mal, Mirzaei A., Ghahremanlou M. Antimicrobial, anticancer, antidiabetic, antineurodegenerative, and antirheumatic activities of thymol: clarification of mechanisms. Micro Nano Bio Aspect. 2023;2(1):1–7. doi: 10.22034/mnba.2023.381107.1019. [DOI] [Google Scholar]
- 24.S.A. Adefegha, A. Salawi, A. Bumrungpert, S. Khorasani, S. Torkaman, M.R. Mozafari, E. Taghavi, Encapsulation of polyphenolic compounds for health promotion and disease prevention: Challenges and opportunities, Nano Micro Biosystem 1 (2) 2022 1-202212, 10.22034/NMBJ.2023.163756. [DOI]
- 25.Alibi S., Crespo D., Navas J. Plant-derivatives small molecules with antibacterial activity. Antibiotics. 2021;10(3):231. doi: 10.3390/antibiotics10030231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aljelehawy Q.H.A., Maroufi Y., Javid H., Mohammadi M.R., Allah Q.R.M., Taheri S.V., Mohammadzade H. Anticancer, antineurodegenerative, antimicrobial, and antidiabetic activities of carvacrol: recent advances and limitations for effective formulations. Nano Micro Biosystems. 2023;2(1):1–10. doi: 10.22034/NMBJ.2023.380207.1009. [DOI] [Google Scholar]
- 27.Rahaiee S., Assadpour E., Esfanjani A.F., Silva A.S., Jafari S.M. Application of nano/microencapsulated phenolic compounds against cancer. Adv. Colloid. Interfac. 2020;279 doi: 10.1016/j.cis.2020.102153. [DOI] [PubMed] [Google Scholar]
- 28.Teng H., Chen L. Polyphenols and bioavailability: an update. Crit. Rev. Food Sci. 2019;59(13):2040–2051. doi: 10.1080/10408398.2018.1437023. [DOI] [PubMed] [Google Scholar]
- 29.Yin C., Cheng L., Zhang X., Wu Z. Nanotechnology improves delivery efficiency and bioavailability of tea polyphenols. J. Food Biochem. 2020;44(9) doi: 10.1111/jfbc.13380. [DOI] [PubMed] [Google Scholar]
- 30.Zou L.Q., Liu W., Liu W.L., Liang R.H., Li T., Liu C.M., Cao Y.L., Niu J., Liu Z. Characterization and bioavailability of tea polyphenol nanoliposome prepared by combining an ethanol injection method with dynamic high-pressure microfluidization. J. Agric. Food Chem. 2014;62(4):934–941. doi: 10.1021/jf402886s. [DOI] [PubMed] [Google Scholar]
- 31.Durazzo A., Lucarini M., Souto E.B., Cicala C., Caiazzo E., Izzo A.A., Novellino E., Santini A. Polyphenols: a concise overview on the chemistry, occurrence, and human health. Phytother Res. 2019;33(9):2221–2243. doi: 10.1002/ptr.6419. [DOI] [PubMed] [Google Scholar]
- 32.Santana-Galvez J., Cisneros-Zevallos L., Jacobo-Velazquez D.A. Chlorogenic acid: recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules. 2017;22(3):358. doi: 10.3390/molecules22030358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tajik N., Tajik M., Mack I., Enck P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: a comprehensive review of the literature. Eur. J. Nutr. 2017;56(7):2215–2244. doi: 10.1007/s00394-017-1379-1. [DOI] [PubMed] [Google Scholar]
- 34.Petersen M. Rosmarinic acid: new aspects. Phytochemistry Rev. 2013;12(1):207–227. doi: 10.1007/s11101-013-9. [DOI] [Google Scholar]
- 35.Nadeem M., Imran M., Gondal T.A., Imran A., Shahbaz M., Muhammad Amir R., Sajid M.W., Qaisrani T.B., Atif M., Hussain G. Therapeutic potential of rosmarinic acid: a comprehensive review. Appl. Sci. 2019;9(15):3139. doi: 10.3390/app9153139. [DOI] [Google Scholar]
- 36.Yang K., Zhang L., Liao P., Xiao Z., Zhang F., Sindaye D., Xin Z., Tan C., Deng J., Yin Y. Impact of gallic acid on gut health: focus on the gut microbiome, immune response, and mechanisms of action. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.580208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin T.H., Wu C.C., Tseng C.Y., Fang J.H., Lin C.T. Effects of gallic acid on capsular polysaccharide biosynthesis in Klebsiella pneumoniae. J. Microbiol. Immunol. 2022;55:1255–1262. doi: 10.1016/j.jmii.2021.07.002. [DOI] [PubMed] [Google Scholar]
- 38.Chen Z., Farag M.A., Zhong Z., Zhang C., Yang Y., Wang S., Wang Y. Multifaceted role of phyto-derived polyphenols in nanodrug delivery systems. Adv. Drug Deliv. Rev. 2021;176 doi: 10.1016/j.addr.2021.113870. [DOI] [PubMed] [Google Scholar]
- 39.Dvorakova M., Landa P. Anti-inflammatory activity of natural stilbenoids: a review. Pharmacol. Res. 2017;124:126–145. doi: 10.1016/j.phrs.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 40.Vestergaard M., Ingmer H. Antibacterial and antifungal properties of resveratrol. Int. J. Antimicrob. Agents. 2019;53(6):716–723. doi: 10.1016/j.ijantimicag.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 41.Guo Z., Xie W., Lu J., Guo X., Xu J., Xu W., Chi Y., Takuya N., Wu H., Zhao L. Tannic acid-based metal phenolic networks for bio-applications: a review. J. Mater. Chem. B. 2021;9(20):4098–4110. doi: 10.1039/d1tb00383f. https://dio.org/10.1039/D1TB00383F [DOI] [PubMed] [Google Scholar]
- 42.Zhang Z.Y., Sun Y., Zheng Y.D., He W., Yang Y.Y., Xie Y.J., Feng Z.X., Qiao K. A biocompatible bacterial cellulose/tannic acid composite with antibacterial and anti-biofilm activities for biomedical application. Mat. Sci. Eng. C-mater. 2020;106 doi: 10.1016/j.msec.2019.110249. [DOI] [PubMed] [Google Scholar]
- 43.Xie Y., Yang W., Tang F., Chen X., Ren L. Antibacterial activities of flavonoids: structure-activity relationship and mechanism. Curr. Med. Chem. 2015;22(1):132–149. doi: 10.2174/0929867321666140916113443. [DOI] [PubMed] [Google Scholar]
- 44.Farhadi F., Khameneh B., Iranshahi M., Iranshahy M. Antibacterial activity of flavonoids and their structure-activity relationship: an update review. Phytother Res. 2019;33(1):13–40. doi: 10.1002/ptr.6208. [DOI] [PubMed] [Google Scholar]
- 45.Alvarez-Martínez F., Barrajon-Catalán E., Herranz-Loopez M., Micol V. Antibacterial plant compounds, extracts and essential oils: an updated review on their effects and putative mechanisms of action. Phytomedicine. 2021;90 doi: 10.1016/j.phymed.2021.153626. [DOI] [PubMed] [Google Scholar]
- 46.Huang J., Chen B., Li H., Zeng Q.H., Wang J.J., Liu H., Pan Y., Zhao Y. Enhanced antibacterial and antibiofilm functions of the curcumin-mediated photodynamic inactivation against Listeria monocytogenes. Food Control. 2020;108 doi: 10.1016/j.foodcont.2019.106886. [DOI] [Google Scholar]
- 47.Renzetti A., Betts J.W., Fukumoto K., Rutherford R.N. Antibacterial green tea catechins from a molecular perspective: mechanisms of action and structure-activity relationships. Food Funct. 2020;11(11):9370–9396. doi: 10.1039/D0FO02054K. [DOI] [PubMed] [Google Scholar]
- 48.Efenberger-Szmechtyk M., Nowak A., Czyzowska A. Plant extracts rich in polyphenols: antibacterial agents and natural preservatives for meat and meat products. Crit. Rev. Food Sci. Nutr. 2021;61(1):149–178. doi: 10.1080/10408398.2020.1722060. [DOI] [PubMed] [Google Scholar]
- 49.Gorniak I., Bartoszewski R., Kroliczewski J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochemistry Rev. 2019;18(1):241–272. doi: 10.1007/s11101-018-9591-z. [DOI] [Google Scholar]
- 50.Gayani B., Dilhari A., Wijesinghe G.K., Kumarage S., Abayaweera G., Samarakoon S.R., Perera I.C., Kottegoda N., Weerasekera M.M. Effect of natural curcuminoids-intercalated layered double hydroxide nanohybrid against Staphylococcus aureus, Pseudomonas aeruginosa, and Enterococcus faecalis: a bactericidal, antibiofilm, and mechanistic study. Microbiology Open. 2019;8(5) doi: 10.1002/mbo3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Memar M.Y., Yekani M., Celenza G., Poortahmasebi V., Naghili B., Bellio P., Baghi H.B. The central role of the SOS DNA repair system in antibiotics resistance: a new target for a new infectious treatment strategy. Life Sci. 2020;262 doi: 10.1016/j.lfs.2020.118562. [DOI] [PubMed] [Google Scholar]
- 52.Oda Y. Inhibitory effect of curcumin on SOS functions induced by UV irradiation. Mutat. Res. 1995;348(2):67–73. doi: 10.1016/0165-7992(95)00048-8. [DOI] [PubMed] [Google Scholar]
- 53.Koh J.J., Qiu S., Zou H., Lakshminarayanan R., Li J., Zhou X., Tang C., Saraswathi P., Verma C., Tan D.T. Rapid bactericidal action of alpha-mangostin against MRSA as an outcome of membrane targeting. Biochim. Biophys. Acta. 2013;1828(2):834–844. doi: 10.1016/j.bbamem.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 54.Mahmud M.M., Zaman S., Perveen A., Jahan R.A., Islam M.F., Arafat M.T. Controlled release of curcumin from electrospun fiber mats with antibacterial activity. J. Drug Deliv. Sci. Technol. 2020;55 doi: 10.1016/j.jddst.2019.101386. [DOI] [Google Scholar]
- 55.Tyagi P., Singh M., Kumari H., Kumari A., Mukhopadhyay K. Bactericidal activity of curcumin I is associated with damaging of bacterial membrane. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0121313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alvarez-Martinez F.J., Barrajon-Catalan E., Encinar J.A., Rodriguez-Diaz J.C., Micol V. Antimicrobial capacity of plant polyphenols against gram-positive bacteria: a comprehensive review. Curr. Med. Chem. 2020;27(15):2576–2606. doi: 10.2174/0929867325666181008115650. [DOI] [PubMed] [Google Scholar]
- 57.Qin Y., Xu L., Teng Y., Wang Y., Ma P. Discovery of novel antibacterial agents: recent developments in D-alanyl-D-alanine ligase inhibitors. Chem. Biol. Drug Des. 2021;98(3):305–322. doi: 10.1111/cbdd.13899. [DOI] [PubMed] [Google Scholar]
- 58.Yang Y., Zhang T. Antimicrobial activities of tea polyphenol on phytopathogens: a review. Molecules. 2019;24(4):816. doi: 10.3390/molecules24040816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li B., Li X., Lin H., Zhou Y. Curcumin as a promising antibacterial agent: effects on metabolism and biofilm formation in S. mutans. BioMed Res. Int. 2018;2018 doi: 10.1155/2018/4508709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cushnie T.T., Lamb A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents. 2005;26(5):343–356. doi: 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Selmani A., Kovacevic D., Bohinc K. Nanoparticles: from synthesis to applications and beyond. Adv. Colloid Interface Sci. 2022;303 doi: 10.1016/j.cis.2022.102640. [DOI] [PubMed] [Google Scholar]
- 62.Liang J., Yan H., Puligundla P., Gao X., Zhou Y., Wan X. Applications of chitosan nanoparticles to enhance absorption and bioavailability of tea polyphenols: a review. Food Hydrocolloids. 2017;69:286–292. doi: 10.1016/j.cis.2022.102640. [DOI] [Google Scholar]
- 63.Guo Y., Sun Q., Wu F.G., Dai Y., Chen X. Polyphenol-containing nanoparticles: synthesis, properties, and therapeutic delivery. Adv. Mater. 2021;33(22) doi: 10.1002/adma.202007356. [DOI] [PubMed] [Google Scholar]
- 64.Nunes S., Madureira A.R., Campos D., Sarmento B., Gomes A.M., Pintado M., Reis F. Solid lipid nanoparticles as oral delivery systems of phenolic compounds: overcoming pharmacokinetic limitations for nutraceutical applications. Crit. Rev. Food Sci. Nutr. 2017;57(9):1863–1873. doi: 10.1080/10408398.2015.1031337. [DOI] [PubMed] [Google Scholar]
- 65.Borges A., De Freitas V., Mateus N., Fernandes I., Oliveira J. Solid lipid nanoparticles as carriers of natural phenolic compounds. Antioxidants. 2020;9(10):998. doi: 10.3390/antiox9100998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mehnert W., Mader K. Solid lipid nanoparticles: production, characterization and applications. Adv. Drug Deliv. Rev. 2012;64:83–101. doi: 10.1016/j.addr.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 67.Shtay R., Keppler J.K., Schrader K., Schwarz K. Encapsulation of (-)-epigallocatechin-3-gallate (EGCG) in solid lipid nanoparticles for food applications. J. Food Eng. 2019;244:91–100. doi: 10.1016/j.jfoodeng.2018.09.008. [DOI] [Google Scholar]
- 68.Santonocito D., Sarpietro M.G., Carbone C., Panico A., Campisi A., Siciliano E.A., Sposito G., Castelli F., Puglia C. Curcumin containing PEGylated solid lipid nanoparticles for systemic administration: a preliminary study. Molecules. 2020;25(13):2991. doi: 10.3390/molecules25132991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jourghanian P., Ghaffari S., Ardjmand M., Haghighat S. M. Sustained release curcumin loaded solid lipid nanoparticles. Pharm. Bull. 2016;6(1):17. doi: 10.15171/apb.2016.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.He J., Huang S., Sun X., Han L., Chang C., Zhang W., Zhong Q. Carvacrol loaded solid lipid nanoparticles of propylene glycol monopalmitate and glyceryl monostearate: preparation, characterization, and synergistic antimicrobial activity. Nanomaterials. 2019;9(8):1162. doi: 10.3390/nano9081162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Luan L., Chi Z., Liu C. Chinese white wax solid lipid nanoparticles as a novel nanocarrier of curcumin for inhibiting the formation of Staphylococcus aureus biofilms. Nanomaterials. 2019;9(5):763. doi: 10.3390/nano9050763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sabir F., Qindeel M., Rehman A.U., Ahmad N.M., Khan G.M., Csoka I., Ahmed N. An efficient approach for development and optimisation of curcumin-loaded solid lipid nanoparticles' patch for transdermal delivery. J. Microencapsul. 2021;38(4):233–248. doi: 10.1080/02652048.2021.1899321. [DOI] [PubMed] [Google Scholar]
- 73.Hazzah H.A., Farid R.M., Nasra M.M., Hazzah W.A., El-Massik M.A., Abdallah O.Y. Gelucire-based nanoparticles for curcumin targeting to oral mucosa: preparation, characterization, and antimicrobial activity assessment. J. Pharm. Sci. 2015;104(11):3913–3924. doi: 10.1002/jps.24590. [DOI] [PubMed] [Google Scholar]
- 74.Sandreschi S., Piras A.M., Batoni G., Chiellini F. Perspectives on polymeric nanostructures for the therapeutic application of antimicrobial peptides. Nanomedicine. 2016;11(13):1729–1744. doi: 10.2217/nnm-2016-0057. [DOI] [PubMed] [Google Scholar]
- 75.Santo M.C.D., Alaimo A., Rubio A.P.D., Matteo R.D., Perez O.E. Biocompatibility analysis of high molecular weight chitosan obtained from Pleoticus muelleri shrimps. evaluation in prokaryotic and eukaryotic cells. Biochem. Biophys. Rep. 2020;24 doi: 10.1016/j.bbrep.2020.100842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Puligundla P., Mok C., Ko S., Liang J., Recharla N. Nanotechnological approaches to enhance the bioavailability and therapeutic efficacy of green tea polyphenols. J. Funct.Foods. 2017;34:139–151. doi: 10.1016/j.jff.2017.04.023. [DOI] [Google Scholar]
- 77.Yanat M., Schroen K. Preparation methods and applications of chitosan nanoparticles; with an outlook toward reinforcement of biodegradable packaging. React. Funct. Polym. 2021;161 doi: 10.1016/j.reactfunctpolym.2021.104849. [DOI] [Google Scholar]
- 78.Quinones J.P., Peniche H., Peniche C. Chitosan based self-assembled nanoparticles in drug delivery. Polymers. 2018;10(3):235. doi: 10.3390/polym10030235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Divya K., Jisha M.S. Chitosan nanoparticles preparation and applications. Environ. Chem. Lett. 2017;16(1):101–112. doi: 10.1007/s10311-017-0670-y. [DOI] [Google Scholar]
- 80.Hu Q., Luo Y. Chitosan-based nanocarriers for encapsulation and delivery of curcumin: a review. Int. J. Biol. Macromol. 2021;179:125–135. doi: 10.1016/j.ijbiomac.2021.02.216. [DOI] [PubMed] [Google Scholar]
- 81.Ma S., D M., Han F., Leonhard M., Schneider-Stickler B., Tan Y.L. Preparation and antibiofilm studies of curcumin loaded chitosan nanoparticles against polymicrobial biofilms of Candida Albicans and Staphylococcus Aureus. Carbohydr. Polym. 2020;241 doi: 10.1016/j.carbpol.2020.116254. [DOI] [PubMed] [Google Scholar]
- 82.Zhao L., Ding X., Khan I.M., Yue L., Zhang Y., Wang Z. Preparation and characterization of curcumin/chitosan conjugate as an efficient photodynamic antibacterial agent. Carbohyd. Polym. 2023;313 doi: 10.1016/j.carbpol.2023.120852. [DOI] [PubMed] [Google Scholar]
- 83.Ta L.P., Bujna E., Kun S., Charalampopoulos D., Khutoryanskiy V.V. Electrosprayed mucoadhesive alginate-chitosan microcapsules for gastrointestinal delivery of probiotics. Int. J. Pharmaceuts. 2021;597 doi: 10.1016/j.ijpharm.2021.120342. [DOI] [PubMed] [Google Scholar]
- 84.Tm M.W., Lau W.M., Khutoryanskiy V.V. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polymers. 2018;10(3):267. doi: 10.3390/polym10030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Madureira A.R., Pereira A., Pintado M. Current state on the development of nanoparticles for use against bacterial gastrointestinal pathogens. Focus on chitosan nanoparticles loaded with phenolic compounds. Carbohyd. Polym. 2015;130:429–439. doi: 10.1016/j.carbpol.2015.05.030. [DOI] [PubMed] [Google Scholar]
- 86.Lin Y.H., Feng C.L., Lai C.H., Lin J.H., Chen H.Y. Preparation of epigallocatechin gallate-loaded nanoparticles and characterization of their inhibitory effects on Helicobacter pylori growth in vitro and in vivo. Sci. Technol. Adv. Mater. 2014 doi: 10.1088/1468-6996/15/4/045006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Costa E., Silva S., Tavaria F., Pintado M. Insights into chitosan antibiofilm activity against methicillin-resistant Staphylococcus aureus. J. Appl. Microbiol. 2017;122(6):1547–1557. doi: 10.1111/jam.13457. [DOI] [PubMed] [Google Scholar]
- 88.Confederat L.G., Tuchilus C.G., Dragan M., Shaat M., Dragostin O.M. Preparation and antimicrobial activity of chitosan and its derivatives: a concise review. Molecules. 2021;26(12):3694. doi: 10.3390/molecules26123694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang H., Jung J., Zhao Y. Preparation, characterization and evaluation of antibacterial activity of catechins and catechins-Zn complex loaded β-chitosan nanoparticles of different particle sizes. Carbohyd. Polym. 2016;137:82–91. doi: 10.1016/j.carbpol.2015.10.036. [DOI] [PubMed] [Google Scholar]
- 90.Madureira A.R., Pereira A., Castro P.M., Pintado M. Production of antimicrobial chitosan nanoparticles against food pathogens. J. Food Eng. 2015;167:210–216. doi: 10.1016/j.jfoodeng.2015.06.010. [DOI] [Google Scholar]
- 91.Sun X., Jia P., Zhe T., Bu T., Liu Y., Wang Q., Wang L. Construction and multifunctionalization of chitosan-based three-phase nano-delivery system. Food Hydrocolloids. 2019;96:402–411. doi: 10.1016/j.foodhyd.2019.05.040. [DOI] [Google Scholar]
- 92.Shetta A., Kegere J., Mamdouh W. Comparative study of encapsulated peppermint and green tea essential oils in chitosan nanoparticles: encapsulation, thermal stability, in-vitro release, Nand antibacterial activities. Int. J. Biol. Macromol. 2019;126:731–742. doi: 10.1016/j.ijbiomac.2018.12.161. [DOI] [PubMed] [Google Scholar]
- 93.Pattnaik S., Barik S., Muralitharan G., Busi S. Ferulic acid encapsulated chitosan-tripolyphosphate nanoparticles attenuate quorum sensing regulated virulence and biofilm formation in Pseudomonas aeruginosa PAO1. IET Nanobiotechnol. 2018;12(8):1056–1061. doi: 10.1049/iet-nbt.2018.5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li F., Jin H., Xiao J., Yin X., Liu X., Li D. Q. The simultaneous loading of catechin and quercetin on chitosan-based nanoparticles as effective antioxidant and antibacterial agent. Food Res. Int. 2018;111:351–360. doi: 10.1016/j.foodres.2018.05.038. [DOI] [PubMed] [Google Scholar]
- 95.Zhang F., Wang B., Jie P., Zhu J., Cheng F. Preparation of chitosan/lignosulfonate for effectively removing Pb (II) in water. Polym. Adv. Technol. 2021;228 doi: 10.1016/j.polymer.2021.123878. [DOI] [Google Scholar]
- 96.Sharma S., Parmar A., Kori S., Sandhir R. PLGA-based nanoparticles: a new paradigm in biomedical applications. Trac. trend Anal. Chem. 2016;80:30–40. doi: 10.1016/j.trac.2015.06.014. [DOI] [Google Scholar]
- 97.Duranoglu D., Uzunoglu D., Mansuroglu B., Arasoglu T., Derman S. Synthesis of hesperetin-loaded PLGA nanoparticles by two different experimental design methods and biological evaluation of optimized nanoparticles. Nanotechnology. 2018;29(39) doi: 10.1088/1361-6528/aad111. http://doi/org 10.1088/1361-6528/aad111 [DOI] [PubMed] [Google Scholar]
- 98.Arasoğlu T., Derman S., Mansuroğlu B., Uzunoğlu D., Koçyiğit B.S., Gümüş B., Acar T., Tuncer B. Preparation, characterization, and enhanced antimicrobial activity: quercetin-loaded PLGA nanoparticles against foodborne pathogens. Turk. J. Biol. 2017;41(1):127–140. doi: 10.3906/biy-1604-80. [DOI] [Google Scholar]
- 99.ElHammadi M.M., Arias J.L. Recent advances in the surface functionalization of PLGA-based nanomedicines. Nanomaterials. 2022;12(3):354. doi: 10.3390/nano12030354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Danhier F., Ansorena E., Silva J.M., Coco R., Breton A.Le, Preat V. PLGA-based nanoparticles: an overview of biomedical applications. J. Contr. Release. 2012;161:505–522. doi: 10.1016/j.jconrel.2012.01.043. [DOI] [PubMed] [Google Scholar]
- 101.Ghitman J., Biru E.I., Stan R., Iovu H. Review of hybrid PLGA nanoparticles: future of smart drug delivery and theranostics medicine. Mater. Des. 2020;193 doi: 10.1016/j.matdes.2020.108805. [DOI] [Google Scholar]
- 102.Pereira M.C., Hill L.E., Zambiazi R.C., Mertens-Talcott S., Talcott S., Gomes C.L. Nanoencapsulation of hydrophobic phytochemicals using poly (dl-lactide-co-glycolide) (PLGA) for antioxidant and antimicrobial delivery applications: guabiroba fruit (Campomanesia xanthocarpa O. Berg) study. LWT--Food Sci. Technol. 2015;63(1):100–107. doi: 10.1016/j.lwt.2015.03.062. [DOI] [Google Scholar]
- 103.Zhi K., Raji B., Nookala A.R., Khan M.M., Nguyen X.H., Sakshi S., Pourmotabbed T., Yallapu M.M., Kochat H., Tadrous E. PLGA nanoparticle-based formulations to cross the blood-brain barrier for drug delivery: from R&D to cGMP. Pharmaceutics. 2021;13(4):500. doi: 10.3390/pharmaceutics13040500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu J., Zhang R., Xu Z.P. Nanoparticle-based nanomedicines to promote cancer immunotherapy: recent advances and future directions. Small. 2019;15(32) doi: 10.1002/smll.201900262. [DOI] [PubMed] [Google Scholar]
- 105.Deepika M.S., Thangam R., Sundarraj S., Sheena T.S., Sivasubramanian S., Kulandaivel J., Thirumurugan R. Co-delivery of diverse therapeutic compounds using PEG-PLGA nanoparticle cargo against drug-resistant bacteria: an improved anti-biofilm strategy. ACS Appl. Bio Mater. 2019;3(1):385–399. doi: 10.1021/acsabm.9b00850. [DOI] [PubMed] [Google Scholar]
- 106.Anbari H., Maghsoudi A., Hosseinpour M., Yazdian F. Acceleration of antibacterial activity of curcumin loaded biopolymers against methicillin-resistant Staphylococcus aureus: synthesis, optimization, and evaluation Eng. Life Sci. 2022;22(2):58–69. doi: 10.1002/elsc.202100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Trigo Gutierrez J.K., Zanatta G.C., Ortega A.L.M., Balastegui M.I.C., Sanita P.V., Pavarina A.C., Barbugli P.A. Mima, Encapsulation of curcumin in polymeric nanoparticles for antimicrobial photodynamic therapy. PLoS One. 2017;12(11) doi: 10.1371/journal.pone.0187418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gu X., Xu Z., Gu L., Xu H., Han F., Chen B., Pan X. Preparation and antibacterial properties of gold nanoparticles: a review. Environ. Chem. Lett. 2021;19(1):167–187. doi: 10.1007/s10311-020-01071-0. [DOI] [Google Scholar]
- 109.Meena J., Gupta A., Ahuja R., Singh M., Bhaskar S., Panda A.K. Inorganic nanoparticles for natural product delivery: a review. Environ. Chem. Lett. 2020;18(6):2107–2118. doi: 10.1007/s10311-020-01061-2. [DOI] [Google Scholar]
- 110.Jaiswal S., Mishra P.J.M.M. Antimicrobial and antibiofilm activity of curcumin-silver nanoparticles with improved stability and selective toxicity to bacteria over mammalian cells. Immunology. 2018;207(1):39–53. doi: 10.1007/s00430-017-0525-y. [DOI] [PubMed] [Google Scholar]
- 111.Song Z., Wu Y., Wang H., Han H. Synergistic antibacterial effects of curcumin modified silver nanoparticles through ROS-mediated pathways. Mater. Sci. Eng. C Mater. Biol. Appl. 2019;99:255–263. doi: 10.1016/j.msec.2018.12.053. [DOI] [PubMed] [Google Scholar]
- 112.Kim T.Y., Cha S.H., Cho S., Park Y. Tannic acid-mediated green synthesis of antibacterial silver nanoparticles. Arch Pharm. Res. (Seoul) 2016;39(4):465–473. doi: 10.1007/s12272-016-0718-8. [DOI] [PubMed] [Google Scholar]
- 113.Fei J., Zhao J., Du C., Wang A., Zhang H., Dai L., Li J. One-pot ultrafast self-assembly of autofluorescent polyphenol-based core@shell nanostructures and their selective antibacterial applications. ACS Nano. 2014;8(8):8529–8536. doi: 10.1021/nn504077c. [DOI] [PubMed] [Google Scholar]
- 114.Kim D.Y., Kim M., Shinde S., Sung J.S., Ghodake G. Cytotoxicity and antibacterial assessment of gallic acid capped gold nanoparticles. Colloids Surf., B. 2017;149:162–167. doi: 10.1016/j.colsurfb.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 115.Park S., Cha S.H., Cho I., Park S., Park Y., Cho S. Park, Antibacterial nanocarriers of resveratrol with gold and silver nanoparticles. Mater. Sci. Eng. C. Mater. Biol. Appl. 2016;58:1160–1169. doi: 10.1016/j.msec.2015.09.068. [DOI] [PubMed] [Google Scholar]
- 116.Onitsuka S., Hamada T., Okamura H. Preparation of antimicrobial gold and silver nanoparticles from tea leaf extracts. Colloids Surf., B. 2019;173:242–248. doi: 10.1016/j.colsurfb.2018.09.055. [DOI] [PubMed] [Google Scholar]
- 117.Liao Y., Yao Y., Yu Y., Zeng Y.J.C. Enhanced antibacterial activity of curcumin by combination with metal ions. Colloid Interface Sci. 2018;25:1–6. doi: 10.1016/j.colcom.2018.04.009. [DOI] [Google Scholar]
- 118.Li Q., Gao Y., Zhang J., Tang Y., Sun Y., Wu L., Wu H., Shen M., Liu X., Han L., Xu Z. Crosslinking and functionalization of acellular patches via the self-assembly of copper@tea polyphenol nanoparticles. Regen. Biomater. 2022;9 doi: 10.1093/rb/rbac030. rbac030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sadani K., Nag P., Pisharody L., Thian X.Y., Bajaj G., Natu G., Mukherji S., Mukherji S. Polyphenol stabilized copper nanoparticle formulations for rapid disinfection of bacteria and virus on diverse surfaces. Nanotechnology. 2021;33(3) doi: 10.1088/1361-6528/ac2e77. [DOI] [PubMed] [Google Scholar]
- 120.Park S., Cha S.H., Cho I., Park S., Park Y., Cho S., Park Y. Antibacterial nanocarriers of resveratrol with gold and silver nanoparticles. Mater. Sci. Eng. C. Mater. Biol. Appl. 2016;58:1160–1169. doi: 10.1016/j.msec.2015.09.068. [DOI] [PubMed] [Google Scholar]
- 121.Panahi Y., Mohammadhosseini M., Nejati-Koshki K., Abadi A.J., Moafi H.F., Akbarzadeh A., Farshbaf M. Preparation, surface properties, and therapeutic applications of gold nanoparticles in biomedicine. Drug Res. 2017;67(2):77–87. doi: 10.1055/s-0042-115171. [DOI] [PubMed] [Google Scholar]
- 122.Yadi M., Mostafavi E., Saleh B., Davaran S., Aliyeva I., Khalilov R., Nikzamir M., Nikzamir N., Akbarzadeh A., Panahi Y., Milani M. Current developments in green synthesis of metallic nanoparticles using plant extracts: a review. Artif. Cells, Nanomed. Biotechnol. 2018;46(sup3):S336–S343. doi: 10.1080/21691401.2018.1492931. [DOI] [PubMed] [Google Scholar]
- 123.Wigginton N.S., Titta A.d., Piccapietra F., Dobias J., Nesatyy V.J., Suter M.J., Bernier-Latmani R. Binding of silver nanoparticles to bacterial proteins depends on surface modifications and inhibits enzymatic activity. Environ. Sci. Technol. 2010;44(6):2163–2168. doi: 10.1021/es903187s. [DOI] [PubMed] [Google Scholar]
- 124.Podstawczyk D., Pawlowska A., Bastrzyk A., Czeryba M., Oszmianski J. Reactivity of (+)-catechin with copper (II) ions: the green synthesis of size-controlled Sub-10 nm copper nanoparticles. ACS Sustainable Chem. Eng. 2019;7(20):17535–17543. doi: 10.1021/acssuschemeng.9b05078. [DOI] [Google Scholar]
- 125.Liao Y., Yao Y., Yu Y., Zeng Y. Enhanced antibacterial activity of curcumin by combination with metal ions. Colloid Interface Sci. 2018;25:1–6. doi: 10.1016/j.colcom.2018.04.009. [DOI] [Google Scholar]
- 126.Li Y., Miao Y., Yang L., Zhao Y., Wu K., Lu Z., Hu Z., Guo J. Recent advances in the development and antimicrobial applications of metal-phenolic networks. Adv. Sci. 2022 doi: 10.1002/advs.202202684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang Z.Y., Sun Y., Zheng Y.D., He W., Yang Y.Y., Xie Y.J., Feng Z.X., Qiao K. A biocompatible bacterial cellulose/tannic acid composite with antibacterial and anti-biofilm activities for biomedical applications. Mater. Sci. Eng. C. Mater. Biol. Appl. 2020;106 doi: 10.1016/j.msec.2019.110249. [DOI] [PubMed] [Google Scholar]
- 128.Qin Y., Wang J., Qiu C., Hu Y., Xu X., Jin Z. Self-assembly of metal-phenolic networks as functional coatings for preparation of antioxidant, antimicrobial, and pH-sensitive-modified starch nanoparticles. ACS Sustainable Chem. Eng. 2019;7(20):17379–17389. doi: 10.1021/acssuschemeng.9b04332. [DOI] [Google Scholar]
- 129.Li D., Li J., Wang S., Wang Q., Teng W. Dually crosslinked copper-poly (tannic acid) nanoparticles with microenvironment-responsiveness for infected wound treatment. Adv. Healthc. Mater. 2023;12(17) doi: 10.1002/adhm.202203063. [DOI] [PubMed] [Google Scholar]
- 130.Wang Y., Zhou J., Yuan L., Wu F., Xie L., Yan X., Li H., Li Y., Shi L., Hu R., Liu Y. Neighboring carboxylic acid boosts peroxidase-like property of metal-phenolic nano-networks in eradicating streptococcus mutans biofilms. Small. 2023;19(3) doi: 10.1002/smll.202206657. [DOI] [PubMed] [Google Scholar]
- 131.Hussain N., Pu H., Sun D.W. Core size optimized silver coated gold nanoparticles for rapid screening of tricyclazole and thiram residues in pear extracts using SERS. Food Chem. 2021;350 doi: 10.1016/j.foodchem.2021.129025. [DOI] [PubMed] [Google Scholar]
- 132.Zhang C., Huang L., Sun D.W., Pu H. Interfacing metal-polyphenolic networks upon photothermal gold nanorods for triplex-evolved biocompatible bactericidal activity. Hazard. Mater. 2022;426 doi: 10.1016/j.jhazmat.2021.127824. [DOI] [PubMed] [Google Scholar]
- 133.Zhou X., Pu H., Sun D.W. DNA functionalized metal and metal oxide nanoparticles: principles and recent advances in food safety detection. Crit. Rev. Food Sci. Nutr. 2021;61(14):2277–2296. doi: 10.1080/10408398.2020.1809343. [DOI] [PubMed] [Google Scholar]
- 134.Yu Y., Li P., Zhu C., Ning N., Zhang S., Vancso G.J. Multifunctional and recyclable photothermally responsive cryogels as efficient platforms for wound healing. Adv. Funct. Mater. 2019;29(35) doi: 10.1002/adfm.201904402. [DOI] [Google Scholar]
- 135.Ahmed T., Wu Z., Jiang H., Luo J., Noman M., Shahid M., Manzoor I., Allemailem K.S., Alrumaihi F., Li B. Bioinspired green synthesis of zinc oxide nanoparticles from a native Bacillus cereus strain RNT6: characterization and antibacterial activity against rice panicle blight pathogens Burkholderia glumae and B. gladioli. Nanomaterials. 2021;11(4):884. doi: 10.3390/nano11040884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gudkov S.V., Burmistrov D.E., Serov D.A., Rebezov M.B., Semenova A.A., Lisitsyn A.B. A mini review of antibacterial properties of ZnO nanoparticles. Front.Phys. 2021;9 doi: 10.3389/fphy.2021.641481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lee J.M., Choi K.H., Min J., Kim H.J., Jee J.P., Park B.J. Functionalized ZnO nanoparticles with gallic acid for antioxidant and antibacterial activity against methicillin-resistant S. aureus. Nanomaterials. 2017;7(11):365. doi: 10.3390/nano7110365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ghaffari S.B., Sarrafzadeh M.H., Salami M., Khorramizadeh M.R. A pH-sensitive delivery system based on N-succinyl chitosan-ZnO nanoparticles for improving antibacterial and anticancer activities of curcumin. Int. J. Biol. Macromol. 2020;151:428–440. doi: 10.1016/j.ijbiomac.2020.02.141. [DOI] [PubMed] [Google Scholar]
- 139.Narayan R., Nayak U.Y., Raichur A.M., Garg S. Mesoporous silica nanoparticles: a comprehensive review on synthesis and recent advances. Pharmaceutics. 2018;10(3):118. doi: 10.3390/pharmaceutics10030118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dessai S., Ayyanar M., Amalraj S., Khanal P., Vijayakumar S., Gurav, Rarokar N., Kalaskar M., Nadaf S., Gurav S. Bioflavonoid mediated synthesis of TiO2 nanoparticles: characterization and their biomedical applications. Mater. Lett. 2022;311 doi: 10.1016/j.matlet.2021.131639. [DOI] [Google Scholar]
- 141.Gudkov S.V., Burmistrov D.E., Serov D.A., Rebezov M.B., Semenova A.A., Lisitsyn A.B. Do iron oxide nanoparticles have significant antibacterial properties? Antibiotics. 2021;10(7):884. doi: 10.3390/antibiotics10070884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Aliya S., Rethinasabapathy M., Yoo J., Kim E., Chung J.Y., Cha J.H., Huh Y.S. Phytogenic fabrication of iron oxide nanoparticles and evaluation of their in vitro antibacterial and cytotoxic activity. Arab. J. Chem. 2023;16(6) doi: 10.1016/j.arabjc.2023.104703. [DOI] [Google Scholar]
- 143.Ke X., Tang S., Dong Z., Wang H., Xu X., Qiu R., Yang J., Luo J., Li J. A silk fibroin based bioadhesive with synergistic photothermal-reinforced antibacterial activity. Int. J. Biol. Macromol. 2022;209:608–617. doi: 10.1016/j.ijbiomac.2022.03.136. [DOI] [PubMed] [Google Scholar]
- 144.Florek J., Caillard R., Kleitz F. Evaluation of mesoporous silica nanoparticles for oral drug delivery-current status and perspective of MSNs drug carriers. Nanoscale. 2017;9(40):15252–15277. doi: 10.1039/C7NR05762H. [DOI] [PubMed] [Google Scholar]
- 145.Vico T.A., Arce V.B., Fangio M.F., Gende L.B., Bertran C.A., Martire D.O., Churio M.S. Two choices for the functionalization of silica nanoparticles with gallic acid: characterization of the nanomaterials and their antimicrobial activity against Paenibacillus larvae. J. Nanopart. Res. 2016;18(11):1–13. doi: 10.1007/s11051-016-3652-2. [DOI] [Google Scholar]
- 146.Petrisor G., Ficai D., Motelica L., Trusca R.D., Birca A.C., Vasile B.S., Voicu G., Oprea O.C., Semenescu A., Ficai A., Popitiu M.I., Fierascu I., Fierascu R.C., Radu E.L., Matei L., Dragu L.D., Pitica I.M., Economescu M., Bleotu C. Mesoporous silica materials loaded with gallic acid with antimicrobial potential. Nanomaterials. 2022;12(10):1648. doi: 10.3390/nano12101648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Alswieleh A.M. Modification of mesoporous silica surface by immobilization of functional groups for controlled drug release. J. Chem. 2020;2020 doi: 10.1155/2020/9176257. [DOI] [Google Scholar]
- 148.Shabana M., Taju G., Majeed A., Karthika M., Ramasubramanian V., As S.H. Preparation and evaluation of mesoporous silica nanoparticles loaded quercetin against bacterial infections in Oreochromis niloticus. Aquacult Rep. 2021;21 doi: 10.1016/j.aqrep.2021.100808. [DOI] [Google Scholar]
- 149.Yang K., Han Q., Chen B., Zheng Y., Zhang K., Li Q., Wang J. Antimicrobial hydrogels: promising materials for medical application. Int. J. Nanomed. 2018;13:2217–2263. doi: 10.2147/IJN.S154748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Liu B., Li J., Zhang Z., Roland J.D., Lee B.P. pH responsive antibacterial hydrogel utilizing catechol-boronate complexation chemistry. Chem. Eng. J. 2022;441 doi: 10.1016/j.cej.2022.135808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Raghuwanshi V.S., Garnier G. Characterisation of hydrogels: linking the nano to the microscale. Adv. Colloid Interfac. 2019;274 doi: 10.1016/j.cis.2019.102044. [DOI] [PubMed] [Google Scholar]
- 152.George D., Maheswari P.U., Begum K.M.S. Synergic formulation of onion peel quercetin loaded chitosan-cellulose hydrogel with green zinc oxide nanoparticles towards controlled release, biocompatibility, antimicrobial and anticancer activity. Int. J. Biol. Macromol. 2019;132:784–794. doi: 10.1016/j.ijbiomac.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 153.Cao C., Yang N., Zhao Y., Yang D., Hu Y., Yang D., Song X., Wang W., Dong X. Biodegradable hydrogel with thermo-response and hemostatic effect for photothermal enhanced anti-infective therapy. Nano Today. 2021;39 doi: 10.1016/j.nantod.2021.101165. [DOI] [Google Scholar]
- 154.Tao B., Lin C., Yuan Z., He Y., Chen M., Li K., Hu J., Yang Y., Xia Z., Cai K. Near infrared light-triggered on-demand Cur release from Gel-PDA@Cur composite hydrogel for antibacterial wound healing. Chem. Eng. J. 2021;403 doi: 10.1016/j.cej.2020.126182. [DOI] [Google Scholar]
- 155.Ninan N., Forget A., Shastri V.P., Voelcker N.H., Blencowe A. Antibacterial and anti-inflammatory pH-responsive tannic acid-carboxylated agarose composite hydrogels for wound healing. ACS Appl. Mater. Interfaces. 2016;8(42):28511–28521. doi: 10.1021/acsami.6b10491. [DOI] [PubMed] [Google Scholar]
- 156.Hu B., Shen Y., Adamcik J., Fischer P., Schneider M., Loessner M.J., Mezzenga R. Polyphenol-binding amyloid fibrils self-assemble into reversible hydrogels with antibacterial activity. ACS Nano. 2018;12(4):3385–3396. doi: 10.1021/acsnano.7b08969. [DOI] [PubMed] [Google Scholar]
- 157.Wang Y., Wu Y., Long L., Yang L., Fu D., Hu C., Kong Q., Wang Y. Inflammation-responsive drug-loaded hydrogels with sequential hemostasis, antibacterial, and anti-inflammatory behavior for chronically infected diabetic wound treatment. ACS Appl. Mater. Interfaces. 2021;13(28):33584–33599. doi: 10.1021/acsami.1c09889. [DOI] [PubMed] [Google Scholar]
- 158.Ni Z., Yu H., Wang L., Huang Y., Lu H., Zhou H., Liu Q. Multistage ros-responsive and natural polyphenol-driven prodrug hydrogels for diabetic wound healing. ACS Appl. Mater. Interfaces. 2022;14(47):52643–52658. doi: 10.1021/acsami.2c15686. [DOI] [PubMed] [Google Scholar]
- 159.Wu Y., Wang Y., Long L., Hu C., Kong Q., Wang Y. A spatiotemporal release platform based on pH/ROS stimuli-responsive hydrogel in wound repairing. J. Contr. Release. 2022;341:147–165. doi: 10.1016/j.jconrel.2021.11.027. [DOI] [PubMed] [Google Scholar]
- 160.El-Aassar M., Ibrahim O.M., Al-Oanzi Z.H.J. Biotechnological applications of polymeric nanofiber platforms loaded with diverse bioactive materials. Polym. Adv. Technol. 2021;13(21):3734. doi: 10.3390/polym13213734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Huesca-Uriostegui K., Garcia-Valderrama E.J., Gutierrez-Uribe J.A., Antunes-Ricardo M., Guajardo-Flores D. Nanofiber systems as herbal bioactive compounds carriers: current applications in healthcare. Pharmaceutics. 2022;14(1):191. doi: 10.3390/pharmaceutics14010191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rahmati M., Mills D.K., Urbanska A.M., Saeb M.R., Venugopal J.R., Ramakrishna S., Mozafari M. Electrospinning for tissue engineering applications. Prog. Mater. Sci. 2021;117 doi: 10.1016/j.pmatsci.2020.100721. [DOI] [Google Scholar]
- 163.Xu Y., Li X., Xiang H.F., Zhang Q.Q., Wang X.X., Yu M., Hao L.Y., Long Y.Z. large-scale preparation of polymer nanofibers for air filtration by a new multineedle electrospinning device. J. Nanomater. 2020;2020 doi: 10.1155/2020/4965438. [DOI] [Google Scholar]
- 164.Hammami M.A., Krifa M., Harzallah O. Centrifugal force spinning of PA6 nanofibers-processability and morphology of solution-spun fibers. J. Text. Inst. 2014;105(6):637–647. doi: 10.1080/00405000.2013.842680. [DOI] [Google Scholar]
- 165.Daristotle J.L., Behrens A.M., Sandler A.D., Kofinas P. A review of the fundamental principles and applications of solution blow spinning. ACS Appl. Mater. Interfaces. 2016;8(51):34951–34963. doi: 10.1021/acsami.6b12994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Suzuki A., Shimba Y. Poly (l-lactic acid) twisted nanofiber yarn prepared by carbon dioxide laser supersonic multi-drawing. Eur. Polym. J. 2019;110:145–154. doi: 10.1016/j.eurpolymj.2018.11.028. [DOI] [Google Scholar]
- 167.Wang H., Hao L., Wang P., Chen M., Jiang S., Jiang S. Release kinetics and antibacterial activity of curcumin loaded zein fibers. Food Hydrocolloids. 2017;63:437–446. doi: 10.1016/j.foodhyd.2016.09.028. [DOI] [Google Scholar]
- 168.Perumal G., Pappuru S., Chakraborty D., Nandkumar A.M., Chand D.K., Doble M. Synthesis and characterization of curcumin loaded PLA-Hyperbranched polyglycerol electrospun blend for wound dressing applications. Mater. Sci. Eng. C. Mater. Biol. Appl. 2017;76:1196–1204. doi: 10.1016/j.msec.2017.03.200. [DOI] [PubMed] [Google Scholar]
- 169.Chouhan D., Janani G., Chakraborty B., Nandi S.K., Mandal Functionalized PVA-silk blended nanofibrous mats promote diabetic wound healing via regulation of extracellular matrix and tissue remodelling. J. Tissue Eng. Regen. M. 2018;12(3):e1559–e1570. doi: 10.1002/term.2581. [DOI] [PubMed] [Google Scholar]
- 170.Ghosal K., Manakhov A., Zajickova L., Thomas S. Structural and surface compatibility study of modified electrospun poly (ε-caprolactone) (PCL) composites for skin tissue engineering. AAPS PharmSciTech. 2017;18(1):72–81. doi: 10.1208/s12249-016-0500-8. [DOI] [PubMed] [Google Scholar]
- 171.Zhang L.H., Shen Q. Fully green poly (vinyl alcohol)/tea polyphenol composites and super anti-ultraviolet and -bacterial properties. Macromol. Mater. Eng. 2020;305(3) doi: 10.1002/mame.201900669. [DOI] [Google Scholar]
- 172.Fallah M., Bahrami S.H., Ranjbar-Mohammadi M. Fabrication and characterization of PCL/gelatin/curcumin nanofibers and their antibacterial properties. J. Ind. Text. 2016;46(2):562–577. doi: 10.1177/1528083715594978. [DOI] [Google Scholar]
- 173.Wang F., Sun Z., Yin J., Xu L. Preparation, characterization and properties of porous PLA/PEG/curcumin composite nanofibers for antibacterial application. Nanomaterials. 2019;9(4):508. doi: 10.3390/nano9040508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wang Y., Xu L. Preparation and characterization of porous core-shell fibers for slow release of tea polyphenols. Polymers. 2018;10(2):144. doi: 10.3390/polym10020144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sebe I., Szabo P., Kallai-Szabo B., Zelko R. Incorporating small molecules or biologics into nanofibers for optimized drug release: a review. Int. J. Pharm. 2015;494(1):516–530. doi: 10.1016/j.ijpharm.2015.08.054. [DOI] [PubMed] [Google Scholar]
- 176.Wu J., Liu F., Chen C., Zhao Z., Du Y., Shi X., Wu Y., Deng H. Long-term antibacterial activity by synergistic release of biosafe lysozyme and chitosan from LBL-structured nanofibers. Carbohyd. polym. 2023;(312) doi: 10.1016/j.carbpol.2023.120791. [DOI] [PubMed] [Google Scholar]
- 177.Wutticharoenmongkol P., Hannirojram P., Nuthong P. Gallic acid-loaded electrospun cellulose acetate nanofibers as potential wound dressing materials. Polym. Adv. Technol. 2019;30(4):1135–1147. doi: 10.1002/pat.4547. [DOI] [Google Scholar]
- 178.Lohith Kumar D., Sarkar P. Encapsulation of bioactive compounds using nanoemulsions. Environ. Chem. Lett. 2018;16(1):59–70. doi: 10.1007/s10311-017-0663-x. [DOI] [Google Scholar]
- 179.Garavand F., Jalai-Jivan M., Assadpour E., Jafari S.M. Encapsulation of phenolic compounds within nano/microemulsion systems: a review. Food Chem. 2021;364 doi: 10.1016/j.foodchem.2021.130376. [DOI] [PubMed] [Google Scholar]
- 180.Jiang S.P., He S.N., Li Y.L., Feng D.L., Lu X.Y., Du Y.Z., Yu H.Y., Hu F.Q., Yuan H. Preparation and characteristics of lipid nanoemulsion formulations loaded with doxorubicin. Int. J. Nanomed. 2013;8:3141. doi: 10.2147/IJN.S47708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hwang Y.Y., Ramalingam K., Bienek D.R., Lee V., You T., Alvarez R. Antimicrobial activity of nanoemulsion in combination with cetylpyridinium chloride in multidrug-resistant Acinetobacter Baumannii. Antimicrob. Agents Ch. 2013;57(8):3568–3575. doi: 10.1128/aac.02109-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ghosh V., Mukherjee A., Chandrasekaran N. Eugenol-loaded antimicrobial nanoemulsion preserves fruit juice against, microbial spoilage. Colloid. Surface B. 2014;114:392–397. doi: 10.1016/j.colsurfb.2013.10.034. [DOI] [PubMed] [Google Scholar]
- 183.McClements D.J., Xiao H. Potential biological fate of ingested nanoemulsions: influence of particle characteristics. Food Funct. 2012;3(3):202–220. doi: 10.1039/c1fo10193e. [DOI] [PubMed] [Google Scholar]
- 184.Joung H.J., Choi M.J., Kim J.T., Park S.H., Park H.J., Shin G.H. Development of food-grade curcumin nanoemulsion and its potential application to food beverage system: antioxidant property and in vitro digestion. J. Food Sci. 2016;81(3):N745–N753. doi: 10.1111/1750-3841.13224. [DOI] [PubMed] [Google Scholar]
- 185.Kaur A., Gabrani R., Dang S. Nanoemulsions of green tea catechins and other natural compounds for the treatment of urinary tract infection: antibacterial analysis. Adv. Pharm. Bull. 2019;9(3):401–408. doi: 10.15171/apb.2019.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Letsididi K.S., Lou Z., Letsididi R., Mohammed K., Maguy B.L. Antimicrobial and antibiofilm effects of trans-cinnamic acid nanoemulsion and its potential application on lettuce. LWT--Food Sci. Technol. 2018;94:25–32. doi: 10.1016/j.lwt.2018.04.018. [DOI] [Google Scholar]
- 187.Singh Y., Meher J.G., Raval K., Khan F.A., Chaurasia M., Jain N.K., Chourasia M.K. Nanoemulsion: concepts, development and applications in drug delivery. J. Contr. Release. 2017;252:28–49. doi: 10.1016/j.jconrel.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 188.Kour P., Afzal S., Gani A., Zargar M.I., Tak U.N., Rashid S., Dar A.A. Effect of nanoemulsion-loaded hybrid biopolymeric hydrogel beads on the release kinetics, antioxidant potential and antibacterial activity of encapsulated curcumin. Food Chem. 2022;376 doi: 10.1016/j.foodchem.2021.131925. [DOI] [PubMed] [Google Scholar]
- 189.Y. Wang, Y. Yang, Y. Shi, H. Song, C. Yu, Antibiotic-free antibacterial strategies enabled by nanomaterials: progress and perspectives, Adv. Mater. 32 (18) 1904106, 10.1080/14712598.2017.1315402. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.