Abstract
Objectives
Almost all patients with systemic sclerosis (SSc) harbour autoantibodies. Anti-topoisomerase antibodies (ATA) and anti-centromere antibodies (ACA) are most prevalent and associate with distinct clinical phenotypes. B cell responses underlying these phenotypes are ill-defined. To understand how B cell autoreactivity and disease pathology connect, we determined phenotypic and functional characteristics of autoreactive B cells in ATA-positive and ACA-positive patients.
Methods
Levels and isotypes of autoantibodies secreted by ex vivo cultured peripheral blood mononuclear cells from patients with ATA-positive (n=22) and ACA-positive (n=20) SSc were determined. Antibody secreting cells (ASCs) were isolated by cell sorting and cultured separately. Correlations were studied between the degree of spontaneous autoantibody production and the presence and degree of interstitial lung disease (ILD).
Results
Circulating B cells secreting either ATA-immunoglobulin G (IgG) or ACA-IgG on stimulation was readily detectable in patients. The ATA response, but not the ACA response, showed additional secretion of autoreactive IgA. ATA-IgG and ATA-IgA were also secreted spontaneously. Additional cell sorting confirmed the presence of ATA-secreting plasmablasts. The degree of spontaneous ATA-secretion was higher in patients with ILD than in those without (p<0.001) and correlated with the degree of pulmonary fibrosis (p<0.001).
Conclusion
In contrast to ACA-positive patients, ATA-positive patients show signs of recent activation of the B cell response that hallmarks this disease. The degree of activation correlates with the presence and severity of ILD, the most deleterious disease manifestation. This could explain differential responsiveness to B cell depleting therapy. The abundant and spontaneous secretion of ATA-IgG and ATA-IgA may point toward a continuously activating trigger.
Keywords: scleroderma, systemic; autoimmunity; B-lymphocytes; pulmonary fibrosis
WHAT IS ALREADY KNOWN ON THIS TOPIC
Deranged B cell immunity is one of the hallmarks of SSc; different autoantibody reactivities associate with distinct clinical phenotypes.
Autoreactive B cells have been detected in patients but it is unclear how such responses are triggered and whether and how such cells are involved in disease pathology.
WHAT THIS STUDY ADDS
B cells against topoisomerase-1 are not just present but circulate as activated, immunoglobulin G (IgG) expressing and IgA-expressing memory B cells and plasmablasts; the degree of activation (plasmablast differentiation) of this B cell response associates with presence and severity of ILD.
In contrast, the anti-centromere B cell response shows far less of this immunological activity and lacks an active IgA compartment.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Differential immunological (B cell) activity in SSc patients may help to explain patient heterogeneity, identify patients that could benefit from specific (B cell targeting) interventions, and inform research on potential triggers that activate the autoreactive B cell compartment.
Introduction
Systemic sclerosis (SSc) is an orphan autoimmune disease, characterised by microangiopathy, immune dysregulation and fibrosis of the skin and visceral organs.1 SSc has the highest mortality burden among the rheumatic diseases and displays remarkable clinical heterogeneity. Disease course can vary from rapidly progressive disease with multiple organs involved to indolent courses with little or no deterioration over time. Currently, the mechanisms responsible for this heterogeneity are poorly understood, nor are there distinctive biomarkers available to reliably predict different phenotypes or to aid in the choice of treatment. A better understanding of the underlying immunological abnormalities and, especially, the differences in disease course between patients, is crucial for improved and more personalised treatment and for a deeper understanding of disease pathogenesis.
Over 95% of patients with SSc harbour antinuclear autoantibodies (ANA), including anti-topoisomerase-1 antibodies (ATA), anti-centromere antibodies (ACA) and anti-RNA polymerase III antibodies. These autoantibodies rarely coexist (<2%), with ATA and ACA being most prevalent.2 3 ATA-immunoglobulin G (IgG) and ACA-IgG associate with distinct clinical phenotypes.2–5 ATA-IgG associate with diffuse skin involvement (diffuse cutaneous SSc, dcSSc), interstitial lung disease (ILD) and a progressive disease course. ACA-IgG associate with limited skin involvement (limited cutaneous SSc), involvement of the gastrointestinal tract and a relatively mild prognosis. SSc-specific autoantibodies can be found before the onset of clinical disease. In several cohorts, levels of individual isotypes were found to associate with risk for disease progression.6 7 In individuals with Raynaud’s phenomenon, the risk of SSc development increases strongly when disease-specific ANA are present.8 Despite these observations, patient stratification based on autoantibody type is limited, as heterogeneity remains high even within these autoantibody-defined subgroups.
The diagnostic and prognostic value of ANA in SSc and their disease-specificity suggest a role for autoreactive B cells in disease pathogenesis. This notion is supported by data from murine disease models and by the therapeutic effects of rituximab, a B cell depleting antibody.9–13 ATA-expressing B cells have been detected in the circulation of patients with SSc using in vitro peripheral blood mononuclear cells (PBMC) and T/B cell coculture systems.14–16 In these studies, topoisomerase-1 (top1) and pokeweed mitogen, a strong B cell activator, were both required to induce and detect ATA production. ATA production was limited to the IgG isotype, even though ATA-IgA and ATA-IgM can be present in the serum of patients.17 18 Follow-up work indicated that the induction of ATA-IgG requires CD4+ T cell help and depends on B/T cell interaction via HLA-DR and CD40L. These studies suggested that the ATA response primarily originates from T cell dependent, germinal centre (GC) derived immune responses. When, to what extent and at which anatomical site such responses occur in patients are unknown. However, active GC responses usually lead to the differentiation of B cells into (mostly short-lived) antibody secreting cells (ASCs; plasmablasts) capable of spontaneously secreting (auto-)antibodies.19 20 Such cells appear transiently in the circulation and can be seen as a proxy for recent, antigen-specific stimulation of the underlying B cell response.19 Indeed, we and others found evidence for such antigen-specific stimulation events of autoreactive B cells in patients with rheumatoid arthritis (RA).21 22 The activated B cell compartment in RA appeared dynamic with regard to disease status and activity, indicating that it may reflect a measure of immunological disease activity that is insufficiently reflected by serum autoantibodies or their levels.23
Here, we set out to study the functional activity and phenotypic characteristics of autoreactive B cell responses in SSc and their relationship with clinical parameters. We hypothesised that also in SSc, autoreactive B cells could show dynamic degrees of activation, which, if linked to clinical phenotype, could be informative as to disease-relevant pathogenetic mechanisms and the identification of patients that might specifically benefit from B cell targeted interventions. We studied ATA and ACA secretion by freshly isolated, ex vivo cultured PBMC from ATA-positive and ACA-positive patients and found remarkable differences between these groups. ATA-positive patients harboured ATA-IgG and ATA-IgA expressing ASCs, indicating recent activation. The ACA-expressing B cell response appeared less activated and lacked the active IgA compartment. In patients with ILD, the degree of spontaneous ATA-IgG secretion was substantially higher than in those without. These findings provide insights into differences in immunological activity between individual patients with SSc that are related to clinical outcome and that might help to guide therapeutic concepts. In addition, the active IgA compartment in ATA-positive patients suggests a mucosal trigger of the ATA response, warranting further research into its origin and the factors that drive these autoreactive responses in individual patients.
Methods
Patients and healthy donors
Peripheral blood was obtained from patients with SSc (n=42) with established disease visiting the outpatient clinic of the Department of Rheumatology at Leiden University Medical Center. All patients with SSc were part of a prospective cohort study (the Leiden Combined Care in Systemic Sclerosis (CCISS) cohort) and fulfilled the ACR/EULAR 2013 classification criteria for SSc.24 25 Exclusion criteria were the use of rituximab and a history of haematopoietic stem cell transplantation. All donors, including the controls (patients with RA and healthy controls (HC)), gave written informed consent for sample acquisition. Patient characteristics are detailed in table 1. More information on immunomodulatory medication is provided in online supplemental table 1. The presence/absence and severity of ILD were determined by high resolution CT (HRCT). Fibrosis scores for ATA-positive patients with lung fibrosis (n=14) were assessed based on HRCT images by one independent investigator (LS), a thoracic radiologist experienced in the evaluation of chest imaging in patients with connective tissue diseases, who was blinded to the outcome of the cell cultures. The following five levels were examined: (1) origin of great vessels, (2) main carina, (3) pulmonary venous confluence, (4) halfway between the third and fifth section and (5) immediately above the right hemi-diaphragm. The mean fibrosis score of the five areas was calculated according to Goh et al and used for the analyses.26 27
Table 1.
Clinical characteristics of included SSc patients (n=42)
| Total N=42 |
ATA N=22 |
ACA N=20 |
|
| Age, years, mean (SD) | 57 (14) | 52 (16) | 62 (11) |
| Female, n (%) | 30 (71%) | 12 (55%) | 18 (90%) |
| Duration since first non-Raynaud’s phenomenon symptom, months, median (IQR) | 98 (50–160) | 63 (39–102) | 120 (95–221) |
| Diffuse cutaneous subset, n (%) | 7 (17%) | 7 (32%) | – |
| mRSS, median (IQR) | 5 (2-7) | 5 (2-7) | 3 (0–8) |
| ILD on HRCT, n (%) | 14 (33%) | 14 (64%) | – |
| Renal crisis, n (%) | – | – | – |
| Pulmonary hypertension, n (%) | 2 (5%) | – | 2 (10%) |
| Current immunosuppressive use, n (%) | 18 (43%) | 12 (55%) | 6 (30%) |
Clinical characteristics were taken from the visit of the sample collection.
Immunosuppressive use included corticosteroids, azathioprine, methotrexate, mycophenolate mofetil, infliximab and hydroxychloroquine (see online supplemental table 1 for details).
ACA, anti-centromere antibodies; ATA, anti-topoisomerase antibodies; HRCT, high resolution CT; ILD, interstitial lung disease; mRSS, modified Rodnan Skin Score; SSc, systemic sclerosis.
rmdopen-2023-003148supp001.pdf (1.9MB, pdf)
Cells and culture conditions
PBMC were isolated directly on blood sampling using Ficoll-Paque gradient centrifugation. To examine antibody secretion on stimulation, PBMC were cultured in 96-well flat bottom plates at a cell density of 2×105/well in Iscove’s Modified Dulbecco’s Medium (IMDM) supplemented with 10% heat-inactivated fetal calf serum, 100 U/mL penicillin/streptomycin and 2 mM Glutamax on a layer of irradiated (7000 RAD) CD40L-expressing mouse fibroblasts in the presence of 100 ng/mL IL-21 (ThermoFisher Scientific) and 100 ng/mL B cell activating factor (BAFF, Miltenyi Biotec) (‘with stimulation’). To examine spontaneous antibody secretion, PBMC were cultured in 96-well flat bottom plates at a cell density of 2×105/well in IMDM supplemented with 10% heat-inactivated fetal calf serum, 100 U/mL penicillin/streptomycin and 2 mM Glutamax without CD40L-expressing mouse fibroblasts and without additional cytokines (‘without stimulation’). For both conditions, supernatants were harvested after 7 days of culture. PBMC were available for additional flow cytometric analysis of B cell subsets for a subset of donors (15/22 SSc ATA-positive patients and 12/20 SSc ACA-positive patients). These PBMC were stained with anti-CD3 (UCHT1, Biolegend), anti-CD14 (M5E2, Biolegend), anti-CD19 (SJ25C1, BD Biosciences), anti-CD20 (2H7, Biolegend), anti-CD27 (M-T271, BD Biosciences), anti-IgG (G18-145, BD Biosciences), anti-IgD (IA6-2, BD Biosciences) and a LIVE/DEAD cell stain kit (ThermoFisher Scientific) and subsequently analysed on an LSR Fortessa (BD Biosciences).
For some experiments, B cell subsets were isolated using a fluorescence activated cell sorter (FACS, Aria III, BD Biosciences). The antibody panel consisted of anti-CD3 (UCHT1, Biolegend), anti-CD14 (M5E2, Biolegend), anti-CD19 (SJ25C1, BD Biosciences), anti-CD20 (2H7, Biolegend), anti-CD27 (M-T271, BD Biosciences) and a LIVE/DEAD cell stain kit (ThermoFisher Scientific). ASCs were defined as LIVE/DEAD-CD3-CD14-CD19+CD20-CD27++ and cultured in 96-well round bottom plates in IMDM supplemented as described above at a maximal cell density of 1×104 cells/well without stimulation. PBMC depleted of ASCs were cultured as described before. Culture supernatants were collected after 13–14 days.
Antibody measurements
Levels of ATA-IgG and ACA-IgG and ACA-IgA in culture supernatant and patient plasma were measured using an in-house ELISA. In short, Corning 384-well microplates (Sigma Aldrich) were coated with recombinant human top1 (ENZ-306, Prospec) or Centromere Protein B (CENP-B, the major reactive antigen of ACA, which is used for diagnostic tests28) (PRO-390, Prospec). Supernatants were tested at a 1:2 dilution, plasma between 1:100 and 1:4000. Antibody binding was detected with horseradish peroxidase-conjugated rabbit-anti-human IgG (P0214, DAKO) or goat-anti-human IgA (A18781, Novex) and subsequently visualised using 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS). Arbitrary units were calculated using titrations of a reference serum pool. For culture supernatants, a cut-off was calculated per experiment based on the average plus two times the SD of all control wells, separately for stimulated and unstimulated conditions. For plasma, a cut-off was calculated based on the average plus two times the SD of all control plasma samples. We did not assess ATA-IgM and ACA-IgM levels in supernatants as the assay to detect this isotype did not yield reliable results above background in our hands. Total IgA and IgG concentrations were assessed by ELISA according to the manufacturer’s protocol (Bethyl Laboratories).
Statistical analysis
Statistical analyses were performed using GraphPad Prism V.9.3.1 for Windows (GraphPad Software, San Diego, CA, USA, www.graphpad.com) and IBM SPSS Statistics for Windows V.25.0. Mann-Whitney U test was used for determining statistical difference between two groups, correlations were assessed by Spearman correlation.
Results
Presence of ATA-isotype and ACA-isotype expressing B cells in the circulation of patients with SSc
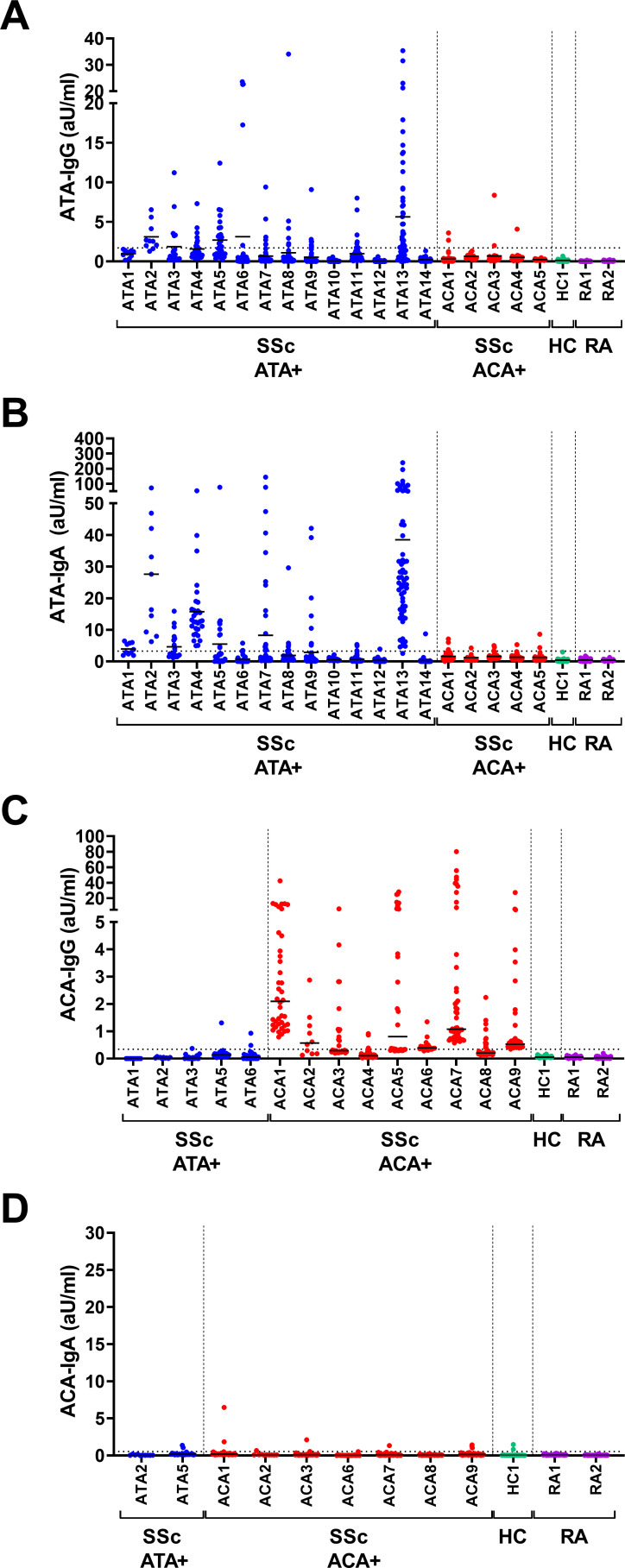
To approach the autoreactive B cell compartment, we first aimed to assess the presence of B cells capable of secreting different ATA and ACA isotypes in peripheral blood. PBMC from ATA-IgG or ACA-IgG positive patients with SSc with established disease were cultured in the presence of CD40L-expressing fibroblasts, IL-21 and BAFF directly on ex vivo isolation.14 15 Frequencies of naive and memory B cells in the starting population were determined by flow cytometry (online supplemental figure 1).29 Both ATA-IgG and ATA-IgA were readily detectable in culture supernatants on stimulation (figure 1A,B). In contrast, PBMC from ACA-positive patients secreted ACA-IgG, while ACA-IgA was practically undetectable (figure 1C,D). The stimulation conditions did induce the secretion of total IgA (online supplemental figure 2). Furthermore, six of the seven ACA-IgG-positive patients tested (figure 1D) were ACA-IgA positive in serum, and ACA-IgA could be measured in highly diluted serum with our assay (dilutions of up to 1:10,000; online supplemental figure 3). Of note, we did not detect apparent differences between treated or untreated patients in the capacity of cells to produce autoantibodies (online supplemental figure 4). Detection of IgM isotypes of either specificity was technically not reliable in patients versus controls and, therefore, omitted from further analysis. Autoantibody secretion was limited to PBMC cultures from patients with SSc and undetectable in patients with RA or in the HC. Incidental wells of ACA-positive patients with SSc showed weak positivity for ATA-IgG and vice versa. The frequencies and levels of such wells were very low. Patients with SSc harbouring more than one autoantibody specificity have been described, although at very low frequency.2 The patients tested here were negative for the respective other autoantibody in serum.
Figure 1.
IgG and IgA ATA and ACA in cell culture supernatants on in vitro PBMC stimulation. PBMC from patients with ATA+SSc, ACA+SSc, HC and RA were cultured on ex vivo isolation in 96-well flat bottom plates at a cell density of 2×105/well in the presence of CD40L-expressing fibroblasts, IL-21 and BAFF. Culture supernatants were assessed for the presence of ATA-IgG (A), ATA-IgA (B), ACA-IgG (C) and ACA-IgA (D) by ELISA after 7 days of culture. Each column represents one patient, each dot one culture well. Lines depict median levels of secretion. Dotted line represents the cut-off based on the mean plus two times the SD of measurements of all controls. Average of 30 wells/donor (10–50 wells/donor). ACA, anti-centromere antibodies; ATA, anti-topoisomerase antibodies; BAFF, B cell activating factor; HC, healthy controls; IgG, immunoglobullin G; IL-21, interleukin 21; PBMC, peripheral blood mononuclear cells; RA, rheumatoid arthritis; SSc, systemic sclerosis.
Together, these findings show the presence of autoreactive ATA-IgG-expressing and ATA-IgA-expressing B cells in the peripheral blood of ATA-positive patients. In the ACA-positive patients tested, the immune response appeared to be dominated by ACA-IgG-expressing B cells, while cells expressing ACA-IgA were practically absent.
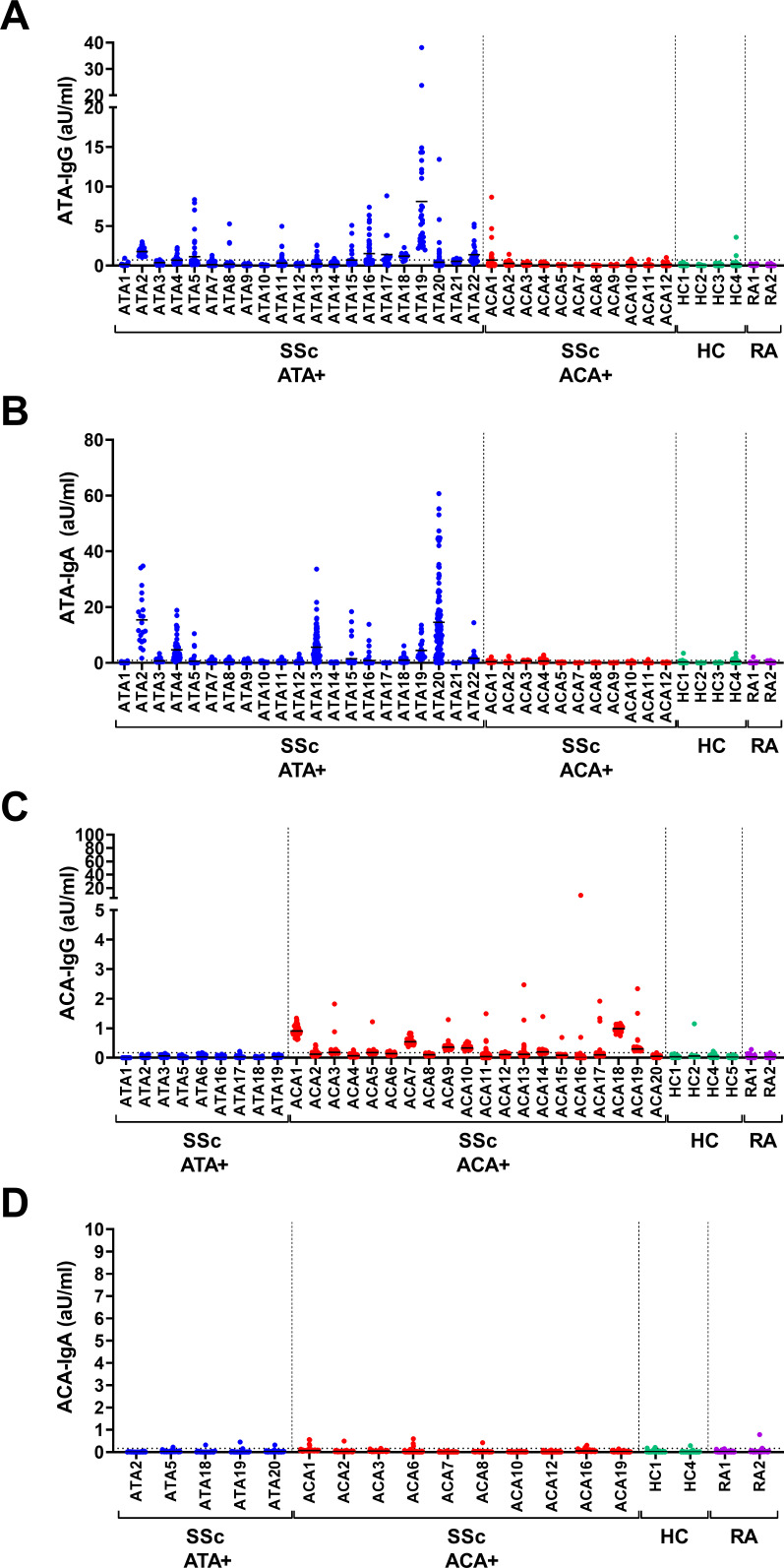
PBMC derived from ATA-positive patients produce high levels of ATA-IgG and ATA-IgA spontaneously
Antigen-specific stimulation of B cells typically results in the induction of mostly short-lived ASCs that are also detectable in peripheral blood. In the absence of antigen or other stimulatory triggers, B cells circulate in a resting state, with ASCs being usually absent.19 20 Therefore, the presence of antigen-specific ASCs can be seen as a sign of recent (or continuous) B cell activation. To assess the presence of autoreactive ASCs, PBMC from ATA-positive and ACA-positive patients were isolated and cultured without additional stimulation. Both ATA-IgG and ATA-IgA were spontaneously secreted in concentrations largely comparable to the conditions with stimulation (figure 2A,B). As before, this secretion was specific for ATA-positive patients and not observed for samples derived from ACA-positive patients or the respective controls. In contrast, spontaneous secretion of ACA-IgG was observed at much lower levels (median: 0.13 aU/mL, range: 0–0.93 aU/mL) than the secretion after stimulation (median: 0.53 aU/mL, range: 0.10–2.06 aU/ml) (figure 2C, p=0.006). As in the stimulated cultures, secretion of ACA-IgA was practically undetectable (figure 2D). Again, we did not detect differential secretion of autoantibodies by PBMC from patients with immunosuppressive treatment compared with those without (online supplemental figure 5). Additionally, we observed a correlation between the secretion of ATA-IgG and ATA-IgA and plasma levels of either isotype (online supplemental figure 6A,B). Of note, the amount of spontaneous secretion of ATA-IgG and ATA-IgA in individual donors did not correlate (online supplemental figure 6C). Finally, the spontaneous secretion of ATA-IgA by unstimulated PBMC did not reflect an enhanced secretion of total IgA (online supplemental figure 7), nor was there a difference in secretion of total IgA by unstimulated PBMC in ATA-positive and ACA-positive patients (online supplemental figure 2). This suggests an expansion of, specifically, the ATA-IgA and ATA-IgG compartment within the total ASC population. Together, these data indicate the presence of ATA-expressing ASCs of both isotypes (IgG and IgA) in the circulation of some but not all ATA-positive patients. In ACA-positive patients, the frequency and/or capacity of ACA-IgG-expressing ASCs to secrete autoantibodies appears low, while ACA-IgA-expressing ASCs were undetectable.
Figure 2.
Spontaneous secretion of IgG and IgA ATA and ACA in cell culture supernatants on in vitro PBMC culture. PBMC from patients with ATA+SSc, ACA+SSc, HC and RA were cultured on ex vivo isolation in 96-well flat bottom plates at a cell density of 2×105/well without CD40L-expressing fibroblasts or cytokines. Culture supernatants were assessed for the presence of ATA-IgG (A), ATA-IgA (B), ACA-IgG (C) and ACA-IgA (D) by ELISA after 7 days of culture. Each column represents one patient, each dot one culture well. Lines depict median levels of secretion. Dotted line represents the cut-off based on the mean plus two times the SD of measurements of all controls. Average of 43 wells/donor (10–120 wells/donor). ACA, anti-centromere antibodies; ATA, anti-topoisomerase antibodies; HC, healthy controls; IgG, immunoglobullin G; IL-21, interleukin 21; PBMC, peripheral blood mononuclear cells; RA, rheumatoid arthritis; SSc, systemic sclerosis.
To further substantiate the findings, we analysed the presence of ATA-secreting ASCs within the expected B cell compartment using FACS. ASCs (defined as CD3-CD14-CD19+CD20-CD27++) were separated from freshly isolated PBMC and cultured separately without stimulation. ATA-IgG and ATA-IgA secretion was assessed after 2 weeks of culture. While spontaneous secretion of ATA-IgG was low in the ASCs compartment of the two donors tested, the secretion of ATA-IgA was remarkable (online supplemental figure 8). Also, we noted spontaneous secretion of ATA-IgA despite depletion of ASCs from PBMC, indicating the presence of memory B cells differentiating to ATA-secreting ASCs in culture. The frequency of total ASCs (as determined by flow cytometry) and the degree of spontaneous ATA secretion did not correlate, suggesting an antigen-specific expansion of the ATA-Ig compartment within the total ASC population (online supplemental figure 9). These data support the notion that ASCs and/or activated memory B cells differentiating toward ASCs circulate in peripheral blood, suggesting recent, repetitive or continuous activation of the top1-specific immune response in individual patients.
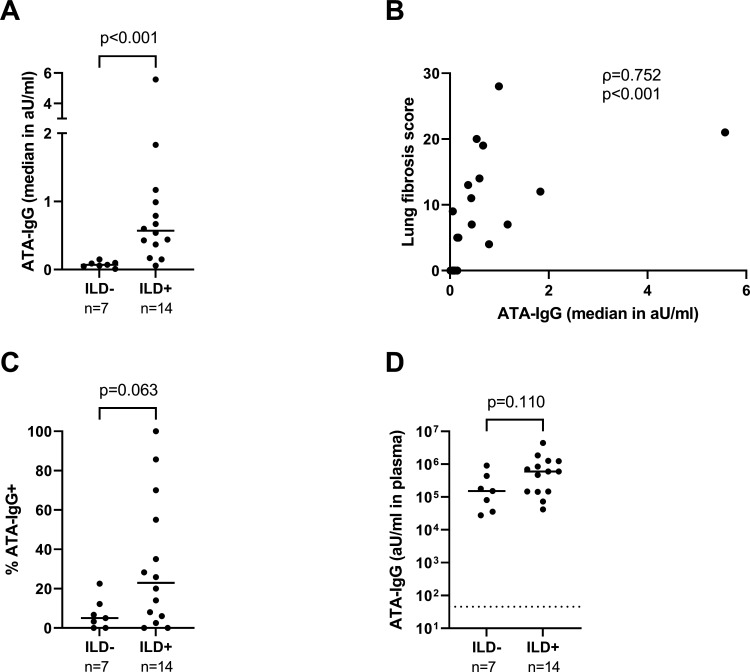
The degree of spontaneous ATA-IgG secretion associates with the presence and degree of pulmonary fibrosis in ATA-positive patients
B cell contribution to disease pathology in human SSc is still incompletely understood. In vivo murine studies and in vitro assays suggest that B cells enhance and/or promote fibroblast activation.9 10 13 30–32 Therapeutic B cell depletion positively affects fibrosis of skin and lungs in most but not all patients with SSc and ameliorates pulmonary fibrosis also in a range of other fibrotic disorders.33–36 ATA-positive patients are prone to develop ILD and B cells have been detected in lung tissue biopsies of patients with SSc.5 36–38 Based on these observations, we hypothesised that the active, autoreactive B cell response we observed in some patients could specifically associate with the presence of ILD and/or the degree of pulmonary fibrosis in ATA-positive patients. Therefore, we assessed the spontaneous secretion of ATA-IgG and ATA-IgA in patients with ILD (n=14) or without ILD (n=7) and assessed the presence and degree of pulmonary fibrosis by HRCT. The latter was assessed as fibrosis score according to the method developed by Goh et al.26 27 Interestingly, the degree of spontaneous ATA-IgG secretion (assessed as the median ATA-IgG concentration secreted per patient in all culture wells of that patient) was significantly higher in patients with ILD than in those without (figure 3A, online supplemental figure 10). Also, the capacity to secrete autoantibodies correlated strongly with the degree of pulmonary fibrosis (figure 3B). The percentage of ATA-IgG positive wells (a measure reflecting the frequency of ATA-IgG ASCs in the circulation) followed a similar trend but did not reach statistical significance (p=0.063, figure 3C). Of note, we observed only a non-significant trend toward higher ATA-IgG plasma levels in donors with ILD compared with those without (figure 3D). Finally, although highly active in individual patients, both frequency and degree of spontaneous ATA-IgA secretion did not correlate with (the degree of) ILD (online supplemental figure 11), nor did the secretion of ATA-IgG or IgA correlate convincingly with skin fibrosis as assessed by modified Rodnan Skin Score (online supplemental figure 12).
Figure 3.
Spontaneous secretion of ATA-IgG by PBMC in relation to the presence and severity of ILD. PBMC from ATA+SSc patients with ILD (n=14) or without ILD (n=7) were cultured without stimulation. ATA-IgG was measured in culture supernatants by ELISA after 7 days. (A) Levels of ATA-IgG (median in aU/mL) were compared between groups. (B) Levels of ATA-IgG produced spontaneously (median in aU/mL) in relation to the lung fibrosis score. (C) Percentage of wells positive for ATA-IgG were compared between groups. (D) ATA-IgG plasma levels in the same patients. Lines depict median values. Dotted line represents the cut-off based on the mean plus two times the SD of measurements of HC (n=11) and patients with RA (n=3). P values and Spearman correlation (ρ) are shown. ATA, anti-topoisomerase antibodies; HC, healthy controls; IgG, immunoglobulin G; ILD, interstitial lung disease; PBMC, peripheral blood mononuclear cells; SSc, systemic sclerosis.
Discussion
The clinical heterogeneity of SSc and its poor prognostic stratification of patients pose a major clinical problem. Immunological processes driving disease and individual disease manifestations are still incompletely understood. Here, we provide evidence for the differential regulation of the two major autoreactive B cell responses representing disease-associated antigen specificities in SSc, those against top1 and CENP-B. This differential regulation on the cellular level may reflect distinct triggers and pathogenetic mechanisms underlying these responses in individual patients and may reflect a previously unexplored layer of immunological disease activity and/or disease phenotype.
In our experiments, ATA-expressing and ACA-expressing B cells differed considerably in phenotype and function. Our results confirm, in part, previous research on the ATA B cell response,14–16 as we also found ATA-IgG-expressing B cells in the circulation. Previous work, however, did not identify an active ATA-IgA compartment. In addition, spontaneous ATA-IgG and ATA-IgA secretion, a measure of recent B cell activation, has not been previously described. Several factors could contribute to these differences. Especially the use of freshly isolated PBMC in our study as opposed to the use of frozen samples in previous work could be relevant, as freeze/thaw cycles compromise the ASC compartment. Also, differences in culture conditions and the overall sensitivity of the detection system may have influenced detectability of individual isotypes. Our data suggest that the ACA response misses a detectable IgA B cell compartment in the circulation, while this was readily detectable for ATA-expressing B cells. Whether ACA-IgA-expressing B cells are indeed absent from the circulation of ACA-positive patients or rather poorly responsive to the stimulation approach chosen here remains to be determined. As ACA-IgA was present in serum of most donors tested, however, it is conceivable that such cells are generated as part of the ACA response. Possibly, such cells primarily reside in tissues as, for example, terminally differentiated plasma cells, while the naïve or memory compartments are rarely triggered. Together, these findings point to distinct dynamics of both autoreactive B cell responses, which might help to understand and identify their underlying triggers. In particular, the prominent ATA-IgA response could reflect continuous immune activation at mucosal sites.
Both PBMC culture and cell sorting experiments showed that, in contrast to the ACA B cell response, ASCs are an important component of the circulating ATA B cell response. Such cells are typically found in the circulation following antigen encounter during GC reactions or on extrafollicular triggering by antigen and danger signals such as DNA or RNA.19 39 ASCs remain in blood only temporarily, however, and are thought to either die or home to sites of inflammation and/or secondary lymphoid organs.19 20 Therefore, the presence of antigen-specific ASCs indicates recent antigen-driven activation of ATA-expressing B cells. This may be clinically relevant, as it may indicate that antigenic, possibly external triggers, are continuously present in some but not all ATA-positive patients. Whether SSc patients with active B cell responses may specifically benefit from B cell depleting therapy remains to be determined. However, the differences in immunological activity between ATA-positive and ACA-positive patients observed here fit well with the observation that rituximab use in patients with SSc impacts on skin and lung fibrosis, two hallmarks mainly observed in the subgroup of ATA-positive SSc. In this context, it is intriguing that we observed a strong correlation between the degree of spontaneous ATA-IgG production and the presence and severity of ILD. It is important to note that ATA-IgG in serum are well recognised as diagnostic biomarkers of SSc–ILD, but that studies addressing their role as prognostic markers for ILD progression have yielded controversial results.40–42 Our observations suggests a dynamic layer within the ATA-IgG positive stratum, and would be in line with a direct interaction between migratory ASCs homing to lung-tissue and tissue-resident fibroblasts, a concept supported by several in vitro studies.30–33 Hence, it will be important to replicate these findings in a larger cohort and, possibly, a prospective study in which the prognostic value of an active ATA B cell response can be evaluated. The lack of an association with skin fibrosis remains unclear, given that ATA-IgG serum levels have been linked to skin involvement in previous work.7 This discrepancy could relate to small sample size, different pathogenetic mechanisms of fibrosis or, possibly, triggers of the B cell response that are present in the lung but not in the skin. Also, skin involvement was relatively low in our patient groups, as we selected for ATA positivity rather than dcSSc.
The abundance of ATA-IgA-expressing ASCs raises questions as to the underlying triggers driving this response. IgA responses are classically thought to originate at mucosal sites, and it is tempting to speculate that bacterial topoisomerases could act as mucosal antigens that such cells recognise. In fact, cross-reactivity of (autoreactive) B and T cells with commensal antigens has been implicated as pathogenic mechanism initiating and driving various autoimmune responses, as has molecular mimicry.43–46 In this context, it is interesting that type 1B topoisomerases expressed by microbes in human microbiota share structural similarities with human top1.47 However, also local inflammatory responses that lead to the release of human top1 could be sufficient to break immunological tolerance, in particular if topoisomerases are bound to DNA. In addition, abundance of transforming growth factor-β (TGF-β), a mediator/amplifier of fibroblast activation, could drive IgA class switch recombination.48 49 In this respect, it will be important to dissect the top1-directed IgA response in more detail, to define its clonal relatedness to ATA-IgG and its dependency on T cell help. This might help to understand why the IgG response closely associated with fibrosis in our study, while the IgA response did not. As we did not observe a correlation between spontaneous ATA-IgG and ATA-IgA secretion in individual donors, these responses could be triggered independently. Eventually, studies addressing ATA-expressing B cells and the local microenvironment in the respiratory, skin and gastrointestinal tract will be of particular relevance. This may also help to understand whether TGF-β produced by autoreactive ASCs is relevant for amplifying and maintaining fibroblast activation in human SSc.30 31
Our study has limitations. Even though we have accessed our well-structured and detailed Leiden CCISS cohort for donor selection, the associations between ATA-IgG-expressing ASCs and pulmonary fibrosis require independent replication. This is a logistically challenging study, as the activity of the B cell response can only be determined in fresh material, as most ASCs do not survive freeze/thaw cycles. Also, as our cell culture approach addressed autoreactive B cells indirectly, the clonal relationship between the ATA-IgG and ATA-IgA responses remains unclear. To gain more detailed insight, it will be important to reliably and specifically identify and isolate ATA-expressing B cells by flow cytometry and to determine their clonal identity, for which first steps have recently been taken.50 Finally, for technical reasons related to the reliable detection of (low levels of) IgM in culture supernatants (see the Methods section), we could not address the ATA-IgM and ACA-IgM B cell compartment in our work. This will be important for a deeper understanding of processes leading to the loss of tolerance. Nonetheless, in previous work, we found that ATA-IgM serum levels associate with disease progression, which, due to the short serum half-life of IgM, is in-line with the signs of recent stimulation of the ATA B cell response noted here.7
In conclusion, we here present first evidence for differential regulation of autoreactive B cell responses in ATA-positive versus ACA-positive patients with SSc and identify intriguing signs of immunological disease activity in ATA-positive patients that relate to clinical phenotype. Such activity may be clinically meaningful for identifying patients that benefit from B cell depleting therapy and may represent an important biomarker for stratifying patients beyond the current clinical standard, that is, the presence or absence of autoantibodies.
Footnotes
Contributors: Conceptualisation and study design: CMW, CMF, TWJH, REMT, JDV-B and HUS. Data acquisition and analysis: CMW, SIEL, NMvL, MB and LS. Data interpretation: CMW, SIEL, NMvL, CMF, TWJH, REMT, JDV-B and HUS. Writing manuscript: CMW and HUS. Guarantor: HUS. All authors read and approved the manuscript.
Funding: This work was supported by Target-to-B LSHM18055-SGF (to REMT), the Dutch Arthritis Foundation 15-2-402 and 18-1-205 (to HUS) and LLP5 (to TWJH), NWO-ZonMW VENI grant 91617107 (to HUS) and NWO-ZonMW VIDI grant 09150172010067 (to HUS).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants. Permission for conduct of the study was granted by the ethical review board of Leiden University Medical Center (protocol numbers: P17.151 and P09.003/SH/sh). Participants gave informed consent to participate in the study before taking part.
References
- 1.Allanore Y, Simms R, Distler O, et al. Systemic sclerosis. Nat Rev Dis Primers 2015;1:15002. 10.1038/nrdp.2015.2 [DOI] [PubMed] [Google Scholar]
- 2.Mierau R, Moinzadeh P, Riemekasten G, et al. Frequency of disease-associated and other nuclear Autoantibodies in patients of the German network for systemic scleroderma: correlation with characteristic clinical features. Arthritis Res Ther 2011;13:R172. 10.1186/ar3495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steen VD. Autoantibodies in systemic sclerosis. Semin Arthritis Rheum 2005;35:35–42. 10.1016/j.semarthrit.2005.03.005 [DOI] [PubMed] [Google Scholar]
- 4.Vanthuyne M, Smith V, De Langhe E, et al. The Belgian systemic sclerosis cohort: correlations between disease severity scores, cutaneous Subsets, and autoantibody profile. J Rheumatol 2012;39:2127–33. 10.3899/jrheum.120283 [DOI] [PubMed] [Google Scholar]
- 5.Liaskos C, Marou E, Simopoulou T, et al. Disease-related autoantibody profile in patients with systemic sclerosis. Autoimmunity 2017;50:414–21. 10.1080/08916934.2017.1357699 [DOI] [PubMed] [Google Scholar]
- 6.Leeuwen NM, Boonstra M, Bakker JA, et al. Anti‐Centromere antibody levels and isotypes and the development of systemic sclerosis. Arthritis Rheumatol 2021;73:2338–47. 10.1002/art.41814 Available: https://onlinelibrary.wiley.com/toc/23265205/73/12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boonstra M, Bakker JA, Grummels A, et al. Association of anti-Topoisomerase I antibodies of the Igm Isotype with disease progression in anti-Topoisomerase I-positive systemic sclerosis. Arthritis Rheumatol 2020;72:1897–904. 10.1002/art.41403 Available: https://onlinelibrary.wiley.com/toc/23265205/72/11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koenig M, Joyal F, Fritzler MJ, et al. Autoantibodies and Microvascular damage are independent predictive factors for the progression of Raynaud’s phenomenon to systemic sclerosis: a twenty-year prospective study of 586 patients, with validation of proposed criteria for early systemic sclerosis. Arthritis Rheum 2008;58:3902–12. 10.1002/art.24038 [DOI] [PubMed] [Google Scholar]
- 9.Hasegawa M, Hamaguchi Y, Yanaba K, et al. B-lymphocyte depletion reduces skin fibrosis and Autoimmunity in the tight-skin mouse model for systemic sclerosis. Am J Pathol 2006;169:954–66. 10.2353/ajpath.2006.060205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshizaki A, Yanaba K, Ogawa A, et al. Immunization with DNA Topoisomerase I and Freund’s complete adjuvant induces skin and lung fibrosis and Autoimmunity via Interleukin-6 signaling. Arthritis Rheum 2011;63:3575–85. 10.1002/art.30539 [DOI] [PubMed] [Google Scholar]
- 11.Mehta H, Goulet P-O, Nguyen V, et al. Topoisomerase I peptide-loaded Dendritic cells induce autoantibody response as well as skin and lung fibrosis. Autoimmunity 2016;49:503–13. 10.1080/08916934.2016.1230848 [DOI] [PubMed] [Google Scholar]
- 12.Goswami RP, Ray A, Chatterjee M, et al. Rituximab in the treatment of systemic sclerosis-related interstitial lung disease: a systematic review and meta-analysis. Rheumatology (Oxford) 2021;60:557–67. 10.1093/rheumatology/keaa550 [DOI] [PubMed] [Google Scholar]
- 13.Matsushita T, Fujimoto M, Hasegawa M, et al. BAFF antagonist attenuates the development of skin fibrosis in tight-skin mice. J Invest Dermatol 2007;127:2772–80. 10.1038/sj.jid.5700919 [DOI] [PubMed] [Google Scholar]
- 14.Kuwana M, Medsger TA, Wright TM. T and B cell collaboration is essential for the autoantibody response to DNA Topoisomerase I in systemic sclerosis. J Immunol 1995;155:2703–14. [PubMed] [Google Scholar]
- 15.Kuwana M, Medsger TA, Wright TM. Analysis of soluble and cell surface factors regulating anti-DNA Topoisomerase I autoantibody production demonstrates synergy between Th1 and Th2 Autoreactive T cells. J Immunol 2000;164:6138–46. 10.4049/jimmunol.164.12.6138 [DOI] [PubMed] [Google Scholar]
- 16.Kuwana M, Feghali CA, Medsger TA, et al. Autoreactive T cells to Topoisomerase I in Monozygotic twins discordant for systemic sclerosis. Arthritis Rheum 2001;44:1654–9. [DOI] [PubMed] [Google Scholar]
- 17.Hildebrandt S, Weiner E, Senécal JL, et al. The IgG, Igm, and IgA isotypes of anti-Topoisomerase I and Anticentromere Autoantibodies. Arthritis Rheum 1990;33:724–7. 10.1002/art.1780330515 [DOI] [PubMed] [Google Scholar]
- 18.Kuwana M, Medsger TA, Wright TM. Detection of anti-DNA Topoisomerase I antibody by an enzyme-linked immunosorbent assay using overlapping recombinant Polypeptides. Clin Immunol Immunopathol 1995;76:266–78. 10.1006/clin.1995.1125 [DOI] [PubMed] [Google Scholar]
- 19.Mei HE, Yoshida T, Sime W, et al. Blood-borne human plasma cells in steady state are derived from Mucosal immune responses. Blood 2009;113:2461–9. 10.1182/blood-2008-04-153544 [DOI] [PubMed] [Google Scholar]
- 20.Ellebedy AH, Jackson KJL, Kissick HT, et al. Defining antigen-specific Plasmablast and memory B cell Subsets in human blood after viral infection or vaccination. Nat Immunol 2016;17:1226–34. 10.1038/ni.3533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kerkman PF, Rombouts Y, van der Voort EIH, et al. Circulating Plasmablasts/Plasmacells as a source of Anticitrullinated protein antibodies in patients with rheumatoid arthritis. Ann Rheum Dis 2013;72:1259–63. 10.1136/annrheumdis-2012-202893 [DOI] [PubMed] [Google Scholar]
- 22.Li S, Yu Y, Yue Y, et al. Autoantibodies from single circulating Plasmablasts react with Citrullinated antigens and Porphyromonas gingivalis in rheumatoid arthritis. Arthritis Rheumatol 2016;68:614–26. 10.1002/art.39455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kristyanto H, Blomberg NJ, Slot LM, et al. Persistently activated, proliferative memory Autoreactive B cells promote inflammation in rheumatoid arthritis. Sci Transl Med 2020;12:eaaz5327. 10.1126/scitranslmed.aaz5327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van den Hoogen F, Khanna D, Fransen J, et al. Classification criteria for systemic sclerosis: an American college of rheumatology/European League against rheumatism collaborative initiative. Ann Rheum Dis 2013;72:1747–55. 10.1136/annrheumdis-2013-204424 [DOI] [PubMed] [Google Scholar]
- 25.Meijs J, Schouffoer AA, Ajmone Marsan N, et al. Therapeutic and diagnostic outcomes of a standardised, comprehensive care pathway for patients with systemic sclerosis. RMD Open 2016;2:e000159. 10.1136/rmdopen-2015-000159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Desai SR, Veeraraghavan S, Hansell DM, et al. CT features of lung disease in patients with systemic sclerosis: comparison with idiopathic pulmonary fibrosis and nonspecific interstitial pneumonia. Radiology 2004;232:560–7. 10.1148/radiol.2322031223 [DOI] [PubMed] [Google Scholar]
- 27.Goh NSL, Desai SR, Veeraraghavan S, et al. Interstitial lung disease in systemic sclerosis: a simple staging system. Am J Respir Crit Care Med 2008;177:1248–54. 10.1164/rccm.200706-877OC [DOI] [PubMed] [Google Scholar]
- 28.Earnshaw W, Bordwell B, Marino C, et al. Three human Chromosomal Autoantigens are recognized by sera from patients with anti-Centromere antibodies. J Clin Invest 1986;77:426–30. 10.1172/JCI112320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simon D, Balogh P, Bognár A, et al. Reduced non-switched memory B cell Subsets cause imbalance in B cell repertoire in systemic sclerosis. Clin Exp Rheumatol 2016;34 Suppl 100:30–6. [PubMed] [Google Scholar]
- 30.Dumoitier N, Chaigne B, Régent A, et al. Scleroderma peripheral B lymphocytes Secrete Interleukin-6 and TGF-beta and activate fibroblasts. Arthritis & Rheumatology 2017;69:1078–89. 10.1002/art.40016 Available: https://onlinelibrary.wiley.com/toc/23265205/69/5 [DOI] [PubMed] [Google Scholar]
- 31.François A, Chatelus E, Wachsmann D, et al. B lymphocytes and B-cell activating factor promote collagen and Profibrotic markers expression by Dermal fibroblasts in systemic sclerosis. Arthritis Res Ther 2013;15:R168. 10.1186/ar4352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heukels P, van Hulst JAC, van Nimwegen M, et al. Enhanced Bruton’s tyrosine kinase in B-cells and Autoreactive IgA in patients with idiopathic pulmonary fibrosis. Respir Res 2019;20:232. 10.1186/s12931-019-1195-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Della-Torre E, Rigamonti E, Perugino C, et al. B lymphocytes directly contribute to tissue fibrosis in patients with IgG4-Related disease. J Allergy Clin Immunol 2020;145:968–81. 10.1016/j.jaci.2019.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keir GJ, Maher TM, Ming D, et al. Rituximab in severe, treatment-refractory interstitial lung disease. Respirology 2014;19:353–9. 10.1111/resp.12214 [DOI] [PubMed] [Google Scholar]
- 35.Xue J, Kass DJ, Bon J, et al. Plasma B lymphocyte Stimulator and B cell differentiation in idiopathic pulmonary fibrosis patients. The Journal of Immunology 2013;191:2089–95. 10.4049/jimmunol.1203476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Todd NW, Scheraga RG, Galvin JR, et al. Lymphocyte aggregates persist and accumulate in the lungs of patients with idiopathic pulmonary fibrosis. J Inflamm Res 2013;6:63–70. 10.2147/JIR.S40673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harrison NK, Myers AR, Corrin B, et al. Structural features of interstitial lung disease in systemic sclerosis. Am Rev Respir Dis 1991;144:706–13. 10.1164/ajrccm/144.3_Pt_1.706 [DOI] [PubMed] [Google Scholar]
- 38.Lafyatis R, O’Hara C, Feghali-Bostwick CA, et al. B cell infiltration in systemic sclerosis-associated interstitial lung disease. Arthritis Rheum 2007;56:3167–8. 10.1002/art.22847 [DOI] [PubMed] [Google Scholar]
- 39.Jenks SA, Cashman KS, Woodruff MC, et al. Extrafollicular responses in humans and SLE. Immunol Rev 2019;288:136–48. 10.1111/imr.12741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Assassi S, Sharif R, Lasky RE, et al. Predictors of interstitial lung disease in early systemic sclerosis: a prospective longitudinal study of the GENISOS cohort. Arthritis Res Ther 2010;12:R166. 10.1186/ar3125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inoue Y, Kaner RJ, Guiot J, et al. Diagnostic and Prognostic biomarkers for chronic Fibrosing interstitial lung diseases with a progressive phenotype. Chest 2020;158:646–59. 10.1016/j.chest.2020.03.037 [DOI] [PubMed] [Google Scholar]
- 42.Distler O, Gahlemann M, Maher TM. Nintedanib for systemic sclerosis-associated interstitial lung disease. reply. N Engl J Med 2019;381:1596–7. 10.1056/NEJMc1910735 [DOI] [PubMed] [Google Scholar]
- 43.Greiling TM, Dehner C, Chen X, et al. Commensal Orthologs of the human Autoantigen Ro60 as triggers of Autoimmunity in lupus. Sci Transl Med 2018;10:eaan2306. 10.1126/scitranslmed.aan2306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruff WE, Dehner C, Kim WJ, et al. Pathogenic Autoreactive T and B cells cross-react with Mimotopes expressed by a common human gut Commensal to trigger Autoimmunity. Cell Host Microbe 2019;26:100–13. 10.1016/j.chom.2019.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lanz TV, Brewer RC, Ho PP, et al. Clonally expanded B cells in multiple sclerosis bind EBV Ebna1 and Glialcam. Nature 2022;603:321–7. 10.1038/s41586-022-04432-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ang CW, Jacobs BC, Laman JD. The Guillain-Barré syndrome: a true case of molecular Mimicry. Trends Immunol 2004;25:61–6. 10.1016/j.it.2003.12.004 [DOI] [PubMed] [Google Scholar]
- 47.Krogh BO, Shuman S. A poxvirus-like type IB topoisomerase family in bacteria. Proc Natl Acad Sci U S A 2002;99:1853–8. 10.1073/pnas.032613199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coffman RL, Lebman DA, Shrader B. Transforming growth factor beta specifically enhances IgA production by Lipopolysaccharide-stimulated murine B lymphocytes. J Exp Med 1989;170:1039–44. 10.1084/jem.170.3.1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sonoda E, Matsumoto R, Hitoshi Y, et al. Transforming growth factor beta induces IgA production and acts Additively with interleukin 5 for IgA production. J Exp Med 1989;170:1415–20. 10.1084/jem.170.4.1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fukasawa T, Yoshizaki A, Ebata S, et al. Single-cell-level protein analysis revealing the roles of Autoantigen-reactive B lymphocytes in autoimmune disease and the murine model. Elife 2021;10:e67209. 10.7554/eLife.67209 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2023-003148supp001.pdf (1.9MB, pdf)
Data Availability Statement
Data are available upon reasonable request.